
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 18 June 2020
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00272
 Na Guo1,2†
Na Guo1,2† Yongchang Chen1,3†
Yongchang Chen1,3† Bin Su1,2
Bin Su1,2 Xiaodong Yang1,2
Xiaodong Yang1,2 Qiuyue Zhang1,2
Qiuyue Zhang1,2 Ting Song1,2
Ting Song1,2 Hao Wu1,2
Hao Wu1,2 Cuie Liu1,3*
Cuie Liu1,3* Lifeng Liu1,2*
Lifeng Liu1,2* Tong Zhang1,2*
Tong Zhang1,2*HIV-1/Treponema pallidum (T. pallidum) coinfection has become a global challenge, and three monocyte subsets express varying levels of the chemokine receptors CCR2 and CX3CR1. We recently evaluated the association between monocyte subsets and regulatory T cells in HIV-infected individuals with syphilis. Currently, the dynamic changes of CCR2 and CX3CR1 on monocyte subsets during HIV-1 and syphilis coinfection have not been fully investigated. In this study, cell surface staining was used to explore CCR2 and CX3CR1 expression on three monocyte subsets during HIV-1/T. pallidum coinfection. We found that CCR2 densities on the classical monocyte subsets decreased in acute HIV-1 infected (AHI) patients, chronic HIV-1-infected individuals without antiviral therapy (ART) (CHI+ ART–), chronic HIV-1-infected individuals receiving ART (CHI+ART+), rapid plasma reagin-positive (RPR+) individuals, CHI+ ART– plus RPR+ (CHI+RPR+ ART–) individuals, and CHI+ART+ plus RPR+ (CHI+RPR+ART+) individuals. CX3CR1 density increased on the three monocyte subsets during HIV-1 and/or T. pallidum infection. CX3CR1 density on the intermediate and non-classical monocyte subsets in CHI+ ART– individuals was lower than that in CHI+ART+ individuals, and CX3CR1 density on the three monocyte subsets in CHI+ART+ individuals was higher than that in CHI+RPR+ART+ individuals. Our data provide new insight into the roles of CCR2 and CX3CR1 on three monocyte subsets in HIV-1 and T. pallidum pathogenesis.
HIV-1/Treponema pallidum (T. pallidum) coinfection has become a global challenge among men who have sex with men (MSM) (1). The prevalence of syphilis and HIV-1 coinfection among MSM was 28.7% in Istanbul, Turkey (2). A total of 12.5% of syphilis infected MSM were HIV-positive in 61 cities in China (3).
HIV-1 and T. pallidum act synergistically to accelerate transmission and disease progression (4, 5). T. pallidum recruits HIV-1 susceptible inflammatory cells, such as activated macrophages, to the infection site (6). Syphilis infection differentially regulates the phenotype and function of gammadelta T cells at different stages of HIV-1 diseases (7). The immunological response to syphilis differs during the course of HIV-1 disease progression (8). T. pallidum-specific antibody activity was reduced in HIV-infected patients with syphilis (9). Approximately 12% of serological treatment response failures occur in early and late syphilis infected patients with HIV-1 because of immunosuppression (10), and repeat syphilis infection is likely responsible for asymptomatic infection in HIV-infected patients (11). An increased titer RPR, delay or failure of titer decline, and clinical relapse have been described during the course of syphilis in HIV-1-infected patients (12).
Based on CD14 and CD16 expression, human monocytes have been subdivided into three subsets: classical monocyte subsets (CD14++CD16−), intermediate monocyte subsets (CD14++CD16+) and non-classical monocyte subsets (CD14+CD16++) (13). We recently found that the frequency of the intermediate monocyte subsets in acute HIV-1-infected individuals was significantly higher than that in healthy controls, and the frequency of the intermediate monocyte subsets was inversely correlated with CD4+ T cell counts during HIV-1 infection (14). We also found that the frequency of the classical monocyte subsets was higher in syphilis patients than that in HCs and in syphilis/HIV-1 coinfected patients (15).
The intermediate and non-classical monocyte subsets express increased levels of CX3CR1, while classical monocytes express increased levels of CCR2 (16). It was reported that CCR2+ monocytes promote colon fibrosis by inhibiting collagen degradation through tissue inhibitor of metalloproteinase (TIMP-1) production in inflammatory bowel disease (IBD) (17). CCR2 on CD14+CD16+ monocytes can act as a novel biomarker of HIV-1-associated neurocognitive disorders (HANDs) (18). The CCL2/CCR2 axis is linked to viral replication and immune activation during HIV-1 infection, and modulation of this axis may have an impact on HIV disease progression. CX3CR1 is the receptor of fractalkine, which is also known as CX3CL1. The fractalkine/CX3CR1 axis plays an important role in the pathogenesis of many diseases with imbalances of immune response. Interruption of the fractalkine/CX3CR1 axis has been shown to ameliorate murine colitis by regulating monocyte behaviors (19, 20). Until now, the alteration of CCR2 and CX3CR1 expression on three monocyte subsets at different stages of HIV-1 and T. pallidum infection has not been fully investigated.
In this study, we investigated the alterations of CCR2 and CX3CR1 on the three monocyte subsets during HIV-1/T. pallidum coinfection.
All subjects in the study provided written consent according to the Declaration of Helsinki. The study was approved by the Beijing Youan Hospital Research Ethics Committee. Seven groups were included in the study: Thirty-four acute HIV-1-infected (AHI, group 1) patients were randomly enrolled from the Beijing PRIMO Clinical Cohort, the cohort was from high-risk HIV-1-negative men who have sex with men (MSM), and the patients in the cohort were tested for HIV-1 antibodies every 3 months. On the basis of laboratory test results, AHI patients were in Fiebig stage III-V (21). Forty-nine male chronic HIV-1-infected individuals were randomly enrolled from the HIV/AIDS clinic of Beijing Youan Hospital, which consisted of 25 chronic HIV-1-infected individuals without ART (CHI+ ART–, group 2) and 24 patients receiving ART (CHI+ART+, group3). Seventeen RPR+ individuals were seropositive in both the TPPA and RPR tests within 1 year (group 4). Forty-six chronic HIV-1-infected individuals were diagnosed with early syphilis: 16 chronic HIV-1-infected individuals without ART patients with syphilis (CHI+RPR+ ART–, group 5) and 30 patients receiving ART with syphilis (CHI+RPR+ART+, group 6). Additionally, we enrolled 23 age-matched HIV-1-negative individuals from the MSM population with high-risk behaviors as healthy controls (HCs, group 7). The inclusion and exclusion criteria for each group were the same as previously described (15). All groups were matched for age. The characteristics of the subjects are presented in Table 1.
Cryopreserved peripheral blood mononuclear cells (PBMCs) were used, and cell viability was evaluated. Cryopreserved PBMCs were thawed in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA), washed with PBS containing 1% BSA, and then incubated at room temperature for 20 min with the cell viability fixable viability stain 510 (BD Biosciences, San Jose, CA, USA). The viability of the PBMCs in the study is above 90%, as is shown in Figure 1. Cell surface staining were performed as previously described (15). After stained with anti-CD14-FITC (eBioscience), anti-CD16-PE (eBioscience), anti-CX3CR1-PE-Cy7 (eBioscience Inc., San Diego, CA) and anti-CCR2-APC-Cy7 (Biolegend Inc., San Diego, CA), monocyte phenotypes were detected by flow cytometry using a BD FACSCanto™ II with Diva software (BD Biosciences, San Jose, CA, USA). Then, data were analyzed by using FlowJo 10.0.7 software (Tree Star Inc., Ashland, OR, USA).
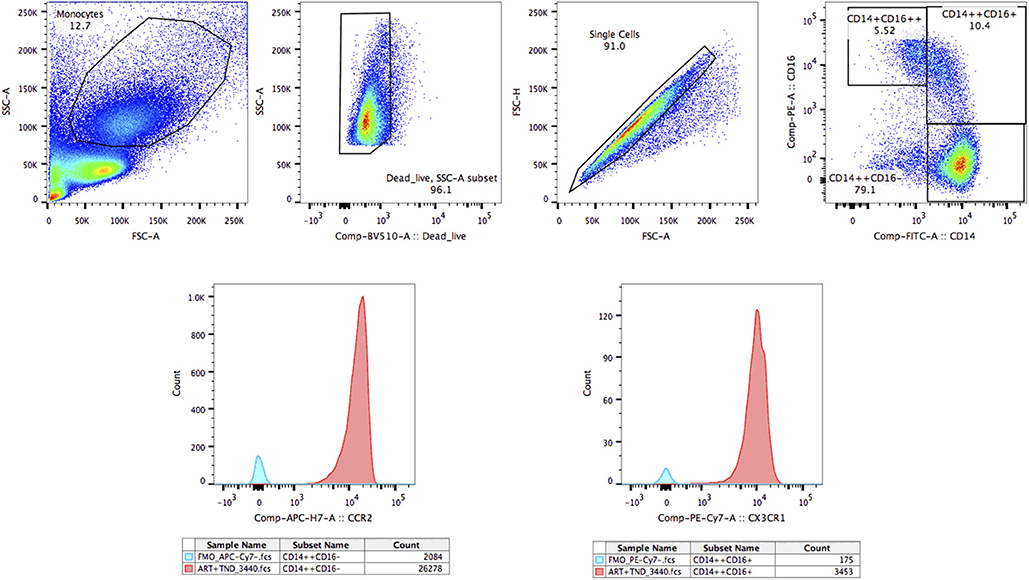
Figure 1. The gating strategy for CCR2 and CX3CR1 on three monocyte subsets. The gating strategy for CCR2 and CX3CR1 on classical monocyte subsets (CD14++CD16–), intermediate monocyte subsets (CD14++CD16+), and non-classical monocyte subsets (CD14+CD16++).
CD4+ T-cell counts were determined by three-color flow cytometry after stained with anti-CD3-APC, anti-CD4-FITC, and anti-CD8-PE monoclonal antibodies (BD Biosciences). The data were analyzed with a BD FACSCanto™ II Flow Cytometry System (BD Biosciences, San Jose, CA, USA). HIV-1 viral loads were measured with an automated real-time PCR-based m2000 System (Abbott Molecular Inc., Des Plaines, IL, USA).
Normal distribution and variance homogeneity were tested using one-way analysis of variance (ANOVA). If a significant effect was found, post hoc comparisons were conducted to evaluate the differences among groups. All reported p-values were two-tailed and were considered significant at p < 0.05. If the data were not normally distributed, non-parametric test was used for pairwise comparison, and a much lower pairwise type I error rate was used in the study. The difference was considered significant if the p-value was below 0.05/6 = 0.0083.
Statistical analysis was performed with GraphPad Prism 6.0 software (San Diego, CA, USA).
There were seven groups included in the study. The characteristics of the participants are listed in Table 1. The ages and sexes of the individuals in the seven groups were matched. The viral loads in HCs, CHI+ART+ individuals, and CHI+RPR+ART+ individuals were target not detected (TND). The mean CD4+ T cell counts in HCs were significantly higher than those in other groups except in RPR+ individuals. The mean CD4+ T cell counts in CHI+ART+ individuals were higher than those in AHI individuals and in CHI+ ART– individuals, the CD4+ T cell counts were higher in RPR+ individuals than those in CHI+RPR+ ART– individuals and CHI+RPR+ART+ individuals. Compared with those in CHI+RPR+ART+ individuals, the mean CD4+ T cell counts were lower in CHI+RPR+ ART– individuals.
The perturbations of three monocyte subsets during HIV and/or T. pallidum infection were characterized by our group (14, 15). The perturbations of CCR2 and CX3CR1-expressing monocyte subsets in the study were shown in Table 2, and the results were similar to our previous reports.
The frequency of the intermediate monocyte subsets in AHI individuals was significantly higher than that in healthy controls. The frequency of the classical monocyte subsets was higher in RPR+ individuals than in HCs and CHI+RPR+ patients. In addition, the frequency of the classical monocyte subsets was higher in RPR+ individuals and was lower in CHI+RPR+ ART– individuals than in CHI+RPR+ART+ individuals. The frequency of the intermediate monocyte subsets was higher in CHI+ART+ and CHI+RPR+ ART– individuals than in CHI+RPR+ART+ individuals. The frequency of the non-classical monocyte subsets in CHI+RPR+ART+ individuals was lower than that in HCs.
The gating strategy for CCR2 on monocyte subsets is shown in Figure 1.
The median fluorescence intensity (MFI) of CCR2 on the classical monocyte subsets (CD14++CD16−) in HCs was significantly higher than that in AHI, CHI+ ART– and CHI+ART+ individuals. In addition, the MFI of CCR2 decreased in the CHI+ ART– group compared to that in the AHI group (Figure 2A). The MFI of CCR2 density in HCs was higher than that in RPR+, CHI+RPRs+ ART– and CHIs+RPR+ART+ individuals (Figure 2D).
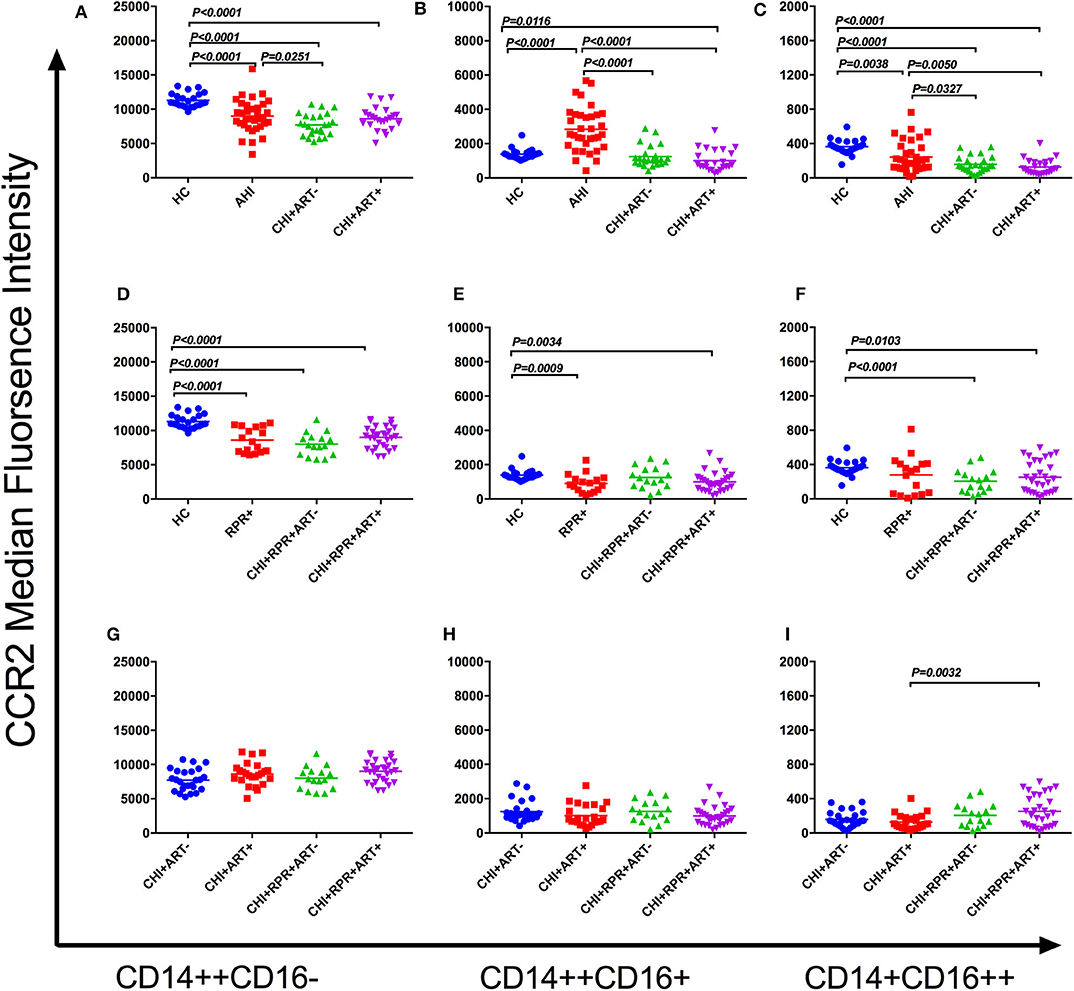
Figure 2. CCR2 perturbations on three monocyte subsets during HIV-1/Treponema pallidum coinfection. The median fluorescence intensity (MFI, density) of surface CCR2 on the classical monocyte subsets (CD14++CD16–) (A), intermediate monocyte subsets (CD14++CD16+) (B), and non-classical monocyte subsets (CD14+CD16++) (C) in HCs, AHI, CHI+ART–, and CHI+ART+ individuals. The MFI of surface CCR2 on the classical monocyte subsets (D), intermediate monocyte subsets (E), and non-classical monocyte subsets (F) in HCs, RPs+, CHI+RPR+ART–, and CHI+RPR+ART+ individuals. The MFI of surface CCR2 on the classical monocyte subsets (G), intermediate monocyte subsets (H), and non-classical monocyte subsets (I) in CHI+ ART–, CHI+ART+, CHI+RPR+ART–, CHI+RPR+ART+ individuals. The differences among groups were analyzed by one-way analysis of variance (data with normal distribution and variance homogeneity) or non-parametric test (data without normal distribution), and the differences were considered significant if p < 0.05 or p < 0.05/6 = 0.0083 (non-parametric test). The solid line indicates the mean or median value.
For CCR2 density on the intermediate monocyte subsets (CD14++CD16+), the MFI of CCR2 in AHI individuals was significantly higher than that in HC, CHI+ ART– and CHI+ART+ individuals, and the density of CCR2 in CHI+ART+ individuals was lower than that in HCs (Figure 2B). The MFI of CCR2 in RPR+ and CHI+RPR+ART+ individuals was lower than that in HCs (Figure 2E).
The MFI of CCR2 on the non-classical monocyte subsets (CD14+CD16++) in HCs was significantly higher than that in AHI, CHI+ ART–, CHI+ART+. In addition, the CCR2 density in AHI individuals was higher than that in CHI+ ART– and CHI+ART+ individuals (Figure 2C). The CCR2 density was higher in HCs than in CHI+RPR+ ART– individuals and CHI+RPR+ART+ individuals (Figure 2F). The CCR2 density in CHI+ART+ individuals was lower than that in CHI+RPR+ART+ individuals (Figure 2I).
The association among CCR2 expression on the three monocyte subsets and viral loads and CD4+ T cell counts was evaluated by Spearman's correlation test. CCR2 density on the intermediate monocyte subsets was inversely correlated with CD4+ T cell counts in the CHI+ ART– group (r = −0.4237, p = 0.0348).
The gating strategy for CX3CR1 on monocyte subsets is shown in Figure 1.
On the classical monocyte subsets, the density of CX3CR1 was significantly higher in AHI, CHI+ ART– and CHI+ART+ individuals than in HCs, and the density of CX3CR1 in AHI individuals was higher than that in CHI+ ART– individuals (Figure 3A). The density of CX3CR1 on the intermediate and non-classical monocyte subsets was significantly higher in AHI, CHI+ ART– and CHI+ART+ individuals than in HCs, and CX3CR1 density on these two subsets was higher in CHI+ART+ individuals than in CHI+ ART– individuals (Figures 3B,C). The CX3CR1 density on the non-classical monocyte subsets was higher in CHI+ART+ individuals than in AHI individuals (Figure 3C).
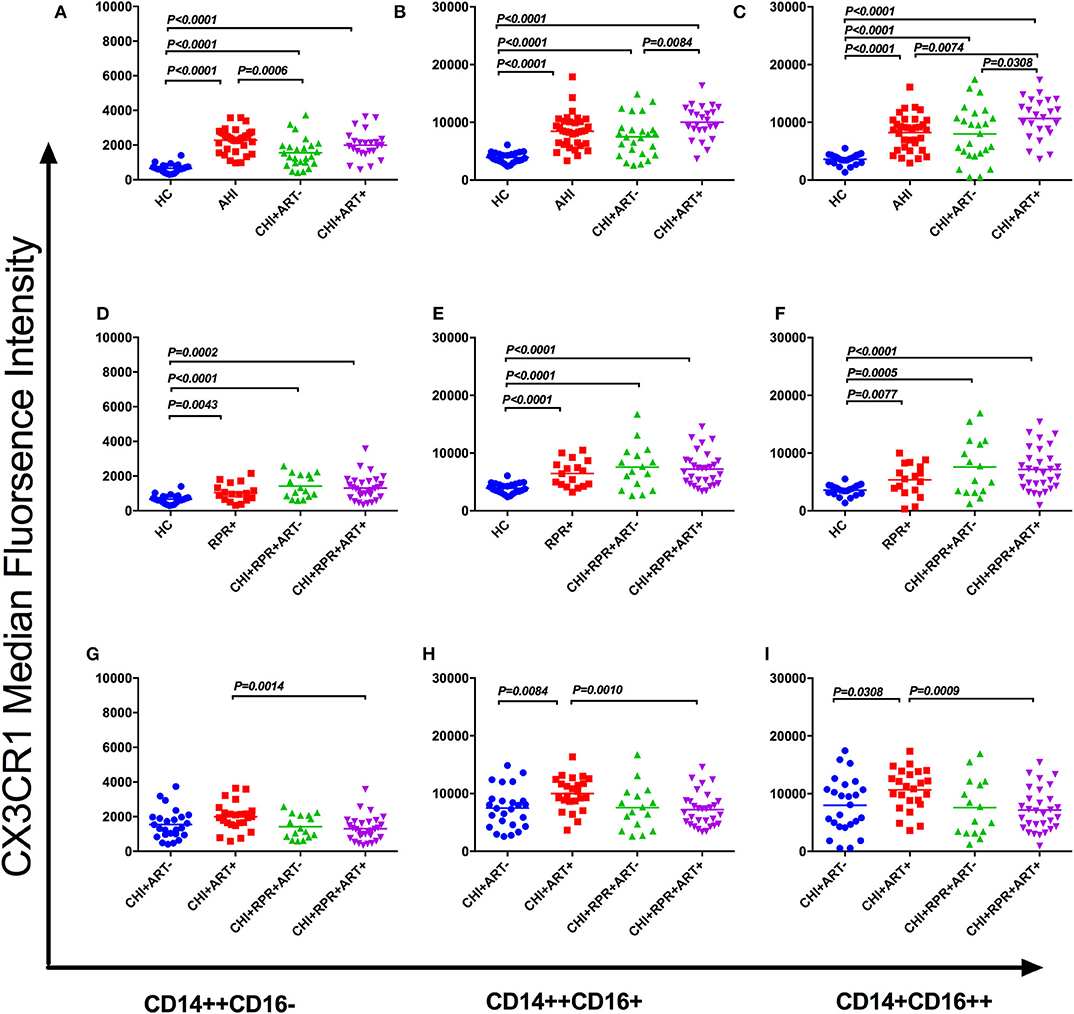
Figure 3. CX3CR1 perturbations on three monocyte subsets during HIV-1/Treponema pallidum coinfection. The median fluorescence intensity (MFI, density) of surface CX3CR1 on classical monocyte subsets (CD14++CD16–) (A), intermediate monocyte subsets (CD14++CD16+) (B), and nonclassical monocyte subsets (CD14+CD16++) (C) in HCs, AHIs CHI+ART–, and CHI+ART+ individuals. The MFI of surface CX3CR1 on classical monocyte subsets (D), intermediate monocyte subsets (E), and non-classical monocyte subsets (F) in HCs, RPR+, CHI+RPR+ART–, and CHI+RPR+ART+ individuals. The MFI of surface CX3CR1 on classical monocyte subsets (G), intermediate monocyte subsets (H), and non-classical monocyte subsets (I) in CHI+ ART–, CHI+ART+, CHI+RPR+ART–, CHI+RPR+ART+ individuals. The differences among groups were analyzed by one-way analysis of variance (data with normal distribution and variance homogeneity) or non-parametric test (data without normal distribution), and the differences were considered significant if p < 0.05 or p < 0.05/6 = 0.0083 (non-parametric test). The solid line indicates the mean or median value.
As shown in Figures 3D–F, the CX3CR1 density on the three monocyte subsets was significantly lower in HCs than in RPR+, CHI+RPR+ ART–, and CHI+RPR+ART+ individuals. According to Figures 3G–I, the density of CX3CR1 on three monocyte subsets was significantly lower in CHI+RPR+ART+ individuals than in CHI+ART+ individuals, and the density of CX3CR1 on the intermediate monocyte subsets and the non-classical monocyte subsets in CHI+ ART– individuals was lower than that in CHI+ART+ individuals (Figures 3H,I).
In this study, we evaluated the changes of CCR2 and CX3CR1 expression on three monocyte subsets at the different stages of HIV-1/T. pallidum coinfection. We found perturbations of CCR2 and CX3CR1 expression on monocyte subsets at the different stages of HIV-1/T. pallidum coinfection. We found that CCR2 was downregulated on the classical monocyte subsets but upregulated on the intermediate monocyte subsets during acute HIV-1 infection. The density of CCR2 in RPR+ individuals decreased on the classical and intermediate monocyte subsets, and the density of CCR2 in CHI+ART+, CHI+RPR+ART+ individuals on three monocyte subsets decreased. We also found that CX3CR1 was upregulated on the three monocyte subsets in all groups compared to that on the monocyte subsets in HCs. Compared to that in CHI+ ART– individuals, the density of CX3CR1 in CHI+ART+ individuals was higher on the intermediate and non-classical monocyte subsets, and the density of CX3CR1 in CHI+ART+ individuals was higher than the density of CX3CR1 in CHI+RPR+ART+ individuals on the three monocyte subsets.
The decreased densities of CCR2 expression on the classical monocyte subsets during HIV-1/T. pallidum coinfection, which reflects the impaired phagocytosis and chemotaxis of monocytes, may impact the death of infected progenitor cells in the bone marrow. The increased expression of CX3CR1 on monocytes implies systemic inflammation during HIV-1/T. pallidum coinfection.
In response to CCL2, CCR2-mediated monocyte recruitment is essential for defense against microbial pathogens (22). CX3CR1 on monocytes results in differentiation into resident tissue cells and is involved in tissue homeostasis (23).
Classical monocyte subsets are involved mostly in preventing pathogen invasion, the intermediate monocyte subsets are involved principally in antigen presentation and inflammatory responses, and the non-classical monocyte subsets are involved mostly in immune surveillance (24). In response to microbial stimuli, inflammatory monocytes secrete CCL2 and traffic to sites of microbial infection via CCR2-mediated emigration to defend against bacteria (25). It was reported that monocytes recruited via CCL2/CCR2, were related to immune activation and inflammation to propagate inflammation and tissue damage in osteoarthritis (OA) (26). Gama et al. demonstrated that CCR2 was downregulated on the classical monocyte subsets during acute HIV-1 infection (27), which was consistent with our findings. In our study, decreased CCR2 expression on the classical monocyte subsets was found in AHI, CHI+ ART– and CHI+ART+ individuals, which indicates impaired chemotaxis. Decreased CCR2 expression may be responsible for the death of infected progenitor cells in the bone marrow which results in a reduction in classical monocyte subsets migrating to the peripheral blood (4, 27) Compared with AHI individuals, there was more CCR2 deficiency in the CHI individuals, which may be responsible for impaired monocyte migration in CHI individuals (28). In this study, we found that CCR2 expression on the intermediate monocyte subsets was higher in AHI individuals than that in HCs, CHI+ ART– and CHI+ART+ individuals. Compared with that in HCs, lower CCR2 expression on monocytes has been found in elite controllers and individuals with suppressed viremia after ART (29).
Our group recently found that the frequency of the classical monocyte subsets increased during syphilis infection (15). However, in this study, the expression of CCR2 on the classical monocyte subsets decreased in RPR+ individuals. Lower proportions of CCR2-expressing cells may reflect increased systemic exposure to their ligand CCL2, causing impaired monocyte migration (28). The levels of CX3CR1 are higher on the non-classical monocyte subsets compared to intermediate monocyte subsets (30). CX3CR1 is required for cellular transendothelial migration and entry into atherogenic plaques, which is associated with cardiovascular disease (CVD) (31). In this study, we found increased expression of CX3CR1 on the three monocyte subsets in HIV-1-infected patients. The increased expression of CX3CR1 on monocytes has been associated with systemic inflammation during HIV-1 infection (4). Although combination antiretroviral therapy (ART) is effective at suppressing HIV viremia to undetectable levels in peripheral blood, HIV-associated inflammation and innate immune activation persists in HIV-1-infected patients with virologic suppression (32). Elite controllers (individuals with suppressed viremia without ART) express higher CX3CR1 levels on monocytes than HIV-negative controls (29). Compared with treated HIV-1 patients, HIV-1 patients with ART initiation have higher CX3CR1 expression on CD16+ monocytes (28). Circulating memory CD8+ T cells that express CX3CR1 are enriched in HIV-infected recipients after ART, CX3CR1+ CD8+ T cells could interact with coagulation elements, CX3CR1+ CD8+ T cells may be associated with CVD risk in HIV-infected ART recipients (33). High levels of CX3CR1 on the intermediate monocyte subsets and non-classical monocyte subsets were found in the study, which may be a risk factor for the development of CVD, perhaps because of the persistent innate immune activation regardless of ART.
In our study, we found increased expression of CX3CR1 on the three monocyte subsets in RPR+, CHI+RPR+ ART– and CHI+RPR+ART+ individuals. It is of interest that the expression of CX3CR1 on the three monocyte subsets was lower in CHI+RPR+ART+ individuals than that in CHI+ART+ individuals. The limited surface antigenicity of T. pallidum is poorly detected by the innate immune system, and may promote the evasion of adaptive immune responses (34).
In summary, we evaluated the alterations of CCR2 and CX3CR1 expression on three monocyte subsets during different stages of HIV-1/T. pallidum coinfection. Our findings provide new insight into the roles of CCR2 and CX3CR1 on three monocyte subsets in HIV-1 and T. pallidum pathogenesis.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Beijing Youan Hospital Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
LL, BS, and TZ conceived the study, designed the experiments, and analyzed the data. NG, YC, XY, QZ, and TS performed the experiments. HW, CL, and TZ contributed to reagents and materials. NG, YC, BS, and LL wrote the article. All authors read and approved the final manuscript.
This work was supported by the National 13th Five-Year Grand Program on Key Infectious Disease Control (2017ZX10202102-005-003 to BS, 2017ZX10202101-004-001 to TZ), the National Natural Science Foundation of China (NSFC, 81772165 and 81974303 to BS, 81571973 to HW), the Beijing Health System High-Level Technical Talents Training Fund (2014-3-085), the Beijing Municipal Administration of Hospitals Incubating Program (PZ2017013), the NSFC-NIH Biomedical collaborative research program (81761128001 to HW), the Beijing Municipal of Science and Technology Major Project (D161100000416003 to HW; Z161100000516148 to BS), and the Beijing Key Laboratory for HIV/AIDS Research (BZ0089).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Yunxia Ji, Xiangyun Guo, Guanxin Li, and Xiaoxue Tian for CD4+ T-cell counting and HIV viral load detecting, and the individuals participated in our study, for their interest and commitment to the project, which made this work possible.
1. Read P, Fairley CK, Chow EP. Increasing trends of syphilis among men who have sex with men in high income countries. Sex Health. (2015) 12:155–63. doi: 10.1071/SH14153
2. Koksal MO, Beka H, Evlice O, Ciftci S, Keskin F, Basaran S, et al. Syphilis seroprevalence among HIV-infected males in Istanbul, Turkey. Rev Argent Microbiol. (2020). doi: 10.1016/j.ram.2020.01.002. [Epub ahead of print].
3. Wu Z, Xu J, Liu E, Mao Y, Xiao Y, Sun X, et al. HIV and syphilis prevalence among men who have sex with men: a cross-sectional survey of 61 cities in China. Clin Infect Dis. (2013) 57:298–309. doi: 10.1093/cid/cit210
4. Pathela P, Braunstein SL, Blank S, Shepard C, Schillinger JA. The high risk of an HIV diagnosis following a diagnosis of syphilis: a population-level analysis of New York City men. Clin Infect Dis. (2015) 61:281–7. doi: 10.1093/cid/civ289
5. Solomon MM, Mayer KH, Glidden DV, Liu AY, McMahan VM, Guanira JV, et al. Syphilis predicts HIV incidence among men and transgender women who have sex with men in a preexposure prophylaxis trial. Clin Infect Dis. (2014) 59:1020–6. doi: 10.1093/cid/ciu450
6. Radolf JD, Deka RK, Anand A, Smajs D, Norgard MV, Yang XF. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nat Rev Microbiol. (2016) 14:744–59. doi: 10.1038/nrmicro.2016.141
7. Li Z, Lu X, Hu Z, Luo Z, Jiang W, Wu H, et al. Syphilis infection differentially regulates the phenotype and function of γδ T cells in HIV-1-infected patients depends on the HIV-1 disease stage. Front Immunol. (2017) 8:991. doi: 10.3389/fimmu.2017.00991
8. Kenyon C, Osbak KK, Crucitti T, Kestens L. The immunological response to syphilis differs by HIV status; a prospective observational cohort study. BMC Infect Dis. (2017) 17:111. doi: 10.1186/s12879-017-2201-7
9. Marra CM, Tantalo LC, Sahi SK, Dunaway SB, Lukehart SA. Reduced Treponema pallidum-specific opsonic antibody activity in HIV-infected patients with syphilis. J Infect Dis. (2016) 213:1348–54. doi: 10.1093/infdis/jiv591
10. Spagnuolo V, Poli A, Galli L, Nozza S, Bossolasco S, Cernuschi M, et al. Incidence and predictors of serological treatment response in early and late syphilis among people living with HIV. Open Forum Infect Dis. (2019) 6:ofy324. doi: 10.1093/ofid/ofy324
11. Kenyon C, Osbak KK, Apers L. Repeat syphilis is more likely to be asymptomatic in HIV-infected individuals: a retrospective cohort analysis with important implications for screening. Open Forum Infect Dis. (2018) 5:ofy096. doi: 10.1093/ofid/ofy096
12. Kassutto S, Doweiko JP. Syphilis in the HIV era. Emerg Infect Dis. (2004) 10:1471–3. doi: 10.3201/eid1008.031107
13. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. (2010) 116:e74–80. doi: 10.1182/blood-2010-02-258558
14. Chen P, Su B, Zhang T, Zhu X, Xia W, Fu Y, et al. Perturbations of monocyte subsets and their association with T helper cell differentiation in acute and chronic HIV-1-infected patients. Front Immunol. (2017) 8:272. doi: 10.3389/fimmu.2017.00272
15. Guo N, Liu L, Yang X, Song T, Li G, Li L, et al. Immunological changes in monocyte subsets and their association with Foxp3(+) regulatory T cells in HIV-1-infected individuals with syphilis: a brief research report. Front Immunol. (2019) 10:714. doi: 10.3389/fimmu.2019.00714
16. van de Veerdonk FL, Netea MG. Diversity: a hallmark of monocyte society. Immunity. (2010) 33:289–91. doi: 10.1016/j.immuni.2010.09.007
17. Kuroda N, Masuya M, Tawara I, Tsuboi J, Yoneda M, Nishikawa K, et al. Infiltrating CCR2(+) monocytes and their progenies, fibrocytes, contribute to colon fibrosis by inhibiting collagen degradation through the production of TIMP-1. Sci Rep. (2019) 9:8568. doi: 10.1038/s41598-019-45012-6
18. Veenstra M, Byrd DA, Inglese M, Buyukturkoglu K, Williams DW, Fleysher L, et al. CCR2 on peripheral blood CD14(+)CD16(+) monocytes correlates with neuronal damage, HIV-associated neurocognitive disorders, and peripheral HIV DNA: reseeding of CNS reservoirs?. J Neuroimmune Pharmacol. (2019) 14:120–33. doi: 10.1007/s11481-018-9792-7
19. D'Haese JG, Friess H, Ceyhan GO. Therapeutic potential of the chemokine-receptor duo fractalkine/CX3CR1: an update. Expert Opin Ther Targets. (2012) 16:613–8. doi: 10.1517/14728222.2012.682574
20. Kuboi Y, Nishimura M, Ikeda W, Nakatani T, Seki Y, Yamaura Y, et al. Blockade of the fractalkine-CX3CR1 axis ameliorates experimental colitis by dislodging venous crawling monocytes. Int Immunol. (2019) 31:287–302. doi: 10.1093/intimm/dxz006
21. Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. (2003) 17:1871–9. doi: 10.1097/00002030-200309050-00005
22. Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. (2008) 26:421–52. doi: 10.1146/annurev.immunol.26.021607.090326
23. Panek CA, Ramos MV, Mejias MP, Abrey-Recalde MJ, Fernandez-Brando RJ, Gori MS, et al. Differential expression of the fractalkine chemokine receptor (CX3CR1) in human monocytes during differentiation. Cell Mol Immunol. (2015) 12:669–80. doi: 10.1038/cmi.2014.116
24. Sprangers S, de Vries TJ, Everts V. Monocyte heterogeneity: consequences for monocyte-derived immune cells. J Immunol Res. (2016) 2016:1475435. doi: 10.1155/2016/1475435
25. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. (2003) 19:71–82. doi: 10.1016/S1074-7613(03)00174-2
26. Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. (2017) 76:914–22. doi: 10.1136/annrheumdis-2016-210426
27. Gama L, Shirk EN, Russell JN, Carvalho KI, Li M, Queen SE, et al. Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV infection. J Leukoc Biol. (2012) 91:803–16. doi: 10.1189/jlb.1111579
28. McCausland MR, Juchnowski SM, Zidar DA, Kuritzkes DR, Andrade A, Sieg SF, et al. Altered monocyte phenotype in HIV-1 infection tends to normalize with integrase-inhibitor-based antiretroviral therapy. PLoS ONE. (2015) 10:e0139474. doi: 10.1371/journal.pone.0139474
29. Krishnan S, Wilson EM, Sheikh V, Rupert A, Mendoza D, Yang J, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis. (2014) 209:931–9. doi: 10.1093/infdis/jit581
30. Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. (2003) 197:1701–7. doi: 10.1084/jem.20022156
31. Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. (2007) 117:185–94. doi: 10.1172/JCI28549
32. Jaworowski A, Hearps AC, Angelovich TA, Hoy JF. How monocytes contribute to increased risk of atherosclerosis in virologically-suppressed HIV-positive individuals receiving combination antiretroviral therapy. Front Immunol. (2019) 10:1378. doi: 10.3389/fimmu.2019.01378
33. Mudd JC, Panigrahi S, Kyi B, Moon SH, Manion MM, Younes SA, et al. Inflammatory function of CX3CR1+ CD8+ T cells in treated HIV infection is modulated by platelet interactions. J Infect Dis. (2016) 214:1808–16. doi: 10.1093/infdis/jiw463
Keywords: HIV-1, syphilis, monocyte subset, CCR2, CX3CR1
Citation: Guo N, Chen Y, Su B, Yang X, Zhang Q, Song T, Wu H, Liu C, Liu L and Zhang T (2020) Alterations of CCR2 and CX3CR1 on Three Monocyte Subsets During HIV-1/Treponema pallidum Coinfection. Front. Med. 7:272. doi: 10.3389/fmed.2020.00272
Received: 03 January 2020; Accepted: 15 May 2020;
Published: 18 June 2020.
Edited by:
Remco P. H. Peters, University of Pretoria, South AfricaReviewed by:
Thomas A. Angelovich, RMIT University, AustraliaCopyright © 2020 Guo, Chen, Su, Yang, Zhang, Song, Wu, Liu, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuie Liu, bGl1Y3VpZTY2NkBzaW5hLmNvbQ==; Lifeng Liu, bGl1bGY2OEBob3RtYWlsLmNvbQ==; Tong Zhang, enRfZG9jQGNjbXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.