
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 23 October 2018
Sec. Plant Pathogen Interactions
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.02535
This article is part of the Research Topic Interactions of Plants with Bacteria and Fungi: Molecular and Epigenetic Plasticity of the Host View all 14 articles
Botrytis cinerea is one of the most notorious pathogenic species that causes serious plant diseases and substantial losses in agriculture throughout the world. We identified BcXyl1 from B. cinerea that exhibited xylanase activity. Expression of the BcXyl1 gene was strongly induced in B. cinerea infecting Nicotiana benthamiana and tomato plants, and BcXyl1 deletion strains severely compromised the virulence of B. cinerea. BcXyl1 induced strong cell death in several plants, and cell death activity of BcXyl1 was independent of its xylanase activity. Purified BcXyl1 triggered typically PAMP-triggered immunity (PTI) responses and conferred resistance to B. cinerea and TMV in tobacco and tomato plants. A 26-amino acid peptide of BcXyl1 was sufficient for elicitor function. Furthermore, the BcXyl1 death-inducing signal was mediated by the plant LRR receptor-like kinases (RLKs) BAK1 and SOBIR1. Our data suggested that BcXyl1 contributed to B. cinerea virulence and induced plant defense responses.
Botrytis cinerea is a necrotrophic pathogen, causing widespread plant diseases and enormous economic losses in a large number of important crops throughout the world (Prins et al., 2000). B. cinerea can infect various organs in plants, including leaves, bulb, flowers, fruits, and root tubers. The infection process of B. cinerea mainly includes two typical stages: local lesions at an early stage and a late stage of fast-spreading lesions.
The plant cell wall is a natural barrier, which provides mechanical strength and rigidity to prevent pathogen infection. To establish successful colonization, B. cinerea, like other fungal pathogen, secretes a large number of cell wall-degrading enzymes (CWDEs) to degrade the plant defensive barriers during the infection process, thereby to permit pathogens to invade plant tissue and supply pathogens with nutrients (Cantarel et al., 2009; Kubicek et al., 2014). These CWDEs, including pectinases, cellulases, cutinases, and xylanases, are generally regarded as important virulence factors through the maceration of host tissues and the degradation of host macromolecules (Prins et al., 2000). The effects of targeted deletion of some genes encoding CWDEs support their direct involvement in the infection process. For example, deletion of the pectate lyase gene CcpelA and the pectate lyase gene PelB in Colletotrichum coccodes, resulted in a substantial loss of virulence on green tomato fruit and reduced virulence on avocado, respectively (Yakoby et al., 2001; Ben-Daniel et al., 2011). Targeted deletion of VdCUT11, a cutinase in V. dahliae, significantly compromised virulence on cotton plants (Gui Y. et al., 2017). However, the specific roles of the majority of CWDEs in pathogen virulence remain largely unknown, especially in B. cinerea.
To ward off microorganisms infection, plants have evolved elaborate systems to provide better immunity against pathogens (Zipfel, 2008). Recognition of conserved pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) located on the cell surface constitutes the first layer of plant innate immunity and is termed as PAMP-triggered immunity (PTI). Intracellular responses associated with PTI include Ca2+ influx, the burst of reactive oxygen species (ROS), the accumulation of defense hormone, the expression of defense-related genes and callose deposition (Boller and Felix, 2009; Couto and Zipfel, 2016). In turn, during the coevolution of hosts and microbes, pathogens also employ numerous effectors to interfere with PTI and establish successful infection, which is regarded as effector-triggered susceptibility (ETS) (Chisholm et al., 2006; Jones and Dangl, 2006; Saijo et al., 2017). As a countermeasure, some plants recruit R proteins to recognize these effectors directly or indirectly termed effector-triggered immunity (ETI) (Houterman et al., 2008; Stergiopoulos and de Wit, 2009). Generally, ETI is often accompanied with stronger immune responses, such as hypersensitive response (HR).
Apart from the role of virulence factor, some CWDEs also function as PAMPs to activate the plant immune responses independent of their enzymatic activity. For instance, VdEG1, VdEG3 and VdVUT11 from Verticillium dahliae, XEG1 from Phytophthora sojae and BcXYG1, a secreted xyloglucanase from B. cinerea contributed to virulence and triggered plant immunity as PAMPs simultaneously (Ma et al., 2015; Gui Y. et al., 2017; Gui Y.-J. et al., 2017; Zhu et al., 2017).
Plants recognizes characteristic microbial molecules classically known as PAMPs by employing a multitier surveillance system, including PRRs (Couto and Zipfel, 2016). Plant PRRs include RLKs and receptor-like proteins (RLPs) (Boutrot and Zipfel, 2017). Currently, a handful of PRRs have been identified as receptors to participate in the recognition of PAMPs. The brassinosteroid insensitive 1 (BRI1)-associated receptor kinase 1 (BAK1) and the LRR receptor-like kinase (LRR-RLK) SUPPRESSOR OF BIR1-1 (SOBIR1) are involved in multiple PRR pathways and signal activation (Liebrand et al., 2014). For example, BcSpl1, XEG1, and VdCUT11 could trigger cell death in the plants, and the resulting immunity signal was mediated by the plant LRR RLKs BAK1 and SOBIR1 (Frías et al., 2011; Ma et al., 2015; Gui Y. et al., 2017).
Xylan is the major component of hemicellulose of the plant cell wall (Collins et al., 2005). Due to the complexity, the degradation of xylan requires several hydrolytic enzymes, of which xylanase is a crucial component for hydrolyzing the 1,4-β-d-xylosidic linkages in xylan. Xylanase has received more attention because of the special role in fungi pathogenicity. For example, a mutation in the xynB endoxylanase gene from Xanthomonas oryzae pv. oryzae resulted in attenuated virulence in rice (Pandey and Sonti, 2010). Moreover, the deletion of xylanases Xyn11A gene had a marked effect on the ability of B. cinerea to infect tomato leaves and grape (Brito et al., 2006). In addition to their roles in virulence, xylanases are regarded as elicitors to induce defense responses in plants. For example, ethylene-inducing xylanase (EIX) is a potent elicitor in tobacco and tomato. However, the function of the majority of xylanases in B. cinerea remains mostly undiscovered. Here, we reported on the identification and characterization of BcXyl1, a xylanase from B. cinerea. BcXyl1 contributes to B. cinerea virulence and triggers PTI responses in plants. Furthermore, a small peptide of BcXyl1 is sufficient for elicitor function. We found that the cell death signal is mediated by BAK1 and SOBIR1, and the xylanases activity is not necessary for the induction of necrosis.
Botrytis cinerea B05.10 was used as wild-type strain and control strain in this study. All B. cinerea strains, including two independent BcXyl1 knockout mutants and two complementary transformants, were routinely maintained in 15% glycerol at -80°C and grown on PDA at 22°C, respectively. Agrobacterium tumefaciens AGL-1 were grown on LB (Kan and Rif) medium at 28°C. To obtain conidia, B. cinerea grown on tomato-PDA plates (39 g of potato dextrose agar plus 250 g of homogenized tomato fruits per liter) as explained previously (Benito et al., 1998). N. benthamiana and tomato (Solanum lycopersicum) plants were grown at 27 °C in a greenhouse with a day/night period of 14/10 h and 60% relative humidity (RH).
The open reading frame of BcXyl1 (amplified with primers BcXyl1 F/BcXyl1 R; Supplementary Table S2) and C130-155 were amplified by PCR from cDNA of the wild-type strain B05.10 and the fragment fused with a myc tag and a 6xHis tag at the C terminus was cloned into the pPICZαA vector at the BamHI and EcoRI sites. The recombinant plasmid pPICZαA-BcXyl1 and pPICZαA-C130-155 were linearized with PmeI and transformed into Pichia pastoris KM71H for expression. The transformed yeasts were grown and induced in BMGY (buffered glycerol complex medium) and BMMY (buffered methanol complex medium), respectively (Easy Select Pichia expression kit; Invitrogen). Then, the supernatant was collected (3000 g for 10 min at 4°C) and purified using nickel affinity chromatography. The purified C130-155, BcXyl1rec, or BcXyl1 were kept in protein buffer (20 mM Tris, pH 8.0) and further detected via SDS-PAGE and Western blotting. The concentration of the purified protein was measured using Easy II Protein Quantitative Kit (BCA) and the protein was then stored at -80°C.
To transiently express truncated mutants of the BcXyl1 protein in leaves, DNA sequences encoding different fragments (BcXyl1, N80, N130, N155 C80, C130, C155, and C130-155) were amplified by PCR from cDNA of the wild-type strain B05.10 and inserted into pYBA1132 vector at the XbaI and BamHI sites and then transformed into the A. tumefaciens strain GV3101. Agroinfiltration assays were performed on N. benthamiana plants. Agrobacterium-mediated transient expression was performed as described (Ma et al., 2015). Leaves were scored and photographed 6 days after initial inoculation. Each assay was performed on six leaves from three individual plants, and repeated at least three times.
To determine the relationship between the enzymatic activity and cell death-inducing activity of BcXyl1, we constructed BcXyl1rec mutant, which abolished the enzymatic activity. According to multiple sequence alignment, two potentially highly conserved catalytic residues (E104 and E157) were the critical catalytic sites of BcXyl1. Next, two glutamic acid residues were substituted by Gln using the Quick ChangeTM Site-Directed Mutagenesis Kit (Stratagene, United States). BcXyl1rec was expressed in P. pastoris and carried out the enzyme assay.
The xylanase activity was assayed via the method as described previously (Biely et al., 1988). The purified BcXyl1rec or BcXyl1 (500 ng) and substrate (1% beechwood xylan) were co-incubated in citrate phosphate McIlvaine buffer, pH 5, at 35°C for 10 min (total volume: 125 μl). All samples were incubated at 100°C for 10 min to end the assays reactions. The amount of reducing sugars released from xylan was quantified using a standard calibration curve obtained with the dinitrosalicylic acid procedure. The experiment was replicated three times.
To confirm whether BcXyl1 was secreted into the apoplast and the relationship between enzymatic activity of BcXyl1 and cell death-inducing activity, transient expression in N. benthamiana was performed. Three sequences (BcXyl1, BcXyl1-ΔSP, and BcXyl1rec) were cloned into the pYBA1132 vector which contained a C-terminal GFP tag at the XbaI and BamHI sites, and then transformed into the A. tumefaciens strain GV3101. All primers are listed in Supplementary Table S2. Plant total protein extractions and immunoblots were assessed as previously described (Yu et al., 2012). All proteins were analyzed by immunoblots using anti-GFP-tag primary monoclonal antibody. The blots were visualized using the Odyssey® LI-COR Imaging System. Rubisco was used to confirm the equal protein loading.
To test the induction of cell death or PTI responses, BcXyl1 and C130-155 were dissolved in PBS and infiltrated into the leaves of N. benthamiana and tomato plants using a syringe. Plants were grown in a greenhouse with a day/night period of 14/10 h. The cell-death response was investigated after 48 h treated with BcXyl1, C130-155, or PEVC (P. pastoris culture supernatant from an empty vector control strain, purified in the same way as BcXyl1). To further investigate cell death, trypan blue staining was performed by boiling leaf tissues in a mixture of phenol, lactic acid, glycerol, and distilled water containing 1 mg/ml trypan blue (1:1:1:1) for 1 min. The samples were then soaked in 2.5 mg/ml chloral hydrate overnight. The accumulation of ROS in plant leaves was stained by 3′3-diaminobenzidine (DAB) and Nitroblue Tetrazolium (NBT) solution as described previously (Bindschedler et al., 2006). To visualize callose deposition, 4-week-old N. benthamiana leaves were infiltrated with 1 μM recombinant proteins and stained with aniline blue at 24 h post-treatment, as described previously (Chen et al., 2012). To assay electrolyte leakage, the N. benthamiana leaves treated with proteins were harvested at different time points and submerged in sterile water at 4°C. Ion conductivity was measured using a conductivity meter. To test whether BcXyl1 could confer plants disease resistance, the purified BcXyl1, C130-155, or PEVC was individually syringe-infiltrated into 4-week-old N. benthamiana and tomato leaves. Five microliters of 2 × 106 conidia/ml B. cinerea and TMV-GFP were placed on the systemic leaves, respectively. The inoculated plants were placed in a greenhouse at 25°C with a day/night period of 14/10 h. Lesion diameter of B. cinerea and the number of TMV-GFP lesions on N. benthamiana leaves were evaluated at 2 and 4 days post-inoculation, respectively. All experiments were performed three times.
To test whether BcXyl1 functioned as a virulence factor of B. cinerea, the wild-type strain and derived mutants, including the BcXyl1 deletion (ΔBcXyl1-1 and ΔBcXyl1-2) and complementary mutants (ΔBcXyl1-1-C and ΔBcXyl1-2-C) were used in this study. Four-week-old N. benthamiana leaves were inoculated with 5 μL of 2 × 106 conidia/ml B. cinerea. The inoculated plants were placed in a greenhouse with a day/night period of 14/10 h. The lesion development of B. cinerea on the N. benthamiana leaves was evaluated at 2 days post-inoculation by determining the average lesion diameter. Tomato, grape, and apple fruits (commercially obtained) were washed under running tap water and surface sterilized by immersion for 5 min in ethanol. After air drying, fruits were inoculated with 5 μL of 2 × 106 conidia/ml B. cinerea. Fruits were incubated at 25°C under conditions of high humidity on water-soaked filter paper in closed containers. The lesion development of B. cinerea on the fruits was evaluated at 3 days post-inoculation by determining the average lesion diameter. All the experiments were performed three times.
To determine whether BAK1 or SOBIR1 participate in induction of cell death by BcXyl1, VIGS was performed. NbBAK1 or NbSOBIR1 gene was silenced using VIGS, as described previously (Kettles et al., 2016). A. tumefaciens strain harboring constructs (pTRV1, pTR::BAK1 or pTRV1, pTRV2::SOBIR1) were infiltrated into the N. benthamiana leaves. pTRV2::GFP was used as the control and the expression levels of BAK1 and SOBIR1 were determined by qRT-PCR. Agroinfiltration assays were performed on N. benthamiana plants using Bcl-2-associated X protein (BAX) as positive controls. Phenotypes were photographed 6 days after infiltration. All the experiments were performed three times.
To measure the expression of BcXyl1 during infection, 4-week-old N. benthamiana or 4-week-old tomato plants were inoculated with B. cinerea 2 × 106 conidia/ml. We selected 10 indicated time points during different stages of post-inoculation to determine expression patterns of BcXyl1 by qPCR. All samples were stored at -80°C. Total RNA of B. cinerea was extracted with the E.Z.N.A.® Total RNA Kit I according to the manufacturer’s instructions and stored at -80°C. For the measurement of defense-related genes expression, leaves of 4-week-old N. benthamiana plants were treated with 1 μM purified BcXyl1, C130-155, or PEVC. The leaves were obtained at the indicated time points, immediately frozen in liquid nitrogen, and stored at -80°C. The EasyPure Plant RNA Kit (TransGen Biotech) was used to extract total RNA. After isolation of total RNA, qPCR was performed using a TransStart Green qPCR SuperMix UDG according to the manufacturer’s instructions (TransGen Biotech). qRT-PCR was performed under the following conditions: an initial 95°C denaturation step for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. N. benthamiana EF-1a (P43643.1) and B. cinerea Bcgpdh gene (BC1G_05277) were used as endogenous plant controls and used to quantify fungal colonization, respectively. qPCR assays were repeated at least twice, each repetition with three independent replicates (Livak and Schmittgen, 2001). All primers are listed in Supplementary Table S2. The relative transcript levels among various samples were determined using the 2-ΔΔCT method with three independent determinations (Livak and Schmittgen, 2001).
BcXyl1 gene and 500 bp flanking sequences of the target gene were amplified from B. cinerea B05.10 wild-type strain genomic DNA. Two flanking sequences of the target gene and hygromycin resistance cassette were constructed into a fusion fragment using a nested PCR reaction, which is subsequently introduced into the binary vector pGKO2 gateway. To generated complementary transformants, the donor vector pCT-HN containing BcXyl1 gene was integrated into the mutant transformants using a previously described Agrobacterium-mediated transformation method (Liu et al., 2013). All mutants were identified using PCR with the corresponding primers. All primers are listed in Supplementary Table S2.
All the experiments and data presented here were performed at least three repeats. The data are presented as the means and standard deviations. Statistical Analysis System (SAS) software was used to perform the statistical analysis via Student’s t-test.
BcXyl1 was identified by searching the B. cinerea genome sequence. The open reading frame of BcXyl1 (GenBank: ATZ53308.1) is 987 bp encoding a 329 aa protein with a predicted N-terminal signal peptide (1–20 aa), and no transmembrane helices of BcXyl1 were found, suggesting that it may be secreted into extracellular space. The bioinformatics analysis suggested that BcXyl1 belongs to SGNH hydrolase subfamily and has a highly strong similarity to fungal endo-β-1,4-xylanases.
Previous studies showed that xylanases in pathogenic microorganisms were implicated in the pathogenicity. In order to assess the role of BcXyl1 to B. cinerea virulence, we first analyzed the expression patterns of BcXyl1 during different stages of post-inoculation. qRT-PCR results suggested that when the spore suspension of B. cinerea was inoculated onto leaves of N. benthamiana and tomato, transcript level of BcXyl1 increased rapidly and reached a maximum of about 26-fold to 28-fold at 2 days post-inoculation, and then rapidly declined and maintained a level that was slightly higher than the initial level during later stages (Figure 1).
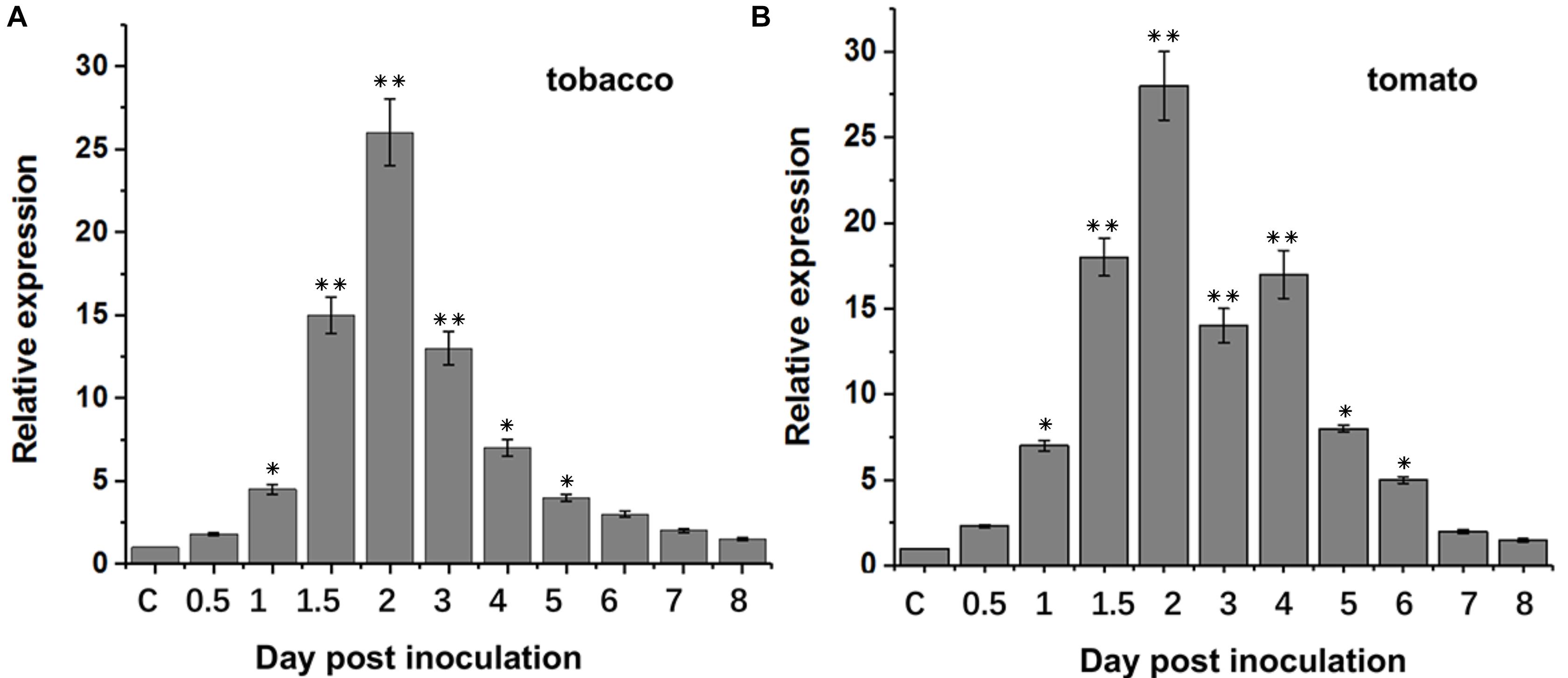
FIGURE 1. BcXyl1 expression analysis during infection of tobacco and tomato plants. Tobacco and tomato leaves were inoculated with B. cinerea spores, and the expression of BcXyl1 was detected by qPCR. The control (C) was mixed with non-inoculated conidia tobacco or tomato leaves. B. cinerea Bcgpdh gene (BC1G_05277) was used as an endogenous control. Error bars represent standard deviation of three independent replicates. Asterisks indicate significant differences with based on Student’s t-test (∗p < 0.05 and ∗∗p < 0.01).
To more directly explore the biological roles of BcXyl1 during infection, we constructed two BcXyl1 deletion mutants in B. cinerea (ΔBcXyl1-1 and ΔBcXyl1-2) and two rescued strains (ΔBcXyl1-1-C and ΔBcXyl1-2-C), and the ability of the resulting mutants to infect various plant organisms was evaluated. All mutants showed no significant differences with the wild-type stain in growth rate and colony morphology on PDA plates (Supplementary Figure S2). N. benthamiana leaves were inoculated with spore suspension of the wild type and mutants, and lesion size was measured 48 h after inoculation. Interestingly, the deletion of BcXyl1 displayed significantly reduced virulence and produced much smaller lesions on leaves of N. benthamiana than the WT strain at 48 hpi (Figures 2A,B). The rescued strains recovered the high virulence phenotypes. And two BcXyl1 deletion mutants displayed much weaker disease symptoms and lesion diameter than the wild-type strain and the complement strains (ΔBcXyl1-1-C and ΔBcXyl1-2-C) on grape, tomato, and apple fruits 72 h post-inoculation (Figures 2A,B). These results indicated that BcXyl1 functioned as a virulence factor that contributes to B. cinerea virulence on host plants.
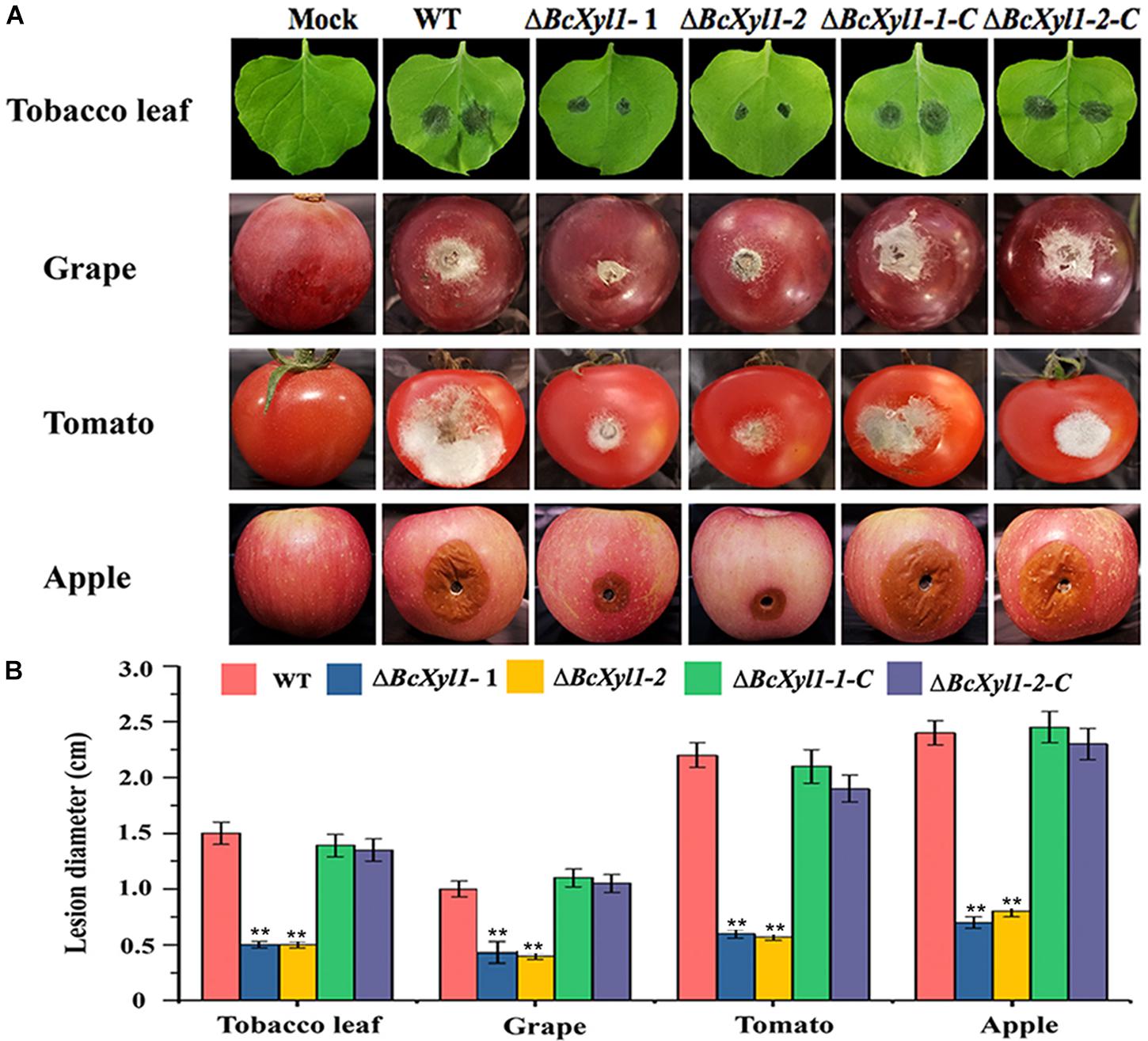
FIGURE 2. Virulence analysis of BcXyl1 mutants on plant organs. (A) Disease symptoms on wounded tobacco leaves, grape fruits, tomato fruits, and apple fruits after 72 h inoculation. (B) Diameter of disease lesion on leaves and fruits was determined. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between mutants and WT stain. Asterisks “∗∗” indicate statistically significant differences at a p-value <0.01.
To further confirm whether BcXyl1 could induce cell death in N. benthamiana, we expressed BcXyl1 in the yeast P. pastoris using the pPICZαA vector (pPICZαA: BcXyl1). Moreover, the recombinant protein BcXyl1, with a size of 35 kDa, was infiltrated into the mesophyll of N. benthamiana leaves with different concentrations (Supplementary Figure S3). The area of necrosis occurred and increased with increasing concentrations of BcXyl1 from 800 nM to 2 μM after infiltration 3 days, whereas no cell death activity was detected in the leaves treated with PEVC (P. pastoris culture supernatant from an empty vector control strain, purified in the same way as BcXyl1) (Figures 3A,B).
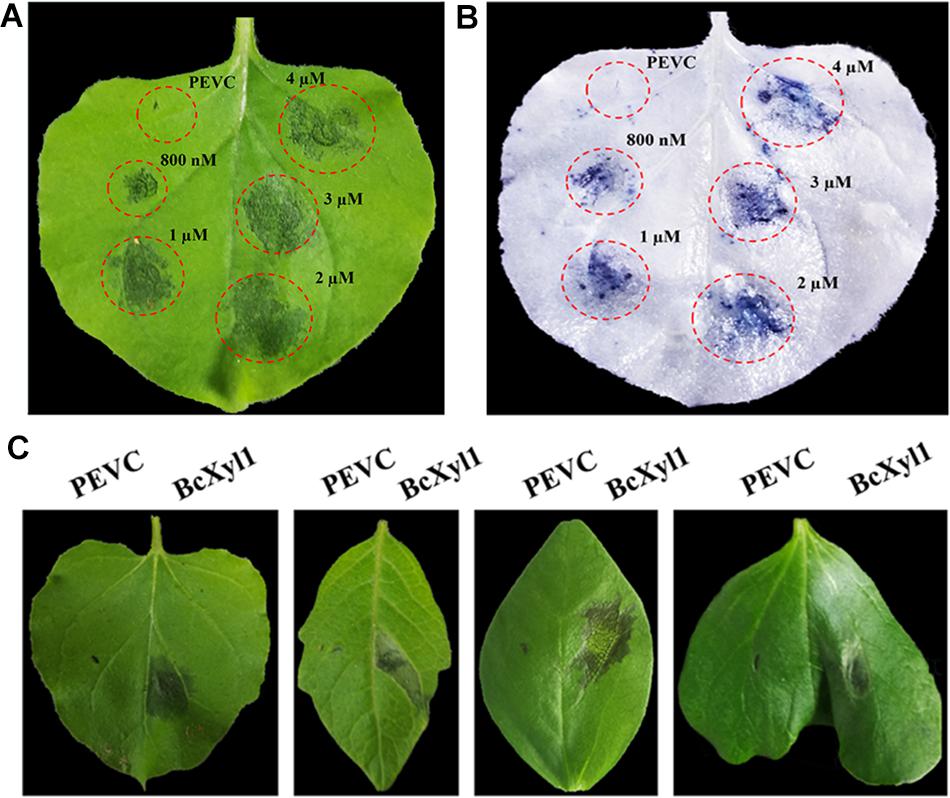
FIGURE 3. BcXyl1 induces cell death in several plants. (A,B) N. benthamiana leaves were infiltrated with purified BcXyl1 protein (800 nM to 2 μM), and PEVC (P. pastoris culture supernatant from an empty vector control strain, purified in the same way as BcXyl1). Two days post-infiltration, the leaves were photographed and stained with trypan blue. (C) The leaves of tomato, soybean, and cotton were infiltrated with purified BcXyl1 (1 μM) and PEVC (1 μM). Two days post-infiltration, different plants leaves were photographed. All the experiments were replicated three times.
To examined the cell death-inducing activity of BcXyl1 in plants other than N. benthamiana, we infiltrated BcXyl1 (1 μM) into the leaves of several plants, including tomato, soybean, and cotton. BcXyl1 could induce significant cell death in these plants while PEVC did not (Figure 3C). So, BcXyl1 has ability to induce cell death in several plant species.
BcXyl1 has a signal peptide with 20 amino acids and no transmembrane helices, implying that BcXyl1 might be a secreted protein. In order to check if, as previously hypothesized, BcXyl1 was secreted into the apoplast to induce cell death response, we transiently expressed the full length BcXyl1 and BcXyl1-ΔSP (lacking the signal peptide) in N. benthamiana by agroinfiltration. The results showed that BcXyl1 containing signal peptide induced cell death in N. benthamiana, whereas BcXyl1-ΔSP lacking signal peptide abolished the ability to trigger cell death at 5 days after agroinfiltration (Supplementary Figure S4A). The protein expression level of BcXyl1 and BcXyl1-ΔSP in N. benthamiana were detected by immunoblot (Supplementary Figure S4B). So, all results showed BcXyl1 was delivered into the apoplast to induce cell death in several plant species.
Previous reports showed that xylanases from fungi had ability to degrade xylan (Brutus et al., 2005). Interestingly, purified BcXyl1 had a xylanase activity using low viscosity xylane (LVX) as substrate (Supplementary Table S1). The sequence alignment results showed that BcXyl1 included two potentially highly conserved catalytic residues (E104 and E157), which are essential for the xylanase activity (Supplementary Figure S1). In addition, the enzymatic activity of CWDEs was required for cell death activity, and in a few cases, the cell death-inducing activity was found to be independent of the enzymatic activity. To determine the relationship between the enzymatic activity and cell death-inducing activity of BcXyl1, we generated a site-directed mutant (BcXyl1rec) that two glutamic acid residues were substituted by Gln using site-directed mutagenesis and expressed the mutant protein in P. pastoris (Figure 4A). Enzymatic assays with purified BcXyl1rec showed the xylan-degrading xylanase activity was abolished (Supplementary Table S1). Surprisingly, although BcXyl1rec absent the ability of xylanase activity, retained the same cell death-inducing activity as the wild-type (BcXyl1) (Figure 4B). Further, A. tumefaciens infiltration assays showed that BcXyl1rec and BcXyl1 induced similar visible cell death symptoms in N. benthamiana leaves 4 days post-inoculation (Figure 4B). Western blot assays showed that the accumulation of BcXyl1 and BcXyl1rec was similar (Figure 4C). These results confirmed that BcXyl1 did not need to xylanase activity to induce cell death in N. benthamiana.
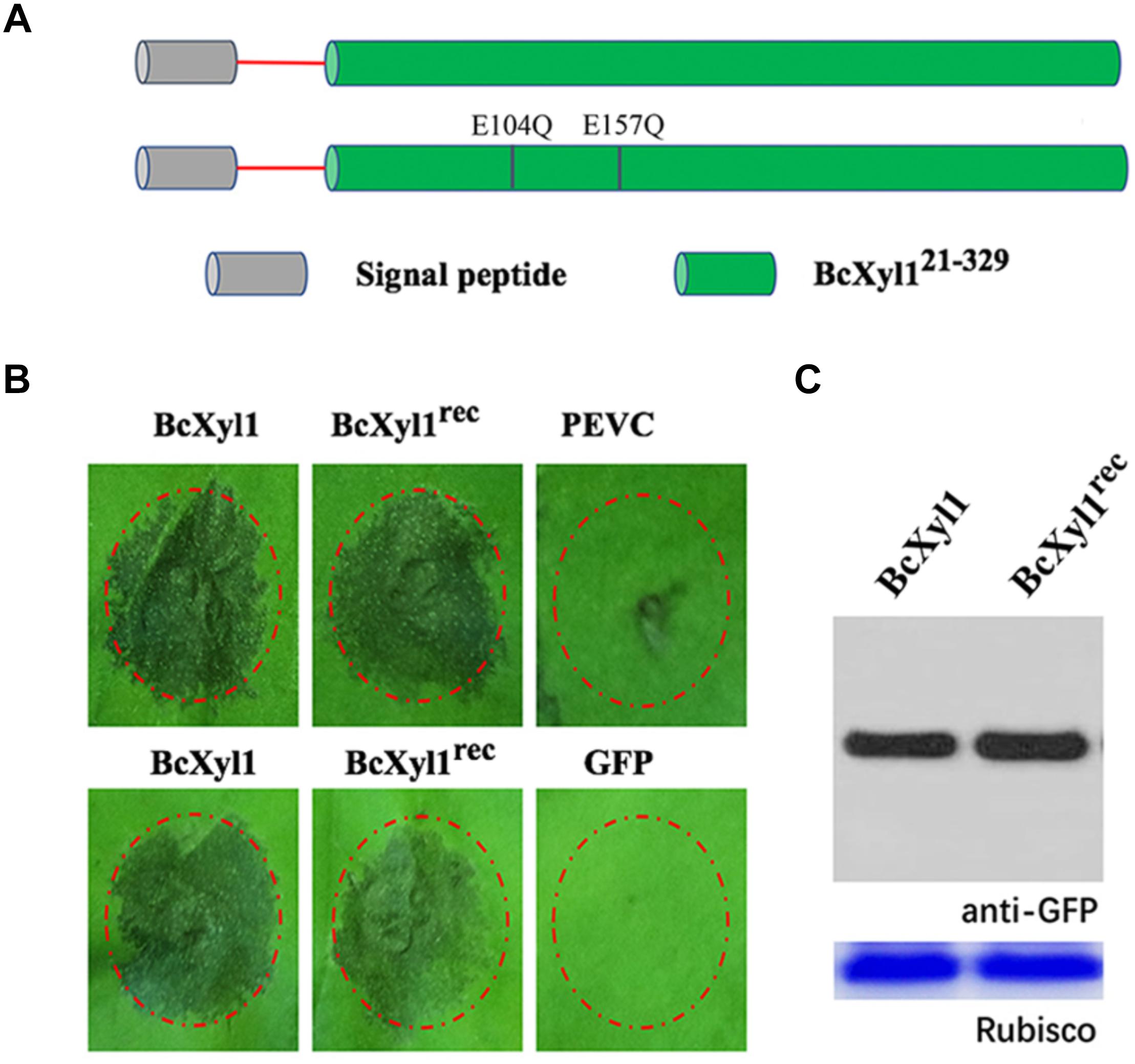
FIGURE 4. Cell death activity of BcXyl1 is independent of its xylanase activity in N. benthamiana. (A) Schematic presentation of the examined constructs. BcXyl1 (the native protein) and BcXyl1rec (replaced E104 and E157 with Gln). (B) Upper pictures: treatment of tomato leaves with 1 μM purified BcXyl1 or BcXyl1rec. Lower pictures: analysis of cell death produced by A. tumefaciens strains transiently expressing BcXyl1 or BcXyl1rec. (C) Immunoblot analysis of proteins from N. benthamiana leaves transiently expressing the examined proteins using a pYBA1132 vector. All the experiments were replicated three times.
Some cell death-inducing proteins are recognized by plant immune system and activate host PTI responses, bring a series of typical characteristics such as accumulation of ROS, leakage of ion electrolytes, expression of defense genes, and callose deposition (Frías et al., 2012; Zhang et al., 2014, 2015). To examine whether BcXyl1 could induce typical PTI responses, the leaves of N. benthamiana and tomato plants were infiltrated with 1 μM BcXyl1. The ability of BcXyl1 to induce the accumulation of ROS in the infiltrated leaves was studied. The hydrogen peroxide (H2O2) and superoxide anion (O2-) production levels were assayed using DAB and NBT, respectively. A clear brown and blue precipitate was observed in leaves treated with BcXyl1, whereas the leaves treated with PEVC showed opposite patterns of DAB and NBT signal (Figure 5A). Meanwhile, BcXyl1 also induced electrolyte leakage and displayed an increase in conductivity, while PEVC exhibited barely change at the same concentration (Figure 5B). BcXyl1 was shown to cause significantly upregulation of seven genes associated with PTI and defense response in N. benthamiana leaves 12 h after treatment with BcXyl1; these genes included PR-1a and PR-5, which are involved in the SA-dependent defense pathway, PAL (phenylalanine ammonia lyase), NPR1 (the non-expressor of pathogenesis related 1), HSR203J and HIN1, which are two HR marker genes in tobacco, and COI1 (CORONATINE INSENSITIVE 1), which is JA responsive (Figure 5C). We finally examined callose deposition in leaves treated with BcXyl1, PEVC, or flg22. Furthermore, N. benthamiana leaves infiltrated with BcXyl1 or flg22 exhibited strong callose deposition compared with those infiltrated with PEVC, which exhibited undetectable levels of callose deposition (Figure 5D). These data indicated that BcXyl1 could induce typical PTI responses.
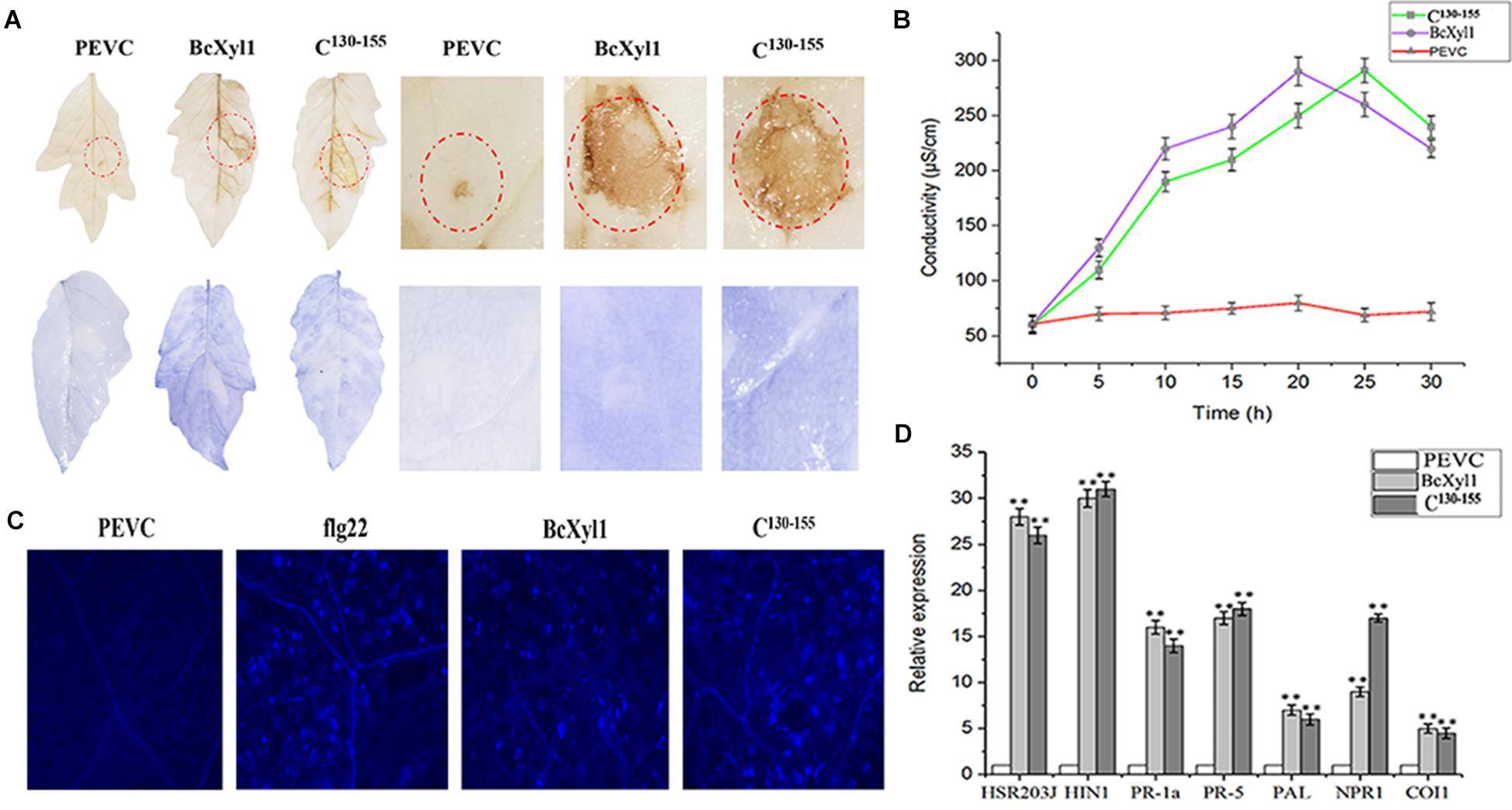
FIGURE 5. BcXyl1 induces PTI responses in plants. N. benthamiana or tomato leaves were infiltrated with 1 μM purified BcXyl1, C130-155, or PEVC. (A) ROS accumulation was detected in tobacco and tomato leaves 12 h after infiltration. The treated leaves were stained with DAB and NBT. All the experiments were replicated three times. (B) The conductivity was measured at the indicated time points. Error bars represent standard errors. All the experiments were replicated three times. (D) The expression of defense response genes was measured in N. benthamiana leaves by qPCR. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between BcXyl1, C130-155, and PEVC. Asterisks “∗∗” indicate statistically significant differences at a p-value <0.01. (C) Callose deposition in N. benthamiana leaves were detected 2 days after infiltration; the treated leaves were stained with aniline blue. All the experiments were replicated three times.
Recent reports showed that fungi CWDEs could confer plants disease resistance (Gui Y. et al., 2017; Gui Y.-J. et al., 2017; Zhu et al., 2017). To further confirm the role of BcXyl1 in conferring resistance to plant diseases, the N. benthamiana leaves were treated with 1 μM BcXyl1or PEVC, and after 2 days, the systemic leaves were inoculated with TMV-GFP and B. cinerea spore suspension. BcXyl1-treated tobacco plants enhanced disease resistance against TMV, and the number of TMV-GFP lesions of BcXyl1-treated leaves was significantly decreased than that of the leaves treated with PEVC (Figure 6A). Meanwhile, BcXyl1 led to more resistance to the B. cinerea infection in N. benthamiana, as significantly lower lesions size on leaves compared with the leaves treated with PEVC controls (Figure 6B). Furthermore, in tomato plants that were pre-infiltrated with BcXyl1, lesion size on the B. cinerea-infected leaves was significant smaller compared with lesions size on leaves in plants that were pre-infiltrated with PEVC (Figure 6C). Together, these results strongly suggested that BcXyl1 conferred plants disease resistance.
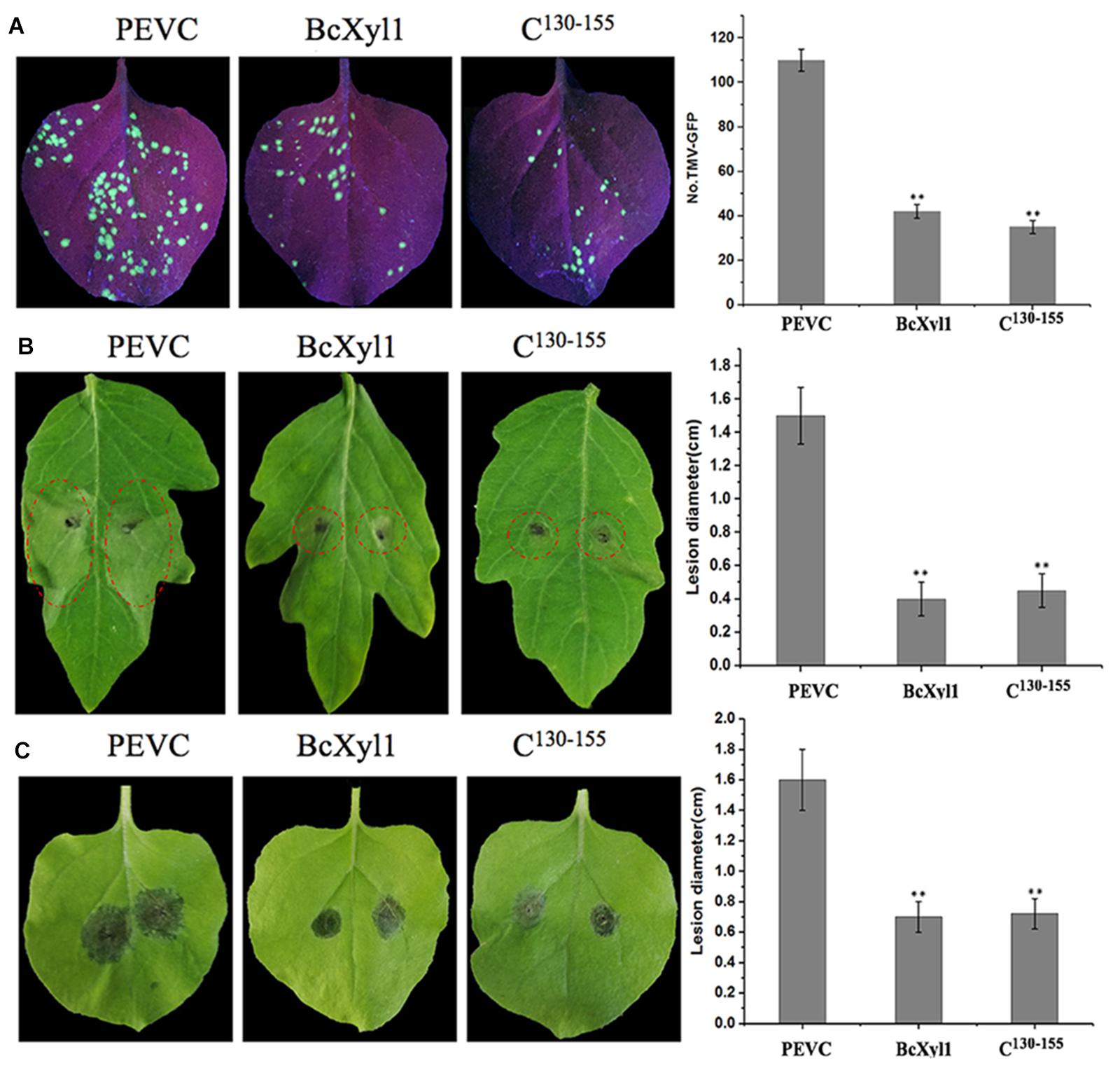
FIGURE 6. BcXyl1 confers disease resistance in plants. N. benthamiana or tomato leaves were infiltrated with 0.5 μM purified BcXyl1, C130-155, or PEVC. (A) The systemic leaves were inoculated with TMV-GFP, and the number of TMV-GFP lesions were measured. (B,C) The N. benthamiana or tomato systemic leaves were inoculated with 5 μL of 2 × 106 conidia/ml Botrytis cinerea. Lesions symptoms and diameter were observed and measured at 2 days post-inoculation, respectively. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between BcXyl1, C130-155, and PEVC. Asterisks “∗∗” indicate statistically significant differences at a p-value <0.01.
The plant receptors often recognize specific small protein epitopes of PAMP to induce plant immunity (Rotblat et al., 2002). To delineate the elicitor active peptide of BcXyl1, we generated N-terminal and C-terminal truncated mutants and detected the ability to induce cell death by agroinfiltration in N. benthamiana leaves (Figure 7A). We found that the N-terminal truncated mutant (N155) maintained the ability of cell death-inducing, whereas expression of N80 and N130 did not trigger cell death. The C-terminal truncated mutants (C80 and C130) induced cell death, but C155 resulted in the loss of cell death-inducing activity in N. benthamiana. Further, C130-155 induced the same cell death symptom compared with full-length BcXyl1 in N. benthamiana (Figure 7B). Hence, C130-155 was identified as the functional peptide of BcXyl1 to induce cell death in N. benthamiana. To probe whether C130-155 induced plant immune responses, purified C130-155 was used to infiltrate plants leaves. We found that like BcXyl1, C130-155 could induce typical PTI responses, including accumulation of ROS, leakage of ion electrolytes, expression of defense genes, and callose deposition (Figure 5). Meanwhile, C130-155 could also enhance resistance to B. cinerea and TMV in plants (Figure 6). These results suggested that a small peptide of BcXyl1 was sufficient for elicitor function.
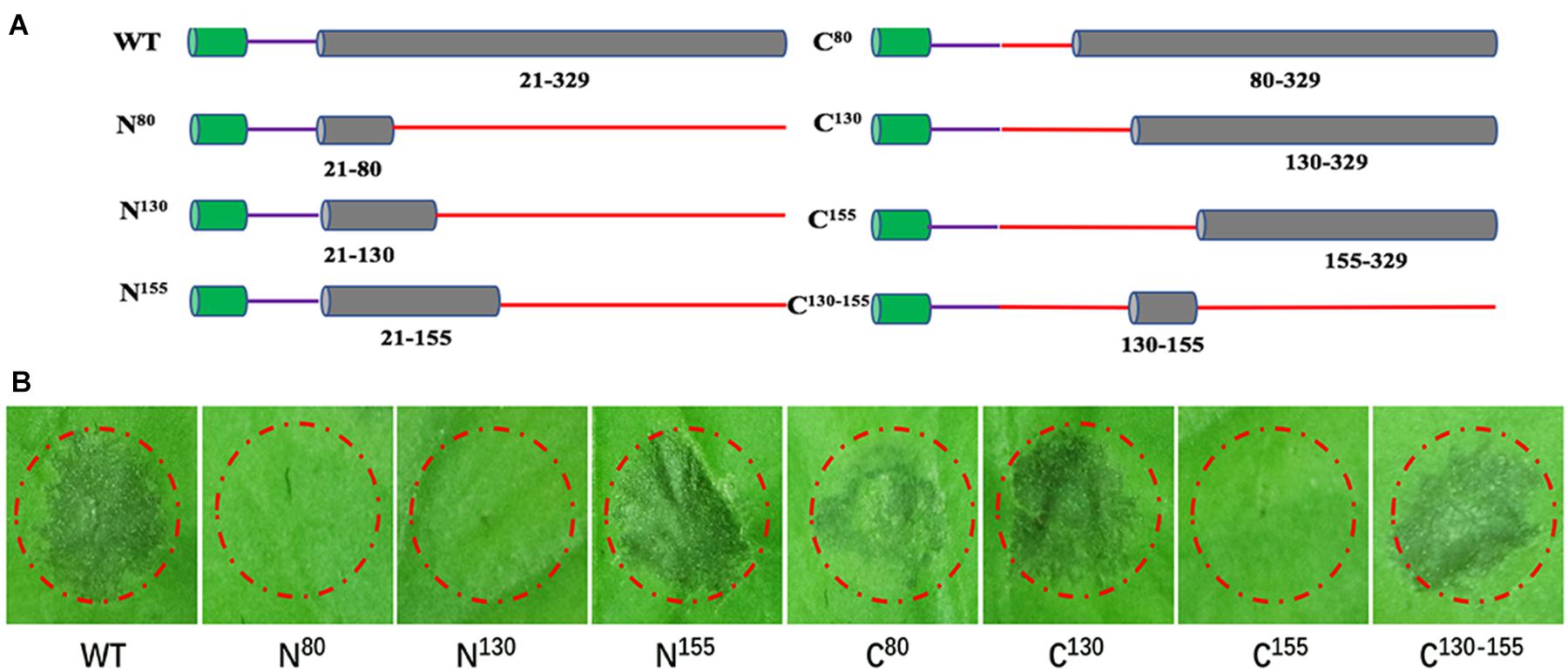
FIGURE 7. A small peptide of BcXyl1 is sufficient for inducing cell death. (A) Schematic presentation of the examined constructs, including N80, N130, N155, C80, C130, C155, and C130-155. (B) Analysis of cell death produced by A. tumefaciens strains transiently expressing various truncated mutants in N. benthamiana leaves. All the experiments were replicated three times.
The plant PRRs, such as the LRR RLKs BAK1 and SOBIR1, were employed to participate in multiple PRR pathways, including cell death induction (Monaghan and Zipfel, 2012; Liebrand et al., 2014; Gravino et al., 2016). For example, BAK1 was required for cell death inducing of GH12 members (Ma et al., 2015; Zhu et al., 2017). As demonstrated above, BcXyl1 was secreted into the apoplast to induce cell death. To determine whether BAK1 and SOBIR1 participated in induction of cell death by BcXyl1, we used virus-induced gene silencing (VIGS) to induce the gene silencing of BAK1 or SOBIR1 in N. benthamiana leaves. Three weeks after viral inoculation to silence BAK1, transient expression of BcXyl1 in N. benthamiana did not result in cell death after agroinfiltration with BcXyl1 expression constructs. Treatment of BAK1-silenced plants with Bcl-2-associated protein X (BAX) was used as a control, which resulted in cell death induction (Figure 8A). The results of SOBIR1-silenced plants were in accordance with BAK1-silenced plants, BcXyl1 did not trigger cell death, while BAX was still capable of inducing cell death (Figure 8A). Immunoblotting confirmed that BcXyl1 were successfully expressed at the expected size in N. benthamiana plants inoculated with TRV::BAK, TRV::SOBIR1, or TRV::GFP (Figure 8B). qPCR analysis confirmed that the expression of BAK1 or SOBIR1 expression was markedly reduced upon inoculation with the TRV::BAK or TRV::SOBIR1, with an expression level about 20% in comparison with inoculation with TRV::GFP (Figure 8C). From these results, we inferred that BAK1 and SOBIR1 (a LRR-RLP/SOBIR1/BAK1 complex) were required for BcXyl1-triggered cell death in N. benthamiana.
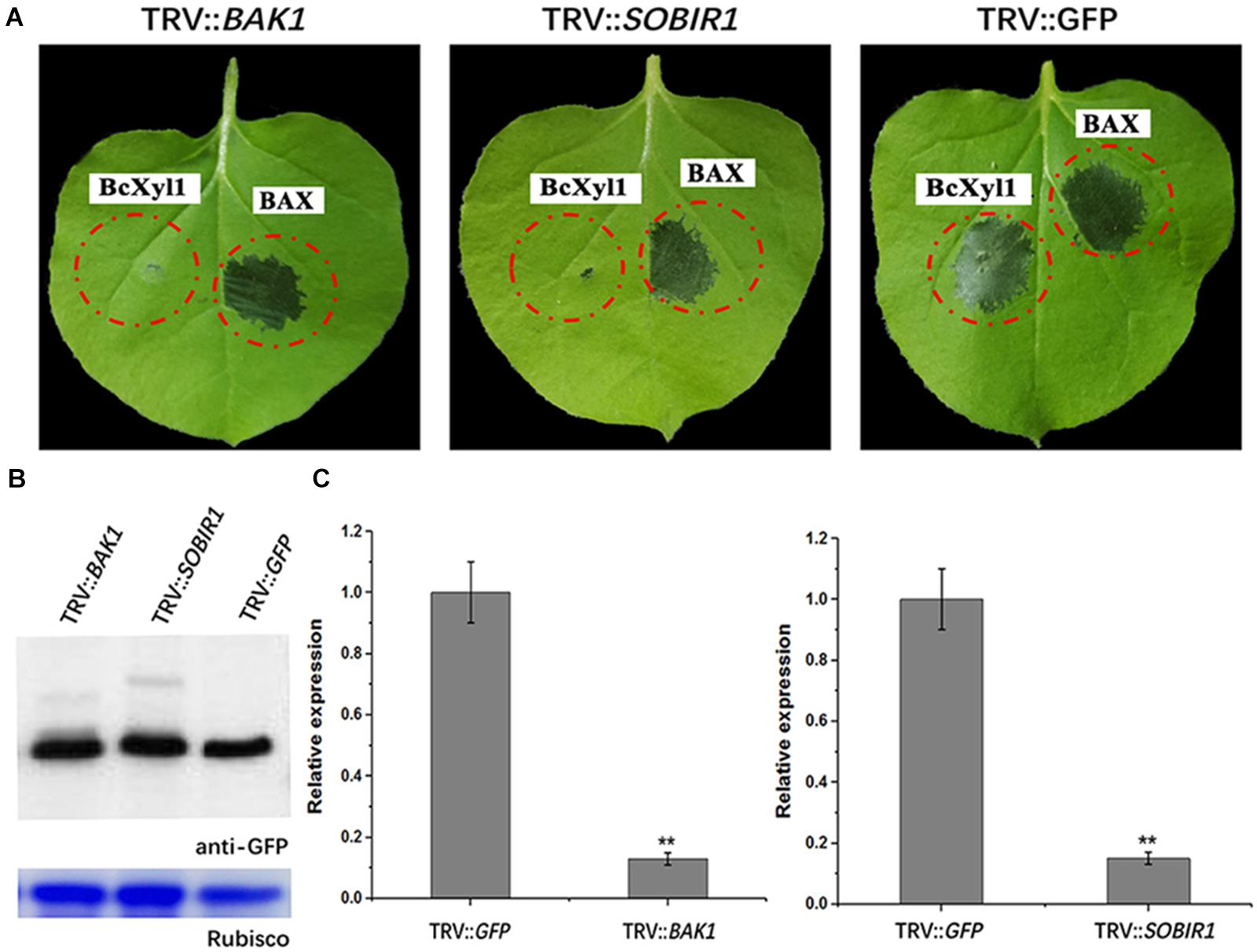
FIGURE 8. BAK1 and SOBIR1 mediates BcXyl1-triggered cell death in N. benthamiana. Three silence constructs (TRV::BAK1, TRV::SOBIR1, and TRV::GFP) were generated. (A) BcXyl1 and BAX (the positive control) were transiently expressed in BAK1 and SOBIR1 silenced tobacco leaves, respectively. The cell death induction was detected and photographed in N. benthamiana leaves 4 days after infiltration. (B) Immunoblot analysis of BcXyl1 transiently expressed in genes-silenced N. benthamiana leaves. (C) The silencing efficiency of BAK1 and SOBIR1 was examined by qPCR. Error bars represent standard errors. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between mutants and WT. Asterisks “∗∗” indicate statistically significant differences at a p-value <0.01.
Botrytis cinerea, a necrotrophic plant pathogen, attacks the plant organs, including leaves, flowers, fruits, bulb, and root tubers, and causes serious plant diseases and substantial losses in agriculture throughout the world (Williamson et al., 2007; Ky et al., 2012). Like other phytopathogenic fungi, B. cinerea secretes vast array of proteins during infection process (Fillinger and Elad, 2016). Cell wall-degrading enzymes (CWDEs) are the largest class of B. cinerea-secreted proteins (Kubicek et al., 2014). Recent studies have revealed that several CWDEs functioned as virulence factors in plant pathogens and were also recognized as PAMPs by plant PRRs to trigger the PTI responses, during plant–pathogen interactions (Ma et al., 2015). In this study, we described the identification and analysis of BcXyl1, a secreted xylanase from B. cinerea. BcXyl1 had the ability to induce cell death and plant PTI responses independent of its enzymatic activity. Furthermore, our study also found that a small peptide from BcXyl1 was sufficient for elicitor activity. VIGS assays showed that a LRR-RLP/SOBIR1/BAK1 complex modulates BcXyl1-triggered cell death in N. benthamiana. We also found that BcXyl1 functions as a virulence factor that contributes to B. cinerea virulence on host plants.
Increasing evidence demonstrated that xylanases are responsible for the pathogenesis of necrotrophic phytopathogens, including B. cinerea (Schouten et al., 2007; Frías et al., 2011). For instance, xyn11A was an endo-β-1,4-xylanase belonging to family 11 of glycoside hydrolase and required for virulence in B. cinerea, and the deletion of the xynB gene encoding an endo-xylanase distinctly reduced the virulence of Xanthomonas oryzae pv. oryzae (Brito et al., 2006; Pandey and Sonti, 2010). In this study, we found BcXyl1 appeared to be a major virulence factor. Strikingly, BcXyl1 was strongly induced and accumulated during the early stage of infection, and the mutation of BcXyl1 had a severe effect on pathogenicity (Figures 1, 2). It is noteworthy that not all fungal xylanases have been conclusively involved in pathogenicity and virulence. So far, gene deletion experiments in Fusarium oxysporum, Fusarium graminearum, Magnaporthe grisea, and Cochliobolus carbonum did not support an essential role for xylanases in fungal pathogenesis (Apel, 1993; Wu et al., 1997; Gómez-Gómez et al., 2002; Santhanam et al., 2013; Sella et al., 2013). In addition, previously study showed that a xylanase from B. cinerea could contribute to virulence by promoting the necrosis of the plant tissue surrounding the infection (Noda et al., 2010). Interestingly, a few nanograms of purified BcXyl1 resulted in a rapid leaf tissue necrosis in soybean, tomato, cotton, and N. benthamiana (Figure 3C). The range of plant species responding to BcXyl1 may be larger than we detected.
Previous studies showed that the enzymatic activity of many fungal CWDEs was required for cell death-inducing activity (Gui Y. et al., 2017). However, in certain cases, the cell death inducing activity was found to be unrelated to the enzymatic activity (Ma et al., 2014, 2015). For instance, Xyn11A, a xylanase from B. cinerea, and the Trichoderma viride EIX could induce cell death in plants independent of the xylanase activity (Furman-Matarasso et al., 1999; Noda et al., 2010). Although BcXyl1 is a xylanase, induction of cell death did not require the enzymatic activity (Figure 4).
Our results showed that BcXyl1 was localized to the plant apoplast by a signal peptide experiment, suggesting that the cell death-inducing activity may be mediated by surface-localized PRRs (Supplementary Figure S5). Plant surface-localized PRRs such as RLKs and RLPs were involved in the recognition of PAMPs (Boutrot and Zipfel, 2017). In addition, BAK1, as a co-receptor, plays a regulatory role in receptor complexes that mediate PTI (Schulze et al., 2010; Liu et al., 2013; Gravino et al., 2016; Yamada et al., 2016). And SOBIR1 is also specifically required for the function of receptor complexes (Liebrand et al., 2014). VIGS assays confirmed that tobacco BAK1 was required for BcXyl1-induced cell death, and the cell-death response also disappeared in VIGS-SOBIR1 plants (Figure 8). Hence, the RLP–SOBIR1–BAK1 complex mediated the cell death-inducing activity of BcXyl1.
The detection of PAMPs by plant PRRs to trigger PTI is a major component of plant defense responses. We confirmed that BcXyl1 triggered typical defense responses, including accumulation of ROS, leakage of ion electrolytes, deposition of callose, and expression of defense genes (Figure 5). We also found that the recombinant BcXyl1 proteins conferred systemic resistance in N. benthamiana, which offered protection against TMV and B. cinerea (Figure 6).
Generally, PAMPs are perceived by PRRs via specific epitopes, and the small peptides located on the surface of the proteins are sufficient to stimulate immune responses. For example, a 35-amino acid peptide of BcIEB1 could trigger necrosis and the PTI responses (González et al., 2017). Similarly, a 30-amino acid peptide of Xyn11A mediated the induction of cell death (Noda et al., 2010). The small peptide of VdEG3 from the GH12 domain was sufficient to induce cell death in N. benthamiana (Gui Y.-J. et al., 2017). In this study, progressive truncation of BcXyl1 confirmed that a region with 26 amino acids was sufficient for elicitor function (Figures 5–7).
Previous studies showed that many fungal xylanases involved in inducing plant defense responses immunity. For instance, the xylanase EIX from T. viride was an elicitor to induce defense responses in tomato, pepper and tomato plants (Rotblat et al., 2002). A xylanase from F. graminearum could induce cell death and hydrogen peroxide accumulation in wheat leaves (Sella et al., 2013; Moscetti et al., 2014). We have also determined that BcXyl1 induced plant defense responses and conferred tobacco and tomato plants disease resistance. Therefore, we speculated that fungal xylanases have the ability to trigger immunity in dicot and monocot plants.
Successful pathogens deliver effectors to interference the host PTI response and establish infection (Jones and Dangl, 2006; Gimenez-Ibanez et al., 2009). For example, a RXLR effector and CBM1 effector suppressed XEG1-triggered immunity in oomycetes and suppressed the GH12 protein and BcXyl1-triggered immunity in V. dahlia, respectively. Whether effectors mediate the suppression of BcXyl1, needs further investigation.
YD and DQ designed the experiments. YY performed most of the experiments and wrote the paper. XY participated in some part of the study and the Graduate Student Innovation Scientific Research Subject of Hainan Province (Hyb2017-17).
This study was supported by the National Natural Science Foundation of China (no. 31701782 and no. 31371984).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to X. F. Dai from the Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences, for the generous gift of the vectors for gene knockout and complementation. We also thank L. Yao from Beijing Academy of Agriculture and Forestry for providing the pYBA1132 plasmid and pPICZαA plasmid. And YY thanks the meticulous care and selfless support of Ms. Nianhua Jiang over the past 4 years.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02535/full#supplementary-material
FIGURE S1 | Sequence alignment of BcXyl1 and xylanases from other fungi. Two red triangles indicated possible catalytic residues of BcXyl1 (E104 and E157). Sequence data of all proteins can be found in the GenBank/EMBL data libraries under accession numbers: XynBc1 (ACF16413.1), BcXyl1 (ATZ53308.1), XynG1 (XP_001258363.1), BcXyl2 (XP_001546507.1), BcXyl3 (ATZ58346.1), BcXyl4 (ATZ51455.1), NpGH11 (EOD46026.1), and SsGH11 (XP_001588545).
FIGURE S2 |BcXyl1 deletion strains do not show developmental defects. BcXyl1 deletion mutants (ΔBcXyl1-1 and ΔBcXyl1-2), rescued strains (ΔBcXyl1-1-C and ΔBcXyl1-2-C). (A) The radial growth and colony morphology were observed after 8 days of incubation on PDA medium at 25°C. (B) Conidial germination rate of each strain was determined after cultivated on Water-Agar media at 25°C for 15 h. (C) Fungi were grown on PDA plates at 25°C. Radial growth was measured every day, and the growth rate was calculated. All the experiments were replicated three times.
FIGURE S3 | SDS-PAGE analysis of BcXyl1 and BcXyl1rec recombinant proteins. BcXyl1 is the native protein; BcXyl1rec is the site-directed mutagenized protein, which E104 and E157 were substituted with Gln. Two recombinant proteins were stained with Coomassie blue.
FIGURE S4 | BcXyl1 is secreted into the apoplast to induce cell death. (A) BcXyl1 (the native protein) and BcXyl1-ΔSP (deleted the signal peptide). Cell death induction was detected in N. benthamiana leaves 5 days after infiltration with the examined various A. tumefaciens strains. (B) Immunoblot analysis of proteins from N. benthamiana leaves transiently expressing the examined proteins using a pYBA1132 vector. All the experiments were replicated three times.
FIGURE S5 | BcXyl1 confers disease resistance in plants. N. benthamiana or tomato leaves were infiltrated with 0.5 μM purified BcXyl1, C130-155, or PEVC. (A) The local leaves were inoculated with TMV-GFP, and the number of TMV-GFP lesions were measured. (B,C) The N. benthamiana or tomato local leaves were inoculated with 5 μL of 2 × 106 conidia/ml Botrytis cinerea. Lesions symptoms and diameter were observed and measured at 2 days post-inoculation, respectively. Error bars represent standard deviation of three independent replicates. Student’s t-test was performed to determine the significant differences between BcXyl1, C130-155 and PEVC. Asterisks “∗∗” indicate statistically significant differences at a p-value <0.01.
TABLE S1 | Hydrolysis activity test.
TABLE S2 | Primers used in this study.
Apel, P. C. (1993). Cloning and targeted gene disruption of XYL1,a β1,4-Xylanase gene from the maize pathogen Cochliobolus carbonum. Mol. Plant Microbe Interact. 6, 467–473. doi: 10.1094/MPMI-6-467
Ben-Daniel, B.-H., Bar-Zvi, D., and Tsror Lahkim, L. (2011). Pectate lyase affects pathogenicity in natural isolates of Colletotrichum coccodes and in pelA gene-disrupted and gene-overexpressing mutant lines. Mol. Plant Pathol. 13, 187–197. doi: 10.1111/j.1364-3703.2011.00740.x
Benito, E. P., ten Have, A., van ’t Klooster, J. W., and van Kan, J. A. L. (1998). Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur. J. Plant Pathol. 104, 207–220. doi: 10.1023/A:1008698116106
Biely, P., Mislovičová, D., and Toman, R. (1988). Remazol brilliant blue-xylan: a soluble chromogenic substrate for xylanases. Meth. Enzymol. 160, 536–541. doi: 10.1016/0076-6879(88)60165-0
Bindschedler, L. V., Dewdney, J., Blee, K. A., Stone, J. M., Asai, T., Plotnikov, J., et al. (2006). Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 47, 851–863. doi: 10.1111/j.1365-313X.2006.02837.x
Boller, T., and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Boutrot, F., and Zipfel, C. (2017). Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu. Rev. Phytopathol. 55, 257–286. doi: 10.1146/annurev-phyto-080614-120106
Brito, N., Espino, J. J., and González, C. (2006). The endo-beta-1,4-xylanase xyn11A is required for virulence in Botrytis cinerea. MPMI 19, 25–32. doi: 10.1094/MPMI-19-0025
Brutus, A., Reca, I. B., Herga, S., Mattei, B., Puigserver, A., Chaix, J.-C., et al. (2005). A family 11 xylanase from the pathogen Botrytis cinerea is inhibited by plant endoxylanase inhibitors XIP-I and TAXI-I. Biochem. Biophys. Res. Commun. 337, 160–166. doi: 10.1016/j.bbrc.2005.09.030
Cantarel, B. L., Coutinho, P. M., Rancurel, C., Bernard, T., Lombard, V., and Henrissat, B. (2009). The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37, D233–D238. doi: 10.1093/nar/gkn663
Chisholm, S. T., Coaker, G., Day, B., and Staskawicz, B. J. (2006). Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814. doi: 10.1016/j.cell.2006.02.008
Chen, M., Zeng, H., Qiu, D., Guo, L., Yang, X., Shi, H., et al. (2012). Purification and characterization of a novel hypersensitive response-inducing elicitor from Magnaporthe oryzae that triggers defense response in rice. PLoS One 7:e37654. doi: 10.1371/journal.pone.0037654
Collins, T., Gerday, C., and Feller, G. (2005). Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 29, 3–23. doi: 10.1016/j.femsre.2004.06.005
Couto, D., and Zipfel, C. (2016). Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. doi: 10.1038/nri.2016.77
Fillinger, S., and Elad, Y. (2016). Botrytis – the Fungus, the Pathogen and its Management in Agricultural Systems. Berlin: Springer International Publishing. doi: 10.1007/978-3-319-23371-0
Frías, M., Brito, N., and González, C. (2012). The Botrytis cinerea cerato-platanin BcSpl1 is a potent inducer of systemic acquired resistance (SAR) in tobacco and generates a wave of salicylic acid expanding from the site of application. Mol. Plant Pathol. 14, 191–196. doi: 10.1111/j.1364-3703.2012.00842.x
Frías, M., González, C., and Brito, N. (2011). BcSpl1, a cerato-platanin family protein, contributes to Botrytis cinerea virulence and elicits the hypersensitive response in the host. New Phytol. 192, 483–495. doi: 10.1111/j.1469-8137.2011.03802.x
Furman-Matarasso, N., Cohen, E., Du, Q., Chejanovsky, N., Hanania, U., and Avni, A. (1999). A point mutation in the ethylene-inducing xylanase elicitor inhibits the beta-1-4-endoxylanase activity but not the elicitation activity. Plant Physiol. 121, 345–351. doi: 10.1104/pp.121.2.345
Gimenez-Ibanez, S., Hann, D. R., Ntoukakis, V., Petutschnig, E., Lipka, V., and Rathjen, J. P. (2009). AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol. 19, 423–429. doi: 10.1016/j.cub.2009.01.054
Gómez-Gómez, E., Ruíz-Roldán, M. C., Di Pietro, A., Roncero, M. I. G., and Hera, C. (2002). Role in pathogenesis of two endo-β-1,4-xylanase genes from the vascular wilt fungus Fusarium oxysporum. Fungal Genet. Biol. 35, 213–222. doi: 10.1006/fgbi.2001.1318
González, M., Brito, N., and González, C. (2017). The Botrytis cinerea elicitor protein BcIEB1 interacts with the tobacco PR5-family protein osmotin and protects the fungus against its antifungal activity. New Phytol. 215, 397–410. doi: 10.1111/nph.14588
Gravino, M., Locci, F., Tundo, S., Cervone, F., Savatin, D. V., and De Lorenzo, G. (2016). Immune responses induced by oligogalacturonides are differentially affected by AvrPto and loss of BAK1/BKK1 and PEPR1/PEPR2. Mol. Plant Pathol. 18, 582–595. doi: 10.1111/mpp.12419
Gui, Y., Zhang, W., Zhang, D., Zhou, L., Short, D. P. G., Wang, J., et al. (2017). A Verticillium dahliae extracellular cutinase modulates plant immune responses. Mol. Plant Microbe Interact. 31, 260–273. doi: 10.1094/MPMI-06-17-0136-R
Gui, Y.-J., Chen, J.-Y., Zhang, D.-D., Li, N.-Y., Li, T.-G., Zhang, W.-Q., et al. (2017). Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate-binding module 1. Environ. Microbiol. 19, 1914–1932. doi: 10.1111/1462-2920.13695
Houterman, P. M., Cornelissen, B. J. C., and Rep, M. (2008). Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 4:e1000061. doi: 10.1371/journal.ppat.1000061
Jones, J. D. G., and Dangl, J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Kettles, G. J., Bayon, C., Canning, G., Rudd, J. J., and Kanyuka, K. (2016). Apoplastic recognition of multiple candidate effectors from the wheat pathogen Zymoseptoria triticiin the nonhost plant Nicotiana benthamiana. New Phytol. 213, 338–350. doi: 10.1111/nph.14215
Kubicek, C. P., Starr, T. L., and Glass, N. L. (2014). Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 52, 427–451. doi: 10.1146/annurev-phyto-102313-045831
Ky, I., Lorrain, B., Jourdes, M., Pasquier, G., Fermaud, M., Gény, L., et al. (2012). Assessment of grey mould (Botrytis cinerea) impact on phenolic and sensory quality of Bordeaux grapes, musts and wines for two consecutive vintages. Aust. J. Grape Wine Res. 18, 215–226. doi: 10.1111/j.1755-0238.2012.00191.x
Liebrand, T. W. H., van den Burg, H. A., and Joosten, M. H. A. J. (2014). Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 19, 123–132. doi: 10.1016/j.tplants.2013.10.003
Liu, Z., Wu, Y., Yang, F., Zhang, Y., Chen, S., Xie, Q., et al. (2013). BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc. Natl. Acad. Sci. U.S.A. 110, 6205–6210. doi: 10.1073/pnas.1215543110
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ma, Y., Han, C., Chen, J., Li, H., He, K., Liu, A., et al. (2014). Fungal cellulase is an elicitor but its enzymatic activity is not required for its elicitor activity. Mol. Plant Pathol. 16, 14–26. doi: 10.1111/mpp.12156
Ma, Z., Song, T., Zhu, L., Ye, W., Wang, Y., Shao, Y., et al. (2015). A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell 27, 2057–2072. doi: 10.1105/tpc.15.00390
Monaghan, J., and Zipfel, C. (2012). Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 15, 349–357. doi: 10.1016/j.pbi.2012.05.006
Moscetti, I., Faoro, F., Moro, S., Sabbadin, D., Sella, L., Favaron, F., et al. (2014). The xylanase inhibitor TAXI-III counteracts the necrotic activity of a Fusarium graminearum xylanase in vitroand in durum wheat transgenic plants. Mol. Plant Pathol. 16, 583–592. doi: 10.1111/mpp.12215
Noda, J., Brito, N., and González, C. (2010). The Botrytis cinerea xylanase Xyn11A contributes to virulence with its necrotizing activity, not with its catalytic activity. BMC Plant Biol. 10:38. doi: 10.1186/1471-2229-10-38
Pandey, A., and Sonti, R. V. (2010). Role of the FeoB protein and siderophore in promoting virulence of Xanthomonas oryzae pv. oryzae on Rice. J. Bacteriol. 192, 3187–3203. doi: 10.1128/JB.01558-09
Prins, T. W., Tudzynski, P., von Tiedemann, A., Tudzynski, B., ten Have, A., Hansen, M. E., et al. (2000). “Infection strategies of Botrytis cinerea and related necrotrophic pathogens,” in Fungal Pathology, ed. J. W. Kronstad (Dordrecht: Kluwer Academic Publishers Group), 33–64.
Rotblat, B., Enshell-Seijffers, D., Gershoni, J. M., Schuster, S., and Avni, A. (2002). Identification of an essential component of the elicitation active site of the EIX protein elicitor. Plant J. 32, 1049–1055. doi: 10.1046/j.1365-313X.2002.01490.x
Saijo, Y., Loo, E. P.-I., and Yasuda, S. (2017). Pattern recognition receptors and signaling in plant-microbe interactions. Plant J. 93, 592–613. doi: 10.1111/tpj.13808
Santhanam, P., van Esse, H. P., Albert, I., Faino, L., Nürnberger, T., and Thomma, B. P. H. J. (2013). Evidence for functional diversification within a fungal NEP1-Like protein family. MPMI 26, 278–286. doi: 10.1094/MPMI-09-12-0222-R
Schouten, A., van Baarlen, P., and van Kan, J. A. L. (2007). Phytotoxic Nep1-like proteins from the necrotrophic fungus Botrytis cinerea associate with membranes and the nucleus of plant cells. New Phytol. 177, 493–505.
Schulze, B., Mentzel, T., Jehle, A. K., Mueller, K., Beeler, S., Boller, T., et al. (2010). Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 285, 9444–9451. doi: 10.1074/jbc.M109.096842
Sella, L., Gazzetti, K., Faoro, F., Odorizzi, S., D’Ovidio, R., Schäfer, W., et al. (2013). A Fusarium graminearum xylanase expressed during wheat infection is a necrotizing factor but is not essential for virulence. Plant Physiol. Biochem. 64, 1–10. doi: 10.1016/j.plaphy.2012.12.008
Stergiopoulos, I., and de Wit, P. J. G. M. (2009). Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263. doi: 10.1146/annurev.phyto.112408.132637
Williamson, B., Tudzynski, B., Tudzynski, P., and van Kan, J. A. L. (2007). Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8, 561–580. doi: 10.1111/j.1364-3703.2007.00417.x
Wu, S.-C., Ham, K.-S., Darvill, A. G., and Albersheim, P. (1997). Deletion of two endo-β-1,4-Xylanase genes reveals additional isozymes secreted by the rice blast fungus. MPMI 10, 700–708. doi: 10.1094/MPMI.1997.10.6.700
Yakoby, N., Beno-Moualem, D., Keen, N. T., Dinoor, A., Pines, O., and Prusky, D. (2001). Colletotrichum gloeosporioides pelBIs an important virulence factor in avocado fruit-fungus interaction. MPMI 14, 988–995. doi: 10.1094/MPMI.2001.14.8.988
Yamada, K., Yamashita-Yamada, M., Hirase, T., Fujiwara, T., Tsuda, K., Hiruma, K., et al. (2016). Danger peptide receptor signaling in plants ensures basal immunity upon pathogen-induced depletion of BAK1. EMBO J. 35, 46–61. doi: 10.15252/embj.201591807
Yu, X., Tang, J., Wang, Q., Ye, W., Tao, K., Duan, S., et al. (2012). The RxLR effector Avh241 from Phytophthora sojae requires plasma membrane localization to induce plant cell death. New Phytol. 196, 247–260. doi: 10.1111/j.1469-8137.2012.04241.x
Zhang, H., Wu, Q., Cao, S., Zhao, T., Chen, L., Zhuang, P., et al. (2014). A novel protein elicitor (SsCut) from Sclerotinia sclerotiorum induces multiple defense responses in plants. Plant Mol. Biol. 86, 495–511. doi: 10.1007/s11103-014-0244-3
Zhang, Y., Zhang, Y., Qiu, D., Zeng, H., Guo, L., and Yang, X. (2015). BcGs1, a glycoprotein from Botrytis cinerea, elicits defence response and improves disease resistance in host plants. Biochem. Biophys. Res. Commun. 457, 627–634. doi: 10.1016/j.bbrc.2015.01.038
Zhu, W., Ronen, M., Gur, Y., Minz-Dub, A., Masrati, G., Ben-Tal, N., et al. (2017). BcXYG1, a secreted xyloglucanase from Botrytis cinerea, triggers both cell death and plant immune responses. Plant Physiol. 175, 438–456. doi: 10.1104/pp.17.00375
Keywords: Botrytis cinerea, xylanase, BcXyl1, plant immunity, virulence
Citation: Yang Y, Yang X, Dong Y and Qiu D (2018) The Botrytis cinerea Xylanase BcXyl1 Modulates Plant Immunity. Front. Microbiol. 9:2535. doi: 10.3389/fmicb.2018.02535
Received: 01 August 2018; Accepted: 04 October 2018;
Published: 23 October 2018.
Edited by:
Luisa Lanfranco, Università degli Studi di Torino, ItalyReviewed by:
Franco Faoro, Università degli Studi di Milano, ItalyCopyright © 2018 Yang, Yang, Dong and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yijie Dong, bmtkb25neWlqaWVAMTYzLmNvbQ== Dewen Qiu, cWl1ZGV3ZW5AY2Fhcy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.