
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 03 July 2020
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00387
This article is part of the Research Topic Coronavirus Disease (COVID-19): Pathophysiology, Epidemiology, Clinical Management and Public Health Response View all 400 articles
We report the clinical features of novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a family setting of 13 people with person-to-person transmission in Yancheng, Jiangsu Province, China.
Since the first case of a novel coronavirus disease 2019 (COVID-19) was detected in early December 2019, it has spread rapidly all over the world (1). As of June 17, 2020, more than 8 million of confirmed cases with 440,000 deaths have been reported globally (2). Several family clusters of infected individuals have been reported, which presents a serious threat to public health (3–6). In previously reported family clusters, most infected individuals have exhibited clinical symptoms, abnormal lymphocyte counts, and chest computed tomography (CT) images and were positive for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on quantitative RT-PCR (qRT-PCR) assays. However, some patients were found to have lung abnormalities on chest CT images and positive qRT-PCR results without any clinical symptoms. Here, we report the clinical characteristics of COVID-19 in a family setting of 13 people with person-to-person transmission in Yancheng, Jiangsu Province, China.
Data were collected from Yancheng Third People's Hospital of Jiangsu Province, China. A total of 13 patients from a family cluster were tested SARS-CoV-2 positive after seven of the family members had been to Wuhan. Patients were hospitalized from January 26, 2020 to February 28, 2020. Throat swab samples were collected, and SARS-CoV-2 was detected using qRT-PCR assay. CT and hematological examinations were performed. Patients were carefully monitored and treated during hospital isolation. This study was approved by the ethics committee of Yancheng Third People's Hospital of Jiangsu Province, and written informed consent was obtained. This study followed the reporting guideline for case series.
As shown in Figure 1, on January 20, 2020, seven family members without any symptoms went back to Yancheng from Wuhan via two cars after participating in activities celebrating the Chinese Spring Festival. In Yancheng, two people from this family were infected after touching one or more out of the seven people at home on January 20 and January 23, respectively. Another four family members were subsequently infected following a family wedding together with the above nine people on January 27. Afterwards, all the 13 family members were tested positive for SARS-CoV-2 infection. Of these 13 patients, six cases had fever, two cases had cough, and one had fatigue and dizziness as the first manifestations; however, case did not present with any symptoms (Table 1). Nine patients developed symptoms after an average of 9 days of exposure, and those four asymptomatic patients tested as qRT-PCR positive after exposure for an average of 15.5 days. CT scans of 10 patients when admitted showed mild or moderate pulmonary fibrosis, but no abnormalities were observed in three patients by chest CT images. During the hospital isolation ward stay, all patients were carefully monitored. Besides supportive oxygen therapy, all the adult patients were treated with intramuscular thymalfasin (1.6 mg per day to twice per week according to patients' response), oral hydroxychloroquine (0.4 mg per day for 7 days), alpha interferon (nebulized inhalation, 500 iu twice per day for 10 days), and oral lopinavir/ritonavir (500 mg twice per day for 6–16 days). All patients were also received traditional Chinese medicine including oral Lianhua Qingwen Granules (1.4 g three times/day for 6–13 days), oral Shufeng Jiedu Capsules (2 g three times/day for 6–13 days), and oral Arbidol (0.2 g three time/day for 6–13 days). No obvious adverse effects of these agents were observed; an exception was an 88-year-old with mild dizziness and unstable walking after taking hydroxychloroquine. All these 13 patients in the family recovered well with symptom-free and pulmonary fibrosis was absorbed by chest CT and qRT-PCR re-evaluations until discharge after around 2 months of hospitalization. Representative changes of chest CT images in Patient 3 were presented in Figure 2 during this time course.
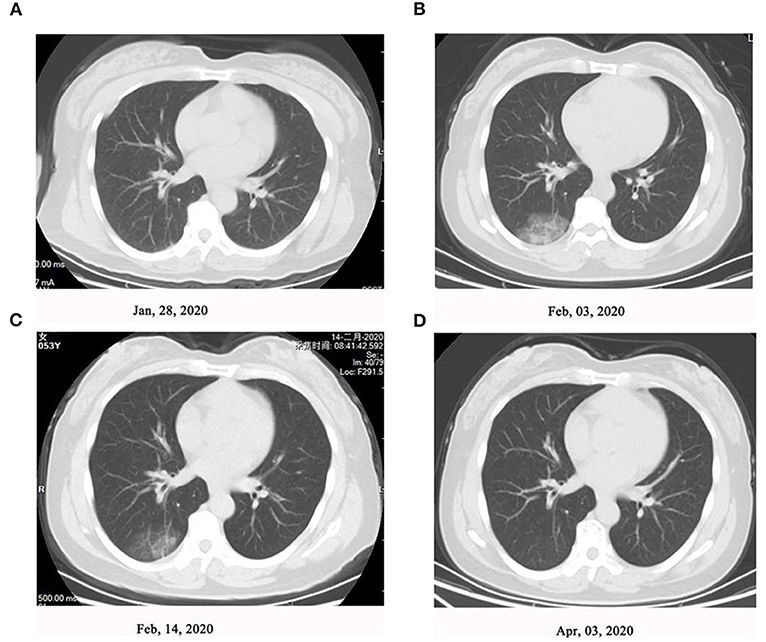
Figure 2. Representative changes of chest computed tomography (CT) images in Patient 3. High-resolution chest CT scan showed (A) normal chest at level of left atrium (January 28, 2020), (B) ground-glass attenuation close to the pleura in the right lower lung (February 03, 2020), (C) fade of ground-glass attenuation after treatment (February 14, 2020), and (D) resolution of lung inflammation after treatment (April 03, 2020).
We reported a cluster of 13 family members of infected with SARS-CoV-2. The uniqueness of this cluster is that only four people were infected during the wedding with so many people attending the wedding. Therefore, it has been assumed that infection of this virus is correlated with the strength of individual immunity (7, 8). Furthermore, although all individuals were tested positive for SARS-CoV-2 infection on qRT-PCR in this family cluster, four patients did not show any clinical symptoms and diagnosis may have been delayed owing to atypical presentations. Asymptomatic patients might be unaware of their disease and therefore not isolate themselves or seek further treatment, or they might be overlooked by health-care professionals and thus unknowingly transmit the virus to others (3, 8, 9). Fortunately, symptoms of the four asymptomatic patients in this family cluster were mild, and all of them were recovered. However, to prevent and control this highly infectious disease as early as possible, people with family members with COVID-19 diagnosis should be closely monitored and tested to rule out the virus infection, even if they do not show any symptoms. It is also important for countries to do active case-findings among close contacts of confirmed patients to prevent symptoms worsen and virus spreading (10). Finally, Chinese medicine was used to as part of the treatment against COVID-19 in this family cluster with good self-reported feedback and no obvious adverse effects, thus recommending a consideration of its use according to our experience, though concrete mechanisms still need further investigation (11).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Yancheng Third People's Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
HZ, JC, RC, and BC: data collection and interpretation. RC and HZ: original draft preparation. RC and BC: review and editing. BC, RC, and JC: supervision. All authors: reviewed and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
3. Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. (2020) 323:1406–7. doi: 10.1001/jama.2020.2565
4. Pan X, Chen D, Xia Y, Wu X, Li T, Ou X, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. (2020) 20:410–1. doi: 10.1016/S1473-3099(20)30114-6
5. Pung R, Chiew CJ, Young BE, Chin S, Chen MI, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. (2020) 395:1039–46. doi: 10.1016/S0140-6736(20)30528-6
6. Zhanwei D, Xiaoke X, Ye W, Lin W, Benjamin JC, Lauren Ancel M. Serial Interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis J. (2020) 26:1341. doi: 10.3201/eid2606.200357
7. Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. (2020) 27:1451–4. doi: 10.1038/s41418-020-0530-3
8. He X, Lau EH, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. (2020) 26:672–5. doi: 10.1038/s41591-020-0869-5
9. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
10. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
Keywords: COVID-19, SARS-CoV-2, family cluster, asymptomatic, transmission, intimate contact
Citation: Zhang H, Chen R, Chen J and Chen B (2020) COVID-19 Transmission Within a Family Cluster in Yancheng, China. Front. Med. 7:387. doi: 10.3389/fmed.2020.00387
Received: 01 May 2020; Accepted: 22 June 2020;
Published: 03 July 2020.
Edited by:
Zisis Kozlakidis, International Agency for Research on Cancer (IARC), FranceCopyright © 2020 Zhang, Chen, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoan Chen, Y2JhODg4OEBob3RtYWlsLmNvbQ==; Runzhe Chen, cmNoZW4yQG1kYW5kZXJzb24ub3Jn; Jibei Chen, MTYxMjhAc29odS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.