- 1Central Caribbean Marine Institute, Princeton, NJ, United States
- 2Marine Science Institute, University of California, Santa Barbara, Santa Barbara, CA, United States
- 3Marine Ecology Laboratory, Department of Oceanography and Limnology, Universidade Federal do Rio Grande do Norte, Natal, Brazil
- 4Department of Ecology, Evolution and Marine Biology, University of California, Santa Barbara, Santa Barbara, CA, United States
Coral reefs have changed radically in the last few decades with reefs in the Caribbean now averaging 13% coral cover and 40% macroalgal cover (mostly Dictyota and Lobophora). So, it is time we re-evaluate which species are key to the process of herbivory in these new conditions. The role herbivorous fishes play in controlling macroalgae is often considered by managers and researchers at a guild or family level, but greater resolution is needed to understand the impact of herbivores more fully. We performed feeding assays and behavioral observations of fish feeding to quantify the removal of the most common macroalgae by different herbivorous fish species. In total, we ran 34 h-long trials using Dictyota and Lobophora across two sites and conducted over 34 h of observation of 105 fish from eight species in the Cayman Islands, Caribbean. We show that many nominal herbivores did not consume macroalgae but instead targeted the epibionts on macroalgae and other substrates. In fact, only three fish taxa consumed macroalgae as a significant proportion of their feeding: one species of surgeonfish (Acanthurus coeruleus), one species of parrotfish (Sparisoma aurofrenatum), and the third, the chubs (Kyphosus spp.), is a group of species which is not consistently considered as part of the herbivore community in the Caribbean. From our observations, an individual A. coeruleus can consume ∼44 g of Dictyota per day, while S. aurofrenatum can consume ∼50 g and Kyphosus spp. can consume ∼100 g. These values are significantly more than all other herbivorous fish species and suggest these three taxa are key macroalgal consumers in the Caribbean. These results highlight that disentangling the role of individual herbivore species is necessary for critical species to be identified and protected. Furthermore, as reef conditions change, we need to re-evaluate the key functions and species to be more effective at protecting and managing these important ecosystems. With far higher macroalgal coverage than in the past, the few browsing species that remove macroalgae may be increasingly important in promoting reef health.
Introduction
In the Caribbean, coral cover has declined to a regional average of 13%, while macroalgal cover is now ∼ 40% of the forereef (between 28 and 45% by ecoregion, AGRRA, 2018). This region-wide decline in reef health has been observed since the 1970s and is likely a result of a combination of factors including hurricanes, coral disease, temperature stress, over-fishing of herbivorous fishes and the die-off of the algal-grazing urchin, Diadema antillarum (Gardner et al., 2003; Jackson et al., 2014). Of the ∼40% macroalgal cover, Dictyota and Lobophora are often the most abundant genera (Cardoso et al., 2009; Diaz-Pulido et al., 2011; Suchley and Alvarez-Filip, 2017). This increase in macroalgae can cause problems for corals and hence the resilience of the reef system by reducing their growth (Tanner, 1995; Box and Mumby, 2007) and fecundity (Kuffner et al., 2006), increasing the prevalence of disease (Birrell et al., 2008) and pre-empting space and inhibiting coral recruitment (McCook, 2001; Birrell et al., 2008; Venera-Ponton et al., 2011). Given these negative effects of macroalgae and their increasing abundance, the herbivore species that remove them are key to promoting reef health (Burkepile and Hay, 2008; Adam et al., 2015).
Since the mass mortality of the urchin Diadema antillarum in 1983 and subsequent lack of recovery of urchin populations to previous densities (Lessios, 1988; Lessios, 2005), fishes are the most abundant herbivores on Caribbean coral reefs. Studies to date suggest that the herbivore guild in the Caribbean is composed almost exclusively of parrotfish (Scarus and Sparisoma) and surgeonfish (Acanthurus; Mumby, 2006; Burkepile and Hay, 2008; Kramer et al., 2017; Longo et al., 2019). Although chubs (Kyphosus), unicornfish (Naso), and rabbitfish (Siganus) are also recognized as important herbivores in the Indo-Pacific (Cheal et al., 2010; Rasher et al., 2013; Knudsen and Clements, 2016; Steneck et al., 2017), they are either absent (unicornfish and rabbitfish) in the Caribbean or not studied in the same intensity as other members of the Caribbean herbivore guild (chubs, see Mumby, 2006; Burkepile et al., 2013; Kramer et al., 2017; Suchley and Alvarez-Filip, 2017 as examples of Kyphosus not being considered). Indeed, research on herbivory in the Caribbean has been heavily focused on parrotfish while other taxa have received far less attention (Duran et al., 2019). This disregard of Acanthurus and Kyphosus is curious because these genera can be abundant in the Caribbean (each genus can form 25% of the herbivorous fish biomass on Caribbean coral reefs, Paddack et al., 2006; Hernández-Landa et al., 2015) and because they are recognized as important herbivores in the Indo-Pacific (for example see Clements and Choat, 1997; Green and Bellwood, 2009; Marshell and Mumby, 2015; Knudsen and Clements, 2016). One potential ramification of this focus is that browsing species (those that consume established macroalgae) may have been understudied and underappreciated in the Caribbean. With macroalgae now covering almost half of the reef, more attention should be paid to the species that consume these algae.
Herbivores are often assessed at the community or family level (for example, see Paddack et al., 2006; Suchley et al., 2016; Steneck et al., 2017), rather than emphasizing the role of individual species or taxa. When distinctions are made, herbivores are typically categorized as a “browser,” “scraper,” or “excavator” (scraper and excavator both remove the epilithic algal matrix, EAM, from the reef; Bellwood and Choat, 1990; Bellwood et al., 2004; Adam et al., 2018). The drawbacks with this generalized approach are that critical species will not be identified and so could be overlooked in management strategies. Without a more detailed understanding of the impact individual species within the guild have on the reef, conservation and management efforts may be misguided. Indeed, recent work investigating herbivore feeding at the species level has found differences within genera that emphasize the species-specific nature of herbivores’ roles and effects (Longo et al., 2019). For example, Ruttenberg et al. (2019) found species identity a critical feature in estimating how much of the reef area is grazed: while Scarus guacamaia was responsible for a considerable proportion of areal grazing in the Florida Keys, Scarus iserti and Scarus taeniopterus had negligible impact in all reef habitats. Likewise, Duran et al. (2019) reported that congenerics Acanthurus tractus and Acanthurus coeruleus, targeted significantly different proportions of food items. Similarly, in the Indo-Pacific, Sargassum was removed almost exclusively by Naso unicornis (Hoey and Bellwood, 2009) and the batfish (Platax pinnatus) was unexpectedly found to be a key herbivore on some reefs (Bellwood et al., 2006). Thus, greater resolution is required to understand herbivory more fully and to understand the impact each species, particularly the browsers, have on Caribbean reefs (Adam et al., 2015). Additionally, since macroalgal cover has increased on Caribbean reefs, we may currently be underappreciating the role browsers play in consuming these macroalgae and thereby promoting reef health and resilience.
Furthermore, Clements et al. (2017) intimated that many nominal herbivores are not targeting macroalgae and are in fact consuming other food sources including epilithic/endolithic cyanobacteria, microbes, and detritus. This would mean that the number of species actively targeting and consuming macroalgae is much smaller than appears from observation and that this role may be performed by fewer species than currently appreciated. Therefore, understanding which herbivorous fishes are targeting the dominant macroalgae in the Caribbean requires information on feeding behavior and food targets at the species level. Consequently, we closely observed the feeding of fish and made a distinction between bites taken from the surface of macroalgae and actual consumption of macroalgal tissue. In addition, we also cleaned two of the most prevalent macroalgae, Dictyota spp. and Lobophora variegata, of particulates and epibionts and presented them in feeding assays so that we could measure consumption of these algae without any confounding food items. We found that only three fish taxa consumed these dominant macroalgae and that many other nominal herbivores were actually feeding on epibionts on the macroalgal surface, rather than the macroalgae themselves.
Materials and Methods
All experiments were conducted with permission from the Cayman Islands Department of Environment (permits awarded 21 May, 2016, 12 June and 23 August, 2017).
Site Description
Our experiments and observations were conducted at three sites in the fringing reef of the Cayman Islands, an island nation situated in the central Caribbean, ∼240 km south of Cuba and ∼220 km north-west of Jamaica (Supplementary Figure S1). The three Cayman Islands are surrounded by a narrow fringing forereef that begins at about 8 m depth and extends for about 400 m to the wall at 25 to 30 m depth. Photographs of the benthos from May 2017 indicated that the forereefs were representative of the Caribbean with coral cover between 9 and 12% and macroalgal cover between 40 and 53% across the three islands. Dictyota and Lobophora in particular dominate the benthos of the Cayman Islands and together comprise ∼38% of the forereef (Supplementary Figure S2). Observations of fish feeding were conducted over a 1.5 km stretch (∼700 m wide) of continuous forereef that was representative of local reefs, on the north side of Little Cayman at 15 m depth. Feeding assays were conducted at two forereef sites at 8 m depth, one on the north side of Little Cayman and one on the north side of Grand Cayman (Supplementary Figure S1). These two sites were selected because they had the lowest macroalgal cover and so were appropriate sites to run feeding assays.
Observations of Fish Feeding
To quantify feeding behavior of common herbivorous fishes, in May 2017 three observers followed between nine and 12 adults across all sizes of the following taxa and phases: Acanthurus coeruleus (blue tang), Acanthurus tractus (ocean surgeonfish), Scarus iserti (striped parrotfish initial phase), Scarus taeniopterus (princess parrotfish initial phase), Scarus vetula (queen parrotfish initial phase), Sparisoma aurofrenatum (redband parrotfish initial phase and terminal phase), Sparisoma viride (stoplight parrotfish initial phase and terminal phase) and Kyphosus spp. (chub). Observations were conducted for 20 min per fish maintaining a distance of about 5 m so that our presence would not affect fish feeding behavior. We haphazardly selected each fish for observation and, before commencing data collection, followed the fish for three minutes so that it could become accustomed to our presence. After this time, we recorded depth, estimated fish standard length to the nearest whole centimeter, then counted the number of bites each fish took off the benthos and identified food items bitten (e.g., algae, sponge, coral, etc.). We focused particularly on consumption of macroalgae (>1 cm thallus length as per Steneck and Dethier, 1994), which we identified to the lowest taxonomic resolution possible in the field, versus the EAM growing on the reef substrate (EAM, the filamentous turf assemblage <1 cm as per Steneck and Dethier, 1994). No distinction was made between scraping and excavating functions in consumption of EAM. Several fish species were observed feeding off algae, gorgonians, and sponges, but on closer inspection no tissue had been removed and there was no evidence of bite marks or damage/striations on the surface. So, these bites were recorded as bites of epibionts on the surface of these larger organisms. Thus, our dataset yielded three major food types: macroalgae, EAM, and epibionts. We did not count bites taken from food items floating in the water column.
To reduce the likelihood of resampling the same individual, upon completion of the first 20-min observation, we immediately switched from the first targeted fish to another of the same species in the immediate vicinity. We then moved to a new location ∼700 m away to repeat this process and observe subsequent fish. All observations commenced after 1000 h and were concluded before 1,600 h. Observations of each species were conducted on the same day or on consecutive days within a 1.5 km stretch of continuous forereef and all observations were concluded within 3 weeks.
Differences in proportion of bites taken between initial and terminal phase parrotfish were analyzed statistically by Pearson Chi Squared test of Homogeneity using SPSS version 22. Differences between species in bite rate and percent consumption of different food items were analyzed by Permutations ANOVA using the package “lmperm” (Wheeler, 2010) in the software R (R Core Team, 2019) because data were non-normal even after transformation.
Feeding Assays With Common Macroalgae
To determine which fish species grazed the most common macroalgae without the confounding presence of epibionts, we ran feeding assays in September 2017 with algal thalli that had been thoroughly cleaned of epibionts and we video the trials so that diver presence would not influence fish behavior. Furthermore, to assess as many different herbivore species as possible, the experiment was conducted at two different sites of equivalent depth but with different species of herbivorous fish present.
In order to acclimatize the fish to the experimental set up, feeding assay equipment was installed on the reef 2 weeks prior to collecting data. A mixture of the macroalgae found in the area were fixed in 50 cm long, three-strand ropes that were deployed on the reef and replaced daily. This was done to attract as many different species as possible to the ropes and so ensure that the resident fish community was familiar with and comfortable with the apparatus. The days prior to running the Dictyota and Lobophora assays, we trialed the experimental set-up with Sargassum fluitans and Laurencia gemmifera which are known to be palatable to herbivores (Littler and Littler, 2000). These assays acted as a control because they were visited by several fish species that did not appear in the Dictyota and Lobophora assays. As a result, we knew the feeding behavior of the fish in the subsequent feeding assays was due to algal species in the trial and not influenced by fear of unfamiliar equipment.
For the videoed feeding trials, Dictyota and Lobophora were collected in shallow areas (1–2 m depth) near the sites where the feeding assays were conducted. All algae were cleaned by shaking in seawater to remove particulates, then by brushing gently with a toothbrush to remove epibionts and picking off any remaining with fine pointed tweezers. This was done in the lab using natural seawater and ensured that any bites would be of the macroalgae and not of associated flora or fauna. Pieces of Dictyota or Lobophora (1.5–2 g Lobophora or 4 g Dictyota) were fastened within the strands of the 50 cm long, three-strand feeding rope and a tripod-mounted GoPro (Hero 4, San Mateo, CA, United States) was secured ∼0.5 m away so that the alga was within the frame the entire trial. Five such assays were installed a minimum of 5 m apart on the forereef at two sites 8 m deep and ran for 1 h between 0700 and 0800 and again between 1200 and 1300, although at site two only the midday trial was possible for Dictyota. This yielded n = 10 for Dictyota and Lobophora at site one, n = 5 for Dictyota at site two and n = 9 for Lobophora at site two because one camera failed. We sampled these two periods because food selectivity is reported to decrease over the course of the day and so sampling earlier and later in the day was expected to cover both high and low food selectivity (Khait et al., 2013). During each of these sessions a snorkeler recorded which fish species >5 cm in length were present in the area for the majority of the hour-long trial period, and also noted whether any large schools visited the area during the trial. The two algal species were run on separate days within the same week. The number of bites taken per hour by each fish species was then counted from each video and averaged across all videos from the day to give a mean number of bites per hour for each species at each site. Because our goal was to measure consumption of algae, bites that were spat out were not included in the count.
These assays provided information on which species consumed the macroalgae, but we then ran a subsequent, separate trial to calculate the wet weight in grams consumed per bite for each fish species. For this trial, a piece of either Sargassum fluitans, Lobophora variegata, or Laurencia gemmifera ∼1 g in size was weighed to within 0.1 g and secured within the three strands of a rope. One snorkeler deposited the rope on the reef while the second snorkeler observed the rope continuously counting the bites taken of the alga and identifying the fish consumer to species. Once a fish had been able to bite the alga a few times, the first snorkeler immediately removed the rope so that only one fish species consumed the alga on each rope. The alga was then re-weighed and the difference in weight divided by the number of bites that had been observed from that rope. We used Sargassum in place of Dictyota because consumption of Dictyota was too low to obtain sufficient bites from only one fish species per trial. We were only able to obtain data for A. coeruleus using Laurencia, because the other fish always consumed Sargassum or Lobophora before A. coeruleus could.
In total, we obtained data for A. coeruleus from nine pieces of Laurencia, for Kyphosus spp. from 10 pieces of Lobophora and 10 of Sargassum, and for S. aurofrenatum from 13 pieces of Sargassum and 11 of Lobophora. We then calculated an average weight (g) of algae removed per bite for each fish species and multiplied this by the number of bites counted in each video to calculate the grams consumed by each fish species on each algal species. Data were highly non-normal and could not be successfully transformed, so analysis of differences in grams consumed by fish species was performed in SPSS version 22 using the Kruskal–Wallis test for site one and Mann Whitney-U test for site two.
Fish Density Surveys at Feeding Assay Sites
To quantify the abundance and diversity of the fish community in the vicinity of the feeding assays, we conducted visual transect surveys immediately following the final feeding assay and recorded the species of each fish >5 cm in length both on the reef and in the water column above. Each transect was 2 m wide, 23 to 30 m in length and were a minimum of 4 m apart (n = 3 at site one, n = 4 at site two). These surveys provided average species density per 100 m2.
To compare with regional reports of fish density (and benthic cover) we accessed the Atlantic and Gulf Rapid Reef Assessment (AGRRA) database 20180310 and averaged the values reported for forereefs for each ecoregion. This yielded results for parrotfish, surgeonfish, and chub families (as well as scaled percent macroalgal cover, scaled percent turf cover and scaled percent live coral cover) from the Bahamian, Eastern Caribbean, Greater Antilles, Southwestern Caribbean and Western Caribbean ecoregions.
Consumption of Dictyota and Lobophora by the Herbivore Community per 100 m2
To estimate the quantity of Dictyota and Lobophora consumed by the herbivore community, we integrated data from our observational fish follows, feeding assays, and fish density surveys. For each of the three species (A. coeruleus, Kyphosus spp., and S. aurofrenatum) that commonly fed on Dictyota and Lobophora, we multiplied the bites taken per hour of each alga from our observations by the mass of alga removed per bite as determined from our assays. We then scaled this consumption per hour by the density estimates from our transect surveys at sites one and two. This calculation allowed us to estimate the quantity of algae removed by each herbivore species per hour per 100 m2 of reef.
Results
Observations of Fish Feeding
In total, we conducted over 34 h of observation of 105 fish from eight species counting the bites from the benthos. All individuals we observed fed from the benthos during these observations except for three Kyphosus spp. which remained in the water column during the observation period. There were no significant differences in proportions of bites between phases for either S. aurofrenatum or S. viride (p = 0.672; p = 0.100, respectively; Pearson Chi Square test for Homogeneity) so these were combined for subsequent analyses giving n = 20 for these two species.
Mean bite rate from the benthos varied significantly among species, ranging from 80 per hour for Kyphosus spp. to 2,036 per hour for A. tractus (p < 0.001, Permutations ANOVA). Kyphosus spp., S. aurofrenatum, and S. viride had significantly lower bite rates than all other species we observed (p < 0.001, Permutations ANOVA, Figure 1), while A. tractus took significantly more bites from the benthos per hour than all other species (p < 0.05, Permutations ANOVA, Figure 1).
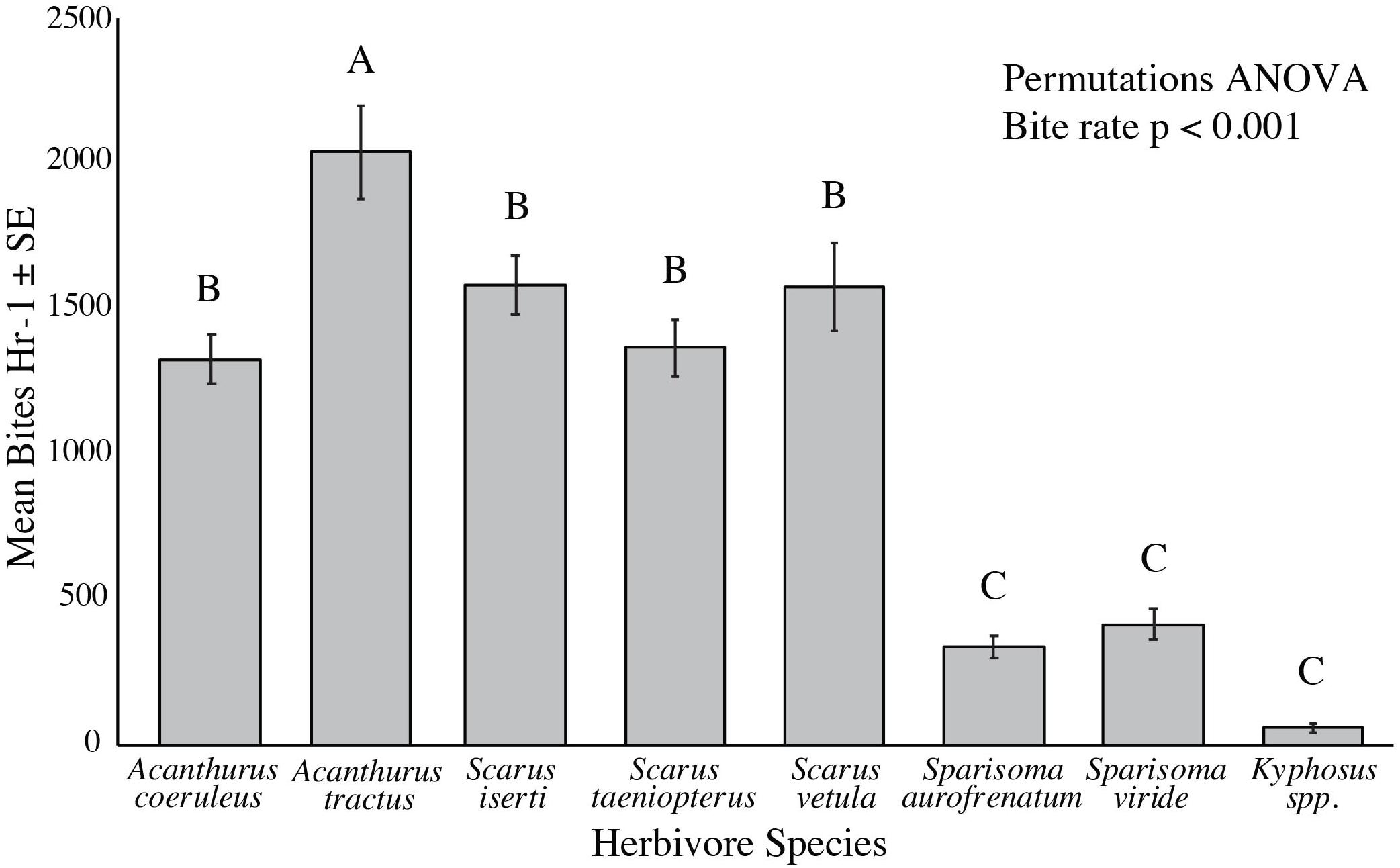
Figure 1. Bite rate from the benthos per hour of the common herbivorous fish species (n = 9 to 20). Individual fish were followed for 20 min counting all bites taken from the benthos. A. tractus had a significantly higher bite rate than all other species (p < 0.05, Permutations ANOVA), while Kyphosus spp., S. aurofrenatum, and S. viride had significantly lower bite rates (p < 0.001, Permutations ANOVA).
For each species we divided the proportion of these bites from the benthos into bites of Dictyota, Lobophora, other macroalgae, bites of EAM off the reef substrate and bites of epibionts on other substrates (sand, sponge, gorgonian, and macroalgae; Figure 2). Kyphosus spp. consumed a significantly higher proportion of total macroalgae (85%, p < 0.001, Permutations ANOVA; Figure 2), followed by A. coeruleus and S. aurofrenatum (35 and 41%, respectively, p < 0.001, Permutations ANOVA; Figure 2) than all other species we observed. For the other species, macroalgae was less than 11% of the bites consumed (p < 0.001, Permutations ANOVA; Figure 2).
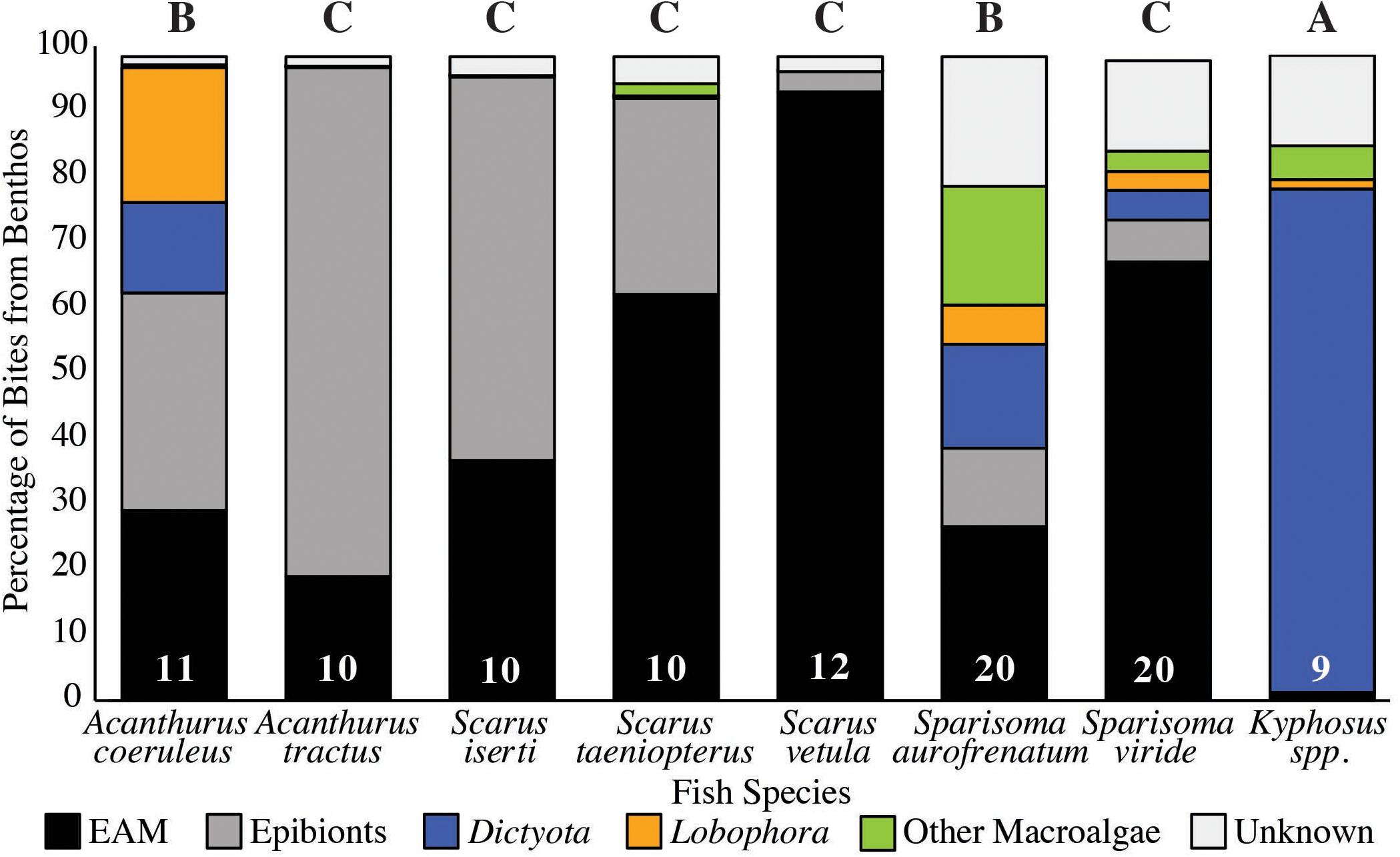
Figure 2. Percentage of Bites Taken of Macroalgae, EAM and Epibionts (n is at the base of each column). Fish were followed for 20 min counting number of bites taken off the benthos, distinction was made between EAM bitten from reef surface indicated in black, epibionts represented in dark gray, and consumption of macroalgae: Dictyota (blue), Lobophora (orange), and other macroalgae (green). Bites that could not be reliably identified are in pale gray. Letters indicate homologous subsets from analysis of bites of total macroalgae using Permutations ANOVA.
Of this macroalgae, Kyphosus spp. consumed the most Dictyota (78% of total bites from the benthos, p < 0.001, Permutations ANOVA; Figure 2), followed by A. coeruleus and S. aurofrenatum (14 and 16% of the total bites of the benthos, respectively, p < 0.01, Permutations ANOVA; Figure 2). A. coeruleus consumed significantly more Lobophora than any other species (21% of total bites of the benthos, p < 0.001, Permutations ANOVA; Figure 2), while Lobophora constituted less that 6% of the bites for the other species. Other macroalgae (predominantly Halimeda but occasionally also Sargassum) comprised 18% of the bites for S. aurofrenatum, significantly more than all other species (p < 0.001, Permutations ANOVA). Other macroalgae were less than 5% of the bites of other species (Figure 2).
Epilithic algal matrix formed a noticeable proportion of the bites of all species except for Kyphosus spp. (1% of total bites). Of the remaining species, bites of EAM ranged from 19% of total bites for A. tractus up to 94% for S. vetula (which consumed significantly more EAM than any other species, p < 0.001, Permutations ANOVA; Figure 2). A. tractus and S. iserti were the only two species whose feeding was primarily of epibionts: A. tractus consumed significantly more than all other species (79%, p < 0.01, Permutations ANOVA; Figure 2), followed by S. iserti (59%, p < 0.01, Permutations ANOVA; Figure 2).
In all these observations only one individual (S. viride initial phase) out of the 105 we followed was observed taking bites of a sponge (one event of four bites which was 3% of the total bites observed), so this food item is omitted from further analyses. All parrotfishes were observed occasionally consuming coral, however, at most this food item constituted <1% of the total bites (S. viride initial phase) so is also excluded from further analysis.
Feeding Assays
In total, we ran 34 h-long trials using Dictyota (n = 15) and Lobophora (n = 19) across two sites and two feeding periods (morning and midday) from which only three fish taxa were observed feeding from the ropes with any regularity: A. coeruleus, Kyphosus spp., and S. aurofrenatum (Figure 3). This pattern was despite seven to 10 herbivore species being present during the trials, including A. tractus, S. iserti, S. taeniopterus, S. vetula, and S. viride at both sites. Moreover, our trials with S. fluitans and L. gemmifera were also consumed by Melichthys niger (black durgeon), Scarus iserti (striped parrotfish), Sparisoma rubripinne (yellowtail parrotfish), and Sparisoma viride (stoplight parrotfish, in addition to A. coeruleus, Kyphosus spp., and S. aurofrenatum), indicating that the experimental set-up did not deter these other species from feeding.
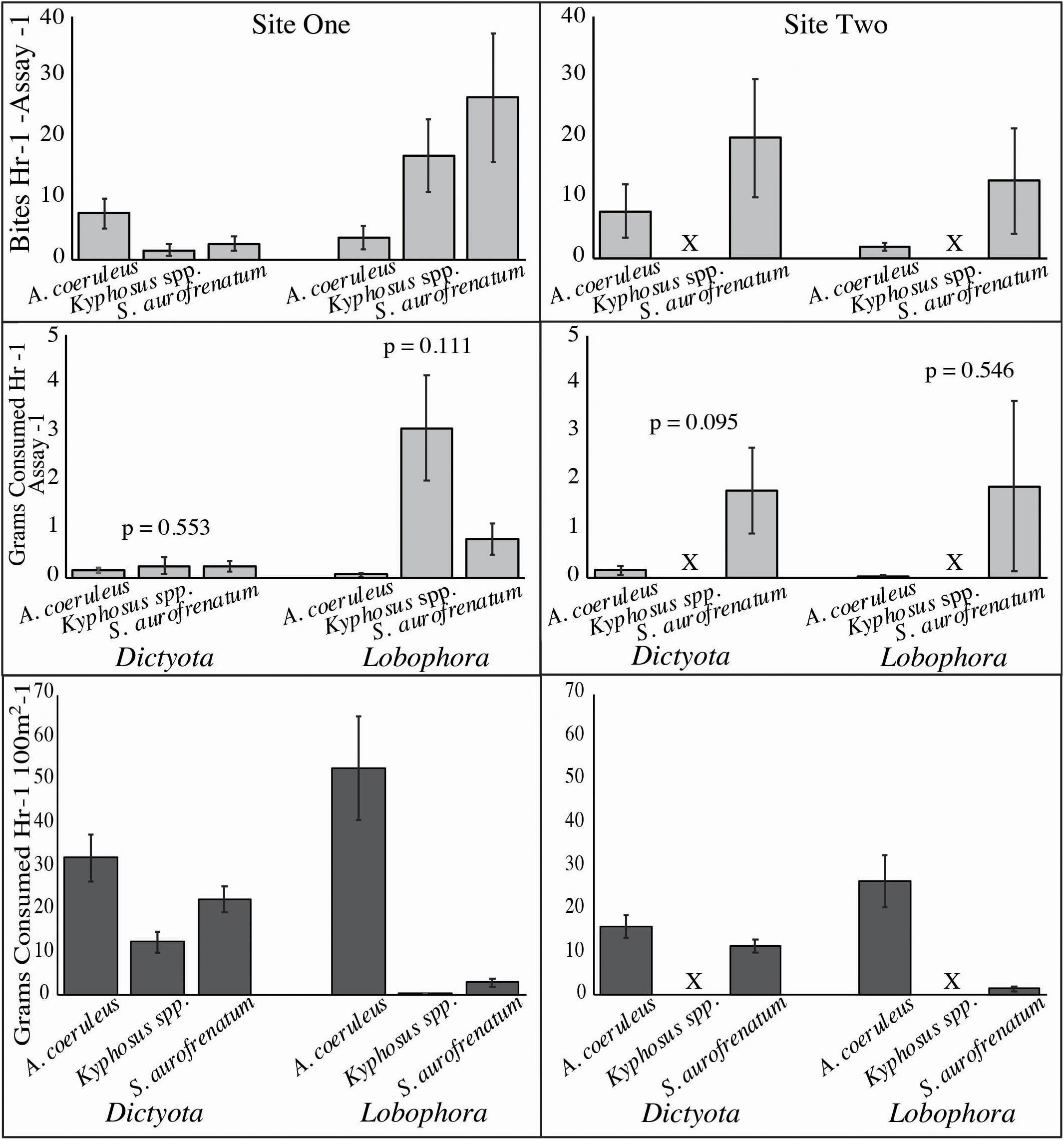
Figure 3. Consumption of Dictyota and Lobophora from video feeding assays ± SE. Mean number of bites taken (upper two graphs) and mean grams consumed (middle two graphs) are shown of the community of Acanthurus coeruleus (blue tang), Kyphosus spp. (chub), and Sparisoma aurofrenatum (redband parrotfish) per assay from site one (left panel) and site two (right panel). Number of bites was counted from 34 h of feeding assay videos and grams consumed was calculated using data in Figure 4. P values are from analysis by Kruskal–Wallis (site one) and Mann–Whitney U (site two). The lower two graphs show consumption (g) per hour per 100 m2 calculated by multiplying the bite rate per hour for each alga (Figure 2) by the grams removed per bite (Figure 4) by the density estimates (Figure 5). X indicates chub were not seen at site two.
To estimate the quantity of algae consumed by each species we calculated the grams consumed per bite for the three main consumers (from our second set of feeding assays; Figure 4). For A. coeruleus, we determined a mean of 0.02 g per bite from Laurencia (n = 9). Kyphosus spp. consumed a mean of 0.16 and 0.18 g per bite of Sargassum and Lobophora, respectively (n = 10), and S. aurofrenatum consumed a mean of 0.09 and 0.03 g per bite of Sargassum (n = 13) and Lobophora (n = 11), respectively.
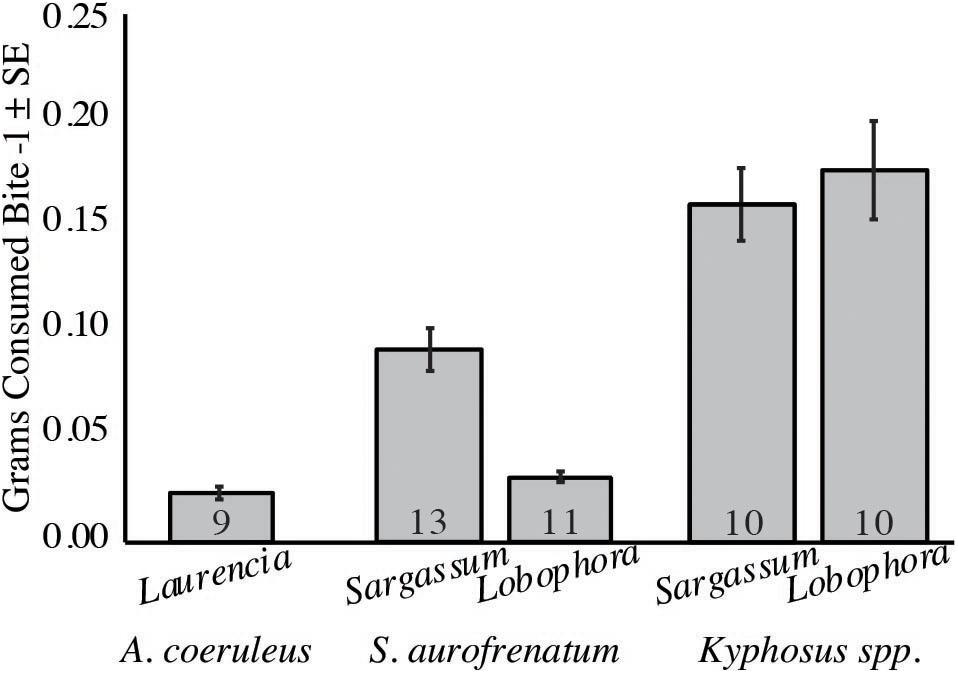
Figure 4. Grams consumed per bite for the three main herbivorous fishes. The number of trials is at the base of each column (n = 9 to 13). Assays were run using Sargassum fluitans and Lobophora variegata for Kyphosus spp. and S. aurofrenatum. S. fluitans was used in place of Dictyota spp. because grazing on Dictyota spp. was too low to obtain reliable results. Laurencia gemmifera was used to measure A. coeruleus consumption because when we used S. fluitans and L. variegata, the other fish consumed the alga before A. coeruleus could.
After multiplying the mean weight (g) of algae removed per bite (Figure 4) by the number of bites counted in each video (Figure 3, upper two panels), we found no significant differences in grams consumed by the three main herbivore species at either site (Site One Dictyota: n = 10, p = 0.553, Kruskal–Wallis; Site One Lobophora: n = 10, p = 0.111, Kruskal–Wallis; Site Two Dictyota: n = 5, p = 0.095, Mann–Whitney U; Site Two Lobophora: n = 9, p = 0.546, Mann–Whitney U; Figure 3, middle two panels).
Herbivorous Fish Density at Feeding Assay Sites
Density of herbivorous fishes at site one was 36.2/100 m2 and at site two was 24.6/100 m2.
Acanthurus coeruleus, Acanthurus tractus, Scarus iserti, Scarus taeniopterus, Scarus vetula, Sparisoma aurofrenatum, and Sparisoma viride were recorded at both sites and S. iserti and A. coeruleus had the highest densities at both sites (S. iserti was 9.6/100 m2 and 8.3/100 m2 at sites one and two, respectively; A. coeruleus was 9.2/100 m2 and 4.6/100 m2 at sites one and two, respectively; Figure 5). On the contrary, Kyphosus spp. was only seen at site one (1.4/100 m2), while Scarus guacamaia (rainbow parrotfish) and Sparisoma rubripinne (yellowtail parrotfish) were only recorded at site two (0.4/100 m2 and 1.25/100 m2, respectively; Figure 5). Density of S. aurofrenatum was 4.9/100 m2 and 2.5/100 m2 at sites one and two, respectively. Densities of these families are in keeping with regional values: we recorded 21.2/100 m2, 7.9/100 m2, and 0.6/100 m2 for parrotfish, surgeonfish and chub respectively, while densities from AGRRA (2018) were 24.3/100 m2, 11.9/100 m2, and 0.6/100 m2 for parrotfish, surgeonfish, and chub, respectively. No Acanthurus chirurgus (doctorfish) were seen at either site and no anomalous large schools of herbivorous fish visited the feedings ropes during any trial.
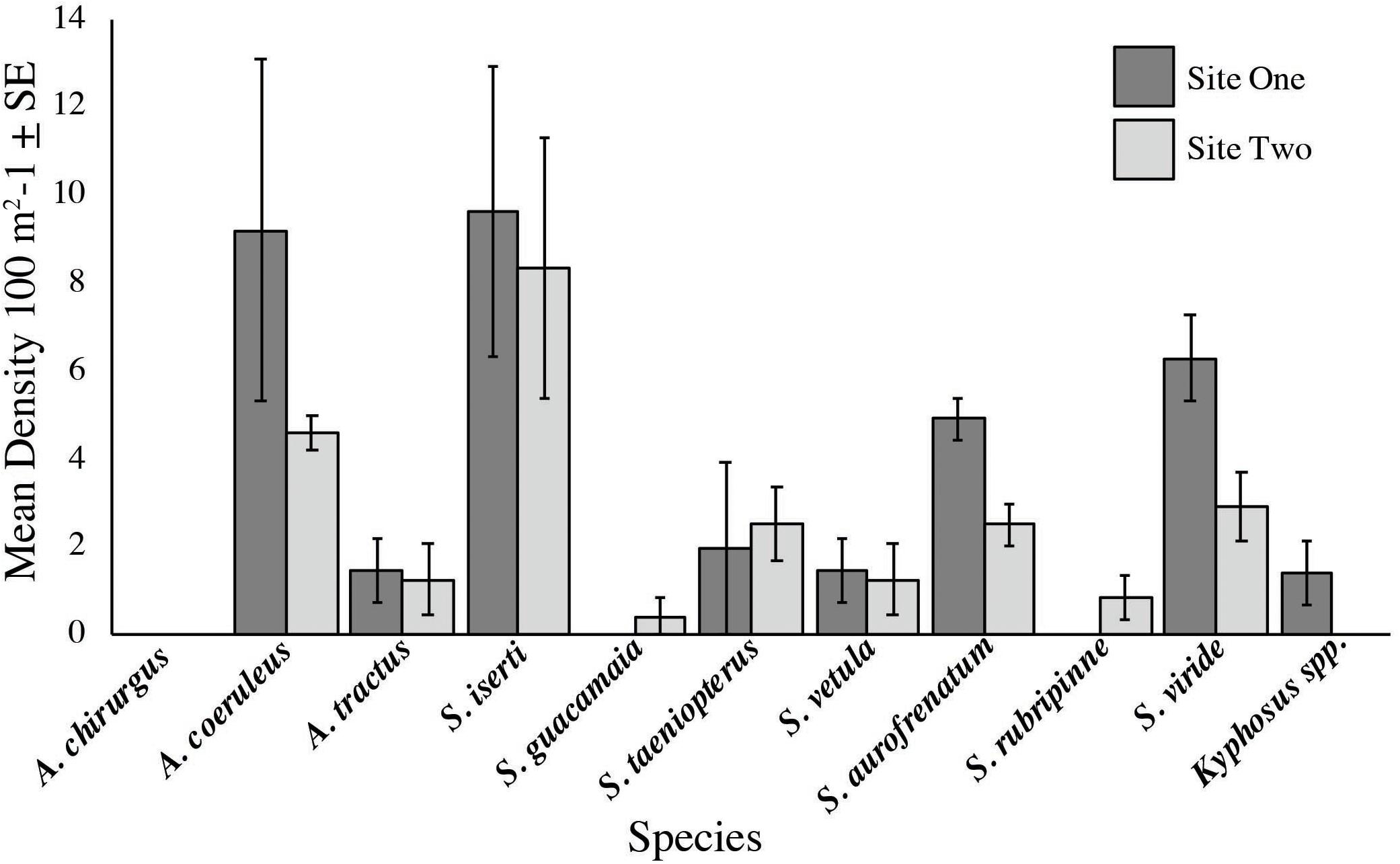
Figure 5. Herbivorous fish densities at the two feeding assay sites. Mean herbivore densities were 3.6/100 and 2.3/100 m2 at sites one and two, respectively. Densities of these families agree with those reported elsewhere in the region (AGRRA, 2018).
Consumption of Dictyota and Lobophora by the Herbivore Community per 100 m2
Consumption of Dictyota and Lobophora from our observations was scaled to consumption per hour per 100 m2 by multiplying the bite rate per hour for each alga (Figure 2) by the grams removed per bite (Figure 4) by the density estimates from site one and site two (Figure 5). At site one this gave 32.1, 12.4, and 22.5 g/h/100m2 of Dictyota consumed by A. coeruleus, Kyphosus spp. and S. aurofrenatum, respectively, and 52.8, 0.3g/h/100m2, and 3.0 g/h/100 m2 of Lobophora consumed by A. coeruleus, Kyphosus spp. and S. aurofrenatum, respectively (Figure 3, lower two graphs). At site two this gave 16.0 and 11.5 g/h/100 m2 of Dictyota consumed by A. coeruleus and S. aurofrenatum, respectively, and 26.4 and 1.5 g/h/100 m2 of Lobophora consumed by A. coeruleus and S. aurofrenatum, respectively (Figure 3, lower two graphs). No Kyphosus spp. were seen at site two.
Discussion
Modern Caribbean reefs are covered by ∼40% macroalgae (AGRRA, 2018), often Dictyota and Lobophora (Diaz-Pulido et al., 2011; Suchley and Alvarez-Filip, 2017), yet in our study only three fish taxa consume these macroalgae as a substantial proportion of their feeding: Acanthurus coeruleus (blue tang surgeonfish), Kyphosus spp. (chub), and Sparisoma aurofrenatum (redband parrotfish; Figures 2, 3). As macroalgal cover has increased on Caribbean reefs it is important to determine which are the browsers that can control it.
We found our observations of fish feeding agreed with our results from the feeding assays. The three taxa we observed feeding most frequently on macroalgae during our observations (A. coeruleus, Kyphosus spp., and S. aurofrenatum) were also the most frequent visitors to our ropes (Figures 2, 3). Similarly, those species which fed predominantly on EAM or epibionts (the other six species we followed) were never seen eating Dictyota or Lobophora from the ropes. However, there is a discrepancy in observations of Kyphosus spp. between our two experiments. Kyphosus that were present at site one were frequent visitors to the Lobophora assay and took 17 bites per hour of this alga. Conversely, of the individuals we followed on the reef only 1% of their bites were of Lobophora (less than one bite per hour). This discrepancy may be because Lobophora growing on the reef was often found in more cryptic habitats than when it was presented in the feeding ropes. The relatively large Kyphosus (adult length ∼30 to 75 cm; Humann and DeLoach, 2002) may not have been able to access naturally occurring Lobophora as easily as the smaller and more agile A. coeruleus and S. aurofrenatum (∼13to 25 cm and ∼15 to 30 cm, respectively; Humann and DeLoach, 2002) and so, the feeding assay could have made Lobophora more accessible to the chub than under natural conditions. It follows then that removal of Lobophora from the reef by Kyphosus spp. is likely to be closer to the values from our observations than from the feeding assays. However, during outbreaks or after dislodgement of Lobophora by storms, Kyphosus spp. may be one of the key consumers. The assay probably would not have affected access to the more conspicuous, upright Dictyota. Consumption of Dictyota and Lobophora from our feeding assays was lower than that calculated from our observations. This discrepancy may be due to difference in experimental design and the less “natural” presentation of algae in the feeding assays, or may suggest that epibionts encourage feeding. However, this needs to be investigated further.
Regardless, both experiments highlight the taxa that are “browsers” and consume established macroalgae as a substantial component of their diet at our sites. While this finding is limited to these sites in the boreal summer, it is important to note that the browsing function is performed by relatively few taxa. Since the browsers are the subset of herbivores that are more likely to reverse macroalgal dominance, their importance on the reef is growing as macroalgal cover rises on reefs (Cheal et al., 2010). It should be determined whether this critical browsing function is performed by other species elsewhere in the region so that herbivory can be more thoroughly understood and effective management strategies can be implemented.
Our findings in this study agree with Dromard et al. (2015) who used stable isotope and stomach content analyses to show that A. coeruleus consumed a mixture of macroalgae, turf, and invertebrates. Similarly, they also found the proportions of food items in the S. aurofrenatum diet were more similar to the Acanthurids than other parrotfishes, which is very much in keeping with our results. Interestingly, they found a higher contribution of macroalgae than we did in the diets of all herbivore species. However, they suggest this could be from consumption of macroalgal propagules growing within the EAM which we could not have detected from our observations. Also similar to our findings, Adam et al. (2018) and Ruttenberg et al. (2019) reported that all parrotfishes in their studies fed on EAM, but only S. aurofrenatum, S. chrysopterum, and S. rubripinne consumed macroalgae regularly. S. chrysopterum was absent from our study reefs and S. rubripinne was only seen at site two where we ran the feeding assays. However, this species did not feed from our Dictyota or Lobophora assays, perhaps because it was an infrequent visitor to the site and was not present in all our trials. Thus, the evidence from multiple sources indicates that S. aurofrenatum and A. coeruleus are key browsers in the Caribbean. It is worth noting that estimates of Dictyota consumption from our observations suggest an individual A. coeruleus could consume almost as much as one S. aurofrenatum per day, assuming they both feed from 0600 to 1600 h (44 g versus 50 g for A. coeruleus and S. aurofrenatum, respectively) although if this were scaled by density, A. coeruleus as a species could consume far more macroalgae at our sites. Thus, it appears that both S. aurofrenatum and A. coeruleus should be considered key browsers (Duran et al., 2019).
Contrary to our results, however, Cardoso et al. (2009) reported a high proportion of bites taken of macroalgae for the seven Caribbean species of parrotfish they observed. However, they made no distinction between bites taken of epibionts off the surface of macroalgae and actual removal of macroalgal tissue. This is a critical distinction to make because species such as A. tractus and S. iserti were observed feeding from the surface of macroalgae, but never once fed off Dictyota or Lobophora after they had been cleaned of epibionts in our assays. It is possible that the diet of these species varies across the region since Burkepile and Hay (2008) also found that A. tractus significantly reduced macroalgae in their experiments, including both Dictyota and Lobophora. Further, feeding assay results may also vary with factors that affect herbivore consumption (see below). It is also possible that a higher epibiont load on macroalgae in some locations allows some species to meet dietary requirements without consuming macroalgae itself. However, our results support those of Clements et al. (2017) who proposed that many nominal herbivores are not targeting macroalgae but instead consume other food items such as detritus, epilithic cyanobacteria, microalgae, or diatoms.
While parrotfish have received most attention in the literature, and surgeonfish to a lesser extent (Duran et al., 2019), the Kyphosidae are not consistently considered part of the herbivore guild in the Caribbean (for example, see Mumby, 2006; Burkepile et al., 2013; Kramer et al., 2017; Suchley and Alvarez-Filip, 2017 as examples of studies omitting Kyphosidae). Here we found that it was one of the top three consumers of macroalgae at one feeding assay site and that macroalgae formed a significantly higher proportion of its diet than any of the more familiar herbivores (Figures 2, 3). From our observations, an individual Kyphosus spp. can consume ∼100 g of Dictyota per day (assuming they feed from 0600 to 1600 h), which is significantly more than all other herbivores in our study. (From our observations, in this time period A. coeruleus and S. aurofrenatum would consume 44 and 50 g of Dictyota, respectively). It should be noted that these calculations are “back-of-the-envelope” estimates from our observations. Consumption on different reefs will likely vary from these values because consumption of algae by fishes is influenced by many factors including benthic community composition (Cvitanovic and Hoey, 2010), associational defenses (Littler et al., 1986), presence of epiphytes (Fong et al., 2006), season (Paddack et al., 2006) and reef structural complexity (Verges et al., 2011) among others. (The other “nominal herbivores” on these reefs consumed primarily either epibionts or EAM; Figure 2).
It should not be surprising that a species of Kyphosus was a frequent consumer of brown macroalgae. In 1967, Randall (1967) reported that the stomach contents of two species in the Caribbean were 100% brown macroalgae and 99.5% brown and red macroalgae (n = 6 and 19, respectively). Further, the family Kyphosidae have long been recognized as important browsers in the Indo-Pacific (Clements and Choat, 1997; Green and Bellwood, 2009; Knudsen and Clements, 2016). This omission may be because traditional survey protocols miss many Kyphosus because of their distribution and behavior. At least on Cayman reefs we see solitary individuals in the shallows (5 to 8 m depth) and large schools of Kyphosus off the drop-off around 25 to 30 m depth. However, the AGRRA protocol for example stipulates fish transects be conducted between 1–5 m and 8–15 m (version 2.2). Similarly, survey protocols specify transects be conducted over the middle of the day (between 1000 and 1400 h in the AGRRA protocol version 2.2). The genus Kyphosus is known to use specific reef areas at specific times of day (Eristhee and Oxenford, 2001) so could often be missed in surveys that only occur in the middle of the day. Consequently, as “roving herbivores” they may be difficult to quantify during fish surveys with commonly used methods, but could still significantly impact algal communities. Thus, measurements of Kyphosus densities may be artificially low and hence their impact may have been underappreciated. While visual transect surveys may provide a reasonable estimation of species that exhibit site fidelity, we suspect that the number of Kyphosus impacting our sites is underestimated. Additionally, bite rates are not always proportional to abundance (Longo et al., 2014). For these reasons we advise caution when interpreting our estimates of consumption per 100 m2. However, these calculations suggest that A. coeruleus may be the most important browser at this location, followed by S. aurofrenatum and Kyphosus spp. We anticipate this result will vary across the Caribbean, given that presence and density of Kyphosus and other herbivores are highly variable across the region (Floeter et al., 2005; Ruttenberg et al., 2019).
The Kyphosus individuals we followed appeared to have divergent feeding strategies. Six of the 12 chub we followed took less than 12 bites/hour from the benthos (average 3 bites/hour) and stayed higher in the water column throughout the 20-min observation period. In contrast, the other six individuals took an average of 107 bites/hour from the benthos. This dichotomy in feeding may be an artifact of the relatively short duration of observation, however, it could also be because these were a different species of Kyphosus. There are four species of Kyphosus in the western Atlantic (Kyphosus bigibbus, K. cinerascens, K. sectatrix, and K. vaigiensis; Knudsen and Clements, 2016), some of which can be very difficult to distinguish (Humann and DeLoach, 2002). So, it is possible that more than one species featured in our observations. Further research will be necessary to determine the identity of Kyphosus on these reefs and if their feeding behavior differs.
Traditionally, herbivores have been assigned to one of three functional groups: the “browsers,” “scrapers,” and “excavators” (Bellwood and Choat, 1990; Bellwood et al., 2004; Adam et al., 2018). Historically, parrotfish may have been key herbivores in the Caribbean because they remove turf and macroalgal propagules off the reef surface by scraping or excavating and this is critical in preventing a shift to macroalgal dominance (Mumby, 2006; Suchley and Alvarez-Filip, 2017). However, in many places macroalgae are now established as the dominant benthic community: indeed in 2018, macroalgae were 40% of Caribbean forereefs, whereas turf was only 15% (AGRRA, 2018). Since many parrotfish do not consume established macroalgae, parrotfish alone are unlikely to reverse a phase shift once it is proceeding (Cheal et al., 2010; Rasher et al., 2013). Thus, while herbivory is still a key process, we contend that the “browsers” rather than the “scrapers” are of increasing importance on modern reefs. From our observations and experiments, we found that the taxa responsible for consuming established macroalgae are Acanthurus coeruleus (blue tang), Sparisoma aurofrenatum (redband parrotfish), and Kyphosus spp. (chub). But, since species composition varies with habitat, it is likely that other species are performing this role on other reefs (Floeter et al., 2005; Ruttenberg et al., 2019). Hence, it will be imperative to determine which are the key browsers elsewhere in the region so that they can be monitored and protected. Governments around the region have implemented bans on fishing parrotfish and some countries, such as Belize, have also banned fishing of surgeonfish (Kramer et al., 2017). However, we encourage region-wide protection of all browsers. At a time when reefs around the world are undergoing rapid change and are at severe risk (Heron et al., 2017), it is vital to revaluate the role species perform in the system and protect those that are key to promoting ecosystem health.
Data Availability Statement
The datasets generated in this study can be found in Zenodo: http://doi.org/10.5281/zenodo.3762238.
Ethics Statement
The animal study was reviewed and approved by the Cayman Islands Department of Environment.
Author Contributions
CD and CM conceived of the study and designed the experiments. CD conducted all field work. CD, GL, and DB formed the concept of the manuscript. CD and GL performed the statistical analyses. CD took lead writing the manuscript with input from all authors and under the supervision of CM and DB. All authors contributed to the article and approved the submitted version.
Funding
All work was supported by the Darwin Initiative (DPLUS061), United Kingdom, CCMI, and an anonymous supporter. GOL is grateful to a research productivity scholarship provided by the Brazilian National Council for Scientific and Technological Development (CNPq; 310517/2019-2).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks go to Lindsay Spiers and CCMI staff for assistance with data collection, Simon Whicker for in-field logistical support, the Cayman Islands Department of Environment for field support and research permission. We also thank the Atlantic Gulf Rapid Reef Assessment (AGRRA) contributors and data managers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00676/full#supplementary-material
References
Adam, T. C., Duran, A., Fuchs, C. E., Roycroft, M. V., Rojas, M. C., Ruttenberg, B. I., et al. (2018). Comparative analysis of foraging behavior and bite mechanics reveals complex functional diversity among Caribbean parrotfishes. Mar. Ecol. Progr. Ser. 597, 207–220. doi: 10.3354/meps12600
Adam, T. C., Kelley, M., Ruttenberg, B. I., and Burkepile, D. E. (2015). Resource partitioning along multiple niche axes drives functional diversity in parrotfishes on Caribbean coral reefs. Oecologia 179, 1173–1185. doi: 10.1007/s00442-015-3406-3
AGRRA (2018). Atlantic and Gulf Rapid Reef Assessment (AGRRA): An Online Database of AGRRA coral reef survey data. Available: http://agrra.org (accessed March 10, 2018).
Bellwood, D. R., and Choat, J. H. (1990). A functional analysis of grazing in parrotfishes (family Scaridae): The ecological implications. Environ. Biol. Fish. 28, 189–214. doi: 10.1007/978-94-009-2065-1_11
Bellwood, D. R., Hughes, T. P., Folke, C., and Nyström, M. (2004). Confronting the coral reef crisis. Nature 429, 827–833. doi: 10.1038/nature02691
Bellwood, D. R., Hughes, T. P., and Hoey, A. S. (2006). Sleeping functional group drives coral-reef recovery. Curr. Biol. 16, 2434–2439. doi: 10.1016/j.cub.2006.10.030
Birrell, C. L., McCook, L. J., Willis, B. L., and Diaz-Pulido, G. A. (2008). “Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs,” in Oceanography and Marine Biology, eds S. J. Hawkins, A. L. Allcock, A. E. Bates, L. B. Firth, I. P. Smith, S. E. Swearer, and P. A. Todd (Boca Raton, FL: CRC Press), 31–70.
Box, S. J., and Mumby, P. J. (2007). Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar. Ecol. Progr. Ser. 342, 139–149. doi: 10.3354/meps342139
Burkepile, D. E., Allgeier, J. E., Shantz, A. A., Pritchard, C. E., Lemoine, N. P., Bhatti, L. H., et al. (2013). Nutrient supply from fishes facilitates macroalgae and suppresses corals in a Caribbean coral reef ecosystem. Sci. Rep. 3:1493.
Burkepile, D. E., and Hay, M. E. (2008). Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. 105, 16201–16206. doi: 10.1073/pnas.0801946105
Cardoso, S. C., Soares, M. C., Oxenford, H. A., and Côté, I. M. (2009). Interspecific differences in foraging behaviour and functional role of Caribbean parrotfish. Mar. Biodivers. Rec. 2:e148.
Cheal, A. J., MacNeil, M. A., Cripps, E., Emslie, M. J., Jonker, M., Schaffelke, B., et al. (2010). Coral–macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29, 1005–1015. doi: 10.1007/s00338-010-0661-y
Clements, K. D., and Choat, J. H. (1997). Comparison of herbivory in the closely-related marine fish genera Girella and Kyphosus. Mar. Biol. 127, 579–586. doi: 10.1007/s002270050048
Clements, K. D., German, D. P., Piché, J., Tribollet, A., and Choat, J. H. (2017). Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linnean Soc. 120, 729–751.
Cvitanovic, C., and Hoey, A. S. (2010). Benthic community composition influences within-habitat variation in macroalgal browsing on the Great Barrier Reef. Mar. Freshw. Res. 61, 999–1005.
Diaz-Pulido, G., Gouezo, M., Tilbrook, B., Dove, S., and Anthony, K. R. (2011). High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 14, 156–162. doi: 10.1111/j.1461-0248.2010.01565.x
Dromard, C. R., Bouchon-Navaro, Y., Harmelin-Vivien, M., and Bouchon, C. (2015). Diversity of trophic niches among herbivorous fishes on a Caribbean reef (Guadeloupe, Lesser Antilles), evidenced by stable isotope and gut content analyses. J. Sea Res. 95, 124–131. doi: 10.1016/j.seares.2014.07.014
Duran, A., Adam, T. C., Palma, L., Moreno, S., Collado-Vides, L., and Burkepile, D. E. (2019). Feeding behavior in Caribbean surgeonfishes varies across fish size, algal abundance, and habitat characteristics. Mar. Ecol. 40:e12561.
Eristhee, N., and Oxenford, H. A. (2001). Home range size and use of space by Bermuda chub Kyphosus sectatrix (L.) in two marine reserves in the Soufriere Marine Management Area. St Lucia, West Indies. J. Fish Biol. 59, 129–151. doi: 10.1111/j.1095-8649.2001.tb01383.x
Floeter, S. R., Behrens, M. D., Ferreira, C. E. L., Paddack, M. J., and Horn, M. H. (2005). Geographical gradients of marine herbivorous fishes: patterns and processes. Mar. Biol. 147, 1435–1447. doi: 10.1007/s00227-005-0027-0
Fong, P., Smith, T. B., and Wartian, M. J. (2006). Epiphytic cyanobacteria maintain shifts to macroalgal dominance on coral reefs following ENSO disturbance. Ecology 87, 1162–1168. doi: 10.1890/0012-9658(2006)87[1162:ecmstm]2.0.co;2
Gardner, T. A., Côté, I. M., Gill, J. A., Grant, A., and Watkinson, A. R. (2003). Long-term region-wide declines in Caribbean corals. Science 301, 958–960. doi: 10.1126/science.1086050
Green, A. L., and Bellwood, D. R. (2009). “Monitoring functional groups of herbivorous reef fishes as indicators of coral reef resilience – A practical guide for coral reef managers in the Asia Pacific region,” in IUCN working group on Climate Change and Coral Reefs (Gland: IUCN), 70.
Hernández-Landa, R. C., Acosta-González, G., Núñez-Lara, E., and Arias-González, J. E. (2015). Spatial distribution of surgeonfish and parrotfish in the north sector of the Mesoamerican Barrier Reef System. Mar. Ecol. 36, 432–446. doi: 10.1111/maec.12152
Heron, S. F., Eakin, C. M., Douvere, F., Anderson, K. L., Day, J. C., Geiger, E., et al. (2017). Impacts of climate change on World Heritage Coral Reefs: A First Global Scientific Assessment. Paris: UNESCO.
Hoey, A. S., and Bellwood, D. R. (2009). Limited functional redundancy in a high diversity system: single species dominates key ecological process on coral reefs. Ecosystems 12, 1316–1328. doi: 10.1007/s10021-009-9291-z
Humann, P., and DeLoach, N. (2002). Reef fish identification, Florida Caribbean Bahamas, 3rd Edn. Florida: New World Publications Inc.
Jackson, J. B. C., Donovan, M. K., Cramer, K. L., and Lam, V. V. (eds) (2014). Status and Trends of Caribbean Coral Reefs: 1970-2012. Gland: Global Coral Reef Monitoring Network, IUCN.
Khait, R., Obolski, U., Hadany, L., and Genin, A. (2013). Food selectivity and diet switch can explain the slow feeding of herbivorous coral-reef fishes during the morning. PLoS One 8:e82391. doi: 10.1371/journal.pone.0082391
Knudsen, S. W., and Clements, K. D. (2016). World-wide species distributions in the family Kyphosidae (Teleostei: Perciformes). Mol. Phylogenet. Evol. 101, 252–266. doi: 10.1016/j.ympev.2016.04.037
Kramer, P., McField, M., Drysdale, I., Rueda, M., GirÃ, A., and Pott, R. (2017). 2015 Report card for the Mesoamerican reef. Washington, DC: The Summit Foundation.
Kuffner, I. B., Walters, L. J., Becerro, M. A., Paul, V. J., Ritson-Williams, R., and Beach, K. S. (2006). Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar. Ecol. Progr. Series 323, 107–117. doi: 10.3354/meps323107
Lessios, H. A. (1988). Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Ann. Rev. Ecol. Syst. 19, 371–393. doi: 10.1146/annurev.es.19.110188.002103
Lessios, H. A. (2005). Diadema antillarum populations in Panama twenty years following mass mortality. Coral Reefs 24, 125–127. doi: 10.1007/s00338-004-0443-5
Littler, D. S., and Littler, M. M. (2000). Caribbean Reef Plants. Washington, DC: OffShore Graphics.
Littler, M. M., Taylor, P. R., and Littler, D. S. (1986). Plant defense associations in the marine environment. Coral Reefs 5, 63–71. doi: 10.1007/bf00270354
Longo, G. O., Ferreira, C. E. L., and Floeter, S. R. (2014). Herbivory drives large-scale spatial variation in reef fish trophic interactions. Ecol. Evol. 4, 4553–4566. doi: 10.1002/ece3.1310
Longo, G. O., Hay, M. E., Ferreira, C. E., and Floeter, S. R. (2019). Trophic interactions across 61 degrees of latitude in the Western Atlantic. Glob. Ecol. Biogeogr. 28, 107–117. doi: 10.1111/geb.12806
Marshell, A., and Mumby, P. J. (2015). The role of surgeonfish (Acanthuridae) in maintaining algal turf biomass on coral reefs. J. Exp. Mar. Biol. Ecol. 473, 152–160. doi: 10.1016/j.jembe.2015.09.002
McCook, L., Jompa, J., and Diaz-Pulido, G. (2001). Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19, 400–417. doi: 10.1007/s003380000129
Mumby, P. J. (2006). The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecol. Appl. 16, 747–769. doi: 10.1890/1051-0761(2006)016[0747:tioegs]2.0.co;2
Paddack, M. J., Cowen, R. K., and Sponaugle, S. (2006). Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs 25, 461–472. doi: 10.1007/s00338-006-0112-y
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Randall, J. E. (1967). “Food habits of reef fishes of the west indies,” in Proceedings of the International Conference on Tropical Oceanography, Miami Beach.
Rasher, D. B., Hoey, A. S., and Hay, M. E. (2013). Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 94, 1347–1358. doi: 10.1890/12-0389.1
Ruttenberg, B. I., Adam, T. C., Duran, A., and Burkepile, D. E. (2019). Identity of coral reef herbivores drives variation in ecological processes over multiple spatial scales. Ecol. Appl. 29:e01893. doi: 10.1002/eap.1893
Steneck, R. S., Bellwood, D. R., and Hay, M. E. (2017). Herbivory in the marine realm. Curr. Biol. 27, R484–R489.
Steneck, R. S., and Dethier, M. N. (1994). A functional group approach to the structure of algal-dominated communities. Oikos 69, 476–498.
Suchley, A., and Alvarez-Filip, L. (2017). Herbivory facilitates growth of a key reef-building Caribbean coral. Ecol. Evol. 7, 11246–11256. doi: 10.1002/ece3.3620
Suchley, A., McField, M. D., and Alvarez-Filip, L. (2016). Rapidly increasing macroalgal cover not related to herbivorous fishes on Mesoamerican reefs. PeerJ 4:e2084. doi: 10.7717/peerj.2084
Tanner, J. E. (1995). Competition between scleractinian corals and macroalgae: an experimental investigation of coral growth, survival and reproduction. J. Exp. Mar. Biol. Ecol. 190, 151–168. doi: 10.1016/0022-0981(95)00027-o
Venera-Ponton, D. E., Diaz-Pulido, G., McCook, L. J., and Rangel-Campo, A. (2011). Macroalgae reduce growth of juvenile corals but protect them from parrotfish damage. Mar. Ecol. Progr. Ser. 421, 109–115. doi: 10.3354/meps08869
Verges, A., Vanderklift, M. A., Doropoulos, C., and Hyndes, G. A. (2011). Spatial patterns in herbivory on a coral reef are influenced by structural complexity but not by algal traits. PLoS One 6:e17115. doi: 10.1371/journal.pone.0017115
Wheeler, R. E. (2010). Permutation tests for linear models in R. Available online at: http://cran.r-project.org/web/packages/lmPerm/vignettes/lmPerm (accessed October 12, 2019). doi: 10.1371/journal.pone.0017115
Keywords: herbivory, macroalgae, Kyphosus, Sparisoma, Acanthurus, browser, Dictyota, Lobophora
Citation: Dell CLA, Longo GO, Burkepile DE and Manfrino C (2020) Few Herbivore Species Consume Dominant Macroalgae on a Caribbean Coral Reef. Front. Mar. Sci. 7:676. doi: 10.3389/fmars.2020.00676
Received: 23 October 2019; Accepted: 24 July 2020;
Published: 12 August 2020.
Edited by:
Jesús Ernesto Arias González, Centro de Investigación y Estudios Avanzados, Instituto Politécnico Nacional de México (CINVESTAV), MexicoReviewed by:
Douglas Fenner, Independent Researcher, Pago Pago, American SamoaMichelle J. Paddack, Santa Barbara City College, United States
Yves-Marie Bozec, The University of Queensland, Australia
Copyright © 2020 Dell, Longo, Burkepile and Manfrino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claire L. A. Dell, ZGVsbEB1Y3NiLmVkdQ==
 Claire L. A. Dell
Claire L. A. Dell Guilherme O. Longo
Guilherme O. Longo Deron E. Burkepile
Deron E. Burkepile Carrie Manfrino
Carrie Manfrino