- 1Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), Rome, Italy
- 2Stazione Zoologica Anton Dohrn (SZN), Napoli, Italy
- 3Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), Ozzano dell’Emilia, Italy
- 4Istituto Nazionale di Oceanografia e Geofisica Sperimentale (OGS), Trieste, Italy
Biogenic reefs are known worldwide to play a key role in benthic ecosystems, enhancing biodiversity and ecosystem functioning at every level, from shallow to deeper waters. Unfortunately, several stressors threaten these vulnerable systems. The widespread presence of marine litter represents one of these. The harmful effects of marine litter on several organisms are known so far. However, only in the last decade, there was increasingly scientific and public attention on the impacts on reef organisms and habitats caused by litter accumulating on the seafloor. This review aims to synthesize literature and discuss the state of current knowledge on the interactions between marine litter and reef organisms in a strongly polluted basin, the Mediterranean Sea. The multiple impacts (e.g., entanglement, ghost-fishing, coverage, etc.) of litter on reef systems, the list of species impacted, and the main litter categories were identified, and a map of the knowledge available so far on this topic was provided. Seventy-eight taxa resulted impacted by marine litter on Mediterranean reefs, and the majority belonged to the phylum Cnidaria (41%), including endangered species like the red coral (Corallium rubrum) and the madrepora coral (Madrepora oculata). Entanglement, caused mainly by abandoned, lost, or otherwise discarded fishing gear (ALDFG), was the most frequent impact, playing a detrimental effect mainly on coralligenous arborescent species and cold-water corals (CWCs). The information was spatially heterogeneous, with some areas almost uncovered by scientific studies (e.g., the Aegean-Levantine Sea and the Southern Mediterranean Sea). Although many legal and policy frameworks have been established to tackle this issue [e.g., marine strategy framework directive (MSFD) and the Barcelona Convention], several gaps still exist concerning the assessment of the impact of marine litter on marine organisms, and in particular on reefs. There is a need for harmonized and standardized monitoring protocols for the collection of quantitative data to assess the impact of litter on reefs and animal forests. At the same time, urgent management measures limiting, for instance, the impact of ALDFG and other marine litter are needed to preserve these valuable and vulnerable marine ecosystems.
Introduction
Biogenic reefs or marine bioconstructions are three-dimensional biogenic structures regulating ecological functions of benthic ecosystems from shallow to deeper waters (Ingrosso et al., 2018). They are shaped by one or few engineer species (benthic bioconstructors) that cause morphological and chemical–physical changes in the primary (abiotic) substrates and provide secondary (biotic) structures to generate biogenic new habitat suitable for a highly diverse associated fauna (Ingrosso et al., 2018).
Bioconstructor frameworks can provide a complex network of ecological niches for a large variety of organisms, including endangered and protected species, as well as species of high commercial value (Chimienti et al., 2020), functioning as habitat for shelter and feeding, spawning and nursery areas, substrata for both larval settlement and juvenile growth (e.g., Tursi et al., 2004; D’Onghia et al., 2010, 2015; Rosso et al., 2010). Since bioconstructions increase spatial complexity and settlement opportunities at every level, they are known worldwide to play a pivotal ecological role in enhancing and maintaining high marine biodiversity. Therefore, they contribute to ecosystems’ goods and services, and to regulate natural resource dynamics (Lo Iacono et al., 2018).
The equilibrium between building and bioerosion processes determines bioconstruction development (Garrabou and Ballesteros, 2000) and can take centuries or even millennia of biological activities. However, some bioconstructions are ephemeral and can rapidly degrade. Bioconstructions can have various shapes and sizes, and they are distributed worldwide along depth gradients. The most known bioconstructions are the popular tropical reefs, noted for their beauty, high-diversity (polytypic), and complexity (Ingrosso et al., 2018). Much less attention has received other similar types of bioconstructions (mono- or oligotypic), found in other seas, included in temperate areas, such as the Mediterranean Sea.
The Mediterranean is a semi-closed basin considered one of the world’s biodiversity hotspots, hosting 7.5% of global biodiversity (Bianchi and Morri, 2000) with a high percentage of endemism (Boudouresque, 2004; Coll et al., 2010), species of conservation concern (such as several cetaceans, sea turtles, monk seal) and endangered and protected habitats [i.e., meadows of the endemic Posidonia oceanica, coralligenous assemblages, animal forests sensu lato (Rossi et al., 2017) and deep-sea cold-water corals (CWCs; Davies et al., 2017)]. Some of these endangered habitats are biogenic reefs that provide structural complexity to seafloor habitats and support unique species and ecosystems (Orejas et al., 2009; Rossi, 2013; Bo et al., 2015; Davies et al., 2017; Rossi et al., 2017). For example, the coralligenous is a key ecosystem recognized as a natural habitat of community interest and Zone of Special Conservation at the European level (92/43/EEC Habitats Directive). From shallow to deeper waters, the Mediterranean Sea hosts a large variety of bioconstructions.
Ingrosso et al. (2018) identified a list of the main biogenic reefs in the Mediterranean Sea: vermetid reefs, which are biogenic formations that border rocky shores at the tide level; Lithophyllum byssoides trottoirs, common in the western and central Mediterranean, forming thick algae concretions that cover the rock surface; coral banks created by the shallow-water corals Cladocora caespitosa or Astroides calycularis formations/reefs, hosting a rich invertebrate fauna and that can cover up to 90% of some rocky areas (Goffredo et al., 2011); coralligenous assemblage, dwelled in rocky bottoms from 15 to 130 m depth (Ballesteros, 2006) developing extraordinary habitats with high biodiversity level; CWCs, that change the structural heterogeneity of the environment, forming large (monospecific or mixed) aggregations, the so-called animal forests, from 200 to 1000 m depth (Freiwald et al., 2009; Chimienti et al., 2018); and sabellariid or serpulid worm reefs, made of calcareous tube, where polychaetes live (Bianchi, 1981) that encrust any hard substrate. Other important and peculiar structures are recorded in the northern Adriatic Sea, and they are called “Tegnùe” or “Trezze” (Melli et al., 2017), subtypes of coralligenous habitats (Falace et al., 2015; Tosi et al., 2017). All these types of biogenic reefs are common throughout the whole Mediterranean Sea. Still, there are several knowledge gaps about their distribution, and knowledge on their biology and ecology is, in some cases, still fragmentary (Ingrosso et al., 2018).
Several direct and indirect anthropogenic pressures (industrial, urban and agricultural pollution, coastal development, marine litter, fishery, increase in sedimentation, organic enrichment, coastal development, deep-sea mineral mining and oil exploration, submarine cable, etc.) threaten these vulnerable bioconstruction systems, as well as climate change and the spread of alien species (Ballesteros, 2006; Coll et al., 2010; Piazzi et al., 2012). In particular, the widespread presence of marine litter represents one of the most important threats to biogenic reefs, which leads to a degradation of these habitats (de Carvalho-Souza et al., 2018) and the associated organisms (Galgani et al., 2018).
The Mediterranean is a densely populated sea with intense use of coasts, and it is notable for its contributions to the global economy and trade. It attracts 25% of international tourism (tens of millions of people descend each year), and about 220,000 vessels of more than 100 tons are estimated cross the Mediterranean annually, carrying 30% of the international maritime traffic (Ramirez-Llodra et al., 2013). Historical and current pressures in many ways have brought irreversible changes in the ecology of the Mediterranean Sea (Micheli et al., 2013). Mediterranean ecosystems are altered and threatened at an increasingly fast rate, and this basin is one of the most affected and polluted areas in the world (Barnes et al., 2009; Costello et al., 2010; Deudero and Alomar, 2015; Jambeck et al., 2015; Ramírez et al., 2018).
Due to its wide distribution, durability, and low biodegradability, marine litter is nowadays a remarkable and persistent threat to ecosystems and wildlife globally (Avio et al., 2017). Litter items, including micro-plastics, contaminate habitats from shallow water to the deep sea and from the poles to the equator (Worm et al., 2017). The most visible effect of marine litter is probably the entanglement of animals, which are hindered in their ability to move, feed, breathe, and reproduce (Li et al., 2016). This phenomenon is typically associated with abandoned, lost, or otherwise discarded fishing gear (ALDFG) entangling marine mammals, sea turtles, seabirds, and fish (ghost-fishing; Galgani et al., 2018; Richardson et al., 2019). However, also many sessile erected species are deeply damaged by entanglement that may cause tissue abrasion, branch breaking, by-catches, etc. (e.g., Yoshikawa and Asoh, 2004; Angiolillo, 2019). These impacts may cause progressive and extended habitat degradation and a reduction in the coverage by biota on the seafloor (Laist, 1997; Fosså et al., 2002; Brown and Macfadyen, 2007). On the seabed, litter objects alter the surrounding habitat, interfering with life, and providing a previously absent hard substrate (UNEP, 2009).
Additionally, litter items can be used as a means of transport by alien invasive species traveling over long distances, both horizontally or vertically (Kühn et al., 2015). Moreover, microparticles of plastic (also known as microplastics when their size is < 5 mm in their largest dimension), specifically realized for various applications (primary microplastics) or deriving from the flaking of larger pieces (secondary microplastics), can be ingested by marine organisms and enter the trophic web (Corcoran et al., 2014; Gall and Thompson, 2015). Litter can be mistaken for food by several marine organisms, and indigestible debris may affect individual fitness, with negative consequences on survival (Kühn et al., 2015). The degradation of plastic, metals, and other litter material can also result in the release of toxic chemicals substances with chronic and sub-lethal consequences, which could likely compromise populations and communities and have long-term effects. Some xenobiotics, including persistent organic pollutants, toxic metals, pesticides, herbicides, pharmaceuticals as well as plastics and microplastics, are resistant to degradation, and deep waters and sediments have been suggested as their final accumulation site (Ramirez-Llodra et al., 2011; Ma et al., 2015). In the Mediterranean Sea, a recent review found that 116 species have ingested plastic, 44 species were found entangled in marine litter, and 178 taxa were found rafting on floating objects or using marine litter as a substratum (Anastasopoulou and Fortibuoni, 2019).
The presence of marine litter and its effects has stimulated increasingly global interest in this issue due to its ubiquity and its potential impact on human health (Hess et al., 1999; UNEP, 2009; Miyake et al., 2011). For several decades, the majority of studies had focused on the distribution of beach and floating litter and its impact by entanglement on charismatic organisms as marine birds, turtles, and mammals, and ingestion, in particular by fishes. Only in the last few years, increasing interest is also focusing on the distribution and injuries to benthic habitats and invertebrates caused by anthropogenic litter accumulating on the seafloor. Increasing awareness on this issue is stimulating scientific research, monitoring programs, NGO activities (Consoli et al., 2019), and it is initiating political action to tackle this environmental problem (Galgani et al., 2013; Galgani et al., 2019; Ronchi et al., 2019).
In 2008, the European Union issued the marine strategy framework directive (MSFD, 2008/56/CE), which is the leading European legal instrument to protect the marine environment in the Baltic Sea, North-east Atlantic Ocean, Mediterranean Sea, and Black Sea. The MSFD foresees 11 Descriptors, and Descriptor 10 states, “properties and quantities of marine litter do not cause harm to the coastal and marine environment.” Four criteria were established to assess the achievement of the good environmental status (GES) of European seas considering Descriptor 10, one of which (D10C4) consists in “the number of individuals of each species which are adversely affected due to litter, such as by entanglement, other types of injury or mortality, or health effects.” In 2016, the 19th Meeting of Contracting Parties agreed on the integrated monitoring and assessment program (IMAP) of the Mediterranean Sea and Coast and Related Assessment Criteria in its Decision IG. 22/7, which laid down the principles for integrated monitoring, including pollution and marine litter. IMAP Ecological Objective (EO) 10 (Marine Litter: “Marine and coastal litter do not adversely affect coastal and marine environment”) includes Candidate Indicator 24: “Trends in the amount of litter ingested by or entangling marine organisms focusing on selected mammals, marine birds, and marine turtles.”
Moreover, the Ecosystem Approach (EcAp), defined as “a strategy for the integrated management of land, water and living resources that promotes conservation and sustainable use in an equitable way” (Morand and Lajaunie, 2018), is now fully integrated into the mediterranean action plan (MAP) – Barcelona Convention System dedicated to the protection of the Mediterranean Sea against pollution and is in line with the MSFD and the decisions of the convention on biological diversity (CBD) and the Aichi targets. Nevertheless, to date, limited and fragmented knowledge is available on the effect of litter on the benthic realm, in particular for reef systems (de Carvalho-Souza et al., 2018) and the Mediterranean Sea (UNEP/MAP and SPA/RAC, 2018). Thus, this review aims to put together, synthesize and discuss the state of the current knowledge about the interactions of macro litter and the Mediterranean reef systems, from shallow to deep waters, investigating: (i) the spatial distribution and qualitative composition of litter causing impact; (ii) the different typologies of interaction and the species involved; (iii) the methods used to trace and identify marine litter on the bottom and its impact on reef species and; (iv) the knowledge gaps to help to find solutions to mitigate marine litter effects. Information provided here may contribute to the crafting of new indicators of entanglement to populate criteria D10C4 of the MSFD and IMAP Indicator 24 and identify main knowledge gaps (e.g., main species and habitats likely to be impacted) and areas where more scientific effort is needed.
Bibliographic Research
Information was collected by consulting scientific databases on marine litter impacts on biota (Litterbase1 and MedBioLitter2) and the search engines Web of Science, Scopus, Google Scholar, and ResearchGate. A list of keywords linked with marine litter and reefs was used, i.e., “marine litter,” “deep marine debris,” “submerged marine litter,” “anthropogenic debris,” “seafloor litter,” “marine pollution,” “garbage,” “derelict fishing gear,” “ADLFG,” “CWCs,” “Mediterranean Sea,” “entanglement,” “ghost-fishing,” “animal forest,” “abrasion,” “marine litter interaction,” “coralligenous,” “bioconstruction,” and “fishing impact.”
Sixty-seven publications reporting evidence of the impact of marine litter on Mediterranean reef systems were found, the first one published in 1994. However, the bulk of papers were published in the last decade (Figure 1). In the majority of publications, the impact of marine litter on animal forests and reefs was not the focus of the study. In this review, we selected only those cases where it was clearly defined that the impact was caused by marine litter, i.e., “any persistent, manufactured or processed solid material that is discarded, disposed of or abandoned in the marine or coastal environment” (UNEP, 2009). Thus, we excluded cases in which it was not clear if the impact was related to fishing activities or active fishing gear.
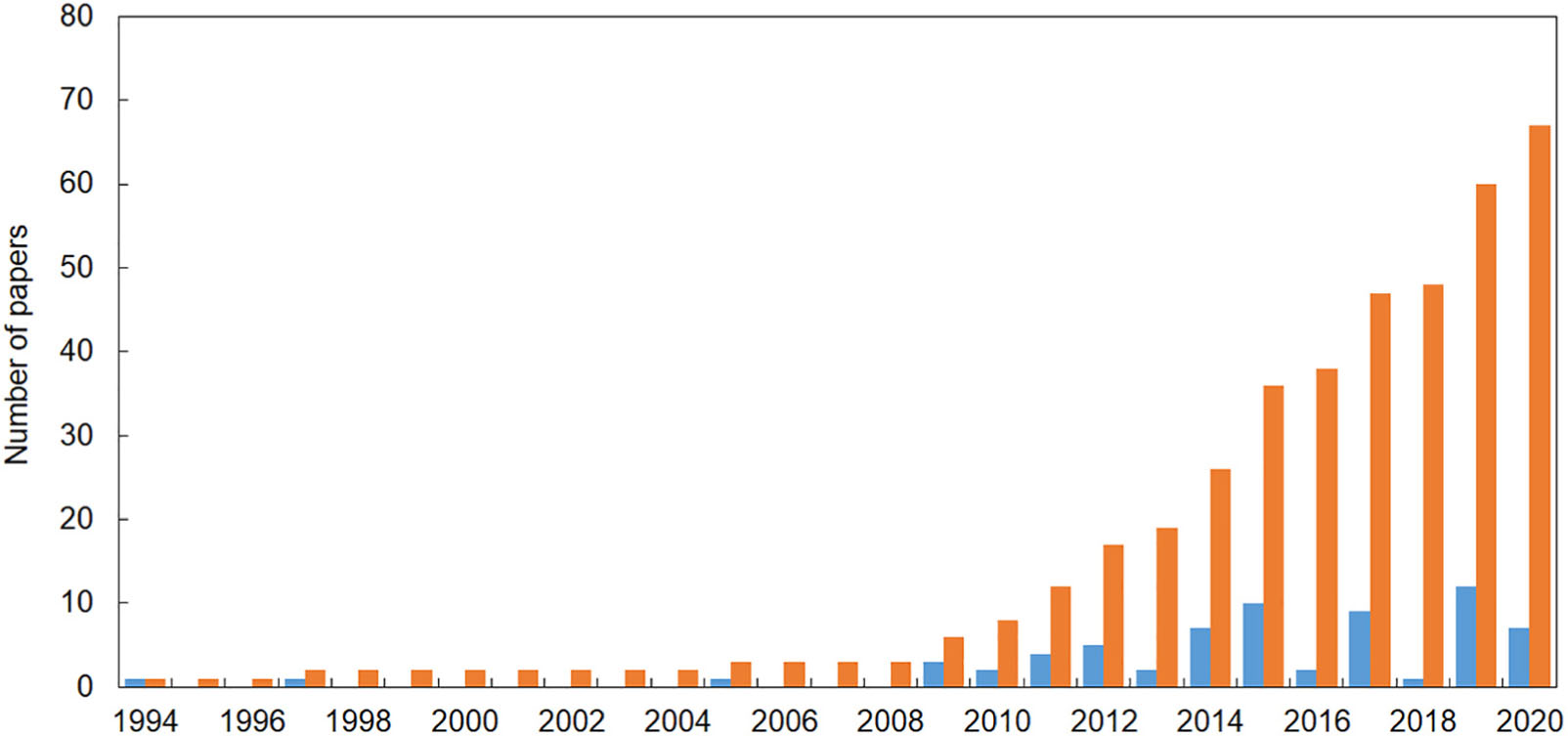
Figure 1. The number of papers reporting evidence of the impact of marine litter on reef systems in the Mediterranean Sea (updated at the end of May 2020). Blue = number of papers published each year; orange = cumulative number of papers published since 1994.
The information gathered from the publications included: the place where the study was conducted (coordinates and depth), the taxa involved, the type of reef impacted, the type of interaction, the type of litter, and the effect on impacted species. Species names were updated using the World Register of Marine Species (WoRMS) database,3 and their conservation status was defined according to the International Union for Conservation of Nature (IUCN) Red List database,4 referring to the Mediterranean subpopulation when specific information was available. Otherwise, we referred to the global assessment or, in some case studies in Italian waters, to the assessment performed by the Italian committee.5 Case studies were subdivided into benthic environments as follows: 0–200 m (littoral), 200–1000 m (archibenthic), and 1000–4000 m (bathybenthic).
A Summary of the Impacts on Reef Species
Study areas were not homogeneously distributed across Mediterranean subregions, and sampling effort was mainly concentrated in the Western Mediterranean Sea (51 papers), followed by the Adriatic Sea and the Ionian and Central Mediterranean Sea (seven papers each), and lastly, the Aegean-Levantine Sea (four papers; Figure 2). Moreover, almost the totality of studies was conducted in the Northern Mediterranean Sea: Italy (n = 39), Spain (n = 9), France (n = 7), Croatia (n = 3), Greece (n = 3), Malta (n = 2), Cyprus (n = 1) and Montenegro (n = 1). One study was conducted close to the Moroccan coast in the Chafarinas Islands (Spain; Figure 2). Case studies included evidence of marine litter impact on reefs from a few meters below the surface down to 1,208 m. Most case studies (n = 40) were focused in the littoral zone, followed by the archibenthic zone (n = 27) and bathybenthic zone (n = 2). The major part of studies was carried out on coralligenous (33%) and CWCs (50%) assemblages, or both coral aggregations (13%). Only two studies were conducted on the “tegnùe” (Melli et al., 2017; Moschino et al., 2019) and one on Astroides calycularis reefs (Terrón-Sigler, 2015). No data were available on the impact of marine litter on other types of Mediterranean biogenic reefs.
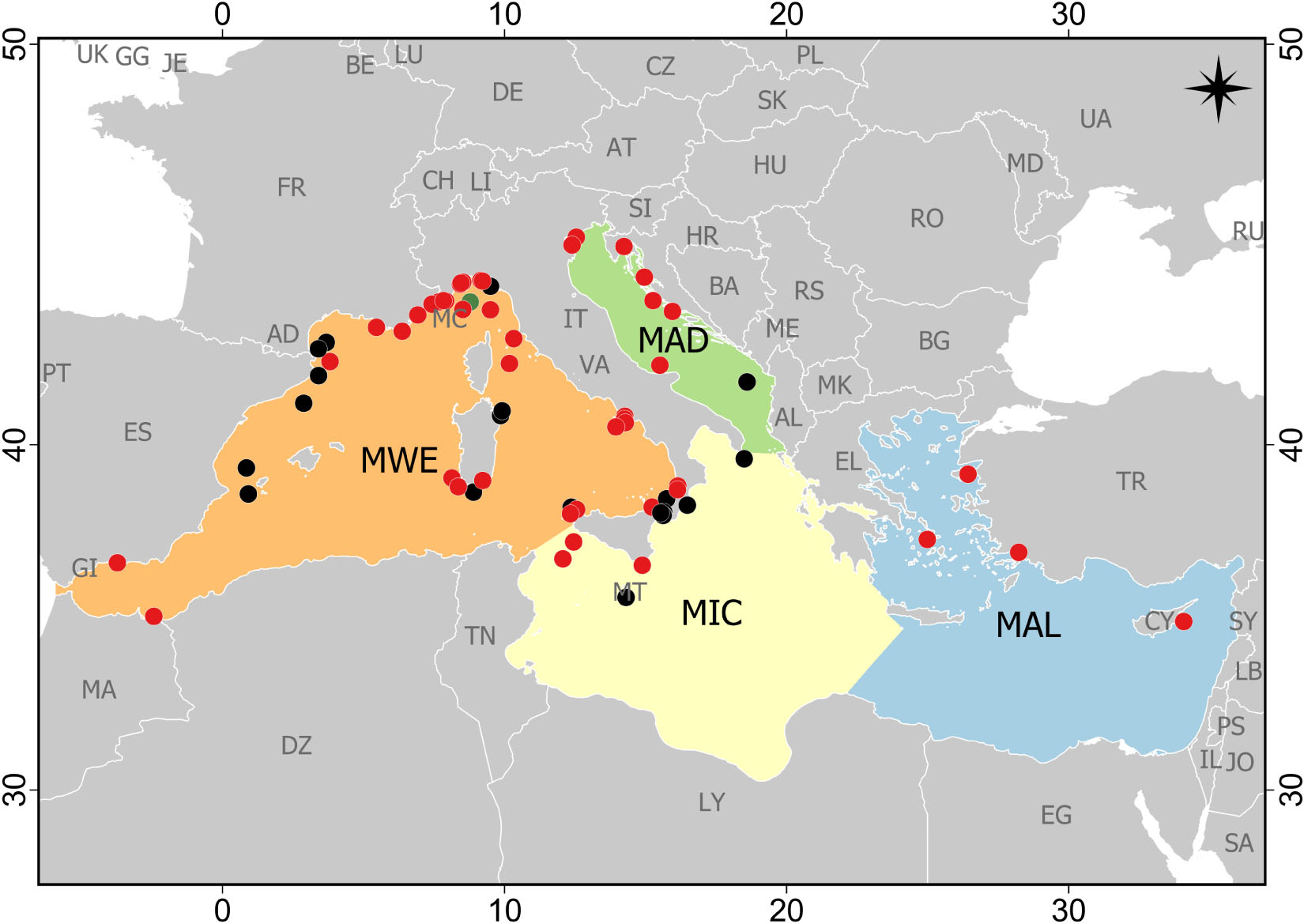
Figure 2. Distribution of the case studies reporting the impact of marine litter on reef systems in the Mediterranean Sea (updated at the end of May 2020). Case studies are reported with different colors according to their benthic environment: littoral (0–200 m) – red dots; archibenthic (200–1000 m) – black dots; bathybenthic (1000–4000 m) – green dots (two case studies were referred to this environment, but one did not report the exact place and thus it was not possible to include it in the map). MWE, Western Mediterranean Sea; MIC, Ionian Sea and the Central Mediterranean Sea; MAD, Adriatic Sea; MAL, Aegean-Levantine Sea.
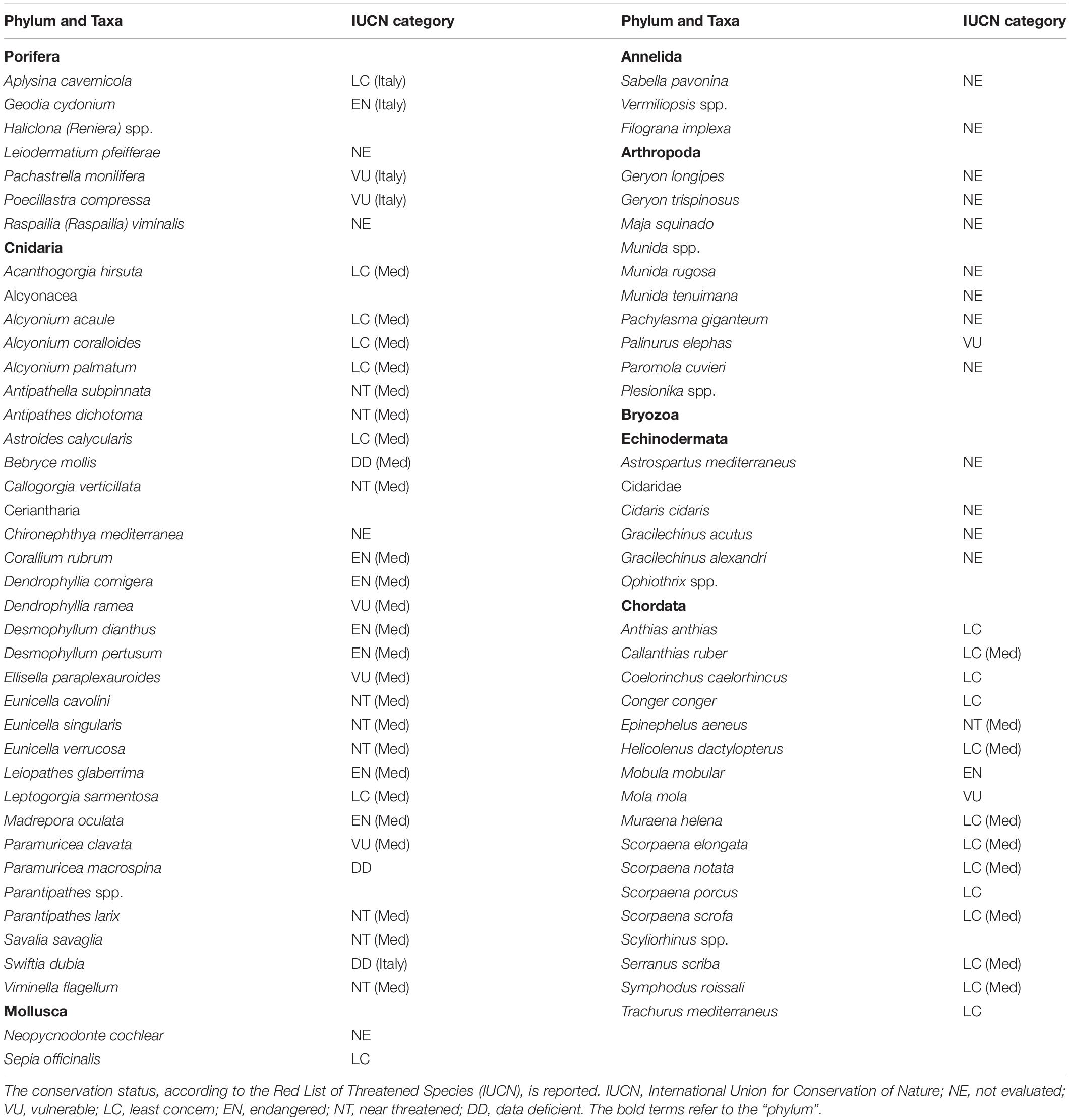
Seventy-eight taxa resulted impacted by marine litter on Mediterranean reefs (Table 1). The large majority belonged to the phylum Cnidaria (41%), followed by Chordata (22%), Arthropoda (13%), Porifera (9%), and Echinodermata (8%). The remaining taxa represented less than 5% (Figure 3). The list of impacted species included eight endangered (EN) species: the red coral (Corallium rubrum), the stony cup coral (Dendrophyllia cornigera), the cockscomb cup coral (Desmophyllum dianthus), the white coral (Desmophyllum pertusum), the smooth black coral (Leiopathes glaberrima), the madrepora coral (Madrepora oculata), the sea-sponge Geodia cydonium, and the giant devil ray (Mobula mobular). This provides evidence for the potential hazard represented by marine litter for threatened reef species. Seven vulnerable (VU) species were also found to be impacted by marine litter in the Mediterranean (Table 1), while the large majority (n = 17) of species were not evaluated (NE) or data deficient (DD; n = 3).
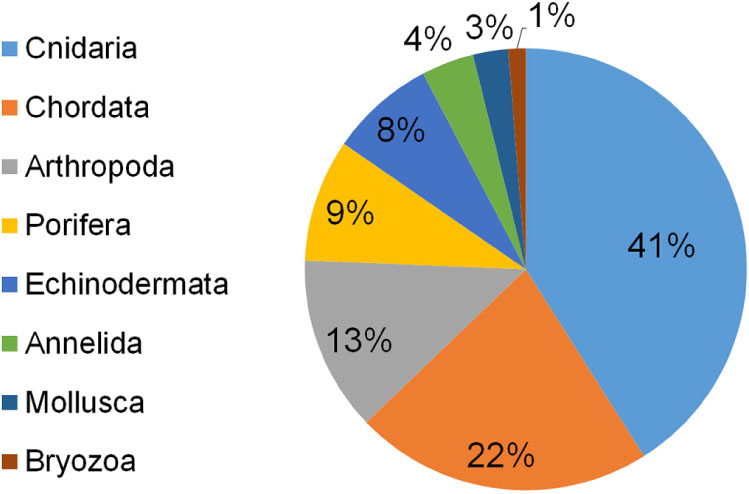
Figure 3. Percentage of reef species impacted by marine litter in the Mediterranean Sea subdivided by phylum.
Methods Used to Detect Impacts
In the Mediterranean Sea, the first scientific evidence of seafloor litter interaction with reefs and biota dates back to the end of the 1990s with visual investigations through SCUBA divers in shallow coastal environments (Harmelin and Marinopoulos, 1994; Bavestrello et al., 1997). The first studies using a remotely operated vehicle (ROV) were published in 2009 (Freiwald et al., 2009; Orejas et al., 2009; Salomidi et al., 2009), and later on, ROV was the method most used both in shallow and deep waters. We also found one case study where a fixed camera was used in the Ionian Sea (D’Onghia et al., 2017), and a paper where ROV and Agassiz trawl were used complementarily for in situ observation and to sample corals in the Blanes Canyon (France), respectively (Aymà et al., 2019).
Seafloor imagery is increasingly being used to study the abundance and distribution of species on the seafloor (Valisano et al., 2019). The advance and availability of exploration technologies, such as ROV-imaging, applied to previously unexplored hard bottoms, have provided a lot of in vivo information for the deep sea and reef systems, that in the last decades have revolutionized and rapidly increased the knowledge on these habitats and its threats. The increasing interest in deep-sea exploration had also caused the copious number of studies on litter compared to shallower waters (references herein).
In the last years, these methods have also been applied to the study of seafloor litter (Spengler and Costa, 2008), and they have allowed to describe and quantify litter interactions with marine organisms. Visual methods can be applied to all sea bottom types from the continental shelf to the bathyal environment, including complex reef habitats (Watters et al., 2010; Angiolillo et al., 2015). Visual data obtained by SCUBA divers, towed systems, ROVs or submersibles, and other camera platforms, equipped with specific scientific tools (HD video and camera, laser points, and geolocalization), have allowed highlighting as marine litter represents one of the major pollution problems harming benthic organisms and habitats (Gilman, 2015). These methods do not cause any impact to the marine environment and are thus applicable in protected and sensitive areas such as marine protected areas (MPAs) and coral reefs (e.g., Betti et al., 2019, 2020; Chimienti et al., 2020). From videos and frame/photo, it is possible to obtain high-resolution quantitative data (depending on optical device), precise geolocalization, and provides in situ information at various depths.
However, the most common method to study seafloor litter is still through trawling hauls (i.e., otter, beam, Agassiz trawl), mainly for its lower costs of operation in comparison with other methods and large scale evaluation (e.g., Galgani et al., 1996, 2000; Stefatos et al., 1999; Moore and Allen, 2000; Lee et al., 2006; Koutsodendris et al., 2008; Keller et al., 2010; Sánchez et al., 2013; Neves et al., 2015; Pasquini et al., 2016; Fortibuoni et al., 2019; Spedicato et al., 2019). In trawl surveys, litter is taken onboard and thus can be directly inspected, measured, counted, and weighed, allowing to obtain quantitative data. Nevertheless, the use of trawls should be avoided/limited on hard substrates such as reefs or in areas protected from fishery activity, since it is a destructive method. Besides, trawling does not enable to directly observe and assess the effects of litter on habitats and species nor the precise localization of litter and impacted organisms.
Types of Interaction
The types of litter interactions were coded into six categories, i.e., entanglement, ghost-fishing, coverage, behavioral, substratum, and incorporation. Marine macrolitter were subdivided according to the MSFD Commission Decision 2017/848, into the following categories: artificial polymer materials, rubber, cloth/textile, paper/cardboard, processed/worked wood, metal, glass/ceramics, chemicals, undefined, and food waste. However, “ALDFG” (including longlines, trammel nets, gillnets, set nets, ropes, FADs, pots, etc.) were considered separately, considering the documented wide presence in biogenic reefs (Galgani et al., 2018). The effects on biota were not always described, and when the information was available, it was classified into nine groups: abrasion, behavioral, colonization, damage, death, detachment, entrapment, epibiosis, and necrosis.
Entanglement
Entanglement resulted in being the major impact of marine litter affecting marine organisms on Mediterranean reefs (Supplementary Table S1). We found evidence of entanglement for thirty-four taxa, most of which were cnidarians. Fishing gears were by large the most common litter type causing entanglement mainly through snagging and catches on reef organisms (Figure 4). Artisanal gears (trammel nets, gillnets, and long and fishing line, ropes, etc.) or lost fishing gear, under the pressure of bottom currents, can indeed easily become entangled in rocks and in all taxa that elevate on the substrate due to their massive or arborescent morphologies (Figures 5A–C). The abrasive action due to the continuous mechanical friction caused by entangled gear can cause breakage of ramifications of all erect biotic structures or a progressive removal of their tissues (Macfadyen et al., 2009; de Carvalho-Souza et al., 2018), making them more vulnerable to parasites or bacterial infections (Bo et al., 2014).
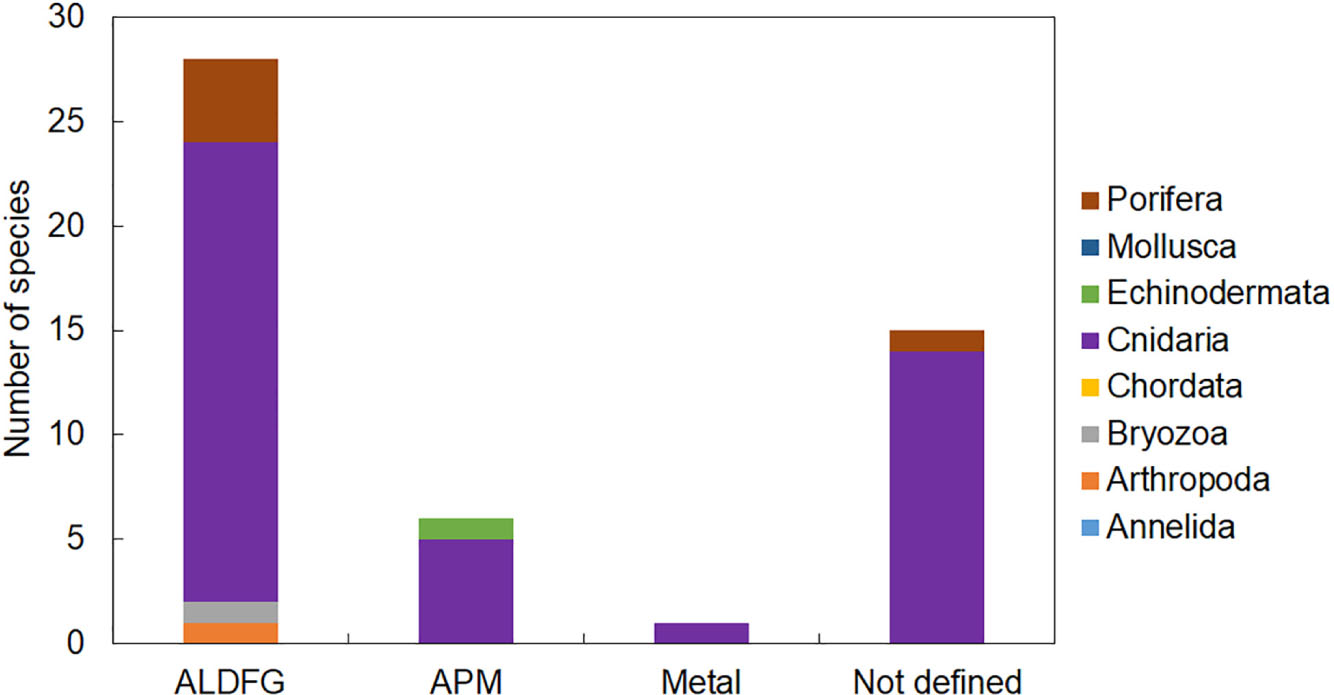
Figure 4. The number of species by taxa found entangled in different typologies of marine litter on Mediterranean reefs. ALDFG, abandoned, lost, or otherwise discarded fishing gear; APM, artificial polymer materials.
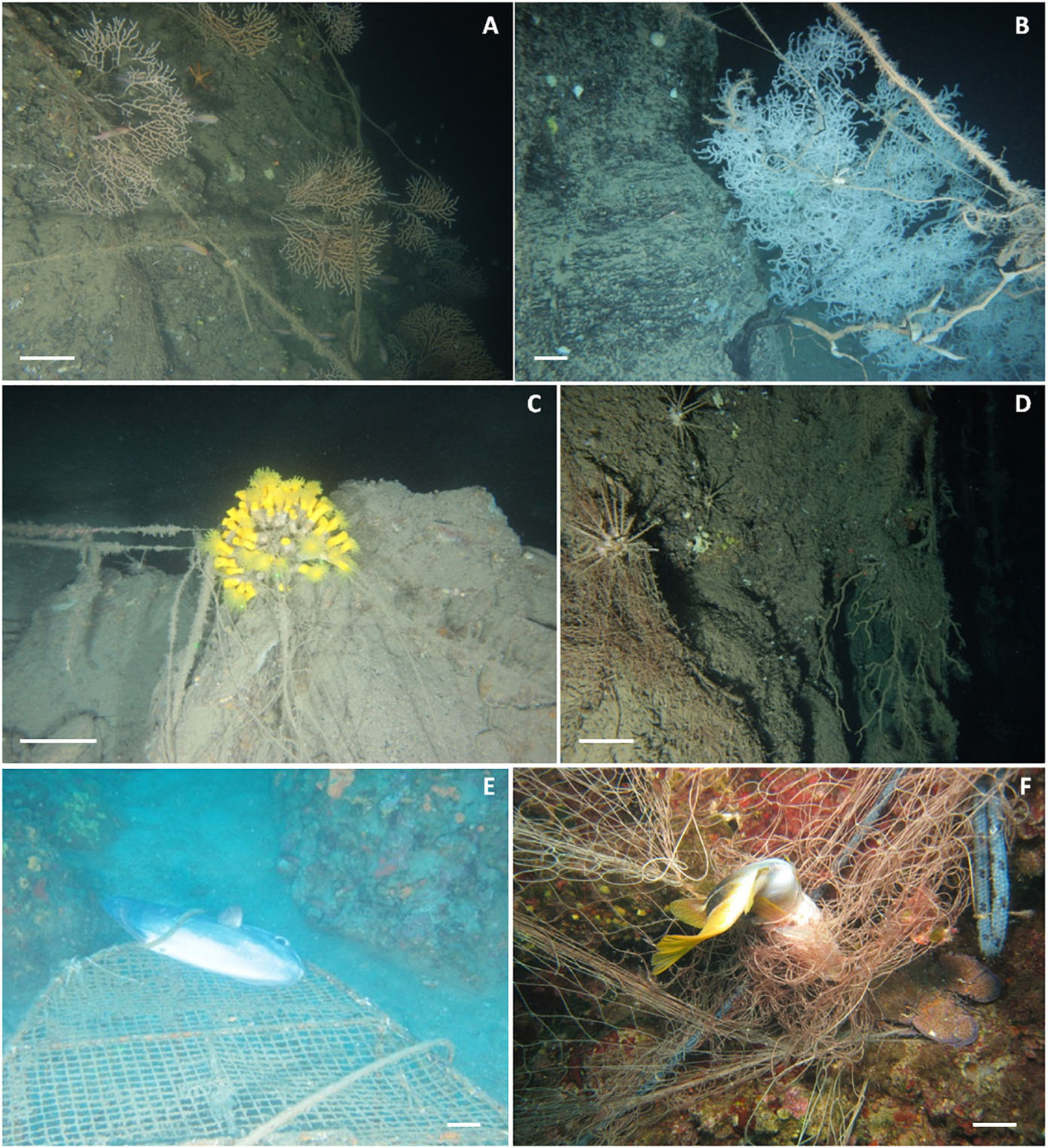
Figure 5. Examples of marine litter impacts on biogenic reefs in the Mediterranean Sea. Scale bar: 10 cm. (A) lines and ropes entangling Eunicella cavolini colonies; (B) old long line and monofilaments entangled on a colony of Leiopathes glaberrima, peeling off part of its tissue (with evidence of necrosis); (C) Dendrophyllia cornigera colony completely entangled in a lost longline; (D) lost nets entrapping a specimen of Cidaridae and a dead gorgonian; (E,F) examples of ghost-fishing: a sunfish (Mola mola) entrapped in a fishing pot and a painted comber (Serranus scriba) entrapped in a lost net.
Abrasion/coenenchyme loss was the most common effect of entanglement by ALDFG reported for animal forests on Mediterranean reefs, and it was observed in all subregions. Eleven cnidarian species resulted impacted, including the endangered Corallium rubrum, as well as the sea-sponge Leiodermatium pfeifferae. Because of abrasion, a naked coral skeleton can be quickly covered by fast-growing epibionts (such as an encrusting sponges, zoanthids, or some alcyonaceans; Bo et al., 2014; Angiolillo et al., 2015; Angiolillo and Canese, 2018) and this phenomenon could cause colony loss (Bavestrello et al., 1997). The high frequency of these opportunistic organisms may suggest a general state of stress of the community (Bo et al., 2014) also due to marine litter impacts.
Necrosis and epibiosis linked to the impacts of marine litter on reefs were, for instance, widely reported by Giusti et al. (2019) in the Ligurian and Western Mediterranean Seas. Direct damages (e.g., broken branches) to coral colonies due to entangling marine litter were also observed on Mediterranean reefs (e.g., Madurell et al., 2012; Maldonado et al., 2013; Angiolillo et al., 2015; Consoli et al., 2018; Moccia et al., 2019). Fishing gear entangled on coral colonies was also shown to cause their detachment from the seafloor (Houard et al., 2012; Tsounis et al., 2012; Angiolillo et al., 2015; Kipson et al., 2015; Maldonado et al., 2015; Cattaneo-Vietti et al., 2017; Consoli et al., 2019) and even their death (Maldonado et al., 2013; Deidun et al., 2015; Consoli et al., 2018; Enrichetti et al., 2019; Figure 5D).
Coral skeletal characteristics, determining the rigidity and fragility of a colony, as well as the size and shape of individuals, determine the resistance to friction, which explains the different responses of the various species of coral to mechanical impacts (Bo et al., 2014; Fabri et al., 2014; Angiolillo and Canese, 2018). In this review, the most impacted species resulted in those that easily remain entangled due to their medium-large colony size, an arborescent morphology, and a flexible skeleton (e.g., Antipathella subpinnata, Leiopathes glaberrima, Callogorgia verticillata, Dendrophyllia cornigera, Paramuricea clavata, Figures 5A–D), as observed in other studies (Asoh et al., 2004; Bo et al., 2014; Valisano et al., 2019). Numerous examples from this review illustrate marine litter impacts on vulnerable (VU) and endangered (EN) species (IUCN criteria, Table 1) and sensitive habitats, such as CWCs (Orejas et al., 2009; Madurell et al., 2012), coral gardens (Bo et al., 2014, 2015; Fabri et al., 2014; Angiolillo et al., 2015) and coralligenous assemblages (Sbrescia et al., 2008; Valisano et al., 2019) that are more vulnerable because of slow growth rate and longevity of their coral species (MacDonald et al., 1996; Consoli et al., 2018).
The impact of the hook-and-line fishery on the seafloor is generally perceived as lower when compared with other fishing methodologies, such as bottom trawling (Macfadyen et al., 2009). However, monofilament fishing line is responsible for the largest part of entanglements observed on Mediterranean reefs and, more in general, represent the major impact in rocky areas, not suitable for trawling (Angiolillo, 2019). Kroodsma et al. (2018) estimated that globally longline fishing is the most widespread activity, detected in 45% of the ocean more than trawling (9.4%). However, longline incidence can strongly vary according to the region, and even if in many areas it may be mainly confined to artisanal fishing grounds, passive gears could drift for long distances driven by currents. Due to its extensive use, often extremely long configuration, and low cost, its cumulative effects over time might be very detrimental (Macfadyen et al., 2009). Moreover, line and longline are now made of non-biodegradable synthetic fibers and, once lost, can persist in the environment for centuries (Carr, 1987; Thompson et al., 2004; Moore, 2008; Barnes et al., 2009; Watters et al., 2010; Bo et al., 2014).
The reduction in the coverage of habitat-forming species and the detrimental effect on the diversity and abundance of reef invertebrates and fishes resulting from human-induced pressure can cause substantial modifications to the structure and functioning of reef ecosystems (Bo et al., 2014; Clark et al., 2016; Valisano et al., 2019). The accumulation of fishing debris represents one of the major causes of habitat degradation of Mediterranean reefs. Thus, destructive practices (e.g., fishing and anchoring) that can threaten arborescent corals should be banned in the proximity of the coral forests (Chimienti et al., 2020).
Ghost-Fishing
Ghost-fishing represents another aspect of entanglement. Crabs, octopus, fishes, and many small invertebrates may be taken in traps, nets, gear, or other litter items that continue to “fish” if lost at sea. In Mediterranean reefs, according to this review, ghost-fishing was exclusively related to fishing gears (Supplementary Table S1) and was observed for four species belonging to the phylum Arthropoda (Geryon trispinosus, Maja squinado, Munida rugosa, and Palinurus elephas) and 10 Chordata (Conger conger, Epinephelus aeneus, Mobula mobular, Mola mola, Scorpaena notata, S. porcus, S. scrofa, Serranus scriba, Scyliorhinus spp., and Symphodus roissali, Figures 5E,F).
Water turbidity, making the litter and the gear less visible, as well as the presence of organisms in or near the nets, are factors that may contribute to organisms being entangled in, or strangled by, abandoned fishing gear, resulting in domino effects of damage. Benthic invertebrates, such as crabs and echinoderms, may become entangled in nets, traps, or other kinds of debris lying on the seafloor while scavenging animals that have already become entangled (Good et al., 2010). In this way, ghost fishing gear may continue to catch for a long time a large variety of organisms (Carr, 1987; Matsuoka et al., 2005; Brown and Macfadyen, 2007). The impossibility of moving and breathing, compromising the ability to acquire food and escaping from predators, might eventually lead to death for stress, starvation, or drowning (Ayaz et al., 2010; Butterworth et al., 2012; Enrichetti et al., 2020). Moreover, entanglement, abrasion, and restricted movements can lead to lesions at risk of infections and amputation (Laist, 1997; Chiappone et al., 2005; Criddle et al., 2009; Gregory, 2009; NOAA, 2014).
It was estimated that in some areas, ghost fishing might remove up to 30% of commercial species, with a significant economic impact on fisheries (Gilman et al., 2016). The time over which lost fishing gears continue to entangle organisms is highly variable, depending on the location and the gear typology (Erzini, 1997; Matsuoka et al., 2005; Erzini et al., 2008). Derelict gill nets and trammel nets, for instance, are estimated to continue catching marine biota for a period ranging between 30 and 568 days (Matsuoka et al., 2005). However, the impact of ghost-fishing on marine populations is difficult to quantify as an unknown number of marine animals die or are consumed by predators at sea and decompose without being recorded (Katsanevakis and Issaris, 2010). Hence, the effects on the population dynamics and the mortality rates of many affected species are probably underestimated (Katsanevakis and Issaris, 2010). No specific studies were carried out on this issue in the Mediterranean Sea reef systems, and only some descriptive observations are available. An experimental study exists only for shallow waters (i.e., Ayaz et al., 2006).
Coverage
Coverage was mainly linked to fishing gear (in particular nets, Figure 6A) and impacted four Cnidaria species (Corallium rubrum, Eunicella cavolini, Madrepora oculata, and Paramuricea clavata) and one Porifera (Geodia cydonium). The white coral (M. oculata) was also found covered by artificial polymer materials (plastic sheets, bags, and objects, Figure 6B) as well as chemicals (bauxite residues). Coverage can induce stress in sessile organisms (e.g., corals and sponges) by depriving them of light and oxygen. de Carvalho-Souza et al. (2018) reported many examples of reef sites that suffered significant losses of coral cover related to suffocation by macrolitter. Marine litter may cover large portions of the settled communities (Saldanha et al., 2003), impeding the recolonization of large organisms (Galgani et al., 2015), preventing gas exchange and oxygenation, and diminishing the feed capacity of the organisms (Kühn et al., 2015).
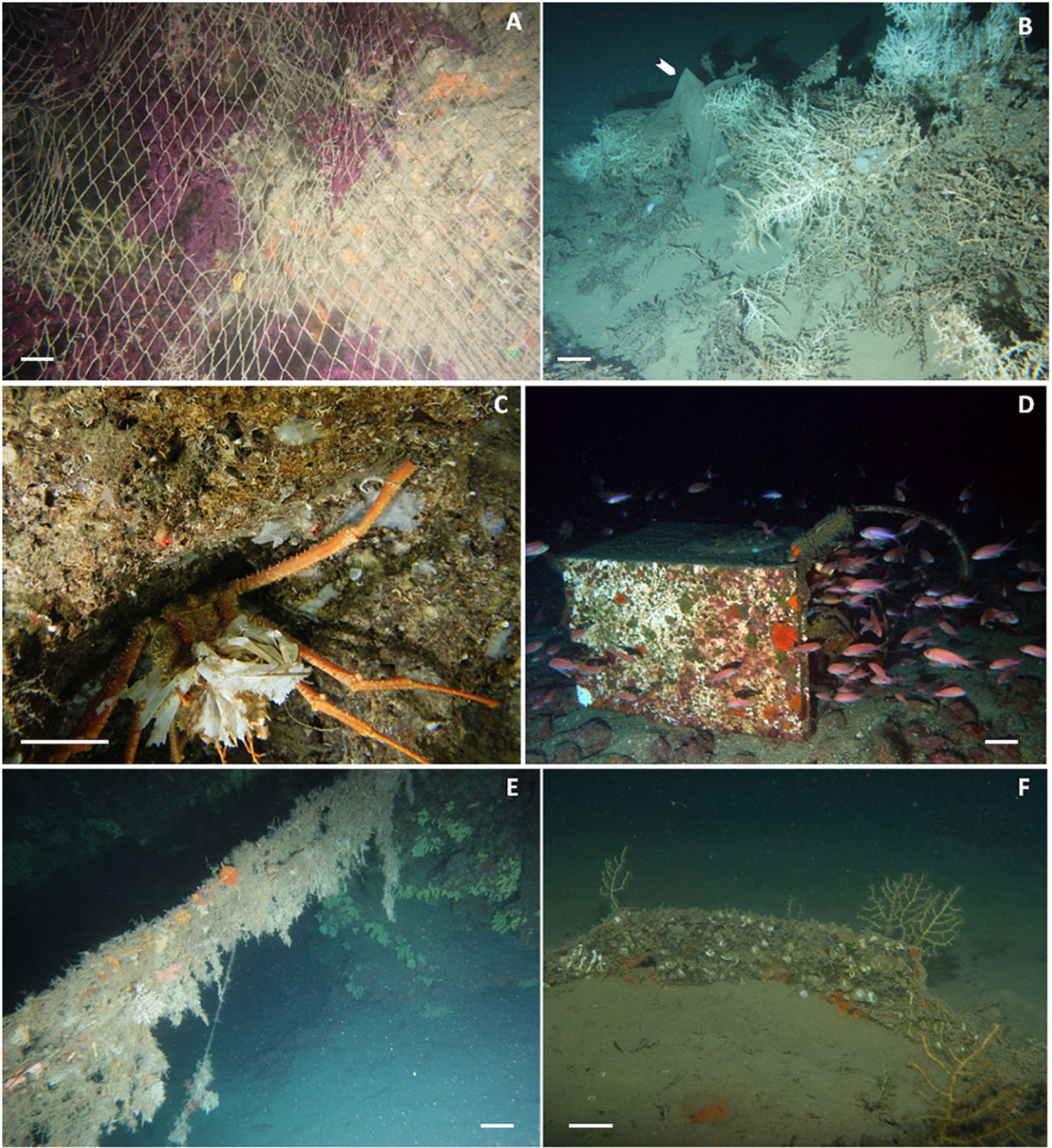
Figure 6. (A) Abandoned or lost net completely covering the sea bottom, suffocating Paramuricea clavata colonies and other sessile organisms; (B) plastic sheet (white arrow) covering colonies of Madrepora oculata; (C) the crustacean Paromola cuvieri carrying plastic on the back, instead of sponges/gorgonians; (D) a washing machine completely covered by encrusting organisms and used by marine goldfish (Anthias anthias) as a refuge; (E) hanging fish net completely overgrown by encrusting and epibenthic organisms such as sponges, hydroids, bryozoans, and ascidians; (F) little gorgonians growing on human-made hard substrata.
Behavioral
Marine litter can also cause behavioral changes in marine organisms and be used as shelter and refuge. We found evidence of 8 fish species using general waste and one species using fishing gears to hide from predators in Mediterranean reefs (Supplementary Table S1). Several authors observed the crab Paromola cuvieri (Angiolillo and Pisapia, 2015; Taviani et al., 2017; Mecho et al., 2018; Angiolillo, 2019; Pierdomenico et al., 2019) to use unusual camouflage shelters carrying on plastic on its exoskeleton, instead of usual sponges or gorgonians (Figure 6C). The high availability of marine litter may indeed result in its use by reef species instead of natural materials, with unknown ecological implications (de Carvalho-Souza et al., 2018). The squat lobster Munida spp. was found hidden in lost fishing gear (Pierdomenico et al., 2018), while some individuals of the shrimp Plesionika spp. were found aggregated on litter accumulations (Pierdomenico et al., 2019).
Some other types of litter such as tires, cans, glass bottles, and larger objects (e.g., washing machines, bins, etc.) can be adopted as shelters and refuge by vagile fauna (Figure 6D). Fishes, or other vagile invertebrates, can take advantage of artificial three-dimensional structures, especially in an otherwise soft-sediment environment (Angiolillo, 2019) or in a degraded environment where natural structuring species functioning as shelter are reduced. Even if these artificial substrata, used as a refuge by organisms, may enhance biodiversity, they interfere with life on the seabed and modify the spatial heterogeneity at different spatial scales, altering the natural environment, the community structure, and possibly ecosystem functioning (Saldanha et al., 2003; UNEP, 2009; Sánchez et al., 2013; de Carvalho-Souza et al., 2018; Angiolillo, 2019).
Other Interactions (Substratum and Incorporation)
Marine litter can also serve as an alternative substratum for sessile species on Mediterranean reefs (Figures 6E,F). Ten cnidarian species were found growing on fishing gears, as well as three Annelida, three Echinodermata, one Mollusca, and one Porifera species (Supplementary Table S1). Also general waste can provide a substratum for benthic reef species (four Cnidaria and one Echinodermata). Moreover, other examples of other interactions with marine litter came from Aymà et al. (2019). They recovered with an Agassiz trawl some fragments of a Desmophyllum pertusum colony growing on a nylon net cords at 752–864 m depth in the Blanes Canyon (Western Mediterranean Sea). Chimienti et al. (2020) observed a steel cable directly within a group of Antipathella subpinnata colonies in the Tremiti Islands MPA. Angiolillo and Canese (2018) observed fishing lines fully incorporated in the yellow scleractinian Dendrophyllia cornigera on the Mantice Shoal in the Ligurian Sea. Savini et al. (2014) found litter incorporated into the skeletons of living coral colonies in the Apulian ridge in the Ionian Sea. We named this interaction – the ability of gorgonians and corals to grow and/or include marine litter in their coral framework – “incorporation.”
Discussion
Globally, de Carvalho-Souza et al. (2018) reported marine litter driven ecological disruptions on 418 reef species belonging to eight reef taxa. The authors found that entanglement and catches in ALDFG represent the most common impacts on marine biota in these environments. In the Mediterranean Sea, the present review reports evidence for 78 taxa impacted by marine litter on reefs (Table 1 and Supplementary Table S1). The large majority belonged to the phylum Cnidaria, followed by Chordata, Arthropoda, Porifera, and Echinodermata. The remaining taxa represented less than 5%. This number is probably underestimated due to the scarcity of specific studies focusing on this topic and the difficulty of working on reefs. The majority of the studies focused on coralligenous and CWC (Figure 7) that are more vulnerable to these types of impacts. No information is available, regarding litter impact, in other reef systems, probably due to the greater sampling effort and increasing scientific interest for the first ones.
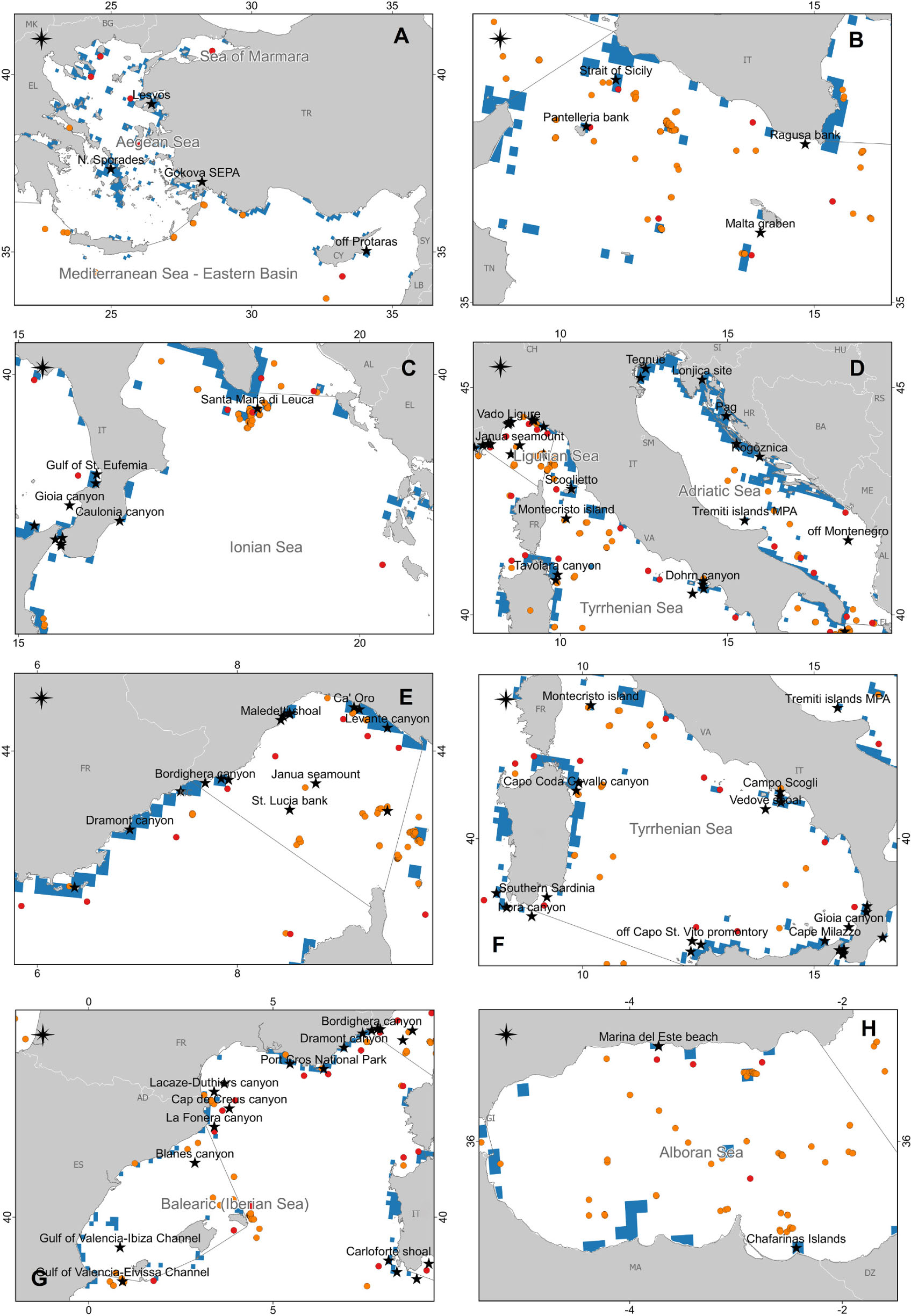
Figure 7. The map reports the case studies collected in this review (black stars) together with the presence of coralligenous areas according to Giakoumi et al. (2013; light blue), and the presence of cold-water corals (CWCs) according to Chimienti et al. (2019; red circles) and Freiwald et al. (2017; orange circles). (A) Aegean and Eastern Mediterranean Seas; (B) Strait of Sicily; (C) Ionian Sea; (D) Adriatic Sea and North Tyrrhenian Sea; (E) Ligurian Sea and the northwestern Mediterranean Sea; (F) South Tyrrhenian Sea; (G) Iberian Sea and the northwestern Mediterranean Sea; (H) Alboran Sea.
A wide diversity of interactions between marine litter and reef organisms was observed in the Mediterranean, including entanglement, coverage, and ghost-fishing. In most cases, impacts were due to ALDFG or other fishery-related waste. The effects of marine litter on animal forests and reef species could be manifold and can be direct (e.g., broken branches for corals and ghost-fishing for fish) or indirect. Parasitic colonization was, for instance, reported by several authors because of the abrasion induced by marine litter. On the other side, some species may take advantage of litter, using it as shelter and refuge.
However, at present, our knowledge of the deleterious effects of marine litter on Mediterranean reefs is limited and heterogeneous in space (Figure 2). Data from the Aegean-Levantine (Figure 7A) and the Adriatic Sea (Figure 7D) subregions are, for instance, very scarce, whereas data from the southern Mediterranean Sea are completely missing (Figure 2). The Aegean Sea is rich in reefs (Giakoumi et al., 2013; Freiwald et al., 2017; Di Camillo et al., 2018; Chimienti et al., 2019) but at the same time, poor in studies regarding marine litter in these habitats (Figure 7A). Now, there is not any routinely monitoring program for seafloor litter on hard substrata in Greece that can fill this gap. The only surveys with ROV are carried out within the frame of research projects (e.g., Ioakeimidis et al., 2015) but not specifically include litter impact on bioconstructions.
This heterogeneous distribution could be due to the greater sampling efforts and technological availability in the western part of the Mediterranean basin respect to the other areas. Only some countries can use non-invasive visual approaches, in particular for deep-sea areas, due to expensive costs. Even if several projects on marine litter have been financed in the last years in Europe (Maes et al., 2019), the major part assessed litter distribution and abundance and not the direct impact on the seafloor and marine organisms, in particular on reefs. In general, the most common approaches to evaluate seafloor litter make use of opportunistic sampling. Litter impact assessments are indeed usually coupled with surveys or programs whose main aim is the study of biodiversity in coral reef assemblages by diving in shallow areas and through ROV in the deep sea since methods for determining seafloor litter distributions can be similar to those used for benthic assessments. The absence of specific monitoring protocols and the different strategies of data collection (sampling methodologies, unit of measures, parameters, etc.) and elaboration have led to obtaining heterogeneous data, often not comparable in a robust way.
The major part of the studies analyzed in this review gives only descriptive information about litter interaction. Very few studies provide a quantification of litter and species abundance and the number of affected individuals (i.e., Angiolillo et al., 2015; Consoli et al., 2019; Enrichetti et al., 2019, 2020). Moreover, several studies have analyzed still images, sub-samples of video surveys, whereas others have analyzed a continuous video to collect quantitative data. Some papers evaluated the occurrence of the impact by considering the percentage of frames showing litter interacting with marine organisms (i.e., Bo et al., 2014, 2020). Thus, there is an increasing need to collect litter data regularly using common templates, harmonized procedures, and joint items categories and types of impacts. Standardization of monitoring approaches and existing datasets is under development across European countries to generate comparable information about the temporal and spatial distribution of marine litter and its impact (Molina Jack et al., 2019). Since the MSFD included marine litter as one of the eleven descriptors to define the GES of European seas (Galgani et al., 2013), seafloor litter data collection has significantly increased in the last years, mainly through trawl-surveys (Maes et al., 2018; Spedicato et al., 2019). Litter items in fishery catches are now regularly recorded in many European countries as part of other environmental monitoring activities (e.g., the MEDITS program; Spedicato et al., 2019). Instead, no uniform programs exist to collect litter data on reefs/hard substrata and to assess/quantify litter harms on marine biota (as requested by D10C4 of the MSFD and Indicator 24 of the EcAp). One of the reasons is due to the not mandatory status of these indicators. Only recently, the MSFD and the UN Environment/MAP Regional Plan on Marine Litter Management in the Mediterranean have begun to take into account the entanglement in their future monitoring. In May 2019, the MSFD TG Litter Working Group (TG-ML) proposed guidelines for the assessment of marine litter interaction and entanglement on benthic organisms using visual methods, applicable to the seafloor and reef systems. This protocol harmonizes the procedures for collecting and reporting marine litter data (distribution, occurrence, abundance, litter, and impact categories) that are gathered on the back of existing biodiversity surveys. The protocol is under review by the TG-ML to provide an accurate methodology applicable for MSFD monitoring to facilitate the identification of sources and trends, data analysis, comparison among countries, etc.
Some effort in this sense has already been made in Italy in the implementation of the first monitoring cycle of the MSFD, in the framework of Descriptor 1 on biodiversity. A unique monitoring protocol was developed to collect data on biodiversity and marine litter simultaneously. The status of populations (species richness, abundance, morphologies, epibiosis, necrosis), litter distribution, and its impact on organisms (focusing in particular on the most structuring species, i.e., corals and sponges), were assessed in coralligenous habitats. In this way, it will be possible to relate the number of entangled individuals/colonies for each structuring species to the total number of individuals/colonies in a defined area. Data are available since 2016 and will represent the first baseline for future comparisons. The data mentioned above could also be used to populate the secondary criteria D10C4 of the MSFD and Candidate Indicator of the IMAP. Indeed, since reef coral communities have a strong potential to be entangled by marine litter, some structuring sessile suspension feeders have already been proposed as indicators. For instance, in 2018, SPA/RAC suggested an approach to study entanglement using benthic invertebrates as indicators of entanglement events, since they offer the possibility of monitoring this impact at a wide range of depths (UNEP/MAP and SPA/RAC, 2018).
Given the difficulties to differentiate entanglement by active fishing gears (by-catch) from entanglement due to ALDFG (ghost-fishing) or other kinds of marine litter for seabird, marine mammals, sea turtles, and fish (Anastasopoulou and Fortibuoni, 2019), Galgani et al. (2018) proposed animal forests as priority elements to monitor the spatio-temporal trends of entanglement in shallow and deep waters. Some previous reviews (de Carvalho-Souza et al., 2018; Anastasopoulou and Fortibuoni, 2019), as well as the present work, indicated cnidarian species as the taxa most affected by entanglement, caused mainly by fishing gear. The vulnerable habitat-forming cnidarian species, characterized by few dominant coral species and by an incredible variety of symbiotic associations, are slow-growing and long-living species (Sheehan et al., 2017) with an extensive distribution and high abundance. They are exposed to marine litter occurring in fishing areas, MPAs, and both coastal and remote areas. The massive and arborescent morphologies of these taxa and their sessile characteristic make them more susceptible to be entangled (Bo et al., 2014; de Carvalho-Souza et al., 2018) and at the same time, allow researchers to obtain an accurate location of the entanglement event, preventing from the misinterpretation in the case of interaction with active fishing gear (by-catch). However, at present, there is not enough data on the differences in entanglement rates among species and life stages, allowing to assess species vulnerability, the frequency of interactions with different marine litter types, and the possible implications in terms of populations. Bo et al. (2014) provided insight into the different responses of some structuring species to physical impacts, depending on the resistance of the coral skeleton, to its morphological and mechanical characteristics. Fragility, recovery ability, as well as reproductive and growth strategies, represent other factors that can determinate different responses to entanglement (MacDonald et al., 1996). The positive correlation between the number of dead colonies and the presence of ALDFG was showed by Angiolillo et al. (2015), indicating the detrimental effects of fisheries. Broken branches, damaged morphologies, and epibiosis can represent secondary consequences of the direct impact during fishing operations, such as the eradication of some specimens from their natural site. Therefore, the sensitivity of a benthic species should be an essential parameter to understand the capability of a species to cope with this impact. For instance, in the north-western Mediterranean Sea, visual surveys have provided evidence of the almost omnipresent incidence of marine litter in all investigated areas, highlighting as in reef systems fishery is a significant source and cause of impact.
Nevertheless, very few studies put in relationship the fishing effort, the rate of losing gear, and the by-catch to assess the entanglement rate (i.e., Enrichetti et al., 2019). Difficulties exist because no official data are available on the patterns of exploitation of artisanal and overall recreational fishing, which contribute in an important way to the impact on reef systems, also in offshore areas. Data on the fishing effort are thus needed to assess this source of impact (e.g., Enrichetti et al., 2019) and define mitigation measures (Bo et al., 2020). An appropriate database targeting fishing effort is needed for small fishery (artisanal and recreational), as suggested by some authors (Bo et al., 2020).
Gear can be lost or discarded due to several intentional and unintentional causes (Richardson et al., 2018), such as contact with other fishing gears, bad weather conditions, tracking systems malfunction, and catching or snagging on submerged features. Fishers can lose their gear also due to improper fishing methods or because of difficulty in retrieving it. On the other hand, gear can be deliberately abandoned when fishers are operating illegally or when the disposal onshore is not practical or economical, especially where reception facilities in the port are unavailable (Gilman, 2015; UNEP, 2015). In the Mediterranean Sea, it was estimated that 0.05% of gillnet and 3.2% of nets were lost per boat per year (Macfadyen et al., 2009). However, the amounts can be highly variable at small spatial scales (Macfadyen et al., 2009; Gilman, 2015).
The high persistence and widespread distribution of fishing gear at sea are due to the use of synthetic, durable, and buoyant materials (Erzini, 1997; Laist, 1997; Sampaio et al., 2012). Moreover, the increasing expansion of fishing effort and fishing grounds determined a wider ALDFG distribution (Macfadyen et al., 2009; Gilardi et al., 2010; Gilman, 2015; Kroodsma et al., 2018). The by-catch of a wide variety of reef species operated by longline and artisanal fishing gear is widely known in the Mediterranean Sea (Mastrototaro et al., 2010; Bo et al., 2014; D’Onghia et al., 2017) and can harm benthic communities (Erzini et al., 1997; Mytilineou et al., 2014; Enrichetti et al., 2019). The analysis of benthic organisms in fishery discards can provide information on reef species sensitivity to fisheries and their probability of being caught in a specific area (Enrichetti et al., 2019). The discard investigation, coupled with the visual approach to assess community structure and the extent of the impact, could represent a useful instrument to gain an overall view of the problem. The identification and mapping of areas where reef species are mostly exposed to the impact of fishing activities (i.e., co-existence of fishing grounds, presence of ALDFG, distribution of sensitive species, probability of encounters between sensitive species and marine litter, etc.) could represent a first step to rationalizing the sampling efforts.
Despite the prevalence of ALDFG, also other types of litter were recorded on biogenic reefs, mainly made of artificial polymer materials (i.e., plastic bottles, bags, and sheets). Oceanographic currents and wind can disperse plastic items over long distances, both vertically and horizontally, depending on plastic lightness, buoyancy, and durability (Watters et al., 2010; Tubau et al., 2015; Vieira et al., 2015). Plastic degrades slowly, and its biological decomposition is negligible. On the seafloor, in particular below the photic zone where the light is absent, and the temperatures and oxygen concentrations are low, the decomposition process is further slowed down. A study carried out in the Saronikos Gulf (Greece) showed that PET bottles can remain intact for approximately 15 years and then began to deteriorate, with the consequent release of chemical compounds (Ioakeimidis et al., 2016). Physical degradation determinates the formation of abundant small plastic fragments, with the consequence of a high persistence of plastic litter, especially on the seafloor (Andrady, 2015; Galgani et al., 2015; Tubau et al., 2015). Another study observed coral polyps ingesting microplastic fragments with detrimental consequences for the colonies (Hall et al., 2015). Therefore, plastic and microplastics represent a significant environmental threat for the marine environment, including reef systems (Lamb et al., 2018).
The other forms of interaction described in this review (colonization, substrate, and behavioral) are under-studied, and they may represent a form of adaptation to a changing environment. The seafloor was considered for a long time an unlimited natural dump for the disposal of any kind of garbage (Ramirez-Llodra et al., 2011; Angiolillo, 2019). Anything that ends in the sea is in a short time colonized and reused. Iconic examples are represented by the wrecks, used as a suitable habitat for a variety of sessile organisms, and as a refuge for vagile fauna. Even if these interactions of organisms with anthropogenic artifacts are often considered neutral, the large evidence of the adaptive behavior of species, such as Paromola cuvieri, should make us reflect on the substantial and irreversible changes that humans are bringing to marine environments.
Conclusion
(1) This review provides evidence that marine litter and specifically ALDFG, threaten Mediterranean reefs. However, the information available is mainly qualitative and geographically unbalanced, and this knowledge gap prevents from quantitatively assessing the phenomenon in a way that could inform policy-makers in MSFD and IMAP implementation. Some areas resulted particularly poor in data and information, i.e., the southern Mediterranean (Figure 2), the Aegean-Levantine Sea (Figure 7A), and the Adriatic Sea subregions (Figure 7D). Thus, an effort should be made to fill these geographical gaps, in particular in the southern basins.
(2) Considering the widespread impact of ALDFG in biogenic reefs, the identification of high exposure risk areas (i.e., fishing grounds), the measure of the impact relating the abundance of litter with entangled target species, the probability of a species to be entangled (sensitivity), the fishing effort, the rates of lost fishing and by-catch are needed for the evaluation of the effects of marine litter on biota.
(3) On the seabed, some invertebrate taxa have a high risk of entanglement and may thus be good candidates for monitoring programs on the temporal and spatial variability of entanglements, giving the possibility to gain long-term data at all depths and significant observations in situ, in particular in areas of intense fishing activity, high density of litter, or high abundance of vulnerable species (Consoli et al., 2018; Galgani et al., 2018).
(4) To date, baseline litter abundance and the effects of marine litter on marine communities and its habitats remain poorly known (Maes et al., 2018) and probably underestimated (de Carvalho-Souza et al., 2018). Litter was present on the seafloor before specific scientific investigations started in the 1990s, and due to the persistence of some litter materials, the monitoring of litter should consider accumulation processes for past decades.
(5) The harmonization of methodologies and regular assessments through opportunistic approaches in long-term benthic biodiversity monitoring is essential to compare data and gain long-term support to the evaluation of accumulation and impacts. Moreover, the development of models on pathways for litter distribution and transfer could represent a useful instrument for tracking litter spread and impact (Galgani et al., 2019).
(6) Several actions are already set on to reduce litter impact, ranging from the removing of litter on the seafloor to the reduction of ALDFG through prevention and mitigation (FAO, 2016). However, to date, these initiatives are mainly limited to shallow waters. The high number of taxa and protected/vulnerable species impacted by marine litter in the Mediterranean Sea reefs, even in the deep sea, indicates that marine biodiversity is under threat. Thus, there is an urgency to implement effective management actions and enforce measures to reduce marine litter inputs.
(7) The impacted reef species are mainly vulnerable and slow-growing species, some of them protected at international levels. It is important to take into account their natural resilience, considering that they may need decades to recover (Clark et al., 2014). Moreover, more complete biodiversity inventories of their spatial distribution are essential to define conservation strategies (Clark et al., 2012; Bo et al., 2020). In particular, the establishment of a network of inshore and offshore protected areas (de Juan and Lleonart, 2010) and the identification of specific fishery restrictions (Bo et al., 2020) to preserve these hotspots of biodiversity and to improve the ecological status of the reef systems, is suggested. All these efforts should be strongly encouraged to develop prevention and mitigation approaches and increase awareness on this topic to preserve and conserve valuable and vulnerable marine ecosystems.
Author Contributions
MA designed the study. MA and TF collected the data, wrote the manuscript, and gave the final approval for publication. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are very grateful to Aikaterini Anastasopoulou from the Hellenic Centre for Marine Research for helping in the bibliographic research and providing information and comments regarding the Aegean Sea. We would like to thank both reviewers for their insightful comments on the paper, as these comments led us to an improvement of the work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.581966/full#supplementary-material
Footnotes
- ^ https://litterbase.awi.de
- ^ http://panaceaweb.adabyron.uma.es/marine-litter/
- ^ www.marinespecies.org
- ^ www.iucnredlist.org
- ^ www.iucn.it
References
Anastasopoulou, A., and Fortibuoni, T. (2019). “Impact of plastic pollution on marine life in the Mediterranean Sea,” in Handbook of Environmental Chemistry, eds F. Stock, G. Reifferscheid, N. Brennholt, and E. Kostianaia (Cham: Springer), 1–12. doi: 10.1007/698_2019_421
Andrady, A. L. (2015). “Persistence of plastic litter in the oceans,” in Marine Anthropogenic Litter, eds M. Bergmann, M. Gutow, and L. Klages (Cham: Springer), 57–72. doi: 10.1007/978-3-319-16510-3
Angiolillo, M. (2019). “Debris in deep water,” in World Seas: an Environmental Evaluation, 2nd Edn, ed. C. Sheppard (Cambridge, MA: Academic Press), 251–268. doi: 10.1016/B978-0-12-805052-1.00015-2
Angiolillo, M., and Canese, S. (2018). “Deep gorgonians and corals of the Mediterranean Sea,” in Corals in a Changing World, eds C. Duque and E. T. Camacho (Rijeka: InTech). doi: 10.5772/intechopen.69686
Angiolillo, M., Lorenzo, B., Farcomeni, A., Bo, M., Bavestrello, G., Santangelo, G., et al. (2015). Distribution and assessment of marine debris in the deep Tyrrhenian Sea (NW Mediterranean Sea, Italy). Mar. Pollut. Bull. 92, 149–159. doi: 10.1016/j.marpolbul.2014.12.044
Asoh, K., Yoshikawa, T., Kosaki, R., and Marschall, E. A. (2004). Damage to cauliflower coral by monofilament fishing lines in Hawaii. Conserv. Biol. 18, 1645–1650. doi: 10.1111/j.1523-1739.2004.00122.x
Avio, C. G., Gorbi, S., and Regoli, F. (2017). Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar. Environ. Res. 128, 2–11. doi: 10.1016/j.marenvres.2016.05.012
Ayaz, A., Acarli, D., Altinagac, U., Ozekinci, U., Kara, A., and Ozen, O. (2006). Ghost fishing by monofilament and multifilament gillnets in Izmir Bay, Turkey. Fish. Res. 79, 267–271. doi: 10.1016/j.fishres.2006.03.029
Ayaz, A., Ünal, V., Acarli, D., and Altinagac, U. (2010). Fishing gear losses in the Gökova Special Environmental Protection Area (SEPA), eastern Mediterranean, Turkey. J. Appl. Ichthyol. 26, 416–419. doi: 10.1111/j.1439-0426.2009.01386.x
Aymà, A., Aguzzi, J., Canals, M., Company, J. B., Lastras, G., Mecho, A., et al. (2019). “Occurrence of living cold-water corals at large depths within submarine canyons of the Northwestern Mediterranean Sea,” in Mediterranean Cold-Water Corals: Past, Present and Future, ed. C. J. C. Orejas (Cham: Springer), 271–284. doi: 10.1007/978-3-319-91608-8_26
Ballesteros, E. (2006). Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr. Mar. Biol. 44, 123–195.
Barnes, D. K. A., Galgani, F., Thompson, R. C., and Barlaz, M. (2009). Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 364, 1985–1998. doi: 10.1098/rstb.2008.0205
Bavestrello, G., Cerrano, C., Zanzi, D., and Cattaneo-Vietti, R. (1997). Damage by fishing activities to the Gorgonian coral Paramuricea clavata in the Ligurian Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 7, 253–262.
Betti, F., Bavestrello, G., Bo, M., Ravanetti, G., Enrichetti, F., Coppari, M., et al. (2020). Evidences of fishing impact on the coastal gorgonian forests inside the Portofino MPA (NW Mediterranean Sea). Ocean Coast. Manag. 187:105105. doi: 10.1016/j.ocecoaman.2020.105105
Betti, F., Bavestrello, G., Fravega, L., Bo, M., Coppari, M., Enrichetti, F., et al. (2019). On the effects of recreational SCUBA diving on fragile benthic species: the Portofino MPA (NW Mediterranean Sea) case study. Ocean Coast. Manag. 182:104926. doi: 10.1016/j.ocecoaman.2019.104926
Bianchi, C. N. (1981). Policheti Serpulidei. Guide per il Riconoscimento Delle specie Animali Delle Acque Lagunari e Costiere Italiane. AQ/1/96. 5. Genova: Consiglio Nazionale delle Ricerche.
Bianchi, C. N., and Morri, C. (2000). Marine biodiversity of the Mediterranean Sea: situation, problems and prospects for future research. Mar. Poll. Bull. 40, 367–376. doi: 10.1016/S0025-326X(00)00027-8
Bo, M., Bava, S., Canese, S., Angiolillo, M., Cattaneo-Vietti, R., and Bavestrello, G. (2014). Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 171, 167–176. doi: 10.1016/j.biocon.2014.01.011
Bo, M., Bavestrello, G., Angiolillo, M., Calcagnile, L., Canese, S., Cannas, R., et al. (2015). Persistence of pristine deep-sea coral gardens in the Mediterranean Sea (SW Sardinia). PLoS One 10:e0119393. doi: 10.1371/journal.pone.0119393
Bo, M., Coppari, M., Betti, F., Massa, F., Gay, G., Cattaneo-Vietti, R., et al. (2020). Unveiling the deep biodiversity of the Janua Seamount (Ligurian Sea): first Mediterranean sighting of the rare Atlantic bamboo coral Chelidonisis aurantiaca Studer, 1890. Deep Res. Part I Oceanogr. Res. Pap. 156:103186. doi: 10.1016/j.dsr.2019.103186
Boudouresque, C. F. (2004). Marine biodiversity in the Mediterranean: status of species, populations and communities. Sci. Rep. Port Cros Natl. 20, 97–146.
Brown, J., and Macfadyen, G. (2007). Ghost fishing in European waters: impacts and management responses. Mar. Policy 31, 488–504. doi: 10.1016/j.marpol.2006.10.007
Butterworth, A., Clegg, I., and Bass, C. (2012). Marine Debris: A Global Picture of the Impact on Animal Welfare and of Animal-Focused Solutions. London: World Society for the Protection of Animals.
Carr, A. (1987). Impact of non degradable marine debris on the ecology and survival outlook of sea turtles. Mar. Pollut. Bull. 18, 352–356. doi: 10.1016/S0025-326X(87)80025-5
Cattaneo-Vietti, R., Bavestrello, G., Bo, M., Canese, S., Vigo, A., and Andaloro, F. (2017). Illegal ingegno fishery and conservation of deep red coral banks in the Sicily Channel (Mediterranean Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 27, 604–616. doi: 10.1002/aqc.2731
Chiappone, M., Dienes, H., Swanson, D. W., and Miller, S. L. (2005). Impacts of lost fishing gear on coral reef sessile invertebrates in the Florida Keys National Marine Sanctuary. Biol. Conserv. 121, 221–230. doi: 10.1016/j.biocon.2004.04.023
Chimienti, G., Bo, M., and Mastrototaro, F. (2018). Know the distribution to assess the changes: mediterranean cold-water coral bioconstructions. Rend. Lincei Sci. Fis. Nat. 29, 583–588. doi: 10.1007/s12210-018-0718-3
Chimienti, G., Bo, M., Taviani, M., and Mastrototaro, F. (2019). “Occurrence and biogeography of mediterranean cold-water corals,” in Mediterranean Cold-Water Corals: Past, Present and Future, ed. C. J. C. Orejas (Springer International Publishing AG), 213–243. doi: 10.1007/978-3-319-91608-8_19
Chimienti, G., De Padova, D., Mossa, M., and Mastrototaro, F. (2020). A mesophotic black coral forest in the Adriatic Sea. Sci. Rep. 10:8504. doi: 10.1038/s41598-020-65266-9
Clark, M. R., Althaus, F., Schlacher, T. A., Williams, A., Bowden, D. A., and Rowden, A. A. (2016). The impacts of deep-sea fisheries on benthic communities: a review. ICES J. Mar. Sci. 73, i51–i69. doi: 10.1093/icesjms/fsv123
Clark, M. R., Rowden, A. A., Schlacher, T. A., Guinotte, J., Dunstan, P. K., Williams, A., et al. (2014). Identifying ecologically or biologically significant areas (EBSA): a systematic method and its application to seamounts in the south Pacific Ocean. Ocean Coast Manag. 91, 65–79. doi: 10.1016/j.ocecoaman.2014.01.016
Clark, M. R., Schlacher, T. A., Rowden, A. A., Stocks, K. I., and Consalvey, M. (2012). Science priorities for seamounts: research links to conservation and management. PLoS One 7:e29232. doi: 10.1371/journal.pone.0029232
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Ben Rais Lasram, F., Aguzzi, J., et al. (2010)). The Biodiversity of the Mediterranean Sea: estimates, Patterns, and Threats. PLoS One 5:e11842. doi: 10.1371/journal.pone.0011842
Consoli, P., Andaloro, F., Altobelli, C., Battaglia, P., Campagnuolo, S., Canese, S., et al. (2018). Marine litter in an EBSA (Ecologically or Biologically Significant Area) of the central Mediterranean Sea: abundance, composition, impact on benthic species and basis for monitoring entanglement. Environ. Pollut. 236, 405–415. doi: 10.1016/j.envpol.2018.01.097
Consoli, P., Scotti, G., Romeo, T., Cristina, M., Esposito, V., Alessandro, M. D., et al. (2019). Characterization of seafloor litter on Mediterranean shallow coastal waters: evidence from Dive Against Debris®, a citizen science monitoring approach. Mar. Pollut. Bull. 150:110763. doi: 10.1016/j.marpolbul.2019.110763
Corcoran, P. L., Moore, C. J., and Jazvac, K. (2014). An anthropogenic marker horizon in the future rock record. GSA Today 24, 4–8. doi: 10.1130/GSAT-G198A.1
Costello, M. J., Coll, M., Danovaro, R., Halpin, P., Ojaveer, H., and Miloslavich, P. (2010). A census of marine biodiversity knowledge, resources, and future challenges. PLoS One 5:e12110. doi: 10.1371/journal.pone.001211
Criddle, K. R., Amos, A. F., Carroll, P., Coe, J. M., Donohue, M. J., Harris, J. H., et al. (2009). Tackling Marine Debris in the 21st Century. Washington DC: The National Academies Press.
Davies, J. S., Guillaumont, B., Tempera, F., Vertino, A., Beuck, L., Ólafsdóttir, S. H., et al. (2017). A new classification scheme of European cold-water coral habitats: implications for ecosystem-based management of the deep sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 145, 102–109. doi: 10.1016/j.dsr2.2017.04.014
de Carvalho-Souza, G. F., Llope, M., Tinôco, M. S., Medeiros, D. V., Maia-Nogueira, R., and Sampaio, C. L. S. (2018). Marine litter disrupts ecological processes in reef systems. Mar. Pollut. Bull. 133, 464–471. doi: 10.1016/j.marpolbul.2018.05.049
de Juan, S., and Lleonart, J. (2010). A conceptual framework for the protection of vulnerable habitats impacted by fishing activities in the Mediterranean high seas. Ocean Coast Manag. 53, 717–723. doi: 10.1016/j.ocecoaman.2010.10.005
Deidun, A., Andaloro, F., Bavestrello, G., Canese, S., Consoli, P., Micallef, A., et al. (2015). First characterisation of a Leiopathes glaberrima (Cnidaria: Anthozoa: Antipatharia) forest in Maltese exploited fishing grounds. Ital. J. Zool. 82, 271–280. doi: 10.1080/11250003.2014.986544
Deudero, S., and Alomar, C. (2015). Mediterranean marine biodiversity under threat: reviewing influence of marine litter on species. Mar. Pollut. Bull. 98, 58–68. doi: 10.1016/j.marpolbul.2015.07.012
Di Camillo, C. G., Ponti, M., Bavestrello, G., Krzelj, M., and Cerrano, C. (2018). Building a baseline for habitat-forming corals by a multi-source approach, including Web Ecological Knowledge. Biodivers. Conserv. 27, 1257–1276. doi: 10.1007/s10531-017-1492-8
D’Onghia, G., Calculli, C., Capezzuto, F., Carlucci, R., Carluccio, A., Grehan, A., et al. (2017). Anthropogenic impact in the Santa Maria di Leuca cold-water coral province (Mediterranean Sea): observations and conservation straits. Deep Sea Res. Part II Top. Stud. Oceanogr. 145, 87–101. doi: 10.1016/j.dsr2.2016.02.012
D’Onghia, G., Capezzuto, F., Cardone, F., Carlucci, R., Carluccio, A., Chimienti, G., et al. (2015). Macro- and megafauna recorded in the submarine Bari Canyon (southern Adriatic, Mediterranean Sea) using different tools. Medit. Mar. Sci. 16, 180–196. doi: 10.12681/mms.1082
D’Onghia, G., Maiorano, P., Sion, L., Giove, A., Capezzuto, F., Carlucci, R., et al. (2010). Effects of deep-water coral banks on the abundance and size structure of the megafauna in the Mediterranean Sea. Deep Sea Res. II 57, 397–411. doi: 10.1016/j.dsr2.2009.08.022
Enrichetti, F., Bava, S., Bavestrello, G., Betti, F., Lanteri, L., and Bo, M. (2019). Artisanal fishing impact on deep coralligenous animal forests: a Mediterranean case study of marine vulnerability. Ocean Coast. Manag. 177, 112–126. doi: 10.1016/j.ocecoaman.2019.04.021
Enrichetti, F., Dominguez-Carrió, C., Toma, M., Bavestrello, G., Canese, S., and Bo, M. (2020). Assessment and distribution of seafloor litter on the deep Ligurian continental shelf and shelf break (NW Mediterranean Sea). Mar. Pollut. Bull. 151:110872. doi: 10.1016/j.marpolbul.2019.110872
Erzini, K. (1997). An experimental study of gill net and trammel net “ghost fishing” off the Algarve (southern Portugal). Mar. Ecol. Prog. Ser. 158, 257–265. doi: 10.3354/meps158257
Erzini, K., Bentes, L., Coelho, R., Lino, P. G., Monteiro, P., Ribeiro, J., et al. (2008). Catches in ghost-fishing octopus and fish traps in the northeastern Atlantic Ocean (Algarve, Portugal). Fish. Bull. 106, 321–327.
Erzini, K., Monteiro, C. C., Ribeiro, J., Santos, M. N., Gaspar, M., Monteiro, P., et al. (1997). An experimental study of gill net and trammel net “ghost fishing” off the Algarve (southern Portugal). Mar. Ecol. Prog. Ser. 158, 257–265. doi: 10.3354/meps158257
Fabri, M. C., Pedel, L., Beuck, L., Galgani, F., Hebbeln, D., Freiwald, A., et al. (2014). Megafauna of vulnerable marine ecosystems in French Mediterranean submarine canyons: spatial distribution and anthropogenic impacts. Deep Res. Part II Top. Stud. Oceanogr. 104, 184–207. doi: 10.1016/j.dsr2.2013.06.016
Falace, A., Kaleb, S., Curiel, D., Miotti, C., Galli, G., Querin, S., et al. (2015). Calcareous bio-concretions in the Northern Adriatic Sea: habitat types, environmental factors that influence Habitat distributions, and predictive modeling. PLoS One 10:e0140931. doi: 10.1371/journal.pone.0140931
FAO (2016). Abandoned, Lost and Discarded Gillnets and Trammel Nets: Methods to Estimate Ghost Fishing Mortality, and the Status of Regional Monitoring and Management, by Eric Gilman, Francis Chopin, Petri Suuronen and Blaise Kuemlangan. FAO Fisheries and Aquaculture Technical Paper No. 600. Rome: FAO.
Fortibuoni, T., Ronchi, F., Mačić, V., Mandić, M., Mazziotti, C., Peterlin, M., et al. (2019). A harmonized and coordinated assessment of the abundance and composition of seafloor litter in the Adriatic-Ionian macroregion (Mediterranean Sea). Mar. Pollut. Bull. 139, 412–426. doi: 10.1016/j.marpolbul.2019.01.017
Fosså, J. H., Mortensen, P. B., and Furevik, D. M. (2002). The deep-water coral Lophelia pertusa in Norwegian waters: distribution and fishery impacts. Hydrobiologia 471, 1–12. doi: 10.1023/A:1016504430684
Freiwald, A., Beuck, L., Ruggeberg, A., Taviani, M., and Hebbeln, D. (2009). The white coral community in the central Mediterranean sea revealed by ROV surveys. Oceanography 22, 58–74.
Freiwald, A., Rogers, A., Hall-Spencer, J., Guinotte, J. M., Davies, A. J., et al. (2017). Global Distribution of Cold-Water Corals (version 5.0). Fifth Update to the Dataset in Freiwald et al. (2004) by UNEP-WCMC, in Collaboration with Andre Freiwald and John Guinotte. Cambridge: UN Environment World Conservation Monitoring Centre.
Galgani, F., Hanke, G., and Maes, T. (2015). “Global distribution, composition and abundance of marine litter,” in Marine anthropogenic Litter, eds M. Bergmann, L. Gutow, and M. Klages (Berlin: Springer Open), 29–56.
Galgani, F., Hanke, G., Werner, S., and De Vrees, L. (2013). Marine litter within the European Marine Strategy Framework Directive. ICES J. Mar. Sci. 70, 1055–1064. doi: 10.1093/icesjms/fst122
Galgani, F., Leaute, J. P., Moguedet, P., Souplet, A., Verin, Y., Carpentier, A., et al. (2000). Litter on the sea floor along European coasts. Mar. Pollut. Bull. 40, 516–527. doi: 10.1016/S0025-326X(99)00234-9
Galgani, F., Pham, C. K., Claro, F., and Consoli, P. (2018). Marine animal forests as useful indicators of entanglement by marine litter. Mar. Pollut. Bull. 135, 735–738. doi: 10.1016/j.marpolbul.2018.08.004
Galgani, F., Souplet, A., and Cadiou, Y. (1996). Accumulation of debris on the deep sea floor off the French Mediterranean coast. Mar. Ecol. Ser. 142, 225–234. doi: 10.3354/meps142225
Galgani, L., Beiras, R., Galgani, F., Panti, C., and Borja, A. (2019). Editorial: “impacts of marine litter.”. Front. Mar. Sci. 6:208. doi: 10.3389/fmars.2019.00208
Gall, S. C., and Thompson, R. C. (2015). The impact of debris on marine life. Mar. Pollut. Bull. 92, 170–179. doi: 10.1016/j.marpolbul.2014.12.041
Garrabou, J., and Ballesteros, E. (2000). Growth of Mesophyllum alternans and Lithophyllum frondosum (Corallinales, Rhodophyta) in the northwestern Mediterranean. Eur. J. Phycol. 35, 1–10. doi: 10.1080/09670260010001735571
Giakoumi, S., Sini, M., Gerovasileiou, V., Mazor, T., Beher, J., Possingham, H. P., et al. (2013). Ecoregion-based conservation planning in the mediterranean: dealing with large-scale heterogeneity. PLoS One 8:e76449. doi: 10.1371/journal.pone.0076449
Gilardi, K. V. K., Carlson-Bremer, D., June, J. A., Antonelis, K., Broadhurst, G., and Cowan, T. (2010). Marine species mortality in derelict fishing nets in Puget Sound, WA and the cost/benefits of derelict net removal. Mar. Pollut. Bull. 60, 376–382. doi: 10.1016/j.marpolbul.2009.10.016
Gilman, E. (2015). Status of international monitoring and management of abandoned, lost and discarded fishing gear and ghost fishing. Mar. Policy 60, 225–239. doi: 10.1016/j.marpol.2015.06.016
Gilman, E., Chopin, F., Suuronen, P., and Kuemlangan, B. (2016). Abandoned, Lost and Discarded Gillnets and Trammel Nets. Methods to Estimate Ghost Fishing Mortality, and Status of Regional Monitoring and Management. FAO Fisheries and Aquaculture Technical Paper 600. Rome: Food and Agriculture Organization of the United Nations, 79.
Giusti, M., Canese, S., Fourt, M., Bo, M., Innocenti, C., Goujard, A., et al. (2019). Coral forests and Derelict Fishing Gears in submarine canyon systems of the Ligurian Sea. Prog. Oceanogr. 178:102186. doi: 10.1016/j.pocean.2019.102186
Goffredo, S., Caroselli, E., Gasparini, G., Marconi, G., Putignano, M. T., Pazzini, C., et al. (2011). Colony and polyp biometry and size structure in the orange coral Astroides calycularis (Scleractinia: Dendrophylliidae). Mar. Biol. Res. 7, 272–280. doi: 10.1080/17451000.2010.492222
Good, T. P., June, J. A., Etnier, M. A., and Broadhurst, G. (2010). Derelict fishing nets in Puget Sound and the Northwest Straits: patterns and threats to marine fauna. Mar. Pollut. Bull. 60, 39–50. doi: 10.1016/j.marpolbul.2009.09.005
Gregory, M. R. (2009). Environmental implications of plastic debris in marine settings—entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 364, 2013–2025. doi: 10.1098/rstb.2008.0265
Hall, N. M., Berry, K. L. E., Rintoul, L., and Hoogenboom, M. O. (2015). Microplastic ingestion by scleractinian corals. Mar. Biol. 162, 725–732. doi: 10.1007/s00227-015-2619-7
Harmelin, J.-G., and Marinopoulos, J. (1994). Population structure and partial mortality of the gorgonian Paramuricea clavata (Risso) in the northwestern Mediterranean (France, Port-Cros Island). Mar. Life 4, 5–13.
Hess, N. A., Ribic, C. A., and Vining, I. (1999). Benthic marine debris, with an emphasis on fishery-related items, surrounding Kodiak Island, Alaska, 1994-1996. Mar. Pollut. Bull. 38, 885–890. doi: 10.1016/S0025-326X(99)00087-9
Houard, T., Boudouresque, C. F., Barcelo, A., Cottalorda, J., Formentin, J., Jullian, E., et al. (2012). Occurrence of a lost fishing net within the marine area of the Port-Cros National Park (Provence, northwestern Mediterranean Sea). Sci. Rep. Port Cros Natl. 118, 109–118.
Ingrosso, G., Abbiati, M., Badalamenti, F., Bavestrello, G., Belmonte, G., Cannas, R., et al. (2018). Mediterranean Bioconstructions along the Italian Coast. Adv. Mar. Biol. 79, 61–136. doi: 10.1016/bs.amb.2018.05.001
Ioakeimidis, C., Fotopoulou, K. N., Karapanagioti, H. K., Geraga, M., Zeri, C., Papathanassiou, E., et al. (2016). The degradation potential of PET bottles in the marine environment: an ATR-FTIR based approach. Sci. Rep. 6:23501. doi: 10.1038/srep23501
Ioakeimidis, C., Papatheodorou, G., Fermeli, G., Streftaris, N., and Papathanassiou, E. (2015). Use of ROV for assessing marine litter on the seafloor of Saronikos Gulf (Greece): a way to fill data gaps and deliver environmental education. Springerplus 4:463. doi: 10.1186/s40064-015-1248-4
Jambeck, J. R., Geyer, R., Wilcox, C., Siegler, T. R., Perryman, M., Andrady, A., et al. (2015). Plastic waste inputs from land into the ocean. Science 347, 768–771. doi: 10.1126/science.1260352
Katsanevakis, S., and Issaris, Y. (2010). Impact of marine litter on sea life: a review. Rapp. Comm. Int. Mer. Médit. 557.
Keller, A. A., Fruh, E. L., Johnson, M. M., Simon, V., and McGourty, C. (2010). Distribution and abundance of anthropogenic marine debris along the shelf and slope of the US West Coast. Mar. Pollut. Bull. 60, 692–700. doi: 10.1016/j.marpolbul.2009.12.006
Kipson, S., Linares, C., Čižmek, H., Cebrián, E., Ballesteros, E., Bakran-Petricioli, T., et al. (2015). Population structure and conservation status of the red gorgonian Paramuricea clavata (Risso, 1826) in the Eastern Adriatic Sea. Mar. Ecol. 36, 982–993. doi: 10.1111/maec.12195
Koutsodendris, A., Papatheodorou, G., Kougiourouki, O., and Georgiadis, M. (2008). Benthic marine litter in four Gulfs in Greece, Eastern Mediterranean; abundance, composition and source identification. Estuar. Coast. Shelf Sci. 77, 501–512. doi: 10.1016/j.ecss.2007.10.011
Kroodsma, D. A., Mayorga, J., Hochberg, T., Miller, N. A., Boerder, K., Ferretti, F., et al. (2018). Tracking the global footprint of fisheries. Science 359, 904–908. doi: 10.1126/science.aao5646
Kühn, S., Bravo Rebolledo, E. L., and van Franeker, J. A. (2015). “Deleterious effects of litter on marine life,” in Marine Anthropogenic Litter, eds M. Bergmann, L. Gutow, and M. Klages (Cham: Springer), 75–116. doi: 10.1007/978-3-319-16510-3_4
Laist, D. W. (1997). “Impacts of marine debris: entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records,” in Marine Debris Sources, Impacts and Solutions, eds J. M. Coe and D. B. Rogers (New York, NY: Springer), 99–413. doi: 10.1007/978-1-4613-8486-1
Lamb, J. B., Willis, B. L., Fiorenza, E. A., Couch, C. S., Howard, R., Rader, D. N., et al. (2018). Plastic waste associated with disease on coral reefs. Science 359, 460–462. doi: 10.1126/science.aar3320
Lee, D. I., Cho, H. S., and Jeong, S. B. (2006). Distribution characteristics of marine litter on the sea bed of the East China Sea and the South Sea of Korea. Estuar. Coast. Shelf Sci. 70, 187–194. doi: 10.1016/j.ecss.2006.06.003
Li, W. C., Tse, H. F., and Fok, L. (2016). Plastic waste in the marine environment: a review of sources, occurrence and effects. Sci. Total Environ. 566–567, 333–349. doi: 10.1016/j.scitotenv.2016.05.084
Lo Iacono, C., Robert, K., Gonzalez-Villanueva, R., Gori, A., Gili, J. M., and Orejas, C. (2018). Predicting cold-water coral distribution in the Cap de Creus Canyon (NW Mediterranean): implications for marine conservation planning. Prog. Oceanogr. 169, 169–180. doi: 10.1016/j.pocean.2018.02.012
Ma, Y., Halsall, C. J., Crosse, J. D., Graf, C., Cai, M., He, J., et al. (2015). Persistent organic pollutants in ocean sediments from the North Pacific to the Arctic Ocean. J. Geophys. Res. Oceans 120, 2723–2735.
MacDonald, D. S., Little, M., Eno, N. C., and Hiscock, K. (1996). Disturbance of benthic species by fishing activities: a sensitivity index. Aquat. Conserv. Mar. Freshw. Ecosyst. 6, 257–268.
Macfadyen, G., Huntington, T., and Cappell, R. (2009). Abandoned, Lost or Otherwise Discarded Fishing Gear. UNEP Regional Seas Reports and Studies No.185; FAO Fisheries and Aquaculture Technical Paper, No. 523. Rome: FAO.
Madurell, T., Orejas, C., Requena, S., Gori, A., Purroy, A., Lo Iacono, C., et al. (2012). “The benthic communities of the Cap de Creus canyon,” in Mediterranean Submarine Canyons: Ecology and Governance, eds M. Würtz, et al. (Gland: IUCN), 123–132.
Maes, T., Barry, J., Leslie, H. A., Vethaak, A. D., Nicolaus, E. E. M., Law, R. J., et al. (2018). Below the surface: twenty-five years of seafloor litter monitoring in coastal seas of North West Europe (1992–2017). Sci. Total Environ. 630, 790–798. doi: 10.1016/j.scitotenv.2018.02.245
Maes, T., Perry, J., Aliji, K., Clarke, C., and Birchenough, S. N. R. (2019). Shades of grey: marine litter research developments in Europe. Mar. Pollut. Bull. 146, 274–281. doi: 10.1016/j.marpolbul.2019.06.019
Maldonado, M., Aguilar, R., Blanco, J., García, S., Serrano, A., and Punzón, A. (2015). Aggregated clumps of lithistid sponges: A singular, reef-like bathyal habitat with relevant paleontological connections. PLoS One 10:e0125378. doi: 10.1371/journal.pone.0125378
Maldonado, M., López-Acosta, M., Sánchez-Tocino, L., and Sitjá, C. (2013). The rare, giant gorgonian Ellisella paraplexauroides: demographics and conservation concerns. Mar. Ecol. Progr. Ser. 479, 127–141. doi: 10.3354/meps10172
Mastrototaro, F., D’Onghia, G., Corriero, G., Matarrese, A., Maiorano, P., Panetta, P., et al. (2010). Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): An update. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 412–430. doi: 10.1016/j.dsr2.2009.08.021
Melli, V., Angiolillo, M., Ronchi, F., Canese, S., Giovanardi, O., Querin, S., et al. (2017). The first assessment of marine debris in a Site of Community Importance in the north-western Adriatic Sea (Mediterranean Sea). Mar. Pollut. Bull. 114, 821–830. doi: 10.1016/j.marpolbul.2016.11.012
Matsuoka, K., Nakashima, T., and Nagasawa, N. (2005). A review of ghost fishing: scientific approaches to evaluation and solutions. Fish. Sci. 71, 691–702. doi: 10.1111/j.1444-2906.2005.01019.x
Mecho, A., Aguzzi, J., De Mol, B., Lastras, G., Ramirez-Llodra, E., Bahamon, N., et al. (2018). Visual faunistic exploration of geomorphological human-impacted deep-sea areas of the north-western Mediterranean Sea. J. Mar. Biol. Assoc. U.K. 98, 1241–1252. doi: 10.1017/S0025315417000431
Micheli, F., Halpern, B. S., Walbridge, S., Ciriaco, S., Ferretti, F., Fraschetti, S., et al. (2013). Cumulative human impacts on Mediterranean and Black Sea marine ecosystems: assessing current pressures and opportunities. PLoS One 8:e79889. doi: 10.1371/journal.pone.0079889
Miyake, H., Shibata, H., and Furushima, Y. (2011). “Deep-sea litter study using deep-sea observation tools,” in Interdisciplinary Studies on Environmental Chemistry-Marine Environmental Modeling & Analysis, eds K. Omori, X. Guo, N. Yoshie, N. Fujii I, C. Handoh, A. Isobe, et al. (Setagaya-ku: TERRAPUB).
Moccia, D., Cau, A., Alvito, A., Canese, S., Cannas, R., Bo, M., et al. (2019). New sites expanding the “Sardinian cold-water coral province” extension: a new potential cold-water coral network? Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 153–160. doi: 10.1002/aqc.2975
Molina Jack, M. E., Chaves Montero, M., del, M., Galgani, F., Giorgetti, A., Vinci, M., et al. (2019). EMODnet marine litter data management at pan-European scale. Ocean Coast. Manag. 181:104930. doi: 10.1016/j.ocecoaman.2019.104930
Moore, C. J. (2008). Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res. 108, 131–139. doi: 10.1016/j.envres.2008.07.025
Moore, S. L., and Allen, M. J. (2000). Distribution of anthropogenic and natural debris on the mainland shelf of the Southern California Bight. Mar. Pollut. Bull. 40, 83–88. doi: 10.1016/S0025-326X(99)00175-7
Morand, S., and Lajaunie, C. (2018). “A brief history on the links between health and biodiversity,” in Biodiversity and Health, 1st Edn, eds S. Morand and C. Lajaunie (London: Iste Press), 1–14. doi: 10.1016/b978-1-78548-115-4.50001-9
Moschino, V., Riccato, F., Fiorin, R., Nesto, N., Picone, M., Boldrin, A., et al. (2019). Is derelict fishing gear impacting the biodiversity of the Northern Adriatic Sea? An answer from unique biogenic reefs. Sci. Total Environ. 663, 387–399. doi: 10.1016/j.scitotenv.2019.01.363
Mytilineou, C., Smith, C. J., Anastasopoulou, A., Papadopoulou, K. N., Christidis, G., Bekas, P., et al. (2014). New cold-water coral occurrences in the Eastern Ionian Sea: results from experimental long line fishing. Deep Sea Res. Part II Top. Stud. Oceanogr. 99, 146–157. doi: 10.1016/j.dsr2.2013.07.007
Neves, D., Sobral, P., Ferreira, J. L., and Pereira, T. (2015). Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 101, 119–126. doi: 10.1016/j.marpolbul.2015.11.008
NOAA (2014). Report on the Entanglement of Marine Species in Marine Debris with an Emphasis on Species in the United States. Available online at: www.MarineDebris.noaa.gov (accessed March 5, 2020).
Orejas, C., Gori, A., Lo Iacono, C., Puig, P., Gili, J. M., and Dale, M. R. T. (2009). Cold-water corals in the Cap de Creus canyon, northwestern Mediterranean: spatial distribution, density and anthropogenic impact. Mar. Ecol. Prog. Ser. 397, 37–51. doi: 10.3354/meps08314
Pasquini, G., Ronchi, F., Strafella, P., Scarcella, G., and Fortibuoni, T. (2016). Seabed litter composition, distribution and sources in the Northern and Central Adriatic Sea (Mediterranean). Waste Manag. 58, 41–51. doi: 10.1016/j.wasman.2016.08.038
Piazzi, L., Gennaro, P., and Balata, D. (2012). Threats to macroalgal coralligenous assemblages in the Mediterranean Sea. Mar. Pollut. Bull. 64, 2623–2629. doi: 10.1016/j.marpolbul.2012.07.027
Pierdomenico, M., Cardone, F., Carluccio, A., Casalbore, D., Chiocci, F., Maiorano, P., et al. (2019). Megafauna distribution along active submarine canyons of the central Mediterranean: relationships with environmental variables. Prog. Oceanogr. 171, 49–69. doi: 10.1016/j.pocean.2018.12.015
Pierdomenico, M., Russo, T., Ambroso, S., Gori, A., Martorelli, E., D’Andrea, L., et al. (2018). Effects of trawling activity on the bamboo-coral Isidella elongata and the sea pen Funiculina quadrangularis along the Gioia Canyon (Western Mediterranean, southern Tyrrhenian Sea). Prog. Oceanogr. 169, 214–226. doi: 10.1016/j.pocean.2018.02.019
Ramírez, F., Coll, M., Navarro, J., Bustamante, J., and Green, A. J. (2018). Spatial congruence between multiple stressors in the Mediterranean Sea may reduce its resilience to climate impacts. Sci. Rep. 8:14871. doi: 10.1038/s41598-018-33237-w
Ramirez-Llodra, E., De Mol, B., Company, J. B., Coll, M., and Sardà, F. (2013). Effects of natural and anthropogenic processes in the distribution of marine litter in the deep Mediterranean Sea. Prog. Oceanogr. 118, 273–287. doi: 10.1016/j.pocean.2013.07.027
Ramirez-Llodra, E., Tyler, P. A., Baker, M. C., Bergstad, O. A., Clark, M. R., Escobar, E., et al. (2011). Man and the Last Great Wilderness: human Impact on the Deep Sea. PLoS One 6:e22588. doi: 10.1371/journal.pone.0022588
Richardson, K., Asmutis-Silvia, R., Drinkwin, J., Gilardi, K. V. K., Giskes, I., Jones, G., et al. (2019). Building evidence around ghost gear: global trends and analysis for sustainable solutions at scale. Mar. Pollut. Bull. 138, 222–229. doi: 10.1016/j.marpolbul.2018.11.031
Richardson, K., Gunn, R., Wilcox, C., and Hardesty, B. D. (2018). Understanding causes of gear loss provides a sound basis for fisheries management. Mar. Policy 96, 278–284. doi: 10.1016/j.marpol.2018.02.021
Ronchi, F., Galgani, F., Binda, F., Mandić, M., Peterlin, M., Tutman, P., et al. (2019). Fishing for Litter in the Adriatic-Ionian macroregion (Mediterranean Sea): strengths, weaknesses, opportunities and threats. Mar. Policy 100, 226–237. doi: 10.1016/j.marpol.2018.11.041
Rossi, S. (2013). The destruction of the “animal forests” in the oceans: towards an over-simplification of the benthic ecosystems. Ocean Coast. Manag. 84, 77–85. doi: 10.1016/j.ocecoaman.2013.07.004
Rossi, S., Bramanti, L., Gori, A., and Orejas, C. (2017). “An overview of the animal forests of the world,” in Marine Animal Forests, The Ecology of Benthic Biodiversity Hotspots, eds S. Rossi, L. Bramanti, A. Gori, and C. Orejas (Cham: Springer), 1–26. doi: 10.1007/978-3-319-17001-5_1-1
Rosso, A., Vertino, A., Di Geronimo, I., Sanfilippo, R., Sciuto, F., Di Geronimo, R., et al. (2010). Hard- and soft-bottom thanatofacies from the Santa Maria di Leuca deep-water coral province, Mediterranean. Deep Sea Res. 57, 360–379. doi: 10.1016/j.dsr2.2009.08.024
Saldanha, H. J., Sancho, G., Santos, M. N., Puente, E., Gaspar, M. B., Bilbao, A., et al. (2003). The use of biofouling for ageing lost nets: a case study. Fish. Res. 64, 141–150. doi: 10.1016/S0165-7836(03)00213-3
Salomidi, M., Smith, C., Katsanevakis, S., and Panayotidis, P. (2009). “Some observations on the structure and distribution of gorgonian assemblages in the Eastern Mediterranean Sea,” in Proceedings of the 1er Symposium sur la Conservation du Coralligène et autres bio concrétions de Méditerranée, Tabarka, 16–17.
Sampaio, I., Braga-Henriques, A., Pham, C., Ocaña, O., De Matos, V., Morato, T., et al. (2012). Cold-water corals landed by bottom longline fisheries in the Azores (north-eastern Atlantic). J. Mar. Biol. Assoc. U.K. 92, 1547–1555. doi: 10.1017/S0025315412000045
Sánchez, P., Masó, M., Sáez, R., De Juan, S., Muntadas, A., and Demestre, M. (2013). Baseline study of the distribution of marine debris on soft-bottom habitats associated with trawling grounds in the northern Mediterranean. Sci. Mar. 77, 247–255. doi: 10.3989/scimar03702.10A
Savini, A., Vertino, A., Marchese, F., Beuck, L., and Freiwald, A. (2014). Mapping cold-water coral habitats at different scales within the Northern Ionian Sea (central Mediterranean): an assessment of coral coverage and associated vulnerability. PLoS One 9:e87108. doi: 10.1371/journal.pone.0087108
Sbrescia, L., Di Stefano, F., Russo, M., and Russo, G. F. (2008). Influence of sport fishing on gorgonians in MPA of Punta Campanella. Biol. Mar. Mediterr. 15, 172–173.
Sheehan, E., Rees, A., Bridger, D., Williams, T., and Hall-Spencer, J. M. (2017). Strandings of NE Atlantic gorgonians. Biol. Conserv. 209 482–487. doi: 10.1016/j.biocon.2017.03.020
Spedicato, M. T., Zupa, W., Carbonara, P., Fiorentino, F., Follesa, M. C., Galgani, F., et al. (2019). Spatial distribution of marine macro-litter on the seafloor in the northern Mediterranean Sea: the MEDITS initiative. Sci. Mar. 83, 257–270. doi: 10.3989/scimar.04987.14A
Spengler, A., and Costa, M. F. (2008). Methods applied in studies of benthic marine debris. Mar. Pollut. Bull. 56, 226–230. doi: 10.1016/j.marpolbul.2007.09.040
Stefatos, A., Charalampakis, M., Papatheodorou, G., and Ferentinos, G. (1999). Marine debris on the seafloor of the Mediterranean Sea: examples from two enclosed gulfs in Western Greece. Mar. Pollut. Bull. 38, 389–393. doi: 10.1016/S0025-326X(98)00141-6
Taviani, M., Angeletti, L., Canese, S., Cannas, R., Cardone, F., Cau, A. B., et al. (2017). The “Sardinian cold-water coral province” in the context of the Mediterranean coral ecosystems. Deep Sea Res. Part II Top. Stud. Oceanogr. 145, 61–78. doi: 10.1016/j.dsr2.2015.12.008
Terrón-Sigler, A. (2015). Conservation Biology of the Endangered Orange Coral Astroides Calycularis. [Ph.D. thesis]. Seville: The University of Seville.
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., et al. (2004). Lost at sea: Where is all the plastic? Science 304:838. doi: 10.1126/science.1094559
Tosi, L., Zecchin, M., Franchi, F., Bergamasco, A., Da Lio, C., Baradello, L., et al. (2017). Paleochannel and beach-bar palimpsest topography as initial substrate for coralligenous buildups offshore Venice, Italy. Sci. Rep. 7:1321. doi: 10.1038/s41598-017-01483-z
Tsounis, G., Martinez, L., Bramanti, L., Viladrich, N., Gili, J. M., Martinez, Á, et al. (2012). Anthropogenic effects on reproductive effort and allocation of energy reserves in the Mediterranean octocoral Paramuricea clavata. Mar. Ecol. Prog. Ser. 449, 161–172. doi: 10.3354/meps09521
Tubau, X., Canals, M., Lastras, G., Rayo, X., Rivera, J., and Amblas, D. (2015). Marine litter on the floor of deep submarine canyons of the Northwestern Mediterranean Sea: the role of hydrodynamic processes. Prog. Oceanogr. 134, 379–403. doi: 10.1016/j.pocean.2015.03.013
Tursi, A., Mastrototaro, F., Matarrese, A., Maiorano, P., and D’Onghia, G. (2004). Biodiversity of the white coral reefs in the Ionian Sea (Central Mediterranean). Chem. Ecol. 20(Suppl.), 107–116. doi: 10.1080/02757540310001629170
UNEP/MAP, and SPA/RAC (2018). Defining the most representative species for IMAP Candidate Indicator 24. Tunis: UNEP.
Valisano, L., Palma, M., Pantaleo, U., Calcinai, B., and Cerrano, C. (2019). Characterization of North-Western Mediterranean coralligenous assemblages by video surveys and evaluation of their structural complexity. Mar. Pollut. Bull. 148, 134–148. doi: 10.1016/j.marpolbul.2019.07.012
Vieira, R. P., Raposo, I. P., Sobral, P., Gonçalves, J. M. S., Bell, K. L. C., and Cunha, M. R. (2015). Lost fishing gear and litter at Gorringe Bank (NE Atlantic). J. Sea Res. 100, 91–98. doi: 10.1016/j.seares.2014.10.005
Watters, D. L., Yoklavich, M. M., Love, M. S., and Schrodeder, D. M. (2010). Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull. 60, 131–138. doi: 10.1016/j.marpolbul.2009.08.019
Worm, B., Lotze, H. K., Jubinville, I., Wilcox, C., and Jambeck, J. (2017). Plastic as a persistent marine pollutant. Annu. Rev. Environ. Resour. 42, 1–26. doi: 10.1146/annurev-environ-102016-060700
Keywords: marine litter, biogenic reefs, Mediterranean Sea, human activities, fishing impacts, vulnerable habitats, threatened species, entanglement
Citation: Angiolillo M and Fortibuoni T (2020) Impacts of Marine Litter on Mediterranean Reef Systems: From Shallow to Deep Waters. Front. Mar. Sci. 7:581966. doi: 10.3389/fmars.2020.581966
Received: 10 July 2020; Accepted: 09 September 2020;
Published: 29 September 2020.
Edited by:
Carlo Cerrano, Marche Polytechnic University, ItalyReviewed by:
Sara Canensi, Polytechnic University of Marche, ItalyLars Gutow, Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research (AWI), Germany
Copyright © 2020 Angiolillo and Fortibuoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michela Angiolillo, michela.angiolillo@isprambiente.it
 Michela Angiolillo
Michela Angiolillo Tomaso Fortibuoni
Tomaso Fortibuoni