- 1Department of Rheumatology and Immunology, Medical University of Graz, Graz, Austria
- 2Department of Rheumatology, Hospital of Brunico (SABES-ASDAA), Brunico, Italy
- 3Trauma Centre Meidling, Ludwig Boltzmann Institute of Osteology at Hanusch Hospital of Oesterreichische Gesundheitskassa and Allgemeine Unfallversicherungsanstalt, First Medical Department Hanusch Hospital, Vienna, Austria
- 4Medical Faculty, Sigmund Freud Private University, Vienna, Austria
In December 2019, a cluster of severe pneumonia was observed in China, with the subsequent discovery of a new beta-coronavirus (SARS-CoV-2) as the causative agent. The elicited disease COVID-19 is characterized by fever, dry cough, myalgia, or fatigue and has a favorable outcome in the majority of cases. However, in some patients COVID-19 leads to severe pneumonia and sepsis with subsequent respiratory failure and gastrointestinal, hematological, neurological, and cardiovascular complications. A higher risk of infection is intrinsic to active rheumatic and musculoskeletal diseases (RMD) and the use of biological disease modifying anti-rheumatic drugs (DMARDs). With an increasing number of reports on COVID-19 in RMD patients, we are beginning to appraise their risks. In this review, we summarize the published cases of COVID-19 infections in RMD patients, including patients with inflammatory arthritis and connective tissue diseases as well as anti-phospholipid syndrome and Kawasaki syndrome. Overall, patients with inflammatory arthritis do not seem to be at a higher risk for infection or a severe course of COVID-19. Risk for critical COVID-19 in patients with systemic inflammatory diseases such as SLE or vasculitis might be increased, but this needs further confirmation. Furthermore, we summarize the data on DMARDs used to fight SARS-CoV-2 infection and hyperinflammation.
Introduction
Since the discovery of the novel coronavirus SARS-CoV-2 causing COVID-19 in December 2019 until June 2020, more than 30,000 reports on the disease or the virus itself have been published. Infections of patients with underlying rheumatic and musculoskeletal diseases (RMD) experiencing a complicated course of COVID-19 have been reported. However, most of these reports are limited by the small numbers of patients. Besides, several studies are still at a pre-publication stage and may therefore not yet meet the rigorous standards of scientific journals. This article summarizes current knowledge on COVID-19 and RMD. It also reviews the general risk of viral infections in patients with RMD, the impact of disease modifying anti-rheumatic drugs (DMARDs) on the outcome of infections, and gives a comparison between present and previous coronavirus pandemics.
SARS-CoV-2 and COVID-19
The first cases of this novel pneumonia were reported in early December 2019 in Wuhan, the capital city of Hubei province, China (1). The genomic characterization of the pathogen causing this infection identified a novel enveloped positive single stranded RNA beta-coronavirus, which has ultimately been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2).
Coronaviruses are RNA-viruses, shielded by an envelope containing membranous proteins such as spike proteins. These spike proteins give the virus a crown like appearance under an electron microscope, coining the name “coronavirus.” The primary function of these proteins is host receptor binding, determining host tropism and transmission capacity (3).
Coronaviruses can cause a broad range of mostly mild infections in humans, mammals, and birds especially in the respiratory and gastrointestinal tracts, and to a lesser extent also in the nervous system. Until the end of 2019, six coronavirus strains (belonging to the alpha or beta-coronavirus genus) causing human disease had been identified (4). Four of these viruses cause common cold symptoms, whereas the two remaining strains elicit severe illnesses, namely the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) (4). Whereas, SARS-CoV was the pathogen leading to the severe acute respiratory syndrome observed in 2002/2003 in Guangdong Province, China (5), MERS-CoV was found to underly the severe respiratory syndrome seen since 2012 in the Middle East (6). Phylogenetically, the present SARS-CoV-2 displays an 88% homogeneity to two bat-derived severe acute respiratory syndrome (SARS)-like coronaviruses, a 79% identity with SARS-CoV and an approximate 50% identity with MERS-CoV (7). An important similarity between SARS-CoV and SARS-CoV-2 is the mechanism of entry into cells of the lower respiratory tract, mediated by the spike protein binding to the ACE2 protein (8).
The novel disease, termed COVID-19, is marked by symptoms reminiscent of other respiratory infections: fever, dry cough, myalgia, or fatigue are commonly observed, whereas sputum production, headache, hemoptysis, and diarrhea are less prevalent (1, 9). While the majority of patients with COVID-19 have a favorable outcome (10–13), some develop severe pneumonia eventually leading to acute respiratory distress syndrome (ARDS), respiratory failure, along with other organ manifestations and sepsis (14). COVID-19 appears to have at least two distinct disease phases: a phase characterized by the immune response against the virus with the aim of eliminating the pathogen. Some patients, even though they may have had only mild initial symptoms, subsequently develop a phase of severe “cytokine storm” (cytokine release syndrome, CRS) instead of the expected phase of re-convalescence leading to fatal autoinflammation of the lung and other organs (15). As the disease spreads throughout the world causing the present pandemic crisis, additional symptoms have been observed, e.g., neurological changes such as anosmia or ageusia or thrombotic complications (16, 17). The development of profound thrombocytopenia (36.2%) and elevated D-dimers (46.4%), which are even higher in patients with severe COVID-19 disease (57.7 and 59.6%, respectively) (9) have become a hallmark of the disease, and suggest a triggered disseminated intravascular coagulation (DIC), reviewed in (18). There are distinctive characteristics of this new disease: it affects more men than women, causes severe disease especially in elderly people. Certain comorbidities including hypertension, diabetes, prior respiratory, and cardiovascular disease as well as obesity have been associated with a worse outcome (9, 19). Based on a developing cohort of 1,590 patients and a validation cohort of 710 patients, a risk score for the development of a critical illness (defined as composite measure of admission to the intensive care unit, invasive ventilation, or death) 10 items emerged as independent predictive factors (12). Three of those were patient characteristics: older age, number of comorbidities, and a history of cancer. However, although immunodeficiency was one of the 72 potential predictors in the beginning, the presence of rheumatic diseases or immunosuppressive medication was not included in the calculation, making the score less applicable from a rheumatological perspective.
According to the first report of the Chinese Center for Disease Control only ~5% of patients out of 72,314 Chinese patients were critically ill, 14% of them suffered from a severe course, while most patients (81%) showed no or mild pneumonic symptoms (10). The overall case-fatality rate (CFR) was 2.3%, which is lower than that during the earlier coronavirus outbreaks with SARS-CoV (9%) and MERS-CoV (36%) (4). Whereas, the comorbidities and risk factors for worse outcomes of COVID-19 are similar throughout international reports, mortality rates diverge largely from 0.06% in Singapore up to 18.3% in France (13). The causes for this disparity are myriad and may include disease factors and genetic susceptibility of the populations, but also differences in health care organization (e.g., number of tests conducted, criteria for testing) and outcome reporting (particularly who is counted as deceased due to/with COVID-19).
Immunological changes generally observed in patients with COVID-19 include lymphocytopenia that pertains to CD4+, CD8+, and Treg cells and increased levels of proinflammatory cytokines [e.g., interleukin (IL)1-β, IL-1RA, IL-7, IL-8, IL-9, IL-10, interferon (IFN)γ, interferon-gamma induced protein (IP)10, monocyte chemoattractant protein (MCP)1, macrophage inflammatory protein (MIP)1α, MIP1β, tumor necrosis factor (TNF) α, granulocyte colony stimulation factor (GCSF), platelet derived growth factor (PDGF) B, and vascular endothelial growth factor (VEGF) A]. A pronounced decrease of CD4+ T cells and higher levels of IL-6, IL-8, and TNFα occur during the phase of hyperinflammation (CRS) (1, 20). Hyperinflammation is considered to initially involve activation of macrophages stimulated by damage-associated molecular patterns (DAMPs) released from dying cells and pathogen-associated molecular patterns (PAMPs) such as viral RNA (21). This leads to massive IL-1 and IL-6 secretion promoting the recruitment of neutrophils and cytotoxic T cells, which in turn induce pneumocyte and endothelial injury, ultimately leading to acute lung injury. Pre-publication reports describe a specific monocyte population in COVID-19 patients, not seen in healthy donors or other viral infections. These monocytes are larger, atypically shaped, vacuolated secreting high levels of both IL-6 and IL-1β and expressing ACE2 (22, 23). Interestingly, interferon type I, a crucial player in the combat of viral infections, seems to be muted for unknown reasons in COVID-19 patients (24), which was especially seen in critically ill patients (25). Furthermore, alterations in the lymphocyte department, including upregulation of markers of T cell exhaustion in both lung-resident and circulating T-lymphocytes, including PD-1 and Tim-3 have been described (26). Furthermore, CD4+ as well as CD8+ T cells of COVID-19 patients display an activated phenotype. In analogy to patients infected with SARS-CoV or MERS-CoV, CD4 Th1-cells expressing GM-CSF, and IL-6 were isolated from patients with COVID-19 CRS (27). In contrast, in patients in the recovery phase an effective adaptive immune response characterized by T cell clonal expansion, activation, and memory formation has also been reported in COVID-19 (23). The higher occurrence of the above described Th1 cells and the COVID-19-typical, highly inflammatory monocyte subset together with the inadequate IFN I answer may be determining factors leading to a worse disease course.
The Infection Risk of Patients With Rheumatic Diseases
Patients with RMD are generally considered to be more prone to bacterial and certain viral infections such as the herpes zoster virus. The susceptibility to infections is multifactorial and is related to disease aspects such as disease activity, disease damage, comorbidities, as well as to treatment. In rheumatoid arthritis (RA), each 0.6 point increment of the disease activity score (DAS) (28), led to a 25% increase in hospital admissions and 4% increase of outpatient infections in a US registry of >16,000 patients (28).
A 6-fold increased susceptibility to infections was seen in systemic lupus erythematosus (SLE) in an insurance database (29) and an incidence rate of 29.2 severe infections/1,000 patients per year in the Spanish SLE-registry (30). Current and past use of HCQ seemed to have a protective effect (RR 0.49, p = 0.0000), whereas glucocorticoids (≥10 mg/d), rituximab, mycophenolate mofetil/mycophenolic acid, and lupus activity/severity were linked to a higher risk of severe infection.
Susceptibility to Infections Through Immunosuppression
According to a Cochrane review, the risk of infection in patients with inflammatory arthritis [IA; including RA, psoriatic arthritis (PsoA) and spondylarthritis (SpA)] is higher under therapy with TNF-blockers than with conventional disease modifying anti-rheumatic drugs (DMARDs) only (31). A meta-analysis of other biological DMARDs (bDMARDs) used in RA indicated a 1.3-fold increased risk of serious infections in standard doses, and an almost 2-fold increased risk at higher doses (any dose higher than the one approved by the regularities) compared to conventional DMARDs (32). Whereas, the risk of serious infections under anti-IL-17 therapy is comparable to that of TNF-blockers; anti-IL-12/23 therapy seems to carry a lower risk (HR = 0.59, 95% CI 0.39–0.90) (33).
Due to their inhibition of interferon-alpha, Januskinase inhibitors (JAKi) increase the risk of viral infections (34), especially of herpes zoster. In contrast, the overall risk for severe infections of RA patients with JAKi is comparable to that of healthy controls according to a recent meta-analysis (35). Whether there is an increased risk of coronavirus infection (or influenza) elicited by JAKi used in rheumatology is currently unknown.
The use of glucocorticoids (GC) in both IA (36) and connective tissue diseases (CTD) leads (29) to an increased rate of infections overall and viral infections in particular, especially for herpes zoster correlating with the actual GC dose, treatment duration, and the cumulative GC dose; however even doses considered relatively safe such as 5 mg prednisone equivalent have been associated with an increased infections risk. Hydroxychloroquine seems to have a protective effect for those who take GCs: in patients with GC monotherapy a HR 3.9 for severe infections was reduced to 0 for those who took GC + hydroxychloroquine.
Among conventional synthetic DMARDs (csDMARDs), methotrexate has not been associated with an increased infection rate according to a study with over 27,000 RA patients (37). In contrast, mycophenolate mofetil, azathioprine, and cyclophosphamide were linked to a higher risk of infections particularly in patients with connective tissue diseases (38).
Rheumatic Diseases and COVID-19
We know little of RMD patients infected in the past outbreaks of coronaviruses like SARS and MERS (39, 40). Also, given the restricted geographical spread and the low number of patients affected, no special containment measures were released affecting diagnosis and the management of patients with RMD, nor were there any difficulties with drug supply for DMARDs. There has been a weak association of coronavirus outbreaks in the past with higher incidence of Kawasaki syndrome, which is often virally triggered. Kawasaki syndrome occurred in <5% of patients with a coronavirus infection in some reports, however, no reports are available for SARS and MERS (41, 42). Single cases of SARS and thrombotic complications have been reported. These complications were considered to be related to the presence of anti-phospholipid antibodies (43).
Occurrence of Rheumatic Diseases in Patients of Large Cohorts of COVID-Patients
In the largest early report on patients with COVID-19 from China there are no data on patients with RMD or any other immunological disorder (10, 44). Guan et al. mention two patients with immunodeficiency without a severe course of COVID-19 infection out of a cohort of 1,099 COVID-19 patients from China (9). Another publication from Wuhan included one patient with pre-existing connective tissue disease, who died (45). The cohort of 5,700 COVID-19 patients from New York City did not list any rheumatologic disease as a comorbidity, although at least CTD should have been captured, given that the Charlson comorbidity index was used in this study (19). Likewise, no RMD was named under the comorbidities in a prospective cohort of 1,150 critically ill patients in New York City or a cohort of 2,070 COVID-19 cases in Brazil (46, 47). In a paper from Lombardy, the Italian region with the highest number of COVID-19 cases (48), there were no patients with immunosuppressive therapy with JAKi or bDMARDs among 700 COVID-19 patients with a severe course of disease.
However, data from the health analytics platform OpenSAFELY, which covers 40% of all patients in England and holds primary care records of 17,278,392 adults pseudonymously linked to 10,926 COVID-19-related deaths found a hazard ratio of 1.19 (1.11–1.27) for the combined autoimmune diseases rheumatoid arthritis, lupus, and psoriasis (49).
Additionally, Arentz et al. reported 21 patients in intensive care units in Washington State. Of these, one person had a “rheumatic disease” as a pre-existing condition and three had immunosuppression due to “rheumatoid disease” or transplantation. However, there is no detailed information about the precise diagnosis, medication, comorbidities, or course/outcome of these patients (50). In that specific cohort, it seems that there was a high rate of co-morbidities (85.7%) and mortality (67%) with only a few discharges (9.5%).
Currently available data do not imply a strikingly increased risk of infection for RMD with or without DMARD therapy in general (51, 52), although differences in the individual diseases have been reported (53). Pablos et al. investigated the prevalence of COVID-19 in seven Spanish hospitals providing medical care for a population of 2.9 million patients, and found a comparable prevalence of the infection in RA and PsoA with the general population (0.58%); in contrast, patients with spondylarthritis (SpA) had a higher prevalence (53). As this was also the case for patients on bDMARDs or JAKi, one might speculate that the higher infection rate of SpA patients was due to a higher percentage of these drugs in SpA patients compared to the other groups. This aspect, however, was not analyzed in the cited paper. Interestingly, patients with Sjögren's syndrome or systemic sclerosis showed a higher prevalence of SARS-CoV-2 infection in comparison with the general population; in contrast, prevalence in SLE patients was similar to that of the reference population. This unexpected discrepancy among CTD patients might be explained at least partly by the younger age and the higher proportion of females among SLE patients in this cohort.
In a report from Lombardy, 1,525 patients with RMD were evaluated by a survey and 8% were found to have either confirmed or suspected COVID-19, with most of them suffering from a form of inflammatory arthritis (54). In addition, the authors used a case-control design to investigate differences between the 117 COVID-19 cases with RMD and control COVID-19 patients without RMD. No differences pertaining to comorbidities or disease course were detected.
Similar findings were described in a case control study from Massachusetts investigating 52 RMD patients, of which 50% had an IA and the remaining patients suffered from different forms of systemic autoimmune diseases. The disease course of COVID-19 of these cases was compared with that in 104 sex- and age-matched controls without underlying RMD (55). The majority of the RMD patients with COVID-19 had active disease (62%) and a higher percentage of coronary and interstitial lung disease. Whereas, the clinical presentation and laboratory values were similar in the two patient groups, RMD patients were at a higher risk for intensive care/mechanical ventilation [adjusted OR for mechanical ventilation 3.11 (95% CI 1.07–9.05), p = 0.04]. However, this did not imply a statistically significant higher mortality (6%) or overall hospital admission rate; the higher rate of ventilation reported in RMD patients is unsettling, considering that the OR was adjusted for the higher cardiorespiratory comorbidities, suggesting an intrinsic effect of the underlying autoimmune disease. Nevertheless, other comorbidities, disease activity or use of immunosuppressive drugs may have contributed to the higher risk of ventilation and need to be addressed in future studies. Similarly, a report from Wuhan found an increased rate of respiratory failure (38%; mortality: 9.52%) in 21 patients with RMD with a similar distribution between IA and CTD (56). In comparison with this Chinese study, a different cohort of 29 RMD patients from Wuhan found a lower need for ventilation (6.9%) and a 3.4% mortality rate (57). Whereas, the disparity of the first Chinese study with the cohort from the US may be attributable to a different disease population, different medication regimes, and also a different genetic background, the reason for the intra-regional difference between the two reports from Wuhan remains elusive. Altogether, there is a dire need for reports with larger cohorts.
To address this need, the Global Rheumatology Alliance has established a registry of RMD patients with COVID-19 infection (https://rheum-covid.org/). This is an international initiative, supported by ACR and the EULAR as well as by several national societies, with the possibility to include RMD patients affected by COVID-19 from all over the world.
The website is updated regularly (summarized in Box 1, accessed on July 19th 2020) and a report of the first 600 patients was recently published (58). The majority (93%) of patients were from North America or Europe, female, and between 50 and 65 years of age. The distribution of IA vs. CTD was approximately 2:1 and 20% displayed moderate/high disease activity. The report described interesting findings:
(1) there was a high rate of hospitalization (46%) and mortality (9%) altogether, with SLE and vasculitis patients showing a higher propensity to be hospitalized than other patients.
(2) the main factor associated with hospitalization was a prednisone dose ≥10 mg/day (OR 2.05, 95% CI 1.06–3.96), whereas DMARD therapy (csDMARD monotherapy or combined with bDMARDS/JAKi) was not associated with hospitalization.
(3) therapy with TNF-blockers reduced the risk of hospitalization (OR 0.40, 95% CI 0.19–0.81) in contrast to therapy with antimalarials, which did not (OR 0.94, 95% CI 0.57–1.57).
(4) established general adverse characteristics/comorbidities as age >65 years, hypertension/cardiovascular disease, lung disease, diabetes as well as chronic renal insufficiency/end-stage renal disease carried a higher risk of hospitalization also in RMD patients.
(5) treatment with bDMARD/JAKi monotherapy just prior to COVID-19 diagnosis reduced the risk of hospitalization compared with no DMARD therapy (OR = 0.46, 95% CI 0.22–0.93; p = 0.03).
Box 1. Data of patients from the rheum-covid registry, assessed on July 19 2020.
• Patients in registry: 3,814
• Patient-characteristics on 1,440: 74.4% female, 76.2% <65 years, 39.5% Caucasian
• Outcome: death 8.06%, hospitalized 41.4%
RMD are distributed as follows:
• Rheumatoid arthritis (RA): 38.5%
• Systemic lupus erythematosus (SLE): 17.2%
• Psoriatic arthritis (PsA): 9.7%
• Vasculitis: 6.7%
• Spondylarthritis (SpA): 6.0%
• Sjögren's syndrome: 4.5%
• Systemic sclerosis: 3.1%
Top comorbidities:
• Arterial hypertension
• Lung diseases
• Diabetes
• Cardiovascular diseases
• Renal disease
• Morbid obesity
Medication prior to COVID-19:
• csDMARDs 62.8%
• bDMARDs 31.1%
• JAKi 4.9%
• CQ/HCQ 26.7%
• GC 33%
csDMARDs, conventional synthetic disease modifying antirheumatic drugs; bDMARDs, biological disease modifying antirheumatic drugs; JAKi, Januskinase inhibitors; CQ/HCQ, Chloroquine/Hydroxychloroquine; GC, glucocorticoids.
Some results, like the role and category of comorbidities, are in line with the general population experiencing COVID-19, others, like the protective effect of TNF-blockers were unexpected. The possible benefit of TNF-blockers was also observed in a cohort from the US (59) and underscores the role of the overabundance of TNF in critically ill COVID-19 patients. Altogether, this report supports the recommendations issued by national and international societies not to withhold DMARD treatment in RMD patients prophylactically in this pandemic. However, important questions as, e.g., a possible difference in disease course in the diverse RMDs have not been answered and await clarification.
The registry has also provided preliminary data on 80 SLE-patients with COVID-19 and the use of HCQ. Sixty-four percent of the SLE-patients were taking antimalarials prior to the infection with SARS-CoV-2 and admission frequency to the hospital did not differ between HCQ users and non-users [55% (16/29) vs. 57% (29/51), p = ns; χ2-test] (60). This argues against a protective role of HCQ (in the usually administered dose for RMD patients) in SARS-CoV-2 infection, which is also supported by pharmacological in vitro data describing a much higher level needed for effective viral inhibition (61). The ineffectiveness of HCQ was also indicated in several RMD and SLE cohorts, in which the prevalence of (confirmed or suspected) COVID-19 was similar in patients with or without HCQ treatment (62–68). In contrast, a large study using databases of general medication use and SARS-CoV-2 infection in Portugal found a protective effect of chronic treatment with HCQ [OR 0.51 (0.37–0.70)] for SARS-CoV-2 infection, even after adjustment for demographic characteristics and immunosuppressive treatment (69). Due to the study design, no data on underlying diseases (including RMD) or other patient data apart from those on medication use and COVID-19 status were retrievable precluding a definite judgement on the efficacy of HCQ for preventing COVID-19. In particular, a selection bias with prescription of HCQ preferably to patients without comorbidities could have influenced the results.
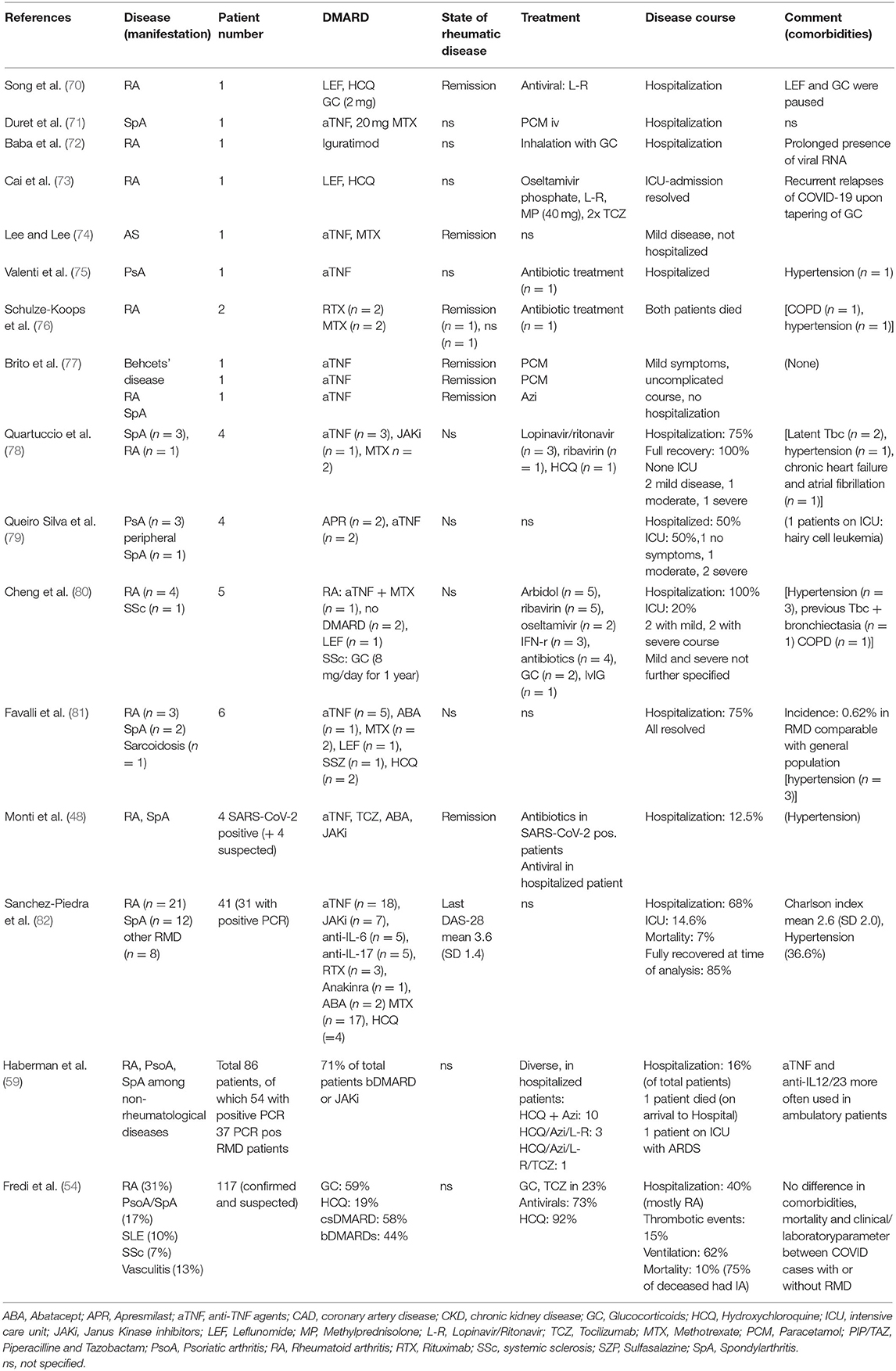
Other descriptions of RMD patients with SARS-CoV-2 infections are often based on case reports or case series (see Tables 1, 2 for summary) with different RMD and are discussed below.
Patients With Inflammatory Arthritis (IA) and COVID-19
The disease course in a series of 86 patients with immune-mediated inflammatory diseases including 50 cases of IA (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis) with confirmed or highly suspected COVID-19 was analyzed (confirmed cases: total: 59, of which 37 IA). A special focus was put on the comparison of patients with an out-patient or in-patient management (16%) (59). Seventy-two percent of patients were receiving bDMARDs (anti-TNF, anti-IL17, anti-IL-23, and anti-IL-12/23 agents) or JAKi. Among hospitalized patients, comorbidities (hypertension, diabetes, or chronic obstructive pulmonary disease) were more common than in out-patients. Besides, oral GC (29 vs. 6%), HCQ (21 vs. 7%), and methotrexate (43 vs. 15%) were more frequently used in the former, whereas certain biologicals (mostly TNF-blockers and anti-IL12/23 agents) were more common in the latter group. Whether this indicates any possible protective effect of these biologics to prevent the occurrence of a CRS in RMD patients remains a matter of speculation.
Another possibility to learn about the course of COVID-19 on patients with RMD is to follow prospectively a cohort of patients with a defined disease and to note symptoms and outcomes of any SARS-CoV-2 infection. In a study from northern Italy with this objective, 320 patients with RA or SpA with an average age of 55 ± 14 years were interviewed and observed prospectively for 2 weeks (48). Among them, 13 patients had a confirmed or suspected SARS-CoV-2 infection or had been in contact with an infected person but had stayed asymptomatic. The use of bDMARDs and JAKi was heterogeneous (4 etanercept, 1 adalimumab, 1 tocilizumab, 2 abatacept, 2 tofacitinib, and 3 barictitinib). Almost half of patients were on regular hydroxychloroquine and/or low-dose glucocorticoid therapy. Only one patient, 65 years old, required hospital admission and was given oxygen and adjuvant therapy with antivirals and HCQ. All other patients paused their DMARDs for the time of the infection and had an uncomplicated course.
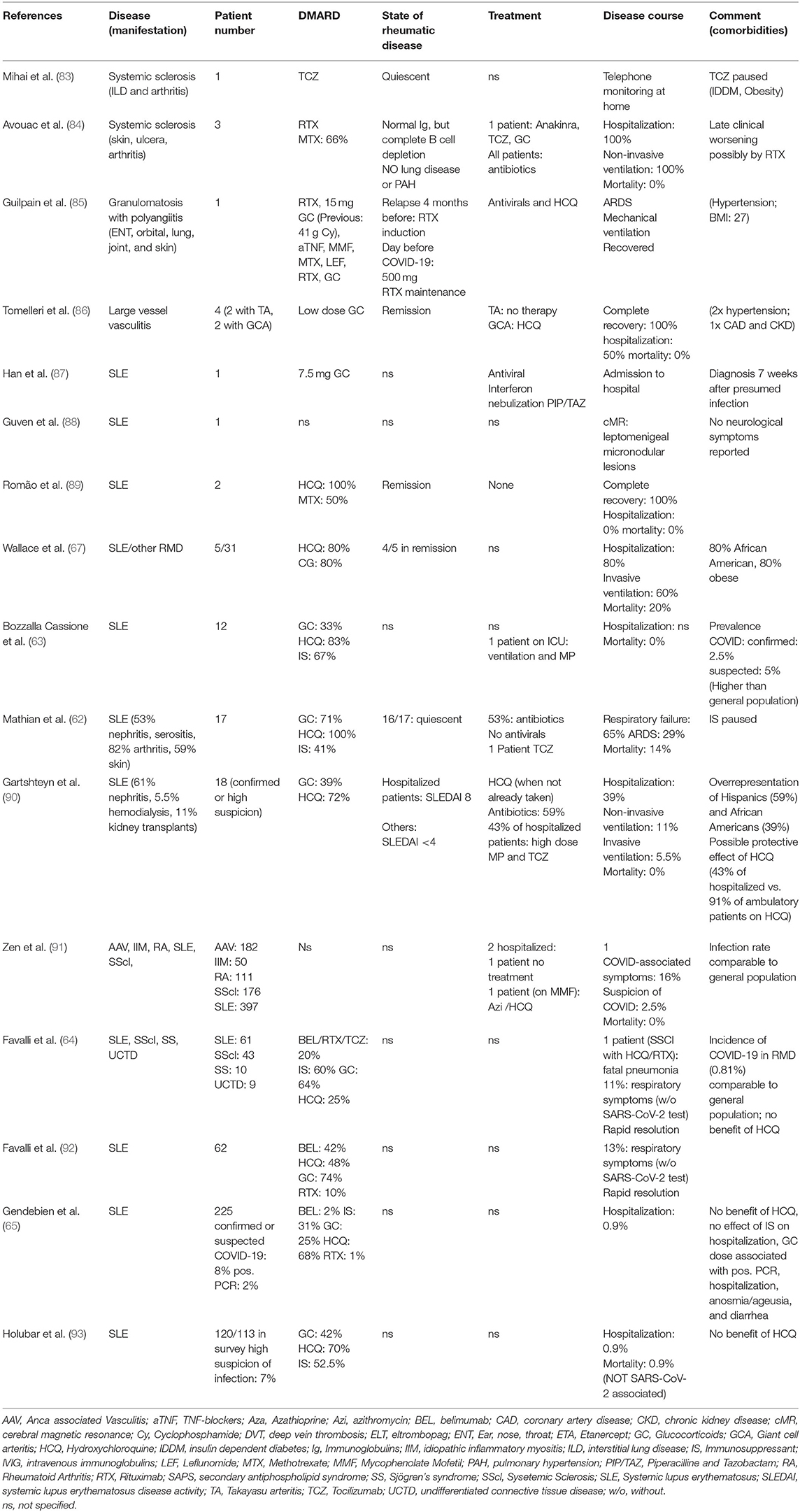
Patients With Connective Tissue Diseases (CTD)
There have been only incidental reports of patients with CTD besides SLE: Of the few cases with systemic sclerosis (Table 2), the most striking common denominator is treatment with Rituximab, which seems to induce a late worsening of symptoms, leading to respiratory complications, also in patients without pre-existing lung disease (64, 84). Likewise, one patient from France with granulomatosis with polyangiitis, who was overweight and had hypertension under rituximab and corticosteroid therapy had a severe COVID-19 course with ARDS requiring intubation. The patient was discharged after 29 days.
The disease course of 17 French patients with SLE [median age: 53.5 (26.6–69.2)] was reported recently (62). Sixteen of the patients had quiescent rheumatic disease, the main comorbidities were obesity (59%) and chronic kidney disease (47%); 24% of them had secondary antiphospholipid syndrome (APS). All were on (different doses) of HCQ, 71% were taking glucocorticoids, most of them below 10 mg prednisone equivalent, and 41% took immunosuppressants. The clinical course of these patients was more severe than expected, 65% of them had signs of respiratory failure needing oxygen therapy. Almost half of these patients were admitted to intensive care and one third developed ARDS. One patient was treated with extracorporeal membrane oxygenation and two of the 17 patients died. Three patients developed acute renal injury; cardiac injury and venous thrombosis occurred in one patient each. An equally severe picture was displayed in five SLE patients from Michigan, were one patient died, and three needed mechanical ventilation (67). The reasons why these SLE patients had a worse outcome than those from other reports of the general population is unclear, however, long-term glucocorticoid treatment and comorbidities might have played a role. Additionally, an epigenetic dysregulation, i.e., DNA hypomethylation of ACE2 resulting in ACE2 overexpression in T cells of SLE patients, has been shown; this phenomenon could be perpetuated by the oxidative stress induced by viral infections or by SLE flares and thus could contribute to the worse disease course seen in this SLE cohort (94).
In contrast, Bozzalla et al. observed an overall mild course of COVID-19 in 12 patients of their cohort of 165 SLE patients, although the incidence of confirmed or suspected COVID-19 was higher in this cohort than in the general population of the area; nevertheless, this might be explained by a bias of higher testing (63).
A special aspect was observed in a 47-year-old Chinese SLE patient, who had been diagnosed with SLE 16 years earlier and had been on 7.5 mg of prednisone ever since, without other immunosuppression. She had experienced mild symptoms for 4 weeks and was diagnosed with COVID-19 7 weeks (87) after her presumed exposure to the virus having infected two family members in the meantime. At the time of diagnosis her nasopharyngeal swabs were negative, and she showed positive SARS-CoV-2 serology, suggesting that the symptoms experienced were part of the second phase. The unusually long incubation period and the supposed prolonged viral shedding might be due to the immunosuppression with GC which also warrants caution in other rheumatic patients on long term GC treatment.
Apart from case series, patient surveys have been carried out in CTD patients. The largest one comprised 916 patients in northeast Italy, of which 88% had underlying CTD (SLE, vasculitis, systemic sclerosis, myositis); 2.5% had a high suspicion of COVID-19, only two patients (0.21%) had a PCR confirmed infection, mortality was 0. The low prevalence of confirmed cases was similar to that of the general population of the Veneto. Overall, discontinuation of DMARD therapy was rare in these patients, with higher percentages in RA (10.8%) and SLE (3.3%) (91). A comparably low prevalence of COVID-19 was seen in other surveys from Milan and Belgium, possibly influenced by a high percentage of patients undertaking precautionary measures early in the pandemic (64, 65, 92, 93). Also in these surveys the ineffectiveness of HCQ as protection against COVID-19 was noted, as well as a negative effect of higher GC doses in terms of hospitalization and clinical features (anosmia/ageusia and diarrhea) (65).
In conclusion, data published in the first 6 months do not consistently describe a higher risk for infection with SARS-CoV-2 or a more severe course of COVID-19 in patients with either inflammatory arthritis or connective tissue diseases. However, due to early case series (62, 67) describing a more severe course of the infection in SLE patients, more data on COVID-19 from cohorts of SLE patients with confirmed SARS-CoV-2 infection are warranted.
Antiphospholipid Syndrome (APS) and COVID-19
The thrombogenic nature of COVID-19 (17) is shared by rheumatic diseases, which should prompt further attention on RMD-associated coagulation problems such as the APS. Analogous to incidental reports of antiphospholipid antibodies (APL) in earlier coronavirus pandemics, these antibodies have occasionally been reported in COVID-19 (95), albeit of an IgA class, which have not yet been included in the classification criteria of APS (96). However, a role for IgA antibodies in clinical APS not meeting the serological criteria and SLE patients has been postulated (97, 98). Lupus anticoagulant (LAC) was present in 45% of a French cohort of 56 patients with COVID-19 without underlying RMD. In this group, 10% also tested positive for anti-cardiolipin (aCL) or anti-beta2glycoprotein1 (ab2gp1) antibodies (mostly associated with LAC) (99). Additional data from London corroborate the higher prevalence of LAC in COVID-19 patients. In 216 samples from COVID-19 patients screened for coagulation abnormalities in 24 h, 44 were found to have a prolonged aPTT, and 91% of the further investigated showed a positive LAC, often associated with reduced factor XII levels (100). Additionally, in a population of COVID-19 patients on the intensive care unit (ICU) 87% of the patients tested had a positive LAC (101). In contrast, antiphospholipid antibodies were commonly detected to a substantially lower degree with a preponderance of antibodies, which are not part of the official classification criteria for APS [IgA aCL; IgA ab2gp1; antibodies against phosphatidylserine/prothrombin (aPS/PT)] (102–104). Due to their disputed role in APS in general, the significance of these antibodies for COVID-19 is not yet clear. The occurrence of APL in viral infection is a well-known phenomenon, however, in most cases these are of IgM or IgG aCL subtypes with an antigen recognition differing from classical APS and are less thrombogenic in nature (105); in COVID-19 the latter finding has also been observed, casting a doubt on the clinical relevance of the characterized antibodies (102). Nevertheless, the pathogenicity of APL from COVID-19 patients was proven elegantly in a pre-publication by Zuo et al., in which IgG aPS/PT antibodies led to NETosis in vitro, thus contributing to thrombus formation, and increased thrombus extension in vivo in a mouse model (104). This finding is corroborated by the observation that APL occur preferably in critically ill patients (103). Altogether, the risk of thrombosis mediated by APS should be kept in mind and a prolonged aPTT should not prevent proper anticoagulation in patients. Besides, it needs to be tested whether the presence of the diverse APL is associated with an increased risk of worse outcomes in patients with COVID-19 (17) or vice versa, and if the emergence of LAC and APL in COVID-19 patients constitute a transient phenomenon or a persistently increased thrombogenic state.
Altogether, reports on patients with RMD infected with SARS-CoV-2 are still insufficient and are partly contradictory in terms of hospitalization and outcome. However, an unequivocal signal of higher risk for infection with SARS-CoV-2 or a more severe disease course of COVID-19 has not been detected. More data, especially larger cohorts of different patient groups are needed to provide a better risk stratification of RMD patients. Additionally, the question, whether patients receiving bDMARDs such as TNF-blockers or anti-IL-12/23 may be protected from a complicated disease course has to be clarified by future research. In conclusion, due to the generally increased susceptibility to infection, patients with rheumatic diseases should be considered at risk as long as the data remain scant.
Does SARS-CoV-2 Increase the Risk of Developing a Rheumatic Disease?
Another yet unresolved question is whether COVID-19 might trigger autoimmune diseases. Although the detection of ANA has been reported in a third of COVID-19 patients in the acute stage (106), the persistence and clinical relevance thereof remains to be investigated.
Whereas, arthralgias and myalgias might be one of the symptoms of COVID-19, data on the incidence of RA or other IA after COVID-19 have not been published yet.
In a Korean study of 24,117 newly diagnosed RA patients, published before the discovery of SARS-CoV-2, an increased risk of developing rheumatoid arthritis after infection with other coronaviruses or influenza was reported. For an increase of 1% of coronavirus-infected patients, a 9.2% increase in the incidence of RA in the following months was observed, especially in women and older patients (107). Whether, there is direct molecular mimicry between coronavirus and host antigens or whether coronaviruses activate other, yet unclear mechanisms leading to chronic autoimmunity has to be investigated further.
Analogous to previous (small) peaks during SARS/MERS, a pediatric inflammatory multisystem syndrome displaying similarities with Kawasaki syndrome has been observed in association with SARS-CoV-2. The first child reported, a 6-month-old infant, received a single dose of 2 g/kg intravenous immunoglobulin (IVIG) and high dose acetylsalicylic acid and was discharged shortly thereafter (108). The unusual accumulation of eight children suffering from a disease reminiscent of Kawasaki syndrome with a combination of rash, conjunctivitis, peripheral oedema, extremity pain, and serious gastrointestinal symptoms prompted a national alert in Great Britain at the end of April (109). Although only two (one of them postmortem) of the children tested positive for SARS-CoV-2, all of them were reported to have antibodies. This suggests a SARS-CoV-2 triggered severe hyperinflammation necessitating the admission to the pediatric ICU, where one 14-year-old child eventually succumbed to a large cerebrovascular infarct. All children experienced either cardiac complications or serositis, were treated with inotropics, and apart from the mentioned 14-year-old are alive at this point. Since the first description, several larger case series have been published describing a clinical spectrum including fever, gastrointestinal symptoms, and rash as well as the complications of myocardial injury, shock, and development of coronary artery aneurysms (110–113). The disease associated with SARS-CoV-2 and the “classical” Kawasaki syndrome differ in terms of age, inflammation markers including ferritin, which are all higher in SARS-CoV-2, as well as more severe cardiac involvement (112, 113). An unusual and alarming treatment resistance to IVIGs was seen in more than 60% of a French cohort of 16 patients. This occurred especially in patients >5 years old with high ferritin levels and should prompt rapid escalation of immunosuppression with tocilizumab or anakinra to avoid serious cardiac sequelae (114). The exact relationship between the virus and the hyperinflammation has yet to be elucidated in order to optimize a treatment regimen.
Should We Use Dmards for the Therapy of COVID-19?
Elevated levels of pro-inflammatory cytokines, including IL-6 are associated with severe COVID-19 and an increase in IL-6 is associated with increased mortality (11, 115). An excessive increase of C-reactive protein (CRP), IL-6, and ferritin observed in COVID-19 cases was termed CRS in analogy to similar findings in hemophagocytic lymphohistiocytosis and CAR-T cell therapy. Blockade of the IL-6 receptor (IL-6R) using tocilizumab is approved by the FDA to alleviate the CRS after hematological CAR-T cell therapy (116). In COVID-19 tocilizumab may be considered in patients with high levels of inflammatory markers such as IL-6 and worsening respiratory insufficiency, requiring non-invasive ventilation or intubation (117). However, evidence for these recommendations from randomized control trials (RCT) is lacking. Several reports have been published: Toniati et al. reported an improvement in 77 out of 100 patients treated with tocilizumab while 20 died (118). Xu et al. described 20 patients with severe COVID-19 and improvement of symptoms after a single administration of tocilizumab (119). Luo et al. reported 15 COVID-19 patients treated with tocilizumab, of whom three died (120). In addition, several retrospective case-control studies on the use of tocilizumab in COVID-19 have been published (121–126), of which a majority reported a favorable outcome of tocilizumab treatment compared to controls. Of these, Campochiaro et al. and Canziani et al. did not find a statistically reduced mortality in tocilizumab treated patients, although both studies showed a respective trend (121, 122) additionally, tocilizumab treated patients required invasive ventilation statistically less frequently than controls (122). In line with this observation the outcomes of several studies suggest that patients may benefit most from tocilizumab if given before invasive ventilation (125, 127). In a prospective open single-arm study investigating 63 COVID-19 patients with signs of CRS, administering tocilizumab within the first 6 days of hospitalization was associated with a better outcome than later in the course of disease, irrespective of the intravenous or subcutaneous route of administration (128). Preliminary results released of a large RCT investigating the use of the IL-6 receptor blocker sarilumab in COVID-19 are ambivalent1. Patients with critical COVID-19 treated with 400 mg of sarilumab tended to less likely need mechanical ventilation and showed clinical improvement more often than the placebo group. However, in patients with non-critical, severe COVID-19 the group receiving sarilumab trended toward unfavorable outcomes when compared to placebo. In this study, the patients had not been pre-stratified for high inflammatory burden. Thus, in the absence of a cytokine storm where IL-6 receptor blockade cannot be effective, adverse events may deteriorate outcomes. Bacterial superinfection, septic shock, gastrointestinal perforation, and viral myocarditis have been reported in COVID-19 after treatment with tocilizumab (118, 126, 129). In summary, IL-6 blockade may be a useful option in COVID-19 when administered early to those with signs of excessive inflammation. A clearer picture will emerge as randomized controlled studies for tocilizumab and sarilumab currently conducted in COVID-19 will be published.
The beneficial role of IL-1 blockade in life-threatening hemophagocytic lymphohistiocytosis (HLH) syndrome has been well-established (130). Due to the pathophysiological similarities pertaining to the macrophage activation between HLH and the hyperinflammation seen in the second phase of COVID-19, a small number of patients have been treated with the IL-1 receptor antagonist anakinra. Two case series report anakinra treatment in 9 and 11 patients, respectively (131, 132). Eight out of nine and seven out of 11 patients treated with anakinra did not require invasive ventilation. Besides a slight elevation of transaminases, anakinra was well-tolerated. However, it is unclear if the patients might have improved without the blockade of IL-1. Cavalli et al. conducted a retrospective case-control study of 29 COVID-19 patients treated with a high dose of intravenous anakinra (10 mg/kg/day) comparing their outcome to seven patients receiving 100 mg of anakinra subcutaneously twice daily and 16 controls on standard treatment (133). All patients suffered from moderate-to-severe ARDS and hyperinflammation. While subcutaneous application of anakinra was terminated due to lack of efficacy, survival in those receiving high dose anakinra treatment was superior to those receiving standard care (90 and 56%, respectively). Although pathophysiologically reasonable, solid evidence from randomized control trials are needed; in this respect at least 10 trials are underway targeting IL-1 in COVID-19.
Corticosteroids have a broad anti-inflammatory potential and are readily available. Therefore, they have been considered for use in critical COVID-19. High-dose corticosteroids at admission were identified as a risk factor for COVID-19 mortality, and they are not recommended by the WHO for viral pneumonia (11)2. In a small observational study from China the use of corticosteroids was not associated with shorter hospitalization or more rapid virus clearance (134). Still, corticosteroids in variable doses are currently used in critical COVID-19 patients, especially those with ARDS (117). This practice is supported by data from the British RECOVERY trial, a randomized, controlled, open-label study including more than 6,000 hospitalized COVID-19 patients (135). A daily dose of 6 mg dexamethasone reduced 28-day mortality by one third in patients receiving invasive mechanical ventilation at randomization. Patients requiring oxygen without invasive mechanical ventilation did benefit from the addition of dexamethasone to a lesser extent, while those not receiving respiratory support of any kind did not benefit at all. In addition, ARDS patients were reported to develop glucocorticoid resistance requiring excess corticosteroids to overcome progressive inflammation (136). This, suggests that dexamethasone is important in the later stage of infection, characterized by CRS, and ARDS, whereas in the first stage, immunosuppression might not be of additional value (137).
The antimalarial drugs chloroquine (CQ) and hydroxychloroquine (HCQ) have been suggested as potential antiviral agents in COVID-19. The rationale arises from the observation that CQ and HCQ can prevent SARS-CoV-2 replication in vitro probably by increasing endosomal pH or increasing intracellular zinc levels (61, 138).
In vivo, chloroquine did not reduce SARS-CoV viral titers in a mouse model (139). Early clinical trials from China, suggested improved clinical outcomes of COVID-19 when treated with chloroquine as compared to controls groups (1, 140). In a controversial French study, 26 patients were treated with HCQ and azithromycin and compared to a cohort receiving standard therapy. Viral load decreased more rapidly in the HCQ and azithromycin group. However, due to methodological shortcomings the results should be interpreted with caution (141). Two large observational studies could not confirm these results (142, 143). In a retrospective analysis of 1,376 propensity-score-matched COVID-19 patients, the rate of intubation or death was similar in those receiving HCQ or standard care (143). Moreover, a retrospective study comparing mortality of HCQ/CQ to standard therapy found higher mortality in those receiving HCQ/CQ (144). In order to find the optimal anti-viral dose of HCQ, a RCT compared two doses of CQ (600 mg bid for 10 days or 450 mg bid on day 1 and once daily for 4 days) in 81 patients with COVID-19 (145). The higher dose of HCQ resulted in an increased lethality. Furthermore, on electrocardiogram QTc-intervals >500 ms were found in 19 and 11 percent in the high- vs. low-dose group. Other studies reported QTc-intervals >500 ms in 5–23% of the patients treated with HCQ and 0–33% in those with azithromycin co-treatment (146–149). Other adverse events of HCQ and azithromycin co-treatment reported in 2.4% were mild (146). In June 2020 an interim analysis of the hydroxychloroquine arm of the RECOVERY trial revealed no benefit of HCQ compared to standard of care (150). A total of 1,542 patients had been randomized to hydroxychloroquine and compared with 3,132 patients receiving standard of care alone. No significant difference in the primary endpoint of 28-day mortality could be found. In summary, evidence for clinical efficacy of CQ and HCQ in COVID-19 is limited. Therefore, antimalarial drugs should not be used in COVID-19 outside clinical trials; accordingly, the FDA revoked the emergency use authorization of these antimalarials in June 2020 and issued a cautionary statement in July (151).
Baricitinib was identified as a potential therapeutic agent for COVID-19 employing a drug repurposing software. The suggested mechanism is specific for baricitinib, not constituting a class-effect of JAKi, and works through the inhibition of virus uptake by the blockade of endocytosis (152). In line with these considerations, a retrospective study compared 173 COVID-19 patients receiving baricitinib to 78 patients on standard therapy (153). Case fatality and intensive care admission was significantly less frequent in the patients receiving baricitinib. Additionally, a higher proportion of patients had negative nasopharyngeal swabs on discharge in the baricitinib arm. Altogether, randomized controlled trials for the use of baricitinib in COVID-19 seem worthwhile and are ongoing.
Colchicine, used in gout due to its effects on the chemotaxis of neutrophils and monocytes, has been proposed as a potential anti-inflammatory therapy in COVID-19 since recruitment of neutrophils is a key factor in severe COVID-19 pneumonia (154). Evidence from a Greek randomized, controlled trial on 105 COVID-19 patients suggests a benefit of colchicine treatment (155). However, the primary outcome measures were focused on inflammatory markers (CRP and high sensitivity cardiac troponin) and overall clinical deterioration, which may have been biased by the open-label design. Larger trials with mortality or invasive ventilation as endpoints are needed to support the findings of this initial trial.
Intravenous gammaglobulins (IVIG) serve as an anti-inflammatory rescue therapy in autoimmune diseases although the precise mode of action is elusive. In addition, commercially available IVIG preparations contain antibodies cross-reactive to SARS CoV-2 or endogenous proteins, such as cytokines (156). Therefore, IVIG have been used in COVID-19 (157–159). However, as no case-control studies or RCT's have been published so far, the therapeutic efficacy of IVIG in COVID-19 is unknown.
Conclusion
Overall, data regarding COVID-19 and RMD are sparse. An increased risk of developing COVID-19 or a complicated course of COVID-19 cannot be deduced from data on COVID-19 or other infections in patients with inflammatory arthritis. Further studies are needed to investigate the course of COVID-19 in connective tissue diseases such as SLE or vasculitis, particularly in patients with already existing organ damage and/or other comorbidities. A further point of consideration is the role of bDMARDs or JAKi. However, more data are needed to disentangle a possible protective role of biological DMARDs or JAKi against a possible CRS as compared to an increased susceptibility for viral infections (summarized in Box 2).
Box 2. Overview of open and resolved research questions.
Issues needing further research and data:
- is there a role for Antiphospholipid antibodies in the thrombogenicity of COVID-19
- is there a protective role of JAKi, IL-6, bDMARDs
- if yes, when should they be applied
- is the risk of a complicated course of CTD/ vasculitis patients heightened?
Issues that have been answered
- there is no protective role of HQ in RMD patients
- RMD patients can continue most of their DMARDs without problems
- there is no increased risk for a complicated course of COVID-19 in patients with inflammatory arthritis.
Author Contributions
All authors critically evaluated the literature and wrote the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^Available online at: https://www.prnewswire.com/news-releases/regeneron-and-sanofi-provide-update-on-us-phase-23-
adaptive-designed-trial-of-kevzara-sarilumab-in-hospitalized-covid-19-patients-301047326.html [press release].
2. ^Available online at: https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
3. Lu G, Wang Q, Gao GF. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. (2015) 23:468–78. doi: 10.1016/j.tim.2015.06.003
4. Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. (2016) 24:490–502. doi: 10.1016/j.tim.2016.03.003
5. Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. (2003) 361:1319–25. doi: 10.1016/S0140-6736(03)13077-2
6. Raj VS, Osterhaus ADME, Fouchier RAM, Haagmans BL. MERS: emergence of a novel human coronavirus. Curr Opin Virol. (2014) 5:58–62. doi: 10.1016/j.coviro.2014.01.010
7. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
8. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e8. doi: 10.1016/j.cell.2020.02.052
9. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–1720. doi: 10.1056/NEJMoa2002032
10. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
11. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. (2020) 146:110–8. doi: 10.1016/j.jaci.2020.04.006
12. Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Int Med. (2020) 180:1–9. doi: 10.1001/jamainternmed.2020.2033
14. Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J Antimicrob Agents. (2020) 2020:105948. doi: 10.1016/j.ijantimicag.2020.105948
15. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
16. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, china. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
17. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. (2020) 191:9–14. doi: 10.1016/j.thromres.2020.04.024
18. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. (2020) 127:104362. doi: 10.1016/j.jcv.2020.104362
19. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with covid-19 in the New York City Area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
20. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. (2020) 71:762–8. doi: 10.1093/cid/ciaa248
21. Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. (2020) 217:678. doi: 10.1084/jem.20200678
22. Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.24.20042655
23. Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. (2020) 6:31. doi: 10.1038/s41421-020-0168-9
24. Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Møller R, Panis M, Sachs D, et al. SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.03.24.004655
25. Trouillet-Assant S, Viel S, Gaymard A, Pons S, Richard J-C, Perret M, et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol. (2020) 146:206–8.e2. doi: 10.1016/j.jaci.2020.04.029
26. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19). medRxiv. (2020) 11:827. doi: 10.3389/fimmu.2020.00827
27. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, qi Y, et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.02.12.945576
28. Au K, Reed G, Curtis JR, Kremer JM, Greenberg JD, Strand V, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. (2011) 70:785–91. doi: 10.1136/ard.2010.128637
29. Herrinton LJ, Liu L, Goldfien R, Michaels MA, Tran TN. Risk of serious infection for patients with systemic lupus erythematosus starting glucocorticoids with or without antimalarials. J Rheumatol. (2016) 43:1503–9. doi: 10.3899/jrheum.150671
30. Rua-Figueroa I, Lopez-Longo J, Galindo-Izquierdo M, Calvo-Alen J, Del Campo V, Olive-Marques A, et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum. (2017) 47:38–45. doi: 10.1016/j.semarthrit.2017.01.010
31. Minozzi S, Bonovas S, Lytras T, Pecoraro V, Gonzalez-Lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. (2016) 15(Suppl. 1):11–34. doi: 10.1080/14740338.2016.1240783
32. Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. (2015) 386:258–65. doi: 10.1016/S0140-6736(14)61704-9
33. Li X, Andersen KM, Chang HY, Curtis JR, Alexander GC. Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann Rheum Dis. (2020) 79:285–91. doi: 10.1136/annrheumdis-2019-216102
34. Boor PPC, de Ruiter PE, Asmawidjaja PS, Lubberts E, van der Laan LJW, Kwekkeboom J. JAK-inhibitor tofacitinib suppresses interferon alfa production by plasmacytoid dendritic cells and inhibits arthrogenic and antiviral effects of interferon alfa. Transl Res. (2017) 188:67–79. doi: 10.1016/j.trsl.2016.11.006
35. Bechman K, Subesinghe S, Norton S, Atzeni F, Galli M, Cope AP, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology. (2019) 58:1755–66. doi: 10.1093/rheumatology/kez087
36. Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. (2011) 306:2331–9. doi: 10.1001/jama.2011.1692
37. Lacaille D, Guh DP, Abrahamowicz M, Anis AH, Esdaile JM. Use of nonbiologic disease-modifying antirheumatic drugs and risk of infection in patients with rheumatoid arthritis. Arthritis Rheum. (2008) 59:1074–81. doi: 10.1002/art.23913
38. Feldman CH, Marty FM, Winkelmayer WC, Guan H, Franklin JM, Solomon DH, et al. Comparative rates of serious infections among patients with systemic lupus erythematosus receiving immunosuppressive medications. Arthr Rheumatol. (2017) 69:387–97. doi: 10.1002/art.39849
39. Zumla A, Hui DS, Perlman S. Middle east respiratory syndrome. Lancet. (2015) 386:995–1007. doi: 10.1016/S0140-6736(15)60454-8
40. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant. (2020) 26:832–834. doi: 10.1002/lt.25756
41. Chang LY, Chiang BL, Kao CL, Wu MH, Chen PJ, Berkhout B, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis. (2006) 193:283–6. doi: 10.1086/498875
42. Turnier JL, Anderson MS, Heizer HR, Jone PN, Glodé MP, Dominguez SR. Concurrent respiratory viruses and kawasaki disease. Pediatrics. (2015) 136:e609–14. doi: 10.1542/peds.2015-0950
43. Chow EY, Chiu WK. Severe acute respiratory syndrome and lupus anticoagulants in children. Br J Haematol. (2003) 123:367–8. doi: 10.1046/j.1365-2141.2003.04608.x
44. The Novel Coronavirus Pneumonia Emergency Response Epidemiology T. Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China. China CDC Weekly (2020). Available online at: http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f512020 (accessed February 20, 2020).
45. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. (2020) 46:846–848. doi: 10.1007/s00134-020-05991-x
46. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
47. Sousa GJB, Garces TS, Cestari VRF, Florêncio RS, Moreira TMM, Pereira MLD. Mortality and survival of COVID-19. Epidemiol Infect. (2020) 148:e123. doi: 10.1017/S0950268820001405
48. Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. (2020) 79:667–8. doi: 10.1136/annrheumdis-2020-217424
49. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. (2020) 71:762–8. doi: 10.1038/s41586-020-2521-4
50. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with covid-19 in washington state. JAMA. (2020) 323:1612–4. doi: 10.1001/jama.2020.4326
51. Salvarani C, Bajocchi G, Mancuso P, Galli E, Muratore F, Boiardi L, et al. Susceptibility and severity of COVID-19 in patients treated with bDMARDS and tsDMARDs: a population-based study. Ann Rheum Dis. (2020) 79:986–8. doi: 10.1136/annrheumdis-2020-217903
52. Michelena X, Borrell H, López-Corbeto M, López-Lasanta M, Moreno E, Pascual-Pastor M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. (2020) 50:564–70. doi: 10.1016/j.semarthrit.2020.05.001
53. Pablos JL, Abasolo L, Alvaro-Gracia JM, Blanco FJ, Blanco R, Castrejón I, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis. (2020) 79:1170–3. doi: 10.1101/2020.05.11.20097808
54. Fredi M, Cavazzana I, Moschetti L, Andreoli L, Franceschini F, Airò P, et al. COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case & control study. Lancet Rheumatol. (2020) 2:e549–e56. doi: 10.1016/S2665-9913(20)30169-7
55. D'Silva KM, Serling-Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. (2020) 2020:217888. doi: 10.1136/annrheumdis-2020-217888
56. Ye C, Cai S, Shen G, Guan H, Zhou L, Hu Y, et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis. (2020) 2020:217627. doi: 10.1136/annrheumdis-2020-217627
57. Zhao J, Pang R, Wu J, Guo Y, Yang Y, Zhang L, et al. Clinical characteristics and outcomes of patients with COVID-19 and rheumatic disease in China ‘hot spot’ versus in US ‘hot spot’: similarities and differences. Ann Rheum Dis. (2020) 2020:218183. doi: 10.1136/annrheumdis-2020-218183
58. Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. (2020) 79:859–66. doi: 10.1136/annrheumdis-2020-217871
59. Haberman R, Axelrad J, Chen A, Castillo R, Yan D, Izmirly P, et al. Covid-19 in immune-mediated inflammatory diseases — case series from New York. N Engl J Med. (2020) 383:85-8. doi: 10.1056/NEJMc2009567
60. Konig MF, Kim AH, Scheetz MH, Graef ER, Liew JW, Simard J, et al. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis. (2020) 2020:217690. doi: 10.1136/annrheumdis-2020-217690
61. Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. (2020) 6:16. doi: 10.1038/s41421-020-0156-0
62. Mathian A, Mahevas M, Rohmer J, Roumier M, Cohen-Aubart F, Amador-Borrero B, et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. (2020) 2020:217566. doi: 10.1136/annrheumdis-2020-217875
63. Bozzalla Cassione E, Zanframundo G, Biglia A, Codullo V, Montecucco C, Cavagna L. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann Rheum Dis. (2020) 2020:217717. doi: 10.1136/annrheumdis-2020-218193
64. Favalli EG, Agape E, Caporali R. Incidence and clinical course of covid-19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol. 2020:200507. doi: 10.3899/jrheum.200507
65. Gendebien Z, von Frenckell C, Ribbens C, André B, Thys M, Gangolf M, et al. Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments. Ann Rheum Dis. (2020) 2020:218244. doi: 10.1136/annrheumdis-2020-218244
66. Konig MF, Gianfrancesco M, Yazdany J, Robinson PC. Patients with systemic lupus erythematosus using hydroxychloroquine or chloroquine develop severe COVID-19 at similar frequency as patients not on antimalarials: need to explore antithrombotic benefits for COVID-19 coagulopathy. Response to: ‘Clinical course of COVID-19 in patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine’ by Carbillon et al. Ann Rheum Dis. (2020). doi: 10.1136/annrheumdis-2020-217990
67. Wallace B, Washer L, Marder W, Kahlenberg JM. Patients with lupus with COVID-19: University of Michigan experience. Ann Rheum Dis. (2020) 2020:217794. doi: 10.1136/annrheumdis-2020-217794
68. Favalli EG, De Lucia O, Biggioggero M, Del Papa N, Caporali R. Role of antimalarials in COVID-19: observational data from a cohort of rheumatic patients. Ann Rheum Dis. (2020) 2020:218068. doi: 10.1136/annrheumdis-2020-218068
69. Ferreira A, Oliveira ESA, Bettencourt P. Chronic treatment with hydroxychloroquine and SARS-CoV-2 infection. J Med Virol. (2020). doi: 10.1002/jmv.26286
70. Song J, Kang S, Choi SW, Seo KW, Lee S, So MW, et al. Coronavirus disease 19 (COVID-19) complicated with pneumonia in a patient with rheumatoid arthritis receiving conventional disease-modifying antirheumatic drugs. Rheumatol Int. (2020) 40:991–5. doi: 10.1007/s00296-020-04584-7
71. Duret P-M, Sebbag E, Mallick A, Gravier S, Spielmann L, Messer L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann Rheum Dis. (2020) 2020:217362. doi: 10.1136/annrheumdis-2020-218147
72. Baba H, Kanamori H, Oshima K, Seike I, Niitsuma-Sugaya I, Takei K, et al. Prolonged presence of SARS-CoV-2 in a COVID-19 case with rheumatoid arthritis taking iguratimod treated with ciclesonide. J Infect Chemother. (2020) 26:1100–3. doi: 10.1016/j.jiac.2020.06.022
73. Cai S, Sun W, Li M, Dong L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin Rheumatol. (2020) 2020:1–6. doi: 10.1007/s10067-020-05234-w
74. Lee J-M, Lee SJ. Olfactory and gustatory dysfunction in a covid-19 patient with ankylosing spondylitis treated with etanercept: case report. J Korean Med Sci. (2020) 35:e201. doi: 10.3346/jkms.2020.35.e201
75. Valenti M, Facheris P, Pavia G, Gargiulo L, Borroni RG, Costanzo A, et al. Non-complicated evolution of COVID-19 infection in a patient with psoriasis and psoriatic arthritis during treatment with adalimumab. Dermatol Ther. (2020) e13708. doi: 10.1111/dth.13708
76. Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. (2020) 2020:218075. doi: 10.1136/annrheumdis-2020-218075
77. Brito CA, Paiva JG, Pimentel FN, Guimarães RS, Moreira MR. COVID-19 in patients with rheumatological diseases treated with anti-TNF. Ann Rheum Dis. (2020). doi: 10.1136/annrheumdis-2020-218171. [Epub ahead of print]
78. Quartuccio L, Valent F, Pasut E, Tascini C, De Vita S. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: A population-based study in the first two months of COVID-19 outbreak in Italy. Joint Bone Spine. (2020) S1297-319X(20)30088-9. doi: 10.1016/j.jbspin.2020.05.003
79. Queiro Silva R, Armesto S, González Vela C, Naharro Fernández C, González-Gay MA. COVID-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy versus apremilast in North Spain. Dermatol Ther. (2020) e13961. doi: 10.1111/dth.13961
80. Cheng C, Li C, Zhao T, Yue J, Yang F, Yan Y, et al. COVID-19 with rheumatic diseases: a report of 5 cases. Clin Rheumatol. (2020) 39:2025–9. doi: 10.1007/s10067-020-05160-x
81. Favalli EG, Monti S, Ingegnoli F, Balduzzi S, Caporali R, Montecucco C. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthr Rheumatol. (2020). doi: 10.1002/art.41388. [Epub ahead of print].
82. Sanchez-Piedra C, Diaz-Torne C, Manero J, Pego-Reigosa JM, Rúa-Figueroa Í, Gonzalez-Gay MA, et al. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis. (2020) 79:988–90. doi: 10.1136/annrheumdis-2020-217948
83. Mihai C, Dobrota R, Schroder M, Garaiman A, Jordan S, Becker MO, et al. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. (2020) 79:668–9. doi: 10.1136/annrheumdis-2020-217442
84. Avouac J, Airó P, Carlier N, Matucci-Cerinic M, Allanore Y. Severe COVID-19-associated pneumonia in 3 patients with systemic sclerosis treated with rituximab. Ann Rheum Dis. (2020) 2020:217864. doi: 10.1136/annrheumdis-2020-217864
85. Guilpain P, Le Bihan C, Foulongne V, Taourel P, Pansu N, Maria ATJ, et al. Rituximab for granulomatosis with polyangiitis in the pandemic of covid-19: lessons from a case with severe pneumonia. Ann Rheum Dis. (2020). doi: 10.1136/annrheumdis-2020-217549. [Epub ahead of print].
86. Tomelleri A, Sartorelli S, Campochiaro C, Baldissera EM, Dagna L. Impact of COVID-19 pandemic on patients with large-vessel vasculitis in Italy: a monocentric survey. Ann Rheum Dis. (2020) 2020:217600. doi: 10.1136/annrheumdis-2020-217600
87. Han Y, Jiang M, Xia D, He L, Lv X, Liao X, et al. COVID-19 in a patient with long-term use of glucocorticoids: a study of a familial cluster. Clin Immunol. (2020) 214:108413. doi: 10.1016/j.clim.2020.108413
88. Guven F, Ogul H, Turgut A, Tezcan A, Kantarci M. Leptomeningeal involvement in a patient with systemic lupus erythematosus infected by COVID-19. Joint Bone Spine. (2020) S1297-319X(20)30112-3. doi: 10.1016/j.jbspin.2020.06.002. [Epub ahead of print].
89. Romão VC, Cruz-Machado AR, Fonseca JE. No evidence so far on the protective effect of hydroxychloroquine to prevent COVID-19: response to the comment by Joob and Wiwanitkit. Ann Rheum Dis. (2020) 2020:217665. doi: 10.1136/annrheumdis-2020-217665
90. Gartshteyn Y, Askanase AD, Schmidt NM, Bernstein EJ, Khalili L, Drolet R, et al. COVID-19 and systemic lupus erythematosus: a case series. Lancet Rheumatol. (2020) 2:e452–e454. doi: 10.1016/S2665-9913(20)30161-2
91. Zen M, Fuzzi E, Astorri D, Saccon F, Padoan R, Ienna L, et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: A cross-sectional study on 916 atients. J Autoimmun. (2020) 2020:102502. doi: 10.1016/j.jaut.2020.102502
92. Favalli EG, Gerosa M, Murgo A, Caporali R. Are patients with systemic lupus erythematosus at increased risk for COVID-19? Ann Rheum Dis. (2020) 2020:217787. doi: 10.1136/annrheumdis-2020-217787
93. Holubar J, Le Quintrec M, Letaief H, Faillie JL, Pers Y-M, Jorgensen C. Monitoring of patients with systemic lupus erythematosus during the COVID-19 outbreak. Ann Rheum Dis. (2020) 2020:217919. doi: 10.1136/annrheumdis-2020-217919
94. Sawalha AH, Zhao M, Coit P, Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol. (2020) 215:108410. doi: 10.1016/j.clim.2020.108410
95. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. (2020) 382:e38. doi: 10.1056/NEJMc2007575
96. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. (2006) 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x
97. Ruiz-Garcia R, Serrano M, Martinez-Flores JA, Mora S, Morillas L, Martin-Mola MA, et al. Isolated IgA anti- beta2 glycoprotein I antibodies in patients with clinical criteria for antiphospholipid syndrome. J Immunol Res. (2014) 2014:704395. doi: 10.1155/2014/704395
98. Murthy V, Willis R, Romay-Penabad Z, Ruiz-Limon P, Martinez-Martinez LA, Jatwani S, et al. Value of isolated IgA anti-beta2 -glycoprotein I positivity in the diagnosis of the antiphospholipid syndrome. Arthritis Rheum. (2013) 65:3186–93. doi: 10.1002/art.38131
99. Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. (2020) 18:2064–2065. doi: 10.1111/jth.14867
100. Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, et al. Lupus anticoagulant and abnormal coagulation tests in patients with covid-19. N Engl J Med. (2020) 383:288–90. doi: 10.1056/NEJMc2013656
101. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens Care Med. (2020) 2020:1–10. doi: 10.1007/s00134-020-06062-x
102. Borghi MO, Beltagy A, Garrafa E, Curreli D, Cecchini G, Bodio C, et al. Prevalence, specificity, and clinical association of anti-phospholipid antibodies in COVID-19 patients: are the antibodies really guilty? medRxiv [Preprint]. (2020). doi: 10.1101/2020.06.17.20134114
103. Xiao M, Zhang Y, Zhang S, Qin X, Xia P, Cao W, et al. Brief report: anti-phospholipid antibodies in critically ill patients with Coronavirus Disease 2019 (COVID-19). Arthr Rheumatol. (2020). doi: 10.1002/art.41425. [Epub ahead of print].
104. Zuo Y, Estes SK, Gandhi AA, Yalavarthi S, Ali RA, Shi H, et al. Prothrombotic antiphospholipid antibodies in COVID-19. medRxiv [Preprint]. (2020). doi: 10.1101/2020.06.15.20131607
105. Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almazor ME. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. (2016) 25:1520–31. doi: 10.1177/0961203316640912
106. Gazzaruso C, Carlo Stella N, Mariani G, Nai C, Coppola A, Naldani D, et al. High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia. Clin Rheumatol. (2020) 39:2095–7. doi: 10.1007/s10067-020-05180-7
107. Joo YB, Lim YH, Kim KJ, Park KS, Park YJ. Respiratory viral infections and the risk of rheumatoid arthritis. Arthritis Res Ther. (2019) 21:199. doi: 10.1186/s13075-019-1977-9
108. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Bradley Segal J, et al. COVID-19 and kawasaki disease: novel virus and novel case. Hospital Pediatr. (2020) 10:537–540. doi: 10.1542/hpeds.2020-0123
109. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395:1607–8. doi: 10.1016/S0140-6736(20)31094-1
110. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. (2020) 383:347–58. doi: 10.1056/NEJMoa2021756
111. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. Children and Adolescents. N Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
112. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 395:1771–8. doi: 10.1016/S0140-6736(20)31103-X
113. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259–69. doi: 10.1001/jama.2020.10369
114. Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. (2020) 79:999–1006. doi: 10.1136/annrheumdis-2020-217960
115. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. (2020). doi: 10.1002/jmv.25948. [Epub ahead of print].
116. Chen H, Wang F, Zhang P, Zhang Y, Chen Y, Fan X, et al. Management of cytokine release syndrome related to CAR-T cell therapy. Front Med. (2019) 13:610–7. doi: 10.1007/s11684-019-0714-8
117. Lombardy Section Italian Society I, Tropical D. Vademecum for the treatment of people with COVID-19. Infez Med. (2020). 28:143–52.
118. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. (2020) 2020:102568. doi: 10.1016/j.autrev.2020.102568
119. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. (2020) 117:10970–5. doi: 10.1073/pnas.2005615117
120. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. (2020) 92:814–8. doi: 10.1002/jmv.25801
121. Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. (2020) 76:43–9. doi: 10.1016/j.ejim.2020.05.021
122. Canziani LM, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J Autoimmun. (2020) 2020:102511. doi: 10.1016/j.jaut.2020.102511
123. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. (2020) 2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9
124. Potere N, Di Nisio M, Cibelli D, Scurti R, Frattari A, Porreca E, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study. Ann Rheum Dis. (2020). doi: 10.1136/annrheumdis-2020-218243. [Epub ahead of print].
125. Rojas-Marte GR, Khalid M, Mukhtar O, Hashmi AT, Waheed MA, Ehrlich S, et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab - a case- controlled study. QJM. (2020) 113:546–50. doi: 10.1093/qjmed/hcaa206
126. Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. medrxiv [Preprint]. (2020). doi: 10.1101/2020.05.29.20117358
127. Morrison AR, Johnson JM, Griebe KM, Jones MC, Stine JJ, Hencken LN, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J Autoimmun. (2020) 2020:102512. doi: 10.1016/j.jaut.2020.102512
128. Sciascia S, Apra F, Baffa A, Baldovino S, Boaro D, Boero R, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. (2020) 38:529–32.
129. Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for covid-19-induced cytokine release syndrome: a cautionary case report. Chest. (2020) 158:e15–9. doi: 10.1016/j.chest.2020.04.024
130. Neel A, Wahbi A, Tessoulin B, Boileau J, Carpentier D, Decaux O, et al. Diagnostic and management of life-threatening adult-onset still disease: a French nationwide multicenter study and systematic literature review. Crit Care. (2018) 22:88. doi: 10.1186/s13054-018-2012-2
131. Aouba A, Baldolli A, Geffray L, Verdon R, Bergot E, Martin-Silva N, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. (2020) 79:1381–2. doi: 10.1136/annrheumdis-2020-217706
132. Navarro-Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe covid-19: a case series. Arthr Rheumatol. (2020). doi: 10.1002/art.41422. [Epub ahead of print].
133. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. (2020) 2:e325–e31. doi: 10.1016/S2665-9913(20)30127-2
134. Zha L, Li S, Pan L, Tefsen B, Li Y, French N, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust. (2020). 212:416–20. doi: 10.5694/mja2.50577
135. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. (2020). doi: 10.1056/NEJMoa2021436. [Epub ahead of print].
136. Meduri GU, Yates CR. Systemic inflammation-associated glucocorticoid resistance and outcome of ARDS. Ann N Y Acad Sci. (2004) 1024:24–53. doi: 10.1196/annals.1321.004
137. Lane HC, Fauci AS. Research in the context of a pandemic. N Engl J Med. (2020). doi: 10.1056/NEJMe2024638. [Epub ahead of print].
138. Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ. Chloroquine is a zinc ionophore. PLoS ONE. (2014) 9:e109180. doi: 10.1371/journal.pone.0109180
139. Barnard DL, Day CW, Bailey K, Heiner M, Montgomery R, Lauridsen L, et al. Evaluation of immunomodulators, interferons and known in vitro SARS-coV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir Chem Chemother. (2006) 17:275–84. doi: 10.1177/095632020601700505
140. Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.22.20040758
141. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. (2020) 2020:101663. doi: 10.1016/j.tmaid.2020.101663
142. Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with covid-19 in New York state. JAMA. (2020) 323-:2493–502. doi: 10.1001/jama.2020.8630
143. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. (2020) 382:2411–2418. doi: 10.1056/NEJMoa2012410
144. Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv [Preprint]. (2020). doi: 10.1016/j.medj.2020.06.001
145. Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infection: a randomized clinical trial. JAMA Netw Open. (2020) 3:e208857. doi: 10.1001/jamanetworkopen.2020.8857
146. Million M, Lagier JC, Gautret P, Colson P, Fournier PE, Amrane S, et al. Full-length title: early treatment of covid-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in marseille, france. Travel Med Infect Dis. (2020) 2020 35:101738. doi: 10.1016/j.tmaid.2020.101738
147. van den Broek MPH, Mohlmann JE, Abeln BGS, Liebregts M, van Dijk VF, van de Garde EMW. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth Heart J. (2020) 2020:1–4. doi: 10.1007/s12471-020-01429-7
148. Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:1036–41. doi: 10.1001/jamacardio.2020.1834
149. Bessiere F, Roccia H, Deliniere A, Charriere R, Chevalier P, Argaud L, et al. Assessment of QT Intervals in a case series of patients with coronavirus disease 2019 (Covid-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. (2020) 1:e201787. doi: 10.1001/jamacardio.2020.1787
150. RECOVERY homepage (2020). Available online at: https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-
recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-covid-19 (accessed July 15, 2020)
151. Swank F. FDA (2020). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/OSE%20Review_Hydroxychloroquine-Cholorquine%20-%2019May2020_Redacted.pdf (accessed May 19, 2020).
152. Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. (2020) 395:e30–e1. doi: 10.1016/S0140-6736(20)30304-4
153. Cantini F, Niccoli L, Nannini C, Matarrese D, Natale MED, Lotti P, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. (2020) 81:647–79. doi: 10.1016/j.jinf.2020.06.052
154. Parra-Medina R, Sarmiento-Monroy JC, Rojas-Villarraga A, Garavito E, Montealegre-Gómez G, Gómez-López A. Colchicine as a possible therapeutic option in COVID-19 infection. Clin Rheumatol. (2020) 39:2485–6. doi: 10.1007/s10067-020-05247-5
155. Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the grecco-19 randomized clinical trial. JAMA Netw Open. (2020) 3:e2013136. doi: 10.1001/jamanetworkopen.2020.13136
156. Díez JM, Romero C, Gajardo R. Currently available intravenous immunoglobulin contains antibodies reacting against severe acute respiratory syndrome coronavirus 2 antigens. Immunotherapy. (2020) 12:571–6. doi: 10.2217/imt-2020-0095
157. Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. (2020) 7:ofaa102. doi: 10.1093/ofid/ofaa102
158. Xie Y, Cao S, Dong H, Li Q, Chen E, Zhang W, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. (2020) 81:318–56. doi: 10.1016/j.jinf.2020.03.044
Keywords: COVID-19, SARS-CoV-2, rheumatic and musculoskeletal disease, rheumatoid arthritis, systemic lupus erythematosus, Kawasaki disease, hydroxychloroquine, IL-6
Citation: Stradner MH, Dejaco C, Zwerina J and Fritsch-Stork RD (2020) Rheumatic Musculoskeletal Diseases and COVID-19 A Review of the First 6 Months of the Pandemic. Front. Med. 7:562142. doi: 10.3389/fmed.2020.562142
Received: 14 May 2020; Accepted: 17 September 2020;
Published: 09 October 2020.
Edited by:
João Eurico Fonseca, University of Lisbon, PortugalReviewed by:
Micaela Fredi, Civil Hospital of Brescia, ItalyPeter Cheung, National University Health System, Singapore
Copyright © 2020 Stradner, Dejaco, Zwerina and Fritsch-Stork. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruth D. Fritsch-Stork, cnV0aC5mcml0c2NoLXN0b3JrQG9lZ2suYXQ=; cnV0aC5mcml0c2NoQG1lZC5zZnUuYWMuYXQ=
†ORCID: Ruth Fritsch-Stork orcid.org/0000-0003-0217-3629
 Martin H. Stradner
Martin H. Stradner Christian Dejaco1,2
Christian Dejaco1,2 Ruth D. Fritsch-Stork
Ruth D. Fritsch-Stork