- 1Department of Neurology, Jena University Hospital, Jena, Germany
- 2Center for Healthy Ageing, Jena University Hospital, Jena, Germany
Background: Detailed knowledge about nonadherence to medication could improve medical care in elderly patients. We aimed to explore patterns and reasons for nonadherence in people with Parkinson's disease (PD) aged 60 years and older.
Methods: Detailed clinical data and adherence (German Stendal Adherence with Medication Score) were assessed in 230 patients with PD (without dementia). Descriptive statistics were used to study reasons for nonadherence in detail, and general linear models were used to study associations between clusters of nonadherence and clinical parameters.
Results: Overall, 14.2% (n = 32) of the patients were fully adherent, 66.8% (n = 151) were moderately nonadherent, and 19.0% (n = 43) showed clinically meaningful nonadherence. In the multivariable analysis, nonadherence was associated with a lower education level, higher motor impairment in activities of daily living, higher number of medications per day, and motor complications of PD. Three clusters of nonadherence were observed: 59 (30.4%) patients reported intentional nonadherence by medication modification; in 72 (37.1%) patients, nonadherence was associated with forgetting to take medication; and 63 (32.5%) patients had poor knowledge about the prescribed medication. A lower education level was mainly associated with modification of medication and poorer knowledge about prescribed medication, but not with forgetting to take medication. Patients with motor complications, which frequently occur in those with advanced disease stages, tend to be intentionally nonadherent by modifying their prescribed medication. Increased motor problems and a higher total number of drugs per day were associated with less knowledge about the names, reasons, and dosages of their prescribed medication.
Conclusions: Elderly patients with PD report many reasons for intentional and non-intentional nonadherence. Understanding the impact of clinical parameters on different patterns of nonadherence may facilitate tailoring of interventions and counseling to improve outcomes.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease (1). Both the prevalence and incidence of Parkinson's disease increase continuously with age. About 0.5% of patients in the age group 60–69 years suffer from PD in western industrial nations, while about 1.6% of patients in the age group 70–79 years are already affected (1). It is well known that nonadherence to medication is a major issue in chronic disorders such as Parkinson's disease (PD) (2–6). The direct and indirect costs of nonadherence are enormous, and nonadherence contributes to poor outcomes and a lower quality of life (7–10). There are various reasons as to why people do not or cannot follow the instructions they are given for prescribed treatments. Systematically, the factors associated with adherence to medications can be divided into patient characteristics, disease-related factors, financial and health system barriers, patient–provider relationship factors, and treatment-related factors (10, 11). Patient-related factors can be further categorized as intentional (when the patient purposefully decides not to adhere to the recommended treatment) or unintentional (when the patient cannot follow the recommendations). Several studies and reviews explored epidemiological and clinical factors associated with nonadherence to medication in PD, such as younger age, education level, marital status, mood disorders, cognition, disease duration, and regimen complexity (2, 4, 6, 11, 12). However, the sample size in many studies was low (2), or, as in a large study by Valldeoriola et al., cognition as an important cofactor was not assessed in detail (it was graded according to a four-point scale). Moreover, patients with cognitive deficits were not excluded from the analysis, which may influence the validity of a self-reported adherence outcome measure (13). Therefore, replication of the observed associations between clinical parameters and nonadherence in larger, well characterized cohorts is required.
While many studies analyzed the relationship between clinical factors and the presence or absence of nonadherence, little is known about how distinct clinical factors (e.g., degree of motor disability and disease duration) modulate the type of nonadherent behavior. For example, do patients with higher functional impairment more frequently modify or forget their prescribed medication than those with better motor performance? (14).
Therefore, our study had the following aims:
- To describe common self-reported reasons for nonadherence in PD.
- To replicate the associations between different degrees of nonadherence and PD-specific clinical parameters.
- To explore the impact of PD-specific clinical parameters on distinct clusters/reasons of nonadherence.
Methods
Participants and Assessments
This observational study was approved by the local ethics committee of the Jena University Hospital (4572-10/15). All subjects gave written informed consent in accordance with the Declaration of Helsinki. Patients with PD were consecutively recruited between June 2017 and December 2018 from the outpatient clinic and the ward of the Department of Neurology of the Jena University Hospital, Germany. The inclusion criteria were PD diagnosis according to Movement Disorder Society (MDS) diagnostic criteria and stability under dopaminergic treatment. The exclusion criteria were cerebrovascular disorders, delirium, deep brain stimulation, enteral levodopa/carbidopa infusion, apomorphine infusion, and inability to complete a questionnaire. All tests were conducted during the medication ON phase. The demographic data collected included age, gender, marital status (single/divorced/widowed or married), and level of education (high: German Abitur or University; medium: German Realschule or General Certificate of Secondary Education; low: German Hauptschule or no school). Several clinical parameters were recorded: the total daily number of medications administered in any pharmaceutical form, the levodopa equivalent daily dose (15), the MDS-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS), the revised non-motor symptoms questionnaire (NMS-Quest), and Hoehn & Yahr staging. The MDS-UPDRS covers the patient's motor experience of daily living (II), clinician's motor examination (III), and motor complications (IV). Cognition was assessed by using the Montreal Cognitive Assessment (MoCa) (16). Beck's depression inventory II (BDI) was used to quantify depressive mood. Adherence was assessed by using the self-reported German Stendal Adherence with Medication Score (SAMS). It includes 18 questions forming a cumulative scale (0–72) in which 0 indicates complete adherence and 72 indicates complete nonadherence (17). It allows the assessments of three common reasons/cluster of nonadherence: modifications of medication, forgetting to take the medication, and lack of knowledge about medications (14). The full SAMS is available online (CC BY NC 3.0 license): https://data.mendeley.com/datasets/ny2krr3vgg/1 (18). It was so far not explicitly used in patients with PD, but in a mixed cohort of patients with different neurological disorders (also including people with PD) (14).
The total number of patients recruited for the study was 300. Seventy-four cases had incomplete or missing data or PD dementia (MoCa < 21) (16) and were excluded. Therefore, 226 patients were included in the final analyses.
Statistical Analysis
The SPSS statistical computer package (version 25.0; IBM Corporation, USA) was used for all statistical analyses. Values are given as mean and standard deviation or median and interquartile range. Categorical variables are presented as numbers or percentages. Although the validity of the historical 80% threshold remains uncertain (19), it is generally considered that suboptimal adherence becomes clinically significant when <80% of the prescribed medication is taken (20, 21). This leads to a study- and sample-specific SAMS cut-off of 13 points for clinically meaningful/significant nonadherence. The patients were then categorized into (1) fully adherent (SAMS = 0), (2) moderately nonadherent (SAMS 1–12.9), and (3) nonadherent (SAMS ≥ 13).
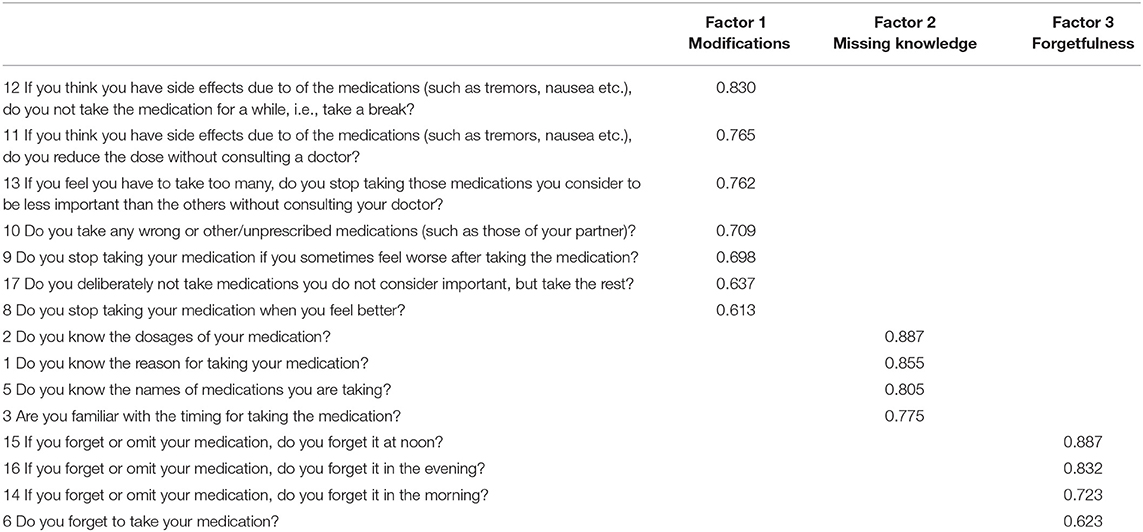
Based on our previous work, each patient was categorized into one cluster of nonadherence: modifications, lack of knowledge, and forgetfulness (14). In the cluster “modifications,” for example, medications were adjusted by patients without consulting their doctor on either experiencing side effects or improvement in health. The cluster “missing knowledge” involved patients who were unaware of the purpose of their medications and/or dosages. The cluster “forgetfulness” included patients who frequently forgot to take their medications. For this purpose, a principal component analysis (PCA) (eigenvalues >1 and varimax rotation) was used to reduce the 18 SAMS items into factors representing these different nonadherence clusters. The structure of the SAMS (items 1–18) was examined by using an exploratory factor analysis. Both the Bartlett test (p < 0.001) and the Kaiser–Meyer–Olkin Measure of Sampling Adequacy (0.80) indicated that the variables were suitable for factor analysis. Three items, item 4 (Do you take your medication regularly?), item 7 (Are you untroubled about taking the medication?) and item 18 (If you take medication from a syringe or in a weekly tablet, have you ever forgotten it?), exhibited a low communality score and were removed from the analysis. A three-factor solution was chosen based on the scree plot, which accounts for 62.0% of the variance. The SAMS items belonging to each factor are displayed in Table 1. For every patient, the regression coefficient for each PCA factor was calculated. Descriptive statistics were used to describe the reasons for nonadherence in each cluster in detail. A general liner model (stepwise forward, Akaike information criterion) was calculated to study the association between a set of clinical variables and overall adherence (SAMS total). The set of clinical variables (independent variables) was derived from the literature and included gender, education level, marital status, total number of prescribed drugs, LEDD, H&Y stage, MDS-UPDRS II, III, and IV, NMS-Quest, MoCa, BDI, and disease duration (2–4, 6, 11–13, 20, 22, 23). The clinical variables which were found to be significantly associated with nonadherence were then entered as independent variables into three additional linear models with (1) degree of modification (regression coefficients for the cluster “modification”), (2) degree of missing knowledge (regression coefficients for the cluster “missing knowledge”), and (3) degree of forgetfulness (regression coefficients for the cluster “forgetfulness”) as dependent variables (stepwise forward, Akaike information criterion). The threshold for statistical significance was set at p < 0.05.
Results
Prevalence of Nonadherence and Its Association With Clinical Parameters
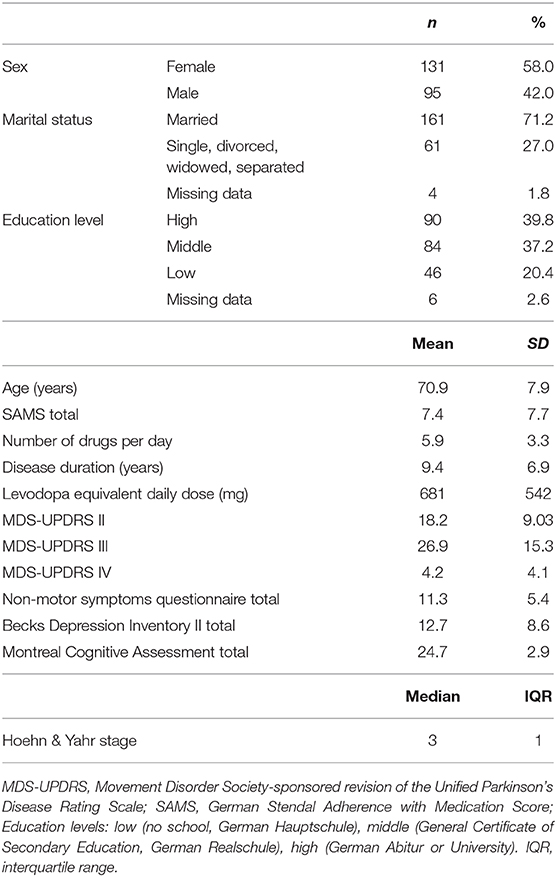
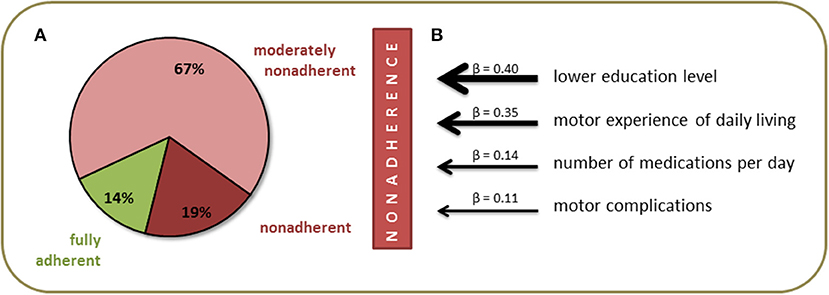
The final sample included 226 patients with PD [95 (41.3%) female] with a mean age of 71.1 ± 7.9 years. The majority of patients was married and had completed middle or high school education. Detailed clinical data are provided in Table 2. The mean total SAMS was 7.4 ± 7.7 points. According to the SAMS, 14.2% (n = 32) of the patients were fully adherent (SAMS = 0), 66.8% (n = 151) were moderately nonadherent (SAMS 1–12.9), and 19.0% (n = 43) showed clinically meaningful nonadherence (SAMS ≥ 13) (Figure 1A). In the multivariable analysis, nonadherence was associated with a lower education level (β = 0.40, p = 0.002), higher rate of motor impairment in activities of daily living (MDS-UPDRS II, β = 0.35, p = 0.004), higher number of medications per day (β = 0.14, p = 0.066), and higher rate of motor complications of PD (MDS-UPDRS IV, β = 0.11, p = 0.104) [F(4, 225) = 7.2, p < 0.001] (Figure 1B).
Reasons for Nonadherence
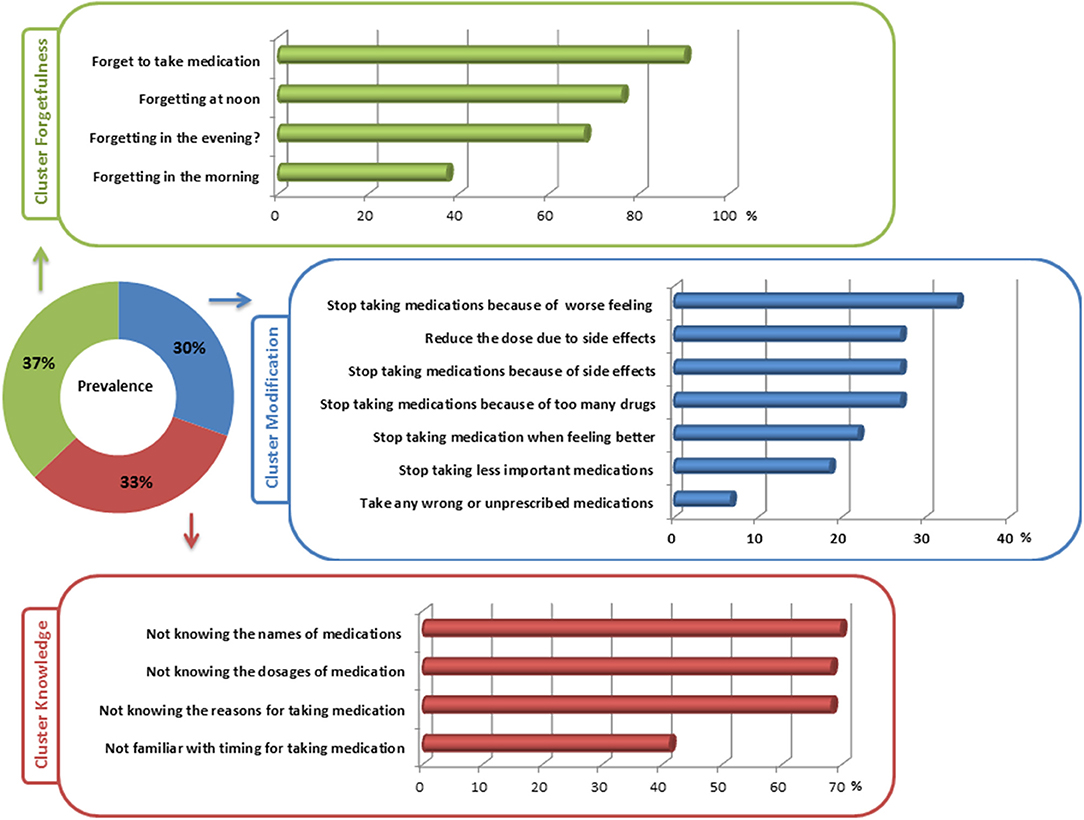
In the nonadherent group (all with SAMS >1), 59 (30.4%) patients reported intentional nonadherence by modification of medication. In this cluster, patients most frequently reported stopping medication because of side effects or feeling worse after taking the medication. Interestingly, 22% discontinued medication when they felt better and 19% stopped taking medication that they considered to be less important (Figure 2). Nonadherence associated with forgetfulness was found in 72 (37.1%) of PD patients. Of note, most patients in this cluster forgot to take their medication at noon. Forgetting to take medication was reported to be less in the morning (Figure 2). In 63 (32.5%) patients, nonadherence was caused by lack of knowledge about the prescribed medication. The majority did not know the exact names, the reasons for, and the dosages of their prescribed medications (Figure 2).
Patterns of Nonadherence and Their Association With Clinical Parameters
In the multivariable analyses, the cluster “modification” was associated with a lower education level (β = 0.63, p = 0.05) and a higher rate of motor complications (MDS-UPDRS IV, β = 0.37, p = 0.14) [F(2, 147) = 3.1, p = 0.049]. The cluster “missing knowledge” was associated with a higher total number of drugs per day (β = 0.37, p = 0.019), a lower education level (β = 0.35, p = 0.022), and higher motor impairment (MDS-UPDRS II, β = 0.28, p = 0.028) [F(4, 147) = 5.8, p = 0.001]. Forgetting to take medication was not significantly associated with any of the clinical parameters.
Discussion
Prevalence of Nonadherence and Its Association With Clinical Factors
Medication adherence in PD, defined as taking >80% of the prescribed dose, ranged widely from 33 to 97.7% in previous studies (11). The prevalence mainly depends on the method used to assess adherence and the study design. Measures of adherence can be classified as direct and indirect. Direct methods (e.g., measurements of the drug or its metabolite concentration in body fluids) are accurate, but have several drawbacks (expensive, improvement of adherence before the upcoming tests, individual metabolic rates, drug-drug or drug-food interactions) (24). Their low cost, simplicity, and real-time feedback make indirect methods (self-reports, interviews) interesting tools to identify individual patient concerns. Estimates of non-adherence prevalence in PD range from 15 to 20% by self-report, to 67% and higher in studies using pharmacy refill data and pill counts (22). Given that self-reports overestimate adherence compared against electronic monitoring, in clinical practice, when self-reports are less than 80%, adherence is extremely likely to be suboptimum (25). However, only self-reports allow a statement about the personal reasons of nonadherence. The prevalence of clinical significant nonadherence observed in our cohort was comparable to that of other studies using self-report in PD patients (2, 22). However, it should be noted that many studies only made a general distinction between adherent/nonadherent. In order to better reflect the broad spectrum of adherence, we have made a classification into three different degrees of adherence (fully, moderate, clinically significant). This shows that nonadherent behavior is very common in elderly people with PD, but it does not always have to be associated with clinical significance.
We found a higher degree of nonadherence in patients with a lower education level, higher motor impairment, a higher number of medications per day, and more motor complications of PD. Associations between a poor clinical state, polypharmacy, or a higher levodopa equivalent dose and nonadherence were reported previously (2–4, 12, 13, 26). Several factors formerly reported to be associated with nonadherence in PD, namely depression, cognitive state, younger age, and disease duration, were not significant predictors of nonadherence in our cohort of elderly PD patients without dementia (2). This requires further consideration. The lack of an association with age may be explained by our focus on elderly subjects and the exclusion of young-onset PD. In contrast, studies which reported that increased age was associated with better overall or timing adherence used electronically monitored bottles to assess adherence (3, 4). These studies are not comparable to our study with a self-report outcome measure, although our sample size was higher. Therefore, we need more sufficient data to substantiate the relationship between adherence and age. We also did not observe a significant association between cognitive disturbances and the overall adherence in the SAMS. The association between cognition and adherence is complex and not yet fully understood (27). While some studies found that cognitive impairment is, in general, a major predictor of nonadherence (28, 29), a recent meta-analysis found no association between cognitive impairment and medication nonadherence in post-stroke patients (23). The Adheson study showed that in relation to the Morisky–Green test, patients with cognitive deterioration were 2.1 times more likely to take the treatment incorrectly (13). Possibly, we did not find an association between adherence and cognition because our study was restricted to PD patients without dementia. This is because we assumed that cognitive deficits may influence the validity of a self-report adherence measure. We also did not observe an association between disease duration and nonadherence. However, we observed an association between motor complications (dyskinesia and dystonia) and nonadherence. Considering that motor complications usually occur in advanced PD disease stages, we conclude that functional impairments are more relevant to nonadherence than disease duration per se.
Self-Reported Reasons for Nonadherence
The use of a comprehensive adherence questionnaire allowed us to gain deeper insight into the reasons for and patterns of nonadherence in PD. A number of studies have shown that intentional nonadherence comprises approximately 50% of all nonadherent behaviors observed among those over the age of 65 years (30). The most common reasons given by our patients for modification of prescribed medication were side effects or feeling worse after taking the medication. However, a relevant proportion of patients do not stop taking medications because they feel worse but because they feel better. Given the plethora of reasons for intentional nonadherence, an individual approach is necessary to positively modulate adherence in elderly people with PD (31, 32). Because many people discontinue medication because of side effects, comprehensive education on side effects and what to do in case of side effects is recommended for every patient. To avoid discontinuation of medication when people feel better, patients must be constantly reminded of the importance of taking medications on a long-term basis in order to positively influence PD symptoms and the course of the disease in the long term.
Forgetting to take medication was the main reason for nonadherence in 37% of patients. Therefore, it is not surprising that interventions to increase adherence which mainly work with reminders are not useful to enhance adherence in general (33). Interestingly, in our study, most patients forgot to take their medication at noon. Therefore, to give long-acting medications, preferably in the morning, seems to be a sensible way to improve adherence.
Lack of knowledge as the main reason for nonadherence was reported in 32.5% of patients. Many patients have problems with the timing of medication (4, 34). However, given the progressive nature of the disease and the occurrence of motor fluctuations during the disease course, correct timing is essential for efficient control of motor symptoms. In line with a former study in older subjects, a relevant proportion of our patients did not exactly know the names, reasons, and dosages of all their prescribed medications (35). Moreover, in other conditions and cohorts, lack of knowledge was found to be a critical determinant of nonadherence independent of education (36, 37).
Factors Associated With Distinct Patterns of Nonadherence
We found that the different clusters of nonadherence were differentially influenced by clinical parameters. A lower education level was mainly associated with modification of medication and poorer knowledge about prescribed medication, but not with forgetting to take medication. We also found that patients with motor complications (higher MDS-UPDRS IV), which frequently occur in advanced disease stages, tended to demonstrate intentional nonadherence by modifying their prescribed medication. This was probably the result of patients' desire to exert control over the treatment and its effects on their bodies (31). Higher motor problems and the consequent higher total number of drugs per day were associated with less knowledge about the names, reasons, and dosages of their prescribed medication.
The study is not free of limitations. Because we were interested in patient-reported and personal-related factors of nonadherence, we did not compare the SAMS with other adherence measures such as electronic monitoring. While this did not affect the results regarding reasons for nonadherence, our estimated prevalence of nonadherence must be considered with caution. This is because self-reports overestimate adherence compared with electronic monitoring (25). Although we included the main known predictors of nonadherence in PD, we were not able to control for all factors influencing adherence (e.g., presence of caregivers, comorbidities etc.). Moreover, our results were restricted to elderly PD patients without PD dementia. Therefore, these results cannot be generalized to PD patients with significant cognitive decline.
Conclusions
Elderly PD patients report many reasons for intentional and non-intentional nonadherence. Forgetting to take medication is common, but it is not the main reason for nonadherence. Several clinical factors were found to be relevant to the question of whether a patient tends to modify or forget medication or has poor knowledge about the prescribed medication. This detailed knowledge about personal factors of nonadherence may positively influence the management of nonadherence in medical care and pave the way toward tailored, patient-centered, theory-driven interventions to improve adherence.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of the Jena University, Jena, Germany. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TP: design of the study. SM, HZ, and TP: collection of data. JG: data management. SM, TP, and HZ: analysis. SM: writing the paper. OW and TP: critical revision of the article.
Funding
This work was supported by a Bundesministerium für Bildung und Forschung grant to TP (01GY1804).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BDI, Becks Depression Inventory; H&Y, Hoehn & Yahr; MDS-UPDRS, Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale; NMS-Quest, non-motor symptoms questionnaire; PD, Parkinson's Disease; SAMS, Stendal Adherence with Medication Score.
References
1. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2014) 29:1583–90. doi: 10.1002/mds.25945
2. Daley DJ, Myint PK, Gray RJ, Deane KH. Systematic review on factors associated with medication non-adherence in Parkinson's disease. Parkinsonism Relat Disord. (2012) 18:1053–61. doi: 10.1016/j.parkreldis.2012.09.004
3. Grosset D, Antonini A, Canesi M, Pezzoli G, Lees A, Shaw K, et al. Adherence to antiparkinson medication in a multicenter European study. Mov Disord. (2009) 24:826–32. doi: 10.1002/mds.22112
4. Grosset KA, Bone I, Grosset DG. Suboptimal medication adherence in Parkinson's disease. Mov Disord. (2005) 20:1502–7. doi: 10.1002/mds.20602
5. Grosset KA, Reid JL, Grosset DG. Medicine-taking behavior: implications of suboptimal compliance in Parkinson's disease. Mov Disord. (2005) 20:1397–404. doi: 10.1002/mds.20525
6. Malek N, Grosset DG. Medication adherence in patients with Parkinson's disease. CNS Drugs. (2015) 29:47–53. doi: 10.1007/s40263-014-0220-0
7. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. (2011) 365:2002–12. doi: 10.1056/NEJMsa1103053
8. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. (2004) 38:303–12. doi: 10.1345/aph.1D252
9. Wimmer BC, Dent E, Bell JS, Wiese MD, Chapman I, Johnell K, et al. Medication regimen complexity and unplanned hospital readmissions in older people. Ann Pharmacother. (2014) 48:1120–8. doi: 10.1177/1060028014537469
10. Yap AF, Thirumoorthy T, Kwan YH. Systematic review of the barriers affecting medication adherence in older adults. Geriatr Gerontol Int. (2016) 16:1093–101. doi: 10.1111/ggi.12616
11. Shin JY, Habermann B. Medication adherence in people with Parkinson disease. J Neurosci Nurs. (2016) 48:185–94. doi: 10.1097/JNN.0000000000000198
12. Straka I, Minar M, Gazova A, Valkovic P, Kyselovic J. Clinical aspects of adherence to pharmacotherapy in Parkinson disease: a PRISMA-compliant systematic review. Medicine (Baltimore). (2018) 97:e10962. doi: 10.1097/MD.0000000000010962
13. Valldeoriola F, Coronell C, Pont C, Buongiorno MT, Camara A, Gaig C, et al. Socio-demographic and clinical factors influencing the adherence to treatment in Parkinson's disease: the ADHESON study. Eur J Neurol. (2011) 18:980–7. doi: 10.1111/j.1468-1331.2010.03320.x
14. Prell T, Grosskreutz J, Mendorf S, Franke GH, Witte OW, Kunze A. Clusters of non-adherence to medication in neurological patients. Res Social Adm Pharm. (2019) 15:1419–24. doi: 10.1016/j.sapharm.2019.01.001
15. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
16. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
17. Prell T, Schaller D, Perner C, Franke GH, Witte OW, Kunze A, et al. Comparison of anonymous versus nonanonymous responses to a medication adherence questionnaire in patients with Parkinson's disease. Patient Prefer Adher. (2019) 13:151–5. doi: 10.2147/PPA.S186732
18. Prell T, Grosskreutz J, Mendorf S, Witte OW, Kunze A. Data on adherence to medication in neurological patients using the German Stendal Adherence to Medication Score (SAMS). Data Brief. (2019) 23:103855. doi: 10.1016/j.dib.2019.103855
19. Baumgartner PC, Haynes RB, Hersberger KE, Arnet I. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front Pharmacol. (2018) 9:1290. doi: 10.3389/fphar.2018.01290
20. DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. (2004) 42:200–9. doi: 10.1097/01.mlr.0000114908.90348.f9
21. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. (2009) 25:2303–10. doi: 10.1185/03007990903126833
22. Fleisher JE, Stern MB. Medication nonadherence in Parkinson's disease. Curr Neurol Neurosci Rep. (2013) 13:382. doi: 10.1007/s11910-013-0382-z
23. Rohde D, Merriman NA, Doyle F, Bennett K, Williams D, Hickey A. Does cognitive impairment impact adherence? A systematic review and meta-analysis of the association between cognitive impairment and medication non-adherence in stroke. PLoS ONE. (2017) 12:e0189339. doi: 10.1371/journal.pone.0189339
24. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. (2015) 2015:217047. doi: 10.1155/2015/217047
25. Grosset KA, Bone I, Reid JL, Grosset D. Measuring therapy adherence in Parkinson's disease: a comparison of methods. J Neurol Neurosurg Psychiatry. (2006) 77:249–51. doi: 10.1136/jnnp.2005.064709
26. Kulkarni AS, Balkrishnan R, Anderson RT, Edin HM, Kirsch J, Stacy MA. Medication adherence and associated outcomes in medicare health maintenance organization-enrolled older adults with Parkinson's disease. Mov Disord. (2008) 23:359–65. doi: 10.1002/mds.21831
27. Smith D, Lovell J, Weller C, Kennedy B, Winbolt M, Young C, et al. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS ONE. (2017) 12:e0170651. doi: 10.1371/journal.pone.0170651
28. Okuno J, Yanagi H, Tomura S. Is cognitive impairment a risk factor for poor compliance among Japanese elderly in the community? Eur J Clin Pharmacol. (2001) 57:589–94. doi: 10.1007/s002280100347
29. Stilley CS, Sereika S, Muldoon MF, Ryan CM, Dunbar-Jacob J. Psychological and cognitive function: predictors of adherence with cholesterol lowering treatment. Ann Behav Med. (2004) 27:117–24. doi: 10.1207/s15324796abm2702_6
30. Mukhtar O, Weinman J, Jackson SH. Intentional non-adherence to medications by older adults. Drugs Aging. (2014) 31:149–57. doi: 10.1007/s40266-014-0153-9
31. Huyard C, Derijks L, Haak H, Lieverse L. Intentional Nonadherence as a Means to Exert Control. Qual Health Res. (2017) 27:1215–24. doi: 10.1177/1049732316688882
32. Shiyanbola OO, Brown CM, Ward EC. “I did not want to take that medicine”: African-Americans' reasons for diabetes medication nonadherence and perceived solutions for enhancing adherence. Patient Prefer Adher. (2018) 12:409–21. doi: 10.2147/PPA.S152146
33. Slomski A. Pill reminders don't improve adherence. JAMA. (2017) 317:2476. doi: 10.1001/jama.2017.7588
34. Buetow S, Henshaw J, Bryant L, O'Sullivan D. Medication timing errors for Parkinson's disease: perspectives held by caregivers and people with Parkinson's in New Zealand. Parkinsons Dis. (2010) 2010:432983. doi: 10.4061/2010/432983
35. Bosch-Lenders D, Maessen DW, Stoffers HE, Knottnerus JA, Winkens B, van den Akker M. Factors associated with appropriate knowledge of the indications for prescribed drugs among community-dwelling older patients with polypharmacy. Age Ageing. (2016) 45:402–8. doi: 10.1093/ageing/afw045
36. Chan YH, Fan MM, Fok CM, Lok ZL, Ni M, Sin CF, et al. Antibiotics nonadherence and knowledge in a community with the world's leading prevalence of antibiotics resistance: implications for public health intervention. Am J Infect Control. (2012) 40:113–7. doi: 10.1016/j.ajic.2011.03.017
Keywords: nonadherence, Parkinson's disease, unified Parkinson's disease rating scale, German stendal adherence with medication score, motor impairment
Citation: Mendorf S, Witte OW, Grosskreutz J, Zipprich HM and Prell T (2020) What Predicts Different Kinds of Nonadherent Behavior in Elderly People With Parkinson's Disease? Front. Med. 7:103. doi: 10.3389/fmed.2020.00103
Received: 14 November 2019; Accepted: 09 March 2020;
Published: 25 March 2020.
Edited by:
Jeremy M. Jacobs, Hadassah Medical Center, IsraelReviewed by:
Rosa Liperoti, Catholic University of the Sacred Heart, ItalyPankaj Gupta, University of Leicester, United Kingdom
Copyright © 2020 Mendorf, Witte, Grosskreutz, Zipprich and Prell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tino Prell, dGluby5wcmVsbCYjeDAwMDQwO21lZC51bmktamVuYS5kZQ==
 Sarah Mendorf
Sarah Mendorf Otto W. Witte
Otto W. Witte Julian Grosskreutz
Julian Grosskreutz Hannah M. Zipprich1
Hannah M. Zipprich1 Tino Prell
Tino Prell