- 1Department of Rheumatology and Immunology, Peking University People's Hospital, Beijing, China
- 2Beijing Key Laboratory for Rheumatism and Immune Diagnosis (BZ0135), Peking University People's Hospital, Beijing, China
Objective: Recent studies on double negative B cells (DN B cells) suggested that they have potential pathogenic roles in systemic lupus erythematosus (SLE). This study aimed to determine the circulating DN B cells in SLE patients and analyzed the clinical significance of this cell subset.
Methods: Fifty-seven SLE patients and fifty healthy controls (HCs) were recruited in this study. Among the 57 SLE patients, 25 had lupus nephritis (LN). All patients were followed up for 24 weeks. Peripheral B cell subsets were analyzed by flow cytometry.
Results: DN B cells were significantly elevated in the SLE patients, especially in the patients with LN (p < 0.01). DN B showed a positive correlation with 24-h urine protein excretion (24 h-UPE) levels (r = 0.444, p = 0.034) in LN patients, and inversely correlated with evaluated glomerular filtration rate (eGFR) (r = −0.351, p = 0.011). DN B cells had a positive correlation with plasma cells (r = 0.484, p < 0.001) and memory B cells (r = 0.703, p < 0.001). After treatment, decreased DN B cells were associated with LN alleviation (p = 0.002). In the follow-up, the remission rate of LN patients with decreased DN B cells was significantly higher than LN patients with increased DN B cells (83.33 vs. 25.00%, p = 0.030) at week 24.
Conclusions: This study suggests that the peripheral DN B cells are positively correlated with the severity of renal damage in LN patients and may potentially be used as a prognostic marker in LN.
Introduction
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease with heterogeneous clinical manifestations that causes organ damage, and is triggered by a loss of self-tolerance, and autoreactive immune responses (1–3). Lupus nephritis (LN) is a frequent complication of SLE which can lead to significant illness and even death, despite great advances in treatment over the recent decades (4–7). The development of LN involves multiple pathogenic pathways, including inflammatory cell infiltration, autoantibody production, aberrant apoptosis, immune complex deposition, and complement activation (4, 8, 9). B cells are prominently involved in the pathogenesis of SLE and connect innate immunity with adaptive immunity, since they can both act as effector cells responding to antigens in humoral immunity and sense the environment and present autoantigens to T cells as antigen-presenting cells (10–12). Hyperactive polyclonal B cells could produce excessive pathogenic autoantibodies, cytokines, and chemokines (10, 13, 14).
The B cell compartment is severely unbalanced in patients with active SLE (15–18). In a previous study, Wei et al. described the expansion of B cells as characterized by lacking both IgD and CD27 (double negative; DN) in SLE and postulated that they represent a novel subset of memory cells (19). Double negative B cells (DN B cells) both in healthy subjects and SLE patients express switched and mutated antibodies. It has been proposed that DN B cells are involved in the pathogenesis of SLE. DN B cells were reported to be correlated with anti-dsDNA and anti-RNP/Sm autoantibodies (19–21). However, the detailed clinical relevance of DN B cells in SLE remains unclear.
In this study, we determined the circulating DN B cells in SLE patients and analyzed the clinical significance of this cell subset. Our study shows that DN B cells are positively correlated with 24-h urine protein excretion (24 h-UPE) in patients with lupus nephritis regardless of disease activity and decreased DN B cells are associated with renal alleviation. Thus, our finding suggests that DN B cells may be potential therapeutic targets for the treatment of lupus nephritis.
Materials and Methods
Patients and Healthy Controls
Patients with SLE satisfying the Systemic Lupus International Collaborating Clinics classification criteria were recruited from the Department of Rheumatology and Immunology in Peking University People's Hospital between February 2016 and January 2017. Fifty age-matched healthy controls (HCs) were enrolled. All participants were older than 16 years of age. All patients received standard-of-care therapy. The medications received by the patients were prednisone and other immunosuppressants, which were shown in Table S1. This study was approved by the ethics committee of Peking University People's Hospital. All patients provided informed consent to donate their blood samples and clinical information for research, and written consent was given by each individual.
Flow Cytometry Analysis
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density gradient centrifugation. For surface staining, PBMCs were stained for 30 min in the dark at 4°C with the monoclonal antibodies Alexa Fluor 700 mouse anti-human CD3 (Biolegend), PE-CF594 mouse anti-human CD19 (BD Biosciences), PE mouse anti-human CD24 (eBioscience), APC mouse anti human CD27 (Biolegend), BV421 mouse anti-human CD38 (BD Biosciences), FITC mouse anti-human IgD (Biolegend), and PerCp-Cy5.5 mouse anti-human CD20 (Biolegend). Based different expression of surface markers, B cell subsets were identified: DN B cells (CD19+CD27–lgD–), memory B cells (CD19+CD27+), and plasma B cells (CD19+CD20–CD27+CD38+). Samples were examined on a BD FACS Aria II flow cytometer and data were analyzed by FlowJo version X.
Clinical and Laboratory Evaluation
Clinical and laboratory data were collected at baseline, week 6, 12, and 24. Patient follow-ups were as scheduled by the treating physician. The following features of SLE were included in this study: rash, alopecia, photosensitivity, ulceration, myositis, fever, arthritis, leukopenia, thrombocytopenia, lupus nephritis, pericarditis, vasculitis, and NPSLE. Disease activity according to the Systemic Lupus Erythematosus Disease Activity Index-2000 (SLEDAI)-2K was recorded. A diagnosis of LN was made if patients fulfilled the American College of Rheumatology renal criteria (persistent proteinuria >0.5 g per day or >3+ by dipstick and/or cellular casts, including red cells, hemoglobin, granular, tubular, or mixed) at any time during the study. White cell and platelet counts <3.50 × 109/L and 125 × 109/L were regarded as leukocytopenia and thrombocytopenia, respectively. All patients underwent extensive serological examinations, including tests of antinuclear antibody (ANA), anti-dsDNA antibody (anti-dsDNA), anti-Sm antibody (anti-Sm), anti-Ro/SSA antibody (anti-SSA), anti-La/SSB antibody (anti-SSB), anti-RNP antibody (anti-RNP), complement component C3, complement component C4, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM). C3, C4, IgA, IgG, and IgM were tested by ELISA with normal ranges of 0.79–1.52 g/L, 0.16–0.38 g/L, 0.82–4.53 g/L, 7.2–16.8 g/L, and 0.46–3.04 g/L. Anti-SSA and anti-SSB were measured by ELISA, and ANA was measured by indirect immunofluorescence. CRP was examined by immunonephelometry assays and values equal to or more than 8 mg/L were considered positive. Serum IFN-alpha2, IL-6, IL-2, IL-21, IL-7, IFN-gamma, IL-12p70, IL-15, IL-17A, BCA-1, IL-10, and TGF-beta were measured by ELISA. Complete renal remission (CR) in this study was defined as 24 h-UPE was <0.5 g/day and absence of hemoglobinuria or pyuria after therapy. Partly remissive (PR) LN was defined as a decrease of 24 h-UPE >50% after therapy.
Statistical Analysis
SPSS 22.0 for windows and GraphPad Prism 7 were used to analyze the data. Data were presented as mean ± standard deviation and statistical significance between the two groups was assessed with the non-parametric Mann-Whitney test, paired t-test, and X2 test. Spearman's rank correlation coefficient was applied to calculate the correlations. Bonferroni correction was performed to adjust multiple comparisons in our correlation analysis. The Kaplan–Meier method was applied to compare the renal remission of the two groups during the follow-up. A value of p < 0.05 was considered to be significant.
Results
Characteristics of SLE Patients
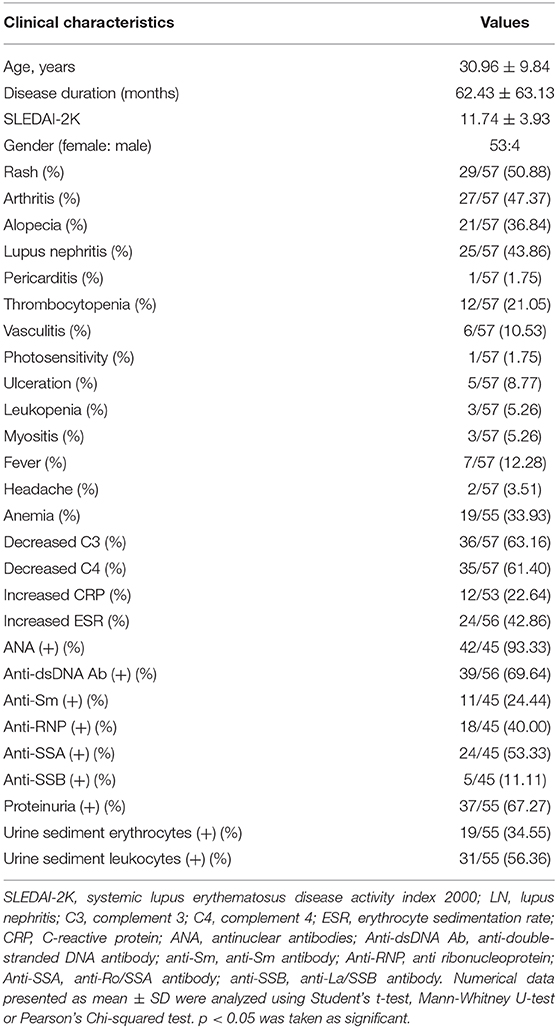
In this study, 57 SLE patients and 50 healthy controls with matched demographic features (age, 30.96 ± 9.84 vs. 30.52 ± 5.66, p = 0.399; male/female, 4/53 versus 7/43, in SLE and HC, respectively, p = 0.386) were recruited. Detailed characteristics of SLE patients are shown in Table 1. The SLE patients had a mean disease duration of 62.43 months, ranging from 0 to 240 months and the mean SLEDAI of the patients was 11.74, ranging from 6 to 27 (Table 1).
Circulating DN B Cells Increased in SLE Patients
As shown in Figures 1A,B, we evaluated the levels of CD19+CD27-IgD- DN B cells in PBMCs of HCs and SLE patients by flow cytometry. Compared with HCs, the SLE patients showed a significant increase of DN B cells (13.70 ± 8.28 vs. 5.95 ± 4.09%, p < 0.01; Figure 1C).
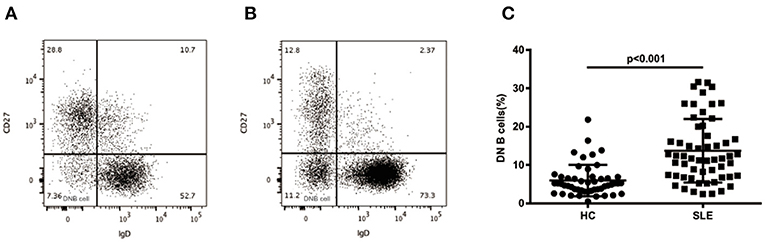
Figure 1. Surface phenotype of peripheral blood DN B cells. Dot plots represent staining of peripheral B cells in healthy subjects (A) and SLE (B) patients with increased frequency of DN B cells. (C) Comparative analysis of blood DN B cells from healthy controls and different groups of SLE patients. Higher levels of DN B cells were detected in SLE patients (n = 57) than in healthy subjects (n = 50). Differences compared to the HC were assessed by Mann-Whitney test.
Increased DN B Cells Were Correlated With Renal Involvement in SLE Patients
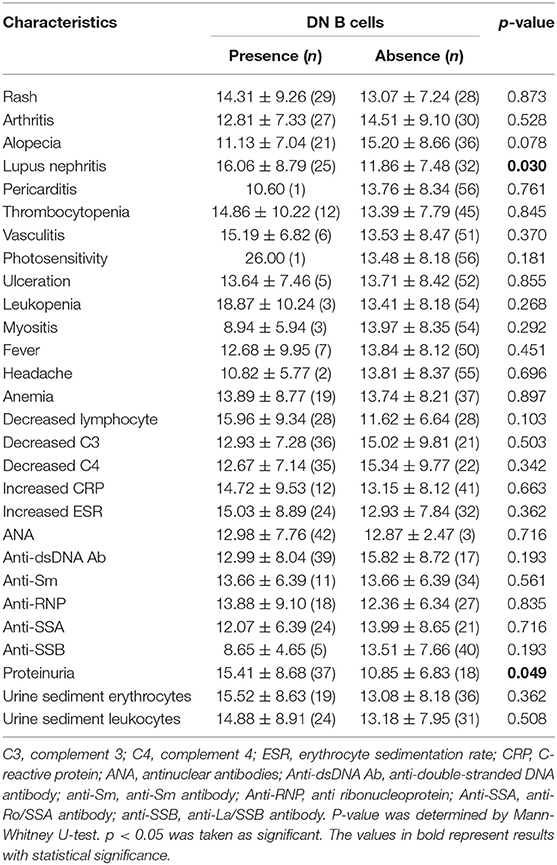
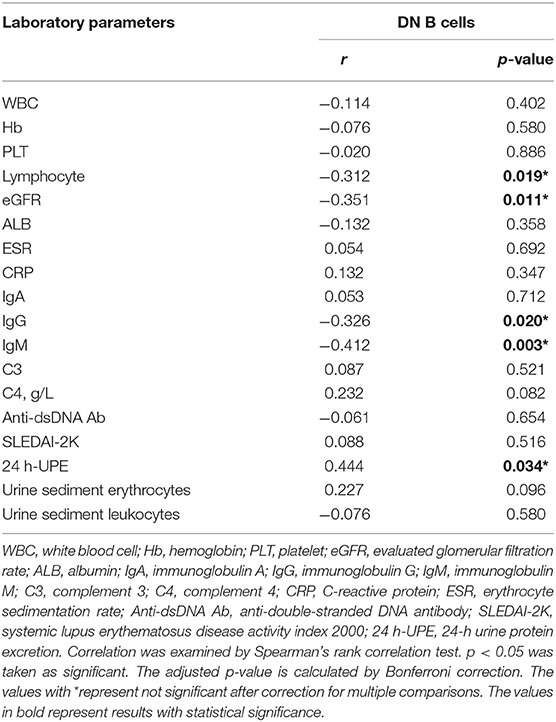
The percentages of DN B cells were compared between patient groups with or without autoimmune clinical and laboratory features. Among the 57 SLE patients in our study (Table 1), 25 were diagnosed as LN. Compared with peripheral blood DN B cell levels of 11.86 ± 7.48 % in non-LN patients, increased peripheral blood DN B cell levels of 16.06 ± 8.79 % in LN patients were observed (p = 0.030; Table 2, Figure 2). We also found the subset from patients with urinary protein was increased significantly (p = 0.018; Table 2, Figure 2). These findings indicated an increase of DN B cell frequency in LN patients. We further analyzed the correlation between DN B cells and laboratory features of SLE. We found that DN B cells were positively correlated with 24 h-UPE levels (r = 0.444, p = 0.034) in LN patients (Table 3, Figure 3). The DN B cells had an inverse correlation with eGFR (r = −0.351, p = 0.011) in SLE patients. These results suggested that high levels of DN B cells were associated with impaired renal function.
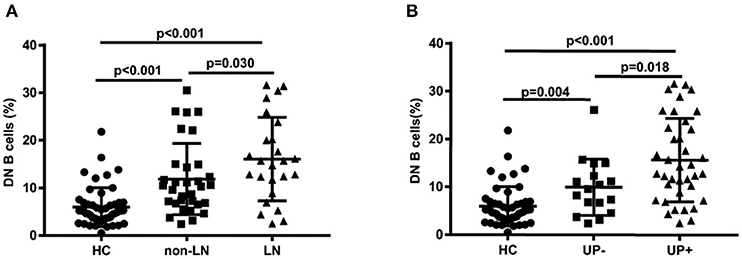
Figure 2. Comparative analysis of blood DN B cells from healthy controls and different groups of SLE patients. (A) Frequency of DN B cells in LN patients, non-LN patients, and healthy controls. Higher frequency of DN B cells was detected in LN patients (n = 25) than in non-LN patients (n = 32). (B) Comparison among healthy controls and SLE patients with proteinuria negative (n = 37) or positive (n = 18). Differences between groups are indicated. Differences were assessed by Mann-Whitney test.
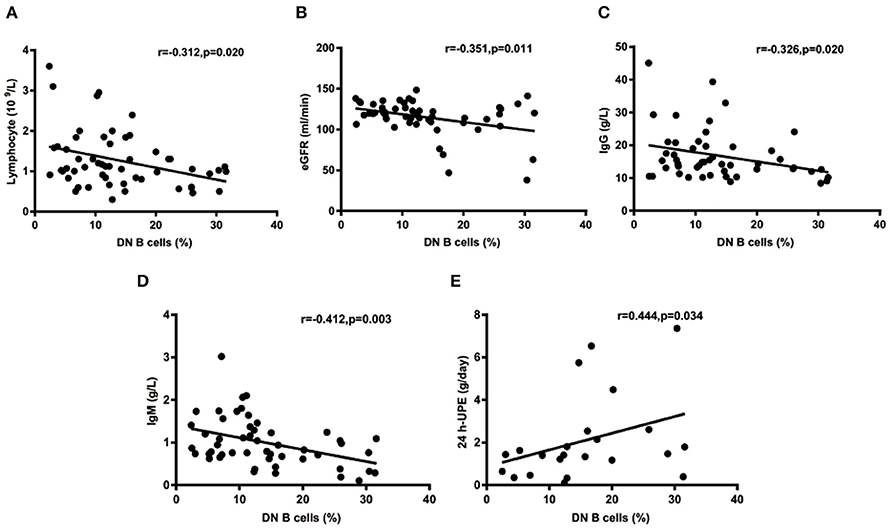
Figure 3. Correlations of circulating DN B cells in SLE with laboratory values. (A–D) DN B cells had an inverse correlation with Lymphocyte, eGFR, IgG, and IgM. (E) DN B cells had a positive correlation with 24 h-UPE. High DN B cells were associated with high 24 h-UPE in LN patients. P was calculated according to Spearman's correlation test.
Since the difference in DN B cells between patients with LN and patients without LN might be associated with variances in the therapies, we summarized the treatments the patients received in Table S1. There was no significant difference in most of the treatments between SLE patients with LN and without LN. Compared with non-LN patients, a higher proportion of LN patients received azathioprine treatment (20 vs. 0%, p = 0.013; Table S1). To exclude the therapeutic influence, the percentages of DN B cells were further compared between LN patients treated with or without azathioprine, and no significant difference was observed (Figure S1, p = 0.362). These results suggested that the significant difference in DN B cell levels between LN and non-LN patients might not be induced by concurrent treatments.
Correlation Between Plasma Cells or Memory B Cells and DN B Cell Subset in SLE Patients
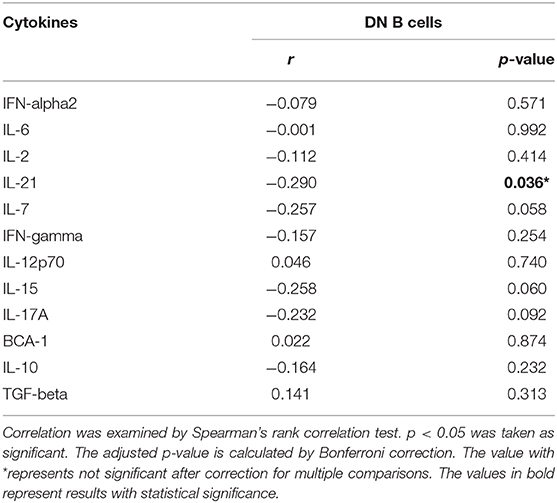
We further analyzed the correlation between DN B cells and a variety of immune cell subsets in SLE. We found that DN B cells had a positive correlation with plasma cells (r = 0.484, p < 0.001) and memory B cells (r = 0.703, p < 0.001) (Figures 4A,B). No association was observed in naïve B cells, non-switched memory B cells, Treg, Tfh, Th1, Th2, Th17, and DN B cells. We also measured the concentration of serum cytokines in SLE patients. There was an inverse correlation between serum IL-21 and DN B cells (Table 4, Figure 4). DN B cells were also inversely correlated with lymphocyte (r = −0.312, p = 0.019), IgG (r = −0.326, p = 0.020), and IgM (r = −0.412, p = 0.003; Table 3, Figure 3). No association was observed between age or disease duration and DN B cells.
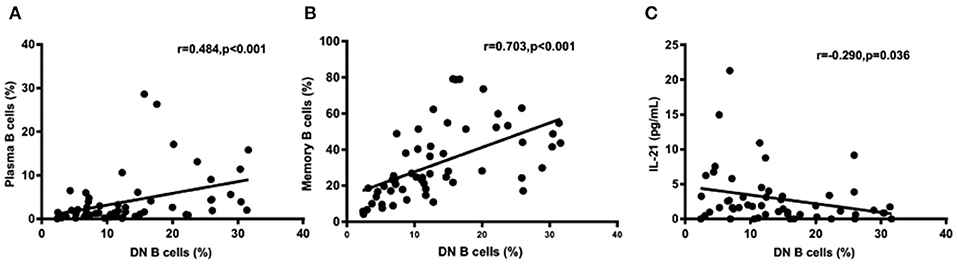
Figure 4. DN B cells in SLE patients are correlated with other subsets and cytokines. DN B cells had a positive correlation with plasma B cells (A) and memory B cells (B). (C) An inverse correlation was observed between DN B cells and IL-21. P was calculated according to Spearman's correlation test.
DN B Cell Decreases After Treatment Was Associated With LN Alleviation and Was a Prognostic Marker for Future LN Remission
In the follow-up, we further assessed the effect of DN B cells on LN. Among the 25 LN patients in our study, 19 LN patients had complete follow-up data. Patients with decreased 24 h-UPE were considered as responsive to therapy (responders, n = 12), and the others were regarded as failing to respond to therapy (non-responders, n = 7). According to the change trends of these subsets at week 6 after treatment, we divided LN patients into two groups: patients with increased DN B cells (Δ DN B cells > 0, n = 8) and patients with decreased DN B cells (Δ DN B cells <0, n = 11).
As the frequency of DN B cells was associated with LN involvement and 24 h-UPE level in LN patients, we speculate that effective therapy for LN might reduce the levels of DN B cells. Among LN patients under treatment, the percentages of DN B cells were indeed significantly decreased from 18.82 ± 8.727 to 15.26 ± 7.034% at week 6 in responsive LN patients (n = 12, p = 0.030; Figure 5A), whereas an obvious increase of DN B cells from 13.6 ± 10.51% at week 0 to 16.11 ± 11.61% at week 6 was observed in non-responsive patients (n = 7, p = 0.080).
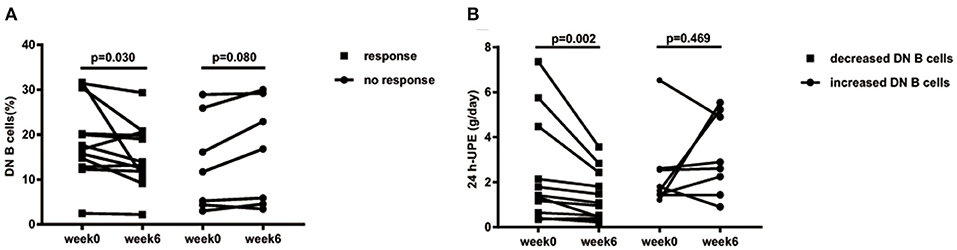
Figure 5. (A) Decreased 24 h-UPE in LN was accompanied by the decrease of DN B cells. Patients who had improvement at week 6 (n = 12) presented significantly decreased DN B cells. Those patients with a poor response (n = 7) showed a trend of increased DN B cells. (B) DN B cells correlated with 24 h-UPE in LN patients. 24 h-UPE decreased in LN patients with decreased DN B cells (n = 11). Differences were assessed by Wilcoxon test.
The 24 h-UPE reduced from 2.44 ± 2.36 g/day at week 0 to 1.44 ± 1.11 g/day at week 6 in LN patients with decreased DN B cells (p = 0.002; Figure 4B); however an increase of 24 h-UPE was found (2.4 ± 1.75 g/day at week 0 vs. 3.22 ± 1.78 g/day at week 6) in LN patients with increased DN B cells (p = 0.469; Figure 5B).
As mentioned above, LN patients were divided into two groups according to the changes of DN B cells at week 6. During the follow-up period of 24 weeks, the remission rate in the group of patients with decreased DN B cells was 81.82%, which was significantly higher than in the other group (25.00%, p = 0.030; Figure 6).
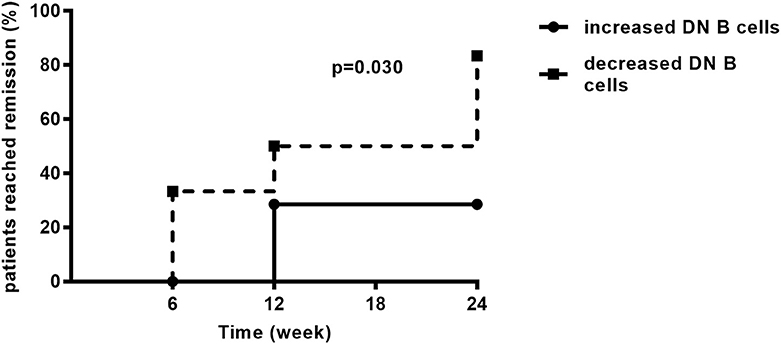
Figure 6. During the follow-up, LN patients achieved remission. The remission rate was higher in LN patients with decreased DN B cells (n = 11) than those with increased DN B cells (n = 8). Differences were assessed by Kaplan–Meier method.
Discussion
Conventional memory B cells can be distinguished from naïve B cells by the presence of somatic hypermutation in their Ig variable gene sequences (22). CD27 is generally used as a marker to identify memory B cells because its expression correlates with the presence of somatic mutations in Ig genes (23, 24). However, further research elucidated that between 10 and 20% of IgG+ class-switched memory B cells were CD27- (25). Absence of IgD expression on DN B cells indicates that they have undergone class switching, though they do not gain the expression of CD27. DN B cells could represent a novel population of memory cells lacking CD27 expression (19). Early studies demonstrated that DN B cells can be detected in healthy persons, but are expanded in elderly people (26), patients with systemic lupus erythematosus, rheumatoid arthritis (27), Alzheimer's disease (28), non-small cell lung cancer (29), rotavirus (30), and HIV (31). Previous work showed that DN B cells are expanded in SLE patients (19). However, its clinical relevance in SLE is not yet clearly understood.
Our study demonstrated that LN patients had significantly higher DN B cells than non-LN patients, suggesting that DN B cells are involved in the renal damage associated with SLE. This result is consistent with previous studies that showed DN B cells expanded in patients with LN (16, 21). In addition, the levels of DN B cells were positively correlated to 24 h-UPE, suggesting that DN B cells may be involved in the severity of renal damage in LN patients. Consistent with this notion, the kidney damage of LN patients was effectively alleviated by drug treatment, accompanied by the downregulation of DN B cells. This result raised the possibility that DN B cells might be used as a prognostic marker in LN. Previous studies indicate that DN B cells are a heterogeneous subpopulation in SLE patients and DN B cells in the peripheral did not correlate significantly with disease activity (32). Our results were in agreement with this study and no correlation between DN B cells and SLEDAI was observed. The relationship between DN B cells and SLE is likely to be complex. Our data presents new evidence that DN B cells may play pathogenic roles in LN specifically. In previous studies, transcriptomic analysis on DN B cell subsets showed altered expression of multiple chemokine receptors including CCR9, CCR7, and CXCR5. In SLE patients, the alteration of the chemokine signaling network might lead to the chemotaxis of DN B cells into inflammatory tissues, like kidneys, to aggravate the local inflammation. These studies also showed that DN B cells were prone to differentiate into plasma cells, which preferentially produced pathogenic autoreactive antibodies in SLE. Altogether, these reports indicate that DN B cells might migrate to the relevant renal tissues and play a pathogenic role either in situ in the kidney or by their active production of autoantibodies.
We found that DN B cells correlated with decreased lymphocytes or eGFRs, which are associated with SLE. As we know, lymphopenia is a typical feature of SLE (33–35). Glomerular filtration rate (GFR) is one of the conventional clinical parameters for detecting ongoing disease activity in lupus-affected kidneys and early relapse of nephritis (36, 37). A low GFR is a dangerous sign of existing kidney disease. Our data presented more evidence that DN B cells were specifically associated with LN development. Previous studies (20, 38) showed that DN B cells were correlated with anti-dsDNA or anti-RNP/Sm autoantibodies, but our study didn't observe such an association. It is possible that the limited cohort size in our study led to the lack of statistical significance. Differences in autoantibody testing technology or genetic background of recruited patients between previous studies and this study might also be possible reasons for this discrepancy.
IL-21 plays a critical role in B-cell differentiation and antibody production (39). Previous studies showed that IL-21 increased the number of transitional B cells, post-switched memory B cells, and plasma cells and promoted serum IgG and IgM production (40, 41). In our study, an inverse correlation was found between serum IL-21 and DN B cells. This may be explained by the possibility that DN B cells consumed IL-21 to differentiate into plasma cells. Our results also showed that increased levels of DN B cells were associated with elevated levels of plasma cells, which also supported the above hypothesis. These results suggest that DN B cells might play a role in the enhanced humoral immune response in SLE. However, the main limitation on our hypothesis is the lack of experimental evidence in this study. Previous studies have shown that the IL-21 receptor (IL-21R) was expressed at high levels in DN B cells, and after binding IL-21 with IL-21R, DN B subsets efficiently differentiated into plasma cells. These studies could provide some supportive experimental clues to our hypothesis.
During the 24-weeks follow-up, the remission rates of LN patients with decreased DN B cells and increased DN B cells at week 6 were 83.33 and 25.00% (p = 0.030), respectively. These results demonstrate that patients with decreased DN B cells under treatment are likely to achieve renal remission, and this is the first study showing that DN B cells might be a prognostic marker in LN.
One limitation of this study is that the SLE patients received different treatments during the study. Since therapy could affect the immune phenotypes, it is possible that the difference in DN B cells between patients with LN and patients without LN is associated with the possible variances in the treatments they received. Our analysis showed that there was no significant difference in most of the treatments between LN patients and non-LN patients. Although 20% of LN patients received azathioprine treatment while non-LN patients did not, no difference in the percentages of DN B cells were observed between LN patients treated with or without azathioprine. These results suggest that different treatments might not lead to different DN B cell levels in LN patients and non-LN patients in the current study. However, it is still possible that differences in DN B cells might be induced by different therapies, and future studies with a larger cohort size should be performed to elucidate the possible effects of different treatments on DN B cells.
Another limitation of this study is shown by the adjusted p-value of correlation analysis with Bonferroni correction (Tables 3, 4). After the correction, no statistical significance was observed in the adjusted p-values. It is possible that the limited cohort size in our study led to the lack of statistical power when correction for multiple comparison was performed.
This study suggests that DN B cells correlate with the severity of renal damage in LN patients. Also, DN B cells may be involved in the pathogenesis of LN. Furthermore, decreased DN B cells are associated with renal alleviation during the follow-up. Specifically, our findings indicate that DN B cells may be used as a prognostic marker in LN.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was approved by the ethics committee of Peking University People's Hospital. All patients provided informed consent to donate their blood samples and clinical information for research, and written consent was given by each individual.
Author Contributions
XY performed the experiment and statistical analysis and wrote the draft of the manuscript. ZL and XS designed the study, analyzed the data, and wrote the manuscript. RZ and MS participated in acquiring clinical data and performed some experiments. JH, JC, JL, XZ, XL, and RJ participated in the analyses. All authors contributed to manuscript revision and have read and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (31530020, 81971520, 31570880, and 81701607), Peking-Tsinghua Center for Life Sciences, State Key Laboratory of Natural and Biomimetic Drugs, and Peking University People's Hospital Research and Development Foundation (RDX2019-03).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all participating patients and healthy controls for contributing to this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00085/full#supplementary-material
References
1. Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. (2016) 2:16039. doi: 10.1038/nrdp.2016.39
2. Tsokos GC. Systemic lupus erythematosus. N Engl J Med. (2011) 365:2110–21. doi: 10.1056/NEJMra1100359
3. Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. (2019) 393:2344–58. doi: 10.1016/S0140-6736(19)30546-X
4. Moroni G, Depetri F, Ponticelli C. Lupus nephritis: when and how often to biopsy and what does it mean? J Autoimmun. (2016) 74:27–40. doi: 10.1016/j.jaut.2016.06.006
5. Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol. (2016) 12:143–53. doi: 10.1038/nrrheum.2015.159
6. Zampeli E, Klinman DM, Gershwin ME, Moutsopoulos HM. A comprehensive evaluation for the treatment of lupus nephritis. J Autoimmun. (2017) 78:1–10. doi: 10.1016/j.jaut.2016.12.011
7. Schreiber J, Eisenberger U, de Groot K. Lupus nephritis. Internist. (2019) 60:468–77. doi: 10.1007/s00108-019-0574-y
8. Yu F, Haas M, Glassock R, Zhao MH. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. (2017) 13:483–95. doi: 10.1038/nrneph.2017.85
9. Castro CHM, Andrade LEC. T regulatory cell-based therapy and renal injury repair: restoring homeostatic balance of the immune response in lupus nephritis. Rheumatology. (2019) 58:1896–7. doi: 10.1093/rheumatology/kez210
10. Dorner T, Lipsky PE. Beyond pan-B-cell-directed therapy—new avenues and insights into the pathogenesis of SLE. Nat Rev Rheumatol. (2016) 12:645–57. doi: 10.1038/nrrheum.2016.158
11. Tipton CM, Hom JR, Fucile CF, Rosenberg AF, Sanz I. Understanding B-cell activation and autoantibody repertoire selection in systemic lupus erythematosus: a B-cell immunomics approach. Immunol Rev. (2018) 284:120–31. doi: 10.1111/imr.12660
12. Liossis SC, Staveri C. B cell-based treatments in SLE: past experience and current directions. Curr Rheumatol Rep. (2017) 19:78. doi: 10.1007/s11926-017-0707-z
13. Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. (2011) 13:243. doi: 10.1186/ar3433
14. Schrezenmeier E, Weissenberg SY, Stefanski AL, Szelinski F, Wiedemann A, Lino AC, et al. Postactivated B cells in systemic lupus erythematosus: update on translational aspects and therapeutic considerations. Curr Opin Rheumatol. (2019) 31:175–84. doi: 10.1097/BOR.0000000000000576
15. Wu C, Fu Q, Guo Q, Chen S, Goswami S, Sun S, et al. Lupus-associated atypical memory B cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis. (2019) 78:1090–100. doi: 10.1136/annrheumdis-2019-215039
16. Kosalka J, Jakiela B, Musial J. Changes of memory B- and T-cell subsets in lupus nephritis patients. Folia Histochem Cytobiol. (2016) 54:32–41. doi: 10.5603/FHC.a2016.0005
17. Rodriguez-Bayona B, Ramos-Amaya A, Perez-Venegas JJ, Rodriguez C, Brieva JA. Decreased frequency and activated phenotype of blood CD27 IgD IgM B lymphocytes is a permanent abnormality in systemic lupus erythematosus patients. Arthritis Res Ther. (2010) 12:R108. doi: 10.1186/ar3042
18. Ma K, Li J, Wang X, Lin X, Du W, Yang X, et al. TLR4(+)CXCR4(+) plasma cells drive nephritis development in systemic lupus erythematosus. Ann Rheum Dis. (2018) 77:1498–506. doi: 10.1136/annrheumdis-2018-213615
19. Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. (2007) 178:6624–33. doi: 10.4049/jimmunol.178.10.6624
20. Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. (2018) 49:725–739.e6. doi: 10.1016/j.immuni.2018.08.015
21. Zhu L, Yin Z, Ju B, Zhang J, Wang Y, Lv X, et al. Altered frequencies of memory B cells in new-onset systemic lupus erythematosus patients. Clin Rheumatol. (2018) 37:205–12. doi: 10.1007/s10067-017-3877-1
22. Klein U, Goossens T, Fischer M, Kanzler H, Braeuninger A, Rajewsky K, et al. Somatic hypermutation in normal and transformed human B cells. Immunol Rev. (1998) 162:261–80. doi: 10.1111/j.1600-065X.1998.tb01447.x
23. Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. (1998) 188:1679–89. doi: 10.1084/jem.188.9.1679
24. Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. (1999) 341:1520–9. doi: 10.1056/NEJM199911113412007
25. Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. (2006) 177:3728–36. doi: 10.4049/jimmunol.177.6.3728
26. Colonna-Romano G, Bulati M, Aquino A, Pellicano M, Vitello S, Lio D, et al. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev. (2009) 130:681–90. doi: 10.1016/j.mad.2009.08.003
27. Mahmood Z, Muhammad K, Schmalzing M, Roll P, Dorner T, Tony HP. CD27-IgD- memory B cells are modulated by in vivo interleukin-6 receptor (IL-6R) blockade in rheumatoid arthritis. Arthritis Res Ther. (2015) 17:61. doi: 10.1186/s13075-015-0580-y
28. Bulati M, Buffa S, Martorana A, Gervasi F, Camarda C, Azzarello DM, et al. Double negative (IgG+IgD-CD27-) B cells are increased in a cohort of moderate-severe Alzheimer's disease patients and show a pro-inflammatory trafficking receptor phenotype. J Alzheimers Dis. (2015) 44:1241–51. doi: 10.3233/JAD-142412
29. Centuori SM, Gomes CJ, Kim SS, Putnam CW, Larsen BT, Garland LL, et al. Double-negative (CD27(-)IgD(-)) B cells are expanded in NSCLC and inversely correlate with affinity-matured B cell populations. J Transl Med. (2018) 16:30. doi: 10.1186/s12967-018-1404-z
30. Wu YC, Kipling D, Dunn-Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol. (2011) 2:81. doi: 10.3389/fimmu.2011.00081
31. Bamford A, Hart M, Lyall H, Goldblatt D, Kelleher P, Kampmann B. The influence of paediatric HIV infection on circulating B cell subsets and CXCR5(+) T helper cells. Clin Exp Immunol. (2015) 181:110–7. doi: 10.1111/cei.12618
32. Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum. (2008) 58:1762–73. doi: 10.1002/art.23498
33. Martin M, Guffroy A, Argemi X, Martin T. Systemic lupus erythematosus and lymphopenia: clinical and pathophysiological features. Rev Med Interne. (2017) 38:603–13. doi: 10.1016/j.revmed.2017.01.005
34. Carli L, Tani C, Vagnani S, Signorini V, Mosca M. Leukopenia, lymphopenia, and neutropenia in systemic lupus erythematosus: prevalence and clinical impact–a systematic literature review. Semin Arthritis Rheum. (2015) 45:190–4. doi: 10.1016/j.semarthrit.2015.05.009
35. Newman K, Owlia MB, El-Hemaidi I, Akhtari M. Management of immune cytopenias in patients with systemic lupus erythematosus—old and new. Autoimmun Rev. (2013) 12:784–91. doi: 10.1016/j.autrev.2013.02.001
36. Soliman S, Mohan C. Lupus nephritis biomarkers. Clin Immunol. (2017) 185:10–20. doi: 10.1016/j.clim.2016.08.001
37. Nived O, Hallengren CS, Alm P, Jonsen A, Sturfelt G, Bengtsson AA. An observational study of outcome in SLE patients with biopsy-verified glomerulonephritis between 1986 and 2004 in a defined area of southern Sweden: the clinical utility of the ACR renal response criteria and predictors for renal outcome. Scand J Rheumatol. (2013) 42:383–9. doi: 10.3109/03009742.2013.799224
38. Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. (2018) 9:1758. doi: 10.1038/s41467-018-03750-7
39. Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. (2008) 223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x
40. Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. (2004) 173:5361–71. doi: 10.4049/jimmunol.173.9.5361
Keywords: double negative B cells, systemic lupus erythematosus, lupus nephritis, 24-h urine protein excretion, remission
Citation: You X, Zhang R, Shao M, He J, Chen J, Liu J, Zhang X, Liu X, Jia R, Sun X and Li Z (2020) Double Negative B Cell Is Associated With Renal Impairment in Systemic Lupus Erythematosus and Acts as a Marker for Nephritis Remission. Front. Med. 7:85. doi: 10.3389/fmed.2020.00085
Received: 24 October 2019; Accepted: 02 March 2020;
Published: 07 April 2020.
Edited by:
Xinhua Yu, Research Center Borstel (LG), GermanyReviewed by:
Feng Yu, Peking University First Hospital, ChinaGuixiu Shi, First Affiliated Hospital of Xiamen University, China
Copyright © 2020 You, Zhang, Shao, He, Chen, Liu, Zhang, Liu, Jia, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Sun, sunxiaolin_sxl@126.com; Zhanguo Li, li99@bjmu.edu.cn
 Xujie You
Xujie You Ruijun Zhang
Ruijun Zhang Miao Shao1,2
Miao Shao1,2 Jing He
Jing He Jiali Chen
Jiali Chen Xu Liu
Xu Liu Xiaolin Sun
Xiaolin Sun Zhanguo Li
Zhanguo Li