
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 14 September 2020
Sec. Deep-Sea Environments and Ecology
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.567428
This article is part of the Research Topic 7th International Symposium on Deep-Sea Corals View all 22 articles
 Daniel Wagner1*
Daniel Wagner1* Alan M. Friedlander2,3
Alan M. Friedlander2,3 Richard L. Pyle4
Richard L. Pyle4 Cassandra M. Brooks5
Cassandra M. Brooks5 Kristina M. Gjerde6
Kristina M. Gjerde6 T. ‘Aulani Wilhelm1
T. ‘Aulani Wilhelm1Coral reefs are widely regarded as one of the top science and conservation priorities globally, as previous research has demonstrated that these ecosystems harbor an extraordinary biodiversity, myriad ecosystem services, and are highly vulnerable to human stressors. However, most of this knowledge is derived from studies on nearshore and shallow-water reefs, with coral reef ecosystems remaining virtually unstudied in marine areas beyond national jurisdiction (ABNJ), commonly known as the high seas. We reviewed information on the spatial distribution of reef-building corals throughout their depth range, and compiled a total of 537,782 records, including 116 unique records from ABNJ at depths between 218–5,647 m. The majority of reef-building coral records in ABNJ were in association with geomorphological features that have steep topographies. These habitats, which include escarpments, seamounts, and submarine ridges accounted for >74% of the records in international waters. Such geomorphological features, particularly those that occur within close proximity to the sea surface, should be prioritized for future scientific exploration. The majority of the reef-building coral records in ABNJ (>77%) were recorded in unprotected waters, and this study discusses the challenges and opportunities for protecting marine biodiversity in ABNJ. Finally, this study offers a definition of high seas coral reefs, and provides a framework to better understand and conserve these fragile ecosystems.
Coral reefs are widely regarded as some of the most biodiverse and productive ecosystems on Earth (Reaka-Kudla, 1997; Small et al., 1998; Knowlton and Jackson, 2001). Often referred to as the rainforests of the sea, coral reef ecosystems account for nearly one quarter of the total marine biodiversity, despite only covering 0.2% of the total seafloor by area (Reaka-Kudla, 1997; Knowlton et al., 2010). In addition to their remarkable biodiversity, coral reefs also provide many other ecosystem services and human benefits, including fisheries, coastal protection, tourism, recreation, and medicines (Costanza et al., 1997; Moberg and Folke, 1999; Adey, 2001; Bruckner, 2002; Cesar et al., 2003; Teh et al., 2013). Coral reef ecosystems are subject to many of the same anthropogenic impacts that affect other marine ecosystems; however, they are considered particularly susceptible to changes in environmental conditions (Knowlton, 2001; Hoegh-Guldberg, 2005; Hoegh-Guldberg et al., 2007; Hughes et al., 2017a,b). Almost 30% of corals have disappeared since the early 1980s (Hoegh-Guldberg, 2005), and up to 90% of coral reefs may be gone in the next few decades in the absence of swift conservation action (Hoegh-Guldberg et al., 2018a,b; Hughes et al., 2018).
As a result of their extraordinary biodiversity, myriad ecosystem services, and global anthropogenic stressors, coral reefs are widely regarded as one of the top conservation and science priorities globally (Dight and Scherl, 1997; Crosby et al., 2002; Klein et al., 2010; Hourigan, 2014). Although most of our current understanding of coral reefs is derived from studies in nearshore and coastal areas, coral reefs are also found in many locations far removed from human population centers. This includes coral reef habitats around remote and uninhabited islands (D’agata et al., 2016; Jones et al., 2018), as well as on seamounts and submarine ridges (Rogers, 1999; Mortensen et al., 2007; Rogers et al., 2007). While these remote coral reefs do not provide some of the ecosystem services of their nearshore counterparts (e.g., subsistence fishing, tourism, recreation, and shoreline protection), they are still considered important biodiversity hotspots (Rogers, 1999; Gove et al., 2016). For instance, coral reefs located near remote islands have been shown to be hotspots of productivity and biodiversity in otherwise barren ocean basins (Gove et al., 2016). Similarly, seamounts and submarine ridges, which can often harbor mesophotic coral ecosystems (MCEs) and deep-water coral reefs (Freiwald et al., 2004; Mortensen et al., 2007; Rogers et al., 2007; Pereira-Filho et al., 2012), are well known for their high food availability and remarkable diversity of invertebrates, fishes, and other open-ocean animals (Genin et al., 1986; Wilson and Kaufmann, 1987; Rogers, 1994, 1999, 2018; Worm et al., 2003; Pitcher et al., 2007; Clark et al., 2010; Morato et al., 2010). Additionally, these unique features facilitate the dispersion of organisms between distant geographic areas by serving as navigational marks and stepping stones for the movement of organisms (Wilson and Kaufmann, 1987; Rogers, 1994; Garrigue et al., 2015; Rogers, 2018). Furthermore, isolated islands and seamounts provide important feeding, resting, and spawning grounds for numerous benthic and pelagic species (Wilson and Kaufmann, 1987; Rogers, 1994, 2018; Pitcher et al., 2007; Clark et al., 2010), and coral reefs in these locations often host a high proportion of endemic species (Kane et al., 2014; Friedlander et al., 2016; Rogers, 2018).
In the simplest sense, coral reefs are massive aggregations of limestone and calcareous sediments that are built by a thin veneer of living organisms (Hubbard, 1997). At least 845 species of corals are known to build reef frameworks in the photic zone (Carpenter et al., 2008), although a wide diversity of coralline algae and invertebrates also contribute to building reef mass, including dead organisms of these taxa (Knowlton and Jackson, 2001). In deeper waters below the photic zone, there are at least six coral species that are known to build massive reef structures to depths exceeding 2,000 m, namely Enallopsammia rostrata, Goniocorella dumosa, Lophelia pertusa, Madrepora oculata, Oculina varicosa, and Solenosmilia variabilis (Rogers, 1999; Freiwald et al., 2004; Davies and Guinotte, 2011). While the diversity of these deep-water, reef-building corals is markedly lower than their shallow-water counterparts, they non-etheless act as ecosystem engineers that create habitat for a multitude of associated species. For instance, Lophelia pertusa reefs in the North East Atlantic are home to nearly 900 species (Rogers, 1999).
For the purposes of this paper, we use the term coral reef to refer to limestone structures that are built by reef-building corals throughout ocean depths, including in shallow water (<40 m), at mesophotic depths (∼40–200 m), and in the deep sea (>200 m). While this definition is somewhat broader than many interpretations of this term, it acknowledges several shared characteristics of coral reefs throughout their depth range, namely the presence of reef-building corals that form the basis of the physical reef structure, which are inhabited by a multitude of associated organisms (e.g., Hubbard, 1997; Rogers, 1999; Knowlton and Jackson, 2001; Reed, 2002). Although dominated by different reef-building corals at different depths, ecosystems throughout these locations share many key characteristics (e.g., high structural complexity, vulnerability and diversity of associated organisms) that unite them in ways that suggest shared strategies for management and conservation.
Coral reefs have been the dedicated focus of science and conservation efforts for decades (Dight and Scherl, 1997; Crosby et al., 2002; Klein et al., 2010; Hourigan, 2014). However, like many other marine ecosystems (Ortuño Crespo et al., 2019), they remain largely unexplored in marine areas beyond national jurisdiction (ABNJ), where the water column is commonly known as the high seas, and the international seabed as the “Area” (Molenaar and Elferink, 2009; Gjerde et al., 2016). ABNJ are ocean areas where no one nation has sole management responsibility and hence international cooperation is essential. The high seas generally begin at the 200-nautical mile exclusive economic zone (EEZ) of countries, whereas the international seabed begins at the outer edge of the extended continental shelf, or the EEZ, whichever is greater (Figure 1). Despite covering nearly two thirds of the ocean and almost half the surface of our planet, only 1.2% of the high seas currently lie within marine protected areas (MPAs), with another 0.61% being closed to bottom-fishing, and 0.41% closed to deep-sea mining activities (Figure 1; see materials and methods section for details). This uneven distribution of ocean protection is in large part due to the patchwork legal framework that is in place for managing ABNJ, as well as the lack of broader awareness that important and fragile ecosystems exist within these remote ocean areas (Molenaar and Elferink, 2009; Gjerde et al., 2016; Durussel et al., 2017).
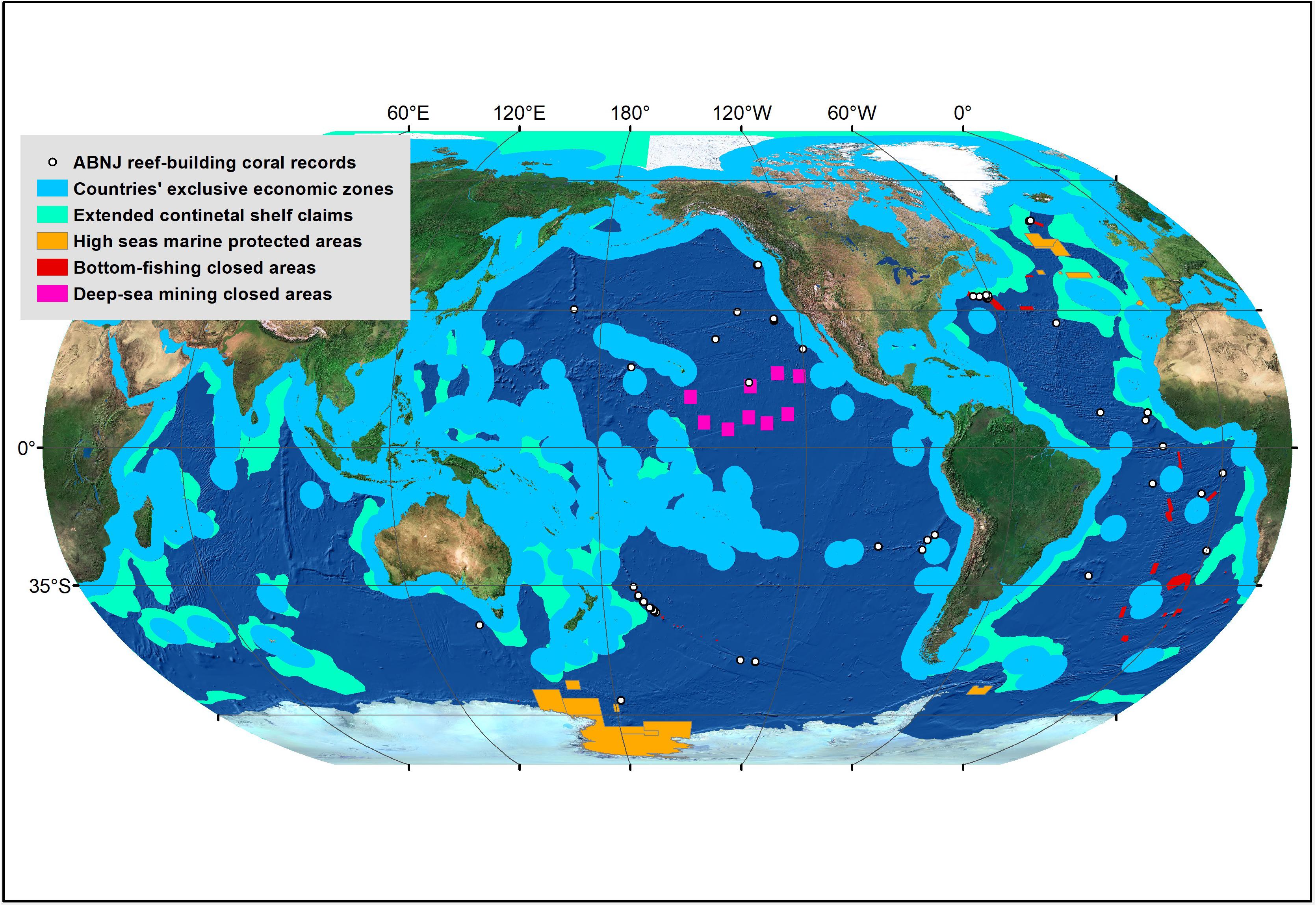
Figure 1. Spatial distribution of the reef-building coral records in marine areas beyond national jurisdiction (ABNJ) examined as part of this study, along with areas where human activities are regulated on the high seas, including marine protected areas (MPAs), areas closed to bottom fishing, and areas closed to deep-sea mining activities. Of the 116 reef-building coral records in ABNJ, none were located in MPAs, with another 25 in bottom-fishing closed areas, and one in areas that are closed to mining activities.
The high seas and international seabed have recently gained increased attention globally, since the United Nations General Assembly committed to developing an international legally binding instrument to protect, conserve, and sustainably use biological diversity in ABNJ (UNGA, 2015). This development illustrates the increased need to identify priority areas to conserve within international waters, and a potential pathway to protect them in the near future. Several studies have recently been conducted to identify priority conservation areas in ABNJ (Harris and Whiteway, 2009; Selig et al., 2014; Visalli et al., 2020; Sala et al., in review). These studies used a combination of various global datasets to identify large ocean areas that should be prioritized for future conservation. Here we provide an alternate approach, which seeks to examine the spatial distribution of one of the most diverse and fragile marine ecosystems (coral reefs) to drive science and conservation efforts on the high seas. Specifically, this study reviews available data sources on the spatial distribution of reef-building corals in ABNJ, and discusses the challenges and opportunities for protecting these unique systems. Finally, this study provides a framework to better understand and conserve these fragile ecosystems.
Records of coral species that are known to build reefs were retrieved from various sources. This includes 845 reef-building species that inhabit the photic zone, primarily scleractinians, but also a few calcified hydrozoans and octocorals (Carpenter et al., 2008), and six scleractinian species that are known to build massive reef structures in the deep sea (see above; Rogers, 1999; Freiwald et al., 2004). Only georeferenced records of reef-building corals were retrieved from publicly available sources, including (1) the Ocean Biogeographic Information System1, (2) the United Nations Environment Programme World Conservation Monitoring Centre2 for shallow-water corals, and3, (3) the United States National Museum of Natural History Museum, Smithsonian Institution4 and (4) the NOAA National Database of Deep-Sea Corals and Sponges5. Retrieved records were entered in a ArcGIS geodatabase and plotted against maritime boundaries of country jurisdictions, which were obtained by combining polygons of each country’s exclusive economic zone (World EEZ v116) and extended continental shelf areas7. The 200 nautical mile limit around Antarctica, as well as any associated extended continental shelf claims, were removed from ABNJ given the unique multi-national jurisdiction of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR) in these waters (Brooks, 2013). Duplicate records were removed from the data, as were other ABNJ records with obvious errors in the spatial data (e.g., description of the locality did not match the reported latitude and longitude; reported depths > 1,000 m off of the predicted depth at the reported position as determined by bathymetry data8; specimens retrieved from gut content of highly mobile fishes). The remaining ABNJ records were compared against the global distribution of seafloor geomorphology (Harris et al., 2014) to determine the broad geophysical feature with which corals were associated. Additionally, high seas coral records were compared against the location of MPAs9, as well as other areas where human activities are regulated in ABNJ, including areas that are closed to bottom-fishing10, exploration areas for deep-sea minerals, and areas of particular environmental interest (APEI) that are closed to deep-sea mining activities11. To examine patterns in the depth distribution of coral records, frequency distributions were plotted and manually binned by looking for breaks in the distribution. For coral records that only had a reported depth range (i.e., no exact depth), the average between minimum and maximum depth was used. Finally, a taxonomic analysis of ABNJ coral records was conducted following taxonomic groupings from the World Register of Marine Species12.
A total of 537,782 geo-referenced records of reef-building corals were available from various publicly available repositories (Table 1). The Ocean Biogeographic Information System provided the largest proportion of coral records used in this study (N = 496,708), followed by the United Nations Environment Programme World Conservation Monitoring Centre (N = 19,860), the NOAA National Database of Deep-Sea Coral and Sponges (N = 16,762), and the United States National Museum of Natural History Museum, Smithsonian Institution (N = 4,456). Of these, 537,666 records (99.98%) were within the jurisdictional boundaries of countries, whereas 116 (0.02%) fell within ABNJ (Table 1; Figure 1). Amongst high seas coral records, 79 (68.10%) were recorded on escarpments, 63 (54.31%) on ridges, 43 (37.07%) on seamounts, 25 (21.55%) on basins, and five (4.31%) on plateaus. The majority of ABNJ coral records were recorded in unprotected waters (77.59%), as none were located in MPAs, 25 (21.51%) in bottom-fishing closed areas, and one (0.86%) in areas closed to mining activities (Figure 1).
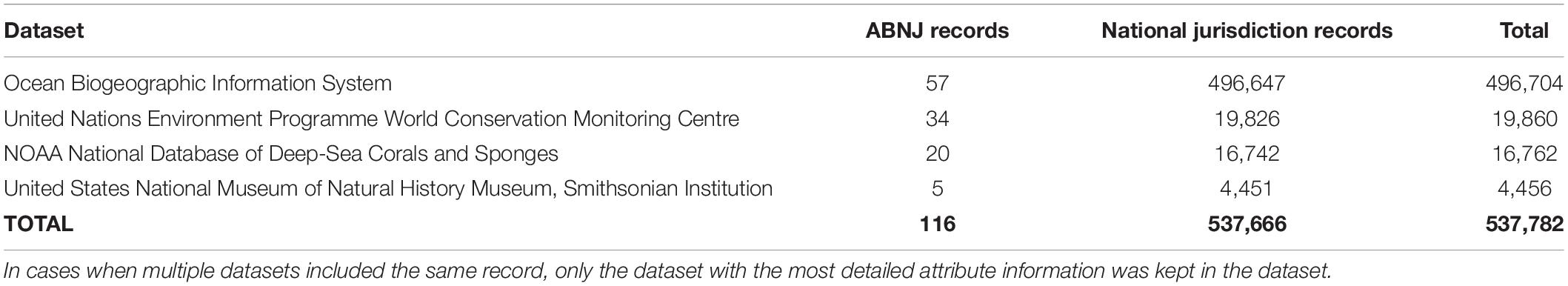
Table 1. Number of unique coral records examined as part of this study by jurisdiction and dataset (ABNJ = areas beyond national jurisdiction).
Depth information was available for 77 of the ABNJ coral records, with an average of 1,058 m, and range of 218–5,567 m (Figure 2). The majority of these records were from depths shallower than 1,200 m (N = 48, 62.34%), with multiple frequency distribution peaks at depths of 301–600 m and 901–1,200 m, respectively (Figure 2). Depths between 1,201–1,800 m represented 31.12% of the ABNJ coral records, which decreased in frequency with increasing depth (Figure 2). Depths below 1,800 m accounted for 6.49% of the ABNJ coral records, and decreased in frequency with increasing depth (Figure 2).
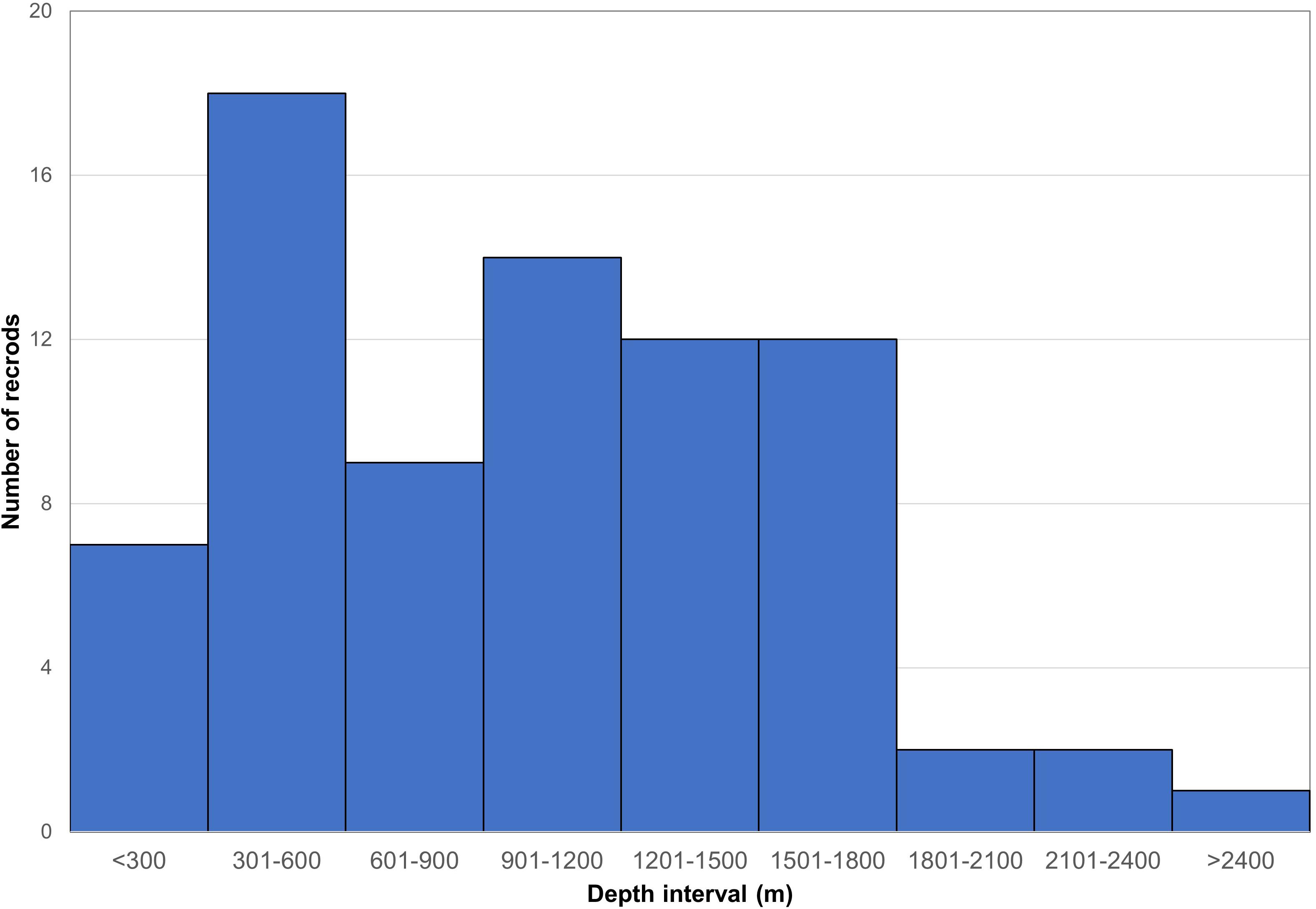
Figure 2. Frequency distribution by depth for the 77 reef-building coral records in marine areas beyond national jurisdiction (ABNJ) for which depth information was available.
All of the 116 high seas records were scleractinian corals, primarily in the families Caryophillidae (N = 68), Oculinidae (N = 31), and Dendrophylliidae (N = 15; Table 2). The most commonly observed ABNJ records were all deep-water, reef-building species (Table 2), and included Solenosmilia variabilis, Madrepora oculata, Lophelia pertusa, and Enallopsammia rostrata in decreasing order of occurrence, respectively (Table 2). Reef-building coral species that are typically known from the photic zone only accounted for two high seas coral records (1.72%), and included a single record of each Agaricia sp. (N = 1) and Acropora humilis (N = 1). Noteworthy, the latter reef-building corals were recorded at depths of 1,800 and 5,647 m respectively, much deeper than where they typically occur.
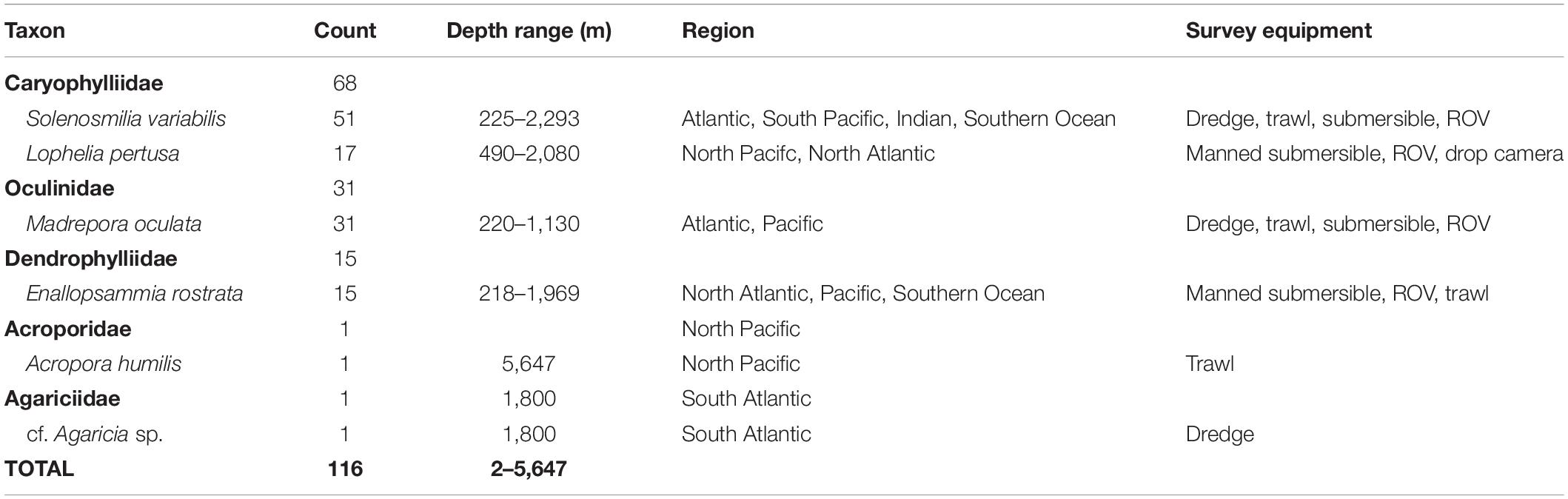
Table 2. Counts by taxonomic groups for the reef-building coral records in marine areas beyond national jurisdiction examined as part of this study.
The high seas are crucial for sustaining life on Earth, as they contain nearly 90% of the total ocean biomass, produce nearly half of the oxygen we breathe, and capture over 1.5 billion tons of carbon dioxide each year (UNEP, 2007; Global Ocean Commission, 2014; Laffoley et al., 2014; Matz-Lück and Fuchs, 2014). These remote ocean areas have played a pivotal role in many seafaring cultures, who for millennia have used them to sustain themselves physically and spiritually (Anderson, 2008). They are also under threat from numerous human impacts, including overfishing, habitat disruption, climate change, ocean acidification, deoxygenation, as well as chemical, noise, and plastic pollution (Halpern et al., 2015). However, despite their enormous size, importance, and vulnerability, the high seas remain largely unstudied and unprotected.
While the terms “coral reef” and “high seas” are rarely combined in the same sentence, our analysis shows that reef-building corals are found within ABNJ (Figure 1). Using publically available data archives, we compiled 116 unique records that fall outside the jurisdiction of countries. While high seas records only comprised an extremely small proportion (0.02%) of all records in this study, this is likely due to the large disparities in sampling effort between ABNJ and national waters, as governments tend to exclusively focus research within their own EEZ. ABNJ account for nearly two thirds of the ocean and ∼70% of the space that is inhabitable to life on Earth (UNEP, 2007; Global Ocean Commission, 2014; Laffoley et al., 2014), and hence these remote areas likely also provide habitat for coral reefs and the myriad species that are associated with them. To date, there have been no dedicated studies on reef-building corals in ABNJ, and this represents an enormous opportunity for future scientific exploration and research.
While this study was only able to confirm an extremely limited number of reef-building coral records (N = 116; 0.02%) when compared to the vast size of ABNJ (Figure 1), it nevertheless provides evidence that reef-building corals, the building blocks for coral reefs, exist in ABNJ. In cases with limited data, habitat suitability models are often used to infer locations where species might be found, and such approaches have been used to predict suitable habitat for various reef-building corals (e.g., Davies and Guinotte, 2011; Rowden et al., 2017; Cryer et al., 2018). Developing such habitat suitability models would be a logical next step for the results of this study; however, in order for these models to be useful, they will require high-quality datasets with good spatial coverage globally, particularly on the high seas. With the exception of data that can be obtained via satellites, data coverage on the high seas is still extremely poor (Fujioka and Halpin, 2014; Ortuño Crespo et al., 2019; Visalli et al., 2020). The Ocean Biogeographic Information System, which is the most comprehensive spatially explicit repository for biodiversity information globally, has disproportionately low data coverage in ABNJ. For instance, almost one third of all species recorded in ABNJ in this repository are represented by a single record (Ortuño Crespo et al., 2019). Therefore, future scientific explorations should not only target ecologically important areas like coral reefs, but also seek to fill in the enormous data gaps that exist across various taxa and disciplines in these remote ocean areas.
The majority of reef-building coral records in ABNJ were in association with geomorphological features that have steep topographies. Escarpments, seamounts and submarine ridges accounted for > 73% of all coral records in ABNJ, despite these features only covering a relatively small portion of the global seafloor. Specifically, escarpments only cover 5.84% of the global seafloor, seamounts 2.17%, and submarine ridges 2.70% (Harris et al., 2014). Such steep geomorphological features are known to accelerate currents around them, as well as generate increased nutrient fluxes (Genin et al., 1986; Lueck and Mudge, 1997; Mortensen et al., 2007; Lavelle and Mohn, 2010). These conditions provide highly suitable habitat for suspension feeders like corals (Genin et al., 1986; Mortensen et al., 2007; Rogers et al., 2007), as well as aggregate biomass at higher trophic levels (Wilson and Kaufmann, 1987; Worm et al., 2003; Morato et al., 2010). A similar pattern has been described for coral reefs on remote ocean islands, where enhanced phytoplankton biomass proximate to island-reef ecosystems can influence food-web dynamics and elicit increases in biomass at higher trophic levels (Gove et al., 2016). By definition ABNJ may not contain emergent land features; however, high seas coral reefs may function in similar ways to reef ecosystems on remote islands (Figure 3). If this is true, then coral aggregations on steep geomorphological features of the high seas likely also represent hotspots of productivity and biodiversity for other taxa (Figure 3).
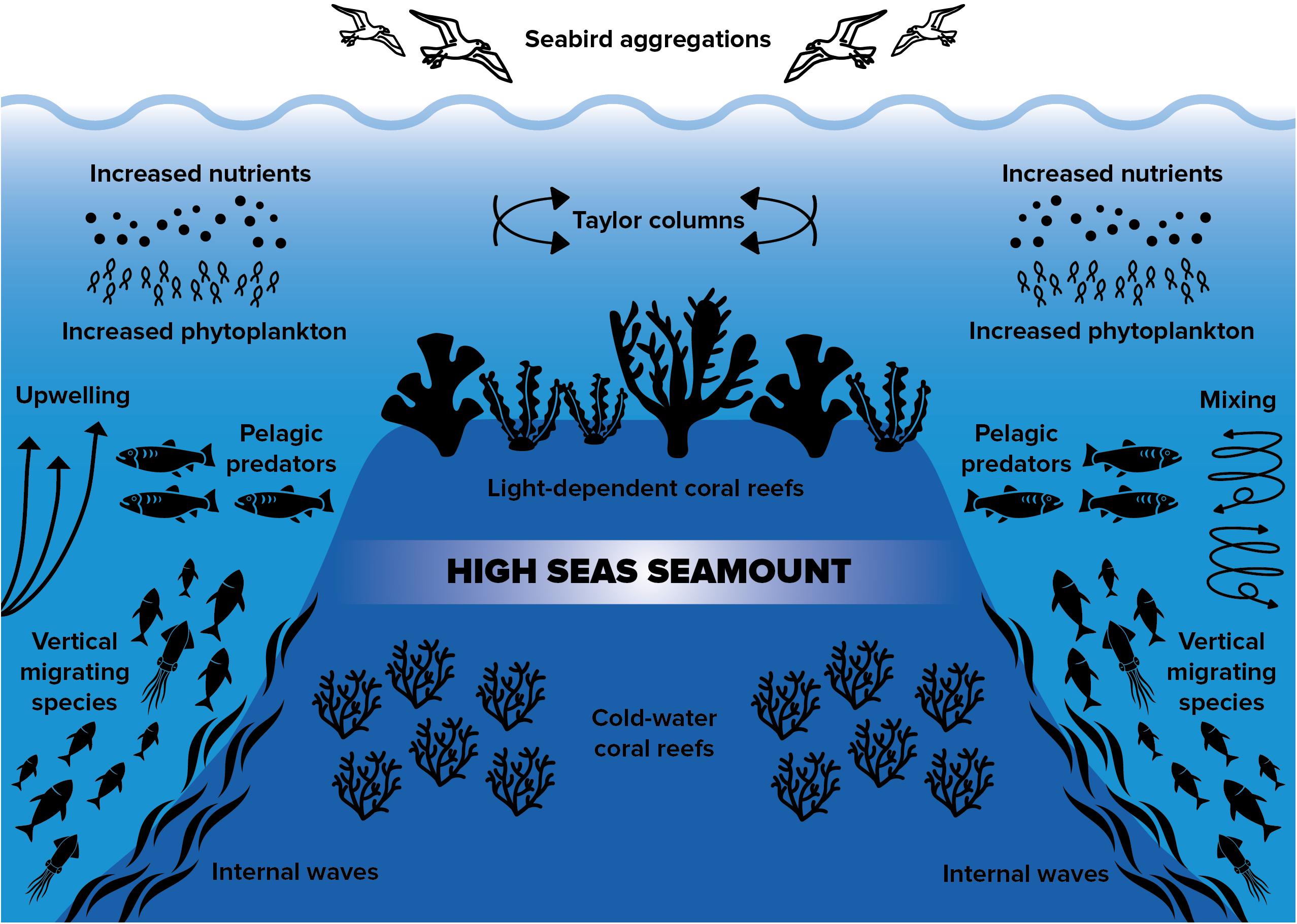
Figure 3. Diagram showing the functional importance of high seas coral reefs, which are associated with seamounts and other steep topographical features in marine areas beyond national jurisdiction. These features create favorable conditions for suspension feeders like corals, and also attract a wide diversity of other organisms.
While the basic structure and function of high seas coral reefs may be similar to those of reef ecosystems on remote islands, they may also represent highly unique ecosystems harboring species and ecological processes that exist nowhere else on Earth. Benthic habitat that is isolated from emergent land may yield a suite of unique environmental conditions. For example, a region of the tropical Pacific Ocean west of Chuuk between about 6.25° N and 9.25° N latitude and 146.5° E and 150.5° E longitude, contains nearly 9,000 km2 of shallow (<100 m) limestone habitat, within which only about 12.25 km2 of emergent land exists. The largest single feature in this region is Gray Feather Bank (sometimes spelled “Grey Feather Bank”), a sunken atoll encompassing approximately 2.700 km2 that summits at a depth of approximately 20–25 m. The nearest emergent land (Poluwat) consists of a small cluster of islets with a combined total area of 3.3 km2, which is located 50 km away and separated by deep ocean. Observations on this remote oceanic seamount by a team of divers in 2007 revealed a habitat that shared some characteristics of shallow reef habitat, but also differed in several fundamental ways (e.g., the composition and depth range of species, and the qualitative nature of the habitat and associated coral cover; see Figure 4) (R. Pyle, unpublished data). Although located within the EEZ of the Federated States of Micronesia, similar coral reefs very likely exist on the summits of seamounts in ABNJ, such as the region north and east of the Marshall Islands (Figure 5).

Figure 4. Diver captures video of coral reef habitat on the summit of Gray Feather Bank at a depth of 25 m. While located within the EEZ of the Federated States of Micronesia, this sunken atoll demonstrates the potential for ABNJ coral reef ecosystems to exist in nearby open-ocean environments where benthic habitat at suitable depths exists (Photo courtesy of B. Cranston).
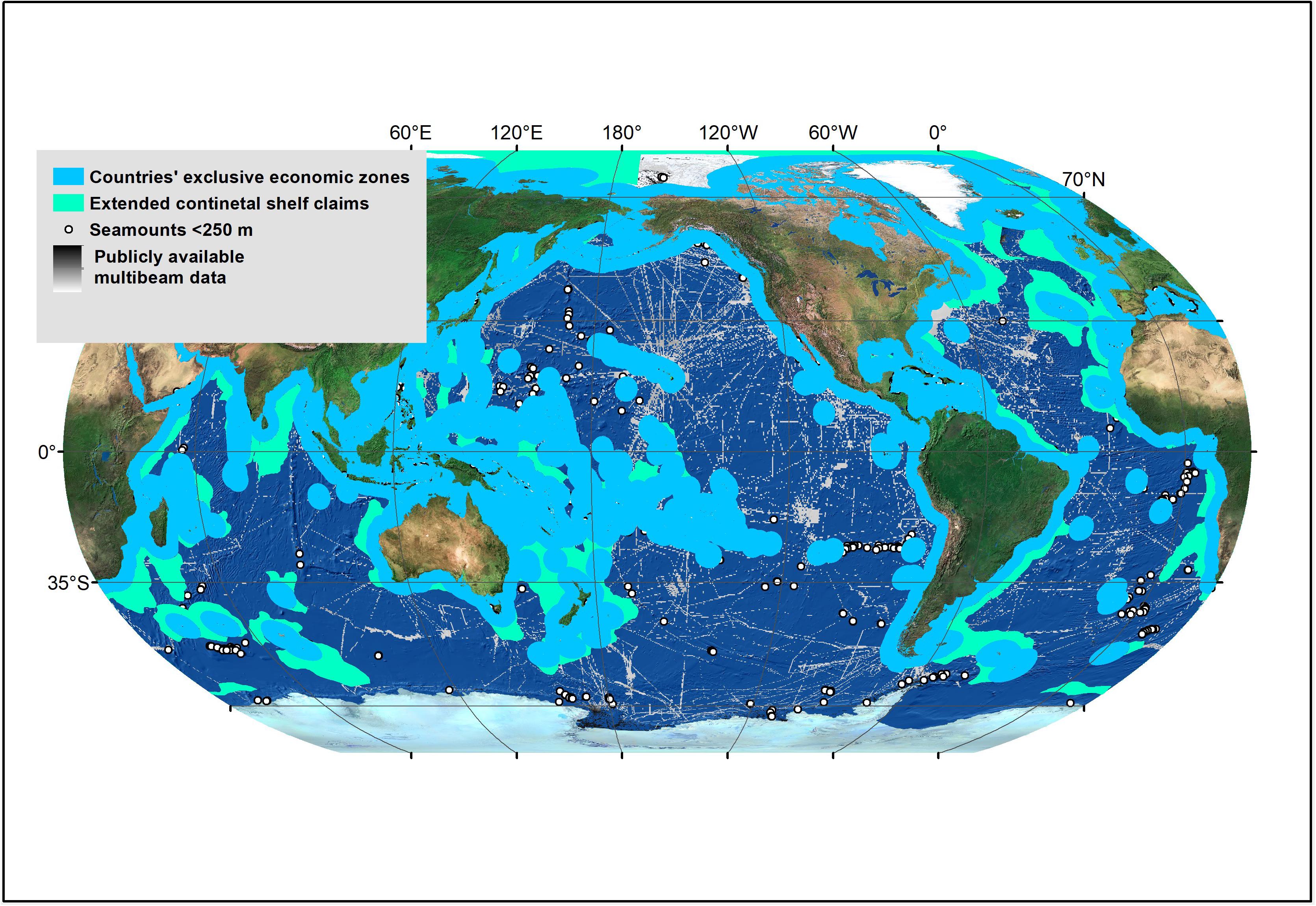
Figure 5. Spatial distribution of seamounts that are predicted to have summits shallower than 250 m in depth based on modeled data in Yesson et al. (2011). These seamounts are likely to host high seas coral reefs and should be prioritized for future scientific explorations and conservation. However, many of these seamounts have not yet been mapped with modern echosounders, and therefore will need to be mapped in detail first in order to ground truth seamount depth data. Publicly available multibeam data from NOAA’s National Centers of Environmental Information.
Beyond these observations, the characteristics and dynamics of open-ocean coral reefs remain almost completely unknown to science due to a historical lack of scientific exploration. However, their existence likely is known to the fishing industry. For example, during the aforementioned exploration of Gray Feather Bank, divers unexpectedly noted the complete absence of sharks, as well as the presence of long-line fishing gear entangled on the reef. Exploration of seamounts and other steep underwater features in ABNJ should be undertaken to examine the similarities between such open-ocean reef ecosystems and their counterparts near emergent land, as well as to understand how these remote habitats are being impacted by human activities.
Future explorations in ABNJ should focus on exploring steep geomorphological features that come within close proximity to the sea surface. Amongst reef-building coral records in ABNJ for which depth information was available (N = 77), this study revealed four records (5.19%) at depths shallower than 250 m (Figure 2). Most of these were found in association with seamounts and ridges. Global seamount inventories (Kim and Wessel, 2011; Yesson et al., 2011; Harris et al., 2014) indicate that ABNJ host several aggregations of seamounts that summit within close proximity of the sea surface in each ocean basin (Figure 5); however, many of these have not yet been explored or even mapped in detail. Because these shallow seamounts could potentially host both shallow-water and cold-water coral assemblages (Figure 3), they should be prioritized for future exploration. Areas that that are particularly promising for such explorations due to their dense aggregations of seamounts are on the Salas y Gómez and Nazca Ridges in the South Pacific, between Hawaii and Guam in Central Pacific, south of the Crozet Islands in the Indian Ocean, off the West Coast of Africa, east of Tristan da Cunha in the South Atlantic, and off the Antarctic Peninsula and Thurston Island in the Southern Ocean (Figure 5). Importantly, many of the locations have not yet been mapped with modern echosounders (Figure 5). Therefore, these locations will need to be mapped in detail first in order to ground truth seamount depth data derived from models (Kim and Wessel, 2011; Yesson et al., 2011).
In addition to providing guidance on future ABNJ that should be prioritized for future scientific explorations, the results of this study have important implications for prioritizing high seas areas for conservation. Due to the remarkable biodiversity and myriad ecosystem services, coral reefs have a long history of protection in many countries. However, they have not had a similar fate in ABNJ, in large part due to the lack of awareness that coral reefs may exist there, as well as the patchwork of a legal framework that is currently in place to protect the high seas (Molenaar and Elferink, 2009; Gjerde et al., 2016; Boteler et al., 2019).
The United Nations Convention on the Law of the Sea (UNCLOS) sets the basic framework for regulating human resource uses throughout the ocean, as well as a specific obligation to protect rare and fragile ecosystems (UNCLOS Article 192 and 194.5). However, it does not specify how States should do so in ABNJ. As a result, a host of regional and global agreements covering different sectors, including fishing, shipping, and mining, were developed both before and after UNCLOS came into effect in 1994 (Molenaar and Elferink, 2009; Gjerde et al., 2016; Durussel et al., 2017; Boteler et al., 2019). For instance, fishing activities on the high seas are regulated by numerous regional fishery management organizations, each one of which is dedicated to managing fisheries in a particular high seas region, or managing fisheries that target highly migratory fish stocks or straddling fish stocks in broader regions that include both national waters and the high seas (Kaitala and Munroe, 1993; Cullis-Suzuki and Pauly, 2010; Auster et al., 2011).
The impacts of bottom-fishing, particularly on seamounts and deep-water coral reefs, are well documented in the literature (e.g., Clark and Koslow, 2007; Clark, 2009; Pitcher et al., 2010). For high seas fisheries that target species associated with the seafloor, the protection of vulnerable marine ecosystems (VME) is an important component of its management framework (Parker et al., 2009; Penney et al., 2009; Auster et al., 2011; Ardron et al., 2014; Rowden et al., 2017). Specifically, requirements of the 2006 United Nations General Assembly Sustainable Fisheries Resolution (61/105) aim to ensure that destructive bottom-fishing activities do not proceed unless measures have been established to prevent significant adverse impacts. While VMEs are embedded in the management regimes of high seas fisheries, there are difficulties of designating these bottom-fisheries protected areas, particularly in poorly explored areas (Auster et al., 2011). VMEs are often found by fishing vessels that accidentally catch corals and other non-target species, and thus these areas often get protected after destruction has already occurred (Auster et al., 2011; Ardron et al., 2014). This is also apparent in our dataset, where many ABNJ coral records were derived from fisheries data (Table 2). Interestingly, 21.51% of all reef-building coral records in ABNJ fell within bottom-fished closed areas, despite the fact that these fishery closures only account for 0.61% of the seafloor area globally.
To address these issues, several regional fishery management organizations have implemented move-on rules, which require fishing vessels to move a minimum distance when a particular catch level of a VME indicator species is encountered (Parker et al., 2009; Penney et al., 2009; Auster et al., 2011; Geange et al., 2020). While the FAO Guidelines call for prior assessment of areas likely to contain VMEs, there are no common approaches for defining threshold metrics for what constitutes evidence for encountering a VME (Auster et al., 2011). As a result, VMEs are not consistently identified in different regions. Only 21.55% of the high seas coral records identified in this study were located inside areas closed to bottom-fishing, and the data of coral reefs outside current closures could provide important guidance on identifying additional areas that should be closed to bottom-fishing in the future. Similar approaches that rely on coral spatial distribution data have already been used to provide practical guidance on the management of bottom fisheries in the South Pacific (Penney et al., 2008; Rowden et al., 2017, 2019; Cryer et al., 2018; Government of New Zealand, 2019).
The International Seabed Authority (ISA) regulates mineral-related activities in the international seabed beyond the limits of national jurisdiction. It does so by setting forth the regulations, standards and procedures for securing exploration and (in the future) exploitation contracts for seabed minerals, as well as for developing the measures to protect the marine environment from the harmful effects of mining (UNCLOS Article 145). Environmental standards are currently under development at the ISA as part of the mining regulations, and to date have included the designation of a network of provisional no-mining areas of particular environmental interest (APEI) as part of a wider regional environmental management plan (Lodge et al., 2014; Wedding et al., 2015). One record in this study was located in an APEI and therefore currently protected from mining activities, whereas two records were found within proximity of areas where there are active exploration contracts for deep-sea minerals. Specifically, reef-building corals were recorded within 56 km of active exploration contracts for cobalt-rich crusts in the South Atlantic, and within 160 km of active exploration contracts for polymetallic sulfides on the Mid-Atlantic Ridge (Figure 1). While the footprint of impacts generated by potential mining activities are still poorly understood (Miller et al., 2018), the results of this study have important implications for the mining regulations that are currently being developed at the ISA, as these should protect sensitive marine organisms from harmful mining effects (UNCLOS Article 145). A precautionary approach to ensuring effective protection means that mining activities should not be allowed to proceed if it cannot be demonstrated that these sensitive ecosystems will not be subjected to lasting harm.
The International Maritime Organization (IMO) regulates international shipping activities across national jurisdictions and on the high seas. IMO manages shipping activities through various measures, including routing, reporting, and discharge requirements. The IMO can also designate particular sensitive sea areas (PSSAs), which may include environmental protection measures, including areas to be avoided by all ships, or by certain classes of ships (Molenaar and Elferink, 2009; Prior et al., 2010; Boteler et al., 2019). While there are currently 17 PSSAs globally, none of these are located in ABNJ, and compared to national waters, relatively few shipping route limitations exist on the high seas (Prior et al., 2010). With over 90% of the world trade now being carried out through international shipping on the high seas (Kaluza et al., 2010), the shipping industry has wide-ranging potential impacts on ABNJ. Protection of marine ecosystems through the designation of PSSAs has already been successfully achieved in the national waters of several countries, and a similar approach could be implemented on the high seas (Prior et al., 2010). Specifically, PSSAs should be established in high seas areas where the risks for potential impacts (e.g., noise, chemical and sewage pollution, invasive species from ballast water or biofouling, ship strike with whales and other marine megafauna) are greatest, such as in places where there may be extensive shallow-water environments and abundant sea life (Figure 5). While some shipping-related threats like noise pollution and ship strikes may be less a concern for reef-building corals, they are still important considerations for many reef-associated organisms.
In addition to intergovernmental bodies that regulate high seas fishing, shipping and mining, there are several international conventions that regulate specific human activities across international borders, including those of the high seas (Ardron and Warner, 2005). Among others these include the International Convention for the Regulation of Whaling, which regulates whaling, the London Convention, which regulates the dumping of wastes in the ocean, the Convention on International Trade in Endangered Species (CITES), which regulates the international trade of endangered species, and the Convention on Migratory Species, which seeks to conserve migratory species, their habitats and migratory routes (Ardron and Warner, 2005). To date, there has been a lack of coordination and cooperation between all of these international bodies. To overcome these challenges, in 2015 the United Nations General Assembly (UNGA) agreed to develop an international legally binding instrument under UNCLOS on the conservation and sustainable use of marine biological diversity in ABNJ (UNGA, 2015). Specifically, this agreement seeks to create new international procedures for: (1) area-based management tools (including MPAs), (2) environmental impact assessments, (3) marine genetic resources, including benefit sharing, and (4) capacity-building and transfer of marine technology. The negotiations for the treaty are still ongoing, with the fourth and final scheduled session being postponed as a result of the international coronavirus crisis.
Until a legally binding instrument under UNCLOS is created, there is currently no comprehensive legal framework for the establishment of MPAs in ABNJ. Rather, initiatives to protect critical habitats on the high seas remain scattered throughout the legal mandates of organizations with different management purposes, such as PSSAs under the IMO, APEIs under the ISA, and temporal or spatial fishing closures by regional fishery management organizations (Ardron and Warner, 2005). Yet, high seas MPAs are possible outside the framework of a legally binding instrument under UNCLOS. For example, the member countries of the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR) and the Convention on the Conservation of Antarctic Marine Life (CCAMLR) have established MPAs in ABNJ of the North Atlantic and Southern Ocean, respectively (Durussel et al., 2017; Smith and Jabour, 2018). While these MPAs provide important advances in protecting biodiversity on the high seas, they still only cover a very small portion of the international ocean and apply only to the States involved, thereby providing little hope to safeguard important ecological processes throughout the ocean. Although most coral reefs occur within national jurisdictions, this study provides evidence that coral reef habitat also exists in ABNJ (Figure 1). Moreover, high seas coral reefs are likely also important for connectivity across large geographic distances.
Existing mechanisms for protecting habitats in ABNJ are scattered and poorly structured, and currently do little to protect particularly important ecosystems such as high seas coral reefs. All of the high seas coral reefs identified through this study fell outside existing MPAs, and are therefore vulnerable to overexploitation in the near future, in addition to impending climate change impacts (Halpern et al., 2015). We urge States and their representatives to take these important ecosystems into consideration when crafting the next round of international mechanisms for protecting marine biodiversity in ABNJ. Specifically, we need explicit mechanisms for the rapid identification and conservation of those ecosystems that are the most important contributors to marine biodiversity globally.
Outside of reducing greenhouse gas emissions, protected areas offer reef-building corals the best chance of thriving in the future. By reducing human stressors and prioritizing conservation, MPAs lead to increases in biomass while safeguarding biodiversity and enhancing global resilience to environmental change (Lester et al., 2009; Barnett and Baskett, 2015; Lubchenco and Grorud-Colvert, 2015; Hopkins et al., 2016; Roberts et al., 2017). At the same time, we need clear messages to sectoral organizations that activities that degrade high seas coral reefs, as has already been agreed for with respect to vulnerable marine ecosystems and bottom fishing, should be managed to avoid harm. While the dearth of available information on the location and assemblages of high seas coral reefs makes conservation planning difficult, the precautionary approach should impel action. High seas coral reefs likely comprise fragile habitats that may not cover large areas, but that are key to global biodiversity and thus warrant protection. Since high seas coral reefs are virtually unexplored, these areas represent enormous opportunities for future scientific discoveries and conservation.
All georeferenced coral datasets used in this study are publicly available from the following sources: (1) Ocean Biogeographic Information System (https://mapper.obis.org/), (2) United Nations Environment Programme World Conservation Monitoring Centre (https://data.unep-wcmc.org/datasets/1 for shallow-water corals, and https://data.unep-wcmc.org/datasets/3 for cold-water corals), (3) United States National Museum of Natural History Museum, Smithsonian Institution (https://collections.nmnh.si.edu/search/iz/), and (4) NOAA National Database of Deep-Sea Corals and Sponges (https://www.ncei.noaa.gov/maps/deep-sea-corals/mapSites.htm).
DW conducted the data analysis and created the original outline for the manuscript. AF, RP, CB, KG, TW, and DW provided input into drafting the manuscript. All authors participated in the editing and final preparation of the manuscript.
This work was funded in part by the Paul M. Angell Family Foundation, Conservation International, and the Gallifrey Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Coral Reefs of the High Seas Coalition for providing invaluable support to this effort, and in particular A. McGowern, A. Smith, A. Khoo, A. Hedlund, A. Miller, C. White, C. Hicks, E. Karan, G. Farmer, G. Cid, I. Irigoyen, J. Custopulos, J. Weller, L. Barrera, L. Van der Meer, M. Gianni, M. Wassum, M. Conathan, N. Clark, N. Ludlow, S. Earle, T. Thomas, T. Mackey, and W. Benchley for all their thoughtful contributions to this work. We further thank S. Cairns and B. Hoeksema for providing invaluable taxonomic guidance. Special thanks to S. Streyle and S. Strauss for assistance with graphic design. We also thank the Association for Marine Exploration and the British Broadcasting Corporation, who provided important resources to facilitate the dives at Gray Feather Bank.
Adey, W. H. (2001). Coral reef ecosystems and human health: biodiversity counts! Ecosyst. Health 6, 227–236. doi: 10.1046/j.1526-0992.2000.006004227.x
Anderson, A. (2008). Traditionalism, interaction, and long-distance seafaring in Polynesia. The J. Island Coast. Archaeol. 3, 240–250. doi: 10.1080/15564890802340000
Ardron, J. A., Clark, M. R., Penney, A. J., Hourigan, T. F., Rowden, A. A., Dunstan, P. K., et al. (2014). A systematic approach towards the identification and protection of vulnerable marine ecosystems. Mar. Pol. 49, 146–154. doi: 10.1016/j.marpol.2013.11.017
Ardron, J. A., and Warner, R. (2005). “International marine governance and protection of biodiversity,” in Routledge Handbook of Ocean Resources and Management, eds H. D. Smith, J. L. Suárez de Vivero, and T. S. Agardy (London: Routledge), 55–72. doi: 10.4324/9780203115398-5
Auster, P. J., Gjerde, K., Heupel, E., Watling, L., Grehan, A., and Rogers, A. D. (2011). Definition and detection of vulnerable marine ecosystems on the high seas: problems with the “move-on” rule. ICES J. Mar. Sci. 68, 254–264. doi: 10.1093/icesjms/fsq074
Barnett, L. A., and Baskett, M. (2015). Marine reserves can enhance ecological resilience. Ecol. Lett. 18, 1301–1310. doi: 10.1111/ele.12524
Boteler, B., Wanless, R., Dias, M., Packeiser, T., Awad, A., Yannicelli, B., et al. (2019). Ecological Baselines for the Southeast Atlantic and Southeast Pacific: Status of Marine Biodiversity and Anthropogenic Pressures in Areas Beyond National Jurisdiction, STRONG High Seas Project. Available online at: https://www.prog-ocean.org/wp-content/uploads/2020/01/STRONG-HS_Ecological-Baselines-Report.pdf
Brooks, C. M. (2013). Competing values on the Antarctic high seas: CCAMLR and the challenge of marine-protected areas. Polar J. 3, 277–300. doi: 10.1080/2154896x.2013.854597
Carpenter, K. E., Abrar, M., Aeby, G., Aronson, R. B., Banks, S., Bruckner, A., et al. (2008). One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. doi: 10.1126/science.1159196
Cesar, H., Burke, L., and Pet-Soede, L. (2003). The Economics of Worldwide Coral Reef Degradation. Zeist: Cesar Environmental Economics Consulting.
Clark, M. R. (2009). Deep-sea seamount fisheries: a review of global status and future prospects. Latin Am. J. Aquat. Res. 37, 501–512. doi: 10.3856/vol37-issue3-fulltex-16
Clark, M. R., and Koslow, J. A. (2007). “Impacts on fisheries on seamounts,” in Seamounts: Ecology, Fisheries, and Conservation, Vol. 12, eds T. J. Pitcher, T. Morato, P. J. B. Hart, M. R. Clark, N. Haggan, and R. S. Santos (Oxford, UK: Blackwell).
Clark, M. R., Rowden, A. A., Schlacher, T., Williams, A., Consalvey, M., Stocks, K. I., et al. (2010). The ecology of seamounts: structure, function, and human impacts. Ann. Rev. Mar. Sci. 2, 253–278. doi: 10.1146/annurev-marine-120308-081109
Costanza, R., d’Arge, R., de Groot, R. S., Farber, S., Grasso, M., Hannon, B., et al. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260.
Crosby, M. P., Brighouse, G., and Pichon, M. (2002). Priorities and strategies for addressing natural and anthropogenic threats to coral reefs in Pacific Island Nations. Ocean Coastal Manag. 45, 121–137. doi: 10.1016/s0964-5691(02)00051-0
Cryer, M., Geange, S., and Bock, T. (2018). Methods for designing spatial management areas using outputs from zonation software and other spatial data. Paper SC6-DW11 for the 6th Meeting of the SPRFMO Scientific Committee, Chile.
Cullis-Suzuki, S., and Pauly, D. (2010). Failing the high seas: a global evaluation of regional fisheries management organizations. Mar. Pol. 34, 1036–1042. doi: 10.1016/j.marpol.2010.03.002
D’agata, S., Mouillot, D., Wantiez, L., Friedlander, A. M., Kulbicki, M., and Vigliola, L. (2016). Marine reserves lag behind wilderness in the conservation of key functional roles. Nat. Commun. 7, 1–10.
Davies, A. J., and Guinotte, J. M. (2011). Global habitat suitability for framework-forming cold-water corals. PLoS One 6:e18483. doi: 10.1371/journal.pone.0018483
Dight, I. J., and Scherl, L. M. (1997). The International Coral Reef Initiative (ICRI): global priorities for the conservation and management of coral reefs and the need for partnerships. Coral Reefs 16, S139–S147.
Durussel, C., Soto, E., and Urrutia, S. A. (2017). Strengthening the legal and institutional framework of the Southeast Pacific: focus on the BBNJ package elements. Int. J. Mar. Coast. Law 32, 635–671. doi: 10.1163/15718085-12324051
Freiwald, A., Fosså, J. H., Grehan, A., Koslow, T., and Roberts, J. M. (2004). Cold-water coral reefs. Cambridge: UNEP-WCMC.
Friedlander, A. M., Ballesteros, E., Caselle, J. E., Gaymer, C. F., Palma, A. T., Petit, I., et al. (2016). Marine biodiversity in juan Fernández and Desventuradas Islands, Chile: global endemism hotspots. PLoS One 11:e0145059. doi: 10.1371/journal.pone.0145059.t005
Fujioka, E., and Halpin, P. N. (2014). Spatio-temporal assessments of biodiversity in the high seas. End. Spec. Res. 24, 181–190. doi: 10.3354/esr00591
Garrigue, C., Clapham, P. J., Geyer, Y., Kennedy, A. S., and Zerbini, A. N. (2015). Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered South Pacific humpback whales. R. Soc. Open Sci. 2:150489. doi: 10.1098/rsos.150489
Geange, S. W., Rowden, A. A., Nicol, S., Bock, T., and Cryer, M. (2020). A data-informed approach for identifying move-on encounter thresholds for vulnerable marine ecosystem indicator taxa. Front. Mar. Sci. 7:155. doi: 10.3389/fmars.2020.00155
Genin, A., Dayton, P. K., Lonsdale, P. F., and Spiess, F. N. (1986). Corals on seamount peaks provide evidence of current acceleration over deep-sea topography. Nature 322, 59–61. doi: 10.1038/322059a0
Gjerde, K. M., Reeve, L. L. N., Harden-Davies, H., Ardron, J., Dolan, R., Durussel, C., et al. (2016). Protecting Earth’s last conservation frontier: scientific, management and legal priorities for MPAs beyond national jurisdiction. Aquat. Conserv. 26(Suppl. 2), 45–60. doi: 10.1002/aqc.2646
Global Ocean Commission. (2014). From Decline to Recovery: A Rescue Package for the Global Ocean. Oxford: Global Ocean Commission.
Gove, J. M., McManus, M. A., Neuheimer, A. B., Polovina, J. J., Drazen, J. C., Smith, C. R., et al. (2016). Near-island biological hotspots in barren ocean basins. Nat. Commun. 7, 1–8.
Government of New Zealand. (2019). A proposal for a revised bottom fishing conservation and management measure forSPRFMO. Paper Prop 03.1 for the 7th meeting of the SPRFMO Commission, Hague, 23–27.
Halpern, B. S., Frazier, M., Potapenko, J., Casey, K. S., Koenig, K., Longo, C., et al. (2015). Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 6:7615.
Harris, P. T., Macmillan-Lawler, M., Rupp, J., and Baker, E. K. (2014). Geomorphology of the oceans. Mar. Geol. 352, 2–24.
Harris, P. T., and Whiteway, T. (2009). High seas marine protected areas: benthic environmental conservation priorities from a GIS analysis of global ocean biophysical data. Ocean Coast. Manag. 52, 22–38. doi: 10.1016/j.ocecoaman.2008.09.009
Hoegh-Guldberg, O., Jacob, D., and Taylor, M. (2018a). “Impacts of 1.5°C global warming on natural and human systems,” in Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C Above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, eds V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, et al. (Geneva: IPCC).
Hoegh-Guldberg, O., Kennedy, E. V., Beyer, H. L., McClennen, C., and Possingham, H. P. (2018b). Securing a long-term future for coral reefs. Trends Ecol. Evol. 33, 936–944. doi: 10.1016/j.tree.2018.09.006
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742.
Hopkins, C. R., Bailey, D. M., and Potts, T. (2016). Perceptions of practitioners: managing marine protected areas for climate change resilience. Ocean Coast. Manag. 128, 18–28. doi: 10.1016/j.ocecoaman.2016.04.014
Hourigan, T. (2014). “A strategic approach to address fisheries impacts on deep-sea coral ecosystems,” in Interrelationships Between Corals and Fisheries, ed. S. Bortoneed (Boca Raton, FL: CRS Press), 127–145. doi: 10.1201/b17159-9
Hubbard, D. K. (1997). “Reefs as dynamic systems,” in Life and Death of Coral Reefs, ed. C. Birkeland (New York, NY: Chapman & Hall), 4937.
Hughes, T. P., Anderson, K. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., et al. (2018). Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. doi: 10.1126/science.aan8048
Hughes, T. P., Barnes, M. L., Bellwood, D. R., Cinner, J. E., Cumming, G. S., Jackson, J. B., et al. (2017a). Coral reefs in the Anthropocene. Nature 546, 82–90.
Hughes, T. P., Kerry, J. T., Álvarez-Noriega, M., Álvarez-Romero, J. G., Anderson, K. D., Baird, A. H., et al. (2017b). Global warming and recurrent mass bleaching of corals. Nature 543, 373–377.
Jones, K. R., Klein, C. J., Halpern, B. S., Venter, O., Grantham, H., Kuempel, C. D., et al. (2018). The location and protection status of Earth’s diminishing marine wilderness. Curr. Biol. 28, 2506–2512. doi: 10.1016/j.cub.2018.06.010
Kaitala, V., and Munroe, G. R. (1993). The management of high seas fisheries. Mar. Resour. Econ. 8, 313–329.
Kaluza, P., Kölzsch, A., Gastner, M. T., and Blasius, B. (2010). The complex network of global cargo ship movements. J. R. Soc. Interf. 7, 1093–1103. doi: 10.1098/rsif.2009.0495
Kane, C., Kosaki, R. K., and Wagner, D. (2014). High levels of mesophotic reef fish endemism in the northwestern hawaiian Islands. Bull. Mar. Sci. 90, 693–703. doi: 10.5343/bms.2013.1053
Kim, S. S., and Wessel, P. (2011). New global seamount census from the altimetry-derived gravity data. Geophys. J. Int. 186, 615–631. doi: 10.1111/j.1365-246x.2011.05076.x
Klein, C. J., Ban, N. C., Halpern, B. S., Beger, M., Game, E. T., Grantham, H. S., et al. (2010). Prioritizing land and sea conservation investments to protect coral reefs. PLoS One 5:e12431. doi: 10.1007/978-1-4302-0647-7_1
Knowlton, N., Brainard, R. E., Fisher, R., Moews, M., Plaisance, L., and Caley, M. J. (2010). “Coral reef biodiversity,” in Life in the World’s Oceans: Diversity, Distribution, and Abundance, ed. A. D. McIntyre (Hoboken, NJ: Wiley-Blackwell), 65–77.
Knowlton, N., and Jackson, J. B. C. (2001). “The ecology of coral reefs,” in Marine community ecology, eds M. D. Bertness, S. Gaines, and M. E. Hay (Sunderland, MA: Sinauer), 395–422.
Laffoley, D., Baxter, J. M., Thevenon, F., and Oliver, J. (2014). The Significance and Management of Natural Carbon Stores in the Open Ocean. Gland: IUCN.
Lavelle, J. W., and Mohn, C. (2010). Motion, commotion, and biophysical connections at deep ocean seamounts. Oceanography 23, 90–103. doi: 10.5670/oceanog.2010.64
Lester, S. E., Halpern, B. S., Grorud-Colvert, K., Lubchenco, J., Ruttenberg, B. I., Gaines, S. D., et al. (2009). Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33–46. doi: 10.3354/meps08029
Lodge, M., Johnson, D., Le Gurun, G., Wengler, M., Weaver, P., and Gunn, V. (2014). Seabed mining: international seabed authority environmental management plan for the Clarion-Clipperton Zone - a partnership approach. Mar. Pol. 49, 66–72. doi: 10.1016/j.marpol.2014.04.006
Lubchenco, J., and Grorud-Colvert, K. (2015). Making waves: the science and politics of ocean protection. Science 350, 382–383. doi: 10.1126/science.aad5443
Lueck, R. G., and Mudge, T. D. (1997). Topographically induced mixing around a shallow seamount. Science 276, 1831–1833. doi: 10.1126/science.276.5320.1831
Matz-Lück, N., and Fuchs, J. (2014). The impact of OSPAR on protected area management beyond national jurisdiction: effective regional cooperation or a network of paper parks? Mar. Pol. 49, 155–166. doi: 10.1016/j.marpol.2013.12.001
Moberg, F., and Folke, C. (1999). Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233. doi: 10.1016/s0921-8009(99)00009-9
Molenaar, E. J., and Elferink, A. G. O. (2009). Marine protected areas in areas beyond national jurisdiction: the pioneering efforts under the OSPAR Convention. Utrecht Law Rev. 5, 5–20. doi: 10.18352/ulr.92
Morato, T., Hoyle, S. D., Allain, V., and Nicol, S. J. (2010). Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl. Acad. Sci. U.S.A. 107, 9707–9711. doi: 10.1073/pnas.0910290107
Mortensen, P. B., Buhl-Mortensen, L., Gebruk, A. V., and Krylova, E. M. (2007). Occurrence of deep-water corals on the Mid-Atlantic Ridge based on MAR-ECO data. Deep Sea Res. II 55, 142–152. doi: 10.1016/j.dsr2.2007.09.018
>Miller, K. A., Thompson, K. F., Johnston, P., and Santillo, D. (2018). An overview of seabed mining including the current state of development, environmental impacts, and knowledge gaps. Front. Mar. Sci. 4:418. doi: 10.3389/fmars.2017.00418
Ortuño Crespo, G., Dunn, D. C., Gianni, M., Gjerde, K., Wright, G., and Halpin, P. N. (2019). High-seas fsh biodiversity is slipping through the governance net. Nat. Ecol. Evol. 3, 1273–1276. doi: 10.1038/s41559-019-0981-4
Parker, S. J., Penney, A. J., and Clark, M. R. (2009). Detection criteria for managing trawl impacts on vulnerable marine ecosystems in high seas fisheries of the South Pacific Ocean. Mar. Ecol. Prog. Ser. 397, 309–317. doi: 10.3354/meps08115
Penney, A., Parker, S., Brown, J., Cryer, M., Clark, M., and Sims, B. (2008). New Zealand implementation of the SPRFMO Interim Measures for high seas bottom trawl fisheries in the SPRFMO Area. Paper presented to the SPRFMO 5 Science Working Group Meeting, Peru, NJ.
Penney, A. J., Parker, S. J., and Brown, J. H. (2009). Protection measures implemented by New Zealand for vulnerable marine ecosystems in the South Pacific Ocean. Mar. Ecol. Prog. Ser. 397, 341–354. doi: 10.3354/meps08300
Pereira-Filho, G., Amado-Filho, G. M., de Moura, R. L., Bastos, A. C., Guimarães, S. M. P. B., Salgado, L. T., et al. (2012). Extensive rhodolith beds cover the summits of southwestern Atlantic Ocean seamounts. J. Coast. Res. 28, 261–269. doi: 10.2112/11t-00007.1
Pitcher, T. J., Clark, M. R., and Watson, R. (2010). Seamount fisheries: do they have a future? Oceanography 23, 134–144. doi: 10.5670/oceanog.2010.66
Pitcher, T. J., Morato, T., Hart, P. J. B., Clark, M. R., Haggan, N., and Santos, R. S. (eds) (2007). Seamounts: Ecology, Fisheries, and Conservation, Vol. 12. Oxford, UK: Blackwell, 527.
Prior, S., Chircop, A., and Roberts, J. (2010). Area-based management on the high seas: possible application of the IMO’s particularly sensitive sea area concept. Int. J. Mar. Coast. Law 25, 483–522. doi: 10.1163/157180810x525403
Reaka-Kudla, M. L. (1997). “The global biodiversity of coral reefs: a comparison with rain forests,” in Biodiversity II: Understanding and Protecting Our Biological Resources, eds M. L. Reaka-Kudla, D. E. Wilson, and E. O. Wilson (Washington, DC: Joseph Henry Press), 83–108.
Reed, J. K. (2002). Comparison of deep-water coral reefs and lithherms of southeastern USA. Hydrobiologia 471, 57–69.
Roberts, C. M., O’Leary, B. C., McCauley, D. J., Cury, P. M., Duarte, C. M., Lubchenco, J., et al. (2017). Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. U.S.A. 114, 6167–6175. doi: 10.1073/pnas.1701262114
Rogers, A. D. (1994). The biology of seamounts. Adv. Mar. Biol. 30, 305–350. doi: 10.1016/s0065-2881(08)60065-6
Rogers, A. D. (1999). The biology of Lophelia pertusa (Linnaeus 1758) and other deep-water reef-forming corals and impacts from human activities. Hydrobiologia 84, 315–406. doi: 10.1002/iroh.199900032
Rogers, A. D. (2018). The biology of seamounts: 25 years on. Adv. Mar. Biol. 79, 137–224. doi: 10.1016/bs.amb.2018.06.001
Rogers, A. D., Baco, A., Griffiths, H., Hart, T., and Hall-Spencer, J. M. (2007). “Corals on seamounts. Supplementary material (including Appendix 8.1),” in Seamounts: Ecology, Conservation and Management Fish and Aquatic Resources Series, eds T. J. Pitcher, T. Morato, P. J. B. Hart, M. R. Clark, N. Haggan, and R. S. Santos (Oxford: Blackwell).
Rowden, A. A., Anderson, O. F., Georgian, S. E., Bowden, D. A., Clark, M. R., Pallentin, A., et al. (2017). High-resolution habitat suitability models for the conservation and management of vulnerable marine ecosystems on the Louisville Seamount Chain, South Pacific Ocean. Front. Mar. Sci. 4:335. doi: 10.3389/fmars.2017.00335
Rowden, A. A., Stephenson, F., Clark, M. R., Anderson, O. F., Guinotte, J. M., Baird, S. J., et al. (2019). Examining the utility of a decision-support tool to develop spatial management options for the protection of vulnerable marine ecosystems on the high seas around New Zealand. Ocean Coast. Manag. 170, 1–16. doi: 10.1016/j.ocecoaman.2018.12.033
Sala, E., Mayorga, J., Bradley, D., Cabral, R., Atwood, T. B., and Auber, A. et al. (in review). Reconciling biodiversity protection, food production, and climate change mitigation in the global ocean. Nature.
Selig, E. R., Turner, W. R., Troëng, S., Wallace, B. P., Halpern, B. S., Kaschner, K., et al. (2014). Global priorities for marine biodiversity conservation. PLoS One 9:e82898. doi: 10.1371/journal.pone.0082898
Small, A. M., Adey, W. H., and Spoon, D. (1998). Are current estimates of coral reef biodiversity too low? The view through the window of a microcosm. Atoll Res. Bull. 458, 1–20. doi: 10.5479/si.00775630.458.1
Smith, D., and Jabour, J. (2018). MPAs in ABNJ: lessons from two high seas regimes. ICES J. Mar. Sci. 75, 417–425. doi: 10.1093/icesjms/fsx189
Teh, L. S. L., Teh, L. C. L., and Sumaila, U. R. (2013). A global estimate of the number of coral reef fishers. PLoS One 8:e65397. doi: 10.1371/journal.pone.0065397
UNEP (2007). Deep Sea Biological Diversity and Ecosystems: A Scoping Report on Their Socio-Economy, Management and Governance. Nairobi: UNEP.
UNGA (2015). Development of an International Legally Binding Instrument Under the United Nations Convention on the Law of the Sea on The Conservation And Sustainable Use Of Marine Biological Diversity Of Areas Beyond National Jurisdictionresolution Adopted By The General Assembly On 19 June 2015. New York, NY: United Nations General Assembly.
Visalli, M. E., Best, B. D., Cabral, R. B., Cheung, W. W. L., Clark, N. A., Garilao, C., et al. (2020). Data-driven approach for highlighting priority areas for protection in marine areas beyond national jurisdiction. Mar. Pol. (in press).
Wedding, L. M., Reiter, S. M., Smith, C. R., Gjerde, K. M., Kittinger, J. N., Friedlander, A. M., et al. (2015). Managing mining of the deep seabed. Science 349, 144–145.
Wilson, R. R., and Kaufmann, R. S. (1987). “Seamount biota and biogeography,” in Seamounts, Islands, and Atolls Geophysical Monograph, Vol. 43, eds B. H. Keating, P. Fryer, R. Batiza, and G. W. Boehlert (Washington, DC: American Geophysical Union), 355–378. doi: 10.1029/gm043p0355
Worm, B., Lotze, H. K., and Myers, R. A. (2003). Predator diversity hotspots in the blue ocean. Proc. Natl. Acad. Sci. U.S.A. 100, 9884–9888. doi: 10.1073/pnas.1333941100
Keywords: areas beyond national jurisdiction, area-based management tools, coral reef, marine protected area, seamount, reef-building, Scleractinia, vulnerable marine ecosystems
Citation: Wagner D, Friedlander AM, Pyle RL, Brooks CM, Gjerde KM and Wilhelm T‘A (2020) Coral Reefs of the High Seas: Hidden Biodiversity Hotspots in Need of Protection. Front. Mar. Sci. 7:567428. doi: 10.3389/fmars.2020.567428
Received: 29 May 2020; Accepted: 25 August 2020;
Published: 14 September 2020.
Edited by:
Santiago Herrera, Lehigh University, United StatesReviewed by:
Jeff A. Ardron, University of Southampton, United KingdomCopyright © 2020 Wagner, Friedlander, Pyle, Brooks, Gjerde and Wilhelm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Wagner, ZHdhZ25lckBjb25zZXJ2YXRpb24ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.