
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 12 June 2018
Sec. Fungi and Their Interactions
Volume 9 - 2018 | https://doi.org/10.3389/fmicb.2018.01246
This article is part of the Research Topic Diagnostic Approaches for Aspergillus Infections View all 20 articles
 Matxalen Vidal-García1,2*
Matxalen Vidal-García1,2* Sergio Redrado3
Sergio Redrado3 M. Pilar Domingo3
M. Pilar Domingo3 Patricia Marquina3
Patricia Marquina3 Cristina Colmenarejo2
Cristina Colmenarejo2 Jacques F. Meis4,5
Jacques F. Meis4,5 Antonio Rezusta2,6
Antonio Rezusta2,6 Julian Pardo1,7,8,9
Julian Pardo1,7,8,9 Eva M. Galvez3*
Eva M. Galvez3*Gliotoxin (GT) is a fungal secondary metabolite that has attracted great interest due to its high biological activity since it was discovered by the 1930s. An inactive derivative of this molecule, bis(methylthio)gliotoxin (bmGT), has been proposed as an invasive aspergillosis (IA) biomarker. Nevertheless, studies regarding bmGT production among common opportunistic fungi, including the Aspergillus genus, are scarce and sometimes discordant. As previously reported, bmGT is produced from GT by a methyl-transferase, named as GtmA, as a negative feedback regulatory system of GT production. In order to analyze the potential of bmGT detection to enable identification of infections caused by different members of the Aspergillus genus we have assessed bmGT production within the genus Aspergillus, including A, fumigatus, A. niger, A. nidulans, and A. flavus, and its correlation with gtmA presence. In order to validate the relevance of our in vitro findings, we compared bmGT during in vitro culture with the presence of bmGT in sera of patients from whom the Aspergillus spp. were isolated. Our results indicate that most A. fumigatus isolates produce GT and bmGT both in vitro and in vivo. In contrast, A. niger and A. nidulans were not able to produce GT or bmGT, although A. niger produced bmGT from a exogenous GT source. The frequency and amount of bmGT production in A. terreus and A. flavus isolates in vitro was lower than in A. fumigatus. Our results suggest that this defect could be related to the in vitro culture conditions, since isolates that did not produce bmGT in vitro were able to synthetize it in vivo. In summary, our study indicates that bmGT could be very useful to specifically detect the presence of A. fumigatus, the most prevalent agent causing IA. Concerning A. terreus and A. flavus a higher number of analyses from sera from infected patients will be required to reach a useful conclusion.
More than 20 years ago the first invasive aspergillosis (IA) biomarker, galactomannan (GM), was developed based on an enzyme linked immunosorbent assay (Stynen et al., 1995). It stirred up the diagnosis of this lethal infectious disease, as it allowed to detect the infection when combined with clinical signs and symptoms (Maertens et al., 1999, 2002). During the last few years, the biomarker weaponry has arisen with the development of a system to detect β-D-glucan, Aspergillus PCR and lateral flow device to detect an Aspergillus-derived protein among others (Odabasi et al., 2004; Thornton, 2008; White et al., 2015). New diagnostic approaches were developed based on the increased accuracy of these tests, such as pre-emptive therapy (Wingard, 2007; Riwes and Wingard, 2012). Despite of these advances, IA management continues to be challenging due to the heterogeneous population at risk, the diversity of clinical and radiological presentations and the lack of a gold standard (Lamoth and Calandra, 2017). Thus, at present, it is required to understand the limitations of each biomarker and the corresponding diagnosis test in order to accurately diagnose these challenging infections (Maertens et al., 2016). In this line, the future directions in IA diagnosis research need to focus on the development of new biomarkers, including a clear understanding of their strengths and limitations, along with the assessment of their utility in well-designed clinical trials (Arvanitis and Mylonakis, 2015; Mercier and Maertens, 2017).
In recent years, bis(methylthio)gliotoxin (bmGT) has generated great interest as an IA biomarker (Maertens et al., 2016; Mercier and Maertens, 2017). Its detection in serum by High Performance Thin Layer Chromatography (HPTLC) was shown to be reliable (Domingo et al., 2012). Moreover, it has been clinically validated in a small prospective study in comparison with GM quantification (Vidal-García et al., 2016). Data suggest a good diagnostic performance (61.5% sensitivity and 93% specificity) and importantly, high positive and negative predictive values when used in combination with GM detection (100% and 97.5%, respectively), which suggest a potential utility in pre-emptive approaches. Pending further validation, unlike GM, bmGT detection could be useful in non-immunocompromised populations as it was previously found to be positive in a non-compromised patient suffering from IA that presented negative GM values (Vidal-García et al., 2017). Nevertheless, data regarding the frequency and distribution of bmGT production by different opportunistic molds are scarce and in most cases based on bioinformatics analysis (Bergmann et al., 2007; Andersen et al., 2013; Dolan et al., 2014). These data would be very important for understanding the specificity and the clinical sensitivity of this biomarker to differentiate between species within the Aspergillus genus and, thus, treat this infection more effectively.
Bis(methylthio)gliotoxin is an inactive derivative of gliotoxin (GT). A. fumigatus is, to date, the most important opportunistic fungi producing bmGT (Li et al., 2006; Guimarães et al., 2010; Domingo et al., 2012; Sun et al., 2012; Liang et al., 2014). BmGT serves as a negative regulator of the GT biosynthesis, and it is produced by methylation of GT by an S-adenosylmethionine-dependent bis-thiomethyltransferase (Dolan et al., 2014, 2015, 2017), eliminating the ability of GT to produce toxic reactive oxygen species (ROS) (Dolan et al., 2015). BmGT formation from an exogenous source of GT has been described in A. niger and A. nidulans (Scharf et al., 2014; Manzanares-Miralles et al., 2016). The enzyme responsible for bmGT biosynthesis, which is an S-adenosylmethionine (SAM)-dependent methyltransferase called GtmA, has been characterized in A. fumigatus; and other orthologs have been found on species like A. niger or A. terreus (Dolan et al., 2014; Scharf et al., 2014; Manzanares-Miralles et al., 2016). The best characterized enzyme is GtmA, which is known to be encoded by the gtmA gene, located in the chromosome 2 (Dolan et al., 2014). Bioinformatics analysis of the Ascomycota phylum showed 124 orthologs of GtmA. However, it is known that toxin production is discontinuous among different species and it is not clear which species within Aspergillus genus are able to produce GT and, subsequently, the inactive derivative bmGT (Gardiner and Howlett, 2005; Patron et al., 2007). The aim of the present study was to assess the frequency and species distribution of bmGT within the Aspergillus genus in cultures in vitro as well as in vivo in sera of patients from whom fungi were isolated. We also characterized the ability of different clinical isolates from Aspergillus genus to methylate GT in cultures in vitro, to confirm the presence and activity of methyltransferases in Aspergillus species isolated from probable and proven cases of IA. Our findings indicate that bmGT could be considered as an specific biomarker to detect infections by A. fumigatus, the most common agent causing IA, excluding the presence of A. nidulans and A. niger.
We analyzed GT and bmGT production within 252 Aspergillus spp. isolates. Most A. fumigatus complex (n = 119) were clinical isolates from Canisius-Wilhelmina Hospital, Nijmegen (Netherlands). Eighteen of those isolates were cryptic species from Section Fumigati from Gregorio Marañón University Hospital, Madrid (Spain). Other Aspergillus species were clinical isolates from Miguel Servet University Hospital, Zaragoza (Spain) and corresponded to 36 A. flavus complex, 35 A. terreus complex, 40 A. niger complex, and 22 A. nidulans complex. One milliliter of 12 McFarland conidial suspension (approximately 3–5 × 107 conidia/mL) was added to 9 mL of liquid medium (Roswell Park Memorial Institute [RPMI] 1640 + glucose 20 g/L + glutamine 2 mM + HEPES 25 mM) in 50 mL culture flasks and incubated at 37°C for 96 h. A 2 mL sample of supernatant was obtained and frozen at -20°C and subsequently used for GT and bmGT detection and quantification by HPTLC as described below. In those cases where GT and/or bmGT was not detected, fungal isolates were cultured employing Czapek Dox Broth (+ glutamine + HEPES) to confirm that this defect was not specific for RPMI medium. Czapek Dox Broth is a medium of a different composition to RPMI1640, and similarly to the last one, it is commonly used in Aspergillus cultures in vitro. Thus, we decided to compare both in order to discard effects relative to specific culture media conditions in vitro.
Bis(methylthio)gliotoxin production from an exogenous source of GT was assessed in a total of 35 isolates of the species complexes A. flavus (n = 12), A. terreus (n = 9), A. niger (n = 8), and A. nidulans (n = 6). Conidial inoculum was prepared as described above and added to 50 mL culture flasks with Czapek Dox Broth (+ glutamine + HEPES). These cultures were incubated at 37°C for 45 h. At 45 h, GT was added to a final concentration of 2.5 mg/L and methanol was added as solvent control. At 0, 3, and 6 h, 2 mL aliquots of supernatant were taken and frozen until GT and bmGT analysis.
We assessed sera from patients hospitalized in the Miguel Servet University Hospital with probable/proven IA according to the EORTC/MSG definitions (De Pauw et al., 2008). We included in the study those cases with Aspergillus spp. growth in clinical samples. Serum were prospectively collected and frozen at -20°C until GT and bmGT detection. All protocols were supervised and approved by the Ethics Committee of Clinical Research from Aragón (CEICA), number PI15/0203.
Gliotoxin and bis(methylthio)gliotoxin detection and quantification were performed both in serum and supernatant samples by HPTLC as described by Domingo et al. (2012). Briefly, GT and bmGT were extracted together using dichloromethane. After agitation and two phase’s separation, non-aqueous phase was added onto silica gel plates. Then, they were developed using a horizontal development chamber (Camag). The mobile phase was a mixture of tetrahydrofuran/n-heptane/acetonitrile (40:58:2 [v/v/v]). After 25 min development, plates were scanned with an ultraviolet scanning densitometry (TLC Scanner 3, Camag; λ = 280 and 367 nm; linear scanning). GT and bmGT identification was performed by retention time and spectral analysis and quantification was performed by peak area under curve analysis using Camag’s personal computer software.
Chromosomal DNA of Aspergillus spp. isolates was extracted using cetyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO, United States). The specie-specific primers used for gtmA detection are summarized in Table 1, along with expected amplicon length. PCR was performed using HotTaq Master Mix, (IBIAN Technologies, Zaragoza, Aragón, Spain). An initial denaturation of 2 min at 94°C was followed by 35 cycles at 94°C for 30 s, 56°C for 15 s, and 72°C for 1 min. DNA amplification products were visualized after electrophoresis on 2% agarose gels. UView 6x loading dye (Bio-Rad, Hercules, CA, United States) was used for nucleic acid staining. As a length standard 0.1–1 kbp molecular mass marker was used.
Statistical analyses were performed using Prism 6, Graphpad (San Diego, CA, United States). GT and bmGT concentration in culture filtrates are given as mean ± standard error of mean (SEM). A significance level of 0.05 was considered statistically relevant for multiple comparison test and chi-square test, as appropriate.
The production of GT and bmGT was tested in culture supernatants of 252 Aspergillus isolates from the species complexes A. fumigatus, A. flavus, A. terreus, A. niger, and A. nidulans after 4 days of incubation. Non-cryptic A. fumigatus isolates (n = 101) produced GT and bmGT at highest frequencies, 77.23% and 84.16%, respectively. Among the five cryptic species analyzed: A. calidoustus (n = 1), A. fumigatiaffinis (n = 3), A. lentulus (n = 11), N. udagawae (n = 2), and A. novofumigatus (n = 1), all but the last produced GT and/or bmGT. A. fumigatiaffinis seemed to be the most frequent GT and bmGT producing species, as the three tested isolates (100%) produced bmGT. In contrast, just two of the eleven isolates (18%) of A. lentulus produced bmGT. BmGT was also more frequently detectable than GT in culture supernatants of A. flavus, 11.11% vs. 8.33%, respectively. In contrast, A. terreus produced GT more frequently than bmGT (22.86% vs. 2.86% respectively) (Figure 1). Notably, none of the A. niger and A. nidulans isolates tested produced GT and/or bmGT. All cultures were analyzed by HPTLC, a method to detect GT and bmGT previously optimized and validated versus LC-MS (Domingo et al., 2012), and some of the results in selected culture isolates of A. fumigatus, A. niger, A. nidulans, and A. flavus, were confirmed by LC-MS (data not shown). The absence of GT and/or bmGT production was not specific for the culture conditions in vitro since culture of GT/bmGT negative fungal isolates employing Czapek Dox Broth yielded similar results (data not shown). In addition, the differences were not due to different fungal growth since cell cultures showed a similar behavior and growth as analyzed by XTT reduction assay. Optimal culture conditions, as well as analytical specificity of the method, were confirmed by employing cell cultures from an A. fumigatus gliP deletion mutant, which is unable to produce GT and bmGT (Sugui et al., 2007).
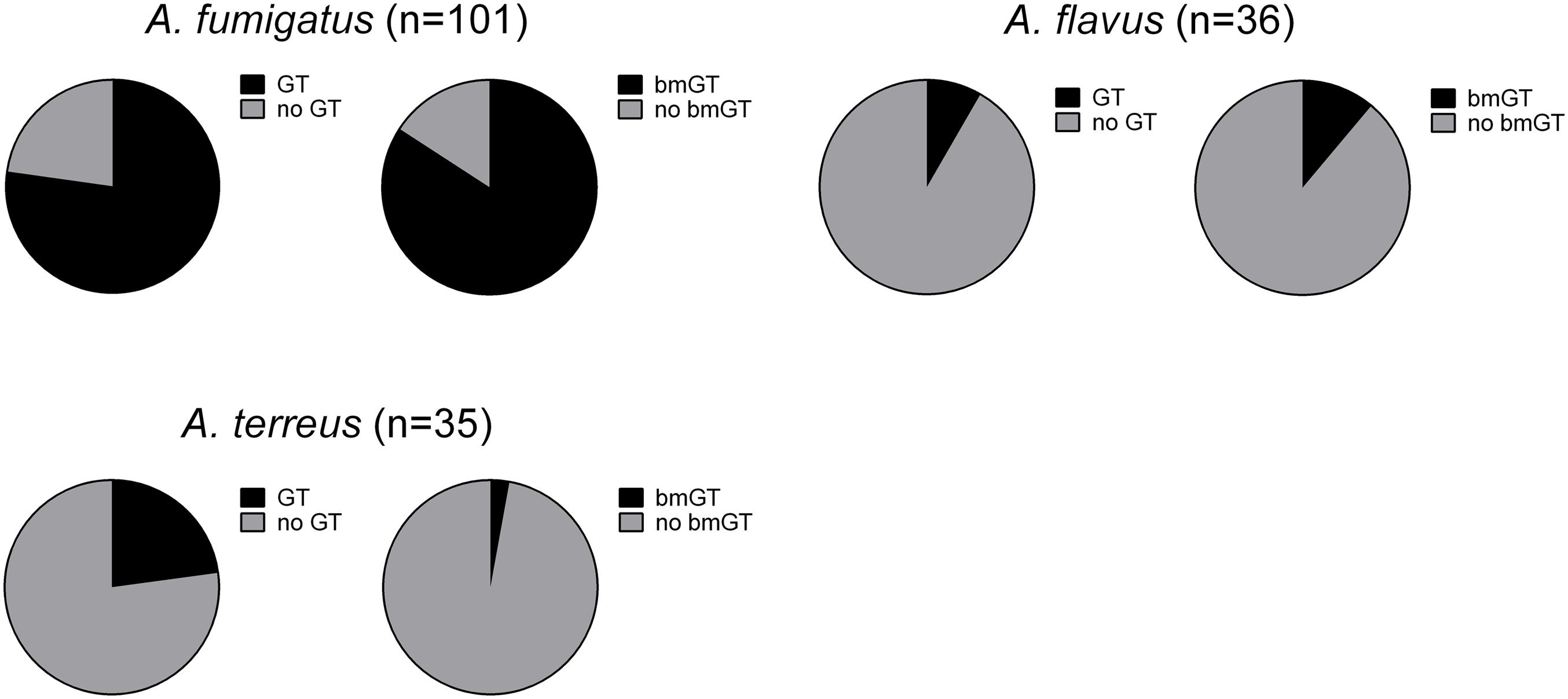
FIGURE 1. Percentage of gliotoxin and bis(methylthio)gliotoxin producing isolates among different Aspergillus species.
A. fumigatus isolates also produced GT and bmGT in higher concentration than other Aspergillus spp. did. These differences were statistically significant (p < 0.05) (Figure 2). The mean concentration of GT was 2.26 ± 0.40 mg/L and of bmGT was 3.45 ± 0.44 mg/L for A. fumigatus. A. flavus isolates yielded a mean concentration of 0.14 ± 0.13 mg/L of GT and 0.39 ± 0.31 mg/L of bmGT. The mean concentration of GT and bmGT were 0.79 ± 0.34 mg/L and 0.07 ± 0.07 for A. terreus.
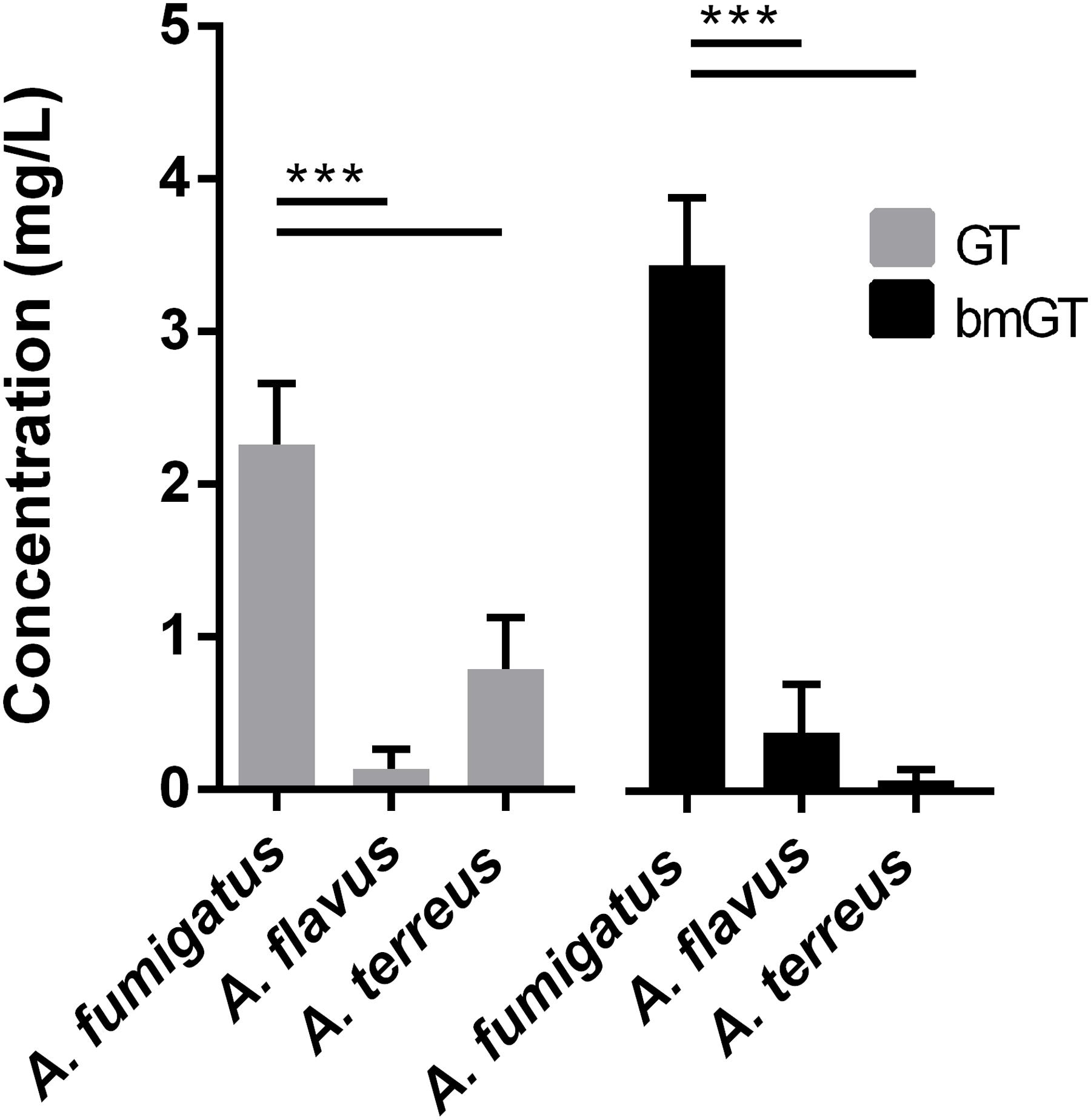
FIGURE 2. Mean concentrations of gliotoxin and bis(methylthio)gliotoxin detected in culture supernatans of different Aspergillus species. ∗∗∗Statistically significant differences (p < 0.001).
In order to confirm the results obtained in cell cultures concerning bmGT production, we analyzed the ability of some fungal isolates to generate bmGT from an external GT source as well as the presence of methyltransferase genes. This is of special utility to find out whether the Aspergillus spp. that did not produce GT and bmGT (A. niger and A. nidulans) are also unable to methylate exogenous GT. This finding would mean that these isolates do not express methyl-transferase activity and, thus, confirm that they are unable to generate bmGT. Moreover, this would indirectly suggest that they are also unable to generate GT, since GT methylation has been proposed as a negative feedback regulatory system, inherent to all GT-producing species.
First, we analyzed if fungal isolates presented GT methyl-transferase activity by adding pure GT and monitoring the generation of bmGT. The ability to produce bmGT from an exogenous source of GT was assessed in the isolates of A. flavus, A. terreus, A. niger, and A. nidulans that did not produce GT or bmGT in the previous experiment. All isolates from the A. flavus (n = 12) and A. terreus species (n = 9) were able to methylate exogenous GT in order to produce bmGT. Among A. niger isolates, this ability was less consistent, nevertheless, 5/8 isolates (62.5%) showed such ability. Finally, none of the A. nidulans isolates (n = 6) methylated GT to generate bmGT (Figure 3). This result confirms that A. nidulans does not express methyl-transferase activity and, thus, it is unable to generate endogenously bmGT, and, likely, GT, in line with the results of Figure 1. Concerning A. niger, some isolates seem to express methyl-transferase activity against exogenous GT. Indeed, it has been previously shown that the methyl-transferase MT-ii is expressed in A. niger and methylates exogenous GT (Dolan et al., 2017). However, since they do not produce GT (Figure 1 and Manzanares-Miralles et al., 2016), this would explain that they are unable to endogenously produce bmGT.
Among bmGT producing isolates, immediately after GT addition (t = 0 h), this was recovered in a mean concentration of 0.86 ± 0.04 mg/L. None of the isolates produced bmGT at this time (Figure 4). At 3 h after GT addition, GT concentration decreased and bmGT concentration increased. This observation continued at 6 h, when the maximum bmGT and the minimum GT concentrations were detected. There were no differences between mean concentration of GT and bmGT among species (p > 0.05) indicating a similar methylating activity.
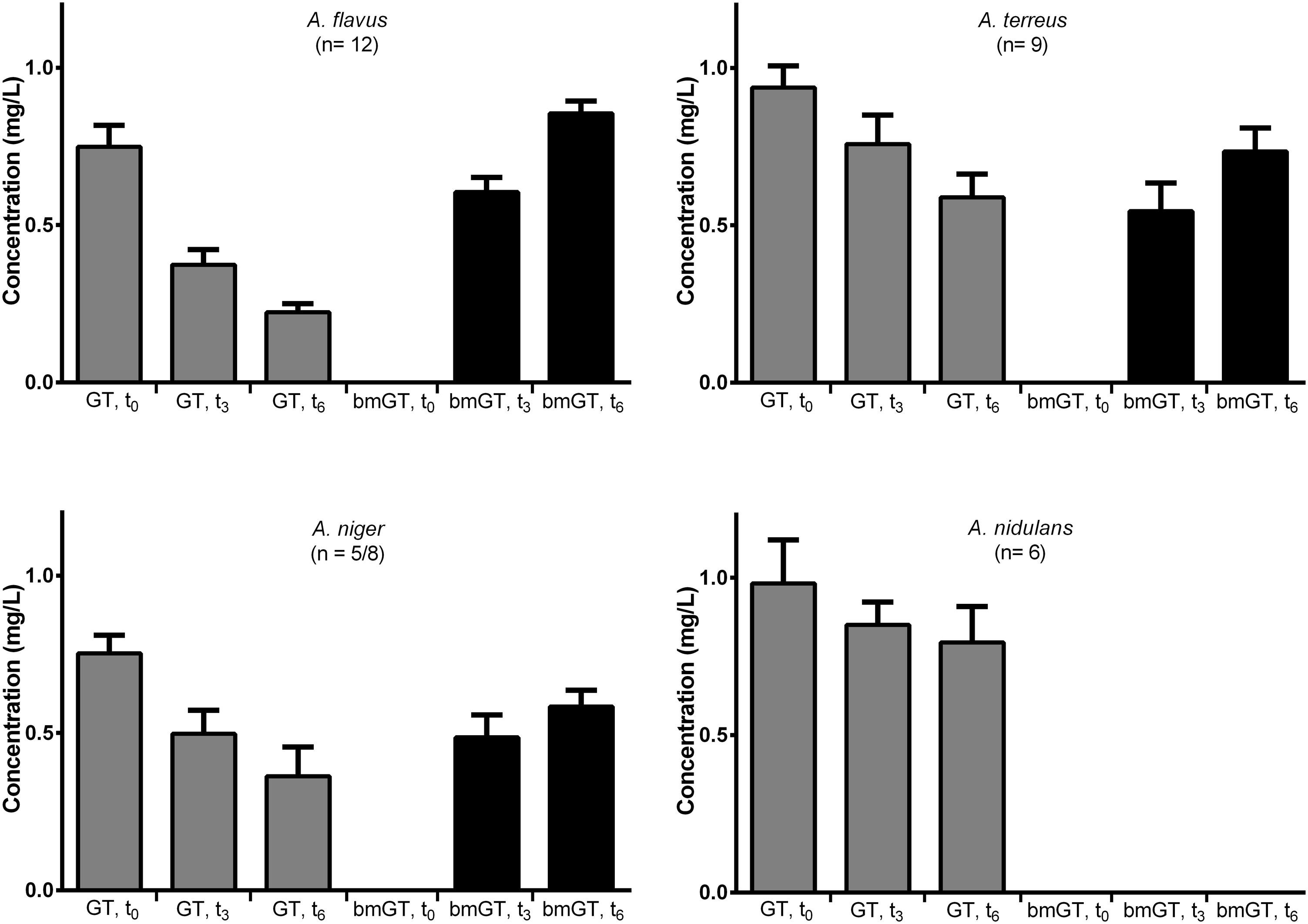
FIGURE 4. Bis(methylthio)gliotoxin production from exogenous gliotoxin among different Aspergillus species. The bars represent mean concentrations with standard error of means.
Our results confiults confirm that most A. fumigatus isolates and some A. terreus and A. flavus isolates were able to endogenously and exogenously produce bmGT. However, the frequency of bmGT production within A. terreus and A. flavus isolates was much less than in A. fumigatus isolates. Since the analyses of the genes involved in GT synthesis is difficult due to the complexity of the pathways involved, we decided to analyze if the isolates that did not produce endogenously GT and bmGT in vitro, were able to synthesis bmGT in humans in vivo. To this aim, we included six cases of probable/proven IA with mycological growth from whom in vitro cultures had been established and analyzed. Serum bmGT concentration for these patients, fungal isolation, sample type and fungal ability to produce bmGT in vitro (de novo and from exogenous GT) as well as detection of gtmA gene and the MT-ii homolog are summarized in Table 2.
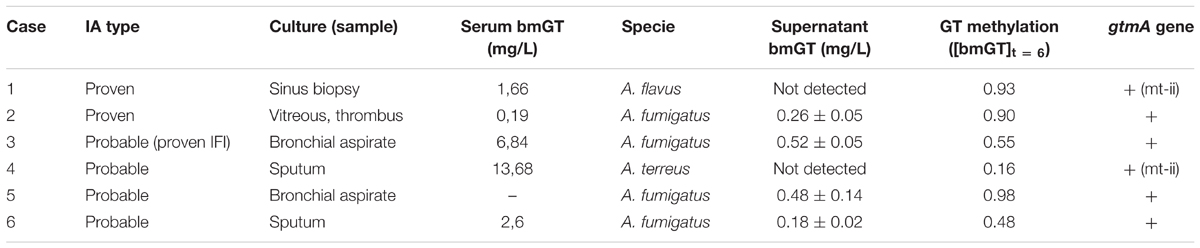
TABLE 2. bmGT production, methylation of exogenous GT and carriage of gtmA or MT-ii genes for Aspergillus spp. isolates from probable and proven invasive aspergillosis cases.
All, but one serum, were positive for bmGT. This serum belonged to a patient with probable IA diagnosed by A. fumigatus growth in bronchial aspirate. The other three patients with A. fumigatus isolation had positive bmGT (range 0.19–13.68 mg/L). There was a case of IA by A. flavus and a case of IA by A. terreus. Both had detectable bmGT in serum. Notably, these isolates corresponded to those ones in which we were not able to detect either endogenous GT or bmGT during in vitro culture. Nevertheless, all of them methylated the exogenous GT and carried the mt-ii methyl-transferase gene, as seen in Figure 5. Of note, those isolates which produced bmGT in higher amounts in vitro, did not correlate with the highest bmGT production in vivo. All the clinical isolates from A. fumigatus showed the ability to methylate exogenous GT and carry the gtmA gene.
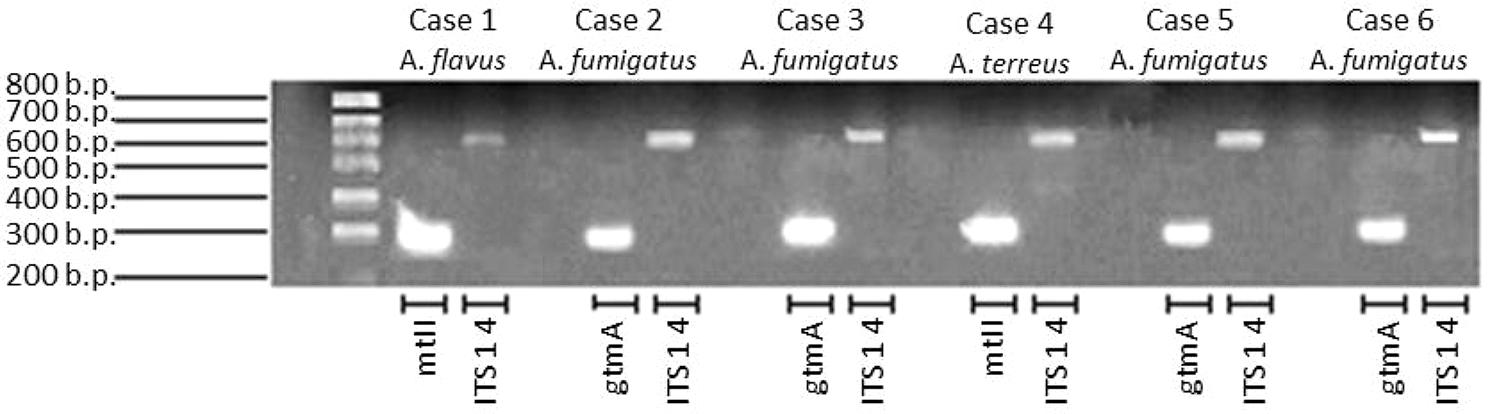
FIGURE 5. Detection of gtmA and mt-ii genes in clinical isolates from probable/proven invasive aspergillosis cases.
Finally, in order to confirm that A. nidulans and A. niger did not produce GT and, thus, are unable to endogenously synthesize bmGT, we analyze the presence of gliP gene (a critical gene within the gli cluster responsible for GT synthesis) by PCR. None of the isolates from A. niger and A. nidulans carry the gliP gene (data not shown) confirming that they are unable to produce GT and bmGT, as found in the cell culture analysis (Figure 1). Moreover, a bioinformatic analyses searching for the presence of gli cluster homology sequences in the genome of sequenced A. niger and A. nidulans strains, yielded negative results, confirming our experimental data and in line with previous findings (De Pauw et al., 2008; Manzanares-Miralles et al., 2016). In contrast, sequences with some homology to gli cluster were found in both A. terreus and A. flavus genomes (data not shown) as previously indicated (Patron et al., 2007).
Despite recent advances, the lack of a single gold standard technique and the limitations of the available ones, make diagnosis of IA still challenging (Maertens et al., 2016; Mercier and Maertens, 2017). In recent years, new metabolite based diagnostic tools have been under research, such as GT or volatile organic compounds (Lewis et al., 2005a; Chambers et al., 2009).
Regarding GT, its high biological reactivity and its potential ability to interact with cells and tissues (Domingo et al., 2012) make it hard to detect in body fluids (Scharf et al., 2012). This limitation is overcame by bmGT, which is more stable and reliably detected in serum (Domingo et al., 2012). It is known that A. fumigatus produces GT in the highest concentrations and more frequently than other Aspergillus species (Lewis et al., 2005b; Kupfahl et al., 2008). This conclusion has been supported by our results, in which 77% of the A. fumigatus isolates were GT-producers in significantly higher concentrations and frequencies than those obtained for A. flavus and A. terreus species complexes, the other Aspergillus-producing GT species. With reference to bmGT, A. fumigatus was also the most common bmGT producing complex. Notably, and in line with previous findings (De Pauw et al., 2008; Manzanares-Miralles et al., 2016), neither A. niger or A. nidulans strains were able to endogenously produce GT and/or bmGT, although methyl-transferase activity was found in A. niger isolates when using exogenous GT. These results are supported by both PCR analyses and bioinformatic studies confirming the absence of the gli cluster in these species. These findings contrast to previous studies in which a high proportion of A. niger isolates were shown to produce GT (Kupfahl et al., 2008). We have no explanation for these contradictory findings although, in line with our findings, Kupfahl et al. (2008) did not detect gliP gene in A. niger, which has been shown to be critical for GT synthesis, at least in A. fumigatus isolates (Sugui et al., 2007).
Some authors have been interested in the secondary metabolite profiles of cryptic species of the Fumigati section. Unlike other authors, we found out that A. lentulus and A. fumigatiaffinis were able to produce GT and bmGT (Larsen et al., 2007; Sugui et al., 2010; Tamiya et al., 2015). Regrettably, we just analyzed one isolate of A. calidoustus and A. novofumigatus. The A. calidoustus isolate was GT and bmGT producer, but A. novofumigatus was not. This result does not rule out the ability to produce GT and bmGT by A. novofumigatus since culture conditions (medium, aeration, temperature, sampling time…) affect to secondary metabolite synthesis (Belkacemi et al., 1999; Watanabe et al., 2004). This could explain the low GT and bmGT detection among non-A. fumigatus species since all experiments were performed in the same conditions and confirmed employing other culture protocols.
Aiming to avoid such a limitation, we analyzed the ability to produce bmGT from an exogenous source of GT among non-toxigenic isolates. We detected bmGT in culture filtrates of all the A. flavus and A. terreus analyzed, thus suggesting a consistent ability to produce bmGT. To our knowledge no specific methyltransferases had been described to date for these species. Nevertheless, it has been described an ortholog and a homolog of GtmA for A. terreus and A. flavus by bioinformatics analysis but it is the first time in which its expression has been described. In our study, A. niger also showed the ability to methylate GT, but less frequently. Curiously, none of the A. nidulans isolates analyzed produced bmGT even when it has been described that a specific methyltransferase (and the encoding gene) able to produce bmGT from GT for this species (Manzanares-Miralles et al., 2016). This discrepancy could be due to the known fact that culture conditions do not reflect the genetic potential and that not all the strains of the same species have the same metabolic profile (Bergmann et al., 2007). Indeed, secondary metabolism confers a survival benefit to the producing isolate and the in vitro culture conditions are not optimal to activate this survival pathway, depending on the Aspergillus spp. and/or isolate.
In order to overcome the limitations of the in vitro culture to analyze secondary metabolism, we have employed some clinical isolates from patients with probable/proven IA and compared bmGT production in in vitro culture with bmGT in serum from those patients. In this scenario, where fungi has to colonize the host and adapt itself to the new environmental conditions, the fungi would activate secondary metabolism and display all potential virulence factors such a GT (Cramer et al., 2006; Sugui et al., 2007) In these conditions, all but one of the seven probable/proven patients had bmGT detectable in serum, even those that did not produce GT and bmGT in vitro. Importantly, all of them were able to produce bmGT from exogenous GT and carried an ortholog of GtmA, MT-ii.
In summary, our findings indicate that bmGT production is useful to diagnose IA caused by A. fumigatus and, at some extent, by A. terreus and A. flavus, although at a much lower frequency, since they present the ability to methylate GT and endogenously produce bmGT in vitro and in vivo. Moreover, and pending of validation with a higher number of samples, our novel findings indicate that conclusions about the expression of molecules that could be used as potential diagnostic biomarkers based on in vitro fungal cultures cannot be reached unless they are confirmed in proper in vivo studies employing animal models or patients suffering from IA.
MV-G and SR carried out the experiments. PM helped with genomic analysis and CC helped with in vitro experiments. MD performed the HPTLC analysis. MV-G wrote the manuscript with support from AR, JP, and EG. JM provided and characterized different isolates of Aspergillus spp. JP and EG conceived the original idea and supervised the project with the support of AR.
This work was supported by Fondo Social Europeo (FSE; Gobierno de Aragón) and by grants MAT2011-26851-C02-02, SAF2014- 54763-C2-1-R, SAF2014-54763-C2-2-R from Spanish Ministry of Economy and Competitiveness. JP was supported by Aragón I+D (ARAID). MV-G was granted by a Río Hortega contract of National Institute of Health Carlos III (CM16/00236).
MD, JP, and EG are co-inventors of a patent licensed to Blackhills Diagnostic Resources S.L. that protects the use of bmGT to diagnose IA (PCT/EP2012/058,247).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We kindly acknowledge Dr. J. F. Meis from Canisius-Wilhelmina Hospital, Nijmegen (Netherlands) and Dr. E. Bouza from Gregorio Marañón University Hospital, Madrid (Spain) for providing clinical isolates from several Aspergillus species and from cryptic species, respectively. We would like to acknowledge ASPANOA (Asociacion de Padres de Niños con Cáncer de Aragón) for its support to develop this work.
Andersen, M. R., Nielsen, J. B., Klitgaard, A., Petersen, L. M., Zachariasen, M., Hansen, T. J., et al. (2013). Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc. Natl. Acad. Sci. U.S.A. 110, E99–E107. doi: 10.1073/pnas.1205532110
Arvanitis, M., and Mylonakis, E. (2015). Diagnosis of invasive aspergillosis: recent developments and ongoing challenges. Eur. J. Clin. Invest. 45, 646–652. doi: 10.1111/eci.12448
Belkacemi, L., Barton, R. C., Hopwood, V., and Evans, E. G. V. (1999). Determination of optimum growth conditions for gliotoxin production by Aspergillus fumigatus and development of a novel method for gliotoxin detection. Med. Mycol. 37, 227–233. doi: 10.1080/j.1365-280X.1999.00225.x
Bergmann, S., Schümann, J., Scherlach, K., Lange, C., Brakhage, A. A., and Hertweck, C. (2007). Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 3, 213–217. doi: 10.1038/nchembio869
Chambers, S. T., Syhre, M., Murdoch, D. R., McCartin, F., and Epton, M. J. (2009). Detection of 2-pentylfuran in the breath of patients with Aspergillus fumigatus. Med. Mycol. 47, 468–476. doi: 10.1080/13693780802475212
Cramer, R. A., Gamcsik, M. P., Brooking, R. M., Najvar, L. K., Kirkpatrick, W. R., Patterson, T. F., et al. (2006). Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell 5, 972–980. doi: 10.1128/EC.00049-06
De Pauw, B., Walsh, T. J., Donnelly, J. P., Stevens, D. A., Edwards, J. E., Calandra, T., et al. (2008). Revised definitions of invasive fungal disease from the european organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (eortc/msg) consensus group. Clin. Infect. Dis. 46, 1813–1821. doi: 10.1086/588660
Dolan, S. K., Bock, T., Hering, V., Owens, R. A., Jones, G. W., Blankenfeldt, W., et al. (2017). Structural, mechanistic and functional insight into gliotoxin bis-thiomethylation in Aspergillus fumigatus. Open Biol. 7:160292. doi: 10.1098/rsob.160292
Dolan, S. K., O’Keeffe, G., Jones, G. W., and Doyle, S. (2015). Resistance is not futile: gliotoxin biosynthesis, functionality and utility. Trends Microbiol. 23, 419–428. doi: 10.1016/j.tim.2015.02.005
Dolan, S. K., Owens, R. A., O’Keeffe, G., Hammel, S., Fitzpatrick, D. A., Jones, G. W., et al. (2014). Regulation of nonribosomal peptide synthesis: bis-thiomethylation attenuates gliotoxin biosynthesis in Aspergillus fumigatus. Chem. Biol. 21, 999–1012. doi: 10.1016/j.chembiol.2014.07.006
Domingo, M. P., Colmenarejo, C., Martínez-Lostao, L., Müllbacher, A., Jarne, C., Revillo, M. J., et al. (2012). Bis(methyl)gliotoxin proves to be a more stable and reliable marker for invasive aspergillosis than gliotoxin and suitable for use in diagnosis. Diagn. Microbiol. Infec. Dis. 73, 57–64. doi: 10.1016/j.diagmicrobio.2012.01.012
Gardiner, D. M., and Howlett, B. J. (2005). Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol. Lett. 248, 241–248. doi: 10.1016/j.femsle.2005.05.046
Guimarães, D. O., Borges, W. S., Vieira, N. J., de Oliveira, L. F., da Silva, C. H., Lopes, N. P., et al. (2010). Diketopiperazines produced by endophytic fungi found in association with two Asteraceae species. Phytochemistry 71, 1423–1429. doi: 10.1016/j.phytochem.2010.05.012
Kupfahl, C., Michalka, A., Lass-Flörl, C., Fischer, G., Haase, G., Ruppert, T., et al. (2008). Gliotoxin production by clinical and environmental Aspergillus fumigatus strains. Int. J. Med. Microbiol. 298, 319–327. doi: 10.1016/j.ijmm.2007.04.006
Lamoth, F., and Calandra, T. (2017). Early diagnosis of invasive mould infections and disease. J. Antimicrob. Chemother. 72(Suppl. 1), i19–i28. doi: 10.1093/jac/dkx030
Larsen, T. O., Smedsgaard, J., Nielsen, K. F., Hansen, M. A. E., Samson, R. A., and Frisvad, J. C. (2007). Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section Fumigati. Med. Mycol. 45, 225–232. doi: 10.1080/13693780601185939
Lewis, R. E., Wiederhold, N. P., Chi, J., Han, X. Y., Komanduri, K. V., Kontoyiannis, D. P., et al. (2005a). Detection of gliotoxin in experimental and human aspergillosis. Infect. Immun. 73, 635–637. doi: 10.1128/IAI.73.1.635-637.2005
Lewis, R. E., Wiederhold, N. P., Lionakis, M. S., Prince, R. A., and Kontoyiannis, D. P. (2005b). Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J. Clin. Microbiol. 43, 6120–6122. doi: 10.1128/JCM.43.12.6120-6122.2005
Li, X., Kim, S.-K., Nam, K. W., Kang, J. S., Choi, H. D., and Son, B. W. (2006). A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J. Antibiot. 59, 248–250. doi: 10.1038/ja.2006.35
Liang, W.-L., Le, X., Li, H.-J., Yang, X.-L., Chen, J.-X., Xu, J., et al. (2014). Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 12, 5657–5676. doi: 10.3390/md12115657
Maertens, J. A., Blennow, O., Duarte, R. F., and Muñoz, P. (2016). The current management landscape: aspergillosis. J. Antimicrob. Chemother. 71(Suppl. 2), ii23–ii29. doi: 10.1093/jac/dkw393
Maertens, J., Van Eldere, J., Verhaegen, J., Verbeken, E., Verschakelen, J., and Boogaerts, M. (2002). Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J. Infect. Dis. 186, 1297–1306. doi: 10.1086/343804
Maertens, J., Verhaegen, J., Demuynck, H., Brock, P., Verhoef, G., Vandenberghe, P., et al. (1999). Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J. Clin. Microbiol. 37, 3223–3228.
Manzanares-Miralles, L., Sarikaya-Bayram,Ö., Smith, E. B., Dolan, S. K., Bayram, Ö., Jones, G. W., et al. (2016). Quantitative proteomics reveals the mechanism and consequence of gliotoxin-mediated dysregulation of the methionine cycle in Aspergillus niger. J. Proteomics 131, 149–162. doi: 10.1016/j.jprot.2015.10.024
Mercier, T., and Maertens, J. (2017). Clinical considerations in the early treatment of invasive mould infections and disease. J. Antimicrob. Chemother. 72(Suppl. 1), i29–i38. doi: 10.1093/jac/dkx031
Odabasi, Z., Mattiuzzi, G., Estey, E., Kantarjian, H., Saeki, F., Ridge, R. J., et al. (2004). Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39, 199–205. doi: 10.1086/421944
Patron, N. J., Waller, R. F., Cozijnsen, A. J., Straney, D. C., Gardiner, D. M., Nierman, W. C., et al. (2007). Origin and distribution of epipolythiodioxopiperazine (ETP) gene clusters in filamentous ascomycetes. BMC Evol. Biol. 7:174. doi: 10.1186/1471-2148-7-174
Riwes, M. M., and Wingard, J. R. (2012). Diagnostic methods for invasive fungal diseases in patients with hematologic malignancies. Expert Rev. Hematol. 5, 661–669. doi: 10.1586/ehm.12.53
Scharf, D. H., Heinekamp, T., Remme, N., Hortschansky, P., Brakhage, A. A., and Hertweck, C. (2012). Biosynthesis and function of gliotoxin in Aspergillus fumigatus. Appl. Microbiol. Biotechnol. 93, 467–472. doi: 10.1007/s00253-011-3689-1
Scharf, D., Habel, A., Heinekamp, T., Brakhage, A., and Hertweck, C. (2014). Opposed effects of enzymatic gliotoxin N- and S-methylations. J. Am. Chem. Soc. 136, 11674–11679. doi: 10.1021/ja5033106
Stynen, D., Goris, A., Sarfati, J., and Latge, J. P. (1995). A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33, 497–500.
Sugui, J. A., Pardo, J., Chang, Y. C., Zarember, K. A., Nardone, G., Galvez, E. M., et al. (2007). Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell 6, 1562–1569. doi: 10.1128/EC.00141-07
Sugui, J. A., Vinh, D. C., Nardone, G., Shea, Y. R., Chang, Y. C., Zelazny, A. M., et al. (2010). Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J. Clin. Microbiol. 48, 220–228. doi: 10.1128/JCM.01556-09
Sun, Y., Takada, K., Takemoto, Y., Yoshida, M., Nogi, Y., Okada, S., et al. (2012). Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J. Nat. Prod. 75, 111–114. doi: 10.1021/np200740e
Tamiya, H., Ochiai, E., Kikuchi, K., Yahiro, M., Toyotome, T., Watanabe, A., et al. (2015). Secondary metabolite profiles and antifungal drug susceptibility of Aspergillus fumigatus and closely related species, Aspergillus lentulus, Aspergillus udagawae, and Aspergillus viridinutans. J. Infect. Chemiother. 21, 385–391. doi: 10.1016/j.jiac.2015.01.005
Thornton, C. R. (2008). Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin. Vaccine Immunol. 15, 1095–1105. doi: 10.1128/CVI.00068-08
Vidal-García, M., Domingo, M. P., De Rueda, B., Roc, L., Delgado, M. P., Revillo, M. J., et al. (2016). Clinical validity of bis(methylthio)gliotoxin for the diagnosis of invasive aspergillosis. Appl. Microbiol. Biotechnol. 100, 2327–2334. doi: 10.1007/s00253-015-7209-6
Vidal-García, M., Sánchez-Chueca, P., Domingo, M. P., Ballester, C., Roc, L., Ferrer, I., et al. (2017). Disseminated aspergillosis in an immunocompetent patient with detectable bis(methylthio)gliotoxin and negative galactomannan. Rev. Iberoam. Micol. 34, 49–52. doi: 10.1016/j.riam.2016.05.007
Watanabe, A., Kamei, K., Sekine, T., Waku, M., Nishimura, K., Miyaji, M., et al. (2004). Effect of aeration on gliotoxin production by Aspergillus fumigatus in its culture filtrate. Mycopathologia 157, 245–254. doi: 10.1023/B:MYCO.0000012224.49131.dd
White, P. L., Wingard, J. R., Bretagne, S., Löffler, J., Patterson, T. F., Slavin, M. A., et al. (2015). Aspergillus polymerase chain reaction: systematic review of evidence for clinical use in comparison with antigen testing. Clin. Infect. Dis. 61, 1293–1303. doi: 10.1093/cid/civ507
Keywords: bis(methylthio)gliotoxin, Aspergillus spp., gtmA, invasive aspergillosis, biomarker
Citation: Vidal-García M, Redrado S, Domingo MP, Marquina P, Colmenarejo C, Meis JF, Rezusta A, Pardo J and Galvez EM (2018) Production of the Invasive Aspergillosis Biomarker Bis(methylthio)gliotoxin Within the Genus Aspergillus: In Vitro and in Vivo Metabolite Quantification and Genomic Analysis. Front. Microbiol. 9:1246. doi: 10.3389/fmicb.2018.01246
Received: 02 March 2018; Accepted: 23 May 2018;
Published: 12 June 2018.
Edited by:
Martin Hoenigl, University of California, San Diego, United StatesReviewed by:
Sean Doyle, Maynooth University, IrelandCopyright © 2017 Vidal-García, Redrado, Domingo, Marquina, Colmenarejo, Meis, Rezusta, Pardo and Galvez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matxalen Vidal-García, bWF0dmlnYXJAZ21haWwuY29t Eva M. Galvez, ZXZhQGljYi5jc2ljLmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.