- 1Ministry of Education Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Hohai University, Nanjing, China
- 2College of Environment, Hohai University, Nanjing, China
- 3Hydrology & Water Resources Bureau of Henan Province, Zhengzhou, China
This study investigates the feasibility of ultrasonic pretreatment for improving treatment efficiency of waste activated sludge (WAS) in microbial electrolysis cell (MEC). Results showed that at applied voltage of 0.5 V, biogas production and cathodic hydrogen recovery enhanced 3.68-fold and 2.56-fold, respectively. Due to the transformation of soluble COD accelerated by the pretreatment, the removal rates of suspended solids and volatile suspended solids were significantly enhanced by 1.38-fold and 1.48-fold, respectively. Various kinds of organics, including VFAs (volatile fatty acids), proteins and carbohydrates, could be utilized in sequence. The primary biodegradable substance in MEC was hydrophilic fraction from sludge organics and the pretreatment effectively resulted in an elevated concentration of this fraction. The 16S rRNA pyrosequencing analysis demonstrated multiple syntrophic interactions between fermentative bacteria, exoelectrogenes, and methanogenic archaea in MEC for WAS.
Introduction
Globally there is a strong consideration toward WAS, which is massively generated by commonly used biological wastewater treatment process, as a source of renewable energy, i.e., named biomass. This conception was emphasized by the reality of shortage of energy and concerns over greenhouse effect (Hasan and Rahman, 2017; Abdulrahman and Huisingh, 2018; Uzoejinwa et al., 2018).
Anaerobic digestion was an anaerobic approach to stabilize sludge, while simultaneously produces biogas to offset part of the consumption. The main constituent of biogas produced by AD is methane (accounted for 55–65%). The other constituents include 30–40% of CO2, fractions of water vapor, traces of H2S and H2 (Appels et al., 2008). Methane is regarded as an important energy source. However, in terms of energy content, hydrogen (141.8 MJ/kg) has a 2.6 times over methane (55.5 MJ/kg), which is a favorable energy carrier. Moreover, “fermentation barrier” is an issue during AD process that causes fermentation dead-end products accumulation (e.g., acetic acid) (Zhu et al., 2014). Typically, a secondary aerobic process was required to decompose these residual organic matters; In this case, energy containing in these organic matters was wasted. MEC, a new anaerobic biotechnology, is reported to be an alternative way to solve this problem and simultaneously recover hydrogen. Various substrates were used in MEC to produce hydrogen, including VFAs, glucose, glycerol, cellulose, protein, and etc. (Kadier et al., 2014). In recent decades, more attentions are paid to real wastes, e.g., domestic wastewater (Ditzig et al., 2007), swine wastewater (Wagner et al., 2009) and sewage sludge (Lu et al., 2012; Guo et al., 2013; Sun R. et al., 2014). These studies indicated the feasibility of waste treatment via MEC technology. Nevertheless, most studies tested sludge fermentation liquid as substrates for the MEC feasibility. These researches separate the fermentation process from microbial electrolysis process. The WAS, with its abundance in nutrients and carbons, is a quite complex waste. Interestingly, a mixed culture was required for achieving better performance rather than pure culture (Liu W.Z. et al., 2016). In this opinion, microbial electrolysis process could combine with fermentation process for sludge treatment. During this process, it is very important to reveal how the multiple groups of microorganisms work together to transform complex substrate to energy products, such as hydrogen or methane.
The main organic compounds in WAS were EPS and IS, both of which included lipid, polysaccharide, protein, and nucleic acid (Appels et al., 2008). These compounds are not readily to hydrolyze. Accordingly, pretreatment method was recommended to disintegrate sludge matrix and release these compounds to the solution. One of the methods gaining increasing interests is the use of ultrasound due to its advantages such as shorter treatment time, no chemical addition, and no generation of by-products (Li et al., 2018). Ultrasonic sludge pretreatment is based on the influence of cavitation derived from collapse of microbubbles, which results in deagglomeration of flocs, microorganism cells disruption (cells lysis), sonochemical effects, and rheological changes (Skórkowski et al., 2018). Most laboratory and industrial scale studies reported enhanced performance by this method before AD or fermentation process (Zhen et al., 2017). The characterization of community structure and community shifts when fed with U-SS is not well understood. However, it starts to attract interest of scientists and engineers.
In this study, ultrasonic pretreatment was chosen to disintegrate WAS and the influence of U-SS on MEC performance was investigated. To achieve this goal, raw sludge was compared with U-SS as substrates in a single-chamber MEC. Besides, the hypothesis that sludge utilization can be directly achieved for hydrogen production in MEC instead of sludge fermentation liquid was explored in the present study. From this aspect, variations of both soluble organics and EBOM in influent and effluent from MEC were examined using hydrophilic-hydrophobic fractionation and EEM fluorescence spectroscopy. The microbial community structure of anode biofilm was analyzed using high-throughput 16S rRNA pyrosequencing in order to relate reactor performance with microbial function, with emphasize on the microbial behavior and interactions.
Materials and Methods
Raw WAS and U-SS
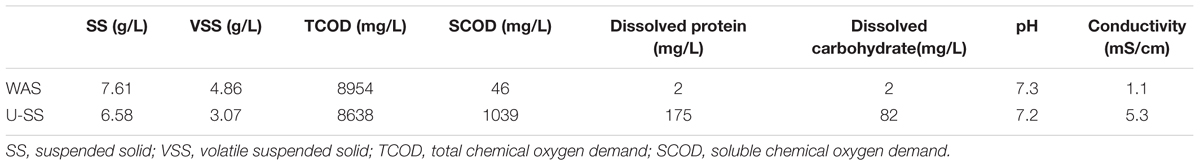
Raw WAS used in this study was collected from a secondary sedimentation tank in a municipal wastewater treatment plant located in Nanjing, China. The ultrasonic pretreatment was conducted as follows: firstly, pH of WAS was adjusted to around 10 using 5 M NaOH solution. This step was on the basis of the fact that alkaline condition promotes the hydrolysis of proteins and fats and could be combined with the ultrasound method to enhance the sludge solubilization (Carrere et al., 2016). Then the WAS was treated for 5 min at an ultrasound energy density of 3 W/mL by a laboratory ultrasonic cell disrupter (JY98-IIIN, Ningbo Scientz Biotechnology Co., Ltd., China). Before being fed into MEC, pH of U-SS was adjusted to approximately 7 using HCl solution. The characteristics of raw WAS and U-SS were presented in Table 1.
Reactors Configuration
At first, a two-chamber MFC was employed for anode biofilm enrichment. The MFC was H-shaped with two identical cylindrical chambers (7 cm diameter × 7 cm length, effective volume of 269 mL) separated by a proton exchange membrane (N117CS, Dupont Co., Ltd., United States). A carbon fiber brush (5 cm diameter × 5 cm length, fiber type: T700SC-24K, Toray Co., Ltd., Japan) was introduced as the anode of MFC and in the case of cathode, a carbon cloth (WOS1002, CeTech Co., Ltd., Taiwan), coated with Pt catalyst (0.5 mg/cm2) was adopted. As for the MEC, the reactor was a rectangular shape (7 cm × 6 cm × 7 cm, effective volume of 294 mL). The MEC anode was replaced by the anode of MFC after enrichment and the cathode of MEC was exactly the same as that of MFC. Therefore, a single-chamber MEC was constructed.
Inoculation and Operation
The MFC was initially inoculated with WAS. Then, a 50 mM nutrient phosphate buffer solution (1.5 g/L of CH3COONa, 2.41 g/L of KH2PO4, 7.35 g/L of K2HPO4⋅3H2O, 0.31 g/L of NH4Cl, 0.13 g/L of KCl, 12.5 mL/L of trace nutrient medium, and 5 mL/L of vitamin solution) was mixed with WAS at the volume ratio of 2:1. The catholyte of MFC was a 50 mM phosphate buffer solution. A resistor (1 kΩ) was connected with the MFC, and the voltage across the resistor was measured through a multimeter (DT-118, Shenzhen Everbest Machinery Industry Co., Ltd., China). Provided that the voltage reached a constant value, the MFC was refilled with new feed of mixed solution of WAS and nutrient phosphate buffer solution. When the maximum voltage was reproducible for at least three batch operation cycles, the biofilm on the anode was acclimated and then was transferred to replace the MEC anode.
The MEC was fed with raw WAS or U-SS in a 50 mM phosphate buffer solution (the same composition as MFC, except for the absence of CH3COONa). A fixed voltage (0.5 V) was applied to the MEC using a direct current power source (LP2002D, Shenzhen Lodestar Precision Tools Co., Ltd., China). A resistor (10 Ω) was connected with the MEC, and the voltage across the resistor was measured. A batch cycle ended when the voltage decreased below 10 mV, then the MEC was refilled. The produced biogas was collected by a gas bag (200 mL, E-Switch, Shanghai Shenyuan Scientific Instrument Co., Ltd., China), and the biogas volume was measured using a glass syringe. All tests were conducted at room temperature (22 ± 3°C).
Analyses and Calculations
The influent and effluent of each batch cycle from MEC were withdrawn. TCOD, SS, VSS, SCOD and NH3-N were determined according to standard methods (APHA, 1998). The soluble protein, soluble carbohydrate and amino acid were examined using coomassie brilliant blue method, anthrone-sulfuric acid colorimetric method and ninhydrin hydrate colorimetric method, respectively, as described (Hu et al., 2018). The DOC content was determined by a total organic carbon analyzer (multi N/C 2100, Analytik Jena Co., Ltd., Germany).
The sludge sample was centrifuged for 10 min at 5000 rpm, then the sludge EBOM was extracted as described by Jiang et al. (2010). The EBOM solution and sludge supernatant were filtrated (0.45 μm) and acidified to pH = 2 using HCl solution. The organic matters in sludge were fractionated into five types: HPI, HPO-A, HPO-N, TPI-A and TPI-N, adopting XAD-4/DAX-8 resins (Supelco Co., Ltd., United States) as described by Xue et al. (2008).
The electrochemical performance of MEC was evaluated in terms of volumetrical current density (IV, A/m3), coulombic efficiency (CE, %), cathodic hydrogen recovery (rcat, %), and electricity efficiency (ηE, %). The gas production was determined through production rate [Q, m3/(m3⋅d)]. The calculation methods for the above indicators were referred to literature (Logan et al., 2008).
High-Throughput 16S rRNA Pyrosequencing
The microbial communities of anode biofilm and raw WAS were examined by high-throughput 16S rRNA pyrosequencing. The biofilm sample was obtained by cutting carbon fiber with a sterilized scissor when the current density reached maximum values. The WAS sample was centrifuged for 1 min at 1000 rpm and the supernatant was drained. All samples were stored below -20°C before analysis. The tests of DNA extraction, PCR amplification and pyrosequencing were conducted by Sangon Biotech Co., Ltd. (Shanghai, China), and the detailed information was available in the literature (Wang et al., 2017).
Results and Discussion
Performances of the MECs
The performance of the U-SS MEC was superior to that of feeding WAS (Figure 1). With 0.5 V applied voltage, during a typical batch operation cycle, the current density increased dramatically at first. The maximum current density in the U-SS MEC (13.4 A/m3) was about 1.8 times of that in the WAS MEC (7.9 A/m3). Then the current density declined with time. At day 4, the current density in the WAS MEC decreased significantly; whereas in the U-SS MEC, the decrement delayed until day 9. Ultrasonic pretreatment resulted in solubilization of various organics from sludge matrix, hence accelerating the microbial electrohydrogenesis. After 11 days, the current densities in both MECs were averaged at 3.2 A/m3.
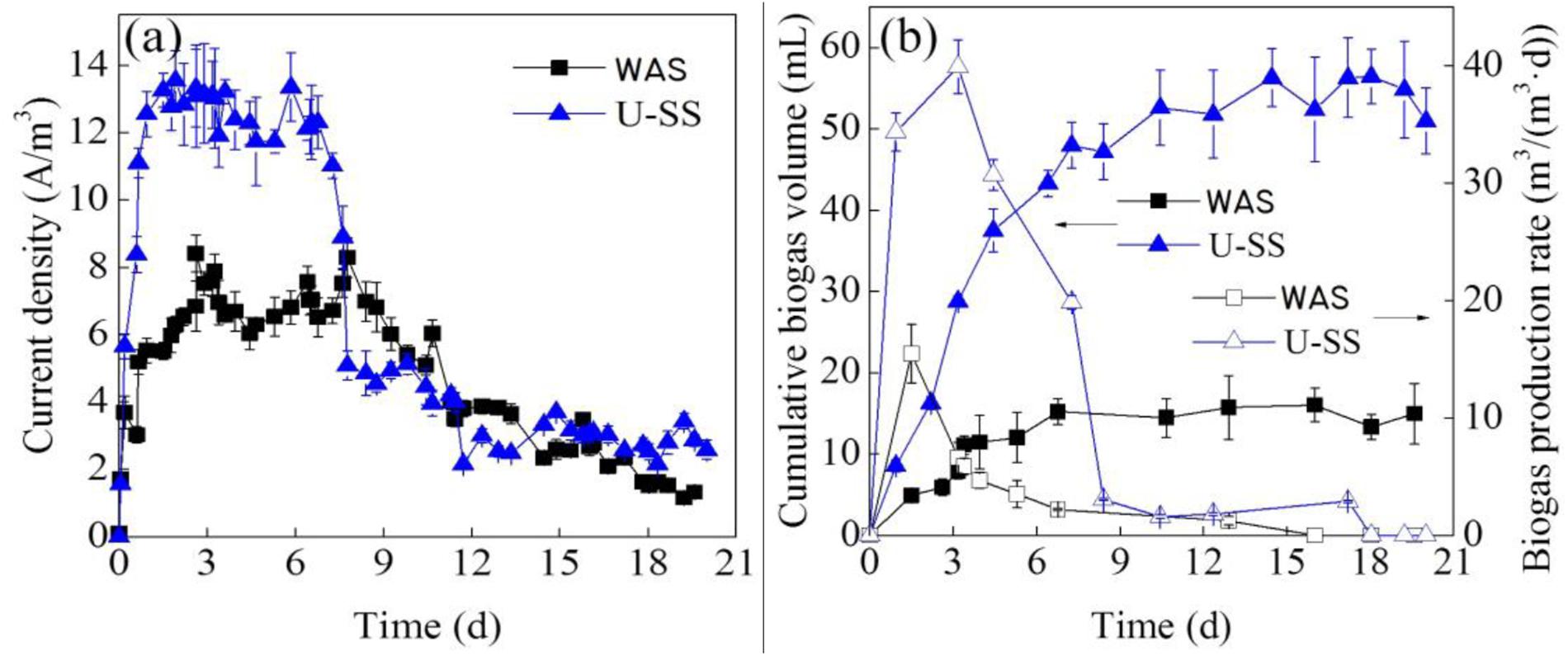
Figure 1. Variations of current density (a), cumulative biogas volume (b), and biogas production rate (b) in the MECs treating WAS and U-SS. (Error bars ± standard deviation were based on three MECs operated in parallel).
As can be seen in Figures 1a,b, the changes of current density were in accordance with the variations of biogas production rate. This result has been proved by the equation (1) (Logan et al., 2008), where Q represented volumetric hydrogen production rate, Iv represented current density, rcat represented cathodic hydrogen recovery, F represented Faraday’s constant, and cg (mol/L) was the molar density of gas at a standard temperature (298.15 K) and standard pressure (1 bar).
The MEC performance was listed in Table 2. The achieved TCOD removal rate was much lower than other studies (Liu W.Z. et al., 2016), which was above 60% adopting integrated system of microbial electrolysis and anaerobic fermentation fed with U-SS supernatant. This result implied the fact that the hydrolysis rate was the limiting factor in MEC in terms of COD reduction. Overall, the CE values of both cells were not very high (33.7% for WAS MEC and 36.8% for U-SS MEC), and were comparable to the reported values of 28% ± 10% to 34% ± 4% (Lu et al., 2012). As a result of the suspended growth of microorganisms in WAS, a part of sludge organics was fermented and degraded regardless of applied voltage, which did not generate current. However, the ultrasonic pretreatment did not improve the CE value.
The rcat value in the U-SS MEC was 2.56 times higher than that in the WAS MEC, but remained at a low level. The reasons could be ascribed as: (1) the consumption of H2 by H2-oxidizing methanogens; (2) the leakage problem; and (3) catalyst activity. Moreover, the complexity of substrate may lead to various reactions, which could influence the rcat value (Cusick and Logan, 2012).
Variations of Dissolved Organics
During microbial electrohydrogenesis process, the SCOD variation is a function of solubilization and degradation of sludge matrix. Figure 2 presents the dissolved matter changes in the MEC.
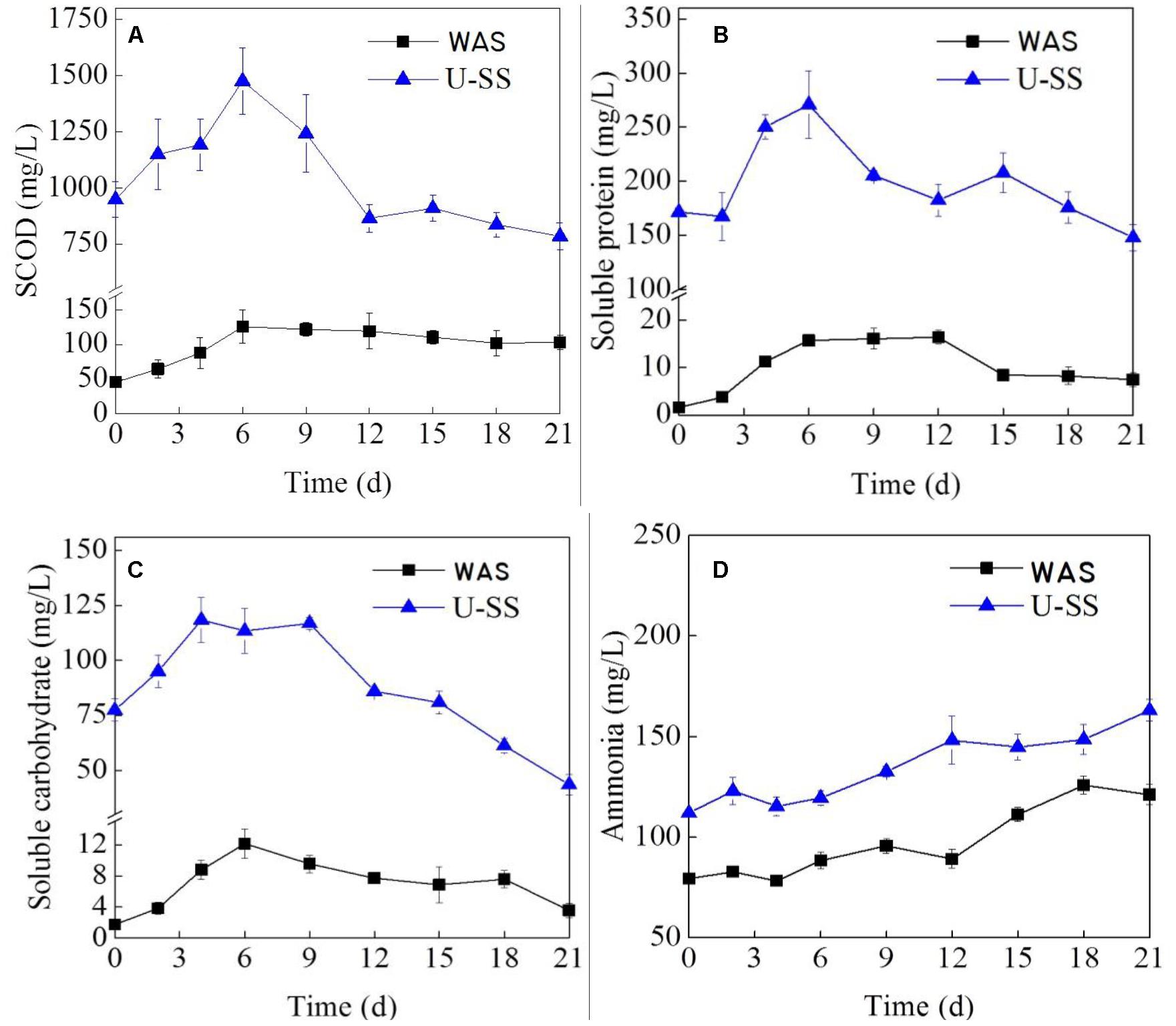
Figure 2. Variations of SCOD (A), soluble protein (B), soluble carbohydrate (C), and ammonia (D) in MECs (Error bars ± standard deviation were based on three MECs operated in parallel).
At the initial stage, SCOD increased with time, implying a higher hydrolysis rate over degradation rate (Figure 2A). Noteworthy, the SCOD of U-SS was much higher than that of WAS and increased more rapidly, which was owing to the ultrasonic pretreatment. At day 9 (for U-SS MEC) and day 6 (for WAS MEC), the SCOD reached peak values and after that gradually decreased. This result was in consistent with the finding by Lu et al. (2012), which stated that from day 0.6 to day 8.8, the SCOD value almost kept unchanged in the WAS MEC and a rapid decrement of acetic acid was the main reason causing the SCOD drop. The SCOD changes were in agreement with the current density changes, demonstrating the relationship between electrochemical characteristics and organics degradation.
As for the soluble protein, the changes were identical to the SCOD changes (Figure 2B). The research conducted by Lu et al. (2010) confirmed the biodegradation availability of protein in the MEC, although obtained with a notable decreasing electrochemical parameters. In this study, in the latter stage of the operation, the protein concentration gradually declined, indicating the degradation rate grow at a higher speed than the hydrolysis rate. At this time, the readily-biodegradable organics concentration was low and protein became a major substrate.
The concentration of soluble carbohydrate rose at first and then declined (Figure 2C). A few part of exoelectrogens was found to directly utilize carbohydrate, such as Rhodoferax ferrireducens, Klebsiella pneumonia, and Aeromonas hydrophila, etc (Logan, 2009). Most exoelectrogens utilized carbohydrate on the condition that this material was subject to hydrolysis or fermentation. Therefore, at the first few days, the hydrolysis rate of carbohydrate was high, as can be seen in Figure 2C that showed an accumulation of carbohydrate. From day 9 for the both cells, due to the low concentration of VFAs, the microorganism turned to metabolize carbohydrates and proteins, resulted in an increment in the degradation rate. The above-mentioned results clearly presented how complex organics in sludge were converted to electrons in MECs, suggesting a cascaded utilization with time. This phenomenon was observed by many studies (Lu et al., 2012; Liu et al., 2012; Liu W.Z. et al., 2016; Wang et al., 2014).
A previous study showed the NH3-N reduction ranged from 174 to 186 mg/L in MEC fed with sludge fermentation liquid (Wang et al., 2014). If the MEC was dosed with complex substrate, for example WAS or U-SS in this study, the recalcitrant nature of complex waste caused an ascending NH3-N concentration (below 200 mg/L for both cells, seen Figure 2D) during the operation. The released NH3-N in the solution could not inhibit the microorganism growth.
Transformations of Organic Fractions
As indicated in Figure 3, the aromatic proteins and soluble microbial by-product-like materials were the principal components in influents and effluents. The influent (WAS or U-SS) EBOM of MEC contained few fulvic acid-like materials, while these materials were not detected in the effluent EBOM. Considering that all the effluent supernatant showed high fluorescent intensity in the region referring to the fulvic acid-like substances, these substances may be derived from the solubilization and metabolism of IS in sludge, not from EBOM. In the influent supernatant, U-SS exhibited higher fluorescent intensities in the regions referring to aromatic proteins and soluble microbial by-product-like materials, which demonstrated the effectiveness of ultrasonic pretreatment to break the sludge matrix and increase the soluble organics.
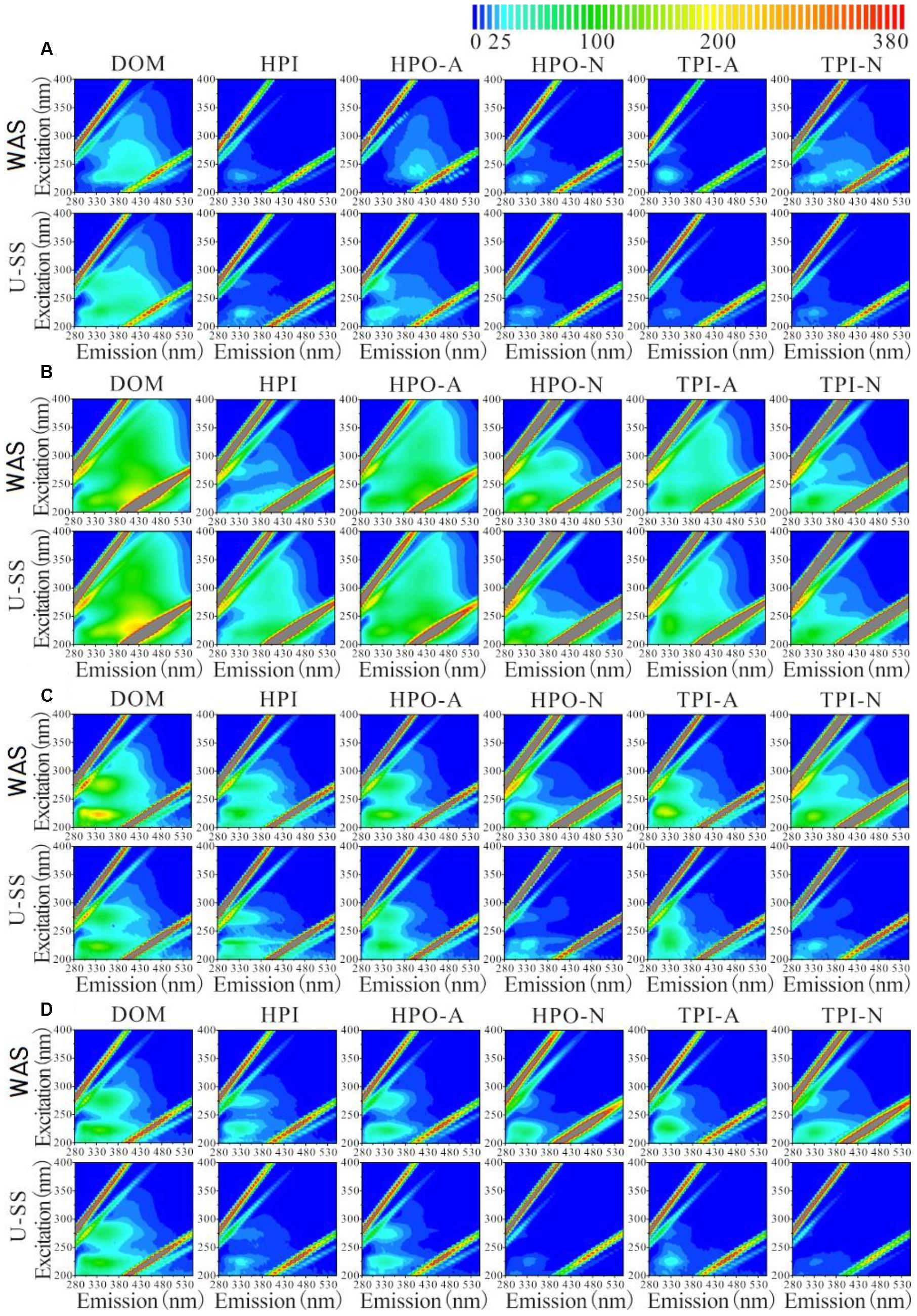
Figure 3. Excitation-emission matrix fluorescence spectra of DOM, HPI, HPO-A, HPO-N, TPI-A, TPI-N obtained from influent supernatant (A), effluent supernatant (B), influent EBOM (C), and effluent EBOM (D) in MECs.
A weak fluorescent intensity of HPI fraction was found in the WAS supernatant. In the effluent of WAS MEC, the supernatant mainly consisted of HPO-N and TPI-N fractions, which were not readily-biodegradable. In comparison, higher fluorescent intensity of HPI fraction was detected in U-SS supernatant, which represented readily-biodegradable materials. This result also proved the effectiveness of ultrasonic pretreatment and explained the higher organic matter removal rate in U-SS MEC.
Regarding the sludge EBOM, the influent EBOM of WAS MEC was rich in aromatic proteins in various fractions. Whereas HPI, HPO-A, and TPI-A fractions were in the majority of the influent EBOM of U-SS MEC. In the effluent EBOM, fluorescent intensity decreased significantly, revealing the degradation of unsaturated and aromatic organic compounds in MEC. Due to the low organics removal rate, the effluent EBOM from WAS MEC showed a relatively high fluorescent intensity.
Microbial Community Analysis
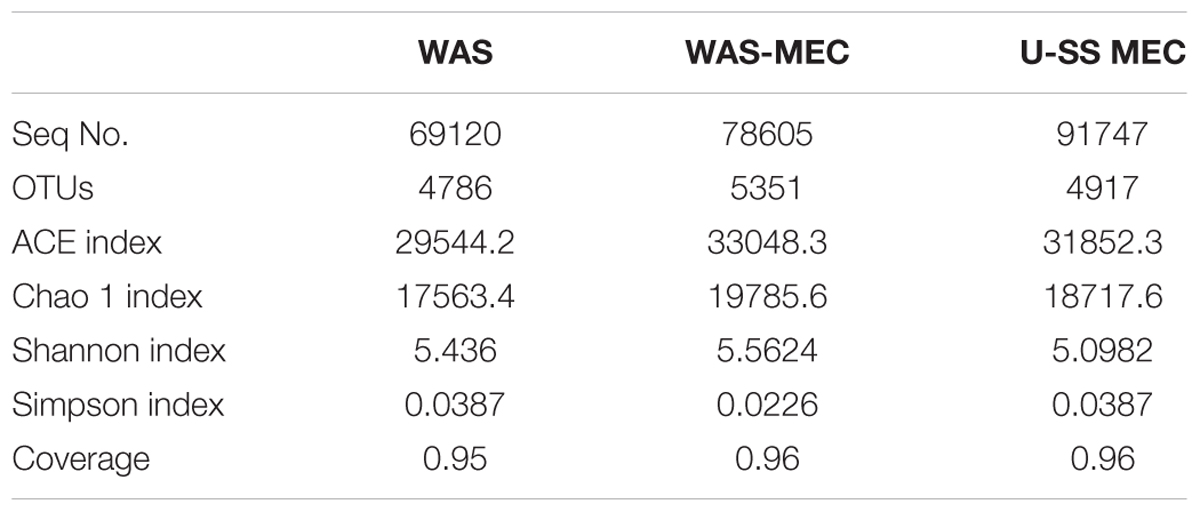
A total of 239,472 sequences were analyzed over three samples (Table 3). OTUs at 3% distance were the most detected ones in the biofilm from WAS MEC (5351), with the highest diversity (Shannon index of 5.5624), whereas being the least detected in the raw sludge (WAS, 4786), showing a reduced diversity (Shannon index of 5.436) (Table 3). Similar results were observed for ACE and Chao1 indices (Table 3). Noteworthy, an increase of diversity was detected in anode biofilm communities, indicating an interactive effect between communities during microbial electrolysis.
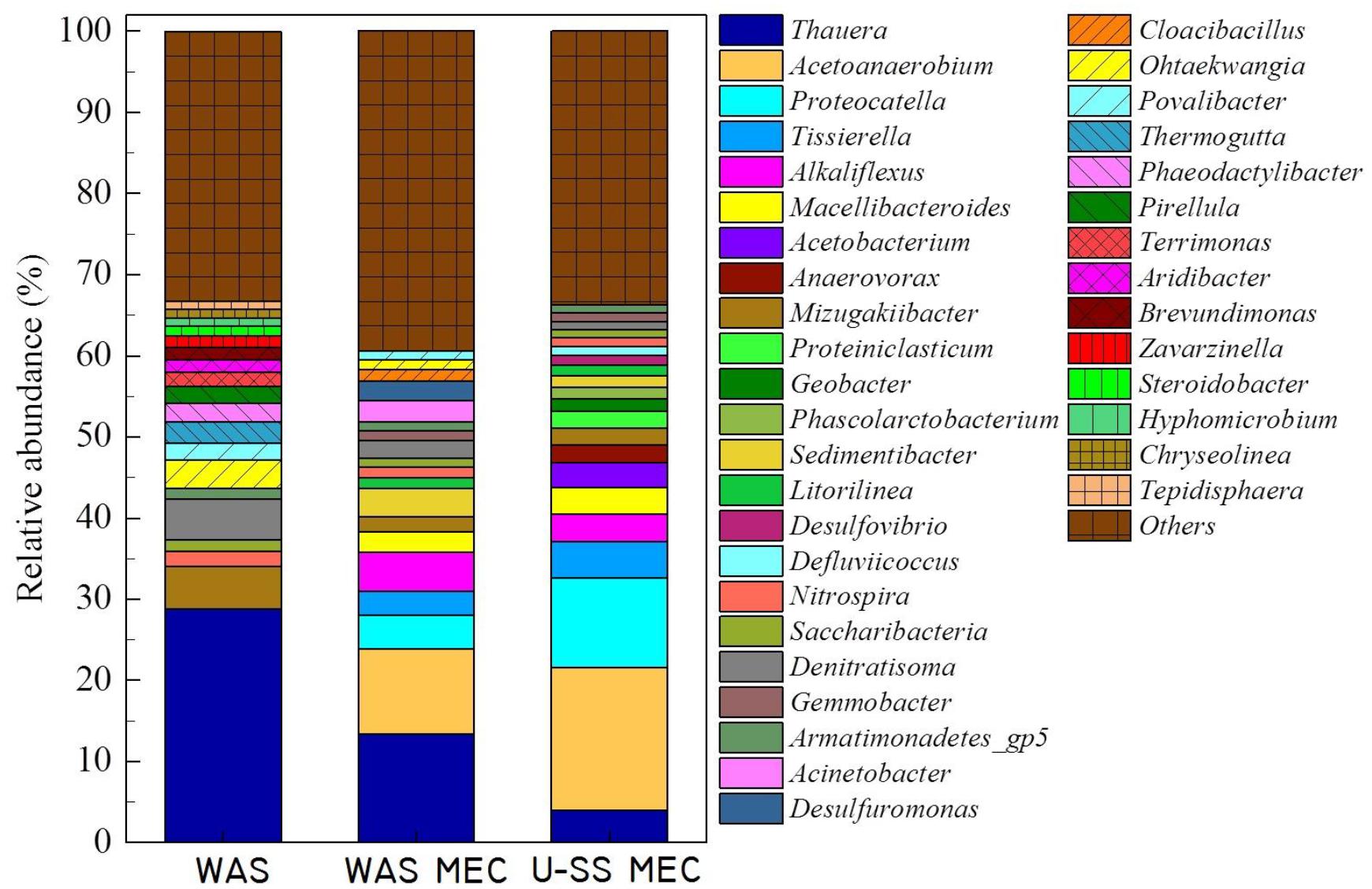
Characterization of the anode biofilm from the MEC fed WAS (Figure 4) showed it was dominated by Thauera (accounted for 10.4% of the population), Acetoanaerobium (accounted for 8.9% of the population), Alkaliflexus (accounted for 4.1% of the population), Proteocatella (accounted for 3.4% of the population), Sedimentibacter (accounted for 3.0% of the population), Tissierella (accounted for 2.5% of the population), Acinetobacter (accounted for 2.2% of the population), Macellibacteroides (accounted for 2.0% of the population), Desulfuromonas (accounted for 2.0% of the population). The anode biofilm sample from U-SS MEC comprised principally of Acetoanaerobium (15.4%), Proteocatella (9.6%), Tissierella (3.9%), Thauera (3.4%), Alkaliflexus (3.0%), Macellibacteroides (2.9%), Acetobacterium (2.6%), Anaerovorax (1.9%). The results showed a different composition of populations for the anode biofilm in MECs compared with raw WAS, i.e., an enrichment of Desulfuromonas, Proteocatella, Alkaliflexus, and Acetoanaerobium along with decrement of Thauera. The Shewanella and Geobacter could convert organic matters to electricity (Lovley, 2006). Low numbers of clones belonged to Geobacter was detected and Shewanella was not found in raw WAS. However, a high relative abundance (1.3%) was gained for Geobacter in the U-SS MEC, which indicated that Geobacter was one of the dominant exoelectrogens.
The major part of raw WAS was found to be aerobic bacterial. For example, Litorilinea and Terrimonas were reported to have capable of oxidizing hydrocarbons (Kale et al., 2013). Similarly, it was reported that Nitrospira has capacity of nitrification (van Kessel et al., 2015). A small portion of anaerobic and anaerobic bacterial was also detected, among which Thauera and Mizugakiibacter were known to proceed denitrification (Mechichi et al., 2002; Watanabe et al., 2015). In contrast, the majority of anode biofilm in MEC was found to be anaerobic and anaerobic bacterial. For example, Proteocatella and Alkaliflexus were capable of fermentation to produce VFAs (Zhilina et al., 2004; Pikuta et al., 2009); Nitrospira had capacity of nitrification (van Kessel et al., 2015); Thauera and Mizugakiibacter were capable of denitrification (Mechichi et al., 2002; Watanabe et al., 2015); Desulfovibrio was recognized as sulfate-reducing bacterium (Gilmour et al., 2011); Desulfuromonas and Geobacter were responsible for the dissimilatory metal reduction (Roden and Lovley, 1993; Sun D. et al., 2014). Moreover, the growth of Geobacter was expected, since acetate presented in sludge SCOD could be adopted as carbon source to grow the exoelectrogenic biofilm on the anode (Yates et al., 2012). The diversity of microorganisms in MEC participated in the degradation of complex organic matters presented in the sludge, as revealed by an important portion of unclassified genus obtained from the anode biofilm sample. More than 11 kinds of aerobic bacterial was also detected on the anode biofilm in WAS-MEC with very low relative abundance (<1%), except for the Acinetobacter with a relative abundance of 2.2%, one specie of which was found to be electrochemically active (Xiao et al., 2013). Nevertheless, fewer types of aerobic bacterial (approximately 7 types) were presented in the biofilm of U-SS MEC, indicating the population dominated by anaerobic types. This was in accordance with Borole et al.’s (2011) statement that exoelectrogens in the BES (bio-electrochemical system) were mostly comprised of anaerobic microorganisms, although some bacterial, such as Shewanella, was able to grow in the anoxic condition. The better performance of U-SS MEC may be ascribed to the high relative abundance of exoelectrogens.
Practical Implications of This Work
Syntrophy is recognized as an essential intermediary step in the anaerobic metabolism, especially for the complete decomposition of complex polymers such as polysaccharides, proteins, nucleic acids, and lipids (Liu Q. et al., 2016). The pyrosequencing analysis revealed the coexistence of fermentative and exoelectrogenic bacteria in the anodes, including proteolytic and saccharolytic bacteria, hydrogen-producing bacteria, and so on. This result was in accordance with the finding by Montpart et al. (2015), which discovered the coexistence of different kinds of bacteria irrespective of the substrate fed into the MEC. Other trophic groups in MECs belonged to acetogenic bacteria, hydrogenotrophic methanogenic archaea (Kouzuma et al., 2015; Liu Q. et al., 2016). It has been found that interaction between microbes can improve system performance and energy recovery efficiency (Liu W.Z. et al., 2016). Future works on the level and mechanism of interactions between these syntrophic consortia needs to be disclosed.
The pyrosequencing results also demonstrated that community composition reassembled with inconsiderable structure changes between WAS-MEC and U-SS MEC, despite appearance of Bellilinea and Longilinea in the U-SS MEC, which may be caused by microbial competition. These results suggest that electron generation and hydrogen production was enhanced by enrichment of cytochrome genes and exoelectrogens involved, rather than community structure adjustments, which arose from the ultrasound pretreatment. Based on this study, the efficiency of a pretreatment method for MEC could be judged from three aspects. The first aspect was the production rate of desirable compounds for the subsequent microbial electrolysis. An effective pretreatment should accelerate the hydrolysis and conversion of organic matter (Beegle and Borole, 2017), for example, VFAs, which were favored for the hydrogen production (Guo et al., 2013; Kadier et al., 2014; Liu Q. et al., 2016). The second aspect was the content of treated sludge, the overall performance of WAS cascade utilization in MEC was substantially related to the microbial community structures, which in turn depended on the content of treated sludge. The U-SS herein resulted in the dominance of Acetoanaerobium and Proteocatella, whilst alkaline pretreated sludge led to the dominance of Proteiniclasticum and Parabacteroides in MECs (Liu W.Z. et al., 2016). In addition, the content of treated sludge would supply higher conductivities to the solution, which facilitated mass transfer in anode biofilm. The third aspect was for the economic consideration. Efforts should be tried to balance the energy recovered from biomass and the input energy, as a high conversion rate of COD into energy and a low energy consumption were favorable. The future prospect of this technology relied greatly on this condition, as anaerobic biomethod is important to realize the potential of energy-sufficient in waste/wastewater treatment.
Conclusion
This study demonstrated bioelectrochemical enhancement of biogas production and organics degradation for WAS by ultrasonic pretreatment in single-chamber MEC. At applied voltage of 0.5 V, biogas production was enhanced 3.68-fold, and cathodic hydrogen recovery was enhanced 2.56-fold, in the MEC fed with ultrasound-pretreated sludge. Meanwhile, the removals of SS and VSS were enhanced by 1.38-fold and 1.48-fold, respectively. Regarding sludge organics, HPI was the main biodegradable substance in MEC, which was improved by the pretreatment. The 16S rRNA pyrosequencing technology was successfully applied to analyze the microbial community, and demonstrated multiple syntrophic interactions between fermentative bacteria, exoelectrogenes, and methanogenic archaea in MEC cascade utilization of WAS and U-SS. The pretreatment facilitated the transformation of SCOD, rather than community structure adjustments during microbial electrolysis.
Author Contributions
KH and S-qJ designed and performed the experiments. KH, WC, WW, and FH wrote the manuscript. All authors read and commented on the draft manuscript and agreed to the revised version.
Funding
The authors are gratefully acknowledge funding from National Science and Technology Major Project (Grant No. 2016YFC0400800-04), Major Science and Technology Program for Water Pollution Control and Treatment (Grant No. 2017ZX07201002), Fundamental Research Funds for the Central Universities (Grant No.2019B14114), Ministry of Education Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Hohai University (Grant No. 2015002), National Natural Science Foundation of China (Grant No. 51408194), Scientific and Technological Project of Henan Province (Grant No. 162102310057), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ηE, electricity efficiency; ACE, abundance-based coverage estimator; AD, anaerobic digestion; CE, coulombic efficiency; DNA, deoxyribonucleic acid; DOC, dissolved organic carbon; DOM, dissolved organic matter; EBOM, extracellular biological organic matter; EEM, excitation-emission matrix; EPS, extracellular polymeric substances; HPI, hydrophilic fraction; HPO-A, hydrophobic acid; HPO-N, hydrophobic neutral; IS, intracellular substance; IV, volumetrical current density; MEC, microbial electrolysis cell; MFC, microbial fuel cell; NH3-N, ammonia nitrogen; OTUs, operational taxonomic units; PCR, polymerase chain reaction; Q, gas production rate; rcat, cathodic hydrogen recovery; rRNA, ribosomal ribonucleic acid; SCOD, soluble chemical oxygen demand; SS, suspended solid; TCOD, total chemical oxygen demand; TPI-A, transphilic acid; TPI-N, transphilic neutral; U-SS, ultrasound-treated WAS; VFAs, volatile fatty acids; VSS, volatile suspended solid; WAS, waste activated sludge.
References
Abdulrahman, A. O., and Huisingh, D. (2018). The role of biomass as a cleaner energy source in Egypt’s energy mix. J. Clean. Production 172, 3918–3930. doi: 10.1016/j.jclepro.2017.05.049
APHA (1998). Standard Methods for the Examination of Water and Wastewater, 20th Edn. Washington, DC: American Public Health Association.
Appels, L., Baeyens, J., Degreve, J., and Dewil, R. (2008). Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 34, 755–781. doi: 10.1016/j.pecs.2008.06.002
Beegle, J. R., and Borole, A. P. (2017). An integrated microbial electrolysis-anaerobic digestion process combined with pretreatment of wastewater solids to improve hydrogen production. Environ. Sci. Water Res. Technol. 3, 1073–1085. doi: 10.1039/C7EW00189D
Borole, A. P., Reguera, G., Ringeisen, B., Wang, Z. W., Feng, Y. J., and Kim, B. H. (2011). Electroactive biofilms: current status and future research needs. Energy Environ. Sci. 4, 4813–4834. doi: 10.1039/c1ee02511b
Carrere, H., Antonopoulou, G., Affes, R., Passos, F., Battimelli, A., Lyberatos, G., et al. (2016). Review of feedstock pretreatment strategies for improved anaerobic digestion: from lab-scale research to full-scale application. Bioresour. Technol. 199, 386–397. doi: 10.1016/j.biortech.2015.09.007
Cusick, R. D., and Logan, B. E. (2012). Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour. Technol. 107, 110–115. doi: 10.1016/j.biortech.2011.12.038
Ditzig, J., Liu, H., and Logan, B. E. (2007). Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int. J. Hydrogen Energy 32, 2296–2304. doi: 10.1016/j.ijhydene.2007.02.035
Gilmour, C. C., Elias, D. A., Kucken, A. M., Brown, S. D., Palumbo, A. V., Schadt, C. W., et al. (2011). Sulfate-reducing bacterium Desulfovibrio desulfuricans ND132 as a model for understanding bacterial mercury methylation. Appl. Environ. Microbiol. 77, 3938–3951. doi: 10.1128/AEM.02993-10
Guo, X., Liu, J., and Xiao, B. (2013). Bioelectrochemical enhancement of hydrogen and methane production from the anaerobic digestion of sewage sludge in single-chamber membrane-free microbial electrolysis cells. Int. J. Hydrogen Energy 38, 1342–1347. doi: 10.1016/j.ijhydene.2012.11.087
Hasan, M. M., and Rahman, M. M. (2017). Performance and emission characteristics of biodiesel-diesel blend and environmental and economic impacts of biodiesel production: a review. Renew. Sustain. Energy Rev. 74, 938–948. doi: 10.1016/j.rser.2017.03.045
Hu, K., Xu, L., Chen, W., Jia, S. Q., Wang, W., and Han, F. (2018). Degradation of organics extracted from dewatered sludge by alkaline pretreatment in microbial electrolysis cell. Environ. Sci. Pollut. Res. 25, 8715–8724. doi: 10.1007/s11356-018-1213-1
Jiang, J. Q., Zhao, Q. L., Wei, L. L., and Wang, K. (2010). Extracellular biological organic matters in microbial fuel cell using sewage sludge as fuel. Water Res. 44, 2163–2170. doi: 10.1016/j.watres.2009.12.033
Kadier, A., Simayi, Y., Kalil, M. S., Abdeshahian, P., and Hamid, A. A. (2014). A review of the substrates used in microbial electrolysis cells (MECs) for producing sustainable and clean hydrogen gas. Renew. Energy 71, 466–472. doi: 10.1016/j.renene.2014.05.052
Kale, V., Björnsdóttir, S. H., Friðjónsson,ÓH., Petursdottir, S. K., Omarsdottir, S., and Hreggviosson, G. O. (2013). Litorilinea aerophila gen. nov., sp. nov., an aerobic member of class Caldilineae, phylum Chloroflexi, isolated from an intertidal hot spring. Int. J. Syst. Evol. Microbiol. 63, 1149–1154. doi: 10.1099/ijs.0.044115-0
Kouzuma, A., Kato, S., and Watanabe, K. (2015). Microbial interspecies interactions: recent findings in syntrophic consortia. Front. Microbiol. 6:477. doi: 10.3389/fmicb.2015.00477
Li, X., Guo, S., Peng, Y., He, Y. L., Wang, S. Y., Li, L. K., et al. (2018). Anaerobic digestion using ultrasound as pretreatment approach: changes in waste activated sludge, anaerobic digestion performances and digestive microbial populations. Biochem. Eng. J. 139, 139–145. doi: 10.1016/j.bej.2017.11.009
Liu, Q., Ren, Z. J., Huang, C., Liu, B. F., Ren, N. Q., and Xing, D. F. (2016). Multiple syntrophic interactions drive biohythane production from waste sludge in microbial electrolysis cells. Biotechnol. Biofuels 9:162. doi: 10.1186/s13068-016-0579-x
Liu, W. Z., He, Z. W., Yang, C. X., Zhou, A. J., Guo, Z. C., Liang, B., et al. (2016). Microbial network for waste activated sludge cascade utilization in an integrated system of microbial electrolysis and anaerobic fermentation. Biotechnol. Biofuels 9:83. doi: 10.1186/s13068-016-0493-2
Liu, W. Z., Huang, S. C., Zhou, A. J., Zhou, G. Y., Ren, N. Q., Wang, A. J., et al. (2012). Hydrogen generation in microbial electrolysis cell feeding with fermentation liquid of waste activated sludge. Int. J. Hydrogen Energy 37, 13859–13864. doi: 10.1186/s13068-016-0493-2
Logan, B. E. (2009). Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 7, 375–381. doi: 10.1038/nrmicro2113
Logan, B. E., Call, D., Cheng, S., Hamelers, H. V. M., Sleutels, T. H. J. A., Jeremiasse, A. W., et al. (2008). Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 42, 8630–8640. doi: 10.1021/es801553z
Lovley, D. R. (2006). Microbial energizers: fuel cells that keep on going. Microbe 1, 323–329. doi: 10.1128/microbe.1.323.1
Lu, L., Xing, D., Liu, B., and Ren, N. Q. (2012). Enhanced hydrogen production from waste activated sludge by cascade utilization of organic matter in microbial electrolysis cells. Water Res. 46, 1015–1026. doi: 10.1016/j.watres.2011.11.073
Lu, L., Xing, D., Xie, T., Ren, N. Q., and Logan, B. E. (2010). Hydrogen production from proteins via electrohydrogenesis in microbial electrolysis cells. Biosens. Bioelectron. 25, 2690–2695. doi: 10.1016/j.bios.2010.05.003
Mechichi, T., Stackebrandt, E., Gad’On, N., and Fuchs, G. (2002). Phylogenetic and metabolic diversity of bacteria degrading aromatic compounds under denitrifying conditions, and description of Thauera phenylacetica sp. nov., Thauera aminoaromatica sp. nov., and Azoarcus buckelii sp. nov. Arch. Microbiol. 178, 26–35. doi: 10.1007/s00203-002-0422-6
Montpart, N., Rago, L., Baeza, J. A., and Guisasola, A. (2015). Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 68, 601–615. doi: 10.1016/j.watres.2014.10.026
Pikuta, E. V., Hoover, R. B., Marsic, D., Whitman, W. B., Lupa, B., Tang, J., et al. (2009). Proteocatella sphenisci gen. nov., sp. nov., a psychrotolerant, spore-forming anaerobe isolated from penguin guano. Int. J. System. Evol. Microbiol. 59, 2302–2307. doi: 10.1099/ijs.0.002816-0
Roden, E. E., and Lovley, D. R. (1993). Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetoxidans. Appl. Environ. Microbiol. 59, 734–742.
Skórkowski, L., Zielewicz, E., Kawczynski, A., and Gil, B. (2018). Assessment of excess sludge ultrasonic, mechanical and hybrid pretreatment in relation to the energy parameters. Water 10:551. doi: 10.3390/w10050551
Sun, D., Wang, A., Cheng, S., Yates, M., and Logan, B. E. (2014). Geobacter anodireducens sp. nov., an exoelectrogenic microbe in bioelectrochemical systems. Int. J. Syst. Evol. Microbiol. 64(Pt 10), 3485–3491. doi: 10.1099/ijs.0.061598-0
Sun, R., Xing, D., Jia, J., Liu, Q., Zhou, A. J., Bai, S. W., et al. (2014). Optimization of high-solid waste activated sludge concentration for hydrogen production in microbial electrolysis cells and microbial community diversity analysis. Int. J. Hydrogen Energy 39, 19912–19920. doi: 10.1016/j.ijhydene.2014.09.163
Uzoejinwa, B. B., He, X. H., Wang, S., Abomohra, A., Hu, Y. M., and Wang, Q. (2018). Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: recent progress and future directions elsewhere worldwide. Energy Convers. Manage. 163, 468–492. doi: 10.1016/j.enconman.2018.02.004
van Kessel, M. A., Speth, D. R., Albertsen, M., Nielsen, P. H., Op den Camp, H. J., Kartal, B., et al. (2015). Complete nitrification by a single microorganism. Nature 528, 555–559. doi: 10.1038/nature16459
Wagner, R. C., Regan, J. M., Oh, S. E., Zuo, Y., and Logan, B. E. (2009). Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 43, 1480–1488. doi: 10.1016/j.watres.2008.12.037
Wang, D., Han, H., Han, Y., Li, K., and Zhu, H. (2017). Enhanced treatment of Fischer-Tropsch (F-T) wastewater using the up-flow anaerobic sludge blanket coupled with bioelectrochemical system: effect of electric field. Bioresour. Technol. 232, 18–26. doi: 10.1016/j.biortech.2017.02.010
Wang, L., Liu, W. Z., Kang, L. L., Yang, C. X., Zhou, A. J., and Wang, A. J. (2014). Enhanced biohydrogen production from waste activated sludge in combined strategy of chemical pretreatment and microbial electrolysis. Int. J. Hydrogen Energy 39, 11913–11919. doi: 10.1016/j.ijhydene.2014.06.006
Watanabe, T., Kojima, H., and Fukui, M. (2015). Draft genome sequence of Mizugakiibacter sediminis skMP5T. Genome Announce. 3:e01185-15. doi: 10.1128/genomeA.01185-15
Xiao, Y., Wu, S., Yang, Z., Zheng, Y., and Zhao, F. (2013). Isolation and identification of electrochemically active microorganisms (in Chinese). Prog. Chem. 25, 1771–1779.
Xue, S., Zhao, Q., Wei, L., and Jia, T. (2008). Trihalomethane formation potential of organic fractions in secondary effluent. J. Environ. Sci. 20, 520–527. doi: 10.1016/S1001-0742(08)62089-6
Yates, M. D., Kiely, P. D., Call, D. F., Rismani-Yazdi, H., Bibby, K., Peccia, J., et al. (2012). Convergent development of anodic bacterial communities in microbial fuel cells. ISME J. 6, 2002–2013. doi: 10.1038/ismej.2012.42
Zhen, G. Y., Lu, X. Q., Kato, H., Zhao, Y. C., and Li, Y. Y. (2017). Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 69, 559–577. doi: 10.1016/j.rser.2016.11.187
Zhilina, T. N., Appel, R., Probian, C., Brossa, E. L., Harder, J., Widdel, F., et al. (2004). Alkaliflexus imshenetskii gen. nov. sp. nov., a new alkaliphilic gliding carbohydrate-fermenting bacterium with propionate formation from a soda lake. Arch. Microbiol. 182, 244–253. doi: 10.1007/s00203-004-0722-0
Keywords: waste activated sludge, microbial electrolysis cell, ultrasonic pretreatment, hydrogen, organics degradation, microbial communities, organics fractionation
Citation: Hu K, Chen W, Jia S-q, Wang W and Han F (2019) Enhanced Degradation of Waste Activated Sludge in Microbial Electrolysis Cell by Ultrasonic Treatment. Front. Microbiol. 10:128. doi: 10.3389/fmicb.2019.00128
Received: 02 October 2018; Accepted: 21 January 2019;
Published: 05 February 2019.
Edited by:
Yang-Chun Yong, Jiangsu University, ChinaReviewed by:
Shanshan Chen, Fujian Agriculture and Forestry University, ChinaAbdul-Sattar Nizami, King Abdulaziz University, Saudi Arabia
Copyright © 2019 Hu, Chen, Jia, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Hu, aHVrYWloaXRAMTYzLmNvbQ==
 Kai Hu
Kai Hu Wei Chen1,2
Wei Chen1,2