
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 30 July 2020
Sec. Gastroenterology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00282
This article is part of the Research TopicHot Topics in Pancreatology from Europe- 2020View all 14 articles
Objective: To evaluate the survival benefit of metformin use for pancreatic cancer (PC) patients underwent pancreatectomy.
Methods: Databases including EMBASE, PubMed, the Cochrane Library were searched to identify studies relevant to the outcomes on the survival benefit of metformin use for the PC patients who underwent pancreatectomy until June 30, 2019. STATA 12.0 software was used to performed the meta-analysis.
Results: 12 studies involving 35,346 PC patients were included in this meta-analysis. With a random-model, there are significant differences in overall survival (HR = 0.85, 95% CI: 0.77–0.94, P = 0.002) between PC patients who were treated with metformin underwent pancreatectomy and those who underwent pancreatectomy without metformin use. Subgroup analyses showed Caucasians (HR = 0.903, 95% CI = 0.825–0.940, P = 0.008) and Asian (HR = 0.691, 95% CI = 0.588–0.813, P = 0.001) PC patients have a significantly reduced risk of death for metformin users. Subgroup analyses also showed a survival benefit for PC patients at stage I-II (HR = 0.762, 95% CI = 0.677–0.858, P = 0.0001).
Conclusions: Metformin use is related to a better survival benefit for PC patients who underwent pancreatectomy, which would be a potential drug for the treatment of PC.
Pancreatic cancer, reported as the 4th death-leading cause worldwide (1) and was predicted to be the second death-leading cause by 2030. For diagnosis of PC, many of them were diagnosed at an unresectable stage or a distant metastasis stage (2). However, the patients with PC had a lower survival rate. The research reported that <20% of PC patients benefit from current surgery and the rate of 5-year survival is not even higher than 5% (3). Despite this, curative resection has improved the survival outcomes for PC patients over decades and tumor further progression and recurrence are still influenced by the great variability of chemotherapeutics resistance and clinical responses even for some patients with appropriate surgery or at early tumor stage (4, 5), which highlights that it is necessary for us to find better treatment strategies and survival risk factors for the patients with PC (6, 7).
Recently, a growing number of evidences suggested that anti-diabetic drug metformin can inhibit the division of cancer cells, down-regulate the level of circulating insulin and activate the immune-system for cancer patients (8). In addition, some hypoglycemic drugs can enhance the therapeutic outcomes by effecting the metabolic pathway with a result of inhibiting the malignant tumor cells, and also can control the blood glucose for individuals (9). Of which, metformin is the most promising adjuvant for cancer therapy (10). Although it was repeatedly reported that metformin plays an important role in decreasing the mortality and incidence of PC by epidemiologic and basic researches, the survival benefit of metformin use for PC patients who underwent pancreatectomy is still unclear. Therefore, we conducted a meta-analysis to evaluate the survival benefit of metformin use for patients with PC who underwent pancreatectomy.
To include studies about the survival benefit of metformin use for patients with pancreatic cancer who underwent pancreatectomy, a comprehensive databases search including PubMed, the Cochrane Library and EMBASE was performed until June 30, 2019. Literature search terms were as follows: “pancreatic cancer,” “Pancreatic Neoplasm,” “Pancreas cancer,” “Pancreatic Ductal Carcinomas,” “PC,” “metformin,” “overall survival.” The language of the included study was limited to English in this meta-analysis.
Selection criteria were listed as follows: (1) the pancreatic cancer was diagnosed by histological or pathological examination; (2) PC patients were treated with pancreatectomy surgery. (3) survival outcomes including overall survival were reported in full text; (4) survival outcomes were reported on hazard ratio (HR) and its 95% confidence interval (95% CI), and (5) the full research was published in English.
The articles including the following were excluded: (1) duplicate studies; (2) conference abstracts, case report, editorial letters and review; (3) without full text; (4) survival outcomes were not reported in full articles.
The relevant data was extracted from included studies by two reviewers (ZJQ and MJC) independently. Data retrieved from included studies as follows: (1) characteristics of studies including publications, authors, year of publication, sample size, country, pancreatectomy strategy, duration of follow-up; (2) clinical outcome: the data of overall survival.
The quality of included studies was assessed according to the Newcastle-Ottawa scale (NOS) (11): (1) the selection of cohorts (0–4 points); (2) comparability of cohorts (0–2 points); (3) the exposure or outcome of the participant (0–3 points). Finally, the total score of each study represented the overall result of quality assessment. Studies with 7–9 points were regarded as “high quality.”
STATA version 12.0 was performed to process all data for this meta-analysis. The heterogeneity between included studies which were evaluated by using I2-based Q-test: if p-value was higher than 0.1 or I2 was lower than 50%, fixed effect model was used to pool the HR and its 95%CI. If not, the random effect model was adopted. Subgroup analyses were performed according to ethnicity and tumor clinical stage. Funnel plots were used to measure the bias of potential publication.
All of the 322 researches were screened, among them, 12 studies (12–23) involving 35346 PC patients were eligible and were included in our meta-analysis. The process of selecting studies is shown in Figure 1.
The baseline of included studies and the characteristics of PC patients were presented in Table 1. The research types of included studies are cohort studies. Patients who were diagnosed with PC were at an advanced or metastatic stage. Both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) patients were included in our meta-analysis. All of these included patients accepted the surgery and metformin treatment. Each of the 12 included trails had calculated the result of a NOS score for each included study which was more than 8 and this presents high methodological quality for this meta-analysis.
HR and its 95%CI of overall survival were reported in all included studies. There is inter-study heterogeneity between included studies (I2= 71.3%, P = 0.000). The random-effect model was adopted to perform the meta-analysis, results of which showed that there is a significant difference in the overall survival (HR = 0.85, 95%CI: 0.77–0.94, P = 0.002) between PC patients treated with metformin who underwent pancreatectomy and PC patients treated without metformin who underwent pancreatectomy (Figure 2).
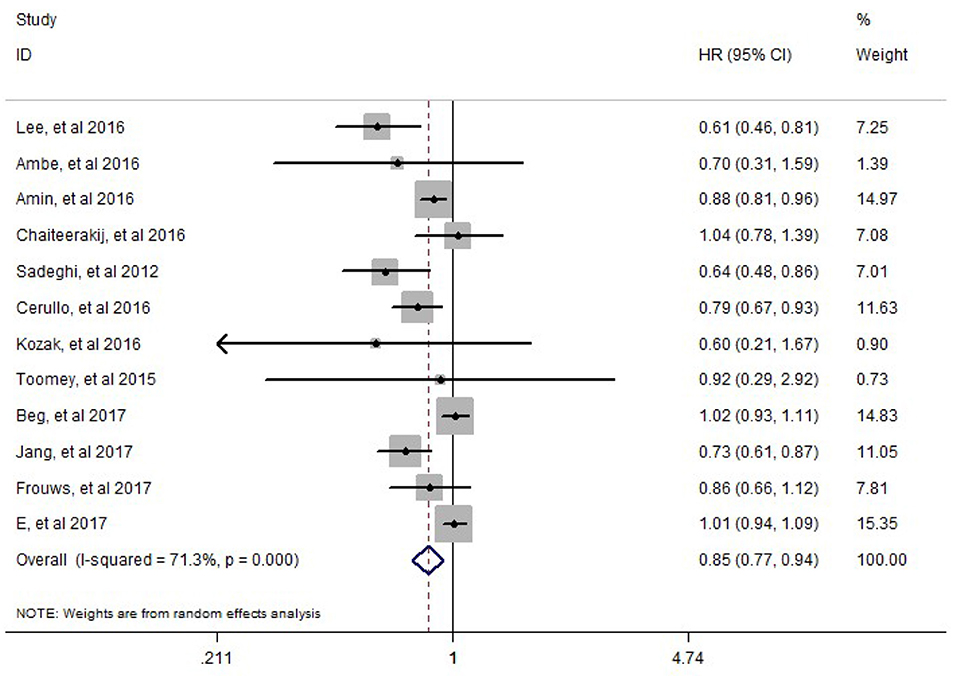
Figure 2. Meta-analysis results of the effect of metformin therapy on the overall survival of pancreatic cancer patients who underwent pancreatectomy.
Subgroup analyses showed Caucasians (HR = 0.903, 95% CI = 0.825–0.940, P = 0.008) and Asian (HR = 0.691, 95% CI = 0.588–0.813, P = 0.001) PC patients have a significantly reduced risk of death for metformin users. Subgroup analyses also showed a survival benefit for PC patients at stage I-II (HR = 0.762, 95% CI = 0.677–0.858, P = 0.0001).
By excluding any specific study, we found no substantial alteration among all included studies (Figure 3).
Currently, with the development of treatment strategies for pancreatic cancer including surgery, radiotherapy, chemotherapy, chemoradiotherapy, gene therapy and new target therapeutic, patients with PC were well-treated (24). However, many patients were diagnosed in an advanced or metastatic stage. Distal pancreatectomy, pancreaticoduodenectomy and total pancreatectomy were regarded as the curative surgical treatments for PC patients (25).
The result of this meta-analysis showed that there is a significant difference in overall survival (HR = 0.85, 95%CI: 0.77–0.94, P = 0.002) between PC patients treated with metformin who underwent pancreatectomy and PC patients treated without metformin who underwent pancreatectomy. Subgroup analyses showed Caucasians (HR = 0.903, 95% CI = 0.825–0.940, P = 0.008) and Asian (HR = 0.691, 95% CI = 0.588–0.813, P = 0.001) PC patients have a significantly reduced risk of death for metformin users. Subgroup analyses also showed a survival benefit for PC patients at stage I-II (HR = 0.762, 95% CI = 0.677–0.858, P = 0.0001). The previous meta-analyses (26) also showed the same survival benefit from metformin for PC patients. A meta-analysis with four studies involving 1,429 PC patients demonstrated that metformin use can improve the prognosis for PC patients (HR = 0.80, 95% CI = 0.62–1.03) (27). Moreover, a study also showed that metformin use can improve the outcomes of survival for cancer patients such as colorectal cancer, breast cancer, and ovarian cancer (28).
Limitations exist in this meta-analysis: (1) studies we included were all retrospective research, which may influence our meta-analysis. (2) the status of diabetes mellitus was not reported clearly in the included studies and a lack of relevant information about the use of metformin. (3) other important factors such as adverse events, tobacco use, cytotoxicity, which may result in the result of overall survival were not mentioned in included studies.
Metformin use is associated with survival benefit for PC patients who underwent pancreatectomy, which would be a potential drug for the treatment of PC.
The datasets are available on request. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
JZ, YL, and ZJ planed and designed the research. JZ, JM, and BY tested the feasibility of the study. JZ, JM, and LG wrote the manuscript. All authors approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Grant No. 81560480), Health Science Research Program of Gansu Province (No. GSWSKY2016-19), Ph.D. Science Research Foundation of Lanzhou University Second Hospital and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. (2017) 67:8–29. doi: 10.3322/caac.21387
2. Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. (2015) 15:8–18. doi: 10.1016/j.pan.2014.10.001
3. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. (2007) 297:267–77. doi: 10.1001/jama.297.3.267
4. Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer: a meta-analysis. JAMA. (1995) 273:1605–9. doi: 10.1001/jama.273.20.1605
5. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. (2008) 134:981–7. doi: 10.1053/j.gastro.2008.01.039
6. Li J, Cao G, Ma Q, Liu H, Li W, Han L. The bidirectional interation between pancreatic cancer and diabetes. World J Surg Oncol. (2012) 10:171. doi: 10.1186/1477-7819-10-171
7. Hart PA, Chari ST. Diabetes mellitus and pancreatic cancer: why the association matters? Pancreas. (2013) 42:1207–9. doi: 10.1097/MPA.0b013e3182a7c963
8. Zhang HH, Guo XL. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother Pharmacol. (2016) 78:13–26. doi: 10.1007/s00280-016-3037-3
9. Bhaw-Luximon A, Jhurry D. Metformin in pancreatic cancer treatment: from clinical trials through basic research to biomarker quantification. J Cancer Res Clin Oncol. (2016) 142:2159–71. doi: 10.1007/s00432-016-2178-4
10. de Souza A, Khawaja KI, Masud F, Saif MW. Metformin and pancreatic cancer: is there a role? Cancer Chemother Pharmacol. (2016) 77:235–42. doi: 10.1007/s00280-015-2948-8
11. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
12. Ambe CM, Mahipal A, Fulp J, Chen L, Malafa MP. Effect of metformin use on survival in resectable pancreatic cancer: a single-institution experience and review of the literature. PLoS ONE. (2016) 11:e0151632. doi: 10.1371/journal.pone.0151632
13. Lee SH, Yoon SH, Lee HS, Chung MJ, Park JY, Park SW, et al. Can metformin change the prognosis of pancreatic cancer? Retrospective study for pancreatic cancer patients with pre-existing diabetes mellitus type 2. Dig Liver Dis. (2016) 48:435–40. doi: 10.1016/j.dld.2015.12.006
14. Amin S, Mhango G, Lin J, Aronson A, Wisnivesky J, Boffetta P, et al. Metformin improves survival in patients with pancreatic ductal adenocarcinoma and pre-existing diabetes: a propensity score analysis. Am J Gastroenterol. (2016) 111:1350–7. doi: 10.1038/ajg.2016.288
15. Chaiteerakij R, Petersen GM, Bamlet WR, Chaffee KG, Zhen DB, Burch PA, et al. Metformin use and survival of patients with pancreatic cancer: a cautionary lesson. J Clin Oncol. (2016) 34:1898–904. doi: 10.1200/JCO.2015.63.3511
16. Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. (2012) 18:2905–12. doi: 10.1158/1078-0432.CCR-11-2994
17. Cerullo M, Gani F, Chen SY, Canner J, Pawlik TM. Metformin use is associated with improved survival in patients undergoing resection for pancreatic cancer. J Gastrointest Surg. (2016) 20:1572–80. doi: 10.1007/s11605-016-3173-4
18. Kozak MM, Anderson EM, von Eyben R, Pai JS, Poultsides GA, Visser BC, et al. Statin and metformin use prolongs survival in patients with resectable pancreatic cancer. Pancreas. (2015) 45:64–70. doi: 10.1097/MPA.0000000000000470
19. Toomey P, Teta A, Patel K, Downs D, Luberice K, Ross S, et al. Sulfonylureas (not metformin) improve survival of patients with diabetes and resectable pancreatic adenocarcinoma. Int J Surg Oncol. (2017) 2:e15. doi: 10.1097/IJ9.0000000000000015
20. Beg MS, Gupta A, Sher D, Ali S, Khan S, Gao A, et al. Impact of concurrent medication use on pancreatic cancer survival—SEER-medicare analysis. Am J Clin Oncol. (2017) 41:766–71. doi: 10.1097/COC.0000000000000359
21. Jang WI, Kim MS, Kang SH, Jo AJ, Kim YJ, Tchoe HJ, et al. Association between metformin use and mortality in patients with type 2 diabetes mellitus and localized resectable pancreatic cancer: a nationwide population-based study in korea. Oncotarget. (2017) 8:9587–96. doi: 10.18632/oncotarget.14525
22. Frouws MA, Sibinga Mulder BG, Bastiaannet E, Zanders MM, van Herk-Sukel MP, de Leede EM, et al. No association between metformin use and survival in patients with pancreatic cancer. Medicine. (2017) 96:e6229. doi: 10.1097/MD.0000000000006229
23. E J-Y, Lu S-E, Lin Y, Graber JM, Rotter D, Zhang L, et al. Differential and joint effects of metformin and statins on overall survival of elderly patients with pancreatic adenocarcinoma: a large population-based study. Cancer Epidemiol Biomarkers Prev. (2017) 26:1225–32. doi: 10.1158/1055-9965.EPI-17-0227
24. Del CM, Rangelova E, Segersvärd R, Arnelo U. Are there still indications for total pancreatectomy? Updates Surg. (2016) 68:257–63. doi: 10.1007/s13304-016-0388-6
25. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. (2003) 26(Suppl. 1):S5–20. doi: 10.2337/diacare.26.2007.S5
26. Zhou DC, Gong H, Tan CQ, Luo JQ. Prognostic significance of anti-diabetic medications in pancreatic cancer: a meta-analysis. Oncotarget. (2017) 8:62349–57. doi: 10.18632/oncotarget.17728
27. Zhang JW, Sun Q. Metformin may improve the prognosis of patients with pancreatic cancer. Asian Pac J Cancer Prev. (2015) 16:3937–40. doi: 10.7314/APJCP.2015.16.9.3937
Keywords: pancreatic cancer, metformin, pancreatectomy, overall survival, meta-analysis
Citation: Zhang J, Ma J, Guo L, Yuan B, Jiao Z and Li Y (2020) Survival Benefit of Metformin Use for Pancreatic Cancer Patients Who Underwent Pancreatectomy: Results From a Meta-Analysis. Front. Med. 7:282. doi: 10.3389/fmed.2020.00282
Received: 22 February 2020; Accepted: 21 May 2020;
Published: 30 July 2020.
Edited by:
Enrique de-Madaria, Hospital General Universitario de Alicante, SpainReviewed by:
Alejandro Piscoya, Universidad San Ignacio de Loyola, PeruCopyright © 2020 Zhang, Ma, Guo, Yuan, Jiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuoyi Jiao, amlhb3p5QGx6dS5lZHUuY24=; Yumin Li, bGl5bUBsenUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.