
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 12 June 2018
Sec. Regulatory Science
Volume 5 - 2018 | https://doi.org/10.3389/fmed.2018.00167
 Yimin Xu1,2
Yimin Xu1,2 Dhavalkumar N. Patel1
Dhavalkumar N. Patel1 Suet-Leng P. Ng3
Suet-Leng P. Ng3 Siew-Har Tan3
Siew-Har Tan3 Dorothy Toh3
Dorothy Toh3 Jalene Poh3
Jalene Poh3 Adena Theen Lim3
Adena Theen Lim3 Cheng-Leng Chan1,3
Cheng-Leng Chan1,3 Min-Yong Low2
Min-Yong Low2 Hwee-Ling Koh1*
Hwee-Ling Koh1*The objective of this study is to collate and analyse adverse event reports associated with the use of complementary health products (CHP) submitted to the Health Sciences Authority (HSA) of Singapore for the period 2010–2016 to identify various trends and signals for pharmacovigilance purposes. A total of 147,215 adverse event reports suspected to be associated with pharmaceutical products and CHP were received by HSA between 2010 and 2016. Of these, 143,191 (97.3%) were associated with chemical drugs, 1,807 (1.2%) with vaccines, 1,324 (0.9%) with biological drugs (biologics), and 893 (0.6%) with CHP. The number of adverse event reports associated with Chinese Proprietary Medicine, other complementary medicine and health supplements are presented. Eight hundred and ninety three adverse event reports associated with CHP in the 7-year period have been successfully collated and analyzed. In agreement with other studies, adverse events related to the “skin and appendages disorders” were the most commonly reported. Most of the cases involved dermal allergies (e.g., rashes) associated with the use of glucosamine products and most of the adulterated products were associated with the illegal addition of undeclared drugs for pain relief. Dexamethasone, chlorpheniramine, and piroxicam were the most common adulterants detected. Reporting suspected adverse events is strongly encouraged even if the causality is not confirmed because any signs of clustering will allow rapid regulatory actions to be taken. The findings from this study help to create greater awareness on the health risks, albeit low, when consuming CHP and dispelling the common misconception that “natural” means “safe.” In particular, healthcare professionals and the general public should be aware of potential adulteration of CHP. The analysis of spontaneously reported adverse events is an important surveillance system in monitoring the safety of CHP and helps in the understanding of the risk associated with the use of such products. Greater collaboration and communication between healthcare professionals, regulators, patients, manufacturers, researchers, and the general public are important to ensure the quality and safety of CHP.
In Singapore, health products are defined as any substance, preparation or device intended for use by humans, solely or principally for a health-related purpose. These include pharmaceutical products, complementary health products (CHP), medical devices, and cosmetic products (Figure 1). CHP consists of Chinese proprietary medicines (CPM), traditional medicines (TM), homeopathic medicines, health supplements (HS), and traditional medicinal materials (1). There have been some concerns about the safety of CHP, in particular adverse effects associated with them. World Health Organization (WHO) defines adverse drug reaction as a reaction which is noxious and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of a disease, or for the modification of a physiological function (2). An adverse event is “a medical occurrence temporally associated with the use of a medicinal product, but not necessarily causally related” (2). For the purpose of this paper, the term “adverse event” will be used.
Although CHP are widely perceived to be safe by consumers on the basis of their natural origin and long term use by various cultures, factors such as poor quality, incorrect or misidentified herbs, incorrect processing methods, variations in the concentration of active ingredients in products, inherent toxicity of herbs (e.g., hepatotoxicity, nephrotoxicity), contamination or deliberate adulteration with harmful substances or synthetic drugs, herb-herb interactions, and herb-drug interactions can affect the quality and safety of these products (3–6).
Regulation of CHP in many developed countries (including Singapore) focuses primarily on post-market strategies like post-market surveillance testing and adverse event monitoring. Recognizing the public health issues and concerns surrounding the use of herbal medicines, the “WHO Traditional Medicine Strategy 2014–2023” was established. The strategy aims to support Member States in developing proactive policies and implementing action plans for monitoring of herbal safety within the existing pharmacovigilance framework (7). Pharmacovigilance has great significance in detecting adverse effects as many herbal products on the market have not been thoroughly tested for their efficacy and safety.
Different methods are used for pharmacovigilance of medicines including spontaneous reporting, prescription event monitoring and case control, and cohort studies (8). As these methods are primarily developed for conventional drugs, they are currently of little use for evaluating the safety of herbal medicines although modified methods have been developed. Spontaneous reporting of adverse events has played a major role in post-market drug surveillance and has led to the withdrawal of pharmaceutical products as well as herbal medicinal products such as Kava kava. Therefore, international and national databases of reported adverse events can serve as an effective tool to identify signals and trends of adverse events associated with the use of herbal medicinal products (9).
The objective of this study is to collate and analyse adverse event reports associated with the use of CHP submitted to the Health Sciences Authority of Singapore (HSA) for the period 2010–2016 to identify various trends and signals for pharmacovigilance purposes.
The data was obtained from adverse event reports submitted to the Vigilance & Compliance Branch (VCB) of the HSA in Singapore using the “Suspected Adverse Drug Reactions” form, via e-mail, facsimile, postal mail, online or electronically through the Critical Medical Information Store component of Electronic Medical Record Exchange. The manual form is shown in Figure 2 (10). The reports were reviewed by regulatory specialists of the VCB and examined for causality of the suspected drug or herb. Further clarifications with the reporters may be conducted. All related materials and documents to each report were included in the Pharmaceutical Regulatory Information System (PRISM) database for aggregate analysis. As a member country of the WHO International Drug Monitoring Programme, these adverse event reports were also submitted to the WHO-Uppsala Monitoring Centre in Sweden for collation into WHO's VigiLyze (11).
The data used for analysis was for the period between January 2010 and December 2016 and was generated on 19th January 2017. The analysis was performed on valid reports that were approved and with VCB assessed causality terms “certain,” “probable,” and “possible.” To be a valid report, the minimum information required includes the following: an identifiable reporter/healthcare professional, an identifiable patient, an adverse event and a suspected product. The focus of the study was on CHP, namely CPM, health supplements and other types of complementary medicines. Information to be used in the study was extracted from the reports and collated on Microsoft® Excel files. Patient's demographic information such as age, gender, ethnicity, hospitalization status, and outcome of the adverse event, seriousness of the adverse event, organ system affected, profession of the reporter, type and description of products involved, route of administration, organ system affected, concomitant use of conventional drugs, testing for adulteration, and test results were collated.
Adverse events in the reports were categorized using the WHO adverse reaction terminology (WHO-ART) involving different system-organ classes (SOC) (12). Hospitalization status of the patients was categorized into “hospitalized,” “not hospitalized,” and “already hospitalized.” The term “already hospitalized” describes patients who have been admitted for other co-morbidities when the adverse event was detected. Outcome of the adverse events associated with the use of CHP was also analyzed by categorizing them into four categories, namely, “patients who recovered,” “not yet recovered at the point of reporting,” “had uncertain outcome,” and “died.” Conventional medicines consumed by the patient at the same time or within 3 months at the time of reporting were considered as concomitant medication. To ascertain the likelihood of the cause of the adverse event, some implicated products were sent to the Pharmaceutical Laboratory of HSA to test for the presence of drug adulterants and toxic heavy metals. Test results of those products were also collated. Information on the type and frequency of adulterants was compiled for products found to be adulterated. The variables collected from the reports were analyzed using descriptive statistics.
A total of 147,215 adverse event reports suspected to be associated with pharmaceutical products and CHP were received by HSA between 2010 and 2016. Of these, 143,191 (97.3%) were associated with chemical drugs, 1,807 (1.2%) with vaccines, 1,324 (0.9%) with biological drugs (biologics), and 893 (0.6%) with CHP. Each adverse event report may involve more than 1 suspected drug or product type, more than one adverse event and more than one system organ class. The CHP surveyed were CPM, other types of complementary medicines and health supplements. Table 1 shows the number of adverse event reports associated with different CHP for the period from 2010 to 2016. Four hundred and forty nine out of the 893 (50%) cases were found to be serious according to the assessment of the VCB including 14 deaths.
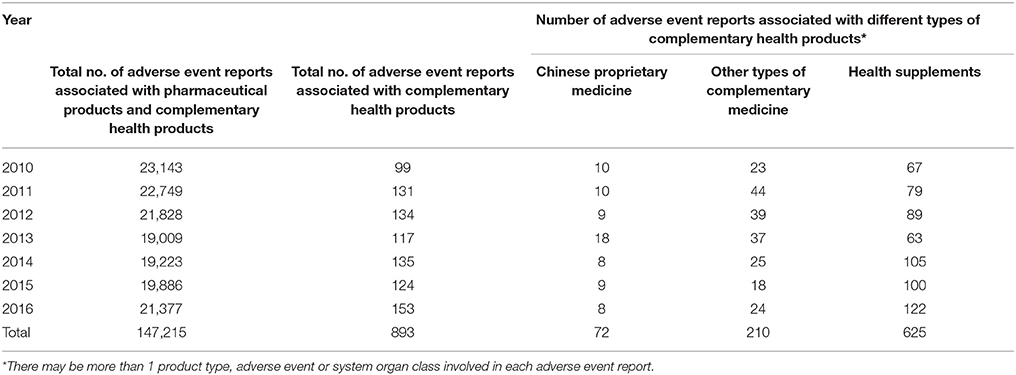
Table 1. Number of adverse event reports associated with different types of complementary health products for the period 2010–2016.
Among the 625 adverse event reports associated with health supplements, 517 (82.7%) were associated with products containing glucosamine and were mostly non-serious hypersensitivity reactions. Three hundred and seventy five of these cases involved female patients, 136 cases involved male patients and 6 cases did not report the gender of the patients. Three hundred and twenty nine cases related to glucosamine indicated that the adverse event was an allergic reaction while 43 cases were deemed not allergic reactions. In 140 cases, it was uncertain whether the adverse event was an allergic reaction while 5 cases did not provide the relevant information.
CPM (72 cases) and other types of complementary medicine (210 cases, out of which 76 were related to Chinese herbal remedies) were also implicated in the adverse events. Only 1 out of 72 CPM was found to be adulterated with undeclared drugs. However, 41 out of the 76 cases associated with other complementary medicines (related to Chinese herbal remedies) were found to be adulterated with 1 or more drugs.
Of the 893 cases of adverse events, only 228 (25.5%) reported the indications of the CHP implicated. Out of these 228 cases, most of the patients used the CHP to relieve pain such as joint and neck pain (75, 32.9%), for sexual performance enhancement (29, 12.7%), and for slimming purposes (22, 9.6%). A total of 270 products (32.2%) were taken orally as powders, capsules or tablets. Six products were used topically. The route of administration was not indicated for the remaining 617 (69.0%) cases. Eighty-six patients (9.6%) took both CHP and concomitant conventional drugs during the study period. For the remaining 807 reports, there was no information on whether the patients were taking any concomitant medicine.
One hundred and fifty four (17.2%) patients were hospitalized due to the adverse events. Ninety-one (10.2%) patients were not hospitalized and 15 (1.7%) patients were already hospitalized at the point of reporting. However, information on hospitalization was not available for 633 (70.9%) cases. Further analysis on the outcomes of the adverse events associated with the use of CHP showed that 112 (12.5%) patients had recovered at the time the reports were made, 120 (13.4%) patients were not yet recovered, 607 (68.0%) had uncertain outcome and 14 died (1.57%). There was no mention of the outcome for the remaining 40 cases. Five out of the 14 fatal cases were associated with the adulteration of CHP with undeclared drugs.
Table 2 shows the patient demographic data, profession of reporters and source of reporting. In the reports where the gender was known, 66.4% (593 cases) involved females and 32.5% (290 cases) involved males. Most of the patients were Chinese (72.8%), followed by Malays (11.2%), and Indians (6.5%). Majority of the reports were contributed by the public hospitals/healthcare organizations and polyclinics (89.8%). The remaining reports were submitted by private hospitals/clinics (8.4%), retail pharmacies (0.3%), pharmaceutical companies (1.1%), and others (0.3%). Reports were submitted mainly by doctors (86.9%) and pharmacists (10.3%).
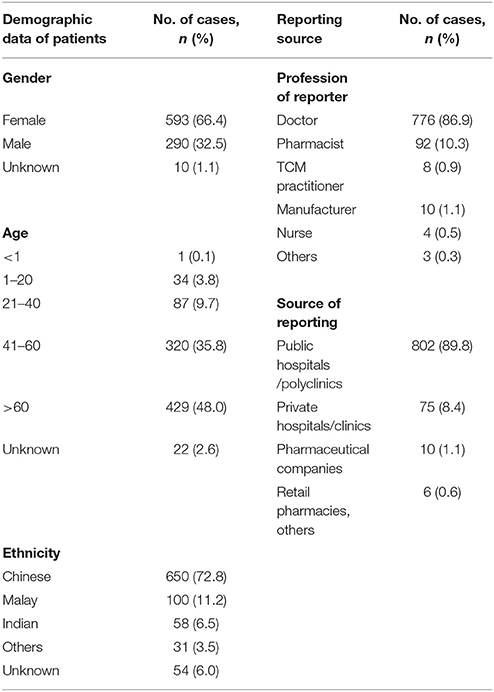
Table 2. Patient demographic data, profession of reporter, and source of reporting of a total of 893 adverse event reports.
Table 3 shows the SOC classification of adverse events associated with the use of CHP. From the 893 reports, 1,111 different adverse events were reported. The two major SOC reported were “skin and appendages disorders,” followed by “body as a whole—general disorders” e.g., anaphylactic reaction, malaise, fever, and pain. Of these, 402 (44.9%) reports were hypersensitivity reactions e.g., rash, pruritus, and periorbital oedema. Serious adverse events during the study period included Cushing's Syndrome (36 reports), Stevens-Johnson Syndrome (5 reports), acute renal failure (6 reports), hepatic failure (11 reports), severe hypoglycemia with coma (6 reports), toxic epidermal necrolysis (3 reports), stroke (2 reports), anaphylaxis (1 report), and exfoliative dermatitis (1 report). Twenty-nine out of the 36 reports with patients experiencing Cushing's Syndrome involved adulteration of products with drugs, in particular steroids. All six reports of severe hypoglycaemia with coma shock involved sexual performance enhancement products adulterated with glibenclamide and sildenafil. Three cases of liver failure were associated with products adulterated with undeclared drugs (chlorpheniramine, dexamethasone, and paracetamol) and one of these products was also found to contain arsenic at almost 25 times above Singapore legal permissible limits of 5 ppm.

Table 3. Adverse events associated with the use of complementary health products classified by System Organ Class (SOC) according to the WHO Adverse Reaction Terminology (WHO-ART) arranged in decreasing order of prevalence.
One hundred and sixty (17.9%) products out of the 893 CHP were sent for laboratory testing during the study period from 2010 to 2016. Eighty-five out of 160 (53.1%) products were found to be adulterated with undeclared drugs. These adulterated products consisted of 1 CPM, 3 health supplements and 81 other types of complementary medicines. Common drug adulterants were not detected in the remaining tested products. The other 734 products were not sent for testing due to various reasons, such as unavailability of the products and absence of strong causation between the adverse events and the products. As can be seen from Table 4, the top 3 drug adulterants found are dexamethasone, chlorpheniramine, and piroxicam. In our study, 63 out of 85 cases associated with adulterated products were found to involve drugs used to alleviate pain.
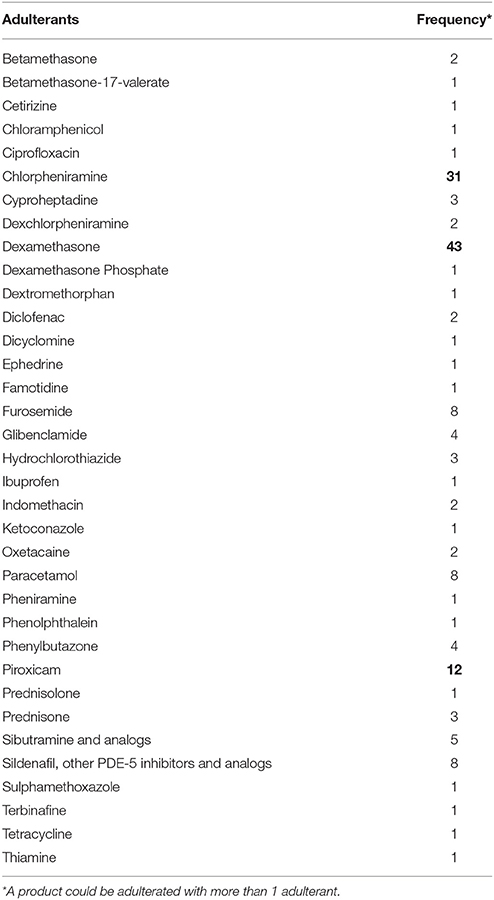
Table 4. Adulterants (in alphabetical order) detected in complementary health products, with the frequencies of the 3 most commonly detected drug adulterants in bold.
As shown in Table 5, 48 out of 85 adulterated products were found to contain more than 1 adulterant. These include “LifeSparks 100% Natural Pain Relief Supplement,” a health product which was found to contain 6 drugs, namely chlorpheniramine, dexamethasone, diclofenac, paracetamol, sulphamethoxazole, and piroxicam (13). Another adulterated product called “Herbal Health Jointcare,” was found to be adulterated with betamethasone-17-valerate, piroxicam, furosemide, chlorpheniramine, and famotidine (14).

Table 5. Frequency of complementary health products with different number of undeclared drug adulterants detected.
In this study, 2 cases involved products with high arsenic content. In one case, a patient had consumed a complementary medicine found to contain 120 ppm of arsenic. The patient had acute liver failure and subsequently died. The coroner's report stated that the cause of death was “multi-organ failure, following liver failure consistent with being due to auto-immune hepatitis. Death was consistent with being due to a natural disease process.” In addition, the report stated that based on the urine test, there was no evidence to suggest acute arsenic poisoning. In another case, a mother reported dramatic improvement in her young child's chronic eczema shortly after applying “TCM Recipe Licozen Ointment” (15), prompting the attending doctor to alert HSA. The product claimed to cure skin disorder and was natural, free of steroids and safe for use. High concentration of arsenic was detected in the product and a press release was issued to alert the public to stop using the potentially harmful product.
This study presents and analyses the pattern of adverse event reports associated with the use of CHP in Singapore during a 7-year period from January 2010 to December 2016. The total number of adverse event reports in Singapore has grown over the years, with a significant increase in 2006 (1,171 reports in 2005 vs. 11,984 reports in 2006) (16, 17) as that year corresponded to the first electronic submission of adverse event reports via the Critical Medical Information Store. Adverse event reporting remains one of the most important ways of monitoring the safety of a health product throughout its marketed life. Singapore has one of the highest number of valid reports per million inhabitants that was submitted to the WHO global pharmacovigilance database from 2010 to 2015 according to the reports published by WHO's Uppsala Monitoring Centre (18).
During the 7-year study period, there was significantly higher number of adverse event reports associated with health supplement products (625 reports) compared to the preceding 12-year period study with 102 reports (19, 20). This may be due to the increasing popularity of health supplements in Singapore over the years, and more importantly, the inclusion of non-serious adverse effect terms (e.g., rash, watery eyes) into the PRISM database with effect from September 2010 (21).
Most of the reports were associated with products containing glucosamine. It might be due to the prevalent usage of glucosamine in public institutions such as the polyclinics and public hospitals. HSA receives adverse event reports mainly from these institutions. Glucosamine health supplements are commonly used for the treatment of osteoarthritis and are generally safe for use (22). Nevertheless, there are still concerns about their potential to cause allergies (23). Glucosamine is available as the sulfate or hydrochloride salt. Glucosamine sulfate used in health supplements is commonly derived from the shells of crustaceans, hence the possibility of contamination with allergens, such as tropomyosin if proper purification is not carried out (24). Tropomyosin is a muscle protein and is one of the major allergens responsible for ingestion-related allergic reactions due to shellfish (25). Hence, it is important for glucosamine products to indicate the presence of shellfish if it is part of the ingredients as most of the 517 reports were reported as allergies to the suspected products. Furthermore, dietary supplements that contain glucosamine often contain additional ingredients such as chondroitin sulfate and methylsulfonylmethane. Although chondroitin sulfate and methylsulfonylmethane have good safety profiles with very few known and mild side effects (26, 27), mild skin and eye irritation have been observed when methylsulfonylmethane is applied topically (28). Gastrointestinal discomfort has been reported with chondroitin sulfate (29).
A number of CPM and other types of complementary medicines related to Chinese herbal remedies were also implicated in the adverse events. This may be due to higher usage of such products by the Chinese population which comprised about 74% of the total population of Singapore (30) and the increasing trend of seeking TCM treatment by other non-Chinese ethnic groups (31).
In this study, almost half of the cases involved patients above the age of 60 and most of the implicated products were for the purpose of relieving and controlling back, joint and neck pain. This could be explained by the correlation between aging and prevalence of pain. About 19.5% of the participants in an epidemiological study of older adults (age 60 and above) experienced pain in the past month (32). In another study, 8.7% of the interviewed adults (age 18–65) had chronic pain issues, and the major cause of pain was due to musculoskeletal conditions, e.g., arthritis (33). Many elderly tend to dismiss joint pain and body aches as part of aging and would rather endure the pain or self-medicate by taking CHP that were marketed for pain relief with the perception that they were natural and safe. It had also been observed in other studies that the incidence of adverse events among older adults were significantly higher than other age groups (34). Older patients are more susceptible to adverse reactions due to comorbid conditions, polypharmacy and sensitivity to drug effects (35). Older people are also more likely to have various health problems including the decreasing functions of liver and kidney (36). Altered drug metabolism and pharmacokinetics may result in the drugs remaining longer in an old person's body, thus prolonging the drug effects and increasing the risk of side effects (37). Furthermore, drugs are also less likely to be studied extensively in the very young and the elderly.
There were more adverse events involving females than males. The results were similar to other reports (38–40). One plausible explanation for this observation could be due to the higher usage of CHP by women than men as most of the cases reported were associated with health supplements containing glucosamine. Gender related differences in the frequencies of adverse event reporting may be due to various reasons including (i) pharmacokinetic or pharmacodynamics factors, (ii) polypharmacy, and (iii) differences in reporting patterns. Higher preference for CHP in women had also been reported in both western and eastern countries (41, 42). The prevalence of glucosamine use among women was 60.5% in an Australian study involving 266,844 participants aged 45 and above (43). Women are more likely to consume glucosamine than men due to musculoskeletal pain (44).
In addition, the higher incidence of adverse events among women may be due to anatomical and physiological differences between females and males. Women have lower bodyweight and organ size, higher body fat, slower gastric emptying time, and slower organ blood flow (45). These differences can affect the absorption, distribution, metabolism and elimination of drugs (46). For example, the higher proportion of body fat in women and slower organ blood flow may result in faster onset of action and prolonged duration of certain lipid-soluble drugs (47). Thus gender factor is expected to play a significant role in the incidence and severity of drug reaction.
Studies had shown that the hepatic enzyme CYP3A4 is more active in females than males, leading to differences in the drug metabolism in the two genders (48). For example, the metabolism of midazolam in women is more than that in men due to the activity of CYP3A4 (49).
Pronounced differences between women and men were seen in a study on the incidence of adverse events caused by cardiovascular drugs (50). In that nationwide investigation, women were found to be more frequently admitted with an adverse event related to high-ceiling diuretics, low-ceiling diuretics, and cardiotoxic glycosides than men. However, adverse event-related admissions associated with coronary vasodilators were more frequently reported in men. Pharmacodynamic differences between men and woman are particularly seen with cardiac drugs. For example, aldosterone is directly related to cardiac wall thickness in women but not in men, suggesting that women are more at risk from the harmful effects of aldosterone (51). Another plausible reason for the gender distribution is that women are more willing to report adverse events in contrast to men (52).
As can be seen from Table 3, majority of the adverse events were classified as “skin and appendages disorders” and the clinical manifestation was mainly rashes. Based on the data, reports involving products containing glucosamine could be due to allergy to possible allergens (e.g., tropomyosin) in the products as discussed earlier in the section Characteristics of the Adverse Event Reports. Further analysis was not possible as the total ingredient listings of each product had not been included in the reports. In addition, 19 adverse events were associated with Ginkgo biloba. Extracts of G. biloba leaf are used to treat various health problems such as dementia, memory deficits, headaches, intermittent claudication, vertigo, and tinnitus (53, 54). Ginkgo Folium (Ginkgo leaf, 银杏叶) and Ginkgo Semen (Ginkgo seed, 白果) are also used in TCM and Japanese Kampo. Ginkgo Folium in TCM is used to “activate the blood, resolve stasis, unblock the collaterals, relieve pain, astringe the lung, relieve wheezing, resolve turbidity, and lower lipid” (Committee of National Pharmacopeia of PR China 2015). Ginkgo Semen is used for the treatment of profuse sputum, wheezing and cough, vaginal discharge with white turbidity, enuresis and frequent urination (Committee of National Pharmacopeia of PR China 2015). In recent years, G. biloba has become a popular herbal supplement marketed to improve age-related physical and cognitive disorders (55). However, due to the presence of trace amounts of ginkgolic acid in the leaves and nuts, G. biloba may provoke allergic reactions (56, 57).
“Body as a whole—general disorders” was found to be the second most commonly reported SOC in the current study. This involves hypersensitivity, periorbital oedema, chest/back/leg pain, fatigue, and malaise etc. “Metabolism and nutritional disorders” include hypoglycaemia, hypertriglyceridaemia, and weight increase. Twenty-five out of the 71 adverse events with “metabolism and nutritional disorders” involved sexual enhancement health supplements adulterated with glibenclamide and sildenafil between the period from 2010 to 2013. These patients experienced hypoglycaemia, some were comatosed and one died.
In the WHO adverse drug reaction database, skin reactions were the most frequently reported adverse events associated with the use of herbal products. In a hospital-based observational study in Korea from April 2012 to December 2014, gastro-intestinal system disorders, followed by skin-related disorders were the most common adverse events reported in 163 herbal-drug-associated cases (58). Likewise in Sweden, the most commonly reported adverse reactions to herbal medicinal products or natural remedies were “skin and subcutaneous tissue disorders” and “gastrointestinal disorders” in a 9-year study on adverse events reported to the Swedish Medical Product Agency (59). “Skin and hypersensitivity reactions” and “gastrointestinal disorders” had also been reported as the two most common health products related adverse events (9, 60, 61).
Besides the skin and “body as a whole”, the liver was found to be one of the most affected organs in the present study. Some herbal medicines or their derived products were found to cause drug-induced liver injury (DILI). These patients experienced increased levels of hepatic enzymes, jaundice and even hepatocellular damages in serious adverse cases. Elevated liver enzymes is one of the more common adverse effects caused by herbal products (62). The National Institutes of Health has also developed a database which includes herbal medicines and dietary supplement ingredients associated with hepatotoxicity (23). Over 30 herbal medicines were reported to cause DILI. Besides prescription drugs, herbal products were ranked as the second most common cause of DILI in USA (63).
In the current study, to the best of our knowledge, no toxic herb was confirmed to be implicated in the hepatotoxic adverse events. Hepatotoxicity cases associated with herbal products on spontaneous adverse events in Singapore from 2010 to 2014 were reported (64). In that study, 35 out of the 57 adverse event reports involved hepatotoxicity, and Chai Hu (Radix bupleuri) was suspected in 11 of these 35 cases. Prevalence of DILI associated with CHP was reported to be 26% (98 out of 371 studied cases) in Korea (65) and 36% (130 out of 361 studied cases) in Taiwan (66). In the USA, 1,219 patients with liver injury from medications and/or dietary supplements between 2004 and 2013 showed that dietary supplement related liver injury increased from 7 to 20% during the study period (67). Furthermore, a population-based survey in Iceland reported that 16% of the 96 cases of DILI diagnosed in 2010–2011 were attributed to dietary supplements. The incidence of DILI in the population was estimated to be 19.1 per 100,000 persons (68). Due to the widespread use of herbal remedies in Asia, the percentage of DILI cases attributed to CHP in Asian countries was higher compared to the West.
Herbs which may cause DILI include Symphytum species (Comfrey), Heliotropium, and Senecio species (Groundsel), Germander, Chaparral, and Kava kava (69–72). Despite the benefits of green tea, green tea extract associated hepatotoxicity has also been reported (73, 74). Hepatotoxicity following the consumption of concentrated green tea extract may be attributed to the catechins due to the collapsing of the mitochondrial membrane potential of hepatocytes leading to cell death and the formation of reactive oxygen species (75). Besides intrinsic toxicity, herbal products contaminated by aflatoxins can result in acute toxic hepatitis associated with high morbidity (76). In the current study, 9 adverse event reports with liver biliary system disorders were found to be associated with CHP adulterated with various drugs. Therefore, besides reviewing the intrinsic toxicity of the herbs, it is also necessary to screen for contaminants and adulterants to assess the safety of the products.
The adulteration of CHP with undeclared drugs appeared to be one of the main causes of the associated adverse events. Majority of the adulterated cases involved products labeled or marketed to treat rheumatic joint pain, backache and numbness of the limbs to relieve pain and reduce inflammation. The adulterants found were mainly non-steroidal anti-inflammatory drugs (NSAIDs) and steroids. This can be very dangerous as the usage of NSAIDs may result in cardiovascular side effects such as myocardial infarction and stroke. In addition, long term unsupervised usage of oral steroids can lead to Cushing's Syndrome, increased blood glucose levels leading to diabetes, high blood pressure, cataracts, muscular and bone disorders, and an increased risk of infections (77, 78).
Dexamethasone is a potent steroid usually prescribed for inflammatory conditions and used under strict medical supervision. Chlorpheniramine is an antihistamine which helps to reduce flu-like symptoms (e.g., watery eyes and runny nose). Piroxicam is an NSAID used for the treatment of painful inflammatory conditions e.g., arthritis. A patient developed Cushing's Syndrome and various complications after consuming a complementary health product (Huo Li Shen Dan) adulterated with pain relief drugs, namely dexamethasone, hydrochlorothiazide, indomethacin, and prednisolone. The patient subsequently died. In a study in Hong Kong on 61 patients using proprietary Chinese medicines adulterated with corticosteroids and NSAIDs from 2008 to 2012 (79), 11.5% of the patients required intensive care and 2 died within 30 days of presentation.
Products used for slimming purposes were also found to be adulterated with synthetic drugs such as sibutramine, benzyl sibutramine, and phenolphthalein. Sibutramine, a prescription medicine previously used in the treatment of exogenous obesity, had been banned for sale in the EU, USA and Singapore markets since 2010 because of safety concerns about its cardiovascular risks (80, 81). The use of sibutramine may cause serious adverse effects, including tachycardia, hypertension, anxiety, heart attacks, and irregular heartbeats. However, the drug continues to be fraudulently added to weight loss products.
According to the U.S. Food and Drug Administration (FDA) list of tainted dietary supplements, 316 out of 781 public alerts (40%) published between 2009 and 2016 involved adulterated weight loss products. Most of the cases (89%) involved the illegal addition of sibutramine (82). In another study, about half of the 52 weight loss products purchased via the Internet contained sibutramine with contents exceeding normal therapeutic dosage (83). Benzyl sibutramine, an analog of sibutramine first reported in 2013, was detected in a weight loss product “Nutri Drops Grape fruit Diet” (84–86). It was found to contain sibutramine, benzyl sibutramine, and phenolphthalein despite claiming to contain all natural ingredients. The patient experienced hallucinations and was hospitalized after consuming the product for more than 1 month to lose weight. As the safety of drug analogs is unknown, there is a potential health risk to consumers because of their structural similarity to sibutramine. Another common adulterant in weight loss products is phenolphthalein, a drug used as a laxative and has been banned due to carcinogenicity concerns. Similar to sibutramine, it is frequently listed in public notifications from the U.S. FDA.
It may be possible that the number of adverse effects of sibutramine and other drugs present as adulterants in slimming products is under reported as many patients will discontinue the use of these products when they feel unwell. The ill effects are often reversible when the adverse effects are mild.
In this study, some products used for sexual performance enhancement were also found to be adulterated with sildenafil (a PDE-5 inhibitor), analogs of PDE-5 inhibitors and glibenclamide. Sildenafil and glibenclamide are prescription drugs that should only be taken under the supervision of a doctor. In one of the 5 fatal cases associated with the adulteration, the patient had taken a sexual enhancement health supplement called “Power 1 Walnut,” found to be adulterated with sildenafil and glibenclamide. The patient experienced hypoglycaemia and subsequently died. The exact reasons for the presence of the anti-diabetic drug glibenclamide in sexual enhancement products are unknown but possible reasons include contamination or mixing up of ingredients during manufacturing (87). The use of such adulterated products may also be associated with side effects and drug-drug interactions as the users may have co-morbid conditions such as diabetes and cardiovascular diseases. Concomitant consumption of sildenafil and nitrate drugs may result in severe hypotension and may be fatal. More than 70 analogs of approved PDE-5 inhibitor drugs had been found to be adulterated in sexual performance enhancement products (87, 88).
Two cases in the current study involved analogs of PDE-5 inhibitors. They were N-cyclopentyl nortadalafil (89) and 3,5-dimethylpiperazinyl dithio-desmethylcarbodenafil (90). They are illegally synthesized and adulterated into herbal products to avoid detection. The USP has published a General Chapter <2251> Screening for Undeclared Drugs and Drug Analogs, which provides guidance on several screening techniques for the detection of adulterants (e.g., PDE-5 inhibitors and their analogs) (91). Future update of the chapter may include methodologies for analysis of adulterated weight loss and sports performance enhancement products. Many of these analogs are not subjected to the thorough pre-clinical and clinical studies needed for drug registration, hence their toxicities remain unknown, and they are of safety concerns to the unknowing public.
The actual number of patients taking both conventional drugs and CHP may be under reported as the patients might be reluctant to share or were unaware of the importance of such information. Concurrent use of conventional drugs and CHP could result in herb-drug interactions which may lead to potentially serious adverse events. Patients most at risk of harmful drug-herb interactions are the children, the elderly, those on polypharmacy, those with chronic illnesses or impaired organ functions and those on drugs with narrow therapeutic windows such as warfarin (92). Coagulation problems arising from drug-herb interaction with warfarin had been reported, sometimes with serious consequences, such as intracranial hematoma (93). The prevalence of the disclosure of CHP usage to physicians is generally low (94–96). Fifty six percent of adult cancer patients in Singapore reported using CHP (97). However, only 54% of them informed their oncologists regarding the use of such products as most patients and caregivers do not readily disclose their use, and healthcare professionals may not routinely ask about such use. Adverse events due to drug-herb interactions may not be recognized if the physician or other health professional is not aware of the concomitant use of herbal products. Therefore, it is important for the patients and their healthcare providers to communicate openly about the usage of herbal products and pharmaceutical products.
Toxic heavy metals are of special health concerns due to their potential toxic effects. Arsenic and mercury can result in increased risk of various disorders such as cardiovascular abnormalities (98, 99), neurotoxicity, hepatotoxicity, nephrotoxicity, and carcinogenicity (100, 101). Cadmium toxicity has been demonstrated in several organs, especially the kidney. It can induce tissue injury through creating oxidative stress, changes in DNA expression and inhibition or upregulation of transport pathway particularly in the proximal segment of the kidney tubule (102). Chronic exposure to lead may cause adverse effects on the central nervous system, blood pressure, kidneys, and vitamin D metabolism mainly through lead's ability to inhibit or mimic the actions of calcium and to interact with proteins (101). Excessive intake of copper can lead to cellular toxicity through free radical-induced oxidative damage. Copper toxicity has been associated with Wilson's disease, renal and hepatic failure when copper homeostasis is disrupted (103).
HSA has in place a product quality surveillance programme to conduct risk-based sampling and testing of CHP on the market. Manufacturers and importers of CHP must ensure that their products are free of undeclared drugs and drug analogs or toxic heavy metals above the permissible legal limits. When adulteration or non-compliance to heavy metal limits have been established, HSA will issue press releases, alert healthcare professionals and/or take actions to remove the affected products from the market.
As the study is based on the retrospective analysis of spontaneous reports of adverse events, certain information such as indications of products, route of administration, concomitant drug administration, and other relevant information regarding the patients' medications were often lacking in the reports. In addition, patients might have been unwilling to share certain information that they deemed sensitive. Also, in many cases, the exact herbal ingredients were not described in the report or a sample of the complementary health product was not available for testing. Therefore, without performing further toxicity tests on the products, it was not possible to determine whether the suspected products might contain herbal ingredients with toxic constituents. In addition, the use of concomitant drugs and the presence of adulterants (e.g., undeclared drugs and drug analogs) in the products could also lead to adverse events (19). Likewise, individual patient's medical conditions may predispose one to adverse events. The classification of an adverse event as being due to a complementary health product (i.e., decision with regards to causality) depends on the reporter's account and assessment, besides the presence of adulterants and toxic heavy metals. If the product is available, it may be sent to HSA laboratories for analysis. Manufacturers are usually unaware of the adverse event or the products may be illegal. The adverse events are usually observed by the healthcare professionals when patients seek treatment and then reported by the healthcare professionals as their ethical duty. The information whether both pharmaceutical products and CHP are used concomitantly may not be present in the adverse event reports. In such situations, there may or may not be concomitant usage of both. Hence, a causal relationship cannot be confidently drawn. Moreover, under-reporting (104) might occur due to various plausible reasons, e.g., (1) patients do not inform their healthcare providers on their usage of CHP, (2) lack of association between the product and adverse effects, (3) mild self-limiting adverse effects upon stopping the use, (4) sensitivity of information (e.g., with slimming and sexual performance enhancement products), (5) healthcare professionals are unaware that herbal adverse events should be reported, and (6) patients may not consider herbal and nutritional products to be “medicines” and hence neither disclose the use nor seek professional advice before using them as most of the products are available over-the-counter. Under reporting is a challenge with spontaneous reporting system (105). Since large post market surveillance studies of herbal medicine will require extensive resources, spontaneous reporting of adverse event is still an essential and effective pharmacovigilance tool. Currently, the Health Products Act in Singapore legislatively mandates the reporting of serious adverse events by the manufacturer, importer, supplier or registrant of therapeutic products (pharmaceutical products), medical devices and cosmetic products. All local manufacturing facilities engaged in the manufacture or assembly of pharmaceutical products and CPM must be licensed with HSA. The manufacturers are expected to comply with the relevant legislative and regulatory requirements, and GMP standard. For imported CPMs, GMP from country of origin is required where applicable (i.e., if imposed by country of origin). Local manufacturers of CPMs must be licensed with HSA.
In the United States, most herbal products are classified under Dietary supplements and regulated under the Dietary Supplement Health and Education Act (DSHEA) 1994 (106). In Australia, medicinal products containing such ingredients as herbs, vitamins, minerals, nutritional supplements, homeopathic, and certain aromatherapy preparations are referred to as “complementary medicines” and are regulated as medicines under the Therapeutic Goods Act 1989 (107). It is beyond the scope of this paper to compare the classification system in Singapore and Western countries. Regardless of classifications, there is no distinction between the way adverse events due to different products (e.g., pharmaceutical products and CHP) are handled. The adverse event reports are managed in the same database and based on botanical names where possible.
In conclusion, 893 adverse event reports associated with CHP in the period between 2010 and 2016 have been successfully collated and analyzed. They constituted about 0.6% of the total number of adverse events reported. The majority of the adverse event cases in that period were still due to conventional drugs. In agreement with other studies, adverse reactions related to the “skin and appendages disorders” were the most commonly reported reactions. Most of the cases involved the use of glucosamine products and most of the adulterated products were associated with the illegal addition of drugs for pain relief. Even if the causality is not confirmed, reporting of suspected adverse events is strongly encouraged as rapid regulatory actions can be taken if there is any sign of clustering. The findings from this study help to create greater awareness on the health risks, albeit low, when consuming CHP and dispelling the common misconception that “natural” means “safe.” In particular, healthcare professionals and the general public should be aware of potential adulteration of CHP. The analysis of spontaneously reported adverse events is an important surveillance system in monitoring the safety of CHP and helps in the understanding of the risk associated with the use of such products. Greater collaboration and communication between healthcare professionals, regulators, patients, manufacturers, researchers, and the general public are important to ensure the quality and safety of CHP, while harnessing their potential benefits.
YX collated the data, analyzed the data and drafted the manuscript; DP, S-LN, S-HT, DT, JP, and ATL corrected the manuscript; C-LC, M-YL, and H-LK conceptualized the project and corrected the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study is supported by a Health Sciences Authority—National University of Singapore research grant (R-148-000-115-490), the Health Manpower Development Plan Fellowship (to YX) and a National University of Singapore graduate research scholarship (to DP). This work is an extension of DP's Ph.D. thesis work (19). Professional discretion and judgement should be exercised in relying on reports received under HSA's AE monitoring programme due to a number of factors such as the reports being based on limited or incomplete data, variable degree of under-reporting and pattern of usage of the product being reported on.
1. Health Sciences Authority (HSA) (2017a). Available online at: https://eservice.hsa.gov.sg/prism/common/enquirepublic/SearchCPProduct.do?action=load (Accessed November 29, 2017).
2. World Health Organization (WHO) Definition (2017a). Available online at: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/trainingcourses/definitions.pdf (Accessed November 29, 2017).
3. Wardle J, Adams J. Indirect and non-health risks associated with complementary and alternative medicine use: an integrative review. Eur J Integr Med. (2014). 6:409–22. doi: 10.1016/j.eujim.2014.01.001
4. Nortier JL, Vanherweghem JL. For patients taking herbal therapy–lessons from aristolochic acid nephropathy. Nephrol Dial Transplant. (2007) 22:1512–7. doi: 10.1093/ndt/gfm167
5. Jha V. Herbal medicines and chronic kidney disease. Nephrology (2010) 15:10–7. doi: 10.1111/j.1440-1797.2010.01305.x
6. Liu C, Fan H, Li Y, Xiao X. Research advances on hepatotoxicity of herbal medicines in China. Biomed Res Int. (2016) 2016:7150391. doi: 10.1155/2016/7150391
7. World Health Organization (WHO). WHO Traditional Medicine Strategy (2014–2023) (2014a). Available online at: http://apps.who.int/iris/bitstream/10665/92455/1/9789241506090_eng.pdf?ua=1 (Accessed November 29, 2017).
8. Shaw D, Graeme L, Pierre D, Elizabeth W, Kelvin C. Pharmacovigilance of herbal medicine. J Ethnopharmacol. (2012) 140:513–8. doi: 10.1016/j.jep.2012.01.051
9. Saokaew S, Suwankesawong W, Permsuwan U, Chaiyakunapruk N. Safety of herbal products in Thailand: an analysis of reports in the thai health product vigilance center database from 2000 to 2008. Drug Saf. (2011) 34:339–50. doi: 10.2165/11586590-000000000-00000
10. Health Sciences Authority (HSA). Adverse Drug Report Form (2017b). Available online at: www.hsa.gov.sg/content/dam/HSA/HPRG/Safety_Alerts_Product_Recalls_Enforcement/Adverse_Drug_Report_Form_Yellow_APR2010%20revised%202017.pdf (Accessed November 29, 2017).
11. World Health Organization (WHO). Uppsala Monitoring Centre - Being a Member of the WHO Programme for International Drug Monitoring (2014b). Available online at: https://www.who-umc.org/media/1434/being-a-member.pdf (Accessed November 29, 2017).
12. World Health Organization (WHO). Introduction Guide MedDRA Version 14.0 (2017b). Avaliable online at: http://www.who.int/medical_devices/innovation/MedDRAintroguide_version14_0_March2011.pdf (Accessed November 29, 2017).
13. Health Science Authority (HSA). HSA Alerts Public to ‘LONGRED Oyster-x' and ‘LifeSparks 100% Natural PAIN RELIEF SUPPLEMENT' Which Contained Undeclared Potent Ingredients and Caused Adverse Effects in a Patient (2016a). Available online at: http://www.hsa.gov.sg/content/hsa/en/News_Events/Press_Releases/2016/longredoysterxandlifesparkspainrelief.html (Accessed November 29, 2017).
14. Health Sciences Authority (HSA). HSA Alerts Public to 'Herbal Health Jointcare' Found to Contain 5 Undeclared Potent Western Medicinal Ingredients, Including Steroid (2014a). Available online at: http://www.hsa.gov.sg/content/hsa/en/News_Events/Press_Releases/2014/HSA_Alerts_Public2.html (Accessed November 29, 2017).
15. Health Sciences Authority (HSA). HSA Alerts Public to ‘TCM Recipe Licozen Ointment' Sold Locally for Eczema and Found to Contain Very High Levels of Arsenic (2015). Available online at: http://www.hsa.gov.sg/content/hsa/en/News_Events/Press_Releases/2015/hsa-alerts-publictotcmrecipelicozenointmentsoldlocallyforeczemaa.html (Accessed November 29, 2017).
16. Health Sciences Authority (HSA). Adverse Drug Reaction news Mar 2006 (2005). Available online at: http://www.hsa.gov.sg/content/dam/HSA/HPRG/Safety_Alerts_Product_Recalls_Enforcement/Adverse_Drug_Reaction_News/2006/ADR_News_Mar2006_Vol8_No1.pdf (Accessed November 29, 2017).
17. Health Sciences Authority (HSA). Adverse Drug Reaction news Mar 2007(2006). Available online at: http://www.hsa.gov.sg/content/dam/HSA/HPRG/Safety_Alerts_Product_Recalls_Enforcement/Adverse_Drug_Reaction_News/2007/ADR_News_Mar2007_Vol9_No1.pdf (Accessed November 29, 2017).
18. Health Sciences Authority (HSA) (2016b). Available online at: http://www.hsa.gov.sg/content/dam/HSA/HPRG/ (Accessed November 29, 2017).
19. Patel DN. Evaluation of Anti-Proliferative Activity and Hepatotoxicity of Botanicals and Botanical Health Products. Dissertation/Ph.D. thesis, National University of Singapore, Singapore (2012).
20. Patel DN, Low WL, Tan LL, Tan MMB, Zhang Q, Low MY, et al. Adverse events associated with the use of complementary medicine and health supplements: an analysis of reports in the Singapore Pharmacovigilance database from 1998 to 2009. Clin Toxicol. (2012). 50:481–9. doi: 10.3109/15563650.2012.700402
21. Health Sciences Authority (HSA) (2011). Available online at: http://www.hsa.gov.sg/content/dam/HSA/HPRG/Safety_Alerts_Product_Recalls_Enforcement/Adverse_Drug_Reaction_News/2016/ADR_News_May2016_Vol18_No1.pdf (Accessed November 29, 2017).
22. Zhang WB, Zhuang CY, Li JM, Yang ZP, Chen XL. Efficacy and safety evaluation of glucosamine hydrochloride in the treatment of osteoarthritis. Zhonghua Wai Ke Za Zhi (2007) 45:998–1001. doi: 10.3760/j.issn:v0529-5815.2007.14.020
23. U.S. National Library of Medicine. LiverTox Clinical and Research Information on Drug-Induced Liver Injury Database (2017). Available online at: https://livertox.nih.gov/ (Accessed November 29, 2017).
24. Villacis J, Rice TR, Bucci LR, El-Dahr JM, Wild L, Demerell D, et al. Do shrimp-allergic individuals tolerate shrimp-derived glucosamine? Clin Exp Allergy (2006) 36:1457–61. doi: 10.1111/j.1365-2222.2006.02590.x
25. Woo CK, Bahna SL. Not all shellfish “allergy” is allergy! Clin Transl Allergy (2011) 1:3. doi: 10.1186/2045-7022-1-3
26. U.S. Food and Drug Administration (FDA). GRAS Notice 666, Chondroitin Sodium Sulfate – FDA (2017a). Available online at: https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm524570.pdf (Accessed November 29, 2017).
27. U.S. Food and Drug Administration FDA. GRAS Notice 000229: Methylsulfonylmethane – FDA (2017b). Available online at: https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm269126.pdf (Accessed November 29, 2017).
28. Butawan M, Benjamin RL, Bloomer RJ. Methylsulfonylmethane: applications and safety of a novel dietary supplement. Nutrients (2017) 9:290. doi: 10.3390/nu9030290
29. Amer NA, Naji AN. Possible adverse effects of once-daily oral therapeutic dose of either glucosamine sulfate or glucosamine/chondroitin sulfate on blood cells count in rats. Int Res J Pharm. (2013) 4:24–9. doi: 10.7897/2230-8407.041007
30. Department of Statistics Singapore Population Trends 2016 (2016). Available online at: https://www.singstat.gov.sg/-/media/files/publications/population/population2016.pdf (Accessed November 29, 2017).
31. The Straits Times. New TCM Clinic Aims to Reach More Non-Chinese Patients (2016). Available online at: https://www.straitstimes.com/singapore/new-tcm-clinic-aims-to-reach-more-non-chinese-patients (Accessed November 29, 2017).
32. Yeo SN, Tay KH. Pain prevalence in Singapore. Ann Acad Med Singapore (2009) 38:937–42. Available online at: http://www.annals.edu.sg/pdf/38VolNo11Nov2009/V38N11p937.pdf
33. Satghare P, Chong SA, Vaingankar J, Picco L, Abdin E, Chua BY, et al. Prevalence and correlates of pain in people aged 60 years and above in Singapore: results from the WiSE study. Pain Res Manag. (2016) 2016:1–7. doi: 10.1155/2016/7852397
34. Jose J, Rao P. Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospital. Pharmacol Res. (2006) 54:226–33. doi: 10.1016/j.phrs.2006.05.003
35. Ahmed B, Nanji K, Mujeeb R, Patel MJ. Effects of polypharmacy on adverse drug reactions among geriatric outpatients at a tertiary care hospital in Karachi: a prospective cohort study. PLoS ONE (2014) 9:e112133. doi: 10.1371/journal.pone.0112133
36. Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. (2007) 147:755–65. doi: 10.7326/0003-4819-147-11-200712040-00006
37. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. (2009) 41:67–76. doi: 10.1080/03602530902722679
38. Montastruc JL, Lapeyre-Mestre M, Bagheri H, Fooladi A. Gender differences in adverse drug reactions: analysis of spontaneous reports to a Regional Pharmacovigilance Centre in France. Fundam Clin Pharmacol. (2002) 16:343–6. doi: 10.1046/j.1472-8206.2002.00100.x
39. Zopf Y, Rabe C, Neubert A, Gaßmann KG, Rascher W, Hahn EG, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. (2008) 64:999–1004. doi: 10.1007/s00228-008-0494-6
40. Zopf Y, Rabe C, Neubert A, Janson C, Brune K, Hahn E, et al. Gender-based differences in drug prescription: relation to adverse drug reactions. Pharmacology (2009) 84:333–9. doi: 10.1159/000248311
41. Bishop F, Lewith G. Who uses CAM? A narrative review of demographic characteristics and health factors associated with CAM use. Evid Based Complement Alternat Med. (2010) 7:11–28. doi: 10.1093/ecam/nen023
42. Chung V, Ma P, Lau C, Wong S, Yeoh E, Griffiths S. Views on traditional Chinese medicine amongst Chinese population: a systematic review of qualitative and quantitative studies. Health Expect. (2012) 17:622–36. doi: 10.1111/j.1369-7625.2012.00794.x.
43. Sibbritt D, Adams J, Lui CW, Broom A, Wardle J. Who uses glucosamine and why? A study of 266,848 Australians aged 45 years and older. PLoS ONE (2012) 7:e41540. doi: 10.1371/journal.pone.0041540
44. Nakua EK, Otupiri E, Dzomeku VM, Owusu-Dabo E, Agyei-Baffou P, Yawson AE, et al. Gender disparities of chronic musculoskeletal disorder burden in the elderly Ghanaian population: study on global ageing and adult health (SAGE WAVE 1). BMC Musculoskelet Disord. (2015) 16:204. doi: 10.1186/s12891-015-0666-3
45. Bergiannaki J, Kostaras P. Pharmacokinetic and pharmacodynamic effects of psychotropic medications: differences between sexes. Psychiatriki (2016) 27:118–26. Available online at: http://europepmc.org/abstract/med/27467032
46. Alomar MJ. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm J. (2014) 22:83–94. doi: 10.1016/j.jsps.2013.02.003
47. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. (2009) 48:143–57.
48. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. (2009) 76:215–28. doi: 10.1124/mol.109.056705
49. Labbé L, Sirois C, Pilote S. Effect of gender, sex hormones, time variables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates. Pharmacogenetics (2000) 10:425–38. Available online at: https://journals.lww.com/jpharmacogenetics/pages/articleviewer.aspx?year=2000&issue=07000&article=00006&type=abstract
50. Rodenburg EM, Stricker BH, Visser LE. Sex differences in cardiovascular drug-induced adverse reactions causing hospital admissions. Br J Clin Pharmacol. (2012) 74:1045–52. doi: 10.1111/j.1365-2125.2012.04310.x
51. Franconi F, Campesi I. Pharmacogenomics, pharmacokinetics and pharmacodynamics: interaction with biological differences between men and women. Br J Pharmacol. (2014) 171:580–94. doi: 10.1111/bph.12362
52. Kristoffersen AE, Stub T, Salamonsen A, Musial F, Hamberg K. Gender differences in prevalence and associations for use of CAM in a large population study. BMC Complement Altern Med. (2014) 14:463. doi: 10.1186/1472-6882-14-463
53. Blumenthal M, Goldberg A, Brinckmann J. Herbal Medicine. Expanded Commission E Monographs: Texas, American Botanical Council (2000).
54. Cybulska-Heinrich AK, Mozaffarieh M, Flammer J. Ginkgo biloba: an adjuvant therapy for progressive normal and high tension glaucoma. Mol Vis. (2012) 18:390–402.
55. Sun ZK, Yang HQ, Chen SD. Traditional Chinese medicine: a promising candidate for the treatment of Alzheimer's disease. Transl Neurodegener. (2013) 2:6. doi: 10.1186/2047-9158-2-6
56. Metz D, Weston P, Barker D. Case report of vasculitic rash induced by Ginkgo biloba and/or Horny Goat Weed. Br Med J. (2009). 2009:bcr07.2008.0399. doi: 10.1136/bcr.07.2008.0399
57. Roland PD, Nergard CS. Ginkgo biloba–effect, adverse events and drug interaction. Tidsskr Nor Laegeforen (2012) 132:956–9. doi: 10.4045/tidsskr.11.0780
58. Kim M, Han CH. Analysis of herbal-drug-associated adverse drug reactions using data from spontaneous reporting system in electronic medical records. J Korean Orient Med. (2015) 36:45–60. doi: 10.13048/jkm.15005
59. Svedlund E, Larsson M, Robert Hagerkvist R. Spontaneously reported adverse reactions for herbal medicinal products and natural remedies in Sweden 2007–15: report from the Medical Products Agency. Drug Real World Outcomes (2017) 4:119–25. doi: 10.1007/s40801-017-0104-y
60. Jacobsson I, Jönsson AK, Gerdén B, Hägg S. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. (2009) 18:1039–47. doi: 10.1002/pds.1818
61. Tabali M, Ostermann T, Jeschke E, Witt CM, Matthes H. Adverse drug reactions for CAM and conventional drugs detected in a network of physicians certified to prescribe CAM drugs. J Manag Care Pharm. (2012) 18:427–38. doi: 10.18553/jmcp.2012.18.6.427
62. Teschke R. Traditional Chinese Medicine induced liver injury. J Clin Translat Hepatol. (2014) 2:80–94. doi: 10.14218/JCTH.2014.00003
63. Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. (2014) 109:950–66. doi: 10.1038/ajg.2014.131
64. Teo CH, Ng SL, Tan SH, Lim AT, Toh SL, Chan SY, et al. Drug-induced liver injury associated with Complementary and Alternative Medicine: a review of adverse event reports in an Asian community from 2009 to 2014. BMC Complement Altern Med. (2016) 16:192. doi: 10.1186/s12906-016-1168-z
65. Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. (2012) 107:1380–7. doi: 10.1038/ajg.2012.138
66. Ou P, Chen Y, Li B, Zhang M, Liu X, Li F, et al. Causes, clinical features and outcomes of drug-induced liver injury in hospitalized patients in a Chinese tertiary care hospital. SpringerPlus (2015) 4:802.
67. Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology (2014) 60:1399–408. doi: 10.1002/hep.27317
68. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology (2013) 144:1419–25. doi: 10.1053/j.gastro.2013.02.006
69. Frenzel C, Teschke R. Herbal hepatotoxicity: clinical characteristics and listing compilation (Review). Int J Mol Sci. (2016) 17:588. doi: 10.3390/ijms17050588
70. Fau D, Lekehal M, Farrell G, Moreau A, Moulis C, Feldmann G, et al. Diterpenoids from germander, an herbal medicine, induce apoptosis in isolated rat hepatocytes. Gastroenterology (1997) 113:1334–6.
72. Tarantino G, Pezzullo MG, di Minno MN, Milone F, Pezzullo LS, Milone M, et al. Drug-induced liver injury due to “natural products” used for weight loss: a case report. World J Gastroenterol. (2009) 15:2414–7. doi: 10.3748/wjg.15.2414
73. Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. (2013) 58:2682–90. doi: 10.1007/s10620-013-2687-9
74. Patel SS. Green tea extract: a potential cause of acute liver failure. World J Gastroenterol. (2013) 19:5174–7. doi: 10.3748/wjg.v19.i31.5174
75. Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Bio Med. (2006) 40:570–80. doi: 10.1016/j.freeradbiomed.2005.09.014
76. Probst C, Njapau H, Cotty PJ. Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the causal agent. Appl Environ Microbiol. (2007) 73:2762–4. doi: 10.1128/AEM.02370-06
77. Pawlosky N. Cardiovascular risk: are all NSAIDs alike? Can Pharm J. (2013) 146:80–83. doi: 10.1177/1715163513481569
78. Ericson-Neilsen W, Kaye AD. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. (2014) 14:203–7.
79. Chong Y, Ching C, Ng S, Mak TW. Corticosteroid adulteration in proprietary Chinese medicines: a recurring problem. Hong Kong Med J. (2015) 21:411–6. doi: 10.12809/hkmj154542
80. European Medicines Agency. European Medicines Agency Recommends Suspension of Marketing Authorisations for Sibutramine (2010). Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/01/WC500069995.pdf (Accessed November 29, 2017).
81. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA Recommends Against the Continued Use of Meridia (sibutramine) (2010). Available online at: https://www.fda.gov/Drugs/DrugSafety/ucm228746.htm (Accessed November 29, 2017).
82. U.S. Food and Drug Administration. Tainted Products Marketed as Dietary Supplements (2017c). Available online at: https://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?filter=&sortColumn=5a&sd=tainted_supplements_cder&page=6&displayAll=false (Accessed November 29, 2017).
83. Mathon C, Ankli A, Reich E, Bieri S, Christen P. Screening and determination of sibutramine in adulterated herbal slimming supplements by HPTLC-UV densitometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2014) 31:15–20. doi: 10.1080/19440049.2013.861934
84. Mans DJ, Gucinski AC, Dunn JD, Gryniewicz-Ruzicka CM, Mecker-Pogue LC, Kao JLF, et al. Rapid screening and structural elucidation of a novel sibutramine analogue in a weight loss supplement: 11-Desisobutyl-11-benzylsibutramine. J Pharm Biomed Anal. (2013) 83:122–8. doi: 10.1016/j.jpba.2013.02.031
85. Health Sciences Authority (HSA). HSA Alerts Public to Two Illegal Weight Loss Products Purchased Online Which Led to Two Patients Being Hospitalised (2014b). Available online at: http://www.hsa.gov.sg/content/hsa/en/News_Events/Press_Releases/2014/TwoIllegalWeightLossProducts.html (Accessed November 29, 2017).
86. Hunsel FV, Venhuis BJ, Keizers PHJ, Kant A. A ‘natural' weight loss product containing sibutramine. Drug Test Anal. (2015) 8:311–4. doi: 10.1002/dta.1925
87. Patel DN, Li L, Kee CL, Ge X, Low MY, Koh HL. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges. J Pharm Biomed Anal. (2014) 87:176–90. doi: 10.1016/j.jpba.2013.04.03
88. Venhuis B, Kaste DD. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: a history, analytical aspects and health risks. J Pharm Biomed Anal. (2012) 69:196–208. doi: 10.1016/j.jpba.2012.02.014
89. Xu Y, Kee CL, Ge X, Low MY, Koh HL. Isolation and characterization of a tadalafil analogue, N-cyclopentyl nortadalafil in health supplement. J Pharm Biomed Anal. (2016) 118:235–41. doi: 10.1016/j.jpba.2015.08.005
90. Kee CL, Low MY, Ge X. Isolation and characterization of a novel dithio-carbodenafil analogue from a health supplement. J Pharm Biomed Anal. (2017) 137:132–8. doi: 10.1016/j.jpba.2017.01.010
91. Sarma N, Giancaspro G, Venema J. Dietary supplements quality analysis tools from the United States Pharmacopeia. Drug Test Anal. (2016) 8:418–23.
92. Boullata J. Natural health product interactions with medication. Nutr Clin Pract. (2005) 20:33–51. doi: 10.1177/011542650502000133
93. Ge B, Zhang Z, Zuo Z. Updates on the clinical evidenced herb-warfarin interactions. Evid Based Complement Alternat Med. (2014) 2014:957362. doi: 10.1155/2014/957362
94. Lim M, Sadarangani P, Chan H, Heng J. Complementary and alternative medicine use in multiracial Singapore. Complement Ther Med. (2005) 13:16–24. doi: 10.1016/j.ctim.2004.11.002
95. Grant SJ, Bin YS, Kiat H, Chang DHT. The use of complementary and alternative medicine by people with cardiovascular disease: a systematic review. BMC Public Health (2012) 12:299. doi: 10.1186/1471-2458-12-299
96. Chen C, Chong YJ, Hie SL, Sultana R, Lee SHD, Chan WSD, et al. Complementary and alternative medicines use among pediatric patients with epilepsy in a multiethnic community. Epilepsy Behav. (2016) 60:68–74. doi: 10.1016/j.yebeh.2016.04.008
97. Shih V, Chiang JYL, Chan A. Complementary and alternative medicine (CAM) usage in Singaporean adult cancer patients. Ann Oncol. (2009) 20:752–7.
98. Moon K, Guallar E, Navas–Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. (2012) 14:542–55. doi: 10.1007/s11883-012-0280-
99. Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury exposure and heart diseases. Int J Environ Res Public Health (2017) 14:74. doi: 10.3390/ijerph14010074
100. Singh AP, Goel RK, Kaur T. Mechanisms pertaining to arsenic toxicity. Toxicol Int. (2011) 18:87–93. doi: 10.4103/0971-6580.84258
101. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metals toxicity and the environment. EXS (2012) 101:133–64. doi: 10.1007/978-3-7643-8340-4_6
102. Bernhoft RA. Cadmium toxicity and treatment. Sci World J. (2013) 2013:394652. doi: 10.1155/2013/394652
103. Gaetke LM, Chow-Johnson HS, Chow CK. Copper: toxicological relevance and mechanisms. Arch Toxicol. (2014) 88:1929–38. doi: 10.1007/s00204-014-1355-y
104. Barnes J. Pharmacovigilance of herbal medicines: a UK perspective. Drug Saf. (2003) 26:829–51. doi: 10.2165/00002018-200326120-00001
105. Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. (2006) 29:385–96. doi: 10.2165/00002018-200629050-00003
106. U.S. Food and Drug Administration FDA. Dietary Supplements (2017d). Available onine at https://www.fda.gov/Food/DietarySupplements/ (Accessed March 29, 2018).
107. Therapeutic Goods Administration TGA. An Overview of the Regulation of Complementary Medicines in Australia (2017). Available online at: https://www.tga.gov.au/overview-regulation-complementary-medicines-australia (Accessed March 29, 2018).
Keywords: complementary health products, adverse events, pharmacovigilance, glucosamine, adulterants
Citation: Xu Y, Patel DN, Ng S-LP, Tan S-H, Toh D, Poh J, Lim AT, Chan C-L, Low M-Y and Koh H-L (2018) Retrospective Study of Reported Adverse Events Due to Complementary Health Products in Singapore From 2010 to 2016. Front. Med. 5:167. doi: 10.3389/fmed.2018.00167
Received: 01 March 2018; Accepted: 14 May 2018;
Published: 12 June 2018.
Edited by:
Steffen Thirstrup, NDA Advisory Services Ltd, United KingdomCopyright © 2018 Xu, Patel, Ng, Tan, Toh, Poh, Lim, Chan, Low and Koh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hwee-Ling Koh, cGhha29oaGxAbnVzLmVkdS5zZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.