
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 25 August 2020
Sec. Marine Megafauna
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00690
 Philip D. Doherty1*
Philip D. Doherty1* Annette Cameron Broderick1
Annette Cameron Broderick1 Brendan John Godley1
Brendan John Godley1 K. A. Hart2
K. A. Hart2 Q. Phillips3
Q. Phillips3 A. Sanghera4
A. Sanghera4 Thomas B. Stringell1
Thomas B. Stringell1 J. T. Walker5
J. T. Walker5 Peter Bradley Richardson4*
Peter Bradley Richardson4*Marine turtles are of conservation concern throughout their range, with past population declines largely due to exploitation through both legal and illegal take, and incidental capture in fisheries. Whilst much research effort has been focused on nesting beaches and elaborating migratory corridors, these species spend the vast majority of their life-cycle in foraging grounds, which are, in some species, quite discrete. To understand and manage these populations, empirical data are needed on distribution, space-use, and habitats to best inform design of protective measures. Here we describe space-use, occupancy, and wide-ranging movements derived from conventional flipper tagging and satellite tracking of sub-adult green turtles (Chelonia mydas) within the coastal waters of the Turks and Caicos Islands (TCI; 2011–2017). 623 turtles were fitted with flipper tags, with 69 subsequently recaptured, five of which in international waters. Sixteen individual turtles of between 63 and 81 cm curved carapace length were satellite tracked for a mean 226 days (range: 38–496). Data revealed extended periods of occupancy in the shallow coastal waters within a RAMSAR protected area. Satellite tracking and flipper tagging showed wide-ranging movements, with flipper tag recaptures occurring in waters off Nicaragua (n = 4), and Venezuela (n = 1). Also, four of 16 satellite tracked turtles exhibiting directed movements away (displaced >450 km) from TCI waters traveling through nine geo-political zones within the Caribbean-Atlantic basin, as well as on the High Seas. One turtle traveled to the Central American coast before settling on inshore habitat in Colombia’s waters for 162 days before transmission ceased, indicating ontogenetic dispersal to a distant foraging habitat. These data highlight connectivity throughout the region, displaying key linkages between countries that have previously only been linked by genetic evidence. This study also provides evidence of the importance of the Turks and Caicos Islands marine protected area network and importance of effective management of the sea turtle fishery for regional green turtle populations.
Satellite telemetry has transformed the study of many marine vertebrate species, although there are challenges with early life stages and animals which rarely surface (Hazen et al., 2012; Hussey et al., 2015; Hays et al., 2016). Insights have been gathered into migratory corridors between important breeding and foraging areas (Schofield et al., 2013) allowing suggestions for threat mitigation (Fossette et al., 2014; Sequeira et al., 2014; McKenna et al., 2015; Trathan et al., 2015), and informing the design of marine protected areas (MPAs; Maxwell et al., 2016; Doherty et al., 2017). With the advancement of bio-logging leading to smaller tags, longer battery duration, and increased location accuracy (Hays et al., 2016), our ability to gather insight into complex life history characteristics and migratory life cycles of many marine vertebrates has improved – essential information to inform conservation efforts (Greene et al., 2009; Hammerschlag et al., 2011; Hazen et al., 2012; Hays et al., 2019).
Marine turtles are a taxon for which satellite tracking has been transformational in unraveling complicated life histories (Godley et al., 2008). For adult turtles, migration patterns have become well elaborated (Fossette et al., 2014; Hays et al., 2014; Dodge et al., 2015) also highlighting important within and between population differences in movement patterns. In line with many aspects of marine turtle ecological studies (Wildermann et al., 2018) juvenile movements have been relatively understudied (Godley et al., 2008; Jeffers and Godley, 2016). However, studies have begun to give important insights into the movements and dispersal of juveniles (Hart and Fujisaki, 2010; Mansfield et al., 2014; Putman and Mansfield, 2015; Chambault et al., 2018).
Globally, marine turtle populations are reduced from historical levels, largely due to direct exploitation (Stoddart, 1980; Broderick et al., 2006; Humber et al., 2014). The take of marine turtles for meat, shell, and other products has occurred for centuries (Groombridge and Luxmore, 1989). Global take of marine turtles peaked in the late 1960s with over 17,000 tonnes being captured (van Dijk and Shepherd, 1995; Fleming, 2001; McClenachan et al., 2006). Despite increasing levels of protection, direct take of turtles has continued legally in many regions (Humber et al., 2014; Barrios-Garrido et al., 2017; Edyvane and Penny, 2017), for example permitted take in the Caribbean accounts for over one third (14,640 turtles year–1) of estimated take globally (Humber et al., 2014). Legal take is often comprised of subsistence use by traditional coastal groups, or small-scale fisheries supplying local communities (Brautigam and Eckert, 2006) providing a source of income, food, and cultural identity (Hamann et al., 2006).
The Turks and Caicos Islands (TCI) are home to large aggregations of foraging green turtles (Chelonia mydas), small numbers of nesting green turtles, moderately large aggregations of foraging and nesting hawksbill turtles (Eretmochelys imbricata), but few foraging and nesting loggerhead turtles (Caretta caretta; Richardson et al., 2009; Stringell et al., 2010, 2013, 2015). Turtles are often targeted by a traditional fishery that, prior to 2014, landed approximately 176–324 green turtles annually (Stringell et al., 2013). Turtles are generally caught and either gifted or sold for domestic consumption, with some commercial re-sale in local restaurants (Richardson et al., 2009; Stringell et al., 2013). In July 2014, new fishery regulations were enacted under the Fisheries Protection Ordinance that include the prohibition of take of green turtles smaller than 18 inches (46 cm) and larger than 24 inches (61 cm) curved carapace length (CCL) to protect breeding adults and sub-adults, that will soon become breeding adults, and maintained a prohibition on the take of any turtle above the low-water mark, and eggs (Stringell et al., 2015). The average number of turtles landed under the current legislation is unknown.
While under-studied and poorly understood, sub-adult individuals represent a key life stage for sea turtle population maintenance and recovery (Crouse et al., 1987). Here we set out to use satellite tracking to understand the habitat utilization of sub-adult green turtles in TCI waters and beyond to effectively inform local and regional marine conservation. Green turtles of all sizes were flipper-tagged during the study, but sub-adults between 60 and 90 cm were specifically targeted for satellite telemetry. These individuals were most likely to be protected by the proposed/enacted legislation, and would therefore be less likely to be landed by the local fishery; and we considered these larger individuals more likely to exhibit ontogenetic dispersal. This study was carried out to help describe this understudied phenomenon and shed further light on the level of ecological connectivity in the Caribbean with respect to regional green turtle populations.
The TCI consists of a low-lying archipelago of eight larger islands and approximately 40 smaller cays situated at the southern end of the Bahamas Lucayan Archipelago (21° 45N, 71° 35W). These islands cover an area of ∼950 km2 at low-tide, divided between the Caicos Bank and the Turks Bank. The area to the south of the Caicos Islands, known as the Caicos Bank, hosts shallow, sand and seagrass habitat, fringed in the north by mangroves and creeks – providing a regionally significant foraging habitat for juvenile, sub-adult, and adult marine turtles. The islands of Grand Turk, Providenciales, and South Caicos host the majority of the human population, and the economy is supported largely through tourism, offshore finance, and fishing (Richardson et al., 2009).
The TCI has an extensive network of 23 MPAs covering approximately 679 km2, representing more than 70% of MPA in all the UK Overseas Territories (UKOTs) in the Caribbean (Martinez et al., 2017). Turks and Caicos Islands’ MPAs are regulated under the National Parks Ordinance (revised 2014) and are categorized as either National Parks, Nature Reserves, Sanctuaries or Areas of Historical Interest, each with differing statutory management regimes, including restrictions on taking flora and fauna, with Sanctuaries providing the highest level of protection (Turks and Caicos Government, 2014). As with most UKOTs in the Caribbean, enforcement of environmental legislation is a challenge, with the responsible authority, the Department of Environment and Coastal Resources (DECR), often under-resourced (Forster et al., 2011; Baker et al., 2015).
This study was carried out exclusively on the Caicos Bank, along the southern coast of Middle and East Caicos and within the boundaries of the North, Middle, and East Caicos Nature Reserve (Figure 1), a RAMSAR site designated in 1990, covering an area of 568 km2 and where the extraction of turtles is prohibited (Martinez et al., 2017). Located within the RAMSAR protection site is a smaller MPA (Vine Point and Ocean Hole Nature Reserve) where the take of any animal or plant by any method is prohibited (Turks and Caicos Government, 2014).
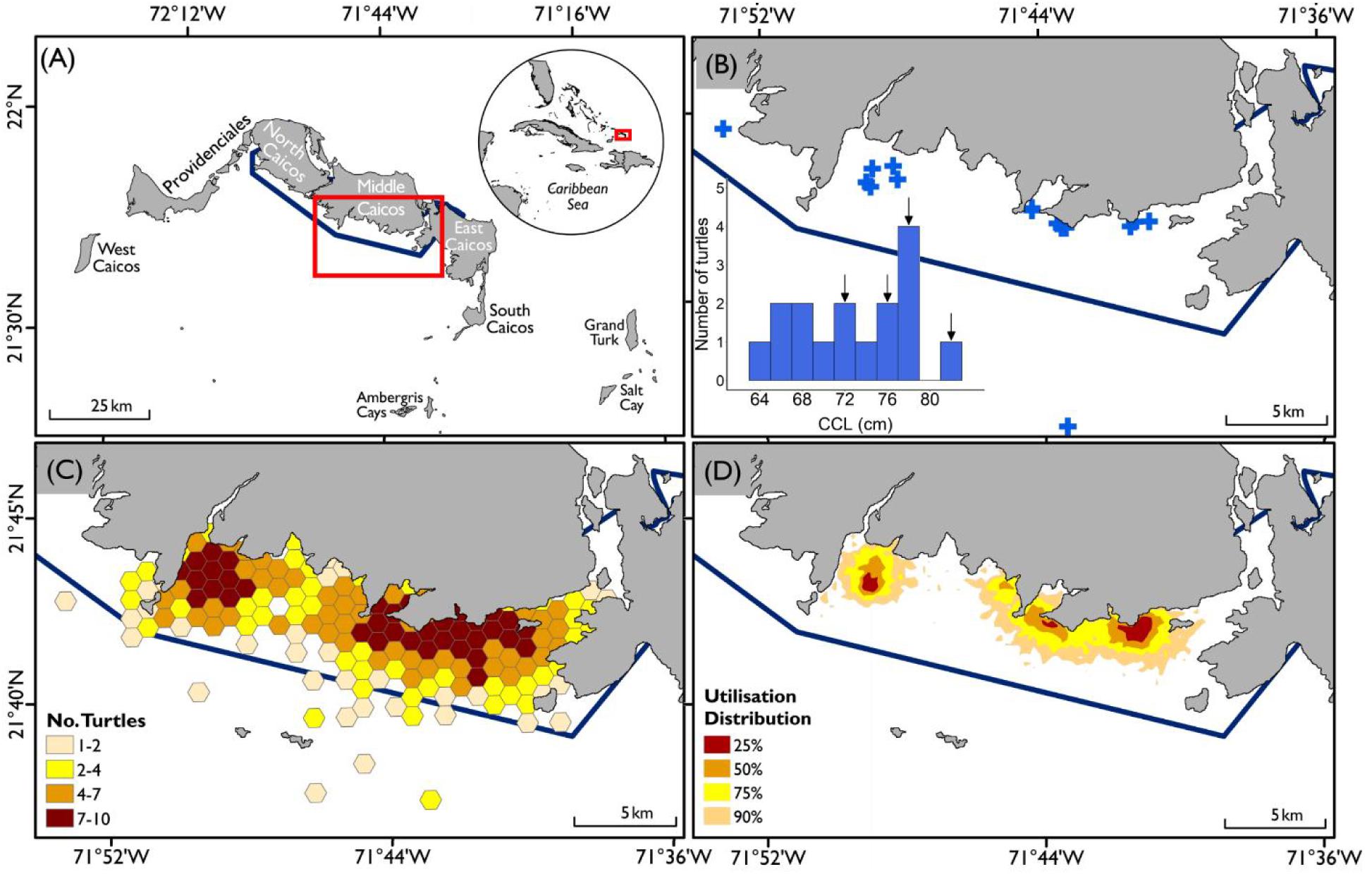
Figure 1. Areas of relative importance for satellite tracked sub-adult green turtles (n = 16) within coastal waters of the Turks and Caicos Islands. (A) Location of Turks and Caicos Islands within wider Caribbean (inset) and area of interest within the Turks and Caicos Islands; (B) locations of turtle capture and release (blue crosses), inset histogram of number of turtles for body size classes (CCL), arrows indicate size classes containing each of the four migratory turtles; (C) Grid enumeration of average (mean) number of turtles present in each 500 m (distance from edge to centroid) grid cell accounting for psuedoreplication of individual, and (D) Percentage Volume Contours (PVC) of 25, 50, 75, and 90% Utilization Distributions (UDs), derived from interpolated locations at 6-h time step using a hierarchical behavioral switching State Space Model (hSSM). Dark blue line in all plots denotes the boundaries of RAMSAR protected area (BirdLife International, 2020).
In-water capture, sampling and release of green turtles in TCI waters was carried out with permission from DECR from 2002 to 2006, and from 2008 to 2017 (Richardson et al., 2009; Stringell et al., 2013). Turtles were either captured using a turtle rodeo method, where turtles were spotted, pursued, and captured by hand from a patrol vessel, or captured by freedivers. The CCL (notch to tip) and curved carapace width (CCW) of all captured turtles were measured using a flexible measuring tape (Bolten, 1999). Turtles were tagged with either monel or inconel flipper tags in the trailing edge of the front flippers following Balazs (1999) to allow for potential re-sighting and long-term observations of life-history events. A GPS waypoint was taken at the point of capture of each turtle when the equipment was available.
From August 2011 to May 2017, captured turtles of between 60 and 90 cm CCL were considered for application of satellite transmitters, and if selected, taken back to secure facilities in Providenciales and South Caicos where satellite transmitters were attached directly to the highest point of the carapace using two-part epoxy. The transmitters and attachment surface were coated with antifouling paint (Richardson et al., 2013), and turtles were released at the GPS waypoint of capture, with the exception of one turtle, which was released as close to the point of capture as the prevailing tide conditions allowed. Sirtrack (Hawkes Bay, New Zealand) F4-G (n = 8) and F4-H (n = 1), and Wildlife Computers (Redmond, CA, United States) SPLASH-F (n = 7) satellite tags were deployed on sixteen sub-adult turtles captured in waters of the Turks and Caicos Islands between 2011 and 2017 (Table 1). All satellite tags were attached to healthy turtles following protocols outlined in Godley et al. (2002b). All tag data were downloaded from CLS-Argos and archived using the Satellite Tracking and Analysis Tool (STAT; Coyne and Godley, 2005).
Argos location data from all tags were subject to filtering, retaining location classes 1 typically accurate to 500–1500 m, 2 accurate to 250–500 m, and 3 accurate to <250 m, “A” (three messages received but no accuracy estimation) and “B” (one or two messages received but no accuracy estimation; Witt et al., 2010). A maximum plausible speed filter and turning angle threshold were applied to tracking data removing locations if speed between two locations exceeded 1.5 ms–1 (5.4 km h–1), and/or if turning angles between locations were less than 25° (Hart et al., 2015; Stokes et al., 2015). All filtering was conducted in R (R Core Team, 2018) using the argosfilter (Freitas, 2012), trip (Sumner, 2016), sf (Pebesma, 2018), and sp (Bivand et al., 2013) packages.
To account for the irregularity in Argos derived location data, a Bayesian state-space model (SSM) was applied as described in Jonsen et al. (2005), implemented with the bsam package (Jonsen et al., 2005; Jonsen, 2016). State-space models are time-series models that consist of two components, one accounting for location error, and one related to the movement dynamics related to the behavioral state of the animal (Jonsen et al., 2003; Patterson et al., 2008; Breed et al., 2009). Tracks with gaps between locations exceeding one week were split into segments and recombined after the SSM was fitted (Acuña-Marrero et al., 2017). A hierarchical, first difference, correlated, random-walk, switching SSM (hDCRWS) was applied (Jonsen et al., 2013) using tracking data from all individuals allowing for parameters to be estimated across all tracks (Breed et al., 2009). The model was run to produce standardized locations at 6-h time steps, chosen as > 95% of temporal gaps between input locations were less than 6 h (Supplementary Figure S1). The hierarchical state-space model (hSSM) was run using two parallel Markov Chain Monte Carlo simulations (MCMC) for 40,000 iterations with an initial burn-in of 100,000, applying a thinning factor of 40 to reduce within chain autocorrelation. Model convergence was assessed by visually inspecting trace plots and calculating the potential scale-reduction factor (PSRF; Supplementary Table S1) – a ratio between the variance and the within chain variability for MCMC simulations, whereby if less than 1.1 then there should be adequate enough observations so that sampling variability is negligible (Supplementary Figure S2; Brooks and Gelman, 1998).
Given the lack of information about how sub-adult green turtles use coastal waters of the Turks and Caicos Islands and areas linked to this early life-stage foraging ground we calculated maximum displacement distance (km) from release location to describe the extent of area these turtles occupy, and applied two techniques to identify core areas of space-use. These techniques were: (i) grid enumeration; and (ii) Utilization Distributions (UDs). Prior to conducting analysis on core activity areas, locations were reduced to those within the EEZ of the Turks and Caicos Islands (Figure 1). Grid enumeration was achieved by spatially intersecting a hexagon grid (500 m from edge to centroid; 0.87 km2 area) with sub-adult turtle locations to calculate the total number of individual turtles present within each grid cell. Utilization Distributions were employed using the adehabitatHR package (Calenge, 2006) to identify core activity areas, and were calculated using methods that apply physical barriers to movement to reduce over-smoothing of kernels (Sprogis et al., 2016; Doherty et al., 2017). A grid cell size of 250 m was applied utilizing the Plug-In smoothing factor (bandwidth; search radius determining which locations to incorporate into the UDs). Percentage Volume Contours (PVC) were created representing the estimated density of turtles that are likely to occur within each grid cell, from which we extracted 95, 75, 50, and 25% UDs. We also generated Minimum Convex Polygons (M; the smallest convex polygon that incorporates all locations within its boundary) for satellite tracking locations (n = 16 turtles) within the Turks and Caicos Islands EEZ, and for initial and recapture locations of individual turtles identified by flipper tags attached (n = 17 turtles).
A total of 623 green turtles were flipper-tagged during the project, including 415 caught within the waters off the North, Middle, and East Caicos Nature Reserve (RAMSAR site). Sixty-nine of these individuals were subsequently recaptured, with GPS locations recorded for 17 of these turtles. All satellite tracked turtles were caught within the RAMSAR site between 2011 and 2017 (Figures 1A,B) and deemed to be sub-adult (<102.6 cm CCL based on minimum size of observed maturity; Stringell et al., 2015); no assignments of sex were possible. Body size (CCL) of satellite tracked individuals ranged from 63.2–81.2 cm (mean ± SD: 72.8 ± 5.1 cm, n = 16; Table 1). These individuals were tracked for between 38 and 496 days (mean ± SD: 226 ± 135 days, n = 16; Table 1), and maximum displacement distance from release locations ranged from 4.8–1783 km (mean ± SD: 324.4 ± 573.6 km, n = 16; Table 1, Supplementary Figure S2). One other sub-adult green turtle was satellite tagged during this study in April 2017 (89 cm CCL), but was subsequently illegally caught by fishers within the RAMSAR site eight days after release. The turtle was landed in South Caicos and the satellite tag removed, however the fishers were consequently apprehended with the turtle being released unharmed and alive. The data from this turtle were not included in the analysis.
Turtles showed some variation in movement patterns; twelve of the sixteen sub-adult green turtles tracked remaining within close proximity of the Turks and Caicos Islands (<100 km displacement; range: 4.8–80.9 km), particularly the waters of Middle and East Caicos Islands (Supplementary Figure S3). The remaining four individuals exhibited directed movements away from the Turks and Caicos Islands (>450 km displacement; range: 493.4–1783 km, Figure 2 and Supplementary Figure S3) after periods of 13, 283, 294, and 339 days post-release. Of these four, one individual traveled from the Turks and Caicos Islands along the coasts of the Dominican Republic and Haiti, then crossing to the coastal region of Colombia, Panama, Costa Rica and back along the coast of Costa Rica to Colombia, passing through eight geo-political zones (TCI, Dominican Republic, Haiti, Cuba, Jamaica, Colombia, Panama, and Costa Rica, Figure 2A). This individual settled at a discrete site in Colombian inshore waters for 162 days before transmissions ceased. Another individual made a directed movement away from the Turks and Caicos Islands, through Bahamian waters following the coast of the USA from Florida to North Carolina, and then into the High Seas, passing through three geo-political zones (TCI, Bahamas, and the United States, Figure 2B). The remaining two of these individuals migrated to the coastal waters of Cuba, passing through the geo-political zone of the Bahamas (Figures 2C,D).
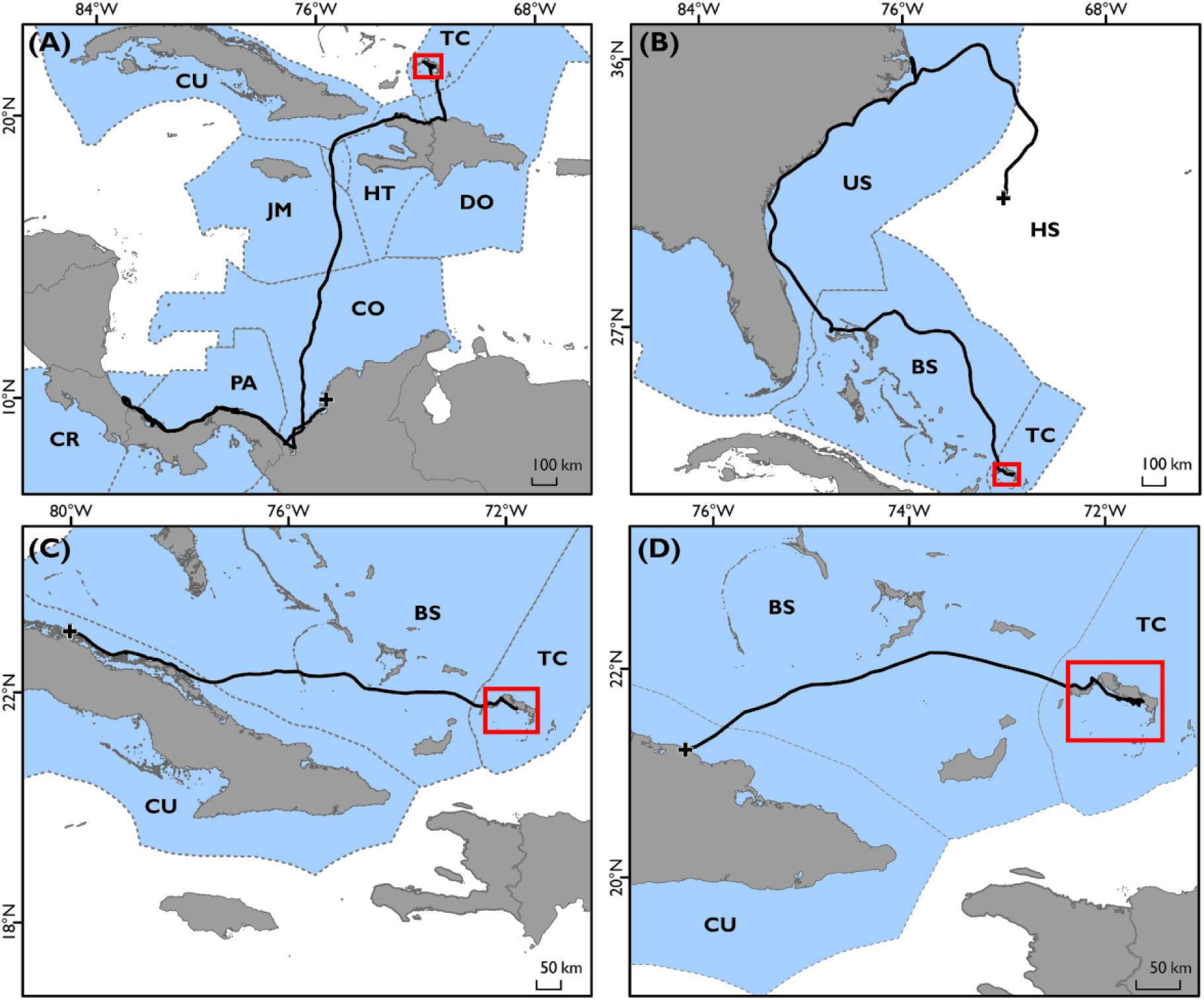
Figure 2. Geo-political zones (blue polygons) occupied by turtles that exhibited wide-ranging movements away from the Turks and Caicos Islands Economic Exclusive Zone (EEZ; n = 4). Each panel shows full track of an individual turtle (black dots); (A) Turtle G, (B) Turtle A, (C) Turtle M, and (D) Turtle B. Two-letter international country codes for each EEZ shown; TC; Turks and Caicos Islands, CU; Cuba, HT; Haiti, DO; Dominican Republic, JM; Jamaica, CO; Colombia, PA; Panama, CR; Costa Rica, BS; The Bahamas, US; United States of America, and HS; High Seas. All tracks originate from Turks and Caicos Islands (red boxes) and end location denoted by black cross.
Satellite tracking data and flipper tag recaptures showed high levels of occupancy within the shallow waters close to the shorelines of Middle Caicos and East Caicos (Figures 1C,D and Supplementary Figure S4). An area of overlap is highlighted between initial flipper tag deployment locations and satellite tag locations, and between recapture locations of flipper tagged individuals and satellite tag locations off the coastline of Middle Caicos, and between Middle Caicos and East Caicos (Supplementary Figure S4). Five individuals fitted with flipper-tags were recaptured internationally at locations throughout the wider Caribbean region (Figure 3). These individuals were originally tagged within the North, Middle, and East Caicos Nature Reserve (recaptured in Nicaragua; n = 4; CCL range: 49.6–73.4 cm, and Venezuela; n = 1; CCL: 65 cm).
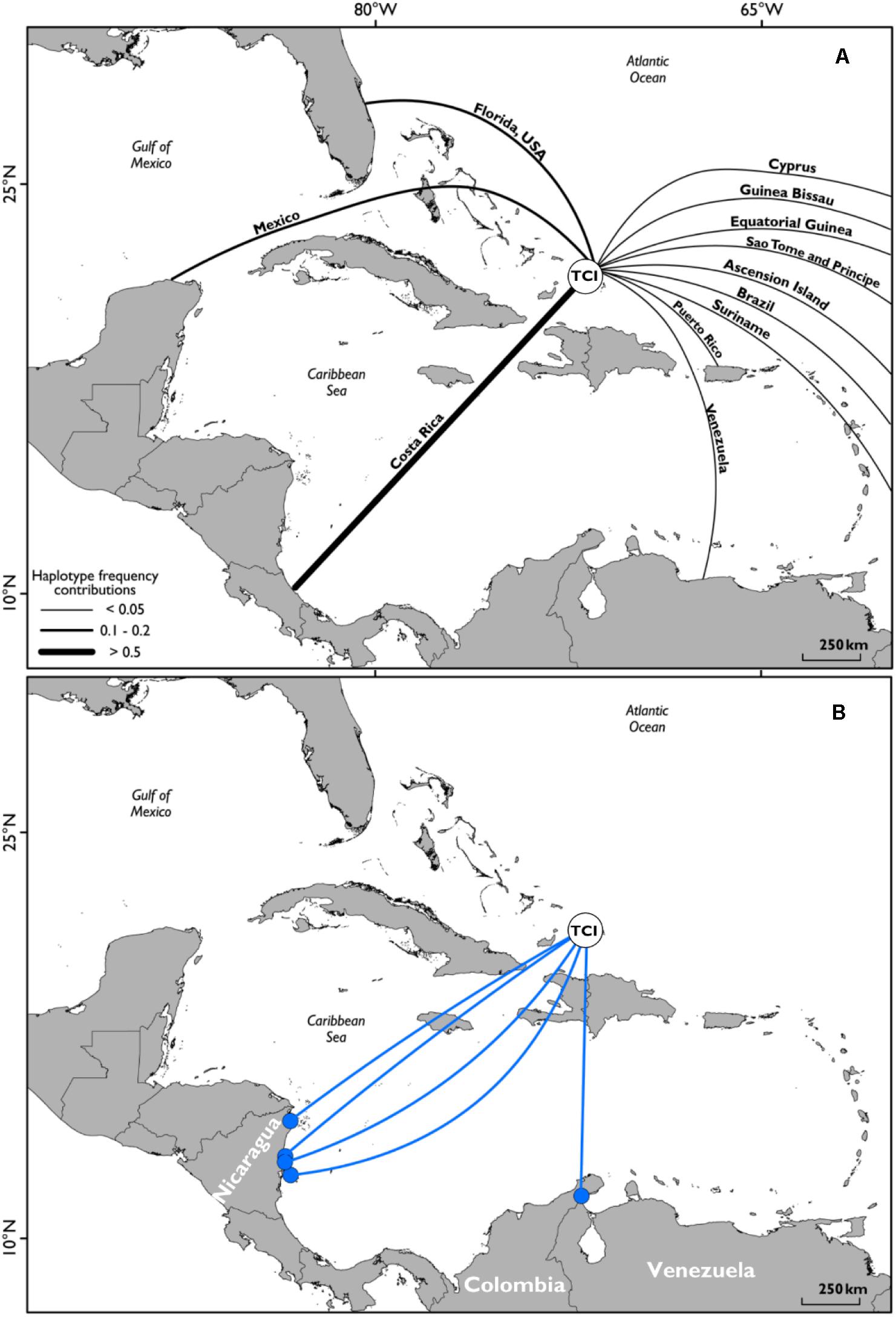
Figure 3. (A) Geographic distribution of mitochondrial DNA haplotype frequency contributions to green turtles captured in Turks and Caicos waters from all known potential rookeries; adapted from data produced in Richardson et al. (2009). (B) Locations of international flipper tag recaptures. Deployment area of all flipper tags on green sea turtles (white filled circle, labeled TCI), and locations of recapture of individuals (n = 5; blue filled circles), with representative linkage routes (blue lines). Countries of recapture locations labeled.
To sufficiently manage and protect species of conservation concern we need to identify areas and habitats that support annual key life-history events (e.g., nesting, breeding, and foraging), which may also overlap with anthropogenic threats. Through a combination of flipper tagging and satellite tracking we have generated a number of important insights into green turtle ecology that can be useful at a range of scales for this long-lived species.
We show two modes of space-use in sub-adult green turtles; remaining in shallow near-shore waters (within a RAMSAR protected area), and wide-ranging movements, which occurred in individuals of larger size classes. Whilst satellite tracking of juvenile/sub-adult green turtles is still in its infancy, other studies have also observed these behaviors. For example, both Hart and Fujisaki (2010), and Wildermann et al. (2019) showed tracked individuals remained in the shallow coastal waters of Florida, United States for the duration of tag attachment. Those individuals were smaller than presented in our study, however, Chambault et al. (2018) showed localized movements near the island of Martinique, with some larger individuals exhibiting more directed movements away from their tagging location. These individuals (mean CCL: 86 cm) were of a similar size to those displaying wide-ranging movements in our study (mean CCL: 77 cm) and may represent individuals nearing maturity and therefore conducting movements toward adult foraging grounds or natal beaches to themselves breed.
We demonstrate the waters of TCI provide critical areas to sub-adult green turtles, likely providing a foraging ground for this size-class. Both flipper tags and satellite tags highlight that many individuals show extended occupancy in TCI waters, most notably in the waters off Middle and East Caicos. These islands host extensive and largely pristine wetlands connected by a complex of tidal creeks vegetated by seagrass and marine algae (Richardson et al., 2009), providing rich foraging grounds supporting developmental and growth phase for sub-adult green turtles. The repeated use of a shared resource by multiple individuals for extended periods identifies the RAMSAR protected area as key habitat for green turtles. Given the nature of similar habitat within the Turks and Caicos Islands’ both within and outside of MPAs, it is likely that this extensive network of marine habitats is of regional importance to Caribbean green turtles (Richardson et al., 2009). However, an illegal capture of a satellite-tagged green turtle during this study suggests the need for a review of enforcement and compliance with national laws to protect green turtles and their critically important habitats in TCI waters.
We provide data that revealed region-wide connectivity across the Caribbean. Movements shown by satellite tracking crossed through nine geo-political zones, and into the High Seas; and international flipper tag recaptures occurring from waters of two other countries within the wider Caribbean as turtles traveled to other foraging grounds or toward natal beaches. These results emphasize the importance of establishing a cooperative network throughout the region to coordinate and support marine turtle management efforts especially when considering likely exposure to multiple fisheries (both legal and illegal), varying in regulation and enforcement levels, that will differ to those in TCI.
The movements displayed in our study corroborate studies describing the Caribbean as having a genetically mixed stock of green turtles inhabiting common developmental habitats but originating from diverse natal beaches (Lahanas et al., 1998; Richardson et al., 2009; Costa Jordao et al., 2017; Chambault et al., 2018). Richardson et al. (2009) highlighted that the main source for TCI green turtles was likely to be Costa Rica, especially for juvenile males (Stringell, 2013), with a significant contribution from nesting populations in Florida. Satellite tracking offers another technique to provide evidence of linkages between nations, as shown by an individual tracked from TCI into the waters off Costa Rica, and another traveling north to the inshore waters of South-East United States. Furthermore, most of the international flipper tag returns were reported from turtles caught by fisheries targeting green turtle foraging grounds in Nicaragua’s waters, known to be important for the Costa Rica nesting population (Troëng et al., 2005; Lagueux et al., 2014). International flipper tag recaptures support other studies showing the seagrass beds of Nicaragua as important foraging grounds for green turtles within the Caribbean Sea (e.g., Cuba, Bjorndal et al., 2003; Moncada et al., 2006), and especially for turtles originating from the large green turtle rookery at Tortuguero, Costa Rica (Troëng et al., 2005; Velez-Espino et al., 2018), with the possibility that these flipper tagged individuals join adults in the foraging ground before nesting in Costa Rica.
Similar green turtle movements have also been shown at a wider Caribbean-Atlantic scale with turtles tracked from Bermuda to the coast of Costa Rica (Meylan et al., 2014). As the waters of the TCI are regionally important foraging grounds, any negative impacts (e.g., fishing pressure; habitat destruction; increased development) here will potentially have consequential effects for nesting locations throughout the Caribbean and more widely in the Atlantic; thus to account for geographic and ecological scales, species conservation should take into account the genetic structure and demographic history of populations (Lande, 1988). Many observations of mature females elsewhere in the Atlantic mostly consist of individuals >90 cm CCL (Godley et al., 2002a, 2003; Hays et al., 2002; Troëng et al., 2005), which suggests the wide-ranging movements shown by four of the 16 tracked green turtles here are nearing a size of sexual maturity, or at least approaching a size threshold at which to begin migration toward developmental foraging, adult foraging grounds, or natal beaches.
This study has increased our knowledge on regional occupancy of green turtles, and linkages between TCI and locations throughout the Caribbean and more widely in the Atlantic. However, there is still a paucity on fine-scale information on movement within TCI waters, which would greatly improve ability to focus management efforts within and outside areas of protection. To obtain such information, higher resolution data needs to be acquired, likely in the form of GPS quality locations, and/or the establishment of an acoustic array in which to monitor turtle presence and movement at local scales continuously (Schofield et al., 2007; Scales et al., 2011). These data, coupled with detailed information on fishing activity may allow us to understand overlap of anthropogenic activity and gain insight into potential sustainability of harvests.
Our results provide new insight into space-use patterns identifying concentrated areas of use by sub-adult green turtles within a RAMSAR designated protected area. We used an integrated approach of techniques to maximize strengths of satellite telemetry (e.g., fine-scale movements, near continuous tracks showing pathways and routes undertaken, and speed of data acquisition) and flipper tagging (e.g., affordability, longevity, and extended temporal scales). Extended periods of occupancy show this area consistently provides suitable conditions for this size-class of green turtle, which are currently protected under national legislation. These findings illustrate the regional value of TCI MPAs, and the importance of ensuring they are effectively managed to support the conservation of Caribbean green turtle populations. This study provides critical spatio-temporal information that increases the knowledge base on this life-stage of green turtles to add to information accessible to policymakers to focus management strategies and enforcement. The protection of pathways linking foraging grounds and nesting beaches presents a challenging intervention. However, with populations, and sub-populations of green turtles in the Atlantic being intrinsically linked, multi-national cooperation to implement agreements on bycatch mitigation and levels of take are recommended where possible.
Raw data will not be made available due to the sensitivity of endangered species location information. Requests to access the datasets should be directed to the corresponding author. All other data used for analyses are presented in the manuscript. Requests to access the datasets should be directed tocGV0ZXIucmljaGFyZHNvbkBtY3N1ay5vcmc=.
The animal study was reviewed and approved by the University of Exeter’s Ethics Commitee in partnership with DECR.
PR and AB conceived the study. PR, AS, TS, KH, QP, and JW managed and facilitated tag deployment and data collection. PD performed the analyses with support and advice from PR, BG, and AB. All authors contributed to the manuscript revision, read and approved the submitted version.
We are grateful for the generous funding for this project from Anne and Simon Notley, the Blavatnik Family, the Wiese Family, the Gerrity Family, Keith Anderson, Kenneth De Regt and Alison Overseth, Patrick and Linda Flockhart, Stephen Meringoff and Kim Charlton, Big Blue Unlimited, Amanyara Resort, The People’s Trust for Endangered Species, Princess Yachts, and the National Marine Aquarium, Plymouth, United Kingdom. PD was funded by a NERC Knowledge Exchange Fellowship (NE/R007039/1).
JW was employed by the Amanyara Resort.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was a collaborative Project between the Department of Environment and Coastal Resources (DECR); Marine Conservation Society (MCS), United Kingdom; University of Exeter, United Kingdom; and the Amanyara Resort. This work would not have been possible without the assistance and cooperation of the Turks and Caicos Islands fishing community. We thank the following people for their help with aspects of this work: Logistics: DECR staff, Kathleen McNary-Wood, Gilbert Jennings, David Claire, The School for Field Studies – South Caicos, Big Blue Unlimited, Eiglys Trejo and Andrew Snead, and staff at the Amanyara Resort, Providenciales. International flipper tag return data: WIDECAST’s Marine Turtle Tagging Centre at the University of the West Indies, Barbados; Dr. Cynthia Lagueux and fisher colleagues in Nicaragua; Dr. Claudia Lombard, USFWS, and Hector Barrios-Garrido, La Universidad del Zulia. We thank Ian Jonsen for additional help with the bsam package.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00690/full#supplementary-material
Acuña-Marrero, D., Smith, A. N. H., Hammerschlag, N., Hearn, A., Anderson, M. J., Calich, H., et al. (2017). Residency and movement patterns of an apex predatory shark (Galeocerdo cuvier) at the Galapagos Marine Reserve. PLoS One 12:e0183669. doi: 10.1371/journal.pone.0183669
Baker, S., Paddock, J., Smith, A. M., Unsworth, R. K. F., Cullen-Unsworth, L. C., and Hertler, H. (2015). An ecosystems perspective for food security in the Caribbean: seagrass meadows in the Turks and Caicos Islands. Ecosyst. Serv. 11, 12–21. doi: 10.1016/j.ecoser.2014.07.011
Balazs, G. H. (1999). “Factors to consider in the tagging of sea turtles,” in Research and Management Techniques for the Conservation of Sea Turtles, eds K. Eckert, K. Bjorndal, F. Abreu-Grobois, and M. Donnelly (Washington, DC: IUCN/SSC Marine Turtle Specialist Group Publication), 101–109.
Barrios-Garrido, H., Espinoza-Rodríguez, N., Rojas-Cañizales, D., Palmar, J., Wildermann, N., Montiel-Villalobos, M. G., et al. (2017). Trade of marine turtles along the Southwestern Coast of the Gulf of Venezuela. Mar. Biodivers. Rec. 10, 1–12. doi: 10.1186/s41200-017-0115-0
BirdLife International (2020). Important Bird and Biodiversity Area (IBA) Digital Boundaries. March 2020 Version. Cambridge: BirdLife International.
Bivand, R. S., Pebesma, E., and Gomez-Rubio, V. (2013). Applied Spatial Data Analysis with R, Second Edition. New York: Springer.
Bjorndal, K. A., Bolten, A. B., and Chaloupka, M. Y. (2003). Survival probability estimates for immature green turtles Chelonia mydas in the Bahamas. Mar. Ecol. Prog. Ser. 252, 273–281. doi: 10.3354/meps252273
Bolten, A. B. (1999). “Techniques for measuring sea turtles,” in Research and Management Techniques for the Conservation of Sea Turtles, eds K. L. Eckert, K. A. Bjorndal, F. A. Abreu-Grobois, and M. Donnelly (Washington, DC: IUCN/SSC Marine Turtle Specialist Group Publication).
Brautigam, A., and Eckert, K. L. (2006). Turning the Tide: Exploitation, Trade and Management of Marine Turtes in the Lesser Antilles, Central America, Colombia and Venezuela. Cambridge: TRAFFIC.
Breed, G. A., Jonsen, I. D., Myers, R. A., Bowen, W. D., and Leonard, M. L. (2009). Sex-specific, seasonal foraging tactics of adult grey seals (Halichoerus grypus) revealed by state-space analysis. Ecology 90, 3209–3221. doi: 10.1890/07-1483.1
Broderick, A. C., Frauenstein, R., Glen, F., Hays, G. C., Jackson, A. L., Pelembe, T., et al. (2006). Are green turtles globally endangered? Glob. Ecol. Biogeogr. 15, 21–26. doi: 10.1111/j.1466-822X.2006.00195.x
Brooks, S. P., and Gelman, A. (1998). General methods for monitoring convergence of iterative simulations. J. Comput. Graph Stat. 7, 434–455. doi: 10.1080/10618600.1998.10474787
Calenge, C. (2006). The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Modell. 197, 516–519. doi: 10.1016/j.ecolmodel.2006.03.017
Chambault, P., de Thoisy, B., Huguin, M., Martin, J., Bonola, M., Etienne, D., et al. (2018). Connecting paths between juvenile and adult habitats in the Atlantic green turtle using genetics and satellite tracking. Ecol. Evol. 8, 12790–12802. doi: 10.1002/ece3.4708
Costa Jordao, J., Bondioli, A. C. V., de Almeida-Toledo, L. F., Bilo, K., Berzins, R., Le Maho, Y., et al. (2017). Mixed-stock analysis in green turtles Chelonia mydas: mtDNA decipher current connections among west Atlantic populations. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 28, 197–207. doi: 10.3109/19401736.2015.1115843
Coyne, M. S., and Godley, B. J. (2005). Satellite tracking and analysis tool (STAT): an integrated system for archiving, analyzing and mapping animal tracking data. Mar. Ecol. Prog. Ser. 301, 1–7. doi: 10.3354/meps301001
Crouse, D. T., Crowder, L. B., and Caswell, H. (1987). A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1423. doi: 10.2307/1939225
Dodge, K. L., Galuardi, B., and Lutcavage, M. E. (2015). Orientation behaviour of leatherback sea turtles within the North Atlantic subtropical gyre. Proc. R. Soc. B Biol. Sci. 282, 1–7. doi: 10.1098/rspb.2014.3129
Doherty, P. D., Baxter, J. M., Godley, B. J., Graham, R. T., Hall, G., Hall, J., et al. (2017). Testing the boundaries: seasonal residency and inter-annual site fidelity of basking sharks in a proposed marine protected area. Biol. Conserv. 209, 68–75. doi: 10.1016/j.biocon.2017.01.018
Edyvane, K. S., and Penny, S. S. (2017). Trends in derelict fishing nets and fishing activity in northern Australia: implications for trans-boundary fisheries management in the shared Arafura and Timor Seas. Fish. Res. 188, 23–37. doi: 10.1016/j.fishres.2016.11.021
Fleming, E. (2001). Swimming Against the Tide: Recent Surveys of Exploitation, Trade, and Management of Marine Turtles in the Northern Caribbean. Washington, DC: TRAFFIC.
Forster, J., Lake, I. R., Watkinson, A. R., and Gill, J. A. (2011). Marine biodiversity in the Caribbean UK overseas territories: perceived threats and constraints to environmental management. Mar. Policy 35, 647–657. doi: 10.1016/j.marpol.2011.02.005
Fossette, S., Witt, M. J., Miller, P., Nalovic, M. A., Albareda, D., Almeida, A. P., et al. (2014). Pan-atlantic analysis of the overlap of a highly migratory species, the leatherback turtle, with pelagic longline fisheries. Proc. R. Soc. B 281:20133065. doi: 10.1098/rspb.2013.3065
Freitas, C. (2012). argosfilter: Argos Location Filter. R Package Version 0.63. Available online at: https://CRAN.R-project.org/package=argosfilter (accessed January 11, 2012).
Godley, B. J., Blumenthal, J. M., Broderick, A. C., Coyne, M. S., Godfrey, M. H., Hawkes, L. A., et al. (2008). Satellite tracking of sea turtles: where have we been and where do we go next? Endanger Species Res. 4, 3–22. doi: 10.1109/TGRS.2014.2344627
Godley, B. J., Broderick, A. C., Frauenstein, R., Glen, F., and Hays, G. C. (2002a). Reproductive seasonality and sexual dimorphism in green turtles. Mar. Ecol. Prog. Ser. 226, 125–133. doi: 10.3354/meps226125
Godley, B. J., Luschi, P., Lima, E. H. S. M., Akesson, S., Broderick, A. C., Glen, F., et al. (2003). Movement patterns of green turtles in Brazilian coastal waters described by satellite tracking and flipper tagging. Mar. Ecol. Prog. Ser. 253, 279–288. doi: 10.3354/meps253279
Godley, B. J., Richardson, S., Broderick, A. C., Coyne, M. S., Glen, F., and Hays, G. C. (2002b). Long-term satellite telemetry of the movements and habitat utilisation by green turtles in the Mediterranean. Ecography (Cop) 25, 352–362. doi: 10.1034/j.1600-0587.2002.250312.x
Greene, C. H., Block, B. A., Welch, D., Jackson, G., Lawson, G. L., and Rechisky, E. L. (2009). Advances in conservation oceanography: new tagging and tracking technologies and their potential for transforming the science underlying fisheries management. Oceanography 22, 210–223. doi: 10.5670/oceanog.2009.21
Groombridge, B., and Luxmore, R. (1989). The Green Turtle and Hawksbill: World Status Exploitation and Trade. Cambridge: CITES.
Hamann, M., Limpus, C., Hughes, G., Mortimer, J., and Pilcher, N. (2006). Assessment of the Conservation Status of the Leatherback Turtle in the Indian Ocean and South East Asia, Including Consideration of the Impacts of the December 2004 Tsunami on Turtles and Turtle Habitats. Bangkok: IOSEA Marine Turtle MoU Secretariat.
Hammerschlag, N., Gallagher, A. J., and Lazarre, D. M. (2011). A review of shark satellite tagging studies. J. Exp. Mar. Bio Ecol. 398, 1–8. doi: 10.1016/j.jembe.2010.12.012
Hart, C. E., Blanco, G. S., Coyne, M. S., Delgado-Trejo, C., Godley, B. J., Jones, T. T., et al. (2015). Multinational tagging efforts illustrate regional scale of distribution and threats for East Pacific green turtles (Chelonia mydas agassizii). PLoS One 10:e0116225. doi: 10.1371/journal.pone.0116225
Hart, K., and Fujisaki, I. (2010). Satellite tracking reveals habitat use by juvenile green sea turtles Chelonia mydas in the Everglades, Florida, USA. Endanger Species Res. 11, 221–232. doi: 10.3354/esr00284
Hays, G. C., Bailey, H., Bograd, S. J., Bowen, W. D., Campagna, C., Carmichael, R. H., et al. (2019). Translating marine animal tracking data into conservation policy and management. Trends Ecol. Evol. 34, 459–473. doi: 10.1016/j.tree.2019.01.009
Hays, G. C., Broderick, A. C., Glen, F., and Godley, B. J. (2002). Change in body mass associated with long-term fasting in a marine reptile: the case of green turtles (Chelonia mydas) at Ascension Island. Can. J. Zool. 80, 1299–1302. doi: 10.1139/z02-110
Hays, G. C., Ferreira, L. C., Sequeira, A. M. M., Meekan, M. G., Duarte, C. M., Bailey, H., et al. (2016). Key questions in marine megafauna movement ecology. Trends Ecol. Evol. 31, 463–475. doi: 10.1016/j.tree.2016.02.015
Hays, G. C., Mortimer, J. A., Ierodiaconou, D., and Esteban, N. (2014). Use of long-distance migration patterns of an endangered species to inform conservation planning for the World’s largest marine protected area. Conserv. Biol. 28, 1636–1644. doi: 10.1111/cobi.12325
Hazen, E., Maxwell, S., Bailey, H., Bograd, S., Hamann, M., Gaspar, P., et al. (2012). Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar. Ecol. Prog. Ser. 457, 221–240. doi: 10.3354/meps09857
Humber, F., Godley, B. J., and Broderick, A. C. (2014). So excellent a fishe: a global overview of legal marine turtle fisheries. Divers. Distrib. 20, 579–590. doi: 10.1111/ddi.12183
Hussey, N. E., Kessel, S., Aarestrup, K., Cooke, S. J., Cowley, P. D., Fisk, A. T., et al. (2015). Aquatic animal telemetry: a panoramic window into the underwater world. Science 348, 1221–1231.
Jeffers, V. F., and Godley, B. J. (2016). Satellite tracking in sea turtles: how do we find our way to the conservation dividends? Biol. Conserv. 199, 172–184. doi: 10.1016/j.biocon.2016.04.032
Jonsen, I. (2016). Joint estimation over multiple individuals improves behavioural state inference from animal movement data. Sci. Rep. 6:20625. doi: 10.1038/srep20625
Jonsen, I. D., Basson, M., Bestley, S., Bravington, M. V., Patterson, T. A., Pedersen, M. W., et al. (2013). State-space models for bio-loggers: a methodological road map. Deep Sea Res. Part II Top. Stud. Oceanogr. 8, 34–46. doi: 10.1016/j.dsr2.2012.07.008
Jonsen, I. D., Flemmings, J. M., Myers, R. A., and Scotia, N. (2005). Robust state – space modeling of animal movement data. Ecology 86, 2874–2880. doi: 10.1890/04-1852
Jonsen, I. D., Myers, R. A., and Flemming, J. M. (2003). Meta-analysis of animal movement using state-space models. Ecology 84, 3055–3063. doi: 10.1890/02-0670
Lagueux, C. J., Campbell, C. L., and Strindberg, S. (2014). Artisanal green turtle, Chelonia mydas, fishery of caribbean Nicaragua: I. Catch rates and trends, 1991-2011. PLoS One 9:e94667. doi: 10.1371/journal.pone.0094667
Lahanas, P. N., Bjorndal, K. A., Bolten, A. B., Encalada, S. E., Miyamoto, M. M., Valverde, R. A., et al. (1998). Genetic composition of a green turtle (Chelonia mydas) feeding ground population: evidence for multiple origins. Mar. Biol. 130, 345–352. doi: 10.1007/s002270050254
Lande, R. (1988). Genetics and demography in biological conservation. Science 241, 1455–1460. doi: 10.1126/science.3420403
Mansfield, K. L., Wyneken, J., Porter, W. P., and Luo, J. (2014). First satellite tracks of neonate sea turtles redefine the “lost years” oceanic niche. Proc. R. Soc. B Biol. Sci. 281:20133039. doi: 10.1098/rspb.2013.3039
Martinez, C., Rockel, S., and Vieux, C. (2017). European Overseas Coastal and Marine Protected Areas: Overview of Coastal and Marine Conservation Efforts in European Outermost Regions and Overseas Countries and Territories. Gland: IUCN.
Maxwell, S. M., Conners, M. G., Sisson, N. B., and Dawson, T. M. (2016). Potential benefits and shortcomings of marine protected areas for small seabirds revealed using miniature tags. Front. Mar. Sci. 3:264. doi: 10.3389/fmars.2016.00264
McClenachan, L., Jackson, J. B. C., and Newman, M. J. H. (2006). Conservation implications of historic sea turtle nesting beach loss. Front. Ecol. Environ. 4, 290–296. doi: 10.1890/1540-929520064[290:CIOHST]2.0.CO;2
McKenna, M. F., Calambokidis, J., Oleson, E. M., Laist, D. W., and Goldbogen, J. A. (2015). Simultaneous tracking of blue whales and large ships demonstrates limited behavioral responses for avoiding collision. Endanger Species Res. 27, 219–232. doi: 10.3354/esr00666
Meylan, A., Arenas, A., Zurita, J. C., Harrison, E., Gray, J., and Meylan, P. (2014). Turtles tagged in developmental habitat in bermuda nest in Mexico and Costa Rica. Mar. Turt. Newsl. 77710, 15–17.
Moncada, F., Abreu-grobois, F. A., Muhlia-melo, A., Bell, C., Tröeng, S., Bjorndal, K. A., et al. (2006). Movement patterns of green turtles (Chelonia mydas) in Cuba and adjacent caribbean waters inferred from flipper tag recaptures published by: the society for the study of amphibians and reptiles your use of this PDF, the BioOne Web site, and all. J. Herpetol. 40, 22–34. doi: 10.1670/39-05a.1
Patterson, T. A., Thomas, L., Wilcox, C., Ovaskainen, O., and Matthiopoulos, J. (2008). State-space models of individual animal movement. Trends Ecol. Evol. 23, 87–94. doi: 10.1016/j.tree.2007.10.009
Pebesma, E. (2018). sf: Simple Features for R. R Package Version 0.6-3. Available online at: https://CRAN.R-project.org/package=sf (accessed July 14, 2020).
Putman, N. F., and Mansfield, K. L. (2015). Direct evidence of swimming demonstrates active dispersal in the sea turtle “lost years.” Curr. Biol. 25, 1221–1227. doi: 10.1016/j.cub.2015.03.014
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Richardson, P. B., Broderick, A. C., Coyne, M. S., Ekanayake, L., Kapurusinghe, T., Premakumara, C., et al. (2013). Satellite telemetry reveals behavioural plasticity in a green turtle population nesting in Sri Lanka. Mar. Biol. 160, 1415–1426. doi: 10.1007/s00227-013-2194-8
Richardson, P. B., Bruford, M. W., Calosso, M. C., Campbell, L. M., Clerveaux, W., Formia, A., et al. (2009). Marine turtles in the Turks and Caicos Islands: remnant rookeries, regionally significant foraging stocks, and a major turtle fishery. Chelonian Conserv. Biol. 8, 192–207. doi: 10.2744/ccb-0871.1
Scales, K. L., Lewis, J. A., Lewis, J. P., Castellanos, D., Godley, B. J., and Graham, R. T. (2011). Insights into habitat utilisation of the hawksbill turtle, Eretmochelys imbricata (Linnaeus, 1766), using acoustic telemetry. J. Exp. Mar. Bio Ecol. 407, 122–129. doi: 10.1016/j.jembe.2011.07.008
Schofield, G., Bishop, C. M., MacLean, G., Brown, P., Baker, M., Katselidis, K. A., et al. (2007). Novel GPS tracking of sea turtles as a tool for conservation management. J. Exp. Mar. Bio Ecol. 347, 58–68. doi: 10.1016/j.jembe.2007.03.009
Schofield, G., Dimadi, A., Fossette, S., Katselidis, K. A., Koutsoubas, D., Lilley, M. K. S., et al. (2013). Satellite tracking large numbers of individuals to infer population level dispersal and core areas for the protection of an endangered species. Divers. Distrib. 19, 834–844. doi: 10.1111/ddi.12077
Sequeira, A. M. M., Mellin, C., Fordham, D. A., Meekan, M. G., and Bradshaw, C. J. A. (2014). Predicting current and future global distributions of whale sharks. Glob. Chang Biol. 20, 778–789. doi: 10.1111/gcb.12343
Sprogis, K. R., Raudino, H. C., Rankin, R., Macleod, C. D., and Bejder, L. (2016). Home range size of adult Indo-Pacific bottlenose dolphins (Tursiops aduncus) in a coastal and estuarine system is habitat and sex-specific. Mar. Mammal. Sci. 32, 287–308. doi: 10.1111/mms.12260
Stoddart, D. R. (1980). Little cayman: ecology and significance. Atoll Res. Bull. 241:171. doi: 10.5479/si.00775630.241-16.171
Stokes, K. L., Broderick, A. C., Canbolat, A. F., Candan, O., Fuller, W. J., Glen, F., et al. (2015). Migratory corridors and foraging hotspots: critical habitats identified for Mediterranean green turtles. Divers. Distrib. 21, 665–674. doi: 10.1111/ddi.12317
Stringell, T. B. (2013). Population Dynamics of Marine Turtles under Harvest. Cornwall: University of Exete.
Stringell, T. B., Calosso, M. C., Claydon, J. A. B., Clerveaux, W., Godley, B. J., Lockhart, K. J., et al. (2013). Marine turtle harvest in a mixed small-scale fishery: evidence for revised management measures. Ocean Coast Manag. 82, 34–42. doi: 10.1016/j.ocecoaman.2013.05.004
Stringell, T. B., Calosso, M. C., Claydon, J. A. B., Clerveaux, W., Godley, B. J., Phillips, Q., et al. (2010). Loggerhead turtles in the Turks and Caicos Islands, Caribbean. Mar. Turt. Newsl. 127, 23–25.
Stringell, T. B., Clerveaux, W. V., Godley, B. J., Phillips, Q., Ranger, S., Richardson, P. B., et al. (2015). Protecting the breeders: research informs legislative change in a marine turtle fishery. Biodivers. Conserv. 24, 1775–1796. doi: 10.1007/s10531-015-0900-1
Sumner, M. D. (2016). trip: Tools for the Analysis of Animal Track Data. R Package Version 1.5.0. Available online at: https://CRAN.R-project.org/package=trip (accessed June 15, 2020).
Trathan, P. N., García-Borboroglu, P., Boersma, D., Bost, C. A., Crawford, R. J. M., Crossin, G. T., et al. (2015). Pollution, habitat loss, fishing, and climate change as critical threats to penguins. Conserv. Biol. 29, 31–41. doi: 10.1111/cobi.12349
Troëng, S., Evans, D. R., Harrison, E., and Lagueux, C. J. (2005). Migration of green turtles Chelonia mydas from Tortuguero, Costa Rica. Mar. Biol. 148, 435–447. doi: 10.1007/s00227-005-0076-4
Turks and Caicos Government (2014). Chapter 10.01. National Parks Ordinance. In Laws of the Turks and Caicos Islands. Available online at: http://online.fliphtml5.com/fizd/jgle/#p=5 (accessed December 31, 2014).
van Dijk, P. P., and Shepherd, C. R. (1995). Shelled Out?: A snapshot of Bekko Trade in Selected locations in South-east-Asia. South-East Asia: TRAFFIC.
Velez-Espino, A., Pheasey, H., Araújo, A., and Fernández, L. M. (2018). Laying on the edge: demography of green sea turtles (Chelonia mydas) nesting on Playa Norte, Tortuguero, Costa Rica. Mar. Biol. 165, 1–12. doi: 10.1007/s00227-018-3305-3
Wildermann, N. E., Gredzens, C., Avens, L., BarriosGarrido, H. A., Bell, I., Blumenthal, J., et al. (2018). Informing research priorities for immature sea turtles through expert elicitation. Endanger Species Res. 37, 55–76. doi: 10.3354/esr00916
Wildermann, N. E., Sasso, C. R., Stokes, L. W., Snodgrass, D., and Fuentes, M. M. P. B. (2019). Habitat use and behavior of multiple species of marine turtles at a foraging area in the Northeastern Gulf of Mexico. Front. Mar. Sci. 6:155. doi: 10.3389/fmars.2019.00155
Keywords: Chelonia mydas, connectivity, marine protected area (MPA), migration, satellite tracking
Citation: Doherty PD, Broderick AC, Godley BJ, Hart KA, Phillips Q, Sanghera A, Stringell TB, Walker JT and Richardson PB (2020) Spatial Ecology of Sub-Adult Green Turtles in Coastal Waters of the Turks and Caicos Islands: Implications for Conservation Management. Front. Mar. Sci. 7:690. doi: 10.3389/fmars.2020.00690
Received: 15 June 2020; Accepted: 29 July 2020;
Published: 25 August 2020.
Edited by:
Michael Paul Jensen, Southwest Fisheries Science Center (NOAA), United StatesReviewed by:
Jeffrey Aleksandr Seminoff, Southwest Fisheries Science Center (NOAA), United StatesCopyright © 2020 Doherty, Broderick, Godley, Hart, Phillips, Sanghera, Stringell, Walker and Richardson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip D. Doherty, cC5kb2hlcnR5QGV4ZXRlci5hYy51aw==; Peter Bradley Richardson, cGV0ZXIucmljaGFyZHNvbkBtY3N1ay5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.