
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 26 February 2019
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.00366
Modulation of the membrane permeability through a decrease in porin-mediated antibiotic entry and/or an increase in antibiotic efflux is one of the resistance mechanisms to antibiotics evolved by Gram-negative bacteria. To assess whether the outer membrane porin OprD and Resistance-Nodulation-Division (RND) efflux pumps were similarly expressed in 33 ciprofloxacin-resistant clinical strains of Pseudomonas aeruginosa and in 30 non-clinical strains originating from the hospital environment (mainly waterborne Pseudomonas aeruginosa), the expression of oprD, mexB, mexF, and mexY genes was investigated. Overall, the expression of oprD was not detected by RT-qPCR in 14 (22%) strains and underexpressed in 35 (56%) more. No significant difference in oprD expression was detected between clinical and non-clinical strains. As for efflux pumps, 23 (70%) of the clinical strains overexpressed at least one of the tested RND genes. Overexpression of mexB, mexF and mexY was detected in 27, 12, and 45% of the clinical strains, respectively. In the 30 non-clinical strains, no overexpression could be found for mexB, mexF, or mexY. On the contrary, a global underexpression of the tested efflux pump genes was recorded. In both clinical and environmental strains, a positive correlation was found between the expressions of oprD and mexB. Similarly, the expressions of oprD and mexF were positively correlated. This result contrasts with the inverse correlation between both MexAB-OprM/MexEF-OprN and OprD previously described in carbapenem-resistant P. aeruginosa strains. The only positive correlation between phenotypic ciprofloxacin minimum inhibitory concentrations (MICs) and the expression of efflux pump gene was witnessed with mexY (analysis on pooled results for clinical and environmental strains). However, in clinical strains, no statistically significant link could be found between the degree of reduction in ciprofloxacin MICs witnessed with Phenylalanine-Arginine β-naphthylamide (PAβN) and the expression of any of the 3 RND genes tested.
Pseudomonas aeruginosa is a Gram-negative ubiquitous microorganism. It can be found in various environmental ecological niches (e.g., water, soil, and plants) but can also infect humans (Botzenhart and Döring, 1993). It more specifically affects individuals with impaired defenses such as patients with severe burns, cancer or cystic fibrosis (Gellatly and Hancock, 2013). P. aeruginosa is innately resistant to a large number of commercially available antibiotics (Poole, 2011) and, like most other species of bacteria, has acquired a wide array of resistance mechanisms, tremendously complicating the clinical handling of P. aeruginosa infections and leading to the emergence of so-called pan resistant strains (Bonomo and Szabo, 2006; Poole, 2011). Modification of the membrane permeability is one of the mechanisms by which Gram-negative bacteria can decrease their susceptibility to antibiotics, by a reduction in the antibiotic entry through outer membrane porins and/or an increase in antibiotic efflux through efflux pumps (EPs). For example, in P. aeruginosa, mutations in the porin OprD can lead to the resistance to carbapenems (Xia et al., 2016; Del Barrio-Tofiño et al., 2017). Overexpression of EPs belonging to the Resistance-Nodulation-Division (RND) family, i.e., MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY-OprM, has also been pointed out as a resistance mechanism toward various antibiotic families such as β-lactams, aminoglycosides or fluoroquinolones (FQ) (Poole, 2011). Additionally, an inverse association between the expressions of oprD and genes encoding RND EPs has been pointed out in carbapenem-resistant P.aeruginosa strains (Köhler et al., 1999; Ikonomidis et al., 2008; Xavier et al., 2010; Lee and Ko, 2012). It was demonstrated that MexT, a positive regulator of mexEF-OprN expression, negatively regulates the expression of OprD in carbapenem-resistant strains (Köhler et al., 1999).
RND EPs are constituted by three proteins: the first one is acting as an outer membrane channel [a protein belonging to the outer membrane factor (OMF) family, e.g., OprM], the pump itself is embedded in the cytoplasmic membrane (the actual RND protein of the pump, e.g., MexB) and, in the periplasmic space, a linker protein is joining the first two proteins [this protein is also referred to as the major facilitator protein (MFP), e.g., MexA] (Piddock, 2006). These pumps are involved in P. aeruginosa physiological functions such as quorum-sensing (Minagawa et al., 2012), biofilm formation (Alav et al., 2018) and virulence factors (Blair and Piddock, 2009; El-Shaer et al., 2016). Expression and overexpression have been described when a mutation occurs in the repressor/activator gene sequences commanding the operons encoding EPs (Poole, 2011; López-Causapé et al., 2018). Useful details on the systems regulating EP expression in P. aeruginosa can be found in reviews such as Poole (2011) and Morita et al. (2012). The aim of the present work was to assess the expression of OprD and RND EPs was different in clinical strains resistant to ciprofloxacin (CIP), a molecule belonging to the fluoroquinolone (FQ) family and in P. aeruginosa strains retrieved from non-clinical (mainly environmental) samples isolated from a hospital background. Phenotypic resistance to CIP was chosen as a tracker for the overexpression of EPs as FQs are good substrates for the four major RND pumps found in P. aeruginosa (Poole, 2011). Assessing whether the CIP phenotypic resistance profile of P. aeruginosa strains could be related to the expression of one or several genes encoding for the major RND EPs could be helpful to evaluate the in vitro efficacy of new potential efflux pump inhibitors on relevant clinical strains resistant to this molecule.
Thirty-three clinical strains of P. aeruginosa resistant to ciprofloxacin (CIP) isolated from 33 patients of Amiens University Hospital (France) were collected over a 5-month period and included in this study. The selection of these clinical strains was made by screening the results of all in-coming clinical samples (no specific unit or ward targeted) and retaining P. aeruginosa strains detected as resistant to CIP (CIP-R) by the disk diffusion method used routinely. If multiple samples yielded a ciprofloxacin-resistant strain for a given subject, only one isolate was included in the panel of strains. Resistance was confirmed through minimum inhibitory concentration (MIC) determination. MICs were determined over a range of 0.0625 to 128 μg/mL on 96-microwell plates using the broth microdilution technique in cation-adjusted Mueller-Hinton (CLSI, 2015). Resistance to CIP was determined for MICs of 1 μg/mL and above, according to EUCAST latest breakpoints (European Committee on Antimicrobial Susceptibility Testing [EUCAST], 2018).
Environmental strains consisted of P. aeruginosa strains previously isolated from non-clinical samples and retrieved from the culture collection of Amiens University Hospital as well as strains prospectively obtained over a 2-year period (May 2016–May 2018) from water samples systematically collected as part of the program for water quality monitoring in Amiens University Hospital and surrounding hospitals. This program includes the detection of P. aeruginosa on a cetrimide agar (Biomérieux, Marcy-l’Etoile, France) according to the current ISO guidelines (EN ISO 16266:2008, 2008) and is based on a bi-annual sampling of critical control points of the hospital water supply system. Overall, 208 critical control points have been identified through an internal risk analysis and are sampled routinely.
A target number of 30 strains for each group (clinical and non-clinical) was set at the beginning of the study. While this target was easily met for clinical strains, the prospective collection of environmental strains originating from the water quality monitoring program was slow going. As only five P. aeruginosa strains had been isolated at the end of 2016, it was decided to include P. aeruginosa strains previously isolated from the environment and as well as non-clinical samples that had been kept in the laboratory collection in addition to the prospective collection of such strains. A total of 30 non-clinical strains was reached in May 2018.
Two strains were considered for the role of reference (calibrator) in RT-qPCR assays: a strain (AM19) from the same clinical background as CIP-R strains but susceptible to CIP and P. aeruginosa DSM (Deutsche Sammlung für Mikroorganismen und Zellkulturen, Braunschweig, Germany) 1117/ATCC (American Type Culture Collection) 27853, a reference P. aeruginosa strain used as quality control in antimicrobial susceptibility testing (Fang et al., 2012). All strains were kept frozen at -80°C on microbeads (WVR International SAS, Fontenay-sous-Bois, France) until use.
PAβN (Sigma-Aldrich, Saint-Quentin-Fallavier, France) has been described as an efflux pump inhibitor (EPI) affecting MexAB-OprM, MexCD-OprJ and MexEF-OprN EPs (Lomovskaya et al., 2001) and has also been shown to reduce levofloxacin MIC in a P. aeruginosa strain with an increased MexXY activity (Mao et al., 2001). This molecule was subsequently used as such to phenotype-wise determine the prevalence of efflux pump-mediated resistance to fluoroquinolones in P. aeruginosa. It was added to the standard MIC determination procedure at a fixed final concentration of 50 μg/mL (Sonnet et al., 2012). MIC determination for PAβN was also carried out for all strains over concentrations ranging from 0.1 to 200 μg/mL, to ascertain that the 50 μg/mL concentration used for EP inhibition was not inhibitory in itself. The phenotypic efflux was deemed significant when the MIC determined in the presence of PAβN was at least fourfold lower than the MIC witnessed in its absence (Piddock, 2006; Sonnet et al., 2012), leading to a calculated phenotypic efflux factor (MIC without PAβN/MIC with PAβN) of four and above.
Strains were cultured in cation adjusted Mueller-Hinton Broth (Merck, Darmstadt, Germany) for 18 to 24 h at 37°C up to the late exponential phase prior to EP expression determination. Total RNA extraction was carried out using the Qiagen RNeasy purification kit with the addition of RNAprotect Bacteria Reagent (Qiagen, Courtaboeuf, France). Briefly, 1 mL of culture medium was centrifuged (8000 g, 5 min), the bacterial pellet resuspended in 1 mL of RNAprotect reagent and left to incubate at room temperature for 5 min. After decantation of the RNAprotect reagent (8000 g, 10 min), 200 μL of Tris 30 mM-EDTA 1 mM (pH 8.0) buffer containing lysozyme (15 mg/mL) and proteinase K (0.56 mg per strain) were added and incubated for 10 min at room temperature to ensure a complete bacterial cell wall lysis. Further precipitation and purification of nucleic acids were performed according to the manufacturer’s recommendations and the final elution step carried out using 50 μL RNase-free water. After checking the RNA extraction quality on a 1% agarose gel and measuring the RNA content (Nanodrop, ThermoFisher Scientific, France), RNA extracts were stored at -20°C until further use.
Prior to cDNA synthesis, genomic DNA was removed from 1 μg of total RNA using the gDNA wipeout buffer included in the Quantitect® Reverse Transcription kit (Qiagen). The reverse transcription was performed under a volume of 20 μL including 14 μL of template RNA (extract concentrations adjusted to contain 1 μg of RNA), 1 μL of reverse transcription master mix, 4 μL RT buffer 5x (containing dNTPs and Mg2+) and 1 μL of RT primer mix containing random hexamers. Reverse transcription was performed in a Veriti PCR Thermal Cycler (Applied Biosystems, France) for 30 min at 42°C followed by a 3 min incubation at 95°C to inactivate the reverse transcriptase.
All reactions including RNA handling were carried out on ice.
Primers used in this study were designed using the Primer3 software available online1 and are listed in Table 1. As the genes encoding EP components are organized in operons (Poole et al., 1993; Morita et al., 2001), this work focused on the expression of oprD and mexB, mexF, mexY genes encoding the actual efflux pump protein of the three main RND EPs found in P. aeruginosa.
Normalization of expression results was carried out using rpsL (Llanes et al., 2004; Yoneda et al., 2005).
A Lightcycler 480 (Roche Diagnostics, Meylan, France) was used for all quantitative PCRs. All PCR amplification reactions were performed in 384-well-plates under a 10 μL final volume containing 2.5 μL of diluted (1:20) template cDNA, 1 μL of each primer (corresponding to a final concentration of 0.5 μM), 5 μL of Quantitect SYBR Green PCR Master Mix (including MgCl2 to reach to final concentration of 2.5 mM) (Qiagen) and 0.5 μL RNase/DNase free water (Qiagen).
The cycling program was set as follows : (1) activation : 1 cycle at 95°C for 15 min, (2) amplification : 45 cycles including a 15-s denaturation at 95°C, a 25-s annealing at 60°C and a 15-s elongation at 72°C, and (3) melting curve : 1 cycle including 5 s at 95°C, 1 min at 65°C and a final increase at 97°C with a transition rate of 0.11°C/s.
Each reaction was carried out in duplicate and the experiment was repeated on two different sets of RNA extracts (biological replicate).
Relative standard curves describing the PCR efficiency (E) for each primer pair were generated for each amplicon (Larionov et al., 2005).
Prior to any other analysis, melting curves were checked for the presence of primer dimers and other artifacts. The relative expression between the target and reference genes, was calculated using the formula (E_goi∧(ΔCt,goi))/(E_ref∧(ΔCt,ref)), where E stands for the PCR efficiency factor, goi for gene of interest and ref for reference.
Results were subsequently expressed as Normalized Calibrated Ratios (NCRs).
Differences in the expression of each gene of interest were tested using the single sample t-test vs. cut-off values of 0.5 for underexpression and 2 for overexpression, respectively (Tomás et al., 2010). Differences in gene detection and expressions between clinical and non-clinical/environmental strains were assessed using Fisher’s exact test.
Correlations between gene expressions, MICs with and without PAβN as well as with the MIC reduction factor were tested using Spearman’s correlation coefficient.
Statistical significance was inferred for p-values below 0.05.
The statistical analysis was performed using VassarStats website2 and XLstat 2015 software (Addinsoft, France).
As strains were isolated from clinical samples routinely prescribed to patients by the medical staff in charge and as no specific sampling in order to collect P. aeruginosa strains was performed, no evaluation by the local ethics committee was sought. Additionally, written informed consent from the patients was not needed as this work was of a retrospective nature and as no information that could be linked to patients was included.
Tables 2, 3 summarize the origins, MICs with and without PAβN and the resulting calculated phenotypic efflux factor for each of the 33 CIP-R clinical strains and 30 non-clinical/environmental strains evaluated in this work, respectively. Only 3 (10%) these non-clinical/environmental strains were classified as resistant to CIP with a MIC of 1 μg/mL according to EUCAST 2018 standards (European Committee on Antimicrobial Susceptibility Testing [EUCAST], 2018).
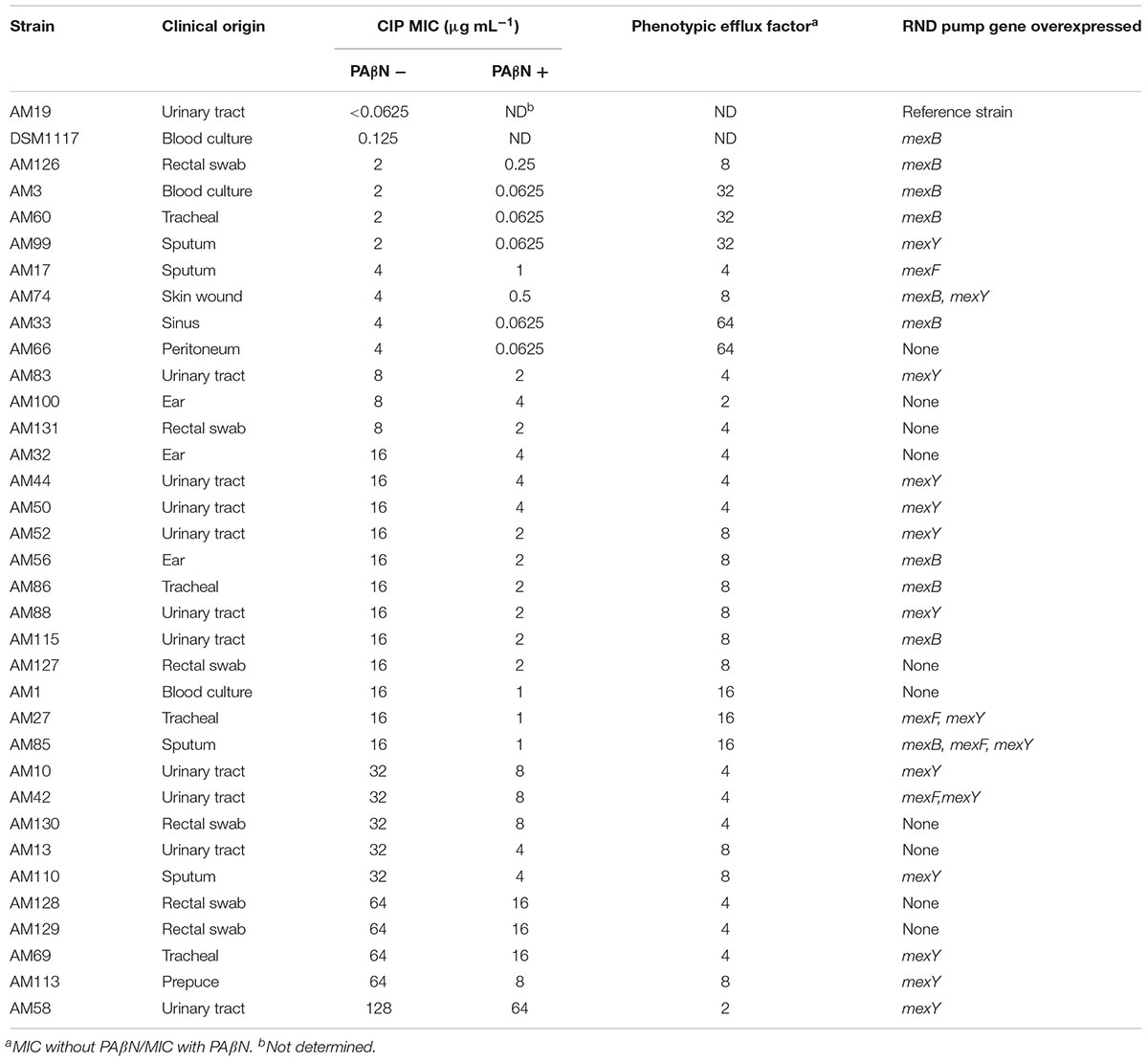
Table 2. Characteristics of the clinical strains included in this study, classified by increasing Ciprofloxacin (CIP) MIC.
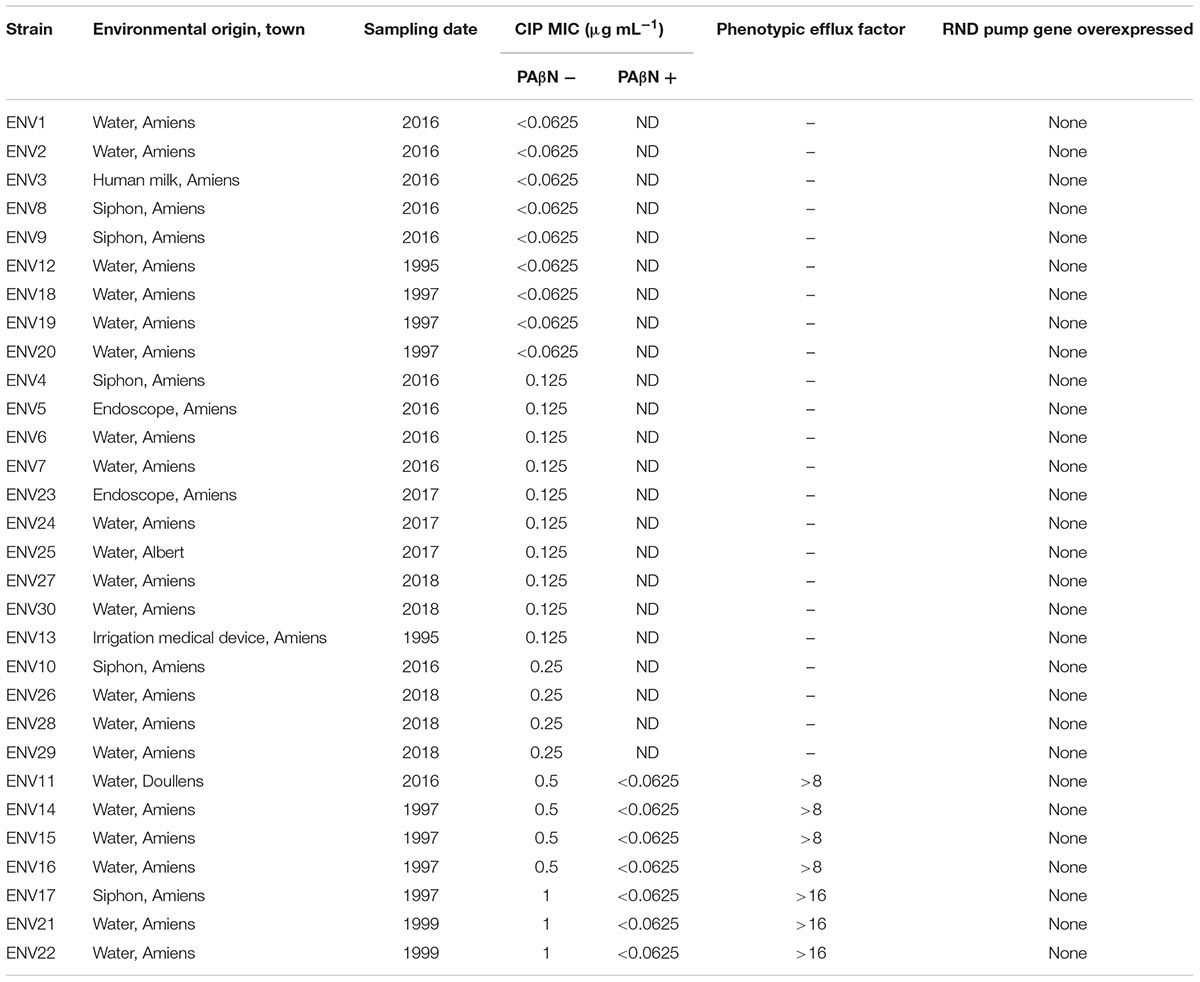
Table 3. Characteristics of non-clinical/environmental strains included in this study, classified by increasing Ciprofloxacin (CIP) MIC.
Most (31/33, ∼94%) CIP-R clinical strains displayed a phenotypic efflux factor of four or above (Table 2). All threeCIP-R environmental strains had a phenotypic efflux factor above 16 (Table 3). The lower phenotypic efflux factor witnessed in clinical strains could be explained by a resistance only partly mediated through an increased efflux. Indeed, the higher MICs (up to 128 μg/mL) in clinical strains might be linked to cumulative mutations in CIP targets, DNA gyrase and topoisomerase IV proteins, as described previously (Bruchmann et al., 2013; Rehman et al., 2019). In the environmental strains, efflux could be the sole mechanism supporting the rise of the MIC up to 1 μg/mL. Determination of QRDR mutation frequencies in the environmental strains included in this study would have reinforced this hypothesis. Nevertheless, the study by Bruchmann et al. (2013) has already shown that most QRDR mutations lead to CIP MICs equal or superior to 1 μg/mL while strains only overexpressing EPs displayed a maximum MIC of 2 μg/mL. Our results are therefore in line with this report. Also, they are in agreement with a theory stating that the overexpression of EPs could act as a first-step mutation, leading to subsequent ones in DNA gyrase and topoisomerase IV proteins and higher levels of CIP resistance (Köhler et al., 1997; Vila and Martínez, 2008).
First, gene expression was compared between the two CIP-susceptible strains included in this work as possible calibrators for NCR calculations. P. aeruginosa DSM1117, a clinical strain from a culture collection used in antibiotic testing (European Committee on Antimicrobial Susceptibility Testing [EUCAST], 2018), displayed a significant overexpression of mexB and underexpression of mexY as compared to the clinical strain AM 19 (Supplementary Table S1). The reasons for choosing P. aeruginosa AM19 over P. aeruginosa DSM1117 as a calibrator in our calculations were that (i) AM19 came from a similar background as the other strains enrolled in this study and (ii) oprD expression was not detected in P. aeruginosa DSM1117 and would therefore not allow the calculation of NCRs for this gene. Thereafter, individual NCRs were calculated as mean ± sem (standard error of the mean) for each strain, using P. aeruginosa AM19 as calibrator (Supplementary Table S1). If P. aeruginosa DSM1117 had been chosen as a calibrator, the potential differences in NCRs results for EP genes would have been (i) a greater frequency of overexpression for mexY gene in clinical strains, as it was underexpressed in P. aeruginosa DSM1117 as compared to P. aeruginosa AM19, and (ii) a lower frequency of mexB overexpression in clinical strains (Supplementary Table S1). As for non-clinical strains, the results would have remained similar, EP gene expressions being overall low among these strains.
At least one EP was overexpressed in 23 (70%) out of the 33 clinical strains included in this work (Table 2). One could argue that the mRNA expression level for a given gene is not directly related to the actual amount of protein produced by the bacteria. However, Yoneda et al. (2005) previously showed a good correlation between the amounts of MexY and MexB proteins and their respective mRNA expressions.
Hocquet et al. (2007) reported a simultaneous overexpression of MexAB-OprM and MexXY in a set of ticarcillin-resistant clinical strains originating from 15 French hospitals. No such joint overexpression was witnessed for the CIP-R strains in this study. Only three clinical strains overexpressed two EPs simultaneously (mexF/mexY for two strains and mexB/mexY for one). Additionally, a single clinical strain overexpressed all three RND genes tested in this work (Table 2).
A high prevalence of mexY overexpression was witnessed in the series of clinical strains (Table 4). MexXY expression has been shown to be regulated by repressor MexZ but also by an independent two-component system, ParRS (Morita et al., 2012). The frequent overexpression of mexY witnessed here might therefore be linked to mutation(s) in this repressor sequence and/or the triggering of ParRS system. MexAB-OprM appeared to be either up- or down-regulated (Table 4). Similar results were reported in multidrug-resistant strains of P. aeruginosa in Bulgaria (Vatcheva-Dobrevska et al., 2013). Only four clinical strains were found to overexpress MexEF-OprN (Table 3), ruling out its role as a major contributor to CIP efflux mediated resistance.
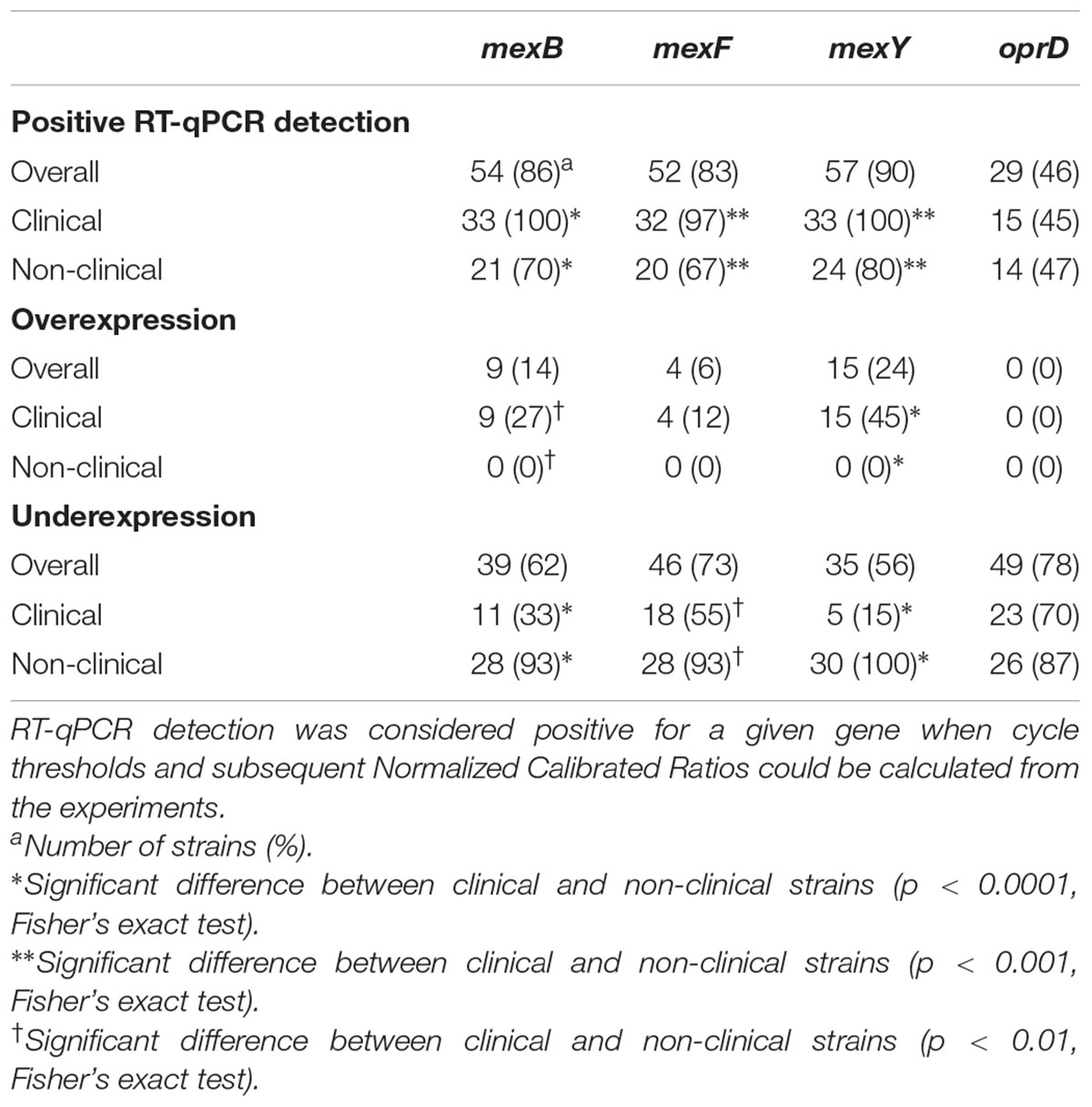
Table 4. Efflux pump genes and oprD detection and expression in clinical (n = 33) and non-clinical (n = 30) Pseudomonas aeruginosa strains isolated in this study.
A positive correlation was found between mexB and mexF expressions (Pearson’s r = 0.557, p = 0.001). This result is at odds with regulation pathways previously described. Indeed, in addition to mutations in its negative regulators MexR, NalC, and NalD, the overexpression of MexB has been linked with quorum-sensing signaling (Maseda et al., 2004; Poole, 2011). Quorum-sensing autoinducers were shown to be implied in an inverse regulation of mexB and mexF expressions along with MexR and MexT regulators (Maseda et al., 2004). However, the interplay between mexF and mexB might not be so straightforward as (i) other reports also describe a simultaneous overexpression of MexAB-OprM and MexEF-OprN (Tomás et al., 2010) and (ii) other regulation pathways might be implied such as those including MexS, another regulator of MexEF-OprN (Uwate et al., 2013).
The results for MexCD-OprJ were not included in this paper, as preliminary results for mexD expression in clinical strains showed that none of the strains displayed changes in the expression of this gene.
In contrast to clinical strains, none of the environmental strains overexpressed any EP (Tables 3, 4 and Supplmentary Table S1). Expression of RND EP genes in environmental P. aeruginosa strains is seldom reported in the literature (Braz et al., 2016; Maravić et al., 2018). These studies showed that MexAB-OprM overexpression was linked to resistance to aztreonam and other β-lactams in P. aeruginosa strains isolated from agricultural soils and marine shellfish, respectively. They showed environmental strains can display features predisposing them to antibiotic resistance, which could, in turn, pose a threat to human and environmental health. This was not the case for the environmental strains included in this study.
Only about 50% of the tested strains displayed detectable levels of oprD mRNA (Table 4). No significant difference was found in the distribution of oprD expression profiles between clinical and non-clinical strains (Table 4). Positive correlations were found with both mexB and mexF expressions (i) in clinical strains: mexB/oprD (Pearson’s r = 0.417, p = 0.016) and mexF/oprD (r = 0.476, p = 0.005) and (ii) in environmental strains: mexB/oprD (Pearson’s r = 0.494, p = 0.006) and mexF/oprD (r = 0.649, p < 0.001, respectively).
An oprD repression has previously been reported as concomitant to MexAB-OprM, MexXY-OprM and MexEF-OprN overexpression in P. aeruginosa strains resistant to various β-lactams (Köhler et al., 1999; Ikonomidis et al., 2008; Xavier et al., 2010). Additionally, the link between mexB and oprD expressions in β-lactam resistant strains has been suggested to reflect an external pressure, such as antibiotic exposure, affecting both systems (Horna et al., 2018). However, the statistical significance of a possible correlation between these EPs and OprD expression remains to be ascertained in strains selected on the basis of other antibiotic resistances. For instance, in this study, no repression of oprD expression could be witnessed either in the CIP-R clinical P. aeruginosa strains when EPs are overexpressed.
As the growth stage has been shown to influence the expression levels of EP genes (Mesaros et al., 2007), P. aeruginosa strains were all grown up to the late exponential phase prior to RNA extraction to minimize variations and standardize culture conditions. This step insured the relevance of the correlation drawn between MICs and gene expression levels as MICs are classically determined after an 18 to 24-h incubation. CIP was used as a marker to detect efflux-mediated resistance to antibiotics as it has been shown to be a substrate for various EPs (e.g., NorA for Staphylococcus aureus, OqxAB for Klebsiella pneumoniae, AcrAB-TolC for Escherichia coli, AdeABC for Acinetobacter baumannii), including P. aeruginosa RND ones (Poole, 2005). When clinical and non-clinical strains were pooled together, CIP MICs were loosely correlated with mexY expression (Pearson’s r = 0.400, p = 0.001). When the statistical analysis was held on clinical and environmental strains separately, no correlation between the expression of EP genes and MICs could be pointed out. For CIP-R clinical strains, a likely explanation for this lack of correlation is the presence of co-existing mutations in gyrase and topoisomerase IV proteins (López-Causapé et al., 2018). Additionally, other parameters might also contribute to this lack of correlation such as resistance to aminoglycosides and/or β-lactams. Indeed, these antibiotic families are also known to be related to EPs’ expression (Poole, 2011). It would therefore be interesting to further evaluate aminoglycosides and carbapenem resistances in these strains and the presence/absence of correlation with their expression of EPs.
To address a possible correlation between the reduction in CIP MICs in the presence of PAβN and EP expression, non-clinical strains were removed from the analysis as their initial low CIP MICs would not allow for a significant reduction (Table 3). Hence, for clinical strains, no statistically significant correlations between EP expression, the MIC reduction or the phenotypic efflux were detected. Therefore, CIP MICs in the presence of PAβN are not predictive of the overexpression of RND EPs in P. aeruginosa. Interfering factors such as a variable expression for other EPs and/or a decrease in MIC with PAβN not entirely linked with efflux inhibition could explain this lack of correlation. Lamers et al. (2013) indeed put forward that PAβN not only blocks EPs but also permeabilizes the outer membrane. In these conditions, entry of large antibiotics such as vancomycin and macrolides are facilitated. Such might also be the case for CIP (even though it is a smaller molecule which would not require outer membrane permeabilization to enter), hence further contributing to a reduction in apparent MICs. This might also explain why a high phenotypic efflux factor could be calculated for the three CIP-R environmental when no RND EP overexpression was detected in those strains. Nevertheless, this work enabled the description of clinical strains with various profiles of RND EP overexpression that will be used as laboratory tools for the evaluation of newly synthesized candidates for efflux pump inhibition.
To gain further insight in the relationships between the expression of RND EPs and phenotypic resistance to CIP in environmental strains, the collection of additional strains displaying such a resistance would be of interest as well as collecting strains from different hospitals to broaden the conclusions drawn from the results of this first study. It would also be interesting to investigate whether additional CIP susceptible clinical strains share the expression profile RND EPs’ of their resistant counterparts or if their profile is closer to the one of environmental strains.
The results obtained on the 33 clinical strains pertaining to this study showed that the main contributor to efflux-mediated CIP resistance was the MexXY EP. MexAB-OprM was either over- or under-expressed in clinical strains. P. aeruginosa strains isolated from Amiens hospital environment did not frequently display a high-level resistance to ciprofloxacin. However, the low-level resistance witnessed for some strains could not be linked with an overexpression of RND EPs. Indeed, RND EPs were significantly underexpressed in nearly all of the 30 non-clinical strains tested in this work. In contrast with what was previously described in carbapenem-resistant strains, a positive correlation between the expression of oprD and efflux pump genes mexB and mexF was witnessed in both CIP-R clinical environmental P. aeruginosa strains. A correlation limited to mexY expression was nevertheless witnessed for CIP MICs in the statistical analysis gathering the 63 clinical and environmental strains. However, no link with a specific EP component could be ascribed for the magnitude of the reduction in MICs generated by PAβN in the clinical strains. Therefore, no direct quantitative correlation can be made between this phenotypic trait and the genotypic overexpression of one or another of the three RND EPs tested here.
CS, BB, SG, and CM performed the experiments. SG and CM analyzed data. AT-M and CM contributed strains. CM conceived and supervised the experiments and wrote the manuscript.
CS benefited from an ERASMUS grant; BB and this work were partly supported by PHC Tassili grant 16MDU974/35118ZF. The Université de Picardie Jules Verne partly supported the financial cost of this publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00366/full#supplementary-material
Alav, I., Sutton, J. M., and Rahman, K. M. (2018). Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 73, 2003–2020. doi: 10.1093/jac/dky042
Blair, J. M., and Piddock, L. J. (2009). Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr. Opin. Microbiol. 12, 512–519. doi: 10.1016/j.mib.2009.07.003
Bonomo, R. A., and Szabo, D. (2006). Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 43, S49–S56. doi: 10.1086/504477
Botzenhart, K., and Döring, G. (1993). “Ecology and epidemiology of Pseudomonas aeruginosa,” in Pseudomonas aeruginosa as an opportunistic pathogen, eds M. Campa, M. Bendinelli, and H. Friedman (New York, NY: Plenum Press), 1–18.
Braz, V. S., Furlan, J. P., Fernandes, A. F., and Stehling, E. G. (2016). Mutations in NalC induce MexAB-OprM overexpression resulting in high level of aztreonam resistance in environmental isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 363:fnw166. doi: 10.1093/femsle/fnw166
Bruchmann, S., Dötsch, A., Nouri, B., Chaberny, I. F., and Häussler, S. (2013). Quantitative contributions of target alteration and decreased drug accumulation to Pseudomonas aeruginosa fluoroquinolone resistance. Antimicrob. Agents Chemother. 57, 1361–1368. doi: 10.1128/AAC.01581-12
CLSI. (2015). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard CLSI document M07-A10, 10th Edn, Wayne, PA: Clinical and Laboratory Standards Institute.
Del Barrio-Tofiño, E., López-Causapé, C., Cabot, G., Rivera, A., Benito, N., Segura, C., et al. (2017). Genomics and susceptibility profiles of extensively drug-resistant Pseudomonas aeruginosa isolates from Spain. Antimicrob. Agents Chemother. 61:e1589-17. doi: 10.1128/AAC.01589-17
El-Shaer, S., Shaaban, M., Barwa, R., and Hassan, R. (2016). Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl β-naphthylamide. J. Med. Microbiol. 65, 1194–1204. doi: 10.1099/jmm.0.000327
EN ISO 16266:2008 (2008). Water Quality- Detection and Enumeration of Pseudomonas aeruginosa. Method by Membrane Filtration. Geneva: ISO.
European Committee on Antimicrobial Susceptibility Testing [EUCAST] (2018). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.1, 2018. Available at: http://www.eucast.org [accessed June 11, 2018].
Fang, X., Fang, Z., Zhao, J., Zou, Y., Li, T., Wang, J., et al. (2012). Draft genome sequence of Pseudomonas aeruginosa strain ATCC 27853. J. Bacteriol. 194:3755. doi: 10.1128/JB.00690-12
Gellatly, S. L., and Hancock, R. E. (2013). Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog. Dis. 67, 159–173. doi: 10.1111/2049-632X.12033
Hocquet, D., Roussel-Delvallez, M., Cavallo, J. D., and Plésiat, P. (2007). MexAB-OprM and MexXY-overproducing mutants are very prevalent among clinical strains of Pseudomonas aeruginosa with reduced susceptibility to ticarcillin. Antimicrob. Agents Chemother. 51, 1582–1583. doi: 10.1128/AAC.01334-06
Horna, G., López, M., Guerra, H., Saénz, Y., and Ruiz, J. (2018). Interplay between MexAB-OprM and MexEF-OprN in clinical isolates of Pseudomonas aeruginosa. Sci. Rep. 8:16463. doi: 10.1038/s41598-018-34694-z
Ikonomidis, A., Tsakris, A., Kantzanou, M., Spanakis, N., Maniatis, A. N., and Pournaras, S. (2008). Efflux system overexpression and decreased OprD contribute to the carbapenem heterogeneity in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 279, 36–39. doi: 10.1111/j.1574-6968.2007.00997.x
Köhler, T., Epp, S. F., Curty, L. K., and Pechère, J. C. (1999). Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 181, 6300–6305.
Köhler, T., Michea-Hamzehpour, M., Plésiat, P., Kahr, A. L., and Pechere, J. C. (1997). Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41, 2540–2543. doi: 10.1128/AAC.41.11.2540
Lamers, R. P., Cavallari, J. F., and Burrows, L. L. (2013). The efflux inhibitor phenylalanine-arginine beta-naphthylamide (PA(N) permeabilizes the outer membrane of Gram-negative bacteria. PLoS One 8:e60666. doi: 10.1371/journal.pone.0060666
Larionov, A., Krause, A., and Miller, W. (2005). A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6:62. doi: 10.1186/1471-2105-6-62
Lee, J. Y., and Ko, K. S. (2012). OprD mutations and inactivation, expression of efflux pumps and AmpC, and metallo-β-lactamases in carbapenem-resistant Pseudomonas aeruginosa isolates from South Korea. Int. J. Antimicrob. Agents 40, 168–172. doi: 10.1016/j.ijantimicag.2012.04.004
Llanes, C., Hocquet, D., Vogne, C., Benali-Baitich, D., Neuwirth, C., and Plésiat, P. (2004). Clinical strains of Pseudomonas aeruginosa overproducing MexAB-OprM and MexXY efflux pumps simultaneously. Antimicrob. Agents Chemother. 48, 1797–1802. doi: 10.1128/AAC.48.5.1797-1802.2004
Lomovskaya, O., Warren, M. S., Lee, A., Galazzo, J., Fronko, R., Lee, M., et al. (2001). Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45, 105–116. doi: 10.1128/AAC.45.1.105-116.2001
López-Causapé, C., Cabot, G., Del Barrio-Tofiño, E., and Oliver, A. (2018). The versatile mutational resistome of Pseudomonas aeruginosa. Front. Microbiol. 9:685. doi: 10.3389/fmicb.2018.00685
Mao, W., Warren, M. S., Lee, A., Mistry, A., and Lomovskaya, O. (2001). MexXY-OprM efflux pump is required for antagonism of aminoglycosides by divalent cations in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45, 2001–2007. doi: 10.1128/AAC.45.7.2001-2007.2001
Maravić, A., Šamanić, I., Šprung, M., Fredotović, Z., Ilić, N., Dragičević, J., et al. (2018). Broad-spectrum resistance of Pseudomonas aeruginosa from shellfish: infrequent acquisition of novel resistance mechanisms. Environ. Monit. Assess. 190:81. doi: 10.1007/s10661-018-6471-3
Maseda, H., Sawada, I., Saito, K., Uchiyama, H., Nakae, T., and Nomura, N. (2004). Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 48, 1320–1328. doi: 10.1128/AAC.48.4.1320-1328.2004
Mesaros, N., Glupczynski, Y., Avrain, L., Caceres, N. E., Tulkens, P. M., and Van Bambeke, F. (2007). A combined phenotypic and genotypic method for the detection of Mex efflux pumps in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 59, 378–386. doi: 10.1093/jac/dkl504
Minagawa, S., Inami, H., Kato, T., Sawada, S., Yasuki, T., Miyairi, S., et al. (2012). RND type efflux pump system MexAB-OprM of Pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol. 12:70. doi: 10.1186/1471-2180-12-70
Morita, Y., Komori, Y., Mima, T., Kuroda, T., Mizushima, T., and Tsuchiya, T. (2001). Construction of a series of mutants lacking all of the four major mex operons for multidrug efflux pumps or possessing each one of the operons from Pseudomonas aeruginosa PAO1: MexCD-OprJ is an inducible pump. FEMS Microbiol. Lett. 202, 139–143. doi: 10.1111/j.1574-6968.2001.tb10794.x
Morita, Y., Tomida, J., and Kawamura, Y. (2012). MexXY multidrug efflux system of Pseudomonas aeruginosa. Front. Microbiol. 3:408. doi: 10.3389/fmicb.2012.00408
Piddock, L. J. V. (2006). Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19, 382–402. doi: 10.1128/CMR.19.2.382-402.2006
Poole, K. (2005). Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56, 20–51. doi: 10.1093/jac/dki171
Poole, K. (2011). Pseudomonas aeruginosa: resistance to the max. Front. Microbiol. 2:65. doi: 10.3389/fmicb.2011.00065
Poole, K., Krebes, K., McNally, C., and Neshat, S. (1993). Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175, 7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993
Rehman, A., Patrick, W. M., and Lamont, I. L. (2019). Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem. J. Med. Microbiol. 68, 1–10. doi: 10.1099/jmm.0.000873
Sonnet, P., Izard, D., and Mullié, C. (2012). Prevalence of efflux-mediated ciprofloxacin and levofloxacin resistance in recent clinical isolates of Pseudomonas aeruginosa and its reversal by the efflux pump inhibitors 1-(1-naphthylmethyl)-piperazine and phenylalanine-arginine-β-naphthylamide. Int. J. Antimicrob. Agents 39, 77–80. doi: 10.1016/j.ijantimicag.2011.08.005
Tomás, M., Doumith, M., Warner, M., Turton, J. F., Beceiro, A., Bou, G., et al. (2010). Efflux pumps, OprD porin, AmpC beta-lactamase, and multiresistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 54, 2219–2224. doi: 10.1128/AAC.00816-09
Uwate, M., Ichise, Y. K., Shirai, A., Omasa, T., Nakae, T., and Maseda, H. (2013). Two routes of MexS-MexT-mediated regulation of MexEF-OprN and MexAB-OprM efflux pump expression in Pseudomonas aeruginosa. Microbiol. Immunol. 57, 263–272. doi: 10.1111/1348-0421.12032
Vatcheva-Dobrevska, R., Mulet, X., Ivanov, I., Zamorano, L., Dobreva, E., Velinov, T., et al. (2013). Molecular epidemiology and multidrug resistance mechanisms of Pseudomonas aeruginosa isolates from Bulgarian hospitals. Microb. Drug Resist. 19, 355–361. doi: 10.1089/mdr.2013.0004
Vila, J., and Martínez, J. L. (2008). Clinical impact of the over-expression of efflux pump in nonfermentative Gram-negative bacilli, development of efflux pump inhibitors. Curr. Drug Targets 9, 797–807. doi: 10.2174/138945008785747806
Xavier, D. E., Picao, R. C., Girardello, R., Fehlberg, L. C. C., and Gales, A. C. (2010). Efflux pumps expression and its association with porin down-regulation and (-lactamase production among Pseudomonas aeruginosa causing bloodstream infections in Brazil. BMC Microbiol. 10:217. doi: 10.1186/1471-2180-10-217
Xia, J., Gao, J., and Tang, W. (2016). Nosocomial infection and its molecular mechanisms of antibiotic resistance. Biosci. Trends 10, 14–21. doi: 10.5582/bst.2016.01020
Keywords: Pseudomonas aeruginosa, efflux pump, overexpression, fluoroquinolone, resistance, environment
Citation: Serra C, Bouharkat B, Tir Touil-Meddah A, Guénin S and Mullié C (2019) MexXY Multidrug Efflux System Is More Frequently Overexpressed in Ciprofloxacin Resistant French Clinical Isolates Compared to Hospital Environment Ones. Front. Microbiol. 10:366. doi: 10.3389/fmicb.2019.00366
Received: 06 August 2018; Accepted: 12 February 2019;
Published: 26 February 2019.
Edited by:
Yuji Morita, Meiji Pharmaceutical University, JapanReviewed by:
Catherine Llanes, University of Franche-Comté, FranceCopyright © 2019 Serra, Bouharkat, Tir Touil-Meddah, Guénin and Mullié. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine Mullié, Y2F0aGVyaW5lLm11bGxpZUB1LXBpY2FyZGllLmZy
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.