
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 13 September 2019
Sec. Pathology
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00200
 Jasmin Zeindler1†
Jasmin Zeindler1† Savas Deniz Soysal2,3†
Savas Deniz Soysal2,3† Salvatore Piscuoglio2,4
Salvatore Piscuoglio2,4 Charlotte K. Y. Ng4
Charlotte K. Y. Ng4 Robert Mechera2,3
Robert Mechera2,3 Andrej Isaak5
Andrej Isaak5 Walter Paul Weber1
Walter Paul Weber1 Simone Muenst1,4*†
Simone Muenst1,4*† Christian Kurzeder1,6†
Christian Kurzeder1,6†Background: Triple-negative breast cancer (TNBC) represents about 10-20% of all invasive breast cancers and is associated with a poor prognosis. The nectin cell adhesion protein 4 (Nectin-4) is a junction protein involved in the formation and maintenance of cell junctions. Nectin-4 has previously shown to be expressed in about 60% of TNBC as well as in TNBC metastases, but to be absent in normal breast tissue, which makes it a potential specific target for TNBC therapy. Previous studies have shown an association of Nectin-4 protein expression with worse prognosis in TNBC in a small patient cohort. The aim of our study was to explore the role of Nectin-4 in TNBC and confirm its impact on survival in a larger TNBC patient cohort.
Material and Methods: We performed immunohistochemical staining for Nectin-4 on a tissue microarray encompassing 148 TNBC cases with detailed clinical annotation and outcomes data.
Results: A high expression of Nectin-4 was present in 86 (58%) of the 148 TNBC cases. In multivariate survival analysis, high expression of Nectin-4 was associated with a significantly better overall survival when compared with low expression of Nectin-4 (p < 0.001). Nectin-4-high expression was also significantly associated with a lower tumor stage (p = 0.025) and pN0 lymph node stage (p = 0.034).
Conclusion: Our results confirm that expression of Nectin-4 serves as a potential prognostic marker in TNBC and is associated with a significantly better overall survival. In addition, Nectin-4 represents a potential target in TNBC, and its role in molecular defined breast cancer subtype should be investigated in larger patient cohorts.
Triple-negative breast cancer (TNBC) is distinguished from other types of breast cancer by a particularly aggressive progression and poor clinical outcomes (1). Responsible for 10-20% of all invasive breast cancers, TNBC tends to affect younger women (2) and shows higher recurrence rates (1) as well as lower survival rates than non-TNBC (3). In fact, the 5-year survival rate for metastatic TNBC is <30%, while the overall survival rate declines close to zero (4). The poor prognosis associated with TNBC is largely owed to TNBC's “adverse” molecular characteristics, which considerably limit the scope of appropriate treatment options. With no expression of either estrogen or progesterone receptors, and no HER2 overexpression, TNBC cells lack the leverage points for efficient hormone therapy and/or HER2-targeted agents, which are successfully used for the treatment of non-TNBC. As a consequence, the only available treatment option for patients with TNBC is cytotoxic chemotherapy, often supplemented by the use of a platinum-based agent, which recent studies suggest enhances response to chemotherapy, especially in neo-adjuvant treatment settings (5). Pathologic complete response after neoadjuvant chemotherapy is considered one of the most important prognostic factors in early stage disease but can only been achieved in approximately one third of patients (6). Alternative treatment strategies in terms of molecularly targeted agents are thus desperately needed.
Although the rise of so-called “omics” technologies (such as genomics, transcriptomics, proteomics and metabolomics) over the past decade has significantly contributed to a better understanding of TNBC's molecular make-up, the search for actionable targets continues to be hampered by the striking molecular heterogeneity that TNBC displays (7). Gene expression analyses have shown that TNBC does not constitute a uniform disease entity to begin with, but can be classified into several subtypes with distinctive molecular ontologies (8). While the exact number of TNBC subtypes remains a subject of discussion, and can be expected to change as research progresses, the existence of at least four major TNBC subtypes seems by now fairly well-established, including a basal-like (BL), a mesenchymal (M), a luminal androgen receptor (LAR), and an immunomodulatory (IM) subtype. The most frequent TNBC subtype by far the BL subtype, accounts for about 70% of all TNBC cases (5, 9). TNBC's strong molecular heterogeneity would seem to offer sufficient potential leverage points for the design of molecularly targeted therapies; yet, despite the identification of several tumor-specific molecular alterations in various subtypes of TNBC, none of them has so far proven to be an actionable oncogenic driver for TNBC (8). At the same time, encouraging results have been achieved with the use of immunotherapeutic agents, e.g., monoclonal antibodies and cytokines, with increasing evidence to suggest the effectiveness of immunotherapy in at least some subgroups of TNBC patients (10, 11). A recent study demonstrated that enrichment levels of 26 immune cell activities were significantly higher in TNBC than in non-TNBC (12, 13). These findings, indicating an overall higher level of immunogenicity in TNBC compared to non-TNBC, underline the need for increased research on TNBC-specific cell surface molecules for the purpose of identifying new biomarkers as well as potential targets for immunotherapeutic agents.
One such TNBC-specific cell surface molecule is Nectin-4 (also known as PVRL4), which has previously been described as a new tumor-associated antigen in a number of different carcinomas (14–19). Being one of at least five members of the Nectin family, a group of cell adhesion molecules within the immunoglobulin superfamily, Nectin-4 consists of three conserved immunoglobulin-like domains (V, C, C) in its extracellular region. Unlike other Nectins, Nectin-4 is not expressed in normal adult tissue; however, several studies have found re-expression of Nectin-4 as a tumor-associated antigen in various cancer tissues, including pancreatic, ovarian, lung and breast cancer (14–19).
In this study, we further explore the role of Nectin-4 in a larger TNBC patient cohort.
We retrospectively recorded the clinicopathological features of all 148 patients included in this study, non-matched and non-stratified. The recorded features included patient age and gender, tumor localization, pT and pN stage, histological subtype, molecular subtype, BRE grade and overall survival. The median event-free follow-up time was 50.4 months. The treatments the patients received were according to the current guidelines at the time of treatment.
We designed a tissue microarray (TMA) of 148 non-consecutive, primary human triple-negative breast cancers, sampled between 1985 until 2015. These samples were collected from the tissue biobank of the Institute of Pathology, University Hospital Basel, with the approval of the Ethics Committee Nordwestschweiz (EKNZ, Nr. 2014-396) and in compliance with ethical standards and medical confidentiality. Histological slides from all patients were collected from the archives of the Institute of Pathology, and all cases were reviewed by an experienced breast pathologist (S.M.). Diagnosis of TNBC was confirmed, and staining of ER, PR and Her2 was repeated when necessary, as described before, and in accordance with the ASCO/CAP guidelines (20). For each block, a morphologically representative area was identified, from which a tissue cylinder of 0.6 mm diameter was punched. Subsequently, a semi-automated tissue arrayer was used to assemble all cylinders into one recipient paraffin block, 30 × 25 mm in size. The punches were taken from the tumor center, thus ensuring that each TMA cylinder contained at least 50% of tumor tissue. The TMA blocks were stored in the certified biobank of the Institute of Pathology.
For immunohistochemistry, 4 μm-sections were cut from the paraffin tissue block. Sections were pretreated with MW Tris/EDTA at 98°C for 30 min for antigen retrieval and then incubated with the primary Nectin-4 antibody (Abcam, ab 192033) overnight at 4°C. DAB was used as chromogen, and counterstaining was performed with Hematoxylin (Roche). Nectin-4 expression was detectable on the cell membrane. The Nectin-4 staining was scored according to the Quick score (QS) by using the following formula: QS = P (percentage of positive cells) × I (intensity), the maximum score being 300. The high Nectin-4 expression group was defined as a QS > 100 and the low Nectin-4 expression group as a QS = or < 100. The entire immunohistochemical analysis was performed by a trained research fellow blinded to the clinicopathological data, and challenging cases were reviewed and discussed together with an experienced breast pathologist, until consensus was reached. Representative pictures of high and low Nectin-4 expression can be found in Figure 1.
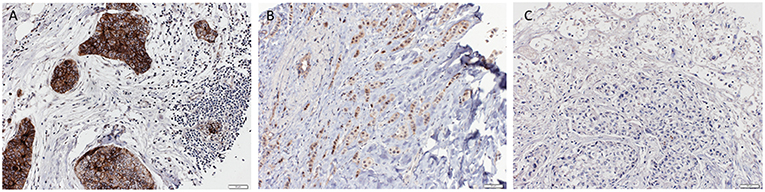
Figure 1. Representative pictures of triple negative breast cancer with strong (A), moderate (B) and negative (C) Nectin-4 expression respectively (Magnification 200x).
We performed a retrospective case selection of 148 primary human triple-negative breast cancers, which were available in our TMA platform. The clinical endpoint was overall survival, defined as percentage of patients in our study group who were alive after the follow-up period. The initially collected variables were median tumor size, mean age at diagnosis, tumor stage, lymph node involvement, tumor grade, histologic subtype, intrinsic subtype, Ki-67, time of death.
We defined two subgroups of high (QS > 100) and low (QS = or < 100) Nectin-4 expression. To correlate Nectin-4 expression with clinicopathological features, we used Chi-Square or Mann-Whitney U-tests, respectively for categorical and non-categorical variables. Overall survival was calculated using the Kaplan-Meier method and differences between groups assessed using log-rank tests.Univariate analyses of the effect of high Nectin-4 expression and of high Nectin-4 expression stratified by intrinsic subtypes were performed using the Cox proportional hazards regression model. For multivariate Cox proportional hazards regression analyses, we evaluated the effects of clinicopathological parameters (age, tumor stage, lymph node involvement, tumor grade), intrinsic subtype and high Nectin-4 expression on overall survival. Hazard ratios and corresponding 95% confidence intervals were estimated. All tests were two-sided. P-values <0.05 were considered statistically significant. All analyses were performed using R v3.4.2. Any missing clinico-pathological information was assumed to be missing at random.
Table 1 shows basic demographic data. Mean age at diagnosis was 62 years (±15 years SD) and median tumor size was 25 mm (± 19 mm SD). The majority of the patients (53.4%) presented with a tumor stage pT2. Almost a third of the patients (29.1%) presented with a pT1 tumor stage. 51.4% of patients had no lymph node involvement, while 28.4% presented with a pN1 lymph node stage. Tumor grade 3 could be observed in the majority of the patients (78.4%). Invasive ductal carcinoma was the most observed histological subtype in 90% of the cases.
High Nectin-4 expression (Nectin-4 high group) was found in in 86 (58%) of 148 cases. The association between high Nectin-4 expression and clinicopathological parameters is shown in Table 2. Mean age and tumor grade did not differ significantly between the Nectin-4-high and Nectin-4-low group, while the distribution of tumor stage was significantlly different between the two groups (p = 0.025), with Nectin-4-high being associated with a lower tumor stage. Furthermore, lymph node involvement was significantlly different between the two groups (p = 0.034), with high Nectin-4 expression being more frequent in patients with pN0 lymph node stage.
In univariate survival analysis, high Nectin-4 expression was associated with a significantly better overall survival (Hazard ratio 0.271, 95% CI 0.0077–0.0952, p < 0.001, Table 3). In multivariate analyses for the effect of clinicopathological parameters and high expression of Nectin-4 on overall survival, age (Hazard ratio 1.0369, 95% CI 1.0068–1.0678, p = 0.0158) and high Nectin-4 expression (Hazard ratio 0.0220, 95% CI 0.0055–0.0889, p<0.001) were associated with significantly better overall survival (Table 4 and Figure 2).
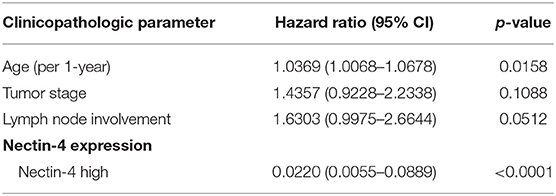
Table 4. Multivariate analysis for the effect of clinicopathologic parameters and Nectin-4 expression on overall survival.
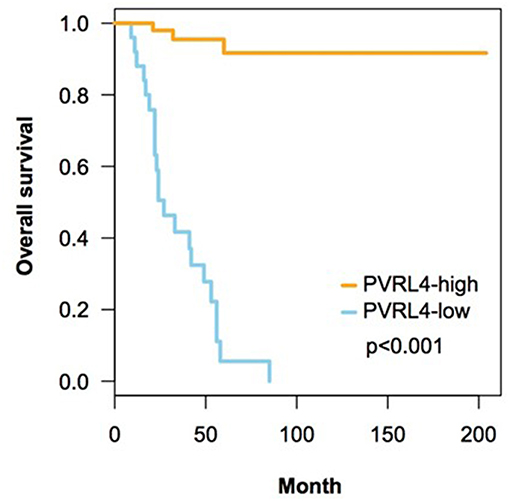
Figure 2. Correlation of expression of Nectin-4 on overall survival. This figure contains Kaplan-Meyer plots depicting the impact of high Nectin-4 expression on overall survival. Statistical analyses were performed using log-rank tests.
Our results show that Nectin-4 expression is an independent prognostic biomarker for better overall survival in TNBC. To our knowledge, this is the first study with a sample size as large as this to investigate Nectin-4 expression in human TNBC. Interestingly, high Nectin-4 expression showed a significant association with lower tumor stage and negative lymph nodes.
Several reports identified Nectin-4 as a new promising tumor antigen in various carcinomas (14–19). Most recently, M-Rabet et al. performed a mRNA- and protein-based analysis of Nectin-4 expression in approximately 5,700 invasive breast cancer samples, showing that Nectin-4 is significantly overexpressed in both triple negative and basal breast cancer samples, with high expression of mRNA being an independent biomarker associated with poor prognosis in TNBC (21). Within the same cohort, protein expression of Nectin-4 was analyzed by immunohistochemistry in 61 TNBC cases and positively correlated with mRNA expression. Two other studies investigated Nectin-4 expression by immunohistochemistry in luminal-A breast cancers (22) and in a mixed cancer cohort (19). Lattanzio et al. (22) showed a significant association of high membranous expression of Nectin-4 with lower disease-free survival as well as lower distant-free survival in the luminal-A breast cancer cohort (T1 and T2, n = 193). Challita-Eid et al. (19) conducted immunohistochemical staining of Nectin-4 on 2394 patient specimens from different tumor entities including cancer of the bladder, breast, lung, pancreas, ovaries, head/neck and esophagus). A positive staining for Nectin−4 was detected in 69% of all specimen. When moderate Nectin-4 expression was defined as a QS > 100 and strong expression as a QS > 200, immunohistochemical analysis of 36 healthy human organs showed homogenous weak to moderate staning, including in the breast. Interestingly, moderate (26%) and strong (27%) Nectin-4 expression was seen most frequently in bladder cancer, followed by breast cancer (53%, n = 654). Whereas 30% of the invasive ductal carcinomas had strong Nectin-4 expression, only 20% of the invasive lobular carcinomas were categorized into this group. In 18% of cancer metastases, strong Nectin-4 expression could be observed. There was no specific investigation of TNBC or association of Nectin-4 expression with overall or recurrence free survival in this study.
In contradiction to the results of M-Rabet et al. (21), our results indicate that high Nectin-4 expression is associated with a better overall survival in TNBC. Our analysis is based on protein expression as determined by immunohistochemistry, whereas M-Rabet et al. analyzed mRNA expression by microarray technology. In their study high Nectin-4 expression in a breast cancer cohort of mixed molecular subtypes and also specifically in TNBC was associated with a lower metastasis free survival. Analyses per molecular subtype indicated a significant association only for TNBC. In contrast, our multivariate analysis shows that high Nectin-4 expression is significantly associated with better overall survival (hazard ratio 0.22 in TNBC). In both series, adjuvant treatment was not specified and comparison of the underlying cohorts is hampered by lack of full clinical data.
Due to the fact that Nectin-4 is mainly expressed during fetal development with a decrease of expression in adult tissues (23), its re-expression during tumor development makes it a tumor-associated antigen with the possibility of developing a targeted therapy. To our knowledge, no studies investigating Nectin-4 expression during progression of cancer exist. Association of Nectin-4 expression with markers of tumor proliferation was analyzed in pancreatic cancer patients (18). Additionally, a significant inhibition of cell proliferation in human pancreatic cells by siRNA-mediated gene silencing could be demonstrated in vitro.
Challita-Eid et al. (19) observed strong membranous Nectin-4 expression in only 18% of the investigated metastases, while it was more often observed in primary tumors. One possible explanation could be that Nectin-4 expression on the cancer cell surface is highly present during tumor formation, but declines during progression. This would explain the better association between high Nectin-4 expression and its association with lower tumor stage and absent lymph node involvement. Furthermore, expression on a DNA or mRNA level might not have the same impact and could still be measured in progressive disease, while the surface protein is not longer necessarily expressed in advanced stages.
While presenting important findings, our study also has several limitations. First, even though the cohort is well-characterized, it is based on a retrospective analysis. Secondly, we did not investigate the role of high expression of Nectin-4 in other molecular breast cancer subtypes and not on a DNA or mRNA level. In addition, our core diameter of 0.6 mm is small, and we did not use duplets of the cancers. However, several studies have shown a high concordance for immunohistochemical stainings between TMA and whole slides sections, even for a core diameter of 0.6 mm (24–28). Finally, the exact molecular mechanisms which could potentially improve prognosis in Nectin-4 expressing TNBC are not established. Further translational studies are needed to investigate the role of membranous Nectin-4 expression during cancer progression and to reveal potential interactions with the immune system or therapeutic interventions.
Despite conflicting results, our data add insightful information to the prognostic significance of high Nectin-4 expression in TNBC. Nectin-4 represents a potential target in TNBC, and its role in molecularly defined breast cancer subtypes should be investigated in larger patient cohorts.
All authors state that they have no financial competing interests in relation to this manuscript.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Ethikkommission Nordwest- und Zentralschweiz (EKNZ). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SS and SM planned and designed the study, and interpreted the data. JZ and SM analyzed the immunohistochemical stainings and both wrote part of the manuscript. SP and CN performed the data analysis and created Figure 2. SM created Figure 1. SS created the tables. WW, CK, AI, and RM critically revised the article for important intellectual content. All authors approved of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
This work was supported by the Holcim Foundation for the Advancement of Scientific Research, the Claudia von Schilling Foundation for Breast Cancer Research, the Freiwillige Akademische Gesellschaft Basel and the Huggenberger-Bischoff Foundation. SP is funded by Swiss National Science Foundation (Ambizione grant number PZ00P3_168165). JZ is funded by the Department of Surgery University Hospital Basel for 1 year of basic research (2017–2018). The funding agencies had no role in any of the stages from study design to submission of the paper for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Petra Hirschmann for the immunohistochemical staining of the TMA slides as well as Martin Portmann for the slide scanning.
1. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. (2010) 28:1684–91. doi: 10.1200/JCO.2009.24.9284
2. Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. (2016) 34:3308–14. doi: 10.1200/JCO.2015.65.8013
3. Millar EK, Graham PH, O'Toole SA, McNeil CM, Browne L, Morey AL, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. (2009) 27:4701–8. doi: 10.1200/JCO.2008.21.7075
4. Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. (2014) 19:608–15. doi: 10.1634/theoncologist.2014-0002
5. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. (2011) 121:2750–67. doi: 10.1172/JCI45014
6. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. (2014) 384:164–72. doi: 10.1016/S0140-6736(13)62422-8
7. Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Curr Mol Med. (2012) 12:96–110. doi: 10.2174/156652412798376134
8. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. (2001) 98:10869–74. doi: 10.1073/pnas.191367098
9. Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. (2013) 18:123–33. doi: 10.1634/theoncologist.2012-0397
10. Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. (2013) 73:3470–80. doi: 10.1158/0008-5472.CAN-12-4524-T
11. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol. (2016) 34:2460–7. doi: 10.1200/JCO.2015.64.8931
12. Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, Tsao MS, et al. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. (2011) 7:e1002240. doi: 10.1371/journal.ppat.1002240
13. Liu Z, Li M, Jiang Z, Wang X. A comprehensive immunologic portrait of triple-negative breast cancer. Transl Oncol. (2018) 11:311–29. doi: 10.1016/j.tranon.2018.01.011
14. Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestier C, Reymond N, et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer. (2007) 7:73. doi: 10.1186/1471-2407-7-73
15. Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. (2009) 69:6694–703. doi: 10.1158/0008-5472.CAN-09-0016
16. Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, Lopez M, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol. (2010) 134:835–45. doi: 10.1309/AJCPGXK0FR4MHIHB
17. Pavlova NN, Pallasch C, Elia AE, Braun CJ, Westbrook TF, Hemann M, et al. A role for PVRL4-driven cell-cell interactions in tumorigenesis. Elife. (2013) 2:e00358. doi: 10.7554/eLife.00358
18. Nishiwada S, Sho M, Yasuda S, Shimada K, Yamato I, Akahori T, et al. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J Exp Clin Cancer Res. (2015) 34:30. doi: 10.1186/s13046-015-0144-7
19. Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. (2016) 76:3003–13. doi: 10.1158/0008-5472.CAN-15-1313
20. Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L. Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract. (2019) 215:251–5. doi: 10.1016/j.prp.2018.10.035
21. M-Rabet M, Cabaud O, Josselin E, Finetti P, Castellano R, Farina A, et al. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann Oncol. (2017) 28:769–776. doi: 10.1093/annonc/mdw678
22. Lattanzio R, Ghasemi R, Brancati F, Sorda RL, Tinari N, Perracchio L, et al. Membranous Nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis. (2014) 3:e118. doi: 10.1038/oncsis.2014.32
23. Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. (2001) 276:43205–15. doi: 10.1074/jbc.M103810200
24. Gulbahce HE, Gamez R, Dvorak L, Forster C, Varghese L. Concordance between tissue microarray and whole-section estrogen receptor expression and intratumoral heterogeneity. Appl Immunohistochem Mol Morphol. (2012) 20:340–3. doi: 10.1097/PAI.0b013e318241ca14
25. Jones S, Prasad ML. Comparative evaluation of high-throughput small-core (0.6-mm) and large-core (2-mm) thyroid tissue microarray: is larger better? Arch Pathol Lab Med. (2012) 136:199–203. doi: 10.5858/arpa.2011-0080-OA
26. Chavan SS, Ravindra S, Prasad M. Breast biomarkers-comparison on whole section and tissue microarray section. J Clin Diagn Res. (2017) 11:EC40–4. doi: 10.7860/JCDR/2017/25088.9573
27. Muftah AA, Aleskandarany MA, Al-Kaabi MM, Sonbul SN, Diez-Rodriguez M, Nolan CC, et al. Ki67 expression in invasive breast cancer: the use of tissue microarrays compared with whole tissue sections. Breast Cancer Res Treat. (2017) 164:341–8. doi: 10.1007/s10549-017-4270-0
Keywords: Nectin-4, breast cancer, biomarkers, prognosis, triple-negative breast cancer
Citation: Zeindler J, Soysal SD, Piscuoglio S, Ng CKY, Mechera R, Isaak A, Weber WP, Muenst S and Kurzeder C (2019) Nectin-4 Expression Is an Independent Prognostic Biomarker and Associated With Better Survival in Triple-Negative Breast Cancer. Front. Med. 6:200. doi: 10.3389/fmed.2019.00200
Received: 22 July 2019; Accepted: 27 August 2019;
Published: 13 September 2019.
Edited by:
Luigi Insabato, University of Naples Federico II, ItalyReviewed by:
Elia Guadagno, University of Naples Federico II, ItalyCopyright © 2019 Zeindler, Soysal, Piscuoglio, Ng, Mechera, Isaak, Weber, Muenst and Kurzeder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simone Muenst, c2ltb25lLm11ZW5zdEB1c2IuY2g=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.