- 1Division of Sea Turtle Science and Recovery, Padre Island National Seashore, National Park Service, Corpus Christi, TX, United States
- 2Sea Turtle, Inc., South Padre Island, TX, United States
Knowledge of the spatial and temporal distribution of green sea turtle (Chelonia mydas) nesting is crucial for management of this species. Limited data exist on the nesting patterns of green turtles along the northwestern Gulf of Mexico (GoM) coast. From 1987 to 2019, 211 green turtle nesting activities were documented on the Texas coast, including 111 confirmed nests and 100 non-nesting emergences. Of the 111 nests, 99 were located on North Padre Island (97 at Padre Island National Seashore (PAIS), two north of PAIS) and 12 on South Padre Island (six within the Laguna Atascosa or Lower Rio Grande Valley National Wildlife Refuges (NWR), six outside of a NWR). Of the 100 non-nesting emergences, 75 were on North Padre Island (70 at PAIS, 5 north of PAIS), 21 on South Padre Island (nine within a NWR, 12 outside of a NWR), one on Boca Chica Beach, two on San Jose Island, and one on Mustang Island. Nearly all of the nests (92.8%) and most of the non-nesting emergences (79.0%) were on property protected by the United States Department of the Interior as PAIS or a NWR, and confirmed nest density was largest at PAIS, highlighting the importance of these federally protected lands as nesting habitat for this threatened species. Of the 111 located nests, eight were predated. Mean hatching success of the 103 non-predated nests was 77.4%, and 9,475 hatchlings were released from the predated and non-predated nests. The largest annual number of green turtle nests documented was 29 in 2017. Nesting appeared to increase since 2010, but at a much lower rate than at other GoM nesting beaches. To aid with recovery, efforts should be undertaken to monitor long-term nesting trends, protect nesting turtles and nests, and investigate potential causes for the slower recovery in Texas. Additionally, the genetic structure of the population that nests in Texas should be determined to reveal if the population warrants recognition as a unique management unit, or if it is part of a broader unit that is a shared nesting resource with Mexico which is already being considered as a unique management unit.
Introduction
Green turtles (Chelonia mydas) are distributed world-wide in tropical and warm temperate oceans. Green turtles in the Gulf of Mexico (GoM), Caribbean, and North Atlantic Ocean are part of the North Atlantic Distinct Population Segment and are classified as threatened under the United States Endangered Species Act (Seminoff et al., 2015; NMFS and USFWS, 2016). Systematic harvest decimated the tens of millions of green turtles that once existed in the Caribbean and GoM, but after decades of conservation, green turtle nesting numbers have increased in many areas (Shamblin et al., 2015, 2018; Cuevas et al., 2018).
In Mexico, the annual number of nests documented in Quintana Roo increased from 500 to more than 23,000 from 1996 to 2017 (Tzeek Tuz et al., 2019), and the most recent total nester abundance for the five Mexican states of Tamaulipas, Veracruz, Campeche, Yucatan, and Quintana Roo was estimated to be 24,330 turtles (Seminoff et al., 2015). Despite this progress, green turtles continue to be vulnerable at inter-nesting sites in Veracruz and the Yucatan Peninsula (Cuevas et al., 2019).
Historic nesting levels of green turtles in Florida are not known. The first green turtle nest scientifically confirmed in peninsular Florida was in 1957 near Vero Beach in Indian River County (Carr and Ingle, 1959). Green turtle nesting was sparse in Florida through the early 1980s and then began to surge on both Atlantic and GoM coast beaches (Chaloupka et al., 2008; Witherington et al., 2009; Weishampel et al., 2016). The epicenter of nesting in Florida is on the Atlantic coast at the Archie Carr National Wildlife Refuge, on Melbourne Beach, which recorded 11,000 nests in 2013 (Shamblin et al., 2015).
In Texas, the green turtle was once abundant and commercially exploited during the mid-1800s (Witzell, 1994a, b). Turtle harvesting peaked during April–November (Hildebrand, 1981), aligning with the breeding season for green turtles in North America (Hirth, 1997). This timing, combined with the average weight of the harvested turtles recorded at 113 kg/turtle (Doughty, 1984), indicates that adult green turtles were likely among the individuals slaughtered. By 1963, when sea turtle fisheries were prohibited in Texas, green turtle catch had already precipitously declined and was almost non-existent (Hildebrand, 1981; Doughty, 1984). Presently, most green turtles inhabiting Texas waters are juveniles, and Texas inshore waters (i.e., bays, lagoons, and passes) serve as important developmental and foraging habitat for them (Metz and Landry, 2013; Shaver et al., 2017b). Some hypothesize that green turtles historically nested in Texas in abundance (Neck, 1978), but the first confirmed record of green turtle nesting in Texas was not until 1987 (Shaver, 1989). Neck (1978) relayed observations by Robert A. F. Penrose of 91–122 cm long turtles nesting near the mouth of the Rio Grande river in south Texas in 1889 (Fairbanks and Berkey, 1952) and concluded that these were likely green turtles. Hildebrand (1981) hypothesized that they were Kemp’s ridley turtles (Lepidochelys kempii), but the size described is more indicative of green turtles, which nest at about 90–120 cm straight carapace length (SCL) (NMFS and USFWS, 2007; Seminoff et al., 2015), than of Kemp’s ridley turtles, which nest at about 58.5–72.5 cm SCL (Márquez-Millán, 1994).
Green turtle nesting is low in Texas, with lower increases in nesting, compared to other GoM nesting beaches. Without historical data, it is unknown whether this nesting population is rebuilding from an exploited, once abundant nesting population or represents a spread of nesting from Mexico. The closest nesting beaches to Texas are in Tamaulipas, Mexico and along the Florida GoM coast. Little has been published in peer-reviewed literature regarding green turtle nesting on the Gulf coast of Mexico and tracking data from adult green turtles in the GoM is very limited. Adults tracked from Veracruz and Quintana Roo, Mexico, have migrated to foraging grounds in southwest Florida or remained in Mexico (Bresette et al., 2010; Méndez et al., 2013; Seminoff et al., 2015). We undertook this study to investigate the spatial and temporal trends of green turtle nesting in Texas, which have not been described in the peer-reviewed literature, and to establish a baseline to compare future green turtle nesting patterns in the northwestern GoM. This analysis is vital for evaluating population status and developing future monitoring strategies and management actions to aid with recovery efforts for this threatened species.
Materials and Methods
Patrol Effort and Study Area
Efforts to find, document, and protect nesting sea turtles and their eggs in Texas were initiated on North Padre Island in 1986 and later expanded to include more Texas GoM beaches, days of the sea turtle nesting season, and hours of the day. The temporal and spatial variations of these patrols are described in NMFS et al. (2011) and Shaver et al. (2016b). Since 1986, daytime patrols have been conducted on the entire 128 km GoM shoreline of North Padre Island, including the southernmost 105 km protected by the United States Department of the Interior (DOI), National Park Service (NPS), as Padre Island National Seashore (PAIS). Established in 1962, PAIS preserves the longest stretch of undeveloped barrier island beach in the United States. Daytime patrols on North Padre Island were conducted a few days each week until 1995–1997, when patrol frequency increased to seven days per week (Shaver, 2005). Beginning in 1998, North Padre Island was repeatedly patrolled each day, from approximately 0630 to 1830 h, from April through mid-July, to target when Kemp’s ridleys typically nest. Kemp’s ridley is the most frequent nesting sea turtle in Texas and is the focus of a long-term, bi-national, multi-agency effort to form a secondary nesting colony at PAIS (Shaver and Caillouet, 2015). In 1999, repeated daytime patrols began on Boca Chica Beach, and on South Padre Island repeated daytime patrols began in 2000 (Shaver et al., 2016b). Of the 55 km long Gulf beachfront of South Padre Island, 15.3 km is currently protected by the DOI, United States Fish and Wildlife Service, as Laguna Atascosa and Lower Rio Grande Valley National Wildlife Refuges (NWRs) in a mosaic of fragmented parcels of land added to the refuges since 2000, and concentrated on the northern end of South Padre Island. Patrols began on other Texas beaches starting in 2003 and have been conducted on most Texas beaches since 2005, however, patrols from the upper Texas coast to San Jose Island were conducted only a few days per week. Patrollers also watched for signs of green turtle nesting activity, especially during their first patrols of the morning, since green turtles nest predominantly at night.
From 2002 to 2004, exploratory patrols were conducted on North Padre Island to determine the nesting season for the five sea turtle species that have been recorded nesting in Texas (Hildebrand, 1981; Shaver, 1989; Shaver and Frandsen, 2019; Shaver et al., 2019a) and protect the nests that were found. In addition to the annual April through mid-July patrols, surveys were conducted once a day from 1 February–24 March and 12 July–30 September 2002, 1 February–29 March and 11 July–30 September 2003, and 1 February–3 April and 18 July–30 September 2004. In the years following the exploratory study, from mid-July through as late as early-October, patrols were sometimes conducted during morning hours to document green turtle and loggerhead (Caretta caretta) nesting activity on North and South Padre Islands. Late-season dawn patrols were conducted with more regularity beginning in 2010, with patrols terminating 2 weeks after the last documented nest each season. Nesting green turtles and green turtle tracks were also located and documented by biologists while they conducted other research and conservation activities, by other personnel working on the beach that were trained to identify signs of nesting, and by the public.
Documentation and Protection of Adult Females and Nests
Locations of all green turtle nesting activity found on the Texas coast from 1987 to 2019 were recorded using a hand-held GPS. Adult females encountered on the beach were measured for SCL using calipers or curved carapace length (CCL) using a flexible tape measure. An attempt was made to find eggs at all nesting activity locations. Due to significant threats from anthropogenic activities, predation, and high tides, eggs from all green turtle nests located were retrieved for protected incubation. Eggs from nests found on North Padre Island were placed into polystyrene foam boxes lined with sand from the nest site and relocated to the PAIS incubation facility (Shaver and Caillouet, 2015). Starting in 2008, eggs from nests found on South Padre Island were incubated in an outdoor screened enclosure called a corral, unless found after 15 July, in which case they were placed into polystyrene foam boxes containing sand from the nest site and transported to the PAIS incubation facility to protect them from the increased threat of hurricane activity from July through October. Eggs were monitored through the incubation period. After hatching, the number of hatchlings produced, number of unhatched eggs, and hatching success were recorded for each clutch. From 1987 to 2006, prior to release, hatchlings from all green turtle nests found in Texas were weighed to the nearest hundredth of a gram using an electronic balance and measured to the nearest hundredth of a millimeter using calipers. Hatchlings from all years were released at the surf line on the islands where they were hatched. All activities were carried out according to protocols approved by the NPS Institutional Animal Care and Use Committee.
Analysis
Locations where eggs were found were categorized as nests and locations where no eggs were found were categorized as non-nesting emergences. The numbers of nests and non-nesting emergences were determined for each year. The numbers of nests and non-nesting emergences found on North and South Padre Islands were categorized as within and outside of PAIS and NWRs, respectively. Density and optimized hot spot analyses were performed in ArcGIS 10.4 to determine preferred nesting areas. Nesting success was defined as:
A chi-squared test was conducted to examine whether the proportion of emergences that were nests differed inside and outside of the DOI protected lands. The numbers of confirmed nests per year were calculated for the entire study period (1987–2019) and linear regression was used to examine the nesting trend between 2010 and 2019, when patrol efforts were more consistent in time and area. Nesting female abundance was determined using the formula developed by Seminoff et al. (2015):
Nesting female abundance is the total number of reproductive females using Texas beaches over time and is not an annual nester count (Seminoff et al., 2015).
Mean SCL was quantified for the females; if only CCL was obtained for a turtle, CCL was converted to SCL using the regression equation published by Teas (1993). Mean weights and lengths were calculated for hatchlings weighed and measured from 1987 to 2006. All statistical analyses were conducted in R version 3.4.4 (R Core Team, 2018).
Results
Two hundred and eleven green turtle nesting activities were documented on the Texas coast from 1987 through 2019, including 111 confirmed nests and 100 non-nesting emergences (Figures 1, 2). Nearly all nests (92.8%) and most non-nesting emergences (79.0%) were on federally protected lands. Of the 111 nests, 99 were found on North Padre Island (97 at PAIS, 2 north of PAIS) and 12 on South Padre Island (6 within the Laguna Atascosa or Lower Rio Grande Valley NWR, 6 outside of a NWR). The 100 non-nesting emergences included 75 on North Padre Island (70 at PAIS, 5 north of PAIS), 21 on South Padre Island (9 within a NWR, 12 outside of a NWR), 1 on Boca Chica Beach, 2 on San Jose Island, and 1 on Mustang Island (Figure 1). Confirmed nest density was greatest at PAIS, particularly between the PAIS 31.1 km (19.3 mile) and 74.8 km (46.5 mile) markers (Figure 1). Optimized hot spot results confirmed density findings with ≥95% confidence for nests to be found within this area. Nesting success for all years and areas combined was 52.6% and was higher at the federally protected lands (56.6%) than outside of them (27.6%) [χ2(1, 211) = 8.44, p = 0.00367]. Of the nests with reported beach positions (n = 102), 81.4% were situated between the embryonic dunes and the top of the first foredune. Nesting appeared to increase from 1987 to 2019, but predictive modeling of this data was deemed inappropriate due to high variability, both in time and space, of patrol effort. Linear modeling of nests laid between 2010 and 2019, when patrol effort was increased and more consistent between years, indicated a 0.7× annual increase of nests, but was not statistically significant with low explanatory power [y = −1359.02 + 0.68x, F(1, 8) = 8.79, R2 = 0.06, p = 0.503]. The largest numbers of nests (n = 29) and non-nesting emergences (n = 47) were documented in 2017 (Figure 2). Nesting occurred from May through September, but was most frequent in July (n = 53), with 47.7% of all confirmed nests documented that month (Figure 3). Non-nesting emergences were documented from June through October and were most frequent in August (n = 46), with 46.0% of non-nesting emergences documented that month (Figure 3). Collectively, from 1987 to 2019, 74.4% of all green turtle nesting activity (nests and non-nesting emergences) occurred between July and August.
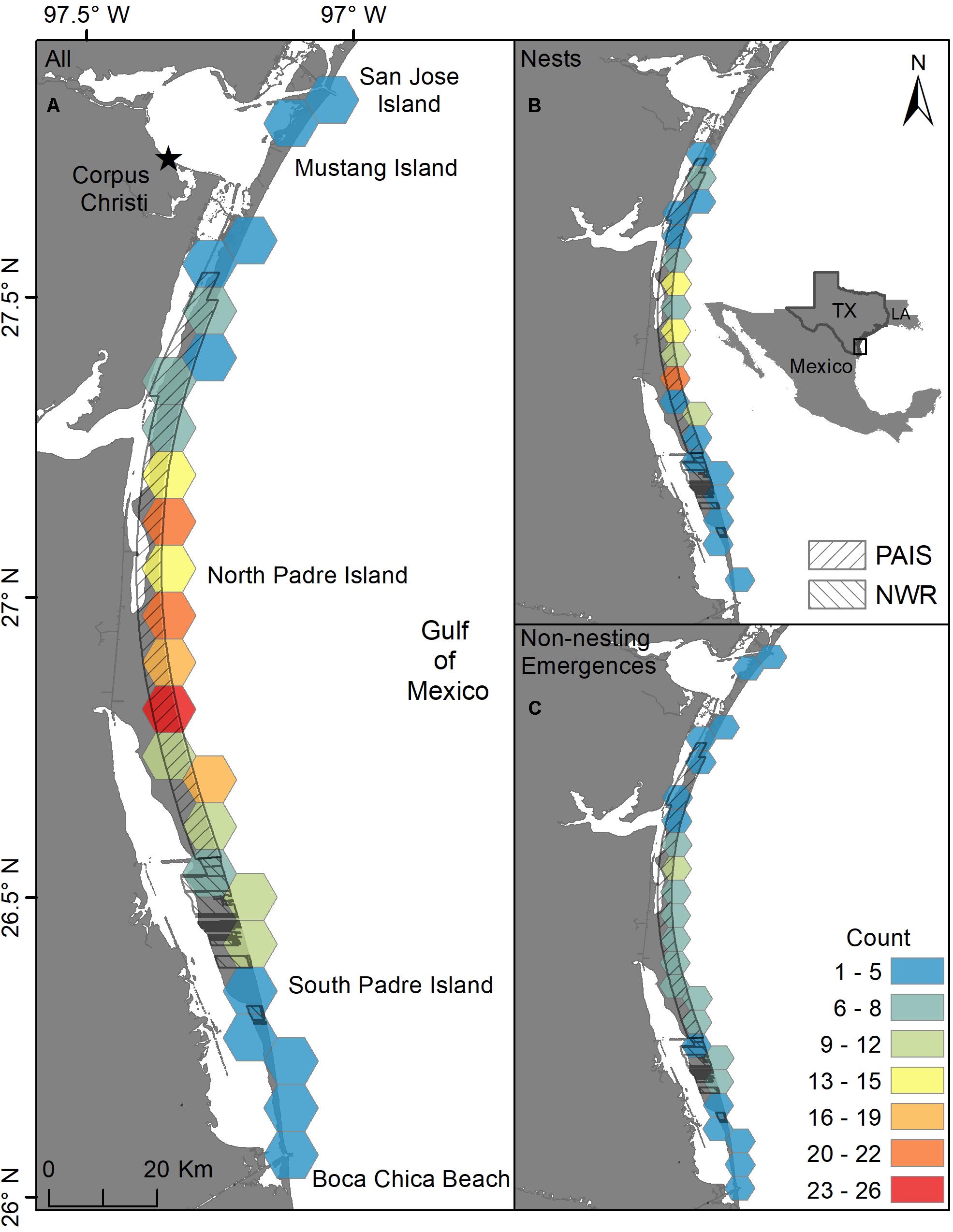
Figure 1. Ten km hex-bin density of all green turtle (Chelonia mydas) nesting activity (nests and non-nesting emergences) in Texas from 1987 to 2019. (A) Density of all nests and non-nesting emergences, (B) density of all nests, (C) density of all non-nesting emergences. PAIS = Padre Island National Seashore, NWR = Laguna Atascosa and Lower Rio Grande Valley National Wildlife Refuges.
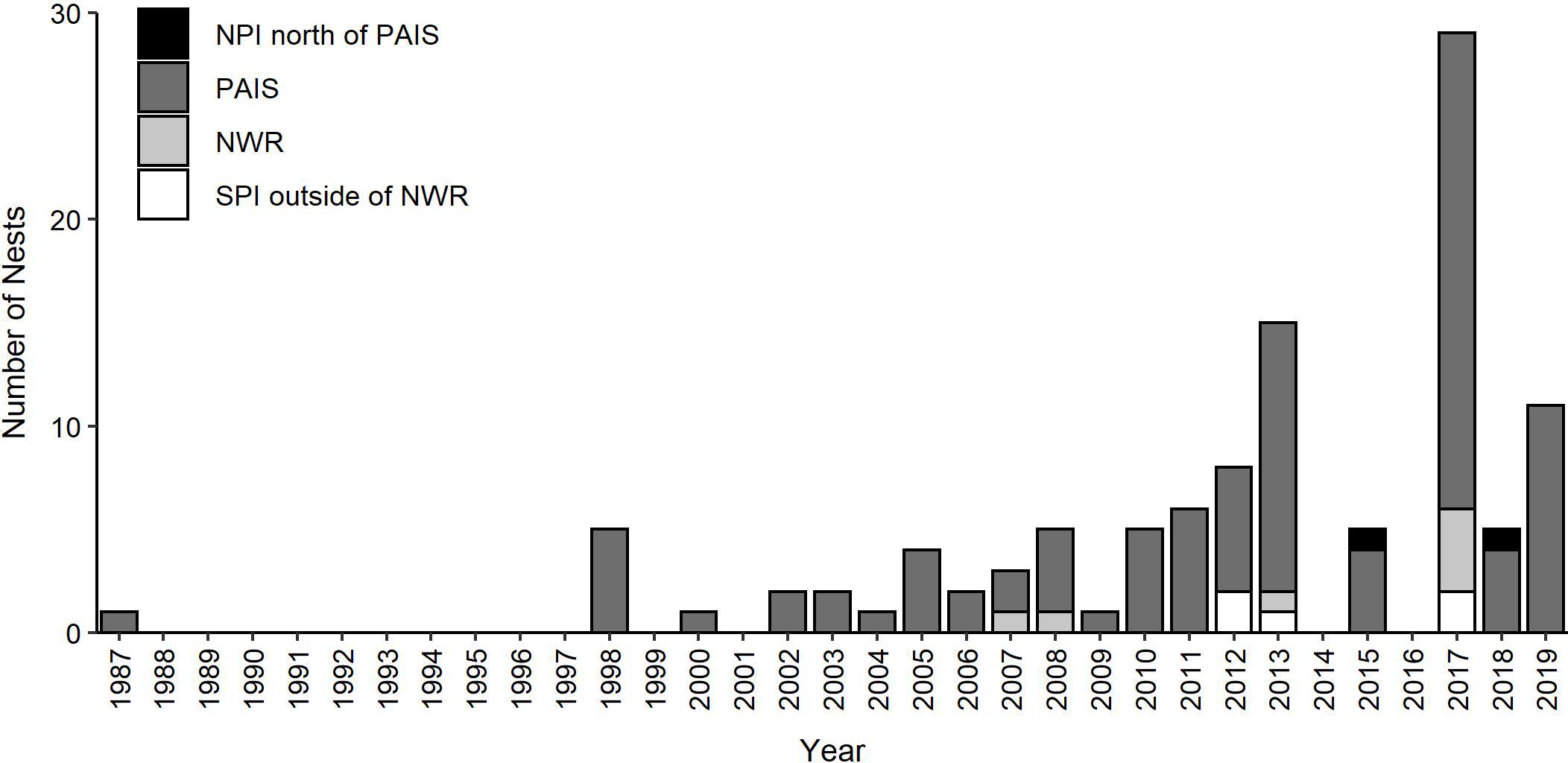
Figure 2. Annual numbers of green turtle (Chelonia mydas) nests documented in Texas from 1987 to 2019 (non-nesting emergences not included). NPI = North Padre Island outside of PAIS, PAIS = Padre Island National Seashore, NWR = Laguna Atascosa and Lower Rio Grande National Wildlife Refuges, SPI = South Padre Island outside of a NWR.
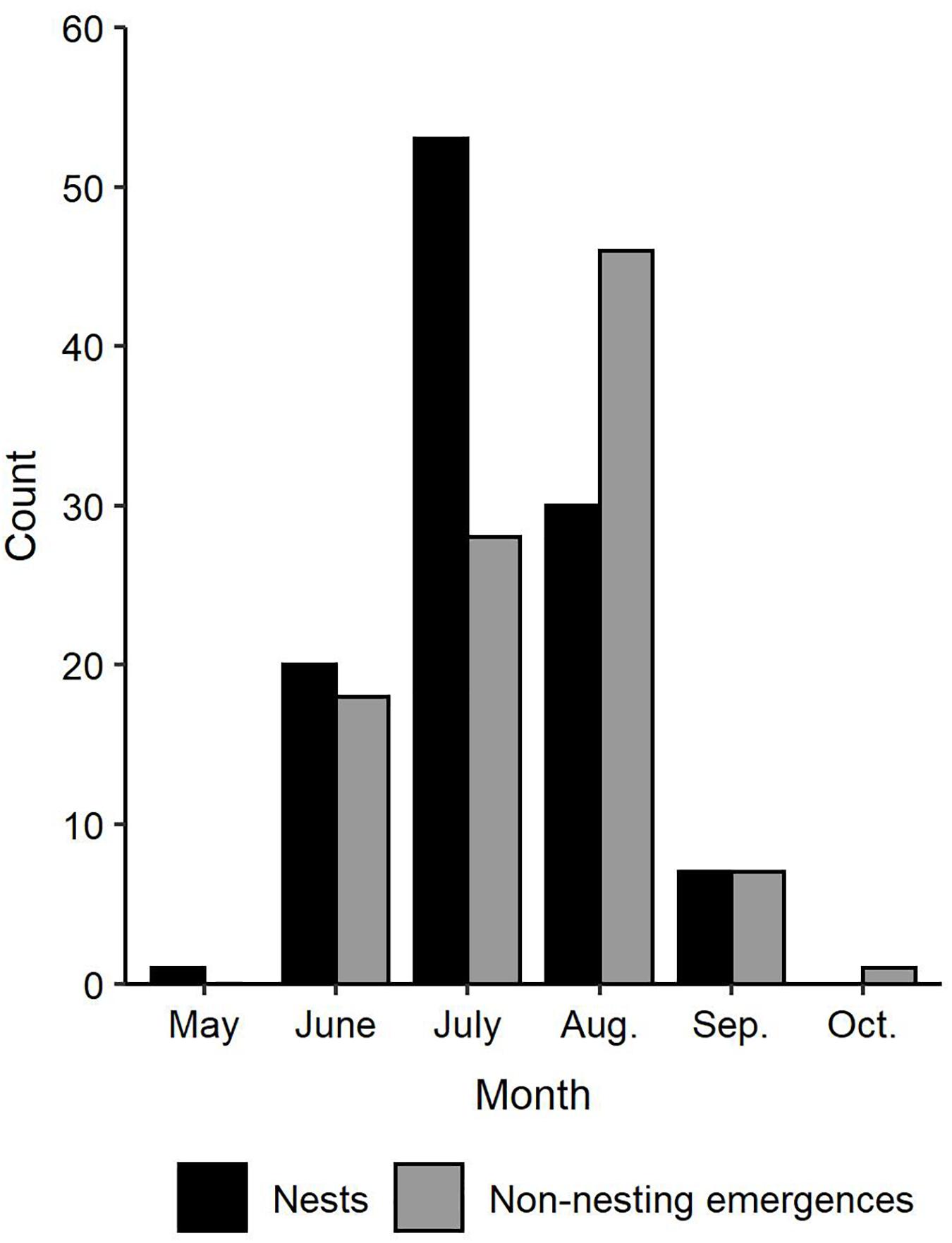
Figure 3. Monthly trends in total number of green turtle (Chelonia mydas) nesting activity (nests and non-nesting emergences) documented in Texas from 1987 to 2019.
Females were observed at 18 of the 211 nesting activity sites, but at all other sites the females had already returned to the GoM by the time that biologists arrived. Nine females were tagged, including four at nest sites and five at non-nesting emergence sites. Mean SCL of the nine females measured was 105.7 cm (SD = 5.3 cm, range = 98.6–113.2 cm). One female tagged in 2019 at a non-nesting emergence site was observed expelling a prolapsed oviduct (Frandsen et al., 2020). Through tag returns, two nesting females (1 in 2017 and 1 in 2018) were confirmed to have nested twice in a season. One female that was tagged and documented nesting in 2006 was observed during a non-nesting emergence in Hampton Bays, New York in 2011 (Shaver et al., 2019b). Based on total nests, a clutch frequency of three (Seminoff et al., 2015), and a re-migration interval of 2.27 years (del Méndez Matos et al., 2019), it is estimated that 84 adult female green turtles have nested in Texas since 1987.
During the 32-year study, 12,598 eggs were located at 111 nests, of which 9,486 hatched. Ten hatchlings died prior to release (0.1%), 1 weak hatchling was recaptured after unsuccessful release and retained for rehabilitation, but later died, and 9,475 hatchlings were successfully released and entered the surf at PAIS (n = 9,078) or South Padre Island (n = 397) (Table 1). The 103 non-predated nests contained 12,038 eggs, with a mean clutch size of 117 eggs (SD = 29.4, range = 2 to 177 eggs). Mean hatching success for the 103 non-predated clutches was 77.4% (SD = 31.4, range = 0.0–99.3%) (Table 1). The 8 nests predated by badgers (Taxidea taxus), coyotes (Canis latrans), and/or ghost crabs (Ocypode quadrata) before nest detection contained 560 identifiable eggs (total does not include shredded egg shells found at nest sites), of which 434 eggs were still intact when found. The 434 eggs were salvaged, incubated, and produced 169 hatchlings. Using our mean clutch size of 117 eggs as a proxy for clutch size in the eight predated nests, estimated mean hatching success for the predated nests was 18.1%. Combining estimates for the eight predated nests with results for the 103 non-predated nests, the estimated mean hatching success of the 111 green turtle nests found in Texas from 1987 to 2019 was 73.1%. For the 1,537 hatchlings weighed and measured from 1987 to 2006, mean SCL was 52.97 mm (SD = 1.90 mm, range = 43.62–58.06 mm) and mean weight was 28.61 g (SD = 2.51 g, range = 19.95–34.07 g) (Figure 4).
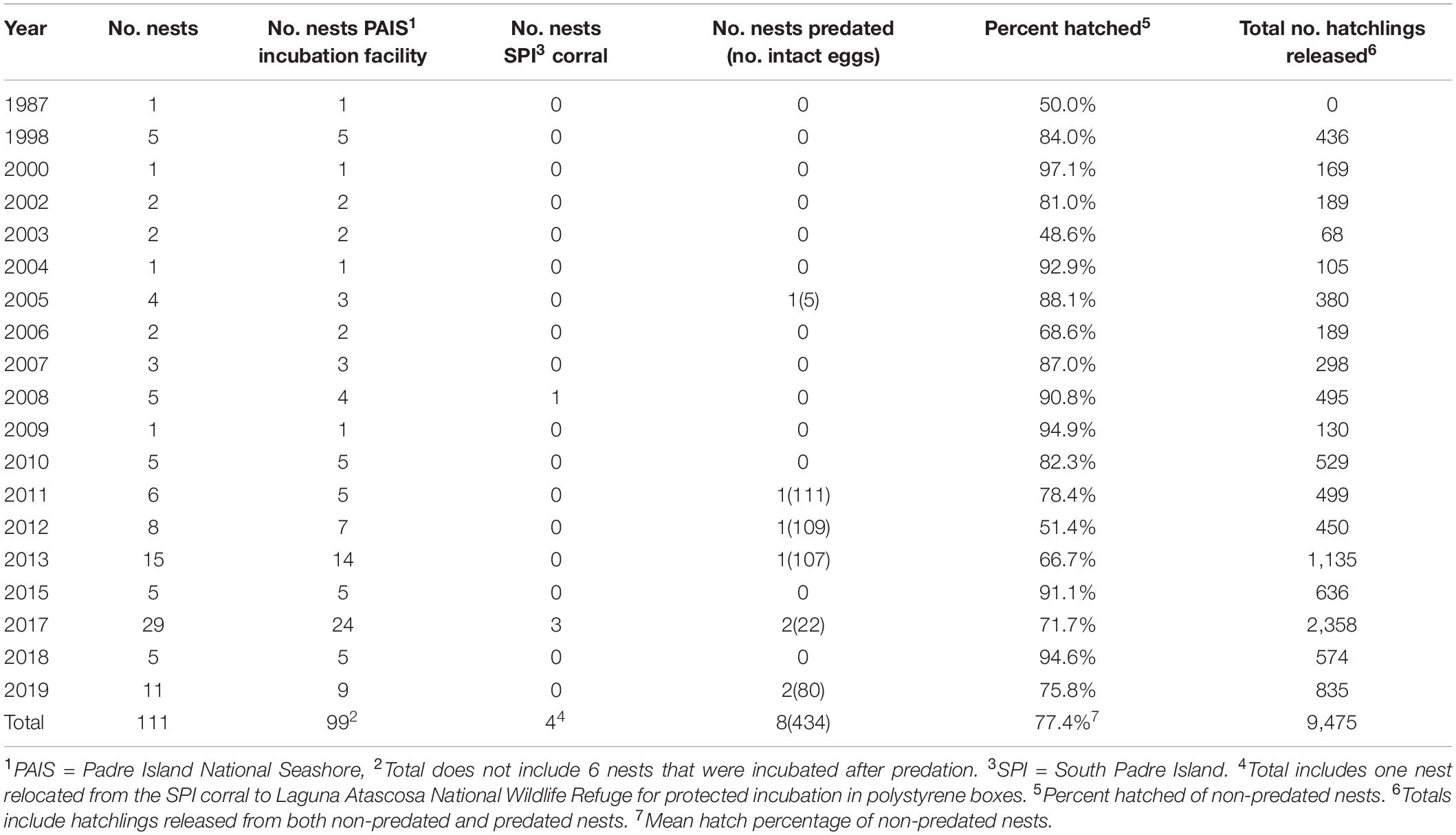
Table 1. Summary of incubation, hatching, and release information for green turtle (Chelonia mydas) nests documented on the Texas coast from 1987 through 2019, including the annual number of nests found, number of nests by incubation method, number of nests predated, hatching success, and number of hatchlings released.
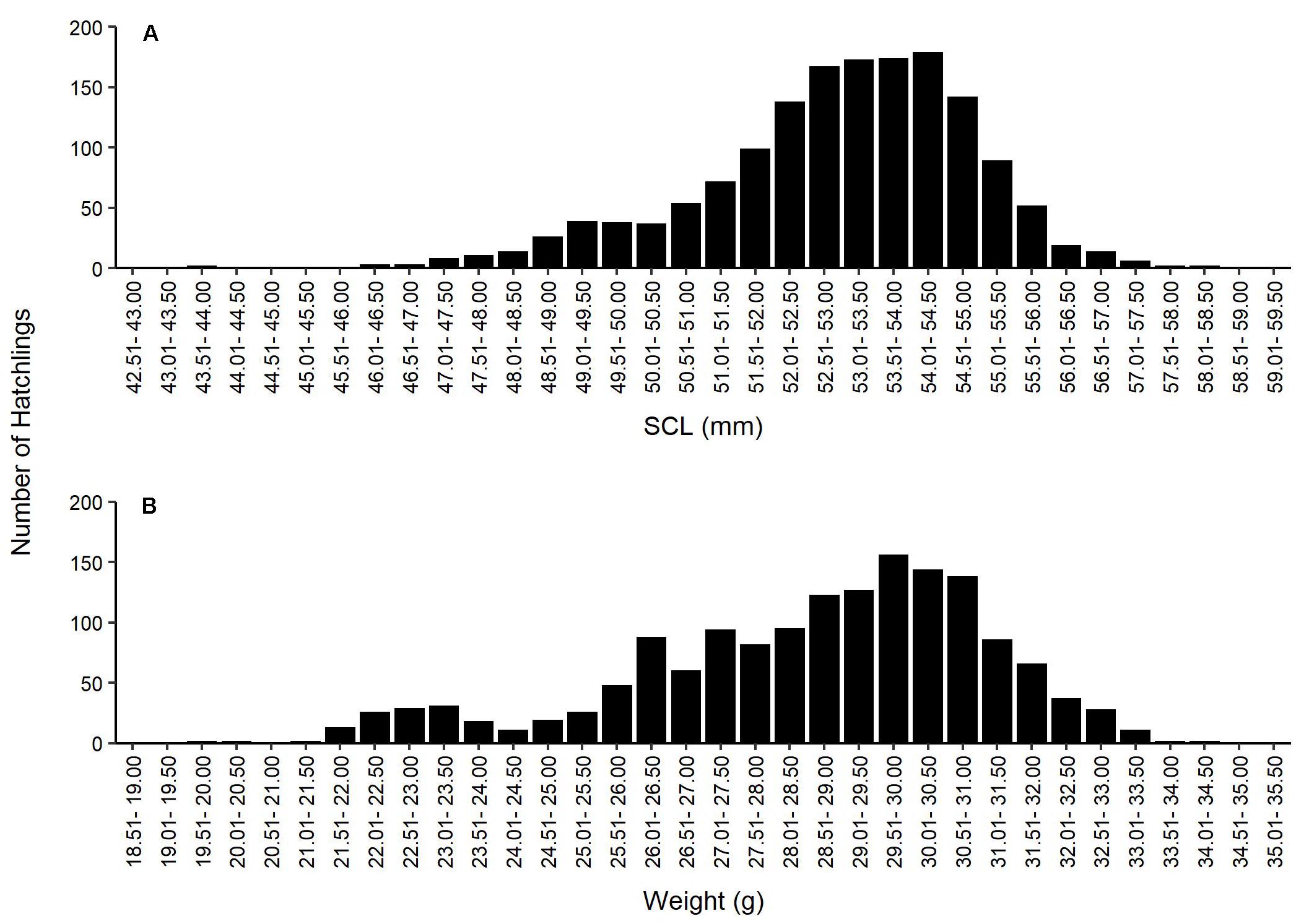
Figure 4. (A) Straight carapace lengths (SCL) (mm) and (B) weights (g) measured for green turtle (Chelonia mydas) hatchlings that emerged from eggs hatched at the Padre Island National Seashore (PAIS) incubation facility, and released on the beach at PAIS, from 1987 to 2006.
Discussion
Spatial Trends
All green turtle nests confirmed on the Texas coast through 2019 were on North and South Padre Islands, with 92.8% of nests documented on lands protected by the DOI as PAIS (n = 97 nests) or NWR (n = 6 nests) (Figures 1, 2). During the study period, nesting success was 52.6%, which is slightly higher than the 50% recorded for green turtles in the southeast United States (Weishampel et al., 2003). Green turtles demonstrated a preference for nesting on DOI property and had higher nesting success at the federally protected lands (56.6%) than outside of them (27.6%). Not only is PAIS the most important green turtle nesting beach in Texas, it is also the most important nesting beach in Texas for loggerhead turtles and the most important nesting beach in the United States for Kemp’s ridley turtles, with more Kemp’s ridley nests recorded there annually than at any other United States beach (Shaver et al., 2016b, 2017a; Figure 5). Although green turtle nests have only been confirmed in Texas on North and South Padre Islands, Kemp’s ridley and loggerhead nests have been recorded state-wide (Shaver et al., 2016b; Shaver pers. obs.), indicating that green turtles strongly prefer to nest on North and South Padre Islands over other Texas beaches that have been used for nesting by other sea turtle species. Additionally, the only hawksbill (Eretmochelys imbricata) and leatherback (Dermochelys coriacea) nests confirmed in Texas were documented on North Padre Island at PAIS (Shaver and Frandsen, 2019; Shaver et al., 2019a), indicating that PAIS is the most important sea turtle nesting beach for all five GoM species on the Texas coast.
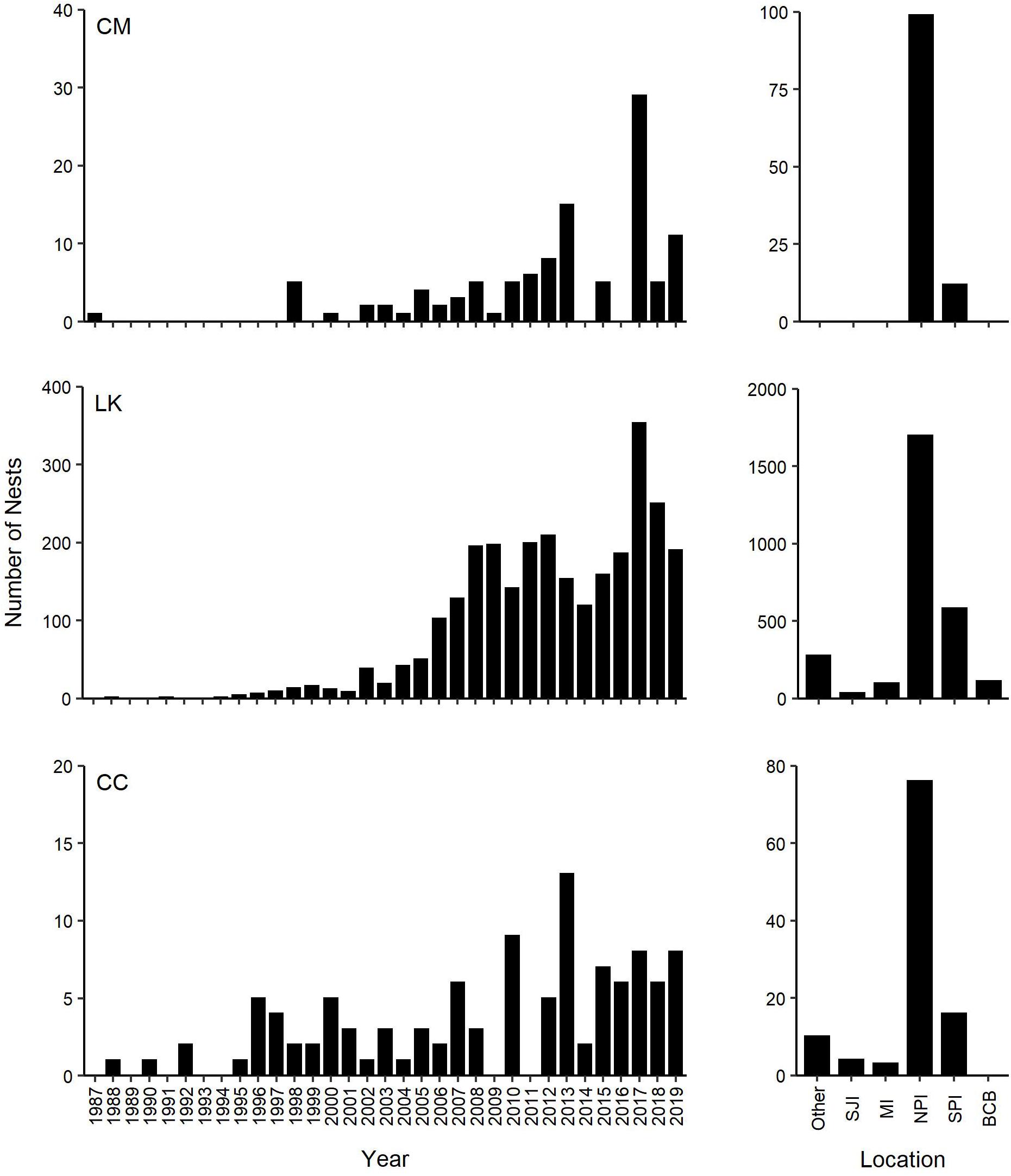
Figure 5. Nesting trends of green (CM, Chelonia mydas), Kemp’s ridley (LK, Lepidochelys kempii), and loggerhead (CC, Caretta caretta) turtles in Texas, United States between 1987 and 2019, with total documented nests each year and total numbers of nests documented on specific Texas beaches. Other: All other Texas beaches where green turtle nesting activity has not been observed that are monitored annually for nesting activity; SJI, San Jose Island; MI, Mustang Island; NPI, North Padre Island; SPI, South Padre Island; BCB, Boca Chica Beach.
Green turtle nest density was highest at PAIS, particularly between the 31.1 km (19.3 mile) and 74.8 km (46.5 mile) markers (Figure 1). This nesting epicenter encompasses the southernmost 25.2 km of “Big Shell Beach”, which extends from the PAIS 27.4 km (17 mile) to 56.3 km (35 mile) markers (Weise and White, 1980; USDA NRCS and NPS, 2005), and 18.5 km immediately south of Big Shell Beach. Culver (2018) found that Kemp’s ridley nest density on North and South Padre Islands was highest between approximately the PAIS 27.4 km (17 mile) and 67.6 km (42 mile) markers, which encompassed all of Big Shell Beach and the 11.3 km stretch of beach immediately south of it. The nesting epicenters for green and Kemp’s ridley sea turtles at PAIS are nearly identical and include beaches with geomorphological characteristics that are unique on the Texas coast, but resemble the geomorphology of beaches at Rancho Nuevo, Tamaulipas, Mexico (Carranza-Edwards et al., 2004; Culver et al., 2020), where Kemp’s ridley and green turtle nesting are prolific. Longshore currents converge near the center of PAIS and cause sediment and shell fragments to accumulate in this area (Davis, 1978).
Multiple factors may influence where sea turtles choose to nest (Mortimer, 1990, 1995; Weishampel et al., 2003, 2006; Cuevas et al., 2010), including magnetic fields (Brothers and Lohmann, 2018), offshore habitat structure (Hughes and Richard, 1974), offshore and near-shore oceanographic conditions (Carr and Carr, 1972; Marcovaldi and Laurent, 1996; Weishampel et al., 2003), beach morphology and covering (Whitmore and Dutton, 1985; Kikukawa et al., 1996; Fujisaki and Lamont, 2016; Maurer and Johnson, 2017), sand characteristics, and anthropogenic factors (Crain et al., 1995; Steinitz et al., 1998; Davis et al., 1999; Kikukawa et al., 1999). However, multiple factors, including human or predator disturbance, lighting, unfavorable topography or sand characteristics, marine debris, and others, can also cause green turtles to abandon nesting attempts.
At other beaches, green turtles tended to nest on beaches with 1–2 mm sand particles (Salleh et al., 2018), moderate to steep slope (Cuevas et al., 2010; Zavaleta-Lizárraga and Morales-Mávil, 2013), vegetated dunes (Whitmore and Dutton, 1985), and little to no development or recreational activity (Weishampel et al., 2003; Zavaleta-Lizárraga and Morales-Mávil, 2013). Though nesting has been observed on highly developed beaches (Shamblin et al., 2015), lighting from development can deter nesting and cause disorientation of hatchlings (Witherington, 1992; Salmon and Witherington, 1995; Salmon et al., 1995; Fuentes et al., 2016; Price et al., 2018).
Beaches on the three DOI properties are more remote and primitive and are less heavily visited by the public. The remoteness of the properties may attract green turtles to nest there due to nearly non-existent levels of development, light pollution, and anthropogenic disturbance (Fuentes et al., 2016). There is no road behind the dunes on most of the DOI property, so visitor activities there are limited to beach driving by 4-wheel drive vehicles, fishing, wildlife watching, and primitive camping; therefore, boating and visitation is often sparse there. Of the combined total length of 120.3 km, only approximately 1.6 km (1.0 miles) on North Padre Island in front of the PAIS Visitor Center and Campground is mechanically raked and graded to remove marine debris and Sargassum spp. when large influxes periodically occur. Large items that wash ashore (i.e., logs, poles) are not removed unless they pose a safety hazard to the public, and marine debris clean-up is restricted to hand work by volunteers. But since these beaches are remote, clean-ups are not as frequent as in the developed areas.
In contrast, developed beaches on the north end of North Padre Island, south end of South Padre Island, and multiple other locations in Texas, are more accessible, manipulated, and visited by the public. Some of these beaches are reinforced with seawalls and these beaches are sometimes re-nourished. Mechanical grading and raking are routinely used to remove marine debris, trash left on the beach by visitors, and Sargassum spp. from the beachfront. Heavy equipment is used to remove loose sand accumulated there to facilitate beach driving, which is permitted on most Texas beaches under the Texas Open Beaches Act.
Temporal Trends and Genetic Structure
Numbers of green turtle nests recorded on the Texas coast appear to have increased from the late-1980s through 2019. However, nesting detection efforts have been incomplete through the years and the 111 nests recorded is a minimum estimate of the total number of nests laid on the Texas coast by this species. Nesting green turtles and their tracks could have been missed as a result of minimal visitation to PAIS prior to 1962 when it was designated as a unit of the National Park System. Sea turtle nest detection patrols did not begin on North Padre Island until 1986, and patrols were limited by the number and proficiency of patrollers until 1995. The documentation of five nests in 1998, and virtually none before then, is likely a result of increased patrol effort and skill. Additionally, for many years, patrols were only conducted from April through mid-July, to target Kemp’s ridley nesting, and thus missed most of the green turtle nesting season, which can extend through early-October. During a three-year exploratory study beginning in 2002, patrollers found green turtle nests (n = 2) during late season surveys, indicating that patrols for this species should be extended through at least July 31. Late season, early-morning patrols were conducted on parts of North and South Padre Islands starting in 2010, but they rarely extended through the entire green turtle nesting season due to funding limitations. Biologists were watchful for nesting activity when they drove on North and South Padre Islands at night to release hatchlings, but this typically was not a full sweep of both islands and did not occur nightly throughout the green turtle nesting season. Nighttime patrols designed to detect nesting green turtles were only conducted on North Padre Island in 2018 and 2019. However, due to grant funding limitations, patrols were only conducted on a portion of North Padre Island during a portion of the nesting season.
To understand nesting trends and inform conservation efforts, the genetic population structure of green turtles nesting in Texas must be identified. It is unknown whether green turtles nesting in Texas are remnants of a formerly much larger nesting population, represent a spread of nesting from Tamaulipas and Veracruz, Mexico, or originate from elsewhere. Furthermore, it is unknown how many of the exponentially increasing numbers of juvenile green turtles now occurring in Texas waters will someday nest on Texas Gulf beaches. Recent increases in documented nests may indicate that this population is recovering from past exploitation and that western GoM beaches served as important nesting habitat for green turtles. Increases in green turtle nesting have been documented in Florida and on the Gulf coast of Mexico (Weishampel et al., 2003; Cuevas et al., 2010). However, the rate of increase in nesting in Texas is less than at these other GoM nesting beaches, which may reflect higher mortality of turtles from this population during one or more of their life stages. It is unknown why green turtle nesting is so sparse in Texas compared to nesting on GoM beaches in Mexico and Florida. It is impossible to reference historical nesting levels since overharvesting of green turtles in GoM waters prior to 1900 likely eliminated nesting activity in Texas before baseline levels could be reported. Additionally, a thriving fishing and shrimping industry in Brownsville and Port Isabel, Texas, may have killed adult green turtles in southern Texas GoM waters where shrimping intensity and sea turtle abundance were high (McDaniel et al., 2000). The industry flourished in the 1940s–1990s and in 1989, Brownsville/Port Isabel was ranked the sixth most valuable commercial fishing port in the United States and the most valuable port in the GoM (Haby et al., 1993). The port potentially sustained the largest offshore shrimping fleet in the world (Haby et al., 1993) and subsequent trawling activities likely exacerbated the precipitous decline of adult green turtles along the Texas coast. When turtle excluder devices (TEDs) were initially implemented in the United States, the opening size required in the GoM was smaller than the opening size required in Atlantic waters (Epperly and Teas, 2002). Thus, larger turtles were more at risk in the GoM until 2003, when regulations were changed requiring larger TED openings to enable escape of leatherback and all other sea turtle species in both Atlantic and GoM waters (Finkbeiner et al., 2011). However, it is unknown whether adult green turtles captured in Texas fisheries were at their foraging grounds or near their nesting beaches. Currently, there are no known adult green turtle foraging grounds along the Texas coastline.
Alternatively, nesting increases in Texas may reflect a spread of nesting northward from Tamaulipas. Although sea turtles are thought to return to their natal beaches for reproduction through geomagnetic imprinting and magnetic navigation (Brothers and Lohmann, 2018), some sea turtles have been recorded nesting on multiple beaches and colonization of nesting beaches occurs over geologic time. Rabon et al. (2003) suggested that the nesting colony of leatherbacks in Florida could have been the source population of the females that nested sporadically between 1981 and 2011 on the Atlantic coast of the United States north of Florida, which was outside the historical nesting range of this species. Carreras et al. (2018) found that sporadic loggerhead nesting events in developmental feeding grounds in the western Mediterranean may be an adaptation to changing environmental conditions and can be considered new populations in this context.
The spread of green turtle nesting northward from Tamaulipas may be a compensatory mechanism that may allow this population to adapt to changing environmental conditions (Shamblin et al., 2018). As ocean surface temperatures increase (Cheng et al., 2017), earlier nesting dates (Hawkes et al., 2007; Weishampel et al., 2010) and smaller clutch sizes (Mazaris et al., 2008) at other sea turtle nesting beaches have been observed. With rising sand temperatures, many nesting beaches are predicted to produce 100% female clutches by 2070 (Fuentes et al., 2011). Sand temperatures recorded on western GoM beaches during the Kemp’s ridley nesting season (April–August) indicate that northern nesting beaches (PAIS and South Padre Island, United States and La Pesca, Mexico) are consistently cooler than southern nesting beaches in Mexico (Bevan et al., 2019). Though modeling by Pike (2013) predicts that habitat suitability in Texas is marginal for green turtle nesting, the preferential use of DOI property by nesting green turtles indicates that these areas along the Texas GoM coast are important for this species. A spread of nesting northward may be evidence that green turtles in the western GoM are able to utilize new nesting habitat as those areas become more suitable due to climatic change (Araújo et al., 2005).
The juvenile green turtle population has increased substantially in Texas since 2010. Although it originates predominantly from nearby rookeries on the western Gulf coast of Mexico (Anderson et al., 2013; Shamblin et al., 2017; Shaver et al., 2017b), more work needs to be done to determine if the nesting adults and juveniles in Texas are of the same genetic stock, and whether some individuals that use Texas waters as juveniles for foraging also use Texas beaches for nesting. Kemp’s ridley is considered one genetic stock and one Regional Management Unit (RMU) (Wallace et al., 2010). Annual counts of Kemp’s ridley nests in Texas and Mexico trended similarly from the 1990’s through 2019, although numbers in Texas were greatly reduced compared to the numbers in Mexico (Shaver et al., 2016b; Shaver pers. obs.).
There is a slight possibility that the green turtles that have nested in Texas were from Operation Green Turtle, where over 130,000 hatchlings and eggs were shipped from Tortuguero, Costa Rica, to 17 Greater Caribbean countries from 1959 to 1968, with the goal of re-establishing decimated green turtle nesting populations (Carr, 1967; Owens et al., 1982; Bjorndal et al., 1999). Numbers released at each site were not well documented, but some hatchlings were released into the GoM in south Texas. Based on estimated age to maturity of about 23–45 years (NMFS and USFWS, 2007; Seminoff et al., 2015), green turtle hatchlings released could have reached maturity by 1987, when the first green turtle nest was confirmed in Texas.
Distinct green turtle rookeries and foraging aggregations must be conserved to effectively protect the genetic diversity of the species (Ng et al., 2017). Green turtles nesting in Texas may represent an emerging subpopulation and warrant recognition as a unique management unit separate from those nesting in Mexico, which are already being considered a unique management unit (Shamblin et al., 2017). Haplotypes CM-A1.1 and CM-A3.1 accounted for 87% of the green turtles sampled within 10 Florida rookeries (Shamblin et al., 2015). Haplotype frequencies were strongly partitioned by latitude and supported recognition of at least two management units based on a genetic break between rookeries separated by a 1 km wide inlet (Shamblin et al., 2015). This population structure was reassessed using the mitochondrial microsatellite short tandem repeat (mtSTR), which further identified four management units in Florida instead of two and demonstrated discrete fine-scale natal homing to island groups (Shamblin et al., 2020). Further study is needed to identify the degree of demographic partitioning among green turtle rookeries along the western GoM coast. Depending on the results, preservation of green turtle nesting in Texas may be vital to conserving rare haplotypes only found in a new management unit there, or in the broader GoM unit shared with Mexico.
Conservation Implications
North and South Padre Islands are the epicenter of sea turtle nesting in the northwestern GoM. All green turtle, hawksbill, and leatherback nests confirmed in Texas, 84.5% of loggerhead, and 81.5% of Kemp’s ridley nests confirmed in Texas through 2019 were located there (Shaver et al., 2016b, 2019a; Shaver and Frandsen, 2019; Shaver pers. obs.). The concentration of green turtle nesting on DOI property on these islands will aid with conservation of this nesting population in the northwestern GoM into the future. DOI properties have prohibitions against future development and restrictions on the activities allowed within their boundaries, whereas development continues on the northern end of North Padre Island and the southern end of South Padre Island. Interestingly, PAIS was initially planned to include all but 22.5 km on the northern end of North Padre Island and 22.5 km on the southern end of South Padre Island, which would be reserved for development (Jones, 1999). Although only a small portion of South Padre Island was preserved within PAIS when it was established (Jones, 1999), over the last 60 years several land parcels on South Padre Island have been added to the Laguna Atascosa and Lower Rio Grande Valley NWRs, now achieving much of the initial intended protection through a mosaic of DOI lands.
The Archie Carr National Wildlife Refuge was established to protect important nesting habitat for the largest nesting rookeries of loggerhead and green turtles on the United States Atlantic coast (Weishampel et al., 2003). About 70.6% of North and South Padre Island is currently preserved as PAIS and NWR, and though these properties were not established to help conserve sea turtles, as green turtle and Kemp’s ridley nesting has increased on the northwestern GoM coast, these properties have become an important sanctuary for sea turtle nesting. The continued purchase of additional parcels of undeveloped land on South Padre Island, and transfer of those parcels to a NWR, could benefit green, Kemp’s ridley, and other sea turtles nesting on South Padre Island by establishing a unified nesting habitat with North Padre Island, similar to the Archie Carr NWR, but for the western GoM.
Establishment of a Marine Protected Area (MPA) surrounding this unified nesting habitat would also help protect green turtles and other marine species from various anthropogenic threats occurring there. Nearshore GoM waters off south Texas are critical for multiple life stages of multiple sea turtle species (Shaver, 1992; Plotkin et al., 1993; [citeskum]BR88,BR84,BR85,BR86,BR83,BR81[citeekum]Shaver et al., 2005, 2013a, 2013b, 2016a, 2017a, 2020b). On 10 July 2019, a green turtle mating pair was observed off the coast of South Padre Island (Mariana Devlin, pers. comm., Sea Turtle, Inc., South Padre Island, Texas, United States). Turtles foraging off the Texas coast are threatened by shipping, commercial fisheries, oil and gas platforms, surface oiling, hypoxia (Hart et al., 2018), entanglement in marine debris and ghost fishing gear (Purvin et al., 2020), and continued illegal red snapper (Lutjanus campechanus) fishing practices in south Texas (Shaver pers. obs.). In addition, Texas hosts the longest-term land-based recreational shark fishery in the United States (Ajemian et al., 2016). MPA’s, if managed and enforced appropriately, can benefit mobile, wide-ranging species and prevent further decline of their populations (Doherty et al., 2017).
The large stretch of undeveloped coastline protected by DOI on North and South Padre Islands could help absorb future changes in shorelines and sea turtle nesting distributions along the western GoM coast due to climate change. Sea level is projected to rise in the GoM over time and at PAIS, sea level is projected to rise 0.46–0.69 m by 2100 (Caffrey et al., 2018). Some important green turtle nesting beaches in the Caribbean are already eroding and being destroyed (Zavaleta-Lizárraga and Morales-Mávil, 2013). In contrast, parts of PAIS accrete sand, with the greatest accretion measured at the center of the park (Pendleton et al., 2004; KellerLynn, 2010). With no development on the beach, the coastline of the DOI properties on North and South Padre Islands can be allowed to move as the beach is eroded or accretes over time. In contrast, at other areas where seawalls, hotels, residences, and other structures line the beach, great lengths are undertaken to maintain the beachfront through sand re-nourishment and other practices. Developed beaches (particularly those with hotels) have been shown to be the most vulnerable to sea-level rise (Fish et al., 2005), indicating that the substantially developed northern end of North Padre Island and southern end of South Padre Island may no longer contain viable nesting habitat in the future.
If the population of green turtles that nests in Texas continues to grow, there could be a surge in nesting in Texas within the next few years as has been documented on the closest other green turtle nesting beaches in the GoM, in Mexico and the east coast of Florida. Though the magnitude of historic nesting in Texas is unknown as late-season patrol effort (July–October) was not conducted with regularity until 2010, and predominantly from North Padre Island (including PAIS) to South Padre Island, data collected from 2010–2019 establishes a baseline for comparison of future nesting levels. However, these estimates only provide minimum estimates of green turtle nesting activity on the south Texas coast and nighttime patrols targeting green turtle nesting were only conducted in 2018 and 2019 on a portion of North Padre Island during a portion of the green turtle nesting season due to grant funding limitations.
This study defined the temporal and spatial trends of green turtle nests confirmed in Texas, which is the first step needed for developing a monitoring and protection program. We recommend that systematic nighttime patrols be instituted on North and South Padre Islands through the green turtle nesting season (June though early-October), to locate, document, and protect nesting green turtles and their nests. Additionally, these nighttime patrols will protect the females and nests of the other GoM species that nest later in the year than Kemp’s ridley turtles. Leaving nests unprotected allows mammalian predators to develop habits of nest predation (Pritchard and Marquez, 1973; Worth and Smith, 1976) and associate females and their tracks with a reliable food source, which has led to predation upon the smaller Kemp’s ridley nesters at PAIS (Shaver et al., 2020a). Non-nesting emergences should also be documented and tracked through the potential incubation and hatching seasons, to confirm whether they are actually non-nesting emergences or nest sites. On-going daytime patrols conducted from April through mid-July that target Kemp’s ridley nesting would not enable detection and protection of nesting green turtles, and would only enable location of green turtle nests during first patrols of the morning in June and early July, and opportunistically during release of hatchlings and through reports from the public. Continued data collection through a systematic program will be essential for developing an accurate assessment of trends and demographics for the population of green turtles that nest in Texas. Continued detection, documentation, and protection efforts will aid with recovery efforts for this threatened species as well as for the other four GoM species that utilize Texas for nesting. Preservation of this genetic stock is vital to population viability. If this green turtle population is a shared stock with Mexico, this highlights the critical need for international collaboration across political boundaries. However, this stock is still poorly defined and could be a rare remnant, essential for preservation of the species.
Data Availability Statement
The datasets presented in this article are not readily available as National Park Service (NPS) policy prohibits disclosure of endangered species location data, and we are unable to provide the GPS locations of the nesting activities presented in the article. Requests to access the datasets should be directed to DS, donna_shaver@nps.gov.
Ethics Statement
The animal study was reviewed and approved by National Park Service Institutional Animal Care and Use Committee.
Author Contributions
DS conceived the study and methodology and acquired funding. DS, HF, JG, and CG provided resources and wrote and reviewed the original draft. DS, HF, and CG performed the data analysis. CG created the figures. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Park Service, Sea Turtle, Inc., the Texas State Aquarium, and the United States Geological Survey. The funders provided support in the form of salaries for authors (DS, HF, JG, and CG) and supplies, but did not have a role in the study design, data collection and analysis, or preparation of the manuscript.
Disclaimer
The statements and findings are those of the authors and do not necessarily reflect the views of the National Park Service or the Department of the Interior.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the individuals and organizations that aided with detection, monitoring, and reporting of the nests included in this analysis. Amos Rehabilitation Keep (ARK), National Biological Survey/Service, National Marine Fisheries Service, National Park Service, Sea Turtle, Inc., Texas A&M University at Galveston, Texas Parks and Wildlife Department, Texas State Aquarium, Turtle Island Restoration Network, United States Fish and Wildlife Service, United States Geological Survey, University of Texas, Western National Parks Association, and others provided assistance or funding for nest detection and protection activities in Texas. Two reviewers provided suggestions that enhanced the manuscript. Work by Padre Island National Seashore personnel was authorized under FWS Permit PRT-840727, FWS Permit TE40727, Texas Parks and Wildlife Department Scientific Permit SPR-0190-122, and NPS Institutional Animal Care Protocols NPS IACUC 2011-15 and IMR_PAIS_Walker_SeaTurtles_2016.A3.
References
Ajemian, M. J., Jose, P. D., Froeschke, J. T., Wildhaber, M. L., and Stunz, G. W. (2016). Was everything bigger in Texas? Characterization and trends of a land-based recreational shark fishery. Mar. Coast. Fish. 8, 553–566. doi: 10.1080/19425120.2016.1227404
Anderson, J. D., Shaver, D. J., and Karel, W. J. (2013). Genetic diversity and natal origins of green turtles (Chelonia mydas) in the western Gulf of Mexico. J. Herpetol. 47, 251–257. doi: 10.1670/12-031
Araújo, M. B., Pearson, R. G., and Rahbek, C. (2005). Equilibrium of species’ distributions with climate. Ecography 28, 693–695. doi: 10.1111/j.2005.0906-7590.04253.x
Bevan, E. M., Wibbels, T., Shaver, D., Walker, J. S., Illescas, F., Montano, J., et al. (2019). Comparison of beach temperatures in the nesting range of Kemp’s ridley sea turtles in the Gulf of Mexico, Mexico and USA. Endang. Spec. Res. 40, 31–40. doi: 10.3354/esr00977
Bjorndal, K. A., Wetherall, J. A., Bolten, A. B., and Mortimer, J. A. (1999). Twenty-six years of green turtle nesting at Tortuguero, Costa Rica: an encouraging trend. Conserv. Biol. 13, 126–134. doi: 10.1046/j.1523-1739.1999.97329.x
Bresette, M. J., Witherington, B. E., Herren, R. M., Bagley, D. A., Gorham, J. C., Traxler, S. L., et al. (2010). Size-class partitioning and herding in a foraging group of green turtles Chelonia mydas. Endang. Spec. Res. 9, 105–116. doi: 10.3354/esr00245
Brothers, J. R., and Lohmann, K. J. (2018). Evidence that magnetic navigation and geomagnetic imprinting shape spatial genetic variation in sea turtles. Curr. Biol. 28, 1–5. doi: 10.1016/j.cub.2018.03.022
Caffrey, M. A., Beavers, R. L., and Hoffman, C. H. (2018). Sea Level Rise and Storm Surge Projections for the National Park Service. Fort Collins, CO: National Park Service.
Carr, A. F. (1967). So Excellent a Fishe: A Natural History of Sea Turtles. New York, NY: Natural History Press.
Carr, A. F., and Carr, M. H. (1972). Site fixity in the Caribbean green turtle. Ecology 53, 425–429. doi: 10.2307/1934228
Carr, A. F., and Ingle, R. M. (1959). The green turtle (Chelonia mydas mydas) in Florida. Bull. Mar. Sci. Gulf Caribbean 9, 315–320.
Carranza-Edwards, A., Rosales-Hoz, L., Chávez, M. C., and de la Garza, E. M. (2004). “Environmental geology of the coastal zone,” in Environmental Analysis of the Gulf of Mexico, eds M. Caso, I. Pisanty, and E. Ezcurra (College Station, TX: Texas A&M University Press), 351–372.
Carreras, C., Pascual, M., Tomás, J., Marco, A., Hochscheid, S., Castillo, J., et al. (2018). Sporadic nesting reveals long distance colonisation in the philopatric loggerhead sea turtle (Caretta caretta). Sci. Rep. 8:1435. doi: 10.1038/s41598-018-19887-w
Chaloupka, M. Y., Bjorndal, K. A., Balazs, G. H., Bolten, A. B., Ehrhart, L. M., Limpus, C. J., et al. (2008). Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob. Ecol. Biogeogr. 17, 297–304. doi: 10.1111/j.1466-8238.2007.00367.x
Cheng, L., Trenberth, K. E., Fasullo, J., Boyer, T., Abraham, J., and Zhu, J. (2017). Improved estimates of ocean heat content from 1960 to 2015. Sci. Adv. 3:e1601545. doi: 10.1126/sciadv.1601545
Crain, D. A., Bolten, A. B., and Bjorndal, K. A. (1995). Effects of beach nourishment on sea turtles: review and research initiatives. Restor. Ecol. 3, 95–104. doi: 10.1111/j.1526-100X.1995.tb00082.x
Cuevas, E. A., Guzmán-Hernández, V., Uribe-Martínez, A., Raymundo-Sánchez, A., and Herrera-Pavon, R. (2018). Identification of potential sea turtle bycatch hotspots using a spatially explicit approach in the Yucatan Peninsula, Mexico. Chelonian Conserv. Biol. 17, 78–93. doi: 10.2744/CCB-1263.1
Cuevas, E. A., Liceaga-Correa, M., de los, Á, and Mariño-Tapia, I. (2010). Influence of beach slope and width on hawksbill (Eretmochelys imbricata) and green turtle (Chelonia mydas) nesting activity in El Cuyo, Yucatán, Mexico. Chelonian Conserv. Biol. 9, 262–267. doi: 10.2744/CCB-0819.1
Cuevas, E. A., Liceaga-Correa, M., de los, Á, and Uribe-Martinez, A. (2019). Ecological vulnerability of two sea turtle species in the Gulf of Mexico: an integrated spatial approach. Endang. Spec. Res. 40, 337–356. doi: 10.3354/esr00984
Culver, M. F. (2018). Beach Geomorphology and Kemp’s ridley (Lepidochelys kempii) Nest Site Selection Along Padre Island, TX, USA. M. Sc. Thesis, Texas A&M University-Corpus Christi, Corpus Christi, TX.
Culver, M., Gibeaut, J. C., Shaver, D. J., Tissot, P., and Starek, M. (2020). Using lidar data to assess the relationship between beach geomorphology and kemp’s ridley (Lepidochelys kempii) nest site selection along Padre Island, TX, United States. Front. Mar. Sci. 7, 1–14. doi: 10.3389/fmars.2020.00214
Davis, R. A. Jr. (1978). Beach sedimentology of Mustang and Padre Islands: a time-series approach. J. Geol. 86, 35–46. doi: 10.1086/649654
Davis, R. A. Jr., FitzGerald, M. V., and Terry, J. (1999). Turtle nesting on adjacent nourished beaches with different construction styles: Pinellas County, Florida. J. Coast. Res. 15, 111–120.
del Méndez Matos, V. C., Guzmán Hernández, V., and Rivas Hernández, G. A. (2019). “Capítulo 17. Dinámica poblacional de hembras de tortuga blanca (Chelonia mydas) en el estado de Campeche, Mexico,” in El Uso del Conocimiento de las Tortugas Marinas Como Herramienta Para la Restauración de sus Poblaciones y Hábitats Asociados México, eds E. A. Cuevas Flores, V. Guzmán Hernández, J. J. Guerra Santos, and G. A. Rivas Hernández (Campeche: Universidad Autónoma del Carmen), 169–185.
Doherty, P. D., Baxter, J. M., Godley, B. J., Graham, R. T., Hall, G., Hall, J., et al. (2017). Testing the boundaries: seasonal residency and inter-annual site fidelity of basking sharks in a proposed marine protected area. Biol. Conserv. 209, 68–75. doi: 10.1016/j.biocon.2017.01.018
Epperly, S. P., and Teas, W. G. (2002). Turtle excluder devices - are the escape openings large enough? Fish. Bull. 100, 466–474.
Fairbanks, H. R., and Berkey, C. P. (1952). Life and Letters of R. A. F. Penrose, Jr. New York, NY: The Geological Society of America.
Finkbeiner, E. M., Wallace, B. P., Moore, J. E., Lewison, R. L., Crowder, L. B., and Read, A. J. (2011). Cumulative estimates of sea turtle bycatch and mortality in USA fisheries between 1990 and 2007. Biol. Conserv. 144, 2719–2727. doi: 10.1016/j.biocon.2011.07.033
Fish, M. R., Côté, I. M., Gill, J. A., Jones, A. P., Renshoff, S., and Watkinson, A. R. (2005). Predicting the impact of sea-level rise on Caribbean sea turtle nesting habitat. Conserv. Biol. 19, 482–491. doi: 10.1111/j.1523-1739.2005.00146.x
Frandsen, H. R., Purvin, C. M., Villalba-Guerra, M. R., and Shaver, D. J. (2020). Chelonia mydas (Green Sea Turtle). Reproductive abnormality. Herpetol. Rev. 51:312.
Fuentes, M. M. P. B., Gredzens, C., Bateman, B. L., Boettcher, R., Ceriani, S. A., Godfrey, M. H., et al. (2016). Conservation hotspots for marine turtle nesting in the United States based on coastal development. Ecol. Appl. 26, 2708–2719. doi: 10.1002/eap.1386
Fuentes, M. M. P. B., Limpus, C., and Hamann, M. (2011). Vulnerability of sea turtle nesting grounds to climate change. Glob. Change Biol. 17, 140–153. doi: 10.1111/j.1365-2486.2010.02192.x
Fujisaki, I., and Lamont, M. M. (2016). The effects of large beach debris on nesting sea turtles. J. Exp. Mar. Biol. Ecol. 482, 33–37. doi: 10.1016/j.jembe.2016.04.005
Haby, M. G., Edwards, R. A., Reisinger, E. A., Tillman, R. E., and Younger, W. R. (1993). The Importance of Seafood-Linked Employment and Payroll in Texas. College Station, TX: Texas A&M University.
Hart, K. M., Iverson, A. R., Fujisaki, I., Lamont, M. M., Bucklin, D., and Shaver, D. J. (2018). Marine threats overlap key foraging habitat for two imperiled sea turtle species in the Gulf of Mexico. Front. Mar. Sci. 5:336. doi: 10.3389/fmars.2018.00336
Hawkes, L. A., Broderick, A. C., Godfrey, M. H., and Godley, B. J. (2007). Investigating the potential impacts of climate change on a marine turtle population. Glob. Change Biol. 13, 1–10. doi: 10.1111/j.1365-2486.2006.01320.x
Hildebrand, H. H. (1981). “A historical review of the status of sea turtle populations in the western Gulf of Mexico,” in Biology and Conservation of Sea Turtles, ed. K. A. Bjorndal (Washington, D.C: Smithsonian Institution Press), 447–453.
Hirth, H. F. (1997). Synopsis of the Biological Data on the Green Turtle Chelonia mydas (Linnaeus 1758). Washington, DC: Fish and Wildlife Service.
Hughes, D. A., and Richard, J. D. (1974). The nesting of the Pacific Ridley turtle Lepidochelys olivacea on Playa Nancite. Costa Rica. Mar. Biol. 24, 97–107. doi: 10.1007/BF00389343
Jones, W. D. (1999). Padre Island National Seashore: An administrative history. Available online at: http://npshistory.com/publications/pais/adhi/index.htm (accessed March 26, 2020)
KellerLynn, K. (2010). Padre Island National Seashore: Geologic Resources Inventory Report. Ft. Collins, Colorado: National Park Service.
Kikukawa, A., Kamezaki, N., Hirate, K., and Ota, H. (1996). Distribution of nesting sites of sea turtles in Okinawajima and adjacent islands of the central Ryukyus, Japan. Chelonian Conserv. Biol. 2, 99–101.
Kikukawa, A., Kamezaki, N., and Ota, H. (1999). Factors affecting nesting beach selection by loggerhead turtles (Caretta caretta): a multiple regression approach. J. Zool. 249, 447–454. doi: 10.1111/j.1469-7998.1999.tb01214.x
Marcovaldi, M. Â, and Laurent, A. (1996). A six season study of marine turtle nesting at Praia do Forte, Bahia, Brazil, with implications for conservation and management. Chelonian Conserv. Biol. 2, 55–59.
Márquez-Millán, R. (1994). Synopsis of Biological Data on the Kemp’s ridley Turtle, Lepidochelys kempi (Garman, 1880). Miami, FL: U.S. Department of Commerce.
Maurer, A. S., and Johnson, M. W. (2017). Loggerhead nesting in the northern Gulf of Mexico: importance of beach slope to nest site selection in the Mississippi barrier islands. Chelonian Conserv. Biol. 16, 250–254. doi: 10.2744/CCB-1256.1
Mazaris, A. D., Kallimanis, A. S., Sgardelis, S. P., and Pantis, J. D. (2008). Do long-term changes in sea surface temperature at the breeding areas affect the breeding dates and reproduction performance of Mediterranean loggerhead turtles? Implications for climate change. J. Exp. Mar. Biol. Ecol. 367, 219–226. doi: 10.1016/j.jembe.2008.09.025
McDaniel, C. J., Crowder, L. B., and Priddy, J. A. (2000). Spatial dynamics of sea turtle abundance and shrimping intensity in the U.S. Gulf of Mexico. Conserv. Ecol. 4:15.
Méndez, D., Cuevas, E. A., Navarro, J., González-Garza, B. I., and Guzmán-Hernández, V. (2013). Satellite tracking of green turtle females Chelonia mydas and the evaluation of their home ranges in the north coast of the Yucatán Peninsula, Mexico. Revist. Biol. Mar. Oceanogr. 48, 497–509. doi: 10.4067/S0718-19572013000300008
Metz, T. L., and Landry, A. M. Jr. (2013). An assessment of green turtle (Chelonia mydas) stocks along the Texas coast, with emphasis on the lower Laguna Madre. Chelonian Conserv. Biol. 12, 293–302. doi: 10.2744/ccb-1046.1
Mortimer, J. A. (1990). The influence of beach sand characteristics on the nesting behavior and clutch survival of green turtles (Chelonia mydas). Copeia 1990, 802–817. doi: 10.2307/1446446
Mortimer, J. A. (1995). “Factors influencing beach selection by nesting sea turtles,” in Biology and Conservation of Sea Turtles, Revised Ed, ed. K. A. Bjorndal (Washington, D.C: Smithsonian Institution Press), 45–51.
Neck, R. W. (1978). Occurrence of marine turtles in the lower Rio Grande of south Texas (Reptilia, Testudines). J. Herpetol. 12, 422–427. doi: 10.2307/1563631
Ng, C. K. Y., Dutton, P. H., Gu, H. X., Li, T. H., Ye, M. B., Xia, Z. R., et al. (2017). Regional conservation implications of green turtle (Chelonia mydas) genetic stock composition in China. Chelonian Conserv. Biol. 16, 139–150. doi: 10.2744/CCB-1253.1
NMFS, and USFWS (2007). Green Sea Turtle (Chelonia mydas) 5-Year Review: Summary and Evaluation. Silver Spring, MD: National Marine Fisheries Service.
NMFS and USFWS (2016). Endangered and Threatened Wildlife and Plants; Final Rule to List Eleven Distinct Population Segments of the Green Sea Turtle (Chelonia mydas) as Endangered or Threatened and Revision of Current Listings Under the Endangered Species Act. Silver Spring, MD: Office of Protected Resources.
NMFS, USFWS, and SEMARNAT (2011). Bi-National Recovery Plan for the Kemp’s Ridley Sea Turtle (Lepidochelys kempii). Second Revision. Silver Spring, MD: National Marine Fisheries Service.
Owens, D. W., Grassman, M. A., and Hendrickson, J. R. (1982). The imprinting hypothesis and sea turtle reproduction. Herpetologica 38, 124–135.
Pendleton, E. A., Thieler, E. R., Williams, S. J., and Beavers, R. S. (2004). Coastal Vulnerability Assessment of Padre Island National Seashore (PAIS) to Sea-Level Rise. Woods Hole, MA: U.S. Geological Survey.
Pike, D. A. (2013). Climate influences the global distribution of sea turtle nesting. Glob. Ecol. Biogeogr. 22, 555–566. doi: 10.1111/geb.12025
Plotkin, P. T., Wicksten, M. K., and Amos, A. F. (1993). Feeding ecology of the loggerhead sea turtle Caretta caretta in the Northwestern Gulf of Mexico. Mar. Biol. 115, 1–15.
Price, J. T., Drye, B., Domangue, R. J., and Paladino, F. V. (2018). Exploring the role of artificial lighting in loggerhead turtle (Caretta caretta) nest-site selection and hatchling disorientation. Herpetol. Conserv. Biol. 13, 415–422.
Pritchard, P. C. H., and Marquez, M. R. (1973). Kemp’s Ridley Turtle or Atlantic Ridley. Lepidochelys kempi. IUCN Monograph No. 2: Marine Turtle Series. Gland: International Union for Conservation of Nature.
Purvin, C. M., Villalba-Guerra, M. R., Frandsen, H. R., and Shaver, D. J. (2020). Chelonia mydas (Green Sea Turtle). Incidental Capture. Herpetol. Rev. 51, 311–312.
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rabon, D. R. Jr., Johnson, S. A., Boettcher, R., Dodd, M., Lyons, M., Murphy, S., et al. (2003). Confirmed leatherback turtle (Dermochelys coriacea) nests from North Carolina, with a summary of leatherback nesting activities north of Florida. Mar. Turtle Newsl. 101, 4–8.
Salleh, S. M., Nishizawa, H., Ishihara, T., Sah, S. A. M., and Chowdhury, A. J. K. (2018). Importance of sand particle size and temperature for nesting success of green turtles in Penang Island, Malaysia. Chelonian Conserv. Biol. 17, 116–122. doi: 10.2744/CCB-1266.1
Salmon, M., Reiners, R., Lavin, C., and Wyneken, J. (1995). Behavior of loggerhead sea turtles on an urban beach. I. correlates of nest placement. J. Herpetol. 29, 560–567. doi: 10.2307/1564739
Salmon, M., and Witherington, B. E. (1995). Artificial lighting and seafinding by loggerhead hatchlings: evidence for lunar modulation. Copeia 1995, 931–938. doi: 10.2307/1447042
Seminoff, J. A., Allen, C. D., Balazs, G. H., Dutton, P. H., Eguchi, T., Haas, H. L., et al. (2015). Status Review of the Green Turtle (Chelonia mydas) Under the U.S. Endangered Species Act. La Jolla, CA: National Marine Fisheries Service.
Shamblin, B. M., Bagley, D. A., Ehrhart, L. M., Desjardin, N. A., Martin, R. E., Hart, K. M., et al. (2015). Genetic structure of Florida green turtle rookeries as indicated by mitochondrial DNA control region sequences. Conserv. Genet. 16, 673–685. doi: 10.1007/s10592-014-0692-y
Shamblin, B. M., Dutton, P. H., Shaver, D. J., Bagley, D. A., Putman, N. F., Mansfield, K. L., et al. (2017). Mexican origins for the Texas green turtle foraging aggregation: a cautionary tale of incomplete baselines and poor marker resolution. J. Exp. Mar. Biol. Ecol. 488, 111–120. doi: 10.1016/j.jembe.2016.11.009
Shamblin, B. M., Godfrey, M. H., Pate, S. M., Thompson, W. P., Sutton, H., Altman, J., et al. (2018). Green turtles nesting at their northern range limit in the United States represent a distinct subpopulation. Chelonian Conserv. Biol. 17:314. doi: 10.2744/CCB-1332.1
Shamblin, B. M., Hart, K. M., Martin, K. J., Ceriani, S. A., Bagley, D. A., Mansfield, K. L., et al. (2020). Green turtle mitochondrial microsatellites indicate finer-scale natal homing to isolated islands than to continental nesting sites. Mar. Ecol. Prog. Ser. 643, 159–171. doi: 10.3354/meps13348
Shaver, D. J. (2005). Analysis of the Kemp’s ridley imprinting and headstart project at Padre Island National Seashore, Texas, 1978–88, with subsequent nesting and stranding records on the Texas coast. Chelonian Conserv. Biol. 4, 846–859.
Shaver, D. J., and Caillouet, C. W. Jr. (2015). Reintroduction of Kemp’s Ridley (Lepidochelys kempii) sea turtle to Padre Island National Seashore, Texas and its connection to head-starting. Herpetol. Conserv. Biol. 10, 378–435.
Shaver, D. J., and Frandsen, H. R. (2019). Eretmochelys imbricata (Hawksbill Sea Turtle). Nesting. Herpetol. Rev. 50, 350–351.
Shaver, D. J., Frandsen, H. R., and Walker, J. S. (2019a). Dermochelys coriacea (Leatherback Sea Turtle). Nesting. Herpetol. Rev. 50:350.
Shaver, D. J., Frandsen, H. R., and Walker, J. S. (2020a). Lepidochelys kempii (Kemp’s Ridley Sea Turtle). Predation. Herpetol. Rev. 51, 110–111.
Shaver, D. J., Frandsen, H. R., Walker, J. S., George, J. A., and Mirón, R. J. G. D. (2020b). Chelonia mydas (Green Sea Turtle). Bi-national recaptures. Herpetol. Rev. 51, 310–311.
Shaver, D. J., Frandsen, H. R., Walker, J. S., Montello, M. A., Thurman, S., Nairn, C. J., et al. (2019b). Chelonia mydas (Green Sea Turtle). Nesting behavior. Herpetol. Rev. 50, 555–556.
Shaver, D. J., Hart, K. M., Fujisaki, I., Bucklin, D., Iverson, A. R., Rubio, C., et al. (2017a). Inter-nesting movements and habitat-use of adult female Kemp’s ridley turtles in the Gulf of Mexico. PLoS One 12:e0174248. doi: 10.1371/journal.pone.0174248
Shaver, D. J., Hart, K. M., Fujisaki, I., Rubio, C., and Sartain, A. R. (2013a). “Movement mysteries unveiled: spatial ecology of juvenile green sea turtles,” in Reptiles in Research, ed. W. I. Lutterschmidt (Hauppauge, NY: Nova Science Publishers, Inc), 465–484.
Shaver, D. J., Hart, K. M., Fujisaki, I., Rubio, C., Sartain, A. R., Peña, J., et al. (2013b). Foraging area fidelity for Kemp’s ridleys in the Gulf of Mexico. Ecol. Evol. 3, 2002–2012. doi: 10.1002/ece3.594
Shaver, D. J., Hart, K. M., Fujisaki, I., Rubio, C., Sartain-Iverson, A. R., Peña, J., et al. (2016a). Migratory corridors of adult female Kemp’s ridley turtles in the Gulf of Mexico. Biol. Conserv. 194, 158–167. doi: 10.1016/j.biocon.2015.12.014
Shaver, D. J., Rubio, C., Walker, J. S., George, J., Amos, A. F., Reich, K. J., et al. (2016b). Kemp’s ridley sea turtle (Lepidochelys kempii) nesting on the Texas coast: geographic, temporal, and demographic trends through 2014. Gulf of Mexico. Science 33, 158–178. doi: 10.18785/goms.3302.04
Shaver, D. J., Schroeder, B. A., Byles, R. A., Burchfield, P. M., Peña, J., Márquez, R., et al. (2005). Movements and home ranges of adult male Kemp’s ridley sea turtles (Lepidochelys kempii) in the Gulf of Mexico investigated by satellite telemetry. Chelonian Conserv. Biol. 4, 817–827.
Shaver, D. J., Tissot, P. E., Streich, M. M., Walker, J. S., Rubio, C., Amos, A. F., et al. (2017b). Hypothermic stunning of green sea turtles in a western Gulf of Mexico foraging habitat. PLoS One 12:e0173920. doi: 10.1371/journal.pone.0173920
Steinitz, M. J., Salmon, M., and Wyneken, J. (1998). Beach renourishment and loggerhead turtle reproduction: a seven year study at Jupiter Island, Florida. J. Coast. Res. 14, 1000–1013.
Teas, W. G. (1993). Species Composition and Size Class Distribution of Marine Turtle Strandings on the Gulf of Mexico and Southeast United States Coasts, 1985-1991. Miami, FL: National Marine Fisheries Service.
Tzeek Tuz, M., Herrera Pavón, R., Quintana Pali, G. P., Barragán Zepeda, A., and Gómez Nieto, L. (2019). “Capítulo 1. programa de conservación de tortugas marinas riviera maya-tulum: resultado de más de tres décadas de protección,” in El Uso del Conocimiento de las Tortugas Marinas Como Herramienta Para la Restauración de sus Poblaciones y Hábitats Asociados México, eds E. A. Cuevas Flores, V. Guzmán Hernández, J. J. Guerra Santos, and G. A. Rivas Hernández (Campeche: Universidad Autónoma del Carmen), 27–34.
USDA NRCS, and NPS (2005). Soil survey of Padre Island National Seashore, Texas, Special Report. Washington, DC: National Cooperative Soil Survey.
Wallace, B. P., DiMatteo, A. D., Hurley, B. J., Finkbeiner, E. M., Bolten, A. B., Chaloupka, M. Y., et al. (2010). Regional management units for marine turtles: a novel framework for prioritizing conservation and research across multiple scales. PLoS One 5:e15465. doi: 10.1371/journal.pone.0015465
Weise, B. R., and White, W. A. (1980). Padre Island National Seashore: A Guide to the Geology, Natural Environments, and History of a Texas Barrier Island (Guidebook 17). Austin, TX: University of Texas.
Weishampel, J. F., Bagley, D. A., and Ehrhart, L. M. (2006). Intra-annual loggerhead and green turtle spatial nesting patterns. Southeast. Natural. 5, 453–462. doi: 10.1656/1528-7092(2006)5[453:ilagts]2.0.co;2
Weishampel, J. F., Bagley, D. A., Ehrhart, L. M., and Rodenbeck, B. L. (2003). Spatiotemporal patterns of annual sea turtle nesting behaviors along an east central Florida beach. Biol. Conserv. 110, 295–303. doi: 10.1016/S0006-3207(02)00232-X
Weishampel, J. F., Bagley, D. A., Ehrhart, L. M., and Weishampel, A. C. (2010). Nesting phenologies of two sympatric sea turtle species related to sea surface temperatures. Endang. Spec. Res. 12, 41–47. doi: 10.3354/esr00290
Weishampel, Z. A., Cheng, W.-H., and Weishampel, J. F. (2016). Sea turtle nesting patterns in Florida vis-à-vis satellite-derived measures of artificial lighting. Rem. Sens. Ecol. Conserv. 2, 59–72. doi: 10.1002/rse2.12
Whitmore, C. P., and Dutton, P. H. (1985). Infertility, embryonic mortality and nest-site selection in leatherback and green sea turtles in Suriname. Biol. Conserv. 34, 251–272. doi: 10.1016/0006-3207(85)90095-3
Witherington, B. E. (1992). Behavioral responses of nesting sea turtles to artificial lighting. Herpetologica 48, 31–39.
Witherington, B. E., Kubilis, P., Brost, B., and Meylan, A. B. (2009). Decreasing annual nest counts in a globally important loggerhead sea turtle population. Ecol. Appl. 19, 30–54. doi: 10.1890/08-0434.1
Witzell, W. N. (1994a). The origin, evolution, and demise of the U.S. sea turtle fisheries. Mar. Fish. Rev. 56, 8–23.
Witzell, W. N. (1994b). The U.S. Commercial Sea Turtle Landings. Miami, FL: National Marine Fisheries Service.
Worth, D. F., and Smith, J. B. (1976). Marine turtle nesting on Hutchinson Island, Florida, in 1973. Florida Mar. Res. Public. 18, 1–17.
Keywords: conservation, endangered species, texas, hatching success, spatial trends
Citation: Shaver DJ, Frandsen HR, George JA and Gredzens C (2020) Green Turtle (Chelonia mydas) Nesting Underscores the Importance of Protected Areas in the Northwestern Gulf of Mexico. Front. Mar. Sci. 7:673. doi: 10.3389/fmars.2020.00673
Received: 27 March 2020; Accepted: 23 July 2020;
Published: 11 August 2020.
Edited by:
Lisa Marie Komoroske, University of Massachusetts Amherst, United StatesReviewed by:
Luis Cardona, University of Barcelona, SpainAntonios D. Mazaris, Aristotle University of Thessaloniki, Greece
Copyright © 2020 Shaver, Frandsen, George and Gredzens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donna J. Shaver, donna_shaver@nps.gov
 Donna J. Shaver
Donna J. Shaver Hilary R. Frandsen
Hilary R. Frandsen Jeffrey A. George2
Jeffrey A. George2 Christian Gredzens
Christian Gredzens