- Division of Nephrology, First Department of Integrated Medicine, Saitama Medical Center, Jichi Medical University, Saitama, Japan
Background: Zinc deficiency is common and is associated with erythropoietin resistant anemia, dysgeusia, and hypogonadism in patients undergoing hemodialysis. However, the prevalence and clinical effects of zinc deficiency in patients undergoing peritoneal dialysis (PD) have not been determined.
Methods: This was a retrospective, cross-sectional study. The prevalence of serum zinc deficiency and the clinical factors related to serum zinc concentration were determined in 49 patients undergoing PD [mean age 59.5 years (±14.8 years), 38/49 were men (78.6%), median PD period 24.0 months (12.5–45.0 months)]. A serum zinc concentration <60 μg/dL was defined as serum zinc deficiency, and a serum zinc concentration between 60 and 80 μg/dL as possible serum zinc deficiency.
Results: Serum zinc deficiency was present in 51% (25/49) of the patients, and possible serum zinc deficiency was present in 45% (22/49) of patients undergoing PD. Multivariate analysis showed that serum zinc concentration significantly correlated with serum ferritin concentration (β = 0.357, P < 0.01).
Conclusions: The prevalences of serum zinc deficiency and possible serum deficiency are high and serum zinc concentration correlates with serum ferritin concentration in patients undergoing PD.
Introduction
Zinc is an essential trace element and is required for important physiological functions, such as immunity, nerve function, growth, skeletal integrity, and endocrine function (1–6). Clinically, zinc deficiency induces various pathological conditions, such as dermatitis, anemia, susceptibility to infection, gastrointestinal disorders, including loss of appetite, dysgeusia, and diarrhea, developmental disorders, and hypogonadism (1–6).
A number of studies have reported a high prevalence of zinc deficiency, and zinc supplementation ameliorates several clinical problems related to zinc deficiency, such as erythropoietin resistant anemia, dysgeusia, and hypogonadism, in patients undergoing hemodialysis (7–15). The causes of zinc deficiency in patients undergoing hemodialysis are thought to be multiple, and include dietary restriction attributable to renal failure, malabsorption, loss into the dialysate, and the effects of oral medications, such as phosphate binders (3, 7, 12, 16–19). However, there have been few studies of zinc deficiency in patients undergoing peritoneal dialysis (PD) (20, 21). In addition, the clinical factors that are associated with zinc status in patients undergoing PD have not been investigated. Therefore, in the present study, we determined the prevalence of zinc deficiency and the clinical factors associated with serum zinc concentration in patients undergoing PD.
Materials and Methods
Study Design
This was a retrospective cross-sectional study of the prevalence of zinc deficiency and the clinical factors associated with zinc status in patients undergoing PD.
Study Population
This study was conducted at Saitama Medical Center, Jichi Medical University. We studied patients who were undergoing PD between April 2018 and March 2019. The inclusion criteria were that the patients were undergoing PD, that they were >18 years old, and they had their serum zinc concentration measured for clinical purposes among all patients undergoing PD in our hospital. The exclusion criteria were zinc supplementation, renal transplantation, and lack of consent to participate in the study. All of the patients had been treated according to the 2009 Japanese Society for Dialysis Therapy Guidelines for PD and the 2015 Japanese Society for Dialysis Therapy Guidelines for Renal Anemia in Chronic Kidney Disease (22, 23). Because this study was observational and retrospective, an opt-out procedure replaced the need for the provision of written consent. This study was approved by the Saitama Medical Center, Jichi Medical University Ethics committee (DAI-RIN 15-34) and was conducted in accordance with the principles of the Declaration of Helsinki.
Data Collection
Data from patient medical records were used for this analysis. Information regarding the age, sex, body mass index, PD period, combination hemodialysis therapy, oral medication (antacids, phosphate binders, vitamin D and loop diuretics), intravenous iron supplementation, oral iron supplementation, PD modality [continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD)], total weekly Kt/V urea, peritoneal weekly Kt/V urea, renal weekly Kt/V urea, 4-h dialysate/plasma creatinine ratio, and laboratory measurements, including serum zinc concentration was obtained for each patient. Information regarding the symptoms associated with zinc deficiency (dysgeusia, dermatitis, and chronic diarrhea) was obtained from medical records. Combination hemodialysis therapy was defined as a combination of hemodialysis once a week in addition to PD. Oral iron supplementation was defined as the administration of ferrous citrate or an iron-containing phosphate binder. CAPD is a method of changing PD fluids manually and APD is a method of changing PD fluids using an automated system (24–27). Peritoneal weekly Kt/V urea is an indicator of the efficacy of PD and renal weekly Kt/V is an indicator of residual renal function (28); they were summarized together as total weekly Kt/V urea (28). Four-hour dialysate/plasma creatinine ratio is used as an indicator of peritoneal permeability (29). The Geriatric Nutritional Risk Index (GNRI) is an indicator of nutritional status calculated by (14.89 × serum albumin [g/dL]) + 41.7 × (body weight/ideal body weight [kg]) (30). Blood parameters and PD fluid parameters were measured by the Department of Clinical Laboratory, Saitama Medical Center.
Definition of Zinc Deficiency
On the basis of the Japanese guidelines for zinc deficiency, a serum zinc concentration <60 μg/dL was defined as serum zinc deficiency, a serum zinc concentration between 60 and 80 μg/dL as possible serum zinc deficiency, and >80 μg/dL as normal (31). These guidelines were published by the Japanese Society of Clinical Nutrition in 2018 (31).
Definition of Erythropoietin Resistance Index
The resistance of erythropoietin stimulating agents to anemia in patients undergoing dialysis was evaluated as erythropoietin resistance index (32, 33). The erythropoietin resistance index was defined as the average weekly dose of recombinant human erythropoietin (IU)/body weight (kg)/hemoglobin (g/dL) (32, 33). The ratio of recombinant human erythropoietin darbepoetin alfa or epoetin beta pegol was converted to 200:1 (34).
Statistical Analysis
The normality of distribution of the data was assessed using the Shapiro–Wilk test. Quantitative variables are expressed as mean ± standard deviation (SD) for normally distributed data and as median and interquartile range [IQR] for non-normally distributed data. Categorical variables are expressed as frequency and percentage. Univariate and multivariate regression analyses were performed to determine the clinical factors associated with serum zinc concentration. Statistical analysis was performed using JMP (SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered to represent statistical significance.
Results
Clinical Characteristics of the Patients
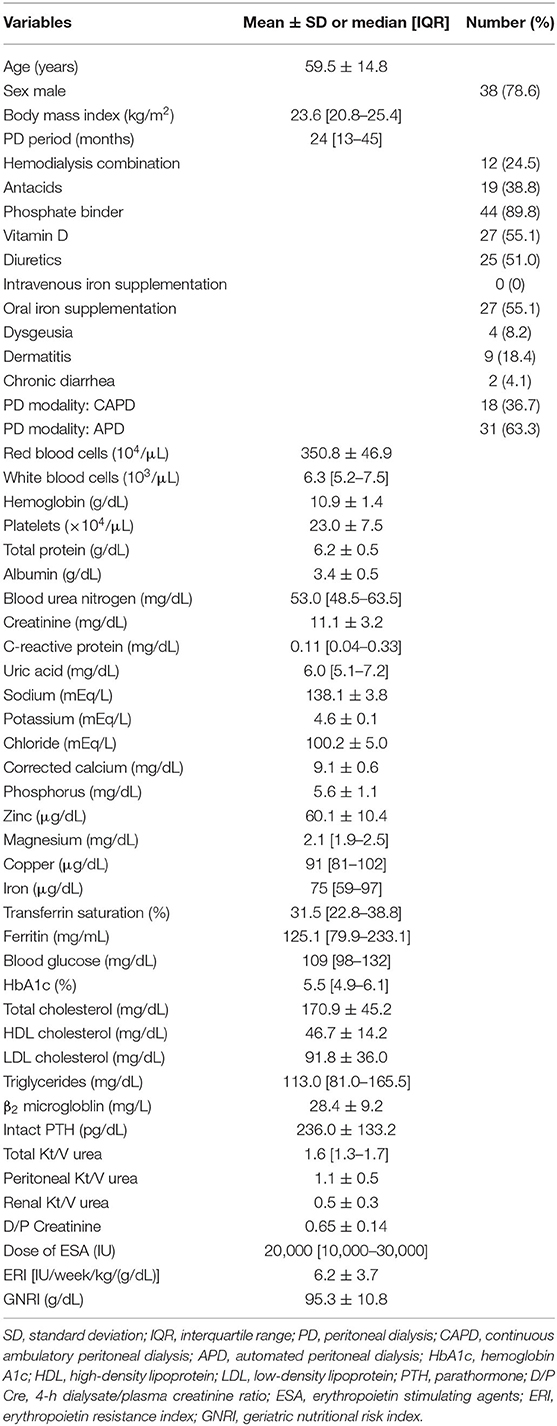
Forty-nine participants were enrolled in the present study. Table 1 shows the clinical characteristics of the study population. The mean age of the participants was 59.5 ± 14.8 years old. Thirty-eight (78.6%) men were enrolled in the study. The participants' median body mass index was 23.6 [20.8–25.4] kg/m2, their median PD period was 23.6 [20.8–25.4] months, and their mean serum zinc concentration was 60.1 ± 10.4 μg/dL. None of the participants had received intravenous iron supplementation, but 27 (55.1%) had received oral iron supplementation. With respect to the PD modality, 18 (36.7%) participants had undergone CAPD and 31 (63.3%) had undergone APD. Eight percent (4/49) of the participants had dysgeusia, 18% (9/49) had dermatitis, and 4% (2/49) had chronic diarrhea. The mean GNRI was 95.3 ± 10.8 (g/dL). The mean erythropoietin resistance index was 6.2 ± 3.7 [IU/week/kg/(g/dL)]. Table 2 and shows the prevalence of serum zinc deficiency and possible serum zinc deficiency in participants who had undergone PD. In this sample, 51% (25/49) of the participants had serum zinc deficiency (serum zinc concentration < 60 μg/dL), 45% (22/49) had possible serum zinc deficiency (60 ≤ serum zinc concentration < 80 μg/dL), and 4% (2/49) had normal zinc concentrations (serum zinc concentration ≥ 80 μg/dL).
Factors Associated With Serum Zinc Concentration in Patients Undergoing PD
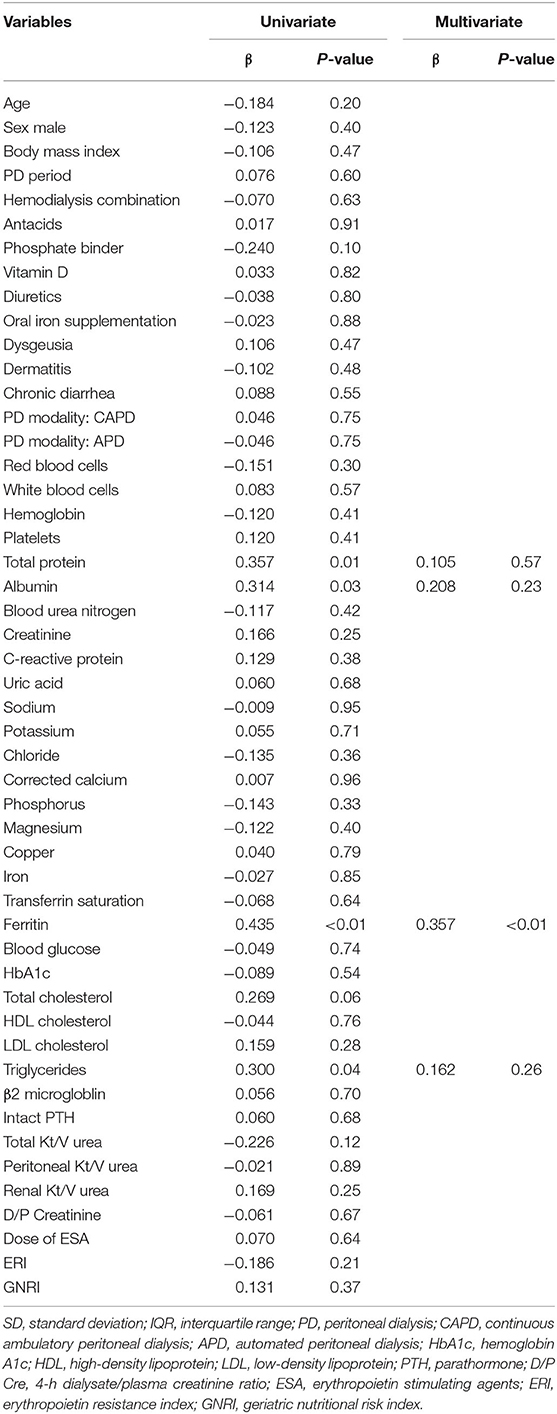
Table 3 shows the correlations between serum zinc concentration and other clinical parameters in patients undergoing PD. In univariate analysis, serum total protein (β = 0.357, P = 0.01), albumin (β = 0.314, P = 0.03), triglyceride (β = 0.300, P = 0.04), and ferritin (β = 0.435, P < 0.01) concentrations significantly correlated with zinc concentration. The correlation between erythropoietin resistance index and serum zinc concentration was not observed. No significant correlations between the symptoms related to zinc deficiency (dysgeusia, dermatitis, and chronic diarrhea) and serum zinc concentration were observed. In multiple regression analysis using these factors as explanatory variables, ferritin concentration (β = 0.357, P < 0.01) significantly correlated with zinc concentration (Table 3), but there were no correlations with other parameters.
Discussion
This study shows that serum zinc deficiency (51%) and possible serum zinc deficiency (45%) are highly prevalent in patients undergoing PD, which consistent with the results of previous studies (20, 21). In addition, we found a significant correlation between serum zinc and ferritin concentrations after excluding confounders in patients undergoing PD. This is the first study to show a positive correlation between zinc and ferritin status in patients undergoing PD.
The prevalences of serum zinc deficiency and possible serum zinc deficiency in patients undergoing PD were high in the present study. Several factors, such as dietary restriction attributable to renal failure (3, 7, 21), lower intestinal zinc absorption (18, 19), and the use of phosphate binders, which are known to inhibit the absorption of zinc in the gastrointestinal tract (16, 17, 21), may contribute to high prevalence of zinc deficiency in patients undergoing PD. In addition, there was a significant correlation between serum zinc and ferritin concentrations after excluding confounders in multivariate analysis. This may be explained by several mechanisms. dietary restriction and malabsorption due to renal failure may affect cause deficiencies of both zinc and iron, but greater zinc usage may also contribute to this positive correlation. Zinc has been reported to be used for the biosynthesis of protoporphyrin IX, a precursor of hemoglobin, instead of iron during iron deficiency (35). Furthermore, there have been several reports of a positive association between serum zinc and ferritin concentrations in adult and infant patients who were not undergoing dialysis (36, 37); however, the mechanism of this association has not been fully determined. Therefore, further studies are required to identify the mechanism of the zinc-ferritin correlation identified in patients undergoing PD. In clinical setting, regular measurement of serum ferritin concentration is recommended for the treatment in patients undergoing dialysis (38). However, serum zinc concentration is only measured when the patient has characteristic symptoms. Our study suggests that iron and zinc are positively correlated in patients undergoing peritoneal dialysis. Therefore, clinicians should be note of the potential presence of patients with zinc deficiency among the patients with iron deficiency.
In this study, 8% (4/49) of the participants had dysgeusia, 18% (9/49) had dermatitis, and 4% (2/49) had chronic diarrhea; however, significant correlations were not observed between these symptoms and serum zinc concentration. This may be because these symptoms in patients undergoing PD were caused by various abnormalities such as uremia, volume overload, electrolyte abnormalities, and prescribed drugs. Further prospective and interventional studies are needed to clarify the relationship between serum zinc concentration and symptoms in these populations. Additionally, the serum zinc concentration was not associated with total protein levels, albumin levels, the GNRI, and C-reactive protein levels on multivariate analysis in this study. These results suggested that the serum zinc level was not associated with malnutrition and inflammatory syndrome in patients undergoing PD. Future large-scale studies are necessary to investigate the relationships among zinc deficiency, malnutrition, and inflammatory syndrome in patients undergoing PD.
The Japanese Society of Clinical Nutrition guidelines recommend oral zinc supplementation for patients with characteristic symptoms and low zinc status (serum zinc deficiency or possible serum zinc deficiency) (31). In addition, these guidelines emphasize the importance of monitoring the improvement of symptoms during zinc supplementation (31). Current guidelines recommend oral zinc supplementation at a dose of 50–100 mg/day for adult patients with zinc deficiency (31). They also recommend oral zinc supplementation at a dose of 1–3 mg/kg/day (otherwise, 25 mg/day if the body weight is <20 kg or 50 mg/day if the body weight is more than 20 kg) for infant patients (31). Thus, further interventional studies are needed to verify the benefits of zinc supplementation in patients undergoing PD.
There were several limitations in this study. First, it was performed at a single facility and it is unclear whether the results are generalizable to other populations. Second, the sample size of this study was small. Third, because this was a cross-sectional study, this study did not show an association between clinical outcomes, such as morbidity and mortality, and zinc deficiency. Future long-term observational studies are necessary to investigate zinc deficiency and outcomes in patients undergoing PD. Thus, further multicenter-interventional studies are required to identify zinc deficiency and determine the effects of zinc supplementation in patients undergoing PD.
Conclusion
The prevalences of serum zinc deficiency and possible serum zinc deficiency are high and serum zinc concentration significantly positively correlates with ferritin concentration in patients undergoing PD. Clinicians should be aware of this when they manage such patients.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to Yoshiyuki Morishita, eW1vcmkmI3gwMDA0MDtqaWNoaS5hYw==.jp.
Ethics Statement
The studies involving human participants were reviewed and approved by Saitama Medical Center, Jichi Medical University Ethics committee (DAI-RIN 15-34). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SK analyzed data and wrote the manuscript. SK, JM, SM, KY, YMu, HI, MM, TK, MS, AA, HM, YU, KI, KH, SO, and YMo contributed to the study design, data collection, and statistical analyses. YMo supervised this study. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the medical staff in the Dialysis department, Saitama Medical Center, Jichi Medical University. We also thank Mark Cleasby, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Abbreviations
APD, Automated peritoneal dialysis; CAPD, Continuous ambulatory peritoneal dialysis; GNRI, Geriatric nutritional risk index; IQR, Interquartile range; PD, Peritoneal dialysis; SD, Standard deviation.
References
1. Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract. (2015) 30:371–82. doi: 10.1177/0884533615570376
2. Prasad AS. Zinc in growth and development and spectrum of human zinc deficiency. J Am Coll Nutr. (1988) 7:377–84. doi: 10.1080/07315724.1988.10720255
3. Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. (2017) 9:624. doi: 10.3390/nu9060624
4. Willoughby JL, Bowen CN. Zinc deficiency and toxicity in pediatric practice. Curr Opin Pediatr. (2014) 26:579–84. doi: 10.1097/MOP.0000000000000132
5. Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. (2013) 4:176–90. doi: 10.3945/an.112.003210
6. Kambe T, Fukue K, Ishida R, Miyazaki S. Overview of inherited zinc deficiency in infants and children. J Nutr Sci Vitaminol. (2015) 61:S44–6. doi: 10.3177/jnsv.61.S44
7. Filler G, Felder S. Trace elements in dialysis. Pediatr Nephrol. (2014) 29:1329–35. doi: 10.1007/s00467-013-2585-6
8. Harshman LA, Lee-Son K, Jetton JG. Vitamin and trace element deficiencies in the pediatric dialysis patient. Pediatr Nephrol. (2018) 33:1133–43. doi: 10.1007/s00467-017-3751-z
9. Tonelli M, Wiebe N, Hemmelgarn B, Klarenbach S, Field C, Manns B, et al. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med. (2009) 7:25. doi: 10.1186/1741-7015-7-25
10. Choi S, Liu X, Pan Z. Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol Sin. (2018) 39:1120–32. doi: 10.1038/aps.2018.25
11. Fukushima T, Horike H, Fujiki S, Kitada S, Sasaki T, Kashihara N. Zinc deficiency anemia and effects of zinc therapy in maintenance hemodialysis patients. Ther Apher Dial. (2009) 13:213–9. doi: 10.1111/j.1744-9987.2009.00656.x
12. Kobayashi H, Abe M, Okada K, Tei R, Maruyama N, Kikuchi F, et al. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients. (2015) 7:3783–95. doi: 10.3390/nu7053783
13. Mahajan SK, Abbasi AA, Prasad AS, Rabbani P, Briggs WA, McDonald FD. Effect of oral zinc therapy on gonadal function in hemodialysis patients. A double-blind study. Ann Intern Med. (1982) 97:357–61. doi: 10.7326/0003-4819-97-3-357
14. Antoniou LD, Shalhoub RJ, Sudhakar T, Smith JC Jr. Reversal of uraemic impotence by zinc. Lancet. (1977) 2:895–8. doi: 10.1016/S0140-6736(77)90832-7
15. Heckmann SM, Hujoel P, Habiger S, Friess W, Wichmann M, Heckmann JG, et al. Zinc gluconate in the treatment of dysgeusia–a randomized clinical trial. J Dent Res. (2005) 84:35–8. doi: 10.1177/154405910508400105
16. Gilli P, Docci D, Baldrati L, Turci F. Plasma zinc decreases in hemodialysis patients treated with calcium carbonate as the phosphate binder. Nephron. (1989) 53:384–5. doi: 10.1159/000185789
17. Takagi K, Masuda K, Yamazaki M, Kiyohara C, Itoh S, Wasaki M, et al. Metal ion and vitamin adsorption profiles of phosphate binder ion-exchange resins. Clin Nephrol. (2010) 73:30–5. doi: 10.5414/CNP73030
18. Mahajan SK, Bowersox EM, Rye DL, Abu-Hamdan DK, Prasad AS, McDonald FD, et al. Factors underlying abnormal zinc metabolism in uremia. Kidney Int Suppl. (1989) 27:S269–73.
19. Foote JW, Hinks LJ. Zinc absorption in haemodialysis patients. Ann Clin Biochem. (1988) 25 (Pt. 4):398–402. doi: 10.1177/000456328802500413
20. Yonova D, Vazelov E, Tzatchev K. Zinc status in patients with chronic renal failure on conservative and peritoneal dialysis treatment. Hippokratia. (2012) 16:356–9.
21. Panorchan K, Davenport A. Incidence and predictors of zinc deficiency in stable peritoneal dialysis patients. Perit Dial Int. (2015) 35:597–9. doi: 10.3747/pdi.2014.00134
22. Working Group Committee for Preparation of Guidelines for Peritoneal Dialysis JSFDT Japanese Society for Dialysis T. 2009 Japanese society for dialysis therapy guidelines for peritoneal dialysis. Ther Apher Dial. (2010) 14:489–504. doi: 10.1111/j.1744-9987.2010.00901.x
23. Yamamoto H, Nishi S, Tomo T, Masakane I, Saito K, Nangaku M, et al. 2015 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Renal Replace Ther. (2017) 3:36. doi: 10.1186/s41100-017-0114-y
24. van Hoeck KJM, Rusthoven E, Vermeylen L, Vandesompel A, Marescau B, Lilien M, et al. Nutritional effects of increasing dialysis dose by adding an icodextrin daytime dwell to Nocturnal Intermittent Peritoneal Dialysis (NIPD) in children. Nephrol Dial Transplant. (2003) 18:1383–7. doi: 10.1093/ndt/gfg120
25. Badve SV, Zimmerman DL, Knoll GA, Burns KD, McCormick BB. Peritoneal phosphate clearance is influenced by peritoneal dialysis modality, independent of peritoneal transport characteristics. Clin J Am Soc Nephrol. (2008) 3:1711–7. doi: 10.2215/CJN.00190108
26. Diaz-Buxo JA, Walker PJ, Chandler JT, Burgess WP, Farmer CD. Experience with intermittent peritoneal dialysis and continuous cyclic peritoneal dialysis. Am J Kidney Dis. (1984) 4:242–8. doi: 10.1016/S0272-6386(84)80099-2
27. Venkataraman V, Nolph KD. Utilization of PD modalities: evolution. Semin Dial. (2002) 15:380–4. doi: 10.1046/j.1525-139X.2002.00095.x
28. Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int. (2006) 26:520–2. doi: 10.1177/089686080602600502
29. Teitelbaum I, Burkart J. Peritoneal dialysis. Am J Kidney Dis. (2003) 42:1082–96. doi: 10.1016/j.ajkd.2003.08.036
30. Ren M, Sheng Q, Xie X, Zhang X, Han F, Chen J. Geriatric nutritional risk index is associated with mortality in peritoneal dialysis patients. Int Med J. (2020) 50:470–6. doi: 10.1111/imj.14680
31. The Japanese Society of Clinical Nutrition. Available online at: http://jscn.gr.jp/pdf/aen2018.pdf (accessed December 11, 2019).
32. Hayashi T, Joki N, Tanaka Y, Iwasaki M, Kubo S, Matsukane A, et al. Resistance to erythropoiesis-stimulating agents in pre-dialysis and post-dialysis mortality in Japanese incident hemodialysis patients. Blood Purif. (2019) 47(Suppl. 1):31–7. doi: 10.1159/000496634
33. Kamei D, Tsuchiya K, Nitta K, Mineshima M, Akiba T. Association between resistance to erythropoiesis-stimulating agents and carnitine profile in patients on maintenance haemodialysis. Nephrology. (2018) 23:737–43. doi: 10.1111/nep.13079
34. Aljama P, Bommer J, Canaud B, Carrera F, Eckardt KU, Hörl WH, et al. Practical guidelines for the use of NESP in treating renal anaemia. Nephrol Dial Transplant. (2001) 16(Suppl. 3):22–8. doi: 10.1093/ndt/16.suppl_3.22
35. Hastka J, Lasserre JJ, Schwarzbeck A, Hehlmann R. Central role of zinc protoporphyrin in staging iron deficiency. Clin Chem. (1994) 40:768–73. doi: 10.1093/clinchem/40.5.768
36. Özhan O, Erdem N, Aydogdu I, Erkurt A, Kuku I. Serum zinc levels in iron deficient women: a case-control study. Turk J Haematol. (2016) 33:156–8. doi: 10.4274/tjh.2015.0206
37. Ece A, Uyanik BS, Işcan A, Ertan P, Yigitoglu MR. Increased serum copper and decreased serum zinc levels in children with iron deficiency anemia. Biol Trace Elem Res. (1997) 59:31–9. doi: 10.1007/BF02783227
Keywords: zinc, ferritin, peritoneal dialysis, dietary restriction, malabsorption
Citation: Kaneko S, Morino J, Minato S, Yanai K, Mutsuyoshi Y, Ishii H, Matsuyama M, Kitano T, Shindo M, Aomatsu A, Miyazawa H, Ueda Y, Ito K, Hirai K, Ookawara S and Morishita Y (2020) Serum Zinc Concentration Correlates With Ferritin Concentration in Patients Undergoing Peritoneal Dialysis: A Cross-Sectional Study. Front. Med. 7:537586. doi: 10.3389/fmed.2020.537586
Received: 02 March 2020; Accepted: 18 August 2020;
Published: 17 September 2020.
Edited by:
Robert P. Woroniecki, Stony Brook Children's Hospital, United StatesReviewed by:
Anthony Michael Valeri, Columbia University, United StatesBernard J. M. Canaud, Université de Montpellier, France
Copyright © 2020 Kaneko, Morino, Minato, Yanai, Mutsuyoshi, Ishii, Matsuyama, Kitano, Shindo, Aomatsu, Miyazawa, Ueda, Ito, Hirai, Ookawara and Morishita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiyuki Morishita, eW1vcmkmI3gwMDA0MDtqaWNoaS5hYy5qcA==
 Shohei Kaneko
Shohei Kaneko Junki Morino
Junki Morino Katsunori Yanai
Katsunori Yanai Hiroki Ishii
Hiroki Ishii Kiyonori Ito
Kiyonori Ito Yoshiyuki Morishita
Yoshiyuki Morishita