
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 26 February 2019
Sec. Microbiotechnology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.00302
This article is part of the Research TopicNovel Research on Metabolites Secreted by Gram-Positive Bacteria View all 11 articles
 Simon Caulier1,2†
Simon Caulier1,2† Catherine Nannan1†
Catherine Nannan1† Annika Gillis1
Annika Gillis1 Florent Licciardi1
Florent Licciardi1 Claude Bragard2*
Claude Bragard2* Jacques Mahillon1*
Jacques Mahillon1*Over the last seven decades, applications using members of the Bacillus subtilis group have emerged in both food processes and crop protection industries. Their ability to form survival endospores and the plethora of antimicrobial compounds they produce has generated an increased industrial interest as food preservatives, therapeutic agents and biopesticides. In the growing context of food biopreservation and biological crop protection, this review suggests a comprehensive way to visualize the antimicrobial spectrum described within the B. subtilis group, including volatile compounds. This classification distinguishes the bioactive metabolites based on their biosynthetic pathways and chemical nature: i.e., ribosomal peptides (RPs), volatile compounds, polyketides (PKs), non-ribosomal peptides (NRPs), and hybrids between PKs and NRPs. For each clade, the chemical structure, biosynthesis and antimicrobial activity are described and exemplified. This review aims at constituting a convenient and updated classification of antimicrobial metabolites from the B. subtilis group, whose complex phylogeny is prone to further development.
The genus Bacillus comprises 377 species1 (last update in January 2019) of Gram-positive, rod-shaped bacteria (Gordon et al., 1973). Their ability to form endospores, their diversity in physiological properties, as well as their capacity to produce numerous antimicrobial compounds (AMCs) favor their ubiquitous distribution in soil, aquatic environments, food and gut microbiota of arthropods and mammals (Nicholson, 2002).
Bacteria from the Bacillus subtilis group consist of small vegetative cells (<1 μm-wide) for which the strain B. subtilis subsp. subtilis 168 is considered as model organism (Barbe et al., 2009). They are usually mesophilic and neutrophilic, although some can tolerate high pH. The four original species of the group (B. subtilis, Bacillus licheniformis, Bacillus pumilus, and Bacillus amyloliquefaciens) were discovered more than 40 years ago (Gordon et al., 1973; Priest et al., 1987). Since then, the evolution of their molecular, chemotaxonomic and physiological characterizations led to regular re-evaluations and (re-)description of numerous novel species and subspecies (see current taxonomy of the group in Figure 1) (Fan et al., 2017).
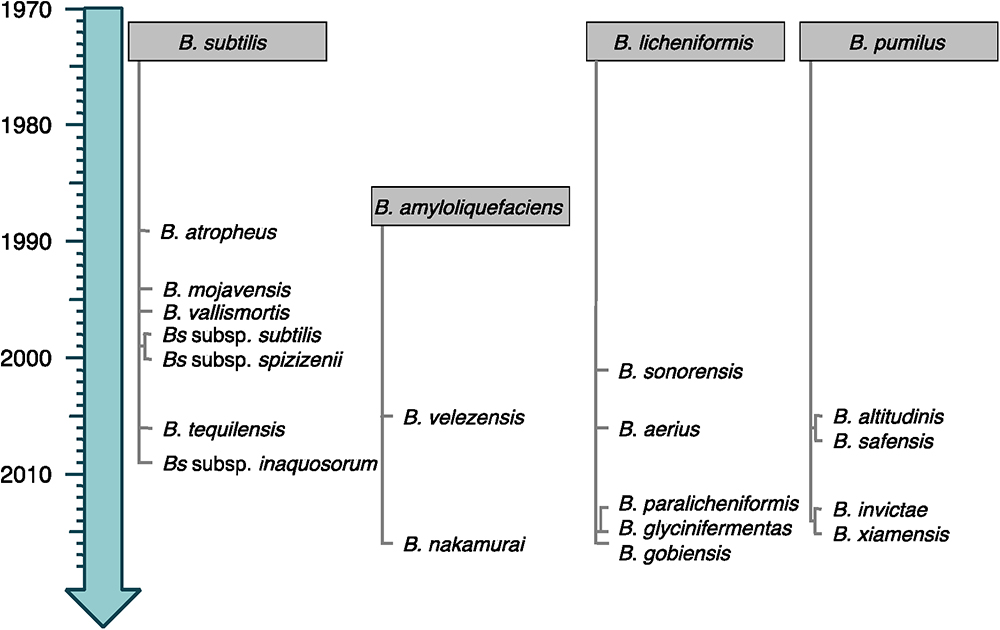
Figure 1. Timeline emergence of the species from the B. subtilis group. The species are classified following their relatedness to the closest original member of the group (gray boxes). Heterotypic synonyms are not shown.
The potential of B. subtilis group strains to produce a wide diversity of secondary metabolites mediating antibiosis was recognized for decades. For any given strain of the B. subtilis group, it is now estimated that at least 4–5% of its genome is devoted to antimicrobial compounds (AMCs) production (Stein, 2005). These molecules are mainly antimicrobial peptides (AMPs). Their structures are usually cyclic, hydrophobic and contain peculiar moieties such as D-amino acids (AA) or intramolecular thioether bonds. In addition to AMPs, volatile metabolites also constitute a large family of antimicrobials exhibiting numerous metabolic and functional roles.
Due to the wide diversity of these molecules, their classification is rather complex and can be based on several criteria such as their biosynthetic machinery, sources, biological functions, properties, three-dimensional structure, covalent bonding pattern or molecular targets (Tagg et al., 1976; Wang et al., 2015). Here a classification of the B. subtilis group antimicrobial molecules is proposed, based on their biosynthetic pathways and their chemical nature as shown in Figure 2. This review will emphasize the biosynthesis pathway and the bioactivity of the main clades of AMCs within the B. subtilis group: i.e., the ribosomal peptides (RPs) (bacteriocins and enzymes), the polyketides (PKs), the non-ribosomal peptides (NRPs) and the volatiles. A full overview of this chart is provided as Supplementary Material (Supplementary Figure S1).
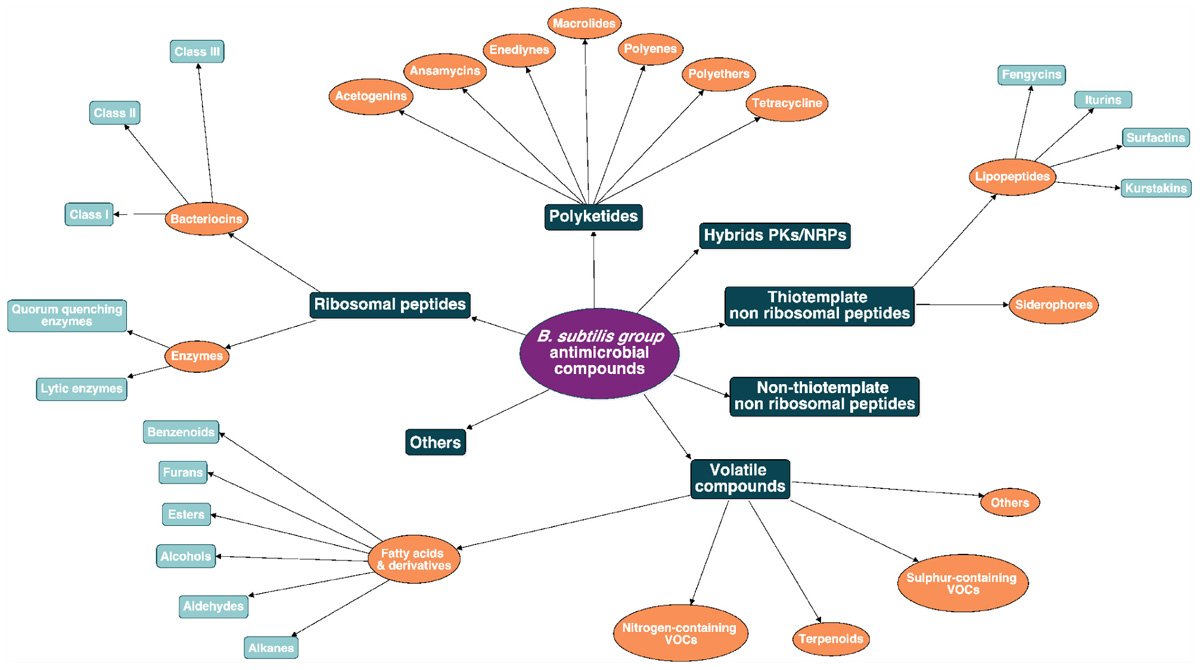
Figure 2. Antimicrobial molecules classes from the B. subtilis group. The subdivision between the classes is based on the biosynthetic pathway (i.e., ribosomal peptides, polyketides, hybrids, non-ribosomal peptides, and volatile compounds).
Ribosomally synthesized peptides (RPs) are usually derived from short precursors (ca. 100 AA) and are processed to mature compounds through post-translational modifications (Oman and van der Donk, 2009). Various enzymes mediate these modifications and therefore generate a wide diversity of chemical structures. Most of these peptides were originally referred to as “bacteriocins,” characterized as low molecular weight molecules that exhibit inhibiting growth activities against bacteria closely related to the producing strain (Klaenhammer, 1988; Chopra et al., 2015). In addition to bacteriocins, other types of enzymes exhibiting antagonistic activities are also ribosomally synthesized. However, those compounds display diverse metabolic activities such as quorum sensing (QS) mediation, cell lysis or induction of genetic competence (Schmidt, 2010; Shafi et al., 2017). It should also be noted that molecules referred to as BLIS (bacteriocins-like inhibitory substances) include AMPs for which the ribosomal synthesis has not been confirmed yet (Abriouel et al., 2011).
It is estimated that 99% of the bacteria and archaea are able to produce at least one bacteriocin. Historically, lactic acid bacteria (LAB) were studied as main bacteriocin producers, mostly because of their long history of safe use in food fermentation (O’Sullivan et al., 2002). Nisin (Figure 3C), produced by Lactobacillus lactis subsp. lactis, was approved as a food additive in the 1960s and has since then been used in over 50 countries for its antimicrobial activity against Gram-positive pathogens such as Clostridium spp. and Bacillus spp. (Klaenhammer, 1988; Delves-Broughton, 1990). However, the search for new bioactive molecules has rapidly expanded to other bacteriocin-producing genera, with a particular attention, in the late 1990s, to the GRAS (generally recognized as safe) Bacillus species whose bacteriocin antimicrobial spectra were broader than those of LAB (Pedersen et al., 2002; Riley and Wertz, 2002; Sumi et al., 2015).
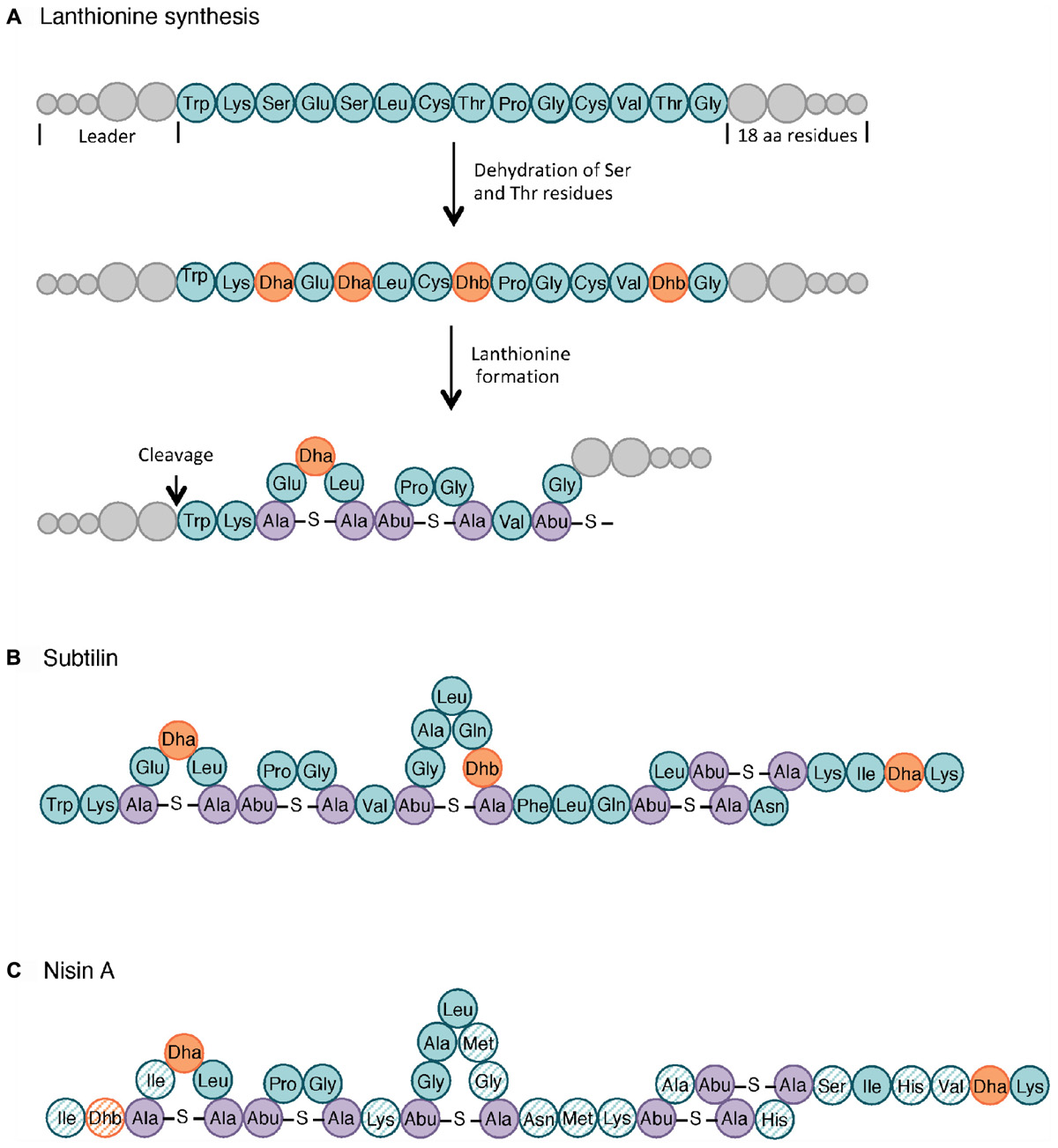
Figure 3. Lanthionine biosynthesis. General pathway of the lanthionine synthesis (A), structure of subtilin (B) and nisin A (C). Non-modified AA are indicated in teal whereas dehydrated serine (Dha, dehydroalanine) and threonine (Dhb, dehydrobutyrine) are colored in orange. The lanthionine (Ala-S-Ala, alanine-S-alanine) and R-methyllanthionine (Abu-S-Ala, aminobutyrate-S-alanine) bridges are shown in purple. The AA of nisin that differ from those in subtilin are highlighted as hatched circles. Adapted from Cotter et al. (2005) and Spieß et al. (2015).
The generic biosynthetic pathway of Bacillus species bacteriocins includes several post-translational modifications, including the proteolytic cleavage of the leader peptide at the N-terminal end (McIntosh et al., 2009). The modifications of active peptides, its secretion and the immunity to the bacteriocin (as described below) vary depending on the bacteriocin class.
While many classifications have been suggested over the years, one reasonable way to cope with the diversity of the Bacillus bacteriocins is to sort them on the basis of their biosynthetic pathway as previously reported for Streptococcus spp. and Enterococcus spp. bacteriocins (Nes et al., 2007) and reviewed in Abriouel et al. (2011). Accordingly, three main classes subdivided into several subclasses can be distinguished for the B. subtilis group. As detailed in Table 1, Class I includes the post-translationally modified peptides such as the lantibiotics whereas the non-modified peptides are grouped in Class II; Class III involved bacteriocins larger than 10 kDa (Abriouel et al., 2011). Supplementary Table S1 summarizes the different RPs produced by the strains belonging to the B. subtilis group, as well as their reported antimicrobial activities.
Class I includes small AMPs (19–38 AA) with extensive post-translational modifications. Subclasses I.1, I.2, and I.3 have in common their lantibiotic structure, which refers to inter-residual thioester bonds made of modified AA residues. As illustrated in Figure 3, lantibiotics involve 2,3-didehydroalanine (Dha) and (Z)-2,3-didehydrobutyrine (Dhb), resulting from the dehydration of serine and threonine residues, respectively. The intra-molecular addition of Dha or Dhb on a cysteine residue leads to the respective formation of lanthionine and methyllanthionine bridges (Willey and Donk, 2007). Subtilin (Figure 3B), from subclass I.1, is one of the most studied bacteriocins from the B. subtilis group. Its structure shares several similarities with nisin A lantibiotics, shown in Figure 3C (Guder et al., 2000; Abriouel et al., 2011). Peptides from subclass I.4 undergo other types of modifications. For instance, subtilosin A is a head-to-tail cyclic peptide with unusual inter-residue linkages (i.e., Cys-Phe bond) (Marx et al., 2001; Kawulka et al., 2004).
Class II bacteriocins include small (<10 kDa), linear and non-modified peptides, resistant to heat and acido-basic treatments. They are divided in three subclasses based on a conserved AA motif near their N-terminus. The YGNGVXC (X is any AA) motif is associated to pediocin-like peptides from subclass II.1 whereas DWTXWSXL is specific to thuricin-like peptides from subclass II.2. Subclass II.3 comprises the small non-modified AMPs without any typical motif in their AA sequence (Abriouel et al., 2011). Finally, class III bacteriocins consist into large and heat labile molecules, generally characterized by a phospholipase activity (Cleveland et al., 2001).
Because of their wide diversity, bacteriocins display different modes of action such as protoplasm vesicularization, pore formation or cell disintegration (Sumi et al., 2015). They are generally bactericidal with some exceptions that exhibit bacteriostatic activities (Gautam and Sharma, 2009). For most class I and II bacteriocins, the target of their activity is the bacterial envelope due to their amphiphilic or hydrophobic properties. For instance, lantibiotics from subclass I.1 have a dual mode of action. On the one hand, they can inhibit the cell wall synthesis of the targeted bacteria through binding to lipid II, the major transporter of peptidoglycan subunits across the inner cell membrane. On the other hand, lipid II can be used as a docking molecule to insert the lantibiotic in the membrane leading to pore formation and ultimately to cell death as well described in Chatterjee et al. (2005) and Cotter et al. (2005). This duality has been reported for subtilin, a class I bacteriocin which is active against a broad range of Gram-positive bacteria such as Staphylococcus simulans, B. subtilis, and Bacillus stearothermophilus (Linnett and Strominger, 1973; Parisot et al., 2008).
Many regulation systems mediate bacteriocin production, secretion and immunity. Bacteriocin production is usually linked to particular cellular events such as stress responses. For instance, subtilin production depends on cell density and is increased under starvation conditions (Abriouel et al., 2011). Lantibiotic production is also mediated by QS. For subtilin, it has been demonstrated that the peptide itself acts as an auto-inducer of its own production (Kleerebezem, 2004). The export of bacteriocins is generally ensured by a dedicated membrane-associated ATP-Binding Cassette (ABC) transporter. For some lantibiotics, the cleavage of the leader peptide often occurs in a proteolytic domain present in the ABC transporter as described in McAuliffe et al. (2001) and Cotter et al. (2005). The immunity of the producing strains to its own active bacteriocin(s) can be achieved by several mechanisms like the secretion of immunity proteins sequestering the peptide, the bacteriocin re-export through an ABC transporter system or the alteration of the targeted peptidoglycans bonds (e.g., modification of the cell wall or cytoplasmic membrane charge) (Cotter et al., 2005; Dubois et al., 2009).
Among the B. subtilis group, two major types of enzymes exhibit antagonistic activities (Supplementary Table S1): the lytic enzymes and those involved in quorum quenching (QQ). Several strains from the B. subtilis group have indeed been identified as capable to produce lytic enzymes with biocontrol potential (Herrera-Estrella and Chet, 1999; Kumar et al., 2012; Shafi et al., 2017). They include cellulases, glucanases, proteases and chitinases and are generally referred to as cell wall degrading enzymes (CWDE) (Ariffin et al., 2006; Alamri, 2015; Caulier et al., 2018). They are particularly active against fungi since chitin and glucan are the major constituents of their cell wall where various glycoproteins are embedded (Bowman and Free, 2006; Geraldine et al., 2013; Gomaa, 2012).
Quorum quenching is able to silence or block QS which is generally defined as the cell-to-cell communication mechanism through the production of signal molecules (Czajkowski and Jafra, 2009). N-acyl-homoserine lactones (AHLs), composed of a fatty acid side chain and a homoserine lactone (Figure 4) are the most characterized signal autoinducers in Gram-negative bacteria. When a bacterial population proliferates, concentration of AHLs increases so that all the cells coordinate their metabolic activities (e.g., biofilm formation, sporulation, virulence factors or antibiotic production) (Dong et al., 2004). As the QS system brings ecological advantages to a coordinate population, QQ is able to counteract QS. Four types of enzymes (i.e., lactonase, decarboxylase, acylase, and deaminase) are able to inactivate AHLs, as illustrated in Figure 4 (Czajkowski and Jafra, 2009). B. subtilis AHL-lactonases have for instance attracted interest for biocontrol since they affect the growth of deleterious microbial pest such as Pectobacterium carotovorum subsp. carotovorum causing potato soft rot (González and Keshavan, 2006).
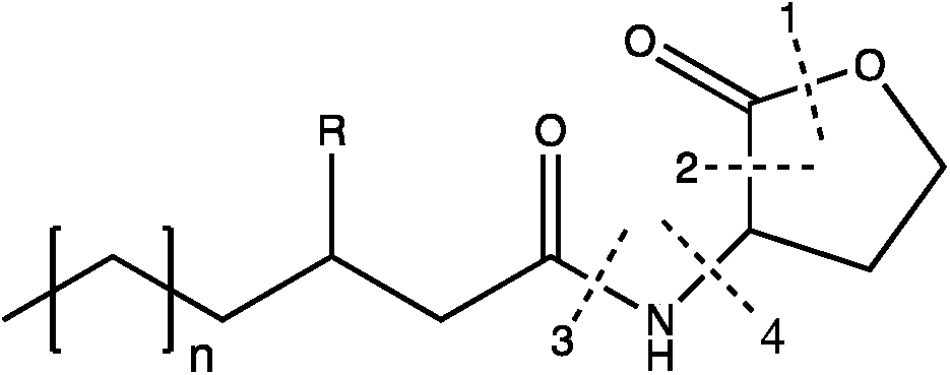
Figure 4. AHLs structure and its corresponding enzymatic degradations by QQ. The broken lines show the cleavages sites of four enzymes: (1) lactonase; (2) decarboxylase; (3) acylase; (4) deaminase. Adapted from Czajkowski and Jafra (2009).
Among the bioactive compounds produced by microorganisms, PKs are well known from the human health sector for their broad spectrum of activity encompassing antibacterial, immunosuppressive, antitumor and many more antagonistic abilities. Typical PKSs structures from the B. subtilis group are presented in Figure 5. They are synthetized from acyl CoA precursors such as malonate and methyl malonate. Their biosynthesis depends on multifunctional polyketide synthases (PKSs). Their structure was first extrapolated from fatty acid synthases (FASs) that share similarities in terms of chain extension mechanisms, precursors and overall architecture design (Smith and Tsai, 2007). As shown in Figure 6A, PKS are composed of a succession of elongation modules, flanked by initiation and termination modules. The reactive mechanism of these three PKS domains is illustrated in Figure 7A and is well summarized in Hertweck (2009). The initiation module is composed of two domains: an acyltransferase (AT) domain that recruits and catalyzes the binding of a monomer substrate to an acyl carrier protein (ACP) domain. The ACP then acts as an arm with a second catalytic domain located on the next elongation module. This domain, a β-ketoacyl synthase (KS), catalyzes the chain-elongation reaction that occurs through a decarboxylative Claisen thioester condensation (Cane and Walsh, 1999; Hertweck, 2009). In addition to the three core domains, auxiliary domains can also be present on elongation modules (gray domains in Figure 6A). These auxiliary domains mediate ketoreduction (KR), dehydration (DH), or enoylacyl reduction (ER) occurring before the chain-elongation reaction. These modifications considerably enrich the structural complexity and diversity of mature PKs (Hertweck, 2009). Finally, a termination module harboring an additional thiosterase (TE) domain catalyzes the macrolactonization and the release of the mature PK (Cane and Walsh, 1999).
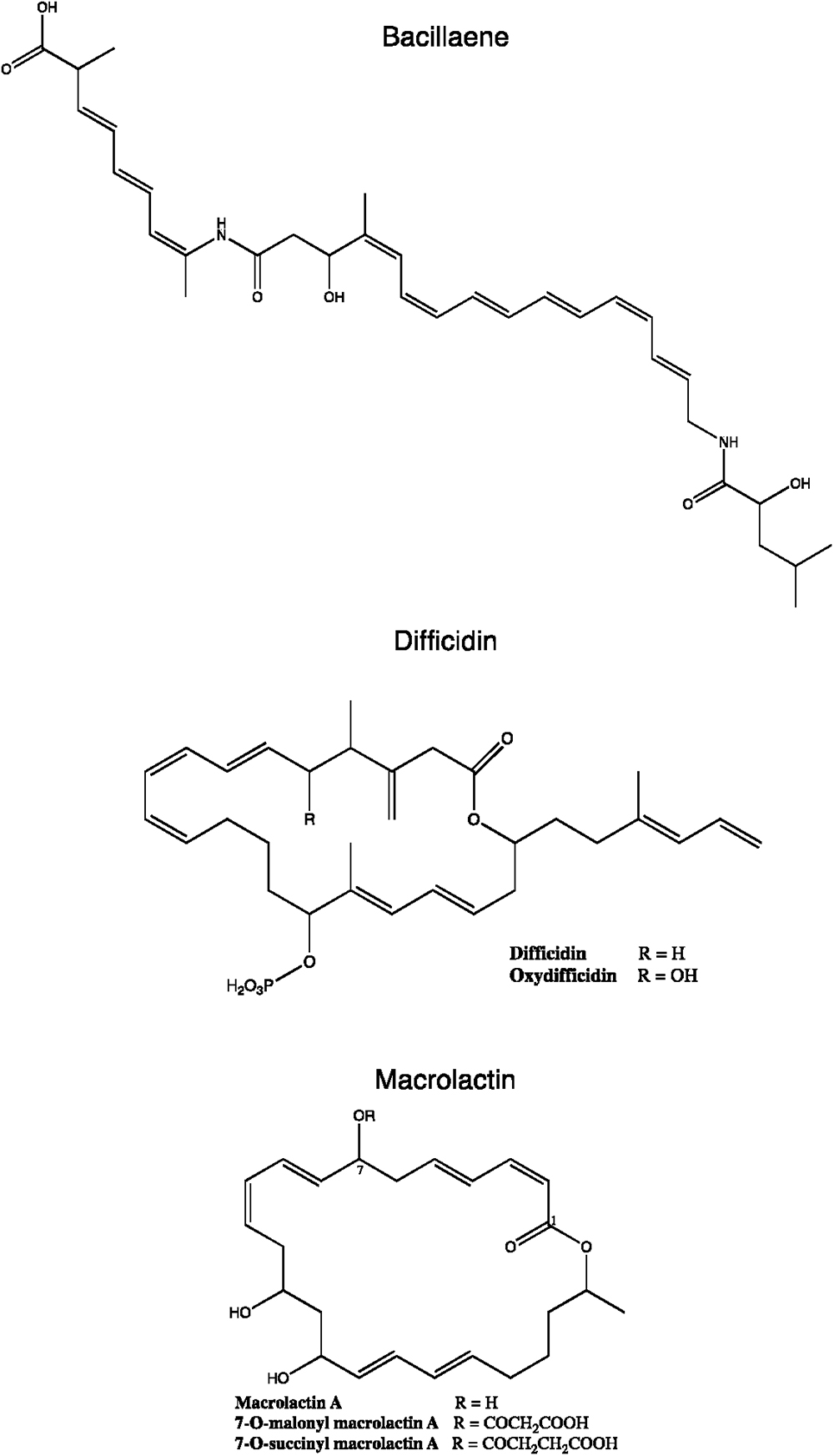
Figure 5. Chemical structures of some B. subtilis group polyketides. Variants from macrolactin and difficidin are presented.
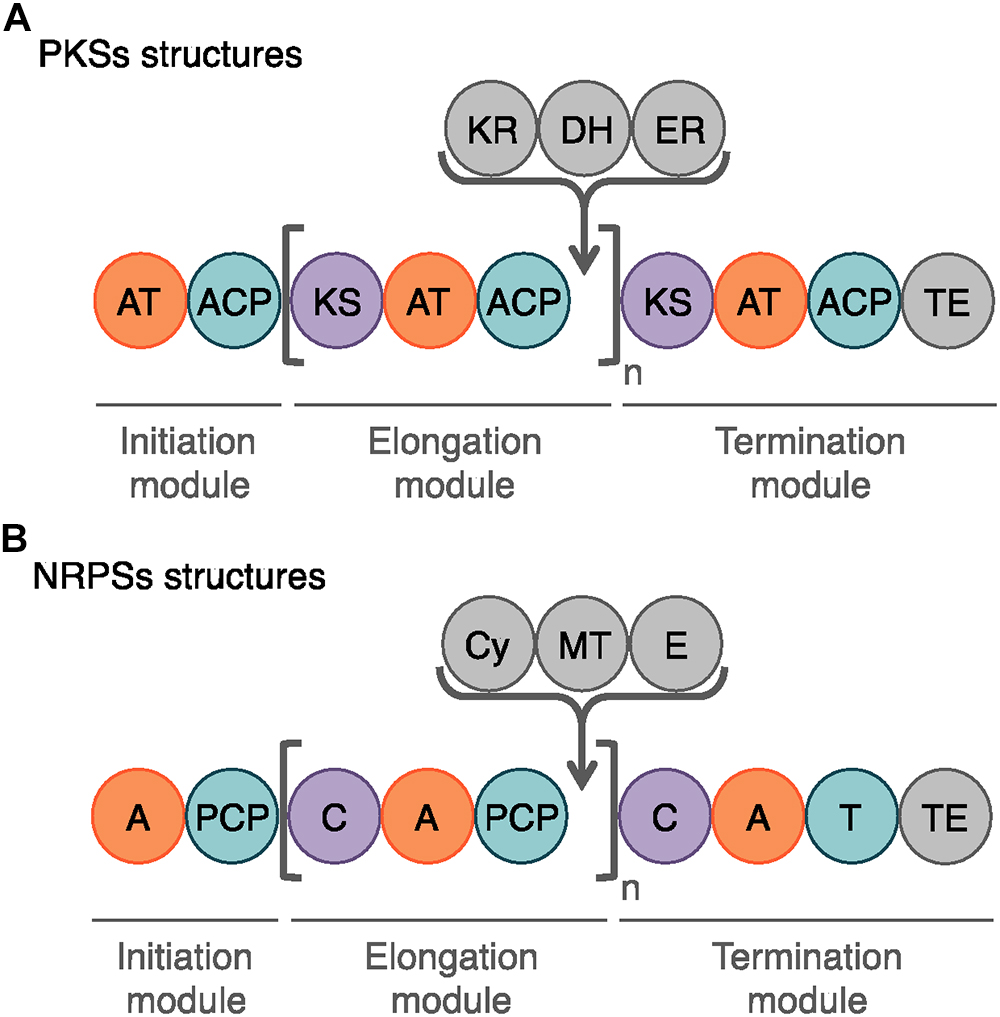
Figure 6. Schematic representation of the modules and domains mediating PKS and NRP biosynthesis. (A) The domains involved in the PK synthesis are the acyltransferase (AT), the acyl carrier protein (ACP), the ketosynthase (KS) and the chain-terminating thiosterase (TE) domains. In gray, the auxiliary domains can mediate ketoreduction (KR), dehydration (DH), and enoylacyl reduction (ER) at each elongation step (n). (B) The core domains for NRP biosynthesis are the adenylation (A), the peptidyl carrier domain (PCP), the condensation (C), and the final thioesterase (TE) domains. The auxiliary domains consist in cyclization (Cy), N-methylation (MT), and epimerization (E) domains.
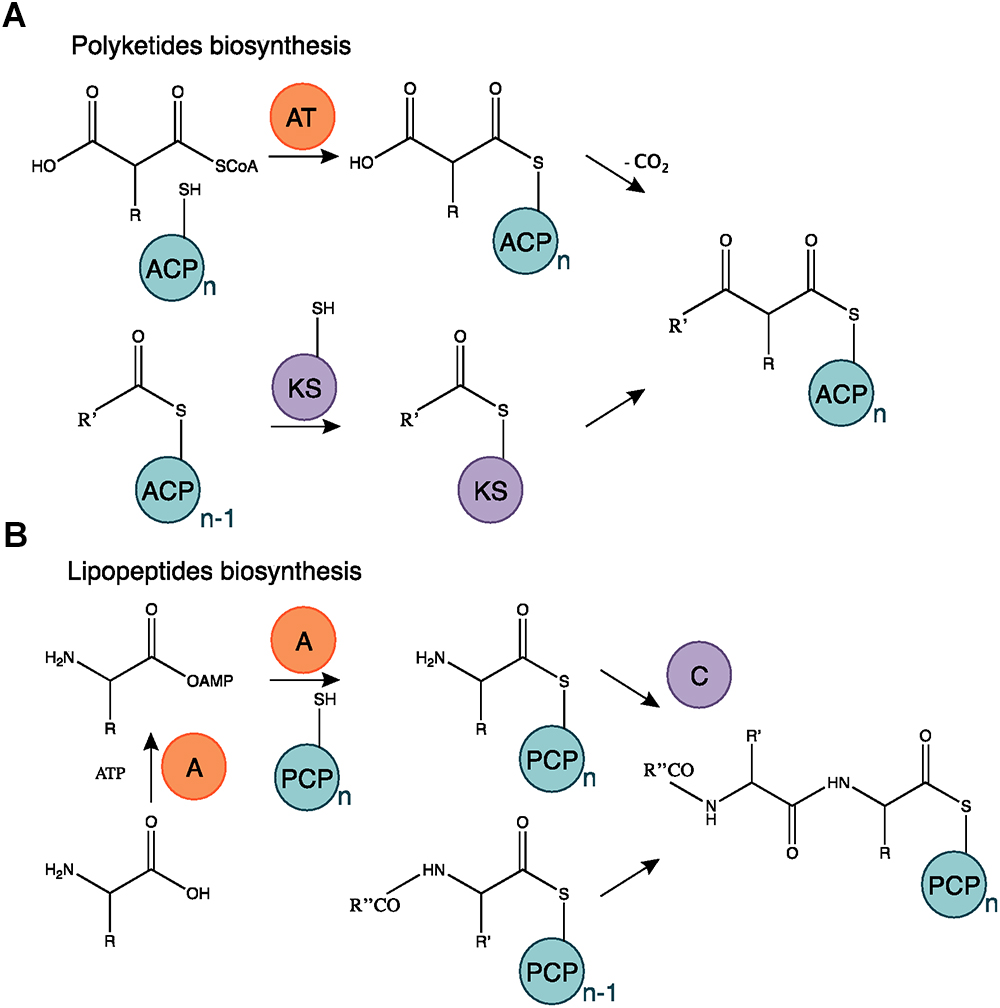
Figure 7. Polyketides and lipopeptides biosynthesis mechanism. (A) The AT domain catalyzes the binding of the monomer substrate and the ACP domain. The KS domain is acetylated on the acyl residue of a polyketide starter or in elongation and catalyzes the transfer of the substrate subunit carried by the ACP. (B) The A domain activates an AA chain extension subunit and its transfer to the PCP carrier domain. The C domain catalyzes the bond mediating the chain elongation. Adapted from Cane and Walsh (1999) and Challis and Naismith (2004).
Polyketide synthases have been classified in three canonical types based on the structural organization of their functional domains. Type I PKSs involve large multifunctional enzymes housing several domains linearly arranged and covalently bonded. Type II PKSs are multienzyme complexes composed of separate monofunctional enzymes combined during the PK synthesis. Type III PKSs are chalcone synthase-like PKSs that operate the acid CoA thioesters directly without any ACP domain (Chen and Du, 2016). Beside these structural differences, PKSs are classified as iterative or non-iterative depending on how many KS domains are used in the biosynthetic process. Within prokaryotes, the non-iterative type I PKSs is the most represented. They produce PK compounds that harbor a one-to-one correspondence with the PKS modular architecture. This conservation of collinearity is used for PKS discovery via genome mining (Challis, 2008).
Due to the diversity of PKSs, many exceptions and transition states between the three main types are observed. In some cases, mixed PKs pathways combine different types of PKSs or can even be associated with FASs or NRP synthetases (NRPSs) to form PK-peptide hybrid metabolites such as bacillaene, compactin, fusarin C or salinosporamide A (Moldenhauer et al., 2007; Hertweck, 2009; Fisch, 2013).
To date, seven PKs families have been recognized based on their carbon skeletons and typical structures, as summarized in Table 2 (Eustáquio et al., 2009). However, to our knowledge, only three antimicrobial PKs and their variants are produced within the B. subtilis group: bacillaene, difficidin, and macrolactin. These compounds exhibit antibacterial activities through selective inhibition of protein synthesis (Table 3). Bacillaene is a polyene PK resulting from a hybrid synthesis by a type I PKS and a NRPS bae operon (baeJ, baeL, baeM, baeN and baeR) (Chen et al., 2006; Moldenhauer et al., 2007). Its exhibits antimicrobial activity against various bacteria (e.g., Myxococcus xanthus or Staphylococcus aureus) and fungi (e.g., Trichoderma spp. or Fusarium spp.) (Patel et al., 1995; Um et al., 2013; Müller et al., 2014). Difficidin, and its oxidized form oxydifficidin, are polyenes synthesized by a type I PKS encoded in the dif operon. They both inhibit bacterial pathogens such as Clostridium perfringens, Erwinia amylovora, Escherichia coli or Xanthomonas oryzae (Zimmerman et al., 1987; Chen et al., 2009; Aleti et al., 2015; Wu et al., 2015b). Finally, macrolactins and their 7-O-succinyl- or 7-O-malonyl-derivatives are synthetized via a type I PKS. They show antibacterial and antifungal activities against Burkholderia cepacia, Ralstonia solanacearum, S. aureus or Fusarium oxysporum (Romero-Tabarez et al., 2006; Yoo et al., 2006; Yuan et al., 2012a). Some macrolactins, such as the macrolactin A, apparently also displays antiviral properties (e.g., against Herpes simplex viruses) (Gustafson et al., 1989).
Non-ribosomal peptides form a versatile family of secondary metabolites with growing interest in many industrial fields as antibiotics, siderophores, surfactants, pigments, immunosuppressors or antitumor molecules (Wang et al., 2014). NRPs show a broad structural diversity, from linear to cyclic or branched structures (Kopp and Marahiel, 2007). As illustration, the Norine database counts almost 1.200 NRP molecules, including their structure, synthesis and evolution2 (last update in January 2019) (Caboche et al., 2008).
Two categories of NRPs can be distinguished whether they are synthetized through a multi-enzyme thio-template mechanism or not (Sumi et al., 2015). The first ones usually result in structures with two to ca. 50 residues and other moieties such as fatty acid chains [i.e., lipopeptides (LPs) and siderophores] whereas the second ones are generally smaller. Figure 8 shows the chemical structures of typical NRPS from the B. subtilis group.
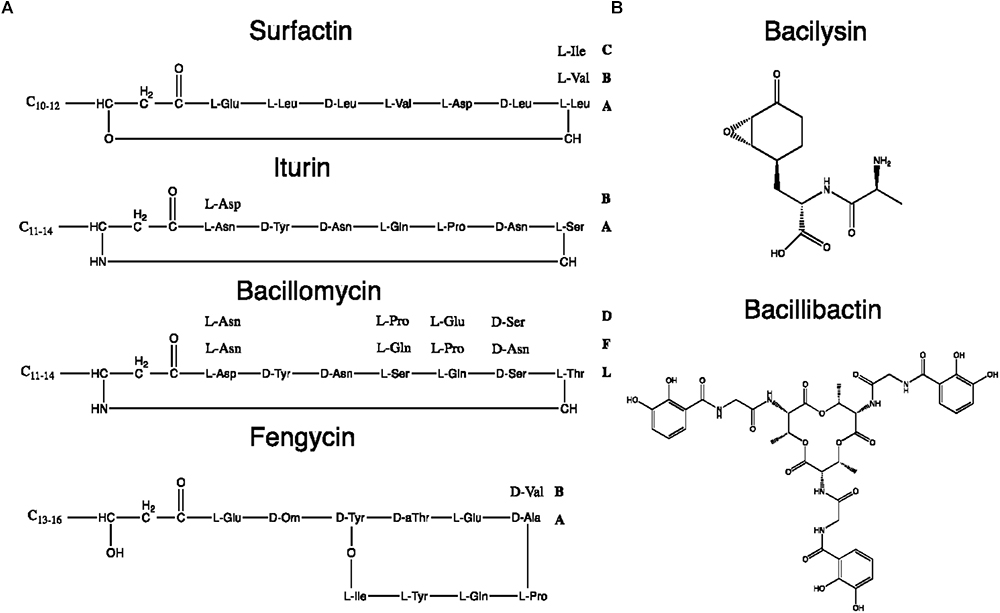
Figure 8. Chemical structures of some B. subtilis group NRPs. (A) Lipopeptides. (B) Miscellaneous NRPs.
Lipopeptides are usually synthetized through a NRPS sequential addition of AA residues, either in an iterative or non-iterative way. Similarly to PKSs, NRPSs have a modular organization implementing the initiation, elongation, and termination modules (Figure 6B). Each module is subdivided in core domains whose catalytic and carrier domains slightly differ from PKSs, as shown in Figure 7B. The biosynthesis which was previously summarized in Ongena and Jacques (2008) and Raaijmakers et al. (2010) starts with an adenylation domain (A domain) that recruits and phosphorylates an AA monomer into an aminoacyl adenylate intermediate. The intermediate is then linked to the corresponding peptidyl carrier protein or thiolation domain (PCP or T domain) through a thioester bond. The PCP acts as a bridge and ensures the link with the condensation domain (C domain) that forms the C–N bond between the recruited aminoacyl and the peptide acyl chain in formation. The termination module contains a thioesterase domain (TE) that catalyzes the release of the final peptide acyl chain (Ongena and Jacques, 2008; Raaijmakers et al., 2010). The elongation modules can be supplemented with accessory domains such as cyclization domain (Cy), epimerization domain (E) and methylation domain (M). Those domains are able to modify the growing peptide chain which leads to diverse mature compounds structure (Cane and Walsh, 1999; Challis and Naismith, 2004).
Since the LP biosynthetic pathways are highly flexible, the range of produced LPs is extremely heterogeneous. Among LPs produced by Bacillus spp., four main families have been distinguished: kurstakins, surfactins, iturins, and fengycins (Jacques, 2011). Each family shares the same structural features based on the nature and organization of the peptide moiety or fatty acid tail, as summarized in Table 4. Strains from the B. subtilis group produce surfactins, iturins and fengycins whereas kurstakins are produced by B. thuringiensis strains (Béchet et al., 2012). Among the three LP families produced by B. subtilis, at least eight fengycins, 13 surfactins and 14 iturins variants have been described so far, as detailed in Supplementary Table S2.
For each LP family, the compounds production is mainly regulated by environmental factors such as carbon sources, oxygen availability, pH and temperatures (Yakimov et al., 1995; Kim et al., 1997; Cosby et al., 1998). Warm temperature (≥37°C) and anaerobic conditions increase the production of surfactins while lower temperatures (25–37°C) and aerated bioreactors favor fengycins and iturins family metabolites (Jacques, 2011). The production of surfactins by B. subtilis is also QS-dependent and involves ComX and PhrC. These pheromones trigger complex cascades regulating cell density-dependent processes such as sporulation and competence (Hamoen et al., 2003; Ongena et al., 2005).
Iturins and fengycins are mainly known for their strong antifungal activity against several plant and human pathogenic fungi (Supplementary Table S2). In addition, iturin-like mycosubtilin, bacillomycin R, subtulene A and eumycin show antibacterial properties (Besson et al., 1976; Leclere et al., 2005; Thasana et al., 2010). Contrary to iturins and fengycins, surfactins mainly display antiviral and antibacterial activities (Ongena and Jacques, 2008). Their antiviral activity essentially targets enveloped viruses (e.g., herpes simplex or porcine epidemic diarrhea viruses). They also inhibit pathogenic bacteria such as Legionella pneumophila, Listeria monocytogenes, R. solanacearum or X. oryzae (Naruse et al., 1990; Yakimov et al., 1995; Sabaté and Audisio, 2013; Loiseau et al., 2015; Luo et al., 2015). However, some surfactins are able to control important fungal plant and human pathogens such as Botrytis cinerea, Candida albicans, F. oxysporum or Rhizoctonia solani (Jenny et al., 1991; Lee et al., 2007; Qi et al., 2010; Dimkić et al., 2013; Romano et al., 2013).
The mere composition of LPs, where a peptide moiety is bound to a lipid tail, gives them an amphiphilic property. This nature makes them excellent surfactants and plays a significant role in their biological functions and antimicrobial properties. Indeed, LPs are able to destabilize the plasma membrane via a pore forming activity leading to the cell death of the target microbes. Their antiviral activity is the result of a similar disintegration of the bi-lipid envelope of virions explaining the weak LPs activity against plant viruses among which very few are enveloped (Ongena and Jacques, 2008).
Bacillus spp. LPs have many other biological and ecological functions as fully documented by Raaijmakers et al. (2010). They are also known to impact other metabolic mechanisms such as biofilm formation, motility, virulence, plant root colonization, and plant defenses. Moreover, it has been suggested that their participation to the degradation of hydrophobic substrates could be used for polluted soils bioremediation (Mulligan et al., 2001). Although some lipopetides have already been exploited as food biopreservatives or crop protection products, the industrial interest for LPs in specific applications is unsurprisingly continuously growing.
Itoic acid is a mono-peptide composed of a 2,3-dihydroxybenzoate (DHB) molecule bound to a glycine. It is used as a precursor by trimodular NRPS machinery to produce bacillibactin which is obtained after a condensation of three units of DHB-glycine-threonine (May et al., 2001). The synthesis of the final hexapeptide is catalyzed by a terminal thioesterase domain leading to the production of a methylated trilactone ring link to three catecholates moieties. It is this cyclic structure that enables the sequestration of the metal atom (Dertz et al., 2006). Itoic acid and bacillibactin are both catecholic siderophores that chelates iron reducing its bioavailability. This is limited access to iron that allows B. subtilis to antagonize the growth of other surrounding microbes such as, for instance, F. oxysporum f. sp. capsici (Yu et al., 2011).
Bacteria from the B. subtilis group are also able to synthesize other antimicrobial NRPs through non-thiotemplate mechanism. Rhizocticins are di- and tri-phosphono-peptides. They are constituted of a L-2-amino-5-phosphono-3-cis-pentenoic acid (APPA) linked to an arginine (rhizocticin A). They can be supplemented with an additional valine (rhizocticin B), isoleucine (rhizocticine C) or leucine (rhizocticine D). After their integration into the target microbes, their cleavage by host cell peptidases releases the fungitoxic L-APPA moiety that interferes with threonine metabolism in fungal cells. Interestingly, rhizocticin A has also an antagonistic activity against nematodes such as Caenorhabditis elegans (Kugler et al., 1990).
In addition to rhizocticin compounds, two other dipeptide NRPs are produced by B. subtilis: bacilysin (also known as tetaine) and its chlorinated derivative, chlorotetain. They contain L-alanine (or chlorine-L-alanine) bound to the non-proteinogenic L-anticapsin (Kenig and Abraham, 1976; Rapp et al., 1988). Despite their simple composition, these bioactive compounds display strong antibacterial activity mediated by the anticapsin moiety that inhibits the glucosamine-6-phosphate synthase. Its inhibition suppresses the biosynthesis of peptidoglycans that are the main constituents of bacterial cell wall (Steinborn et al., 2005; Mahlstedt and Walsh, 2010). For the fungi, it has been proposed that because anticapsin is able to inhibit the production of chitin and fungal membrane mannoproteins, bacilysin and chlorotetain exhibit antifungal activity against Aspergillus fumigatus or C. albicans (Milewski et al., 1986; Rapp et al., 1988).
Finally, bacitracin and mycobacillin are two cyclic polypeptides produced by B. subtilis. Bacitracins are dodecapeptides containing a cyclic heptapeptide linked to a thiazoline ring (Johnson et al., 1945). They are mostly active against Gram-positive bacteria where they inhibit the bacterial cell-wall biosynthesis by preventing the lipid carrier from re-entering in the reaction cycle of peptidoglycan synthesis (Siewert and Strominger, 1967). Besides this primary mode of action, bacitracin might also act through other mechanisms affecting membrane functions, hydrolytic enzymes and/or the biosynthesis of ubiquinone precursors (Konz et al., 1997). Mycobacillin is an antifungal cyclic tridecapeptide altering the membrane of fungi like Aspergillus niger (Majumdar and Bose, 1958). Interestingly, its biosynthesis is rather peculiar. Although it is catalyzed by a large NRPS complex, it is divided in three fractions (A, B, and C) and does not use a thio-template mechanism (Zuber et al., 1993). Each fraction of the enzymatic complex contains a single enzyme polypeptide that catalyzes the polymerization of a first pentapeptide (A), a second nonapeptide (B) and the final tridecapeptide.
Besides RPs, NRPs and PKs, strains from the B. subtilis group are able to produce a wide diversity of volatile compounds encompassing important roles especially in soil, one of the major habitats of this group (Supplementary Figure S1). Volatiles are notably involved in the bioconversion of the food chain, in the biogeochemical cycles of essential elements, in many physiological and metabolic reactions (e.g., nitrification, nitrogen mineralization, electron acceptor or donor reactions) as well as in communication signals triggering QS/QQ or defense mechanisms well reviewed in Effmert et al. (2012). Volatile compounds are generally classified into inorganic (VICs) and organic (VOCs) categories.
Volatile inorganic compounds synthesized by microorganisms are mainly by-products of primary metabolism. They are carbonated, hydrogenated, sulfur or nitrogen-containing compounds such as CO2, CO, H2, HCN, H2S, N2, NH3 and NO. Nitrogen-containing compounds are mostly released in aerated upper sediments layers by denitrifying bacteria. In this process, nitric oxide is enzymatically produced by the nitric-oxide reductase or the nitric-oxide synthase (Adak et al., 2002). The range of antimicrobial activities exhibited by VIC nitrogen-containing compounds from the B. subtilis group is wide. For instance, NO is able to induce systemic acquired resistance (SAR) in plants against bacterial pathogens such as R. solanacearum (Wang et al., 2005). A contrario, ammonia, a secondary metabolite from the catabolism of the amino acids L-aspartate, is known to be active against soil-borne Oomycetes such as Pythium spp. (Howell et al., 1988). Hydrogen cyanide, derived from the glycine catabolism, shows a direct antagonistic activity against aerobic microorganisms by inhibiting metal-containing enzymes such as the cytochrome c oxidase active in the respiration chain (Cherif-Silini et al., 2016).
Deeper in the soil, under low oxygen concentration, bacteria tend to produce different VICs such as H2 or H2S. Those compounds can serve as electron acceptors, AA precursors or antimicrobial metabolites. Hydrogen sulfide could be produced by B. subtilis from sulfate reduction or as a by-product of L-methionine and L-cysteine catabolism via a direct cleavage of L-methionine or a transamination followed by reductive demethiolations (Even et al., 2006; Schulz and Dickschat, 2007). It is known to exhibit antifungal activity against several plant pathogens such as A. niger or Penicillium italicum but also against some food-borne bacteria or human pathogens (Fu et al., 2014). Curiously, it is also known to act as a bacterial defense mechanism against antibiotics (Shatalin et al., 2011). Interestingly, ammonia increases the resistance of several Gram-negative and Gram-positive bacteria to antibiotics too (Bernier et al., 2011).
Volatile organic compounds are small compounds with fewer than 20 carbon atoms and are characterized by low molecular mass (100–500 Da), high vapor pressure, low boiling point and a lipophilic moiety. These features ensure an easy evaporation and a long distance distribution which is convenient in a complex matrix like soil (Schmidt et al., 2015). Their diffusion and production by soil-borne microbes are strongly dependent on various factors such as nutrient and oxygen availability, temperature, pH, physiological state of microorganisms, soil moisture, texture and architecture (McNeal and Herbert, 2009; Insam and Seewald, 2010; Effmert et al., 2012). The majority of VOCs derives from glucose oxidation involving glycolysis and the subsequent cycles such as the tricarboxylic acid cycle (TCA) as it has been well summarized in Korpi et al. (2009) and Schmidt et al. (2015). However, their production can also result from various other pathways such as aerobic heterotrophic carbon metabolism, fermentations, AA degradation, terpenes synthesis or sulfur reduction (Peñuelas et al., 2014). Based on previous reviews presented in Schulz and Dickschat (2007); Peñuelas et al. (2014) and Audrain et al. (2015), five categories of VOCs can be distinguished: (1) fatty acids and derivatives, (2) terpenoids, (3) nitrogen-containing VOCs, (4) sulfur-containing VOCs, and (5) metalloid- or halogenated-containing VOCs. To date, about 2,000 compounds produced by almost 1,000 species of microorganisms have been listed in the mVOC 2.0 database (Lemfack et al., 2018). According to this database, almost 70% of recorded Bacillus VOCs are fatty acids derivatives (alcohols, ketones, alkanes, aldehydes, alkenes, and acids) followed by sulfur- and nitrogen-containing compounds. Supplementary Table S3 displays the VOCs produced within the B. subtilis group and their antimicrobial activity.
Since many volatile fatty acids and their derivatives result from the glucose metabolism, their precursors mostly derive from the Embden-Meyerhof (glycolysis), Entner-Doudoroff, heterolactic and homolactic fermentation pathways (Peñuelas et al., 2014). B. subtilis bacteria, for instance, ferment pyruvate to produce ketone compounds such as acetoin (3-hydroxy-2-butanone) or 2,3-butanedione under anaerobic conditions (Ryu et al., 2003). Other intermediates coming from fatty acid biosyntheses or their β-oxydations are also used as precursors by microbes and transformed into VOCs through a decarboxylation reaction or a reduction of their carboxyl group (Schulz and Dickschat, 2007). They provide essential hydrocarbons but also other fatty acid derivatives. An oxidative deamination of several amino acids can lead to the production of aldehyde, ketone or alcohol volatile too. For instance, the degradation of L-phenylalanine or L-tyrosine can be the first step of the aromatic volatile compounds synthesis such as benzene or its carbohydrate derivatives. Finally, benzenoid volatiles can also be synthesized by microbes through the shikimate pathway that leads to the formation of chorismate, a natural precursor of aromatic amino acids (Bentley and Haslam, 1990). Degradation of intermediates from the shikimate pathway or aromatic amino acids can also lead to the production of benzenoid volatiles (Dickschat et al., 2005).
This wide variety of volatile fatty acids and their derivatives make them the most important group of VOCs produce by microbes and represent up to 87% of known antimicrobial VOCs produced by B. subtilis bacteria (Supplementary Table S3). They can be divided in two main categories: hydrocarbons (alkanes, alkenes, alkynes) or carbohydrates (acids, alcohols, aldehydes, esters, furans, ketones, lactones, benzenoids). Among them, benzenoids is the most represented sub-category followed by alkanes, aldehydes, ketones, acids, and alcohols. Even though benzenoids could be considered as an individual category, they can also be seen as fatty acids derivatives because a large majority of antimicrobial benzenoid volatile produced by B. subtilis harbor a benzene core linked to a fatty acid derivatives.
There is an important diversity of benzenoids, sometimes linked with carbohydrate chains containing nitrogen, sulfur or both. Most of these antimicrobial volatile exert fungicidal activities but some have been characterized for their antibacterial or nematicidal abilities, too. Their mode of action is rarely fully characterized. For instance, morphological abnormalities on fungal and bacterial cells have been documented after an exposition to B. subtilis VOCs (Tahir et al., 2017). Volatile such as 1,3-butadiene or 2,3-butanediol are also known to induce modifications in the expression of genes linked to the pathogenicity of R. solanacearum and Pectobacterium carotovorum (Marquez-Villavicencio et al., 2011; Tahir et al., 2017). In addition to direct antimicrobial activities, fatty acids volatile have also several other biological functions. For instance, acetoin and 2-butanone have the ability to stimulate plant defenses or to induce plant stress tolerance which then promote plant growth (Ryu et al., 2003; Ryu et al., 2004; Ryu, 2015). They are essentially produced by strains of B. amyloliquefaciens, B. velezensis or B. subtilis (Audrain et al., 2015).
Terpenes and their derivatives (also known as terpenoids or isoprenoids) are among the most abundant secondary metabolites found in living systems (Fisher et al., 2001; Gershenzon and Dudareva, 2007). They originate from two main precursors: isopentenyl pyrophosphate (IPP) and its allylic isomer the dimethylallyl pyrophosphate (DMAPP) (Schulz and Dickschat, 2007). IPP and DMAPP are also the end-products of the deoxy-xylulose phosphate pathway (DOXP) starting with pyruvate and glyceraldehyde-3-phosphate originating from the glucose metabolism (Fisher et al., 2001). Terpenoids can be synthesized from isoprene molecules too. Julsing et al. (2007) showed that, in B. subtilis, isoprene is not formed by the MVA or DOXP pathways but, as in plant systems, might be a product of the methylerythritol phosphate (MEP) pathway (Guan et al., 2015).
Isoprenoid compounds are produced by all living organisms for essential physiological functions such as electron transport, membrane fluidity, light harvesting, photoprotection, anchoring of molecules to specific membranes and signaling (Fisher et al., 2001). The signaling ability is particularly important and is associated with several antagonistic, mutualistic or multi-trophic interactions (Shrivastava et al., 2015). More than 25,000 terpenic compounds have been listed and, for the vast majority, their biological functions and roles remain unknown (Buckingham, 1997). Volatile terpenes are generally recognized for their ability to inhibit bacteria (Scortichini and Rossi, 1991), fungi (Hammer et al., 2003; Dambolena et al., 2008), nematodes (Gu et al., 2007) or insects (Lee et al., 2003; Justicia et al., 2005). They can be classified in three categories: isoprene, monoterpenes (C10) and sesquiterpenes (C15) (Schmidt et al., 2015).
The mode of action of these compounds might be linked to their lipophilic nature allowing them to destabilize the cell membrane integrity (Cox et al., 2000; Inoue et al., 2004). To our knowledge, only two terpenes produced by B. subtilis show antimicrobial abilities: isoprene and monoterpene α-terpineol exhibit antagonistic activities against cyanobacteria and nematodes (Wright and Thompson, 1985; Gu et al., 2007).
Little is known about the biosynthetic pathways of nitrogen-containing VOCs. Nevertheless, it is accepted that two main routes can be used: a non-enzymatic amination of acyloins, that can lead to the formation of pyrazines (Schulz and Dickschat, 2007) or derived from α-aminoketone intermediates resulting from AA catabolism (Owens et al., 1997; Zhu et al., 2010).
Nitrogen-containing VOCs can be distinguished based on their cyclization rate. Within non-cyclic compounds, three groups are identified (amides, amines and imines) while there are five categories of cyclic compounds (azoles, pyrazines, pyridines, pyridazines, and pyrimidines). Pyrazines are strongly represented among microbial volatile and are separated in two classes: lower-alkylated and higher-alkylated pyrazines (Schulz and Dickschat, 2007). These compounds are characterized by a strong odor and several B. subtilis coming from the rhizosphere or from food fermentations have already been recognized as pyrazines producers (Sugawara et al., 1985; Kosuge and Kamiya, 1962; Larroche et al., 1999; Leejeerajumnean et al., 2001). Pyrazines from B. subtilis strains are known to exhibit antifungal and nematicidal activities (Gu et al., 2007; Chen et al., 2008; Chaves-López et al., 2015; Haidar et al., 2016). For instance, tetramethylpyrazine inhibits the growth of Moniliophthora perniciosa and F. oxysporum f. sp. lactucae. Additionally, it acts on sporulation and elongation of the germ-tube of B. cinerea (Chen et al., 2008; Chaves-López et al., 2015). It is interesting to note that B. subtilis pyrazines can also exhibit antibacterial activities such as pulcherriminic acid which inhibits the growth of S. aureus, E. coli and Proteus vulgaris (Coutts et al., 1965). Beside pyrazines, strains from the B. subtilis group are able to produce other nitrogen VOCs such as 1H-imidazole,1-ethyl showing antifungal activities against numerous soil-borne phytopathogens (Lupetti et al., 2002; Liu et al., 2008; Snelders et al., 2009; Schmidt et al., 2015).
Microbial VOCs containing sulfur (VSCs) derive from two main pathways originated from inorganic or organic sources (Schulz and Dickschat, 2007): inorganic sulfate reduction in methylated inorganic sulfides compounds or, for some microbial VSCs, originate from catabolism of AA such as L-methionine or more rarely, L-cysteine (Schulz and Dickschat, 2007). Some VSCs are produced as secondary volatiles via the production of hydrogen sulfide or methanethiol. Indeed, these two compounds are important precursors for subsequent VSCs synthesis (Schulz and Dickschat, 2007; Sourabié et al., 2012). Within the B. subtilis group, multiple VSCs such as dimethyl disulfide (DMDS), dimethyl trisulfide (DMTS), S-methyl thioacetate or S-methyl butanethioate have been characterized for their antifungal and nematicidal activities (Coosemans, 2005; Gerik, 2005; Gu et al., 2007; Kai et al., 2009; Wang et al., 2009; de Vrieze et al., 2015; Schmidt et al., 2015; Velivelli et al., 2015; Gotor-Vila et al., 2017). A putative antibacterial effect of DMDS is not to exclude. Indeed, DMDS is known to affect the bacterial cell-to-cell communications through a decrease in the amount of N-acyl homoserine lactone (AHL) mediating QS (Chernin et al., 2011).
Other volatile organic compounds such as halogenated, metalloids, tellurium or selenium compounds have also been described. However, at the time of writing, no B. subtilis strains have been proved to produce these type of VOCs (Schulz and Dickschat, 2007), although related bacteria, like Bacillus arsenicoselenatis, have been shown to generate them (Switzer Blum et al., 1998).
The B. subtilis group offers a plethora of antagonistic compounds displaying a broad range of biological functions. This huge versatility increases the industrial and environmental interest of B. subtilis strains, especially when considering their range of action against foodborne or phytopathogenic flora as well as their history of safe use in food. The present review on known AMCs from the B. subtilis group proposes a consistent classification frame based on their biosynthetic pathways (i.e., RPs, PKs, NRPs, volatiles) and chemical nature.
The present classification suggests to establish systematic approaches for novel molecules discoveries and characterizations (biosynthesis, chemical nature and activity). Indeed, most current publications report antimicrobial activity of partially purified fractions which can involve mixtures of bioactive compounds. To assess the activity of an unique compound, implementations of genetic confirmation such as knockout strategy are needed. Besides, very few studies have focused on the putative synergistic effects within these bio-active mixtures. Also, the concentration of purified or semi-purified compound(s) often remains uncharacterized or biologically irrelevant. Finally, there is no doubt that novel AMCs originating from B. subtilis bacteria remain to be identified, characterized and properly classified.
SC, CN, and FL conducted the bibliographic search. SC and CN wrote the manuscript. AG, CB, and JM edited and reviewed the manuscript. All authors have read and approved the final version.
This work was supported by the National Fund for Scientific Research (FNRS), the Université catholique de Louvain (UCLouvain), and the Brussels Institute for Research and Innovation (Innoviris, Doctiris programme to CN). SC was supported by the Foundation for Training in Industrial and Agricultural Research (FRIA, FNRS), AG holds a Chargé de Recherche fellowship from the FNRS (Grant 1.B.208.16F).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully acknowledge members of SC’s Ph.D. committee Prof A. Legrève, UCLouvain, Prof. M. Ongena, ULiège and Dr. J.-P. Goffart, CRAw for their valuable comments on this manuscript. We also acknowledge the Walloon Region for the long-term financial support through the WACOBI and ANTAGONIST projects (Conventions N∘ DGO3-D31-1330 and N∘ DGO3-D31-1383/S1, respectively).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00302/full#supplementary-material
Abriouel, H., Franz, C. M., Omar, N. B., and Gálvez, A. (2011). Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 35, 201–232. doi: 10.1111/j.1574-6976.2010.00244.x
Adak, S., Aulak, K. S., and Stuehr, D. J. (2002). Direct evidence for nitric oxide production by a nitric-oxide synthase-like protein from Bacillus subtilis. J. Biol. Chem. 277, 16167–16171. doi: 10.1074/jbc.M201136200
Agrios, G. N. (1988). “1 - INTRODUCTION TO PLANT PATHOLOGY,” in Plant Pathology, 3rdEdition Edn, ed. G. N. Agrios (New York, NY: Academic Press), 3–39. doi: 10.1016/B978-0-12-044563-9.50005-0
Alamri, S. A. (2015). Enhancing the efficiency of the bioagent Bacillus subtilis JF419701 against soil-borne phytopathogens by increasing the productivity of fungal cell wall degrading enzymes. Arch. Phytopathol. Plant Prot. 48, 159–170. doi: 10.1080/03235408.2014.884671
Aleti, G., Sessitsch, A., and Brader, G. (2015). Genome mining: prediction of lipopeptides and polyketides from Bacillus and related Firmicutes. Comput. Struct. Biotechnol. J. 13, 192–203. doi: 10.1016/j.csbj.2015.03.003
An, J., Zhu, W., Liu, Y., Zhang, X., Sun, L., Hong, P., et al. (2015). Purification and characterization of a novel bacteriocin CAMT2 produced by Bacillus amyloliquefaciens isolated from marine fish Epinephelus areolatus. Food Control 51, 278–282. doi: 10.1016/j.foodcont.2014.11.038
Arguelles Arias, A., Ongena, M., Devreese, B., Terrak, M., Joris, B., and Fickers, P. (2013). Characterization of amylolysin, a novel lantibiotic from Bacillus amyloliquefaciens GA1. PLoS One 8:e83037. doi: 10.1371/journal.pone.0083037
Ariffin, H., Abdullah, N., Md Shah, U. K., Shirai, Y., and Hassan, M. A. (2006). Production and characterization of cellulases by Bacillus pumilus EB3. Int. J. Eng. Technol. 3, 47–53.
Arrebola, E., Sivakumar, D., and Korsten, L. (2010). Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control 53, 122–128. doi: 10.1016/j.biocontrol.2009.11.010
Audrain, B., Farag, M. A., Ryu, C.-M., and Ghigo, J.-M. (2015). Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 39, 222–233. doi: 10.1093/femsre/fuu013
Bais, H. P., Fall, R., and Vivanco, J. M. (2004). Biocontrol of Bacillus subtilis against Infection of Arabidopsis Roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin Production. Plant Physiol. 134, 307–319. doi: 10.1104/pp.103.028712
Barbe, V., Cruveiller, S., Kunst, F., Lenoble, P., Meurice, G., Sekowska, A., et al. (2009). From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology 155, 1758–1775. doi: 10.1099/mic.0.027839-0
Béchet, M., Caradec, T., Hussein, W., Abderrahmani, A., Chollet, M., Leclère, V., et al. (2012). Structure, biosynthesis, and properties of kurstakins, nonribosomal lipopeptides from Bacillus spp. Appl. Microbiol. Biotechnol. 95, 593–600. doi: 10.1007/s00253-012-4181-2
Begley, M., Cotter, P. D., Hill, C., and Ross, R. P. (2009). Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl. Environ. Microbiol. 75, 5451–5460. doi: 10.1128/aem.00730-09
Bentley, R., and Haslam, E. (1990). The shikimate pathway — a metabolic tree with many branche. Crit. Rev. Biochem. Mol. Biol. 25, 307–384. doi: 10.3109/10409239009090615
Bernier, S. P., Létoffé, S., Delepierre, M., and Ghigo, J.-M. (2011). Biogenic ammonia modifies antibiotic resistance at a distance in physically separated bacteria. Mol. Microbiol. 81, 705–716. doi: 10.1111/j.1365-2958.2011.07724.x
Besson, F., Peypoux, F., Michel, G., and Delcambe, L. (1976). Characterization of iturin A in antibiotics from various strains of Bacillus subtilis. J. Antibiot. 29, 1043–1049. doi: 10.7164/antibiotics.29.1043
Besson, F., Peypoux, F., Michel, G., and Delcambe, L. (1979). Antifungal activity upon Saccharomyces cerevisiae of iturin A, mycosubtilin, bacillomycin L and of their derivatives; inhibition of this antifungal activity by lipid antagonists. J. Antibiot. 32, 828–833. doi: 10.7164/antibiotics.32.828
Bowman, S. M., and Free, S. J. (2006). The structure and synthesis of the fungal cell wall. Bioessays 28, 799–808. doi: 10.1002/bies.20441
Brötz, H., Bierbaum, G., Markus, A., Molitor, E., and Sahl, H. G. (1995). Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob. Agents Chemother. 39, 714–719. doi: 10.1128/aac.39.3.714
Buckingham, J. (1997). Dictionary of Natural Products. London: CRC press. doi: 10.1007/978-1-4899-6850-0
Caboche, S., Pupin, M., Leclère, V., Fontaine, A., Jacques, P., and Kucherov, G. (2008). NORINE: a database of nonribosomal peptides. Nucleic Acids Res. 36, D326–D331. doi: 10.1093/nar/gkm792
Cane, D. E., and Walsh, C. T. (1999). The parallel and convergent universes of polyketide synthases and nonribosomal peptide synthetases. Chem. Biol. 6, R319–R325. doi: 10.1016/S1074-5521(00)80001-0
Caulier, S., Gillis, A., Colau, G., Licciardi, F., Liépin, M., Desoignies, N., et al. (2018). Versatile antagonistic activities of soil-borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Front. Microbiol. 9:143. doi: 10.3389/fmicb.2018.00143
Cawoy, H., Debois, D., Franzil, L., De Pauw, E., Thonart, P., and Ongena, M. (2015). Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 8, 281–295. doi: 10.1111/1751-7915.12238
Challis, G. L. (2008). Genome mining for novel natural product discovery. J. Med. Chem. 51, 2618–2628. doi: 10.1021/jm700948z
Challis, G. L., and Naismith, J. H. (2004). Structural aspects of non-ribosomal peptide biosynthesis. Curr. Opin. Struct. Biol. 14, 748–756. doi: 10.1016/j.sbi.2004.10.005
Chatterjee, C., Paul, M., Xie, L., and van der Donk, W. A. (2005). Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105, 633–684. doi: 10.1021/cr030105v
Chaves-López, C., Serio, A., Gianotti, A., Sacchetti, G., Ndagijimana, M., Ciccarone, C., et al. (2015). Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 119, 487–499. doi: 10.1111/jam.12847
Chen, H., and Du, L. (2016). Iterative polyketide biosynthesis by modular polyketide synthases in bacteria. Appl. Microbiol. Biotechnol. 100, 541–557. doi: 10.1007/s00253-015-7093-0
Chen, H., Xiao, X., Wang, J., Wu, L., Zheng, Z., and Yu, Z. (2008). Antagonistic effects of volatiles generated by Bacillus subtilis on spore germination and hyphal growth of the plant pathogen, Botrytis cinerea. Biotechnol. Lett. 30, 919–923. doi: 10.1007/s10529-007-9626-9
Chen, X., Scholz, R., Borriss, M., Junge, H., Mogel, G., Kunz, S., et al. (2009). Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens Dare efficient in controlling fire blight disease. J. Biotechnol. 140, 38–44. doi: 10.1016/j.jbiotec.2008.10.015
Chen, X., Vater, J., Piel, J., Franke, P., Scholz, R., Schneider, K., et al. (2006). Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. J. Bacteriol. 188, 4024–4036. doi: 10.1128/jb.00052-06
Cherif-Silini, H., Silini, A., Yahiaoui, B., Ouzari, I., and Boudabous, A. (2016). Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 66, 1087–1097. doi: 10.1007/s13213-016-1194-6
Chernin, L., Toklikishvili, N., Ovadis, M., Kim, S., Ben-Ari, J., Khmel, I., et al. (2011). Quorum-sensing quenching by rhizobacterial volatiles. Environ. Microbiol. Rep. 3, 698–704. doi: 10.1111/j.1758-2229.2011.00284.x
Chopra, L., Singh, G., Choudhary, V., and Sahoo, D. K. (2014). Sonorensin: an antimicrobial peptide, belonging to the heterocycloanthracin subfamily of bacteriocins, from a new marine isolate, Bacillus sonorensis MT93. Appl. Environ. Microbiol. 80, 2981–2990. doi: 10.1128/aem.04259-13
Chopra, L., Singh, G., Jena, K. K., Verma, H., and Sahoo, D. K. (2015). Bioprocess development for the production of sonorensin by Bacillus sonorensis MT93 and its application as a food preservative. Bioresour. Technol. 175, 358–366. doi: 10.1016/j.biortech.2014.10.105
Cleveland, J., Montville, T. J., Nes, I. F., and Chikindas, M. L. (2001). Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71, 1–20. doi: 10.1016/S0168-1605(01)00560-8
Coosemans, J. (2005). Dimethyl disulphide (DMDS): a potential novel nematicide and soil disinfectant. Acta Hortic. 698, 57–64. doi: 10.17660/ActaHortic.2005.698.6
Cosby, W. M., Vollenbroich, D., Lee, O. H., and Zuber, P. (1998). Altered srf expression in Bacillus subtilis resulting from changes in culture pH is dependent on the Spo0K oligopeptide permease and the ComQX system of extracellular control. J. Bacteriol. 180, 1438–1445.
Cotter, P. D., Hill, C., and Ross, R. P. (2005). Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3, 777–788. doi: 10.1038/nrmicro1273
Coutts, R. T., Pitkethly, W. N., and Wibberley, D. G. (1965). Antibacterial activity of some quinolines containing a cyclic hydroxamic acid group. J. Pharm. Sci. 54, 792–795. doi: 10.1002/jps.2600540530
Cox, S. D., Mann, C. M., Markham, J. L., Bell, H. C., Gustafson, J. E., Warmington, J. R., et al. (2000). The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88, 170–175. doi: 10.1046/j.1365-2672.2000.00943.x
Crane, J. M., Gibson, D. M., Vaughan, R. H., and Bergstrom, G. C. (2012). Iturin levels on wheat spikes linked to biological control of fusarium head blight by Bacillus amyloliquefaciens. Phytopathology 103, 146–155. doi: 10.1094/PHYTO-07-12-0154-R
Czajkowski, R., and Jafra, S. (2009). Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 56, 1–16.
Dambolena, J. S., López, A. G., Cánepa, M. C., Theumer, M. G., Zygadlo, J. A., and Rubinstein, H. R. (2008). Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis. Toxicon 51, 37–44. doi: 10.1016/j.toxicon.2007.07.005
de Vrieze, M., Pandey, P., Bucheli, T. D., Varadarajan, A. R., Ahrens, C. H., Weisskopf, L., et al. (2015). Volatile organic compounds from native potato-associated Pseudomonas as potential anti-oomycete agents. Front. Microbiol. 6:1295. doi: 10.3389/fmicb.2015.01295
Delves-Broughton, J. (1990). Nisin and its application as a food preservative. Int. J. Dairy Technol. 43, 73–76. doi: 10.1111/j.1471-0307.1990.tb02449.x
Dertz, E. A., Xu, J., Stintzi, A., and Raymond, K. N. (2006). Bacillibactin-mediated iron transport in Bacillus subtilis. J. Am. Chem. Soc. 128, 22–23. doi: 10.1021/ja055898c
Dickschat, J. S., Bode, H. B., Wenzel, S. C., Müller, R., and Schulz, S. (2005). Biosynthesis and identification of volatiles released by the myxobacterium Stigmatella aurantiaca. Chembiochem 6, 2023–2033. doi: 10.1002/cbic.200500174
Dimkić, I., Živković, S., Berić, T., Ivanović,Ž, Gavrilović, V., Stanković, S., et al. (2013). Characterization and evaluation of two Bacillus strains, SS-12.6 and SS-13.1, as potential agents for the control of phytopathogenic bacteria and fungi. Biol. Control 65, 312–321. doi: 10.1016/j.biocontrol.2013.03.012
Dong, Y.-H., Zhang, X.-F., Xu, J.-L., and Zhang, L.-H. (2004). Insecticidal Bacillus thuringiensis silences Erwinia carotovora virulence by a new form of microbial antagonism, signal interference. Appl. Environ. Microbiol. 70, 954–960. doi: 10.1128/aem.70.2.954-960.2004
Dubois, J. Y. F., Kouwen, T. R., Schurich, A. K. C., Reis, C. R., Ensing, H. T., Trip, E. N., et al. (2009). Immunity to the bacteriocin sublancin 168 is determined by the SunI (YolF) protein of Bacillus subtilis. Antimicrob. Agents Chemother. 53, 651–661. doi: 10.1128/aac.01189-08
Dunlap, C. A., Schisler, D. A., Price, N. P., and Vaughn, S. F. (2011). Cyclic lipopeptide profile of three Bacillus subtilis strains; antagonists of Fusarium head blight. J. Microbiol. 49, 603–609. doi: 10.1007/s12275-011-1044-y
Effmert, U., Kalderás, J., Warnke, R., and Piechulla, B. (2012). Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 38, 665–703. doi: 10.1007/s10886-012-0135-5
Eshita, S. M., Roberto, N. H., Beale, J. M., Mamiya, B. M., and Workman, R. F. (1995). Bacillomycin Lc, a new antibiotic of the iturin group: isolations, structures, and antifungal activities of the congeners. J. Antibiot. 48, 1240–1247. doi: 10.7164/antibiotics.48.1240
Eustáquio, A. S., McGlinchey, R. P., Liu, Y., Hazzard, C., Beer, L. L., Florova, G., et al. (2009). Biosynthesis of the salinosporamide A polyketide synthase substrate chloroethylmalonyl-coenzyme A from S-adenosyl-L-methionine. Proc. Natl. Acad. Sci. U.S.A. 106, 12295–12300. doi: 10.1073/pnas.0901237106
Even, S., Burguière, P., Auger, S., Soutourina, O., Danchin, A., and Martin-Verstraete, I. (2006). Global control of cysteine metabolism by CymR in Bacillus subtilis. J. Bacteriol. 188, 2184–2197. doi: 10.1128/jb.188.6.2184-2197.2006
Fan, B., Blom, J., Klenk, H.-P., and Borriss, R. (2017). Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 8:22. doi: 10.3389/fmicb.2017.00022
Fickers, P., Guez, J.-S., Damblon, C., Leclère, V., Béchet, M., Jacques, P., et al. (2009). High-level biosynthesis of the anteiso-C17 isoform of the antibiotic mycosubtilin in Bacillus subtilis and characterization of its candidacidal activity. Appl. Environ. Microbiol. 75, 4636–4640. doi: 10.1128/aem.00548-09
Fisch, K. M. (2013). Biosynthesis of natural products by microbial iterative hybrid PKS–NRPS. RSC Adv. 3, 18228–18247. doi: 10.1039/C3RA42661K
Fisher, A. J., Rosenstiel, T. N., Shirk, M. C., and Fall, R. (2001). Nonradioactive assay for cellular dimethylallyl diphosphate. Anal. Biochem. 292, 272–279. doi: 10.1006/abio.2001.5079
Fu, L.-H., Hu, K.-D., Hu, L.-Y., Li, Y.-H., Hu, L.-B., Yan, H., et al. (2014). An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS One 9:e104206. doi: 10.1371/journal.pone.0104206
Fuchs, S. W., Jaskolla, T. W., Bochmann, S., Kötter, P., Wichelhaus, T., Karas, M., et al. (2011). Entianin, a novel subtilin-like lantibiotic from Bacillus subtilis DSM 15029T with high antimicrobial activity. Appl. Environ. Microbiol. 77, 1698–1707. doi: 10.1128/aem.01962-10
Furuta, Y., Harima, H., Ito, E., Maruyama, F., Ohnishi, N., Osaki, K., et al. (2018). Loss of bacitracin resistance due to a large genomic deletion among Bacillus anthracis strains. mSystems 3:e00182-18. doi: 10.1128/mSystems.00182-18
Gao, L., Han, J., Liu, H., Qu, X., Lu, Z., and Bie, X. (2017). Plipastatin and surfactin coproduction by Bacillus subtilis pB2-L and their effects on microorganisms. Antonie Van Leeuwenhoek 110, 1007–1018. doi: 10.1007/s10482-017-0874-y
Gao, Z., Zhang, B., Liu, H., Han, J., and Zhang, Y. (2017). Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 105, 27–39. doi: 10.1016/j.biocontrol.2016.11.007
Gautam, N., and Sharma, N. (2009). Bacteriocin: safest approach to preserve food products. Indian J. Microbiol. 49, 204–211. doi: 10.1007/s12088-009-0048-3
Geraldine, A. M., Lopes, F. A. C., Carvalho, D. D. C., Barbosa, E. T., Rodrigues, A. R., Brandão, R. S., et al. (2013). Cell wall-degrading enzymes and parasitism of sclerotia are key factors on field biocontrol of white mold by Trichoderma spp. Biol. Control 67, 308–316. doi: 10.1016/j.biocontrol.2013.09.013
Gerik, J. S. (2005). Evaluation of soil fumigants applied by drip irrigation for liatris production. Plant Dis. 89, 883–887. doi: 10.1094/PD-89-0883
Gershenzon, J., and Dudareva, N. (2007). The function of terpene natural products in the natural world. Nat. Chem. Biol. 3, 408–414. doi: 10.1038/nchembio.2007.5
Giorgio, A., De Stradis, A., Lo Cantore, P., and Iacobellis, N. S. (2015). Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 6:1056. doi: 10.3389/fmicb.2015.01056
Gomaa, E. Z. (2012). Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: their potential in antifungal biocontrol. J. Microbiol. 50, 103–111. doi: 10.1007/s12275-012-1343-y
Gong, A.-D., Li, H.-P., Yuan, Q.-S., Song, X.-S., Yao, W., He, W.-J., et al. (2015). Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS One 10:e0116871. doi: 10.1371/journal.pone.0116871
Gong, Q., Zhang, C., Lu, F., Zhao, H., Bie, X., and Lu, Z. (2014). Identification of bacillomycin D from Bacillus subtilis fmbJ and its inhibition effects against Aspergillus flavus. Food Control 36, 8–14. doi: 10.1016/j.foodcont.2013.07.034
González, J. E., and Keshavan, N. D. (2006). Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 70, 859–875. doi: 10.1128/mmbr.00002-06
Gordon, R., Haynes, W., Pang, C., and Smith, N. (1973). The Genus Bacillus. Washington, DC: United States Department of Agriculture, 109–126.
Gotor-Vila, A., Teixidó, N., Di Francesco, A., Usall, J., Ugolini, L., Torres, R., et al. (2017). Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 64, 219–225. doi: 10.1016/j.fm.2017.01.006
Gu, Y.-Q., Mo, M.-H., Zhou, J.-P., Zou, C.-S., and Zhang, K.-Q. (2007). Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 39, 2567–2575. doi: 10.1016/j.soilbio.2007.05.011
Guan, Z., Xue, D., Abdallah, I. I., Dijkshoorn, L., Setroikromo, R., Lv, G., et al. (2015). Metabolic engineering of Bacillus subtilis for terpenoid production. Appl. Microbiol. Biotechnol. 99, 9395–9406. doi: 10.1007/s00253-015-6950-1
Guder, A., Wiedemann, I., and Sahl, H.-G. (2000). Posttranslationally modified bacteriocins—the lantibiotics. Biopolymers 55, 62–73. doi: 10.1002/1097-0282(2000)55:1<62::AID-BIP60>3.0.CO;2-Y
Guo, Q., Dong, W., Li, S., Lu, X., Wang, P., Zhang, X., et al. (2014). Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol. Res. 169, 533–540. doi: 10.1016/j.micres.2013.12.001
Gustafson, K., Roman, M., and Fenical, W. (1989). The macrolactin, a novel class of antiviral and cytotoxic macrolides from a deep-sea marine bacterium. J. Am. Chem. Soc. 111, 7519–7524. doi: 10.1021/ja00201a036
Haidar, R., Roudet, J., Bonnard, O., Dufour, M. C., Corio-Costet, M. F., Fert, M., et al. (2016). Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiol. Res. 192, 172–184. doi: 10.1016/j.micres.2016.07.003
Hammami, I., Jaouadi, B., Bacha, A. B., Rebai, A., Bejar, S., Nesme, X., et al. (2012). Bacillus subtilis bacteriocin Bac 14B with a broad inhibitory spectrum: purification, amino acid sequence analysis, and physicochemical characterization. Biotechnol. Bioprocess Eng. 17, 41–49. doi: 10.1007/s12257-010-0401-8
Hammer, K. A., Carson, C. F., and Riley, T. V. (2003). Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 95, 853–860. doi: 10.1046/j.1365-2672.2003.02059.x
Hamoen, L. W., Venema, G., and Kuipers, O. P. (2003). Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology 149, 9–17. doi: 10.1099/mic.0.26003-0
Heinzmann, S., Entian, K.-D., and Stein, T. (2006). Engineering Bacillus subtilis ATCC 6633 for improved production of the lantibiotic subtilin. Appl. Microbiol. Biotechnol. 69, 532–536. doi: 10.1007/s00253-005-0023-9
Herrera-Estrella, A., and Chet, I. (1999). Chitinases in biological control. EXS 87, 171–184. doi: 10.1007/978-3-0348-8757-1_12
Hertweck, C. (2009). The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 48, 4688–4716. doi: 10.1002/anie.200806121
Horsman, G. P., Van Lanen, S. G., and Shen, B. (2009). Chapter 5 Iterative type I polyketide synthases for enediyne core biosynthesis. Methods Enzymol. 459, 97–112. doi: 10.1016/S0076-6879(09)04605-9
Howell, C., Beier, R., and Stipanovic, R. (1988). Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Phytopathology 78, 1075–1078. doi: 10.1094/Phyto-78-1075
Hsieh, F.-C., Lin, T.-C., Meng, M., and Kao, S.-S. (2008). Comparing methods for identifying Bacillus strains capable of producing the antifungal lipopeptide iturin A. Curr. Microbiol. 56, 1–5. doi: 10.1007/s00284-007-9003-x
Hussein, A., and Al-Janabi, S. (2006). Identification of bacitracin produced by local isolate of Bacillus licheniformis. Afr. J. Biotechnol. 5, 1600–1601.
Inoue, Y., Shiraishi, A., Hada, T., Hirose, K., Hamashima, H., and Shimada, J. (2004). The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 237, 325–331. doi: 10.1111/j.1574-6968.2004.tb09714.x
Insam, H., and Seewald, M. S. A. (2010). Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 46, 199–213. doi: 10.1007/s00374-010-0442-3
Jacques, P. (2011). “Surfactin and other lipopeptides from Bacillus spp,” in Biosurfactants: From Genes to Applications, ed. G. Soberón-Chávez (Berlin: Springer), 57–91. doi: 10.1007/978-3-642-14490-5_3
Janiak, A. M., and Milewski, S. (2001). Mechanism of antifungal action of kanosamine. Med. Mycol. 39, 401–408. doi: 10.1080/mmy.39.5.401.408
Jaruchoktaweechai, C., Suwanborirux, K., Tanasupawatt, S., Kittakoop, P., and Menasveta, P. (2000). New Macrolactins from a Marine Bacillus sp. Sc026. J. Nat. Prod. 63, 984–986. doi: 10.1021/np990605c
Jenny, K., Käppeli, O., and Fiechter, A. (1991). Biosurfactants from Bacillus licheniformis: structural analysis and characterization. Appl. Microbiol. Biotechnol. 36, 5–13. doi: 10.1007/bf00164690
Johnson, B. A., Anker, H., and Meleney, F. L. (1945). Bacitracin: a new antibiotic produced by a member of the B. subtilis group. Science 102, 376–377. doi: 10.1126/science.102.2650.376
Julsing, M. K., Rijpkema, M., Woerdenbag, H. J., Quax, W. J., and Kayser, O. (2007). Functional analysis of genes involved in the biosynthesis of isoprene in Bacillus subtilis. Appl. Microbiol. Biotechnol. 75, 1377–1384. doi: 10.1007/s00253-007-0953-5
Justicia, J., Oltra, J. E., Barrero, A. F., Guadaño, A., González-Coloma, A., and Cuerva, J. M. (2005). Total synthesis of 3-hydroxydrimanes mediated by titanocene(III) – evaluation of their antifeedant activity. Eur. J. Org. Chem. 2005, 712–718. doi: 10.1002/ejoc.200400634
Kai, M., Haustein, M., Molina, F., Petri, A., Scholz, B., and Piechulla, B. (2009). Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81, 1001–1012. doi: 10.1007/s00253-008-1760-3
Kawulka, K. E., Sprules, T., Diaper, C. M., Whittal, R. M., McKay, R. T., Mercier, P., et al. (2004). Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to (-carbon cross-links: formation and reduction of (-Thio-(-Amino Acid derivatives. Biochemistry 43, 3385–3395. doi: 10.1021/bi0359527
Kenig, M., and Abraham, E. P. (1976). Antimicrobial activities and antagonists of bacilysin and anticapsin. Microbiology 94, 37–45. doi: 10.1099/00221287-94-1-37
Kenig, M., Vandamme, E., and Abraham, E. P. (1976). The mode of action of bacilysin and anticapsin and biochemical properties of bacilysin-resistant mutants. Microbiology 94, 46–54. doi: 10.1099/00221287-94-1-46
Kim, H.-S., Yoon, B.-D., Lee, C.-H., Suh, H.-H., Oh, H.-M., Katsuragi, T., et al. (1997). Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. J. Ferment. Bioeng. 84, 41–46. doi: 10.1016/S0922-338X(97)82784-5
Kim, P. I., Ryu, J., Kim, Y. H., and Chi, Y.-T. (2010). Production of biosurfactant lipopeptides iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 20, 138–145. doi: 10.4014/jmb.0905.05007
Klaenhammer, T. R. (1988). Bacteriocins of lactic acid bacteria. Biochimie 70, 337–349. doi: 10.1016/0300-9084(88)90206-4
Kleerebezem, M. (2004). Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. Peptides 25, 1405–1414. doi: 10.1016/j.peptides.2003.10.021
Konz, D., Klens, A., Schörgendorfer, K., and Marahiel, M. A. (1997). The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: molecular characterization of three multi-modular peptide synthetases. Chem. Biol. 4, 927–937. doi: 10.1016/S1074-5521(97)90301-X
Kopp, F., and Marahiel, M. A. (2007). Macrocyclization strategies in polyketide and nonribosomal peptide biosynthesis. Nat. Prod. Rep. 24, 735–749. doi: 10.1039/B613652B
Korpi, A., Järnberg, J., and Pasanen, A.-L. (2009). Microbial volatile organic compounds. Crit. Rev. Toxicol. 39, 139–193. doi: 10.1080/10408440802291497
Kosuge, T., and Kamiya, H. (1962). Discovery of a pyrazine in a natural product: tetramethylpyrazine from cultures of a strain of Bacillus subtilis. Nature 193:776. doi: 10.1038/193776a0
Kugler, M., Loeffler, W., Rapp, C., Kern, A., and Jung, G. (1990). Rhizocticin A, an antifungal phosphono-oligopeptide of Bacillus subtilis ATCC 6633: biological properties. Arch. Microbiol. 153, 276–281. doi: 10.1007/bf00249082
Kumar, P., Dubey, R. C., and Maheshwari, D. K. (2012). Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 167, 493–499. doi: 10.1016/j.micres.2012.05.002
Kwon, J. W., and Kim, S. D. (2014). Characterization of an antibiotic produced by Bacillus subtilis JW-1 that suppresses Ralstonia solanacearum. J. Microbiol. Biotechnol. 24, 13–18. doi: 10.4014/jmb.1308.08060
Landy, M., Warren, G. H., RosenmanM, S. B., and Colio, L. G. (1948). Bacillomycin: an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc. Soc. Exp. Biol. Med. 67, 539–541. doi: 10.3181/00379727-67-16367
Larroche, C., Besson, I., and Gros, J.-B. (1999). High pyrazine production by Bacillus subtilis in solid substrate fermentation on ground soybeans. Process Biochem. 34, 667–674. doi: 10.1016/S0032-9592(98)00141-1
Leclere, V., Bechet, M., Adam, A., Guez, J. S., Wathelet, B., Ongena, M., et al. (2005). Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 71, 4577–4584. doi: 10.1128/aem.71.8.4577-4584.2005
Lee, D. W., and Kim, B. S. (2015). Antimicrobial cyclic peptides for plant disease control. Plant Pathol. J. 31, 1–11. doi: 10.5423/PPJ.RW.08.2014.0074
Lee, H., Churey, J. J., and Worobo, R. W. (2008). Purification and structural characterization of bacillomycin F produced by a bacterial honey isolate active against Byssochlamys fulva H25. J. Appl. Microbiol. 105, 663–673. doi: 10.1111/j.1365-2672.2008.03797.x
Lee, S., Peterson, C. J., and Coats, J. R. (2003). Fumigation toxicity of monoterpenoids to several stored product insects. J. Stored Prod. Res. 39, 77–85. doi: 10.1016/S0022-474X(02)00020-6
Lee, S.-C., Kim, S.-H., Park, I.-H., Chung, S.-Y., and Choi, Y.-L. (2007). Isolation and structural analysis of bamylocin A, novel lipopeptide from Bacillus amyloliquefaciens LP03 having antagonistic and crude oil-emulsifying activity. Arch. Microbiol. 188, 307–312. doi: 10.1007/s00203-007-0250-9
Leejeerajumnean, A., Duckham, S. C., Owens, J. D., and Ames, J. M. (2001). Volatile compounds in Bacillus-fermented soybeans. J. Sci. Food Agric. 81, 525–529. doi: 10.1002/jsfa.843
Lemfack, M. C., Gohlke, B.-O., Toguem, S. M. T., Preissner, S., Piechulla, B., and Preissner, R. (2018). mVOC 2.0: a database of microbial volatiles. Nucleic Acids Res. 46, D1261–D1265. doi: 10.1093/nar/gkx1016
Li, L., Ma, M., Huang, R., Qu, Q., Li, G., Zhou, J., et al. (2012). Induction of chlamydospore formation in Fusarium by cyclic lipopeptide antibiotics from Bacillus subtilis C2. J. Chem. Ecol. 38, 966–974. doi: 10.1007/s10886-012-0171-1
Li, N., Shi, Z., Tang, Y., Chen, J., and Li, X. (2008). Recent progress on the total synthesis of acetogenins from Annonaceae. Beilstein J. Org. Chem. 4:48. doi: 10.3762/bjoc.4.48
Linnett, P. E., and Strominger, J. L. (1973). Additional antibiotic inhibitors of peptidoglycan synthesis. Antimicrob. Agents Chemother. 4, 231–236. doi: 10.1128/aac.4.3.231
Liu, W. W., Mu, W., Zhu, B. Y., Du, Y. C., and Liu, F. (2008). Antagonistic activities of volatiles from four strains of Bacillus spp. and Paenibacillus spp. against soil-borne plant pathogens. Agric. Sci. China 7, 1104–1114. doi: 10.1016/S1671-2927(08)60153-4
Liu, Y., Chen, Z., Ng, T. B., Zhang, J., Zhou, M., Song, F., et al. (2007). Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides 28, 553–559. doi: 10.1016/j.peptides.2006.10.009
Loeffler, W., Tschen, J. S.-M., Vanittanakom, N., Kugler, M., Knorpp, E., Hsieh, T. F., et al. (1986). Antifungal effects of bacilysin and fengymycin from Bacillus subtilis F-29-3 a comparison with activities of other Bacillus antibiotics. J. Phytopathol. 115, 204–213. doi: 10.1111/j.1439-0434.1986.tb00878.x
Loiseau, C., Schlusselhuber, M., Bigot, R., Bertaux, J., Berjeaud, J.-M., and Verdon, J. (2015). Surfactin from Bacillus subtilis displays an unexpected anti-Legionella activity. Appl. Microbiol. Biotechnol. 99, 5083–5093. doi: 10.1007/s00253-014-6317-z
Lu, X. L., Xu, Q. Z., Shen, Y. H., Liu, X. Y., Jiao, B. H., Zhang, W. D.et al. (2008). Macrolactin S, a novel macrolactin antibiotic from marine Bacillus sp. Nat. Prod. Res. 22, 342–347. doi: 10.1080/14786410701768162
Luo, C., Liu, X., Zhou, X., Guo, J., Truong, J., Wang, X., et al. (2015). Unusual biosynthesis and structure of locillomycins from Bacillus subtilis 916. Appl. Environ. Microbiol. 81, 6601–6609. doi: 10.1128/aem.01639-15
Lupetti, A., Danesi, R., Campa, M., Tacca, M. D., and Kelly, S. (2002). Molecular basis of resistance to azole antifungals. Trends Mol. Med. 8, 76–81. doi: 10.1016/S1471-4914(02)02280-3
Ma, Z., Hu, J., Wang, X., and Wang, S. (2013). NMR spectroscopic and MS/MS spectrometric characterization of a new lipopeptide antibiotic bacillopeptin B1 produced by a marine sediment-derived Bacillus amyloliquefaciens SH-B74. J. Antibiot. 67, 175–178. doi: 10.1038/ja.2013.89
Mahlstedt, S. A., and Walsh, C. T. (2010). Investigation of anticapsin biosynthesis reveals a four-enzyme pathway to tetrahydrotyrosine in Bacillus subtilis. Biochemistry 49, 912–923. doi: 10.1021/bi9021186
Majumdar, S. K., and Bose, S. K. (1958). Mycobacillin, a new antifungal antibiotic produced by B. subtilis. Nature 181, 134–135. doi: 10.1038/181134a0
Malfanova, N., Franzil, L., Lugtenberg, B., Chebotar, V., and Ongena, M. (2012). Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch. Microbiol. 194, 893–899. doi: 10.1007/s00203-012-0823-0
Marquez-Villavicencio, M. D. P., Weber, B., Witherell, R. A., Willis, D. K., and Charkowski, A. O. (2011). The 3-Hydroxy-2-Butanone pathway is required for Pectobacterium carotovorum pathogenesis. PLoS One 6:e22974. doi: 10.1371/journal.pone.0022974
Marx, R., Stein, T., Entian, K.-D., and Glaser, S. J. (2001). Structure of the Bacillus subtilis peptide antibiotic subtilosin A determined by 1H-NMR and matrix assisted laser desorption/ionization time-of-flight mass spectrometry. J. Protein Chem. 20, 501–506. doi: 10.1023/a:1012562631268
May, J. J., Wendrich, T. M., and Marahiel, M. A. (2001). The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-Dihydroxybenzoate-Glycine-Threonine trimeric ester bacillibactin. J. Biol. Chem. 276, 7209–7217. doi: 10.1074/jbc.M009140200
McAuliffe, O., Ross, R. P., and Hill, C. (2001). Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol. Rev. 25, 285–308. doi: 10.1111/j.1574-6976.2001.tb00579.x
McIntosh, J. A., Donia, M. S., and Schmidt, E. W. (2009). Ribosomal peptide natural products: bridging the ribosomal and nonribosomal worlds. Nat. Prod. Rep. 26, 537–559. doi: 10.1039/B714132G
McNeal, K. S., and Herbert, B. E. (2009). Volatile organic metabolites as indicators of soil microbial activity and community composition shifts. Soil Sci. Soc. Am. J. 73, 579–588. doi: 10.2136/sssaj2007.0245
Mhammedi, A., Peypoux, F., Besson, F., and Michel, G. (1982). Bacillomycin F, a new antibiotic of iturin group: isolation and characterization. J. Antibiot. 35, 306–311. doi: 10.7164/antibiotics.35.306
Milewski, S., Chmara, H., and Borowski, E. (1986). Antibiotic tetaine — a selective inhibitor of chitin and mannoprotein biosynthesis in Candida albicans. Arch. Microbiol. 145, 234–240. doi: 10.1007/bf00443651
Mojid Mondol, M. A., Kim, J. H., Lee, H.-S., Lee, Y.-J., and Shin, H. J. (2011). Macrolactin W, a new antibacterial macrolide from a marine Bacillus sp. Bioorg. Med. Chem. Lett. 21, 3832–3835. doi: 10.1016/j.bmcl.2010.12.050
Moldenhauer, J., Chen, X.-H., Borriss, R., and Piel, J. (2007). Biosynthesis of the antibiotic bacillaene, the product of a giant polyketide synthase complex of the trans-AT family. Angew. Chem. Int. Ed. Engl. 46, 8195–8197. doi: 10.1002/anie.200703386
Moyne, A.-L., Shelby, R., Cleveland, T. E., and Tuzun, S. (2001). Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J. Appl. Microbiol. 90, 622–629. doi: 10.1046/j.1365-2672.2001.01290.x
Müller, S., Strack, S. N., Hoefler, B. C., Straight, P., Kearns, D. B., and Kirby, J. R. (2014). Bacillaene and sporulation protect Bacillus subtilis from predation by Myxococcus xanthus. Appl. Environ. Microbiol. 80, 5603–5610. doi: 10.1128/aem.01621-14
Mulligan, C. N., Yong, R. N., and Gibbs, B. F. (2001). Heavy metal removal from sediments by biosurfactants. J. Hazard. Mater. 85, 111–125. doi: 10.1016/S0304-3894(01)00224-2
Nagao, T., Adachi, K., Sakai, M., Nishijima, M., and Sano, H. (2001). Novel macrolactins as antibiotic lactones from a marine bacterium. J. Antibiot. 54, 333–339. doi: 10.7164/antibiotics.54.333
Naruse, N., Tenmyo, O., Kobaru, S., Kamei, H., Miyaki, T., Konishi, M., et al. (1990). Pumilacidin, a complex of new antiviral antibiotics. J. Antibiot. 43, 267–280. doi: 10.7164/antibiotics.43.267
Nes, I. F., Diep, D. B., and Holo, H. (2007). Bacteriocin diversity in Streptococcus and Enterococcus. J. Bacteriol. 189, 1189–1198. doi: 10.1128/jb.01254-06
Nicholson, W. L. (2002). Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 59, 410–416. doi: 10.1007/s00018-002-8433-7
Oman, T. J., and van der Donk, W. A. (2009). Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat. Chem. Biol. 6, 9–18. doi: 10.1038/nchembio.286
Ongena, M., Duby, F., Jourdan, E., Beaudry, T., Jadin, V., Dommes, J., et al. (2005). Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl. Microbiol. Biotechnol. 67, 692–698. doi: 10.1007/s00253-004-1741-0
Ongena, M., and Jacques, P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. doi: 10.1016/j.tim.2007.12.009
O’Sullivan, L., Ross, R. P., and Hill, C. (2002). Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 84, 593–604. doi: 10.1016/S0300-9084(02)01457-8
Owens, J. D., Allagheny, N., Kipping, G., and Ames, J. M. (1997). Formation of volatile compounds during Bacillus subtilis fermentation of soya beans. J. Sci. Food Agric. 74, 132–140. doi: 10.1002/(SICI)1097-0010(199705)74:1<132::AID-JSFA779>3.0.CO;2-8
Paik, S. H., Chakicherla, A., and Hansen, J. N. (1998). Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of, sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168. J. Biol. Chem. 273, 23134–23142. doi: 10.1074/jbc.273.36.23134
Parisot, J., Carey, S., Breukink, E., Chan, W. C., Narbad, A., and Bonev, B. (2008). Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic. Antimicrob. Agents Chemother. 52, 612–618. doi: 10.1128/aac.00836-07
Patel, P. S., Huang, S., Fisher, S., Pirnik, D., Aklonis, C., Dean, L., et al. (1995). Bacillaene, a novel inhibitor of procaryotic protein synthesis produced by Bacillus subtilis: production, taxonomy, isolation, physico-chemical characterization and biological activity. J. Antibiot. 48, 997–1003. doi: 10.7164/antibiotics.48.997
Pattnaik, P., Kaushik, J. K., Grover, S., and Batish, V. K. (2001). Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J. Appl. Microbiol. 91, 636–645. doi: 10.1046/j.1365-2672.2001.01429.x
Pedersen, P. B., Bjørnvad, M. E., Rasmussen, M. D., and Petersen, J. N. (2002). Cytotoxic potential of industrial strains of Bacillus sp. Regul. Toxicol. Pharmacol. 36, 155–161. doi: 10.1006/rtph.2002.1574
Peñuelas, J., Asensio, D., Tholl, D., Wenke, K., Rosenkranz, M., Piechulla, B., et al. (2014). Biogenic volatile emissions from the soil. Plant Cell Environ. 37, 1866–1891. doi: 10.1111/pce.12340
Podile, A. R., and Prakash, A. P. (1996). Lysis and biological control of Aspergillus niger by Bacillus subtilis AF 1. Can. J. Microbiol. 42, 533–538. doi: 10.1139/m96-072
Preecha, C., Sadowsky, M. J., and Prathuangwong, S. (2010). Lipopeptide surfactin produced by Bacillus amyloliquefaciens KPS46 is required for biocontrol efficacy against Xanthomonas axonopodis pv. Glycines. Kasetsart J. Nat. Sci. 44, 84–99.
Priest, F. G., Goodfellow, M., Shute, L. A., and Berkeley, R. C. W. (1987). Bacillus amyloliquefaciens sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 37, 69–71. doi: 10.1099/00207713-37-1-69
Qi, G., Zhu, F., Du, P., Yang, X., Qiu, D., Yu, Z., et al. (2010). Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 31, 1978–1986. doi: 10.1016/j.peptides.2010.08.003
Raaijmakers, J. M., De Bruijn, I., Nybroe, O., and Ongena, M. (2010). Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol. Rev. 34, 1037–1062. doi: 10.1111/j.1574-6976.2010.00221.x
Ramarathnam, R., Bo, S., Chen, Y., Dilantha Fernando, W. G., Xuewen, G., and Kievit, T. (2007). Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can. J. Microbiol. 53, 901–911. doi: 10.1139/w07-049
Rapp, C., Jung, G., Katzer, W., and Loeffler, W. (1988). Chlorotetain from Bacillus subtilis, an antifungal dipeptide with an unusual chlorine-containing amino acid. Angew. Chem. Int. Ed. Engl. 27, 1733–1734. doi: 10.1002/anie.198817331
Raubitschek, F., and Dostrovsky, A. (1950). An antibiotic active against dermatophytes, derived from Bacillus Subtilis. Dermatology 100, 45–49. doi: 10.1159/000257151
Raza, W., Ling, N., Yang, L., Huang, Q., and Shen, Q. (2016). Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 6:24856. doi: 10.1038/srep24856
Riley, M. A., and Wertz, J. E. (2002). Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56, 117–137. doi: 10.1146/annurev.micro.56.012302.161024
Rohr, J. (1992). Comparison of multicyclic polyketides by folding analysis: a novel approach to recognize biosynthetic and/or evolutionary interrelationships of the natural products or intermediates and its exemplification on hepta-, octa-, and decaketides. J. Org. Chem. 57, 5217–5223. doi: 10.1021/jo00045a040
Romano, A., Vitullo, D., Senatore, M., Lima, G., and Lanzotti, V. (2013). Antifungal cyclic lipopeptides from Bacillus amyloliquefaciens strain BO5A. J. Nat. Prod. 76, 2019–2025. doi: 10.1021/np400119n
Romero, D., de Vicente, A., Rakotoaly, R. H., Dufour, S. E., Veening, J.-W., Arrebola, E., et al. (2007). The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis Toward Podosphaera fusca. Mol. Plant Microbe Interact. 20, 430–440. doi: 10.1094/MPMI-20-4-0430
Romero-Tabarez, M., Jansen, R., Sylla, M., Lünsdorf, H., Häußler, S., Santosa, D. A., et al. (2006). 7-O-Malonyl macrolactin A, a new macrolactin antibiotic from Bacillus subtilis active against methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, and a small-colony variant of Burkholderia cepacia. Antimicrob. Agents Chemother. 50, 1701–1709. doi: 10.1128/aac.50.5.1701-1709.2006
Ryu, C.-M. (2015). “Bacterial volatiles as airborne signals for plants and bacteria,” in Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture, ed. B. Lugtenberg (Cham: Springer International Publishing), 53–61.
Ryu, C.-M., Farag, M. A., Hu, C.-H., Reddy, M. S., Kloepper, J. W., and Paré, P. W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134, 1017–1026. doi: 10.1104/pp.103.026583
Ryu, C.-M., Farag, M. A., Hu, C.-H., Reddy, M. S., Wei, H.-X., Paré, P. W., et al. (2003). Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100, 4927–4932. doi: 10.1073/pnas.0730845100
Sabaté, D. C., and Audisio, M. C. (2013). Inhibitory activity of surfactin, produced by different Bacillus subtilis subsp. subtilis strains, against Listeria monocytogenes sensitive and bacteriocin-resistant strains. Microbiol. Res. 168, 125–129. doi: 10.1016/j.micres.2012.11.004
Saggese, A., Culurciello, R., Casillo, A., Corsaro, M., Ricca, E., and Baccigalupi, L. (2018). A marine isolate of Bacillus pumilus secretes a pumilacidin active against Staphylococcus aureus. Mar. Drugs 16:E180. doi: 10.3390/md16060180
Schmidt, E. W. (2010). The hidden diversity of ribosomal peptide natural products. BMC Biol. 8:83. doi: 10.1186/1741-7007-8-83
Schmidt, R., Cordovez, V., de Boer, W., Raaijmakers, J., and Garbeva, P. (2015). Volatile affairs in microbial interactions. ISME J. 9, 2329–2335. doi: 10.1038/ismej.2015.42
Scholz, R., Molohon, K. J., Nachtigall, J., Vater, J., Markley, A. L., Süssmuth, R. D., et al. (2011). Plantazolicin, a novel microcin B17/Streptolysin S-Like natural product from Bacillus amyloliquefaciens FZB42. J. Bacteriol. 193, 215–224. doi: 10.1128/jb.00784-10
Scholz, R., Vater, J., Budiharjo, A., Wang, Z., He, Y., Dietel, K., et al. (2014). Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 196, 1842–1852. doi: 10.1128/jb.01474-14
Schulz, S., and Dickschat, J. S. (2007). Bacterial volatiles: the smell of small organisms. Nat. Prod. Rep. 24, 814–842. doi: 10.1039/b507392h
Scortichini, M., and Rossi, M. P. (1991). Preliminary in vitro evaluation of the antimicrobial activity of terpenes and terpenoids towards Erwinia amylovora. J. Appl. Bacteriol. 71, 109–112. doi: 10.1111/j.1365-2672.1991.tb02963.x
Shafi, J., Tian, H., and Ji, M. (2017). Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol. Biotechnol. Equ. 31, 446–459. doi: 10.1080/13102818.2017.1286950
Shatalin, K., Shatalina, E., Mironov, A., and Nudler, E. (2011). H2S: a universal defense against antibiotics in bacteria. Science 334, 986–990. doi: 10.1126/science.1209855
Shelburne, C. E., An, F. Y., Dholpe, V., Ramamoorthy, A., Lopatin, D. E., and Lantz, M. S. (2007). The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J. Antimicrob. Chemother. 59, 297–300. doi: 10.1093/jac/dkl495
Shrivastava, G., Ownley, B. H., Augé, R. M., Toler, H., Dee, M., Vu, A., et al. (2015). Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 65, 65–74. doi: 10.1007/s13199-015-0319-1
Siewert, G., and Strominger, J. L. (1967). Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc. Natl. Acad. Sci. U.S.A. 57, 767–773. doi: 10.1073/pnas.57.3.767
Smith, S., and Tsai, S.-C. (2007). The type I fatty acid and polyketide synthases: a tale of two megasynthases. Nat. Prod. Rep. 24, 1041–1072. doi: 10.1039/B603600G
Snelders, E., Huis in ’t Veld, R. A., Rijs, A. J., Kema, G. H. J., Melchers, W. J. G., and Verweij, P. E. (2009). Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microbiol. 75, 4053–4057. doi: 10.1128/aem.00231-09
Sourabié, A. M., Spinnler, H.-E., Bourdat-Deschamps, M., Tallon, R., Landaud, S., and Bonnarme, P. (2012). S-methyl thioesters are produced from fatty acids and branched-chain amino acids by brevibacteria: focus on l-leucine catabolic pathway and identification of acyl–CoA intermediates. Appl. Microbiol. Biotechnol. 93, 1673–1683. doi: 10.1007/s00253-011-3500-3
Spieß, T., Korn, S. M., Kötter, P., and Entian, K.-D. (2015). Autoinduction Specificities of the Lantibiotics Subtilin and Nisin. Appl. Environ. Microbiol. 81, 7914–7923. doi: 10.1128/aem.02392-15
Stein, T. (2005). Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 56, 845–857. doi: 10.1111/j.1365-2958.2005.04587.x
Stein, T., Borchert, S., Conrad, B., Feesche, J., Hofemeister, B., Hofemeister, J., et al. (2002). Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J. Bacteriol. 184, 1703–1711. doi: 10.1128/JB.184.6.1703-1711.2002
Steinborn, G., Hajirezaei, M.-R., and Hofemeister, J. (2005). bac genes for recombinant bacilysin and anticapsin production in Bacillus host strains. Arch. Microbiol. 183, 71–79. doi: 10.1007/s00203-004-0743-8
Sugawara, E., Ito, T., Odagiri, S., Kubota, K., and Kobayashi, A. (1985). Comparison of compositions of odor components of natto and cooked soybeans. Agric. Biol. Chem. 49, 311–317. doi: 10.1271/bbb1961.49.311
Sumi, C. D., Yang, B. W., Yeo, I.-C., and Hahm, Y. T. (2015). Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can. J. Microbiol. 61, 93–103. doi: 10.1139/cjm-2014-0613
Switzer Blum, J., Burns Bindi, A., Buzzelli, J., Stolz, J. F., and Oremland, R. S. (1998). Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens, sp. nov.: two haloalkaliphiles from Mono Lake, California that respire oxyanions of selenium and arsenic. Arch. Microbiol. 171, 19–30. doi: 10.1007/s002030050673
Tagg, J. R., Dajani, A. S., and Wannamaker, L. W. (1976). Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 40, 722–756.
Tahir, H. A. S., Gu, Q., Wu, H., Niu, Y., Huo, R., and Gao, X. (2017). Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 7:40481. doi: 10.1038/srep40481
Tanaka, K., Ishihara, A., and Nakajima, H. (2014). Isolation of anteiso-C17, iso-C17, iso-C16, and iso-C15 bacillomycin D from Bacillus amyloliquefaciens SD-32 and their antifungal activities against plant pathogens. J. Agric. Food Chem. 62, 1469–1476. doi: 10.1021/jf404531t
Thasana, N., Prapagdee, B., Rangkadilok, N., Sallabhan, R., Aye, S. L., Ruchirawat, S., et al. (2010). Bacillus subtilis SSE4 produces subtulene A, a new lipopeptide antibiotic possessing an unusual C15 unsaturated (-amino acid. FEBS Lett. 584, 3209–3214. doi: 10.1016/j.febslet.2010.06.005
Thimon, L., Peyoux, F., Maget-Dana, R., and Michel, G. (1992). Surface-active properties of antifungal lipopeptides produced by Bacillus subtilis. J. Am. Oil Chem. Soc. 69, 92–93. doi: 10.1007/bf02635884
Um, S., Fraimout, A., Sapountzis, P., Oh, D.-C., and Poulsen, M. (2013). The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci. Rep. 3:3250. doi: 10.1038/srep03250
van Straaten, K. E., Ko, J. B., Jagdhane, R., Anjum, S., Palmer, D. R. J., and Sanders, D. A. R. (2013). The structure of NtdA, a sugar aminotransferase involved in the kanosamine biosynthetic pathway in Bacillus subtilis, reveals a new sub-class of aminotransferases. J. Biol. Chem. 288, 34121–34130. doi: 10.1074/jbc.M113.500637
Velivelli, S. L. S., Kromann, P., Lojan, P., Rojas, M., Franco, J., Suarez, J. P., et al. (2015). Identification of mVOCs from andean rhizobacteria and field evaluation of bacterial and mycorrhizal inoculants on growth of potato in its center of origin. Microb. Ecol. 69, 652–667. doi: 10.1007/s00248-014-0514-2
Wang, B., Yuan, J., Zhang, J., Shen, Z., Zhang, M., Li, R., et al. (2013). Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fertil. Soils 49, 435–446. doi: 10.1007/s00374-012-0739-5
Wang, D., Rosen, C., Kinkel, L., Cao, A., Tharayil, N., and Gerik, J. (2009). Production of methyl sulfide and dimethyl disulfide from soil-incorporated plant materials and implications for controlling soilborne pathogens. Plant Soil 324, 185–197. doi: 10.1007/s11104-009-9943-y
Wang, H., Fewer, D. P., Holm, L., Rouhiainen, L., and Sivonen, K. (2014). Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc. Natl. Acad. Sci. U.S.A. 111, 9259–9264. doi: 10.1073/pnas.1401734111
Wang, T., Liang, Y., Wu, M., Chen, Z., Lin, J., and Yang, L. (2015). Natural products from Bacillus subtilis with antimicrobial properties. Chin. J. Chem. Eng. 23, 744–754. doi: 10.1016/j.cjche.2014.05.020
Wang, T., Wu, M.-B., Chen, Z.-J., Lin, J.-P., and Yang, L.-R. (2016). Separation, determination and antifungal activity test of the products from a new Bacillus amyloliquefaciens. Nat. Prod. Res. 30, 1215–1218. doi: 10.1080/14786419.2015.1048246
Wang, Y., Yang, Q., Tosa, Y., Nakayashiki, H., and Mayama, S. (2005). Nitric oxide-overproducing transformants of Pseudomonas fluorescens with enhanced biocontrol of tomato bacterial wilt. J. Gen. Plant Pathol. 71, 33–38. doi: 10.1007/s10327-004-0157-0
Willey, J. M., and Donk, W. A. V. D. (2007). Lantibiotics: peptides of diverse structure and function. Annu. Rev. Microbiol. 61, 477–501. doi: 10.1146/annurev.micro.61.080706.093501
Williams, T. H. (1975). Naphthomycin, a novel ansa macrocyclic antimetabolite. Proton NMR spectra and structure elucidation using lanthanide shift reagent. J. Antibiot. 28, 85–86. doi: 10.7164/antibiotics.28.85
Wise, C., Falardeau, J., Hagberg, I., and Avis, T. J. (2014). Cellular lipid composition affects sensitivity of plant pathogens to fengycin, an antifungal compound produced by Bacillus subtilis strain CU12. Phytopathology 104, 1036–1041. doi: 10.1094/PHYTO-12-13-0336-R
Wong, J. H., Hao, J., Cao, Z., Qiao, M., Xu, H., Bai, Y., et al. (2008). An antifungal protein from Bacillus amyloliquefaciens. J. Appl. Microbiol. 105, 1888–1898. doi: 10.1111/j.1365-2672.2008.03917.x
Wright, S. J. L., and Thompson, R. J. (1985). Bacillus volatiles antagonize cyanobacteria. FEMS Microbiol. Lett. 30, 263–267. doi: 10.1111/j.1574-6968.1985.tb01093.x
Wu, L., Wu, H., Chen, L., Lin, L., Borriss, R., and Gao, X. (2015a). Bacilysin overproduction in Bacillus amyloliquefaciens FZB42 markerless derivative strains FZBREP and FZBSPA enhances antibacterial activity. Appl. Microbiol. Biotechnol. 99, 4255–4263. doi: 10.1007/s00253-014-6251-0
Wu, L., Wu, H., Chen, L., Yu, X., Borriss, R., and Gao, X. (2015b). Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 5:12975. doi: 10.1038/srep12975
Wu, L., Wu, H., Chen, L., Xie, S., Zang, H., Borriss, R., et al. (2014). Bacilysin from Bacillus amyloliquefaciens FZB42 has specific bactericidal activity against harmful algal bloom species. Appl. Environ. Microbiol. 80, 7512–7520. doi: 10.1128/aem.02605-14
Wu, S., Jia, S., Sun, D., Chen, M., Chen, X., Zhong, J., et al. (2005). Purification and characterization of two novel antimicrobial peptides subpeptin JM4-A and subpeptin JM4-B produced by Bacillus subtilis JM4. Curr. Microbiol. 51, 292–296. doi: 10.1007/s00284-005-0004-3
Xiu, P., Liu, R., Zhang, D., and Sun, C. (2017). Pumilacidin-like lipopeptides derived from marine bacterium Bacillus sp. strain 176 suppress the motility of Vibrio alginolyticus. Appl. Environ. Microbiol. 83:e00450-17. doi: 10.1128/aem.00450-17
Xue, C., Tian, L., Xu, M., Deng, Z., and Lin, W. (2008). A new 24-membered lactone and a new polyene (-lactone from the marine bacterium Bacillus marinus. J. Antibiot. 61, 668–674. doi: 10.1038/ja.2008.94
Yakimov, M. M., Timmis, K. N., Wray, V., and Fredrickson, H. L. (1995). Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol. 61, 1706–1713.
Yoo, J.-S., Zheng, C.-J., Lee, S., Kwak, J.-H., and Kim, W.-G. (2006). Macrolactin N, a new peptide deformylase inhibitor produced by Bacillus subtilis. Bioorg. Med. Chem. Lett. 16, 4889–4892. doi: 10.1016/j.bmcl.2006.06.058
Yu, X., Ai, C., Xin, L., and Zhou, G. (2011). The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 47, 138–145. doi: 10.1016/j.ejsobi.2010.11.001
Yuan, J., Li, B., Zhang, N., Waseem, R., Shen, Q., and Huang, Q. (2012a). Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 60, 2976–2981. doi: 10.1021/jf204868z
Yuan, J., Raza, W., Shen, Q., and Huang, Q. (2012b). Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 78, 5942–5944. doi: 10.1128/aem.01357-12
Zhang, B., Dong, C., Shang, Q., Han, Y., and Li, P. (2013). New insights into membrane-active action in plasma membrane of fungal hyphae by the lipopeptide antibiotic bacillomycin L. Biochim. Biophys. Acta 1828, 2230–2237. doi: 10.1016/j.bbamem.2013.05.033
Zhao, P., Quan, C., Wang, Y., Wang, J., and Fan, S. (2014). Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J. Basic Microbiol. 54, 448–456. doi: 10.1002/jobm.201200414
Zhao, Z., Wang, Q., Wang, K., Brian, K., Liu, C., and Gu, Y. (2010). Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour. Technol. 101, 292–297. doi: 10.1016/j.biortech.2009.07.071
Zheng, M., Shi, J., Shi, J., Wang, Q., and Li, Y. (2013). Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biol. Control 65, 200–206. doi: 10.1016/j.biocontrol.2013.02.004
Zhu, B.-F., Xu, Y., and Fan, W.-L. (2010). High-yield fermentative preparation of tetramethylpyrazine by Bacillus sp. using an endogenous precursor approach. J. Ind. Microbiol. Biotechnol. 37, 179–186. doi: 10.1007/s10295-009-0661-5
Zimmerman, S., Schwartz, C., Monaghan, R., Pelak, B., Weissberger, B., Gilfillan, E., et al. (1987). Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. I. Production, taxonomy and antibacterial activity. J. Antibiot. 40, 1677–1681. doi: 10.7164/antibiotics.40.1677
Keywords: Bacillus subtilis group, bacteriocins, biocontrol, biosynthetic pathways, lipopeptides, polyketides, siderophores, volatile
Citation: Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C and Mahillon J (2019) Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 10:302. doi: 10.3389/fmicb.2019.00302
Received: 05 November 2018; Accepted: 05 February 2019;
Published: 26 February 2019.
Edited by:
José E. Barboza-Corona, Universidad de Guanajuato, MexicoReviewed by:
Stefan Junne, Technische Universität Berlin, GermanyCopyright © 2019 Caulier, Nannan, Gillis, Licciardi, Bragard and Mahillon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claude Bragard, Y2xhdWRlLmJyYWdhcmRAdWNsb3V2YWluLmJl Jacques Mahillon, amFjcXVlcy5tYWhpbGxvbkB1Y2xvdXZhaW4uYmU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.