- 1Dakshin Foundation, Bengaluru, India
- 2Department of Zoology, Division of Mathematical, Physical and Life Sciences, University of Oxford, Oxford, United Kingdom
- 3Centre for Ecological Sciences, Indian Institute of Science, Bengaluru, India
Bycatch poses a significant threat to marine megafauna, such as elasmobranchs. India has one of the highest elasmobranch landings globally, through both targeted catch and bycatch. As elasmobranchs contribute to food and livelihood security, there is a need for holistic approaches to bycatch mitigation. We adopt an interdisciplinary approach to critically assess a range of hypothetical measures for reducing elasmobranch capture in a trawler fishery on India’s west coast, using a risk-based mitigation hierarchy framework. Data were collected through landing surveys, interviews and a literature review, to assess the following potential management options for their technical effectiveness and socio-economic feasibility: (1) spatio-temporal closures; (2) net restrictions; (3) bycatch reduction devices (BRDs); and (4) live onboard release. Our study provides the first evidence-based and nuanced understanding of elasmobranch bycatch management for this fishery, and suggestions for future conservation and research efforts. Onboard release may be viable for species like guitarfish, with moderate chances of survival, and was the favored option among interview respondents due to minimal impact on earnings. While closures, net restrictions and BRDs may reduce elasmobranch capture, implementation will be challenging under present circumstances due to the potentially high impact on fisher income. Interventions for live release can therefore be used as a step toward ameliorating bycatch, while initiating longer-term engagement with the fishing community. Participatory monitoring can help address critical knowledge gaps in elasmobranch ecology. Spatio-temporal closures and gear restriction measures may then be developed through a bottom-up approach in the long term. Overall, the framework facilitated a holistic assessment of bycatch management to guide decision-making. Scaling-up and integrating such case studies across different species, fisheries and sites would support the formulation of a meaningful management plan for elasmobranch fisheries in India.
Introduction
Fisheries constitute one of the biggest pressures on oceans today, due to their impact on marine habitats, overexploitation of fish stocks and bycatch of non-target species (Dayton et al., 1995; Myers et al., 1997; Davies et al., 2009). Bycatch threatens marine megafauna, fish and invertebrates through capture in non-selective fishing gear (Alverson et al., 1994; Hall et al., 2000). At least 20 million endangered, threatened and protected marine animals are estimated to be caught as bycatch annually (Pérez Roda et al., 2019). Traditionally discarded, bycatch is increasingly retained and sold due to dwindling catches of target species and rising demand for seafood products (Kelleher, 2005). As such, bycaught species contribute significantly to livelihood stability and food security in fishery-dependent developing nations like India (Lobo, 2007; Gupta et al., 2019). Given the socio-economic importance of bycatch and the vulnerability of many bycaught species, it is imperative to regulate and manage this complex dimension of fisheries.
Elasmobranchs (sharks and rays) are a highly threatened species group (Dulvy et al., 2014) with more than half of global fishing mortality attributed to bycatch (Stevens et al., 2000). Due to their slow growth, late maturity and low fecundity, elasmobranchs are highly susceptible to fishing pressure, with a limited capacity to recover from overexploitation (Bonfil, 1994). Elasmobranchs play important roles in marine ecosystems as top and meso-predators, and provide socio-economic value to coastal communities through fisheries and tourism, making their conservation a top priority (Ferretti et al., 2010; Gallagher and Hammerschlag, 2011; Dent and Clarke, 2015).
India is among the top three elasmobranch fishing nations in the world (Dent and Clarke, 2015). While artisanal fishers in India have practiced targeted shark fishing since at least the early 1900s (Fernando et al., 2017), the advent of mechanized fishing for shrimp and high value finfish has led to increases in total elasmobranch capture in bycatch (Kizhakudan et al., 2015). Though many elasmobranchs landed in India today are caught as bycatch (Kizhakudan et al., 2015), they are seldom discarded as their meat forms a cheap and widely consumed protein source (Dulvy et al., 2017; Jabado et al., 2018). Therefore, domestic elasmobranch meat consumption may be a major driver of their fishing pressure in India (Karnad et al., 2019). Although we use the term bycatch here, we emphasize that these species are retained, and have some commercial and socio-economic importance.
Landings of elasmobranchs have declined in India in recent decades, from 75,262 tons in 1998 (CMFRI, 1999) to 42,117 tons in 2018 (CMFRI, 2019). This reduction is despite increasing fishing effort, which suggests that elasmobranch populations are overexploited (Kizhakudan et al., 2015), and corresponds with global trends (Davidson et al., 2016). With over half the elasmobranch species in the Arabian Sea region assessed as threatened (Jabado et al., 2018), there is an urgent need for improved management of fisheries that impact these species. While India has imposed a ban on shark fin trade and protected ten species under the Wildlife (Protection) Act, 1972 (Kizhakudan et al., 2015), these regulations are hampered by limited capacity for monitoring and enforcement. Furthermore, with incidental catch being a major issue, such regulations likely have limited success in reducing fishing mortality. They need to be accompanied by practical measures to reduce capture of priority species at the fishery level (Booth et al., 2019a).
The complex issue of elasmobranch bycatch leads to trade-offs between elasmobranch conservation and livelihoods of fishers (Booth et al., 2019a). Trawlers, in particular, have high levels of elasmobranch catch (Kizhakudan et al., 2015). Trawling in India is increasingly driven by exports of shrimp and other high value species, as well as high demand for fishmeal (Bhathal, 2005; Gupta et al., 2019). This is producing a biomass-based fishery with trawlers frequently fishing in shallow inshore waters with small mesh sizes to catch large volumes of fish (Kumar and Deepthi, 2006). Coastal elasmobranchs are collateral damage in this complex, multispecies trawl fishery, and form a small percentage of the total catch (Kizhakudan et al., 2015). However, conservation measures for elasmobranchs are likely to impact catches of high-value species, and hence reduce earnings of fishers. Given that there are 3.8 million active fishers in India (Department of Fisheries, Ministry of Fisheries, Animal Husbandry and Dairying, 2019); it is critical to develop shark management strategies that are science-based, economically viable and socially just.
The mitigation hierarchy is a framework for preventing and compensating for the negative impacts of development projects on biodiversity (BBOP, 2012). It has recently been proposed as a framework for mitigation of fisheries bycatch (Milner-Gulland et al., 2018), and follows four sequential steps: (1) avoidance of bycatch, e.g., through fisheries closures, (2) minimization of fisheries impacts, e.g., through gear modifications, (3) remediation of bycaught species, e.g., through live release protocols, and (4) offsetting of the residual impact through conservation measures elsewhere (Squires et al., 2018). The framework assembles a range of mitigation measures under each step, and assesses their effectiveness in meeting a quantitative bycatch reduction target (Milner-Gulland et al., 2018). It aims to balance conservation with economic development, by facilitating the sustainable use of natural resources with minimal or no net loss of biodiversity (Arlidge et al., 2018). Booth et al. (2019b) expanded and adapted the mitigation hierarchy for shark fisheries management. They provide a risk-based framework which integrates biological and operational aspects of species and fisheries with socio-economic context to manage potential trade-offs between conservation objectives and human needs. Set within the overarching framework of the mitigation hierarchy, this approach can be applied to develop holistic, context-specific and adaptive measures for shark fisheries management.
We used the mitigation hierarchy for sharks (Booth et al., 2019b) to assess options for shark and ray catch mitigation in an Indian trawl fishery. Our study was conducted at Malvan, a fishing town with a coastal, mixed species fishery, making this the first practical application of such an approach to managing elasmobranch bycatch for a data-limited, fisheries-dependent site. Our specific aims were to: (1) evaluate reduction measures for elasmobranch bycatch in the Malvan trawl fishery using the mitigation hierarchy framework, and (2) assess the applicability of this framework for bycatch reduction in a multi-species fishery with a complex socio-economic context.
Following the process outlined in Booth et al. (2019b) we first present an overview of elasmobranch fisheries at the study site and risk factors to the study species. We then propose different options for bycatch mitigation and assess them in terms of their technical effectiveness and socio-economic feasibility. Finally, we discuss the outcomes of the framework and its applicability as a decision-making tool for bycatch management, and propose recommendations for interventions and further research. We do not intend for this to be a complete assessment of management options; rather, we aim to initiate structured and interdisciplinary thinking for elasmobranch bycatch mitigation in India, identify data gaps and highlight potential management solutions going forward.
Materials and Methods
Study Site and Fishery
Malvan is a region on the west coast of India (16.052027°N, 73.468247°E; Figure 1). Its coastline is interspersed with a range of marine habitats including estuaries, mangrove forests, coral outcrops and a shallow shelf ranging from about 20 to 30 m in depth to about 20 km offshore (UNDP, 2013). This shallow shelf forms a habitat for many marine species, as well as highly productive fishing grounds. Malvan’s waters also host the Malvan Marine Sanctuary, one of India’s marine protected areas (Sundaramoorthy et al., 2001). However, while the Marine Sanctuary has been designated in 1987, it is not functional as it is yet to be implemented on ground (UNDP, 2013).
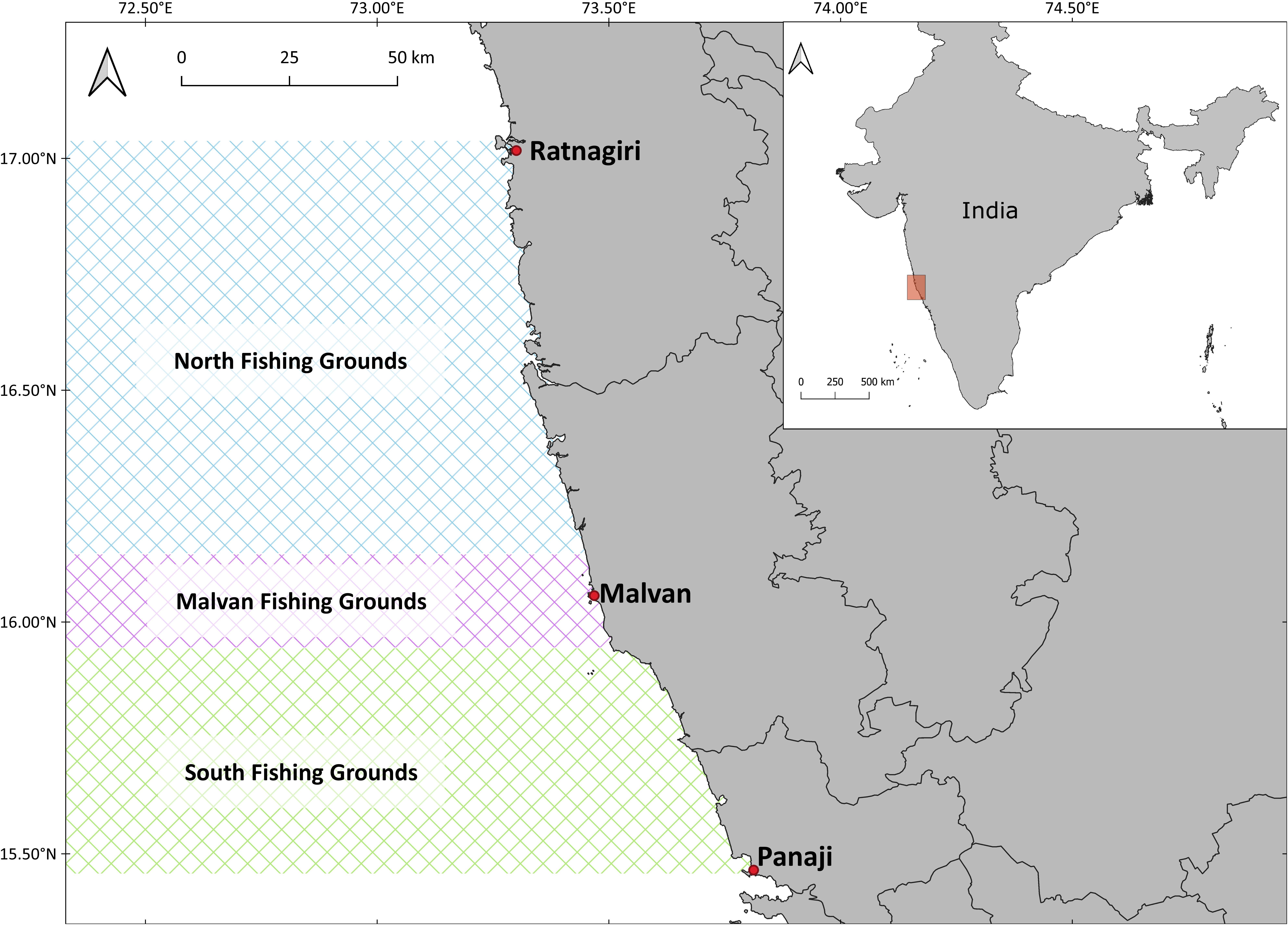
Figure 1. The study site and spatial extent of the fishery, showing the different fishing grounds categorized by this study (north, Malvan, and south). The fishing grounds delimited here represent the latitudinal extend of the fishing areas only, the distances traveled offshore are unknown. Black lines in the map represent the borders of each district, while the red dots represent the main fishing towns in this region.
There are 22 fishing villages in the greater Malvan region (i.e., the Malvan Taluka), with 10,635 resident fishers as well as a significant population of migrant fishers (CMFRI, 2012). This region is home to diverse fisheries, with 80–100 trawlers, at least 600 gillnet boats, and some artisanal fisheries, including those using shore seines. Our study was based at the main town in this region, also known as Malvan. All fishing boats, owned by fishers from different villages throughout the Malvan region, land and sell their catch at this site. Trawlers in Malvan constitute a multi-species fishery that target a range of species: prawn, crabs, and demersal fish using a benthic net (i.e., bottom trawl net) or pomfret (Pampus sp.), mackerel (Rastrelliger kanagurta) and other pelagic fish using a pelagic net (i.e., mid-water trawl net). Trawl fishing takes place between August to May, with a mandatory seasonal ban imposed by the government during June and July to protect spawning fish (Narayanakumar et al., 2017). Trawlers operate across the region, from Panaji in Goa State in the south to Ratnagiri in the north (approx. 180km; Figure 1). They are relatively small-sized (100–140 HP, 40–55 feet vessel length), fishing nearshore within a depth of 100m. Fishing trips typically last 1–5 days. Elasmobranchs are frequently captured as non-target or secondary catch, particularly in trawlers, but also in gillnets and artisanal fisheries. Most elasmobranch catch is retained and sold for meat, which is salted, dried and consumed within the region. Elasmobranchs are generally considered low-value products, with sharks relatively more profitable than rays.
Process
We adopted the risk-based mitigation hierarchy for elasmobranchs developed by Booth et al. (2019b) for this study (Table 1). The process involves understanding the fishery and assessing the risk to the species of concern; developing mitigation measures for incidental catch under the 4 mitigation hierarchy steps; and assessment of these measures in terms of technical and socio-economic feasibility. We used a mixed-methods approach to collect data for this process (section “Data Collection”), which were analyzed and assessed to populate the framework and identify management measures for elasmobranchs (section “Analysis and Assessment”).
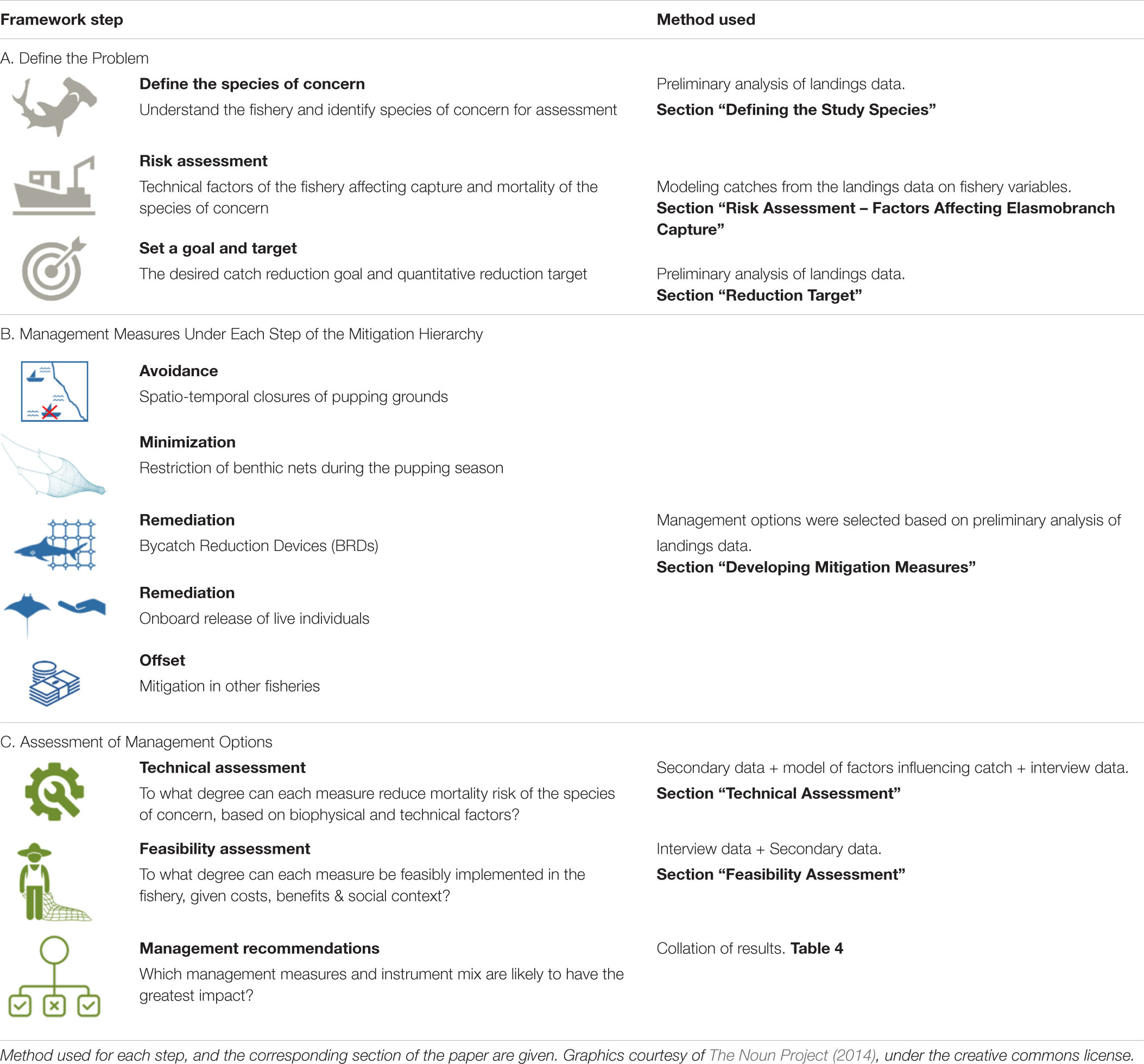
Table 1. The risk-based mitigation hierarchy framework for elasmobranchs adapted from Booth et al. (2019b).
A combination of landing site surveys and interviews were used to collect primary data for the framework. In addition, secondary data were used in the assessment of mitigation measures where no primary data were available (explained in section “Technical Assessment”).
Data Collection
Trawler Landing Surveys
To understand the biological and operational aspects of the fishery, elasmobranch landings from trawlers were sampled over two seasons: March–May 2018 and October 2018–May 2019. Sampling was conducted at the Malvan landing center 3 days per week on alternate days, starting on different days to avoid any bias in sampling the same 3 days. Every boat that had landed any elasmobranchs was sampled. Biological data on the elasmobranchs (species, abundance, size, and sex) were recorded, and operational data on the fishing trip (effort, fishing location, depth, and gear) were collected through informal interviews with the fishers. Some captured elasmobranchs, particularly juveniles, are discarded at sea. We were not able to estimate these discards, and our data are therefore restricted and potentially biased to landed elasmobranchs only. We also acknowledge that our sampling was conducted over a relatively short time period; however, based on informal discussions with key informants, we believe it to be representative of the present fishery scenario in Malvan.
Interviews
To supplement the biological data and understand the socio-economic context of the fishery, interviews were conducted with fishing community stakeholders (fishers and trawler owners). Owners (n = 11) were selected at the landing center through convenience sampling, and represented about 20–25% of the trawlers in Malvan. Fishers (n = 7, two of whom were also boat owners) were selected through purposive sampling, as we intended to interview key informants with in-depth knowledge about elasmobranchs. Although our sample size of fishers was small, we believe it was sufficient as saturation was reached in terms of the information provided.
We used a semi-structured questionnaire in our interviews (Milner-Gulland and Rowcliffe, 2007), with the following sections: (1) background information, fishing experience and behavior; (2) catches and trends in sharks and rays (fishers only); (3) costs and revenues for an average fishing trip (owners only); and (4) opinions of and preferences for the proposed mitigation measures for elasmobranch catch (Supplementary Table 1 and Supplementary Figure 1). Respondents were asked to quantify their opinions about the impact of each mitigation measure on elasmobranchs, on their target species, and on their profits using a Likert scale (Likert, 1932). The scale ranged from −2: More than a 50% decrease, −1: Up to a 50% decrease, 0: No impact, +1: Up to a 50% increase, +2: More than a 50% increase. Interpretation of the Likert scale responses was carried out with some caution to avoid any potential bias arising from the respondents’ understanding of the scale.
Analysis and Assessment
Defining the Study Species
Multiple elasmobranch species are caught and landed by trawlers in Malvan. Given the lack of species-specific data and their similar economic values, this study first considers elasmobranchs in two broad groups of sharks and rays to assess management measures, particularly from an economic and feasibility perspective. However, elasmobranchs are highly diverse in their biological and ecological characteristics, and the same strategy will not fit all species (Dulvy et al., 2017). Therefore, we also focus on a few priority taxa (IUCN, 2019); scalloped hammerhead sharks (Sphyrna lewini), sharpnose guitarfish (Glaucostegus granulatus), and widenose guitarfish (Glaucostegus obtusus). These were chosen due to their threatened status and vulnerability to fishing. S. lewini is a long-lived species with late maturity, slow growth (Miller et al., 2013; Zacharia et al., 2017) and low intrinsic potential to recover from fishing pressure (Smith et al., 1998). It has a higher risk of capture in fishing gear due to the unique shape of its head and the aggregating behavior of juveniles in nearshore waters (Gallagher et al., 2014). S. lewini is listed as Critically Endangered by the International Union for Conservation of Nature (IUCN; Rigby et al., 2019a), and previous studies have noted an apparent decline of this species in Malvan (Karnad et al., 2019). Giant guitarfish (Glaucostegidae) have relatively high population productivities with moderate recovery potential if fishing mortality is kept low (D’Alberto et al., 2019). However, due to the high levels of exploitation throughout their range, both study species (G. granulatus and G. obtusus) have recently been listed as Critically Endangered (Compagno and Marshall, 2019; Kyne and Jabado, 2019), with a need for urgent global action for their conservation and recovery (Kyne et al., 2019).
Due to small sample sizes and their similar biology, we grouped the two guitarfish species for analysis (hereafter guitarfish). We use the term “hammerhead” to refer to the scalloped hammerhead shark, “sharks” to all shark species (i.e., Selachimorpha – including scalloped hammerhead sharks) surveyed at the study site, and “rays” refers to all ray species (i.e., Batoidea – including guitarfish).
Risk Assessment – Factors Affecting Elasmobranch Capture
We first evaluated factors affecting the number of sharks and rays captured in trawlers, by modelling catches from the landings data against a number of operational fishing variables and their interactions (Table 2; see Figure 1 for the locations of the fishing grounds). We used a lognormal linear mixed model (package: lmerTest, Kuznetsova et al., 2017), where the response variable was log-transformed to meet model assumptions. The best fitting model was selected using AIC model selection. Following this, differences in catches associated with the explanatory variables retained in this best fitting model were assessed, using t-tests within the lmerTest program. For the categorical explanatory variables, the coefficients and p-values were calculated with respect to a reference category – for location: south, for gear: benthic, for depth: shallow and for season: pre-monsoon. For example, p-values for the north and Malvan fishing grounds presented in the results are each in comparison to the south fishing grounds. These models were separately constructed for sharks and rays.
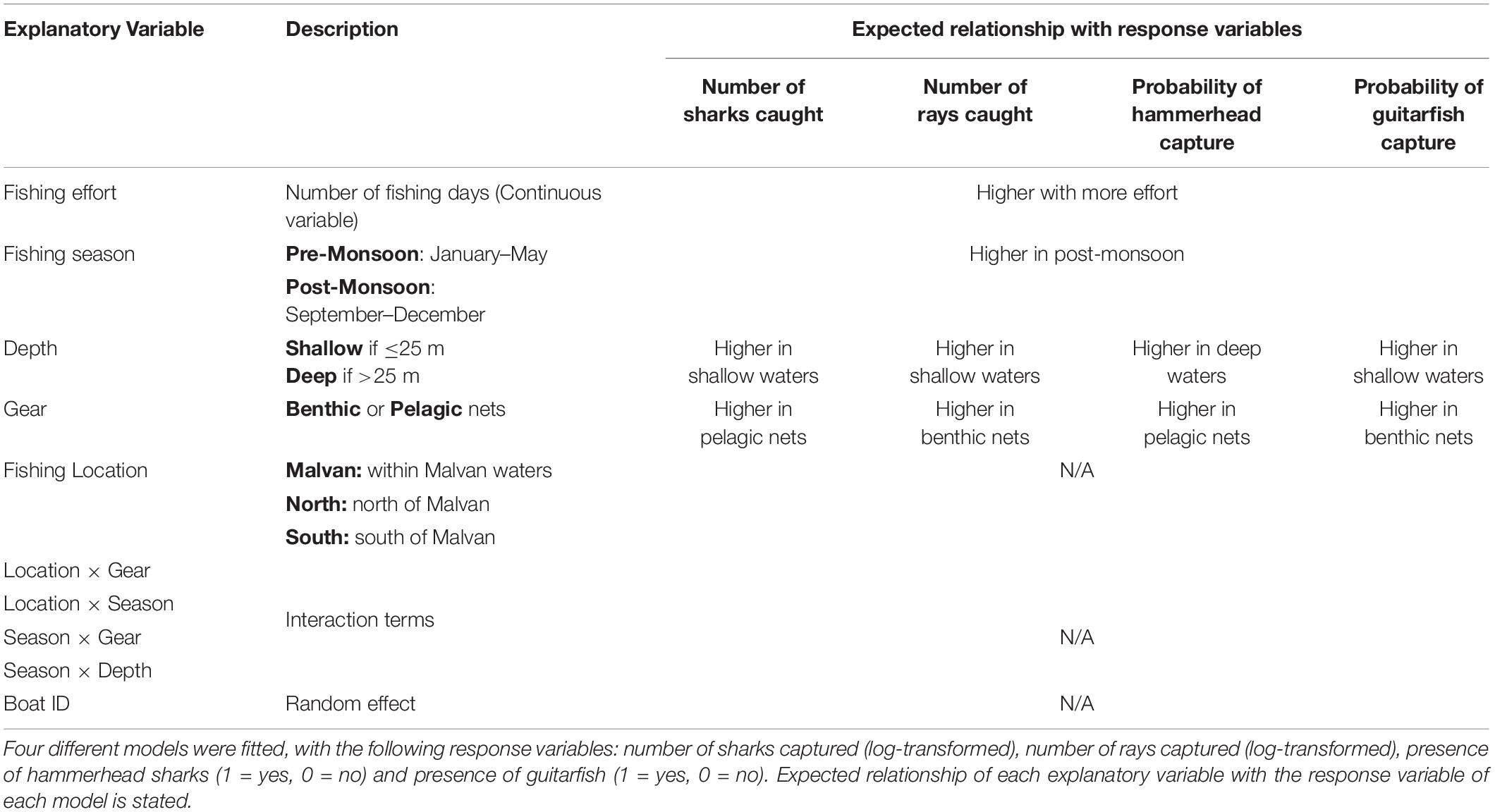
Table 2. The different fishing factors and interactions used as explanatory variables in the models.
We then evaluated factors influencing capture of hammerheads and guitarfish. We created a binary response variable for whether these species had been captured in each of the fishing trips sampled (1 = yes, 0 = no). As we had only sampled trawlers that had captured elasmobranchs, this gave us the probability of capturing a hammerhead or a guitarfish, given that an elasmobranch had been captured. We fitted a generalized linear mixed model (GLMM; package: lme4, Bates et al., 2015) with a binomial logit distribution to this binary response variable, with AIC model selection. The same explanatory variables as before were used (Table 2), and coefficients and p-values calculated similarly. The models were constructed separately for hammerheads and guitarfish. All analyses were conducted in RStudio version 1.1.463 (R Core Team, 2014; RStudio Team, 2015).
Reduction Target
The mitigation hierarchy calls for defining a goal in terms of a desired change in biodiversity, accompanied by a quantitative catch reduction target against which the mitigation measures can be evaluated. The target can be defined using a metric such as population growth rate or Potential Biological Removal (PBR) threshold (Milner-Gulland et al., 2018).
We defined the overarching conservation goal for this study as the minimization of incidental catch of the study species within the socio-economic constraints of the study site. However, we were unable to specify a quantitative reduction target due to the data-limited nature of the site and species. We instead set a relative target, which is a reduction in the number of animals caught of the study species as compared to current catch rates, which is more realistic in the present scenario.
Developing Mitigation Measures
A number of potential bycatch reduction measures can be categorized under each step of the framework (Milner-Gulland et al., 2018; Booth et al., 2019b). Using a preliminary analysis of the landings data, as well as an understanding of the logistics and socio-economic context of the study site, we proposed the following potential management measures for assessment:
Avoidance: spatio-temporal closures of pupping or nursery grounds
Based on pilot surveys, the southern fishing ground (Figure 1) was identified as a possible nursery ground with high catches of juvenile sharks and rays in the post-monsoon season. We therefore proposed a closure of these grounds for 2 months from October to November, which may be the pupping season (when elasmobranchs give birth to their young) for many species. However, due to lack of data we did not define the exact spatial extent of the closure area.
Minimization: restriction of benthic net use during the pupping season
Pilot interviews and landing site surveys found a higher catch of juvenile elasmobranchs in benthic nets as compared to pelagic nets. We proposed a restriction in the use of benthic nets for trawlers during the same time period (October–November) to minimize elasmobranch catch.
Remediation: bycatch reduction devices (BRDs)
Based on studies elsewhere, and trials being undertaken in India, we proposed the use of BRDs such as turtle excluder devices (TEDs) and other similar designs with escape panels as the third option to reduce mortality of elasmobranchs as a result of bycatch.
Remediation: onboard release of live individuals
We proposed the safe handling and release of all captured elasmobranchs, if alive, onboard the trawler to reduce mortality.
Offset: mitigating elasmobranch catch offsite
We proposed to mitigate elasmobranch mortality through improving management measures in other fisheries in the region that are likely to target the same populations as Malvan.
Technical Assessment
The hypothetical effectiveness of the proposed mitigation measures was assessed through a combination of primary and secondary data. For avoidance and minimization, model coefficient values from 2.4.2 were used to evaluate whether changes in particular operational fishing variables would have an impact on elasmobranch catches. This was supplemented with fisher perceptions of the impact of the proposed measures on the populations of elasmobranchs, and of their target fish species, using the Likert scale described in section “Interviews.”
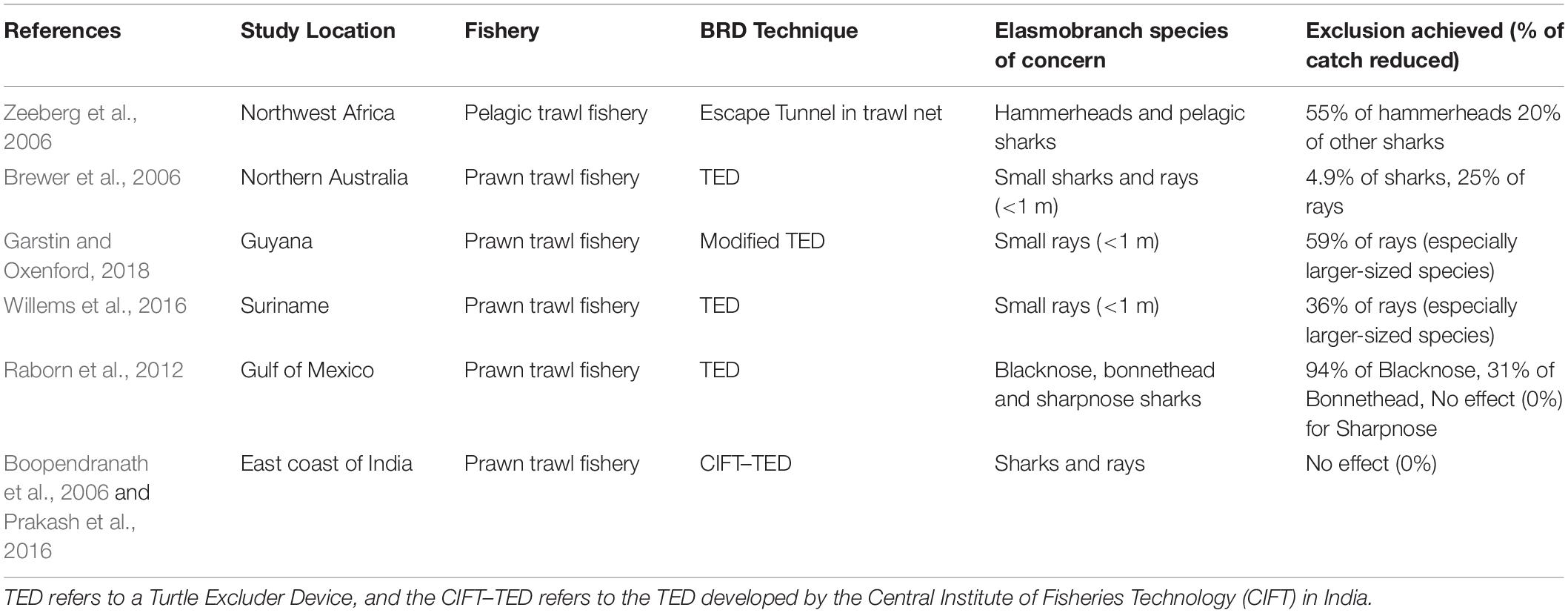
Due to a lack of data on the effectiveness of BRDs and live release for the study species in Malvan trawlers, we assessed the hypothetical impacts of these two measures using secondary data from previous studies in tropical trawl fisheries. We used Google Scholar to search for studies that have assessed the impact of BRDs and live release on bycatch rates and survival of the study species. Search terms included “bycatch reduction device,” “brd,” “turtle excluder device,” “ted,” “fishing mortality,” “post-capture survival” or “release” combined with “elasmobranch,” “shark,” “ray,” “hammerhead,” or “guitarfish.” We found a total of 10 relevant studies to help infer the effectiveness of these measures.
Feasibility Assessment
The feasibility of each mitigation measure was assessed using perceptions of boat owners, who quantified the potential impact of each measure on their income using a Likert scale. All respondents (fishers and boat owners) were also asked for their overall opinion of each mitigation measure, and to select their most preferred option. Feasibility of the measures and compliance of the fishing community were also discussed. The qualitative responses obtained for these sections were noted and used to understand why each measure would or would not work.
Permits and Ethics
No permits were required for landing site surveys. Ethics clearance for the interviews was obtained through an institutional ethic committee review (Reference number: DF_Ethics committee_HS_2019_May_01). The interviews were conducted and voice recorded only after obtaining informed verbal consent from the participants, and assuring them that they could omit questions or end the interview at any stage. All interview data were kept confidential and anonymous.
Results
Risk Assessment
We sampled a total of 985 fishing trips over the two sampling seasons. November and December were the peak months for shark capture (Table 3). Hammerhead sharks were captured only between November and January, and all recorded individuals were juveniles. Catch rates of rays were more consistent throughout the year, with November being the peak month. The two guitarfish species were sporadically captured in low numbers throughout the year (Table 3). In general, most of the sharks and rays captured in trawlers were <1 m in size (Table 3), as they were composed of small-sized coastal species and juveniles of larger species like hammerheads. Adults of large ray species were infrequently captured, whereas those of larger sharks were never captured by trawlers.
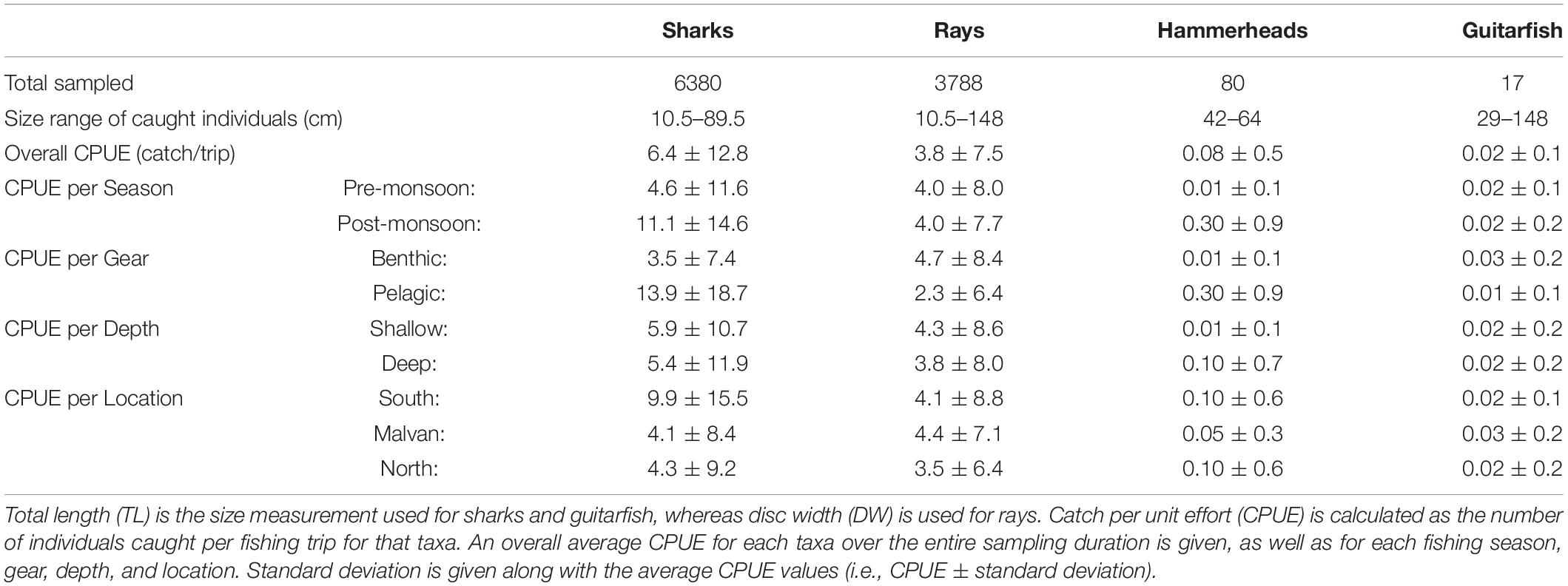
Table 3. Summary of the elasmobranch landings data, given for sharks, rays, hammerheads and guitarfish.
On modeling factors affecting elasmobranch capture, the number of sharks captured was found to be strongly influenced by season, with the post-monsoon having significantly higher catches (p < 0.001). To disentangle the effect of season from the effects of the other fishing variables shark catches were separately modeled for each season. For the post-monsoon season, fishing location was the only significant variable, with the south fishing grounds having higher captures of sharks as compared to Malvan (p < 0.001) and the north grounds (p = 0.01). For the pre-monsoon season several variables were found to be significant. Pelagic nets had higher shark catches than benthic nets (p < 0.001), and higher fishing effort was linked to higher catches (p < 0.001). Deep waters had slightly higher shark captures than shallow waters (p = 0.03; Figure 2 and Supplementary Table 2).
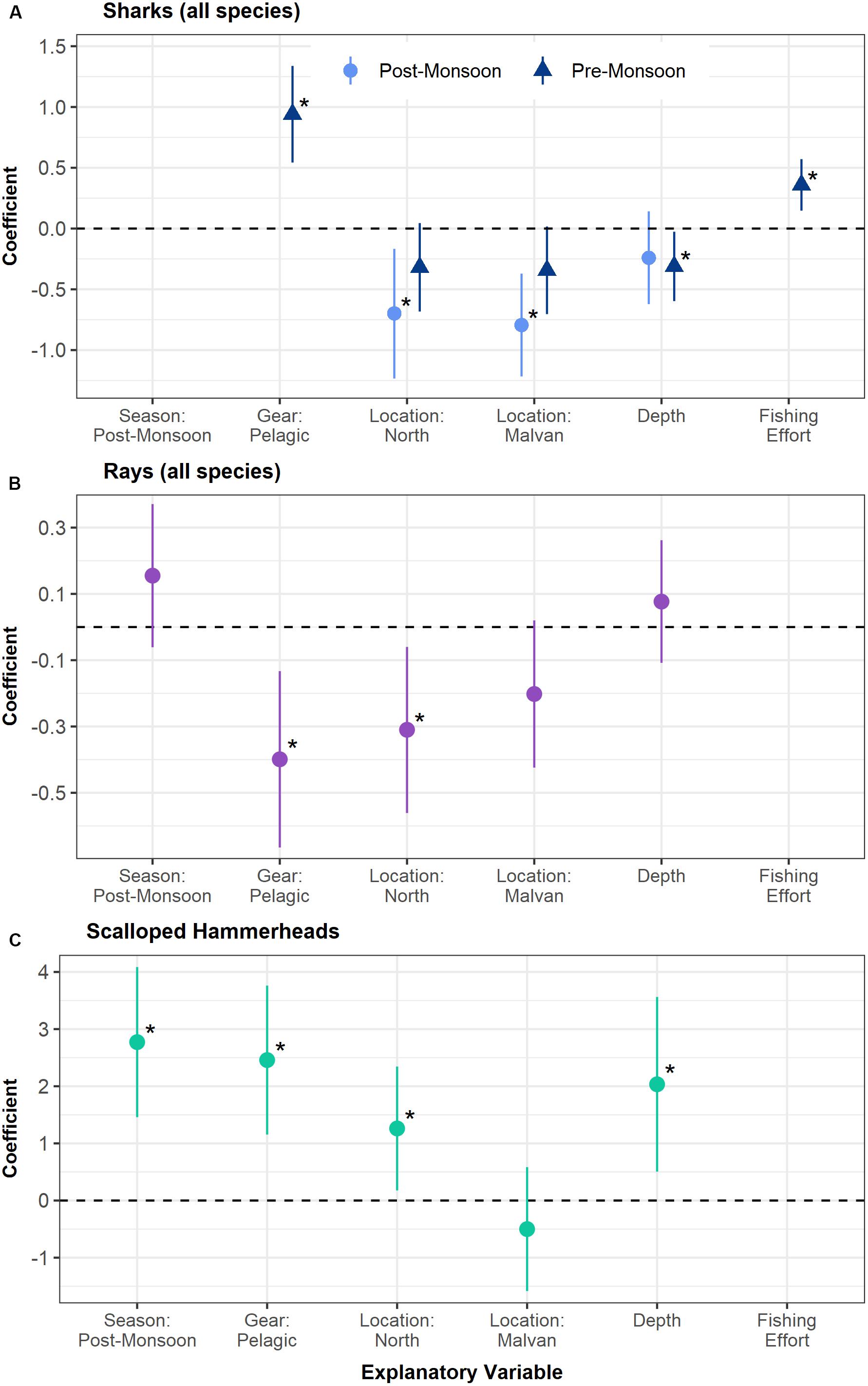
Figure 2. Coefficients and confidence intervals of the best fit mixed models of the number of sharks captured (A), number of rays captured (B), and probability of hammerhead shark capture (C) plotted against various fishing variables. Panels (A,B) are lognormal models, whereas panel (C) is a binomial model. The coefficients of the categorical variables are given with respect to a reference category – for Location: South, for Gear: Benthic, for Season: Pre-monsoon, for Depth: Shallow. A positive coefficient for a category (for example, pelagic gear in panel (A)) indicates a higher catch of that taxa as compared to the reference category (i.e., benthic gear). Similarly, positive coefficients of a continuous variable such as fishing effort indicate a higher catch at greater effort. Significant variables are indicated with an Asterix (*). Shark catches were modeled separately for the pre- and post-monsoon seasons.
Hammerhead sharks also had a significantly higher probability of capture in the post-monsoon season (p < 0.001), in pelagic nets (p < 0.001) and in deep waters (p = 0.009). Contrary to the trends for pooled shark species, the probability of catching hammerheads was slightly higher in the northern fishing grounds (p = 0.02 with reference to the south; Figure 2 and Supplementary Table 2). For rays, the numbers captured were not significantly related to season. Benthic nets had significantly higher catches of rays than pelagic nets (p = 0.003), as expected. Like sharks, the southern fishing grounds had significantly higher captures of rays than the northern grounds (p = 0.01, Figure 2 and Supplementary Table 2).
No interaction terms were included in any of the best fit models. The full set of models and coefficients are presented in the Supplementary Tables 2, 3. Due to the small numbers of guitarfish encountered over the sampling period (n = 17), we were unable to model fishing variables affecting their capture.
In order to deduce potential population trends in the study species, fishers and boat owners were asked about changes (if any) in elasmobranch catches over the past decade. All respondents stated that catches of all species, including elasmobranchs, had significantly reduced over the past 10 years. Most respondents (11 of 16) suggested that poor fishing practices like purse seining, light-emitting diode (LED) fishing (an illegal fishing technique where mechanized vessels use strong LED lights to attract and capture large volumes of fish) and high-speed trawling were the primary reasons for this decline (Supplementary Table 4). Other reasons suggested were overfishing (i.e., high fishing effort) and environmental factors (e.g., climate change). Most fishers were aware of the impacts of their fishing practices on fish populations:
“Because of overfishing and constant killing, the fish have reduced. If there are 50 fish that have been produced and we kill 40–50 of them, then how are they supposed to replenish?”
– A fisher, age 52.
Technical Assessment of Mitigation Measures
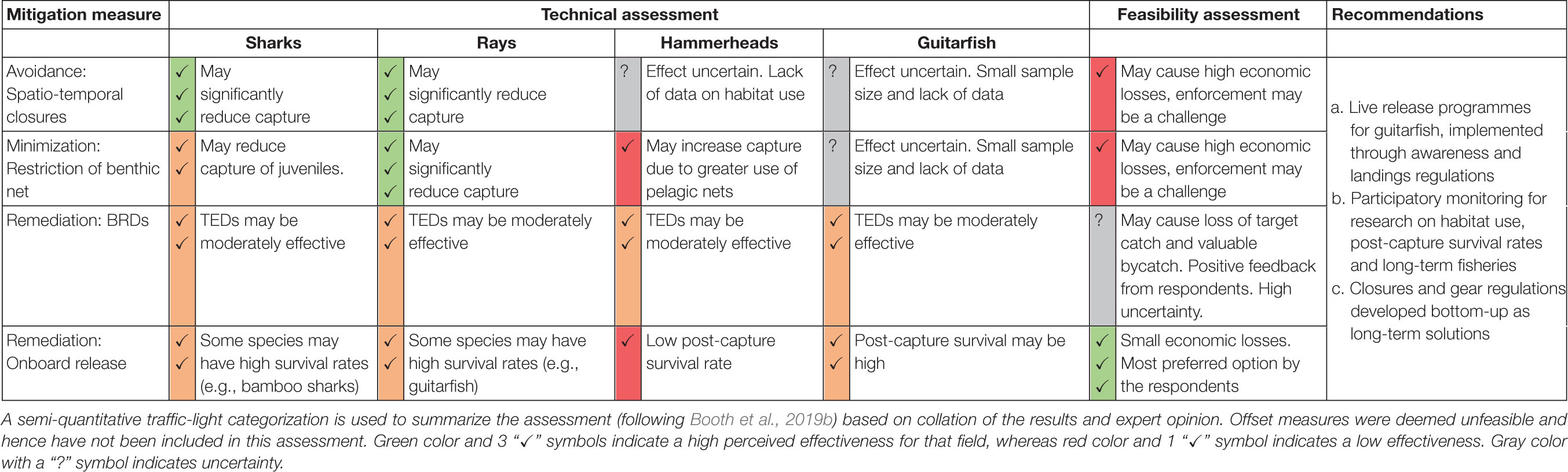
The technical assessment focused on the potential impact of the proposed measures on elasmobranch populations, if fully implemented (Table 4).
Avoidance: Spatio-temporal closure of the southern fishing ground for 2 months during the pupping season.
Model coefficients indicate that this measure is likely to have a significant positive impact for shark populations, due to the higher captures of sharks in the southern fishing grounds and post-monsoon months for which the closure is proposed. This holds true for rays as well, as the southern fishing grounds were related to higher captures (Figure 2). Although the likelihood of catching a scalloped hammerhead was significantly higher in the post-monsoon season, the southern fishing grounds (where the closure was proposed) had a lower likelihood of catching this species (Figure 2). Therefore, the impact of a spatio-temporal closure of the southern grounds on hammerhead catch in the post-monsoon season is somewhat uncertain.
Most fishers perceived that there would be a positive impact of spatio-temporal closures on both elasmobranch populations and populations of their target species (Figure 3). A summary of the fisher responses to all the proposed mitigation measures can be found in Supplementary Table 4.
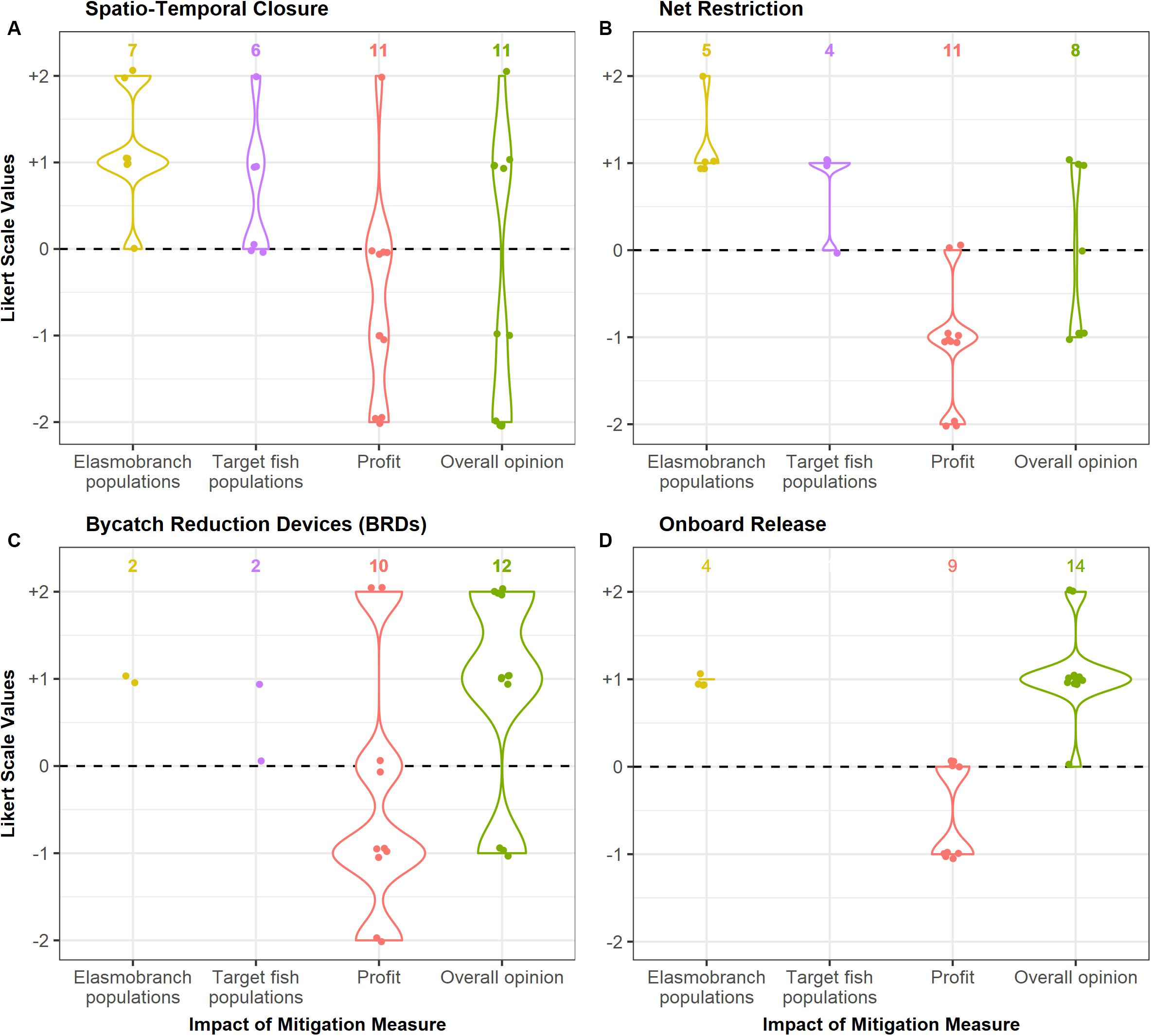
Figure 3. Perceived impact of each mitigation measure (A–D) on elasmobranch populations, target species populations and profit of the boat owners, obtained through interviews. Data is represented on a five-point scale from –2 (greater than a 50% decrease) to +2 (greater than a 50% increase). The overall opinion of the respondents on each mitigation measure is also presented here on a similar scale (–2: Very negative opinion to +2: Very positive opinion). Sample size for each response is given above the plot. Questions on the impact of each measure on elasmobranch populations and target fish populations were directed to fishers (total sample size = 7), whereas questions on the impact on profits were directed toward boat owners (total sample size = 11). An overall opinion on the mitigation measure was asked to both fishers and owners. Not all respondents were able to provide answers for every question, hence the sample size represented on the graph may be lower than the total in some cases.
Minimization: Restriction of benthic net use for 2 months during the pupping season.
Gear was not included in the best fit model during the post-monsoon for sharks (Figure 2). This may be because, while shark catches are higher in pelagic nets on the whole, juveniles of most species are largely caught in benthic nets in the post-monsoon season. Hence, the variation due to size and age may be affecting this result, and benthic net restriction during this period may in fact be effective in reducing juvenile shark capture. This measure is likely to have a positive impact for rays due to the significantly higher captures in benthic nets. However, the opposite relationship was found for hammerheads, indicating that this measure may increase hammerhead capture due to a potential switch to higher use of pelagic nets (Figure 2).
Most fishers believed that this measure could be beneficial for populations of elasmobranchs and of their target species (Figure 3).
Remediation: Bycatch Reduction Devices (BRDs).
This measure was assessed through a literature review of BRDs tested for elasmobranchs in other tropical trawl fisheries, most of these being modifications of TEDs (Table 5). A wide range of impacts on shark catches was observed across the studies, from 4.9 to 94% reduction (Table 5). Bycatch reduction rates for small sharks were on the lower side. A reduction of 25–59% of bycatch of small rays was observed from tropical prawn trawl fisheries in South America (Table 5). For hammerheads, TEDs achieved a reduction rate of 55%, whereas another study found a reduction of 31% in bycatch of the closely related bonnethead sharks (Table 5). Courtney et al. (2007) found mixed and limited effectiveness of TEDs for bycatch reduction of guitarfish and wedgefish.
Locally designed TEDs have been developed for sea turtles caught in Indian trawl fisheries by the Central Institute of Fisheries Technology (CIFT) and are undergoing testing and improvement. However, their effect on elasmobranchs has not been specifically assessed, with one case study mentioning a lack of exclusion achieved (Table 5).
As fishers were not aware of bycatch reduction devices and techniques, they were not able to estimate their impact on elasmobranch and target fish populations (Figure 3).
Remediation: Onboard release of live individuals.
This measure was also assessed through a literature review. Survival rates upon capture and post-release is species-specific, hence sharks and rays as collective groups could not be assessed, and we focused on hammerheads and guitarfish. Hammerheads had high mortality rates of up to 98% upon capture in trawlers in South Africa and Northwest Africa (Fennessy, 1994; Zeeberg et al., 2006). Furthermore, hammerheads captured in the present study are all juveniles (<70 cm TL) and are likely to have very high mortality rates upon capture, with little scope for live release. Although we could not find any literature on the post-capture survival of the guitarfish species under study, related species like Rhinobatos sp. and Rhynchobatus djiddensis were found to have low to moderate mortality rates of 10–53% upon capture by trawlers (Fennessy, 1994; Stobutzki et al., 2002).
Fishers indicated that release of elasmobranchs will only have a slight positive impact on their populations, due to the high mortality rates when captured (Figure 3).
Offset: Mitigating elasmobranch catch offsite.
The final step of the mitigation hierarchy involves compensating for fishing mortality of the species of concern, by investing in actions which increase the probability of another individual in the same stock living to the same age. In other applications of the mitigation hierarchy, this typically involves a financial offset, such as a “bycatch tax,” which is invested in conservation elsewhere (Squires and Garcia, 2018; Booth et al., 2019b). However, the low socio-economic status of fishers in Malvan renders such measures unfeasible at present, and we did not assess them further for this analysis.
Feasibility Assessment of Mitigation Measures
This assessment evaluated the feasibility of implementing the measures, both in terms of its social and economic impact on the fishers, and the likelihood of compliance (Table 4).
Avoidance: Spatio-temporal closure of the southern fishing ground for 2 months during the pupping season.
Most boat owners (6 of 11) indicated that this measure would negatively affect their incomes. A few believed it would have no impact (n = 4), as they could fish in other locations, and one respondent suggested that his profits would increase once the closure was lifted. The overall opinion of the respondents to this measure was mixed; most respondents had a negative view of this measure, whereas a few indicated their willingness to follow it as it may benefit them in the long term (Figure 3).
The months of the post-monsoon season (August–December) were also cited by most owners (n = 8) as the peak months for catch and profit. Hence the closure of a fishing ground for 2 months during this period would likely significantly affect their incomes.
“If any fishing grounds are closed, we’ll have to shut down our boats and go hungry”.
– a trawl fisher, age 61.
Some respondents (n = 3) raised concerns regarding the impact of spatial closures on small-scale fishers in the closure region, as their boats would not have to capacity to travel further to fish. Respondents also indicated that compliance may be a problem, as it would be difficult to monitor and enforce such a closure. A summary of the respondents’ opinion on all the proposed mitigation measures is provided in Supplementary Table 4.
Minimization: Restriction of benthic net use for 2 months during the pupping season.
Respondents stated that although pelagic nets were the primary gear used during the post-monsoon season, benthic nets would occasionally be deployed as well. Most boat owners (n = 9 out of 11) believed that restricting benthic net use would negatively affect their profits. Due to the highly variable nature of the catch, they believed that restriction of any gear may result in a severe loss for them. The overall opinion toward this measure was negative or mixed (Figure 3).
Remediation: Bycatch Reduction Devices (BRDs).
Due to differences in socio-economic contexts, most of the reviewed studies would not serve as suitable proxies to understand the feasibility of BRDs for Malvan. We therefore focused on the Indian case studies testing TEDs (Table 5), which found losses of 0.5–3.3% of prawns and target fish at different sites under highly controlled usage by experts. This number is likely to be higher in Malvan due to the commercial value of non-elasmobranch bycatch species that may also escape through the TED, which was not evaluated by these studies. Furthermore, adoption of TEDs by Indian fishers has been extremely limited despite their mandatory use in some states, probably due to their perceived impact on catch and profits (Rao, 2011).
The general concept of a BRD was explained to boat owners, and its possible effects on target catch and bycatch described. Given this information, most (6 of 11) believed that it could significantly reduce their profits. However, a few owners (n = 2) indicated that their profits may increase over the long-term. The overall opinion toward this measure was mostly positive, with nine respondents indicating their willingness to try it and four selecting BRDs as their most preferred of the four options. Respondents were concerned that buying nets with BRDs would be costly, especially as trawl nets need regular replacement due to wear and tear, and suggested these devices be given to boats for free. Some also believed that any technological modification to reduce bycatch may not suit their boats, and would be willing to use a BRD only if it had been developed for and tested in local conditions.
We also referred to the square-mesh trawl nets introduced in Malvan in 2015 to reduce bycatch of juvenile fish (UNDP, 2017), and discussed this with interview respondents as another type of BRD. Although most boat owners (n = 7) had received this net when it was being promoted, only a few (n = 3) stated that they occasionally used it.
“The problem is, we also catch fish that are small to begin with, like anchovies and sole fish. The square mesh nets reduce our catch of these, and decreases our profits”.
– a boat owner, age 28.
Remediation: Onboard release of live individuals.
5 of the 11 boat owners said that release of live animals would have a small negative impact on their profits, while 4 indicated that it would have no effect (Figure 3). Sharks and rays were considered low value catch and formed only 1–2% (n = 7) or 5% (n = 3) of their income. Overall opinion toward this measure was positive, and it was the most preferred option among the four proposed measures by most respondents (n = 8) as it caused minimal economic loss and involved little time and effort. Some owners (n = 2) stated that their crew already released live juveniles of many species whenever possible.
Key Uncertainties
There is a high degree of uncertainty associated with the proposed mitigation measures. Lack of data regarding critical habitats of elasmobranchs, and spatio-temporal variation in their use of different habitats, is a major hindrance to designating effective avoidance and minimization strategies. Few bycatch reduction technologies have been developed specifically for elasmobranchs in trawl fisheries, and none in India. Similarly, survival of the study species, if released onboard or even through a BRD, is not specifically known for this fishery, but is likely to be low to moderate. Overall, there is limited understanding of the effectiveness of these measures with respect to both catch and mortality reduction of elasmobranchs, as well as their socio-economic impact. Future research needs to focus on these specific data gaps to address this uncertainty (Table 4). Moreover, our dataset was collected over a relatively short time period, and lacks onboard data on discards. Long-term landings and discards data are essential in developing optimal management strategies.
Compliance with the management measures, if implemented, is another major challenge. Only 5 out of the 16 respondents believed that the fishing community would comply with any of the proposed measures, and only if some form of compensation was provided. Respondents mentioned the prevalence of illegal fishing activities such as LED fishing around Malvan, indicating that compliance with any new measures would be unlikely given the challenges in enforcing these existing regulations.
Discussion
Insights From the Mitigation Hierarchy Assessment
Bycaught elasmobranchs in India have a social and economic value (Jabado et al., 2018), which makes bycatch mitigation highly challenging. This study used a novel framework that allowed the systematic assessment of management measures for elasmobranch bycatch mitigation, based on a range of evidence sources. Landings surveys indicated how operational fishery variables affected elasmobranch capture toward designing effective mitigation measures, while interviews provided insights on the perceptions of local stakeholders on the proposed measures. Our study provides the first evidence-based, nuanced and case-specific understanding of elasmobranch bycatch for this fishery, and suggests ways forward for management.
Area-based strategies like Marine Protected Areas (MPAs) are widely used in marine conservation (Shiffman and Hammerschlag, 2016; MacKeracher et al., 2019) and are generally advocated for shark protection (e.g., shark sanctuaries; Ward-Paige, 2017). However, such strategies have had little success in India where they tend to be strict MPAs with little inclusion of the fishing community in the design, implementation or access to the area, leading to violations of MPA rules and conflict between fishers and managers (Rajagopalan, 2009; Bijoor et al., 2018; Muralidharan and Rai, 2020). The Malvan Marine Sanctuary is not yet operational as it has faced considerable opposition from the fishing community due to their exclusion from the entire process (Rajagopalan, 2009). It is clear that area-based strategies in their present format have little scope for success, and need to be approached differently. Our findings suggest that if flexible and case-specific closures or gear regulations were designed with the local community as partners and co-managers, they may be effective (see also Karnad et al., 2019; Rigby et al., 2019b).
Bycatch reduction technologies (BRDs) are generally plagued with implementation challenges (Campbell and Cornwell, 2008). Although some respondents in Malvan provided positive feedback about the adoption of BRDs, their perception may be biased by lack of knowledge regarding this measure. The limited use of square-mesh trawl nets in Malvan to reduce bycatch reported by interview respondents suggests that other BRDs may face a similar response. Furthermore, high levels of uncertainty regarding the effectiveness of BRDs for elasmobranchs, combined with the increasing commercial value of most non-target species, makes this measure somewhat unfeasible at present.
Onboard release of live individuals, particularly species like guitarfish, appears to be the most viable option from a socio-economic perspective. Given that catch rates of guitarfish in trawlers is low, this may be the most cost-effective method to potentially minimize fisheries mortality of these species. The whale shark (Rhincodon typus) conservation campaign in Gujarat, on the north-west coast of India, is an example of a successful intervention where fishers have released several hundred sharks caught in their nets, receiving compensation for any damage (Matwal et al., 2014). However, this measure may be applicable to a few species only; post-capture mortality rates for obligate ram ventilators like scalloped hammerheads are too high to support live release (Ellis et al., 2017). Nonetheless, the greater feasibility of this measure should be taken into consideration, even if its direct impacts are low. In a situation where fishers are generally excluded from management decision-making, and there is high uncertainty and a conservation need, building trust and engagement through feasible management options such as release of live individuals is an important first step (Redpath et al., 2013). This can be followed with solutions that have better conservation outcomes.
Our results provide clear evidence for the need for species-specific management strategies, due to the diversity in elasmobranch species characteristics (Dulvy et al., 2017). Capture trends varied between and within taxa; for instance, hammerheads had a higher likelihood of capture in the northern fishing grounds, but when all shark species were pooled, catches were found to be higher in the southern fishing grounds. We highlight the need for different and complementary management measures, which would together provide conservation benefit to a range of vulnerable species (Shiffman and Hammerschlag, 2016).
Market-based approaches, such as economic incentives, form an important component of the mitigation hierarchy as conceptualized for fisheries (Squires and Garcia, 2018). Our study has not considered these due to the lack of such approaches in Indian fisheries at present. However, incentives in the form of eco-labeling schemes or compensation for lost catch can effectively produce behavioral change in fishers (Gjertsen et al., 2010). For instance, an incentive scheme to give premium prices to fishers abiding by bycatch regulations is currently under trial in a small-scale fishery in Peru (Arlidge et al., 2020). Such mechanisms could be explored to encourage uptake of mitigation measures and compensate for lost profits, once motivations and constraints of fishers are better understood (Booth et al., 2019a). Furthermore, biodiversity offset measures can be made more feasible through market-based approaches like taxation of traders or other nodes of the supply chain.
In summary, our study identifies potential steps to ameliorate the complex and seemingly intractable issue of elasmobranch bycatch in Indian coastal fisheries (Table 4). On the whole, stakeholders in Malvan were not opposed to elasmobranch conservation as long as it did not compromise their earnings. A good first step would be to promote the live release of guitarfish, through extensive outreach and workshops. Participatory monitoring could aid in addressing research gaps for elasmobranchs and collecting long-term fisheries data, while further building community engagement (Estrella and Gaventa, 1998; Sheil and Lawrence, 2004). Development and implementation of fishery closures or gear modifications using a bottom-up approach may then be successful as long-term management measures. The findings and recommendations of this study will be presented to the local Fisheries and Forest Departments and will also be disseminated among the fishing community in Malvan in the local language.
The Mitigation Hierarchy Framework as a Tool for Bycatch Management
Although the mitigation hierarchy has been discussed conceptually for marine bycatch management (Milner-Gulland et al., 2018; Squires and Garcia, 2018), it has only previously been applied to one case study (Arlidge et al., 2020), which investigated marine turtle bycatch in a coastal gillnet fishery in Peru. While still considered a data-limited fishery compared to large-scale industrialized fisheries, there was richer bycatch data available than the current Malvan case study. In addition, unlike Malvan’s elasmobranchs, the marine turtles in that case were not commercially sold. Therefore, our study serves as an important test of its benefits as a decision-making framework for a very challenging situation and identifies scope for improvement.
Given the complexity of the bycatch problem, a single mitigation strategy following a one-size-fits-all approach is not an effective solution (Momigliano and Harcourt, 2014; Shiffman and Hammerschlag, 2016; Squires and Garcia, 2018). The framework facilitated the systematic compilation and critical assessment of multiple strategies to identify nuanced, case-specific, solutions. Moreover, we were able to better understand the challenges associated with classic management measures such as space-time closures. Therefore, the mitigation hierarchy was a useful framework for structuring thinking toward bycatch management of threatened species.
However, there were challenges with applying the mitigation hierarchy to our case study. For instance, setting a quantitative bycatch reduction target was difficult, as elasmobranchs are an exceptionally data-limited group with limited understanding of population dynamics and true fishing mortality for many species, particularly in developing countries (Booth et al., 2019b). Using a less quantitative, more feasible target of reducing elasmobranch bycatch over current observed levels was adequate for this preliminary exploration. Nevertheless, it emphasized the need to better adapt the framework for multi-species fisheries in developing nations with complex socio-economic contexts. In India, this is further complicated by local differences in social, political and economic contexts. For Indian fisheries, it may be more useful to start with a socio-economic assessment of what degree of bycatch mitigation is feasible, followed by the risk and technical assessment to identify priority species for conservation and develop effective management measures. We suggest an adaptive approach iterating the framework over time as trust and capacity, as well as the information base, are developed.
Elasmobranch and Bycatch Management in India
Our assessment began to unpack the problem of elasmobranch conservation at a case study site, and lessons learnt can be applied to elasmobranch management in India more broadly, as well as in other developing countries facing similar challenges. The present study site represents a very small fraction of Indian fisheries, and studies such as this need to be scaled up for sharks and rays across sites and gear types, to develop meaningful mitigation and conservation strategies. Research efforts are currently patchy, and frameworks such as the mitigation hierarchy can guide systematic research to produce scientific data that is relevant to policy making and management (Momigliano and Harcourt, 2014; Shiffman and Hammerschlag, 2016; Milner-Gulland et al., 2018). Most importantly, the human dimensions need to be explicitly studied. Our findings establish that socio-economic feasibility and stakeholder perceptions, rather than technical effectiveness, may be the deciding factors for management. Therefore, understanding the views and socio-economic characteristics of fishing communities is critical to developing conservation interventions (Karnad et al., 2014; Mason et al., 2020).
Lastly, it is important to consider the broader picture. Elasmobranchs form a small component of the incidental catch in Indian fisheries, which ranges from sea snakes, marine turtles and cetaceans to juvenile fish and invertebrates that are either discarded or retained for various commercial uses (Lobo, 2012). The fisheries and gears are equally complex, with a wide assortment of small and large-scale fisheries targeting a variety of species, which often overlap spatially and temporally. Bycatch management will need to integrate specific strategies for these different species and fisheries into a comprehensive action plan at multiple jurisdictional scales. Such an approach needs to be supplemented by research on the drivers of unsustainable fisheries, such as exports and fishmeal production for aquaculture. This improved understanding can then feed into regulatory changes at the local, national and international levels. Interdisciplinary frameworks like the mitigation hierarchy can play a role in operationalizing conservation and fisheries management goals, and shaping policy that integrates environmental sustainability and social justice.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by an institutional ethics committee affiliated with the Dakshin Foundation. The clearance reference number is DF_Ethics committee_HS_2019_May_01. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Ethical review and approval was not required for the animal study because this study conducted research on sharks and rays captured by fishing vessels for the purpose of sale for food. The sharks and rays sampled were hence already dead. None of the study species are Protected under India’s Wildlife Protection Act (WPA). Therefore no permits or animal ethics approval was required, in accordance with national legislation and institutional policy.
Author Contributions
TG, HB, WA, and EM-G conceptualized this study. TG carried out the data collection and analysis and wrote the first draft. All authors contributed significantly to revising the manuscript.
Funding
This work has been supported by a Pew Marine Fellowship awarded to EM-G, a Duleep Matthai Nature Conservation Trust Fellowship awarded to TG and institutional support from the Dakshin Foundation. WA was supported by a Ph.D. Commonwealth Scholarship from the Commonwealth Scholarship Commission in the United Kingdom and the University of Oxford (Ph.D. scholarship NZCR-2015-174).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the fishers and boat owners in Malvan for participating in this study. We acknowledge Paloma Chandrachud for her assistance in conducting interviews, Yogesh Waghmare and Shawn Dsouza for their assistance in the landing surveys and all the interns who helped with data collection for this study. Landings datasets of this study will be made publicly available by the Dakshin Foundation; please contact TG for more information.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00571/full#supplementary-material
References
Alverson, D. L., Freeberg, M. H., Murawski, S. A., and Pope, J. G. (1994). A Global Assessment of Fisheries Bycatch and Discards, Vol. 339. Rome: Food & Agriculture Org, 233.
Arlidge, W. N., Bull, J. W., Addison, P. F., Burgass, M. J., Gianuca, D., Gorham, T. M., et al. (2018). A global mitigation hierarchy for nature conservation. BioScience 68, 336–347. doi: 10.1093/biosci/biy029
Arlidge, W. N., Squires, D. E., Alfaro-Shigueto, J., Booth, H., Mangel, J., and Milner-Gulland, E. J. (2020). A mitigation hierarchy approach for managing sea turtle captures in small-scale fisheries. Front. Mar. Sci. 7:49. doi: 10.3389/fmars.2020.00049
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Statist. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bhathal, B. (2005). Historical reconstruction of indian marine fisheries catches, 1950–200, as a basis for testing the ‘marine trophic index. Fisher. Centre Res. Rep. 13:126.
Bijoor, S., Sharma, D., and Ramesh, M. (2018). Management of Marine Protected Areas in the Andaman Islands: Two Case Studies. Bangalore: Dakshin Foundation, 40.
Bonfil, R. (1994). Overview of World Elasmobranch Fisheries. No. 341. Rome: Food & Agriculture Org, 119.
Boopendranath, M. R., Dawson, P., Pravin, P., Remesan, M. P., Raghu, P. R., Vijayan, V., et al. (2006). “Design and development of the TED for indian fisheries,” in Marine Turtles of the Indian Subcontinent, eds K. Shanker and B. C. Choudhury (Hyderabad: Universities Press), 244–261.
Booth, H., Squires, D., and Milner-Gulland, E. J. (2019a). The neglected complexities of shark fisheries, and priorities for holistic risk-based management. Ocean Coast. Manag. 182:104994. doi: 10.1016/j.ocecoaman.2019.104994
Booth, H., Squires, D., and Milner−Gulland, E. J. (2019b). The mitigation hierarchy for sharks: A risk−based framework for reconciling trade−offs between shark conservation and fisheries objectives. Fish Fish. 19, 1–21. doi: 10.1111/faf.12429
Brewer, D., Heales, D., Milton, D., Dell, Q., Fry, G., Venables, B., et al. (2006). The impact of turtle excluder devices and bycatch reduction devices on diverse tropical marine communities in Australia’s northern prawn trawl fishery. Fish. Res. 81, 176–188. doi: 10.1016/j.fishres.2006.07.009
Campbell, L. M., and Cornwell, M. L. (2008). Human dimensions of bycatch reduction technology: current assumptions and directions for future research. Endang Species Res. 5, 325–334. doi: 10.3354/esr00172
CMFRI. (2012). Marine Fisheries Census 2010 Part II. 9 Maharashtra. Kochi: Central Marine Fisheries Research Institute, 336.
Compagno, L. J. V., and Marshall, A. D. (2019). Glaucostegus Obtusus. The IUCN Red List of Threatened Species 2019: e.T60170A124447244. Available online at: https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T60170A124447244.en (accessed October 31, 2019).
Courtney, A. J., Haddy, J. A., Campbell, M. J., Roy, D. P., Tonks, M. L., Gaddes, S. W., et al. (2007). Bycatch Weight, Composition and Preliminary Estimates of the Impact of Bycatch Reduction Devices in Queensland’s trawl Fishery. Parkhurst QLD: The State of Queensland, Department of Primary Industries and Fisheries, 307.
D’Alberto, B. M., Carlson, J. K., Pardo, S. A., and Simpfendorfer, C. A. (2019). Population productivity of wedgefishes, guitarfishes, and banjo rays: inferring the potential for recovery. bioRvix [Preprint]. doi: 10.1101/584557
Davidson, L. N., Krawchuk, M. A., and Dulvy, N. K. (2016). Why have global shark and ray landings declined: improved management or overfishing? Fish Fisher. 17, 438–458. doi: 10.1111/faf.12119
Davies, R. W. D., Cripps, S. J., Nickson, A., and Porter, G. (2009). Defining and estimating global marine fisheries bycatch. Marine Policy 33, 661–672. doi: 10.1016/j.marpol.2009.01.003
Dayton, P. K., Thrush, S. F., Agardy, M. T., and Hofman, R. J. (1995). Environmental effects of marine fishing. Aquat. Conserv. 5, 205–232. doi: 10.1002/aqc.3270050305
Dent, F., and Clarke, S. (2015). State of the Global Market for Shark Products. FAO Technical Paper 590. Rome: FAO.
Department of Fisheries, Ministry of Fisheries, Animal Husbandry and Dairying (2019). Handbook on Fisheries Statistics 2018. New Delhi: Department of Fisheries, Ministry of Fisheries, Animal Husbandry and Dairying, 190.
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world’s sharks and rays. elife 3:e00590. doi: 10.7554/eLife.00590
Dulvy, N. K., Simpfendorfer, C. A., Davidson, L. N., Fordham, S. V., Bräutigam, A., Sant, G., et al. (2017). Challenges and priorities in shark and ray conservation. Curr. Biol. 27, R565–R572. doi: 10.1016/j.cub.2017.04.038
Ellis, J. R., McCully Phillips, S. R., and Poisson, F. (2017). A review of capture and post−release mortality of elasmobranchs. J. Fish Biol. 90, 653–722. doi: 10.1111/jfb.13197
Estrella, M., and Gaventa, J. (1998). Who Counts Reality? Participatory Monitoring and Evaluation: A Literature Review. Brighton: Institute of Development Studies, University of Sussex, 70.
Fennessy, S. T. (1994). Incidental capture of elasmobranchs by commercial prawn trawlers on the Tugela Bank, Natal, South Africa. South Afr. J. Mar. Sci. 14, 287–296. doi: 10.2989/025776194784287094
Fernando, H., Ananthan, P. S., and Daniel, N. (2017). Shark fishing–a unique traditional fishing practice in Thoothoor, Tamil Nadu, India. Ecol. Environ. Conserv. 23, S10–S15.
Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R., and Lotze, H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x
Gallagher, A. J., and Hammerschlag, N. (2011). Global shark currency: the distribution, frequency, and economic value of shark ecotourism. Curr. Issues Tour. 14, 797–812. doi: 10.1080/13683500.2011.585227
Gallagher, A. J., Hammerschlag, N., Shiffman, D. S., and Giery, S. T. (2014). Evolved for extinction: the cost and conservation implications of specialization in hammerhead sharks. BioScience. 64, 619–624. doi: 10.1093/biosci/biu071
Garstin, A., and Oxenford, H. A. (2018). Reducing elasmobranch bycatch in the atlantic seabob (Xiphopenaeus kroyeri) trawl fishery of guyana. Gulf Caribb. Res. 29, GCFI10–GCFI20. doi: 10.18785/gcr.2901.04
Gjertsen, H., Hall, M., and Squires, D. (2010). Incentives to address bycatch issues. Conserv. Manag. Trans. Tuna Fisher. 10, 225–248. doi: 10.1002/9780813820262.ch14
Gupta, T., Manuel, M., Manoharakrishnan, M., Namboothri, N., and Shanker, K. (2019). Conservation and livelihood implications of trawler bycatch: towards improved management. J. Govern. 19, 55–63.
Hall, M. A., Alverson, D. L., and Metuzals, K. I. (2000). By-catch: problems and solutions. Mar. Pollut. Bullet. 41, 204–219. doi: 10.1016/s0025-326x(00)00111-9
IUCN (2019). The IUCN Red List of Threatened Species. Version 2019-2. Available online at: http://www.iucnredlist.org (accessed July 18, 2019).
Jabado, R. W., Kyne, P. M., Pollom, R. A., Ebert, D. A., Simpfendorfer, C. A., Ralph, G. M., et al. (2018). Troubled waters: threats and extinction risk of the sharks, rays, and chimaeras of the arabian sea and adjacent waters. Fish Fisher. 19, 1043–1062. doi: 10.1111/faf.12311
Karnad, D., Gangal, M., and Karanth, K. K. (2014). Perceptions matter: how fishermen’s perceptions affect trends of sustainability in indian fisheries. Oryx 48, 218–227. doi: 10.1017/s0030605312001251
Karnad, D., Sutaria, D., and Jabado, R. W. (2019). Local drivers of declining shark fisheries in India. Ambio 19, 1–12. doi: 10.1007/s13280-019-01203-z
Kelleher, K. (2005). Discards in the World’s Marine Fisheries: An Update. Rome: Food & Agriculture Org, 470.
Kizhakudan, S. J., Zacharia, P. U., Thomas, S., Vivekanandan, E., and Menon, M. (2015). CMFRI marine fisheries policy series-2; guidance on national plan of action for Sharks in India. CMFRI Mar. Fisher. Policy Ser. 2, 1–102.
Kumar, A. B., and Deepthi, G. R. (2006). Trawling and By-Catch: implications on marine ecosystem. Curr. Sci. 90:7.
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Statist. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Kyne, P. M., and Jabado, R. W. (2019). Glaucostegus Granulatus. The IUCN Red List of Threatened Species 2019: e.T60166A68623788. Available online at: https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T60166A68623788.en (accessed October 31, 2019).
Kyne, P. M., Jabado, R. W., Rigby, C. L., Dharmadi, G., Pollock, C. M., Herman, K. B., et al. (2019). The thin edge of the wedge: extremely high extinction risk in wedgefishes and giant guitarfishes. bioRxiv. [preprint]. doi: 10.1101/595462
Lobo, A. S. (2012). Managing Fisheries in an Ocean of Bycatch. Position Paper for CBD-COP 11. Bengaluru: Dakshin Foundation, Bengaluru and Foundation for Ecological Security, 12.
MacKeracher, T., Diedrich, A., and Simpfendorfer, C. A. (2019). Sharks, rays and marine protected areas: A critical evaluation of current perspectives. Fish Fisher. 20, 255–267. doi: 10.1111/faf.12337
Mason, J. G., Alfaro-Shigueto, J., Mangel, J. C., Crowder, L. B., and Ardoin, N. M. (2020). Fishers’ solutions for hammerhead shark conservation in Peru. Biolo. Conserv. 243:108460. doi: 10.1016/j.biocon.2020.108460
Matwal, M., Premjothi, P. V. R., Joshi, D., Praveenkumar, B. M., Farukhkha, B., Goutham, S., et al. (2014). Gujarat’s Gentle Giant–Conservation of Whale Shark (Rhincodon typus) in Gujarat. Noida: Wildlife Trust of India, 174.
Miller, M., Carlson, J., Cooper, P., Kobayashi, D., Nammack, M., and Wilson, J. (2013). Status Review Report: Scalloped Hammerhead Shark (Sphyrna lewini). Silver Spring, MY: NOAA Fisheries.
Milner-Gulland, E. J., and Rowcliffe, J. M. (2007). Conservation and Sustainable Use: A Handbook of Techniques. Oxford: Oxford University Press, 328.
Milner-Gulland, E. J., Garcia, S., Arlidge, W., Bull, J., Charles, A., Dagorn, L., et al. (2018). Translating the terrestrial mitigation hierarchy to marine megafauna by-catch. Fish Fisher. 19, 547–561. doi: 10.1111/faf.12273
Momigliano, P., and Harcourt, R. (2014). “Shark conservation, governance and management: the science-law disconnect,” in Sharks: Conservation, Governance and Management, eds N. Klein and E. Techera (Abingdon: Routledge), 89–106.
Muralidharan, R., and Rai, N. D. (2020). Violent maritime spaces: conservation and security in gulf of Mannar Marine National Park, India. Polit. Geogr. 80:102160. doi: 10.1016/j.polgeo.2020.102160
Myers, R. A., Hutchings, J. A., and Barrowman, N. J. (1997). Why do fish stocks collapse? The example of cod in Atlantic Canada. Ecol. Appli. 7, 91–106. doi: 10.1890/1051-0761(1997)007[0091:wdfsct]2.0.co;2
Narayanakumar, R., Jayasankar, J., Salim, S. S., Ganga, U., and Vivekanandan, E. (2017). Economic valuation of net social benefit of seasonal fishing ban in selected maritime states of India. Ind. J. Fisher. 64, 85–92.
Pérez Roda, M. A., Gilman, E., Huntington, T., Kennelly, S. J., Suuronen, P., Chaloupka, M., et al. (2019). A Third Assessment of Global Marine Fisheries Discards. FAO Fisheries and Aquaculture Technical Paper No. 633. Rome: Food and Agricultural Organization of the United Nations, 79.
Prakash, R. R., Boopendranath, M. R., and Vinod, M. (2016). Performance evaluation of turtle excluder device off dhamra in bay of Bengal. Fish. Technol. 53, 183–189.
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Raborn, S. W., Gallaway, B. J., Cole, J. G., Gazey, W. J., and Andrews, K. I. (2012). Effects of turtle excluder devices (TEDS) on the bycatch of three small coastal sharks in the gulf of mexico penaeid shrimp fishery? North Am. J. Fisher. Manag. 32, 333–345. doi: 10.1080/02755947.2012.678962
Rao, G. S. (2011). Turtle excluder device (TED) in trawl nets: applicability in indian trawl fishery.”. Ind. J. Fisher. 58, 115–124.
Redpath, S. M., Young, J., Evely, A., Adams, W. M., Sutherland, W. J., Whitehouse, A., et al. (2013). Understanding and managing conservation conflicts. Trends Ecol. Evol. 28, 100–109. doi: 10.1016/j.tree.2012.08.021
Rigby, C. L., Dulvy, N. K., Barreto, R., Carlson, J., Fernando, D., Fordham, S., et al. (2019a). Sphyrna lewini. The IUCN Red List of Threatened Species 2019: e.T39385A2918526. Available online at: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T39386A2920499.en (accessed January 21, 2019).
Rigby, C. L., Simpfendorfer, C. A., and Cornish, A. (2019b). A Practical Guide to Effective Design and Management of MPAs for Sharks and Rays. Gland: WWF, 64.
Sheil, D., and Lawrence, A. (2004). Tropical biologists, local people and conservation: new opportunities for collaboration. Trends Ecol. Evol. 19, 634–638. doi: 10.1016/j.tree.2004.09.019
Shiffman, D. S., and Hammerschlag, N. (2016). Shark conservation and management policy: a review and primer for non-specialists. Anim. Conserv. 19, 401–412. doi: 10.1111/acv.12265
Smith, S. E., Au, D. W., and Show, C. (1998). Intrinsic rebound potentials of 26 species of pacific sharks. Mar. Freshw. Res. 49:663. doi: 10.1071/mf97135
Squires, D., and Garcia, S. (2018). The least-cost biodiversity impact mitigation hierarchy with a focus on marine fisheries and bycatch issues. Conserv. Biol. 32, 989–997. doi: 10.1111/cobi.13155
Squires, D., Restrepo, V., Garcia, S., and Dutton, P. (2018). Fisheries bycatch reduction within the least-cost biodiversity mitigation hierarchy: conservatory offsets with an application to sea turtles. Mar. Policy 93, 55–61. doi: 10.1016/j.marpol.2018.03.018
Stevens, J. D., Bonfil, R., Dulvy, N. K., and Walker, P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494. doi: 10.1006/jmsc.2000.0724
Stobutzki, I. C., Miller, M. J., Heales, D. S., and Brewer, D. T. (2002). Sustainability of elasmobranchs caught as bycatch in a tropical prawn (shrimp) trawl fishery. Fish. Bull. 100, 800–821.
Sundaramoorthy, S., Shunmugaraj, T., and Ramanathan, V. (2001). Critical Habitat Information System of Malvan (Maharashtra - India). Chennai: Integrated Coastal and Marine Area Management, 29.
The Noun Project (2014). Available online at: www.thenounproject.com (accessed November 25, 2019).
UNDP (2013). Mainstreaming Coastal and Marine Biodiversity Conservation into Production Sectors in the Sindhudurg Coast, Maharashtra. Progress Report 2013. Maharashtra: United Nations Development Programme (UNDP), 116.
UNDP (2017). Mainstreaming Coastal and Marine Biodiversity Conservation into Production Sectors in the Sindhudurg Coast, Maharashtra. Project Implementation Review. Maharashtra: United Nations Development Programme (UNDP).
Ward-Paige, C. A. (2017). A global overview of shark sanctuary regulations and their impact on shark fisheries. Mar. Policy 82, 87–97. doi: 10.1016/j.marpol.2017.05.004
Willems, T., Depestele, J., De Backer, A., and Hostens, K. (2016). Ray bycatch in a tropical shrimp fishery: do bycatch reduction devices and turtle excluder devices effectively exclude Rays? Fish. Res. 175, 35–42. doi: 10.1016/j.fishres.2015.11.009
Zacharia, P. U., Kizhakudan, S. J., Thomas, S., Manojkumar, P. P., Nair, R. J., Najmudeen, T. M., et al. (2017). Non-detriment findings (ndf) for the export of shark and ray species listed in appendix II of the CITES and harvested from Indian waters. CMFRI Mar. Fisher. Policy Ser. 6, 1–102.
Keywords: sharks, rays, scalloped hammerheads, guitarfish, mitigation hierarchy, bycatch, management, sustainability
Citation: Gupta T, Booth H, Arlidge W, Rao C, Manoharakrishnan M, Namboothri N, Shanker K and Milner-Gulland EJ (2020) Mitigation of Elasmobranch Bycatch in Trawlers: A Case Study in Indian Fisheries. Front. Mar. Sci. 7:571. doi: 10.3389/fmars.2020.00571
Received: 18 April 2020; Accepted: 22 June 2020;
Published: 15 July 2020.
Edited by:
Rebecca Lent, International Whaling Commission (IWC), United StatesReviewed by:
Matthew David Tietbohl, King Abdullah University of Science and Technology, Saudi ArabiaNarriman Saleh Jiddawi, State University of Zanzibar, Tanzania
Copyright © 2020 Gupta, Booth, Arlidge, Rao, Manoharakrishnan, Namboothri, Shanker and Milner-Gulland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Trisha Gupta, dHJpc2hhZ3VwdGEwNDA1QGdtYWlsLmNvbQ==
 Trisha Gupta
Trisha Gupta Hollie Booth
Hollie Booth William Arlidge
William Arlidge Chetan Rao
Chetan Rao Muralidharan Manoharakrishnan
Muralidharan Manoharakrishnan Naveen Namboothri
Naveen Namboothri Kartik Shanker
Kartik Shanker E. J. Milner-Gulland2
E. J. Milner-Gulland2