
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 15 October 2020
Sec. Nephrology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.576925
This article is part of the Research Topic Management of Patients with Non-Dialysis Dependent Chronic Kidney Disease (ND-CKD) View all 22 articles
 Yang Xun1†
Yang Xun1† Mingzhen Chen2†
Mingzhen Chen2† Ping Liang2
Ping Liang2 Pratik Tripathi2
Pratik Tripathi2 Huchuan Deng3
Huchuan Deng3 Ziling Zhou3
Ziling Zhou3 Qingguo Xie3
Qingguo Xie3 Cong Li1*
Cong Li1* Shaogang Wang1*
Shaogang Wang1* Zhen Li2*
Zhen Li2* Daoyu Hu2
Daoyu Hu2 Ihab Kamel4
Ihab Kamel4Purpose: The purpose of the study is to develop and validate a novel clinical–radiomics nomogram model for pre-operatively predicting the stone-free rate of flexible ureteroscopy (fURS) in kidney stone patients.
Patients and Methods: Altogether, 2,129 fURS cases with kidney stones were retrospectively analyzed, and 264 patients with a solitary kidney stone were included in a further study. For lower calyx calculi, a radiomics model was generated in a primary cohort of 99 patients who underwent non-contrast-enhanced computed tomography (NCCT). Radiomics feature selection and signature building were conducted by using the least absolute shrinkage and selection operator (LASSO) method. Multivariate logistic regression analysis was employed to build a model incorporating radiomics and potential clinical factors. Model performance was evaluated by its discrimination, calibration, and clinical utility. The model was internally validated in 43 patients.
Results: The overall success rate of fURS was 72%, while the stone-free rate (SFR) for lower calyx calculi and non-lower calyx calculi was 56.3 and 90.16%, respectively. On multivariate logistic regression analysis of the primary cohort, independent predictors for SFR were radiomics signature, stone volume, operator experience, and hydronephrosis level, which were all selected into the nomogram. The area under the curve (AUC) of clinical–radiomics model was 0.949 and 0.947 in the primary and validation cohorts, respectively. Moreover, the calibration curve showed a satisfactory predictive accuracy, and the decision curve analysis indicated that the nomogram has superior clinical application value.
Conclusion: In this novel clinical–radiomics model, the radiomics scores, stone volume, hydronephrosis level, and operator experience were crucial for the flexible ureteroscopy strategy.
As a common urological disease, the prevalence rates for kidney stones vary from 1 to 20%. Especially in western developed countries such as the USA, kidney stone prevalence is notably high (>10%) (1). Related treatment costs are estimated to be several billion dollars per year in such countries (2). With the continuous development of the flexible ureteroscope and auxiliary equipment, flexible ureteroscopy (fURS) is considered to be one of the first-line treatments for the active removal of renal stones smaller than 2 cm (3). Furthermore, recent studies have shown fURS to be less dangerous for severe complications than percutaneous nephrolithotomy (PCNL) and also has low possibility for retreatment compared with shock wave lithotripsy (SWL) (4, 5).
However, the complete stone-free rate (SFR) for fURS is relatively low compared with PCNL (6). Numerous factors such as stone characteristics, stone location, renal anatomy, and hydronephrosis level may affect the success rate of fURS. Among these factors, the characteristics of the stone are very crucial. Several articles documented that the composition or Hounsfield units (HU) of the stone have a great impact on the efficacy of fURS (7, 8). However, HU based on computed tomography (CT) only represents the average value of the stone and thus cannot reflect the intracalculi structure, which is eminently related to the SFR. In addition, our previous research discovered that CT texture analysis (CTTA) of urinary tract stones may better predict the SFR on ESWL patients (9). Apparently, the limitations of this previous study included solitary enrollment of simple first-order parameters, and failure to combine clinical factors and lack multivariate analysis.
Recent advances in computer-assisted imaging techniques have enabled the high-throughput extraction of quantitative features from digital medical images. Actually, this new methodology, named radiomics, has been proven to be capable of influencing and altering the diagnosis and treatment strategies in the field of tumors (10, 11). Moreover, several investigations showed that predictive model based on radiomics or machine learning can better predict the post-operative outcome of certain surgical treatments (PCNL or SWL) (12, 13). It is important to develop a novel predictive model for fURS that combines radiomics features and clinical indicators, particularly for the lower renal stones.
Accordingly, the objective of this study was to develop and validate a novel clinical–radiomics nomogram model for pre-operative assessment and prediction SFR of fURS.
Ethical approval was obtained for this retrospective analysis, and the requirement for informed consent was waived. In the present study, we retrospectively enrolled 2,129 fURS for renal stone removal performed between December 2014 and March 2019 (Supplementary Figure 1 shows flowchart of patient selection). In total, 264 patients with solitary kidney stone met the inclusion criterion, including 122 cases of non-lower calyx calculi and 142 cases of lower calyx calculi. Lower calyx calculi treated with fURS were included in further research and randomly divided into two independent cohorts: primary cohort and validation cohort with a ratio of 7:3 based on the 10-fold cross-validation principle.
Meanwhile, patients' pertinent clinical data and stone characteristics were extracted pre-operatively, including age, sex, hydronephrosis level, the burden of calculi, pre-operative catheterization, the experience of the surgeon, etc. The follow-up procedure was conducted on the basis of the results of the CT scan or X-ray of kidney–ureter–bladder (KUB) review 3 months after operation. The standard of stone-free status was defined as free from stones or residual stone fragments <2 mm (14).
Rigid ureteroscopy was routinely used for ureteral dilation before fURS. Thereafter, a 0.035-mm straight guidewire was placed through the ureteric orifice to the renal pelvis under direct rigid ureteroscope vision. Then, we placed a 14-F ureteric access sheath (Cook Medical, Bloomington, IN, USA) by a straight guidewire. A 7.5-F flexible ureteroscope (Flex-X2, Karl Storz, Germany) was passed through the ureteric access sheath to access the stone. Once the location of stones was confirmed, the Ho:YAG laser was used to fragment stones. After lithotripsy, 6-F double-J stent was routinely left in all cases for 2–4 weeks.
All included patients underwent NCCT using a 64-slice MDCT scanner (Discovery CT750 HD, GE Healthcare, USA). The related CT imaging acquisition parameters are as follows: tube voltage, 100–120 kV; automatic tube current, 200–350 mA; rotation time, 0.5 s; scan slice thickness, 5 mm; and reconstruction thickness, 1.25 mm.
Stone regions of interest (ROIs) were manually segmented on each transverse slice CT images, in the format of DICOM, using an open-source software 3D Slicer (version 4.9.0; www.slicer.org). Then, the following features were documented: (a) stone size, defined as maximum diameter on images; (b) stone volume, the ROIs would be fused and become the volume of interest (VOI); (c) stone location, subclassified as lower and non-lower calyx calculi; and (d) the degree of hydronephrosis, defined as severe and non-severe.
Radiomics feature extraction was performed using in-house texture analysis software with algorithms implemented in Matlab 2015a (MathWorks, Natick, Mass). In our research, a total of 604 radiomics features, including first-order statistics, shape- and size-based features, textural features, and wavelet features, were generated from each original CT image. Specific details of feature algorithms are shown in Supplement 1 in Supplementary Material.
Dimension reduction and signature building process were arranged by LASSO logistic regression algorithm (15). With penalty parameter tuning conducted by 10-fold cross-validation, LASSO was performed to select robust and non-redundant features from the primary cohort. A radiomics signature was created by a linear combination of selected features weighted by their respective coefficients, and the relevant radiomics score (Rad-score) was calculated for each patient.
A model that incorporated the radiomics signature and clinical factors for predicting stone-free (SF) status was built based on multivariate logistic regression analysis in the primary set. We initially excluded some variables from the multivariate model for multicolinearity using variance inflation factor (VIF).
To provide a visual tool for clinical decision-making, a clinical–radiomics nomogram was then generated based on multivariate logistic regression analysis of corresponding pre-operative factors. Model performance was typically measured in terms of discrimination and calibration. The area under the curve (AUC), calculated by receiver operating characteristic (ROC) curve, was used to quantify the discrimination performance of established models (16). Calibration curves were portrayed to evaluate the predictive accuracy of the clinical–radiomics nomogram, followed by the Hosmer–Lemeshow goodness-of-fit test (a significant test statistic means that the model does not calibrate perfectly) (17).
To evaluate its predictive accuracy, the performance of nomogram was tested in the validation cohort. The logistic regression formula formed in the primary cohort was applied to all patients of the validation cohort, with total points for each patient calculated. Finally, the ROC and calibration plot were generated based on the regression analysis.
Finally, to determine the clinical value of the radiomics model that incorporates clinical consequences, the decision curve analysis (DCA) was conducted to demonstrate a quantification of the net benefits at different threshold probabilities (18).
Statistical analysis was conducted with Statistical Package for Social Sciences (SPSS) software version 24.0 and R (version 3.4.4) with R packages listed in Supplement 2 in Supplementary Material. The normality of all continuous variables was evaluated by the Kolmogorov–Smirnov test. Univariate analysis (chi-square test for categorical variables and t-test or rank sum test for continuous variables) and multivariate statistical analysis (logistic regression) were performed to identify significant independent predictors. A two-sided p < 0.05 was described as significant.
Baseline characteristics of all patients are shown in Table 1. A total of 264 patients, 160 (60.6%) men and 104 (39.4%) women, were enrolled in this study. The overall rate of SF was about 72%. Significant difference was revealed between the SF and non-SF groups in the following indicators: stone location (p < 0.001), hydronephrosis (p < 0.001), operator experience (p < 0.001), and stone volume (p < 0.001).
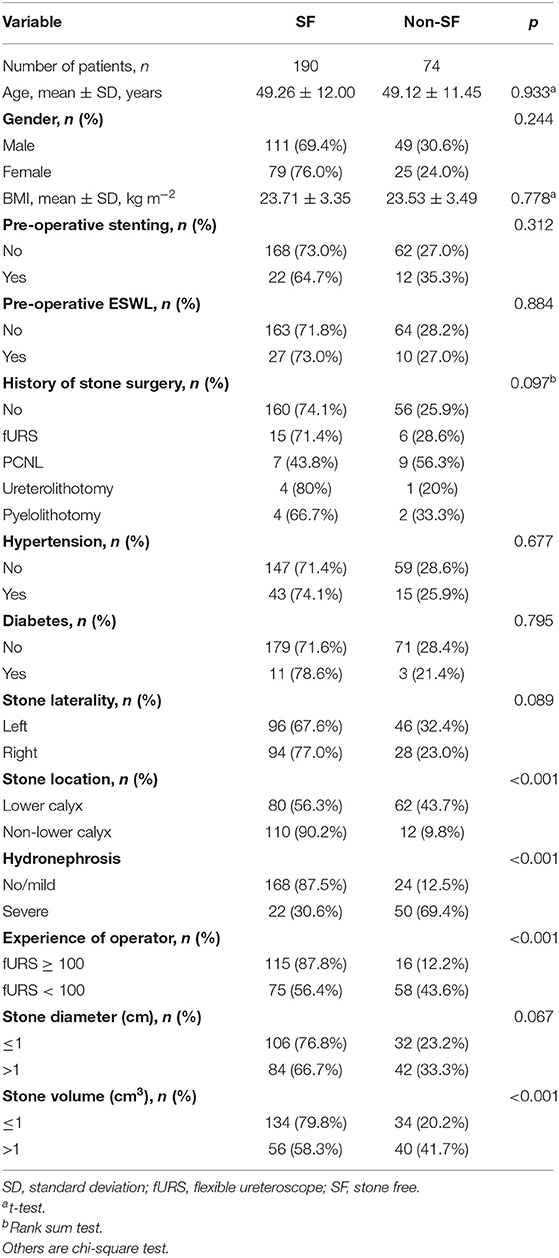
Table 1. Comparison of single kidney stone patient and stone characteristics according to SF at 3 months after fURS.
However, in the subgroup analysis of stone location, the SFR for lower calyx calculi and non-lower calyx calculi was 56.3% (80/142) and 90.16% (110/122), respectively (Table 2). In the lower calyx group, stone diameter, hydronephrosis, experience of operator, and stone volume (all p < 0.001) were found to be the significant factors effecting fURS results, while SF status was significantly associated with hydronephrosis (p = 0.008) and experience of operator (p = 0.003) in the non-lower calyx group.
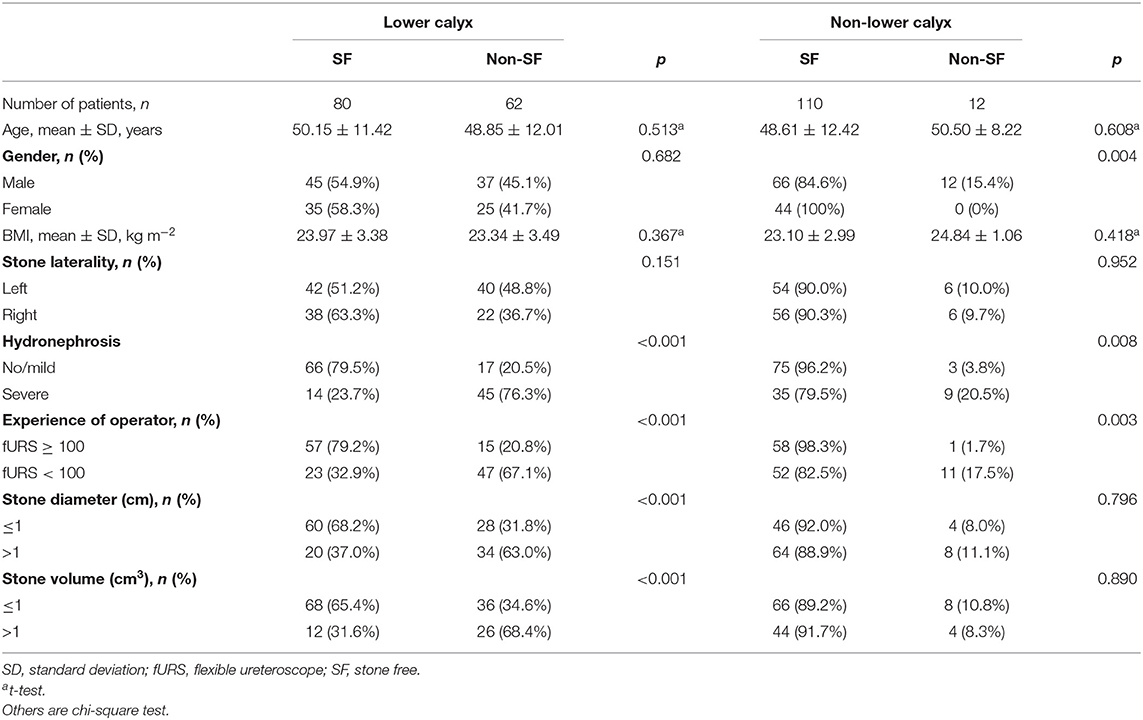
Table 2. Influencing factors on the success rate after fURS between lower calyx stone and non-lower calyx stone patients.
Subsequently, we further conducted studies on the lower calyx cases. Ninety-nine patients were included in primary groups to establish a predictive model, and 43 patients were enrolled in the validation groups to verify the accuracy and reliability of the generated model. Both two groups were further divided into SF and non-SF cases separately in accordance with the results of follow-up of each patient. Univariate analysis revealed the possible association in five factors (hydronephrosis level, operator experience, stone diameter, stone volume, and radiomics score) and the SFR, as presented in Table 3.
Using the 604 extracted radiomics features, LASSO analysis was performed, and 28 features with non-zero coefficients were screened based on the primary group (Figure 1).
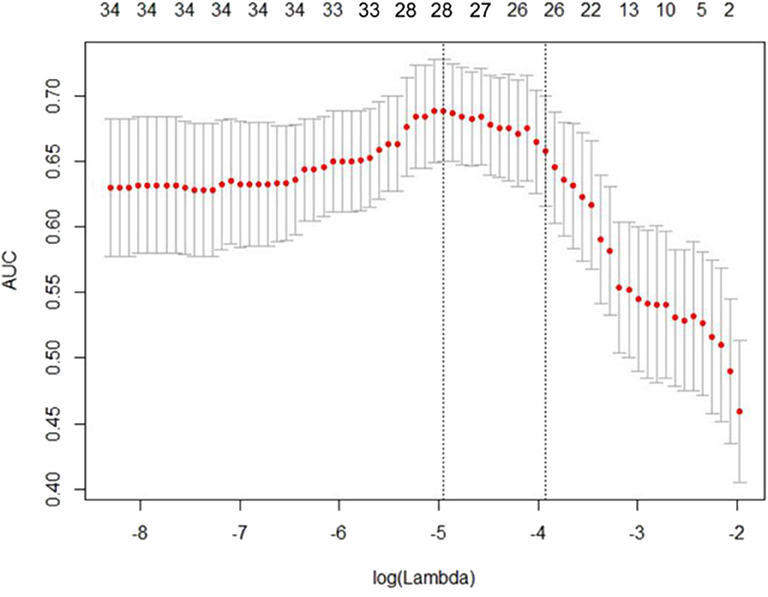
Figure 1. Least absolute shrinkage and selection operator (LASSO) regression analysis uses the minimum standard and a 10-fold cross-validation method. The coefficients of the model are compressed by introducing a penalty adjustment parameter (λ) so that the coefficients of the irrelevant variables tend to be zero, and then, the automatic screening of the variables is realized.
A radiomics score calculation formula was then constructed by using corresponding coefficients of the chosen signature, presented in Supplement 3 in Supplementary Material. Distributions of the radiomics score and post-operative outcome for each patient in the primary and validation groups are shown in Supplementary Figure 2.
A significant difference in radiomics score was initially evidenced between SF and non-SF patients in the primary group (p < 0.001), and later confirmed in the validation group (p < 0.001), which can be noted in Table 3. The results show that the index based on radiomics analysis is significantly and positively correlated with post-operative SFR. Namely, patients with an SF outcome generally had higher Rad scores in the primary cohort.
Results of multivariate regression analysis are shown in Table 4. The VIFs of three potential predictors ranged from 1.2 to 1.42, showing that there was no multicolinearity. A radiomics model that is composed of four different parameters (stone volume, operator experience, hydronephrosis level, and radiomics signature) was constructed and presented as a nomogram (Figure 2). Higher total point reflects corresponding case with higher probability for post-operative treatment success.
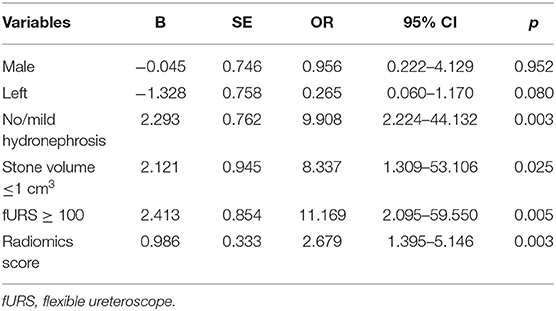
Table 4. Multivariate analysis of the influencing factors on the success rate after fURS in lower calyx patients.
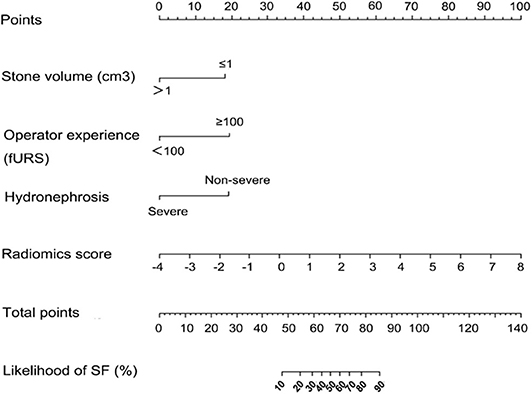
Figure 2. Established clinical–radiomics nomogram model. The clinical–radiomics nomogram was generated in the primary cohort, with the radiomics signature, stone volume, operator experience, and hydronephrosis level incorporated.
The observed AUC value for nomogram predictions was 0.949 (95% CI, 0.910–0.989). The established nomogram was then verified in a validation cohort, with AUC of 0.947 (95% CI, 0.883–1) supported the increased predictive efficacy (Figure 3). The calibration curve and the Hosmer–Lemeshow test (p = 0.344) demonstrated favorable calibration of the nomogram in the primary group (Figure 4A). In the validation cohort, the calibration curve showed that there was a good agreement between nomogram predicted probability of SF and actual SF rate (Figure 4B). Meanwhile, the Hosmer–Lemeshow test yielded a non-significant statistic (p = 0.099), which suggested that there was no departure from perfect fit. Figure 5 showed a specific clinical case decision procedure utilizing the generated clinical–radiomics nomogram.
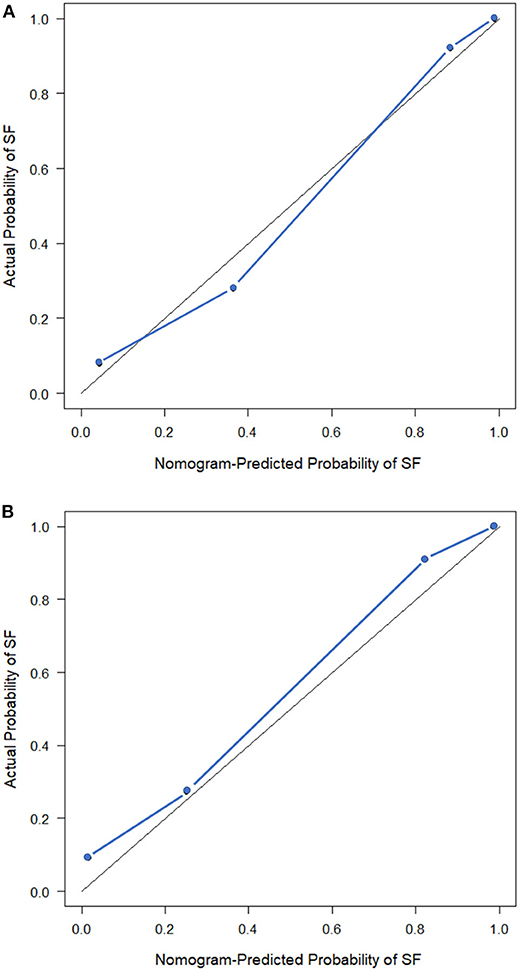
Figure 4. Calibration curves of the clinical–radiomics nomogram. (A) Calibration curve of the clinical–radiomics nomogram in the primary cohort. (B) Calibration curve of the clinical–radiomics nomogram in the validation cohort. Calibration curves describe the calibration of the model with respect to the agreement between nomogram predicted probability of stone free (SF) and actual SF rate. The y-axis depicts actual SF rate. The x-axis depicts the nomogram predicted probability of SF. The diagonal solid line depicts an excellent prediction by a supreme model. The blue dotted line represents the performance of the nomogram.
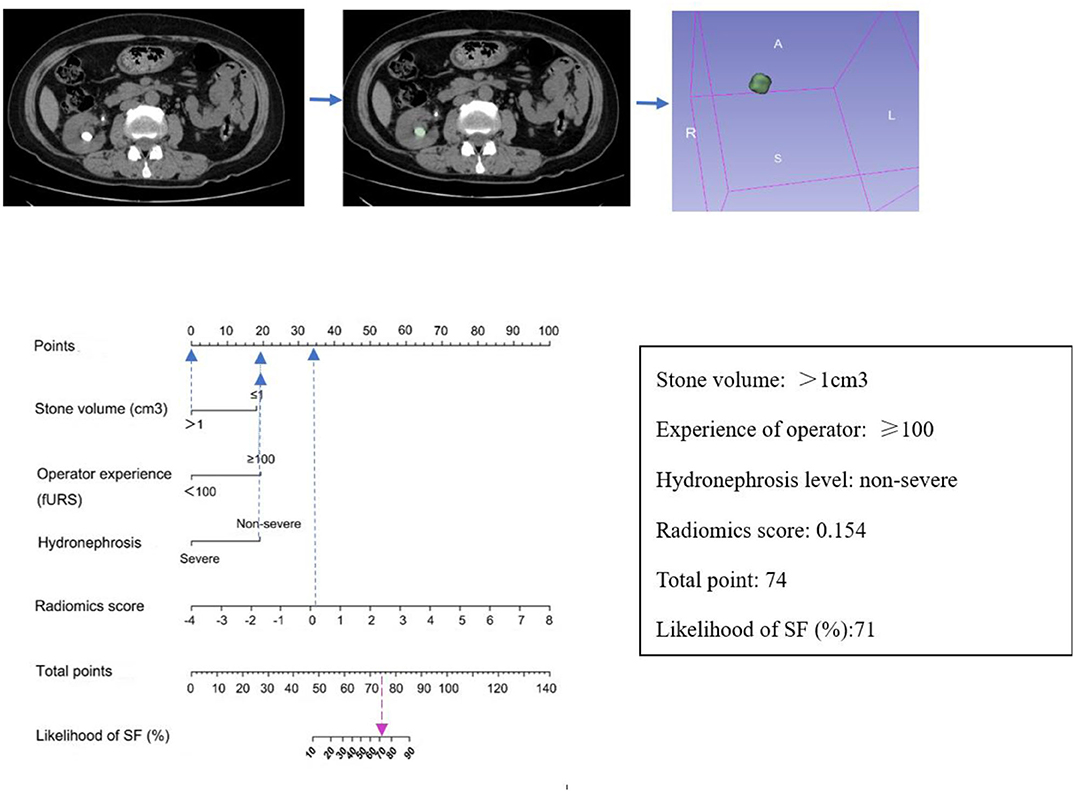
Figure 5. An example of how to use the clinical–radiomics nomogram to predict SF status in a 58-year-old female patient with SF outcome after flexible ureteroscopy (fURS). Locate the patient's Rad-score on the Rad-score axis. Draw a line straight upward to the points' axis to determine how many points the patient receives for his or her Rad-score. Conduct a similar process for other indicators. Sum the points calculated for each of the risk factors and track down the added sum on the total points axis. Draw a line straight down to find the patient's likelihood of SF.
The DCA for the clinical–radiomics nomogram is portrayed in Figure 6. The decision curve showed that if the threshold probability of a patient is above 0.1, using the predictive model to predict SFR provides a better net benefit than either the treat-all-patients scheme or the treat-none scheme.
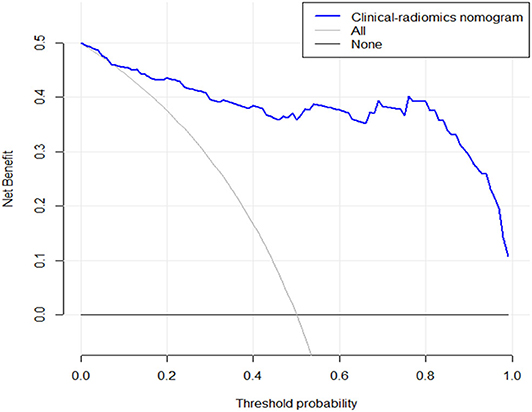
Figure 6. The y-axis measures the net benefit. The blue line represents the clinical–radiomics nomogram. The gray line represents the assumption that all patients have SF outcome. The black line represents the presumption that no patients have SF status. The decision curve showed that if the threshold probability is above 0.1, then using an imaging colinearity chart to predict post-operative result is more beneficial for making clinical decisions. For example, if the personal threshold probability of a patient is 50% (i.e., the patient would opt for treatment if his probability of SF was 50%); then, the net benefit is 0.36 when using the clinical–radiomics nomogram to make the decision of whether to undergo treatment, with added benefit than the treat-all scheme or the treat-none scheme).
In recent years, fURS has become a mainstream surgical treatment for upper urinary calculi with its advantages of non-invasiveness, safety, and having a short learning curve. The indications for fURS have gradually expanded, and some scholars have even tried to use it for the treatment of staghorn calculi. It is very likely to be an alternative to ESWL and PCNL and to become the preferred surgical procedure for upper urinary calculi (19–21). After the craze, some urologists began to reflect on whether this technique is so widely applicable. It is undeniable that some patients have unsatisfactory results after receiving fURS and even fail to touch the stones, causing patients to undergo ineffective surgery and need to be treated again, which brings huge safety risks and waste of medical resources (22). Therefore, it is particularly important to pre-operatively assess the effect of fURS surgery and accurately select patients who are suitable for fURS.
Stone characteristics have been considered as a significant factor determining the final efficacy because various stone characteristics can result in different times and extents of pulverization under the function of laser (7, 8, 23). Stones with longer pulverization times are more likely to reposition during the process, which may lead to an unsuccessful outcome. However, comprehensively identifying stone characteristics prior to surgery still remains an intractable challenge. On the one hand, traditional stone composition analysis can only depend on post-operative or intraoperative vitro testing, which is not feasible for pre-operative assessment. On the other hand, simple measurement of Hounsfield unit or density is unilateral, since the situation of intracalculi is often uneven and complicated. This explains why many studies failed to include this crucial factor into the evaluation scoring system (24, 25).
In this study, a radiomics signature consisting of 28 robust features was identified to be an independent factor for the SFR of fURS in patients with the lower calyx calculi. This multifeature-based radiomics signature also successfully stratified patients into successful and unsuccessful groups in the validation dataset. Our previous study on SWL also found that CTTA, a quantitative analysis method, may be useful in improving medical decision-making on ESWL patients (9). Similarly, several pieces of research showed that establishing a prediction model utilizing radiomics or machine learning may contribute to a better predictive efficacy for pre-operative estimation of PCNL or SWL outcomes (12, 13).
Obviously, the radiological features alone were not enough, and it has been considered that relying on a solitary strong risk indicator could fail to evaluate the comprehensive post-operative outcome of individual patients (26). Therefore, we generated a clinical–radiomics model, which is the combination of the radiomics signature and potential clinical indicators. The established clinical–radiomics nomogram demonstrated superior discrimination and calibration in both training and validation cohort, with an AUC of 0.949 and 0.947, respectively. Likewise, the decision curve analysis indicated that the clinical–radiomics nomogram was more beneficial than the treat-all scheme or the treat-none scheme across the majority of the scope of rational threshold probabilities.
Indeed, with respect to clinical predictors, there are some similarities between our study and Ito's original study (24). The two studies both agreed that the location, stone volume, hydronephrosis level, and surgeon's experience are significant predictors of post-operative SF status. Many articles have reported that the stone volume greatly affects the success rate of fURS (27), and our research also showed similar results. With the increase in stone burden, it would require a longer pulverization time, and the stone fragments are more likely to move. At the same time, it may increase the probability of intraoperative bleeding, which can lead to blurred vision, affecting normal surgical procedure and thus leading to stone residues after surgery. In addition, hydronephrosis causes enlargement of the renal pelvis and calyces, which makes breaking and basketing stones trickier, thus increasing the likelihood of stone residues after the procedure. Furthermore, Ito et al. reported on urologists with experience of >100 fURS that were associated with a satisfactory post-operative outcome. Meanwhile, Cho et al. reported that 56 cases were required for reaching a plateau in the learning curve (28). Another study indicated that surgeon experience affects the outcomes of fURS mainly in terms of safety (29). Similarly, in our department, we found that operators with experience of more than 100 procedures can achieve more skilled surgical techniques and deal with complications more efficiently. Being proficient in using ureteroscopy and its ancillary equipment, they can reduce some dangerous complications, such as intraoperative bleeding, or even prevent them from happening.
Unlike prior prognostic investigations that mostly analyzed all kinds of patients regardless of stone location, our current study focused exclusively on patients with the lower calyx calculi. Among various treatments for single lower calyceal stone, Bozzini et al. (30) reported that fURS and PCNL were more effective than SWL to obtain a better SFR and a lower auxiliary and retreatment rate. fURS, compared with PCNL, offers the best outcome in terms of procedure length, radiation exposure, and hospital stay. De et al. (31) suggested that PCNL provides overall significantly higher stone-free rates than fURS, at the expense of higher complication rates, blood loss, and a longer length of stay. Nevertheless, fURS can provide higher stone-free rates compared with minimally invasive percutaneous procedures. In our study, the overall rates of SF of fURS process was about 72%, which is consistent with other studies (ranging between 65 and 92%) (32). However, in the subgroup analysis based on location, the SF rate for the lower calyx calculi only reached 56.3%, which was obviously lower than the figure for the non-lower calyx calculi (90.16%). It is also verified in another study that the stone treatment in the lower pole is less effective compared to elsewhere in the kidney (33). Compared to middle and upper pole stones, the fURS treatment of lower pole stones is more complicated because of the anatomical factors and the limited deflection angle of flexible ureteroscopes (34). Additionally, Tonyali et al. reported that patients with lower pole stones are 2.25 times more likely to have residual stones after fURS compared to patients having stones at other locations (35). Evidently, the spontaneous passing of stone fragments after the surgery was more difficult due to the position of the lower pole. Therefore, we mainly focused on investigating the factors affecting the success rate of the lower calyx calculi.
Nevertheless, in the studies focusing on lower calyceal stones, some researchers have looked at specific factors that can affect the SFR for fURS. Several studies used the Elbahnasy method to calculate the infundibular pelvic angle (IPA) and reported that IPA and other pelvicaliceal anatomy-correlated parameters associate with a lower SFR (36). However, Danuser et al. (37) reported that influence of the collecting system anatomy on disintegrate clearance from the lower calyx could not be demonstrated. The effect of the lower calyx anatomy on the stone-free rate of fURS is still controversial. In addition, controversies also exist on the measurement of pelvicaliceal anatomy. To measure the IPA, urologists need to rely on intravenous urography (IVU) or contrast-enhanced CT (CCT), which are dependent on the use of a contrast agent. First of all, patients with kidney stones generally do not perform these two tests. Moreover, patients with a contrast agent allergy or moderate to severe renal insufficiency are not suitable for these tests. Last but not least, the anatomy of the renal pelvis and calyx will expand with the perfusion of water during the operation, and the pre-operative measurements cannot play an accurate predictive role in the operation. Herein, the IPA was not analyzed in our present study because of the low feasibility in most of the patients.
The current study included several limitations. First, this was a retrospective study, which may cause possible selection bias. Second, the sampling included was relatively inadequate. Moreover, the model was established based on single-center data, and prospective multicenter studies are needed to further validate our results. Finally, due to practical constraints, this paper cannot provide a comprehensive review of multiple stones and lower calyx anatomy, which may be the aim of a future prospective study.
In conclusion, this study indicated that patients with higher radiomics score, smaller stone volume, non-severe hydronephrosis level, and with a more experienced operator were more likely to reach a successful outcome when choosing the flexible ureteroscopy strategy. This clinical–radiomics model may serve as an effective pre-operative prediction method for clinical decision-making for kidney stone patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SW and DH devised the experiment. CL and ZL developed and organized the paper. PL and ZZ designed the tables and figures. QX and HD performed the data analysis. IK and PT participated in the revision of the manuscript. MC and YX wrote the original draft. All authors read and approved the final manuscript.
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81771801, 81571642, 81701657, and 81801695).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.576925/full#supplementary-material
1. Türk C, Neisius A, Petrik A, Seitz C, Skolarikos A, Thomas K. EAU Guideline on Urolithiasis. EAU Annual Congress Barcelona 2019: EAU Guidelines Office, Arnhem, The Netherlands (2019). Available online at: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed December 14, 2019).
2. Türk C, Petrík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. (2016) 69:475–82. doi: 10.1016/j.eururo.2015.07.041
3. Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American urological association/endourological society guideline, part I. J Urol. (2016) 196:1153–60. doi: 10.1016/j.juro.2016.05.090
4. De S, Autorino R, Kim FJ, Zargar H, Laydner H, Balsamo R, et al. Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. Eur Urol. (2015) 67:125–37. doi: 10.1016/j.eururo.2014.07.003
5. El-Nahas AR, Ibrahim HM, Youssef RF, Sheir KZ. Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for treatment of lower pole stones of 10–20 mm. BJU Int. (2012) 110:898–902. doi: 10.1111/j.1464-410X.2012.10961.x
6. Zhang Y, Wu Y, Li J, Zhang G. Comparison of percutaneous nephrolithotomy and retrograde intrarenal surgery for the treatment of lower calyceal calculi of 2-3 cm in patients with solitary kidney. Urology. (2018) 115:65–70. doi: 10.1016/j.urology.2017.11.063
7. Xue Y, Zhang P, Yang X, Chong T. The effect of stone composition on the efficacy of retrograde intrarenal surgery: kidney stones 1 - 3 cm in diameter. J Endourol. (2015) 29:537–41. doi: 10.1089/end.2014.0778
8. Gucuk A, Uyeturk U. Usefulness of hounsfield unit and density in the assessment and treatment of urinary stones. World J Nephrol. (2014) 3:282–6. doi: 10.5527/wjn.v3.i4.282
9. Xun Y, Li J, Geng Y, Liu Z, Yu X, Wang X, et al. Single extracorporeal shock-wave lithotripsy for proximal ureter stones: can CT texture analysis technique help predict the therapeutic effect? Eur J Radiol. (2018) 107:84–9. doi: 10.1016/j.ejrad.2018.08.018
10. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. (2017) 14:749–62. doi: 10.1038/nrclinonc.2017.141
11. Limkin EJ, Sun R, Dercle L, Zacharaki EI, Robert C, Reuzé S, et al. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol. (2017) 28:1191–206. doi: 10.1093/annonc/mdx034
12. Shabaniyan T, Parsaei H, Aminsharifi A, Movahedi MM, Jahromi AT, Pouyesh S, et al. An artificial intelligence-based clinical decision support system for large kidney stone treatment. Australas Phys Eng Sci Med. (2019) 42:771–9. doi: 10.1007/s13246-019-00780-3
13. Choo MS, Uhmn S, Kim JK, Han JH, Kim D, Kim J, et al. A prediction model using machine learning algorithm for assessing stone-free status after single session shock wave lithotripsy to treat ureteral stones. J Urol. (2018) 200:1371–7. doi: 10.1016/j.juro.2018.06.077
14. Schulster M, Small AC, Silva MV, Abbott JE, Davalos JG. Endoscopic combined intrarenal surgery can accurately predict high stone clearance rates on postoperative CT. Urology. (2019) 133:46–9. doi: 10.1016/j.urology.2019.08.026
15. Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J Royal Stat Soc B. (2011) 73:273–82. doi: 10.1111/j.1467-9868.2011.00771.x
16. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. (1982) 143:29–36. doi: 10.1148/radiology.143.1.7063747
17. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the hosmer-lemeshow test revisited. Crit Care Med. (2007) 35:2052–6. doi: 10.1097/01.CCM.0000275267.64078.B0
18. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. (2008) 8:53. doi: 10.1186/1472-6947-8-53
19. Mariani AJ. Combined electrohydraulic and holmium: YAG laser ureteroscopic nephrolithotripsy of large (greater than 4 cm) renal calculi. J Urol. (2007) 177:168–73. doi: 10.1016/j.juro.2006.08.066
20. Breda A, Ogunyemi O, Leppert JT, Lam JS, Schulam PG. Flexible ureteroscopy and laser lithotripsy for single intrarenal stones 2 cm or greater–is this the new frontier? J Urol. (2008) 179:981–4. doi: 10.1016/j.juro.2007.10.083
21. Cohen J, Cohen S, Grasso M. Ureteropyeloscopic treatment of large, complex intrarenal and proximal ureteral calculi. BJU Int. (2013) 111:E127–31. doi: 10.1111/j.1464-410X.2012.11352.x
22. Karakoyunlu N, Goktug G, Sener NC, Zengin K, Nalbant I, Ozturk U, et al. A comparison of standard PCNL and staged retrograde FURS in pelvis stones over 2 cm in diameter: a prospective randomized study. Urolithiasis. (2015) 43:283. doi: 10.1007/s00240-015-0768-2
23. Gücük A, Kemahli E, Üyetürk U, Tuygun C, Yildiz M, Metin A. Routine flexible nephroscopy for percutaneous nephrolithotomy for renal stones with low density: a prospective, randomized study. J Urol. (2013) 190:144–8. doi: 10.1016/j.juro.2013.01.009
24. Ito H, Sakamaki K, Kawahara T, Terao H, Yasuda K, Kuroda S, et al. Development and internal validation of a nomogram for predicting stone-free status after flexible ureteroscopy for renal stones. BJU Int. (2015) 115:446–51. doi: 10.1111/bju.12775
25. Resorlu B, Unsal A, Gulec H, Oztuna D. A new scoring system for predicting stone-free rate after retrograde intrarenal surgery: the “resorlu-unsal stone score”. Urology. (2012) 80:512–8. doi: 10.1016/j.urology.2012.02.072
26. Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. (2003) 95:634–5. doi: 10.1093/jnci/95.9.634
27. Sorokin I, Cardona-Grau DK, Rehfuss A, Birney A, Stavrakis C, Leinwand G, et al. Stone volume is best predictor of operative time required in retrograde intrarenal surgery for renal calculi: implications for surgical planning and quality improvement. Urolithiasis. (2016) 44:545–50. doi: 10.1007/s00240-016-0875-8
28. Cho SY, Choo MS, Jung JH, Jeong CW, Oh S, Lee SB, et al. Cumulative sum analysis for experiences of a single-session retrograde intrarenal stone surgery and analysis of predictors for stone-free status. PLoS ONE. (2014) 9:e84878. doi: 10.1371/journal.pone.0084878
29. Berardinelli F, Cindolo L, De Francesco P, Proietti S, Hennessey D, Dalpiaz O, et al. The surgical experience influences the safety of retrograde intrarenal surgery for kidney stones: a propensity score analysis. Urolithiasis. (2017) 45:387–92. doi: 10.1007/s00240-016-0919-0
30. Bozzini G, Verze P, Arcaniolo D, Dal Piaz O, Buffi NM, Guazzoni G, et al. A prospective randomized comparison among SWL, PCNL and RIRS for lower calyceal stones less than 2 cm: a multicenter experience: a better understanding on the treatment options for lower pole stones. World J Urol. (2017) 35:1967–75. doi: 10.1007/s00345-017-2084-7
31. De S, Autorino R, Kim FJ, Zargar H, Laydner H, Balsamo R, et al. Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. Eur Urol. (2016) 69:e85. doi: 10.1016/j.eururo.2015.12.046
32. Kilic O, Akand M, Van Cleynenbreugel B. Retrograde intrarenal surgery for renal stones-part 2. Turk J Urol. (2017) 43:252–60. doi: 10.5152/tud.2017.22697
33. Geraghty R, Burr J, Simmonds N, Somani BK. Shock wave lithotripsy outcomes for lower pole and non-lower pole stones from a university teaching hospital: parallel group comparison during the same time period. Urol Ann. (2015) 7:46–8. doi: 10.4103/0974-7796.148601
34. Jessen JP, Honeck P, Knoll T, Wendt-Nordahl G. Flexible ureterorenoscopy for lower pole stones: influence of the collecting system's anatomy. J Endourol. (2014) 28:146–51. doi: 10.1089/end.2013.0401
35. Tonyali S, Yilmaz M, Karaaslan M, Ceylan C, Isikay L. Prediction of stone-free status after single-session retrograde intrarenal surgery for renal stones. Turk J Urol. (2018) 44:473–7. doi: 10.5152/tud.2018.88615
36. Inoue T, Murota T, Okada S, Hamamoto S, Muguruma K, Kinoshita H, et al. Influence of pelvicaliceal anatomy on stone clearance after flexible ureteroscopy and holmium laser lithotripsy for large renal stones. J Endourol. (2015) 29:998–1005. doi: 10.1089/end.2015.0071
Keywords: clinical-radiomics model, flexible ureteroscopy, kidney stone, computed tomography, lithotripsy
Citation: Xun Y, Chen M, Liang P, Tripathi P, Deng H, Zhou Z, Xie Q, Li C, Wang S, Li Z, Hu D and Kamel I (2020) A Novel Clinical-Radiomics Model Pre-operatively Predicted the Stone-Free Rate of Flexible Ureteroscopy Strategy in Kidney Stone Patients. Front. Med. 7:576925. doi: 10.3389/fmed.2020.576925
Received: 27 June 2020; Accepted: 11 September 2020;
Published: 15 October 2020.
Edited by:
Carlo Garofalo, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Marco De Sio, University of Campania Luigi Vanvitelli, ItalyCopyright © 2020 Xun, Chen, Liang, Tripathi, Deng, Zhou, Xie, Li, Wang, Li, Hu and Kamel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Li, bGljb25ndGptQDE2My5jb20=; Shaogang Wang, c2d3YW5ndGptQDE2My5jb20=; Zhen Li, emhlbmxpQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.