- 1Division of Virology, Department of Microbiology and Immunology, Institute of Medical Science, University of Tokyo, Tokyo, Japan
- 2State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin, China
- 3TAUNS Laboratories, Inc., Shizuoka, Japan
- 4Influenza Research Institute, Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI, United States
- 5Department of Special Pathogens, International Research Center for Infectious Diseases, Institute of Medical Science, University of Tokyo, Tokyo, Japan
Since the spring of 2013, human infections with H7N9 viruses have been detected in China. Some of these viruses have become highly pathogenic. Highly and low pathogenic avian influenza H7N9 viruses are currently co-circulating with the seasonal influenza A viruses H3N2 and H1N1pdm09. Prompt identification and isolation of H7N9 patients is one measure to prevent the spread of H7N9 virus and help prevent a pandemic. The majority of commercially available point-of-care rapid influenza diagnostic kits can differentiate between influenza A and B viruses, but cannot distinguish between H7N9 viruses and seasonal influenza A viruses. Accordingly, we have developed a rapid diagnostic kit specific for the H7 subtype that is accessible, easy to use. Although the detection limit of this H7 kit is one-tenth lower than that of a commercially available rapid influenza A and B diagnostic kit of similar design, except for the specificity of the monoclonal antibodies used, this kit is highly specific, detecting only H7-subtype influenza viruses, including the recent highly pathogenic H7N9 viruses from humans, and does not show any non-specific reactions with other HA subtypes. This H7 kit will be of value for the early detection of H7N9-infected patients.
Introduction
Human infections with low pathogenic avian influenza (LPAI) H7N9 virus were first reported in the spring of 2013 in China (Centers for Disease Control and Prevention, 2013; Gao R. et al., 2013). As of 13 February 2018, 1625 human cases of infection and 621 deaths have been attributed to this virus (Food and Agriculture Organization of the United Nations, 2018). The major sources of these human cases are believed to be H7N9 virus-infected live poultry or contaminated environments, especially live poultry markets (Gao R. et al., 2013; Shi et al., 2013; Zhang et al., 2013; Yu et al., 2014; Lam et al., 2015).
In the 2016–2017 influenza season, the fifth and largest wave of LPAI H7N9 occurred in southern China (World Health Organization, 2017c). In addition, highly pathogenic avian influenza (HPAI) H7N9 viruses emerged and infected humans during the fifth wave (World Health Organization, 2017b). Phylogenetically, the HPAI H7N9 viruses were derived from the LPAI H7N9 viruses circulating among domestic poultry (Ke et al., 2017; Shi et al., 2017; Zhang et al., 2017). Although sustained human-to-human transmission of the virus has not yet been reported, several mammalian-adaptive mutations have been detected in H7N9 viruses (Wang D. et al., 2014; Wang Y.R. et al., 2014; Watanabe et al., 2014; Xiao et al., 2016). These mutations may contribute to the ability of these viruses to infect mammals. Shi et al. (2017) found that the H7N9 HPAI readily obtained the 627K or 701N mutation in its PB2 segment upon replication in ferrets, causing it to become highly lethal in mice and ferrets and to be transmitted efficiently in ferrets by respiratory droplet. In addition, we found that HPAI H7N9 viruses isolated from humans are able to transmit among ferrets (Imai et al., 2017). If H7N9 viruses gain the ability to transmit efficiently from human-to-human, they could cause a pandemic. In China, seasonal influenza A viruses, H3N2 and H1N1pdm09, are co-circulating with HPAI and LPAI H7N9 viruses (China CDC, 2017). The continued circulation of these viruses increases the possibility for not only the incorporation of further human adaptive-mutations but also for reassortment with circulating human viruses of the H1N1pdm09 or H3N2 subtypes. H7N9 viruses thus pose a potential pandemic threat.
Prompt identification and isolation of H7N9 patients is one means to prevent the spread of H7N9 virus. However, we cannot distinguish influenza virus subtypes based on the symptoms of patients. Although the severity of H7N9 virus infection is generally higher than that of seasonal H3N2 and pdmH1N1 virus, but lower than that of H5N1 virus, the initial symptoms of human infection with avian H7N9 virus are similar to those caused by other subtypes (Gao H.N. et al., 2013; Yang et al., 2013; Yu et al., 2013). In addition, asymptomatic or mild infection of humans with H7N9 virus has also been reported (Cowling et al., 2013; Yu et al., 2013; Chen et al., 2014; Watanabe et al., 2014). Although rapid influenza diagnosis kits are commercially available, they cannot differentiate between H7N9 viruses and seasonal influenza viruses. To make a definite diagnosis, we currently need to perform real-time PCR, which requires specialized equipment and facilities; such equipment and facilities are not universally available at the bedside. In this study, we developed a rapid diagnostic test that is specific for the H7 subtype and is easy to use, and does not require special equipment. We also report our analysis of the performance of this kit with various H7N9 isolates.
Materials and Methods
Ethics and Biosafety Statements
The research protocol for experiments with mice was approved by and is in accordance with the TAUNS Laboratories, Inc., Shizuoka, Japan (approval number: 201306FLUH7). We used swabs from two healthy volunteers under a research protocol approved by the Research Ethics Review Committee of the Institute of Medical Science, University of Tokyo (approval number 26-42-0822). Written informed consents were obtained from both subjects. All experiments with H7N9 viruses were performed in biosafety level 3 (BSL3) laboratories at the University of Tokyo (approved for such use by the Ministry of Agriculture, Forestry, and Fisheries, Japan) and at the Harbin Veterinary Research Institute, the Chinese Academy of Agricultural Sciences (approved by the Review Board of Harbin Veterinary Research Institute, China).
Preparation of H7 Antigen to Produce MAbs Against H7 HA
A/Anhui/1/2013(H7N9) (Anhui/1) virus was engineered by using reverse genetics as described previously (Neumann et al., 1999). Briefly, the cDNA of each of the eight segments of viral RNA of Anhui/1 were synthesized based on the sequences in GISAID [Global Initiative on Sharing All Influenza Data (accession numbers; PB2, EPI439504; PB1, EPI439508; PA, EPI439503; HA, EPI439507; NP, EPI439505; NA, EPI439509; M, EPI439506; NS, EPI439510)]. The resulting products were cloned into pHH21 plasmids. All constructs were subsequently sequenced to verify the absence of unwanted mutations. Then, Anhui/1 was generated by use of plasmid-based reverse genetics. Transfectant virus was collected and amplified in Madin–Darby canine kidney (MDCK) cells, cultured in Eagle’s minimal essential medium (MEM) containing 0.3% bovine serum albumin (BSA) and 0.75 μg/ml tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin. Amplified Anhui/1 virus was purified through a 10–50% sucrose density gradient and resuspended in phosphate-buffered saline (PBS). To inactivate Anhui/1, β-propiolactone (final concentration, 0.1%; WAKO, Osaka, Japan) was added to the viruses, and incubated at 4°C for 16–24 h. Inactivation of Anhui/1 was confirmed by passaging the viruses twice in 10-day-old specific pathogen-free embryonated chicken eggs and then performing hemagglutination assays.
Development of MAbs for the H7 Kit
To produce monoclonal antibodies (MAbs), 7-week-old female BALB/c mice (Charles River Laboratories, Yokohama, Japan) were immunized with inactivated Anhui/1 plus an adjuvant (unpublished data) four times at 2-week intervals and boosted at 5 or 6 days after the final immunization. Two or three days after boosting, hybridoma cell lines were established with the spleen cells of the immunized mice and myeloma cells (Sp2/O-Ag14) by using methods previously described (Yen et al., 1979). The reactivity of the hybridoma cell lines with purified HA proteins of A/California/07/2009(H1N1pdm09), A/Canada/720/2005(H2N2), A/Perth/16/2009(H3N2), A/Indonesia/5/2005(H5N1), A/northern shoveler/California/HKWF115/2007(H6N1), A/Anhui/1/2013(H7N9), and A/Hong Kong/358 20/2009(H9N2) viruses (details of the purified HA proteins are shown in Supplementary Table 1) was assessed by use of an ELISA.
Viruses
Influenza viruses of various subtypes (Table 1): A/Chicken/Egypt/119S-NLQP/2011(H5N1) was kindly provided by Dr. A.-S. Arafa, Animal Health Research Institute, Egypt; A/duck/Gunma/466/2011(H7N9) was kindly provided by Dr. T. Saito, National Institute of Animal Health, Japan; A/Anhui/1/2013(H7N9) and A/Shanghai/1/2013(H7N9) were kindly provided by Dr. M. Tashiro, National Institute of Infectious Diseases, Japan; A/Guangdong/17SF003/2016(H7N9) and A/Taiwan/1/2017(H7N9) were kindly provided by Dr. T. Odagiri, National Institute of Infectious Diseases, Japan; A/duck/Hong Kong/301/78(H7N2) and A/seal/Massachusetts/1/80(H7N7) were kindly provided by Dr. H. Kida, Hokkaido University, Japan; all other viruses were isolated in our laboratory) were propagated in MDCK cells with MEM containing 0.3% BSA and 0.75 μg/ml TPCK-trypsin. The titers of these viruses were determined by using 50% tissue culture infectious dose (TCID50) in MDCK cells (World Health Organization, 2002). Recent H7N9 viruses isolated from birds and the environment in China (Table 2) were propagated in the allantoic cavity of 10-day-old embryonated chicken eggs. The titers of these viruses were determined by using 50% egg infectious dose (EID50) in embryonated chicken eggs. The EID50 was calculated by using the method of Reed and Muench (1938).
Swabs
We tested six frozen swabs (four from chickens and two from the surrounding environment) that were collected between 2014 and 2015 in China as part of our surveillance activities. H7N9 viruses were isolated from all six frozen swabs (data not shown). Six other swabs, collected in 2013 in Indonesia, were used to demonstrate the lack of reactivity with the sample matrix: two from chickens, two from ducks, and two from quails. No viruses were isolated from these six swabs (data not shown). We also tested clinical swabs from two healthy volunteers as negative controls.
Testing of the H7 Kit
The sensitivity and specificity of the H7 kit was compared with that of the commercially available influenza rapid diagnostic kit ImunoAce Flu (TAUNS Laboratories, Inc., Shizuoka, Japan), which can detect both influenza A and B viruses. Tenfold serially diluted (with MEM containing 0.3% BSA or PBS) viruses (101–106 TCID50/100 μl or 101–106 EID50/100 μl) or undiluted virus solution were tested. One hundred microliters of sample was mixed with 700 μl of the extraction buffer (TAUNS Laboratories, Inc.). Three drops (80–120 μl) of the mixed solution were then dropped on to the sample placement region of the kit. Results were visualized after 3–8 min of incubation at room temperature. The minimum viral titers required for a positive reaction were determined twice or three times independently. The average titers of these two or three experiments were then considered the detection limit of the kit.
Results
Development of the H7 Kit
The detection mechanism of the H7 kit is based on an immunochromatographic method that is almost identical to that of the commercially available rapid diagnostic kit ImunoAce Flu (TAUNS Laboratories, Inc., Shizuoka, Japan). ImunoAce Flu uses colloidal-platinum-gold beads, which are platinum-coated colloidal-gold beads; the detection sensitivity of these beads is more than twice that of colloidal-gold beads (Isizuka et al., 2007; Miyashita et al., 2010). Briefly, the kit consists of three regions: a sample placement region, a reagent pad region, and a result region. The reagent pad region contains colloidal-platinum-gold-labeled anti-H7 HA MAbs. The result region contains nitrocellulose membrane-immobilized anti-H7 HA MAbs and anti-mouse immunoglobulin polyclonal antibodies as capture antibodies in the H7-positive and control lines, respectively (Figure 1). To develop the kit, two MAbs, BL-101 and BL-102, produced from inactivated Anhui/1-immunized mice were selected on the basis of the result from the immunochromatographic assay. MAb BL-101 was conjugated with platinum-gold-colloid (2.5 μg of the antibody and platinum-gold-colloid complex/kit) to make a complex with the H7 antigens in the samples at the reagent pad region of the kit. MAb BL-102 was immobilized to the nitrocellulose membrane to capture the antibody at the result region (0.2 μg of antibody/kit) (Figure 1).
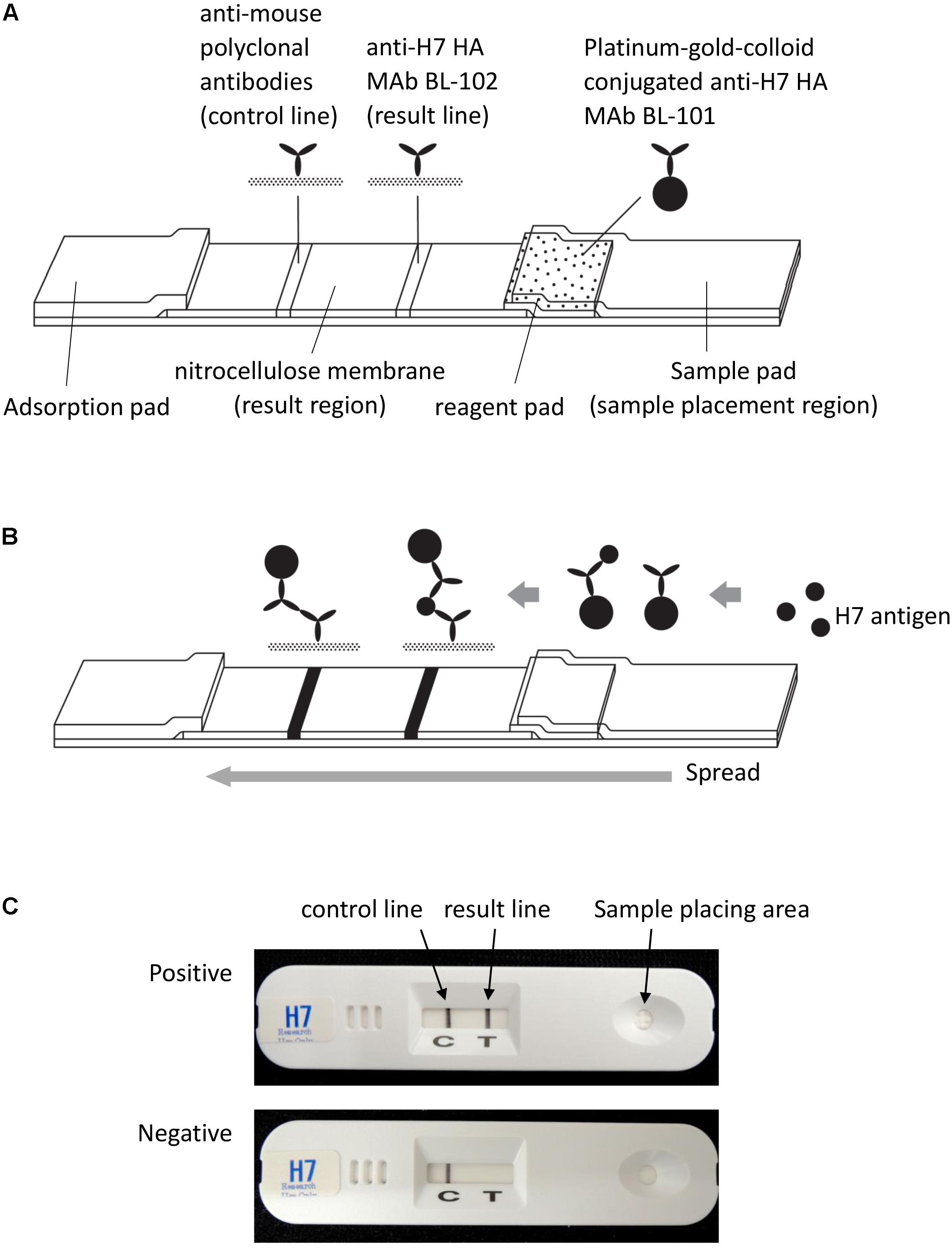
FIGURE 1. Schematic representation of the H7 kit. (A) The kit consists of three regions: a sample placement region, a reagent pad region, and a result region. The reagent pad contains colloidal-platinum-gold-labeled anti-H7 HA MAb BL-101. The result region contains nitrocellulose membrane-immobilized anti-H7 HA MAb BL-102 and anti-mouse immunoglobulin polyclonal antibodies, which serve as capture antibodies in the H7-positive and control lines, respectively. (B) When the sample is dropped on the sample pad of the kit, the platinum-gold-colloid-conjugated anti-H7 HA MAb BL-101 dissolves and binds with antigen in the sample. This complex then migrates through the nitrocellulose membrane, and is captured by the immobilized anti-H7 MAb BL-102. Then, the “result” line turns black in the presence of the platinum-gold colloid. Regardless of the presence or absence of H7 HA antigen, the platinum-gold-colloid-conjugated anti-H7 HA MAb BL-101 is captured by the immobilized anti-mouse polyclonal antibodies and produces the control black line. (C) Photographs of the H7 kit. Upper image shows an H7-positive result; lower image shows an H7-negative result.
Specificity and Sensitivity of the H7 Kit Against Various Influenza Virus Subtypes
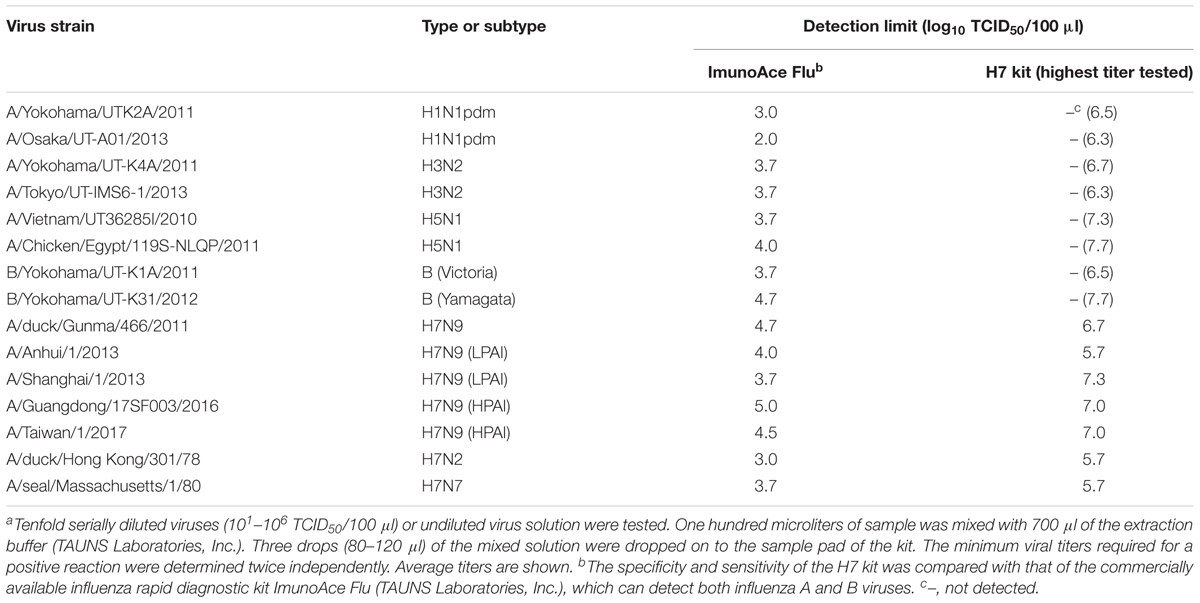
The specificity and sensitivity of the H7 kit were tested against 15 strains of various subtypes (Table 1). All of the viruses reacted with ImunoAce Flu, which can detect type A and B viruses, whereas the H7 kit detected only H7 subtype influenza viruses and did not show any non-specific reactions with any other HA subtypes (Table 1). The detection sensitivity of the H7 kit was significantly lower than that of ImunoAce Flu. ImunoAce Flu gave positive results with 102–104.7 TCID50 of the viruses, whereas the H7 kit required 105.7–107.3 TCID50 of viruses (Table 1).
Detection Sensitivity of the H7 Kit for Recent H7N9 Isolates From Birds and the Environment in China
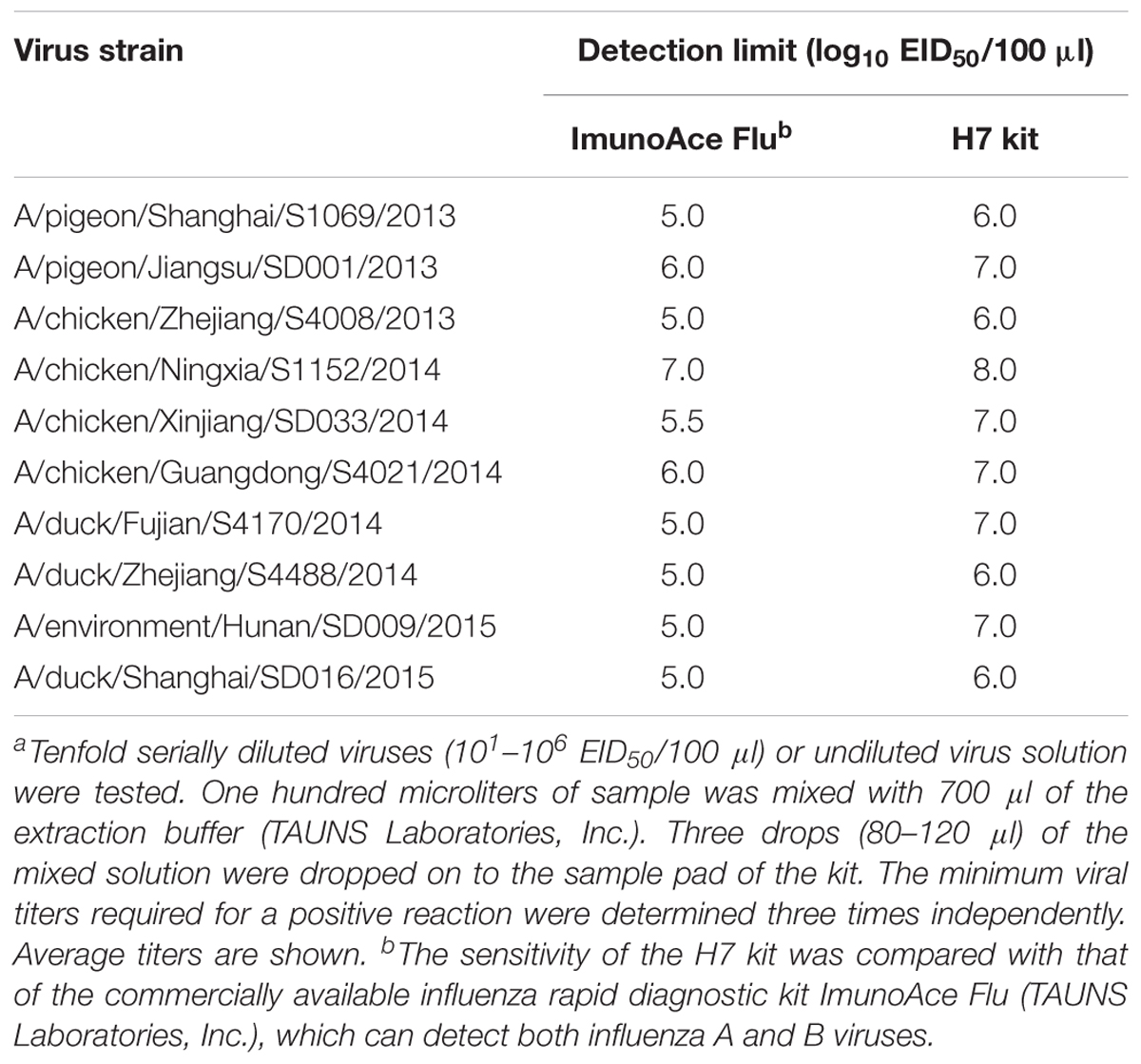
We analyzed the detection sensitivity of the H7 kit for currently circulating H7N9 viruses in China. Both ImunoAce Flu and the H7 kit gave positive results with all of the viruses tested. The detection limit of ImunoAce Flu ranged from 105.0 to 107.0 EID50/100 μl, whereas that for the H7 kit ranged from 106.0 to 108.0 EID50/100 μl. The sensitivity of the H7 kit was almost one-tenth lower than that of ImunoAce Flu (Table 2).
Detection Sensitivity of the H7 Kit for Swabs
We also performed a retrospective analysis on frozen swabs from chickens and the surrounding environment that were collected between 2014 and 2015 in China. We selected six frozen swabs from which H7N9 viruses had been previously isolated, and which were positive with ImunoAce Flu. The H7 kit gave positive results with tow out of these swabs (Table 3). Both kits gave negative results with six frozen swabs from birds (two from ducks, two from chickens, and two from quails) from which we could not isolate influenza viruses (data not shown). In addition, both kits gave negative results with fresh clinical swabs from two healthy volunteers (data not shown). To examine whether materials in the clinical swabs interfered with the test results, we tested the detection limits of the ImunoAce Flu and H7 kits against A/Guangdong/17SF003/2016 (H7N9) with a fresh swab from a healthy volunteer. We found that the detection limits of both kits were identical to those for the virus alone as described in Table 1 (data not shown). This result indicates that materials in clinical swabs do not affect the test results.
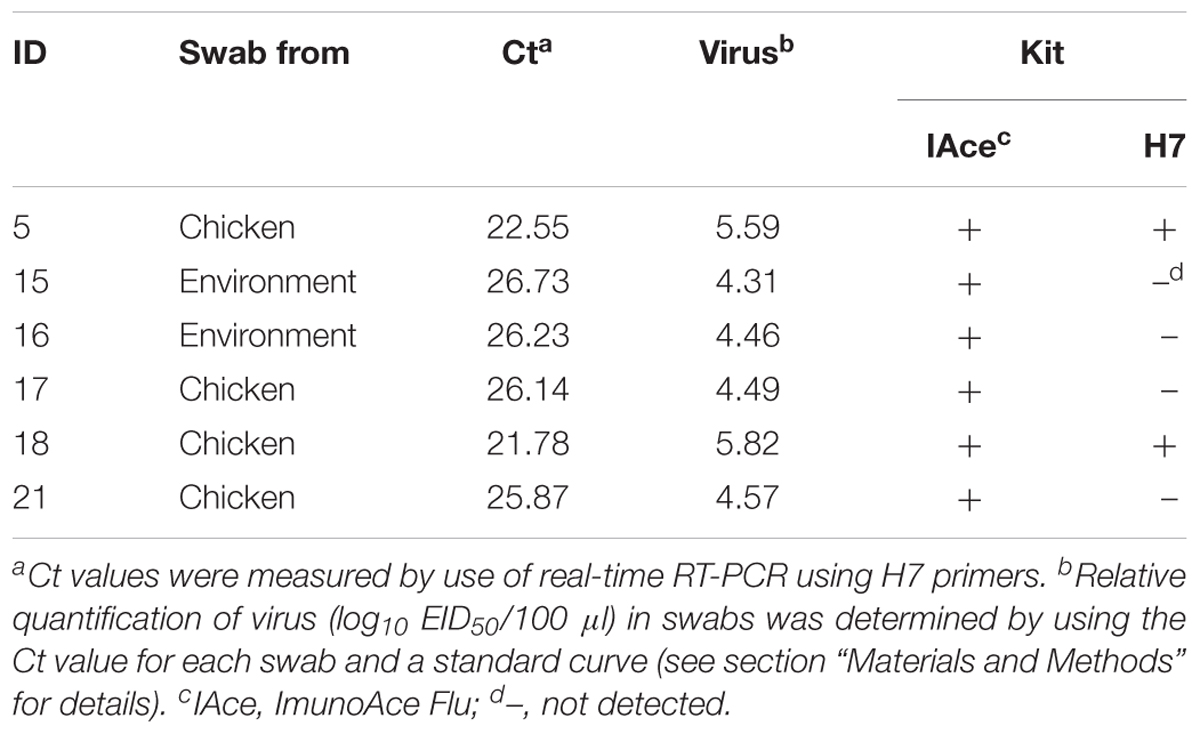
TABLE 3. Detection sensitivity of the H7 kit for swabs from H7N9-infected birds and the surrounding environment.
To determine the relative quantity of virus in each frozen swab, we performed real-time RT-PCR. Due to the limited amounts of samples available, we were able to perform real-time PCR only once. The cycle threshold (Ct) values of three H7N9 isolates, A/pigeon/Shanghai/S1069/2013, A/chicken/Guangdong/S4021/2014, and A/duck/Shanghai/SD016/2015, were used to generate a standard curve (Supplementary Figure 1), and the relative quantification of virus in each frozen swab was calculated based on the Ct value of each frozen swab by using the standard curve. Frozen swabs containing more than 105.59 EID50/100 μl of virus were detected by the H7 kit, and ImunoAce Flu reacted with frozen swabs containing more than 104.31 EID50/100 μl (Table 3). Although we did not confirm how many live viruses there were in each frozen swab, the detection sensitivity of the H7 kit for viruses in frozen swabs was almost the same as that for isolated viruses (Tables 2, 3).
Discussion
In this study, we developed an influenza diagnosis kit specific for the H7 subtype. Although the detection sensitivity of this H7 kit was almost one-tenth lower than that of ImunoAce Flu, which detects the NP of influenza A or B virus, the kit reacted with only H7 subtypes and did not show any non-specific reactions with other subtypes. The difference in sensitivity between the two kits might be influenced by their different detection antigens: ImunoAce Flu detects NP antigens, whereas the H7 kit detects HA antigens, and the amount of NP in virions and infected cells is higher than that of HA (Robert and Lamb, 2001). The reactivity of the antibodies used in the H7 kit against the virus antigens might also be lower. Modification of the antibodies might therefore improve the sensitivity of the kit.
Manzoor et al. (2008) have also reported the development of a rapid diagnosis kit for H7 avian influenza virus. They used A/duck/Hokkaido/Vac-2/2004 (H7N7) as the antigen in their kit. The detection limits of the Manzoor kit for H7N3 and H7N7 viruses were high (103.7–105.3 EID50/test) and almost the same as that of their control kit, Capilia® Flu A+B (103.1–104.4 EID50/test) (Manzoor et al., 2008). We previously compared the sensitivity between Capilia Flu A+B and ImunoAce Flu (which we used for comparison with our H7 kit study), and found that the sensitivity of Capilia Flu A+B was 10 times lower than that of ImunoAce Flu (Sakai-Tagawa et al., 2014). This indicates that the sensitivity of Manzoor’s H7 kit is 10 times lower than that of ImunoAce Flu. Since the sensitivity of our H7 kit was also 10 times lower than that of ImunoAce Flu, Manzoor’s kit and our kit may have similar sensitivities against H7 viruses. A direct comparison is needed to further evaluate these differences in detection sensitivity. Jin et al. (2014) and Kang et al. (2014) also developed immunochromatographic tests for rapid diagnosis using anti-H7 MAbs immediately after the first wave of LPAI H7N9; the sensitivity of those tests against LPAI H7N9 viruses was higher than ours. However, since 2013, the H7N9 viruses have antigenically evolved with substantial mutations in their HA protein (World Health Organization, 2017a; Zhu et al., 2017). Here we show that our kit detects not only LPAI but also HPAI H7N9 strains; whether the kits developed by Manzoor et al. (2008), Jin et al. (2014), and Kang et al. (2014) detect HPAI H7N9 strains remain unknown. We therefore believe our kit will be useful for monitoring H7N9 virus outbreaks.
In our retrospective analysis of six H7N9-positive frozen swabs, the H7 kit detected virus in two of these six swab samples that were positive with ImunoAce Flu (Table 3). Although the sensitivity of the H7 kit was 10 times lower (>105.59 EID50/100 μl) than that of ImunoAce Flu (>104.31 EID50/100 μl) (Table 3), these results demonstrate that the H7 kit could be used to detect H7 virus in swabs from patients. In addition, this H7 kit can detect viruses of different H7 subtypes (Table 1). This H7 kit will therefore be useful not only for analyzing human samples in the clinical setting but also in the field of veterinary medicine for earlier detection of HPAI H7 viruses. We tested only one H7N2 and one H7N7 virus and did not test other H7 viruses. Therefore, although this H7 kit has potential as a diagnostic kit for H7 viruses in general, its ability to detect H7 viruses other than H7N9 viruses needs to be tested in the future. In addition, identification of the epitopes of the MAbs used in our kit will help us to understand the potential changes in the sensitivity of the kit to H7 HA as the virus continues to change.
Since 2016, human infections with HPAI H7N9 viruses have been detected in China (World Health Organization, 2017b). To date, the H7N9 viruses have not caused a pandemic because of their limited human-to-human transmissibility. However, we found that HPAI H7N9 viruses can transmit among ferrets (Imai et al., 2017). Moreover, there have now been many reports of mammalian-adaptive mutations in H7N9 viruses that could confer pandemic potential (Wang D. et al., 2014; Wang Y.R. et al., 2014; Watanabe et al., 2014; Xiao et al., 2016). Prompt identification and isolation of H7N9 patients would help prevent the spread of H7N9 viruses and potentially a pandemic. Although improvements to optimize its sensitivity are required, the H7 kit developed in this study represents a valuable tool for the early detection of H7N9-infected patients.
Author Contributions
KI-H, JS, XW, YS-T, MI, KM, KN, SY, SW, HC, and YK conceived the experiments. KI-H, JS, XW, YS-T, MI, KM, TSL, KN, SY, and SW conducted the experiments. KI-H and YK wrote the manuscript. All authors reviewed the manuscript.
Funding
This research was supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, and from the Japan Agency for Medical Research and Development (AMED) (JP17fm018006); by Leading Advanced Projects for Medical Innovation (LEAP) from AMED (JP17am001007); and by a Grant-in-Aid for Scientific Research on Innovative Areas from the MEXT of Japan (No. 16H06429, 16K21723, and 16H06434).
Conflict of Interest Statement
YK has received speaker’s honoraria from Toyama Chemical Co., Ltd. and Astellas Pharma Inc., has received grant support from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Company, Limited, Toyama Chemical Co., Ltd., TAUNS Laboratories, Inc., Otsuka Pharmaceutical Co., Ltd., and Denka Seiken Co., Ltd., and is a co-founder of FluGen Inc. KM, KN, and SW are employed by TAUNS Laboratories, Inc. TAUNS Laboratories, Inc. has patents “Japan patent JP3886000B” and “United States patent US7713328.”
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Susan Watson for editing the manuscript and Ryuta Uraki for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01346/full#supplementary-material
References
Centers for Disease Control and Prevention (2013). Emergence of avian influenza A(H7N9) virus causing severe human illness - China, February-April 2013. MMWR Morb. Mortal. Wkly. Rep. 62, 366–371.
Chen, Z., Liu, H., Lu, J., Luo, L., Li, K., Liu, Y., et al. (2014). Asymptomatic, mild, and severe influenza A(H7N9) virus infection in humans, Guangzhou, China. Emerg. Infect. Dis. 20, 1535–1540. doi: 10.3201/eid2009.140424
China CDC (2017). Available: http://www.chinaivdc.cn/cnic/en/Surveillance/WeeklyReport/201706/P020170630775099133221.pdf [accessed July 20, 2017].
Cowling, B. J., Freeman, G., Wong, J. Y., Wu, P., Liao, Q., Lau, E. H., et al. (2013). Preliminary inferences on the age-specific seriousness of human disease caused by avian influenza A(H7N9) infections in China, March to April 2013. Euro. Surveill. 18:20475.
Food and Agriculture Organization of the United Nations (2018). H7N9 Situation Update. Available at: http://www.fao.org/ag/againfo/programmes/en/empres/h7n9/situation_update.html
Gao, H. N., Lu, H. Z., Cao, B., Du, B., Shang, H., Gan, J. H., et al. (2013). Clinical findings in 111 cases of influenza A (H7N9) virus infection. N. Engl. J. Med. 368, 2277–2285. doi: 10.1056/NEJMoa1305584
Gao, R., Cao, B., Hu, Y., Feng, Z., Wang, D., Hu, W., et al. (2013). Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368, 1888–1897. doi: 10.1056/NEJMoa1304459
Imai, M., Watanabe, T., Kiso, M., Nakajima, N., Yamayoshi, S., Iwatsuki-Horimoto, K., et al. (2017). A highly pathogenic avian H7N9 influenza virus isolated from a human is lethal in some ferrets infected via respiratory droplets. Cell Host Microbe 22, 615.e8–626.e8. doi: 10.1016/j.chom.2017.09.008
Isizuka, Y., Hasumi, F., Namba, Y., Nonaka, U., and Okura, I. (2007). Metallic Colloidal Particle. Japan Patent Application JP3886000B. doi: 10.1155/2014/425051
Jin, C., Wu, N., Peng, X., Yao, H., Lu, X., Chen, Y., et al. (2014). Comparison of a new gold immunochromatographic assay for the rapid diagnosis of the novel influenza A (H7N9) virus with cell culture and a real-time reverse-transcription PCR assay. Biomed Res. Int. 2014:425051. doi: 10.1155/2014/425051
Kang, K., Chen, L., Zhao, X., Qin, C., Zhan, Z., Wang, J., et al. (2014). Development of rapid immunochromatographic test for hemagglutinin antigen of H7 subtype in patients infected with novel avian influenza A (H7N9) virus. PLoS One 9:e92306. doi: 10.1371/journal.pone.0092306
Ke, C., Mok, C. K. P., Zhu, W., Zhou, H., He, J., Guan, W., et al. (2017). Human infection with highly pathogenic avian influenza A(H7N9) virus, China. Emerg. Infect. Dis. 23, 1332–1340. doi: 10.3201/eid2308.170600
Lam, T. T., Zhou, B., Wang, J., Chai, Y., Shen, Y., Chen, X., et al. (2015). Dissemination, divergence and establishment of H7N9 influenza viruses in China. Nature 522, 102–105. doi: 10.1038/nature14348
Manzoor, R., Sakoda, Y., Sakabe, S., Mochizuki, T., Namba, Y., Tsuda, Y., et al. (2008). Development of a pen-site test kit for the rapid diagnosis of H7 highly pathogenic avian influenza. J. Vet. Med. Sci. 70, 557–562.
Miyashita, K., Ogawa, R., and Kezuka, M. (2010). Metallic colloid particles and process for producing same. U.S. Patent No 0,186,129. Washington, DC: U.S. Patent and Trademark Office. doi: 10.1073/pnas.96.16.9345
Neumann, G., Watanabe, T., Ito, H., Watanabe, S., Goto, H., Gao, P., et al. (1999). Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. U.S.A. 96, 9345–9350. doi: 10.1073/pnas.96.16.9345
Reed, L. J., and Muench, H. (1938). A simple method of estimating fifty percent endpoint. Am. J. Hyg. 27, 493–497.
Robert, A., and Lamb, R. M. K. (2001). “Orthomyxoviridae: the viruses and their replication,” in Fields Virology, Fourth Edn, eds P. M. H. David and M. Knipe (Philadelphia, PA: Lippincott-Raven Publishers), 1487–1531. doi: 10.1038/cr.2017.129
Sakai-Tagawa, Y., Ozawa, M., Yamada, S., Uchida, Y., Saito, T., Takahashi, K., et al. (2014). Detection sensitivity of influenza rapid diagnostic tests. Microbiol. Immunol. 58, 600–606. doi: 10.1111/1348-0421.12185
Shi, J., Deng, G., Kong, H., Gu, C., Ma, S., Yin, X., et al. (2017). H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res. 27, 1409–1421. doi: 10.1038/cr.2017.129
Shi, J., Deng, G., Liu, P., Zhou, J., Guan, L., Li, W., et al. (2013). Isolation and characterization of H7N9 viruses from live poultry markets — Implication of the source of current H7N9 infection in humans. Chin. Sci. Bull. 58, 1857–1863. doi: 10.1007/s11434-013-5873-4
Wang, D., Yang, L., Gao, R., Zhang, X., Tan, Y., Wu, A., et al. (2014). Genetic tuning of the novel avian influenza A(H7N9) virus during interspecies transmission, China, 2013. Euro. Surveill. 19:20836. doi: 10.1016/j.ijid.2014.05.007
Wang, Y. R., Li, J. M., and Wang, X. F. (2014). Clinical and epidemiological analysis of the first case of human infection with avian influenza A (H7N9) virus in Shenzhen, China. Int. J. Infect. Dis. 25, 177–179. doi: 10.1016/j.ijid.2014.05.007
Watanabe, T., Watanabe, S., Maher, E. A., Neumann, G., and Kawaoka, Y. (2014). Pandemic potential of avian influenza A (H7N9) viruses. Trends Microbiol. 22, 623–631. doi: 10.1016/j.tim.2014.08.008
World Health Organization (2002). WHO Manual on Animal Influenza Diagnosis and Serveillance. Geneva: WHO.
World Health Organization (2017a). Analysis Of Recent Scientific Information on Avian Influenza A(H7N9) Virus. Available at: http://www.who.int/influenza/human_animal_interface/avian_influenza/riskassessment_AH7N9_201702/en/ [accessed May 09, 2018].
World Health Organization (2017b). Human Infection with Avian Influenza A(H7N9) Virus-China. Available at: http://www.who.int/csr/don/27-february-2017-ah7n9-china/en/ [accessed May 09, 2018].
World Health Organization (2017c). Influenza at the Human-Animal Interface. Available at: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_06_15_2017.pdf?ua=1 [accessed May 09, 2018] doi: 10.1038/srep19474
Xiao, C., Ma, W., Sun, N., Huang, L., Li, Y., Zeng, Z., et al. (2016). PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci. Rep. 6:19474. doi: 10.1038/srep19474
Yang, P., Pang, X., Deng, Y., Ma, C., Zhang, D., Sun, Y., et al. (2013). Surveillance for avian influenza A(H7N9), Beijing, China, 2013. Emerg. Infect. Dis. 19, 2041–2043. doi: 10.3201/eid1912.130983
Yen, M. Y., Helstrom, I., Brown, J. P., Warner, G. A., Hansen, J. A., and Hellstrom, K. E. (1979). Cell surface antigens of human melanoma identified by monoclonal antibody. Proc. Natl. Acad. Sci. U.S.A. 76, 2927–2931. doi: 10.1016/S0140-6736(13)61207-6
Yu, H., Cowling, B. J., Feng, L., Lau, E. H. Y., Liao, Q., Tsang, T. K., et al. (2013). Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet 382, 138–145. doi: 10.1016/s0140-6736(13)61207-6
Yu, X., Jin, T., Cui, Y., Pu, X., Li, J., Xu, J., et al. (2014). Influenza H7N9 and H9N2 viruses: coexistence in poultry linked to human H7N9 infection and genome characteristics. J. Virol. 88, 3423–3431. doi: 10.1128/jvi.02059-13
Zhang, F., Bi, Y., Wang, J., Wong, G., Shi, W., Hu, F., et al. (2017). Human infections with recently-emerging highly pathogenic H7N9 avian influenza virus in China. J. Infect. 75, 71–75. doi: 10.1016/j.jinf.2017.04.001
Zhang, Q., Shi, J., Deng, G., Guo, J., Zeng, X., He, X., et al. (2013). H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341, 410–414. doi: 10.1126/science.1240532
Keywords: influenza virus, rapid diagnostic kit, H7 subtype, highly pathogenic avian influenza, monoclonal antibody
Citation: Iwatsuki-Horimoto K, Shi J, Wang X, Sakai-Tagawa Y, Ito M, Murakami K, da Silva Lopes TJ, Nakaishi K, Yamayoshi S, Watabe S, Chen H and Kawaoka Y (2018) Development of an Influenza Rapid Diagnostic Kit Specific for the H7 Subtype. Front. Microbiol. 9:1346. doi: 10.3389/fmicb.2018.01346
Received: 26 February 2018; Accepted: 31 May 2018;
Published: 25 June 2018.
Edited by:
Masako Nomaguchi, Tokushima University Graduate School of Medical Sciences, JapanReviewed by:
Yasuko Tsunetsugu Yokota, Tokyo University of Technology, JapanThomas Paul Fabrizio, St. Jude Children’s Research Hospital, United States
Tianlei Ying, Fudan University, China
Copyright © 2018 Iwatsuki-Horimoto, Shi, Wang, Sakai-Tagawa, Ito, Murakami, da Silva Lopes, Nakaishi, Yamayoshi, Watabe, Chen and Kawaoka. This is an openaccess article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshihiro Kawaoka, eW9zaGloaXJvLmthd2Fva2FAd2lzYy5lZHU=
 Kiyoko Iwatsuki-Horimoto
Kiyoko Iwatsuki-Horimoto Jianzhong Shi
Jianzhong Shi Xiurong Wang2
Xiurong Wang2 Kazushi Murakami
Kazushi Murakami Kazunari Nakaishi
Kazunari Nakaishi Seiya Yamayoshi
Seiya Yamayoshi Satoshi Watabe
Satoshi Watabe Hualan Chen
Hualan Chen