- 1Center for Reproductive Medicine, Shandong University, Jinan, China
- 2National Research Center for Assisted Reproductive Technology and Reproductive Genetics, Shandong University, Jinan, China
- 3Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan, China
- 4Shandong Provincial Clinical Medicine Research Center for Reproductive Health, Shandong University, Jinan, China
- 5Department of Reproductive Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang, China
In recent years, the freeze-all strategy has been widely adopted and applied. However, with the exception of age, the factors that affect the outcomes of frozen embryo transfer are still unclear. Therefore, the identification and mitigation of factors that influence the live birth rate after frozen embryo transfer is a good way to increase the “take-home-baby” rate of frozen embryo transfer. The objective of this study was to identify factors affecting the live birth rate after cleavage-stage frozen embryo transfer in young ovulatory women. This was a secondary analysis from a previously published multicenter randomized controlled trial (ChiCTR-IOR-14005406) that was originally designed to compare the live birth rate and perinatal complications after fresh embryo transfer to those after frozen embryo transfer among ovulatory women. This study was carried out using a portion of the data from the original randomized controlled trial, which included 917 young women who underwent cleavage-stage frozen embryo transfer. The 16 clinical candidate variables potentially affecting the live birth rate after frozen embryo transfer were analyzed. Univariable analysis and multivariable analysis were performed to assess the relationship between predictive factors and outcomes, with the aim of identifying independent predictors of live birth after frozen embryo transfer. In this study, the live birth rate was 53.0% (486/917). Three independent predictors were ultimately identified as the main factors affecting the live birth rate of ovulatory young women. Infertility duration [odds ratio (OR): 0.933, 95% confidence interval (CI): 0.876–0.995, p = 0.033], endometrial thickness before frozen embryo transfer (OR: 3.375, 95% CI: 1.556–7.321 p = 0.002), and the number of embryos transferred (OR: 2.653, 95% CI:1.226–5,743, p = 0.013) were the major factors contributing to the live birth rate after cleavage-stage frozen embryo transfer among young women. The cut-off point for infertility duration was 4.5 years, and the cut-off point for endometrial thickness was 0.89 cm. Infertility duration, endometrial thickness and number of embryos transferred might affect the live birth rate after frozen embryo transfer among young women. This result could help inform clinical decisions and counseling to increase the live birth rate after frozen embryo transfer among young women.
Introduction
In 1983, the first successful pregnancy after frozen embryo transfer (FET) was reported, and since then, FET has become an important procedure for infertility treatment (1, 2). However, compared with that of fresh embryo transfer, the pregnancy outcome of FET is controversial. To verify the effects of freezing embryos on pregnancy outcomes, many studies have compared the pregnancy outcomes of FET with those of fresh embryo transfer (3–6). Several studies have reported no significant difference between the live birth rate (LBR) after FET and that after fresh embryo transfer in ovulatory women (3, 4). Other studies reported that the LBR after FET was better than that of fresh embryo transfer (5, 6). Additionally, FET provides many advantages the ability to achieve maximum oocyte retrieval by storing additional viable embryos (7), reduce the incidence of ovarian hyperstimulation syndrome, to decrease the multiple pregnancy risk by reducing the number of transferred embryos (8), test the embryos of couples with a genetic disease (9), and arrange opportunities for unsynchronized embryo donations (10). Therefore, although fresh embryo transfer is still routine, in recent years, the freeze-all strategy has been widely adopted and applied. Identifying and influencing the factors that influence FET outcomes can help to increase the “take-home-baby” rate of FET. Hence, the focus of research has recently shifted toward identifying relevant factors that affect FET outcomes. Various clinical factors, including female age, body mass index (BMI), infertility duration, infertility etiology, protocol type, endometrial preparation regimen for FET, and so on, are thought to affect FET outcomes (11, 12). Age is a well-known factor that affects the LBR after FET. However, other factors that could affect the outcome of FET are still unclear. Although the number of older women with infertility has increased annually, young women with infertility still constitute most of the infertility population. The factors that affect the FET outcomes of young women are common concerns among physicians. Hence, we chose young patients (age ≤ 35 years) as the target population to identify the factors affecting the LBR after FET in young women.
We recently completed a large multicenter randomized controlled trial (RCT) that compared the pregnancy outcome of fresh embryo transfer to that of FET in ovulatory women (3). The results of this RCT indicated no significant difference in the LBR between fresh embryo transfer and FET. The current study is a secondary analysis of the FET portion of the RCT. The purpose of this study was to determine the factors affecting the outcome of FET, increase the LBR, and provide information for clinical decisions and consultations.
Materials and Methods
Study Population
This secondary analysis data was derived from a previously published multicenter RCT in China. The original study was carried out by 20 participating clinical sites in China and recruited 2157 ovulatory women with infertility who underwent their first in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycle from March 2015 to November 2015. The original study obtained ethics approval from Ethics Committee at the Center for Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong University ([2014] No. 18) and each participating center. All patients signed informed consent forms before inclusion in the study and were identified by the participant number only. We included only 917 participants who ultimately underwent the cleavage-stage frozen embryo transfer procedure (excluding those with missing data) (Supplemental Figure 1). The details of the administration and main outcome of the RCT have been reported (3).
The inclusion criteria were as follows: ovulatory women, aged 25–35 years, who had infertility for more than 1 year, were diagnosed with tubal factor or male factor infertility, and had undergone their first in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycle. The exclusion criteria were unilateral oophorectomy history, polycystic ovary syndrome (PCOS) history, uterine abnormality, abnormal parental karyotypes, recurrent spontaneous abortion, or chronic medical conditions that contraindicated pregnancy.
Study Procedures
All participants underwent a standard ovarian stimulation protocol and luteal phase support protocol according to a routine method. The complete details of the trial design and the main outcomes have been previously reported (3). Controlled ovarian hyperstimulation and oocyte retrieval protocols have been extensively described. Treatment with recombinant follicle-stimulating hormone (rFSH, Puregon, MSD, Ravensburg, Germany) 75~225 IU was initiated on days 1–3 of the menstrual cycle and continued for 5 days. Then, the rFSH dose was adjusted, and human menopausal gonadotropin (HMG, Menopur, Ferring, Switzerland) was added to the regimen according to the patient's ovarian response at the discretion of the local investigators. Gonadotropin-releasing hormone antagonist (Ganirelix, Orgalutran, MSD, Ravensburg, Germany) 0.25 mg was started when the leading follicle was ≥12 mm. Human chorionic gonadotropin (HCG 4,000–8,000 IU) was administered when at least 3 follicles were ≥18 mm, and follicle aspiration was performed 34–36 h later. After follicular aspiration, IVF/ICSI or rescue ICSI was performed. The embryos were assessed by the number and regularity of the blastomeres and the percentage of fragmentation. In the primary RCT, participants were randomly divided into a fresh embryo group and a frozen embryo group. In this analysis, we included only women who had ultimately transferred D3 frozen embryos and excluded those who were lost to follow-up and those with missing data. All the embryos of these patients were cryopreserved by vitrification. After the oocyte was aspirated, endometrial preparation began as early as the next menstrual cycle. For endometrial preparation, a natural or artificial cycle was chosen by local physicians. The natural cycle was the first choice. If the natural ovulation cycle was canceled, an artificial cycle was used in next the cycle. For natural cycles, ovulation in the participants was monitored by ultrasound. Two embryos were transferred 3 days after ovulation. For luteal phase support, after ovulation, participants started oral dydrogesterone 10 mg twice daily until 10 weeks of gestation. For artificial cycles, the participants were given 4–8 mg estradiol valerate per day, and then endometrial thickness was monitored by ultrasound. When the endometrial thickness was ≥7 mm, two embryos were transferred according to their cryopreservation stages. The participants began their use of vaginal progesterone gel 90 mg/d and oral dydrogesterone 10 mg twice daily. When the patient became pregnant, estradiol (E2) use was gradually stopped. Vaginal progesterone gel use continued until the fetal heartbeat was heard, and oral dydrogesterone was stopped at 10 weeks of gestation. All pregnancies were included in the follow-up assessment until delivery.
Statistical Analysis
Live birth was the primary endpoint of the study. Sixteen candidate variables that may affect the LBR after FET were selected according to clinical experience and the literature. First, descriptive statistics and univariate analyses were performed. Continuous variables were summarized as the mean ± SD and compared between the live birth group and no live birth group by t-test. Qualitative variables were summarized with percentages and compared between live birth cycles and no live birth cycles by the chi-square test or Fisher's exact test. When the individual expected count was <5, we used Fisher's exact test. Then, univariate and multivariate logistic regression were used to assess the relationships between predictive factors and outcomes. Clinically relevant predictive variables and univariable analysis covariates with p < 0.15 were included in the multivariable analysis. Finally, 8 predictors were analyzed in the multivariable analysis, including the infertility duration, causes of infertility, endometrial thickness, endometrial preparation regimen for FET, LH level, number of embryos transferred, number of top-quality embryos, and oocyte fertilization method. To avoid collinearity, the variance inflation factor (VIF) was used to detect the presence of collinearity between two or more independent variables in this multivariable model. No collinearity was detected among variables. In the multivariate logistic regression analysis, independent factors were determined by a backward variable selection approach in which the least significant variable was removed one by one. Only variables with p < 0.05 were considered for inclusion in the final multivariable model. The Hosmer-Lemeshow goodness-of-fit statistic was used to examine the performance of the final model. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the performance of infertility duration and endometrial thickness in FET. The optimal cut-off points were determined using the Youden index. All statistical analyses were performed using SPSS statistics software (SPSS Inc., version 22.0; Chicago, IL, USA).
Results
In this study, 1,803 embryos were transferred into 917 young women. The overall LBR was 53% (486/917), and the all twins birth rate was 36.8% (179/486). A total of 886 patients transferred two embryos, that the twins birth rate was 37.6% (179/476) after transfer of two frozen embryos. All first FET cycles were completed <1 year after cryopreservation.
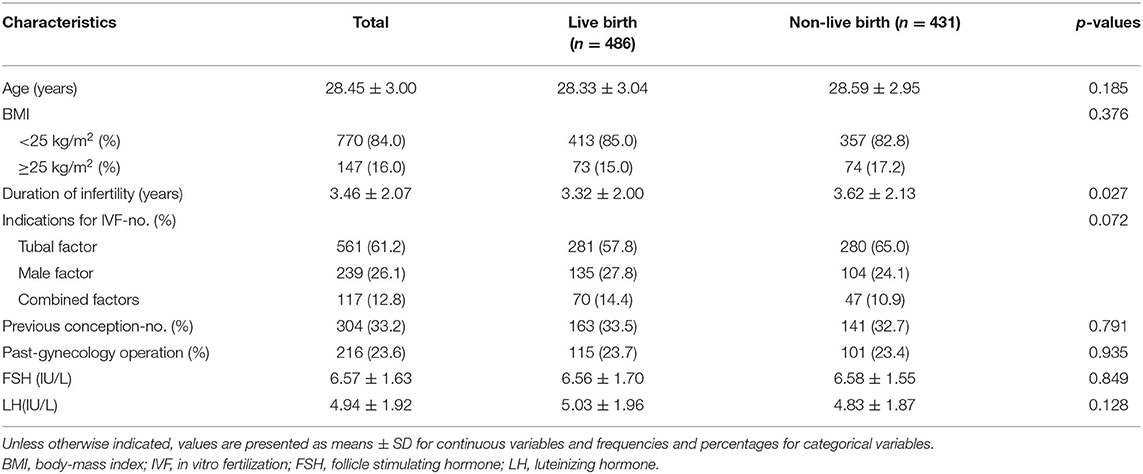
The baseline maternal characteristics that were examined for their effects on the LBR after FET are presented in Table 1. According to our analysis, significant differences were found in infertility duration between the live-birth and non-live-birth groups (3.32 ± 2.00 years vs. 3.62 ± 2.13 years p = 0.027). The longer the infertility time was, the lower the LBR would be. Within the BMI < 25 kg/m2 and BMI ≥ 25 kg/m2 groups, there were no significant differences in BMI between the live-birth and non-live-birth subgroups. Regarding the other factors, the differences were not statistically significant between the live-birth group and non-live-birth group (Table 1).
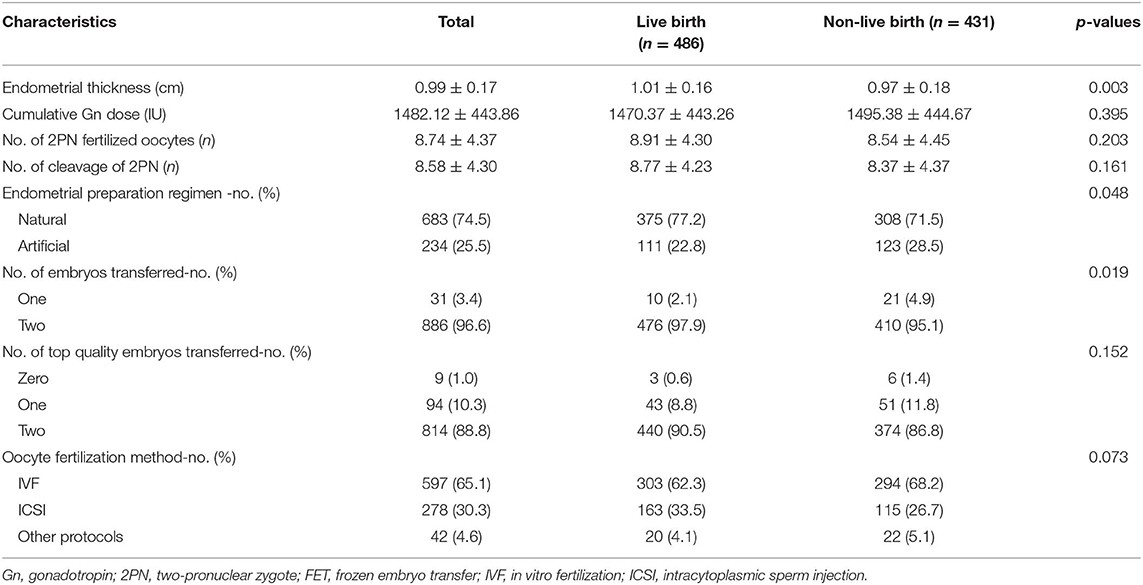
The embryological and clinical factors that may affect the LBR after FET cycles are shown in Table 2. According to the statistical analysis, there were no statistically significant differences in the cumulative gonadotropin (Gn) dose, number of two-pronuclear zygote (2PN) fertilized oocytes, number of cleavage-stage 2PN embryos or number of top-quality embryos transferred between the live-birth group and non-live-birth groups (Table 2). We found that the LBR of the natural cycles was higher than that of the artificial cycles, and that the difference was statistically significant (54.9 vs. 47.4% p = 0.048). A significant difference in endometrial thickness (1.01 ± 0.16 cm vs. 0.97 ± 0.18 cm, p = 0.003) was observed between the two groups. We observed a statistically significant difference in the LBR between FET with one frozen embryo and FET with two embryos. The LBR of the transfer of two frozen embryos was higher than that of a single transfer (53.3 vs. 32.3% p = 0.015).
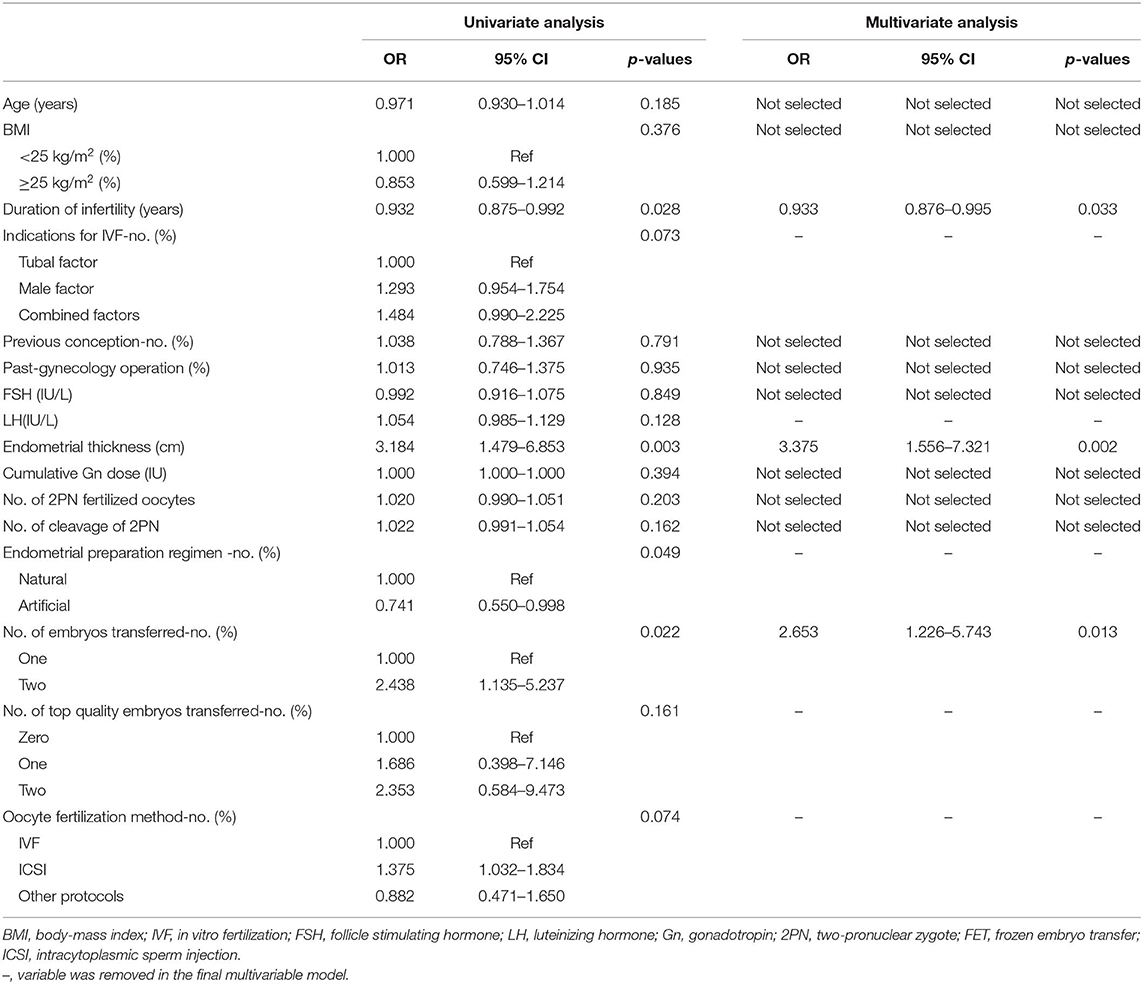
The results of the univariate and multivariate logistic regression analyses of predictive factors for FET pregnancy outcome are summarized in Table 3. Finally, based on the univariate analysis results, infertility duration, endometrial thickness, endometrial preparation regimen for FET, timing of embryo transfer, number of embryos transferred, number of top-quality embryos, and oocyte fertilization method were included in the multivariable analysis. The Hosmer–Lemer goodness-of-fit test showed p = 0.313, which indicated that the data fit the model well. The multivariable logistic regression showed that infertility duration (OR: 0.933, 95% Cl: 0.876–0.995, p = 0.033), endometrial thickness (OR: 3.375, 95% Cl: 1.556–7.321 p = 0.002), and number of embryos transferred (OR: 2.653, 95% Cl: 1.226–5.743 p = 0.013) were the major factors affecting the “take-home-baby” rate of FET.
ROC curve analysis of infertility duration in FET and endometrial thickness in FET was performed for the predictive assessment of live birth (Figure 1). For infertility duration, the area under the curve (AUC) was 0.543. Youden's index for infertility duration was 0.07656, the cut-off point was 4.5 years, and the specificity and sensitivity were 29.47% and 78.19%, respectively. For endometrial thickness, the AUC was 0.562. Youden's index of endometrial thickness was 0.1037, the cut-off point was 0.89 cm, and the specificity and sensitivity were 33.41% and 76.95%, respectively.
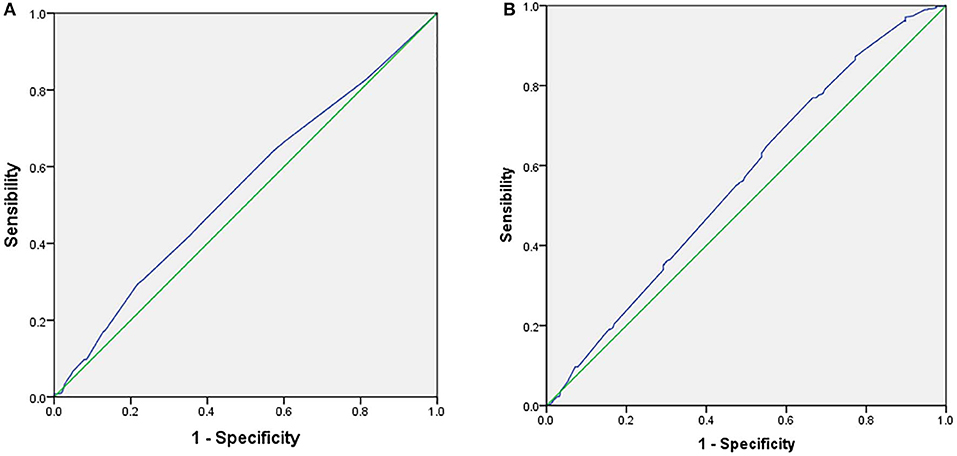
Figure 1. Receiver operator characteristic curve (ROC) for the predictive parameters of live birth rate in frozen embryo transfer. (A) ROC for duration of infertility as predictors of LBR in FET. (B) Receiver operator characteristic curve (ROC) for endometrial thickness as predictors of LBR in FET.
Discussion
This study assessed the LBR of 917 FET participants using a portion of the data from a large multicenter RCT. According to our study, in ovulating women, the infertility duration, endometrial thickness, and number of embryos transferred were important factors that affected the LBR after FET among young women.
Female age was a critical factor in predicting the LBR after FET. Recently, many large studies have shown that oocyte quality is poor in women who are older than 35 years and that this age group has lower ongoing pregnancy and clinical pregnancy rates than younger women (13–15). In this study, all included women were below 35 years of age. We still evaluated the effect of young age on the LBR, aiming to eliminate the effect of age on the pregnancy outcomes of women below 35 years of age. The results of this study showed no correlation between age and LBR among patients under 35 years of age.
There has been controversy among recent studies regarding whether an increase in BMI has an impact on the outcome of FET. Farhi et al. (16) found that the implantation and pregnancy rates of women with a BMI ≥ 25 kg/m2 were not significantly different from those of women with a BMI < 25 kg/m2 when high-quality embryos were transferred. Insogna et al. (17) studied 461 FET patients with good-quality day-5 or day-6 embryos following identical hormone replacement endometrial therapy. They found no statistically significant differences in the implantation rate or live-birth rate among underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2) women. In contrast, a retrospective study found that obesity was associated with declines in the clinical pregnancy rate and LBR (18). In our study, the number of patients with a BMI ≥ 30 kg/m2 was only 14, which was a small number of patients. Therefore, all patients were divided into two groups (BMI < 25 and ≥25.0 kg/m2). The results showed the absence of an association between the LBR and overweight. Whether a BMI ≥ 30 kg/m2 affects the LBR among young ovulating women still needs to be confirmed in a future study.
In this study, we found that prolonged infertility was associated with a decrease in the LBR after FET. Cai et al. (19) and Nelson et al. (20) reported that the infertility duration was a predictive factor of the FET outcome after IVF/ICSI. The pathogenesis of the adverse effect of prolonged infertility on pregnancy outcome remains unknown. However, this factor suggested that extending the waiting time for treatment during early infertility might hinder the outcome of assisted reproduction. This study showed that the cut-off point for infertility duration was 4.5 years, meaning that when the infertility time exceeded 4.5 years, the LBR after FET would be affected. Physicians need to advise women with long-term infertility to receive treatment by assisted reproductive technology and undergo FET as soon as possible.
Endometrial thickness is a crucial factor in determining the timing of FET. Bu et al. (21) found that endometrial thickness made a significant difference in the outcomes of FET cycles. El-Toukhy et al. (22) found significantly higher implantation and pregnancy rates for women with an endometrial thickness of 9–14 mm than in women with an endometrial thickness of 7–8 mm in exogenous hormone replacement cycles. In contrast, some studies found no correlation between FET cycle endometrial thickness and the LBR after FET (23, 24). Our results showed that endometrial thickness was one of the most important major predictive factors of a successful outcome following fresh transfers and FETs. The ROC curves showed that the cut-off point for endometrial thickness was 8.9 mm. This value is consistent with the clinical reality. Thus, when the endometrial thickness of a patient is ≥9 mm, the probability of a live birth is observed to be higher.
We observed that the LBR for natural cycles is higher than that for artificial cycles. However, we did not find a relationship between the LBR and the endometrial preparation regimen for FET after multivariate logistic regression analysis. Morozov et al. (25) showed that hormone replacement treatment for FET was associated with a lower endometrial thickness than that of natural cycles. Endometrial thickness is a major factor that can affect the LBR. Therefore, the two different endometrial preparation methods for FET might affect the FET outcome. Kawamura et al. (26) reported a prospective trial showing that natural cycles had a higher LBR than cycles with hormone replacement treatment for FET, but the difference was not statistically significant. A retrospective analysis showed that the natural cycle for the cleavage-stage frozen embryo was associated with a higher LBR than the cycle with hormone replacement treatment (27). Therefore, whether the two different endometrial preparation methods for FET affect the FET outcome remains unclear. Further research is necessary and warranted to clarify this issue.
In accordance with previous studies, the number of embryos, and number of top-quality embryos transferred affect the pregnancy outcome (28, 29). According to our results, only the number of frozen embryos transferred was an important clinical factor influencing LBR. The LBR associated with the transfer of two frozen embryos was higher than that associated with the transfer of one embryo. We did not find an association between the LBR and the number of top-quality frozen embryos transferred. However, these unrelated conclusions should be carefully considered because the original RCT protocol for the majority of FET cycles specified the selection of two top-quality D3 frozen-thawed embryos. Nevertheless, due to an insufficient number of embryos, poor embryo quality, or patient preference, a small proportion of patients had only one or zero D3 top-quality frozen-thawed embryo to transfer. Hence, further studies are needed to determine whether the timing of embryo transfer and the number of top-quality embryos transferred affect the LBR after FET.
The original data for this study were obtained from our previous RCT. All the patients were randomly assigned to the study, which should reduce the risk of bias. The relatively large sample size should lead to the most reliable and accurate results. To our knowledge, few studies addressing the factors affecting the LBR after FET, especially among young women, are available. Most publications report biochemical pregnancy or clinical pregnancy rather than live birth. We chose the LBR as the endpoint to better help guide clinical practice, and this is the first study to analyze the independent risk factors that could affect the LBR after FET in the young female population. There are also limitations in our study. The original data were from our previous RCT, in which the conditions for enrollment were strictly controlled. The original RCT selected a population of patients in whom two good embryos were transferred, which might lead to a high twin pregnancy rate. Therefore, it was difficult to represent the broader population. Thus, in the future, a large series of patients will be necessary to confirm the factors influencing the FET outcome.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee at Center for Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YS and Z-JC designed the trial and were in charge of the trial conduct. YP, GH, and YS designed the study. YS, HL, YP, GH, and ZW acquired the data. ZW, YP, and QW performed the statistical analyses. YP, HL, ZW, QJ, and QW interpreted the data. YP wrote the first draft of the report with inputs from GH, HL, YS, and Z-JC provided comments, participated in additional discussions, and revised the paper. All authors approved the final version.
Funding
This work was supported by National Key R&D Program of China (2018YFC1003202 and 2017YFC1001004) and Taishan scholar project special funds (No. ts201712103).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors wish to thank all patients, all investigators, and all participants who assisted with the data collection in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00094/full#supplementary-material
References
1. Roque M, Valle M, Sampaio M, Geber S. Obstetric outcomes after fresh versus frozen-thawed embryo transfers: a systematic review and meta-analysis. JBRA Assist Reprod. (2018) 22:253–60. doi: 10.5935/1518-0557.20180049
2. Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. (1983) 305:707–9. doi: 10.1038/305707a0
3. Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. (2018) 378:126–36. doi: 10.1056/NEJMoa1705334
4. Vuong LN, Dang VQ, Ho TM, Huynh BG, Ha DT, Pham TD, et al. IVF transfer of fresh or frozen embryos in women without polycystic ovaries. N Engl J Med. (2018) 378:137–47. doi: 10.1056/NEJMoa1703768
5. Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. (2019) 393:1310–8. doi: 10.1016/S0140-6736(18)32843-5
6. Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, et al. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. (2017) 107:723–30.e3. doi: 10.1016/j.fertnstert.2016.12.022
7. Tiitinen A, Hydén-Granskog C, Gissler M. What is the most relevant standard of success in assisted reproduction? The value of cryopreservation on cumulative pregnancy rates per single oocyte retrieval should not be forgotten. Hum Reprod. (2004) 19:2439–41. doi: 10.1093/humrep/deh446
8. Tiitinen A, Halttunen M, Härkki P, Vuoristo P, Hyden-Granskog C. Elective single embryo transfer: the value of cryopreservation. Hum Reprod. (2001) 16:1140–4. doi: 10.1093/humrep/16.6.1140
9. Chen HH, Huang CC, Cheng EH, Lee TH, Chien LF, Lee MS. Optimal timing of blastocyst vitrification after trophectoderm biopsy for preimplantation genetic screening. PLoS ONE. (2017) 12:e0185747. doi: 10.1371/journal.pone.0185747
10. Sills ES, McLoughlin LJ, Genton MG, Walsh DJ, Coull GD, Walsh AP. Ovarian hyperstimulation syndrome and prophylactic human embryo cryopreservation: analysis of reproductive outcome following thawed embryo transfer. J Ovarian Res. (2008) 1:7. doi: 10.1186/1757-2215-1-7
11. Eftekhar M, Rahmani E, Pourmasumi S. Evaluation of clinical factors influencing pregnancy rate in frozen embryo transfer. Iran J Reprod Med. (2014) 12:513–8.
12. Veleva Z, Orava M, Nuojua-Huttunen S, Tapanainen JS, Martikainen H. Factors affecting the outcome of frozen-thawed embryo transfer. Hum Reprod. (2013) 28:2425–31. doi: 10.1093/humrep/det251
13. Karlström PO, Bergh T, Forsberg AS, Sandkvist U, Wikland M. Prognostic factors for the success rate of embryo freezing. Hum Reprod. (1997) 12:1263–6. doi: 10.1093/humrep/12.6.1263
14. Berin I, McLellan ST, Macklin EA, Toth TL, Wright DL. Frozen-thawed embryo transfer cycles: clinical outcomes of single and double blastocyst transfers. J Assist Reprod Genet. (2011) 28:575–81. doi: 10.1007/s10815-011-9551-7
15. Wang JX, Yap YY, Matthews CD. Frozen-thawed embryo transfer: influence of clinical factors on implantation rate and risk of multiple conception. Hum Reprod. (2001) 16:2316–9. doi: 10.1093/humrep/16.11.2316
16. Farhi J, Ben-Haroush A, Sapir O, Fisch B, Ashkenazi J. High-quality embryos retain their implantation capability in overweight women. Reprod Biomed Online. (2010) 21:706–11. doi: 10.1016/j.rbmo.2010.06.040
17. Insogna IG, Lee MS, Reimers RM, Toth TL. Neutral effect of body mass index on implantation rate after frozen-thawed blastocyst transfer. Fertil Steril. (2017) 108:770–6.e1. doi: 10.1016/j.fertnstert.2017.08.024
18. Zhang J, Liu H, Mao X, Chen Q, Fan Y, Xiao Y, et al. Effect of body mass index on pregnancy outcomes in a freeze-all policy: an analysis of 22,043 first autologous frozen-thawed embryo transfer cycles in China. BMC Med. (2019) 17:114. doi: 10.1186/s12916-019-1354-1
19. Cai QF, Wan F, Huang R, Zhang HW. Factors predicting the cumulative outcome of IVF/ICSI treatment: a multivariable analysis of 2450 patients. Hum Reprod. (2011) 26:2532–40. doi: 10.1093/humrep/der228
20. Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Med. (2011) 8:e1000386. doi: 10.1371/journal.pmed.1000386
21. Bu Z, Wang K, Dai W, Sun Y. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. Gynecol Endocrinol. (2016) 32:524–8. doi: 10.3109/09513590.2015.1136616
22. El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. (2008) 89:832–9. doi: 10.1016/j.fertnstert.2007.04.031
23. Yang W, Zhang T, Li Z, Ren X, Huang B, Zhu G, et al. Combined analysis of endometrial thickness and pattern in predicting clinical outcomes of frozen embryo transfer cycles with morphological good-quality blastocyst: A retrospective cohort study. Medicine. (2018) 97:e9577. doi: 10.1097/MD.0000000000009577
24. Zhang T, Li Z, Ren X, Huang B, Zhu G, Yang W, et al. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: a retrospective cohort study of 1512. IVF cycles with morphologically good-quality blastocyst. Medicine. (2018) 97:e9689. doi: 10.1097/MD.0000000000009689
25. Morozov V, Ruman J, Kenigsberg D, Moodie G, Brenner S. Natural cycle cryo-thaw transfer may improve pregnancy outcome. J Assist Reprod Genet. (2007) 24:119–23. doi: 10.1007/s10815-006-9100-y
26. Kawamura T, Motoyama H, Yanaihara A, Yorimitsu T, Arichi A, Karasawa Y, et al. Clinical outcomes of two different endometrial preparation methods for cryopreserved-thawed embryo transfer in patients with a normal menstrual cycle. Reprod Med Biol. (2007) 6:53–7. doi: 10.1111/j.1447-0578.2007.00165.x
27. Guan Y, Fan H, Styer AK, Xiao Z, Li Z, Zhang J, et al. A modified natural cycle results in higher live birth rate in vitrified-thawed embryo transfer for women with regular menstruation. Syst Biol Reprod Med. (2016) 62:335–42. doi: 10.1080/19396368.2016.1199064
28. Salumets A, Suikkari AM, Mäkinen S, Karro H, Roos A, Tuuri T. Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod. (2006) 21:2368–74. doi: 10.1093/humrep/del151
Keywords: duration of infertility, endometrial thickness, frozen embryo transfer, factors, live birth rate
Citation: Pan Y, Hao G, Wang Q, Liu H, Wang Z, Jiang Q, Shi Y and Chen Z-J (2020) Major Factors Affecting the Live Birth Rate After Frozen Embryo Transfer Among Young Women. Front. Med. 7:94. doi: 10.3389/fmed.2020.00094
Received: 16 December 2019; Accepted: 04 March 2020;
Published: 24 March 2020.
Edited by:
Johnny S. Younis, The Baruch Padeh Medical Center, Poriya, IsraelReviewed by:
Ahmad Hosseini, Independent Researcher, Tehran, IranHannu Kullervo Martikainen, University of Oulu, Finland
Copyright © 2020 Pan, Hao, Wang, Liu, Wang, Jiang, Shi and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhua Shi, c2hpeXVodWEyMDAzQDEyNi5jb20=
†These authors have contributed equally to this work
 Ye Pan
Ye Pan Guimin Hao
Guimin Hao Qiumin Wang
Qiumin Wang Hong Liu
Hong Liu Ze Wang
Ze Wang Qi Jiang
Qi Jiang Yuhua Shi
Yuhua Shi Zi-Jiang Chen
Zi-Jiang Chen