
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 25 June 2020
Sec. Translational Medicine
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00298
This article is part of the Research TopicTargeting Thyroid Hormone-dependent Pathways in Proliferative and Degenerative DisordersView all 5 articles
 Jin San Lee1
Jin San Lee1 Yunsoo Soh2
Yunsoo Soh2 Hyug-Gi Kim3
Hyug-Gi Kim3 Kyung Mi Lee3
Kyung Mi Lee3 Young Nam Kwon4
Young Nam Kwon4 Sung Sang Yoon1
Sung Sang Yoon1 Key-Chung Park1
Key-Chung Park1 Hak Young Rhee5*
Hak Young Rhee5*Background: The aim of the present study was to investigate the associations between thyroid hormones, cognitive performance, and apolipoprotein E (APOE) genotype in euthyroid patients with subjective cognitive decline (SCD).
Methods: We recruited 197 euthyroid patients that fulfilled the criteria for SCD. All participants were classified into APOE ε4 carriers and non-carriers based on the presence of the APOE ε4 allele. Patients with SCD who had the APOE ε2/ε4 genotype were excluded from the study. We then performed correlation and regression analyses to evaluate the associations between cognitive performance and thyroid hormones in APOE ε4 carriers and non-carriers.
Results: We found no significant differences in cognitive function between APOE ε4 carriers and non-carriers. However, higher levels of triiodothyronine (T3) were associated with better verbal memory performance (immediate and delayed recall tasks) in APOE ε4 carriers, whereas a negative association was found in APOE ε4 non-carriers. Furthermore, there was a significant interactive effect of APOE ε4 status and T3 levels on verbal memory performance (immediate and delayed recall tasks).
Conclusions: These findings suggest that in patients with SCD, T3 might have a protective effect on memory in those who are APOE ε4 carriers. The differential susceptibility hypothesis would thus support a gene-by-hormone crossover interaction between APOE ε4 allele and T3 in this study. Early identification and intervention of high-risk individuals for cognitive decline is important to establish new strategies for preventing dementia.
Thyroid hormones have been demonstrated to play an important role in cellular metabolism, growth, and differentiation of human organ systems. Thyrotropin-releasing hormone, produced by the hypothalamus, stimulates the release of thyroid-stimulating hormone (TSH) in the pituitary gland, which, in turn, induces the release of triiodothyronine (T3) and thyroxine (T4) in the thyroid gland (1). T4 is a major form of thyroid hormone in the blood, and has a longer half-life than T3. T4 is converted to the active T3 (three to four times more potent than T4), and this regulatory process is maintained by a neuroendocrine feedback mechanism in healthy individuals. Thyroid hormones are also essential for the development of the nervous system and play crucial roles in the maintenance of brain function (2). Common causes of reversible cognitive impairment include clinical hypothyroidism and hyperthyroidism (3, 4), and the thyroid function test has thus become a standard screening test in individuals who complain of cognitive decline (5).
Subjective cognitive decline (SCD) is the self-reported experience of worsening memory decline without objective cognitive deterioration (6). Previous studies have reported that SCD may represent the early symptomatic stage of Alzheimer's disease (AD) and related dementias (6, 7). SCD is part of a heterogeneous group of disorders, which includes preclinical AD and various conditions that can affect cognition such as depression and anxiety (6). Growing interest in strategies to maintain cognitive health in midlife has led many people who experience cognitive decline to visit a memory clinic (8, 9). However, lifestyle modifications, such as a healthy diet, adequate exercise, limiting alcohol, and abstaining from smoking are mainly recommended for most patients who have been diagnosed with SCD, unless other causes of cognitive deterioration are found.
Previous studies have reported interesting findings on the association between thyroid hormones and cognitive function in healthy euthyroid subjects. For instance, higher levels of T4 correlated positively with better general cognition in elderly men (10), and lower levels of T4 were related to a greater risk of cognitive worsening in elderly women (11)However, higher levels of free T3 were negatively correlated with executive functions in elderly women (1). In patients with mild cognitive impairment (MCI), the classically defined prodromal stage of dementia, higher levels of T3 were also negatively associated with cognitive performance across all cognitive domains (12), while lower levels of free T3 were associated with worse cognitive functioning in patients with coronary artery disease (13). While the investigation of thyroid hormones may be useful for assessments of cognitive performance in the elderly population, to date, knowledge regarding the relationship between thyroid hormones within the normal range and cognitive function in patients with SCD is limited.
Understanding the hormonal interrelationships that occur in SCD can provide opportunities for earlier interventions in patients who are progressing to MCI or dementia. The objective of this study was to investigate the relationship between thyroid hormones as well as TSH and cognitive performance in euthyroid patients with SCD. Specifically, considering that the apolipoprotein E (APOE) ε4 allele is not only a genetic risk factor for sporadic AD but also for earlier stages such as MCI or even SCD (14, 15), we hypothesized that thyroid hormones as well as TSH may have distinct effects on cognitive performance in participants depending on their APOE ε4 status.
The flow chart of the study participants is presented in Figure 1. We consecutively recruited 232 patients with SCD at age 50 or older from the Memory Clinic at Kyung Hee University Hospital (Seoul, Korea) from March 2016 to June 2018, in line with the following criteria (16): (1) subjective memory complaints by patients or caregivers, (2) no objective cognitive dysfunction in any cognitive domain in detailed neuropsychological tests, and (3) no dementia. All participants underwent a standardized diagnostic assessment protocol for cognitive impairment and dementia including high-resolution 3.0T magnetic resonance imaging (MRI) as well as detailed neuropsychological tests. Brain MRI confirmed the absence of structural lesions including cerebral hemorrhage or infarction, hippocampal sclerosis, brain tumors, traumatic encephalomalacia, and vascular malformation. The exclusion criteria included a history of thyroid axis disorders, thyroid hormone replacement therapy, psychological disease, stroke, brain surgery, seizure, head trauma, severe cerebral white matter hyperintensities (deep white matter ≥ 25 mm, and caps or band ≥ 10 mm), other medication that could interfere with thyroid hormone metabolism (such as amiodarone), and current systemic medical diseases that could affect cognition.
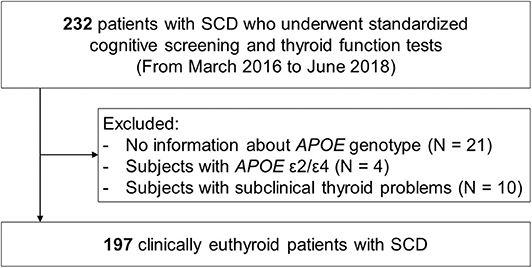
Figure 1. Flow chart of the study participants. SCD, subjective cognitive decline; MRI, magnetic resonance imaging; APOE, apolipoprotein E.
Laboratory tests were conducted to exclude other causes of cognitive impairment, and included thyroid function tests (T3, free T4 [fT4], and TSH), a metabolite profile, vitamin B12 and folate levels, complete blood counts, blood chemistry, and syphilis serology. APOE genotyping was performed in 211 (90.9%) of the 232 participants. We excluded four patients who had the APOE ε2/ε4 genotype from the study, since the putative opposing effects of the ε4 and ε2 alleles could result in some confusion in the interpretation of results (17, 18). All study participants were clinically euthyroid, but 10 participants showed subclinical thyroid problems in endocrinological assessments, and these subjects were also excluded from the study. The final sample size was 197.
Written informed consent was obtained from all participants before inclusion in the study. The study was approved by the Institutional Review Board (IRB) of Kyung Hee University Hospital (IRB file number: 2018-01-023). All procedures were carried out in accordance with approved guidelines.
All participants underwent detailed neuropsychological tests using the standardized Seoul Neuropsychological Screening Battery (19, 20). The battery contains tests for attention (the Digit Span Forward and Backward), language (the Korean version of the Boston Naming Test [K-BNT]), visuospatial function (the Rey-Osterrieth Complex Figure Test [RCFT]; copying), verbal and visual memory (the Seoul Verbal Learning Test [SVLT] and RCFT; immediate and 20-min delayed recall, and recognition), and frontal/executive function (the phonemic and semantic Controlled Oral Word Association Test [COWAT] and a Stroop Test; word and color reading). Cognitive functions associated with each neuropsychological test are presented in Table 1. Age- and education-specific norms for each test based on 447 cognitively normal individuals were used for comparison. Z-scores lower than −1.0 standard deviation (SD) of the age- and education-adjusted norms were considered abnormal. We also used the Mini-Mental Status Examination (MMSE), the Clinical Dementia Rating, the Clinical Dementia Rating Sum of Boxes, and the Geriatric Depression Scale.
Genomic DNA was extracted from peripheral blood leukocytes using the QIAamp DSP DNA Mini Kit following the manufacturer's instructions (QIAGEN GmbH, Hilden, Germany). Two single nucleotide polymorphisms (rs429358 for codon 112 and rs7412 for codon 158) in the APOE gene were genotyped using LG AdvanSureTM apoE Genotyping real-time PCR (LG Lifescience, Korea) on a SLAN real-time PCR Detection System (LG Lifescience, Korea) according to the manufacturer's instructions. Subjects with at least one APOE ε4 allele were identified as ε4 carriers. In addition, subjects with ε2/ε2, ε2/ε3, and ε3/ε3 alleles were identified as ε4 non-carriers.
Serum levels of total T3, fT4, and TSH were evaluated with a chemiluminescence immunoassay using the STRATEC SR 300 analyzer (Brahms, Berlin, Germany). According to our laboratory-verified reference ranges, the normal serum T3, fT4, and TSH intervals were 76–170, 0.9–1.8, and 0.5–4.5 μIU/mL, respectively.
Continuous variables were presented as means ± SD and were compared using the Student's t-test. Categorical variables were compared using a Chi-square test or Fisher's exact test. For comparisons of neuropsychological performance between APOE ε4 carriers and non-carriers, we used age- and education-specific Z-scores. To evaluate correlations between the results of neuropsychological tests and thyroid hormones as well as TSH according to APOE ε4 status, bivariate relationships were calculated using Pearson's correlation coefficient. Multiple linear regression was performed using sex, vascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, cardiovascular disease, and history of stroke), APOE ε4 status, thyroid hormones including TSH, and an interaction term (thyroid hormones including TSH by APOE ε4 status) as independent variables. All tests were two-tailed, and statistical significance was set at p < 0.05. The Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS Inc., Chicago, IL, USA) was used to perform all statistical analyses.
Demographic and clinical characteristics of the study participants are presented in Table 2. The mean age of our participants was 65.5 years, and 142 (72.1%) were female. We identified 49 (24.9%) APOE ε4 carriers, and there were no significant differences in demographics between the carriers and the non-carriers except for education level. Furthermore, there were no significant differences in thyroid hormones and TSH levels between the two groups.
Table 3 shows comparisons of neuropsychological performance between APOE ε4 carriers and non-carriers among our patients with SCD. Although the mean Z-scores of neuropsychological tests were higher in non-carriers than in carriers, except for the Digit Span Forward Test, there were no statistically significant differences between the two groups.

Table 3. Comparisons of neuropsychological performance between APOE ε4 carriers and non-carriers in patients with SCD.
The results of the correlation analyses between neuropsychological performance and thyroid hormones as well as TSH are presented in Table 4. There was a negative correlation between T3 levels and Digit Span Forward and RCFT recognition task Z-scores in our patients with SCD. Levels of T3 and TSH correlated negatively with the Z-score of the Digit Span Forward task in APOE ε4 carriers. Levels of T3 correlated negatively with the Z-scores of the SVLT immediate and delayed recall as well as the RCFT recognition task in APOE ε4 non-carriers, while in APOE ε4 carriers, levels of T3 correlated positively with the Z-scores of the SVLT immediate and delayed recall tasks. In addition, there was a positive overall correlation between fT4 levels and Z-scores of the COWAT phonemic total task and the MMSE in our patients with SCD. Specifically, levels of fT4 correlated positively with the Z-score of the COWAT phonemic total task in APOE ε4 non-carriers. In APOE ε4 carriers, levels of TSH correlated negatively with the Z-score of the Digit Span Forward task.
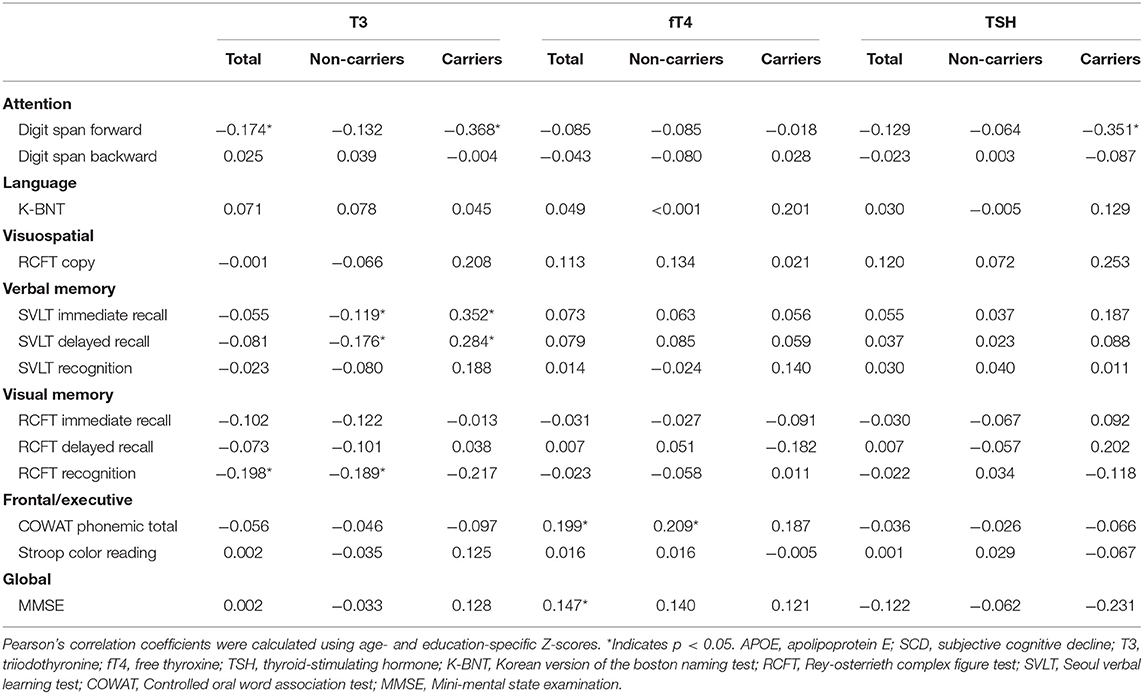
Table 4. Correlation coefficient (r) between neuropsychological performance and thyroid hormone levels as well as TSH according to APOE ε4 status.
Multiple linear regressions were performed to evaluate the interactive effects of APOE ε4 status and thyroid hormones as well as TSH on neuropsychological performance (Table 5). There were significant interactive effects of APOE ε4 status and T3 level on the RCFT copy (p = 0.045), SVLT immediate (p = 0.032), and delayed recall (p = 0.004) tasks, suggesting that higher levels of T3 were associated with better memory performance in APOE ε4 carriers. Figure 2 shows scatter plots investigating the relation between neuropsychological performance and T3 levels in our patients with SCD according to APOE ε4 status.
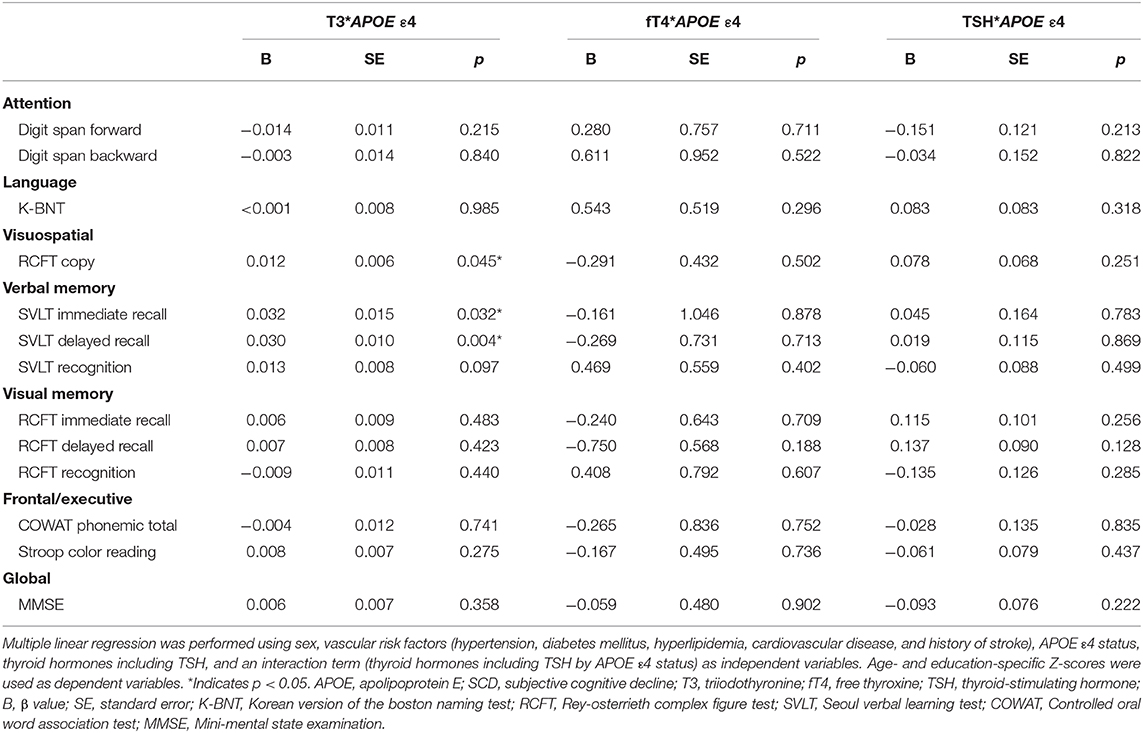
Table 5. Interaction effects of APOE ε4 status and thyroid hormone levels as well as TSH on neuropsychological performance.
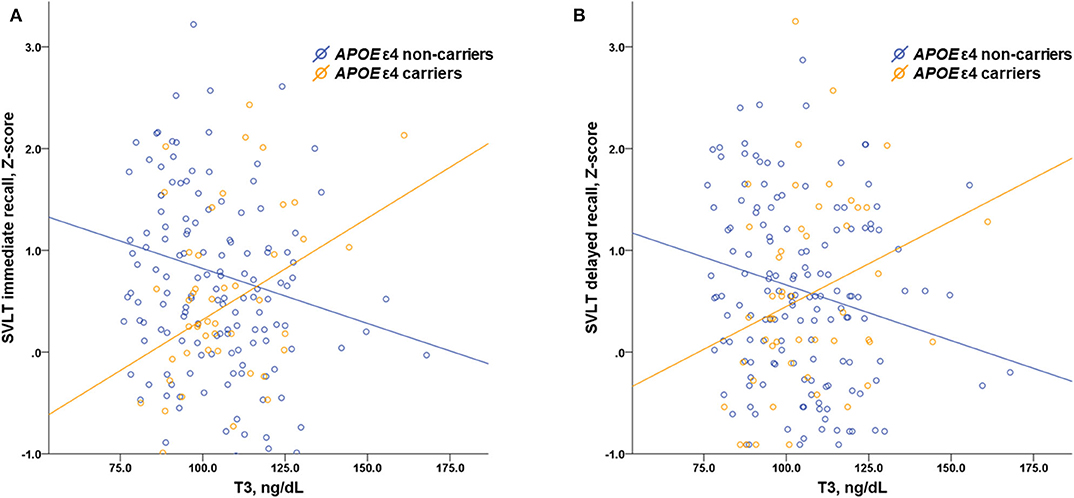
Figure 2. Scatter plots investigating the relation between memory performance and serum T3 levels in patients with SCD according to APOE ε4 status showing interaction effects of APOE ε4 status and T3 level on the results of (A) the SVLT immediate recall (p = 0.032) and (B) the SVLT delayed recall (p = 0.004) task. T3, triiodothyronine; SCD, subjective cognitive decline; APOE, apolipoprotein E; SVLT, Seoul verbal learning test.
In this study, we investigated the effect of thyroid hormones as well as TSH on cognitive performance in APOE ε4 carriers and non-carriers among euthyroid patients with SCD. The key findings of our study are that levels of T3 correlated positively with memory performance in APOE ε4 carriers, whereas a negative correlation was found in APOE ε4 non-carriers. Moreover, there was a significant interactive effect of APOE ε4 status and T3 level on memory performance. This suggests that T3 has a protective effect on memory in APOE ε4 carriers with SCD, which might represent a risk group for cognitive deterioration and development of AD.
Individuals with SCD have generally been regarded as the “worried well,” used to describe individuals who are at risk of developing disease, given the lack of objective evidence of cognitive impairment. However, previous studies suggest that, for substantial numbers of individuals with SCD, self-reported experience of worsening memory decline may indeed herald the development of cognitive decline to dementia (16, 21). Apolipoprotein E, on the other hand, is a plasma lipoprotein that has functions in Aβ clearance (22). APOE ε4 has been well-established as an important risk factor for developing AD (23), whereas APOE ε2 reduces the risk of AD (24). In general, APOE ε4 has been an established risk factor for memory decline, despite ongoing cognitively normal status (25–27). In a recent meta-analysis, the presence of APOE ε4 increased the risk of conversion to AD from 0.78% per year among non-carriers to 3.24% per year among carriers among patients with SCD (15). Therefore, APOE ε4 carriers with SCD have an additional risk for dementia (25, 28).
Current evidence have allowed for a shift in the definition of AD from a syndromal to a biological construct, based on biomarkers that are proxies of pathology (29). However, little is known about mechanisms underlying the disease progression at its early stages, such as SCD. To date, various clinical trials focusing on multimodal interventions (nutritional, physical, cognitive, and medical) have attempted to prevent the progression of dementia in patients with SCD. However, the available evidence with regards to lifestyle interventions for SCD is limited (30). Our finding that T3 may have a protective effect on memory in APOE ε4 carriers with SCD has clinical significance in terms of prevention of cognitive deterioration and dementia. Although not much research on the supplementation of thyroid hormones in patients with SCD has been reported to date, we speculate that supplementing T3 in APOE ε4 carriers with SCD might help prevent cognitive decline.
Indeed, we have found some evidence to support our speculation in a few previous studies on several neuropsychological diseases: in depressed patients with normal levels of thyroid hormones, the addition of T3 to antidepressant drugs had some benefit in the treatment of both manic and depressed phases of mood disorders (31); application of T3 orally improved performance on a verbal fluency task in healthy subjects (32); and partial substitution of T3 for T4 led to improved neuropsychological performance and mood in patients with hypothyroidism (33). However, these findings need to be interpreted with caution, since these results are derived from groups with other diseases and small sample sizes. Contrary to our suggestion, a previous report demonstrated that the use of thyroid drugs was associated with the incidence of AD dementia (34). Thyroid hormone therapy with levothyroxine also provided no benefit with regard to executive cognitive function in older persons with subclinical hypothyroidism (35). Moreover, elderly subjects who had high levels of thyrotropin, above the normal range, were found to have an elongated life span (36).
The detrimental effect of T3 on memory in APOE ε4 non-carriers could be explained by several reports of a potential direct action of T3 on cognitive performance. Aggravation of the cholinergic deficit and related cognitive dysfunction observed in patients with AD has been suggested to be due to thyroid hormone-induced depletion of acetylcholine (37). Thyroid hormone-induced oxidative damage and reduced antioxidative defense enzyme levels have been associated with progressive neurodegeneration (38). However, there is little biological evidence for the protective effect of T3 on memory in APOE ε4 carriers with SCD. Thyroid hormones modulate gene expression and intracellular signal transduction by regulating the synthesis of enzymes necessary for the production of neurotransmitters (2). We suggest that the differential susceptibility hypothesis (39)—genetic factors that are supposed to confer vulnerability may lead to differential susceptibility to both the negative and positive effects of some other factor—supports a gene-by-hormone crossover interaction between the APOE ε4 allele and T3 in this study. In other words, APOE ε4 carriers with higher levels of T3 (beneficial circumstances) may function better than APOE ε4 non-carriers, which is consistent with the results from a previous study reporting on testosterone (40). Alternatively, as the APOE ε4 allele has been reported to be positively associated with hypothyroidism (41), this mechanism might also be related to compensation.
The strength of our study is the sample size that enabled us to conduct substantial statistical analyses. However, there are some limitations. First, our study has a cross-sectional design, which prevents us from making claims of causality. Second, the study participants were recruited from a memory disorder clinic, and the sample might thus not be representative of the general population. Third, the interpretation of our findings is limited since serum concentrations of thyroid hormones do not accurately reflect the metabolism of thyroid hormones in the brain. Fourth, although we confirmed that our participants had no crucial metabolic problems, it is possible that other factors or metabolic pathways influenced the effects of thyroid hormones on cognitive function we observed. Finally, although sex is regarded to be an important parameter in estimating the risk of AD or thyroid disease (42), we did not perform analyses according to sex, due to the relatively small number of men in our sample. Nevertheless, our findings provide important insights that help our understanding of the associations between thyroid hormones, cognitive performance, and APOE genotypes in patients with SCD. Early identification of high-risk individuals and interventions for cognitive decline are important in the quest to establish new strategies for preventing dementia, in keeping with the paradigm shift in focus from AD dementia to preclinical AD in the development of therapeutic interventions. However, further evidence for the supplementation of T3 in APOE ε4 carriers with SCD to prevent cognitive decline can only be gathered from a well-designed, randomized clinical trial.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by institutional review board at the Kyung Hee university hospital. The patients/participants provided their written informed consent to participate in this study.
JL and HR: conception and design of the study and final approval of the manuscript. JL, SY, K-CP, and HR: acquisition of data. JL, YS, H-GK, KL, YK, SY, K-CP, and HR: analysis and interpretation of the data. JL, YK, and HR: drafting and revising the manuscript for content.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2020R1C1C1006623) and Basic Science Research Program through the Ministry of Education of the Republic of Korea (NRF-2018R1D1A1B07041308).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This paper extends the results published in the proceedings version (43) in substantial and meaningful ways.
1. Grigorova M, Sherwin BB. Thyroid hormones and cognitive functioning in healthy, euthyroid women: a correlational study. Horm Behav. (2012) 61:617–22. doi: 10.1016/j.yhbeh.2012.02.014
2. Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. (2002) 25:268–88. doi: 10.1007/BF03344003
3. Cummings J, Benson DF, LoVerme S Jr. Reversible dementia. Illustrative cases, definition, and review. JAMA. (1980) 243:2434–9. doi: 10.1001/jama.1980.03300490052031
4. Smith JS, Kiloh LG. The investigation of dementia: results in 200 consecutive admissions. Lancet. (1981) 1:824–7. doi: 10.1016/S0140-6736(81)92692-1
5. Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for cognitive impairment in older adults: US preventive services task force recommendation statement. JAMA. (2020) 323:757–63. doi: 10.1001/jama.2020.0435
6. Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. (2014) 10:844–52. doi: 10.1016/j.jalz.2014.01.001
7. Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. (2017) 13:369–96. doi: 10.1146/annurev-clinpsy-032816-045136
8. Blackburn DJ, Wakefield S, Shanks MF, Harkness K, Reuber M, Venneri A. Memory difficulties are not always a sign of incipient dementia: a review of the possible causes of loss of memory efficiency. Br Med Bull. (2014) 112:71–81. doi: 10.1093/bmb/ldu029
9. Xanthopoulou P, McCabe R. Subjective experiences of cognitive decline and receiving a diagnosis of dementia: qualitative interviews with people recently diagnosed in memory clinics in the UK. BMJ Open. (2019) 9:e026071. doi: 10.1136/bmjopen-2018-026071
10. Prinz PN, Scanlan JM, Vitaliano PP, Moe KE, Borson S, Toivola B, et al. Thyroid hormones: positive relationships with cognition in healthy, euthyroid older men. J Gerontol A Biol Sci Med Sci. (1999) 54:M111–6. doi: 10.1093/gerona/54.3.M111
11. Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology. (2002) 58:1055–61. doi: 10.1212/WNL.58.7.1055
12. Quinlan P, Nordlund A, Lind K, Gustafson D, Edman A, Wallin A. Thyroid hormones are associated with poorer cognition in mild cognitive impairment. Dement Geriatr Cogn Disord. (2010) 30:205–11. doi: 10.1159/000319746
13. Burkauskas J, Bunevicius A, Brozaitiene J, Neverauskas J, Lang P, Duwors R, et al. Cognitive functioning in coronary artery disease patients: associations with thyroid hormones, N-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein. Arch Clin Neuropsychol. (2017) 32:245–51. doi: 10.1093/arclin/acx004
14. Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. (2006) 66:828–32. doi: 10.1212/01.wnl.0000203264.71880.45
15. Ali JI, Smart CM, Gawryluk JR. Subjective cognitive decline and APOE varepsilon4: a systematic review. J Alzheimers Dis. (2018) 65:303–20. doi: 10.3233/JAD-180248
16. Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis. (2015) 48 (Suppl. 1):S63–86. doi: 10.3233/JAD-150154
17. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. (1997) 278:1349–56. doi: 10.1001/jama.1997.03550160069041
18. Lovati C, Galimberti D, Albani D, Bertora P, Venturelli E, Cislaghi G, et al. APOE epsilon2 and epsilon4 influence the susceptibility for Alzheimer's disease but not other dementias. Int J Mol Epidemiol Genet. (2010) 1:193–200. Available online at: http://ijmeg.org/files/IJMEG1002005.pdf.
19. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul neuropsychological screening battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. (2010) 25:1071–6. doi: 10.3346/jkms.2010.25.7.1071
20. Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, et al. The cortical neuroanatomy related to specific neuropsychological deficits in alzheimer's continuum. Dement Neurocogn Disord. (2019) 18:77–95. doi: 10.12779/dnd.2019.18.3.77
21. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. (2010) 6:11–24. doi: 10.1016/j.jalz.2009.10.002
22. Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. (2009) 10:333–44. doi: 10.1038/nrn2620
23. Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. (2013) 9:106–18. doi: 10.1038/nrneurol.2012.263
24. Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PCJr, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. (1994) 7:180–4. doi: 10.1038/ng0694-180
25. Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, van Kamp GJ, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. (2001) 57:2217–22. doi: 10.1212/WNL.57.12.2217
26. Kang JH, Logroscino G, De Vivo I, Hunter D, Grodstein F. Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiol Aging. (2005) 26:475–84. doi: 10.1016/j.neurobiolaging.2004.05.003
27. Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. (2009) 361:255–63. doi: 10.1056/NEJMoa0809437
28. Muller-Gerards D, Weimar C, Abramowski J, Tebrugge S, Jokisch M, Dragano N, et al. Subjective cognitive decline, APOE epsilon4, and incident mild cognitive impairment in men and women. Alzheimers Dement. (2019) 11:221–30. doi: 10.1016/j.dadm.2019.01.007
29. Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. (2018) 14:535–62. doi: 10.1016/j.jalz.2018.02.018
30. Bhome R, Berry AJ, Huntley JD, Howard RJ. Interventions for subjective cognitive decline: systematic review and meta-analysis. BMJ Open. (2018) 8:e021610. doi: 10.1136/bmjopen-2018-021610
31. Esposito S, Prange AJ Jr, Golden RN. The thyroid axis and mood disorders: overview and future prospects. Psychopharmacol Bull. (1997) 33:205–17.
32. Kathmann N, Kuisle U, Bommer M, Naber D, Muller OA, Engel RR. Effects of elevated triiodothyronine on cognitive performance and mood in healthy subjects. Neuropsychobiology. (1994) 29:136–42. doi: 10.1159/000119076
33. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med. (1999) 340:424–9. doi: 10.1056/NEJM199902113400603
34. Harper PC, Roe CM. Thyroid medication use and subsequent development of dementia of the Alzheimer type. J Geriatr Psychiatry Neurol. (2010) 23:63–9. doi: 10.1177/0891988709342723
35. Stott DJ, Rodondi N, Kearney PM, Ford I, Westendorp RGJ, Mooijaart SP, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. (2017) 376:2534–44. doi: 10.1056/NEJMoa1603825
36. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. (2004) 292:2591–9. doi: 10.1001/jama.292.21.2591
37. Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, et al. Thyroid function and the risk of Alzheimer disease: the framingham study. Arch Intern Med. (2008) 168:1514–20. doi: 10.1001/archinte.168.14.1514
38. Bianchi G, Solaroli E, Zaccheroni V, Grossi G, Bargossi AM, Melchionda N, et al. Oxidative stress and anti-oxidant metabolites in patients with hyperthyroidism: effect of treatment. Horm Metab Res. (1999) 31:620–4. doi: 10.1055/s-2007-978808
39. Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatr. (2009) 14:746–54. doi: 10.1038/mp.2009.44
40. Panizzon MS, Hauger R, Xian H, Vuoksimaa E, Spoon KM, Mendoza SP, et al. Interaction of APOE genotype and testosterone on episodic memory in middle-aged men. Neurobiol Aging. (2014) 35:1778.e1771–1778. doi: 10.1016/j.neurobiolaging.2013.12.025
41. Percy ME, Potyomkina Z, Dalton AJ, Fedor B, Mehta P, Andrews DF, et al. Relation between apolipoprotein E genotype, hepatitis B virus status, and thyroid status in a sample of older persons with down syndrome. Am J Med Genet A. (2003) 120a:191–8. doi: 10.1002/ajmg.a.20099
42. Bojar I, Stasiak M, Cyniak-Magierska A, Raczkiewicz D, Lewinski A. Cognitive function, APOE gene polymorphisms, and thyroid status associations in postmenopausal women in poland. Dement Geriatr Cogn Disord. (2016) 42:169–85. doi: 10.1159/000449373
Keywords: thyroid hormone, apolipoprotein E, subjective cognitive decline, Alzheimer's disease, triiodothyronine
Citation: Lee JS, Soh Y, Kim H-G, Lee KM, Kwon YN, Yoon SS, Park K-C and Rhee HY (2020) Interactive Effects of Apolipoprotein E ε4 and Triiodothyronine on Memory Performance in Patients With Subjective Cognitive Decline. Front. Med. 7:298. doi: 10.3389/fmed.2020.00298
Received: 13 January 2020; Accepted: 26 May 2020;
Published: 25 June 2020.
Edited by:
Andrea Perra, University of Cagliari, ItalyReviewed by:
DR Nicholas Ekow Thomford, University of Cape Coast, GhanaCopyright © 2020 Lee, Soh, Kim, Lee, Kwon, Yoon, Park and Rhee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hak Young Rhee, YXp6bzczQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.