- 1Center for Clinical Epidemiology and Methodology (CCEM), Guangdong Second Provincial General Hospital, Guangzhou, China
- 2Department of Health Research Methods, Evidence, and Impact (HEI), McMaster University, Hamilton, ON, Canada
- 3Biostatistics Unit, St. Joseph Healthcare-Hamilton, Hamilton, ON, Canada
Background: There is no direct comparison from clinical trials amongst the direct oral anticoagulants (DOACs) in patients with acute venous thromboembolism (VTE), leaving an evidence gap in decision-making regarding the choice of a DOAC.
Methods: We performed a systematic review for an indirect comparison from randomized controlled trials (RCTs) for comparative effects amongst DOACs in the patients with acute VTE.
Results: A total of 16 RCTs were included for analyses, among which three for dabigatran (n = 7,963 patients), six rivaroxaban (n = 17,935), five apixaban (n = 12,823), and two edoxaban (n = 9,286). There was no significant difference in risk of recurrent VTE (evidence quality: low) and major bleeding (evidence quality: very low) for treatment effects between the four DOACs. Albeit non-significantly, apixaban seemed to have a lowest risk of major bleeding while rivaroxaban had a smallest risk of VTE. Although in general all the included trials were comparable, data from the included trials indicated that the assumption of transitivity may be challenged. Further methodological research including simulation studies, using a net-benefit or benefit-harm approach, running ranking probability analysis, and developing decision aids with machine-learning may be a worthwhile endeavor to help with the choice of DOACs in patients with acute VTE.
Conclusions: To conclude, based on results from the indirect comparison no significant difference in the efficacy and safety was found among the DOACs in patients with acute VTE. More evidence from direct comparative trials is needed to further inform the choice of DOACs in patients with acute VTE.
Introduction
Venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is the third most common cause of vascular death following myocardial infarction and stroke (1). To treat patients with acute VTE, current guidelines recommend using a parenteral anticoagulant for at least 5 days and subsequently an oral vitamin K antagonist (VKA) for at least 3 months (2, 3). Nevertheless, concerns are raised about the use of parenteral anticoagulant due to its inconvenient administrations and the use of VKA because of its close laboratory monitoring.
Direct oral anticoagulants (DOACs), including the direct thrombin inhibitor (dabigatran) and factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban), have been recommended to treat acute VTE in the current guidelines, given their promising benefit-harm profiles, more predictable pharmacodynamics effects, less food and drug interactions, and less need for coagulation monitoring (4, 5). However, there is no direct comparison from clinical trials amongst the four DOACs, leaving an evidence gap in decision-making regarding the choice of a DOAC. In this study, we aimed to conduct a systematic review to perform an indirect comparison from randomized controlled trials (RCTs) for comparative effects amongst DOACs in the patients with acute VTE. We also appraised advantages and disadvantages of the indirect comparison technique, and provided methodological insights into the potential clinical implications based on the findings.
Methods
We conducted this systematic review based on guidance from the Cochrane Handbook of Systematic Reviews and reported results according to PRISMA (the Preferred Reporting Items for Systematic Reviews and Meta-analyses) (6, 7).
Search Strategy and Study Selection
We searched MEDLINE, CINAHL, CENTRAL, and EMBASE up to November 30th, 2019 to retrieve eligible RCTs. We used descriptors including synonyms for trials, DOACs, and VTE in various combinations for the search process (Supplemental Table 1 shows the terms used in the search). The website www.clinicaltrials.gov was also searched for potential unpublished and ongoing studies (up to December 8th, 2019).
RCTs assessing efficacy and safety of DOACs vs. LMWH (low molecular weight heparin), UFH (unfractionated heparin), or VKA for treatment of acute VTE in adults were eligible for inclusion, where an acute VTE referred to a period within the first six months from the index VTE that had an objectively confirmed diagnosis (3). If patients in a same trial were reported in multiple publications or at different time points, only the study with the largest sample size and longest follow-up was included. We excluded those trials that compared DOACs vs. heparin or VKA to treat acute VTE but did not focus on the efficacy or safety profiles. We also excluded those studies without sufficient information for data extraction. Ongoing studies were included if the authors contacted by us could provide detailed information for our analyses.
Outcomes
Primary outcomes included the efficacy outcome (recurrent symptomatic VTE including new episode of DVT or PE), and the safety outcome (composite of major bleeding). Secondary outcomes were clinically relevant non-major bleeding (CRNB) and all-cause death.
Data Extraction
Two reviewers (GL and JZ) screened and chose studies for inclusion independently. We used the kappa statistics to quantify the agreement between the two reviewers (8, 9). A third reviewer (LT) was resorted if disagreement between the two reviewers could not be resolved by discussion. Two reviewers (GL and JZ) independently extracted data from the included RCTs on patient characteristics, intervention details, control groups, outcome measurement, study time, and study design information including sequence generation, allocation, blinding method, and type of trial design (parallel, cross-over, or factorial).
We used the Risk of Bias evaluation tool from Cochrane Collaboration to assess the quality of individual included RCTs (6). The Risk of Bias tool included sequence generation, allocation concealment, blinding, outcome data completeness, outcome reporting, and other issues.
Statistical Analyses
Due to no head-to-head comparisons from RCTs amongst DOACs, we performed an adjusted indirect comparison using a common comparator based on the Bucher technique (10, 11). We calculated the pooled hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) for comparative efficacy and safety amongst DOACs. We used the highest dose of the DOACs for comparisons if more than one dose was reported in the included RCTs. If more than one trial investigating a same DOAC, we first ran a random-effects model to pool the trials before performing the indirect comparison.
Two predefined subgroup analyses were conducted by type of VTE (DVT and PE) and type of controls (VKA, UFH, and LMWH). If a conventional meta-analysis was performed before the indirect comparison, we carried out three pre-specified sensitivity analyses (1) by using the fixed-effects model, (2) by excluding the high-risk-of-bias trials from the meta-analysis, and (3) by excluding the trials in which the percentages of patients with cancer were over 10%.
Assessment of Transitivity
We assessed the transitivity of the included trials by comparing their patients, interventions, controls, and outcomes (12). We compared the distributions of potential effect modifiers across the different comparisons including age, gender, comorbidity, dosage, time in therapeutic range (TTR), setting, outcome definition and adjudication, and study time duration (13).
Assessment of Certainty of Evidence
We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to rate the certainty in evidence for primary outcomes (14, 15). We first rated the certainty of evidence in direct comparison (DOACs vs. non-DOACs) according to risk of bias, directness of evidence, impression, inconsistency, and publication bias. Indirect comparison was subsequently rated based on results from rating of direct comparison and assessment of transitivity in the included trials (16).
Results
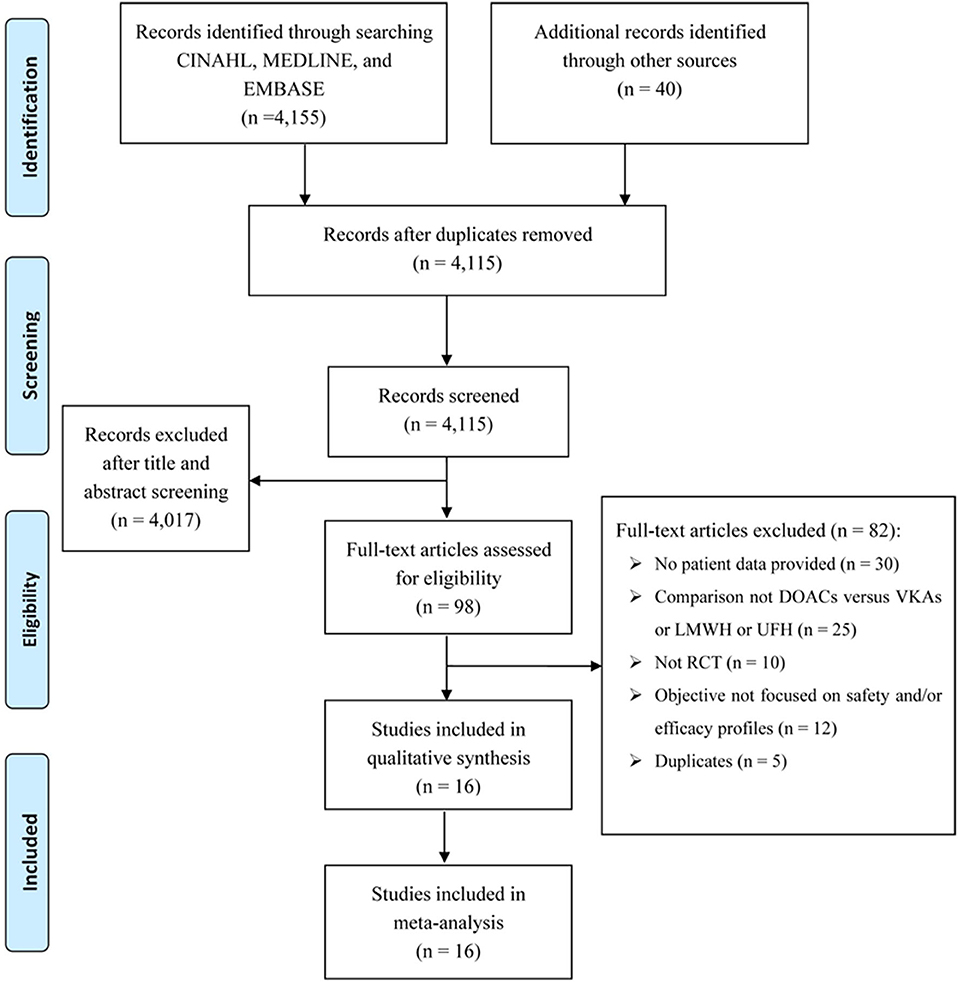
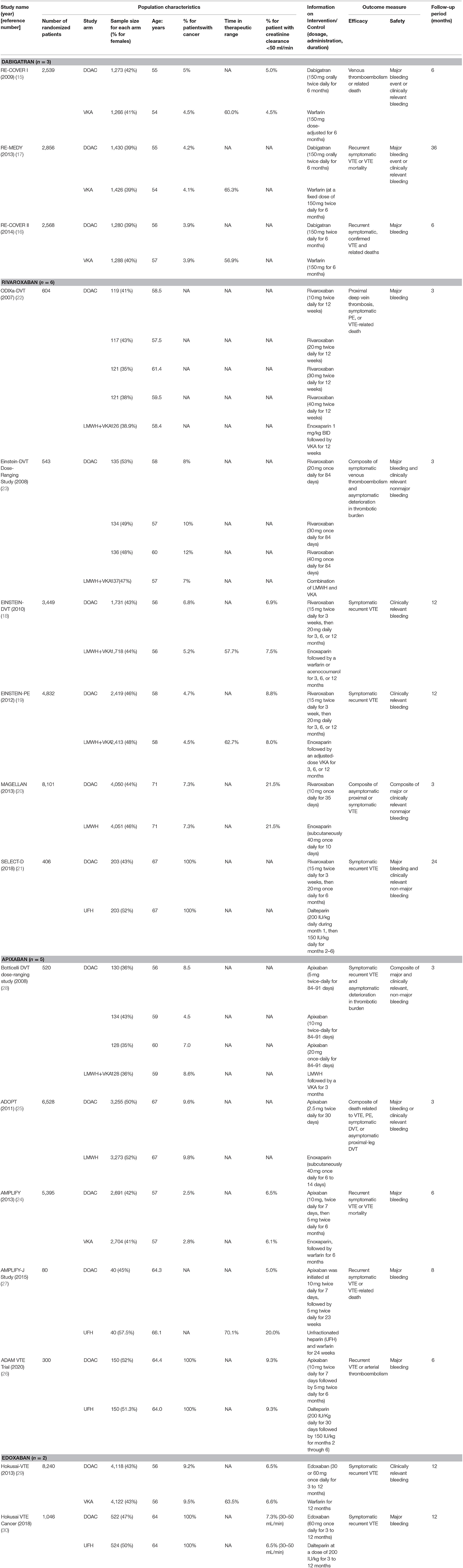
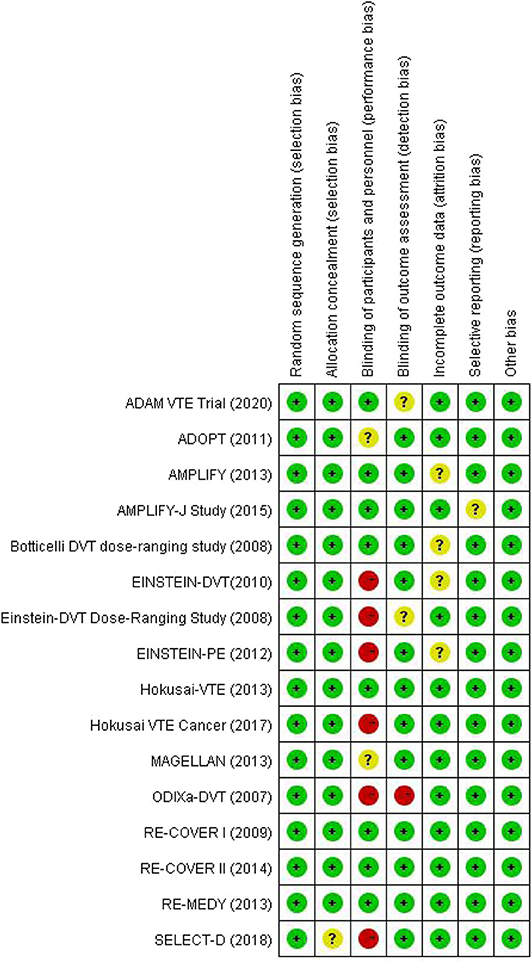
There were 4,155 records identified for screening. A total of 16 studies were included for analyses, among which three for dabigatran (17–19), six rivaroxaban (20–25), five apixaban (26–30), and two edoxaban (31, 32) (Figure 1 shows the flow diagram for study selection). The numbers of participants were 7,963 (40% for females) in RCTs assessing dabigatran, 17,935 (44% females) for rivaroxaban, 12,823 (46% females) for apixaban, and 9,286 (44% females) for edoxaban, respectively. Table 1 displays the characteristics of participants in the included trials. The average age ranged from 54 to 71 years. Three trials included patients with cancer exclusively; i.e., the percentages of patients with cancer were 100% in SELECT-D (23), ADAM VTE (28), and Hokusai VTE Cancer (32). The percentages of patients with creatinine clearance < 50 ml/min ranged from 5 to 22%. Results of the quality assessment for individual trials are presented in Figure 2. Six trials were rated high risk of bias regarding the domain of blinding.
We first performed random-effects pooled analyses for the effects of different DOACs compared with their controls (Supplemental Figure 1 presents findings from the individual meta-analyses). Results are shown in Figure 3 regarding the effects of DOACs compared with non-DOACs on VTE, major bleeding, CRNB, and death. When compared with non-DOACs, a smallest HR for risk of VTE was observed in the pooled analysis for rivaroxaban (HR = 0.75, 95% CI: 0.54–1.04), while a smallest HR for risk of major bleeding was found for dabigatran (HR = 0.67, 95% CI: 0.47–0.96). Certainty of evidence was rated as moderate for VTE of all the comparisons due to risk of bias in the included trials. Regarding major bleeding, certainty of evidence was moderate for dabigatran because of risk of bias in included trials, while it was rated as low for rivaroxaban, apixaban, and edoxaban due to risk of bias and inconsistency of results. Dabigatran was related with the lowest risk of CRNB, and rivaroxaban seemed to have a smallest risk of death among the four comparisons between DOACs and non-DOACs.
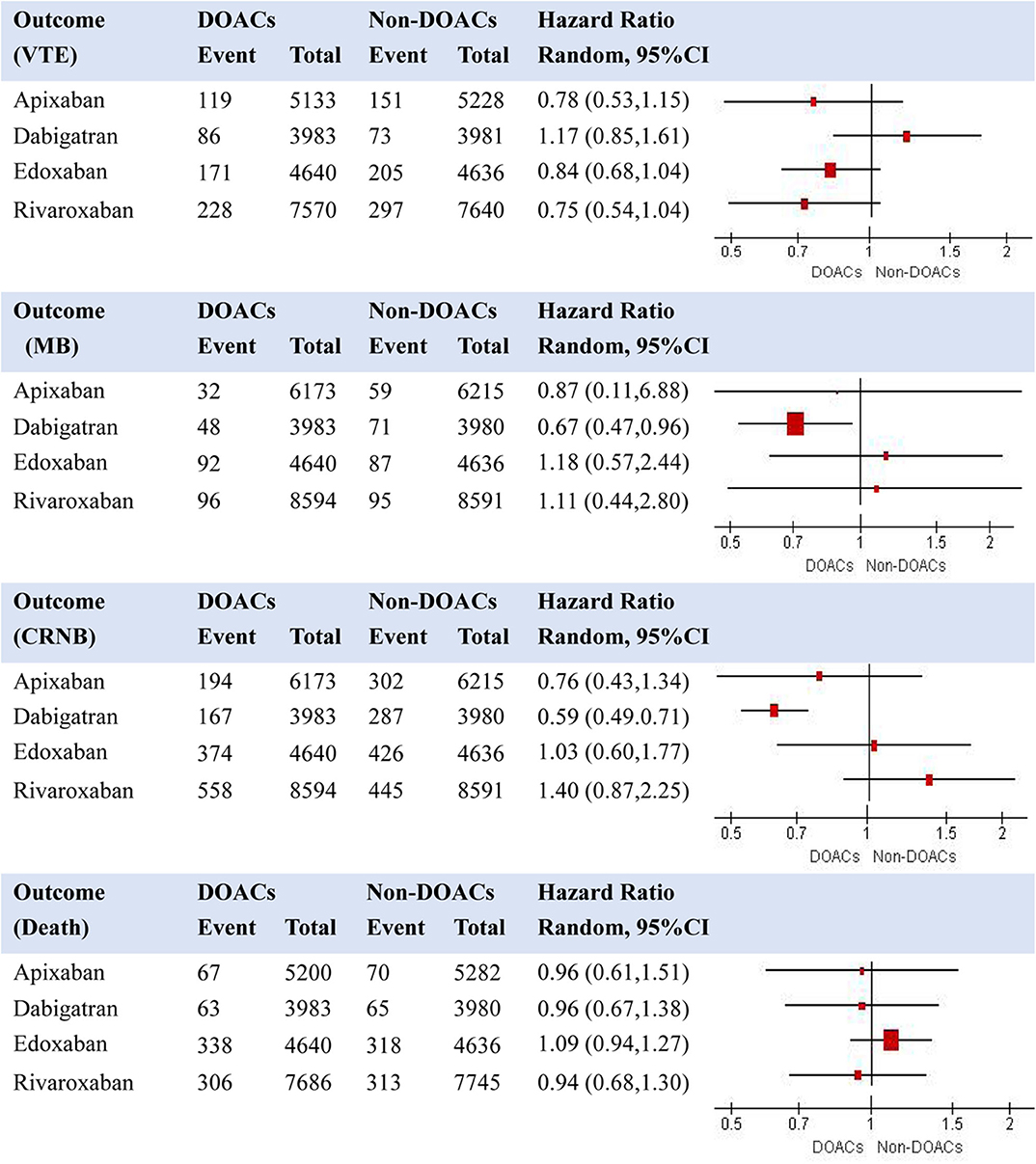
Figure 3. Effects of individual DOACs compared with non-DOACs on risk of recurrent VTE, MB, CRNB, and death (note: VTE, venous thromboembolism; MB, major bleeding; CRNB, clinically relevant non-major bleeding).
Table 2 displays the summarized characteristics of trials included in indirect comparisons for assessment of transitivity. In general, all the included trials were comparable. The average ages, sex composition, and percentages of patients with renal dysfunction were highly similar among the RCTs. Their outcome definitions and evaluations kept consistent with each other. When comparison groups were VKA or LMWH plus VKA, the time in therapeutic range in the control arms ranged from 60 to 70%. The treatment administration and duration varied among the four DOACs. Different follow-up periods were also observed for the included RCTs.
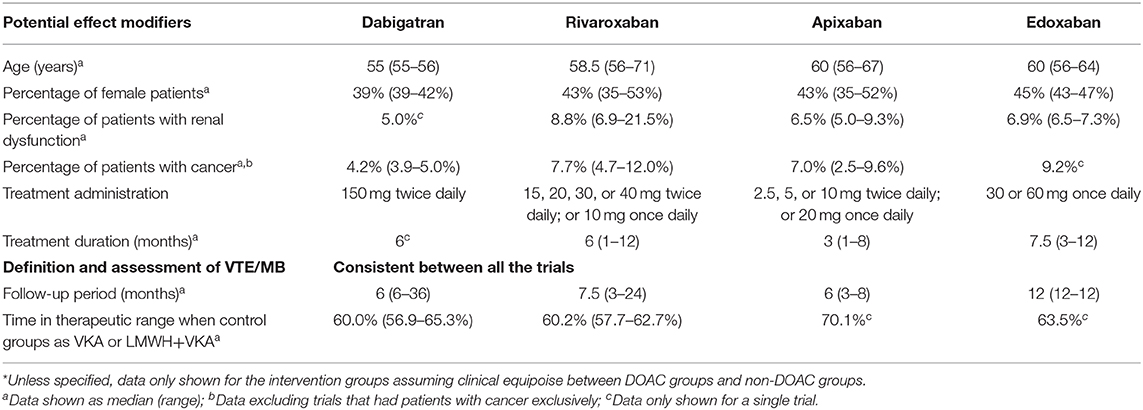
Table 2. Summarized characteristics of trials included in indirect comparisons for assessment of transitivity*.
Figures 4, 5 present results for the indirect comparison. Figure 4 shows findings from the pairwise comparisons for primary outcomes, where all the results were not statistically significant. Regarding risk of recurrent VTE, rivaroxaban seemed to be the most effective among the four DOACs, followed by apixaban, edoxaban, and dabigatran. Certainty of evidence for VTE was rated as low in all the pairwise indirect comparisons due to concerns for intransitivity and the moderate certainty of evidence in direct comparison. For the safety effect, apixaban was non-significantly related to the lowest risk of major bleeding, followed by dabigatran, rivaroxaban, and edoxaban. All the quality of evidence for major bleeding was rated as very low. Figure 5 displays results for secondary outcomes. Apixaban tended to be associated with the smallest risk of CRNB, while rivaroxaban had the highest CRNB risk. Only the dabigatran-rivaroxaban comparison yielded a significant result for risk of CRNB (HR = 0.42, 95% CI: 0.25–0.70, rivaroxaban taken as reference). As regards death, no significant difference in treatment effect was found among the four DOACs.
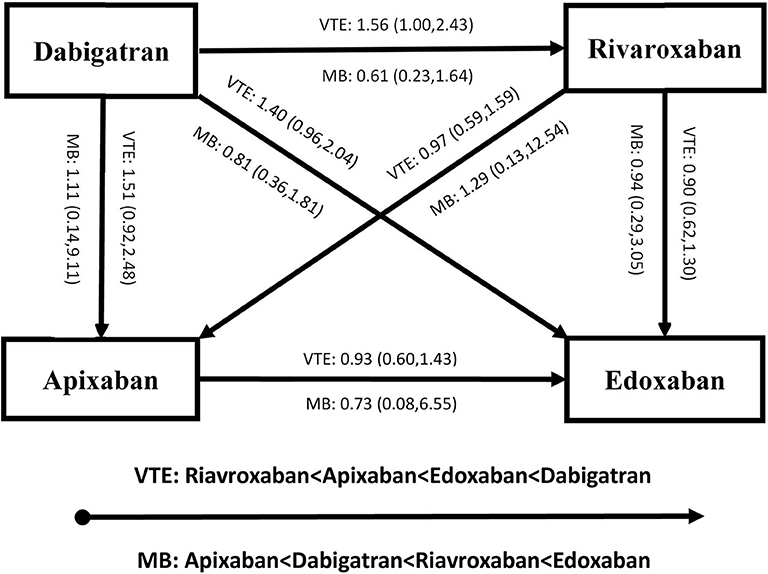
Figure 4. Results of indirect comparison for risk of recurrent VTE and MB (note: arrowhead taken as reference; VTE, venous thromboembolism; MB, major bleeding).
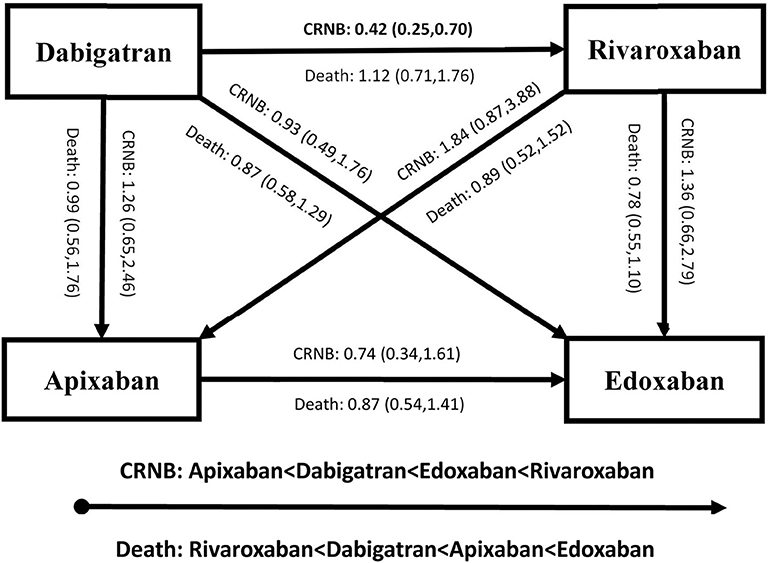
Figure 5. Results of indirect comparison for risk of CRNB and death (note: arrowhead taken as reference; CRNB, clinically relevant non-major bleeding).
Results from the sensitivity analyses were in general consistent with the main findings (Supplemental Table 2). Likewise, subgroup analyses yielded similar results between DVT and PE, and between different types of control groups, in which all the subgroup effect tests were not significant.
Discussion
In our study using indirect comparison technique, we found no significant difference in treatment effect among DOACs in patients with acute VTE. The quality of evidence was rated as low for VTE and very low for major bleeding in all the indirect comparison among DOACs. The use of a DOAC for acute VTE required more evidence to support decision-making in clinical practice, where head-to-head trials would be ultimately needed to confirm the choice of DOACs.
Current guidelines do not recommend a specific DOAC with a best benefit-harm profile, leaving an important gap in evidence-based decision aid. Cohort studies including retrospective and registry research, could provide direct comparative evidence among the DOACs. Nevertheless, their methodology is limited and challenging to inform an optimal DOAC choice (33). While the choice of a DOAC remained largely dependent on physician and/or patient preferences, expense reimbursement and medication availability, employing evidence from high-quality RCTs to perform an indirect comparison may provide some assistance to the DOAC prescription. In the literature, there had been some published studies based on an indirect comparison approach from RCTs. Two indirect comparison studies focusing on non-cancer patients with acute VTE included six RCTs and reported no difference in treatment effect of DOACs (34, 35). Another study comparing three DOACs (apixaban, rivaroxaban, and edoxaban) with dalteparin also found no significant difference among the DOACs for patients with cancer, based on data from three RCTs (36). Our findings were in line with these previous research. However, we found that dabigatran seemed to have a highest risk of recurrent VTE, but a second lowest risk of major bleeding. Alert being difficult to interpret this phenomenon, part of the reasons may be the different pharmacological effect between dabigatran (thrombin inhibitor) and others (factor Xa inhibitors). Different patient characteristics, various treatment administration and study periods may also play a role in the different effect of dabigatran when compared with the factor Xa inhibitors.
Regarding the assessment of transitivity between the included trials, one of the major differences may exist in the treatment for the control groups in the lead-in phases. While some trials used a heparinoid initially before the random assignment, other trials assigned patients to DOAC or control group immediately after their diagnosis of acute VTE (Table 1). Another potential incomparability may be the blinding of study design between the included studies (Figure 2). However, it remained uncertain about whether and to what extent the lack of blinding or different types of blinding would make an impact on participant performance and outcome assessment. Likewise, little would be known about the influence of blinding on our results from the indirect comparison. Patients with cancer and acute VTE may respond differently from those without cancer when treating with a DOAC (37). Therefore, even though the percentages of patients with cancer were similar in the trials that did not enroll cancer-patients exclusively (Table 2), the subtle difference may yield an unmeasurable impact on the transitivity among the included trials. We ran a sensitivity analysis excluding those trials that had patients with cancer exclusively (SELECT-D, ADAM VTE, and Hokusai VTE Cancer), and observed similar results to main findings (Supplemental Table 2). Nevertheless, it remained difficult to fully assess the assumption of transitivity between the included RCTs in our indirect comparison analysis.
Our study has some limitations. An indirect comparison technique is prone to potential and unquantified bias that could be minimized in head-to-head comparative RCTs (11). The number of included trials was small to restrict our further analyses. While the transitivity assumption could not be firmly explored due to lack of formal tests available, data from the included RCTs indicated that the assumption may be challenged or violated, even though it had been argued that the challenging similarity among the included trials may reflect variations in populations in real-world practice (38). We included all trials investigating efficacy and safety of the DOACs in patients with acute VTE, aiming to provide a comprehensive picture for the comparative treatment effect of DOACs based on all the available data. While heterogeneity was introduced especially regarding populations and controls, subgroup and sensitivity analyses were conducted to mitigate this concern. Some trials did not provide information on HRs; we used the reported RRs (relative risks) for the pooled analyses even given the obvious difference between these two effect measures. A post-hoc sensitivity analysis was performed restricting the data of HRs for pooled analyses, yielding the results largely consistent with the main findings. Due to the limited availability of data, no more exploratory analyses could be conducted. For instance, no subgroup analyses by age, sex, history of VTE, treatment administration and duration, or follow-up period could be further performed. The evidence quality of all indirect comparison was low for VTE and very low for major bleeding, reflecting that the current evidence could not provide sufficient information to aid with the selection of a DOAC in clinical practice.
Our results could not provide evidence on favored effects for a specific DOAC; and results from indirect comparison should be interpreted with cautions. However, this study presented the current available evidence from RCTs and highlighted the need for head-to-head comparative trials. While data from the direct comparative trials could not be available in the near future, more methodological research including simulation studies, using a net-benefit or benefit-harm approach, running ranking probability analysis, and developing decision aids with machine-learning may be a worthwhile endeavor to help with the choice of DOACs in patients with acute VTE.
To conclude, no significant difference in the efficacy and safety was found among the DOACs in patients with acute VTE, based on results from the indirect comparison. More evidence from direct comparative trials is needed to further inform the choice of DOACs in patients with acute VTE in clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
GL and JZe: conceptualization. GL and JZe: data curation; GL, JZe, and JZh: formal analysis. GL: funding acquisition. GL and LT: methodology. GL: supervision. GL and LT: validation. GL and JZe: writing—original draft. JZh and LT: writing—review and editing. All authors: contributed to the article and approved the submitted version.
Funding
Research grants from the Science Foundation of Guangdong Second Provincial General Hospital (YY2018-002) and Medical Scientific Research Foundation of Guangdong Province of China (A2020453).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00280/full#supplementary-material
References
1. Di Nisio M, van Es N, Buller HR. Deep vein thrombosis and pulmonary embolism. Lancet. (2016) 388:3060–73. doi: 10.1016/S0140-6736(16)30514-1
2. Tran HA, Gibbs H, Merriman E, Curnow JL, Young L, Bennett A, et al. New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med J Aust. (2019) 210:227–35. doi: 10.5694/mja2.50004
3. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149:315–52. doi: 10.1016/j.chest.2015.11.026
4. Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. (2016) 41:206–32. doi: 10.1007/s11239-015-1310-7
5. Gomez-Outes A, Luisa Suarez-Gea M, Calvo-Rojas G, Lecumberri R, Rocha E, Pozo-Hernández C, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. (2012) 9:83–104. doi: 10.2174/1570163811209020083
6. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions 5.1.0. (2019). Available online at: http://handbook.cochrane.org (accessed October) [updated March 2011].
7. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
8. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. (2005) 37:360–3. Available online at: https://fammedarchives.blob.core.windows.net/imagesandpdfs/fmhub/fm2005/May/Anthony360.pdf
9. Peinemann F, Unverzagt S, Hadjinicolaou AV, Moldenhauer I. Immunotherapy for metastatic renal cell carcinoma: a systematic review. J Evid Based Med. (2019) 12:253–62. doi: 10.1111/jebm.12362
10. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. (1997) 50:683–91. doi: 10.1016/S0895-4356(97)00049-8
11. Edwards SJ, Clarke MJ, Wordsworth S, Borrill J. Indirect comparisons of treatments based on systematic reviews of randomised controlled trials. Int J Clin Pract. (2009) 63:841–54. doi: 10.1111/j.1742-1241.2009.02072.x
12. Caldwell DM. An overview of conducting systematic reviews with network meta-analysis. Syst Rev. (2014) 3:109. doi: 10.1186/2046-4053-3-109
13. Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. (2013) 11:159. doi: 10.1186/1741-7015-11-159
14. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. JCE. (2011). 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
15. Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med. (2013) 6:50–4. doi: 10.1111/jebm.12018
16. Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. (2014) 349:g5630. doi: 10.1136/bmj.g5630
17. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. (2009) 361:2342–52. doi: 10.1056/NEJMoa0906598
18. Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. (2014) 129:764–72. doi: 10.1161/CIRCULATIONAHA.113.004450
19. Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. (2013) 368:709–18. doi: 10.1056/NEJMoa1113697
20. Investigators E. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. (2010) 363:2499–510. doi: 10.1056/NEJMoa1007903
21. Investigators EP. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. (2012) 366:1287–97. doi: 10.1056/NEJMoa1113572
22. Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. (2013) 368:513–23. doi: 10.1056/NEJMoa1111096
23. Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. (2018) 36:2017–23. doi: 10.1200/JCO.2018.78.8034
24. Agnelli G, Gallus A, Goldhaber S, Haas S, Huisman M, Hull R, et al. ODIXa-DVT study investigators: treatment of proximal deep-vein thrombosis with the oral direct Factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (oral direct Factor Xa inhibitor BAY 59-7939 in patients with acute symptomatic deep-vein thrombosis) study. Circulation. (2007) 116:180–7. doi: 10.1161/CIRCULATIONAHA.106.668020
25. Buller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood. (2008) 112:2242–7. doi: 10.1182/blood-2008-05-160143
26. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. (2013) 369:799–808. doi: 10.1056/NEJMoa1302507
27. Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. (2011) 365:2167–77. doi: 10.1056/NEJMoa1110899
28. McBane RD 2nd, Wysokinski WE, Le-Rademacher JG, Zemla T, Ashrani A, Tafur A, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. (2020) 18:411–21. doi: 10.1111/jth.14662
29. Nakamura M, Nishikawa M, Komuro I, Kitajima I, Uetsuka Y, Yamagami T, et al. Apixaban for the treatment of Japanese subjects with acute venous thromboembolism (AMPLIFY-J Study). Circ J. (2015) 79:1230–6. doi: 10.1253/circj.CJ-15-0195
30. Botticelli investigators, Writing Committe, Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost. (2008) 6:1313–8. doi: 10.1111/j.1538-7836.2008.03054.x
31. Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. (2013) 369:1406–15. doi: 10.1056/NEJMoa1306638
32. Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. (2018) 378:615–24. doi: 10.1056/NEJMoa1711948
33. Tritschler T, Castellucci LA. It's time for head-to-head trials with direct oral anticoagulants. Thromb Res. (2019) 180:64–9. doi: 10.1016/j.thromres.2019.05.019
34. Wells GA, Kelly S, Elliott J, Carrier M, Hsieh S, Chen L, et al. Direct Oral Anticoagulants for the Treatment of Venous Thromboembolic Events: A Systematic Review and Network Meta-Analysis. Ottawa Heart Institute Scientif Report. (2016). Available online at: https://www.ottawaheart.ca/sites/default/files/uploads/documents/Researchers/gwells-doac-vte-scientific-report-2015-2016.pdf (Assessed Mar 15, 2020).
35. Hirschl M, Kundi M. New oral anticoagulants in the treatment of acute venous thromboembolism-a systematic review with indirect comparisons. Vasa. (2014) 43:353–64. doi: 10.1024/0301-1526/a000373
36. Fuentes HE, McBane II RD, Wysokinski WE, Tafur AJ, Loprinzi CL, Murad MH, et al. Direct oral factor Xa inhibitors for the treatment of acute cancer-associated venous thromboembolism: a systematic review and network meta-analysis. Mayo Clin Proc. (2019) 94:2444–54. doi: 10.1016/j.mayocp.2019.05.035
37. Bertoletti L, Ollier E, Duvillard C, Delavenne X, Beyens M-N, De Magalhaes E, et al. Direct oral anticoagulants: current indications and unmet needs in the treatment of venous thromboembolism. Pharmacol Res. (2017) 118:33–42. doi: 10.1016/j.phrs.2016.06.023
38. Cohen A, Hamilton M, Mitchell S, Phatak H, Liu X, Bird A, et al. Comparison of the novel oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in the initial and long-term treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PLoS One. (2015) 10:e0144856. doi: 10.1371/journal.pone.0144856
Keywords: venous thromboembolism, direct oral anticoagulant, major bleeding, comparative effect, efficacy, safety
Citation: Li G, Zeng J, Zhang J and Thabane L (2020) Comparative Effects Between Direct Oral Anticoagulants for Acute Venous Thromboembolism: Indirect Comparison From Randomized Controlled Trials. Front. Med. 7:280. doi: 10.3389/fmed.2020.00280
Received: 30 March 2020; Accepted: 21 May 2020;
Published: 19 June 2020.
Edited by:
Yonggang Zhang, Sichuan University, ChinaReviewed by:
Ai Zhao, Peking University, ChinaGao Yan Hui, Guangdong Pharmaceutical University, China
Copyright © 2020 Li, Zeng, Zhang and Thabane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guowei Li, bGlnMjhAbWNtYXN0ZXIuY2E=
†These authors have contributed equally to this work
 Guowei Li
Guowei Li Jie Zeng
Jie Zeng Junguo Zhang
Junguo Zhang Lehana Thabane
Lehana Thabane