- Department of Dermatology, Ruhr-University Bochum, Bochum, Germany
In this article we describe efficacy and safety aspects of ultraviolet A1 (UV-A1) phototherapy in fibrosing conditions. UV-A1 is a specific phototherapeutic modality that is defined by a selective spectral range (340–400 nm). UV-A1 includes distinct modes of action qualifying this method for therapy of a variety of conditions, in particular fibrosing skin diseases. Concerning efficacy of UV-A1 phototherapy in fibrosing conditions, the best evidence obtained from randomized controlled trials exists for localized scleroderma. Moreover, fibrosing disorders such as lichen sclerosus and graft-vs.-host disease can be treated successfully by means of UV- A1. Regarding the optimal dosage regimen medium-dose UV-A1 seems to be linked to the best benefit/risk ratio. Possible acute adverse events of UV-A1 phototherapy include erythema and provocation of photodermatoses. Skin ageing and skin cancer formation belong to the chronic adverse events that may occur after long-term UV-A1 phototherapy.
Introduction
In order to reduce adverse effects such as erythema UV-A1 (340–400 nm) light sources were previously developed by eliminating the UV-A2 wavelengths (320–340 nm) which range to the UV-B (280–320 nm) spectrum (1, 2). Compared to UV-B and UV-A2, UV-A1 is thus less erythematogenic and does penetrate deeper into the skin (3). UV-A1 is a beneficial phototherapeutic modality for the treatment of disorders including eczema, urticaria pigmentosa, cutaneous T cell lymphoma, and in particular, fibrosing skin diseases (4–14). The present review focuses only on the fibrosing skin diseases and, although UVA1 may be beneficial in other conditions, they are not the focus of this review. We will also summarize the evidence in table format for each of the diseases discussed in the following review.
UV-A1 Light Sources and Regimens
UV-A1 Devices
Fluorescent bulbs (i.e., TL10R 100W, Philips, Eindhoven, Netherlands) and high-output metal halide lamps (i.e., Sellamed 4,000W, Sellas Medizinische Geräte GmbH, Ennepetal, Germany) belong to the commercially available UV-A1 sources. For practical reasons, fluorescent lamp cubicles are rather used for low to medium-dose UV-A1 phototherapy. By contrast, high-output metal halide lamps can also be used for high-dose UV-A1 since they deliver doses up to 130 J/cm2 in acceptable time per treatment session. UV-A1 light sources designed for phototherapy have to fulfill some technical requirements. Hence, the amount of wavelengths smaller than 340 nm must be smaller than five percent of the total erythema-effective fluence. Furthermore, wavelengths smaller 320 nm as well as infrared should also be widely filtered out. Thus, irradiance of wavelengths between 800 nm and 1 mm must not be greater than five percent of the total fluence (15). Fluorescent lamp whole- body devices are relatively inexpensive but have considerably lower spectral output as compared to metal halide devices (15). Nevertheless, duration of irradiation using high-output UV-A1 beds may also be long as the patient must usually treat subsequently to two body sides (15). Using UV-A1 metal halide lamps exposure times of thirty to sixty minutes per session are not uncommon, of course depending on fluence, indication and dosage regimen (14, 16).
Dosage Regimens
In order to be consistent with previous publications that are discussed in the present review we use the dosage categories as follows: low-dose UV-A1 (10–20 J/cm2), medium-dose UV-A1 (>20–70 J/cm2), and high-dose UV-A1 (>70–130 J/cm2). Before starting UV-A1 phototherapy the medical history (i.e., photo skin-type, sun sensitivity, skin cancer) of the patient has to be checked also including the use of photo-allergic medications and immune-mediated photodermatoses. Importantly, immunosuppressants, including azathioprine, must not be combined with UV-A1 (17).
Given that there may be considerable variability in individual susceptibility to UVA1 erythema, undertaking an MED prior to starting treatment is preferred where feasible. If this proves not to be the case then a fixed start dose, for example, 20 J/cm2 would usually be a safe approach, but there would then be the concern of potential under-treatment if running at doses that are well below the erythemal threshold (18, 19). UV-A1-MED data of two recent studies indicate that 20 J/cm2 do usually not lead to erythema (20–22). Regular UV-A1 dosimetry is highly recommendable. The irradiance of the light sources should be assessed at different test sites whereby the mean value of all measurements defines the irradiance used for dose calculations (23).
Localized Scleroderma
High-dose UV-A1 therapy of localized scleroderma (LoS) was first reported by German researchers in 1997 (24). Stege et al. compared 10 patients receiving high-dose UV-A1 therapy with seven patients who were exposed to low- dose UV-A1 therapy. Stege et al. showed that UV-A1 significantly increased skin elasticity and decreased thickness and stiffness of the skin–these effects were particularly seen following high-dose UV-A1 (24). The latter findings are supported by in vitro analyses showing UV-A1 to reduce cell proliferation and dose-dependently decrease of collagen and hydroxyproline levels.
Moreover, a mouse model of scleroderma showed for high-dose UV-A1 a marked therapeutic effect on scleroderma. An improvement of dermal sclerosis and softened skin tissue could be observed (25). These results are in line with another mouse model study by Karpec and colleagues who investigated in scleroderma patients the impact of high-dose UV-A1 on dermal sclerosis. They could demonstrate that a total dose of 1,200 J/cm2 does obviously not only prevent worsening of dermal fibrosis but also leads to a decrease of fibrotic skin changes (26). A further study by this working group showed in an animal model employing bleomycin- induced scleroderma that UV-A1 (cumulative doses: 1,200 J/cm2 and 600 J/cm2) is effective as well safe in the management of scleroderma (27).
By contrast, there is a wealth of data confirming the efficacy of low-dose UV-A1 therapy. Kerscher et al. (28) reported for the first time on a successful low-dose UV-A1 treatment in LoS patients (n = 10). Later, they conducted a study including 20 LoS patients who were treated with low-dose UV-A1 over 12 weeks. UV-A1 resulted in remarkable clinical improvement in 80% of the patients (29). However, patients (n = 2) with subcutaneous LoS did not respond to treatment. In a small study performed by Gruss and co-workers, the results mentioned above were supported as well (29). Moreover, LoS patients were treated three times per week using UV-A1 phototherapy (30 J/cm2, treatment duration 10 weeks) (30). In all patients, softening of skin lesions was reported by the authors (30).
de Rie et al. reported on a controlled medium-dose UV-A1 trial including eight patients suffering from LoS (31). UV-A1 was given four times weekly over three months resulting in a decrease of skin fibrosis (cumulative dose: of 2,304 J/cm2 UV-A1). We previously performed a comparative trial investigating low- dose UV-A1 (20 J/cm2), medium-dose UV-A1 (50 J/cm2), and narrowband UV-B for patients with LoS (32). Sixty-four patients suffering from LoS were treated in a randomized controlled trial including three treatment arms (15). Severity of LoS was evaluated using a simple clinical score. Phototherapy was performed five times weekly over two months. Kreuter (32) observed a significant improvement of LoS in all patients who completed the study which was shown by a decrease of clinical symptoms in all study arms assessed (15, 32). However, medium-dose UV-A1 was significantly more effective than narrowband UV-B (32). While low-dose and medium-dose UV-A1 were equally beneficial, substantial differences between low-dose UV-A1 and narrowband UV-B and medium-dose UV-A1 could not be observed.
Sator et al. (33) treated three clinically comparable LoS plaques in sixteen patients using 20 J/cm2 UV-A1, 70 J/cm2 UV-A1, or non-irradiation (32). Thirty therapy sessions were applied in total. Sator et al. (33) assessed thickness of the skin using high-frequency sonography and clinical score. Sonography revealed a significantly greater decrease of skin thickness for medium-dose UV-A1 when compared to low-dose regimen. By contrast, clinical scoring of fibrotic lesions irradiated also decreased markedly but did not show a clinically meaningful difference between medium-dose and low-dose UV-A1 (32). Together, the authors found that medium-dose UV-A1 for LoS resulted in more favorable long-term results when compared to low-dose UV-A1 as confirmed by sonographic assessments. High-frequency sonography is likely a more sensitive tool for the assessment of UV-A1-induced skin changes in LoS patients (33).
A recent cohort study by Vasquez and colleagues investigated recurrence risk of morphea after successful UV-A1 therapy–they observed the duration of LoS prior to therapy as the only associated variable. There was no difference in recurrence risk between different subtypes of morphea, skin types, adults and children, and medium to high dose regimens. Thus, the authors conclude that treatment doses in the medium- and high-dose UV-A1 range are adequate regarding the frequency of recurrence (34). Su et al. (35) treated 35 LoS patients with medium-dose UV-A1 (30 J/cm2). Medium-dose UV-A1 therapy improved fibrotic lesions in all patients. A substantial treatment success was found in 29 of 35 patients. Ultrasound measurements demonstrated that the thickness of skin significantly decreased after medium-dose UV-A1. There were no detectable treatment related adverse events.
Moreover, Andres et al. (36) demonstrated in LoS patients a favorable short-and long-term effect through medium-dose UV-A1 therapy, including diminishment of fibrotic lesions, improvement of skin elasticity, and decrease of skin thickness. Furthermore, Pereira et al. (37) conducted a retrospective evaluation of LoS patients who had underwent low-dose UV-A1 (average dose: 31 J/cm2) phototherapy (32). They treated 18 patients with LoS showing a substantial improvement in more than three-fourth of patients and a modest improvement in 12% of patients (37). Moreover, Gruss et al. (38) reported on disabling pansclerotic morphea of childhood who was successfully treated with low-dose UV-A1 (cumulative dose: 640 J/cm2 UV-A1) four times weekly over two months resulting in substantial reduction of skin fibrosis.
Together, medium UV-A1, based on the evidence base would be considered as the phototherapeutic treatment of choice for patients with LoS (Table 1). However, it is worth emphasizing that there is no head-to-head comparison between UV-A1 and psoralen plus UV-A (PUVA) for scleroderma, and this would be an important study with regards to establishing the place of UVA1 in the phototherapeutic approaches of scleroderma, as at present we do not know whether UVA1 is equivalent, inferior or superior to PUVA.
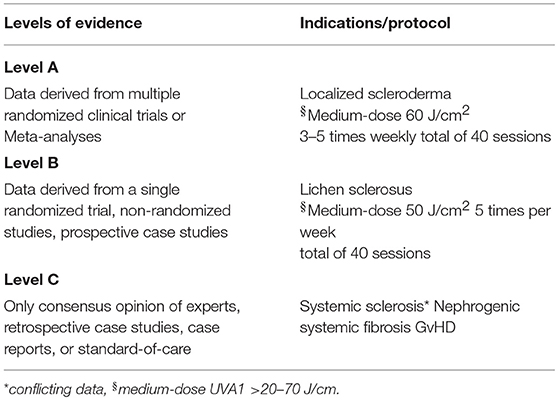
Table 1. UV-A1 treatment for fibrosing conditions—levels of evidence as proposed by the American College of Cardiology and the American Heart Association (39).
Systemic Sclerosis
von Kobyletzki et al. (40) reported on eight patients suffering from systemic sclerosis (SSc) whose acrosclerosis was treated with low-dose UV-A1. They used 30 J/cm2 UV-A1 four times per week over two months and thereafter three times weekly over a six week period (50 treatment sessions in total, cumulative dose: 1,500 J/cm2) (40). Morita et al. (41) also observed UV-A1-induced softening of skin fibrosis (cumulative dose: 510 to 1,740 J/cm2) in four patients with SSc. In another paper they also found UV-A1-induced decrease of dermal decorin expression in SSc patients (41, 42). In an open non-randomized study we previously treated 18 patients with acrosclerosis and underlying SSc. Applying the UV-A1 regimen described by von Kobyletzki et al. (40), Kreuter et al. (43) observed skin softening, enhancement of skin distension, decrease of thickness of skin, and increase of cutaneous collagenase activity in 16 of 18 patients (32).
Pereira et al. (37) reported three SSc patients who were treated with medium-dose UV-A1. In two patients, acrosclerosis improved significantly (37). Moreover, Rose et al. reported on eight SSc patients (diffuse type, n = 5; limited type, n = 3) who showed skin fibrosis predominantly on acral and proximal extremity sites. The patients were treated using UV-A1 (30–40 J/cm2) 3 times per week. Skin fibrosis improved as indicated by a decrease of the modified Rodnan skin score (32). Hence, this study also demonstrated that UV-A1 treatment is effective in SSc patients, particularly for acrosclerosis (44). In contrast, Durand et al. (45) reported a randomized observer-blinded half-side controlled trial on UV-A1 treatment of acrosclerosis. They used low-dose UV-A1 (40 J/cm2) three times per week (14 weeks treatment period). Although a marked improvement of the clinical scores was observed, no difference could be detected regarding the clinical outcome of irradiated and non-irradiated extremities (32).
In contrast to the aforementioned results, the data of Durand et al. (45), which was based on a controlled investigation, suggest that UV-A1 therapy is ineffective in acrosclerosis (45). Otherwise one must consider a systemic UV-A1 effect that could explain the results of Durand et al. (45). Moreover, Tewari et al. reported medium-dose UV-A1-induced reduction of microstomia in a SSc patient (46). Jacobe et al. (9) effectively treated 34 SSc patients. On the basis of their data, medium- to high-dose UV-A1 therapy seems to be similarly effective independently of patients photo-skin types. Nevertheless, outcome measures were not reported in detail (9). In another study on 16 SSc patients, a statistically significant dose-response association was found between low-, medium-, and high-dose treatment regimens (47). Notably, Comte et al. reported UV-A1- induced improvement of Raynaud's phenomenon observed in over 80% of patients (n = 11) with autoimmune disorders including SS (48).
In contrast to the well-documented evidence of beneficial UV-A1 efficacy in LoS the data for SSc are pretty contradictory and of much poorer quality. Hence, UV-A1 should not be considered a first-line treatment modality for SSc patients.
Lichen Sclerosus
In a prospective non-controlled study, we treated ten patients suffering from extragenital lichen sclerosus (LiS) with low-dose UV-A1 (20 J/cm2) therapy 4 times weekly (32). After low-dose UV-A1 therapy a remarkable decrease of the clinical score and normalization of skin texture was observed as also confirmed by sonography. The patients noticed substantial skin softening and repigmentation in pre-existing lesions. It was suggested that similar to therapy outcomes in LoS, low- dose UV-A1 therapy seems to be a beneficial and well-tolerated therapy modality for extragenital LiS (32). Rombold et al. (11) also observed beneficial outcome for LiS patients managed with medium-dose UV-A1 (cumulative dose: 1,018 ± 575.3 J/cm2).
Beattie et al. (49) evaluated the efficacy of UV-A1 in genital LiS. Seven females were exposed to UV-A1 (low- to high-dose protocol according to MED). Five patients responded to treatment, three patients showed modest clinical improvement, and two experienced only slight therapy success. Of the five responders, one had disease relapse within three months and another after one year. The latter patients were re-treated by means of UV-A1 therapy – one had minimal improvement, the other had moderate treatment success. In the other responders, the condition substantially improved and was controllable using topical glucocorticosteroids. The authors suggested that UV-A1 is potentially an effective treatment approach for genital LiS, particularly considering that this disease is frequently poorly manageable (49).
Data of a randomized controlled trial performed in our department comparing the efficacy of high-potent topical glucocorticosteroids (clobetasol propionate 0.05%) with UV-A1 therapy (50 J/cm2, 4 times per week over 12 weeks) in the management of 30 patients with genital LiS showed a significant improvement of symptoms. Nevertheless, the current gold standard, say high-potent glucocorticosteroids, was superior to UV-A1, particularly with respect to practical considerations, reduction of pruritus, and quality of life improvement. However, we suggested to consider UV-A1 phototherapy as potential second-line treatment for VLiS (50). Moreover, our study group investigated epigenetic changes in 10 patients with LiS before and after a medium-dose UV-A1 (up to 50 J/cm2, 4 times weekly for 3 month) treatment compared to healthy controls. It could be shown that UV-A1 phototherapy may cause a normalization of 5-hydroxymethylcytosine levels–epigenetic factors may also contribute to LiS pathophysiology (15, 51).
Conclusively, based on data derived from a single randomized trial, non-randomized studies, and prospective case studies UV-A1 appears to be a treatment option for genital and extragenital forms of LiS.
Graft-vs.-Host Disease
Previously, Grundmann-Kollmann et al. (52) reported a patient suffering from chronic sclerodermic graft-vs.-host disease (GvHD) who was refractory to conventional therapies (32). In the combination with oral mycophenolate mofetil low-dose UV-A1 (20 J/cm2) four times weekly was beneficial (cumulative dose: 480 J/cm2 UV-A1). Furthermore, Stander et al. (53) studied five GvHD patients receiving 50 J/cm2 UV-A1 (5 times per week) over eight weeks followed by subsequent diminishment of UV-A1 doses toward 3 times weekly (32). Notably, one patient was irradiated using a fix dose of 20 J/cm2 UV-A1 combined with immunosuppressants and extracorporeal photopheresis (ECP). In all patients, treatment resulted in skin softening of pre-existing lesions (53). Calzavara-Pinton et al. (54) treated five patients with sclerodermoid GvHD (localized: 4; generalized: 1) with medium-dose UV-A1 (50 J/cm2) therapy three times weekly. Therapy was successful with complete responses observed in three patients and partial responses in two (54).
In contrast, a study of 25 GvHD patients by Connolly et al. found clinical improvement in patients who received high-dose UV-A1 phototherapy (47). In a small trial, 7 patients were exposed to UV-A1 as primary treatment for acute cutaneous GvHD. In 5 patients, a complete response was noticed, in 2 patients were non-responders and requiring systemic steroids (32). In 2010, Schlaak et al. (55) studied 70 patients suffering from acute cutaneous GvHD. Following a median therapy period of 10 months, the authors achieved complete and partial responses in 70% and 24.3% of patients, respectively. Following a median follow-up of 18 (range 10–60) months, non-melanoma skin cancer occurred in three patients. The authors concluded that UV-A1 therapy can be a beneficial therapy for acute GvHD affecting the skin (32). Avoiding chronic use of systemic glucocorticosteroids and/or allowing a faster tapering of immunosuppressants in a substantial number of patients, UV-A1 appears to be an interesting therapy option for GvHD (55). Moreover, Ziemer et al. treated two children with chronic cutaneous GvHD who improved after UV- A1 therapy with regard to cutaneous lesions, joint mobility, and quality of life (32).
The benefit of UV-A1 for GvHD patients has only been documented in small retrospective case series and case reports making it difficult to give a definitive recommendation for this photherapeutic modality in GvHD. Moreover, there are no comparison studies with UV-A1 and ECP–a frequently recommended photochemotherapeutic option for GvHD patients.
Nephrogenic Systemic Fibrosis
Tran et al. (56) recently treated nephrogenic systemic fibrosis (NSF) with UV-A1 phototherapy. All patients (n = 4) received hemodialysis before, during, and after high-dose UV-A1 (32). All patients noticed softening of their skin, and two patients experienced increase of mobility of the limbs. The therapeutic was significant in all cases, even though none patient complete clearance of fibrosis could be achieved. Hence, UV-A1 represents a feasible therapy modality for NSF, in particular in cases in which kidney transplantation is no option or in delay (56). Interestingly, UV-A1 does not only improve clinically NSF but also induce procollagen synthesis and reduce profibrotic cytokine and growth factor expression (32). Using a medium-dose regimen, however, we could not observe beneficial effects after UV-A1 therapy in patients (n = 3) with NSF (32). These results are supported by an analysis of 17 patients with NSF which found high-dose regimens to be more effective than medium- and low-dose regimens for NSF (47). By the way, Gazi et al. (57) performed a survey, and found that an reduction of 3 to 7.5 points of the modified Rodnan skin score does reflect a clinically meaningful treatment outcome (32).
In conclusion, UV-A1 may work in NSF; however, this statement is only based on a few case series and retrospective observations.
Miscellaneous
Moreover, positive results following UV-A1 phototherapy of fibrosing conditions have been documented in case reports on patients with scleromyxedema, scleredema adultorum Buschke, and pansclerotic porphyria tarda. Variable data have been reported for UV-A1 therapy of keloids and eosinophilic fasciitis (5, 7, 11, 58–63).
Mechanisms of Action, Limitations, and Adverse Events
Photo-Skin Type Status
It is still controversially discussed whether patients with photo-skin type > III respond worse to UV-A1 therapy (7, 45, 64). Wang et al. (64) demonstrated that a single UV-A1 dose can markedly reduce procollagen mRNA gene expression and substantially enhance matrix metalloproteinase 1 and 3 gene expression in controls (15). Their results showed that such anti-fibrotic effects likely decrease after repeated UV-A1 irradiation sessions (15). By contrast, skin darkening usually depends on dosage (15). Stronger pigmentation resulted in a decrease of the anti-fibrotic effects of UV-A1 (15). Hence, individuals with dark skin show only marginal or even no decrease of procollagen when compared to individuals with fair skin (15). The aforementioned results could have significant implications on patient stratification for therapy, proposing that patients with fair skin are better candidates for UV-A1 therapy (15). Wang et al. (64) speculated that the aforementioned observation may be the reason for more favorable outcomes reported in previous UV-A1 trials on sclerotic skin diseases predominantly including European Caucasians (15).
Tuchinda et al. (7) reported (n = 92) that patients with fair skin likely respond to UV-A1 better than patients with darker skin (15). However, Jacobe et al. (9) performed a study on 101 patients who were treated with UV-A1 treatment. Photo-skin types and total UV-A1 doses were analyzed. The evaluation of therapy outcome was based on clinical parameters such body surface area, fibrosis and subjective symptoms such as itch (15). Interestingly, clinical response to UV-A1 was not dependent on skin complexion in this population assessed.
Mode of Action Aspects
More infrequent types of LoS including linear LoS an deep morphea and severe cases of acrosclerosis and sGVHD frequently affect deeper anatomical structures such as fascias, muscles, and bones (15). Because UV-A1 penetrates into the subcutis only, the aforementioned conditions rather require systemic immunosuppressive treatment such as methotrexate (65). Evidence indicates that UV-A1 phototherapy acts through diminishment of cutaneous T cell infiltrates, down-regulation of pro-inflammatory cytokines, changes in endothelial cell function, and induction of programmed cell death. Nevertheless, the most important mode of action of UV-A1 in fibrotic conditions is the induction of matrix metalloproteinases and inhibition of collagen synthesis (15). Furthermore, UV-A1 exerts changes in fibroblast cytokine production (15) such as transforming growth factor- ß/Smad signaling and interleukin (IL) and IL-6, leading to an upregulation of collagenase activity (15). It was shown in vitro that UV-A1 irradiation of cultured fibroblasts obtained from LoS patients resulted in increased collagenase gene and protein expression. After UV-A1 irradiation, it was also observed a fast production of interleukin 1 (IL-1) stimulating the release of IL-6 which mediates an upregulation of collagenase synthesis by fibroblasts (14, 65–71).
Side Effects
The most common acute adverse events of UV-A1 include increased pigmentation, erythema, and itch (5, 10, 12, 14, 72). UV-A1 treatment usually needs long exposure times, resulting in considerable heat, which might be intolerable for patients. Phototoxic reactions may occur, in particular in patients with fair skin (20). Notably, UV-A1 absorbing substances of the skin, such as porphyrins and riboflavins, can cause oxidative stress resulting in phototoxic reactions (73). Beside the aforementioned side effects, Wang et al. (74) investigated the effects following a limited number of low-dose UV-A1 irradiation sessions as usually experienced in daily life. They observed that these UV-A1 exposures potentially promoted photoaging by affecting breakdown, rather than synthesis, of collagen. In fair skinned individuals, increasing skin pigmentation due to low-dose UV-A1 did not prevent collagenolytic alterations usually induced by UV-A1. They concluded that sunscreens must block sufficiently UV-A1 wavelengths as well (74). Furthermore, UV-A1 can induce photodermatoses or reactivate herpes flares (16, 75). A recent case study reported a 37-years-old female with a persistent polymorphous light eruption lasting for 5 weeks following UV-A1 phototherapy (76).
Skin cancer and premature skin aging belong to the most important chronic side effects linked to broadband UV-A radiation. UV-A can suppress skin immunity in a bell-shaped dose response (15). Long-wave UV-A corresponding to dose equivalents of 20 min sun exposure contributes to about 75% immunosuppression caused by sun irradiation (15). It was shown that UV-A1 but not UV-A ranging from 320 to 350 nm induces immunosuppression in humans, indicating a significant role for reactive oxygen species (77). Moreover, UV-A induces an energy crisis in cells, can activate alternative complement pathways, and alters the development of memory T cells (15). Skin cancers are associated with p53 and BRM mutations, which can be induced by UV-A1 as well (77–79).
Of importance is also research of Tewari et al. who recently reported a study indicating the induction of DNA dimers at the basal layer and in the upper dermis after UV-A1 exposure (80).
Principally, patients treated with UV-A1 must have regular skin checks and should avoid the use of sunbeds and/or additional sun exposure (15). UV-A1 contraindications may include conditions of UV sensitivity (i.e., xeroderma pigmentosum, porphyrias), use of UV sensitizing substances, history of skin cancers, radiotherapy, and chronic immunosuppresssion (15). For example, azathioprine leads to increased UV-A sensitivity and thus is a well-known photocarcinogen (81).
Overall Conclusion
The best evidence of efficacy for UV-A1 therapy exists in LoS. We consider medium UV-A1 the first-line modality for disseminated forms of this disease, in particular given the fact that there is a lack of effective standard treatments. The latter does also apply to LiS which is closely related to LoS. Hence, UV-A1 represents an attractive treatment option for widespread LiS as well. In the other conditions discussed above UV-A1 may represent an alternative treatment option. About 6 years ago, Kerr et al. (23) considered that UVA1 should only be available through specialist services until we have more evidence. With regard to efficacy of UV-A1 we think that this phototherapeutic option should be widely available in all dermatology centers. However, the price for high-output UV-A1 devices is still very high. Hence, we are afraid that UV-A will predominantly remain a more specialized unit tertiary service.
Author Contributions
Both authors contributed to the literature search, data extraction, interpretation of results, and preparation of the manuscript. Manuscript approval was performed by both authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Plewig G, Hofmann C, Braun-Falco O, Nath G, Kreitmair A. A new apparatus for the delivery of high intensity UVA and UVA+UVB irradiation, and some dermatological applications. Br J Dermatol. (1978) 98:15–24. doi: 10.1111/j.1365-2133.1978.tb07328.x
2. Mutzhas MF, Holzle E, Hofmann C, Plewig G. A new apparatus with high radiation energy between 320-460 nm: physical description and dermatological applications. J Invest Dermatol. (1981) 76:42–7. doi: 10.1111/1523-1747.ep12524813
3. Krutmann J. Phototherapy for atopic dermatitis. Clin Exp Dermatol. (2000) 25:552–8. doi: 10.1046/j.1365-2230.2000.00700.x
4. Baron ED, Stevens SR. Phototherapy for cutaneous T-cell lymphoma. Dermatol Ther. (2003) 16:303–10. doi: 10.1111/j.1396-0296.2003.01642.x
5. Dawe RS. Ultraviolet A1 phototherapy. Br J Dermatol. (2003) 148:626–37. doi: 10.1046/j.1365-2133.2003.05261.x
6. Pavel S. Light therapy (with UVA-1) for SLE patients: is it a good or bad idea? Rheumatology (2006) 45:653–5. doi: 10.1093/rheumatology/kel063
7. Tuchinda C, Kerr HA, Taylor CR, Jacobe H, Bergamo BM, Elmets C, et al. UVA1 phototherapy for cutaneous diseases: an experience of 92 cases in the United States. Photodermatol Photoimmunol Photomed. (2006) 22:247–53. doi: 10.1111/j.1600-0781.2006.00245.x
8. Meduri NB, Vandergriff T, Rasmussen H, Jacobe H. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. (2007) 23:106–12. doi: 10.1111/j.1600-0781.2007.00291.x
9. Jacobe HT, Cayce R, Nguyen J. UVA1 phototherapy is effective in darker skin: a review of 101 patients of Fitzpatrick skin types I-V. Br J Dermatol. (2008) 159:691–6. doi: 10.1111/j.1365-2133.2008.08672.x
10. Kroft EB, Berkhof NJ, van de Kerkhof PC, Gerritsen RM, de Jong EM. Ultraviolet A phototherapy for sclerotic skin diseases: a systematic review. J Am Acad Dermatol. (2008a) 59:1017–30. doi: 10.1016/j.jaad.2008.07.042
11. Rombold S, Lobisch K, Katzer K, Grazziotin TC, Ring J, Eberlein B. Efficacy of UVA1 phototherapy in 230 patients with various skin diseases. Photodermatol Photoimmunol Photomed. (2008) 24:19–23. doi: 10.1111/j.1600-0781.2008.00328.x
12. Gambichler T. Management of atopic dermatitis using photo(chemo)therapy. Arch Dermatol Res. (2009) 301:197–203. doi: 10.1007/s00403-008-0923-5
13. Suh KS, Kang JS, Baek JW, Kim TK, Lee JW, Jeon YS, et al. Efficacy of ultraviolet A1 phototherapy in recalcitrant skin diseases. Ann Dermatol. (2010) 22:1–8. doi: 10.5021/ad.2010.22.1.1
14. York NR, Jacobe HT. UVA1 phototherapy: a review of mechanism and therapeutic application. Int J Dermatol. (2010) 49:623–30. doi: 10.1111/j.1365-4632.2009.04427.x
15. Gambichler T, Terras S, Kreuter A. Treatment regimens, protocols, dosage, and indications for UVA1 phototherapy: facts and controversies. Clin Dermatol. (2013) 31:438–54. doi: 10.1016/j.clindermatol.2013.01.011
16. Lim HW, Hönigsmann H, Hawk JLM. Photodermatology. New York, NY: Informa Healthcare USA Inc (2007).
17. Brem R, Karran P. Multiple forms of DNA damage caused by UVA photoactivation of DNA 6-thioguanine. Photochem Photobiol. (2011) 88:5–13. doi: 10.1111/j.1751-1097.2011.01043.x
18. Calzavara-Pinton PG, Caravello S. A practical approach to the initial dose and subsequent increments for ultraviolet A1 phototherapy. Br J Dermatol. (2017) 177:19–20. doi: 10.1111/bjd.15486
19. Gambichler T, Majert J, Pljakic A, Rooms I, Wolf P. Determination of the minimal erythema dose for ultraviolet A1 radiation. Br J Dermatol. (2017) 177:238–44. doi: 10.1111/bjd.15245
20. Beattie PE, Dawe RS, Ferguson J, Ibbotson SH. Dose-response and time-course characteristics of UV-A1 erythema. Arch Dermatol. (2005) 141:1549–55. doi: 10.1001/archderm.141.12.1549
21. Eadie E, Ibbotson SH, Dawe RS. Irradiance, as well as body site and timing of readings, is important in determining ultraviolet A minimal erythema dose. Br J Dermatol. (2018) 178:297–8. doi: 10.1111/bjd.16005
22. Gambichler T, Wolf P. Irradiance, as well as body site and timing of readings, is important in determining ultraviolet A minimal erythemal dose: reply from the authors. Br J Dermatol. (2018) 178:298–9. doi: 10.1111/bjd.16044
23. Kerr AC, Ferguson J, Attili SK, Beattie PE, Coleman AJ, Dawe RS, et al. Ultraviolet A1 phototherapy: a British Photodermatology Group workshop report. Clin Exp Dermatol. (2012) 37:219–26. doi: 10.1111/j.1365-2230.2011.04256.x
24. Stege H, Berneburg M, Humke S, Klammer M, Grewe M, Grether-Beck S, et al. High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol. (1997) 36(6 Pt 1):938–44. doi: 10.1016/S0190-9622(97)80277-0
25. Ju M, Chen K, Chang B, Gu, H. UVA1 irradiation inhibits fibroblast proliferation and alleviates pathological changes of scleroderma in a mouse model. J Biomed Res. (2012) 26:135–42. doi: 10.1016/S1674-8301(12)60023-2
26. Karpec D, Rudys R, Leonaviciene L, Mackiewicz Z, Bradunaite R, Kirdaite G, et al. The impact of high-dose narrowband ultraviolet A1 on dermal thickness, collagen and matrix- metalloproteinases in animal model of scleroderma. J Photochem Photobiol B. (2017a) 173:448–55. doi: 10.1016/j.jphotobiol.2017.06.021
27. Karpec D, Rudys R, Leonaviciene L, Mackiewicz Z, Bradunaite R, Kirdaite G, et al. The safety and efficacy of light emitting diodes-based ultraviolet A1 phototherapy in bleomycin- induced scleroderma in mice. Adv Med Sci. (2017b) 63:152–9. doi: 10.1016/j.advms.2017.09.001
28. Kerscher M, Dirschka T, Volkenandt M. Treatment of localised scleroderma by UVA1 phototherapy. Lancet (1995) 346:1166. doi: 10.1016/S0140-6736(95)91843-4
29. Gruss CJ, Von Kobyletzki G, Behrens-Williams SC, Lininger J, Reuther T, Kerscher M, et al. Effects of low dose ultraviolet A-1 phototherapy on morphea. Photodermatol Photoimmunol Photomed. (2001) 17:149–55. doi: 10.1034/j.1600-0781.2001.170401.x
30. Camacho NR, Sanchez JE, Martin RF, Gonzalez JR, Sanchez JL. Medium-dose UVA1 phototherapy in localized scleroderma and its effect in CD34-positive dendritic cells. J Am Acad Dermatol. (2001) 45:697–9. doi: 10.1067/mjd.2001.117735
31. de Rie MA, Enomoto DN, de Vries HJ, Bos JD. Evaluation of medium-dose UVA1 phototherapy in localized scleroderma with the cutometer and fast Fourier transform method. Dermatology (2003) 207:298–301. doi: 10.1159/000073093
32. Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. (2006c) 54:440–7. doi: 10.1016/j.jaad.2005.11.1063
33. Sator PG, Radakovic S, Schulmeister K, Honigsmann H, Tanew A. Medium-dose is more effective than low-dose ultraviolet A1 phototherapy for localized scleroderma as shown by 20-MHz ultrasound assessment. J Am Acad Dermatol. (2009) 60:786–91. doi: 10.1016/j.jaad.2008.12.013
34. Vasquez R, Jabbar A, Khan F, Buethe D, Ahn C, Jacobe H. Recurrence of morphea after successful ultraviolet A1 phototherapy: a cohort study. J Am Acad Dermatol. (2014) 70:481–88. doi: 10.1016/j.jaad.2013.10.018
35. Su O, Onsun N, Onay HK, Erdemoglu Y, Ozkaya DB, Cebeci F, et al. Effectiveness of medium-dose ultraviolet A1 phototherapy in localized scleroderma. Int J Dermatol. (2011) 50:1006–13. doi: 10.1111/j.1365-4632.2010.04843.x
36. Andres C, Kollmar A, Mempel M, Hein R, Ring J, Eberlein B. Successful ultraviolet A1 phototherapy in the treatment of localized scleroderma: a retrospective and prospective study. Br J Dermatol. (2009) 162:445–7. doi: 10.1111/j.1365-2133.2009.09438.x
37. Pereira N, Santiago F, Oliveira H, Figueiredo A. Low-dose UVA(1) phototherapy for scleroderma: what benefit can we expect? J Eur Acad Dermatol Venereol. (2011) 26:619–26. doi: 10.1111/j.1468-3083.2011.04137.x
38. Gruss C, Stucker M, Kobyletzki G, Schreiber D, Altmeyer P, Kerscher M. Low dose UVA1 phototherapy in disabling pansclerotic morphoea of childhood. Br J Dermatol. (1997) 136:293–4. doi: 10.1111/j.1365-2133.1997.tb14925.x
39. Silber S. A new and rapid scoring system to assess the scientific evidence from clinical trials. J Interv Cardiol. (2006) 19:485–92. doi: 10.1111/j.1540-8183.2006.00205.x
40. von Kobyletzki G, Uhle A, Pieck C, Hoffmann K, Altmeyer P. Acrosclerosis in patients with systemic sclerosis responds to low-dose UV-A1 phototherapy. Arch Dermatol. (2000) 136:275–6. doi: 10.1001/archderm.136.2.275
41. Morita A, Kobayashi K, Isomura I, Tsuji T, Krutmann J. Ultraviolet A1 (340-400 nm) phototherapy for scleroderma in systemic sclerosis. J Am Acad Dermatol. (2000) 43:670–4. doi: 10.1067/mjd.2000.105165
42. Sawada H, Isogai Z, Morita A. Altered decorin expression of systemic sclerosis by UVA1 (340-400 nm) phototherapy: immunohistochemical analysis of 3 cases. BMC Dermatol. (2003) 3:2. doi: 10.1186/1471-5945-3-2
43. Kreuter A, Breuckmann F, Uhle A, Brockmeyer N, Von Kobyletzki G, Freitag M, et al. Low-dose UVA1 phototherapy in systemic sclerosis: effects on acrosclerosis. J Am Acad Dermatol. (2004) 50:740–7.
44. Rose RF, Turner D, Goodfield MJ, Goulden V. Low-dose UVA1 phototherapy for proximal and acral scleroderma in systemic sclerosis. Photodermatol Photoimmunol Photomed. (2009) 25:153–5. doi: 10.1111/j.1600-0781.2009.00422.x
45. Durand F, Staumont D, Bonnevalle A, Hachulla E, Hatron PY, Thomas P. Ultraviolet A1 phototherapy for treatment of acrosclerosis in systemic sclerosis: controlled study with half-side comparison analysis. Photodermatol Photoimmunol Photomed. (2007) 23:215–21. doi: 10.1111/j.1600-0781.2007.00308.x
46. Tewari A, Garibaldinos T, Lai-Cheong J, Groves R, Sarkany R, Branislav Novakovic L. Successful treatment of microstomia with UVA1 phototherapy in systemic sclerosis. Photodermatol Photoimmunol Photomed. (2011) 27:113–4. doi: 10.1111/j.1600-0781.2011.00570.x
47. Connolly KL, Griffith JL, McEvoy M, Lim HW. Ultraviolet A1 phototherapy beyond morphea: experience in 83 patients. Photodermatol Photoimmunol Photomed. (2015) 31:289–95. doi: 10.1111/phpp.12185
48. Comte C, Bessis D, Picot E, Peyron JL, Guillot B, Dereure O. [Treatment of connective tissue disorder-related acral syndromes using UVA-1 phototherapy. An open study of 11 cases]. Ann Dermatol Venereol. (2009) 136:323–9. doi: 10.1016/j.annder.2008.12.022
49. Beattie PE, Dawe RS, Ferguson J, Ibbotson SH. UVA1 phototherapy for genital lichen sclerosus. Clin Exp Dermatol. (2006) 31:343–7. doi: 10.1111/j.1365-2230.2006.02082.x
50. Terras S, Gambichler T, Moritz RK, Stucker M, Kreuter A. UV-A1 phototherapy vs clobetasol propionate, 0.05%, in the treatment of vulvar lichen sclerosus: a randomized clinical trial. JAMA Dermatol. (2014). 150:621–7. doi: 10.1001/jamadermatol.2013.7733
51. Gambichler T, Terras S, Kreuter A, Skrygan M. Altered global methylation and hydroxymethylation status in vulvar lichen sclerosus: further support for epigenetic mechanisms. Br J Dermatol. (2014) 170:687–93. doi: 10.1111/bjd.12702
52. Grundmann-Kollmann M, Behrens S, Gruss C, Gottlober P, Peter RU, Kerscher M. Chronic sclerodermic graft-versus-host disease refractory to immunosuppressive treatment responds to UVA1 phototherapy. J Am Acad Dermatol. (2000) 42(1 Pt 1), 134–6. doi: 10.1016/S0190-9622(00)90023-9
53. Stander H, Schiller M, Schwarz T. UVA1 therapy for sclerodermic graft-versus-host disease of the skin. J Am Acad Dermatol. (2002) 46:799–800. doi: 10.1067/mjd.2002.121352
54. Calzavara Pinton P, Porta F, Izzi T, Venturini M, Capezzera R, Zane C, et al. Prospects for ultraviolet A1 phototherapy as a treatment for chronic cutaneous graft-versus-host disease. Haematologica (2003) 88:1169–75.
55. Schlaak M, Schwind S, Wetzig T, Maschke J, Treudler R, Basara N, et al. UVA (UVA-1) therapy for the treatment of acute GVHD of the skin. Bone Marrow Transpl. (2010) 45:1741–8. doi: 10.1038/bmt.2010.230
56. Tran KT, Prather HB, Cockerell CJ, Jacobe H. UV-A1 therapy for nephrogenic systemic fibrosis. Arch Dermatol. (2009) 145:1170–4. doi: 10.1001/archdermatol.2009.245
57. Gazi H, Pope JE, Clements P, Medsger TA, Martin RW, Merkel PA, et al. Outcome measurements in scleroderma: results from a delphi exercise. J Rheumatol. (2007) 34:501–9.
58. Asawanonda P, Khoo LS, Fitzpatrick TB, Taylor CR. UV-A1 for keloid. Arch Dermatol. (1999) 135:348–9. doi: 10.1001/archderm.135.3.348
59. Hannuksela-Svahn A, Grandal OJ, Thorstensen T, Christensen OB. UVA1 for treatment of keloids. Acta Derm Venereol. (1999) 79:490. doi: 10.1080/000155599750010076
60. Janiga JJ, Ward DH, Lim HW. UVA-1 as a treatment for scleredema. Photodermatol Photoimmunol Photomed. (2004) 20:210–1. doi: 10.1111/j.1600-0781.2004.00106.x
61. Kroft EB, van de Kerkhof PC, Gerritsen MJ, de Jong EM. Period of remission after treatment with UVA-1 in sclerodermic skin diseases. J Eur Acad Dermatol Venereol. (2008b) 22:839–44. doi: 10.1111/j.1468-3083.2007.02576.x
62. Silny W, Osmola-Mankowska A, Czarnecka-Operacz M, Zaba R, Danczak-Pazdrowska A, Marciniak A. Eosinophilic fascitis: a report of two cases treated with ultraviolet A1 phototherapy. Photodermatol Photoimmunol Photomed. (2009) 25:325–7. doi: 10.1111/j.1600-0781.2009.00463.x
63. Polat M, Kaya H, Sahin A. A new approach in the treatment of keloids: UVA-1 Laser. Photomed Laser Surg. (2016) 34:130–3. doi: 10.1089/pho.2015.4046
64. Wang F, Garza LA, Cho S, Kafi R, Hammerberg C, Quan T, et al. Effect of increased pigmentation on the antifibrotic response of human skin to UV-A1 phototherapy. Arch Dermatol. (2008) 144:851–8. doi: 10.1001/archderm.144.7.851
65. Kreuter A, Gambichler T. UV-A1 phototherapy for sclerotic skin diseases: implications for optimizing patient selection and management. Arch Dermatol. (2008) 144:912–6. doi: 10.1001/archderm.144.7.912
66. Yin L, Yamauchi R, Tsuji T, Krutmann J, Morita A. The expression of matrix metalloproteinase-1 mRNA induced by ultraviolet A1 (340-400 nm) is phototherapy relevant to the glutathione (GSH) content in skin fibroblasts of systemic sclerosis. J Dermatol. (2003) 30:173–80. doi: 10.1111/j.1346-8138.2003.tb00368.x
67. Breuckmann F, Gambichler T, Altmeyer P, Kreuter A. UVA/UVA1 phototherapy and PUVA photochemotherapy in connective tissue diseases and related disorders: a research based review. BMC Dermatol. (2004) 4:11. doi: 10.1186/1471-5945-4-11
68. Kreuter A, Hyun J, Skrygan M, Sommer A, Bastian A, Altmeyer P, et al. Ultraviolet A1- induced downregulation of human beta-defensins and interleukin-6 and interleukin-8 correlates with clinical improvement in localized scleroderma. Br J Dermatol. (2006a) 155:600–7. doi: 10.1111/j.1365-2133.2006.07391.x
69. Kreuter A, Hyun J, Skrygan M, Sommer A, Tomi NS, Breuckmann F, et al. Ultraviolet A1 phototherapy decreases inhibitory SMAD7 gene expression in localized scleroderma. Arch Dermatol Res. (2006b) 298:265–72. doi: 10.1007/s00403-006-0695-8
70. Gambichler T, Skrygan M, Tomi NS, Altmeyer P, Kreuter A. Differential expression of decorin in localized scleroderma following ultraviolet-A1 irradiation. J Am Acad Dermatol. (2007a) 56:956–9. doi: 10.1016/j.jaad.2006.10.961
71. Gambichler T, Skrygan M, Tomi NS, Breuksch S, Altmeyer P, Kreuter A. Significant downregulation of transforming growth factor-beta signal transducers in human skin following ultraviolet-A1 irradiation. Br J Dermatol. (2007b) 156:951–6. doi: 10.1111/j.1365-2133.2007.07802.x
72. Richer V, Lui H. Cross-sectional evaluation of acute adverse reactions during ultraviolet A1 phototherapy. Br J Dermatol. (2017) 177:258–9. doi: 10.1111/bjd.15319
73. Besaratinia A, Kim SI, Bates SE, Pfeifer GP. Riboflavin activated by ultraviolet A1 irradiation induces oxidative DNA damage-mediated mutations inhibited by vitamin C. Proc Natl Acad Sci USA. (2007) 104:5953–8. doi: 10.1073/pnas.0610534104
74. Wang F, Smith NR, Tran BA, Kang S, Voorhees JJ, Fisher GJ. Dermal damage promoted by repeated low-level UV-A1 exposure despite tanning response in human skin. JAMA Dermatol. (2014) 150:401–6. doi: 10.1001/jamadermatol.2013.8417
75. Gambichler T, Al-Muhammadi R, Boms S. Immunologically mediated photodermatoses: diagnosis and treatment. Am J Clin Dermatol. (2009) 10:169–80. doi: 10.2165/00128071-200910030-00003
76. Aljasser MI, Lui H, Ball NJ, Kalia S. Persistent polymorphous light eruption after ultraviolet A1 phototherapy. Photodermatol Photoimmunol Photomed. (2013) 29:52–4. doi: 10.1111/phpp.12020
77. Damian DL, Matthews YJ, Phan TA, Halliday GM. An action spectrum for ultraviolet radiation-induced immunosuppression in humans. Br J Dermatol. (2011) 164:657–9. doi: 10.1111/j.1365-2133.2010.10161.x
78. Park J, Halliday GM, Surjana D, Damian DL. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem Photobiol. (2010) 86:942–8. doi: 10.1111/j.1751-1097.2010.00746.x
79. Halliday GM, Byrne SN, Damian DL. Ultraviolet A radiation: its role in immunosuppression and carcinogenesis. Semin Cutan Med Surg. (2011) 30:214–21. doi: 10.1016/j.sder.2011.08.002
80. Tewari A, Sarkany RP, Young AR. UVA1 induces cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo. J Invest Dermatol. (2012) 132:394–400. doi: 10.1038/jid.2011.283
Keywords: UV-A1, ultraviolet A1, UVA1, irradiation, sclerosis, fibrosis, phototherapy
Citation: Gambichler T and Schmitz L (2018) Ultraviolet A1 Phototherapy for Fibrosing Conditions. Front. Med. 5:237. doi: 10.3389/fmed.2018.00237
Received: 22 February 2018; Accepted: 03 August 2018;
Published: 27 August 2018.
Edited by:
Frank Ronald De Gruijl, Leiden University Medical Center, NetherlandsReviewed by:
Sally Helen Ibbotson, University of Dundee, United KingdomVijaykumar Patra, Medizinische Universität Graz, Austria
Copyright © 2018 Gambichler and Schmitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thilo Gambichler, dC5nYW1iaWNobGVyQGtsaW5pa3VtLWJvY2h1bS5kZQ==
 Thilo Gambichler
Thilo Gambichler Lutz Schmitz
Lutz Schmitz