- 1Physics Department, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia
- 2Center of Research Excellence in Nanotechnology, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia
In this work, sputtered tin oxide films, decorated with silver nanoparticles were fabricated as hydrogen sensors. The fabricated thin films were characterized for their structural, compositional, morphological properties using various characterization techniques including X-ray photoelectron spectroscopy, UV-Vis absorption, X-ray diffraction, field emission scanning electron microscope, and atomic force microscopy. The morphological characterization confirmed the formation of nanoparticle-decorated SnO2 thin films. X-ray photoelectron spectroscopy analysis established the presence of silver/silver oxide on SnO2 thin films. The gas sensing properties of the fabricated sensors were investigated at different concentrations of hydrogen gas, over an operating temperature range of room temperature to 500°C. It was found that the prepared sensor can detect a low hydrogen concentration (50 ppm) at high operation temperature, while the higher concentration (starting from 600 ppm) can be detected even at room temperature. Furthermore, on the basis of the electronic interaction between the SnO2 and the Ag nanoparticles, we propose a reaction model to explain the qualitative findings of the study.
Introduction
Intense research is ongoing for green “future fuel” due to its extraordinary abilities including availability in the natural resources and zero CO2 emission (Crabtree et al., 2004). Since hydrogen (H2) has these characteristics, research is taking place in order to produce H2-fueled engines, in order to reduce atmospheric pollution and harmful emissions (Crabtree et al., 2004; Pal and Agarwal, 2018; Tsujimura and Suzuki, 2019). In addition, H2 is used in petrochemical processes, including the hydrocracking process (Lippke et al., 2018) for example.
H2 is tasteless, colorless, and odorless. With a very low ignition energy of 0.02 mJ, it inflames easily in the air at volume concentrations ranging from 4 to 75%. Therefore, detecting H2 by a proper sensor prevents the threat of fire and explosions. So far, several different types of H2 sensors have been established such as optical fibers (Wu et al., 2018) and surface acoustic wave (D'amico et al., 1982). Metal oxide sensors (Moseley, 1997) have been widely studied as H2 sensors due to their excellent qualities in terms of low cost, high sensitivity, fast response, and compact size. Furthermore, their non-stoichiometric nature allows them to absorb the ambient oxygen and exchange electrons during the adsorption and desorption processes. However, these metal oxide gas sensors have some limitations such as their inability to detect extremely low concentrations of the target gas and their requirement to operate at high temperatures. This usually puts restrictions on their portability.
Currently, a lot of research focuses on improving the sensing features of the metal oxide gas sensors. Doping of the metal oxide sensors with noble metal nanoparticles is a powerful method that improves selectivity and sensitivity, even at low operation temperatures (Kohl, 1990; Ippolito et al., 2005). Among the various metal oxide sensors, tin oxide (SnO2), which is an n-type semiconductor, is one of the most promising materials for gas sensing applications, and especially for H2 detection. H2 sensors based on SnO2 nanowires, nanorods, and nanotubes have good sensitivity, but they are still unsuitable for commercial purposes due to limitations in their fabrication techniques (Van Duy et al., 2012). Indeed, for commercialization purposes, thin films are the most investigated gas sensor nanostructures due to their simplicity in configuration and scalability of fabrication. As sensitivity is one of the most important parameters in H2 gas sensing, many efforts have been made to enhance the sensitivity of the SnO2 thin-film sensors. The decoration of SnO2 with noble metals leads to charge transfer between their two surfaces, hence modifying the Fermi level of the metal oxide and affecting the charge depletion layer. This leads to increasing the electrons in the metal oxide conduction band, which in turn increases the surface interaction between the sensor and the ambient oxygen, and hence enhancing the performance of the sensor.
Korotcenkov et al. (2007) reported that by doping SnO2 with Pd, both response time and sensitivity to CO and H2 increase. Kocemba and Rynkowski (2011), in addition, noticed that sputtered SnO2 thin-film doping with Pt as a catalyst gives a high sensitivity to H2 as a function of different operating temperatures. Chen et al. (2011) reported that the synthesis of SnO2 and Ag2O nanocomposites demonstrate an enhancement in their gas sensing properties. Wu et al. (2013) formed Ag/SnO2 core shells and noticed an improvement in the sensor response to ethanol at room temperature.
For improved H2 gas sensing properties, such as higher sensing and stability capabilities, we have developed an H2 sensor using catalytic particles (a noble metal loading). In particular, we decorated the surface of sputtered SnO2 thin films with Ag nanoparticles. More specifically, to maximize the catalytic effect of the sensor, the Ag nanoparticles were homogeneously decorated, covering the whole area of the surface of SnO2 thin films through a new synthetic method. The details about H2-gas sensing mechanism are discussed in the results section.
Experimental
Synthesis of Ag Nanoparticle-Decorated SnO2 Thin Films
Three steps were used to prepare Ag nanoparticle-decorated SnO2 thin films for fabricating H2 sensors. Firstly, a target of 99.995% SnO2 (purchased from ACI Alloys INC) was utilized to fabricate SnO2 thin films using the RF sputtering technique (model NSC-4000, Nanomaster). Before deposition of the thin films, the deposition chamber and targets were cleaned with acetone and isopropanol while the substrates were sonicated in ethanol for 30 min. For sputtering deposition, the base pressure and deposition pressure were adjusted at 6 × 10−6 and 4.8 × 10−3 torr, respectively. The SnO2 thin films with 250 nm thickness were deposited at 60 standard cubic centimeters per minute (sccm) argon and 10 sccm oxygen gas flow rate using 100 W RF sputtering power for 120 min. The film was deposited on SiO2 substrates with pre-interdigitized Au electrodes (IDEs) to be used as hydrogen gas sensor, and also deposited on glass substrate to study the structural, compositional, morphological, and optical characterization of the fabricated films. The IDEs have 22 electrodes that are 200 nm thick, with 250 μm interspacing distance, connected to two pads used for electrical measurements. Secondly, a metallic target of 99.995% Ag (purchased from Semiconductor wafer) was used to deposit a thin layer of Ag using 20 W DC sputtering power for 30 s. The Ar flow was kept at 70 sccm during the Ag layer deposition. Finally, the obtained thin films were heated at 600°C in a sealed tubular furnace (OTF-1200X from MTI corp.) with a temperature ramp rate of 20°C/min in a flowing argon atmosphere for 2 h to convert Ag layer into nanoparticles in the case of the Ag nanoparticle-decorated SnO2 film and to enhance the crystallinity and stabilize the based SnO2 deposited film for both pure SnO2 film (termed as annealed SnO2) and Ag nanoparticle-decorated SnO2 film (termed as AgNPs/SnO2). In addition to these two samples, one sample was left as-deposited (termed as as-deposited SnO2) in order to study the effect of the annealing process.
Characterization
The synthesized thin films were characterized using different techniques. X-ray diffraction [Rigaku Miniflex 600 X-Ray Diffraction (XRD), with Cu K irradiation at λ = 1.5406 Å] was used to examine the crystalline phases, lattice parameter, and the crystalline size. X-ray photoelectron spectroscopy (Model: ESCALAB250Xi, XPS) was utilized to determine the chemical composition of the fabricated thin films. The structural morphology was investigated using a field-emission scanning electron microscope (FE-SEM; Tescan Lyra 3) and atomic force microscopy (AFM; Dimension Icon, Bruker). Optical transmittance and optical band gap measurements were carried out using double beam UV/Vis spectrophotometer (Jasco V-570) in the wavelength range of 200–1,400 nm.
Gas Sensing Measurements
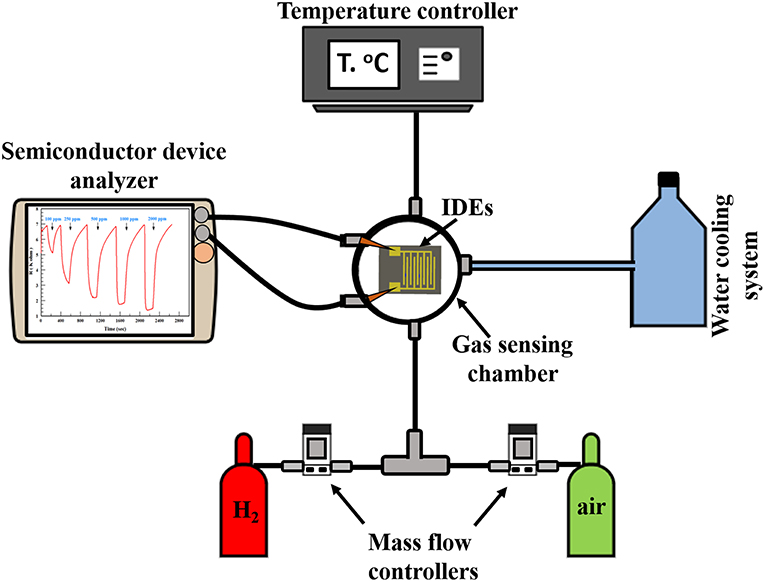
The H2 sensing measurements of the thin-film sensors were conducted by a flow-through technique. The measurement system consists of a gas sensing chamber (Linkam stage, Model HFS-600E-PB4, UK). It contains two metal needles as electrical connections between the IDE pads (i.e., the sensor) and the electrical measurement setup. In addition, the Linkam stage involves a heating unit that has the ability to reach up to 600°C. A mass flow controller (MFC, Horiba, USA) was utilized to control the gas injection into the testing chamber. The sensor resistance was measured through an Agilent B1500A Semiconductor Device Analyzer (SDA) using I/V-t measurements. Dry air, from a cylinder, was used as the background reference gas. H2 gas cylinder (1% H2-bal. N2) was used as the test-gas source. The resistance across the IDE pads would change depending on the H2 concentration changing inside the Linkam stage. The gas sensing system layout is shown in Figure 1.
Results and Discussion
Structure, Morphology, and Composition Test
FE-SEM and AFM imaging techniques were used to investigate the morphological properties of the fabricated thin films. Figures 2a,b shows the FE-SEM micrograph of the as-deposited SnO2 and the annealed SnO2 thin films. Both micrographs show the uniformity of the SnO2 thin film though there seems to be a slight difference in the roughness of SnO2 surface before and after the annealing. Figure 2c displays the annealed AgNPs/SnO2 films at 600°C. The Ag nanoparticles were nearly uniformly distributed over the SnO2 film surface with different particle sizes (Drmosh et al., 2015).
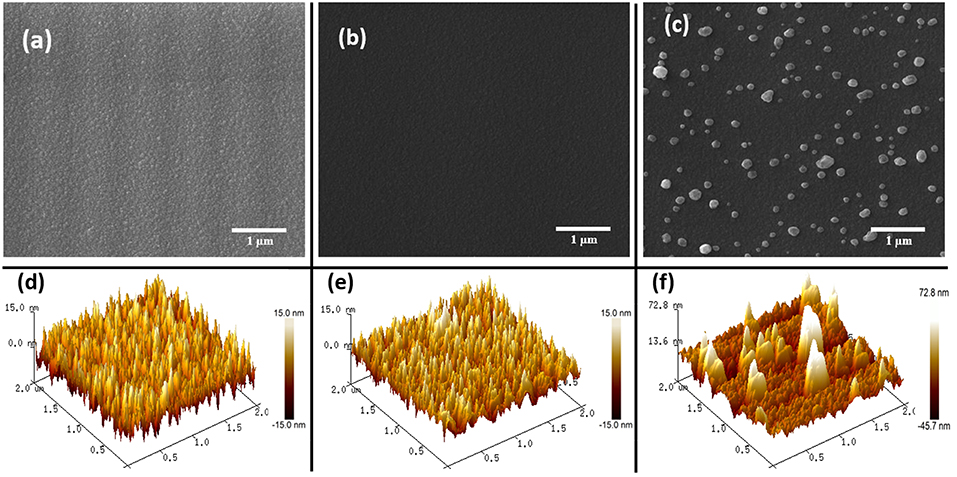
Figure 2. (a–c) FESEM images of as-deposited SnO2, annealed SnO2, and AgNPs/SnO2, respectively. (d–f) AFM images of as-deposited SnO2, annealed SnO2, and AgNPs/SnO2, respectively.
Figures 2d,e are 2 μ m × 2 μm AFM topography images of the as-deposited SnO2 and annealed SnO2 thin films. As presented by SEM, the AFM images show a rough surface for the pristine film, and a more mild roughness for the film after annealing. This accentuates that annealing the SnO2 thin film makes it denser and smoother. This is a result of the film obtaining sufficient energy from the annealing process enabling the atoms to rearrange their position, and hence become closer packed and crystalline (Shinde et al., 2005), as confirmed below by the XRD results. The AFM image of the AgNPs/SnO2 film (Figure 2f) shows the growth of large, separated, and spaced Ag particles over the smooth SnO2. The AgNPs size ranged approximately from 50 to 250 nm.
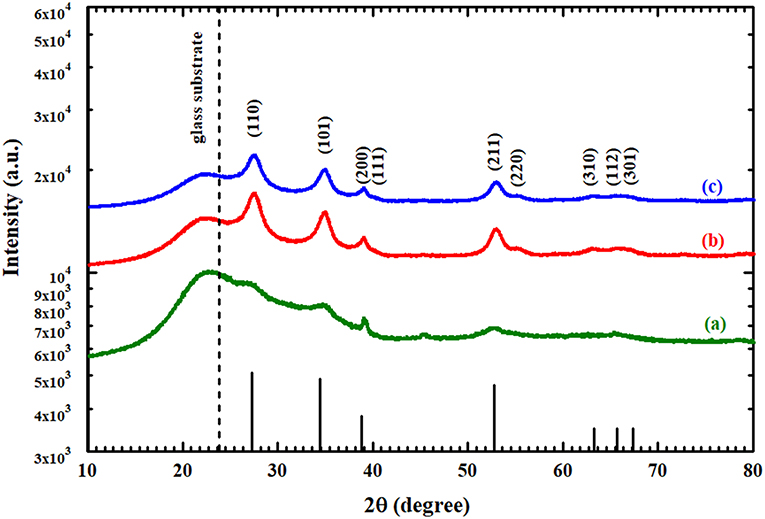
Figure 3 shows the XRD patterns of the pure SnO2 thin film before and after the heat treatment and the annealed AgNPs/SnO2. The wide peaks that appear at ~24°C correspond to the substrate (Chakraborty et al., 2010). All XRD peaks identify the standard crystal planes of tetragonal SnO2 (Ansari et al., 1997). From Figure 3a, the observed low and wide peaks correspond to the diffractions peaks (110), (101), and (200) (Ansari et al., 1997). This confirms the amorphous nature of the as-deposited SnO2. After heating the as-deposited SnO2 to 600°C, it is observed that the peaks become sharper, as displayed in Figure 3b. This is attributed to the transformation of the SnO2 from an amorphous to a polycrystalline structure. No diffraction peaks were found related to Ag nanoparticles as there is no change in the diffraction pattern between Figures 3a,c. This might be due to the random distribution and low concentration of Ag nanoparticles at the surface of the SnO2 film (Yang et al., 2014). No impurity peaks or other phases were observed, confirming the purity of the prepared films.
UV-Vis spectra of the synthesized thin films were obtained within a wavelength range of 200–1,400 nm, as shown in Figure 4A. In the IR and visible region, the optical transmittance fluctuated due to the interference of light being sandwiched inside the thin film between the substrate and air (Cho, 2014). It can be observed that the transmittance of the AgNPs/SnO2 slightly decreased below the pure and annealed SnO2. Certainly, this is attributed to the existence of Ag nanoparticles on the SnO2 surface (Dasgupta et al., 2010) which has considerable absorption to electromagnetic radiation. Tauc relation was used to calculate the optical band gap of these films by plotting the values of (αE)2 against E and extrapolating the linear portion of the curves, where E and Eg are the photon energy of incident light and optical band gap, respectively, A is a constant, and α is the absorption coefficient (Kumar et al., 1999). As can be seen from the extrapolation in Figure 4B, the optical band gap of the both annealed SnO2 and AgNPs/SnO2 increased in comparison with the as-deposited SnO2 sample, and hence, the absorption edge was shifted to shorter wavelength as shown in Figure 4A (Reddy et al., 2013). As an effect of the annealing process, the Ag diffuses into the SnO2 crystal. As a result, the band gap of the Ag/NPs/SnO2 sample is smaller than the annealed SnO2 (Hwang et al., 2011).
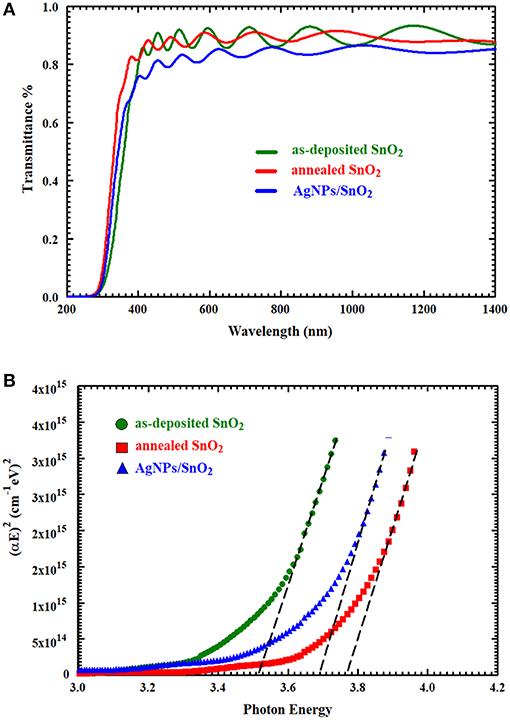
Figure 4. (A) The UV–Vis transmittance spectra of as-deposited SnO2, annealed SnO2, and AgNPs/ SnO2 films. (B) Optical band gap of as-deposited SnO2, annealed SnO2, and AgNPs/SnO2 films.
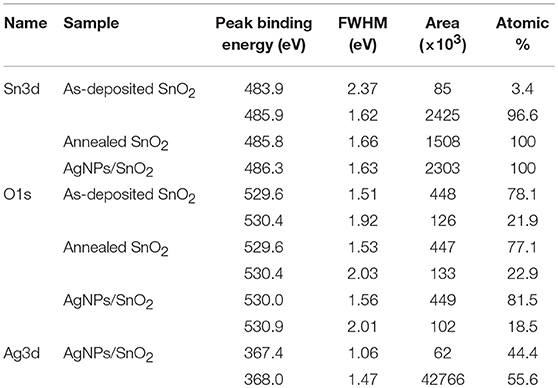
XPS analysis provided information about the thin film's composition. It also determined the oxidation states of the elements. From example, Figure 5 relates to the annealed AgNPs/SnO2 sample. The survey scan (Figure 5A) confirms the presence of the Sn 3d, O 1s, and the Ag 3d. The binding energy and atomic ratio of each element are demonstrated in Table 1. Figure 5B shows two peaks in the range from 483 to 489 eV and from 492 to 498 eV. These relate to the binding energies of the Sn 3d5/2 and Sn 3d3/2, respectively (Wang et al., 2005). Figure 5C shows the Ag 3d5/2 and Ag 3d3/2 located in the range of 365 to 370 eV and 372 to 376 eV, respectively. In each of the two peaks, two Ag oxidization states are identified; the Ag0 peaks are centered at 367.8 and 373.8 eV, while the Ag+ peaks are centered at 367.5 and 373.4 eV (Zielinska et al., 2010). The Ag+ peak is due to the presence of the Ag2O layer on top of Ag nanoparticles that are exposed to ambient oxygen (Matsushima et al., 1988). The O 1s spectrum is shown in Figure 5D. The convolution peak centered at 529.9 eV is ascribed to surface lattice oxygen , while the Oads peak, centered at 530.8 eV, is attributed to water species adsorbed on the surface of the film (Wang et al., 2016).
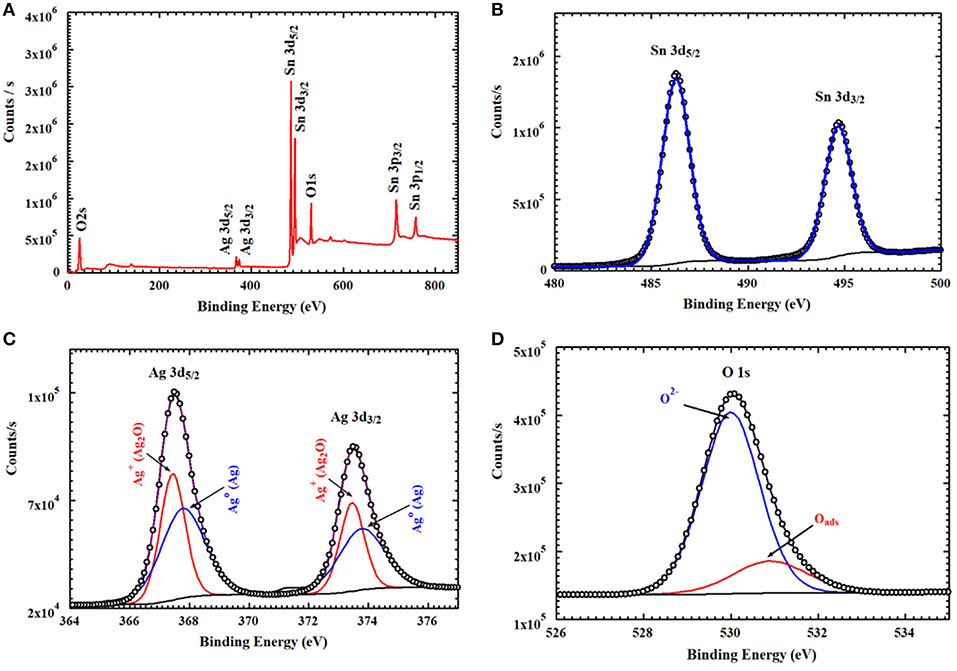
Figure 5. XPS spectra of AgNPs/SnO2 (A) survey spectrum, (B) XPS peaks of Sn3d, (C) XPS peaks of Ag 3d, and (D) XPS peaks of O1s.
Gas Sensing Studies
The gas sensing performance of the AgNPs/SnO2 thin film was tested toward H2, and the results were compared with that of the annealed SnO2. Figures 6a,b shows the response of annealed SnO2 and AgNPs/SnO2 for different H2 concentrations (50–1,800 ppm) at 500°C operation temperature. The comparison of H2 sensing performance of the two sensors reveals that addition of Ag nanoparticles to the SnO2 thin film results in a remarkable enhancement in the sensitivity of the sensor. For example, the response of the SnO2 decorated by Ag toward 50 ppm of H2 is around 21% while that for pure SnO2 is 14%. That is, Ag decoration improved the sensitivity by about 67%.
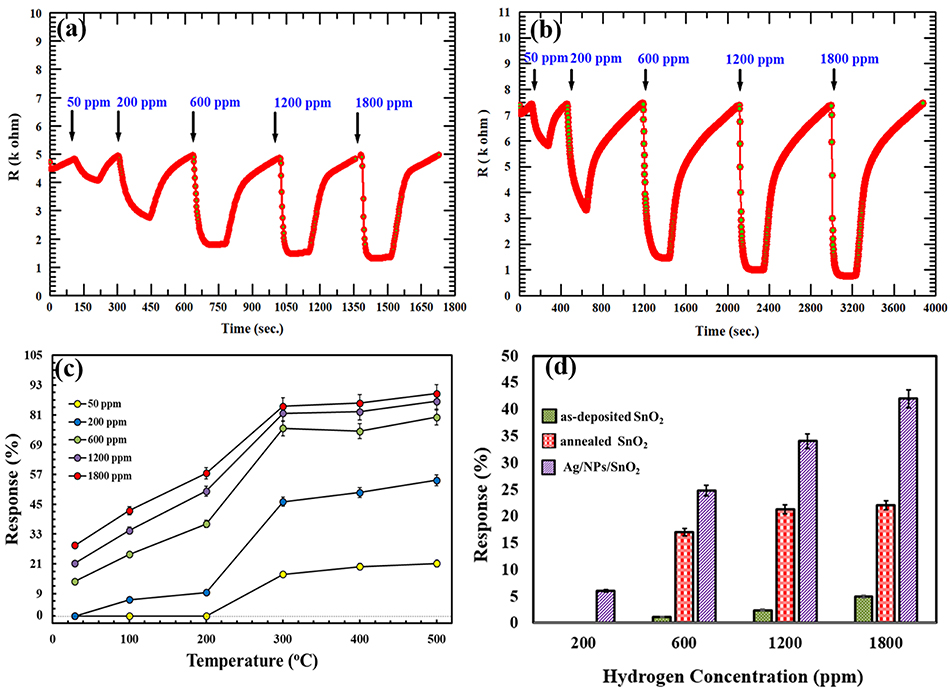
Figure 6. (a,b) Dynamic responses of annealed SnO2 and AgNPs/SnO2 (respectively) gas sensor to different H2 concentrations (50–1,800) ppm at 500°C. (c) Gas responses of AgNPs/SnO2 sensor toward different H2 concentrations (50–1,800) ppm as a function of operating temperature ranging from room temperature to 500°C. (d) A comparison between the as-deposited SnO2, annealed SnO2, and AgNPs/SnO2 at different H2 concentrations (200, 600, 1,200, and 1,800) at 100°C.
The synthesized Ag-decorated sensor was studied at wide range of operating temperatures starting from room temperature (RT = 29°C) and up to 500°C as shown in Figure 6c. For low concentrations (i.e., 50 and 200 ppm), the film showed no response at RT and only a small response at 100 and 200°C for 200 ppm. At higher concentrations (600, 1,200, and 1,800 ppm), the films gave an acceptable response at RT, with the response increasing by increasing the operating temperature to 500°C.
Figure 6d shows the comparison between the as-deposited SnO2, annealed SnO2, and AgNPs/SnO2 sensors toward various H2 concentrations (200–1,800 ppm) at low operation temperature (100°C). The annealed AgNPs/SnO2 sensor exhibits higher response for all H2 concentrations. For example, at 1,800 ppm, the response of as-deposited and annealed SnO2 was ~6 and ~20%, respectively, while the response reached ~42% from the AgNPs/SnO2 sensor. On the other hand, at low concentration (200 ppm), both as-deposited and annealed SnO2 had no response and yet AgNPs/SnO2 gave an acceptable response of ~7%, which confirms the value of using Ag to boost the sensitivity of the hydrogen sensor.
The reproducibility of measurements was tested in order to study the stability of the AgNPs/SnO2 sensor, which is one of the vital properties for H2 sensors. Figure 7a shows that two cycles of three different concentrations (600, 1,200, and 1,800 ppm) were used in this test. Clearly, the sensor has the ability to recover to the same initial response (600 ppm) after introducing different amounts of H2 concentrations (1,200, 1,800 ppm), which confirms the stability of the fabricated sensor. The staircase behavior was also obtained, as shown in Figure 7b. The AgNPs/SnO2 sensor shows excellent resistance descending mode from 200 to 2,400 ppm besides its ability to recover the initial resistance level, as it should for a stable sensor, when decreasing the concentration back to 200 ppm. Table 2 compares the H2 sensing properties of the fabricated AgNPs/SnO2 sensor with the different sensors, operating in the dynamic mode, reported in the literature.
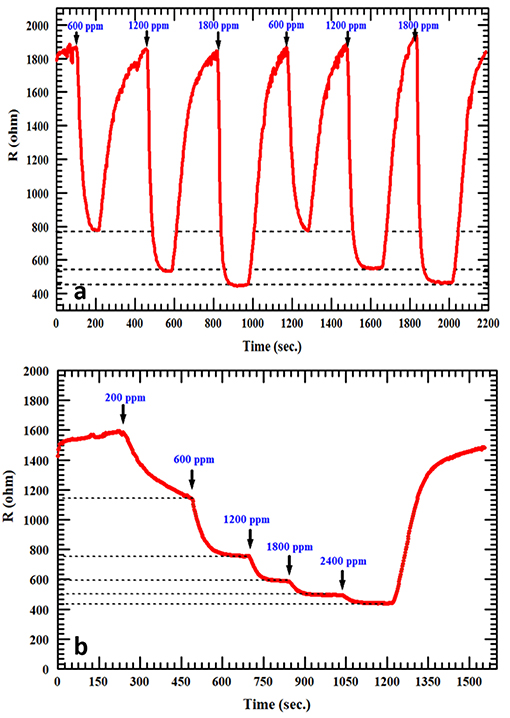
Figure 7. (a) Repeatability test at 600, 1,200, and 1,800 ppm of H2 for AgNPs/SnO2. (b) Staircase behavior of AgNPs/SnO2 sensor.
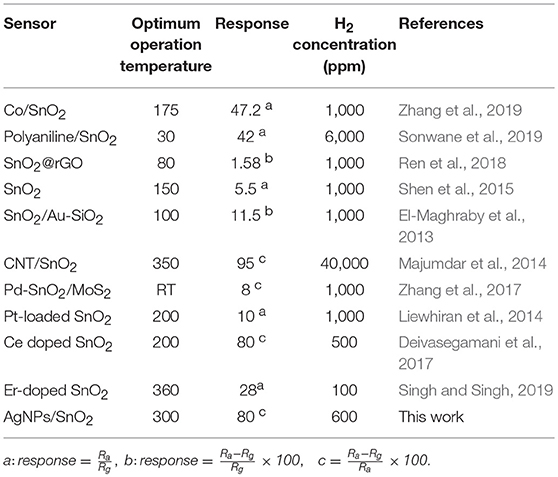
Table 2. A comparison between the fabricated AgNPs/SnO2 sensor with the different sensors reported in the literature.
Sensing Mechanism
SnO2 was used as a gas sensor-based material that has the ability to sense reducing gases such as H2. In the atmosphere, the surface of the SnO2 film interacts with the oxygen molecules in the air. The adsorbed oxygen molecules form O−, O−2, and moieties, resulting in the formation of depletion layers at the surface of the SnO2 film (Chang, 1980). As a result, the SnO2 becomes more resistive (due to lack of electrons in the film conduction band). Figure 8A describes the resulting band bending and the formation of the electron barrier at the SnO2 film surface. In the presence of H2, the H2 molecules capture the oxygen moieties and release electrons back into the conduction band of the SnO2 thin film, therefore decreasing its resistance. This process is described by the following equation:
The enhanced gas-sensing performances of the AgNPs/SnO2 sensor can be explained by the electronic sensitization mechanism, which is based on the controlled carrier concentration (Yamazoe et al., 1983). The surface region of Ag nanoparticles contains two oxidation states Ag0 and Ag+ (Ag2O), as confirmed by the XPS analysis. This mixture leads to the production of the redox couple Ag+/Ag0 (Matsushima et al., 1988). The couple has an electronic potential of 5.3 eV (Matsushima et al., 1988). When a contact is formed between Ag NPs and n n-type semiconductor oxide whose electron affinity is <5.3 eV, the Fermi level of the n-type semiconductor is pinned to the Ag+/Ag0 potential. This leads to the increase of the electron depletion region and to a decrease of the semiconductor oxide conductivity. In our case, the electron affinity (Φs) of the SnO2 is 4.60 eV (Li et al., 2016). Therefore, the Fermi level of the SnO2 becomes aligned with the work function and electron affinity of Ag0 and Ag+, respectively. As a result of electron flows from the SnO2 surface, the energy bands of the SnO2 will bend downward to reach the equilibrium level with the energy of the Ag+/Ag0, and hence, the AgNPs/SnO2 film will have a higher resistance than that of pure SnO2 as shown in Figure 8B. The electron barrier at the surface of the SnO2 film now depends on the energy of the barriers eV1 and eV2, which result from oxygen adsorption and Ag decoration, respectively, as in Figure 8C. Nevertheless, the significance conurbation would be from the larger energy eV2. Now, when H2 is introduced, and following the same mechanism mentioned above as a result of oxygen removal, the Ag2O changes into metallic Ag, which has a work function of ΦAg 4.33 (Luth, 1993). Consequently, the energy bends upward to reach the level of the metallic Ag Fermi energy as a result of restoring electrons from Ag to SnO2 surface as described in Figure 8D. Thus, the resistance of the SnO2 film is more significantly decreased as an indication of H2 presence.
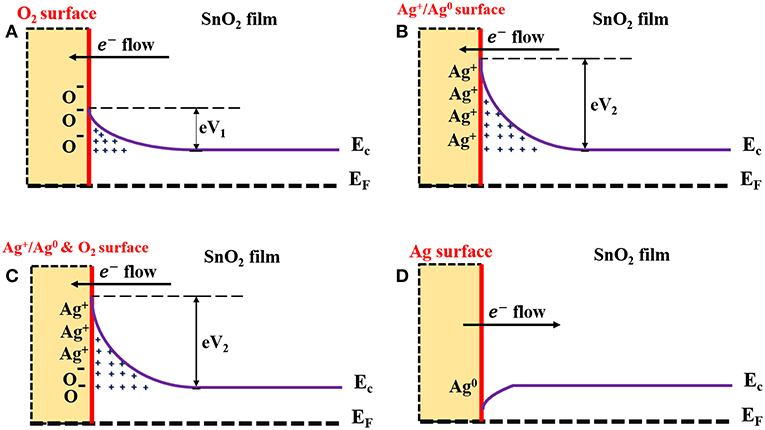
Figure 8. Schematic diagram describes mechanism underlying enhanced H2 sensing by AgNPs/SnO2 nanocomposites. (A) Formation of the electrons barrier at the SnO2 film surface. (B) Formation of the electrons barrier at the AgNPs/SnO2 film surface. (C) Formation of the electrons barrier at the AgNPs/SnO2 film surface in the ambient air. (D) Formation of the electrons barrier at the AgNPs/SnO2 film surface after introducing H2 gas.
Conclusion
Pristine and post-annealed SnO2 thin films as well as silver-decorated SnO2 thin films were fabricated using the DC/RF sputtering system. The structural, morphological, and compositional properties of the as-deposited SnO2, annealed SnO2, and AgNPs/SnO2 were studied by various characterization techniques. The gas sensing properties of the silver-decorated tin oxide film were tested at different operating temperatures (RT to 500°C), and with varying H2 concentration (50 to 1,800 ppm), in order to investigate the sensor sensitivity effect. It was found that the addition of the Ag nanoparticles has significantly increased the sensitivity by 67% compared with annealed SnO2 at 500°C operation temperature. The observed improvement in H2 sensing was explained according to an electronic sensitization mechanism.
Data Availability
All datasets generated for this study are included in the manuscript/supplementary files.
Author Contributions
AM took the measurements, analyzed the data, contributed to the writing of the manuscript, synthesized the films, characterized the materials, and evaluated the sensors. QD designed the experiment, characterized the materials, evaluated the sensors, synthesized the films, and contributed to the writing of the manuscript. ZY designed the experiment, analyzed the data, and contributed to the editing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Center of Excellence in Nanotechnology (CENT) and the Physics department at King Fahd University of Petroleum and Minerals (KFUPM) are acknowledged for their support.
References
Ansari, S. G., Boroojerdian, P., Sainkar, S. R., Karekar, R. N., Aiyer, R. C., and Kulkarni, S. K. (1997). Grain size effects on H2 gas sensitivity of thick film resistor using SnO2 nanoparticles. Thin Solid Films 295, 271–276. doi: 10.1016/S0040-6090(96)09152-3
Chakraborty, R., Dey, A., and Mukhopadhyay, A. K. (2010). Loading rate effect on nanohardness of soda-lime-silica glass. Metall. Mater. Trans. A 41, 1301–1312. doi: 10.1007/s11661-010-0176-8
Chang, S. C. (1980). Oxygen chemisorption on tin oxide: correlation between electrical conductivity and EPR measurements. J. Vac. Sci. Technol. 17, 366–369. doi: 10.1116/1.570389
Chen, X., Guo, Z., Xu, W. H., Yao, H. B., Li, M. Q., Liu, J. H., et al. (2011). Templating synthesis of SnO2 nanotubes loaded with Ag2O nanoparticles and their enhanced gas sensing properties. Adv. Funct. Mater. 21, 2049–2056. doi: 10.1002/adfm.201002701
Cho, S. (2014). Effect of growth temperature on structural, electrical, and optical properties of Gd-doped zinc oxide films. Phys. Status Solidi A Appl. Res. 211, 709–713. doi: 10.1002/pssa.201330345
Crabtree, G. W., Dresselhaus, M. S., and Buchanan, M. V. (2004). The hydrogen economy. Phys. Today 57, 39–44. doi: 10.1063/1.1878333
D'amico, A., Palma, A., and Verona, E. (1982). Surface acoustic wave hydrogen sensor. Sens. Actuators 3, 31–39. doi: 10.1016/0250-6874(82)80004-8
Dasgupta, N. P., Neubert, S., Lee, W., Trejo, O., Lee, J. R., and Prinz, F. B. (2010). Atomic layer deposition of Al-doped ZnO films: effect of grain orientation on conductivity. Chem. Mater. 22, 4769–4775. doi: 10.1021/cm101227h
Deivasegamani, R., Karunanidhi, G., Santhosh, C., Gopal, T., Neogi, A., Nivetha, R., et al. (2017). Chemoresistive sensor for hydrogen using thin films of tin dioxide doped with cerium and palladium. Microchim. Acta 184, 4765–4773. doi: 10.1007/s00604-017-2514-7
Drmosh, Q. A., Hossain, M. K., Alharbi, F. H., and Tabet, N. (2015). Morphological, structural and optical properties of silver treated zinc oxide thin film. J. Mater. Sci-Mater. El. 26, 139–148. doi: 10.1007/s10854-014-2375-3
El-Maghraby, E. M., Qurashi, A., and Yamazaki, T. (2013). Synthesis of SnO2 nanowires their structural and H2 gas sensing properties. Ceram. Int. 39, 8475–8480. doi: 10.1016/j.ceramint.2013.01.112
Hwang, I. S., Choi, J. K., Woo, H. S., Kim, S. J., Jung, S. Y., Seong, T. Y., et al. (2011). Facile control of C2H5OH sensing characteristics by decorating discrete Ag nanoclusters on SnO2 nanowire networks. ACS Appl. Mater. Interfaces 3, 3140–3145. doi: 10.1021/am200647f
Ippolito, S. J., Kandasamy, S., Kalantar-Zadeh, K., and Wlodarski, W. (2005). Hydrogen sensing characteristics of WO3 thin film conductometric sensors activated by Pt and Au catalysts. Sens. Actuators B Chem. 108, 154–158. doi: 10.1016/j.snb.2004.11.092
Kocemba, I., and Rynkowski, J. (2011). The influence of catalytic activity on the response of Pt/SnO2 gas sensors to carbon monoxide and hydrogen. Sens. Actuators B Chem. 155, 659–666. doi: 10.1016/j.snb.2011.01.026
Kohl, D. (1990). The role of noble metals in the chemistry of solid-state gas sensors. Sens. Actuators B Chem. 1, 158–165. doi: 10.1016/0925-4005(90)80193-4
Korotcenkov, G., Blinov, I., Ivanov, M., and Stetter, J. R. (2007). Ozone sensors on the base of SnO2 films deposited by spray pyrolysis. Sens. Actuators B Chem. 120, 679–686. doi: 10.1016/j.snb.2006.03.029
Kumar, V., Sharma, S. K., Sharma, T. P., and Singh, V. (1999). Band gap determination in thick films from reflectance measurements. Opt. Mater. 12, 115–119. doi: 10.1016/S0925-3467(98)00052-4
Li, H. H., He, Y., Jin, P. P., Cao, Y., Fan, M. H., Zou, X., et al. (2016). Highly selective detection of trace hydrogen against CO and CH4 by Ag/Ag2O–SnO2 composite microstructures. Sens. Actuators B Chem. 228, 515–522. doi: 10.1016/j.snb.2016.01.078
Liewhiran, C., Tamaekong, N., Tuantranont, A., Wisitsoraat, A., and Phanichphant, S. (2014). The effect of Pt nanoparticles loading on H2 sensing properties of flame-spray-made SnO2 sensing films. Mater. Chem. Phys. 147, 661–672. doi: 10.1016/j.matchemphys.2014.06.005
Lippke, G. M. G., Singh, S., and Hoehn, R. K. (2018). Staged Hydrotreating and Hydrocracking Process and Apparatus. United States Patent Application No. US20180223198A1.
Luth, H. (1993). Surfaces and Interfaces of Solids. Berlin: Springer. doi: 10.1007/978-3-662-10159-9
Majumdar, S., Nag, P., and Devi, P. S. (2014). Enhanced performance of CNT/SnO2 thick film gas sensors towards hydrogen. Mater. Chem. Phys. 147, 79–85. doi: 10.1016/j.matchemphys.2014.04.009
Matsushima, S., Teraoka, Y., Miura, N., and Yamazoe, N. (1988). Electronic interaction between metal additives and tin dioxide in tin dioxide-based gas sensors. Jpn. J. Appl. Phys. 27:1798. doi: 10.1143/JJAP.27.1798
Moseley, P. T. (1997). Solid state gas sensors. Meas. Sci. Technol. 8, 223–237. doi: 10.1088/0957-0233/8/3/003
Pal, A., and Agarwal, A. K. (2018). “Hydrogen for internal combustion engines,” in Prospects of Alternative Transportation Fuels (Singapore: Springer), 39–54. doi: 10.1007/978-981-10-7518-6_4
Reddy, A. S., Figueiredo, N. M., and Cavaleiro, A. (2013). Nanocrystalline Au: Ag: SnO2 films prepared by pulsed magnetron sputtering. J. Phys. Chem. Solids 74, 825–829. doi: 10.1016/j.jpcs.2013.01.023
Ren, H., Gu, C., Joo, S. W., Cui, J., Sun, Y., and Huang, J. (2018). Preparation of SnO2 nanorods on reduced graphene oxide and sensing properties of as-grown nanocomposites towards hydrogen at low working temperature. Mater. Express 8, 263–271. doi: 10.1166/mex.2018.1428
Shen, Y., Wang, W., Fan, A., Wei, D., Liu, W., Han, C., et al. (2015). Highly sensitive hydrogen sensors based on SnO2 nanomaterials with different morphologies. Int. J. Hydrogen Energy 40, 15773–15779. doi: 10.1016/j.ijhydene.2015.09.077
Shinde, V. R., Lokhande, C. D., Mane, R. S., and Han, S. H. (2005). Hydrophobic and textured ZnO films deposited by chemical bath deposition: annealing effect. Appl. Surf. Sci. 245, 407–413. doi: 10.1016/j.apsusc.2004.10.036
Singh, G., and Singh, R. C. (2019). Highly sensitive gas sensor based on Er-doped SnO2 nanostructures and its temperature dependent selectivity towards hydrogen and ethanol. Sens. Actuators B Chem. 282, 373–383. doi: 10.1016/j.snb.2018.11.086
Sonwane, N. D., Maity, M. D., and Kondawar, S. B. (2019). Conducting polyaniline/SnO2 nanocomposite for room temperature hydrogen gas sensing. Mater. Today 15, 447–453. doi: 10.1016/j.matpr.2019.04.106
Tsujimura, T., and Suzuki, Y. (2019). Development of a large-sized direct injection hydrogen engine for a stationary power generator. Int. J. Hydrogen Energy 44, 11355–11369. doi: 10.1016/j.ijhydene.2018.09.178
Van Duy, N., Hoa, N. D., and Van Hieu, N. (2012). Effective hydrogen gas nanosensor based on bead-like nanowires of platinum-decorated tin oxide. Sens. Actuators B Chem. 173, 211–217. doi: 10.1016/j.snb.2012.06.079
Wang, L., Li, J., Wang, Y., Yu, K., Tang, X., Zhang, Y., et al. (2016). Construction of 1D SnO2-coated ZnO nanowire heterojunction for their improved n-butylamine sensing performances. Sci. Rep. 6:35079. doi: 10.1038/srep35079
Wang, Y., Ma, J., Ji, F., Yu, X., and Ma, H. (2005). Structural and photoluminescence characters of SnO2: Sb films deposited by RF magnetron sputtering. J. Lumin. 114, 71–76. doi: 10.1016/j.jlumin.2004.12.003
Wu, B., Zhao, C., Xu, B., and Li, Y. (2018). Optical fiber hydrogen sensor with single Sagnac interferometer loop based on Vernier effect. Sens. Actuators B Chem. 255, 3011–3016. doi: 10.1016/j.snb.2017.09.124
Wu, R. J., Lin, D. J., Yu, M. R., Chen, M. H., and Lai, H. F. (2013). Ag@ SnO2 core–shell material for use in fast-response ethanol sensor at room operating temperature. Sens. Actuators B Chem. 178, 185–191. doi: 10.1016/j.snb.2012.12.052
Yamazoe, N., Kurokawa, Y., and Seiyama, T. (1983). Effects of additives on semiconductor gas sensors. Sens. Actuators 4, 283–289. doi: 10.1016/0250-6874(83)85034-3
Yang, L., Yin, C., Zhang, Z., and Zhu, B. (2014). A study of hydrogen sensing properties and microstructure for highly dispersed Pd SnO2 thin films with high response magnitude. Appl. Surf. Sci. 311, 74–82. doi: 10.1016/j.apsusc.2014.05.003
Zhang, D., Jiang, C., and Zhang, Y. (2017). Room temperature hydrogen gas sensor based on palladium decorated tin oxide/molybdenum disulfide ternary hybrid via hydrothermal route. Sens. Actuators B Chem. 242, 15–24. doi: 10.1016/j.snb.2016.11.005
Zhang, Z., Yin, C., Yang, L., Jiang, J., and Guo, Y. (2019). Optimizing the gas sensing characteristics of Co-doped SnO2 thin film based hydrogen sensor. J. Alloys Compd. 785, 819–825. doi: 10.1016/j.jallcom.2019.01.244
Keywords: tin oxide, silver, silver oxide, thin film, gas sensor, hydrogen
Citation: Mohamedkhair AK, Drmosh QA and Yamani ZH (2019) Silver Nanoparticle-Decorated Tin Oxide Thin Films: Synthesis, Characterization, and Hydrogen Gas Sensing. Front. Mater. 6:188. doi: 10.3389/fmats.2019.00188
Received: 20 June 2019; Accepted: 24 July 2019;
Published: 16 August 2019.
Edited by:
Kalisadhan Mukherjee, Pandit Deendayal Petroleum University, IndiaReviewed by:
Tarapada Sarkar, University of Maryland, College Park, United StatesSubhasis Roy, University of Calcutta, India
Copyright © 2019 Mohamedkhair, Drmosh and Yamani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zain H. Yamani, emh5YW1hbmlAa2Z1cG0uZWR1LnNh
 Amar K. Mohamedkhair
Amar K. Mohamedkhair Q. A. Drmosh
Q. A. Drmosh Zain H. Yamani
Zain H. Yamani