- 1Sorbonne Université, CNRS UMR 7144, Diversity and Interactions in Oceanic Plankton - Station Biologique de Roscoff, Roscoff, France
- 2Sorbonne Université, CNRS Fédération de Recherche FR2424 - Station Biologique de Roscoff, Roscoff, France
- 3Laboratoire des Sciences du Numérique de Nantes (LS2N), CNRS UMR 6004 – Université de Nantes, Nantes, France
- 4Univ Rennes, Inserm, EHESP, Irset (Institut de recherche en santé, environnement et travail) - UMR_S 1085, Rennes, France
- 5Protim, Univ Rennes, Rennes, France
The marine diatom Guinardia delicatula is a cosmopolitan species that dominates seasonal blooms in the English Channel and the North Sea. Several eukaryotic parasites are known to induce the mortality of this species. Here, we report the isolation and characterization of the first viruses that infect G. delicatula. Viruses were isolated from the Western English Channel (SOMLIT-Astan station) during the late summer bloom decline of G. delicatula. A combination of laboratory approaches revealed that these lytic viruses (GdelRNAV) are small tailless particles of 35–38 nm in diameter that replicate in the host cytoplasm where both unordered particles and crystalline arrays are formed. GdelRNAV display a linear single-stranded RNA genome of ~9 kb, including two open reading frames encoding for replication and structural polyproteins. Phylogenetic relationships based on the RNA-dependent-RNA-polymerase gene marker showed that GdelRNAV are new members of the Bacillarnavirus, a monophyletic genus belonging to the order Picornavirales. GdelRNAV are specific to several strains of G. delicatula. They were rapidly and largely produced (<12 h, 9.34 × 104 virions per host cell). Our analysis points out the host's variable viral susceptibilities during the early exponential growth phase. Interestingly, we consistently failed to isolate viruses during spring and early summer while G. delicatula developed important blooms. While our study suggests that viruses do contribute to the decline of G. delicatula's late summer bloom, they may not be the primary mortality agents during the remaining blooms at SOMLIT-Astan. Future studies should focus on the relative contribution of the viral and eukaryotic pathogens to the control of Guinardia's blooms to understand the fate of these prominent organisms in marine systems.
Introduction
Diatoms are a major component of phytoplankton communities. They have a worldwide distribution (Mann and Droop, 1996; Malviya et al., 2016), occurring in freshwaters and marine habitats from the poles to the tropics (Takano, 1981; Kellogg and Kellogg, 1996; Sarno et al., 2005; Hernández-Becerril et al., 2010; Balzano et al., 2017). They are responsible for 35–75% of the marine primary production in the oceans (Nelson et al., 1995) and they play a fundamental role in the transfer of carbon to consumers (Armbrust, 2009). They are also important drivers in the ocean's export production due to their high sinking rate, thus playing a key-role in the functioning of the biological carbon pump (Falkowski et al., 1998; Smetacek, 1999). In nutrient rich coastal ecosystems, diatoms produce recurrent seasonal successions of species and blooms (Assmy and Smetacek, 2009; Sommer et al., 2012). The marine diatom genus Guinardia is described as a considerable contributor to micro-phytoplankton assemblages along the Atlantic coasts, in the English Channel (Grall, 1972; Gómez and Souissi, 2007; Guilloux et al., 2013), North Sea (Wiltshire et al., 2010) and western Irish Sea (Gowen et al., 1999). Especially, in the German Bight at Helgoland Roads time series, the bloom-forming species Guinardia delicatula is one of the most abundant diatom species, with highest abundances in early summer and autumn. However, a trend toward an earlier and wider period of development in response to environmental variables has been detected (Wiltshire et al., 2010; Schlüter et al., 2012). In the Western English Channel (WEC), G. delicatula dominates the seasonal cycle production, where its spring-summer development occurs commonly from May to August/September (Grall, 1972; Guilloux et al., 2013; Simon et al., personal communication).
Decades of research have emphasized the decisive role of physical factors (e.g., light, turbulence, and sedimentation), nutrient limitations and predation by zooplankton in pacing the seasonal development of marine diatoms (Smetacek, 1985; Sarthou et al., 2005; Schlüter et al., 2012; Sommer et al., 2012). Parasites have also been identified as potential primary agents that could shape diatom population dynamics (Tillmann et al., 1999; Gleason et al., 2015; Scholz et al., 2015). In the literature, several eukaryotic parasites of the genera Pirsonia (Kühn et al., 1996), Cryothecomonas (Drebes et al., 1996) and Rhizamoeba (Kühn, 1996) were described associated with G. delicatula. More recently, viruses have been identified as mortality agents involved in the control of diatoms dynamics. Up to date, about 20 diatom viruses have been described. They are separated into two groups: the single-stranded RNA (ssRNA) viruses (Shirai et al., 2008; Tomaru et al., 2009; Kimura and Tomaru, 2015) and the single-stranded DNA (ssDNA) viruses (Toyoda et al., 2012; Tomaru et al., 2013b; Kimura and Tomaru, 2015). Viruses of diatoms are also highly specific to their hosts, with species-specificity or even strain-specificity (Nagasaki et al., 2004; Tomaru et al., 2008; Toyoda et al., 2012). Different viruses infecting the diatom Chaetoceros tenuissimus can display variable environmental optima suggesting a niche partitioning in the nature (Kimura and Tomaru, 2017). As a consequence, viruses may control diatoms over a broad environmental range and play a key role in species or infra-specific groups successions. Nevertheless, more isolations and characterizations are needed to better understand the role of viruses in the regulation of host populations. To our knowledge, no virus is known to infect G. delicatula.
In this study, we isolated four ssRNA viruses causing lysis of G. delicatula from the long-term monitoring SOMLIT-Astan station located off Roscoff (Western English Channel, WEC). The host range, morphological features, lytic cycle, genome structure and phylogenetic position of the representative GdelRNAV-01 (G. delicatula ssRNA virus 01) were fully described. These viruses are new members of the Bacillarnavirus genus within the Picornavirales and share common features with other viruses infecting diatoms. Due to the ecological importance of its host, this discovery raises new questions about the contribution of viruses and other parasites to the interaction network associated with G. delicatula in the WEC.
Experimental Procedures
Growth Conditions of Algal Cultures
The marine diatom G. delicatula RCC3083 has been used in this study for the isolation of viruses. This xenic clonal strain was provided by the Roscoff Culture Collection (RCC, http://roscoff-culture-collection.org/) and was isolated the 19th September 2012 from surface water at the Roscoff Estacade station in the Western English Channel (48:43:56 N, 3:58:58 W). G. delicatula RCC3083 was maintained in sterile condition in K+Si medium (Keller et al., 1987) at 18°C, under a 12:12 h light:dark cycle of 100 μmol photons·m−2·s−1 provided by a white fluorescent light (Philips Master TL_D 18W/865). These culture conditions were used for all the following experimentations.
Genetic Variations Among G. delicatula Strains
Intraspecific variability within G. delicatula was examined in the SSU-18S, ITS and partial LSU-28S rDNA genes markers. The primers used were 63F (ACGCTTGTCTCAAAGATTA) and 1818R (ACGGAAACCTTGTTACGA) (Lepere et al., 2011) for the 18S, 329F (GTGAACCTGCRGAAGGATCA) (Guillou et al., 2004) and D1R-R (TATGCTTAAATTCAGCGGGT) (Lenaers et al., 1989) for the ITS, and D1R-F (ACCCGCTGAATTTAAGCATA) (Lenaers et al., 1989) and D3Ca (ACGAACGATTTGCACGTCAG) (Orsini et al., 2002) for the partial 28S D1-D3 region. Briefly, aliquots (2.25 μL) of G. delicatula cultures (Table 1) were submitted to 95°C for 5 min. The reaction mixture (30 μL final volume) was then added: Phusion Master Mix (1× final concentration, Thermo Scientific), 3% DMSO and 0.25 μM of each primer. PCR amplifications were performed with the following conditions: an initial incubation step at 95°C for 5 min, followed by 35 or 40 cycles (respectively for 18S−28S and ITS genes) of denaturation at 95°C for 1 min, annealing step for 30 s at 55, 52, and 57° for the amplifications of the SSU, ITS, and LSU, respectively, and extension at 72°C for 1 min 30. The cycles were followed by a final extension step at 72°C for 10 min. PCR products were sent for Sanger sequencing to GATC Biotech (https://www.gatc-biotech.com/en/index.html, Constance, Germany). Sequences were analyzed and aligned using Geneious 9.1.3.
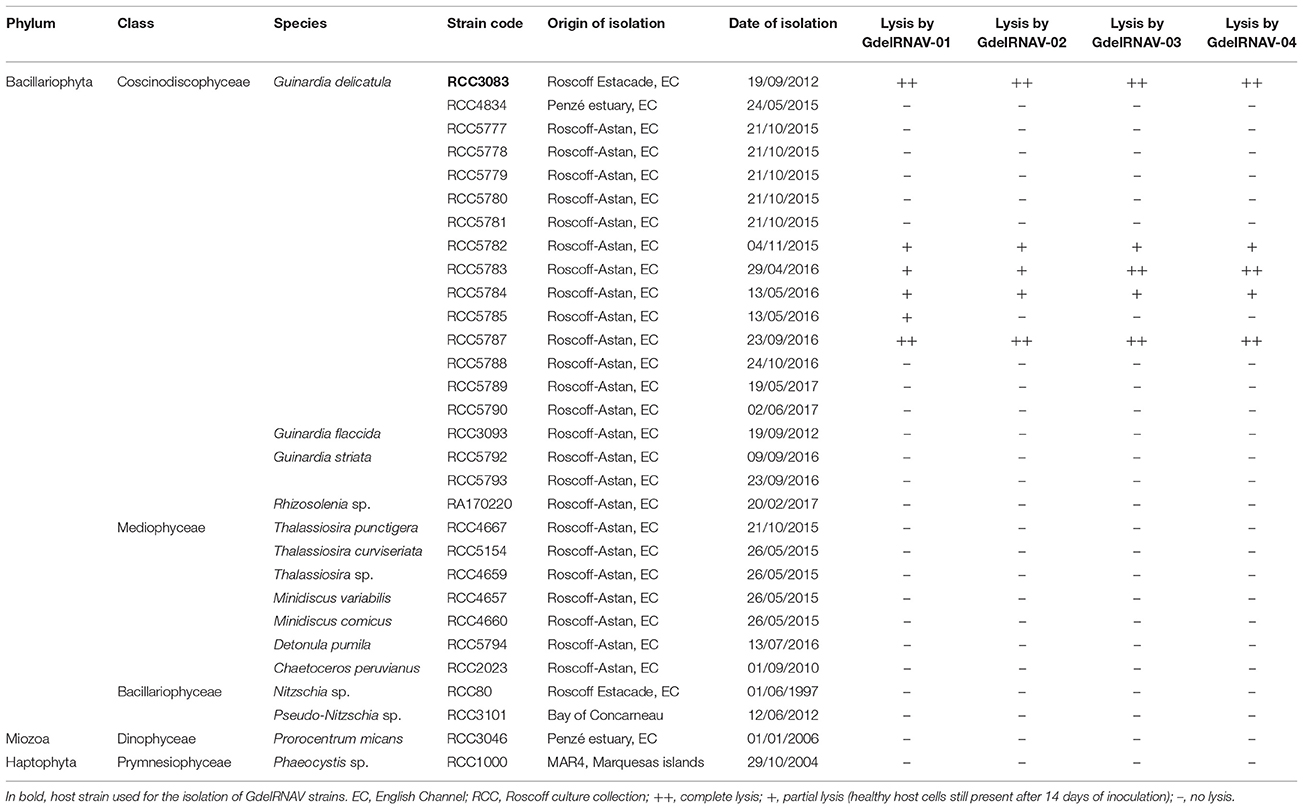
Table 1. Host range of GdelRNAV viral strains: lytic activity recorded within the species Guinardia delicatula and for other phytoplankton species.
Temporal Dynamics of G. delicatula
Seasonal variations in abundance of the diatom G. delicatula at the long-term monitoring station SOMLIT-Astan off Roscoff (48:46:18 N, 3:58:6 W) were obtained from microscopic counts data (RESOMAR-Pelagos database, http://abims.sb-roscoff.fr/pelagos). Briefly, surface seawater (1 meter depth) collected bi-monthly was preserved in acidic Lugol's iodine solution. After sedimentation in Utermöhl chambers, cell counts and identifications were performed under an inverted light microscope (Guilloux et al., 2013).
Isolation of Viruses
Samplings were conducted every fortnight between October 2015 and October 2016 at SOMLIT-Astan station. This station is representative of the permanently mixed water column of the Western English Channel (Wafar et al., 1983; L'Helguen et al., 1996). Seawater samples of 3L were collected at 1 m depth using a 5 L Niskin bottle. Back in the laboratory, samples were immediately pre-filtered through a 150 μm pore-size nylon filter to remove most of the micro- and mesozooplankton. 250 mL of pre-filtered samples were enriched with F/2 medium (10% v/v) and 5 mL of culture of G. delicatula RCC3083. After 2 weeks of incubation, the enriched samples were successively filtered through a GF/F filter (Whatman) and 0.22 μm PES filter (Whatman) to isolate the viral community.
Aliquot (0.5 mL) of the 0.22 μm-filtered samples were inoculated into 1.5 mL exponentially growing host culture in 24-multiwell plates under the host culture conditions as described above. Untreated host cultures served as controls.
Cultures were inspected by light microscopy 2 weeks after inoculation. If algal lysis was observed, 3 extinction dilution cycles were carried out to clone the pathogens (Suttle, 1993). Briefly, 100 μL aliquots of the lysates were serially diluted in 10-fold increment in 900 μL of exponentially growing culture of G. delicatula RCC3083 (900 μL). Lysates in the last dilution before extinction were transferred to another exponentially growing culture of G. delicatula RCC3083 and a new filtration on 0.22 μm was repeated to verify the transferability.
From this procedure, 4 clonal viral isolates lytic to G. delicatula strain RCC3083 have been obtained: GdelRNAV-01 (RCC5809), GdelRNAV-02 (RCC5810), GdelRNAV-03 (RCC5811), and GdelRNAV-04 (RCC5812). They were isolated from natural samples collected respectively on the 23th September, 26th August, 09th September, and 24th October 2016. They were maintained in culture by bimonthly transfers in G. delicatula RCC3083 under the host culture conditions described previously.
Host Ranges
To study the host ranges of GdelRNAV-01, GdelRNAV-02, GdelRNAV-03, and GdelRNAV-04, the viral suspensions were filtered on 0.45 μm PES filter (Whatman) and were added to 29 exponentially growing phytoplankton cultures (10% vol/vol), including 27 diatom cultures (Table 1). Untreated phytoplankton cultures served as controls. The experiment has been carried out in triplicate.
Algal growth and lysis were monitored after 7 and 14 days post-inoculation (dpi) under light microscopy. After 2 weeks incubation, phytoplanktonic cultures where no lysis was detected were not considered as susceptible hosts for these clonal viruses.
Transmission Electronic Microscopy (TEM)
To inspect the replication site of GdelRNAV-01, an exponential culture of G. delicatula RCC3083 host strain was inoculated with a fresh 0.45 μm filtered virus lysate (5% vol/vol). Uninfected host served as control. Aliquots of the cell suspensions were sampled every 12 h post-inoculation (hpi), and the algal abundances in the control and infected cultures were monitored by optical microscopy (Sedgewick Rafter, Hausser Scientific, USA). 10 mL of the aliquots were fixed with 1% glutaraldehyde and stored at 4°C until treatment. Pluronic F68 (final concentration 0.01%, Gibco) was added and cells were pelleted by centrifugation. Samples were rinsed twice in K+Si medium and 0.2 M cacodylate buffer (pH = 7.53) containing 2% of NaCl were added. Samples were then fixed with 1% OsO4 for 1 h at 4°C. After three washings with the cacodylate buffer, samples were progressively dehydrated in ethanol series (from 30 to 100%). Samples were embedded in Spurr's epoxy resin (Low viscosity, Electron Microscopy Sciences) and were polymerized over a week-end at 60°C. Thin sections (40–70 nm) were cut using a Leica ultracut UCT microtome and mounted on copper grids. Sections were stained with 0.4% uranyl acetate and viewed with a JEOL-JEM 1400 electron microscope (JEOL Ltd., Tokyo, Japan) operating at 80 kV.
Morphological features of the virions were also determined by TEM. Briefly, a fresh viral lysate of each four viral strains was filtered through 0.22 μm pore size filter and concentrated by centrifugation (Vivaspin 50 kDa, Sartorius). Concentrated viral suspension was negative stained for 40 s using uranyl acetate (2% w/v) on a copper grid. Appropriate controls (filtrates from uninfected hosts) have also been examined by TEM.
Growth Experiment
In order to study the virus growth kinetics, triplicates of exponentially growing cultures of G. delicatula RCC3083 were inoculated with a fresh 0.1 μm filtered suspension of GdelRNAV-01 (10% v/v, with a multiplicity of infection of 359.5). An untreated culture of G. delicatula RCC3083 served as control. Samples were taken every 12 h for 8 days to monitor host and virus parameters. Diatom counts were obtained using a Sedgewick Rafter cell (Hausser Scientific, USA) on an inverted microscope. Epifluorescence microscopy (U-MNB2 filter, Olympus BX51, Tokyo, Japan) was used to monitor morphological changes occurring in chloroplasts (using the fluorescence of Chl a) and in PicoGreen stained nuclei (Picogreen, final concentration 1×, Molecular Probes).
Viral titer was measured using the extinction dilution method (Suttle, 1993) and was estimated with the software Most Probable Number (MPN; version 2.0, Avineon, U.S Environmental Protection Agency). This experiment was performed in duplicate.
Viral latent period was calculated as the period of time between the viral inoculation and the first increase in viral titer. Burst size (number of viral progenies produced per one host cell lysed) was estimated from the ratio between the increase in viral titer and the decline in host cell concentration for a given period (from 72 to 96 h in our case), as:
where Vmax and Vmin are the maximal and minimal viral concentrations, respectively, and Hmax and Hmin the maximal and minimal host abundances.
Virions Thermal Stability
The stability of the virions to temperature was determined by incubating 0.5 mL of a 0.2 μm filtered GdelRNAV-01 suspension at −196, −80, −20, 4, 10, 18, 25, 30, 40, 50, and 60°C. After 24 h, samples were thawed or cooled down for 30 min at room temperature and inoculated with G. delicatula RCC3083 (10% v/v) in triplicates in 48-multiwell plates. Cultures were inspected by light microscopy at 7 and 14 days dpi to detect lysis.
Sensitivity to Chloroform
In order to determine whether GdelRNAV-01 is enveloped by a lipid membrane, 10% and 50% (v/v) of chloroform were added to aliquots (1.5 mL) of 0.2 μm filtered lysate. The mixtures were vigorously homogenized by inversion and incubated for 60 min at room temperature. Chloroform was removed by centrifugation, 2,200 × g for 20 min at room temperature and the aqueous layers, containing the virions, were transferred to new tubes. Samples were left overnight for evaporation to remove any chloroform contamination. Negative controls of K + Si medium were included. Samples were inoculated with G. delicatula RCC3083 (10% v/v) in triplicates in 48-multiwell plates. Cultures were inspected by light microscopy at 7 and 14 days dpi.
Virus Purification
A freshly produced GdelRNAV-01 lysate (500 mL) was filtered through 0.45 and 0.1 μm PES filters to remove cellular debris and bacteria. Polyethylene glycol 6000 (PEG, Sigma Aldrich) was added to the filtrate (final concentration 10% wt/v) and stored at 4°C overnight as described in Tomaru et al. (2004). The mixture was centrifuged at 30,100 × g, 4°C, for 2h15 (Avanti J-26XP, Beckman Coulter) and the pellet containing the viruses was washed with 10 mM phosphate buffer (10 mM KH2PO4 and 10 mM Na2HPO4, pH 7.2). The suspension was transferred to a Falcon tube (polypropylene) and an equal volume of chloroform was added. The sample was vigorously vortexed and centrifuged at 2,200 × g, for 20 min at room temperature. The aqueous layer was recovered and the chloroform procedure was repeated 7 times. After ultracentrifugation (207,870 × g, 4 h, 4°C, 70 Ti rotor, Optima XPN-80, Beckman Coulter) of the last aqueous phase, the viral pellet was collected and resuspended in 500 μL of Nuclease-Free Water (Life Technologies). This purified virus sample was used for analysis of nucleic acids, genome sequencing and analyses of structural proteins.
Viral Nucleic Acids
The nucleic acids (300 μL) of the four viral strain suspensions were extracted using the Kit MasterPure complete DNA and RNA purification (Epicenter) according to the manufacturer's instructions. This extraction was performed on a purified viral suspension for GdelRNAV-01 and on non-purified suspensions of GdelRNA-02, GdelRNA-03 and GdelRNA-04. Around 50 mL of lysates were filtered through 0.45 μm and 0.1 μm PES filters and concentrated by centrifugation (Vivaspin 50 kDa, Sartorius).
To determine the nature of viral nucleic acids, enzymatic digestions of nucleic acids extracts were conducted. Aliquots of 4 μL were digested with DNase I (final concentration 0.05 U·μL−1, Epicenter) at 37°C for 1h, with RNase A (final concentration 0.025 μg·μL−1, Epicenter) or with S1 nuclease (final concentration 0.03 U·μL−1, Promega) that degrades single stranded nucleic acids, for 30 min at room temperature. An untreated aliquot was kept on ice to serve as control. After incubation, samples were resolved on 1.2% agarose gel, stained with ethidium bromide and electrophoresed at 100 V for 50 min. The gel was visualized on Imagequant LAS4000 (GE Healthcare, Waukesha, WI, USA).
The ssRNA viral nucleic acids were converted to cDNA using the SuperScript III Reverse Transcriptase (Invitrogen) with random primers (Hexamers, 250 ng/μL) following the manufacturer's protocol.
Viral Genome Sequencing and Analyses
The complete viral genome of GdelRNAV-01 was obtained from a 2 × 150 bp paired-end run sequencing on an Illumina NextSeq platform performed by Fasteris (https://www.fasteris.com/dna/, Plan-les-Ouates, Switzerland). A total of 42,872,641 paired reads of 150 nt were quality trimmed using Trimmomatic v. 0.33 with default parameters (Bolger et al., 2014) and normalized using the Diginorm script accessible in the Trinity assembler package (Grabherr et al., 2011). The 443,025 remaining reads were de novo assembled into scaffolds with SPAdes version 3.11.0 using a combination of Kmer size 21, 33, 55 and 77 (Bankevich et al., 2012). Scaffolds sequences larger than 8,000 nucleotides were analyzed by megablast against nr database (release February 2018) and blastx against viral section of nr database (release February 2018) leading to the detection of a unique scaffold of 9,233 nucleotides matching viral sequences. Genes prediction was performed using NCBI ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder/) and validated by NCBI SmartBLAST (http://blast.ncbi.nlm.nih.gov/smartblast/).
Viral Proteins
An aliquot of purified viral suspension (75 μL) was boiled in 4× Laemmli buffer (25 μL) for 5 min and incubated on ice for 30 min. The mixture was then resolved on SDS-PAGE gel (NuPAGE 4–12% Bis-Tris Protein Gel, Life Technologies) using an XCell SureLock Mini-Cell (Invitrogen, Carlsbad, CA, USA) at 200 V for 45 min. After migration, the gel was rinsed 3 times in MilliQ water and stained overnight with ProSieve EX Safe Stain (Lonza Rockland, Inc). The gel was destained in MilliQ water baths and visualized with a white light table. Bands were excised and digested with trypsin for analysis by mass spectrometry using an Orbitrap instrument (LTQ-OrbitrpXL, Thermo Scientific) on Protim platform (https://www.protim.eu/, Rennes, France) as previously described (Lavigne et al., 2012) (see Supplementary Material). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Vizcaíno et al., 2016) partner repository with the dataset identifier PXD010967 (project accession) and 10.6019/PXD010967 (project DOI).
Phylogenetic Analysis of ssRNA Viruses
In order to determine the taxonomic position of GdelRNAV-01, its closest relatives were searched in NCBI non-redundant database (release 01 February 2018) using the helicase, RdRp and ORF2 amino acid sequences as query with the BLASTP tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The phylogenetic position of GdelRNAV-01 was inferred from comparative analyses of amino acid sequences encoding the RNA dependent RNA polymerase (RdRp) domain of the replicase polyprotein. The sequence of the GdelRNAV-01 RdRp domain was retrieved from the whole genome sequence using the Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov/Blast.cgi). RdRp domain sequences of a selection of Picornavirales that are representative of different families (International Committee on Taxonomy of Viruses, ICTV, https://talk.ictvonline.org/) were selected. The sequence alignment was generated by the MAFFT version 7 program and the E-INS-I iterative refinement method (https://mafft.cbrc.jp/alignment/server/, Katoh et al., 2017). A phylogenetic tree was constructed by maximum likelihood with PhyML 3.0 (http://www.atgc-montpellier.fr/phyml/, Guindon et al., 2010) with the automatic model selection by SMS (Lefort et al., 2017) and 1,000 bootstrap replicates. MEGA7 (Kumar et al., 2016) was used to visualize the final tree.
Comparative Analyses of the RdRp Gene Sequences Between G. delicatula Viruses
Degenerated primers, RdRp_F (TCTTCGTATGCCAGCACAACT) and RdRp_R (WAGAGCTCCATGAATCATYCC), were designed based on the RdRp regions of GdelRNAV-01 and of Csp03RNAV that infects Chaetoceros sp. strain SS08-C03 (AB639040), using Geneious 9.1.3 (Biomatters Ltd, NZ). These primers were used to amplify about 500 bp of the RdRp domains of GdelRNA-02, GdelRNA-03 and GdelRNA-04. The PCR reaction mixture (25 μL final volume) consisted of 1× Platinum Taq buffer (final concentration, Invitrogen), 2 mM MgCl2, 0.2 mM dNTP, 1 μM of each primer, 2U of Platinum Taq and 1 μL of cDNA. PCR amplifications were performed with the following conditions: an initial incubation step at 94°C for 75 s, followed by 40 cycles of denaturation at 94°C for 45 s, annealing step at 56°C for 45 s and extension at 72°C for 1 min. The 40 cycles were followed by a final extension step at 72°C for 9 min. PCR products were sent to Fasteris (https://www.fasteris.com/dna/, Plan-les-Ouates, Switzerland) for an enzymatic purification and for Sanger Sequencing using the degenerated primers RdRp_F and RdRp_R. The sequence alignment was generated by the MAFFT version 7.222 available on Geneious 9.1.3 (Biomatters Ltd, NZ).
Biogeography of GdelRNAV-01 in Natural Environments
To determine the distribution of GdelRNAV-01, environmental sequences data were downloaded from public databases and bioinformatics workflows were designed under Galaxy instance of the ABIMS platform: http://galaxy3.sb-roscoff.fr (Giardine et al., 2005) in order to search for homologs of GdelRNAV-01 genome sequences. Briefly, when necessary, data were trimmed and quality filtered and reads were mapped against GdelRNAV-01 genome using the Bowtie2 tool with default parameters (Langmead and Salzberg, 2012). In some cases, the GdelRNAV-01 genome or RdRp domain was directly blasted against environmental sequences using Geneious 9.1.3. This was the case for sequences obtained by Culley et al. (2003, 2006, 2007) and Culley and Steward (2007) with data volume <200 sequences.
Accession Numbers
The nucleotide sequences of the GdelRNAV-01 genome, GdelRNAV-02, GdelRNAV-03, and GdelRNAV-04 RdRp domains were deposited in the NCBI database under accession number MH706768, MH706769, MH706770 and MH706771, respectively.
Sequences obtained from the eukaryotic nuclear rRNA/ITS for G. delicatula strains were also deposited in the NCBI database: RCC3083 (MH712327), RCC4834 (MH712328), RCC5777–RCC5789 (MH712329–MH712341) for the 18S, RCC3083 (MH712342), RCC4834 (MH712343), RCC5777–RCC5787 (MH712344–MH712354) for the partial 28S and RCC3083 (MH714686), RCC4834 (MH714687), RCC5777–RCC5788 (MH714688–MH714699) for the ITS gene marker.
Results
In situ G. delicatula Dynamics and Cultural Diversity
During the sampling course (Sept 2015–Oct 2016), we recorded several blooming episodes of G. delicatula. A first small peak (2,560 cells·L−1) was detected in October 2015 (Figure 1). In 2016, the diatom bloom that occurred mid-June was dominated by G. delicatula (86,960 cells·L−1, 84.7% of the total diatom counts) while two smaller peaks were observed during the end of summer (12,540 cells·L−1 on the 28th of July, 8,500 cells·L−1 on the 26th of August).
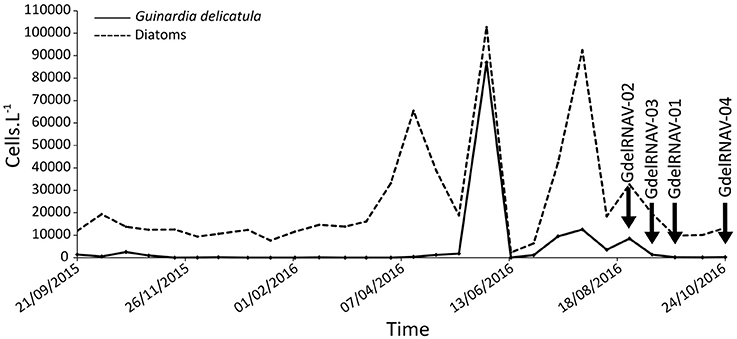
Figure 1. Temporal dynamics of Guinardia delicatula (solid line) and all diatoms (dash line) at SOMLIT-Astan station (Western English Channel) during the September 2015–October 2016 period. All along this period a protocol designed for the isolation of viruses lytic to G. delicatula was applied to seawater samples collected every fortnight. The arrows point to sampling dates on which the virus strains GdelRNAV-01 to 04 were successfully isolated.
During the sampling period, a total of 13 new G. delicatula strains were successfully isolated and maintained in culture (RCC5777-RCC5790, Table 1). Sequencing of the SSU-18S, ITS, and partial LSU-28S gene markers revealed very low variability among strains. For the 18S rDNA gene, the 1,655 bp alignment of the 14 strains indicated 100% of identity between sequences. The ITS gene sequences (646 bp) of 11 of the strains studied were identical while the sequences of RCC4834 (MH714687) and 5783 (MH714694) differed respectively by 2 and 1 position. Concerning the 28S D1-D3 region, 11 strains had identical sequences (833 bp) while a deletion was detected in the RCC5780 sequence (MH712347). The 28S D1-D3 region of strains RCC5788 and RCC5789 were not sequenced (strains lost). Strain RCC5790 was lost before amplification by all three gene markers.
Viral Isolation
Viruses lytic to G. delicatula RCC3083 were isolated between end August 2016 and end October 2016. During this period, four clonal viral strains were successfully isolated and maintained in culture. The inoculation of these viral isolates into fresh host cultures caused the clearance of infected cultures after 2 weeks and led to complete cell degradations (Figure 2).

Figure 2. Aspect of healthy cultures of G. delicatula RCC3083 (A,C) and infected cultures by GdelRNAV-01 (B,D) that show disintegration of host cells. (A,B) Pictures of the flasks, (C,D) Light microscopy micrographs showing heathy cells with golden brown plastids (C) or cells totally degraded (D). All pictures were taken 14 days post-infection. Scale bars on pictures (C,D) 50 μm.
Host Ranges
Cross infection experiments, using 15 phytoplankton species, indicated that G. delicatula was the only species lysed by the four viral isolates. However, all viruses showed clear strain specificity patterns (Table 1). Besides their isolation host (RCC3083), the 4 viruses infected strains RCC5782, RCC5783, RCC5784, and RCC5787 (isolated between October 2015 and September 2016). GdelRNAV-01 was the only virus able to cause lysis of G. delicatula RCC5785 (isolated in May 2016). For some host-virus combinations, lysis was not complete (cells with plastids observed in addition to empty frustules 14 dpi). Due to a boarder host range, GdelRNAV-01 that was isolated on the 23rd of September 2016, was chosen for detailed morphological, physiological and genetic characterization.
Morphological Features of Infected Cells and GdelRNAV Particles
Thin sections of G. delicatula cells showed clear signs of degradation of the cell ultrastructure (few remaining organelles, dispersed traces of cytoplasm) 72 h after the inoculation of GdelRNAV-01 compared to a healthy host (Figures 3A,B). GdelRNAV-01 accumulates in the host cytoplasm where it forms both crystalline arrays and unordered groups of particles (Figures 3C,D). No viral particle was observed in the control (Figure 3A).
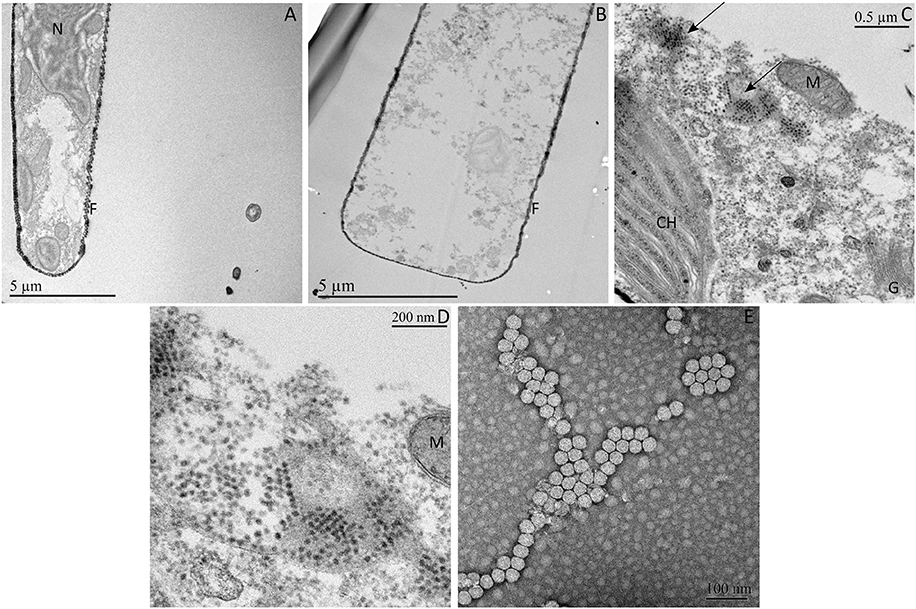
Figure 3. Ultrathin sections of Guinardia delicatula RCC3083 and negatively stained GdelRNAV-01 particles obtained by TEM. (A) Healthy control. (B–E) G. delicatula infected by GdelRNAV-01 at 72 hpi. (C) Crystalline arrays and dispersed viral particles accumulated in the host cytoplasm. (D) Higher magnification of panel C of GdelRNAV-01 in the host cytoplasm. (E) Negatively stained GdelRNAV-01 particles. Arrows: Crystalline arrays. F, frustule; G, Golgi apparatus; M, mitochondrion; N, nucleus, CH, chloroplast.
The TEM examination of GdelRNAV-01 progenies revealed untailed particles of 35 ± 2 nm in diameter (n = 173) with a hexagonal outline suggesting an icosahedral symmetry and the absence of outer membrane (Figure 3E).
Virions of GdelRNAV-02, GdelRNAV-03, and GdelRNAV-04 displayed the same morphological features as GdelRNAV-01 with particles diameter of 38 ± 2 nm (n = 98), 36 ± 2 nm (n = 105) and 38 ± 1.5 nm (n = 97), respectively (data not shown).
Infection Dynamic of GdelRNAV-01
After inoculation of GdelRNAV-01 in cultures of G. delicatula strain RCC3083, infected cells grew exponentially as in control cultures until 72 h post-inoculation (5,344 cell·mL−1 in infected cultured, Figure 4). Cell morphology was similar in infected and control cultures (cells forming colonies with amoeboid-shaped chloroplasts) (Figure 4, optical and epifluorescence micrographs). Then, diatom cell abundance decreased rapidly in infected cultures, with a stabilization step between night and day measurements. At the end of the experiment (168 h), diatom abundance in infected cultures reached 860 cell·mL−1 (mean of the three replicates) and was lower than at T0 (2,140 cell·mL−1). Nuclei and chloroplasts showed signs of degradation (rounded-shaped chloroplasts), and broken frustules heavily colonized by bacteria were observed (Figure 4, optical and epifluorescence pictures). In comparison, diatom cells in control culture exhibited an exponential growth during all the experiment.
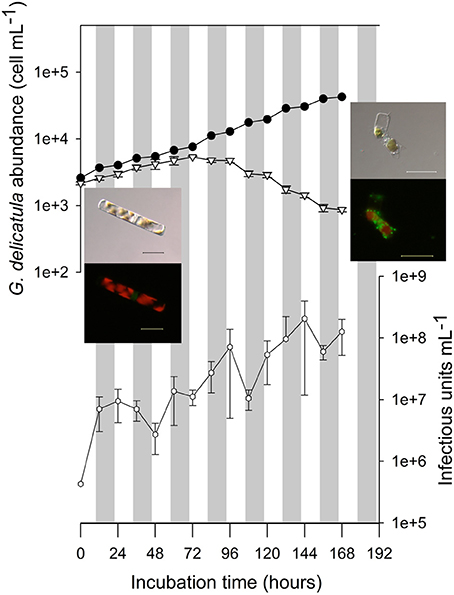
Figure 4. Infection kinetic of Guinardia delicatula RCC3083 by GdelRNAV-01. Abundances of diatom hosts in the control culture (black circles) and in infected cultures (open triangles) were obtained using optical microscopy. Viral titers (open hexagons) were estimated using the MPN method. Error bars were estimated based on counts obtained in triplicates of infected cultures. Gray rectangles represent the dark phases. Pictures obtained using transmitted-light and epifluorescence microscopy illustrate the morphology of G. delicatula cells in control and infected cultures at T0 and Tfinal. With epifluorescence microscopy the red natural fluorescence of chloroplasts and green fluorescence of PicoGreen stained nucleic acids are observed. At Tfinal, the green fluorescence is due to the presence of bacteria. Scale bars: 20 μm.
The first increase of viral titer occurred at 12 hpi, suggesting that the latent period is shorter than 12 h. Periods of increase in virus titer alternated with periods of stagnation suggesting multiple cycles in spite of the high MOI (multiplicity of infection).
The burst size, calculated as the number of viral particles produced per host cell, for a given period, was estimated to be 9.34 × 104 infectious units·cell−1.
Stability of the Viral Particles
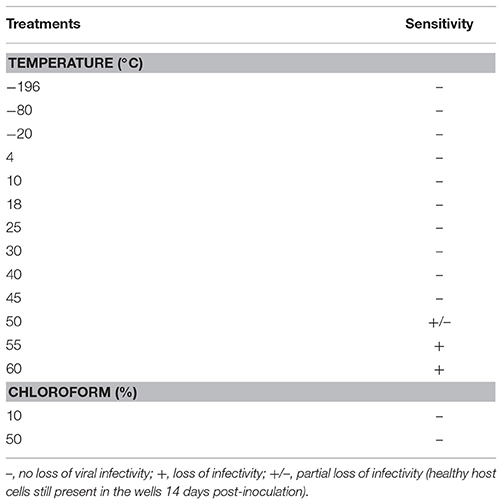
The viral suspension of GdelRNAV-01 has been exposed during 24 h to a broad range of temperatures (Table 2). The virus remained infectious from −196 to 45°C showing a high thermal stability. No viral lysis was recorded above 50°C.
GdelRNAV-01 was not susceptible to chloroform since no loss of viral infectivity was reported regardless of the chloroform concentration (Table 2).
GdelRNAV-01 Genome
Gel electrophoresis of purified GdelRNAV-01 nucleic acids and enzymatic digestions tests with DNase, RNase, and S1 Nuclease, indicated that GdelRNAV-01 possesses a single-stranded RNA genome of about 9 kb (Figure 5). Comparable results were obtained for GdelRNAV-02, GdelRNAV-03 and GdelRNAV-04 (data not shown), implying that these viruses possess a single-stranded RNA genomes of 9 kb.
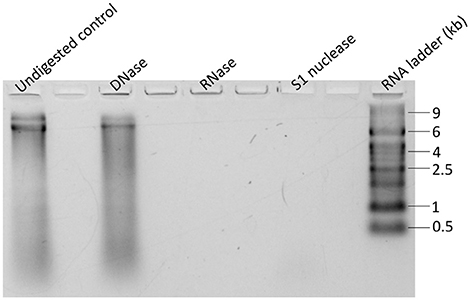
Figure 5. Nucleic acids type of GdelRNAV-01 after extraction. Extracts were treated with DNase treatment, with RNase treatment, or with S1 nuclease treatment.
The assembled genome of GdelRNAV-01 was 9,233 nt in length, excluding a poly(A) tail. The adenine, cytosine, guanine and uracil richness were estimated to be 30.1, 17.6, 19.3, and 33%, respectively.
The GdelRNAV-01 genome consisted of a 5′ untranslated region (UTR, 1,008 nt), two ORFS separated by an intergenic region (IGR, 574 nt) and a 3′ UTR of 367 nt (Figure 6). The size 5′ and 3′ UTR may not be completed as we did not do RACE. The first ORF was 4,959 nt long, representing 53.7% of the whole genome. It clustered two replication-related proteins: a helicase domain (110 amino acids) and a RNA-dependent RNA polymerase (RdRp) domain (291 amino acids) (Figure 6). The BLAST searches (Table 3) revealed that both proteins were closely related to Chaetoceros sp. RNA virus 03, Chaetoceros tenuissimus RNA virus type-II, to Marine RNA virus JP-A (Culley et al., 2007), and to Beihai picorna-like viruses and Wenzhou picorna-like virus 50, that infect invertebrates (Shi et al., 2016).
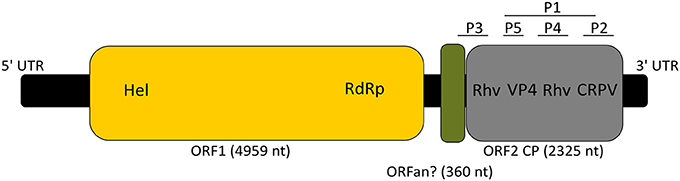
Figure 6. Schematic genome organization of GdRNAV-01 (9,233 nt). 5′UTR: 5′ untranslated region (1,008 nt), 3′ UTR: 3′ untranslated region (367 nt). The yellow box indicates the replication polyprotein with Hel: Helicase and RdRp: RNA-dependent RNA polymerase. The gray box represents the capsid proteins (CP) with domains corresponding to the Rhv_like superfamily interspaced by the Dicistro_VP4, and the CRPV_capsid superfamily. The green box indicates the possible ORFan. P1 to P5: structural proteins. Segments corresponding to P1 to P5 are not scaled on the genome sequence.
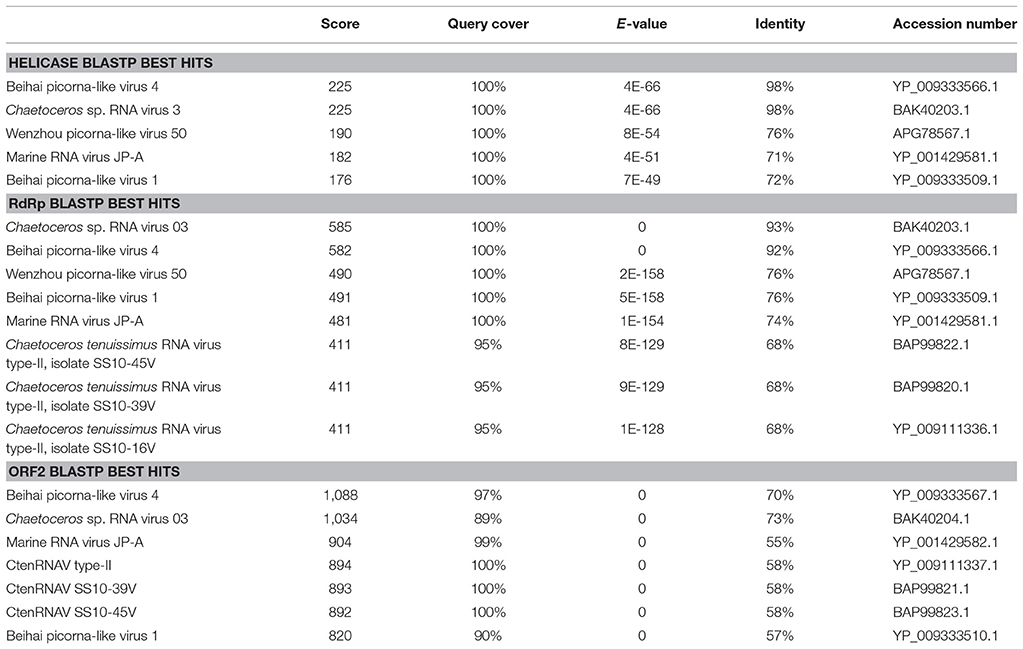
Table 3. Best hits from BLASTP results showing significant alignments with the helicase and RdRp domains and the ORF-2 of GdelRNAV-01.
The second ORF (2325 nt, 25.2% of the viral genome) encoded for putative structural proteins of the capsid based on the detection of 4 conserved capsid domains. This ORF contained two domains that belong to the Picornavirus capsid protein domain_like (Rhv1 and 2), one to the Cricket paralysis virus VP4 domain from the Dicistroviridae family (Dicistro_VP4) and the last domain shared significant homology with the cricket paralysis virus (CRPV) capsid protein like (Figure 6). As with the first ORF, best hits of ORF2 using BLASTP (774 amino acids) corresponded to sequences of ssRNA viruses (Table 3).
In the IGR, a small conserved region was detected (360 nt, 4% of the whole genome). BLASTn searches revealed two hits belonging to Chaetoceros sp. RNA virus 03 (E-value: 2e-88) and Beihai picorna-like virus 4 (E-value: 5e-54). However, no statistically significant matching protein sequence was found using BLASTP searches (119 amino acids).
Structural Proteins
The SDS PAGE showed five proteins of variable staining intensity (Figure 7). Four proteins of respectively 33.9, 29.8, 27, and 6.8 kDa (P2, P3, P4, and P5, respectively) were intensively stained while the largest protein of 38.6 kDa (P1) had a weaker intensity (Figure 7). The amino acid sequences of each protein analyzed by mass spectrometry (MS) were found in the predicted sequence of the ORF2 (Figure 6 and Tables S1, S2). The smallest protein (P5) (6.8 kDa predicted from the gel and 4.7 kDa from the amino acid sequence) corresponded to the Dicistro_VP4 domain. The predicted peptides of P2 (33.9 kDa on the SDS-PAGE gel, 16.8 kDa based on the amino acid sequence) matched the C-ter region of the CRPV domain. The protein P4 was more central and peptides analyzed by MS corresponded to the N-terminal region of Rhv2 domain. MS analysis of P3 peptides revealed that they matched the upstream region of ORF2 up to Rhv1 domain. The largest protein P1 (38.6, 51.7 kDa predicted) encompassed VP4, Rhv2, and CRPV domains.
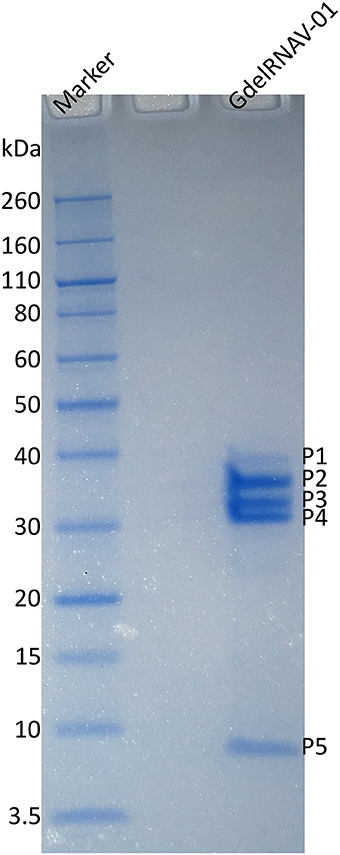
Figure 7. Analysis of the structural proteins of GdelRNAV-01 using SDS-PAGE. Lane Marker: Novex sharp unstained protein standard marker (kDa). Lane GdelRNAV-01: Denatured proteins of purified GdelRNAV-01. P1 to P5 represent the proteins 1 to 5.
Phylogenetic Analysis of the Picornavirales
Phylogenetic reconstructions based on the analysis of the RdRp amino acid sequences of a selection of Picornavirales revealed that GdelRNAV-01 clusters among the monophyletic genus Bacillarnavirus (Figure 8). Sequences of these viruses, that infect diatom species, gathered in a clade supported by a high bootstrap value (98%). GdelRNAV-01 was most closely related to Chaetoceros sp. number03 RNA virus (Csp03RNAV), a virus infecting the marine diatom Chaetoceros sp. (Tomaru et al., 2013a).
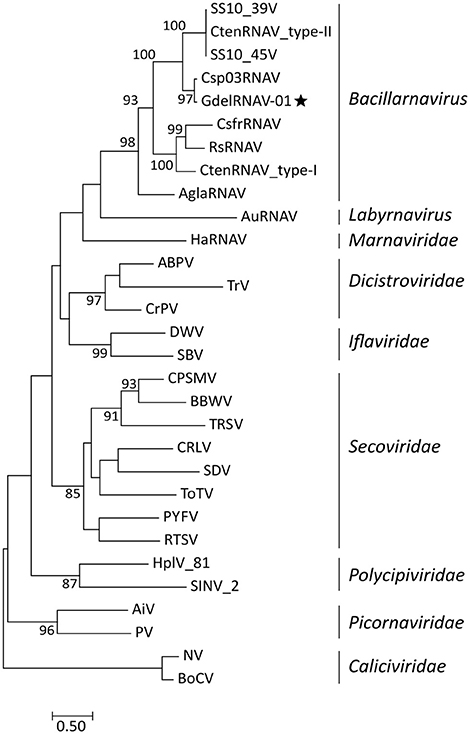
Figure 8. Phylogenetic rooted tree based on RdRp sequences of representative viruses from the Picornavirales order. Caliciviridae viruses were taken as outgroup. The star indicates the position of GdelRNAV-01 in the genus Bacillarnavirus. The Maximum Likelihood tree was generated using PhyML 3.0 with 1,000 replications and a LG + G + I + F substitution model according to the SMS analyses. Bootstraps values (%) >80 are shown. Scale bar indicates the number of substitutions per site. Virus abbreviations: ABPV, acute bee paralysis virus NC_002548.1; AiV, Aichi virus, AB010145; AglaRNAV, Asterionellopsis glacialis RNA virus NC_024489; AuRNAV, Aurantiochytrium single-stranded RNA virus), BAE47143; BoCV, Bovine enteric calicivirus, AJ011099; BBW, broad bean wilt virus 1 NC_005289.1; CsfrRNAV, Chaetoceros socialis f. radians RNA virus, AB469874; Csp03RNAV, Chaetoceros sp. number03 RNA virus, AB639040; CtenRNAV type-I, Chaetoceros tenuissimus RNA virus, AB375474; CtenRNAV type-II, AB971661; CtenRNAV_SS10V-39V, AB971662; CtenRNAV_SS10V-45V, AB971663; CRLV, cherry rasp leaf virus, NC_006271.1; CPSMV, cowpea severe mosaic virus, NC_003545; CrPV, cricket paralysis virus, NC_003924; DWV, deformed wing virus, NC_004830; HaRNAV, Heterosigma akashiwo RNA virus, NC_005281; HplV-81, Hubei picorna-like virus 81 strain CJLX25805, KX884540.1; HplV-82, Hubei picorna-like virus 82 KX883688.1; PV, human poliovirus 1 Mahoney, V01149; IFV, infectious flacherie virus NC_003781.1; NV, Norwalk virus, M87661; PYFV, Parsnip yellow fleck virus, D14066; RsRNAV, Rhizosolenia setigera RNA virus, AB243297; RTSV, rice tungro spherical virus, AAA66056; SBV, sacbrood virus, NC_002066; SDV, Satsuma dwarf virus RNA 1 NC_003785.2; SINV-2, Solenopsis invicta virus 2 EF428566.1; TRSV, tobacco ringspot virus RNA 1 NC_005097.1; ToTV, tomato torrado virus RNA 1 NC_009013.1; TrV, triatoma virus, NC_003783.
Intraspecific Comparison of GdelRNAV Viruses
Nucleotide partial sequences of the RNA-dependent RNA polymerase (RdRp) of GdelRNAV-01, GdelRNAV-02, GdelRNAV-03 and GdelRNAV-04 were highly similar, with 474 identical sites on 478 bp, representing 99.2% of identity (Figure S1). The slight differences between these four RdRp sequences are shown Figure S1 in red frames.
Distribution of GdelRNAV-01 in Natural Environments
Environmental surveys allowed us to study the natural distribution of GdelRNAV-01 across marine and fresh water environments (Table 4). In total, 18,858 homologous reads (488.2 bp on average) mapped against the GdelRNAV-01 RdRp gene marker. They were exclusively found in temperate coastal water stations off British Columbia. At these stations, deep-sequencing of the RdRp has been carried out to assess the diversity and composition of the ssRNA viral community (Gustavsen et al., 2014).
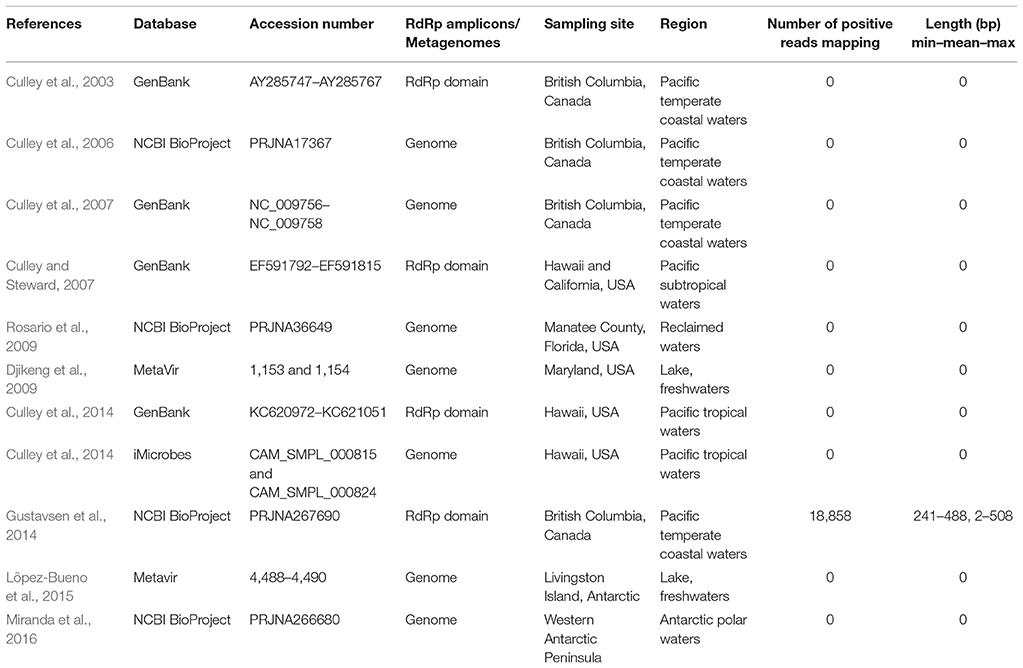
Table 4. Results of the mapping of the GdelRNAV-01 genome or gene sequences onto environmental data.
Discussion
The marine diatom G. delicatula is a cosmopolitan species that dominates seasonal blooms in the English Channel and the North Sea. In the environment, this species is known to be infected by several eukaryotic parasites. In this study, we described for the first time viruses that infect G. delicatula, and probably contribute to the control of its bloom dynamics.
Morphological and genomic analyses indicated that the new G. delicatula viruses isolated during this study belong to the unassigned genus Bacillarnavirus within the order Picornavirales (Tomaru et al., 2015). This genus includes ssRNA viruses that infect diatoms and includes three species to date (ICTV). Like other members of the unassigned genus Bacillarnavirus, virions are small naked particles (35 nm in diameter) with a hexagonal outline, suggesting an icosahedral symmetry, and they contain a positive-sense ssRNA genome. During the infection, viral progenies accumulate in the host cytoplasm, where they form both crystalline arrays and unordered particles, before their release by cell lysis. The genome architecture of GdelRNAV-01 is similar to that of other Picornavirales (Koonin et al., 2008). The 9 kb genome of GdelRNAV-01 comprises 2 ORFs, coding respectively for replication and structural polyproteins with best hits to sequences of ssRNA viruses of the diatom Chaetoceros spp., as well as marine environmental virus genomes (Culley et al., 2007) and viral sequences assembled from transcriptomics (Shi et al., 2016). More precisely, the first ORF includes domains coding for the RNA-dependent RNA-polymerase (RdRp) as well as a helicase. The RdRp is traditionally used as a diversity marker for RNA viruses (Koonin et al., 1993; Culley et al., 2003). The sequencing of this gene showed low genetic variability between the four GdelRNAV isolates, suggesting that the four strains belong to the same virus. The second ORF encodes for the structural polyprotein showing the same conserved protein domains (Rhv_like, Dicistro_VP4, CRPV_capsid) and architecture as the other diatom viruses. According to our proteomic analyses, a large protein (P1), whose amino acid sequence appeared to overlap that of the three protein domains, was detected. This protein may correspond to an immature form associated to provirions. It is likely that this precursor capsid protein cleaves into smaller proteins after a maturation process as described or speculated for other members of the order Picornavirales (Lang et al., 2004; Mullapudi et al., 2016). Apart from this putative immature protein, our MS results suggest that GdelRNAV virions are constituted of four structural proteins that matched with each of the four conserved domains predicted in the genome sequence. Interestingly, other known members of Bacillarnavirus display only three structural proteins (Tomaru et al., 2015).
The functional characterization showed that GdelRNAV is strain specific, virions are produced rapidly (in our case, latent period <12 h) and infection ultimately induced host mortality through cell lysis, as reported for other algal viruses including both RNA and dsDNA viruses (for review see Brussaard and Martínez, 2008 and reference therein). For GdelRNAV, the estimated burst size reached 9.34 × 104 virions per host cell, which is higher than reported values for other ssRNA viruses [<100–104 virions per cell (Tomaru et al., 2015)]. One divergent feature of ssRNA diatom viruses compared to known algal viruses is the simultaneous increase in host and viral concentrations during the first days of incubation (72 h in our case) and the occurrence of multiple viral cycles (Shirai et al., 2008; Tomaru et al., 2009, 2011b, 2014; Kimura and Tomaru, 2013). A theoretical calculation suggests that only 3.3% of G. delicatula cells produced viral progenies at the initial time of the kinetics and that the percentage of permissive cells increases along the growth curve (see Table S3). It is thus likely that the host cell culture, although clonal, exhibited different degrees of viral susceptibility.
Diverse mechanisms of host resistance to viral infection have been described in marine microalgae. For example, in the picoeukaryote Ostreococcus tauri, some proteins encoded in chromosome 19 were shown to be involved in the host defense against viral attack (Yau et al., 2016). Unfortunately, G. delicatula's genome sequence is not available to speculate on similar mechanisms operating in our virus-host model system. However, previous studies demonstrated that the physiological status of diatom host cells can also determine the outcome of viral infection. Chaetoceros host population generally became more permissive to viral infection with the progression of the stationary growth (Tomaru et al., 2014). This led to the hypothesis that diatoms with a high growth rate may tolerate viral infection while cells with less vigorous growth rate undergo rapid lysis and do not participate to the bloom formation (Tomaru et al., 2015). Interestingly, we attempted to isolate G. delicatula viruses throughout the year but isolations were successful only with samples collected in late summer. We cannot rule out that the host strain used for viral isolation was not permissive to the spring and summer viral populations. Yet, the amplitude of G. delicatula late summer bloom is consistently lower compared to spring and early summer blooms at SOMLIT-Astan (our study and RESOMAR Pelagos database). It is tempting to speculate that the late summer environmental conditions were less favorable for the growth of G. delicatula, which, in turn, may have been more vulnerable to viral attack. G. delicatula is also known to be infected by diverse parasites, such as Pirsonia (Kühn et al., 1996) and Cryothecomonas (Drebes et al., 1996). G. delicatula blooms may thus be controlled by a complex network of pathogens, among which viruses may not be the primary cause of bloom disintegration, as already reported for Chaetoceros spp. (Tomaru et al., 2011a, 2018). In any case, variability in viral susceptibility of the host is probably contributing to the sustainability of these diatom bloom events in natural habitats.
Isolating and characterizing new viruses infecting ecologically relevant hosts is a prerequisite to advance our understanding of the large amount of environmental sequences collected worldwide. Data-mining of RNA viromes that are publicly available showed that the genome of GdelRNAV-01 recruited homologs in environments where G. delicatula is known to develop [based on Ocean Biogeographic Information System (OBIS) database and Hobson and McQuoid (1997)]. Although very few RNA viromes are available, these preliminary results suggest that GdelRNAV occur in different temperate coastal waters. Seasonal metagenomic monitoring in the Western English Channel should be considered to investigate the composition, the prevalence and the temporal dynamics of this relevant virus–host model system. It will contribute to have a closer look at the relative contribution of the different pathogens to the control of diatom blooms, necessary to understand the fate of these prominent organisms in marine systems.
Author Contributions
LA designed and conducted the experiments and analyses and wrote the manuscript. NS designed the study, contributed to the experiments, wrote the manuscript. FR-J helped for sampling and for kinetic experiments, and carried out taxonomic counts in the frame of the Roscoff SOMLIT-Astan time series. FL isolated Guinardia hosts, performed the PCR and participated to the analyses of the eukaryotic gene marker. SC designed the genome recruitment analysis. ErC assembled the viral genome. EmC performed and analyzed the proteomics data. EB provided technical support. A-CB designed the study, contributed to the experiments, wrote the manuscript.
Funding
This study was supported by PhD fellowships from the Université Pierre et Marie Curie (Sorbonne Universités) and the Région Bretagne (ARED), the ANR CALYSPO (ANR-15-CE01-0009) and the CNRS-INSU EC2CO CYCLOBS project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the crew of the Neomysis ship for their help during the samplings at SOMLIT-Astan station. We are also grateful to Sophie Le Panse from the microscopy platform, for the transmission electron micrographs, to the RCC for the phytoplankton strains provided and also to Sarah Romac for her assistance with molecular biology. Yuji Tomaru who provided protocols and advices on viral purification is acknowledged. Laurianne Gerin is thanked for her English proofreading. We thank the three reviewers for their comments on a previous version of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.03235/full#supplementary-material
References
Armbrust, E. V. (2009). The life of diatoms in the world's oceans. Nature 459, 185–192. doi: 10.1038/nature08057
Assmy, P., and Smetacek, V. (2009). “Algal blooms,” in Encyclopedia of Microbiology, ed M. Schaechter (Oxford: Elsevier), 27–41.
Balzano, S., Percopo, I., Siano, R., Gourvil, P., Chanoine, M., Marie, D., et al. (2017). Morphological and genetic diversity of Beaufort Sea diatoms with high contributions from the Chaetoceros neogracilis species complex. J. Phycol. 53, 161–187. doi: 10.1111/jpy.12489
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Brussaard, C. P. D., and Martínez, J. (2008). Algal bloom viruses. Plant Viruses 2, 1–10. Available online at: http://www.globalsciencebooks.info/JournalsSup/08PV_2_1.html
Culley, A. I., Lang, A. S., and Suttle, C. A. (2003). High diversity of unknown picorna-like viruses in the sea. Nature 424, 1054–1057. doi: 10.1038/nature01886
Culley, A. I., Lang, A. S., and Suttle, C. A. (2006). Metagenomic analysis of coastal RNA virus communities. Science 312, 1795–1798. doi: 10.1126/science.1127404
Culley, A. I., Lang, A. S., and Suttle, C. A. (2007). The complete genomes of three viruses assembled from shotgun libraries of marine RNA virus communities. Virol. J. 4, 69. doi: 10.1186/1743-422X-4-69
Culley, A. I., Mueller, J. A., Belcaid, M., Wood-charlson, E. M., Poisson, G., and Steward, G. F. (2014). The characterization of RNA viruses in tropical seawater using targeted PCR and metagenomics. MBio 5, e01210–e01214. doi: 10.1128/mBio.01210-14
Culley, A. I., and Steward, G. F. (2007). New genera of RNA viruses in subtropical seawater, inferred from polymerase gene sequences. Appl. Environ. Microbiol. 73, 5937–5944. doi: 10.1128/AEM.01065-07
Djikeng, A., Kuzmickas, R., Anderson, N. G., and Spiro, D. J. (2009). Metagenomic analysis of RNA viruses in a fresh water lake. PLoS ONE 4:e7264. doi: 10.1371/journal.pone.0007264
Drebes, G., Kühn, S. F., Gmelch, A., and Schnepf, E. (1996). Cryothecomonas aestivalis sp. nov., a colourless nanoflagellate feeding on the marine centric diatom Guinardia delicatula (Cleve) Hasle. Helgoländ. Meeresuntersuchung. 50, 497–515. doi: 10.1007/BF02367163
Falkowski, P. G., Barber, R. T., and Smetacek, V. (1998). Biogeochemical controls and feedbacks on ocean primary production. Sci. New Ser. 281, 200–206. doi: 10.1126/science.281.5374.200
Giardine, B., Riemer, C., Hardison, R. C., Burhans, R., Elnitski, L., Shah, P., et al. (2005). Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 15, 1451–1455. doi: 10.1101/gr.4086505
Gleason, F. H., Jephcott, T. G., Küpper, F. C., Gerphagnon, M., Sime-Ngando, T., Karpov, S. A., et al. (2015). Potential roles for recently discovered chytrid parasites in the dynamics of harmful algal blooms. Fungal Biol. Rev. 29, 20–33. doi: 10.1016/j.fbr.2015.03.002
Gómez, F., and Souissi, S. (2007). Unusual diatoms linked to climatic events in the northeastern English Channel. J. Sea Res. 58, 283–290. doi: 10.1016/j.seares.2007.08.002
Gowen, R. J., McCullough, G., Kleppel, G. S., Houchin, L., and Elliott, P. (1999). Are copepods important grazers of the spring phytoplankton bloom in the western Irish Sea? J. Plankton Res. 21, 465–483. doi: 10.1093/plankt/21.3.465
Grabherr, M. G., Haas, B. J., Yassour, M., Levin, J. Z., Thompson, D. A., Amit, I., et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652. doi: 10.1038/nbt.1883
Grall, J. R. (1972). Développement “printanier” de la Diatomée Rhizosolenia delicatula près de Roscoff. Mar. Biol. 16, 41–48.
Guillou, L., Eikrem, W., Chrétiennot-Dinet, M.-J., Le Gall, F., Massana, R., Romari, K., et al. (2004). Diversity of picoplanktonic prasinophytes assessed by direct nuclear SSU rDNA sequencing of environmental samples and novel isolates retrieved from oceanic and coastal marine ecosystems. Protist 155, 193–214. doi: 10.1078/143446104774199592
Guilloux, L., Rigaut-Jalabert, F., Jouenne, F., Ristori, S., Viprey, M., Not, F., et al. (2013). An annotated checklist of Marine Phytoplankton taxa at the SOMLIT-Astan time series off Roscoff (Western English Channel, France): data collected from 2000 to 2010. Cah. Biol. Mar. 54, 247–256.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. doi: 10.1093/sysbio/syq010
Gustavsen, J. A., Winget, D. M., Tian, X., and Suttle, C. A. (2014). High temporal and spatial diversity in marine RNA viruses implies that they have an important role in mortality and structuring plankton communities. Front. Microbiol. 5:703. doi: 10.3389/fmicb.2014.00703
Hernández-Becerril, D. U., Herrera-Hernández, P., Pérez-Mendoza, A., and Gerardo A Ceballos-Corona, J. (2010). Marine planktonic diatoms of the order Rhizosoleniales (Bacillariophyta) From the tropical Mexican pacific. Vie Milieu Life Environ. 60, 95–107.
Hobson, L. A., and McQuoid, M. R. (1997). Temporal variations among planktonic diatom asseblages in a turbulent environment of the southern Strait of Georgia, British Columbia, Canada. Mar. Ecol. Prog. Ser. 150, 263–274. doi: 10.3354/meps150263
Katoh, K., Rozewicki, J., and Yamada, K. D. (2017). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. bbx108. doi: 10.1093/bib/bbx108
Keller, M. D., Seluin, R. C., Claus, W., and Guillard, R. R. L. (1987). Media for the culture of oceanic ultraphytoplankton. J. Phycol. 23, 633–638. doi: 10.1016/0198-0254(88)92621-0
Kellogg, D. E., and Kellogg, T. B. (1996). Diatoms in South Pole ice: implications for eolian contamination of Sirius Group deposits. Geology 24, 115–118. doi: 10.1130/0091-7613(1996)024<0115:DISPII>2.3.CO;2
Kimura, K., and Tomaru, Y. (2013). Isolation and characterization of a single-stranded DNA virus infecting the marine diatom Chaetoceros sp. strain SS628-11 isolated from western Japan. PLoS ONE 8:e82013. doi: 10.1371/journal.pone.0082013
Kimura, K., and Tomaru, Y. (2015). Discovery of two novel viruses expands the diversity of single-stranded DNA and single-stranded RNA viruses infecting a cosmopolitan marine diatom. Appl. Environ. Microbiol. 81, 1120–1131. doi: 10.1128/AEM.02380-14
Kimura, K., and Tomaru, Y. (2017). Effects of temperature and salinity on diatom cell lysis by DNA and RNA viruses. Aquat. Microb. Ecol. 79, 79–83. doi: 10.3354/ame01818
Koonin, E. V, Wolf, Y. I., Nagasaki, K., and Dolja, V. V (2008). The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat. Rev. Microbiol. 6, 925–939. doi: 10.1038/nrmicro2030
Koonin, E. V., Dolja, V. V., and Morris, T. J. (1993). Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 28, 375–430. doi: 10.3109/10409239309078440
Kühn, S. F. (1996). Rhizamoeba schnepfii sp. nov, a naked amoeba feeding on marine diatoms (North Sea, German Bight). Arch. Fur Protistenkd. 147, 277–282. doi: 10.1016/S0003-9365(97)80054-1
Kühn, S. F., Drebes, G., and Schnepf, E. (1996). Five new species of the nanoflagellate Pirsonia in the German Bight, North Sea, feeding on planktonic diatoms. Helgoländ. Meeresuntersuchung. 50, 205–222 doi: 10.1007/BF02367152
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lang, A. S., Culley, A. I., and Suttle, C. A. (2004). Genome sequence and characterization of a virus (HaRNAV) related to picorna-like viruses that infects the marine toxic bloom-forming alga Heterosigma akashiwo. Virology 320, 206–217. doi: 10.1016/j.virol.2003.10.015
Langmead, B., and Salzberg, S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. doi: 10.1038/nmeth.1923.Fast
Lavigne, R., Becker, E., Liu, Y., Evrard, B., Lardenois, A., Primig, M., et al. (2012). Direct iterative protein profiling (DIPP)–an innovative method for large-scale protein detection applied to budding yeast mitosis. Mol. Cell. Proteomics 11:M111.012682. doi: 10.1074/mcp.M111.012682
Lefort, V., Longueville, J. E., and Gascuel, O. (2017). SMS: smart model selection in PhyML. Mol. Biol. Evol. 34, 2422–2424. doi: 10.1093/molbev/msx149
Lenaers, G., Maroteaux, L., Michot, B., and Herzog, M. (1989). Dinoflagellates in evolution. A molecular phylogenetic analysis of large subunit ribosomal RNA. J. Mol. Evol. 29, 40–51. doi: 10.1007/BF02106180
Lepere, C., Demura, M., Kawachi, M., Romac, S., Probert, I., and Vaulot, D. (2011). Whole-genome amplification (WGA) of marine photosynthetic eukaryote populations. FEMS Microbiol. Ecol. 76, 513–523. doi: 10.1111/j.1574-6941.2011.01072.x
L'Helguen, S., Madec, C., and Le Corre, P. (1996). Nitrogen uptake in permanently well-mixed temperate coastal waters. Estuar. Coast. Shelf Sci. 42, 803–818. doi: 10.1006/ecss.1996.0051
Lõpez-Bueno, A., Rastrojo, A., Peirõ, R., Arenas, M., and Alcamí, A. (2015). Ecological connectivity shapes quasispecies structure of RNA viruses in an Antarctic lake. Mol. Ecol. 24, 4812–4825. doi: 10.1111/mec.13321
Malviya, S., Scalco, E., Audic, S., Vincent, F., Veluchamy, A., Poulain, J., et al. (2016). Insights into global diatom distribution and diversity in the world's ocean. Proc. Natl. Acad. Sci. U.S.A. 113, 1516–1525. doi: 10.1073/pnas.1509523113
Mann, D. G., and Droop, S. J. M. (1996). 3. Biodiversity, biogeography and conservation of diatoms. Hydrobiologia 336, 19–32. doi: 10.1007/BF00010816
Miranda, J. A., Culley, A. I., Schvarcz, C. R., and Steward, G. F. (2016). RNA viruses as major contributors to Antarctic virioplankton. Environ. Microbiol. 18, 3714–3727. doi: 10.1111/1462-2920.13291
Mullapudi, E., Pridal, A., Pálková, L., de Miranda, J. R., and Plevka, P. (2016). Virion structure of Israeli acute bee paralysis virus. J. Virol. 90, 8150–8159. doi: 10.1128/JVI.00854-16
Nagasaki, K., Tomaru, Y., Katanozaka, N., Shirai, Y., Nishida, K., Itakura, S., et al. (2004). Isolation and characterization of a novel single-stranded RNA virus infecting the bloom-forming diatom Rhizosolenia setigera. Appl. Environ. Microbiol. 70, 704–711. doi: 10.1128/AEM.70.2.704
Nelson, D. M., Tréguer, P., Brzezinski, M. A., Leynaert, A., and Quéguiner, B. (1995). Production and dissolution of biogenic silica in the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem. Cycles 9, 359–372. doi: 10.1029/95GB01070
Orsini, L., Sarno, D., Procaccini, G., Poletti, R., Dahlmann, J., and Montresor, M. (2002). Toxic Pseudo-nitzschia multistriata (Bacillariophyceae) from the Gulf of Naples: morphology, toxin analysis and phylogenetic relationships with other Pseudo-nitzschia species. Eur. J. Phycol. 37, 247–257. doi: 10.1017/S0967026202003608
Rosario, K., Nilsson, C., Lim, Y. W., Ruan, Y., and Breitbart, M. (2009). Metagenomic analysis of viruses in reclaimed water. Environ. Microbiol. 11, 2806–2820. doi: 10.1111/j.1462-2920.2009.01964.x
Sarno, D., Kooistra, W. H. C. F., Medlin, L. K., Percopo, I., and Zingone, A. (2005). Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species with the description of four new species. J. Phycol. 41, 151–176. doi: 10.1111/j.1529-8817.2005.04067.x
Sarthou, G., Timmermans, K. R., Blain, S., and Tréguer, P. (2005). Growth physiology and fate of diatoms in the ocean: a review. J. Sea Res. 53, 25–42. doi: 10.1016/j.seares.2004.01.007
Schlüter, M. H., Kraberg, A., and Wiltshire, K. H. (2012). Long-term changes in the seasonality of selected diatoms related to grazers and environmental conditions. J. Sea Res. 67, 91–97. doi: 10.1016/j.seares.2011.11.001
Scholz, B., Guillou, L., Marano, A. V., Neuhauser, S., Sullivan, B. K., Karsten, U., et al. (2015). Zoosporic parasites infecting marine diatoms – a black box that needs to be opened. Fungal Ecol. 19, 59–76. doi: 10.1016/j.funeco.2015.09.002
Shi, M., Lin, X. D., Tian, J. H., Chen, L. J., Chen, X., Li, C. X., et al. (2016). Redefining the invertebrate RNA virosphere. Nature 540, 539–543. doi: 10.1038/nature20167
Shirai, Y., Tomaru, Y., Takao, Y., Suzuki, H., Nagumo, T., and Nagasaki, K. (2008). Isolation and characterization of a single-stranded RNA virus infecting the marine planktonic diatom Chaetoceros tenuissimus Meunier. Appl. Environ. Microbiol. 74, 4022–4027. doi: 10.1128/AEM.00509-08
Smetacek, V. (1999). Diatoms and the ocean carbon cycle. Protist 150, 25–32. doi: 10.1016/S1434-4610(99)70006-4
Smetacek, V. S. (1985). Role of sinking in diatom life-history cycles: ecological, evolutionary and geological significance. Mar. Biol. 84, 239–251. doi: 10.1007/BF00392493
Sommer, U., Adrian, R., De Senerpont Domis, L., Elser, J. J., Gaedke, U., Ibelings, B., et al. (2012). Beyond the Plankton Ecology Group (PEG) model: mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 43, 429–448. doi: 10.1146/annurev-ecolsys-110411-160251
Suttle, C. A. (1993). “Enumeration and isolation of viruses,” in Handbook of Methods in Aquatic Microbial Ecology, eds P. F. Kemp, B. F. Sherr, E. B. Sherr, and J. J. Cole (Boca Raton, FL: Lewis Publisher), 121–137.
Takano, H. (1981). New and rare diatoms from Japanese marine waters – VI. Three new species in Thalassiosiraceae. Bull. Tokai Reg. Fish. Res. Lab. 105, 31–43.
Tillmann, U., Hesse, K. J., and Tillmann, A. (1999). Large-scale parasitic infection of diatoms in the Northfrisian Wadden Sea. J. Sea Res. 42, 255–261. doi: 10.1016/S1385-1101(99)00029-5
Tomaru, Y., Fujii, N., Oda, S., Toyoda, K., and Nagasaki, K. (2011a). Dynamics of diatom viruses on the western coast of Japan. Aquat. Microb. Ecol. 63, 223–230. doi: 10.3354/ame01496
Tomaru, Y., Katanozaka, N., Nishida, K., Shirai, Y., Tarutani, K., Yamaguchi, M., et al. (2004). Isolation and characterization of two distinct types of HcRNAV , a single-stranded RNA virus infecting the bivalve-killing microalga Heterocapsa circularisquama. Aquat. Microb. Ecol. 34, 207–218. doi: 10.3354/ame034207
Tomaru, Y., Kimura, K., and Yamaguchi, H. (2014). Temperature alters algicidal activity of DNA and RNA viruses infecting Chaetoceros tenuissimus. Aquat. Microb. Ecol. 73, 171–183. doi: 10.3354/ame01713
Tomaru, Y., Shirai, Y., Suzuki, H., Nagumo, T., and Nagasaki, K. (2008). Isolation and characterization of a single-stranded DNA virus infecting the marine planktonic diatom Chaetoceros debilis. Aquat. Microb. Ecol. 50, 103–112. doi: 10.3354/ame01170
Tomaru, Y., Takao, Y., Suzuki, H., Nagumo, T., Koike, K., and Nagasaki, K. (2011b). Isolation and characterization of a single-stranded DNA virus infecting Chaetoceros lorenzianus Grunow. Appl. Environ. Microbiol. 77, 5285–5293. doi: 10.1128/AEM.00202-11
Tomaru, Y., Takao, Y., Suzuki, H., Nagumo, T., and Nagasaki, K. (2009). Isolation and characterization of a single-stranded RNA virus infecting the bloom-forming diatom Chaetoceros socialis. Appl. Environ. Microbiol. 75, 2375–2381. doi: 10.1128/AEM.02580-08
Tomaru, Y., Toyoda, K., and Kimura, K. (2015). Marine diatom viruses and their hosts: resistance mechanisms and population dynamics. Perspect. Phycol. 2, 69–81. doi: 10.1127/pip/2015/0023
Tomaru, Y., Toyoda, K., and Kimura, K. (2018). Occurrence of the planktonic bloom-forming marine diatom Chaetoceros tenuissimus Meunier and its infectious viruses in western Japan. Hydrobiologia 805, 221–230. doi: 10.1007/s10750-017-3306-0
Tomaru, Y., Toyoda, K., Kimura, K., Takao, Y., Sakurada, K., Nakayama, N., et al. (2013a). Isolation and characterization of a single-stranded RNA virus that infects the marine planktonic diatom Chaetoceros sp. (SS08-C03). Phycol. Res. 61, 27–36. doi: 10.1111/j.1440-1835.2012.00670.x
Tomaru, Y., Toyoda, K., Suzuki, H., Nagumo, T., Kimura, K., and Takao, Y. (2013b). New single-stranded DNA virus with a unique genomic structure that infects marine diatom Chaetoceros setoensis. Sci. Rep. 3, 3337. doi: 10.1038/srep03337
Toyoda, K., Kimura, K., Hata, N., Nakayama, N., Nagasaki, K., and Tomaru, Y. (2012). Isolation and characterization of a single-stranded DNA virus infecting the marine planktonic diatom Chaetoceros sp. (strain TG07-C28). Plankt. Benthos Res. 7, 20–28. doi: 10.3800/pbr.7.20
Vizcaíno, J. A., Csordas, A., Del-Toro, N., Dianes, J. A., Griss, J., Lavidas, I., et al. (2016). 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456. doi: 10.1093/nar/gkv1145
Wafar, M. V. M., Le Corre, P., and Birrien, J. L. (1983). Nutrients and primary production in permanently well-mixed temperate coastal waters. Estuar. Coast. Shelf Sci. 17, 431–446. doi: 10.1016/0272-7714(83)90128-2
Wiltshire, K. H., Kraberg, A., Bartsch, I., Boersma, M., Franke, H.-D., Freund, J., et al. (2010). Helgoland Roads, North Sea: 45 years of change. Estuaries Coasts 33, 295–310. doi: 10.1007/s12237-009-9228-y
Keywords: single-stranded RNA viruses, diatoms, genomics, host-virus dynamics, Western English Channel
Citation: Arsenieff L, Simon N, Rigaut-Jalabert F, Le Gall F, Chaffron S, Corre E, Com E, Bigeard E and Baudoux A-C (2019) First Viruses Infecting the Marine Diatom Guinardia delicatula. Front. Microbiol. 9:3235. doi: 10.3389/fmicb.2018.03235
Received: 06 September 2018; Accepted: 12 December 2018;
Published: 09 January 2019.
Edited by:
Curtis A. Suttle, University of British Columbia, CanadaReviewed by:
Karen Dawn Weynberg, The University of Queensland, AustraliaYuji Tomaru, National Research Institute of Fisheries and Environment of Inland Sea (FEIS), Japan
Marli Vlok, University of British Columbia, Canada
Copyright © 2019 Arsenieff, Simon, Rigaut-Jalabert, Le Gall, Chaffron, Corre, Com, Bigeard and Baudoux. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laure Arsenieff, bGFyc2VuaWVmZkBzYi1yb3Njb2ZmLmZy
 Laure Arsenieff
Laure Arsenieff Nathalie Simon
Nathalie Simon Fabienne Rigaut-Jalabert2
Fabienne Rigaut-Jalabert2 Samuel Chaffron
Samuel Chaffron Erwan Corre
Erwan Corre Emmanuelle Com
Emmanuelle Com Estelle Bigeard
Estelle Bigeard Anne-Claire Baudoux
Anne-Claire Baudoux