
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 08 May 2020
Sec. Nephrology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00153
This article is part of the Research Topic Immune Regulation in Kidney Diseases: Importance, Mechanism and Translation View all 16 articles
Background: N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a useful cardiac biomarker that is associated with acute kidney injury (AKI) and mortality after cardiac surgery. However, its prognostic value in cardiac surgical patients receiving renal replacement therapy (RRT) remains unclear.
Objectives: Our study aimed to assess the prognostic value of NT-proBNP in patients with established AKI receiving RRT after cardiac surgery.
Methods: A total of 163 cardiac surgical patients with AKI requiring RRT were enrolled in this study. Baseline characteristics, hemodynamic variables at RRT initiation, and NT-proBNP level before surgery, at RRT initiation, and on the first day after RRT were collected. The primary outcome was 28-day mortality after RRT initiation.
Results: Serum NT-proBNP levels in non-survivors was markedly higher than survivors before surgery (median: 4,096 [IQR, 962.0–9583.8] vs. 1,339 [IQR, 446–5,173] pg/mL; P < 0.01), at RRT initiation (median: 10,366 [IQR, 5,668–20,646] vs. 3,779 [IQR, 1,799–11,256] pg/mL; P < 0.001), and on the first day after RRT (median: 9,055.0 [IQR, 4,392–24,348] vs. 5,255 [IQR, 2,134–9,175] pg/mL; P < 0.001). The area under the receiver operating characteristic curve of NT-proBNP before surgery, at RRT initiation, and on the first day after RRT for predicting 28-day mortality was 0.64 (95% CI, 0.55–0.73), 0.71 (95% CI, 0.63–0.79), and 0.68 (95% CI, 0.60–0.76), respectively. Consistently, Cox regression revealed that NT-proBNP levels before surgery (HR: 1.27, 95% CI, 1.06–1.52), at RRT initiation (HR: 1.11, 95% CI, 1.06–1.17), and on the first day after RRT (HR: 1.17, 95% CI, 1.11–1.23) were independently associated with 28-day mortality.
Conclusions: Serum NT-proBNP was an independent predictor of 28-day mortality in cardiac surgical patients with AKI requiring RRT. The prognostic role of NT-proBNP needs to be confirmed in the future.
Acute kidney injury (AKI) is a frequent but serious complication for patients undergoing cardiac surgery, with an increased risk of hospital mortality and prolonged length of hospital stay (1). Patients who develop severe AKI requiring renal replacement therapy (RRT) represent nearly 2–6% of patients after cardiac surgery, and RRT dependency results in high mortality (2–6). However, few biomarkers and effective scoring systems have been validated as prognostic factors in this high risk population (7, 8).
Serum N-terminal pro-B-type natriuretic peptide (NT-proBNP), as an inactive polypeptide of the pre-prohormone brain natriuretic peptide (BNP), is synthesized and released by cardiomyocytes in response to pressure and volume overload (9–11). Increasing evidence has shown that the NT-proBNP level is associated with AKI after cardiac surgery (12–15), medical (non-surgical) patients in cardiac intensive care units (16), or unselected critically ill patients (17). Several studies have also validated NT-proBNP as a predictor of mortality in cardiac surgery patients (12, 14, 18, 19). However, little is known about the prognostic value of NT-proBNP in cardiac surgery patients with established AKI. Therefore, the purpose of this study was to investigate the prognostic value of NT-proBNP in cardiac surgery patients with established AKI requiring RRT.
From January 2018 to October 2019, consecutive AKI patients after cardiac surgery who required RRT in the cardiac surgical intensive care unit (ICU) of Zhongshan Hospital, Fudan University, Shanghai, China, were prospectively enrolled.
AKI was diagnosed according to the Kidney Disease Improving Global Outcomes (KDIGO) classification (20). Patients were excluded if they met the following criteria: age < 18 years, ICU length of stay < 48 h, pre-admission chronic RRT or previous history of end-stage renal disease [defined by an estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2], and receiving RRT before cardiac surgery.
Indications for RRT included metabolic acidosis (pH < 7.2), hyperkalemia > 6.0 mmol/L, evidence of fluid overload refractory to diuretics, urine output < 0.5 mL/kg/h for more than 6 h under optimized conditions (preload optimization, titration of vasopressors, and use of diuretics), and severe azotemia (serum creatinine level > 4 mg/dL and/or >3-fold increase in serum creatinine level compared with baseline). RRT was initiated within 6 h of meeting the above criteria. The method of RRT (continuous or intermittent technique, duration, and interval between sessions) depended on the clinical state of individual patients, usually the continuous technique in unsteady hemodynamic phase and then succeeded by intermittent techniques after stabilization. The modality of RRT included continuous venovenous hemofiltration (CVVH), continuous venovenous hemodiafiltration (CVVHDF), intermittent hemodialysis (IHD), and intermittent sustained low efficiency dialysis (SLED), which were used based on the discretion of clinicians to achieve optimal hemodynamic status and metabolic control. Blood flow was usually kept between 180 to 220 mL per minute. The prescribed effluent flow was kept above 25 mL per kilogram per hour. The replacement or dialysate solution used was bicarbonate. Femoral or internal jugular double-lumen dialysis catheter was used for vascular access.
Baseline demographics, co-morbidities, and pre-operative laboratory data including NT-proBNP, serum creatinine (sCr), and blood urea nitrogen (BUN) were recorded. Information regarding the surgical procedure was obtained. The eGFR was calculated based on the Modification of Diet in Renal Disease (MDRD) equation. Day 0 was defined as the day of RRT initiation, and day 1 was defined as the first day after RRT initiation. RRT indications, hemodynamic variables, and clinical characteristics at RRT initiation which included central venous pressure (CVP), mean artery pressure (MAP), vasoactive agent dosages, and Acute Physiology and Chronic Health Evaluation II (APACHE-II) scores were collected.
Serum samples for laboratory assessments were obtained for each patient on day 0 (within 6 h before RRT initiation) and day 1 (within 18 h after RRT initiation). The NT-proBNP levels were measured using the Elecsys Electro-chemo luminescent assay (Cobase 411 analyzer, Roche Diagnostics, Mannheim, Germany) in the clinical chemistry laboratory of the Zhongshan Hospital. The measurement range was 5 to 35,000 pg/mL and the total coefficient of variation was 3.9–4.4% according to multicenter measurements of the automated Roche NT-proBNP assay (21).
The primary outcome of this study was 28-day mortality from the day of RRT initiation. Patients who survived to day 28 were censored at day 28. Secondary outcomes were duration of invasive mechanical ventilation, length of stay in the ICU and hospital, ICU mortality, hospital mortality, and RRT dependency at day 28 in survivors.
The normality of distribution of continuous variables was evaluated using the Kolmogorov-Smirnov test. Continuous variables are shown as the mean ± standard deviation (SD) or median interquartile range [IQR], as appropriate. Categorical variables were presented as numbers and percentages. For skewed data, ln transformation of NT-proBNP, troponin T, and APACHE II score was performed (presented as ln-NT-proBNP, ln-troponin T, and ln-APACHE II score, respectively). Baseline characteristics were compared using the Student's t-test or Mann-Whitney U-test for continuous variables and the chi-square test or Fisher's exact test for categorical variables. We used receiver operating characteristic (ROC) curves to examine the performance of variables to predict 28-day mortality. The area under the curve (AUC) was derived from ROC curves. Survival curves were plotted and compared across different NT-proBNP levels. Cox proportional hazards regressions were performed to evaluate prognostic values of NT-proBNP. Variables with a P < 0.1 in univariate analyses were introduced into multivariable models (stepwise variable-selection method). Analyses were performed using the SPSS software package, version 13.0 (SPSS, Inc., Chicago, IL, USA). Two-sided p < 0.05 was defined as statistically significant.
From January 2018 to October 2019, a total of 8,429 patients undergoing cardiac surgery were screened for inclusion. Of these, 2,903 patients had AKI after cardiac surgery and 186 patients required RRT. Of those, 23 patients were excluded, including 12 patients receiving RRT before surgery, eight patients who died within 48 h after surgery, and three patients with missing data. Finally, 163 patients that received RRT were included for analysis (Figure 1).
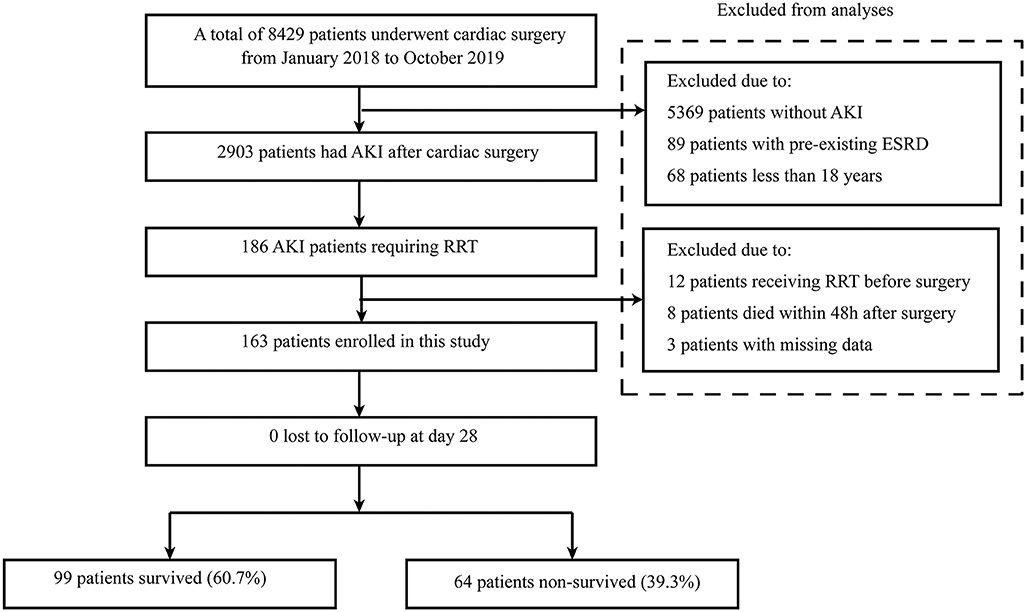
Figure 1. Flow chart of this study. AKI, acute kidney injury; RRT, renal replacement therapy; ESRD, end-stage renal disease.
The baseline characteristics grouped by 28-day mortality are presented in Table 1. There were no significant differences in age, sex, hypertension, and diabetes mellitus. Non-survivors had higher pre-operative troponin T (median: 0.03 [IQR, 0.02–0.14] vs. 0.02 [IQR, 0.01–0.05] ng/mL; P = 0.03), NT-proBNP (median: 4,096 [IQR, 962.0–9,583.8] vs. 1,339 [IQR, 446–5,173] pg/mL; P < 0.01), sCr levels (mean: 173.4, SD ± 92.1 vs. 141.3 SD ± 78.1 μmol/L; P = 0.03) and lower BMI (mean: 22.7, SD ± 4.0 vs. 24.1, SD ± 4.4 kg/m2; P = 0.04) compared with survivors. The type of surgery was comparable between the two groups. The cardiopulmonary bypass (CPB) time and aortic clamp time were higher in non-survivors compared to survivors (all P < 0.05).
The clinical characteristics at RRT initiation are shown in Table 2. The indications for RRT included severe azotemia (25.8%), oliguria (97.5%), metabolic acidosis (6.1%), and electrolyte disorders (3.1%). There were no differences in indications of RRT, urine output before RRT initiation, and hemodynamic variables including MAP, CVP, and the dosage of vasoactive drugs between two groups. The APACHE II score (mean: 18, SD ± 8 vs. 15, SD ± 6; P < 0.01), NT-proBNP (median: 10,366 [IQR, 5,668–20,646] vs. 3,779 [IQR, 1,799–11,256] pg/mL; P < 0.001) and serum lactate level (mean: 5.88, SD ± 5.19 vs. 3.76, SD ± 3.63 mmol/L; P < 0.01) at RRT initiation were higher in non-survivors, while the eGFR (mean: 20.9, SD ± 10.3 vs. 25.2, SD ± 15.7 mL/min/1.73 m2; P = 0.04) and serum bicarbonate level (mean: 23.2, SD ± 4.1 vs. 25.7, SD ± 4.2 mmol/L; P < 0.001) were lower. On day 1 after RRT initiation, NT-proBNP (median: 9,055.0 [IQR, 4,392–24,348] vs. 5,255 [IQR, 2,134–9,175] pg/mL; P < 0.001) and serum lactate level (mean: 4.74, SD ± 4.68 vs. 1.90, SD ± 1.30 mmol/L; P < 0.001) were still higher in non-survivors, while serum bicarbonate level (mean: 23.0, SD ± 4.2 vs. 25.6, SD ± 3.7 mmol/L; P < 0.001) was lower.
The 28-day mortality of our cohort was 39.3% (64/163; Table 3). Concerning the clinical course, 43.6% patients (71/163) received tracheostomy during the ICU stay. The mean duration of invasive mechanical ventilation was 11, SD ± 11 days. The rate of dependence on RRT among survivors at 28 days was 12.1% (12/99). The length of ICU and hospital stay were longer in non-survivors compared with survivors (mean: 21.0, SD ± 16.0 vs.14, SD ± 10 days and 39, SD ± 26 vs. 21, SD ± 13 days, all P < 0.01; Table 3).
ROC curves were constructed to evaluate the performance of variables to predict 28-day mortality (Figure 2). The AUC, optimal cutoff value, sensitivity, and specificity of each variable are shown in Table 4. The APACHE II score (AUC 0.60, SD ± 0.05), NT-proBNPpre−op (AUC 0.64, SD ± 0.05), NT-proBNPday0 (AUC 0.71, SD ± 0.04), and NT-proBNPday1 (AUC 0.68, SD ± 0.04) had a modest power for prediction of 28-day mortality (all P < 0.05). There were no statistically significant differences in the AUC among above indicators (all P > 0.05). A NT-proBNPpre−op cutoff value of ≥3,632.5 pg/mL had a sensitivity of 53.1% and a specificity of 71.4%. A NT-proBNPday0 threshold of ≥5,539 pg/mL had a sensitivity of 78.1% and a specificity of 62.2%. A NT-proBNPday1 threshold of ≥7,841 pg/mL had a sensitivity of 64.1% and a specificity of 71.4%.
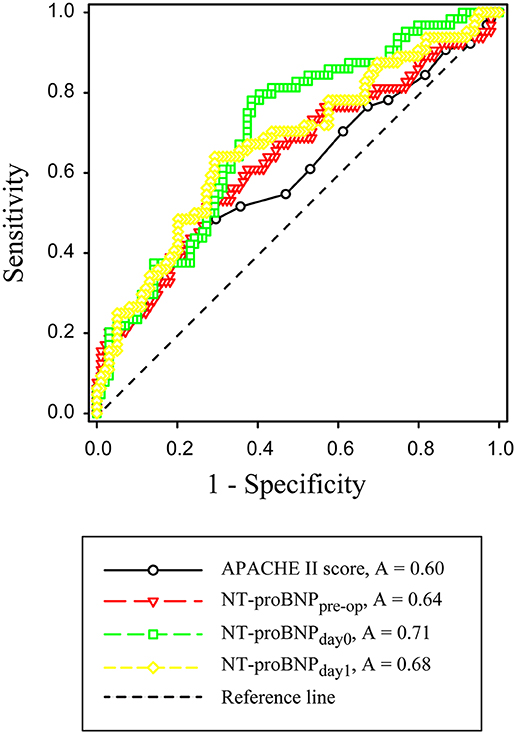
Figure 2. Receiver operating characteristic curves for APACHE II score, NT-proBNPpre−op, NT-proBNPday0 and NT-proBNPday1. APACHE-II, Acute Physiology and Chronic Health Evaluation II; NT-proBNPpre−op, preoperative NT-proBNP level; NT-proBNPday0, NT-proBNP level at RRT initiation; NT-proBNPday1, NT-proBNP level on the first day after RRT initiation.
According to the ROC curves, the cut-off value of NT-proBNPpre−op, NT-proBNPday0, and NT-proBNPday1 levels were set at 3,632.5, 5,539, and 7,841 pg/mL, respectively (Table 4). Patients with higher NT-proBNPpre−op levels (≥3,632.5 pg/mL), NT-proBNPday0 (≥5,539 pg/mL), and NT-proBNPday1 (≥7,841 pg/mL) levels were at a higher risk of mortality (all P < 0.001, log-rank test). Survival curves for 28-day mortality are shown in Figure 3.
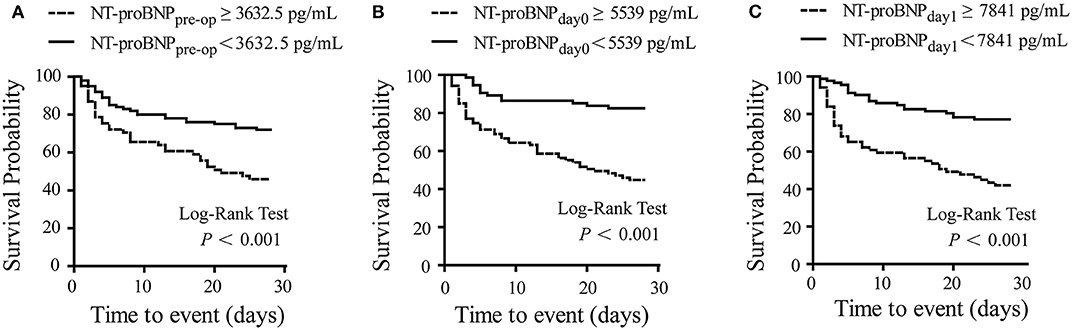
Figure 3. Kaplan-Meier curve for survival. Survival probability in patients with low and high NT-proBNP levels. A significant difference was observed with higher risk of survival events in patients with NT-proBNPpre−op ≥ 3,632.5 pg/mL (A), NT-proBNPday0 ≥ 5,539 pg/mL (B), and NT-proBNPday1 ≥7,841 pg/mL (C). Log-rank P-value shown on graphs. NT-proBNPpre−op, preoperative NT-proBNP level; NT-proBNPday0, NT-proBNP level at RRT initiation; NT-proBNPday1, NT-proBNP level on the first day after RRT initiation.
The predictive variables of 28-day mortality were evaluated using Cox proportional hazards regression. In univariable analysis, ln-APACHE II score, ln-NT -proBNPpre−op, ln-NT -proBNPday0, ln-NT -proBNPday1, lactate day0, lactate day1, and were significantly associated with 28-day mortality (Table 5). To account for the possible influence of NT-proBNP levels on 28-day mortality, multivariable models were constructed (Table 6). In multivariable analysis, ln NT -proBNPpre−op, ln NT-proBNPday0, and ln NT-proBNPday1 were independently associated with 28-day mortality (all P < 0.05).
To our knowledge, this is the first study investigating the prognostic value of NT-proBNP in cardiac surgery patients with established AKI requiring RRT. Serum NT-proBNP levels in non-survivors was markedly higher than survivors before surgery, at RRT initiation and on the first day after RRT initiation. Consistently, ROC curves revealed that NT-pro-BNP levels (pre-op, day 0 or day 1) had a modest power for predicting 28-day mortality. Cox proportional hazards regression analyses revealed that NT-proBNP levels before surgery, at RRT initiation, and on the first day after RRT initiation were independently associated with 28-day mortality.
Recent studies have reported several biomarkers to predict mortality at RRT initiation in critically ill patients. Serum neutrophil gelatinase associated lipocalin (NGAL) at initiation of RRT was identified as a prognostic biomarker in unselected critically ill patients (22). Plasma c-terminal FGF-23 (cFGF-23) at inception of RRT was correlated with higher 90-day overall mortality and predicted worse kidney recovery in survivors in critically ill patients with AKI (23). NT-proBNP and procalcitonin (PCT) have also been identified as prognostic markers in septic AKI patients requiring RRT (24). However, there are no definitive biomarkers for outcome prediction in AKI patients requiring RRT after cardiac surgery.
Natriuretic peptides (NPs), specifically NT-proBNP and B-type natriuretic peptide (BNP), are released by cardiomyocytes in response to stress and pressure overload. NT-proBNP is an inactive N-terminal fragment produced from the cleavage of proBNP (9–11). Elevated NT-proBNP levels are usually associated with cardiac dysfunction or heart failure after cardiac surgery and portend a poor outcome (14, 25–27). Decreases in NT-proBNP during follow-up were associated with reduced morbidity and mortality in patients with heart failure (28–30). In addition, recent evidence showed that NT-proBNP also represents a useful prognostic biomarker in septic patients (31–33), non-cardiac surgery patients (34), patients without heart failure (35), and an unselected cohort of critically ill patients (36, 37).
In the present study, NT-pro-BNP levels (pre-op, day 0 or day 1) had a modest power for prediction of 28-day mortality. Although the AUC of NT-proBNPday0 was larger than NT-proBNPpre−op or NT-proBNPday1, the differences were not statistically significant. Multivariable COX regression analysis also confirmed that serum NT-proBNP levels (pre-op, day 0 or day 1) were independent predictors of 28-day mortality. Several mechanisms may explain the relationship between NT-proBNP and mortality in cardiac surgery patients requiring RRT. First, elevated NT-proBNP are most likely released from stressed myocardium, suggesting that patients with higher NT-proBNP levels may have more severe cardiac dysfunction (27). In this study, the patients receiving RRT tended to be hemodynamically unstable with vasoactive drugs maintenance and high CVP level at RRT initiation, which indicated a reduced cardiac function. Second, all patients in this study had impaired renal function and low GFR at the initiation of RRT. Elevated NT-proBNP levels in this cohort may be partially due to decreased clearance by the kidneys or decreased renal responsiveness to BNP (9). It has been demonstrated that NT-proBNP is associated significantly with progression to end-stage renal disease in patients with chronic kidney disease (38, 39). Even in critically ill patients without underlying cardiac disease, NT-proBNP was independently associated with the maximum stage of AKI and need for RRT (17), which was correlated with high mortality. Third, inflammatory status might also account for increased levels of BNPs, apart from cardiac and renal dysfunction (40). In this study, non-survivors had longer CPB time and aortic clamp time than survivors, which led to a more severe systemic inflammatory reaction and consequently increased the risk of mortality (41, 42).
Several limitations in this study should be addressed. First, the conclusion may be restricted by the small sample size. Second, as the frequency of NT-proBNP measurement is low, the change in NT-proBNP during the clinical course was not analyzed in this study. Finally, as the cohort included patients with severe AKI requiring RRT, we should be cautious to interpret the prognostic capacity in cardiac surgical patients with mild or moderate AKI.
Serum NT-proBNP was an independent predictor of 28-day mortality in cardiac surgical patients with AKI requiring RRT. Large studies are needed to validate the prognostic role of NT-proBNP.
All datasets generated for this study are included in the article/supplementary material.
This prospective study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University (No. B2016-147R) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from legal representatives of participants.
YS, GT, and ZL contributed to study design. YS, JH, YZ, GM, GH, and JL contributed to participants enrollment. JH and YZ contributed to study management and data collection. YS and GT contributed to manuscript writing. GT and ZL contributed to data analyses and manuscript revision. All authors have read and approved the final manuscript.
This article was supported by grants from the Research Funds of Zhongshan Hospital (2019ZSYXQN34, 2019ZSQN13, 2018ZSQN53, and XYYX201922) and the Research Fund of Shanghai Municipal Health Commission (2019ZB0105).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. (2008) 23:1970–4. doi: 10.1093/ndt/gfm908
2. Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. (2015) 10:500–14. doi: 10.2215/CJN.07830814
3. Tu GW, Xu JR, Liu L, Zhu DM, Yang XM, Wang CS, et al. Preemptive renal replacement therapy in post-cardiotomy cardiogenic shock patients: a historically controlled cohort study. Ann Transl Med. (2019) 7:534. doi: 10.21037/atm.2019.09.140
4. Yang XM, Tu GW, Gao J, Wang CS, Zhu DM, Shen B, et al. A comparison of preemptive versus standard renal replacement therapy for acute kidney injury after cardiac surgery. J Surg Res. (2016) 204:205–12. doi: 10.1016/j.jss.2016.04.073
5. Bastin AJ, Ostermann M, Slack AJ, Diller GP, Finney SJ, Evans TW. Acute kidney injury after cardiac surgery according to risk/injury/failure/loss/end-stage, acute kidney injury network, and kidney disease: improving global outcomes classifications. J Crit Care. (2013) 28:389–96. doi: 10.1016/j.jcrc.2012.12.008
6. Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. (2009) 119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011
7. Maccariello E, Soares M, Valente C, Nogueira L, Valenca RV, Machado JE, et al. RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med. (2007) 33:597–605. doi: 10.1007/s00134-007-0535-0
8. Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, et al. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. (2006) 70:1120–6. doi: 10.1038/sj.ki.5001579
9. Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. (2007) 50:2357–68. doi: 10.1016/j.jacc.2007.09.021
10. Lugnier C, Meyer A, Charloux A, Andres E, Geny B, Talha S. The endocrine function of the heart: physiology and involvements of natriuretic peptides and cyclic nucleotide phosphodiesterases in heart failure. J Clin Med. (2019) 8:1746. doi: 10.3390/jcm8101746
11. Nishikimi T, Nakagawa Y. Does impaired processing of pro-B-type (or brain) natriuretic peptide cause decreased plasma BNP levels in obese heart failure patients? Ann Transl Med. (2019) 7:S221. doi: 10.21037/atm.2019.08.56
12. Belley-Cote EP, Parikh CR, Shortt CR, Coca SG, Garg AX, Eikelboom JW, et al. Association of cardiac biomarkers with acute kidney injury after cardiac surgery: a multicenter cohort study. J Thorac Cardiovasc Surg. (2016) 152:245–51. doi: 10.1016/j.jtcvs.2016.02.029
13. Patel UD, Garg AX, Krumholz HM, Shlipak MG, Coca SG, Sint K, et al. Preoperative serum brain natriuretic peptide and risk of acute kidney injury after cardiac surgery. Circulation. (2012) 125:1347–55. doi: 10.1161/CIRCULATIONAHA.111.029686
14. Lurati BG, Bolliger D, Seeberger E, Kasper J, Grapow M, Koller MT, et al. Troponin T and B-type natriuretic peptide after on-pump cardiac surgery: prognostic impact on 12-month mortality and major cardiac events after adjustment for postoperative complications. Circulation. (2014) 130:948–57. doi: 10.1161/CIRCULATIONAHA.113.007253
15. Zelt J, Mielniczuk LM, Liu PP, Dupuis JY, Chih S, Akbari A, et al. Utility of novel cardiorenal biomarkers in the prediction and early detection of congestive kidney injury following cardiac surgery. J Clin Med. (2018) 7:540. doi: 10.3390/jcm7120540
16. Naruse H, Ishii J, Takahashi H, Kitagawa F, Nishimura H, Kawai H, et al. Predicting acute kidney injury using urinary liver-type fatty-acid binding protein and serum N-terminal pro-B-type natriuretic peptide levels in patients treated at medical cardiac intensive care units. Crit Care. (2018) 22:197. doi: 10.1186/s13054-018-2120-z
17. Haines R, Crichton S, Wilson J, Treacher D, Ostermann M. Cardiac biomarkers are associated with maximum stage of acute kidney injury in critically ill patients: a prospective analysis. Crit Care. (2017) 21:88. doi: 10.1186/s13054-017-1674-5
18. Litton E, Ho KM. The use of pre-operative brain natriuretic peptides as a predictor of adverse outcomes after cardiac surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. (2012) 41:525–34. doi: 10.1093/ejcts/ezr007
19. Holm J, Vidlund M, Vanky F, Friberg O, Hakanson E, Walther S, et al. EuroSCORE II and N-terminal pro-B-type natriuretic peptide for risk evaluation: an observational longitudinal study in patients undergoing coronary artery bypass graft surgery. Br J Anaesth. (2014) 113:75–82. doi: 10.1093/bja/aeu088
20. Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. (2013) 17:204. doi: 10.1186/cc11454
21. Yeo KT, Wu AH, Apple FS, Kroll MH, Christenson RH, Lewandrowski KB, et al. Multicenter evaluation of the Roche NT-proBNP assay and comparison to the Biosite Triage BNP assay. Clin Chim Acta. (2003) 338:107–15. doi: 10.1016/j.cccn.2003.08.016
22. Kumpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D, et al. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care. (2010) 14:R9. doi: 10.1186/cc8861
23. Wu VC, Shiao CC, Chi NH, Wang CH, Chueh SJ, Liou HH, et al. Outcome prediction of acute kidney injury biomarkers at initiation of dialysis in critical units. J Clin Med. (2018) 7:202. doi: 10.3390/jcm7080202
24. Sheng X, Yang J, Yu G, Fei Y, Bao H, Yin J, et al. Procalcitonin and N-terminal pro-B-type natriuretic peptide for prognosis in septic acute kidney injury Patients receiving renal replacement therapy. Blood Purif. (2019) 48:262–71. doi: 10.1159/000501388
25. Seoudy H, Kuhn C, Frank J, Eden M, Rangrez AY, Lutter G, et al. Prognostic implications of N-terminal pro-B-type natriuretic peptide in patients with normal left ventricular ejection fraction undergoing transcatheter aortic valve implantation. Int J Cardiol. (2019) 301:195–9. doi: 10.1016/j.ijcard.2019.11.101
26. Ramkumar N, Jacobs JP, Berman RB, Parker DM, MacKenzie TA, Likosky DS, et al. Cardiac biomarkers predict long-term survival after cardiac surgery. Ann Thorac Surg. (2019) 108:1776–82. doi: 10.1016/j.athoracsur.2019.04.123
27. Palazuelos J, Rubio AM, Clares MP. Prognostic implications of baseline NT-proBNP before cardiac surgery. J Thorac Cardiovasc Surg. (2016) 152:252–3. doi: 10.1016/j.jtcvs.2016.03.011
28. Savarese G, Musella F, D'Amore C, Vassallo E, Losco T, Gambardella F, et al. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: a meta-analysis. JACC Heart Fail. (2014) 2:148–58. doi: 10.1016/j.jchf.2013.11.007
29. Felker GM, Ahmad T. Natriuretic peptides and primary prevention: the new world? J Am Coll Cardiol. (2013) 62:1373–5. doi: 10.1016/j.jacc.2013.06.009
30. Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. (2016) 68:2425–36. doi: 10.1016/j.jacc.2016.09.931
31. Wang F, Wu Y, Tang L, Zhu W, Chen F, Xu T, et al. Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta-analysis. Crit Care. (2012) 16:R74. doi: 10.1186/cc11331
32. Varpula M, Pulkki K, Karlsson S, Ruokonen E, Pettila V. Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med. (2007) 35:1277–83. doi: 10.1097/01.CCM.0000261893.72811.0F
33. Brueckmann M, Huhle G, Lang S, Haase KK, Bertsch T, Weiss C, et al. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis. Circulation. (2005) 112:527–34. doi: 10.1161/CIRCULATIONAHA.104.472050
34. Duceppe E, Patel A, Chan M, Berwanger O, Ackland G, Kavsak PA, et al. Preoperative N-terminal pro-B-type natriuretic peptide and cardiovascular events after noncardiac surgery: a cohort study. Ann Intern Med. (2019). doi: 10.7326/M19-2501. [Epub ahead of print].
35. Fukushima N. Is B-type natriuretic peptide (BNP) similarly associated with mortality in patients with and without heart failure? Ann Transl Med. (2018) 6:S9. doi: 10.21037/atm.2018.08.46
36. Wang F, Pan W, Pan S, Wang S, Ge Q, Ge J. Usefulness of N-terminal pro-brain natriuretic peptide and C-reactive protein to predict ICU mortality in unselected medical ICU patients: a prospective, observational study. Crit Care. (2011) 15:R42. doi: 10.1186/cc10004
37. Meyer B, Huelsmann M, Wexberg P, Delle KG, Berger R, Moertl D, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of outcome in an unselected cohort of critically ill patients. Crit Care Med. (2007) 35:2268–73. doi: 10.1097/01.CCM.0000284509.23439.5B
38. Sundqvist S, Larson T, Cauliez B, Bauer F, Dumont A, Le Roy F, et al. Clinical value of natriuretic peptides in predicting time to dialysis in Stage 4 and 5 chronic kidney disease patients. PLoS ONE. (2016) 11:e159914. doi: 10.1371/journal.pone.0159914
39. Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. (2011) 58:717–28. doi: 10.1053/j.ajkd.2011.05.020
40. Rudiger A, Fischler M, Harpes P, Gasser S, Hornemann T, von Eckardstein A, et al. In critically ill patients, B-type natriuretic peptide (BNP) and N-terminal pro-BNP levels correlate with C-reactive protein values and leukocyte counts. Int J Cardiol. (2008) 126:28–31. doi: 10.1016/j.ijcard.2007.03.108
41. Madhavan S, Chan SP, Tan WC, Eng J, Li B, Luo HD, et al. Cardiopulmonary bypass time: every minute counts. J Cardiovasc Surg. (2018) 59:274–81. doi: 10.23736/S0021-9509.17.09864-0
Keywords: acute kidney injury, renal replacement therapy, biomarker, N-terminal pro-B-type natriuretic peptide, mortality
Citation: Su Y, Hou J, Zhang Y, Ma G, Hao G, Luo J, Luo Z and Tu G (2020) Serum N-terminal Pro-B-type Natriuretic Peptide Predicts Mortality in Cardiac Surgery Patients Receiving Renal Replacement Therapy. Front. Med. 7:153. doi: 10.3389/fmed.2020.00153
Received: 22 January 2020; Accepted: 07 April 2020;
Published: 08 May 2020.
Edited by:
Songjie Cai, Brigham and Women's Hospital, United StatesReviewed by:
Maria-Eleni Roumelioti, University of New Mexico, United StatesCopyright © 2020 Su, Hou, Zhang, Ma, Hao, Luo, Luo and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Luo, bHVvLnpoZUB6cy1ob3NwaXRhbC5zaC5jbg==; Guo-wei Tu, dHUuZ3Vvd2VpQHpzLWhvc3BpdGFsLnNoLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.