- 1GEOMAR Helmholtz Centre for Ocean Research Kiel, Kiel, Germany
- 2Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, TAS, Australia
Coccolithophores are an important group of marine phytoplankton which cover themselves with the coccosphere – a shell composed of numerous calcium carbonate (CaCO3) platelets. Despite more than a century of coccolithophore research, it remains speculative why coccolithophores calcify. Resolving this question is essential to assess the competitive fitness of coccolithophores in the future ocean where changes in calcification are expected. Here, we used the Emiliania huxleyi – Emiliania huxleyi Virus 86 host-virus model system to test the hypothesis that the coccosphere serves as a physical barrier reducing viral infection. Therefore, we removed the coccosphere from living E. huxleyi cells and compared the infection progress relative to calcified cells in a series of 6 experiments under different growth conditions. We found that the coccosphere does not constitute an effective physical barrier against viral penetration, since non-growing calcified cells were susceptible to viral infection and lysis (growth stopped by light limitation). However, we also found that protection against the virus may depend on the daily growth cycle. E. huxleyi reached higher peak abundances when decalcified cells were allowed to rebuild their coccosphere before entering cell division phase and being exposed to the virus, thereby suggesting that rates of viral infection could be reduced by the coccosphere during the critical phase in the cell cycle. However, the benefit of this potential protection is arguably of limited ecological significance since the concentrations of both, calcified and decalcified E. huxleyi approached similar values until the end of the bloom. We conclude that the coccosphere provides at best a limited protection against infection with the EhV86.
Introduction
Coccolithophores are a group of marine planktonic algae which cover themselves with a shell (coccosphere) composed of multiple calcified platelets (coccoliths). They appear for the first time in the late Triassic and are present with variable diversity ever since (Bown et al., 2004). Coccolithophores contribute ∼1–10% to marine primary production (Poulton et al., 2007) and ∼50% to open ocean calcium carbonate (CaCO3) sediments (Broecker and Clark, 2009).
Research on coccolithophore calcification has mostly focused on the intracellular mechanisms controlling calcification, the environmental factors influencing calcification, and the biogeochemical processes involving CaCO3 (Riebesell et al., 2000; Brownlee and Taylor, 2004; Mackinder et al., 2010; Riebesell and Tortell, 2011). The question why coccolithophores calcify has received much less attention so far but was recently highlighted as one of the most critical knowledge gaps in coccolithophore research (Monteiro et al., 2016). Without understanding the purpose of this key trait, it will be difficult to determine the relevance of projected changes in calcification rates for the competitive fitness of these organisms in the future ocean (Bach et al., 2015). Numerous hypotheses exist why coccolithophores calcify (Young, 1994; Raven and Crawfurd, 2012; Taylor et al., 2017; Müller, 2019). Recently, Monteiro et al. (2016) pointed out that the coccosphere may have different functions in different coccolithophore species but that the protection against grazing and/or virus and bacterial infection is potentially the one function that could be of universal benefit to most of them.
Emiliania huxleyi is the most abundant coccolithophore in the contemporary oceans (Tyrrell and Young, 2009) and regularly forms large blooms which are often terminated by viral infections (Bratbak et al., 1993; Brussaard et al., 1996; Wilson et al., 2002b; Schroeder et al., 2003). Some E. huxleyi viruses (EhVs) have been isolated from water samples during those algal blooms (Castberg et al., 2002; Schroeder et al., 2002; Wilson et al., 2002a) and the E. huxleyi-EhV system has been frequently studied as a representative host-virus model within the eukaryotic phytoplankton.
The EhVs are coccolithoviruses which belong to the Phycodnaviridae and are nucleocytoplasmic large double-stranded DNA viruses (Schroeder et al., 2002). The genetic material in the EhV virion is encased in a capsid of icosahedral shape and surrounded by a lipid membrane. The virion is thought to enter the host via membrane fusion (Mackinder et al., 2009) where the attachment takes place at distinct lipid-raft-microdomains in the cell membrane characterized by aggregations of specific glycosphingoplipids (Vardi et al., 2009, 2012; Bidle and Vardi, 2011). The virus possesses RNA polymerase genes and presumably replicates at least partly in the cytoplasm (Wilson et al., 2005). In the course of its replication cycle the virus modifies its host’s lipid synthesis (Evans et al., 2009; Fulton et al., 2014; Rose et al., 2014; Rosenwasser et al., 2014; Hunter et al., 2015), makes use of its programed cell death pathway (Bidle et al., 2007; Vardi et al., 2009; Bidle and Vardi, 2011), and affects autophagy-like processes to generate viral progeny (Schatz et al., 2014). New virions are released by a budding mechanism (Mackinder et al., 2009) which ultimately leads to the lysis of the host cell.
It has been suggested that the coccoliths serve as a physical barrier which block the virus from entering the cell (Castberg et al., 2002; Mackinder et al., 2009). Indeed, microscopic observations indicated that viral particles were blocked by the coccosphere and detached again quickly from calcified cells of E. huxleyi (Mackinder et al., 2009). Johns et al. (2019) recently provided evidence that loose coccoliths in the water column protect against viral infection by binding free virions which then become unable to infect further hosts.
To further clarify the role of the coccosphere in viral infection we conducted several culture experiments to test whether the coccosphere could improve growth and resistance of E. huxleyi when exposed to the E. huxleyi Virus 86. We removed the coccospheres of the cells by a short acid-base “decalcification” treatment or by growing cells in medium with depleted calcium ion concentration. Naked and calcified cells were then exposed to the virus to test whether coccolith bearing cells are better protected against infection. Our hypothesis tested in this study is that the coccosphere of E. huxleyi reduces viral penetration into the host cell thereby increasing their survival.
Materials and Methods
Overview
We conducted 6 experiments in which we exposed cultures of E. huxleyi (CCMP1516) to the EhV86 and monitored the abundances of the host and the virus over time. In each experiment we compared the course of infection between calcified cells and naked cells, of which the coccospheres were removed by either an acid-base treatment or by growing them in Ca2+-depleted medium (detailed description below). We then exposed naked and calcified cells at equal cell densities to the EhV and measured the abundances of host cells and viral particles over the following days. At the same time, we monitored the abundance of E. huxleyi in cultures without virus to examine whether the respective coccosphere-removal procedure influenced the development of E. huxleyi. Consequently, each experiment was conducted in a 2 × 2 factorial design (calcified without virus, naked without virus, calcified with virus, naked with virus). The 6 experiments described in this paper differ (i) in the way the coccoliths were removed (acid-base treatment or low [Ca2+] medium) and (ii) whether the virus was added to actively growing cultures in light conditions, or to cultures where growth was stopped by keeping them in the dark. Each of the two coccosphere-removal methods (acid-base treatment or low [Ca2+] medium) was conducted once in a light-dark cycle and once in permanent darkness. Thereafter, we conducted a further acid-base experiment in a light-dark cycle, but modified the timing of the coccosphere removal and the virus addition. At the same time, we tested whether coccoliths that detached from the cells surface reduce the number of infective particles.
A detailed description of each of the 6 experiments is provided later in the “Materials and Methods” section after we have described the applied methodology. Table 1 provides an overview of the experiments.
Basic Culturing Conditions
The culture medium was prepared with sterile filtered (0.2 μm) artificial seawater (Kester et al., 1967). We added sodium bicarbonate to gain a total alkalinity of 2350 μmol kg–1 and aerated the artificial seawater over night to ensure atmospheric equilibrium regarding the carbonate system. The artificial seawater was enriched with 64 μmol kg–1 NaNO3, 4 μmol kg–1 NaH2PO4, 10 nmol kg–1 SeO2, vitamins and trace metals according to the f/8 medium (Guillard and Ryther, 1962). Cultures of E. huxleyi were grown in a 12:12 h light-dark cycle with a photon flux density (PAR) of 230 μmol photons m–2 s–1 (measured with a LI-COR LI-250A light meter) at 15°C until they were used for the respective experiments.
Procedures to Remove the Coccospheres
In the low [Ca2+] experiments, naked cells of E. huxleyi were obtained by using artificial seawater with a 100-fold lower Ca2+ concentration (0.1 mmol kg–1) than in the usual recipe by Kester et al. (1967). Apart from the calcium concentration (and therefore a slightly lower salinity of 33.7) the culture medium and the culture conditions were the same as mentioned above.
For the decalcification experiments, we conducted previous tests to ascertain the gentlest way to dissolve the calcite shell with acid and base without causing too much harm to the cells. The best results, in terms of a complete removal of the coccosphere at a minimum number of cell death, were obtained by using 2.5 mL of 1N hydrochloric acid (HCl) per L of E. huxleyi culture with ∼50 × 103 cells mL–1. After the addition of the acid, the culture bottle was mixed gently, but thoroughly for 1 min. Thereafter, the pH was brought back to the value before the acid addition by adding 1N sodium hydroxide (NaOH) solution. The culture bottle was gently mixed once more after the NaOH addition until all flocculation from the addition of NaOH was dissolved (∼2 min of mixing was required for this). The decalcification procedure was conducted with a large culture volume (2.3 L) to keep the headspace at a minimum relative to the volume of the culture. This was done to prevent elevated outgassing of CO2 during the brief low pH/high CO2 period. The addition of HCl and NaOH caused a decrease in the cell concentrations of 7–10%, likely because cells died in consequence of the direct contact with the concentrated acid and base during injections. To assure no further cell death after the treatment, we determined the cell concentration repeatedly by flow cytometry (see below) over a period of 30 min to ensure that it remained stable. To account for the fraction of dead cells in the decalcified cultures, the cell concentration in the calcified culture was diluted to the same level prior to the start of the experiments. This was done by filtering out the excess cells with a 0.2 μm syringe filter.
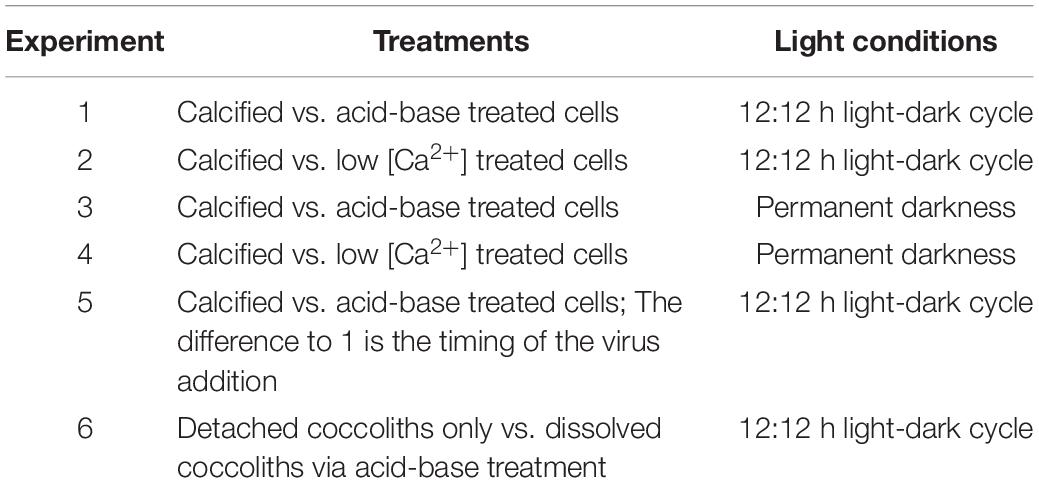
The absence of coccoliths after the acid base treatment was checked by flow cytometry and by cross polarized light microscopy using an inverted microscope (Carl Zeiss Axiovert 100). This microscopy method reveals calcium carbonate as bright shining crystals where calcite in and outside E. huxleyi can be easily seen (Figure 1).
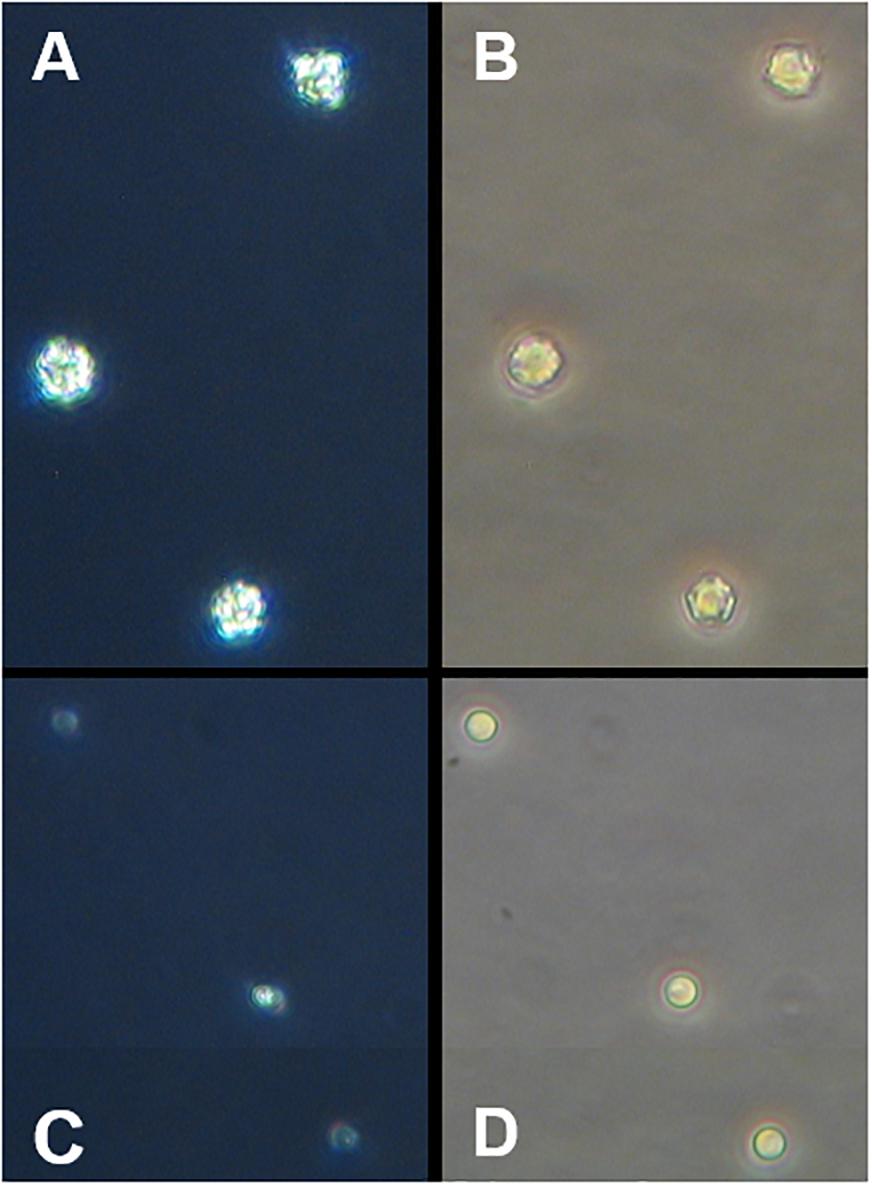
Figure 1. Microscopic images of E. huxleyi (400x magnification). (A) Calcified cells under polarized light, (B) light microscopic picture from the same cells, (C,D) display cells after the treatment with acid and base.
Preparation of Virus Lysate
Fresh isolates of the EhV86 were produced with E. huxleyi CCMP1516 cultures. Therefore, cultures of the host cells were grown to a concentration ∼ 250 × 103 cells mL–1 in 15°C and then inoculated with 0.5 mL viral lysate. The original virus lysate was kindly provided by Dr. Declan Schroeder. The E. huxleyi population crashed within 5 days due to viral infection. The lysate was 0.45 μm filtered and stored at 4°C in the dark until it was used for the experiments (the storage time of lysate was between 1–5 days).
Enumeration of Algal Cells and Viral Particles
Subsamples for cell counts were taken by transferring 1 mL of the cultures into Eppendorf tubes. Algal cell concentrations were measured at a flow rate of 66 μL min–1 in an Accuri C6 flow cytometer (Becton Dickinson). E. huxleyi could be determined based on the chlorophyll fluorescence and the side-scatter signal (SSC). The SSC is the light scattered at right angle when the cells pass the laser beam of the flow cytometer and it is expressed in values without a unit. Calcified cells scatter more light at right angle and thus induce higher SSC signals compared to naked cells (Olson et al., 1989).
Viral particles were quantified following Brussaard (2004). Briefly, 1 mL subsamples were transferred into Cryovials and fixed with electron microscopy grade glutaraldehyde (0.25% final concentration). The samples were incubated at 4°C for 30 min then flash-frozen in liquid nitrogen and stored at -80°C. For the analysis the samples were diluted in TE (Tris-EDTA, pH 8) buffer, stained with SYBR Green I, heated for 10 min at 80°C and measured with a FACSCalibur flow cytometer (Becton Dickinson) at low flow rate of ca. 15 μL min–1. The viral particles in the samples could be identified based on the SYBR Green labeled DNA fluorescence and the SSC.
Experimental Setup
Experiment 1 (Acid-Base/Light-Dark Cycle)
In Experiment 1 calcifying E. huxleyi (CCMP1516) were grown in a 12:12 h light-dark-cycle and naked cells were obtained with the acid-base treatment. E. huxleyi was grown in a volume of 5 L to a concentration of ∼35 × 103 cells mL–1. Thereupon the culture was split into two 2.3 L polycarbonate bottles. One of the bottles was treated with acid and base as described above to remove the coccoliths, while the other bottle that contained calcified cells was only diluted with growth medium to adjust the cell concentrations. Both cultures were further split into six 250 mL glass bottles. Three of these smaller bottles were inoculated with viral lysate while no virus was added into the other three bottles. All these steps were conducted at the end of the dark period under low light conditions (< 0.01 μmol photons m–2 s–1). The virus was inoculated just minutes before the following light period began (please note that the timing of the coccoliths removal and subsequent virus addition is important as will be discussed later). The virus lysate was pipetted in equal amounts into the replicate bottles. The cultures were homogenized every 1.5 h over the day by gently turning the bottles and mounted on a plankton wheel (one round min–1) during night time in order to prevent unequal sedimentation of the algae among the treatments.
We performed Experiment 1 also (and simultaneously) with a non-calcifying strain of E. huxleyi (RCC 1242). This was done to investigate the consequences of the acid-base treatment on a strain that does not form coccoliths and to reveal possible effects of the acid-base treatment on the interplay between the host and the virus irrespective of calcification.
Experiment 2 (Low [Ca2+]/Light-Dark Cycle)
In Experiment 2 naked cells of E. huxleyi were obtained using low [Ca2+] growth medium. Apart from this, the procedures were similar as in Experiment 1. E. huxleyi was grown in two bottles (2.3 L), one of which contained normal medium and the other contained medium with a low [Ca2+]. Both cultures were grown in a 12:12 h light-dark cycle to 35 – 40 × 103 cells mL–1. The E. huxleyi concentrations in both bottles were diluted to the same level with the respective medium and then each culture was further subdivided into six 250 mL glass bottles. These steps were performed at the end of the dark phase under very low light conditions. Virus lysate was added in equal amounts into three bottles with low [Ca2+] and three bottles with normal growth medium. Thereafter, all 12 culture bottles were put back into the light.
Experiment 2 was also conducted simultaneously with the non-calcifying strain of E. huxleyi to test whether the low [Ca2+] only prevented calcification, or had further impacts on the host-virus interaction.
Experiment 3 (Acid-Base/Permanent Darkness)
In Experiment 3 E. huxleyi was raised in a 5 L bottle up to ∼40 × 103 cells mL–1 in a 12:12 h light-dark-cycle. When this concentration was reached the culture was kept in the dark for the remainder of the experiment. After 36 h in darkness, the culture was split into two 2.3 L bottles and one was treated with acid and base as described above. The 36 h period in the dark ensured that all metabolic energy reserves for calcification were consumed before the cells were decalcified. Previous experiments had shown that the decalcified cells were able to rebuild their coccosphere in the dark when the decalcification treatment was applied after a regular 12 h dark phase.
Subsequently, both cultures were further split into the six replicate bottles and the virus was added to three of them.
Experiment 4 (Low [Ca2+]/Permanent Darkness)
Experiment 4 was identical to Experiment 3 except that the naked cells were obtained by growth in low [Ca2+] medium. E. huxleyi was grown in two cultures (2.3 L), one with low [Ca2+] medium and the other with normal growth medium. E. huxleyi was grown to ∼70 × 103 cells mL–1 in a 12:12 h light-dark cycle whereupon the culture was kept in the dark from then onward. After 36 h the cell concentrations in both bottles were adjusted to the same level by diluting the calcified culture with the respective medium. Subsequently, both cultures were further split into the 6 replicate bottles and the virus was added to three of them.
Experiment 5 (Acid-Base/Light-Dark Cycle)
In Experiment 5 naked E. huxleyi were obtained with the acid-base treatment. E. huxleyi was grown to ∼100 × 103 cells mL–1 in a 12:12 h light-dark cycle. The culture was split into two bottles and one was treated with acid and base. Then, both cultures were brought to the same cell concentration and further split into the replicate bottles. Experiment 5 was conducted in the same way as Experiment 1 except of one difference. The dissolution of the calcite and the subsequent addition of the virus were carried out in the middle of the light period, instead of the end of the dark period. That way, E. huxleyi was able to calcify for half of the light period (6 h) before the experiment started.
Experiment 6 (Absorption of Viral Particles by Coccoliths)
In Experiment 6 we tested the influence of coccoliths that are lost from the coccosphere on the viral particle concentration. For this purpose, a fraction of the initial 5 L stock culture from Experiment 5 was gently filtered through a 5 μm syringe filter. The filtrate contained no E. huxleyi cells but detached coccoliths which are smaller than 5 μm. The suspension with detached coccoliths was split into two bottles one of which was treated with acid and base (see above) in order to dissolve the coccoliths. Both bottles were further separated into triplicates and virus stock culture was added in equal amounts. The number of viral particles was measured in both treatments after 24 and 48 h to test whether the viral particle concentrations were lower in the replicates that contained detached coccoliths.
Data Analysis
In each experiment the concentrations of E. huxleyi in the four treatment groups were compared over time based on the sample mean () ± 95% confidence interval (CI), which was calculated with the standard error (se) and the respective t-distribution ( ± tn–1 ∗ se). Each treatment group contained n = 3 replicates. We calculated the mean logarithmic response ratio (L) ± 95% CI (Hedges et al., 1999) to measure the effect the virus caused within the treatments at a given sampling time point:
where the subscripts p and a describe whether the virus was present or absent and SD is the sample standard deviation. L standardizes the ratio of the mean cell concentration in the infected cultures and the mean cell concentration from the respective replicates without virus. This was done because both methods that were used to remove the coccosphere had the potential to affect the concentration of E. huxleyi. For example, when L = 0 = ln (1), there is no effect and the mean cell concentration of the replicates with virus did not differ from the mean host abundance of the respective replicates without virus. A value of L = −0.69 = ln (0.5) could illustrate that E. huxleyi reached only half the concentration in the cultures that were exposed to the virus than in the respective cultures without virus. The effect of the virus within the treatments, calcified vs. naked cells, was then compared based on the effect size and the corresponding 95% CI between the treatments. We consider the effect size to be significantly different between the treatments when the confidence intervals do not overlap (α = 0.05).
Results
Experiment 1 (Acid-Base/Light-Dark Cycle)
When no virus was added, the concentrations of the calcified and decalcified cells did not differ (Figure 2A). In both groups the cells continued to grow exponentially and the acid-base treatment did not affect the growth of E. huxleyi. When the virus was present, the maximum abundances of E. huxleyi were measured 24 h post infection. Afterwards, the cell concentrations decreased due to infection and death in both treatments (Figure 2B). An effect of the virus was observed 24 h post infection in both, the calcified (Lcontrol = −0.12 ± 0.05) and the decalcified (Lacid–base = −0.20 ± 0.02) cultures and the decalcified cells showed a marginally, but significantly lower mean abundance. After 2 days, however, the concentrations were equal again in both treatments and showed the same temporal development until the end of the experiment (Figure 2B). The number of viral particles increased drastically between day one and two post infection and did not differ between the treatments (Figure 2C).

Figure 2. Addition of virus to normal cells of E. huxleyi (controls = blue symbols) and to cells treated with acid and base (red symbols). Virus was added at the beginning of the light period (12:12 h light-dark cycle). Symbols represent the sample means and ribbons illustrate the 95% CI of the sample mean (n = 3). The acid-base treatment was conducted at the end of the dark phase before virus addition. (A) Concentration of calcifying E. huxleyi CCMP1516 without virus; (B) cell concentration when virus was added; (C) concentration of viral particles. (D) Concentrations of non-calcifying E. huxleyi RCC1242 without virus and (E) when virus was added; (F) concentration of viral particles. Light-dark cycle is indicated in (A,D).
The acid-base treatment had a positive effect on the growth of the non-calcifying E. huxleyi strain (RCC1242) (Figures 2D,E). The cells which were treated with acid and base showed higher abundances than the controls. This difference seemed to be more pronounced in the cultures that were exposed to the virus (Figure 2E) than in those without virus (Figure 2D). However, a significant difference in effect size between the treatments could be observed at no time. When the cell concentrations in the infected cultures were set in relation to the concentrations the cells reached without virus, the effect of the virus was similar in both treatments. The collapse of the cultures in consequence of viral infection was much slower compared to the calcifying E. huxleyi CCMP1516. The numbers of viral particles increased in both treatments after 2 days and it was slightly higher in the untreated control cultures (Figure 2F). On day 4 and 5 post infection, the concentration of viral particles was higher in the acid-base treatment. However, at this time there were also more E. huxleyi cells present. Thus, there were more hosts for viral replication.
Figure 3 shows the concentrations of the calcified E. huxleyi CCMP1516 within the first 24 h in Experiment 1, as well as the respective side scatter (SSC) of the cells from the flow cytometry measurements. The SSC is indicative for the degree of calcification of a cell (Hansen et al., 1996) and it increases with an increasing amount of coccoliths on the cells surfaces. The data show a typical pattern of the SSC in relation to the daily light cycle in all treatment groups. The values increased over the course of the light period and were highest at the beginning of the dark phase (Figure 3A). The cells divided in the night (Figure 3B) which caused a decrease of the SSC because the coccoliths were shared between two daughter cells. The mean SSC of the decalcified cells was about 68% lower right after the acid-base treatment compared to the calcified cells. In the course of the subsequent light period the decalcified cells reconstructed their coccospheres and their SSC increased again. They apparently formed a new coccosphere within 6 h, because at that time their SSC reached the same values as measured from the calcified cells at the start of the experiment (Figure 3A). After 24 h, the differences in the SSC between the decalcified and the calcified cells were almost compensated.
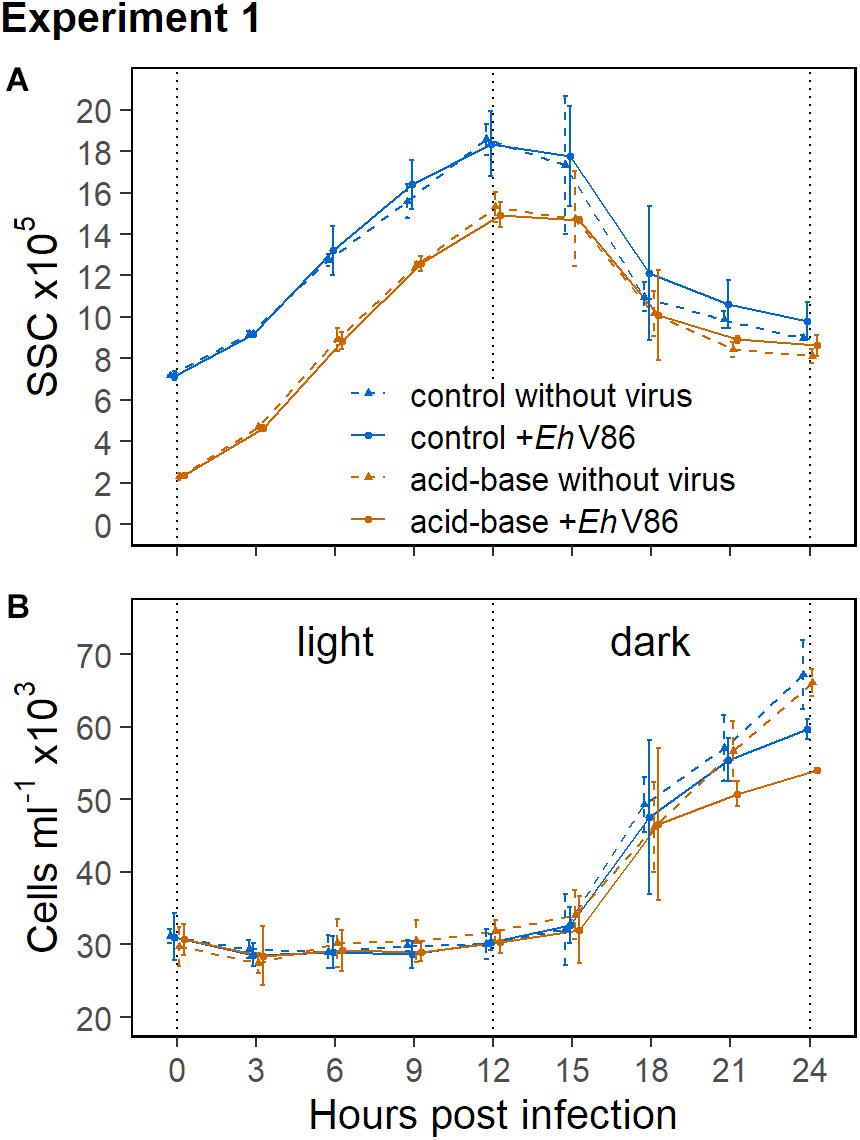
Figure 3. Results of the calcifying E. huxleyi (CCMP1516) within the first 24 h of Experiment 1. Decalcified cells scatter less light (SSC) than calcified cells (A). The SSC increased during the light period and cell division took place in the dark (B). Mean ± 1 SD (n = 3).
Experiment 2 (Low [Ca2+]/Light-Dark Cycle)
E. huxleyi grew minimally slower in the low [Ca2+] medium and reached lower maximum abundances than in normal growth medium (Figure 4A). The cells did not produce coccoliths under low [Ca2+]. The virus stopped growth of the calcified cells after 24 h (Figure 4B) (Lcontrol = −0.39 ± 0.09, Llow[Ca2+] = −0.02 ± 0.26) whereas it took 48 h in the low [Ca2+] treatment (Lcontrol = −1.53 ± 0.05, Llow[Ca2+] = −0.27 ± 0.10). E. huxleyi reached substantially higher abundances under viral infection in the low [Ca2+] treatment (Figure 4B). Concomitantly, the production of viral particles was initially lower under low [Ca2+], but toward the end of the experiment the concentration of viral particles was higher (Figure 4C) in line with a higher concentration of host cells.
Figure 4. Addition of virus to normal cells of E. huxleyi (controls = blue symbols) and to cells that were grown under low [Ca2+] (red symbols). Virus was added at the beginning of the light period (12:12 h light-dark cycle). Symbols represent the sample means, ribbons the 95% confidence interval (n = 3). (A) Concentration of calcifying E. huxleyi CCMP1516 without virus and (B) when virus was added; (C) concentration of viral particles. (D) Concentration of non-calcifying E. huxleyi RCC1242 without virus and (E) when virus was added; (F) concentration of viral particles.
The non-calcifying E. huxleyi (RCC1242) showed equal growth in both treatments when no virus was added (Figure 4D). When the virus was present, non-calcifying E. huxleyi reached higher abundances in the low [Ca2+] treatment which is similar to the response observed in the calcifying strain (compare Figures 4B,E).
Overall, we observed that the effect of the virus was weakened under low [Ca2+] in both, the calcifying and the non-calcifying strain of E. huxleyi. Thus, [Ca2+] influenced the infection of E. huxleyi by EhV86 irrespective of whether the cells possessed a coccosphere or not.
Experiment 3 (Acid-Base/Permanent Darkness)
Without virus, the concentrations of the calcified E. huxleyi remained stable throughout the prolonged darkness (Figure 5A). A significant decrease of the calcified cells due to viral infection could be observed from day 6 post infection (Lcontrol = −0.13 ± 0.06) (Figure 5B). Thus, the virus was able to infect E. huxleyi through the coccosphere even though cell division did apparently not occur.
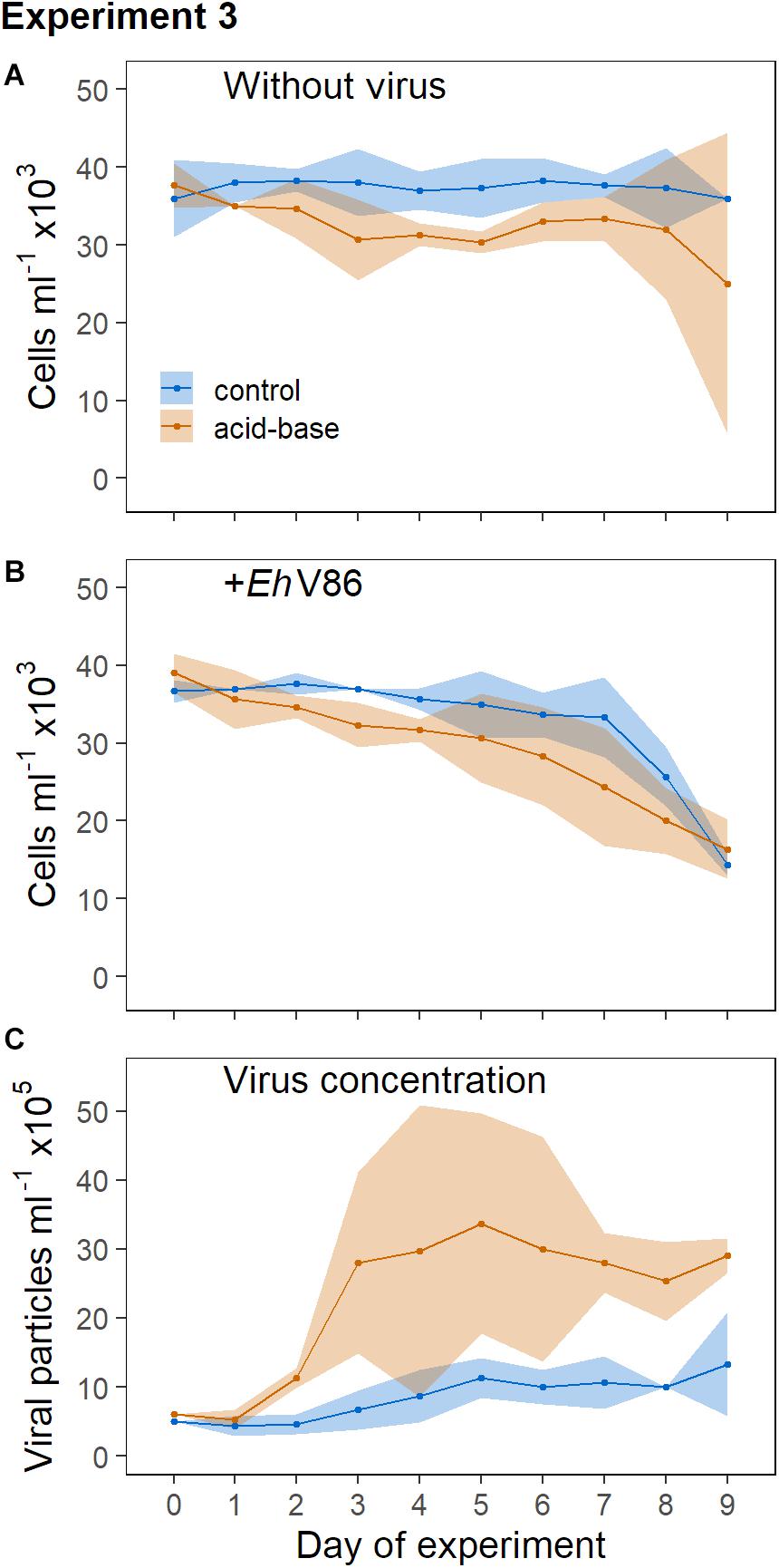
Figure 5. Addition of virus to calcified and decalcified cells in permanent darkness. Sample mean ± 95% CI (n = 3). (A) Concentration of calcifying E. huxleyi CCMP1516 without virus and (B) when virus was added; (C) concentration of viral particles.
In the dark, E. huxleyi was not able to cope with the acid-base treatment as good as in the light (where growth was basically unaffected, Figure 2A), as can be seen in the declining cell numbers (Figure 5A). An effect of the virus on the decalcified cells could be measured on day 7 (Lacid–base = −0.31 ± 0.19) and 8 (Lacid–base = −0.47 ± 0.22), but on day 9 the variability of the cell concentrations in the cultures without virus increased considerably (Figure 5A). The decrease of the cell concentrations due to the virus did not differ between the treatments. However, the production of new viral particles was higher in the cultures that contained decalcified cells (Figure 5C).
Experiment 4 (Low [Ca2 + ]/Permanent Darkness)
The concentrations of the calcified E. huxleyi remained stable for 6 days in the dark without virus, while the low [Ca2+] cells decreased in concentration (Figure 6A). Experiment 4 confirmed that the virus was able to infect the calcified cells in the darkness when cell division ceased (Figure 6B). The concentrations showed a strong decrease on day 4 post infection (Lcontrol = −0.60 ± 0.12) and declined steadily thereafter. The concentration of viral particles increased (Figure 6C). Interestingly, from day 4 post infection onward we measured a positive effect of the virus on the algal concentration under low [Ca2+] (L = 0.20 ± 0.04, L = 0.31 ± 0.07 on day 9). The E. huxleyi concentration was higher when the virus was present (compare Figures 6A,B). Thus, the treatment did not only prevent calcification, but in some way influenced the constitution of the host cells, or the interplay between the host and the virus. In the low [Ca2+] treatment, the presence of the virus seemed to promote the survival of the host cells in permanent darkness. From day 4 until the end of the experiment the cell concentrations were consistently higher in the infected cultures than in those without virus (Llow [Ca2+] = 0.20 ± 0.04, Llow [Ca2+] = 0.31 ± 0.07 on day 9). The viral particle concentration remained stable under low [Ca2+] over the course of the experiment.

Figure 6. Addition of virus to calcified cells and cells under low [Ca2+] in permanent darkness. Sample mean ± 95% CI (n = 3). (A) Concentration of calcifying E. huxleyi CCMP1516 without virus and (B) when virus was added; (C) concentration of viral particles.
Experiment 5 (Acid-Base/Light-Dark Cycle)
In Experiment 5 we tested whether the calcifying E. huxleyi were better protected against viral infection when the cells were able to calcify for 6 h in the light before the virus was added. Therefore viruses were not added directly at the beginning of the light period (as it was done in Experiment 1) when the population had just gone through cell division. Instead, the virus was added 6 h after the light phase had begun. During this period the cells were able to produce additional coccoliths.
Without virus, the concentrations of the decalcified and the calcified cells showed a similar development (Figure 7A). However, in the presence of the virus the decalcified cells reached substantially lower peak concentrations than the calcified cells (Figure 7B; but note that the mean cell concentration at the start of the experiment was about 3% lower in the acid-base treatment, because the balancing of the concentrations of both treaments did not work out precisely). Indeed, at the onset of the following light period (18 h post infection) the virus had a stronger effect on the decalcified cells (Lacid–base = −0.56 ± 0.03) than on the calcified cells in the control treatment (Lcontrol = −0.20 ± 0.03). The differences between the treatments increased even further 24 h post infection (Lacid–base = −0.76 ± 0.09, Lcontrol = −0.28 ± 0.02). Additionally, the initial production of viral particles was higher in the replicates which contained decalcified cells (Figure 7C). Please note that the samples for the quantification of viral particles taken at the start of this experiment (day 0) were lost. Thus, the value shown at day 0 is the mean of three subsamples taken from the initial virus lysate solution, which was pipetted in equal amounts into the replicates, as starting point for both treatments (Figure 7C). (Cytograms of Experiment 5 are provided as Supplementary Material).
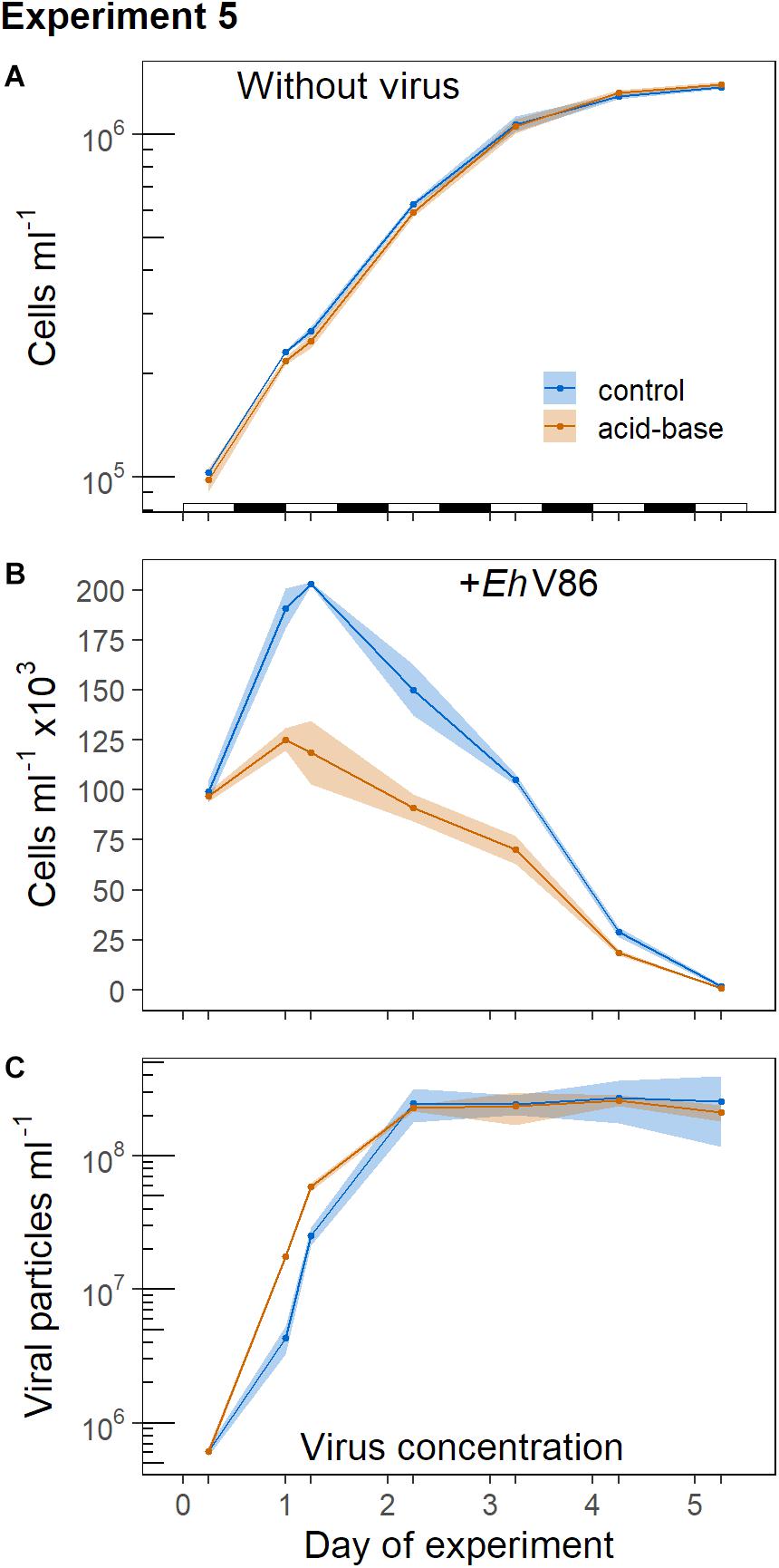
Figure 7. Virus addition to calcified and decalcified cells in the middle of the light period, subsequent to the acid-base treatment. Sample means and 95% CI (n = 3). (A) Concentration of calcifying E. huxleyi CCMP1516 without virus and when virus was added (B); (C) concentration of viral particles. The alteration of the light and dark phases is indicated in (A).
Experiment 6 (Absorption of Viral Particles by Coccoliths)
In Experiment 6 we tested whether the difference in viral infection between calcified and decalcified cells was due to the adsorption of viral particles to detached coccoliths. Therefore, viral lysate was added to a suspension containing only detached coccoliths and the number of viral particles was compared to cultures in which the coccoliths in the suspension were dissolved with acid and base, before the virus was added. The coccolith suspension was obtained from the initial culture used in Experiment 5, prior to its separation into the treatments, by filtering out the E. huxleyi cells. The idea was to set up the same ratio of detached coccoliths to viral particles as in Experiment 5, in which the protective effect of the coccosphere was tested. The number of viral particles did not decrease stronger in the replicates in which coccoliths were present (Figure 8). An effect of the coccoliths on the viral abundance could not be observed with this approach.
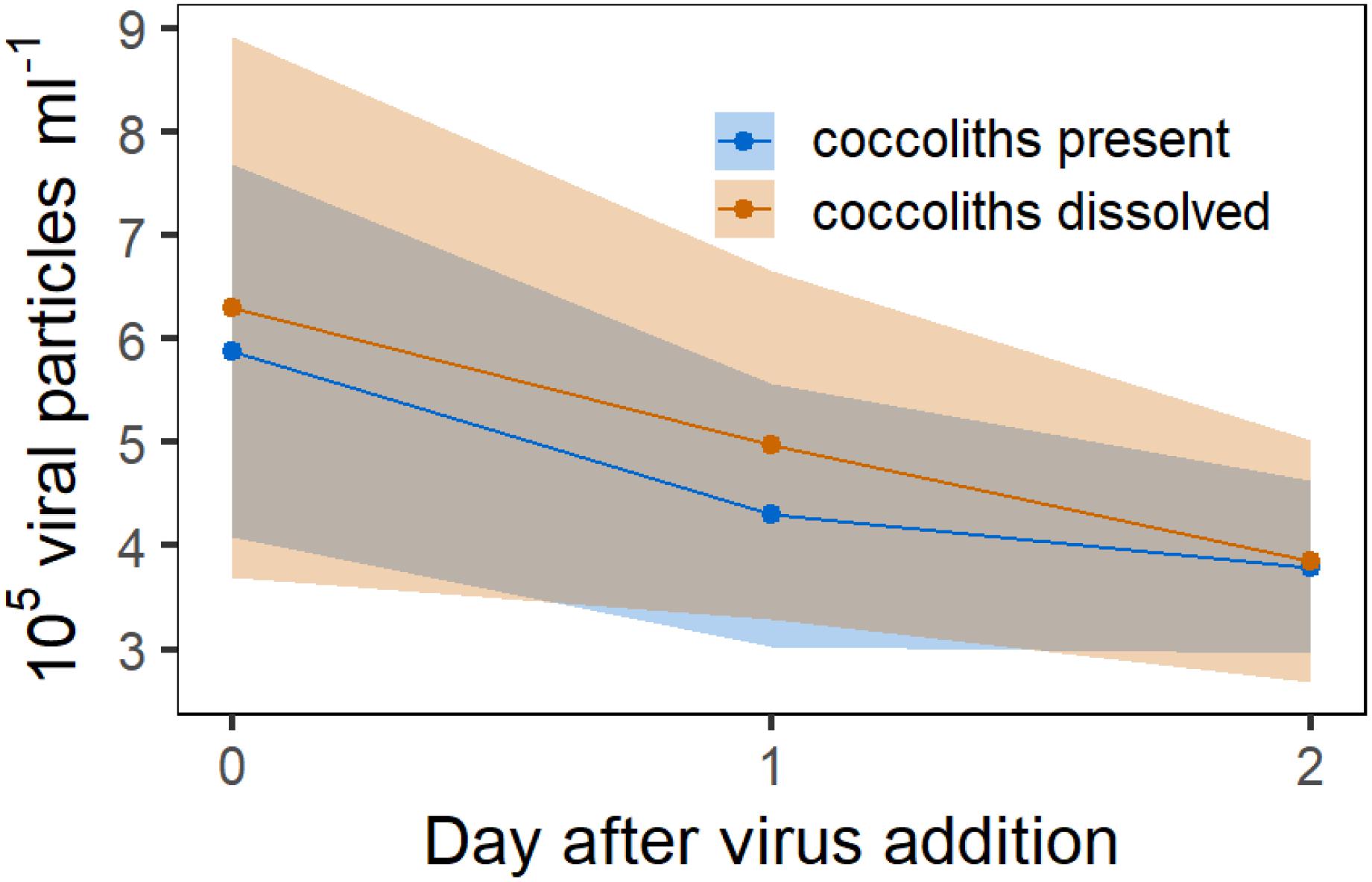
Figure 8. Experiment 6. Counts of viral particles in a 5 μm filtered culture of E. huxleyi which contained no algal cells but detached coccoliths. Red symbols represent mean concentration of viral particles in the solution in which the coccoliths were dissolved with acid and base. Ribbons represent the 95% CI.
Discussion
The Efficiency of the Coccosphere to Reduce Viral Infection
It has been hypothesized that the coccosphere can protect E. huxleyi from becoming infected with the virus and that the viral particles mainly attack the host during cell division (Castberg et al., 2002; Mackinder et al., 2009). When the cell divides, parts of the cell surface are not covered with coccoliths so that the virus should more easily reach and attach to the hosts plasma membrane to enter the cell. Nevertheless, infection can still occur through an intact coccosphere, probably due to gaps between the coccoliths (Mackinder et al., 2009). In both experiments that were conducted in permanent darkness, the virus was able to infect the calcified E. huxleyi, although cell division did not occur (Figures 5, 6). These findings support the notion that the EhV86 particles can penetrate the coccosphere to reach the cell membrane. However, the dark experiments did not conclusively show if the coccosphere could reduce viral infection rates because both, the acid-base as well as the low [Ca2+] treatment also influenced the concentrations of the naked cells that were not exposed to the virus. In the light-dark cycle experiments, the low [Ca2+] mitigated the course of infection in both strains of E. huxleyi, but the acid-base treatment did not alter the effect of the virus on non-calcifying E. huxleyi. We therefore had a closer look at the decalcification experiments in the light as will be discussed in the following.
Exponentially growing cells of E. huxleyi commonly show a synchronized cell cycle along with the light-dark-cycle, whereby the cells grow during the day and divide in the dark phase (Paasche, 1967; Jacquet et al., 2002; Müller et al., 2008). This was also the case in the light-dark cycle Experiment 1, where the virus was added to the growing culture at the onset of the light period and thus right after the majority of the population had just divided (Figure 3). At this point the coccospheres had just been distributed between the dividing cells. We hypothesized that viral particles could more easily reach the organic part of the cells in this phase because the coccosphere would have more gaps between the coccoliths directly after cell division. And indeed, the difference in peak abundance between the calcified and the decalcified E. huxleyi cells was relatively small (Figure 2). To understand if the assumed gaps influence infection we conducted Experiment 5, where the cells had 6 h in the light to calcify before the acid-base treatment was conducted and the virus was added. However, Experiment 5 did not confirm this hypothesis. The effect of the virus on the calcified cells was the same as in Experiment 1 (compare Figures 2B, 7B). In both experiments, the calcified E. huxleyi approximately doubled in number before the concentrations declined. Thus, the infection of calcified cells does rather not depend on the length of time the cells have in the light to build the coccosphere. Paasche (2002) noted that cells of E. huxleyi are completely covered with coccoliths directly after cell division so that “no part of the cell surface is left exposed.” This is confirmed by recent observations on dividing cells of Coccolithus braarudii, which show that the coccosphere is largely maintained throughout cell division with no obvious gaps being left when the cells divided (Walker et al., 2018). It was further shown that the cellular ratio of calcium carbonate to organic carbon remains relatively stable throughout the cell cycle of E. huxleyi (Kottmeier et al., 2020). The production of calcium carbonate during the light phase is closely linked with the increase in biomass and volume of exponentially growing cells (Müller et al., 2008; Kottmeier et al., 2020). Thus, the number of coccoliths remains relatively stable in relation to the volume of the cells throughout the cell cycle. Cells that had just divided are small, but fully covered with coccoliths. The protective effect of the coccosphere against viral infection was consequently either equally good, or equally poor in Experiment 1 and 5 where the virus was added in the beginning or in the middle of the light period, respectively. In contrast to the calcified cells, we observed a pronounced effect of the timing of virus addition on the decalcified cells. In Experiment 1, the decalcified cells nearly doubled whereas their concentrations increased only by about 25% in Experiment 5 (compare Figures 2B, 7B). Either, the decalcified cells were more vulnerable to the virus in the middle of the light period due to the acid-base treatment. Alternatively, the extended time the decalcified cells had in the light when they were decalcified in the morning may have given them opportunity to reconstruct their coccosphere before the most vulnerable point in their cell cycle, e.g., during the dark phase when the cells replicate their DNA and divide (Müller et al., 2008; Kottmeier et al., 2020). In contrast, the cells that were decalcified in the middle of the light period were unable to fully reconstruct their coccosphere and thus poorly protected at this point. Clearly, our results from the dark experiments show that infection and viral lysis is not restricted to the cell division. Nevertheless, the results lend some support to the hypothesis that coccoliths can prevent viruses from reaching the organic part of the cell and the coccosphere can reduce infection. However, at the same time our results indicate that a potential protective effect of the coccosphere against the EhV86 is probably of minor ecological relevance since the cell numbers of calcified and decalcified cells were almost identical at the end of the experiment (Figures 2, 7).
The development of the E. huxleyi concentrations showed a characteristic pattern in all experiments in which actively growing cells in a light-dark cycle were exposed to the virus. The higher the maximum abundance of E. huxleyi was, the steeper was the subsequent decline of the cell concentrations. The results of the calcifying strain (CCMP1516) in particular suggests that, already after 2 days there should had been enough viruses present to infect the entire population. The net growth of E. huxleyi was stopped already within 2 days after virus addition and the numbers of viral particles increased drastically, but the concentrations of the host cells declined only gradually. Thyrhaug et al. (2003) discovered a dynamic feedback mechanism in the E. huxleyi-EhV system. The authors showed that the infection rate decreased when the abundance of the host cells declined in consequence of viral lysis. In our experiments the decline of the cell concentrations also seemed to be dependent on the relative abundance of E. huxleyi and the rate of cell lysis decreased the further the infection progressed. These observations suggest that calcification plays rather a minor protective role in the mutual succession of E. huxleyi and the EhV.
Impacts of the Coccosphere Removal Procedures on Viral Infection
Our findings on the protective role of the coccosphere against viral infection critically depend on whether or not the methods that were applied to remove the coccosphere (acid-base treatment or low [Ca2+] medium) affected the infection process as such. If these procedures somehow changed the susceptibility of the host to the virus, or the viral replication machinery it would be a confounding factor that is hard to distinguish from the protective role of the coccosphere.
The reduction of the [Ca2+] in the growth medium clearly affected the interaction between the host and the virus. The cells of both, the calcifying (CCMP1516) and the non-calcifying strain (RCC1242) of E. huxleyi were less susceptible to infection when they were grown under low [Ca2+] (Experiment 2, Figure 4). Furthermore, in the medium with low [Ca2+] E. huxleyi was able to withstand the prolonged darkness even better when the virus was present (Experiment 4, Figure 6). Calcium plays important role in cell signaling and in certain structures of cell membranes (Verret et al., 2010) and there are various possibilities how a depletion in calcium ions may influence the biological interaction between the host and the virus. Regardless of the physiological mechanism, however, it is clear that the low [Ca2+] had a confounding effect on the virus infection, which restricts us from using these experiments to interpret the role of the coccosphere in viral infection.
Johns et al. (2019) observed the contrary effect of a low [Ca2+]. In their experiments, host cells were more susceptible to viral infection under low [Ca2+] in most of the E. huxleyi strains the authors tested. It is therefore unclear whether calcium plays a direct role in the infection or replication process of the virus or whether the contradictory results attribute to the specific strain or other differences between the experiments, like the cell concentrations and related factors e.g., nutrient concentrations, carbonate chemistry etc. In general, the susceptibility of the host as well as the infectivity of the virus vary strongly depending on the examined strains of the host and the virus (Kegel et al., 2013; Nissimov et al., 2016). In this context, it is important to note that we examined only two E. huxleyi strains and a single strain of the EhV, which does not allow us to generalize our results widely.
The acid-base treatment seemed to have a smaller effect on the cell physiology. Admittedly, the actual addition of acid and base caused a 10% decrease in the cell concentrations, but this decrease occurred within a short period after the procedure was conducted and it was likely due to the direct contact of the cells with the highly concentrated chemicals. The majority of the cells survived and their concentrations remained stable. The growth of the decalcified cells of E. huxleyi was equal to the calcified cells when light was supplied and no virus was added (Experiment 1, Figure 2 and Experiment 5, Figure 7). However, in the dark experiment, the concentrations of the decalcified cells decreased also in absence of the virus (Figure 5). Without light, E. huxleyi was not able to compensate the acid-base treatment as effectively. However, the concentration of viral particles was higher in the cultures that contained decalcified cells. This raises the question whether the higher release of viral particles was due to an increased infection of the decalcified cells, or whether the treatment itself affected the replication of the virus. Strom et al. (2018) showed that the treatment of E. huxleyi with acid and base caused an elevated release of hydrogen peroxide (H2O2) of E. huxleyi into the surrounding medium. An enhanced excretion of H2O2 was also found during the lytic phase of infected E. huxleyi, concomitant with elevated intracellular concentrations of other reactive oxygen species (Evans et al., 2006). Reactive oxygen species play a role in the programed cell death pathway, which is linked to the replication cycle of the EhV (Bidle et al., 2007; Sheyn et al., 2016). It is therefore possible that the acid-base treatment accelerated viral replication. However, when the acid-base method was tested with the non-calcifying strain (RCC1242), it led to enhanced the growth of the host cells, but the effect of the virus on the cell concentrations did not differ between the control and the acid-base treated cells (Experiment 1, Figure 2).
Another important aspect to consider is that the acid-base treatment did not only dissolve the coccospheres, but also loose coccoliths in the medium which detached from the cells. Typically, E. huxleyi produces more coccoliths than necessary to construct a single-layered coccosphere. The additional coccoliths are arranged in multiple layers around the cell, but also detach from the cell and spread into the surrounding medium (Paasche, 2002). Johns et al. (2019) found that free coccoliths can adsorb viral particles. Thus, the reduced infection observed in Experiments 1 and 5 could potentially be explained by the absorption of viruses by free coccoliths, which could have led to a reduced number of infective particles in the treatment with calcified cells.
To test if this mechanism shown by Johns et al. (2019) also occurred in our experiments, we exposed viral particles to a coccolith suspension and compared the development of the viral particle concentration relative to a suspension in which the detached coccoliths were dissolved prior to virus addition (Experiment 6, Figure 8). For Experiment 6, we used the E. huxleyi culture and the same virus stock solution from Experiment 5 to test for the adsorption of viral particles to free coccoliths. Thus, the number of detached coccoliths as well as the quantity of viral particles was equal in both experiments. Our measurements showed no difference in the virus concentration between the treatments, although we acknowledge the large variability in the results of this experiment. Nevertheless, these findings suggest that the absorption of viral particles by detached coccoliths was not the main mechanism explaining the large differences in the cell concentrations between calcified and decalcified E. huxleyi in Experiment 5.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
MH and LB designed the experiments. MH conducted the experiments and performed the measurements and data evaluation. All authors contributed to the data discussion and to the drafting of the manuscript.
Funding
This research was funded by the German Research Foundation (DFG) (BA5188/1-1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jana Meyer for her support on the virus measurements and Jan Taucher for valuable discussions about the dataset. Furthermore, we are grateful to Glen L. Wheeler and Marius N. Müller for their valuable comments, which significantly improved the manuscript. We also thank Declan C. Schroeder for the comprehensive advice on working with the E. huxleyi Virus 86 and for providing viral lysate.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.530757/full#supplementary-material
References
Bach, L. T., Riebesell, U., Gutowska, M. A., Federwisch, L., and Schulz, K. G. (2015). A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework. Prog. Oceanogr. 135, 125–138. doi: 10.1016/j.pocean.2015.04.012
Bidle, K. D., Haramaty, L., Barcelos Ramos, J., and Falkowski, P. (2007). Viral activation and recruitment of metacaspases in the unicellular coccolithophore, Emiliania huxleyi. Proc. Natl. Acad. Sci. U.S.A. 104, 6049–6054. doi: 10.1073/pnas.0701240104
Bidle, K. D., and Vardi, A. (2011). A chemical arms race at sea mediates algal host–virus interactions. Curr. Opin. Microbiol. 14, 449–457. doi: 10.1016/j.mib.2011.07.013
Bown, P. R., Lees, J. A., and Young, J. R. (2004). “Calcareous nannoplankton evolution and diversity through time BT - coccolithophores: from molecular processes to global impact,” in Coccolithophores-From Molecular Processes to Global Impact, eds H. R. Thierstein and J. R. Young (Berlin: Springer), 481–508. doi: 10.1007/978-3-662-06278-4_18
Bratbak, G., Egge, J. K., and Heldal, M. (1993). Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar. Ecol. Prog. Ser. 93, 39–48. doi: 10.3354/meps093039
Broecker, W., and Clark, E. (2009). Ratio of coccolith CaCO3 to foraminifera CaCO3 in late Holocene deep sea sediments. Paleoceanography 24:PA3205. doi: 10.1029/2009PA001731
Brownlee, C., and Taylor, A. (2004). “Calcification in coccolithophores: a cellular perspective BT - coccolithophores: from molecular processes to global impact,” in Coccolithophores-From Molecular Processes to Global Impact, eds H. R. Thierstein and J. R. Young (Berlin: Springer), 31–49. doi: 10.1007/978-3-662-06278-4_2
Brussaard, C. (2004). Optimization of procedures for counting viruses by flow cytometry. Appl. Environ. Microbiol. 70, 1506–1513. doi: 10.1128/AEM.70.3.1506
Brussaard, C., Kempers, R., Kop, A., Riegman, R., and Heldal, M. (1996). Virus-like particles in a summer bloom of Emiliania huxleyi in the North Sea. Aquat. Microb. Ecol. 10, 105–113. doi: 10.3354/ame010105
Castberg, T., Thyrhaug, R., Larsen, A., Sandaa, R.-A., Heldal, M., Van Etten, J. L., et al. (2002). Isolation and characterization of a virus that infects Emiliania huxleyi (haptophyta)1. J. Phycol. 38, 767–774. doi: 10.1046/j.1529-8817.2002.02015.x
Evans, C., Malin, G., Mills, G. P., and Wilson, W. H. (2006). Viral infection of Emiliania huxleyi (prymnesiophyceae) leads to elevated production of reactive oxygen species1. J. Phycol. 42, 1040–1047. doi: 10.1111/j.1529-8817.2006.00256.x
Evans, C., Pond, D. W., and Wilson, W. H. (2009). Changes in Emiliania huxleyi fatty acid profiles during infection with E. huxleyi virus 86: physiological and ecological implications. Aquat. Microb. Ecol. 55, 219–228. doi: 10.3354/ame01295
Fulton, J. M., Fredricks, H. F., Bidle, K. D., Vardi, A., Kendrick, B. J., DiTullio, G. R., et al. (2014). Novel molecular determinants of viral susceptibility and resistance in the lipidome of Emiliania huxleyi. Environ. Microbiol. 16, 1137–1149. doi: 10.1111/1462-2920.12358
Guillard, R. R. L., and Ryther, J. H. (1962). Studies of marine planktonic diatoms: I. Cyclotella nana hustedt, and detonula confervacea (cleve) gran. Can. J. Microbiol. 8, 229–239. doi: 10.1139/m62-029
Hansen, F. C., Witte, H. J., and Passarge, J. (1996). Grazing in the heterotrophic dinoflagellate Oxyrrhis marina: size selectivity and preference for calcified Emiliania huxleyi cells. Aquat. Microb. Ecol. 10, 307–313. doi: 10.3354/ame010307
Hedges, L. V., Gurevitch, J., and Curtis, P. S. (1999). The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. doi: 10.1890/0012-9658(1999)080[1150:tmaorr]2.0.co;2
Hunter, J. E., Frada, M. J., Fredricks, H. F., Vardi, A., and Van Mooy, B. A. S. (2015). Targeted and untargeted lipidomics of Emiliania huxleyi viral infection and life cycle phases highlights molecular biomarkers of infection, susceptibility, and ploidy. Front. Mar. Sci. 2:81. doi: 10.3389/fmars.2015.00081
Jacquet, S., Iglesias-rodriguez, D., Wilson, W., Jacquet, S., Heldal, M., Iglesias-rodriguez, D., et al. (2002). Flow cytometric analysis of an Emiliania huxleyi bloom terminated by viral infection. Aquat. Microb. Ecol. 27, 111–124. doi: 10.3354/ame027111
Johns, C. T., Grubb, A. R., Nissimov, J. I., Natale, F., Knapp, V., and Mui, A. (2019). The mutual interplay between calcification and coccolithovirus infection. Environ. Microbiol. 21, 1896–1915. doi: 10.1111/1462-2920.14362
Kegel, J. U., John, U., Valentin, K., and Frickenhaus, S. (2013). Genome variations associated with viral susceptibility and calcification in Emiliania huxleyi. PLoS One 8:e80684. doi: 10.1371/journal.pone.0080684
Kester, D. R., Duedall, I. W., Connors, D. N., and Pytkowicz, R. M. (1967). Preparation of artificial seawater1. Limnol. Oceanogr. 12, 176–179. doi: 10.4319/lo.1967.12.1.0176
Kottmeier, D. M., Terbrüggen, A., Wolf-Gladrow, D. A., and Thoms, S. (2020). Diel variations in cell division and biomass production of Emiliania huxleyi—Consequences for the calculation of physiological cell parameters. Limnol. Oceanogr. 65, 1781–1800. doi: 10.1002/lno.11418
Mackinder, L., Wheeler, G., Schroeder, D., Riebesell, U., and Brownlee, C. (2010). Molecular mechanisms underlying calcification in coccolithophores. Geomicrobiol. J. 27, 585–595. doi: 10.1080/01490451003703014
Mackinder, L. C. M., Worthy, C. A., Biggi, G., Hall, M., Ryan, K. P., and Varsani, A. (2009). A unicellular algal virus, Emiliania huxleyi virus 86, exploits an animal-like infection strategy. J. Gen. Virol. 90, 2306–2316. doi: 10.1099/vir.0.011635-0
Monteiro, F. M., Bach, L. T., Brownlee, C., Bown, P., Rickaby, R. E., and Poult, A. J. (2016). Why marine phytoplankton calcify. Sci. Adv. 2:e1501822. doi: 10.1126/sciadv.1501822
Müller, M. N. (2019). On the genesis and function of coccolithophore calcification. Front. Mar. Sci. 6:49. doi: 10.3389/fmars.2019.00049
Müller, M. N., Antia, A. N., and LaRoche, J. (2008). Influence of cell cycle phase on calcification in the coccolithophore Emiliania huxleyi. Limnol. Oceanogr. 53, 506–512. doi: 10.4319/lo.2008.53.2.0506
Nissimov, J. I., Napier, J. A., Allen, M. J., and Kimmance, S. A. (2016). Intragenus competition between coccolithoviruses: an insight on how a select few can come to dominate many. Environ. Microbiol. 18, 133–145. doi: 10.1111/1462-2920.12902
Olson, R. J., Zettler, E. R., and Anderson, O. K. (1989). Discrimination of eukaryotic phytoplankton cell types from light scatter and autofluorescence properties measured by flow cytometry. Cytometry 10, 636–643. doi: 10.1002/cyto.990100520
Paasche, E. (1967). Marine plankton algae grown with light-dark cycles. I. Coccolithus huxleyi. Physiol. Plant. 20, 946–956. doi: 10.1111/j.1399-3054.1967.tb08382.x
Paasche, E. (2002). A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions. Phycologia 40, 503–529. doi: 10.2216/i0031-8884-40-6-503.1
Poulton, A. J., Adey, T. R., Balch, W. M., and Holligan, P. M. (2007). Relating coccolithophore calcification rates to phytoplankton community dynamics: regional differences and implications for carbon export. Deep Sea Res. Part II Top. Stud. Oceanogr. 54, 538–557. doi: 10.1016/j.dsr2.2006.12.003
Raven, J., and Crawfurd, K. (2012). Environmental controls on coccolithophore calcification. Mar. Ecol. Prog. Ser. 470, 137–166. doi: 10.3354/meps09993
Riebesell, U., and Tortell, P. D. (2011). “Effects of ocean acidification on pelagic organisms and ecosystems,” in Ocean Acidification, eds J.-P. Gattuso and L. Hansson (Oxford: Oxford University Press), 99–121.
Riebesell, U., Zondervan, I., Rost, B., Tortell, P. D., Zeebe, R. E., and Morel, F. M. M. (2000). Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407, 364–367. doi: 10.1038/35030078
Rose, S. L., Fulton, J. M., Brown, C. M., Natale, F., Van Mooy, B. A. S., and Bidle, K. D. (2014). Isolation and characterization of lipid rafts in Emiliania huxleyi: a role for membrane microdomains in host–virus interactions. Environ. Microbiol. 16, 1150–1166. doi: 10.1111/1462-2920.12357
Rosenwasser, S., Mausz, M. A., Schatz, D., Sheyn, U., Malitsky, S., and Aharoni, A. (2014). Rewiring host lipid metabolism by large viruses determines the fate of Emiliania huxleyi, a bloom-forming alga in the Ocean. Plant Cell 26, 2689–2707. doi: 10.1105/tpc.114.125641
Schatz, D., Shemi, A., Rosenwasser, S., Sabanay, H., Wolf, S. G., Ben-Dor, S., et al. (2014). Hijacking of an autophagy-like process is critical for the life cycle of a DNA virus infecting oceanic algal blooms. New Phytol. 204, 854–863. doi: 10.1111/nph.13008
Schroeder, D. C., Oke, J., Hall, M., Malin, G., and Wilson, W. H. (2003). Virus succession observed during an Emiliania huxleyi bloom. Appl. Environ. Microbiol. 69, 2484–2490. doi: 10.1128/AEM.69.5.2484
Schroeder, D. C., Oke, J., Malin, G., and Wilson, W. H. (2002). Coccolithovirus (Phycodnaviridae): characterisation of a new large dsDNA algal virus that infects Emiliania huxleyi. Arch. Virol. 147, 1685–1698. doi: 10.1007/s00705-002-0841-3
Sheyn, U., Rosenwasser, S., Ben-Dor, S., Porat, Z., and Vardi, A. (2016). Modulation of host ROS metabolism is essential for viral infection of a bloom-forming coccolithophore in the ocean. ISME J. 10, 1742–1754. doi: 10.1038/ismej.2015.228
Strom, S. L., Bright, K. J., Fredrickson, K. A., and Cooney, E. C. (2018). Phytoplankton defenses: do Emiliania huxleyi coccoliths protect against microzooplankton predators? Limnol. Oceanogr. 63, 617–627. doi: 10.1002/lno.10655
Taylor, A. R., Brownlee, C., and Wheeler, G. (2017). Coccolithophore cell biology: chalking up progress. Ann. Rev. Mar. Sci. 9, 283–310. doi: 10.1146/annurev-marine-122414-034032
Thyrhaug, R., Larsen, A., Thingstad, T. F., and Bratbak, G. (2003). Stable coexistence in marine algal host-virus systems. Mar. Ecol. Prog. Ser. 254, 27–35. doi: 10.3354/meps254027
Tyrrell, T., and Young, J. R. (2009). “Coccolithophores,” in Encyclopedia of Ocean Sciences, eds J. Steele, S. Thorpe, and K. Turekian (Cambridge, MA: Academic Press), 606–614.
Vardi, A., Haramaty, L., Van Mooy, B. A. S., Fredricks, H. F., Kimmance, S. A., Larsen, A., et al. (2012). Host–virus dynamics and subcellular controls of cell fate in a natural coccolithophore population. Proc. Natl. Acad. Sci. U.S.A. 109, 19327–19332. doi: 10.1073/pnas.1208895109
Vardi, A., Van Mooy, B. A. S., Fredricks, H. F., Popendorf, K. J., Ossolinski, J. E., Haramaty, L., et al. (2009). Viral glycosphingolipids induce lytic infection and cell death in marine phytoplankton. Science 326, 861–865. doi: 10.1126/science.1177322
Verret, F., Wheeler, G., Taylor, A. R., Farnham, G., and Brownlee, C. (2010). Calcium channels in photosynthetic eukaryotes: implications for evolution of calcium-based signalling. New Phytol. 187, 23–43. doi: 10.1111/j.1469-8137.2010.03271.x
Walker, C. E., Taylor, A. R., Langer, G., Durak, G. M., Heath, S., and Probert, I. (2018). The requirement for calcification differs between ecologically important coccolithophore species. New Phytol. 220, 147–162. doi: 10.1111/nph.15272
Wilson, W. H., Schroeder, D. C., Allen, M. J., Holden, M. T., Parkhill, J., and Barrell, B. (2005). Complete genome sequence and lytic phase transcription profile of a coccolithovirus. Science 309, 1090–1092. doi: 10.1126/science.1113109
Wilson, W. H., Tarran, G. A., Schroeder, D., Cox, M., Oke, J., and Malin, G. (2002a). Isolation of viruses responsible for the demise of an Emiliania huxleyi bloom in the english channel. J. Mar. Biol. Assoc. U.K. 82, 369–377. doi: 10.1017/S002531540200560X
Wilson, W. H., Tarran, G., and Zubkov, M. V. (2002b). Virus dynamics in a coccolithophore-dominated bloom in the North Sea. Deep Sea Res. II Top. Stud. Oceanogr. 49, 2951–2963. doi: 10.1016/s0967-0645(02)00065-6
Keywords: calcification, coccolithophores, Emiliania huxleyi, virus, phytoplankton, infection
Citation: Haunost M, Riebesell U and Bach LT (2020) The Calcium Carbonate Shell of Emiliania huxleyi Provides Limited Protection Against Viral Infection. Front. Mar. Sci. 7:530757. doi: 10.3389/fmars.2020.530757
Received: 30 January 2020; Accepted: 12 August 2020;
Published: 11 September 2020.
Edited by:
Jonathan P. Zehr, University of California, Santa Cruz, United StatesReviewed by:
Glen Lee Wheeler, Marine Biological Association of the United Kingdom, United KingdomMarius Nils Müller, Federal University of Pernambuco, Brazil
Copyright © 2020 Haunost, Riebesell and Bach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mathias Haunost, bWhhdW5vc3RAZ2VvbWFyLmRl
 Mathias Haunost
Mathias Haunost Ulf Riebesell
Ulf Riebesell Lennart T. Bach
Lennart T. Bach