- 1Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 2Department of Laboratory Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 3Chang Gung University College of Medicine, Kaohsiung, Taiwan
This study aimed to determine the predictors of persistent candidemia and examine the impact of biofilm formation by Candida isolates in adult patients with candidemia. Of the adult patients with candidemia in Kaohsiung Chang Gung Memorial Hospital between January 2007 and December 2012, 68 case patients with persistent candidemia (repeated candidemia after a 3-day systemic antifungal therapy) and 68 control patients with non-persistent candidemia (Candida clearance from the bloodstream after a 3-day systemic antifungal therapy) were included based on propensity score matching and matching for the Candida species isolated. Biofilm formation by the Candida species was assessed in vitro using standard biomass assays. Presence of central venous catheters (CVCs) at diagnosis (adjusted odd ratio [AOR], 3.77; 95% confidence interval [CI], 1.09–13.00, p = 0.04), infection with higher biofilm forming strains of Candida species (AOR, 8.03; 95% CI, 2.50–25.81; p < 0.01), and receipt of suboptimal fluconazole doses as initial therapy (AOR, 5.54; 95% CI, 1.53–20.10; p < 0.01) were independently associated with persistent candidemia. Biofilm formation by Candida albicans, C. tropicalis, and C. glabrata strains was significantly higher in the case patients than in the controls. There were no significant differences in the overall mortality and duration of hospitalization between the two groups. Our data suggest that, other than presence of retained CVCs and use of suboptimal doses of fluconazole, biofilm formation was highly associated with development of persistent candidemia.
Introduction
The mortality among patients with invasive candidiasis is as high as 40%, which has been shown to be related to severity of the underlying disease, presence of retained infected vascular catheters, and delayed initiation of appropriate antifungal therapy (Kullberg and Arendrup, 2015; Pappas et al., 2016). To improve therapeutic strategies for candidemia, the most common manifestation of invasive candidiasis (Kullberg and Arendrup, 2015), the Infectious Disease Society of America (IDSA) treatment guidelines recommend follow-up blood cultures every day or every other day in patients with candidemia until clearance of Candida from the bloodstream (Pappas et al., 2016).
Persistent fungemia, an increasingly recognized complication of candidemia, has been reported in 8–15% of patients with candidemia (Nucci, 2011). In addition to host factors, such as poor general condition, several possible mechanisms have been suggested for persistent candidemia: drug resistance, low serum drug levels, endovascular infection, deep-tissue abscesses, and infections associated with prosthetics (Nucci, 2011). Unlike bacteremia, in which persistent positive blood cultures after initiation of antibiotic therapy indicates poor clinical outcome, persistent candidemia has not consistently been associated with increased mortality compared with non-persistent candidemia (Nucci, 2011). However, the case fatality rate among neonates with persistent candidemia was significantly higher than among those with non-persistent candidemia (Hammoud et al., 2013). Currently, the clinical significance of persistent candidemia remains debatable. The intensive care unit (ICU) stay, the presence of retained vascular catheters, and severity of illness were independent predictors of death (Nucci, 2011; Hammoud et al., 2013; Kang et al., 2017). In contrast, early mortality might preclude repetitive blood sampling from being performed for cultures during candidemia management, which may lead to immortal bias when examining the relationship between persistent candidemia and mortality. Well-designed studies with blood cultures performed in a timely fashion in the patients with candidemia are warranted to address this issue.
A biofilm is mainly composed of sessile cells and extracellular matrix, and its formation is initiated by the adherence of free-moving planktonic cells to surfaces of the human endothelium or medical implants (Donlan and Costerton, 2002). Biofilm formation is considered a virulence factor because it might lead to antifungal resistance and protection of fungal cells from immune responses. Biofilm formation by the Candida species may also be responsible for these fungi being well adapted to colonization of tissues and indwelling devices, which may lead to difficulties in eradicating these micro-organisms from the blood (Ramage et al., 2012; Taff et al., 2013). Biofilm formation is considered an independent predictor of mortality among patients with candidemia (Tumbarello et al., 2007). To our knowledge, little is known about the relationship between the microbiological characteristics of Candida isolates, such as biofilm formation, and the risk of persistent candidemia. Thus, this study aimed to identify the factors associated with persistent candidemia and to examine the impact of biofilm formation among adult patients with persistent candidemia.
Materials and Methods
Hospital Setting and Patients
This study was conducted at Kaohsiung Chang Gung Memorial Hospital, a 2,700-bed facility serving as a primary care and tertiary referral center in southern Taiwan, between January 2007 and December 2012. This retrospective study with a waiver of patient consent was approved by the Institutional Review Board of Chang Gung Memorial Hospital [No. 2016-01495B0].
Adult patients (aged ≥ 18 years) with candidemia who had blood cultures repeated after antifungal therapy had been continued for at least 3 days were included in this study. If patients experienced more than one candidemia episode, only the first episode was analyzed. All of the blood cultures were obtained directly from peripheral vessels of patients, not from any retained vessel catheters. The demographic and clinical information were retrieved, including age, gender, underlying diseases, laboratory data [neutrophil counts, albumin, and creatinine for estimated glomerular filtration rate (eGFR)], candidemia severity at onset [acute physiology and chronic health evaluation (APACHE) II score and the sequential organ failure assessment (SOFA) score], exposure to antibiotic and antifungal agents within 1 month before onset of candidemia, treatment regimens, and clinical outcomes. Patients’ outcomes included duration of antifungal treatment after onset of candidemia, early mortality, overall mortality, and duration of hospital stay.
Definitions
Persistent candidemia was defined as presence of candidemia at repeat blood cultures in patients who had received systemic antifungal agents for at least 3 days (Safdar et al., 2004), and the Candida species identified from initial and repeat blood cultures were identical. The underlying diseases/conditions included type 2 diabetes mellitus (Handelsman et al., 2011), chronic kidney disease (eGFR < 60 mL/min per 1.73 m2) (Levey et al., 2003), liver cirrhosis (diagnosed by an abdominal sonography) (Hung et al., 2003), solid tumors, hematologic diseases, organ transplantation, and high-dose steroid use (≥0.3 mg/kg prednisolone or its equivalent for more than 3 weeks) (Lionakis and Kontoyiannis, 2003). Neutropenia was considered to occur when the granulocyte count was <1000 mm3 (Nucci and Colombo, 2002), and malnutrition was defined as albumin level of <3.0 g/dl. Recent chemotherapy and abdominal surgery were defined as receiving therapy within 1 month before onset of candidemia. Acute kidney injury was defined as a decreased eGFR below 50% of the normal range (Bellomo et al., 2004). Presence of a central venous catheter (CVC) at diagnosis of candidemia was recorded, and early CVC removal was defined as removing CVC within 48 h after detection of candidemia (Nucci et al., 2010). Shock was diagnosed as having a mean arterial pressure <65 mmHg, the occurrence of peripheral hypoperfusion, and the need for vasopressors (Rhodes et al., 2017). Infectious disease (ID) consultation within 48 h after blood culture collection referred to both the initial consultation and the period when patients were already followed by an ID specialist (Farmakiotis et al., 2015). Antifungal therapy was considered inappropriate when (i) Candida isolates were resistant to empirical antifungal therapy according to the CLSI M27-S4 document (Clinical and Laboratory Standards Institute [CLSI], 2012), (ii) the loading dose or/and maintenance dose of antifungal agents was insufficient (Gilbert et al., 2017), or/and (iii) the first dose of antifungal agents was used more than 48 h after positive blood cultures. The antimicrobial regimens and dosage were selected at the discretion of the attending physician. The early and overall mortality were defined as death from any cause within 14 days from the onset of candidemia and during hospitalization, respectively.
Identification and Antifungal Susceptibility
Microorganisms isolated from the blood cultures were detected using the BD BACTEC FX system (Becton, Dickinson Microbiology System, Sparks, MD, United States). These cultures were incubated for 5 days at 35°C. A yeast-like microorganism was detected by microscopic inspection after Gram staining. A subsequent identification of Candida spp. was performed with the use of CHROMagar Candida (Bectons, Dickson and Company Co.) or API-ID32C (BioMerieux, Vitek, Hazelwood, MO, United States). Candida isolates were stored at -70°C in a skim milk with glycerol until they were tested (Diagnostic Systems, Sparks, MD, United States).
The antifungal susceptibility of the isolates was classified in accordance with the Clinical and Laboratory Standards Institute (CLSI) M27-S4 document (Clinical and Laboratory Standards Institute [CLSI], 2012). For antifungal susceptibility testing, all isolates were thawed and inoculated onto Sabouraud dextrose agars (SDA) and incubated at 35°C for 24 h. Subculture from SDA was performed to obtain a pure 24-h culture of the Candida isolate and to prepare a suspension with an organism density of approximately 1.5 × 103 CFU/mL. The susceptibility of all tested isolates to six antifungal agents (amphotericin B, fluconazole, voriconazole, anidulafungin, caspofungin, and micafungin) was determined using the broth microdilution method with the Sensititre YeastOne (SYO) system (part YO-09; Trek Diagnostic System) according to the manufacturer’s instructions. The broth microdilution method with SYO system was formed in a 96-well microtiter plate and prepared with 12 dilutions of each drug: amphotericin B concentration ranges from 0.12 to 4 mg/L, fluconazole0.12 to 256 mg/L, voriconazole 0.008 to 8 mg/L, anidulafungin 0.015 to 8 mg/L, micafungin 0.008 to 8 mg/L, and caspofungin 0.008 to 8 mg/L. Plates were inoculated using a prepared suspension of the organism. Alamar Blue was incorporated in each well, which displayed a change in color from blue to pink in the presence of a microbial growth. Colorimetric MIC results for all agents tested were defined as the lowest concentration of antifungal agent that prevented the development of red colonies. Quality control was performed by testing the CLSI recommended strains, C. krusei ATCC 6258, and C. parapsilosis ATCC 22019.
Biofilm Testing and Antifungal Susceptibility on Biofilm Formation of C. albicans
Candida isolates displayed heterogeneity with respect to their biofilm biomass when grown in RPMI. Standardized Candida isolates in RPMI-1640 were grown in flat-bottomed 96-well microtiter plates for 24 h at 37°C. Mature biofilms were carefully washed with PBS, allowed to air dry, and biomass quantified by staining with 0.05% w/v crystal violet (CV) solution. The biofilms were washed and distained with 100% ethanol. The biomass was quantified spectrophotometrically by reading the absorbance at 550 nm in a microtiter plate reader (FluoStar Omega BMG Labtech, Aylesbury, United Kingdom). Three replicates were used for each isolate and were carried out on one occasion. Isolates of each species were categorized as low biofilm formers (LBFs) or high biofilm formers (HBFs) if their biomass absorbance values were less than the first quartile (Q1) or greater than the third quartile (Q3), respectively. Those isolates in between the first and third quartiles (Q1–Q3) were defined as intermediate biofilm formers (IBF) (Sherry et al., 2014).
Furthermore, the C. albicans’ biofilm formation to different classes of antifungal agents was also assessed. The biofilms were treated with either 4 mg/L or 256 mg/L fluconazole, caspofungin, or amphotericin B. After incubation for 24 h, the metabolic activity was measured with the XTT assay, with the optical density being read at 492 nm (Pierce et al., 2008). The percentage viability was calculated relative to the untreated controls, and the data were presented as mean ± standard deviation. Three replicates were used for each isolate and were carried out on one occasion.
Testing for Invasiveness of Candida Isolates
Candida strains were streaked out with a flat toothpick to form single colonies (typically 50 colonies/plate) on YPD plates (yeast extract “Difco” 2%, Bacto Peptone “Difco” 4%, glucose 2%, Bacto-agar “Difco” 2%, and tryptophan 0.003%). All plates were incubated at 37°C, and the extent of invasion was scored after 2 days. Invasion was determined as follows: (i) cells that had not invaded the agar were washed away by rubbing the plate with a gloved finger while rinsing under running water, and (ii) cells that had invaded the agar remained as macroscopically visible microcolonies on the surface of the washed plate and confirmed by a microscopical examination (with a bright light field, 10× magnification) after washing. The invasiveness of Candida isolation was defined as follows: (i) strong invasiveness: more than two-thirds of the colonies kept retained in the agar after washing, (ii) moderate invasiveness: one-third to two-thirds of the colonies kept retained in the agar after washing, and (iii) weakness invasiveness: less than one-third of the colonies kept retained in the agar after washing (Supplementary Figure S1) (Kontoyiannis et al., 2001).
Statistical Analysis
The included patients with candidemia were divided into two groups for analyses: patients with and those without persistent candidemia. Furthermore, the propensity score described the probability, on the basis of age, gender, underlying disease, and disease severity. Subsequently, patients with and those without persistent candidemia were matched (1:1) by propensity score with a caliper radius of 0.2 sigma, Candida species (forced match variable), APACHE II score, and SOFA score (covariate variables) with the use of NCSS (Version 11, LLC, Kaysville, UT). Dichotomous variables were analyzed with a x2 test or Fisher’s exact test appropriately, and continuous variables were analyzed using the Mann–Whitney U-test. The variables with a p-value ≤ 0.2 in univariate analysis were then included in the forward stepwise conditional logistic regression analysis. The discriminatory power of this derivation model was tested using the receiver operating curve (ROC) analysis by assessing the area under the curve (AUC), and the calibration efficiency was tested using the Hosmer–Lemeshow goodness-of-fit test for estimating goodness of fits to the data. A p-value ≤ 0.05 was considered statistically significant. A statistical analysis was conducted using the SPSS statistical analysis system (Version 22.0, IBM Corp, Armonk, NY, United States).
Results
A total of 558 adult patients with candidemia were hospitalized between 2007 and 2012, and 424 (76.0%) had blood cultures repeated after the first growth of Candida spp. Patients aged <18 years (n = 21), those with less than 3-day antifungal therapy before repeating blood cultures (n = 154), and those with incomplete clinical data (n = 11) were excluded. As a result, 238 patients were eligible for case-control study, including 73 with persistent candidemia. Among these 73 patients with persistent candidemia, 33 were infected by C. albicans, 18 by C. tropicalis, 11 by C. parapsilosis, 10 by C. glabrata, and 1 by C. krusei. To reduce the bias of some cases in which blood cultures were not obtained in a timely fashion, we matched 68 patients with persistent candidemia (case patients) with 68 patients without persistent candidemia (control patients) by propensity score, Candida species, APACHE II, and SOFA score (Figure 1). The standardized difference was 42.85% before propensity-score matching and was 1.59% after matching.
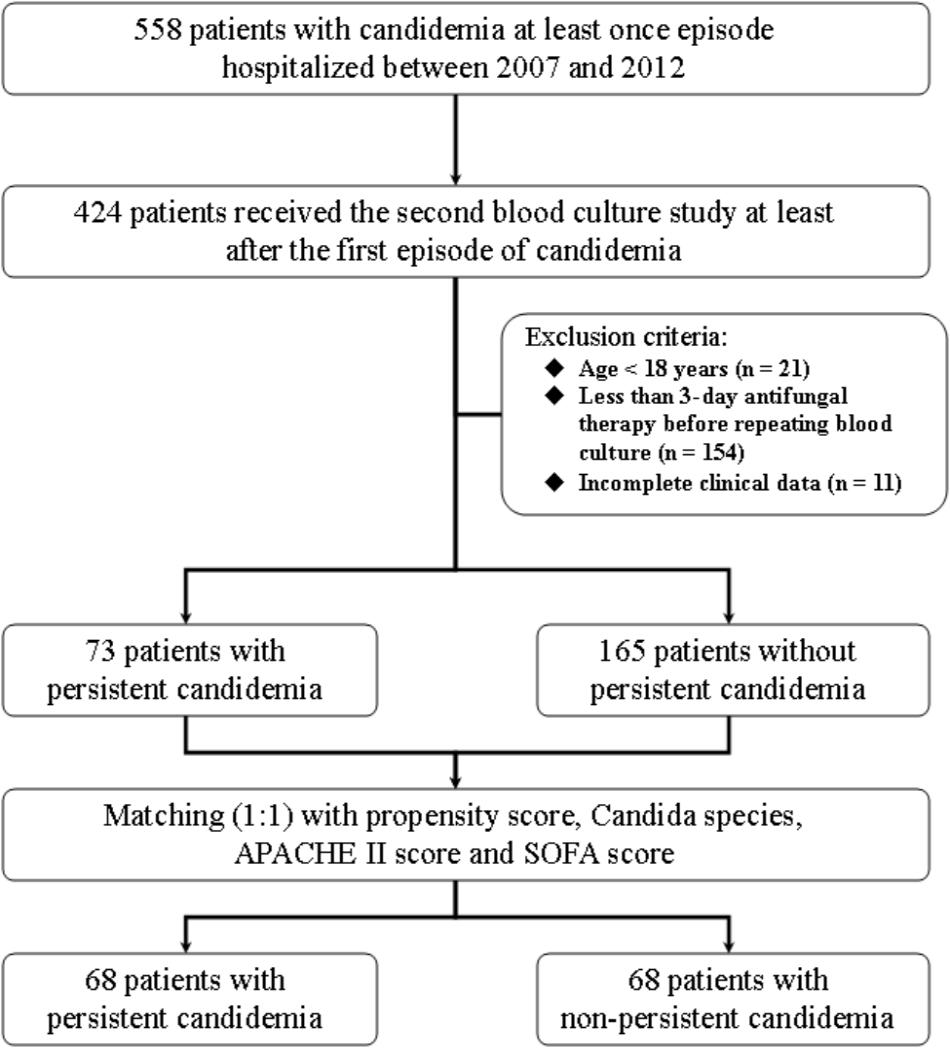
FIGURE 1. Patient selection flowchart. APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
After propensity-score matching, there were no significant differences in terms of age, gender, underlying disease, disease severity, usage of total parenteral nutrition, concomitant bacteremia, and recent exposure to antibiotics, echinocandins, or azoles between the case patients and controls (Table 1). Case patients were more likely to have CVCs at the onset of candidemia but it did not reach significant difference (86.8% vs. 73.5%, p = 0.06). In the univariate analysis, case patients were less likely to receive empirical therapy with echinocandin (26.5% vs. 44.1%, p = 0.03), more received inappropriate antifungal therapy within 48 h after blood culture collection (64.7% vs. 41.2%, p = 0.01), including ineffective use of antifungal agents due to resistance (13.2% vs. 2.9%, p = 0.01) and suboptimal dose of antifungal agents (33.8% vs. 7.4%, p < 0.01). The antifungal agents with suboptimal dose all were prescribing fluconazole. There was no significant distinction in ID consultation within 48 h after blood culture collection, early CVC removal and early and overall mortality rates between case and control patients (Table 2).
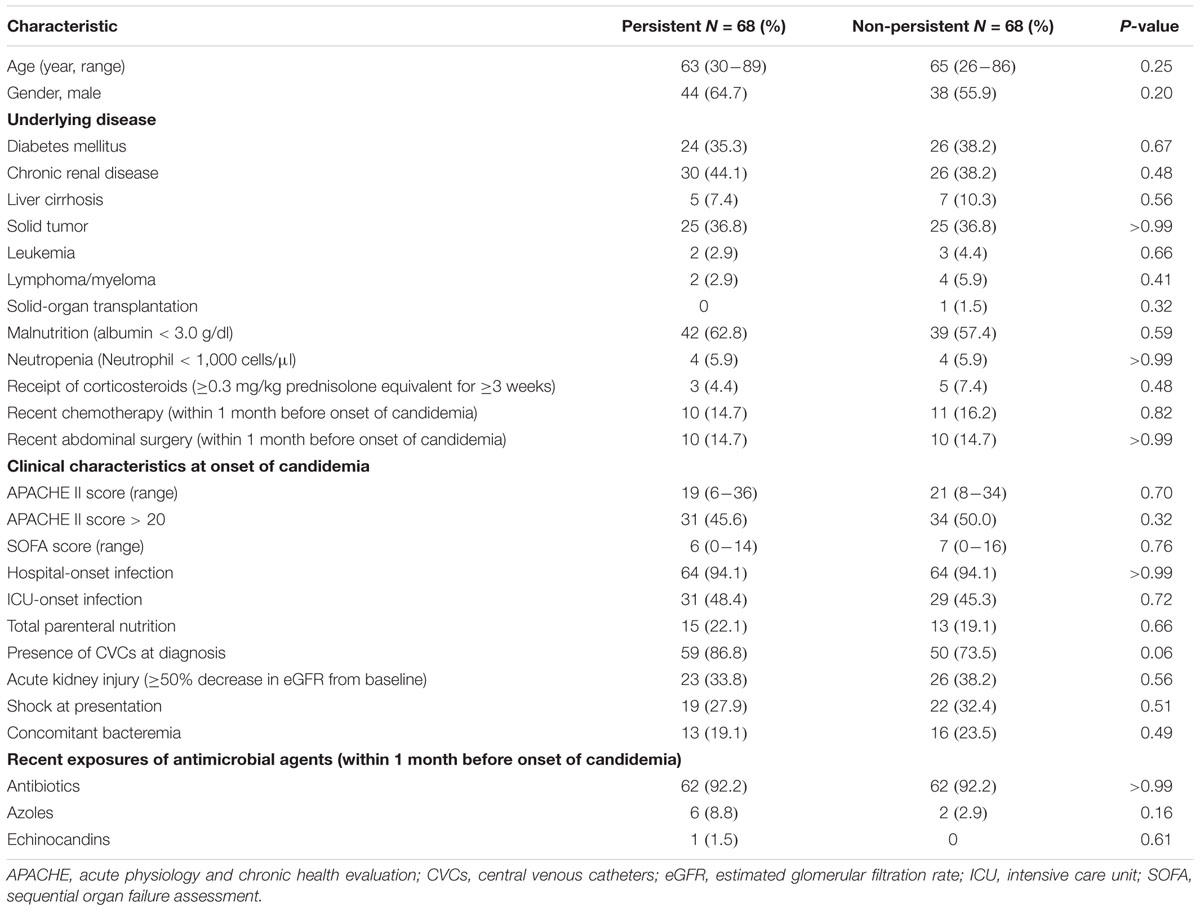
TABLE 1. Characteristics of the 136 adult patients with persistent and non-persistent candidemia after propensity-score matching.
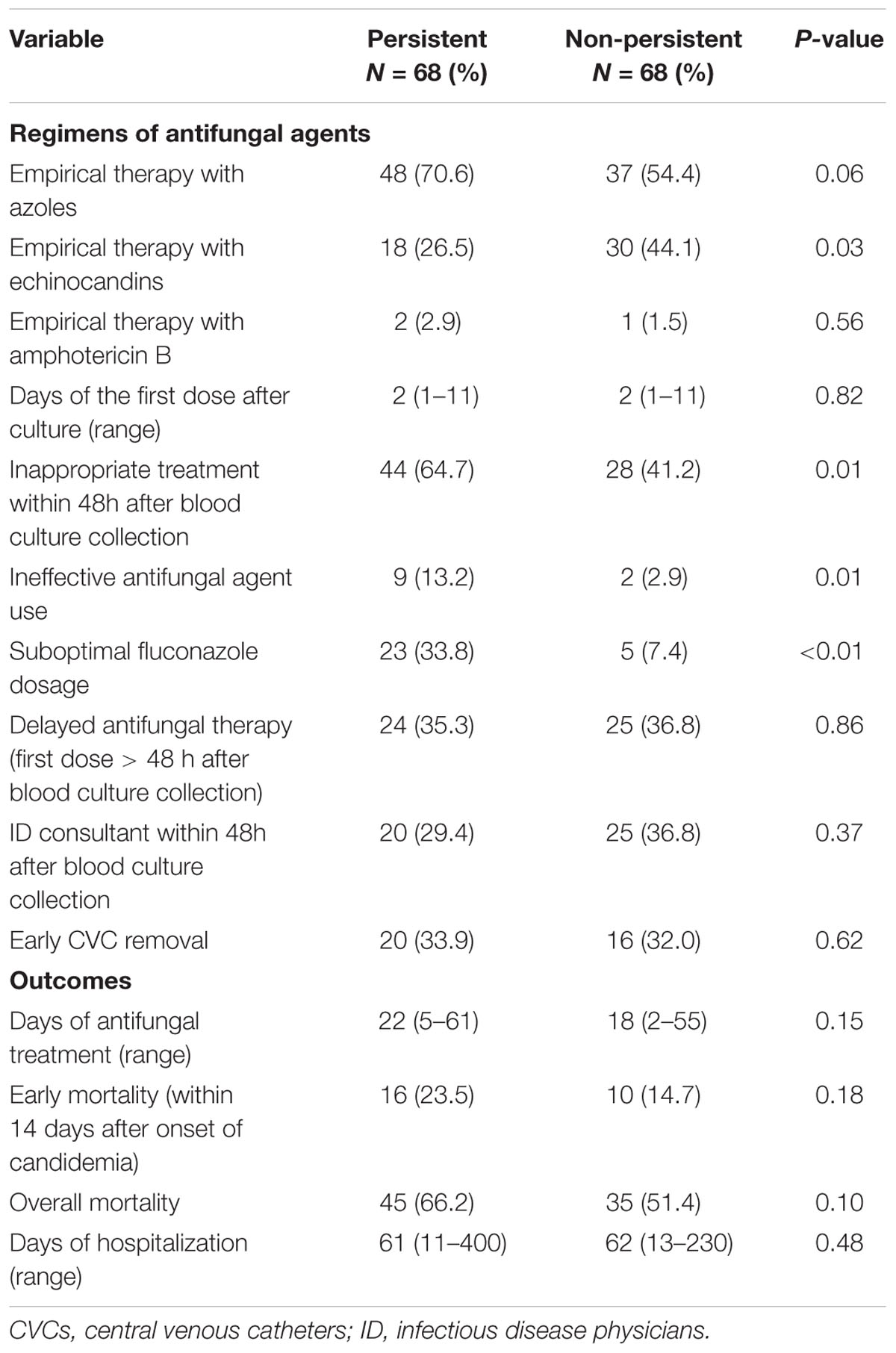
TABLE 2. Treatment regimens and outcomes of the 136 adult patients with persistent and non-persistent candidemia after propensity-score matching.
There was no difference in the resistance to fluconazole or caspofungin among the Candida strains in both groups. The susceptibility of the initial and subsequent blood isolates of Candida species to antifungal agents in each case patient was determined, which revealed that the MICs of antifungal agents for the initial and subsequent isolates remained unchanged. The proportions of HBF and high-IBF were significantly higher among Candida strains isolated from the case patients than those isolated from controls (60.3% vs. 22.1%, p < 0.01; and 92.6% vs. 75.0%, p < 0.01, respectively) (Table 3). The levels of biofilm formation quantified by the CV assay for the 136 Candida isolates were listed in Supplementary Table S1. Of all Candida isolates identified (n = 238), we found that biofilm formation in the isolates of C. albicans, C. tropicalis, and C. glabrata from the case patients were higher than those from the controls (Figure 2).
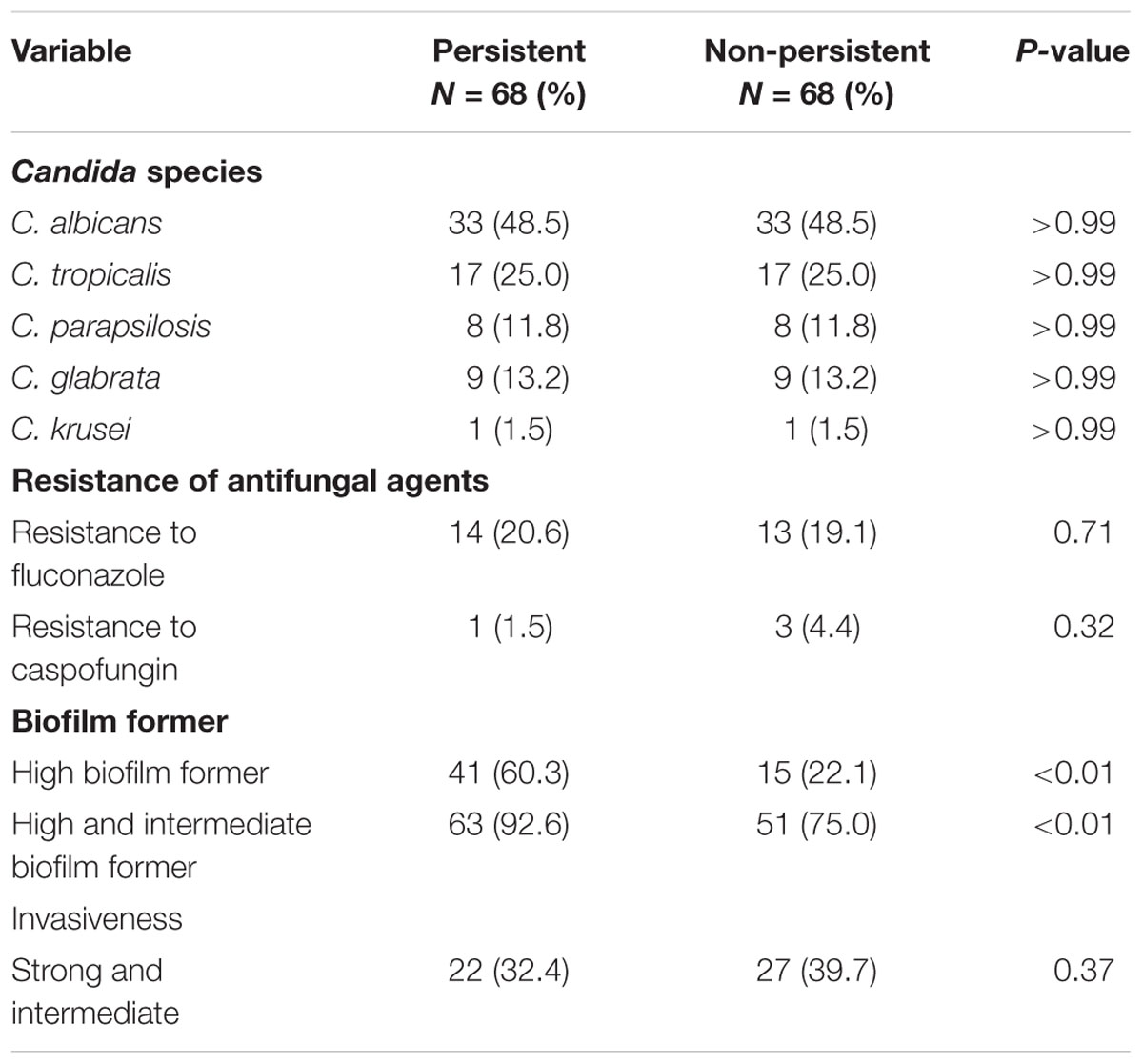
TABLE 3. The microbiological characteristics of Candida strains isolated from the 136 adult patients with persistent and non-persistent candidemia after propensity-score matching.
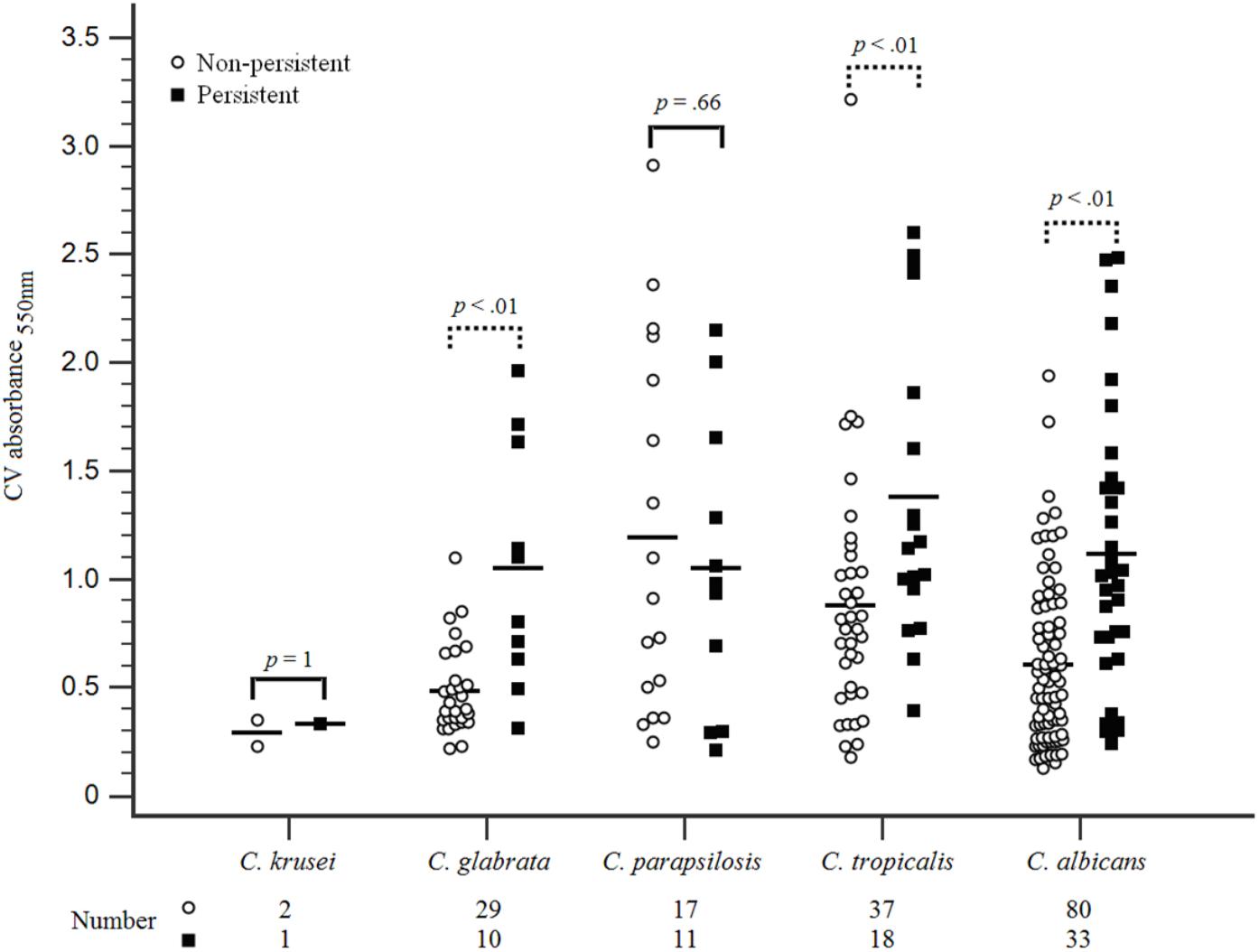
FIGURE 2. Biofilm formation on Candida species isolated from patients with persistent and non-persistent candidemia. Candida bloodstream strains isolated from all included patients were evaluated for biofilm formation with standardized methods. The first Candida spp. isolate was conducted for biofilm formation evaluation when multiple isolates were collected from some of these patients within the study period. Candida isolates standardized (1 × 106 cells/mL) in RPMI-1640 were grown in flat-bottomed 96-well microtiter plates for 24 h at 37°C. The biomass quantified by staining with 0.05% w/v crystal violet solution. Biomass was quantified spectrophotometrically by reading the optical density (OD) at 550 nm in a microtiter plate reader (FluoStar Omega BMG Labtech, Aylesbury, United Kingdom). Three replicates were used for each isolate. The outlier was abandoned and the average of the 2 remaining values was represented. Higher biofilm formation in C. albicans, C. tropicalis, and C. glabrata were found among isolates in the persistent candidemia group compared with those in the non-persistent candidemia group.
The C. albicans isolates of LBFs (n = 10) and HBFs (n = 10) were tested for their susceptibility to azoles (fluconazole), polyenes (amphotericin B), and echinocandins (caspofungin) at low (4 mg/L) and high (256 mg/L) concentrations. Although fluconazole at a concentration of 4 and 256 mg/L were equally ineffective against mature HBFs and LBFs biofilms (Figure 3), a significant difference in the overall activity was observed between HBFs and LBFs at both 4 and 256 mg/L fluconazole (p < 0.05). Conversely, caspofungin and amphotericin B were effective against both HBFs and LBFs, although the levels of biofilm formation significantly impacted on the effectiveness of caspofungin between HBFs and LBFs (4 mg/L, p < 0.05; 256 mg/L, p < 0.01). Amphotericin B was shown to be equally effective against LBFs and HBFs, and no significant difference was found between HBFs and LBFs at 4 mg/L or 256 mg/L concentration. Furthermore, a 4 mg/L concentration of fluconazole and caspofungin was significantly less effective against both LBFs and HBFs than a 256 mg/L concentration of fluconazole and caspofungin.
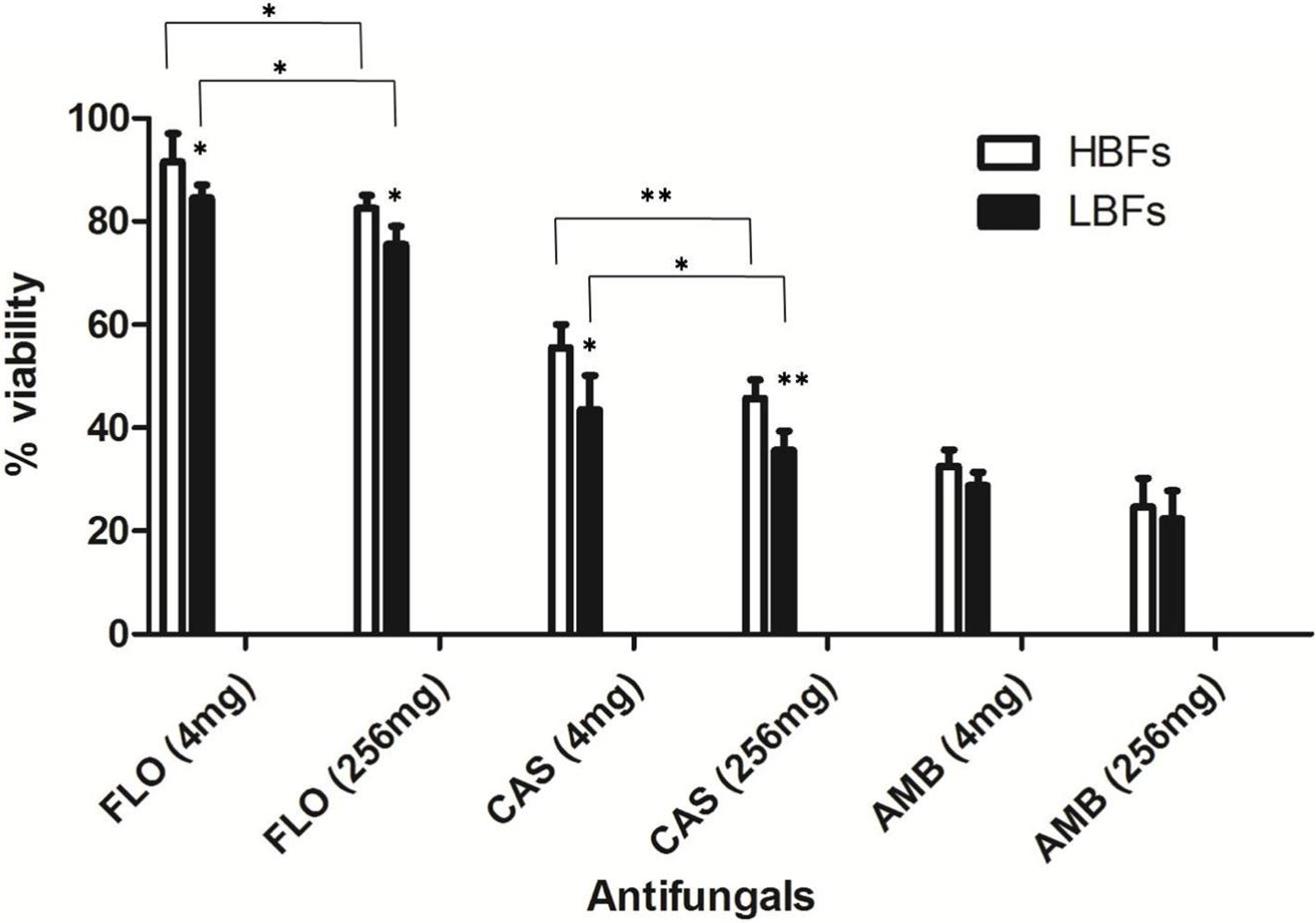
FIGURE 3. Impact of Candida albicans biofilm formation on antifungal susceptibility. Ten low biofilm formers (LBFs) and high biofilm formers (HBFs) were standardized to 1 × 106 cells/mL in RPMI-1640, and grown as biofilms in flat-bottomed 96-well microtiter plates for 24 h. Biofilms were washed with phosphate-buffered saline before being treated with 4 mg/L or 256 mg/L fluconazole (FLO), caspofungin (CAS), and amphotericin B (AMB). Following treatment, the proportional viability was compared with that untreated control by use of an XTT metabolic assay. ∗p < 0.05, ∗∗p < 0.01.
Invasiveness was tested on 136 Candida strains isolated from the two groups of patients after propensity-score matching. With respect to the differences between biofilm formers, strains with strong/intermediate invasiveness were not significantly different among the isolates from the case patients and controls (32.4% vs. 39.7%, p = 0.37) (Table 3), even if classified by each Candida species (Figure 4). Of the 136 Candida strains (Figure 4), C. tropicalis (31/34; 91.2%) accounted for the highest proportion of isolates with strong invasiveness, followed by C. parapsilosis (8/16; 50%) and C. albicans (9/66; 13.6%).
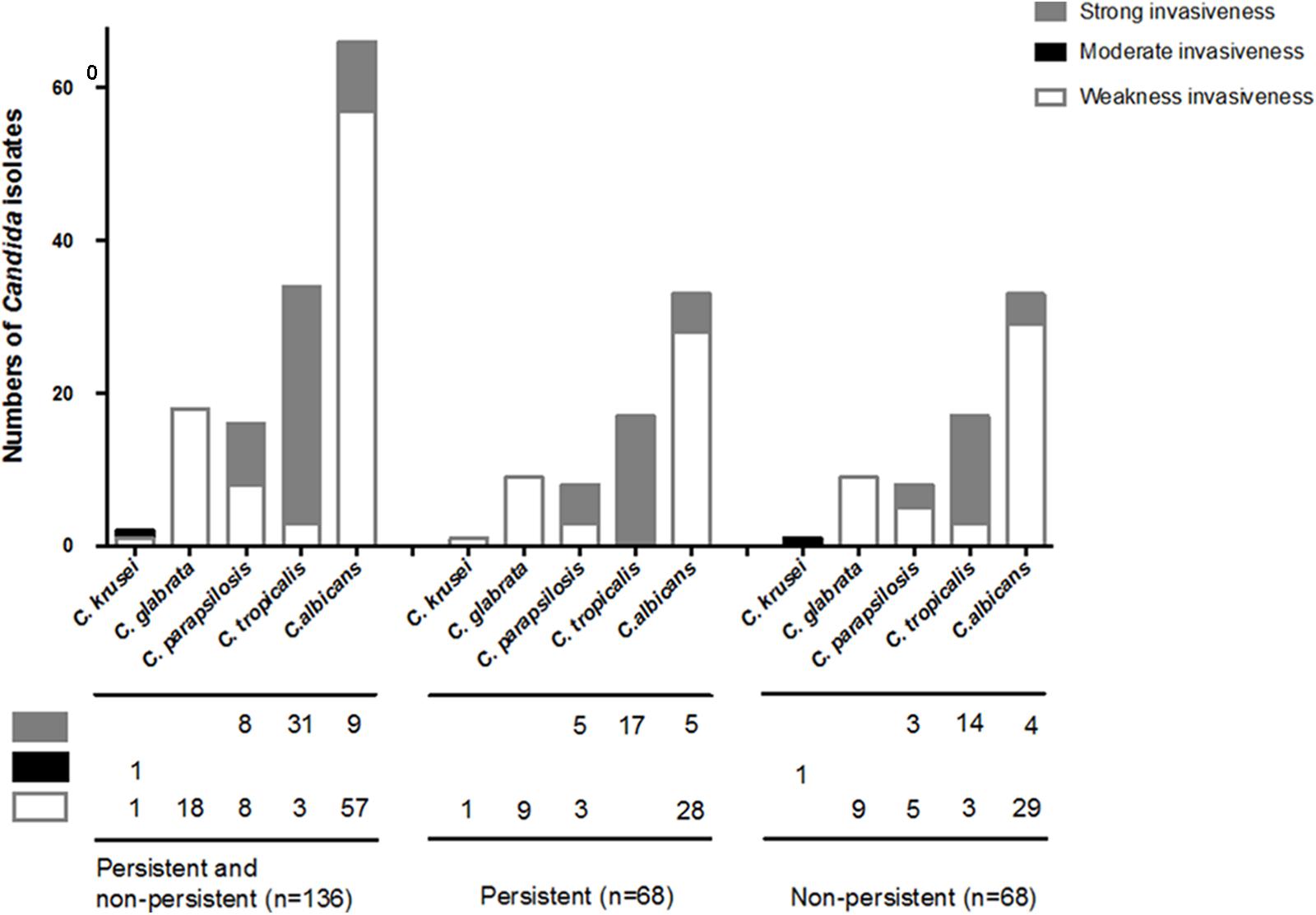
FIGURE 4. Invasiveness on Candida species isolated from persistent and non-persistent candidemia. Invasiveness was tested on 136 Candida strains isolated from including patients after propensity-score matching. Candida strains were streaked out with a flat toothpick to form single colonies (typically 50 colonies/plate) on YPD plates (yeast extract “Difco” 2%, Bacto Peptone “Difco” 4%, glucose 2%, Bacto-agar “Difco” 2%, and tryptophan 0.003%). All plates were incubated at 37°C and the extent of invasion was scored after 2 days. Invasion was determined as (i) cells that had not invaded the agar were washed away by rubbing the plate with a gloves finger while rinsing under running water, (ii) cells that had invaded the agar remained as macroscopically visible micro-colonies on the surface of the washed plate, and confirmed by micro-scopical examination (with a bright light field, 10× magnification) after washing. Candida strains with strong/intermediate invasiveness were not significantly different among the isolates from the patients between persistent and non-persistent group. In the 136 Candida strains, C. tropicalis (31/34; 91.2%) was the highest proportion of strains with strong invasiveness, followed by C. parapsilosis (8/16; 50%) and C. albicans (9/66; 13.6%).
A multivariate analysis showed that Candida isolates with HBFs (p < 0.01; odd ratio [OR] = 8.03; 95% confidence interval [CI] 2.50–25.81), initial treatment with suboptimal dosage of fluconazole (p < 0.01; OR = 5.54; 95% CI 1.53–20.10), and presence of CVCs at diagnosis of candidemia (p = 0.04; OR = 3.77; 95% CI 1.09–13.00) were the independent factors associated with persistent candidemia (Table 4). There was adequate goodness of fit (Hosmer–Lemeshow test, χ2 = 2.74, p = 0.25). The receiver operating characteristic analysis indicated that the predictive performance of logistic regression model was adequate (area under the curve = 0.76).
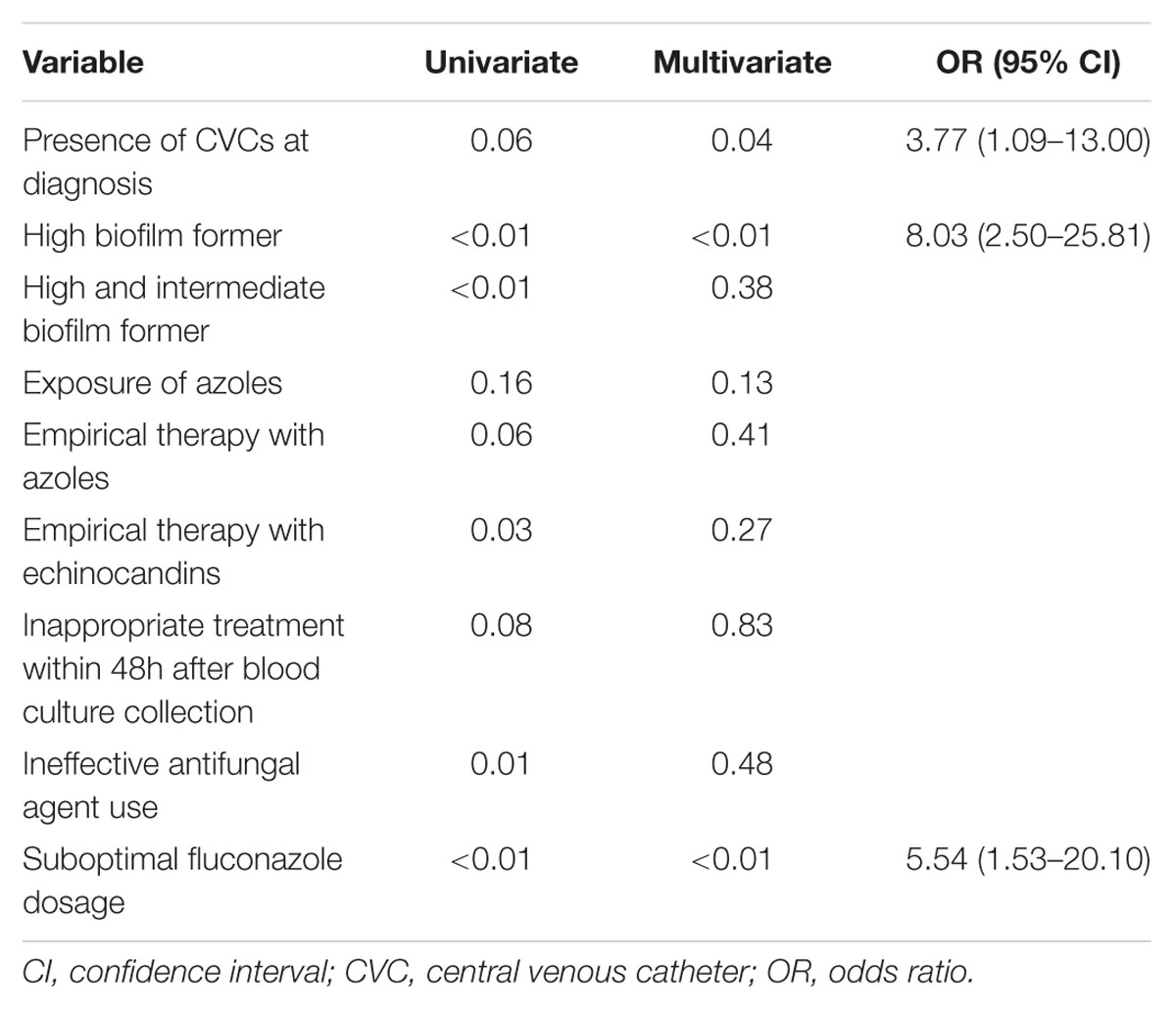
TABLE 4. Multivariate logistic regression analysis for independent factors of persistent candidemia.
Discussion
By comparisons of the clinical characteristics, microbiological features, and outcomes between patients with and those without persistent candidemia, we found that infection by Candida species with HBFs, presence of CVCs, and initial treatment with suboptimal dose of fluconazole were independently associated with persistent candidemia. Other than the presence of CVCs as a risk factor of persistent candidemia demonstrated in previous studies (Chen et al., 2012; Kang et al., 2017), our study also supports the current concept that biofilms formation are very important virulence factors for the establishment of recurrent candidiasis (Cuellar-Cruz et al., 2012) by directly demonstrating that HBFs are highly related to persistent candidemia. However, in the present study, we could not identify a beneficial effect of early CVC removal on prevention of persistent candidemia. A subgroup analysis of 2 randomized controlled trials also suggested that early CVC removal within 24 h or 48 h had no effect on persistent or recurrent candidemia (Nucci et al., 2010). In the retrospective study, blood cultures might not be obtained in a timely fashion to precisely establish the time point at which candidemia has been cleared. Understanding of the role of early CVC removal on persistent candidemia should rely on prospective studies with serial blood cultures performed at predefined intervals.
As already reported (Kang et al., 2017) and shown here, there were no serial MIC changes in the repeatedly cultured Candida strains from patients with persistent candidemia. While the early and overall mortality rates were not significantly different between the patients with and those without persistent candidemia after propensity-score matching that took into consideration the underlying diseases and severity at the onset of candidemia. Our findings illustrate that use of suboptimal dose of fluconazole was significantly higher among patients with persistent candidemia. It has been reported that suboptimal dosage of fluconazole was associated with poor response to treatment, defined as non-clearance of Candida species from bloodstream and mortality on day 7 after the onset of candidemia (Brosh-Nissimov and Ben-Ami, 2015). Therefore, pharmacodynamic-directed fluconazole dosing may help optimize outcomes for patients with candidemia.
Among the 238 Candida isolates (Figure 2), C. parapsilosis (11/28; 39.3%) was the most common pathogen associated with persistent candidemia, followed by C. tropicalis (18/55; 32.7%). We found that HBFs in C. albicans, C. tropicalis, and C. glabrata were higher in the patients with persistent candidemia than those without persistent candidemia, but the phenomenon was not shown in C. parapsilosis isolates. The biofilm-forming ability, structure, and matrix composition are highly species dependent with an additional strain variability occurring with C. parapsilosis (Silva et al., 2009). The C. parapsilosis complex is composed of three genetically distinct species, namely C. parapsilosis sensu stricto, C. orthopsilosis and C. metapsilosis, which are physiologically and morphologically indistinguishable (Tavanti et al., 2005). Previous data have shown that these three species exhibit different prevalence rates, virulence, and in vitro antifungal susceptibility. The species with the highest biofilm production was C. parapsilosis sensu stricto, followed by C. orthopsilosis and further by C. metapsilosis (Neji et al., 2017). However, in the present study, three genomic species of the C. parapsilosis species complex were not performed to classify which might contribute to the finding of no significant difference observed of biofilm formation in current C. parapsilosis isolates between case and control group.
We observed that caspofungin and amphotericin B rather than fluconazole were more effective against C. albicans with HBFs and LBFs. The viability of C. albicans was significantly decreased in high concentrations of fluconazole and caspofungin in comparison with exposure to low concentrations. However, amphotericin B is equally effective against both HBFs and LBFs groups regardless of the drug concentrations. The observation is in line with that of a previous report (Rajendran et al., 2016). It is noteworthy that persisters in Candida biofilms, the mean survivors after 24 h of treatment with high concentration of antifungal agents such as fluconazole, may have the capacity to invoke a specific adaptive strategy against anti-fungals (Li et al., 2015). Biofilm recalcitrance partially accounts for drug-tolerant persisters (Li et al., 2015). It was well known that echinocandins and lipid polyenes possess more activity against biofilm yeasts than do azoles (Uppuluri et al., 2011). In univariate analysis of the present study, patients without persistent candidemia were more likely to receive empirical antifungal with echinocandins than those with persistent candidemia. It was also demonstrated that, among the patients with biofilm-forming Candida bloodstream infection, those treated with highly active anti-biofilm agents (e.g., caspofungin) had significantly shorter hospital length of stay post-Candida bloodstream infection than those treated with non-highly active anti-biofilm agents antifungal agents (e.g., fluconazole) (Tumbarello et al., 2012).
The ability to generate the filamentous form of Candida is important for tissue invasion which is dependent upon species and influenced by environment. We found that C. tropicalis was the highest proportion of strains with strong invasiveness, followed by C. parapsilosis. The finding was consistent with previous study. C. tropicalis showed more extensive colonization and invasion in reconstituted human oral epithelium than C. parapsilosis and C. glabrata (Silva et al., 2011). In our study, the grade of invasiveness behaviors of Candida isolates did not affect the occurrence of persistent candidemia. It was revealed that biofilm formation might be more influential than invasiveness on the development of persistent candidemia.
Our study still has several limitations. It is a retrospective study. Clinical characteristics of the two groups of patients might not be completely captured in the medical records. The follow-up blood cultures in some cases were not obtained timely based on the recommendation of the IDSA to establish the time point at which candidemia has been cleared. However, we used a propensity score to match the disease severity between the two study groups with follow-up blood cultures, which may help alleviate the concerns about immortal bias. In addition, we did not perform further analysis to classify three genomic species of the C. parapsilosis complex. C. parapsilosis strains are heterogeneous in terms of the level of biofilm formation with a 1301-fold difference between the highest and lowest biofilm-producing strains while C. orthopsilosis and C. metapsilosis strains exhibit a more homogeneous behavior (Neji et al., 2017). Therefore, we cannot demonstrate if there existed difference in biofilm formation between case and control group caused by C. parapsilosis after distinguishing the three species. Finally, testing for invasiveness of Candida isolates was only performed in agar plates. A further ex vivo model to assess the invasiveness is needed to confirm the findings of this in vitro method.
In summary, HBFs, presence of CVCs at diagnosis, and initial treatment with suboptimal dose of fluconazole were independent factors associated with persistent candidemia. Using an adequate dose of fluconazole to maximize the effectiveness or use of highly active anti-biofilm agents, such as echinocandin or amphotericin B are strategies that warrant more investigations to prevent persistent candidemia.
Author Contributions
W-SL, Y-CC, and C-HL conceptualized and designed the study, and drafted the manuscript. C-HL assisted with acquiring funding. W-SL collected clinical data. S-FK and F-JC conducted laboratory analyses. Y-CC and C-HL reviewed and validated the laboratory analysis. W-SL conducted the statistical analysis. All authors assisted with critically reviewing the manuscript.
Funding
This study was sponsored by Merck & Co., Ltd. and was supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG 8E1581). C-HL had received research support from Merck.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for the statistics work. We also thank Dr. Chien-Ching Hung at the Department of Internal Medicine, National Taiwan University Hospital, for his critical review of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01196/full#supplementary-material
References
Bellomo, R., Ronco, C., Kellum, J. A., Mehta, R. L., Palevsky, P., and Acute Dialysis Quality Initiative Workgroup (2004). Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 8, R204–R212. doi: 10.1186/cc2872
Brosh-Nissimov, T., and Ben-Ami, R. (2015). Differential association of fluconazole dose and dose/MIC ratio with mortality in patients with Candida albicans and non-albicans bloodstream infection. Clin. Microbiol. Infect. 21, 1011–1017. doi: 10.1016/j.cmi.2015.07.005
Chen, C. Y., Huang, S. Y., Tsay, W., Yao, M., Tang, J. L., Ko, B. S., et al. (2012). Clinical characteristics of candidaemia in adults with haematological malignancy, and antimicrobial susceptibilities of the isolates at a medical centre in Taiwan, 2001-2010. Int. J. Antimicrob. Agents 40, 533–538. doi: 10.1016/j.ijantimicag.2012.07.022
Clinical and Laboratory Standards Institute [CLSI] (2012). Clinical and Laboratory Standards Institute: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, 4th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
Cuellar-Cruz, M., Lopez-Romero, E., Villagomez-Castro, J. C., and Ruiz-Baca, E. (2012). Candida species: new insights into biofilm formation. Future Microbiol. 7, 755–771. doi: 10.2217/fmb.12.48
Donlan, R. M., and Costerton, J. W. (2002). Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193. doi: 10.1128/CMR.15.2.167-193.2002
Farmakiotis, D., Kyvernitakis, A., Tarrand, J. J., and Kontoyiannis, D. P. (2015). Early initiation of appropriate treatment is associated with increased survival in cancer patients with Candida glabrata fungaemia: a potential benefit from infectious disease consultation. Clin. Microbiol. Infect. 21, 79–86. doi: 10.1016/j.cmi.2014.07.006
Gilbert, D. N., Chambers, H. F., Eliopoulos, G. M., Saag, M. S., and Pavia, A. T. (2017). The Sanford Guides to Antimicrobial Therapy 2017, 47th Edn. Sperryville, VA: Antimicrobial Therapy, Inc., 121–124.
Hammoud, M. S., Al-Taiar, A., Fouad, M., Raina, A., and Khan, Z. (2013). Persistent candidemia in neonatal care units: risk factors and clinical significance. Int. J. Infect. Dis. 17, e624–e628. doi: 10.1016/j.ijid.2012.11.020
Handelsman, Y., Mechanick, J. I., Blonde, L., Grunberger, G., Bloomgarden, Z. T., Bray, G. A., et al. (2011). American association of clinical endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr. Pract. 17(Suppl. 2), 1–53. doi: 10.4158/EP.17.S2.1
Hung, C. H., Lu, S. N., Wang, J. H., Lee, C. M., Chen, T. M., Tung, H. D., et al. (2003). Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J. Gastroenterol. 38, 153–157. doi: 10.1007/s005350300025
Kang, S. J., Kim, S. E., Kim, U. J., Jang, H. C., Park, K. H., Shin, J. H., et al. (2017). Clinical characteristics and risk factors for mortality in adult patients with persistent candidemia. J. Infect. 75, 246–253. doi: 10.1016/j.jinf.2017.05.019
Kontoyiannis, D. P., Tarrand, J., Prince, R., Samonis, G., and Rolston, K. V. (2001). Effect of fluconazole on agar invasion by Candida albicans. J. Med. Microbiol. 50, 78–82. doi: 10.1099/0022-1317-50-1-78
Kullberg, B. J., and Arendrup, M. C. (2015). Invasive candidiasis. N. Engl. J. Med. 373, 1445–1456. doi: 10.1056/NEJMra1315399
Levey, A. S., Coresh, J., Balk, E., Kausz, A. T., Levin, A., Steffes, M. W., et al. (2003). National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann. Intern. Med. 139, 137–147. doi: 10.7326/0003-4819-139-2-200307150-00013
Li, P., Seneviratne, C. J., Alpi, E., Vizcaino, J. A., and Jin, L. (2015). Delicate metabolic control and coordinated stress response critically determine antifungal tolerance of Candida albicans biofilm persisters. Antimicrob. Agents Chemother. 59, 6101–6112. doi: 10.1128/AAC.00543-15
Lionakis, M. S., and Kontoyiannis, D. P. (2003). Glucocorticoids and invasive fungal infections. Lancet 362, 1828–1838. doi: 10.1016/S0140-6736(03)14904-5
Neji, S., Hadrich, I., Trabelsi, H., Abbes, S., Cheikhrouhou, F., Sellami, H., et al. (2017). Virulence factors, antifungal susceptibility and molecular mechanisms of azole resistance among Candida parapsilosis complex isolates recovered from clinical specimens. J. Biomed. Sci. 24:67. doi: 10.1186/s12929-017-0376-2
Nucci, M. (2011). Persistent candidemia: causes and investigations. Curr. Fungal Infect. Rep. 5, 3–11. doi: 10.1007/s12281-010-0039-1
Nucci, M., Anaissie, E., Betts, R. F., Dupont, B. F., Wu, C., Buell, D. N., et al. (2010). Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin. Infect. Dis. 51, 295–303. doi: 10.1086/653935
Nucci, M., and Colombo, A. L. (2002). Risk factors for breakthrough candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 21, 209–211. doi: 10.1007/s10096-002-0697-1
Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., et al. (2016). Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 62, e1–e50. doi: 10.1093/cid/civ933
Pierce, C. G., Uppuluri, P., Tristan, A. R., Wormley, F. L. Jr., Mowat, E., Ramage, G., et al. (2008). A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3, 1494–1500. doi: 10.1038/nport.2008.141
Rajendran, R., Sherry, L., Nile, C. J., Sherriff, A., Johnson, E. M., Hanson, M. F., et al. (2016). Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012-2013. Clin. Microbiol. Infect. 22, 87–93. doi: 10.1016/j.cmi.2015.09.018
Ramage, G., Rajendran, R., Sherry, L., and Williams, C. (2012). Fungal biofilm resistance. Int. J. Microbiol. 2012:528521. doi: 10.1155/2012/528521
Rhodes, A., Evans, L. E., Alhazzani, W., Levy, M. M., Antonelli, M., Ferrer, R., et al. (2017). Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 43, 304–377. doi: 10.1007/s00134-017-4683-6
Safdar, A., Bannister, T. W., and Safdar, Z. (2004). The predictors of outcome in immunocompetent patients with hematogenous candidiasis. Int. J. Infect. Dis. 8, 180–186. doi: 10.1016/j.ijid.2003.05.003
Sherry, L., Rajendran, R., Lappin, D. F., Borghi, E., Perdoni, F., Falleni, M., et al. (2014). Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 14:182. doi: 10.1186/1471-2180-14-182
Silva, S., Henriques, M., Martins, A., Oliveira, R., Williams, D., and Azeredo, J. (2009). Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med. Mycol. 47, 681–689. doi: 10.3109/13693780802549594
Silva, S., Negri, M., Henriques, M., Oliveira, R., Williams, D. W., and Azeredo, J. (2011). Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 19, 241–247. doi: 10.1016/j.tim.2011.02.003
Taff, H. T., Mitchell, K. F., Edward, J. A., and Andes, D. R. (2013). Mechanisms of Candida biofilm drug resistance. Future Microbiol. 8, 1325–1337. doi: 10.2217/fmb.13.101
Tavanti, A., Davidson, A. D., Gow, N. A., Maiden, M. C., and Odds, F. C. (2005). Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43, 284–292. doi: 10.1128/JCM.43.1.284-292.2005
Tumbarello, M., Fiori, B., Trecarichi, E. M., Posteraro, P., Losito, A. R., De Luca, A., et al. (2012). Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 7:e33705. doi: 10.1371/journal.pone.0033705
Tumbarello, M., Posteraro, B., Trecarichi, E. M., Fiori, B., Rossi, M., Porta, R., et al. (2007). Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45, 1843–1850. doi: 10.1128/JCM.00131-07
Keywords: candidemia, biofilm, invasive candidiasis, antifungal susceptibility, vascular catheter
Citation: Li W-S, Chen Y-C, Kuo S-F, Chen F-J and Lee C-H (2018) The Impact of Biofilm Formation on the Persistence of Candidemia. Front. Microbiol. 9:1196. doi: 10.3389/fmicb.2018.01196
Received: 21 February 2018; Accepted: 16 May 2018;
Published: 04 June 2018.
Edited by:
George Grant, University of Aberdeen, United KingdomReviewed by:
Maria Rapala-Kozik, Jagiellonian University, PolandNuno Pereira Mira, Instituto de Bioengenharia e Biociências (IBB), Portugal
David Andes, University of Wisconsin–Madison, United States
Chaminda Jayampath Seneviratne, National University of Singapore, Singapore
Copyright © 2018 Li, Chen, Kuo, Chen and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen-Hsiang Lee, bGVlOTAwQGFkbS5jZ21oLm9yZy50dw==
†These authors have contributed equally to this work.
 Wei-Sin Li1†
Wei-Sin Li1† Shu-Fang Kuo
Shu-Fang Kuo Chen-Hsiang Lee
Chen-Hsiang Lee