
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 16 October 2018
Sec. Plant Pathogen Interactions
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.02491
This article is part of the Research TopicHarnessing Useful Rhizosphere Microorganisms for Pathogen and Pest Biocontrol, Volume IIView all 33 articles
A correction has been applied to this article in:
Corrigendum: Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol
Bacillus velezensis FZB42, the model strain for Gram-positive plant-growth-promoting and biocontrol rhizobacteria, has been isolated in 1998 and sequenced in 2007. In order to celebrate these anniversaries, we summarize here the recent knowledge about FZB42. In last 20 years, more than 140 articles devoted to FZB42 have been published. At first, research was mainly focused on antimicrobial compounds, apparently responsible for biocontrol effects against plant pathogens, recent research is increasingly directed to expression of genes involved in bacteria–plant interaction, regulatory small RNAs (sRNAs), and on modification of enzymes involved in synthesis of antimicrobial compounds by processes such as acetylation and malonylation. Till now, 13 gene clusters involved in non-ribosomal and ribosomal synthesis of secondary metabolites with putative antimicrobial action have been identified within the genome of FZB42. These gene clusters cover around 10% of the whole genome. Antimicrobial compounds suppress not only growth of plant pathogenic bacteria and fungi, but could also stimulate induced systemic resistance (ISR) in plants. It has been found that besides secondary metabolites also volatile organic compounds are involved in the biocontrol effect exerted by FZB42 under biotic (plant pathogens) and abiotic stress conditions. In order to facilitate easy access to the genomic data, we have established an integrating data bank ‘AmyloWiki’ containing accumulated information about the genes present in FZB42, available mutant strains, and other aspects of FZB42 research, which is structured similar as the famous SubtiWiki data bank.
Bacteria that are associated with plant roots and exert beneficial effects on plant development are referred to as plant-growth-promoting rhizobacteria (PGPR; Kloepper et al., 1980). It is well accepted today, that numerous PGPR are also enabled to control plant diseases.
Main subject of present and past research about microbial inoculants with beneficial action on plant health and growth are plant-associated representatives of the bacterial genus Pseudomonas, known as strong and persistent colonizer of plant roots (Burr et al., 1978). However, its commercial use is limited by difficulties in preparing stable and long-living bioformulations. As early as at the end of the 19th century a bacterial soil-fertilizing preparation Alinit® consisting of spores of the soil bacterium Bacillus ellenbachensis, later reclassified as Bacillus subtilis, was introduced by the German landowner Albert Caron (1853–1933) on his estate in Ellenbach (Caron, 1897). Alinit was marketed as “bacteriological fertilizer for the inoculation of cereals” by “Farbenfabriken former Friedrich Bayer,” the later Bayer AG, in Elberfeld, Germany. The history of these early attempts in using bacterial inoculants is comprehensively described by Kolbe (1993). After a long period of silence, the plant-growth-promoting effect of Bacillus spp. was rediscovered in Broadbent et al. (1977). Today, formulations based on plant-beneficial endospore-forming Bacilli are by far the most widely used agents on the biopesticide market (Borriss, 2011). Especially, members of the B. subtilis species complex (rRNA group 1) which includes at present more than 20 closely related species (Fan et al., 2017a), and, to a minor extent, of the genus Paenibacillus spp., are able to suppress efficiently plant pathogens, such as viruses, bacteria, fungi and nematodes in vicinity of plant roots. This review describes the current ‘state of the art’ of the model strain for PGPR – and biocontrol, Bacillus velezensis FZB42, and the integrative data bank ‘AmyloWiki,’ recently established for this bacterium.
FZB42 (=BGSC 10A6, DSM23117), the prototype of gram-positive bacteria with phytostimulatory and biocontrol action, has been genome sequenced in Chen et al. (2007) and is subject of intensive research. Since its isolation from beet rhizosphere (Krebs et al., 1998) more than 140 articles about FZB42 have been published1. FZB42 and its closely related ‘cousin’ FZB24, are successfully used as biofertilizer and biocontrol bacteria in agriculture being especially efficient against fungal and bacterial pathogens2. Beneficial effects of FZB42/FZB24 on plant growth and disease suppression in field trials were reported for potato (Schmiedeknecht et al., 1998), cotton (Yao et al., 2006), strawberry (Sylla et al., 2013), wheat (Talboys et al., 2014), lettuce (Chowdhury et al., 2013), and tomato (Elanchezhiyan et al., 2018), for example.
In past, FZB42 and related phytostimulatory Bacilli were subjects of intensive efforts to clarify their taxonomic position. The group of plant-associated, endo-spore forming rhizobacteria (Reva et al., 2004) is known as member of the B. subtilis species complex (Fritze, 2004), which included originally B. subtilis, B. licheniformis, and B. pumilus (Gordon et al., 1973). In 1987, the species B. amyloliquefaciens (Priest et al., 1987) was added, and FZB42 and some other biocontrol bacteria were found as belong to this species (Idriss et al., 2002). By taking advantage of availability of an increasing number of genome sequences, we distinguished two subspecies: B. amyloliquefaciens subsp. amyloliquefaciens (type strain DSM7T) and B. amyloliquefaciens subsp. plantarum (type strain FZB42T) (Borriss et al., 2011). According to extended phylogenomic analysis B. amyloliquefaciens subsp. plantarum was shown as a later heterotypic synonym of B. velezensis (Dunlap et al., 2016), Recently, we proposed to establish an “operational group B. amyloliquefaciens,” which includes B. amyloliquefaciens, known for its ability to produce industrial enzymes (amylases, glucanases and proteases), B. siamensis, mainly occurring in Asian food, and PGPR B. velezensis, the main source for bioformulations increasingly used in agriculture for protecting plant health and to stimulate plant growth (Fan et al., 2017a, Figure 1).
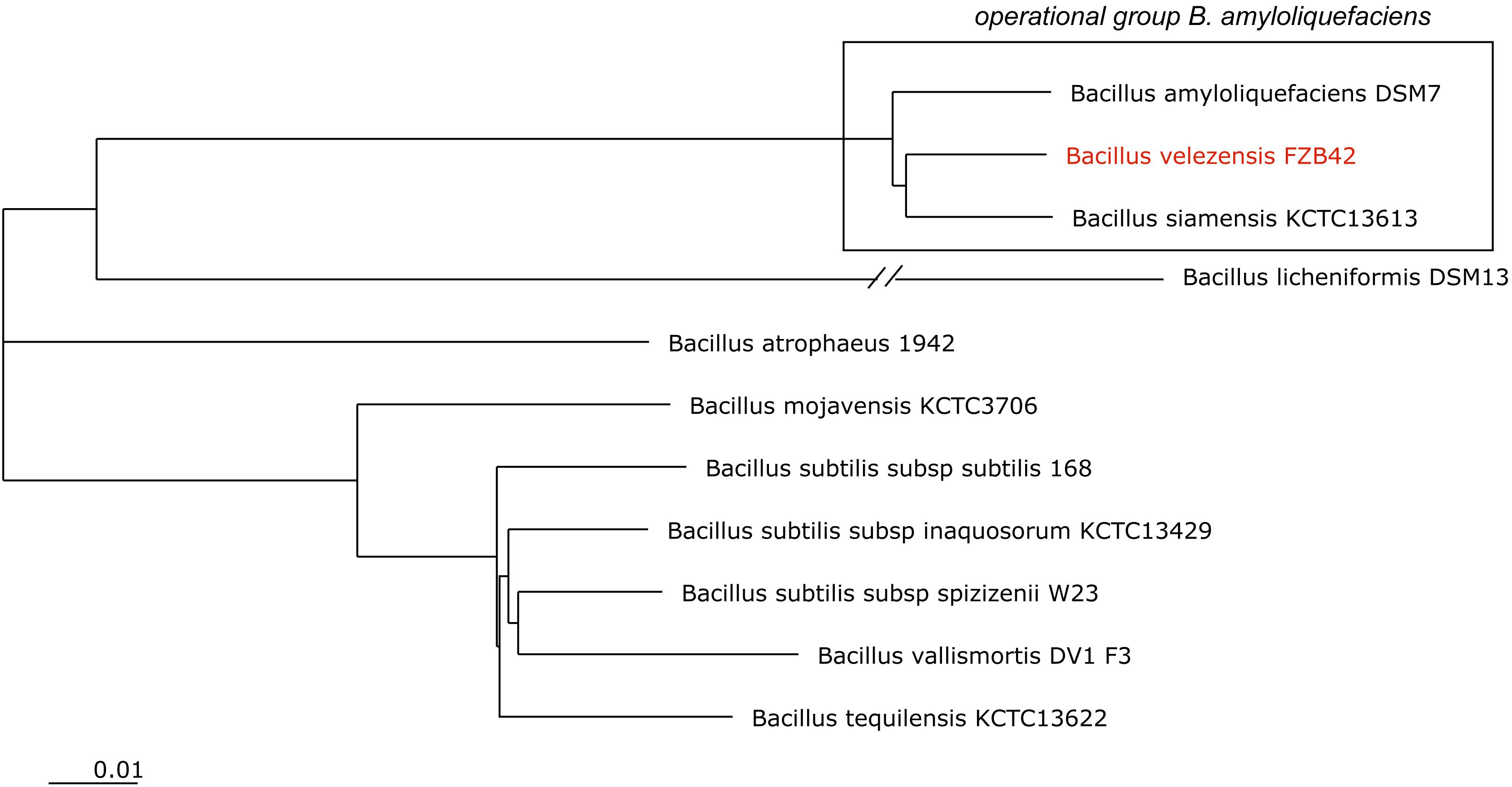
FIGURE 1. NJ phylogenomic tree, constructed from 11 type strain genomes with highest similarity to B. subtilis 168T. The genome of B. licheniformis DSM13 was used as outgroup. The tree was build out of a core of 1946 genes per genome, 21406 in total. The core has 586283 AA-residues/bp per genome, 6449113 in total. B. velezensis FZB42 (labeled in red) is a member of the operational group B. amyloliquefaciens (boxed). The scale bar corresponds to 0.01 substitutions per site.
Biocontrol effects exerted by B. velezensis FZB42 and other antagonistic acting Bacilli are due to different mechanisms: besides direct antibiosis and competition by secretion of a spectrum of secondary metabolites in the rhizosphere (Borriss, 2011), the beneficial action on the host-plant microbiome (Erlacher et al., 2014), and stimulation of plant induced systemic resistance (ISR, Kloepper et al., 2004; Chowdhury et al., 2015a) are of similar importance.
Remarkably, in contrast to Gram-negative biocontrol bacteria and fungal plant pathogens, application of FZB42 did not lead to durable changes in composition of rhizosphere microbial community (Chowdhury et al., 2013; Kröber et al., 2014). Moreover, application of FZB42 was shown to compensate negative changes within composition of the root microbiome caused by plant pathogens (Erlacher et al., 2014).
Induced systemic resistance is triggered by a range of secondary metabolites, which are called ‘elicitors.’ Different signaling pathways, such as jasmonic acid (JA), ethylene (ET), and salicylic acid (SA) are activated to induce plant resistance. Mutant strains of FZB42, devoid in synthesis of surfactin (srf), were found impaired in triggering of JA/ET dependent ISR in lettuce plants, when challenged with plant pathogen Rhizoctonia solani (Chowdhury et al., 2015b). The lower expression of the JA/ET-inducible plant defensin factor (PDF1.2) in a sfp mutant strain, completely devoid in non-ribosomal synthesis of lipopeptides and polyketides, compared to the srf mutant strain, only impaired in surfactin synthesis, suggests that secondary metabolites other than surfactin might also trigger plant response.
Gray leaf spot disease caused by Magnaporthe oryzae is a serious disease in perennial ryegrass (Lolium perenne). A mutant strain of FZB42 (AK3) only able to produce surfactin but no other lipopeptides such as bacillomycin D, and fengycin was shown to induce systemic resistance (ISR). Similarly, treatment with crude surfactin suppressed the disease in perennial ryegrass. ISR defense response was found connected with enhanced hydrogen peroxide (H2O2) development, elevated cell wall/apoplastic peroxidase activity, and deposition of callose and phenolic/polyphenolic compounds. Moreover, a hypersensitive response reaction and enhanced expression of different defense factors, such as peroxidase, oxalate oxidase, phenylalanine ammonia lyase, lipoxygenase, and defensins were caused by surfactin and also the surfactin producing mutant strain (Rahman et al., 2015).
Recent studies performed with mutant strains of B. velezensis SQR9, which is closely related with FZB42, revealed that non-ribosomal synthesized lipopeptides fengycin and bacillomycinD, the non-ribosomal synthesized polyketides macrolactin, difficidin, and bacillaene, the dipeptide bacilysin, exopolysaccharides, and volatile organic compounds (VOCs) contribute to ISR response in Arabidopsis plantlets after infection with plant pathogens Pseudomonas syringae pv. tomato and Botrytis cinerea (Wu G. et al., 2018).
Volatile organic compounds produced by B. velezensis GB03 have been reported to trigger synthesis of ET/JA-responsive plant defense gene PDF1.2 (Ryu et al., 2004; Sharifi and Ryu, 2016). Thirteen VOCs produced by FZB42 were identified using gas chromatography-mass spectrometry analysis. A direct effect against plant pathogens was registered: benzaldehyde, 1,2-benzisothiazol-3(2 H)-one and 1,3-butadiene significantly inhibited the colony size, cell viability, and motility of Ralstonia solanacearum, the causative agent of bacterial wilt in a wide variety of potential host plants (Tahir et al., 2017). Furthermore, transcription of type III (T3SS) and type IV secretion (T4SS) systems were down regulated. In addition, synthesis of other genes contributing to pathogenicity, such as eps-genes responsible for extracellular polysaccharides, and genes involved in chemotaxis (motA, fliT) were found repressed. Simultaneously, the VOCs significantly up-regulated the expression of plant genes related to wilt resistance and pathogen defense. Over-expression of plant defense genes EDS1 and NPR1 suggested that the SA pathway is involved in the ISR response elicited by surfactin (Tahir et al., 2017).
A recent analysis performed with FZB42 VOCs confirmed that signal pathways involved in plant systemic resistance were positively affected. JA response (VSP1 and PDF1.2) and SA response genes (PR1 and FMO1) were triggered in Arabidopsis plantlets after incubation with the volatiles. Noteworthy, defense against nematodes were elicited by volatiles in Arabidopsis roots (Hao et al., 2016).
An interesting mechanism of FZB42 to avoid leaf pathogen infection has been recently described. The foliar pathogen Phytophthora nicotianae is able to penetrate inside of plant tissues by using natural entry sites, such as stomata. Recently it was shown that colonizing of plant roots by FZB42 restricted entry of the pathogen into leave tissues of Nicotiana benthamiana. It was found that FZB52 turned on the abscisic acid (ABA) and SA-regulated pathways to induce stomatal closure after pathogen infection. In addition, it was shown, that several SA- and JA/ET-responsive genes in the leaves became activated in presence of FZB42, suggesting that these signaling pathways are also contributing to plant defenses against P. nicotianae (Wu L. et al., 2018).
Besides their indirect action against pathogens via triggering of ISR, polyketides and lipopeptides act directly against bacterial and fungal plant pathogens. They comprise two families of secondary metabolites non-ribosomally synthesized by multimodular enzymes, polyketide synthases (PKSs) and Peptide synthetases (NRPS), acting in assembly line arrays. The monomeric building blocks are either organic acids (polyketides) or amino acids (lipopeptides), respectively (Walsh, 2004). Their synthesis is depending on an enzyme (Sfp) that transfers 4′-phosphopantheine from coenzyme A to the carrier proteins of nascent peptide or polyketide chains. In Bacilli, e.g., FZB42, a special class of PKSs that lacks the cognate AT domain and require a discrete AT enzyme acting iteratively in trans (trans AT) was detected (Shen, 2003). The broadly conserved antiterminator protein LoaP (Nus G family) was identified as regulator of macrolactin and difficidin gene clusters in B. velezensis FZB42 on the level of transcription elongation (Goodson et al., 2017). Unfortunately, structural instability of these polyketides excluded their use as antibacterial agents.
Lipopeptides are another important class of secondary metabolites, also non-ribosomally synthesized by giant multifunctional enzymes (peptide synthetases, NRPS). Similar to PKS, three catalytic domains are involved in each elongation cycle: (1) The A-domain (adenylation domain) select its cognate amino acid; (2) The PCP domain (peptidyl-carrier domain) is equipped with a PPan prosthetic group to which the adenylated amino acid substrate is transferred and bound as thioester; (3) The condensation domain (C-domain) catalyzes formation of a new peptide bond (Duitman et al., 1999). The lipopeptide bacillomycin D is an efficient antifungal compound produced by FZB42. Its 50% effective concentration against the fungal pathogen Fusarium graminearum was determined to be approximately 30 μg/ml. Bacillomycin D induced morphological changes in the plasma membranes and cell walls of F. graminearum hyphae and conidia. Furthermore, bacillomycin D induced the accumulation of reactive oxygen species and caused cell death in F. graminearum hyphae and conidia. Bacillomycin D suppresses F. graminearum on corn silks, wheat seedlings, and wheat heads (Gu et al., 2017).
Today, B. subtilis is considered as being a plant-associated bacterium (Wipat and Harwood, 1999; Borriss et al., 2018). A direct comparison between the genomes of B. subtilis 168 and B. velezensis FZB42 (Table 1) revealed that 534 FZB42 genes are not occurring in B. subtilis 168, but 3158 genes are shared between both species. By contrast, there are only 423 singletons defined for FZB42 vs. Bacillus subtilis 168. In this context one has to mention, that the singleton numbers don’t correspond to the numbers in the Venn diagram. The Venn diagram (Figure 2) shows the numbers of reciprocal best hits between subsets of genomes. However, a gene without reciprocal best hit to another genome does not necessarily have to be a singleton. A singleton is defined as a gene without any hit against any other genome than the own one.
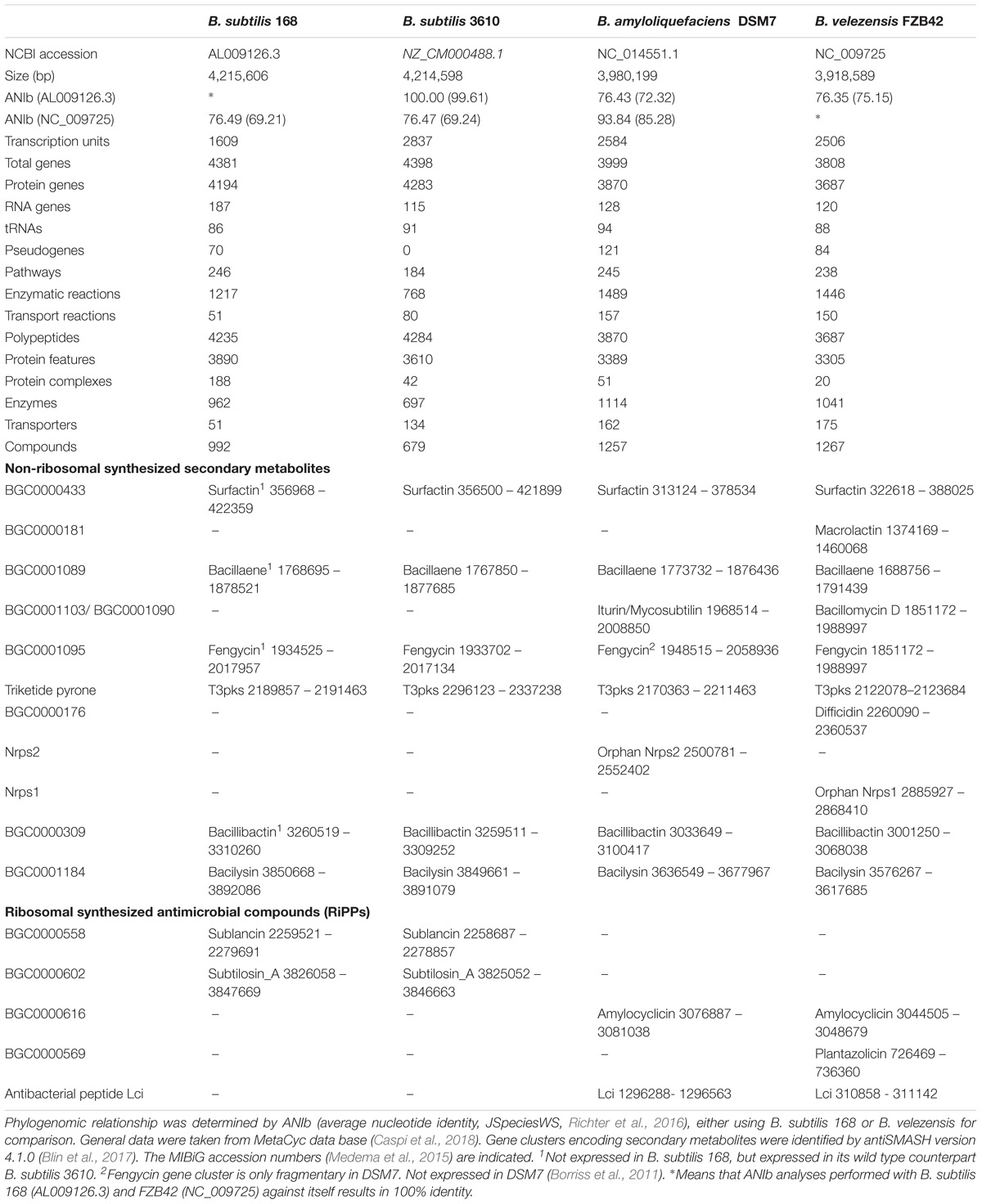
TABLE 1. Comparison of the genomes of Bacillus subtilis 168 (domesticated), Bacillus subtilis 3610 (wild type), Bacillus amyloliquefaciens DSM7 (non-plant associated), and Bacillus velezensis FZB42 (plant associated).
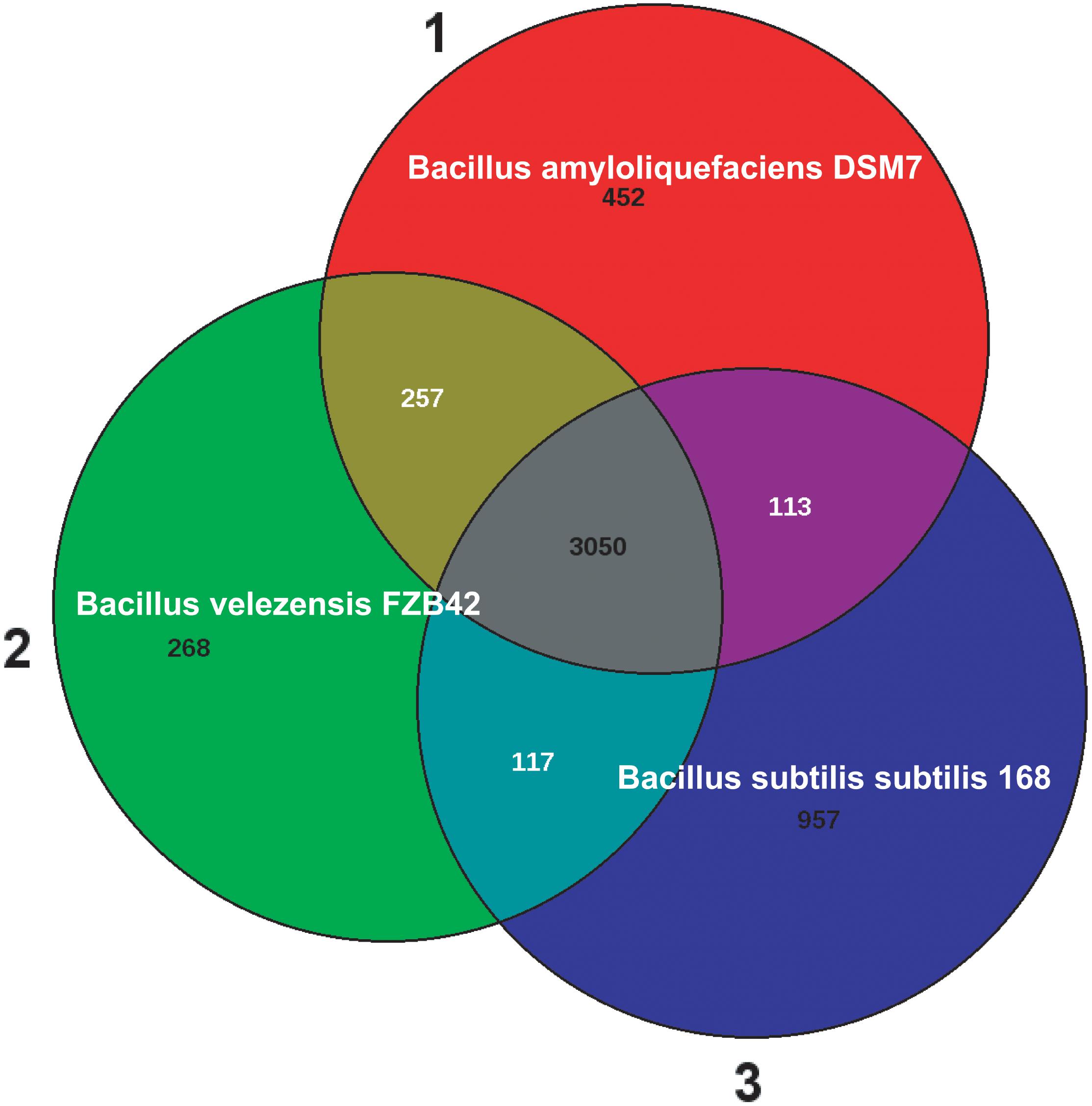
FIGURE 2. The Venn diagram of Bacillus amyloliquefaciens DSM7 (1), Bacillus velezensis FZB42 (2), and Bacillus subtilis subtilis 168 (3). The numbers of reciprocal best hits between subsets of genomes are shown. Note, 100% identical paralogous genes were not counted in the Venn diagram numbers (Blom et al., 2016). The three strains share 3050 genes according to the best hit calculation, whilst 268 genes were found unique in FZB42. A direct comparison between FZB42 and B. subtilis 168 revealed that they have 3122 genes in common, whilst 522 genes were found unique in FZB42.
Many genes, essential for a plant-associated lifestyle, are shared between B. subtilis 168 and FZB42 as well. Relevant examples are YfmS, a chemotaxis sensory transducer, which is involved in plant root colonization (Allard-Massicotte et al., 2017), and BlrA (formerly YtvA) a blue light receptor related to plant phototropins (Borriss et al., 2018). However, due to a century of ‘domestication’ under laboratory conditions, the type strain B. subtilis 168 has lost its ability to colonize roots and to control plant diseases. Its ability to form biofilms on solid surfaces (e.g., rhizoplane) is attenuated by several mutations detected in the genes sfp (necessary for production of lipopeptides and polyketides), epsC (required for extracellular polysaccharide synthesis), swrA (essential for swarming differentiation on solid surfaces), and degQ, which stimulates phosphorylation of DegU. By contrast, the closely related wild type B. subtilis 3610 forms robust biofilms and is able to produce antimicrobial compounds (Table 1). It was shown that by introducing wild type alleles of these four genes and the spo0F phosphatase encoding rapP gene, residing on a large plasmid occurring in B. subtilis 3610 but not in B. subtilis 168, the laboratory strain 168 forms biofilms which are essentially the same as in 3610. This demonstrates that domestication of B. subtilis 168 is only due to the four gene mutations mentioned above and loss of the plasmid occurring in strain 3610 (McLoon et al., 2011). Notably, FZB42 does not harbor a rapP containing plasmid, but is able to produce robust biofilm similar to B. subtilis 3610.
FZB42 releases several cellulases and hemicellulases degrading the external cellulosic and hemicellulosic substrates present in plant cell walls. Final products of enzymatic hydrolysis are free oligosaccharides, which act as elicitors of plant defense (Ebel and Scheel, 1997). Some genes encoding extracellular hydrolases, such as amyE (alpha-amylase), eglS (endo-1,4-β-glucanase), and xynA (xylanase) occurred only in the plant-associated representatives of the ‘B. amyloliquefaciens operational group’ but not in their soil-associated counterparts (Borriss et al., 2011; Zhang et al., 2016). Similarly, an operon involved in xylan degradation (xylA, xynP, xynB, xylR) is present in B. subtilis 168 and B. velezensis FZB42 but not in B. amyloliquefaciens DSM7T suggesting that both strains have in common some genes involved in plant macromolecule degradation (Rückert et al., 2011).
Bacillus velezensis harbored additional genes involved in hexuronate (galacturonate and fructuronate) degradation. Three genes were found unique for B. velezensis FZB42 and other members of this species: kdgK1, (2-dehydro-3-deoxygluconokinase), kdgA (2-dehydro-3-deoxyphosphogluconate aldolase), and the transcription regulator kdgR. They are part of the six-gene kdgKAR operon (He et al., 2012). In addition yjmD, a gene with putative galacticol-1-phosphate dehydrogenase function and two further genes: uxuA encoding mannonate dehydratase, and uxuB encoding mannonate oxidoreductase are part of the six-gene transcription unit. A second operon, containing the genes uxaC, uxaB, and uxaA encoding enzymes for metabolizing different hexuronates to D-altronate and D-fructuronate, occurs distantly from the kdgAR operon. In Escherichia coli K12 UxuA, KdgK, and KdgA are involved in a degradative pathway of aldohexuronates (Portalier et al., 1980). Whilst the complete biochemical pathway from galacturonate to KDG is present, no gene encoding D-glucuronate isomerase was detected, suggesting that B. velezensis is not able to metabolize D-glucuronate (He et al., 2012).
Nearly 10% of the FZB42 genome is involved in synthesizing antimicrobial compounds, such as the polyketides bacillaene, macrolactin and difficidin (Chen et al., 2006; Schneider et al., 2007) and the lipopeptides surfactin, bacillomycin D and fengycin (Koumoutsi et al., 2004). In total, the FZB42 genome harbors 13 gene clusters devoted to non-ribosomal and ribosomal synthesis of secondary metabolites with putative antimicrobial action. In two cases, in the nrs gene cluster and in the type III polyketide gene cluster their products are not identified till now (Table 1). Similar to B. subtilis 168T, the genome of the non-plant associated soil bacterium B. amyloliquefaciens DSM7T possesses a much lower number of gene clusters involved in synthesis of antimicrobial compounds than FZB42 (Table 1).
Notably, the gene clusters involved in non-ribosomal synthesis of the antifungal lipopeptides bacillomycin D and fengycin, and the polyketides difficidin and macrolactin are missing or fragmentary in DSM7T and other representatives of B. amyloliquefaciens suggesting that synthesis of these secondary metabolites might be important for the plant associated life style. Five out of a total of 13 gene clusters are located within variable regions of the FZB42 chromosome, suggesting that they might be acquired via horizontal gene transfer (Rückert et al., 2011). Most of them (bacillomycin D, macrolactin, difficidin, plantazolicin, and the orphan nrsA-F gene cluster) are without counterpart in DSM7T and B. subtilis 168T. Moreover, it has been shown experimentally that DSM7T, due to a deletion in the fengycin gene cluster, is unable to produce fengycin (Borriss et al., 2011), notably the gene cluster for synthesis of iturinA is present in the DSM7T genome (Table 1).
Besides type I PKS also genes encoding type III polyketide synthases are present in the genome of FZB42. By contrast to type I PKSs, type III PKSs catalyze priming, extension, and cyclization reactions iteratively to form a huge array of different polyketide products (Yu et al., 2012). In B. subtilis gene products of bspA-bspB operon were functionally characterized, and found to be involved in synthesis of triketide pyrones. The type III PKS BspA catalyzes synthesis of triketide pyrones and BspB (YpbQ) is a methyltransferase catalyzing its posttranslational modification to alkylpyrones ethers (Nakano et al., 2009). However, their biological role needs further elucidation. Orthologs of bspA and bspB are present in FZB42 and DSM7T (Table 1).
Another group of secondary metabolites are bacteriocins, which represent a class of post-translationally modified peptide antibiotics (Schnell et al., 1988). Together with peptides without antibiotic activity, they are generally termed RiPPs (ribosomally synthesized and post-translationally modified peptides). RiPP precursor peptides are usually bipartite, being composed of an N-terminal leader and C-terminal core regions. RiPP precursor peptides can undergo extensive posttranslational modification, yielding structurally and functionally diverse products (Burkhart et al., 2015). In recent years, two RiPPs with antibacterial activity (bacteriocins) were identified in FZB42: plantazolicin (Scholz et al., 2011) and amylocyclicin (Scholz et al., 2014).
An antibacterial substance still produced by a FZB42 mutant strain, unable to synthesize non-ribosomally any antimicrobial compound, was identified together with the gene cluster responsible for its biosynthesis. The pzn genes cluster encodes a small precursor peptide PznA that is post-translationally modified to contain thiazole and oxazole heterocycles. These rings are derived from Cys and Ser/Thr residues through the action of a modifying “BCD” synthetase complex, which consists of a cyclodehydratase (C), a dehydrogenase (B), and a docking protein (D) (Scholz et al., 2011). After modification and processing of the precursor peptide plantazolicin contains an unusual number of thiazoles and oxazoles (Kalyon et al., 2011). The structure variant plantazolicin A inhibits selectively Bacillus anthracis (Molohon et al., 2016), and is efficient against plant pathogenic nematodes (Liu et al., 2013), whilst the precursor molecule PZNB is inactive (Kalyon et al., 2011).
The head-to-tail cyclized bacteriocin amylocyclicin was firstly described in B. amyloliquefaciens FZB42 (Scholz et al., 2014). Circular bacteriocins are non-lanthionine containing bacteriocins with antimicrobial activity against Gram-positive food-borne pathogens (van Belkum et al., 2011). Amylocyclicin was highly efficient against Bacilli, especially against a sigW mutant of B. subtilis (Y2) (Butcher and Helmann, 2006). An orthologous gene cluster was also detected in B. amyloliquefaciens DSM7T (Table 1).
Lci was reported as an antimicrobial peptide synthesized by a B. subtilis strain with strong antimicrobial activity against plant pathogens, e.g., Xanthomonas campestris pv. oryzae and Pseudomonas solanacearum PE1. Its solution structure has a novel topology, containing a four-strand antiparallel β-sheet as the dominant secondary structure (Gong et al., 2011). The gene is not present in the B. subtilis 168 genome, but was detected in FZB42 and B. amyloliquefaciens DSM7T (Table 1). Lci was found highly expressed in FZB42 biofilms (Kröber et al., 2016).
Nowadays, global gene expression studies were increasingly performed to enlarge our knowledge base about effect of plants on gene expression in Gram-positive plant associated bacteria (Borriss, 2015a). The first combined transcriptome- and proteome analysis in Bacillus, using both, DNA-microarrays and 2-D protein gel electrophoresis, was conducted with B. subtilis 168 (Yoshida et al., 2001). Plant-bacteria interactions were studied with B. subtilis OKB105 in presence of rice seedlings. Transcriptome analysis revealed that expression of 176 bacterial genes was affected by the host plant (Shanshan et al., 2015).
In this context several studies were performed with FZB42, too. Transcription of many genes involved in carbon and amino acid metabolism was turned on, when maize root exudates were added to FZB42 cells growing in planktonic culture suggesting that nutrients present in root exudates are utilized by bacteria cells (Fan et al., 2012). Dependency of FZB42 from nutrient sources present in root exudates was corroborated in a second transcriptome study performed with DNA-microarrays. In this case root exudates with different composition obtained from maize plantlets growing under stress conditions (N, P, Fe, and K limitation) were used. In case of root exudates obtained from N-deprived maize plantlets containing decreased amounts of aspartate, valine and glutamate, FZB42 cells were found to be downregulated in transcription of genes involved in protein synthesis indicating a general stress response. By contrast, P-limited root exudates led to enhanced transcription of FZB42 genes involved in motility and chemotaxis, possibly suggesting a chemotactic response toward carbohydrates in root exudates (Carvalhais et al., 2013). Transcriptional profiling via RNA-sequencing in the taxonomically related B. velezensis SQR9 revealed that maize root exudates stimulated at first expression of metabolism-relevant genes and then genes involved in production of the extracellular matrix (Zhang et al., 2015).
Response of FZB42 on maize root exudates during late exponential and stationary growth phase was also investigated on the level of protein synthesis applying 2-D gel electrophoresis and MALDI TOF MS for protein identification. Elicitors of plant innate immunity such as flagellins, elongation factor Tu, and cold shock proteins were detected in the extracellular fluid (Kierul et al., 2015). Corresponding to the results obtained in our transcriptome studies, we found that the expression of genes involved in utilization of nutrients and transport was enhanced in presence of root exudates. The protein with the highest secretion in presence of maize root exudates was acetolactate synthase AlsS, an enzyme involved in post-exponential phase synthesis of acetoin and 2,3 butandiol (Kierul et al., 2015).
On the other hand, plants are also affected in their gene expression, when colonized by bacteria including representatives of the B. amyloliquefaciens operational group. Transcript analysis of rape seedlings confronted with a root-colonizing B. velezensis strain revealed that gene expression was more affected in leaves than in roots. Altogether the treatment caused a metabolic reprogramming in plant leaves (Bejai et al., 2009; Sarosh et al., 2009). Similar effects on plant gene expression were reported for root-colonizing B. subtilis FB17. A microarray study performed with Arabidopsis plantlets exposed to FB17 showed that expression of auxin-regulated genes and genes involved in metabolism, stress response and plant defense were up-regulated. Some Arabidopsis mutants deficient in three of the up-regulated genes, were found less colonized by FB17 (Lakshmanan et al., 2013). Further papers reporting about triggering of ISR response in plants by lipopeptides and VOCs from B. velezensis (Chowdhury et al., 2015b; Wu G. et al., 2018) were already discussed in a previous section.
Another study performed with FZB42 revealed that gene expression is dependent on life style. Ability to form biofilms is essential for colonizing plant root surfaces. Differential gene expression suggested that under biofilm-forming conditions transcription of 331 genes was increased and of 230 genes was decreased (Kröber et al., 2016).
The differential RNA-sequencing (dRNA-seq) technology was employed to unveil the structure of the FZB42 transcriptome (Fan et al., 2015). The unique feature of this technique is that two libraries split from the same RNA sample are compared. One library is subjected to terminator exonuclease that preferentially degrades processed RNAs with 5′-monophosphate group, thus primary transcripts with 5′-triphosphate group are enriched in relative terms (Sharma et al., 2010). Applying this method, we obtained the first global transcription start sites (TSSs) map of a PGPR Bacillus species. We determined a comprehensive transcriptome profile for FZB42 by identifying 4,877 TSSs for protein-coding genes. This includes >2,000 primary TSSs, >700 secondary TSSs, and nearly 200 orphan TSSs. The primary TSSs have been identified for 60% of all FZB42 genes. In addition, >1,300 internal TSSs and >1,400 antisense TSSs were also identified. A lot of coding genes were shown to be transcribed from multiple TSSs and perhaps own different UTRs. Some mRNAs contained overlapped transcripts (Fan et al., 2015). The global charting of FZB42 TSSs can favor the identification of promoter regions, cis-acting regulatory elements, and cognate transcriptional regulators.
By applying the dRNA-seq technique differentially expressed genes under different growth conditions were identified. For example, a large group of genes that are specifically regulated by root exudates during stationary growth were identified. The results obtained extended and corroborated our previous results obtained by using microarrays (Fan et al., 2012). Knowledge of the genes affected in their expression by plant root exudates contributes to our understanding of rhizobacterial physiology and its interaction with their host plants. They are listed as ‘Interaction with plants’ in AmyloWiki3. Moreover, this study allowed us to propose 46 previously unrecognized genes. 78 polycistronic transcripts covering 210 genes were identified and 10 previously mis-annotated genes were corrected (Fan et al., 2015).
Over the last decade, a growing number of non-coding regulatory small RNAs (sRNAs) have been identified in bacteria (Li et al., 2013), although the functions of most of them are still unknown. Most of sRNAs do not encode a protein, but function as an RNA regulator directly targeting multiple mRNAs. It is revealed that many sRNAs contribute to bacterial adaptation to changing environments and growth conditions (Thomason et al., 2012), therefore it is feasible to expect that sRNAs may also coordinate mutual effects of rhizobacteria on plants.
Besides graphing the profile of expressed protein-coding genes, dRNA-seq technology also offers a possibility to identify genome-wide sRNAs. We detected hundreds of non-coding RNAs in FZB42, including 136 antisense RNAs, 53 cis-encoded leader sequence or riboswitches, and 86 sRNA candidates (Fan et al., 2015). Among them 21 sRNAs were further validated by Northern blotting. According to their gene positions, the majority of the sRNAs perhaps act in-trans targeting the mRNAs encoded from a distant locus. Generally, sRNAs often binds to their target mRNAs, at 5′UTR in many cases, and thus modulate mRNA translation (Waters and Storz, 2009). Since the genome-wide TSS annotation of FZB42 informs about potential sRNA target sites of mRNAs, our study has provided a valuable basis for studying rhizobacterial sRNA regulation.
The function of the identified sRNAs has not been characterized in detail. However, some of the sRNAs were found related to a specific growth phase or to respond to environmental cues (soil extract or maize root exudates) (Fan et al., 2015). Furthermore, one sRNA was found to be involved in Bacillus sporulation and biofilm formation (data not shown). Since, sRNAs are more studied in Gram-negative than in Gram-positive bacteria, systematic detection of sRNAs in FZB42 extends our knowledge base about plant-associated Gram-positive bacteria, especially to rhizobacteria–plant interactions.
In recent years post-translational modifications (PTM) of proteins, such as protein phosphorylation, acetylation, methylation, and succinylation, attracted increasing attention due to their important physiological significance in organisms (Choudhary et al., 2014). Whereas most studies of PTM were performed in eukaryotic cells, nowadays the role of PTM in prokaryotes is increasingly investigated. Acetylation of lysine residues in FZB42 was studied using a combination of immune-affinity purification and high- resolution LC-MS/MS. A total of 3,268 acetylated lysine residues were detected in 1254 proteins, accounting for 32.9% of the entire proteins of FZB42. Remarkably, a high proportion (71.1 and 78.6%) of the proteins related to the synthesis of polyketides and lipopeptides were found acetylated. The finding implies an important role of lysine acetylation in the regulation of FZB42 antibiotic biosynthesis (Liu et al., 2016).
Using a similar technique, we profiled lysine malonylation of proteins in FZB42. In total, we identified 809 malonyl-lysine sites in 382 proteins (Figure 3). Lysine malonylation targets the proteins implicated in a wide range of biological functions, such as fatty acid biosynthesis and metabolism, central carbon metabolism, translation processes, and NAD(P) binding. A group of proteins involved in bacterium-plant interaction was also malonylated. Moreover, malonylation seems to occur on proteins with higher surface accessibility, although the significance of the site preference remains unclear. Similar to lysine acetylation, 33 polyketide synthases (PKS) and polypeptide synthetases (NRPS) involved in non-ribosomal synthesis of bacillaene, difficidin, macrolactin, and bacillomycinD, fengycin and surfactin, were found highly malonylated. They account for 8.6% of all malonylated proteins. The PKSs and NRPSs possessed 128 malonylation sites, averagely 3.8 sites per protein, which is significantly higher than the mean of 2.1 malonylation sites per protein. The polyketide synthases, BmyA, BaeM, BaeN, and BaeR contain more than 10 malonylation sites. BaeR is the most highly malonylated protein carrying 17 malonylation sites (Fan et al., 2017b,c).
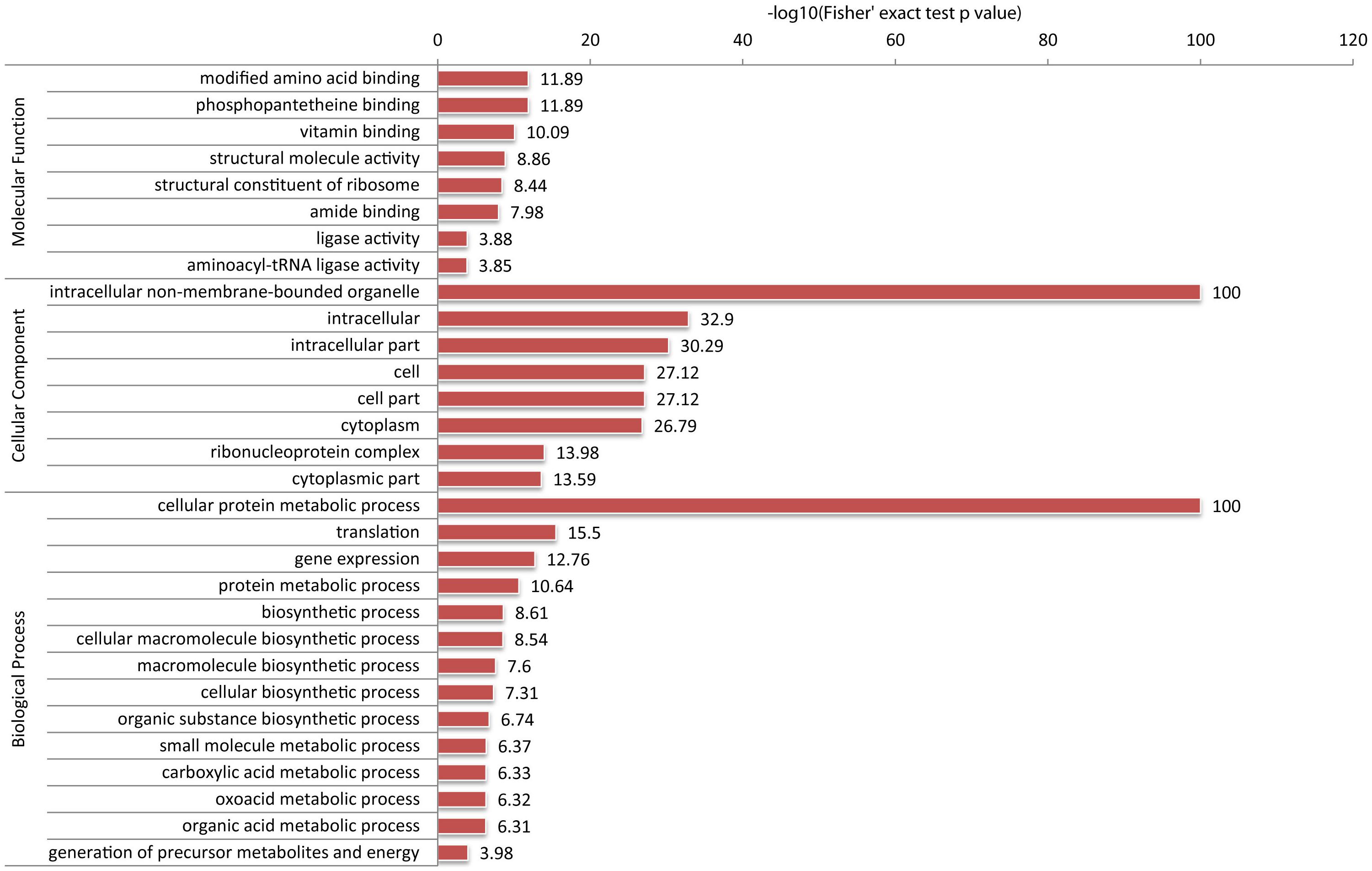
FIGURE 3. Distribution of FZB42 malonylated proteins in various functional categories according to the GO database. The ratio of Kmal sites located in the protein to all KmaI sites was compared with the ratio of malonylated proteins to all proteins in the database. The one-tailed Fisher’s exact test was used to test the enrichment and the result with p-value < 0.05 is considered significant.
Together with the data obtained for acetylation, the high malonylation rate of PKSs and NRPSs indicates a potential effect of protein modification on biosynthesis of antibiotics in FZB42. Better understanding of the underlying mechanism of how PTM affects PKSs and NRPSs may facilitate the development of FZB42 antibiotic production and application.
With the increasing reception of FZB42 as a model organism for Gram-positive PGPR, and in order to celebrate its whole genome sequencing around 10 years ago (Chen et al., 2007), we have established an integrated database ‘AmyloWiki’4 for collecting and gathering all the information known to date about this bacterium (Figure 4). More than 140 articles about FZB42 can be found in AmyloWiki5 and are in part assigned to the corresponding genes/proteins. AmyloWiki centers the achievement of FZB42 studies till now including diverse information such as its 3979 genes, its transcriptome structure, protein regulators and their targets. 595 genes of FZB42 involved in plant-bacteria interactions were listed6. It informs also about recently identified sRNA genes and post-translational modification sites (see previous sections). A growing list of FZB42-site directed mutant strains, available for scientific community, is also presented. AmyloWiki shares some features with SubtiWiki, the popular database for B. subtilis 168 (Zhu and Stülke, 2018); however, specific features of FZB42 such as genes not occurring in B. subtilis 168, and genes involved in antagonism against plant pathogens and plant-microbe interaction, are highlighted in AmyloWiki. To facilitate communication and information exchange, a growing list of groups studying FZB42 is available, and many possibilities for interactive data exchange and feedback with the users are given.
AmyloWiki is configured to be a comprehensive and user-friendly database, built upon typical XAMPP (X-Windows, Linux or Mac OS + Apache + MySQL + PHP + Perl) environment. Apache 2.4.23 was used to construct a webserver. All data sets were processed and stored in MySQL (5.0.11). PHP language (version 5.6.28) was used to built database management system and interface. Webpages were designed with HTML5, CSS3 and JavaScript techniques. AmyloWiki provides a series of functions such as data submission, resource downloading, searching, advanced retrieval, and feedback.
Briefly, most information of user’s interest can be returned by performing a searching. User can search with different of the query strings, such as gene name, gene locus, and PubMed ID. The items that matched the query string will be returned in the result page. This can be exemplified by searching a gene, as happens most often. The basic information of the gene such as its product, locus, synonyms, homolog in B. subtilis, position, length and others, will be provided on the top of the result page. The genomic context of the gene can be viewed in a visualized window with scrollable function to check its neighbor genes. The organization of the gene, if it is present in an operon, the functions the gene involved, and its functional categories/subcategories are offered next. Other associated information includes the phenotypes of the mutant, its transcriptional start sites, protein/non-coding RNA regulators, sigma factors, PTM sites and so on. The references concerning the gene are listed at the bottom of the retrieval page.
For the convenience of the user, all datasets of AmyloWiki can be downloaded at the “Download” page. The data can be downloaded in an Excel-compatible format for their specific analysis. AmyloWiki will be maintained by us with a frequent update to improve its configuration and to keep the information comprehensive. For example, it is planned to add in future experimental protocols specifically worked out and used for FZB42, like transformation and bioassay. Here, support given by experienced groups dealing with FZB42 is highly welcomed. The pages for data submission and correction are designed for authorized users in order to update relevant information. Unauthorized users are encouraged to submit their latest data via E-mail to the authors of the website. Then their information will be verified and included in AmyloWiki.
In order to improve consistency in performance of bioinoculants in a sustainable agriculture we have to integrate them as part of modern crop management programs allowing to decrease the amount of agrochemicals, including harmful chemopesticides. A full understanding of the complex relationship between plant, soil, climate, microbiota, and the microbial inoculant is a necessary precondition for application success of biologicals. Basic research which has been restricted in past to selected representatives of taxonomic groups (‘model organisms’) such as B. subtilis 168, E. coli K12, Saccharomyces cerevisiae, and Drosophila melanogaster has considerably deepen our understanding of those groups in general. We recommend using FZB42 as a model for research on Gram-positive rhizobacteria. This will greatly enhance scientific progress in the field and might contribute to a better consistency in application of environmental friendly beneficial Bacilli in modern agriculture. After 20 years of basic and applied research FZB42 has been proven as suitable for selecting to this task. The following features favor use of FZB42 as model organism:
(1) Apathogenicity: Concerning biosafety issues, no representatives of the B. subtilis species complex including B. velezensis have been listed as risk group in ‘The Approved List of biological agents’ (Advisory Committee on Dangerous Pathogens, 2013). However, B. cereus and B. anthracis were listed in human pathogen hazard group 3, excluding their use as biocontrol agents in agriculture.
(2) long term successful application of a commercialized product based on FZB42 in agriculture
(3) genetic amenable and a large collection of defined mutant is available for interested scientists
(4) large body of scientific knowledge (>140 articles) about FZB42 is already available
(5) an integrative data base ‘AmyloWiki’ has been established about FZB42 aimed to enhance collaboration of several groups dealing with Gram-positive PGPR and biocontrol bacteria
Most of the biocontrol agents currently in use are based on living microbes. Representatives of the B. subtilis species complex, including B. velezensis, B. subtilis, and B. pumilus are increasingly used for commercial production of biofungicides (Borriss, 2016). Most of them are stabilized liquid suspensions or dried formulations prepared from durable endo-spores. They are developed for seed coating, soil or leave application. Unfortunately, it is very unlikely that concentration of Bacillus synthesized cyclic lipopeptides in their natural environment is sufficient for antibiosis (Debois et al., 2014). A possibility for circumventing this problem are combined bioformulations consisting of both, Bacillus spores and antagonistic acting metabolites. However, only a few bioformulations currently on the market, such as SERENADE® prepared from B. subtilis QST713 and Double Nickel 55 prepared from B. amyloliquefaciens D747 (both strain names need to be corrected as B. velezensis, Fan et al., 2017a), contain together with living spores antimicrobial compounds, such as cyclic lipopeptides (iturins, fengycin). Unfortunately, also in these products only the number of spores is declared as active ingredient of the biofungicide, but concentration of the metabolites is not indicated, excluding an exact treatment of pathogen infected plant parts. Labeling a fixed concentration of the active principle for suppressing the target would allow a better comparison of chemical and biological pesticides (Borriss, 2015b). To the best of our knowledge, no bioformulations containing exclusively antimicrobial metabolites are commercially available, although companies like ABiTEP performed extended large scale trials with concentrated and stabilized Bacillus supernatants in order to suppress plant pathogens.
BF and RB outlined and wrote the manuscript. BF, CW, XLD, XFS, and RB developed the integrated data base AmyloWiki. LW, HW, and XG contributed essential scientific results reported in this review. All authors have approved and corrected the final version of the manuscript.
The financial support by the National Natural Science Foundation of China (Nos. 61571223, 61171191, and 31100081), Natural Science Foundation of Jiangsu Province (No. BK20151514), and Key Program for Natural Science of the Higher Education Institutions in Jiangsu Province (No. 17KJA220001) is gratefully acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This article is dedicated to Prof. Yoav Bashan, founder and president of the Bashan foundation, a great scientist and a great personality, who passed away unexpectedly on September 20th 2018. We thank Jörg Stülke and BingYao Zhu, University of Göttingen, for help and the permission to follow the famous SubtiWiki frame in order to present the genomic data known for FZB42 in AmyloWiki. We are thankful for any comment aimed to improve this website.
Advisory Committee on Dangerous Pathogens (2013). The Approved List of Biological Agents. Available at: www.hse.gov.uk/pubns/misc208.htm
Allard-Massicotte, R., Tessier, L., Lécuyer, F., Lakshmanan, V., Lucier, J. F., Garneau, D., et al. (2017). Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio 7:e01664-16. doi: 10.1128/mBio.01664-16
Bejai, S. R., Danielsson, J., and Meijer, J. (2009). Transcript profiling of oilseed rape (Brassica napus) primed for biocontrol differentiate genes involved in microbial interactions with beneficial Bacillus amyloliquefaciens from pathogenic Botrytis cinerea. Plant Mol. Biol. 70, 31–45. doi: 10.1007/s11103-009-9455-4
Blin, K., Medema, M. H., Kottmann, R., Lee, S. Y., and Weber, T. (2017). The antiSMASH database, a comprehensive database of microbial secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 45, D555–D559. doi: 10.1093/nar/gkw960
Blom, J., Kreis, J., Spänig, S., Juhre, T., Bertelli, C., Ernst, C., et al. (2016). EDGAR 2.0: an enhanced software platform for comparative gene content analyses. Nucleic Acids Res. 44, W22–W28. doi: 10.1093/nar/gkw255
Borriss, R. (2011). “Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents,” in Bacteria in Agrobiology: Plant Growth Responses, ed. D. K. Maheshwari (Berlin: Springer), 41–76. doi: 10.1007/978-3-642-20332-9
Borriss, R. (2015a). Transcriptome and proteome profiling for analyzing fates of global gene expression inplant-beneficial Bacilli. Transcriptomics 3:e110. doi: 10.4172/2329-8936.1000e110
Borriss, R. (2015b). “Towards a new generation of commercial microbial disease control and plant growth promotion products,” in Principles of Plant-Microbe Interactions. Microbes for Sustainable Agriculture, ed. B. Lugtenberg (Berlin: Springer), 329–337. doi: 10.1007/978-3-319-08575-3
Borriss, R. (2016). “Phytostimulation and biocontrol by the plant-associated Bacillus amyloliquefaciens FZB42: an update,” in Bacilli and Agrobiotechnology, ed. A. Aeron (Berlin: Springer Int. Publ), 163–184.
Borriss, R., Chen, X., Rueckert, C., Blom, J., Becker, A., Baumgarth, B., et al. (2011). Relationship of Bacillus amyloliquefaciens clades associated with strains DSM7T and FZB42: a proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on their discriminating complete genome sequences. Int. J. Syst. Evol. Microbiol. 61, 1786–1801. doi: 10.1099/ijs.0.023267-0
Borriss, R., Danchin, A., Harwood, C. R., Médigue, C., Rocha, E. P. C., Sekowska, A., et al. (2018). Bacillus subtilis, the model gram-positive bacterium: 20 years of annotation refinement. Microb. Biotechnol. 11, 3–17. doi: 10.1111/1751-7915.13043
Broadbent, P., Baker, K., Franks, N., and Holland, J. (1977). Effect of Bacillus spp. on increased growth of seedlings in steamed and in nontreated soil. Phytopathology 67, 1027–1034. doi: 10.1094/Phyto-67-1027
Burkhart, B. J., Hudson, G. A., Dunbar, K. L., and Mitchell, D. A. (2015). A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat. Chem. Biol. 11, 564–570. doi: 10.1038/nchembio.1856
Burr, T. J., Schroth, M. N., and Suslow, T. (1978). Increased potato yields by treatment of seedpieces with specific strains of Pseudomonas fluorescens and Pseudomonas putida. Phytopathology 68, 1377–1383. doi: 10.1094/Phyto-68-1377
Butcher, B. G., and Helmann, J. D. (2006). Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol. Microbiol. 60, 765–782. doi: 10.1111/j.1365-2958.2006.05131.x
Caron, A. (1897). Culture of bacteria, Albert Caron of Haus Ellenbach, Germany, Assignor to the Farbenfabriken of Elberfeld Co. U.S. Patent No 679600. Washington, DC: United States Patent and Trademark Office.
Carvalhais, L. C., Dennis, P. G., Fan, B., Fedoseyenko, D., Kierul, K., Becker, A., et al. (2013). Linking plant nutritional status to plant-microbe interactions. PLoS One 8:e68555. doi: 10.1371/journal.pone.0068555
Caspi, R., Billington, R., Fulcher, C. A., Keseler, I. M., Kothari, A., Krummenacker, M., et al. (2018). The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 46, D633–D639. doi: 10.1093/nar/gkx935
Chen, X. H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., et al. (2007). Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 25, 1007–1014. doi: 10.1038/nbt1325
Chen, X. H., Vater, J., Piel, J., Franke, P., Scholz, R., Schneider, K., et al. (2006). Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. J. Bacteriol. 188, 4024–4036. doi: 10.1128/JB.00052-06
Choudhary, C., Weinert, B. T., Nishida, Y., Verdin, E., and Mann, M. (2014). The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550. doi: 10.1038/nrm3841
Chowdhury, S. P., Dietel, K., Rändler, M., Schmid, M., Junge, H., Borriss, R., et al. (2013). Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS One 8:e68818. doi: 10.1371/journal.pone.0068818
Chowdhury, S. P., Hartmann, A., Gao, X., and Borriss, R. (2015a). Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 - a review. Front. Microbiol. 6:780. doi: 10.3389/fmicb.2015.00780
Chowdhury, S. P., Uhl, J., Grosch, R., Alquéres, S., Pittroff, S., Dietel, K., et al. (2015b). Cyclic lipopeptides of Bacillus amyloliquefaciens FZB42 subsp. plantarum colonizing the lettuce rhizosphere enhance plant defence responses towards the bottom rot pathogen Rhizoctonia solani. Mol. Plant Microbe Interact. 28, 984–995. doi: 10.1094/MPMI-03-15-0066-R
Debois, D., Jourdan, E., Smargiasso, N., Thonart, P., de Pauw, E., and Ongena, M. (2014). Spatiotemporal monitoring of the antibiome secreted by Bacillus biofilms on plant roots using MALDI Mass Spectrometry imaging. Anal. Chem. 86, 4431–4438. doi: 10.1021/ac500290s
Duitman, E. H., Hamoen, L. W., Rembold, M., Venema, G., Seitz, H., Saenger, W., et al. (1999). The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl. Acad. Sci. U.S.A. 96, 13294–13299. doi: 10.1073/pnas.96.23.13294
Dunlap, C. A., Kim, S. J., Kwon, S. W., and Rooney, A. P. (2016). Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp plantarum and ’Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int. J. Syst. Evol. Microbiol. 24:000858. doi: 10.1099/ijsem.0.000858
Elanchezhiyan, K., Keerthana, U., Nagendran, K., Prabhukarthikeyan, S. R., Prabakar, K., Raguchander, T., et al. (2018). Multifaceted benefits of Bacillus amyloliquefaciens strain FZB24 in the management of wilt disease in tomato caused by Fusarium oxysporum f. sp. lycopersici. Physiol. Mol. Plant Pathol. 103, 92–101. doi: 10.1016/j.pmpp.2018.05.008
Erlacher, A., Cardinale, M., Grosch, R., Grube, M., and Berg, G. (2014). The impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Front. Microbiol. 5:175. doi: 10.3389/fmicb.2014.00175
Fan, B., Blom, J., Klenk, H. P., and Borriss, R. (2017a). Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “Operational Group B. amyloliquefaciens” within the B. subtilis Species Complex. Front. Microbiol. 8:22. doi: 10.3389/fmicb.2017.00022
Fan, B., Li, Y.-L., Li, L., Peng, X.-J., Bu, C., Wu, X.-Q., et al. (2017b). Malonylome analysis of rhizobacterium Bacillus amyloliquefaciens FZB42 reveals involvement of lysine malonylation in polyketide synthesis and plant-bacteria interactions. J. Proteomics 154, 1–12. doi: 10.1016/j.jprot.2016.11.022
Fan, B., Li, Y.-L., Li, L., Peng, X.-J., Bu, C., Wu, X.-Q., et al. (2017c). Malonylome of the plant growth promoting rhizobacterium with potent biocontrol activity, Bacillus amyloliquefaciens FZB42. Data Brief 10, 548–550. doi: 10.1016/j.dib.2016.12.029
Fan, B., Carvalhais, L. C., Becker, A., Fedoseyenko, D., von, Wirén N, and Borriss, R. (2012). Transcriptomic profiling of Bacillus amyloliquefaciens FZB42 in response to maize root exudates. BMC Microbiol. 12:116. doi: 10.1186/1471-2180-12-116
Fan, B., Li, L., Chao, Y., Forstner, K., Vogel, J., Borriss, R., et al. (2015). dRNA-Seq reveals genomewide TSSs and noncoding RNAs of plant beneficial rhizobacterium Bacillus amyloliquefaciens FZB42. PLoS One 10:e0142002. doi: 10.1371/journal.pone.0142002
Fritze, D. (2004). Taxonomy of the genus Bacillus and related genera: the aerobic, endospore-forming bacteria. Phytopathology 94, 1245–1248. doi: 10.1094/PHYTO.2004.94.11.1245
Gong, W., Wang, J., Chen, Z., Xia, B., and Lu, G. (2011). Solution structure of LCI, a novel antimicrobial peptide from Bacillus subtilis. Biochemistry 50, 3621–3627. doi: 10.1021/bi200123w
Goodson, J. R., Klupt, S., Zhang, C., Straight, P., and Winkler, W. C. (2017). LoaP is a broadly conserved antiterminator protein that regulates antibiotic gene clusters in Bacillus amyloliquefaciens. Nat. Microbiol. 2:17003. doi: 10.1038/nmicrobiol.2017.3
Gordon, R. E., Haynes, W. C., and Pang, C. H. N. (1973). The Genus Bacillus. Washington, DC: United States Department of Agriculture.
Gu, Q., Yang, Y., Yuan, Q., Shi, G., Wu, L., Lou, Z., et al. (2017). Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the plant-pathogenic fungus Fusarium graminearum. Appl. Environ. Microbiol. 83:e01075-17. doi: 10.1128/AEM.01075-17
Hao, H.-T., Zhao, X., Shang, Q.-H., Wang, Y., Guo, Z.-H., Zhang, Y.-B., et al. (2016). Comparative digital gene expression analysis of the Arabidopsis response to volatiles emitted by Bacillus amyloliquefaciens. PLoS One 11:0158621. doi: 10.1371/journal.pone.0158621
He, P., Hao, K., Blom, J., Rückert, C., Vater, J., Mao, Z., et al. (2012). Genome sequence of the plant growth promoting strain Bacillus amyloliquefaciens subsp. plantarum B9601-Y2 and expression of mersacidin and other secondary metabolites. J. Biotechnol. 164, 281–291. doi: 10.1016/j.jbiotec.2012.12.014
Idriss, E. E., Makarewicz, O., Farouk, A., Rosner, K., Greiner, R., Bochow, H., et al. (2002). Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology 148, 2097–2109. doi: 10.1099/00221287-148-7-2097
Kalyon, B., Helaly, S. E., Scholz, R., Nachtigall, J., Vater, J., Borriss, R., et al. (2011). Plantazolicin A and B: structure of ribosomally synthesized thiazole/oxazole peptides from Bacillus amyloliquefaciens FZB42. Org. Lett. 13, 2996–2999. doi: 10.1021/ol200809m
Kierul, K., Voigt, B., Albrecht, D., Chen, X. H., Carvalhais, L. C., and Borriss, R. (2015). Influence of root exudates on the extracellular proteome of the plant-growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Microbiology 161, 131–147. doi: 10.1099/mic.0.083576-0
Kloepper, J. W., Leong, J., Teintze, M., and Schroth, M. N. (1980). Enhancing plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286, 885–886. doi: 10.1038/286885a0
Kloepper, J. W., Ryu, C. M., and Zhang, S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94, 1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259
Kolbe, W. (1993). Bakterien und Brache im Haushalt der Natur, ed. Dr. W. A. Kolbe Burscheid, Berlin: Verlag.
Koumoutsi, A., Chen, X. H., Henne, A., Liesegang, H., Hitzeroth, G., Franke, P., et al. (2004). Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 186, 1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004
Krebs, B., Höding, B., Kübart, S., Workie, M. A., Junge, H., Schmiedeknecht, G., et al. (1998). Use of Bacillus subtilis as biocontrol agent. I. Activities and characterization of Bacillus subtilis strains. J. Plant Dis. Prot. 105, 181–197.
Kröber, M., Verwaaijen, B., Wibberg, D., Winkler, A., Pühler, A., and Schlüter, A. (2016). Comparative transcriptome analysis of the biocontrol strain Bacillus amyloliquefaciens FZB42 as response to biofilm formation analyzed by RNA sequencing. J. Biotechnol. 10, 212–223. doi: 10.1016/j.jbiotec.2016.06.013
Kröber, M., Wibberg, D., Grosch, R., Eikmeyer, F., Verwaaijen, B., Chowdhury, S. P., et al. (2014). Effect of the strain Bacillus amyloliquefaciens FZB42 on the microbial community in the rhizosphere of lettuce under field conditions analyzed by whole metagenome sequencing. Front. Microbiol. 5:252. doi: 10.3389/fmicb.2014.00252
Lakshmanan, V., Castaneda, R., Rudrappa, T., and Bais, H. P. (2013). Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta 238, 657–668. doi: 10.1007/s00425-013-1920-2
Li, L., Huang, D., Cheung, M. K., Nong, W., Huang, Q., and Kwan, H. S. (2013). BSRD: a repository for bacterial small regulatory RNA. Nucleic Acids Res. 41, D233–D238. doi: 10.1093/nar/gks1264
Liu, L., Wang, G., Song, L., Lv, B., and Liang, W. (2016). Acetylome analysis reveals the involvement of lysine acetylation in biosynthesis of antibiotics in Bacillus amyloliquefaciens. Sci. Rep. 29:20108. doi: 10.1038/srep20108
Liu, Z., Budiharjo, A., Wang, P., Shi, H., Fang, J., Borriss, R., et al. (2013). The highly modified microcin peptide plantazolicin is associated with nematicidal activity of Bacillus amyloliquefaciens FZB42. Appl. Microbiol. Biotechnol. 97, 10081–10090. doi: 10.1007/s00253-013-5247-5
McLoon, A. L., Guttenplan, S. B., Kearns, D. B., Kolter, R., and Losick, R. (2011). Tracing the domestication of a biofilm-forming bacterium. J. Bacteriol. 193, 2027–2034. doi: 10.1128/JB.01542-10
Medema, M. H., Kottmann, R., Yilmaz, P., Cummings, M., Biggins, J. B., Blin, K., et al. (2015). Minimum information about a biosynthetic gene cluster. Nat. Chem. Biol. 11, 625–631. doi: 10.1038/nchembio.1890
Molohon, K. J., Blair, P. M., Park, S., Doroghazi, J. R., Maxson, T., Hershfield, J. R., et al. (2016). Plantazolicin is an ultra-narrow spectrum antibiotic that targets the Bacillus anthracis membrane. ACS Infect Dis. 2, 207–220. doi: 10.1021/acsinfecdis.5b00115
Nakano, C., Ozawa, H., Akanuma, G., Funa, N., and Horinouchi, S. (2009). Biosynthesis of aliphatic polyketides by type III polyketide synthase and methyltransferase in Bacillus subtilis. J. Bacteriol. 191, 4916–4923. doi: 10.1128/JB.00407-09
Portalier, R., Robert-Baudouy, J., and Stoeber, F. (1980). Regulation of Escherichia coli K-12 hexauronate system genes: exu regulon. J. Bacteriol. 143, 1095–1107.
Priest, F. G., Goodfellow, M., Shute, L. A., and Berkeley, W. (1987). Bacillus amyloliquefaciens sp. nov., nom. rev. Int. J. Syst. Bact. 37, 69–71. doi: 10.1099/00207713-37-1-69
Rahman, A., Uddin, W., and Wenner, N. G. (2015). Induced systemic resistance responses in perennial ryegrass against Magnaporthe oryzae elicited by semi-purified surfactin lipopeptides and live cells of Bacillus amyloliquefaciens. Mol. Plant Pathol. 16, 546–558. doi: 10.1111/mpp.12209
Reva, O. N., Dixelius, C., Meijer, J., and Priest, F. G. (2004). Taxonomic characterization and plant colonizing abilities of some bacteria related to Bacillus amyloliquefaciens and Bacillus subtilis. FEMS Microbiol. Ecol. 48,249–259. doi: 10.1016/j.femsec.2004.02.003
Richter, M., Rosselló-Móra, R., Glöckner, F. O., and Peplies, J. (2016). JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32, 929–931. doi: 10.1093/bioinformatics/btv681
Rückert, C., Blom, J., Chen, X. H., Reva, O., and Borriss, R. (2011). Genome sequence of Bacillus amyloliquefaciens type strain DSM7T reveals differences to plant-associated Bacillus amyloliquefaciens FZB42. J. Biotechnol. 155, 78–85. doi: 10.1016/j.jbiotec.2011.01.006
Ryu, C.-M., Farag, M. A., Hu, C.-H., Reddy, M. S., Kloepper, J. W., and Pare, P. W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134, 1017–1026. doi: 10.1104/pp.103.026583
Sarosh, B. R., Danielsson, J., and Meijer, J. (2009). Transcript profiling of oilseed rape (Brassica napus) primed for biocontrol differentiate genes involved in microbial interactions with beneficial Bacillus amyloliquefaciens from pathogenic Botrytis cinerea. Plant Mol. Biol. 70, 31–45. doi: 10.1007/s11103-009-9455-4
Schmiedeknecht, G., Bochow, H., and Junge, H. (1998). Use of Bacillus subtilis biocontrol agent. II. Biological control of potato diseases. Z. Pflanzenkr. Pflanzenschutz 1998, 376–386.
Schneider, K., Chen, X. H., Vater, J., Franke, P., Nicholson, G., Borriss, R., et al. (2007). Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J. Nat. Prod. 70, 1417–1423. doi: 10.1021/np070070k
Schnell, N., Entian, K. D., Schneider, U., Götz, F., Zähner, H., Kellner, R., et al. (1988). Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333, 276–278. doi: 10.1038/333276a0
Scholz, R., Molohon, K. J., Nachtigall, J., Vater, J., Markley, A. L., Süssmuth, R. D., et al. (2011). Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J. Bacteriol. 193, 215–224. doi: 10.1128/JB.00784-10
Scholz, R., Vater, J., Budiharjo, A., Wang, Z., He, Y., Dietel, K., et al. (2014). Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 196, 1842–1852. doi: 10.1128/JB.01474-14
Shanshan, X., Wu, H., Chen, L., Zang, H., Xie, Y., and Gao, X. (2015). Transcriptome profiling of Bacillus subtilis OKB105 in response to rice seedlings. BMC Microbiol. 15:21. doi: 10.1186/s12866-015-0353-4
Sharifi, R., and Ryu, C. M. (2016). Are bacterial volatile compounds poisonous odors to a fungal pathogen Botrytis cinerea, alarmsignals to Arabidopsis seedlings for eliciting induced resistance, or both? Front. Microbiol. 7:196. doi: 10.3389/fmicb.2016.00196
Sharma, C. M., Hoffmann, S., Darfeuille, F., Reignier, J., Findeiss, S., Sittka, A., et al. (2010). The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464, 250–255. doi: 10.1038/nature08756
Shen, B. (2003). Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr. Opin. Chem. Biol. 7, 285–295. doi: 10.1016/S1367-5931(03)00020-6
Sylla, J., Alsanius, B. W., Krüger, E., Reineke, A., Strohmeier, S., and Wohanka, W. (2013). Leaf microbiota of strawberries as affected by biological control agents. Phytopathology 103, 1001–1011. doi: 10.1094/PHYTO-01-13-0014-R
Tahir, H. A. S., Gu, Q., Wu, H., Niu, Y., Huo, R., and Gao, X. (2017). Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 7:40481. doi: 10.1038/srep40481
Talboys, P. J., Owen, D. W., Healey, J. R., Withers, P. J., and Jones, D. L. (2014). Auxin secretion by Bacillus amyloliquefaciens FZB42 both stimulates root exudation and limits phosphorus uptake in Triticum aestivum. BMC Plant Biol. 14:51. doi: 10.1186/1471-2229-14-51
Thomason, M. K., Fontaine, F., De Lay, N., and Storz, G. (2012). A small RNA that regulates motility and biofilm formation in response to changes in nutrient availability in Escherichia coli. Mol. Microbiol. 84, 17–35. doi: 10.1111/j.1365-2958.2012.07965.x
van Belkum, M. J., Martin-Visscher, L. A., and Vederas, J. C. (2011). Structure and genetics of circular bacteriocins. Trends Microbiol. 19, 411–418. doi: 10.1016/j.tim.2011.04.004
Walsh, C. T. (2004). Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science 303, 1805–1810. doi: 10.1126/science.1094318
Waters, L. S., and Storz, G. (2009). Regulatory RNAs in bacteria. Cell 136, 615–628. doi: 10.1016/j.cell.2009.01.043
Wipat, A., and Harwood, C. R. (1999). The Bacillus subtilis genome sequence: the molecular blueprint of a soil bacterium. FEMS Microbiol. Ecol. 28, 1–9. doi: 10.1111/j.1574-6941.1999.tb00555.x
Wu, G., Liu, Y., Xu, Y., Zhang, G., Shen, Q., and Zhang, R. (2018). Exploring elicitors of the beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 to induce plant systemic resistance and their interactions with plant signaling pathways. Mol. Plant Microbe Interact. 5, 560–567. doi: 10.1094/MPMI-11-17-0273-R
Wu, L., Huang, Z., Li, X., Ma, L., Gu, Q., Wu, H., et al. (2018). Stomatal closure and SA-, JA/ET- signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Front. Microbiol. 9:847. doi: 10.3389/fmicb.2018.00847
Yao, A. V., Bochow, H., Karimov, S., Boturov, U., Sanginboy, S., and Sharipov, A. K. (2006). Effect of FZB 24 Bacillus subtilis as a biofertilizer on cotton yields in field tests. Arch. Phytopathol. Plant Prot. 39, 323–328. doi: 10.1080/03235400600655347
Yoshida, K., Kobayashi, K., Miwa, Y., Kang, C. M., Matsunaga, M., Yamaguchi, H., et al. (2001). Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res. 29, 683–692. doi: 10.1093/nar/29.3.683
Yu, D., Xu, F., Zeng, J., and Zhan, J. (2012). Type III polyketide synthases in natural product biosynthesis. UBMB Life 64, 285–295. doi: 10.1002/iub.1005
Zhang, N., Yang, D., Kendall, J. R. A., Borriss, R., Druzhinina, I. S., Kubicek, C. P., et al. (2016). Comparative genomic analysis of Bacillus amyloliquefaciens and Bacillus subtilis reveals evolutional traits for adaptation to plant-associated habitats. Front. Microbiol. 7:2039. doi: 10.3389/fmicb.2017.00022
Zhang, N., Yang, D., Wang, D., Miao, Y., Shao, J., Zhou, X., et al. (2015). Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genomics 16:685. doi: 10.1186/s12864-015-1825-5
Keywords: Bacillus velezensis, FZB42, AmyloWiki, induced systemic resistance (ISR), non-ribosomal synthesized lipopeptides (NRPS), non-ribosomal synthesized polyketides (PKS), volatiles, plant growth promoting bacteria (PGPR)
Citation: Fan B, Wang C, Song X, Ding X, Wu L, Wu H, Gao X and Borriss R (2018) Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 9:2491. doi: 10.3389/fmicb.2018.02491
Received: 15 June 2018; Accepted: 28 September 2018;
Published: 16 October 2018.
Edited by:
Essaid Ait Barka, Université de Reims Champagne-Ardenne, FranceReviewed by:
Gerardo Puopolo, Fondazione Edmund Mach, ItalyCopyright © 2018 Fan, Wang, Song, Ding, Wu, Wu, Gao and Borriss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ben Fan, ZmFuYmVuMjAwMEBnbWFpbC5jb20= Rainer Borriss, cmFpbmVyLmJvcnJpc3NAcnouaHUtYmVybGluLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.