
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 18 June 2020
Sec. Dermatology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00236
 Yehong Kuang1,2,3†
Yehong Kuang1,2,3† Yi Xiao1,2,3†
Yi Xiao1,2,3† Zhiqin Fang1,2,3†
Zhiqin Fang1,2,3† Yichi Zhang4
Yichi Zhang4 Minxue Shen1,2,3
Minxue Shen1,2,3 Xiang Chen1
Xiang Chen1 Mingliang Chen1
Mingliang Chen1 Chengzhi Lv5*
Chengzhi Lv5* Wu Zhu1,2,3*
Wu Zhu1,2,3*Background: Psoriasis is a chronic recurrent inflammatory disease involving many common mechanisms associated with obesity, such as systemic inflammation and vitamin D deficiency. This study aimed to examine the association of the serum concentration of 25-hydroxyvitamin D with psoriasis and the effect modification by obesity among the affected patients.
Methods: A mixed cross-section study was conducted. We consecutively included untreated psoriasis patients from the outpatients who visited the Department of Dermatology of Xiangya Hospital and recruited 205 gender-matched healthy controls from the Hunan Civil Servant Cohort. In both groups, we measured the serum 25-hydroxyvitamin D level, body mass index (BMI), waist-hip-ratio (WHR) and other psoriasis-related clinical indicators.
Results: A total of 203 psoriasis outpatients and 205 gender-matched cohort participants with complete data of serum vitamin D concentration were included in the analysis. The serum vitamin D levels of the two groups were close to each other, while the mean WHR of the psoriasis outpatients was significantly higher. Compared with the controls, the risk of psoriasis increased significantly when the vitamin D level decreased from 20 to 10 nmol/L. A significant interaction between the serum vitamin D level and the obesity category (BMI × WHR) was identified. After stratification by WHR, vitamin D was not associated with psoriasis in subjects with normal WHR. In contrast, the association between vitamin D deficiency and psoriasis retained and the effect size augmented in patients with central obesity.
Conclusions: WHR may modify the association between serum vitamin D and psoriasis. Treatment advocating Vitamin D supplements may tailor to psoriasis patients with metabolic disorders.
Psoriasis is not only a T-helper-1 (Th1)/Th17-mediated chronic inflammatory skin disease (1) but also a systemic disease involving higher risks of metabolic disorders such as obesity (2), and other metabolic-related diseases. Vitamin D plays a vital role in both bone health and non-skeletal diseases. In addition, vitamin D insufficiency has been reported in numerous major non-communicable diseases (3), such as diabetes (4), cardiovascular diseases (5), obesity (6), and other metabolic disorders. Meanwhile, there were sporadic hospital-based studies showing that the serum vitamin D concentration also decreased in psoriasis patients (7–13) based on relatively small samples.
The 25-Hydroxy vitamin D [25(OH)D] is currently considered the most accurate biomarker of the serum vitamin D level. It is derived from the daily dietary intake (14) and the cutaneous synthesis. A majority of researchers have agreed that 25(OH)D level <20 ng/mL can be used to define vitamin D insufficiency or even deficiency (15), which may implicate adverse health effects. Among numerous factors, the role of obesity in decreasing the serum 25(OH)D level (16) has been well-acknowledged (17). However, to our best knowledge, no study has been designed to evaluate the relationship between obesity and the serum concentration of 25(OH)D in patients with psoriasis. It is worth comparing the vitamin D status between patients with psoriasis and the control population stratified by obesity as measured by BMI and WHR. Thus, we conducted a case-control study to uncover the association of vitamin D with psoriasis and to examine the effect modification by obesity.
This is a case-control study targeting at a population comprised of psoriasis outpatients from the Department of Dermatology of Xiangya Hospital. Demographic information was collected after the recruitment of subjects.
This study was conducted according to the guidelines established in the Declaration of Helsinki. All patients-involved procedures had been approved by the institutional research ethics board of Xiangya Hospital and Xiangya School of Public Health, Central South University, Changsha, China (approval number: XYGW-2016-10). Informed consent had been obtained from all subjects before the investigation. All the recruited patients were diagnosed of psoriasis by an associate professor or above, and had not received any treatment. The main target range of age was 18–70 years. The control group was comprised of the participants from the Hunan Civil Servant Cohort. An even distribution of sex and age was ensured by stratified random sampling from the cohort participants.
Participants who had acute or chronic infection, digestive diseases, kidney diseases, metabolic and nutritional diseases, rheumatic diseases, endocrine diseases, or circulation system diseases, and who had undergone surgery or received medication such as vitamin supplements, blood donation or transfusion within the previous 6 months were excluded.
The serum 25(OH)D level was measured by radioimmunoassay (RIA) (18, 19). Briefly, 5 ml of serum sample was drawn from the whole blood sample obtained from the antecubital vein of the participant to be tested. The process was operated by a qualified phlebotomist in the morning under a specified centrifugal force (2,500 rpm for 10 min at room temperature). Within 2 h, the serum sample would be then collected and stored in a refrigerator at −80°C until further detection. A commercial 125I-25(OH)D RIA kit (DiaSorin, Stillwater, Minnesota, USA) was used to analyze the serum 25(OH)D level in the same laboratory.
Psoriasis outpatients were consecutively recruited from the dermatology clinic. Psoriasis in the cohort participants was diagnosed by certified dermatologists during field survey and the diagnosis results have been added in the final analysis. The age- and sex-matched healthy controls were randomly selected from the cohort participants.
Height, weight, waist circumference, and hip circumference were measured by a research nurse using standardized methods. BMI was calculated as weight (kg)/height2 (m2). WHR was calculated as waist circumferences (cm)/hip circumference (cm). BMI was categorized by the cut-off of 24 kg/m2 (20), and WHR was categorized by the cut-off of 0.85 for females and 0.9 for males (21).
Continuous data was presented as the mean ± standard deviation, and the between-group differences were tested using analysis of variance (ANOVA). Categorical data was presented as percentage (%), and the between-group differences were tested using the chi-square test. Generalized additive models were used to explore the association of vitamin D with psoriasis. Because U-shape associations were identified, quadratic regression models were further used for parametric estimation. Interactions between vitamin D and obesity indices were detected, and stratification analyses by obesity category were performed for any significant interactions. A P < 0.05 was considered statistically significant. Analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina, United States).
A total of 203 psoriasis outpatients and 205 gender-matched cohort participants with complete data of serum vitamin D concentration were included in our analysis. Two subjects with psoriasis identified in the cohort were also included (the prevalence of psoriasis among the cohort participants was 0.98%). The serum vitamin D levels of the two groups were close to each other, while the mean WHR of the psoriasis outpatients was significantly higher (Table 1).
The vitamin D level showed a non-linear U-shape association with psoriasis according to the generalized additive model (Figure 1A). The risk of psoriasis significantly decreased when the vitamin D level increased from 10 to 20 nmol/L, and then slightly increased after that. Because significant interactions between the vitamin D level and obesity category (BMI × WHR) were identified, stratification analyses were then performed. As shown in Figure 1B, vitamin D was not associated with psoriasis in subjects with normal WHR despite the BMI category. In contrast, the association exhibited a U-shape in those with central obesity (large WHR) despite the BMI category.
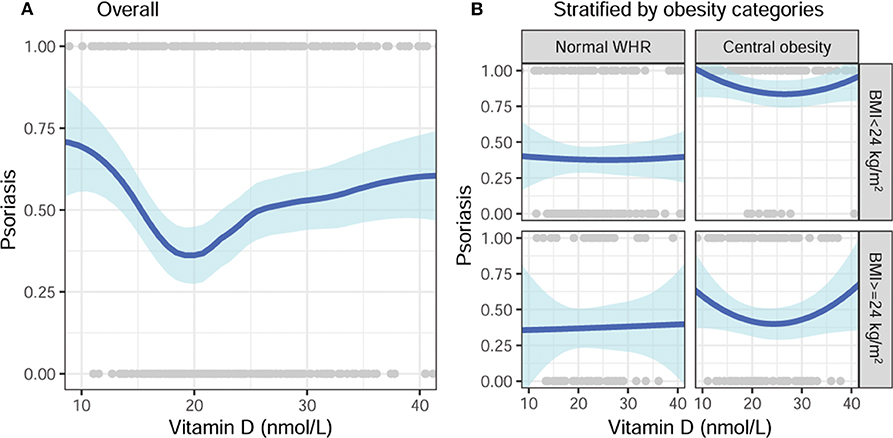
Figure 1. Unadjusted association of vitamin D with psoriasis, stratified by obesity categories. (A) Overall and (B) stratified by BMI and WHR categories. BMI, body mass index; WHR, waist-hip ratio.
After fitting data with quadratic regression, the parametric estimation of the effect size of vitamin D is shown in Table 2. With stratification by obesity category, vitamin D was not significantly associated with psoriasis in subjects with normal WHR. Consistently, the quadratic terms of vitamin D were marginally significant in subjects with central obesity, despite the BMI category. After adjustments for age and gender, the associations remained consistent, although some were no longer statistically significant (Figure 2A). In subjects with central obesity, the risk of psoriasis was the lowest when the serum 25(OH)D was around 25 nmol/L (Figure 2B).
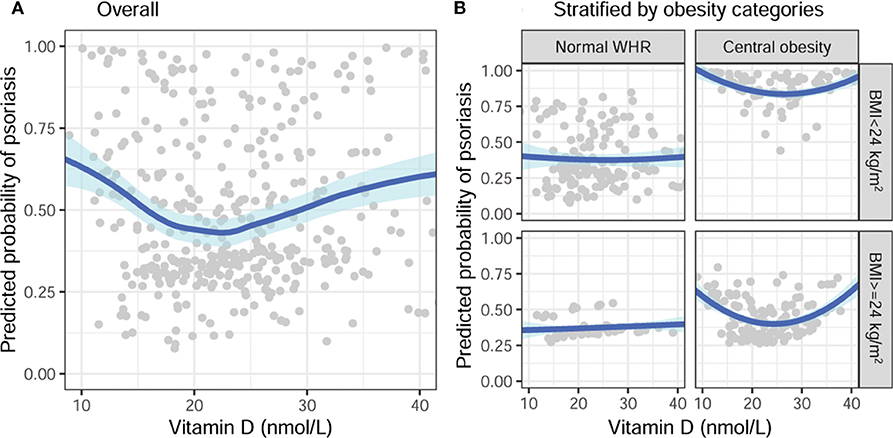
Figure 2. Adjusted association of vitamin D with a predicted probability of psoriasis, stratified by obesity categories, adjusted for age and gender. (A) Overall and (B) stratified by BMI and WHR categories. BMI, body mass index; WHR, waist-hip ratio.
Our study showed that the association between serum vitamin D and psoriasis might be modified by central obesity, indicated by WHR. Inconsistent with the findings in the majority of previous studies (7, 22), our results showed that there was no significant difference in serum vitamin D level between the psoriasis patients and healthy controls. However, a significant interaction between the serum vitamin D level and obesity was identified. This is the first time that the serum vitamin D level in Chinese psoriasis patients was reported. Our results also showed that, interestingly, serum 25(OH)D deficiency was associated with a higher risk of psoriasis only in the subgroup of abnormal WHR. This inconsistent finding suggested that the impact of decreased vitamin D on psoriasis pathogenesis might be related to central obesity, a well-acknowledged comorbidity of psoriasis.
Unfortunately, no previous study has explored the modification effects of WHR on the association between serum vitamin D and psoriasis. Nevertheless, vitamin D deficiency has been multi-dimensionally confirmed to correlate with obesity (6, 23–26), especially central obesity. The reported relationship between psoriasis and decreased serum Vitamin D may be mediated by the shared mechanism of the coexistence of psoriasis and central obesity (27).
Another explanation for our result lies in the specialty for the studied population. After a long time of poverty, the Chinese population has been increasingly experiencing a non-communicable tsunami, especially for central obesity (28) and diabetes (29), since the opening-and-reform-up from the 1980s. Within the past four decades, the number of psoriasis patients has been significantly increased (30), and the epidemic of psoriasis has shifted from a genetic-dominated to a comorbidity-driven epidemic in China. The climbing prevalence of central obesity among Chinese psoriasis patients might help us distinguish the relationship among psoriasis, central obesity, and serum vitamin D. Besides, the average serum vitamin D level in the healthy Chinese population has been reported to be lower than that in the western population (31, 32). A multi-center research in 2013 showed that the average serum vitamin D level (31) in the general Chinese population was around 25 nmol/L, lower than the reported average according to the western standard (33). The fact that vitamin D insufficiency in the Chinese population is too common to show any difference between the psoriasis patients without central obesity and the healthy controls might be another explanation for the inconsistent finding.
One limitation of this study is selection bias. The healthy controls were recruited from an ongoing cohort, and the participants who received vitamin D test were mostly the elderly people (generally >40 years). Another significant limitation of our study is that the participants were recruited across a 1-year period, which cannot erase the confounding effect from the seasonal change of serum vitamin D status owing to the difference in exposure to sunlight (34). However, all participants in our study resided in Hunan province, and the difference due to latitude can be ignored.
All datasets generated for this study are included in the article/supplementary material.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
YK and YX performed study design, data analysis, and manuscript writing. YZ, ZF, XC, MC, and MS contributed to data collection and validation. MC and XC performed clinical diagnosis and samples collection. CL and WZ: clinical experts and manuscript revision. All authors read and approved the final version of the manuscript.
This work was supported by the National Key R&D Project—Precision Medicine Program of China (2016YFC0900802), the National Natural Science Foundation of China (Grant Nos. 81974479, 81773329, and 81830096), Xiangya Hospital Management Research Fund of Central South University (2016GL03), and Dalian Science and Technology Innovation Fund Project (2019J13SN93).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to sincerely appreciate all coordinators, dermatologists, and investigators that participated in this study.
1. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. (2007) 370:263–71. doi: 10.1016/S0140-6736(07)61128-3
2. Kimball AB, Gladman D, Gelfand JM, Gordon K, Horn EJ, Korman NJ, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. (2008) 58:1031–42. doi: 10.1016/j.jaad.2008.01.006
3. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. (2011) 364:248–54. doi: 10.1056/NEJMcp1009570
4. Soderstrom LH, Johnson SP, Diaz VA, Mainous AG III. Association between vitamin D diabetic neuropathy in a nationally representative sample: results from 2001-2004 NHANES. Diabet Med. (2012). 29:50–5. doi: 10.1111/j.1464-5491.2011.03379.x
5. Playford MP, Dey AK, Zierold C, Joshi AA, Blocki F, Bonelli F, et al. Serum active 1,25(OH)(2)D, but not inactive 25(OH)D vitamin D levels are associated with cardiometabolic and cardiovascular disease risk in psoriasis. Atherosclerosis. (2019) 289:44–50. doi: 10.1016/j.atherosclerosis.2019.08.006
7. Gisondi P, Rossini M, Di Cesare A, Idolazzi L, Farina S, Beltrami G, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. (2012) 166:505–10. doi: 10.1111/j.1365-2133.2011.10699.x
8. Morimoto S, Yoshikawa K, Kozuka T, Kitano Y, Imanaka S, Fukuo K, et al. An open study of vitamin D3 treatment in psoriasis vulgaris. Br J Dermatol. (1986) 115:421–9. doi: 10.1111/j.1365-2133.1986.tb06236.x
9. Dayangac-Erden D, Karaduman A, Erdem-Yurter H. Polymorphisms of vitamin D receptor gene in Turkish familial psoriasis patients. Arch Dermatol Res. (2007) 299:487–91. doi: 10.1007/s00403-007-0782-5
10. Staberg B, Oxholm A, Klemp P, Christiansen C. Abnormal vitamin D metabolism in patients with psoriasis. Acta Derm Venereol. (1987) 67:65–8.
11. Solak B, Dikicier BS, Celik HD, Erdem T. Bone mineral density, 25-OH vitamin D and inflammation in patients with psoriasis. Photodermatol Photoimmunol Photomed. (2016) 32:153–60. doi: 10.1111/phpp.12239
12. Bergler-Czop B, Brzezinska-Wcislo L. Serum vitamin D level - the effect on the clinical course of psoriasis. Postepy Dermatol Alergol. (2016) 33:445–9. doi: 10.5114/ada.2016.63883
13. Hambly R, Kirby B. The relevance of serum vitamin D in psoriasis: a review. Archiv Dermatol Res. (2017) 309:499–517. doi: 10.1007/s00403-017-1751-2
14. Hall LM, Kimlin MG, Aronov PA, Hammock BD, Slusser JR, Woodhouse LR, et al. Vitamin D intake needed to maintain target serum 25-hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. J Nutr. (2010) 140:542–50. doi: 10.3945/jn.109.115253
15. Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, et al. Controversies in vitamin D: summary statement from an international conference. J Clin Endocrinol Metab. (2019) 104:234–40. doi: 10.1210/jc.2018-01414
16. Carlin AM, Rao DS, Meslemani AM, Genaw JA, Parikh NJ, Levy S, et al. Prevalence of vitamin D depletion among morbidly obese patients seeking gastric bypass surgery. Surg Obes Relat Dis. (2006) 2:98–103; discussion: 4. doi: 10.1016/j.soard.2005.12.001
17. Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. (2015) 16:341–9. doi: 10.1111/obr.12239
18. Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM, Vitamin DSP. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. (2012) 243:32–40. doi: 10.3109/00365513.2012.681935
19. Snellman G, Melhus H, Gedeborg R, Byberg L, Berglund L, Wernroth L, et al. Determining vitamin D status: a comparison between commercially available assays. PLoS ONE. (2010) 5:e11555. doi: 10.1371/journal.pone.0011555
20. Zhou BF, Cooperative Meta Anal Grp W. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pacific J. Clin. Nutr. (2002) 11:S685–S93. doi: 10.1046/j.1440-6047.11.s8.9.x
21. Ho RC, Niti M, Kua EH, Ng TP. Body mass index, waist circumference, waist-hip ratio and depressive symptoms in Chinese elderly: a population-based study. Int J Geriatr Psychiatry. (2008) 23:401–8. doi: 10.1002/gps.1893
22. Orgaz-Molina J, Buendia-Eisman A, Arrabal-Polo MA, Ruiz JC, Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. (2012) 67:931–8. doi: 10.1016/j.jaad.2012.01.040
23. Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am Coll Nutr. (2002) 21:146–51S. doi: 10.1080/07315724.2002.10719212
24. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. (2000) 72:690–3. doi: 10.1093/ajcn/72.3.690
25. Harel Z, Flanagan P, Forcier M, Harel D. Low vitamin D status among obese adolescents: prevalence and response to treatment. J Adolesc Health. (2011) 48:448–52. doi: 10.1016/j.jadohealth.2011.01.011
26. Moore CE, Liu Y. Low serum 25-hydroxyvitamin D concentrations are associated with total adiposity of children in the United States: National Health and Examination Survey 2005 to (2006). Nutr Res. (2016) 36:72–9. doi: 10.1016/j.nutres.2015.11.003
27. Barrea L, Savanelli MC, Di Somma C, Napolitano M, Megna M, Colao A, et al. Vitamin D and its role in psoriasis: an overview of the dermatologist and nutritionist. Rev Endocr Metab Disord. (2017) 18:195–205. doi: 10.1007/s11154-017-9411-6
28. Xi B, Liang Y, He T, Reilly KH, Hu Y, Wang Q, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993-2009. Obes Rev. (2012) 13:287–96. doi: 10.1111/j.1467-789X.2011.00944.x
29. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. (2010) 362:1090–101. doi: 10.1056/NEJMoa0908292
30. Ding X, Wang T, Shen Y, Wang X, Zhou C, Tian S, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. (2012) 22:663–7. doi: 10.1684/ejd.2012.1802
31. Yu S, Fang H, Han J, Cheng X, Xia L, Li S, et al. The high prevalence of hypovitaminosis D in China: a multicenter vitamin D status survey. Medicine. (2015) 94:e585. doi: 10.1097/MD.0000000000000585
32. Zhen D, Liu L, Guan C, Zhao N, Tang X. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: its relationship to osteoporosis and lifestyle factors. Bone. (2015) 71:1–6. doi: 10.1016/j.bone.2014.09.024
33. Schleicher RL, Sternberg MR, Lacher DA, Sempos CT, Looker AC, Durazo-Arvizu RA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. (2016) 104:454–61. doi: 10.3945/ajcn.115.127985
Keywords: effect modification, obesity, psoriasis, serum vitamin D, waist-hip ratio
Citation: Kuang Y, Xiao Y, Fang Z, Zhang Y, Shen M, Chen X, Chen M, Lv C and Zhu W (2020) Association of Serum Vitamin D With Psoriasis and Effect Modification by Central Obesity. Front. Med. 7:236. doi: 10.3389/fmed.2020.00236
Received: 05 March 2020; Accepted: 06 May 2020;
Published: 18 June 2020.
Edited by:
Ivan V. Litvinov, McGill University, CanadaReviewed by:
Irina Khamaganova, Pirogov Russian National Research Medical University, RussiaCopyright © 2020 Kuang, Xiao, Fang, Zhang, Shen, Chen, Chen, Lv and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengzhi Lv, ZGxwZmJAMTI2LmNvbQ==; Wu Zhu, emh1d3U3MEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.