- 1Ifremer, CNRS, UMR 6308, AMURE, IUEM University of Western Brittany, Plouzané, France
- 2World Wildlife Fund, Global Science, Washington, DC, United States
- 3Nicholas School for Environmental Policy Solutions, Duke University, Durham, NC, United States
- 4Global Change Institute, The University of Queensland, Brisbane, QLD, Australia
- 5Ocean Data Foundation, Lysaker, Norway
- 6World Wide Fund for Nature, Wellington, New Zealand
- 7World Wide Fund for Nature, Hong Kong, Hong Kong
- 8Tribal Leader, Ngāti Kuri, Kaitaia, New Zealand
Climate change is expected to dramatically alter the distribution of many marine megafauna, impacting the people and economies that depend upon them. We build on the recent literature by developing a framework to describe the effects these changes will have on marine megafauna. With the goal to assist policymakers and grass roots organizers, we identify three illustrative pathways by which climate change drives these range shifts: (1) effects on habitat and shelter, (2) impacts on reproduction and disease, and (3) changing distribution of sources of food. We examine non-climate factors that may constrain or enable megafauna to adapt, creating winners and losers both for the species and the people dependent upon them. Finally, we comment on what management strategies exist at international and local scales that could help mitigate these impacts of climate change so that we, as a global community, can ensure that marine megafauna and people can best co-exist in a changing world.
Introduction
Megafauna are species that are much larger than the other species in their group (Ripple et al., 2019). In marine habitats, this includes cetaceans, sharks and rays, turtles, and birds (Sievers et al., 2019). These organisms often play outsized ecological roles (Bornatowski et al., 2014; Roman et al., 2014), support wildlife tourism, and even some fisheries. In many places, marine megafauna are culturally important and can be considered iconic, the embodiment of gods (Thaman et al., 2010), symbols of power (Rudrud, 2010) or play a myriad of other roles (McDavitt, 2005; Robillard and Séret, 2006; Deutsch, 2017).
In spite of their importance to people, marine megafauna have been particularly susceptible to human threats, including pollution (air and water), toxins in streams and rivers, coastal development, shipping, fishing, and hunting. Added to this, these species tend to be slow growing, take years to reach reproductive maturity, and have low fecundity (Pistevos et al., 2015; Thomas et al., 2016; Moore et al., 2017). Thus, they are slow to recover from harvesting, population crashes, and persistent mortality. In order to ensure the survival of these species, it is important to address and reduce these threats.
Climate change will exacerbate the effects of non-climate, human-induced stresses on ocean ecosystems. Increased atmospheric CO2 and associated greenhouse gases are predicted to dramatically alter the chemistry and physical properties of the ocean (Hoegh-Guldberg et al., 2018). Even under moderate carbon emissions scenarios, the world’s ocean will experience significant change. Sea surface temperature (SST) has already increased at an average of 0.11°C per decade since the 1970s (IPCC, 2019). Warmer waters lead to thermal expansion, resulting in sea level rise (SLR) that is amplified by melting land ice. Warming waters are also linked to increased storm magnitude and shifting patterns in the various oscillation cycles (Hoegh-Guldberg et al., 2018). Quantities of freshwater great enough to alter local salinity levels will be added to some coastal areas from melting glaciers and increased precipitation (Hoegh-Guldberg et al., 2018). Increased SST also will lead to increased stratification of the water column, deoxygenation, and changes in patterns of ocean circulation. Finally, as CO2 concentrations in the atmosphere increase, the ocean absorbs more CO2 thus acidifying the water (Hoegh-Guldberg et al., 2018).
To mitigate the effects of climate change on megafauna, and most importantly reduce the impact on the people dependent upon these organisms, policymakers will need to make informed decisions quickly. Our goal here is to provide a framework for decision makers that facilitates evidence-based decision-making, and illustrates the role of place-based conservation in dealing with the impacts of climate change on marine megafauna. We build on the literature recently reviewed in Chapter 5 of the UN Special Report on the Oceans and Cryosphere (Bindoff et al., 2019). Specifically, we create a more general framing of the impacts of climate change by identifying three illustrative pathways by which climate will affect marine megafauna in general. Most of these impacts ultimately result in a change in species distributions due to changes in: (1) where they can find shelter, (2) factors affecting reproduction and disease, and (3) their sources of food. Each category of impact determines, in part, the range of management and adaptive responses needed to deal with these changes. Here we examine how climate change acts through these three pathways to affect species, using specific case studies to highlight each (Figure 1). Species for the case studies were selected to illustrate a wide variety of well documented climate change impacts and provide compelling examples relevant to policymakers and other organizers. We examine non-climate factors that may constrain or enable megafauna to adapt, creating winners and losers both for the species and the people reliant upon them. Finally, we comment on what management strategies exist at international and local scales that could help mitigate impacts.
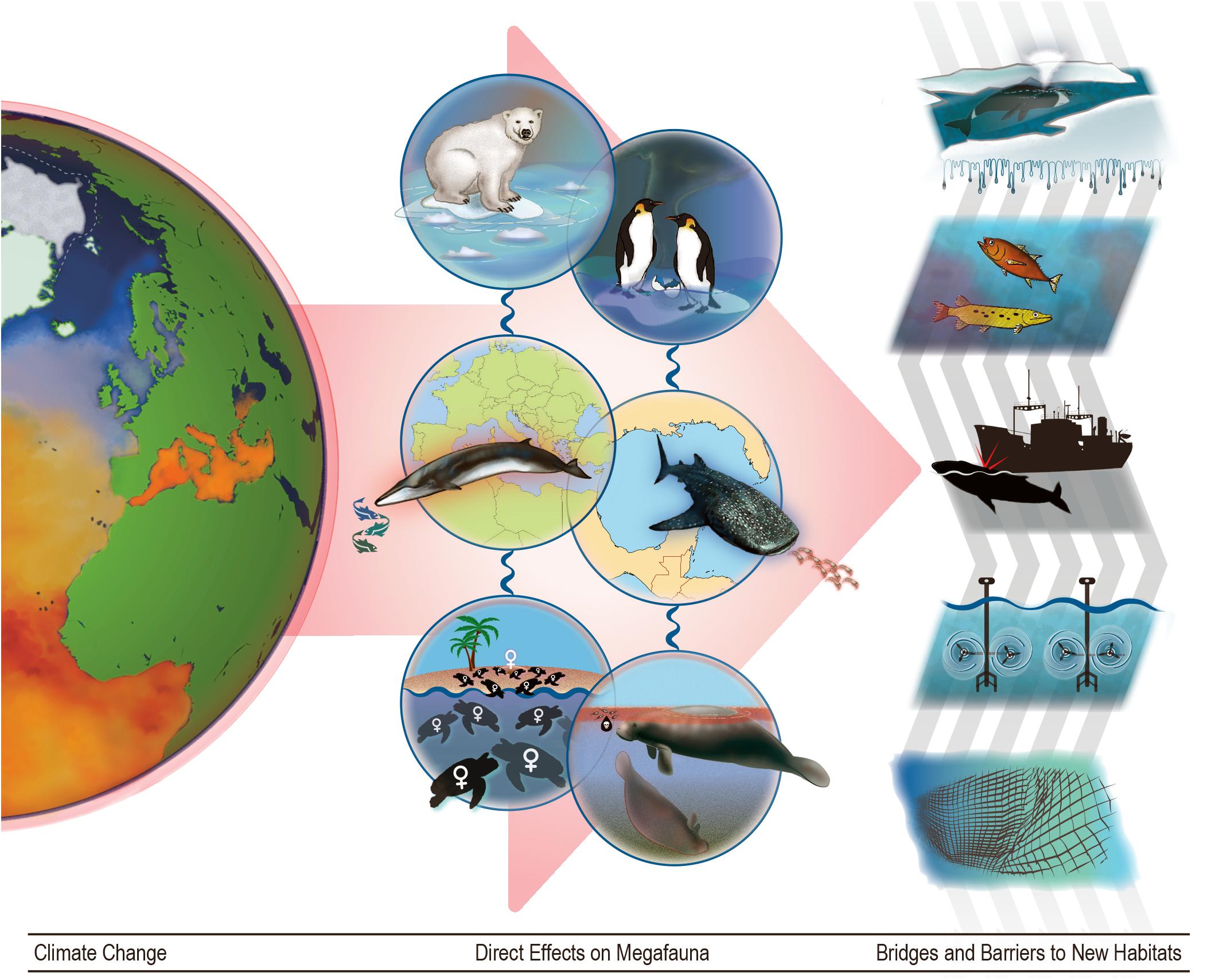
Figure 1. The effects of climate change on marine megafauna. As climate change continues to affect the world’s ocean (column 1), the combined effects will push many species out of their current ranges (column 2) via three illustrative pathways each here represented by two case study species. From top to bottom: via loss of shelter (polar bears and emperor penguins), via range shifts in prey (Mediterranean fin whales and whale sharks), and via changing demographics due to effects on embryonic development (sea turtles) and increased exposure to toxins (sirenians). The third column shows some bridges and barriers that respectively facilitate or impede range shifts in megafauna: ice melt in the arctic, changes in water temperature along migration routes, interactions with ships, tidal mills, and fishing gear.
A Closer Look at the Three Illustrative Pathways
Climate change will dramatically alter the distribution of many marine megafauna, impacting people and economies worldwide. Generally speaking, species are moving poleward as sea temperatures rise (Polovina et al., 2011; Poloczanska et al., 2016; Pecl et al., 2017). Not all species will succeed in shifting their ranges - some will go extinct, either locally or globally. Those species that do relocate will do as a result of: loss of shelter, impacts of climate change on reproduction and disease, and shifting food sources.
Loss of Shelter Due to Climate Change
In the marine setting, shelter refers to an ocean space that provides the necessary conditions for physiological and physical well-being. It is a place where a given individual is within its range of thermal tolerance, its metabolism is efficient, and has resources to meet its needs. A large part of what forms shelter is the physical structure in or upon which an organism lives. Ice caps and coral reefs are often cited as examples of critical habitat, and thus shelter, for which climate change poses immediate threats to megafauna. Loss of sea ice affects where seals, walruses and other marine mammals can find shelter from the sea and predators. Climate change will also reduce the quality of marine shelter as winds and violent storms unearth and bury seagrass beds (Marsh et al., 2017) and erode shoreline habitats. Escalating intensity and possible frequency of storms (Bindoff et al., 2019) will increase the magnitude of habitat destruction over successive weather events. If these storms are so frequent that the habitat does not have time to recover, neither will the local megafauna populations (Pistevos et al., 2015; Thomas et al., 2016; Moore et al., 2017). In extreme cases, for example species living on the melting sea ice or dying coral reefs, some megafauna species will be left with no remaining habitat, and thus face extinction.
Climate change will also affect the metabolic and physiological conditions that determine where megafauna can survive. Some marine megafauna species (e.g., sharks and rays) are limited by the salinity of the water in which they live. Climate change will likely freshen ocean waters in areas where freshwater run-off is likely to increase (Doney et al., 2012). Every species has its unique suite of thermal constraints. Ectotherms such as fish and turtles cannot control their temperatures internally, so must seek habitats within their thermo-neutral zone. In regions where individuals are living at or near their thermal limit, rising water temperatures could put organisms at risk of overheating, causing individuals to seek out cooler waters poleward (Dell’Apa et al., 2018). Warm-blooded, endothermic megafauna, including mammals and birds, are also susceptible to these physiological limitations. Male bottle-nosed dolphins (Tursiops truncatus) are known to have trouble dissipating heat and move into cooler waters during the warmer periods of the year (Barbieri, 2005). In the Gulf of México, tuna and billfish have recently been recorded at deeper than usual depths, presumably to access cooler waters (Dell’Apa et al., 2018). Rising temperatures also affect deep-sea species such as giant squid (Architeuthis spp.). Studies have shown that water above 10°C is linked with decreased ability of squid blood to bind O2, resulting in suffocation (Guerra et al., 2011). In the case of the giant squid, rising acidity is also a concern. Too much acidity causes malformations in the structures squid use to sense their position and direction of movement, compromising an individual’s ability to navigate (Guerra et al., 2011). Guerra et al. (2011) suggested that the combined physiological effects of climate change may be responsible for the increased number of strandings observed worldwide.
Warming waters also will allow tropical marine species to live at higher latitudes, further contributing to a poleward shift. Increasing numbers of tropical and subtropical fish species recorded in the Tasmanian Sea (Last et al., 2011) and in the North Pacific (Polovina et al., 2011) provide evidence for this shift. Similarly, there are more frequent sightings of warm-water cetacean species in northwestern Scotland (MacLeod et al., 2005), while eastern Australian bull sharks (Carcharhinus leucas) have extended their range to the south and are present for longer (Niella et al., 2020).
The situation at the poles is even more clear-cut. As the Arctic ice sheet diminishes, mortality in the Atlantic walrus (Odobenus rosmarus) calves is increasing, as larger herds are being crowded into smaller available sites for hauling out (Kovacs et al., 2015). Likewise, ringed seals (Pusa hispida) in Svalbard, faced with a lack of ice have changed their behavior to haul out onto terrestrial sites, a phenomenon never before observed, with as yet unknown consequences for the population.
Life at the Edge-Emperor Penguins
Some marine megafauna already live at their physiological limits. Emperor penguins (Aptenodytes forsteri, IUCN- Vulnerable), are among these species. Like many Antarctic species, emperor penguins require large areas of sea ice for breeding- areas that are too small have been linked to lowered breeding success (Jenouvrier et al., 2009). Increased numbers of extreme weather events in the Antarctic have been recorded in recent years, a trend expected to continue under most climate change scenarios (Jenouvrier et al., 2009; Ropert-Coudert et al., 2019). These extreme weather events can cause sea ice to rapidly break up and disappear, as happened during the 1970s (Jenouvrier et al., 2009) and again in 2016 (Turner et al., 2017). Both times the emperor penguin population crashed due to loss of breeding grounds. Violent weather and storms can also force large icebergs up on the shore, blocking easy access to feeding grounds from the nesting sites, causing longer, strenuous overland treks to ocean access points. This has a cascade of effects starting with increased energetic costs for the parents leading to lowered hatchling provisioning and ultimately increased hatchling failure (Lescroel et al., 2014). As the frequency of extreme events increases, it is not known if emperor penguins will able to disperse to new habitats (Ropert-Coudert et al., 2019) or where those habitats would be.
Losing the Ice Beneath Their Paws- Polar Bears
Polar bears (Ursus maritimus, IUCN- Vulnerable) live in the Arctic, spending the majority of their time on sea ice from which they prey on various pinniped species. Their prey include bearded seals (Erignathus barbatus), ringed seals, harp seals (Pagophilus groenlandicus), and occasionally beluga whales (Delphinapterus leucas) (Galicia et al., 2016; Durner et al., 2017). Increases in air and water temperatures are causing a loss of sea ice affecting both the bears and their prey (Galicia et al., 2016). Smaller pieces of ice are more readily carried by the ocean currents (Durner et al., 2017), requiring the bears and their young to swim more to stay within their home ranges and to hunt (Durner et al., 2017). Swimming requires approximately five times more energy than walking (Lone et al., 2018), so the bears need to find more prey to maintain their metabolism. Cubs of the year are particularly impacted by lengthening swims; they have reduced body fat making them more susceptible to hypothermia than adults. Their lower muscle development and endurance put them at increased risk of drowning, especially in rough waters (Lone et al., 2018). Warming temperatures and reduced ice also appear to affect the denning habits of pregnant females. Female polar bears use dens to give birth and raise their cubs for the first months of life. In Baffin Bay, west Greenland, females are more frequently selecting terrestrial locations at higher elevations on steeper slopes than in the past (Escajeda et al., 2018). Shifts in the timing of melting sea ice and changes in suitable snow conditions are hypothesized to be the cause of this change (Escajeda et al., 2018). Females are also denning later, likely due to the increased distance required to travel to these new sites combined with the need to remain at the hunting grounds for longer periods (Escajeda et al., 2018). The dates when the bears emerge with their cubs have not shifted, resulting a shorter overall denning time (Escajeda et al., 2018). The effects of changes in denning behavior on cubs and adults is unknown (Escajeda et al., 2018).
Impact of Climate Change on Reproduction and Disease
The eggs and larvae of megafauna are particularly sensitive to changing ocean conditions. Climate change will affect sea temperature, turbidity, and marine chemical conditions (Hoegh-Guldberg et al., 2018; Bindoff et al., 2019). Increasing SST and the increasing inflow of freshwater from melting ice will alter water column structure, that in turn will affect circulation patterns and nutrient availability. Atlantic yellowfin tuna (Thunnus albacares) in the Gulf of México preferentially lay their eggs in turbulent waters of the loop current and its associated eddies. Instabilities associated with climate change will create current fluctuations which could introduce water that is too cold for tuna eggs and larvae to survive (Dell’Apa et al., 2018). The effect on eggs and larvae is intensified by more frequent hypoxic events and acidification (Dell’Apa et al., 2018). Sharks eggs are similarly affected (Pistevos et al., 2015; Rosa et al., 2017).
Extreme weather also affects reproductive success. During very hot or stormy years, seabirds such as Magellanic penguins (Spheniscus magellanicus) (Boersma and Rebstock, 2014), razorbills (Alca torda) (Newell et al., 2015), and African penguins (S. demersus) (Traisnel and Pichegru, 2018) can lose all of their eggs, suffer massive mortalities, or forego nesting altogether, resulting in zero recruitment for that year. Likewise, cetaceans prefer calmer, reef protected waters for calving, presumably because rougher, storm-blown waters are disadvantageous for the young (Whitehead and Moore, 1982). Decreased recruitment over successive generations puts already vulnerable species at an even greater risk. Increases in frequency of extreme events is predicted under all the climate change models, diminishing the time these populations will have to recover from such events (Vargas et al., 2005).
Warming sea temperatures also are correlated with increased frequency of disease outbreaks and harmful algae blooms (HAB) (Gambaiani et al., 2009; Doney et al., 2012). Increased stress, due to the effects of climate change (deteriorating habitat, reduced food) will cause some megafauna species to become more susceptible to disease. Furthermore, warming climate is linked with increased pathogen survival and transmission (Barbosa and Palacios, 2009). Mass mortalities in arctic pinnipeds due to pathogens and parasites have been observed after periods of warmer than average weather when sea-ice is reduced. Less ice results in less space at haul out sites and higher densities, while more open water allows increased connections between previously isolated populations, both factors that contribute to increased transmission rates (Burek et al., 2008; VanWormer et al., 2019). Feather loss disorder, known originally from African penguins has spread to more poleward distributions of Magellanic penguins in Argentina. This has been attributed to warmer ambient temperatures allowing the pathogen’s survival (Ropert-Coudert et al., 2019). Increased frequency of storms and associated flooding are also hypothesized to increase exposure to parasites and pathogens (Schumann et al., 2013 and references therein).
Higher SST – Skewed Sex Ratios in Sea Turtles
All sea turtles (IUCN-Vulnerable to Critically Endangered depending on species) are long-lived, species (Hamann et al., 2013). Sea temperature affects their metabolisms while air temperature affects the sex-ratios of hatchlings (Hamann et al., 2013) and thus population demographics. Warmer temperatures feminize embryos, skewing the male to female ratio in the population. Moreover, increasing beach erosion and destruction yields smaller beach areas for nesting, resulting in crowding of nests that also is linked to the increased feminization of eggs, and increased nest destruction as females inadvertently dig up previously created nests when preparing their own (Katselidis et al., 2014). Crowded nesting sites also attract predators that feed on both eggs and hatchlings.
Predicting the turtles’ potential response to the loss of nesting sites is complicated by their reliance on ocean currents for migrations. Hatchlings spend approximately the first 3 years of their lives drifting in the ocean currents. Evidence suggests that adults base their future migration routes on the dispersal routes they followed during this drift (Scott et al., 2014). There is still much uncertainty about how current patterns will change under the various climate change scenarios (Hamann et al., 2013). Turtles have a very long life span, so any alterations in current patterns have the potential to impair the ability of older individuals to navigate, putting at risk their ability to return to their breeding habitats (Hamann et al., 2013). If they do return to their home beaches, over time they will find that some beaches have disappeared due to climate-driven effects. It is also possible that climate change will cause phenological changes in turtle behavior. There is some evidence that seasonal patterns of nesting could be shifting to cooler periods, being driven by temperature changes in nearshore waters (Hawkes et al., 2009), but there is no published evidence, as of yet, that female turtles will move to new beaches (Katselidis et al., 2014).
Toxic Algae and Sirenians
The micro-organisms that can adversely affect marine megafauna are also shifting their ranges to higher latitudes. Harmful algal blooms (HAB) such as those of the dinoflagellate Karenia brevis, that causes red tides, have increased in frequency and expanded spatially over the past 20 years, as a response to warmer water temperatures (Burek et al., 2008; Marsh et al., 2017; Martin et al., 2017). Sirenians (manatees, Trichechus spp. IUCN-Vulnerable and dugongs Dugong dugon IUCN-Vulnerable) are exposed to the toxins created by HABs when they ingest toxin-laden sea water, seagrasses, and epiphytes (Marsh et al., 2017). HAB events are linked to mass-die offs, but even during non-significant bloom years HAB can cause deaths in marine megafauna (Martin et al., 2017). During years with large blooms, the baseline mortality rate among adult manatees in southwest Florida is estimated to increase by 7% (Martin et al., 2017). Because sirenians are slow breeders, only attaining reproductive maturity between 10 and 15 years of age, producing one calf at a time, and having long periods between pregnancies (Moore et al., 2017), repeated years of elevated mortality can lead to population crashes. With HABs predicted to increase over the next century under climate change (Anderson et al., 2015) sirenians are at further risk of extinction.
Shifting Food Sources Due to Climate Change
Climate change will affect many of the species upon which marine megafauna rely for food. The ability of megafauna to survive will depend on their ability to shift their ranges accordingly. Such range shifts of megafauna, however, may be limited by the reasons already described. For megafauna that undergo annual migrations to feed, successful adaptation would require changes such as the timing of migrations, an extension of time spent at the feeding grounds (Ramp et al., 2015; Thomas et al., 2016), a relocation of feeding grounds, and/or breeding grounds (Meyer-Gutbrod et al., 2018). In migratory baleen whales, extended stays at feeding grounds can have negative consequences for newborns, if there is not enough time left prior to birth for the whales to arrive at their breeding grounds. The risk is that newborns arrive in rough, open ocean waters, where it is difficult for them to breathe, rather than the calm, protected waters of their breeding grounds. This phenomenon has already been documented in gray whales (Eschrichtius robustus), where mothers have been observed with newborns prior to arrival at their breeding grounds (Thomas et al., 2016). To the authors’ knowledge, it is not yet known how many of the calves born prior to the mothers’ arrival at the breeding grounds survive. Just in the past few years, North Atlantic right whales (Eubalaena glacialis) were observed feeding in the Gulf of St. Laurence, north of their usual feeding grounds off of Maine (United States) in response to a shift northward of the copepods upon which they prey (Meyer-Gutbrod et al., 2018). This northward shift of feeding grounds creates a longer migration to the breeding grounds off the South Eastern US coastline.
Non-migrating species are also at risk. Scientists have noted marked responses in the spatial distribution of the endangered Māui dolphins (Cephalorhynchus hectori ssp. maui) off the West Coast of the North Island, New Zealand during marine heat wave years (Baker et al., 2018). This may be driven by shifting prey distributions, given the tight spatial coupling between Hector’s/Māui dolphins and their main prey species, and the limited palate of prey species available to Māui dolphin (Roberts et al., 2019). Temperature extremes are increasing in intensity and frequency (Salinger et al., 2019), and may impede the future recovery of Māui dolphins (Weir, 2018; Roberts et al., 2019).
In seabirds, increasing distance between the nesting sites to the location of prey puts hatchlings at risk, as observed in emperor and Adélie penguins (Pygoscelis adeliae) (Barbraud and Weimerskirch, 2006; Lescroel et al., 2014), and Hawaiian seabirds (Polovina et al., 2011). Increasing sea surface temperature since the mid-1990s has affected the abundance and quality of prey for yellow-eyed penguins (Megadyptes antipodes) in New Zealand, and correlates strongly with reduction in survival rates of both chicks and adults, and population decline (Mattern et al., 2017). Increasing sea surface temperatures and decreases in primary productivity in the Southern Ocean are also linked to the decline in the populations of rockhopper penguin (Eudyptes chrysocome) throughout most of its circumpolar breeding distribution. Stable isotope analyses show a shift in rockhopper diet to prey of lower trophic status over time and in warm years (Hilton et al., 2006).
Shifting Prey and Isolated Populations- Mediterranean Fin Whales
Shifting prey could pose challenges for populations of megafauna living in semi-enclosed and marginal seas. Fin whales (Balaenoptera physalus, IUCN-Vulnerable) are globally distributed, with two known restricted, non-migrating populations, one each in the Mediterranean Sea and the Gulf of California (López et al., 2019). These two populations are considered at risk from climate change primarily because of expected impacts on the availability of their food source (Gambaiani et al., 2009). Mediterranean fin whales eat only krill of the species Meganyctiphanes norvegica which are at their thermal limit (18°C) in the Mediterranean Sea (Gambaiani et al., 2009). These krill are susceptible to rising SST, declining pH, and changes in the circulation patterns of the Mediterranean, all of which will impact demographic patterns, dispersal, and survival rates (Gambaiani et al., 2009; Constable et al., 2014). As the effects of climate change progress in the Mediterranean, this krill species, and thus Mediterranean fin whales, are at risk of local extinction.
It is unknown to what degree whales can change their range in pursuit of changing patterns of food distribution. There is evidence that some cetacean migrations are based on culture and knowledge passed from one generation to the next (Whitehead et al., 2004). As the whales that know how to migrate die, so does the knowledge in the population. Direct observation and photographic evidence suggests that fin whales from the North East North Atlantic population migrate annually into the Mediterranean (Gauffier et al., 2018), yet genetic evidence suggests that the resident population does not mix with these itinerant whales, remaining genetically distinct (Bérubé et al., 1998; Panigada et al., 2017). It is possible that this population has been isolated long enough for the knowledge about migration possibilities and routes to have been lost. If this does prove to be the case, and if the krill species succumb to climate change, so may the Mediterranean fin whales.
Shifting Prey and Shifting Migrations- Whale Sharks
Whale sharks (Rhincodon typus, IUCN vulnerable), the largest species of fish, are among those marine megafauna likely to be affected by shifting food resources. These filter feeders undergo long, poorly understood migrations (Hueter et al., 2013) between sites where they aggregate and feed (Bradshaw et al., 2008; Graham, 2015). Scientists know little about what factors, including those related to climate, influence these migrations, although recent studies suggest food as a likely driver (Hueter et al., 2013; Rohner et al., 2015).
Whale sharks need to consume large quantities of prey (Nelson and Eckert, 2007; Rohner et al., 2015), yet paradoxically, live in nutrient-poor waters of the tropical and subtropical oceans where large concentrations of macro-zooplankton are generally scarce (Rohner et al., 2015). Those tropical areas that are productive attract large aggregations of whale sharks at predictable times throughout the year (Bradshaw et al., 2008; Graham, 2015). Whale sharks will return to the same sites on periodic bases (Hueter et al., 2013; McCoy et al., 2018) coincident with blooms of their prey. Evidence indicates that juvenile whale sharks feed on a variety of macro-zooplankton species, including shrimp, copepods, krill and fish larvae (Nelson and Eckert, 2007; Rohner et al., 2015), all of which will be affected by climate change. As ocean water warms and patterns of prey species shift, it is likely that juvenile whale sharks will attempt to follow these prey, thus shifting their range, and the locations of feeding aggregations.
Will Megafauna Move or Go Extinct?
As climate change alters the opportunities for shelter, reproduction, survival, and food, some marine megafauna will undergo range shifts while others risk extinction. Those populations for which ranges are not blocked by land or other obstacles have the best chance to adapt to climate change. Humpback whales (Megaptera novaeangliae) are plastic in their habitat use, and are considered to have relatively high adaptive capacity to ocean warming, yet modeling predicts that many Oceania breeding grounds will become unsuitably warm for this species (>28°C) by the end of the twenty-first century. Breeding grounds are predicted to move to the archipelagos and seamounts of southern Oceania (Derville et al., 2019).
Range shifts in some megafauna taxa will be enabled via new or existing environmental bridges – corridors that allow for the movement from one part of the ocean to another. Some bridges will become available due to changing climactic conditions. Reduced Arctic sea ice is allowing access to novel feeding grounds (e.g., bowhead whales, Balaena mysticetus in the Arctic) (McKeon et al., 2016), while thermal isoclines in the ocean create dynamic pathways of oceanic conditions in which fish can survive and move (Briscoe et al., 2017).
Geographical, environmental and human-made barriers may limit the adaptive capacity and ability for species to move. For megafauna living in marginal seas, land masses and bathymetric conditions make escape routes difficult to navigate. For instance, in the Mediterranean Sea, fin whales can leave the western Mediterranean Sea through the Straits of Gibraltar, while the 12 species of cetaceans living in the Hellenic Trench (Frantzis et al., 2003) are surrounded by much shallower seas that could make leaving difficult. Land masses (e.g., Asia and the Indian sub-continent) prevent poleward range shifts for many northern hemisphere megafauna species (Polovina et al., 2011; Constable et al., 2014). Climate drivers already affect the foraging distribution and success (and hence the health and reproduction) of pinniped species in the subAntarctic and Southern Oceans including: subAntarctic fur seals (Arctocephalus tropicalis) (Oosthuizen et al., 2016) and southern elephant seals (Mirounga leonina) on Marion Island (McIntyre et al., 2011), Antarctic fur seals (Arctocephalus gazella) at South Georgia (Forcada and Hoffman, 2014), and southern elephant seals at Macquarie Is (van den Hoff et al., 2014). Options to shift distributions and to find new suitable habitats are limited for many of these populations by vast expanses of oceans and lack of coastlines. For example, long term population decline of the endangered New Zealand sea lions (Phocarctos hookeri) is partly driven by nutritional stress (Roberts and Doonan, 2016; Roberts et al., 2018). New Zealand sea lions are constrained in their ability to follow poleward shifts in temperature and prey. There are no new feeding grounds south from their home on the subAntarctic Islands, only a vast stretch of ocean to Antarctica.
Water temperature can also be a barrier to range shifts. Oceanic fronts of cool water that extend across equatorial seas during La Niña events are known to prevent the normal migration of blue marlin (Makaira nigricans) across the equator (Briscoe et al., 2017). One species’ bridge, may be another’s barrier. Melting Arctic sea ice allows whales to move between the Atlantic and Pacific oceans (McKeon et al., 2016), but the bears and pinnipeds that depend on that ice for their habitat will find themselves with a diminishing range area and fractured populations.
Human created barriers also exist limiting options for marine megafauna to move. Shipping lanes and fishing grounds can be gauntlets for many types of marine megafauna. There is a high risk of being hit by ships, or tangled in fishing gear. Of the 70 North Atlantic right whale mortalities recorded between 2003 and 2018, 42% were due to ship strikes while 58% were due to entanglement in fishing lines (Sharp et al., 2019). Although many whale strikes go unreported (Jensen et al., 2004), numbers are known to be high for numerous whale species throughout the world (Monnahan et al., 2015; Di-Méglio et al., 2018). Moreover, shipping lanes create concentrated areas of noise and chemical pollution. Even though they are non-physical structures, they are comparable to terrestrial roads, and may create the same type of barrier (Pirotta et al., 2019).
Offshore renewable energy may also create new physical barriers for marine megafauna. Like their wind counterparts, numerous tide turbines will be clustered in discrete areas. Interactions with the rotating units tend to be lethal, making it difficult to forage around turbines (Fox et al., 2018). As more wind, wave, and tidal energy projects are approved and constructed, mortality of marine megafauna due to these lethal interactions will likely increase. In a phenomenon known as “coastal squeeze,” coastal developments, especially those that include hard-armored shorelines, will prevent lateral and landward shifts of critical coastal habitats such as beaches, rocky platforms, seagrass meadows, mangroves, etc. (Katselidis et al., 2014). Coastal squeeze could be particularly problematic for megafauna that depend directly on coastal habitats for nesting or nurseries and feeding areas (Gittman et al., 2016; Sievers et al., 2019).
These bridges and barriers will shape new species assemblages as populations and species are separated or brought together. There will be effects on predator/prey relationships behavior, habitat use and resource competition. Reduced sea ice in Svalbard is separating polar bears from one prey species, ringed seals, and bringing them into increased contact with a different group of prey, ground-nesting birds (Hamilton et al., 2017). Similarly, reduced sea ice is allowing orcas (Orcinus orca) more frequent access to the Hudson Bay, where they prey on the local populations of narwhal (Monodon monoceros) and bowhead whales (Eubalaena mysticetus) (Higdon and Ferguson, 2009). Recent studies of the behavior of these two prey species in the presence of orcas suggests that this will cause changes in behavior and habitat use, leading to increased stress and decreased fitness (Breed et al., 2017; Matthews et al., 2020), and certainly will cause downstream changes in the local ecosystem.
Human Winners and Losers
Climate-induced shifts in the ranges of megafauna will undoubtedly create economic winners and losers (Pecl et al., 2017). Tropical seas are predicted to lose biodiversity as their water temperatures increase beyond the thermal maxima of the species living there (Poloczanska et al., 2016) resulting in adverse economic impacts. The subtropical zone is expected to expand under climate change (Polovina et al., 2011), bringing new biodiversity to people in higher latitudes and with it, new megafauna ecosystem services. People living in the Arctic will need to adapt to a new suite of megafauna as the species they traditionally use are displaced or go extinct.
Many people depend on marine megafauna for their nutrition, livelihood, and cultural well-being. This is especially true in non-temperate coastal areas where the rural, coastal poor are highly dependent upon the oceans for nutrition, materials, and income (Barbier, 2015; Pecl et al., 2017; Ripple et al., 2019). Local megafauna contribute to tourism industries (Tisdell and Wilson, 2005; Hobday et al., 2015; Moore et al., 2017) that are based on such opportunities as watching marine turtles lay their eggs, swimming with whale sharks, catching marlin, and going whale watching. Tourism is a non-negligible economic resource for many coastal areas; exploiting opportunities for marine megafauna-based tourism can be a particularly important source of much needed foreign income for developing economies (Bindoff et al., 2019, p. 110).
Marine megafauna also play an important role in the culture and history of local communities. Cetaceans in particular have long attracted humans’ attention. From cultures originating around the Mediterranean, consider Jonah’s misadventures in the Old Testament, Captain Ahab’s relationship with Moby Dick, and the sea monsters (“Cetus”) slain by Perseus and Hercules. Pacific Island navigators and way finders followed migrating whales to Aotearoa-New Zealand, and whales continue to have an important place in modern Māori culture specifically (see following case study and Figure 2). Polynesian culture in general has a rich and complex relationship with cetaceans, where they are symbols of religious power, political power and honor (Whimp, 2008). Outside the oceanic island communities, the peoples of the Pacific Northwest have long hunted gray whales, and continue to see this as an important part of maintaining their cultural traditions and ties to their ancestors (Deutsch, 2017). Many coastal indigenous groups in the Arctic also depend on marine megafauna for nutrition, cultural identity and subsistence livelihoods.
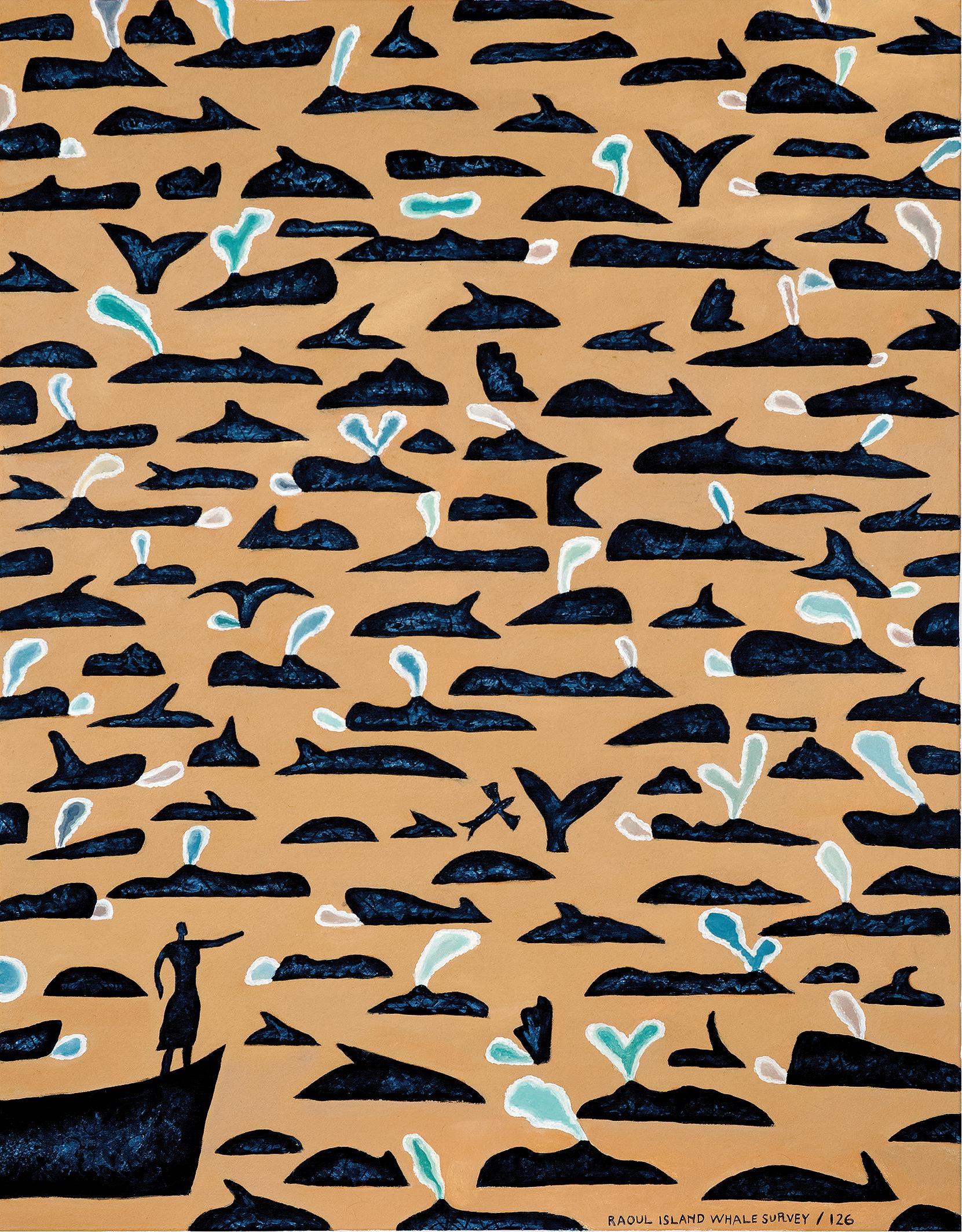
Figure 2. Original artwork: Raoul Island Whale Survey 126, 2012 by Gregory O’Brien demonstrating the relationships between Indigenous peoples and marine megafauna, in this case the importance of whales to Māori people of Aotearoa – New Zealand. Used with permission. ©Gregory O’Brien.
Other groups of megafauna also figure into various cultures’ spiritual worlds. Sharks and rays symbolize bravery and tenacity in many cultures, from Western Africa where sawfish rostra are used as home decorations and mask decorations for rituals to symbolize and recognize acts of bravery (Robillard and Séret, 2006), to Arnhemland in Australia where sawfish are integral to the local tribes’ creation myths and their stories of the landscape formation (McDavitt, 2005). Polynesian traditions, sea turtles are considered aumakua (embodiment of ancestors) and are symbolic of the social hierarchy, as evidenced by the complex rules pertaining to their consumption and the wearing/use of their shells (Rudrud, 2010).
The Cultural Connection Between People and Marine Megafauna, Example From Ngāti Kuri and Rangitāhua (Kermadec Islands) Ocean Mothers
Ngāti Kuri’s ancestors were Polynesian voyagers who traveled on some of the first waka (large canoes) to arrive in Aotearoa (New Zealand). They navigated the south Pacific using traditional methods including winds, stars, the moon, the sun and following migrating birds and whales via Rangitāhua (the Kermedec Islands). Ngāti Kuri are a tribe descended from the founding peoples of the northernmost peninsula of Aotearoa. Their ancestors knew these animals intimately and trusted them implicitly to show them to new lands, new lives and new opportunities. A saying of theirs is: “Tere Tohorahā, Tere Tangata” (where whales travel, people follow).
Rangitāhua is an archipelago northwest of the North Island of Aotearoa that is a point of convergence for many migratory species, such as turtles, whales and seabirds (Clark et al., 2017). Ngāti Kuri hold these islands sacred; for they served as a haven on the southward migration of discovery. Today they are revered as a nursery for all creatures. Central to our case here, they are a place where the annual migratory pathways of several different populations of humpback whales overlap. Individual whales stay in these islands on average about 4.5 days, during which time they sing and exchange songs between different populations (Owen et al., 2019). Ngāti Kuri identify humpack whales as Ocean Mothers – creators and guardians that protect and nurture. The relationship between Ngāti Kuri and the humpback whales is one of kinship, linked by genealogy, and shared ancestry. In these family ties, there is love, respect, reverence and responsibility. The following account, shared and written by Sheridan Waitai of Ngāti Kuri describes some of the ancient links between Rangitāhua, whales and people.
RangitāHua-Kermadec Ocean Mothers
Rangitāhua (Kermadec) is where the sweetest sounds are sung by our Ocean Mothers. They are ancient hymns, like Oriori (traditional waiata) stories, sung to their children. They tell of journeys, geography, and whakapapa (genealogy). Their voices mark historical events, speak of hapuatanga (pregnancy/births), reciprocity, and retribution. They chronicle essential wisdom for their young, so they will mind their mothers. Rangitāhua is where Hine Rakautauri, the songstress, Hine Te Iwaiwa, the weaver, and their sister Hinemoana reside in the moontimes of Koanga (springtime). These are our Hines (females) who give the rhythm and the weaving of sounds, who support the ocean mothers and their young as they travel, so all beneath the ocean crests may savor “Te Reo Aroha” (the language of love). Ocean Mother’s odyssey, her song, is why Ngāti Kuri says Rangitāhua is the Kohanga of all Kohanga (the nesting ground above all). Our beloved Rangitāhua is the breath of heaven, the guiding light, the burning sky, flickering in Te Moana Nui A Kiwa (Pacific Ocean) so creation may find sanctuary to raise their young.
Like other species discussed in this paper, climate change is affecting the distribution of humpback whales in the Pacific Ocean in as yet unknown ways (Derville et al., 2019). If a consequence of climate change were alterations of the migration patterns of the whales, so they no longer came to Rangitāhua, it would be at great cost to the Ngāti Kuri people. As Sheridan Waitai explains- “Humpback whales are the taonga (sacred) species of Ngāti Kuri, loss of these whales would be a personal and emotional tragedy like the loss of a beloved grandmother, but not just one grandmother – an entire familial line, an entire tribe. This would be a collective tragedy to Ngāti Kuri, and a tohu – a sign that we, all who live on earth have gone beyond the point of return. Extinction of these sacred species would be a tear in the fabric of life, a desecration, signaling global scale crisis.”
Managing and Adapting to Changes in Marine Megafauna
While CO2 mitigation continues to be the best and most complete solution to reduce the impacts of climate change on marine organisms (Gattuso et al., 2018), more immediate, regional actions, including improved regional co-operation, will be needed to deal with the inevitable impacts of climate change on marine megafauna, and will require a multidisciplinary approach (Pecl et al., 2017; Bonebrake et al., 2018). Networks of marine protected areas and other area-based management approaches, will need to account for shifting megafauna ranges. In many places existing marine megafauna migratory routes traverse national boundaries and international waters. In such areas international coordination and agreement is necessary to improve MPAs and enforce high seas regulations (e.g., RFMO agreements, Harrison et al., 2018). Tagging and tracking technology is already used to identify areas of ecological significance for marine megafauna and to highlight potential areas of conflict with the fishing industry (Hindell et al., 2020). This same technology could identify areas with a high risk of ship strikes, and over time allow managers to track the expansion of megafauna ranges to inform regional action plans. Increasingly sophisticated habitat models that predict where important habitats may be in the future, can help to “climate-proof” marine spatial planning and MPA network design (Derville et al., 2019), and identify which nations will need to come together in collaboration.
Similarly, managers need to begin anticipating how and where other local measures will be needed to help megafauna thrive in their new ranges. These local measures include reducing pollution, protecting the integrity of the shoreline (i.e., restricting coastal squeeze to locations where it already is), restoring hydrological regimes, reducing overfishing, establishing more protected areas, enforcing rules in those areas already protected (Gattuso et al., 2018; Harrison et al., 2018), and anticipating future conflicts between megafauna and fishing gear and ships. These local actions could buy time for marine megafauna that will ultimately have to shift their ranges, and also can pave the way for healthy environmental conditions in the new ranges where marine megafauna will live. Reducing overfishing will increase food resources for megafauna while pollution reduction will help prevent eutrophication and harmful algae blooms. To ensure future options for nesting sites and breeding grounds, managers will need to account for changing beaches and other coastal habitats. For example, conservation managers in New Zealand are increasing efforts to protect a newly established colony of New Zealand sea lions on Otago Peninsula which has access to rich foraging grounds, and is growing; compared to the declining subAntarctic Islands populations affected by climate-driven nutritional stress (New Zealand Department of Conservation and New Zealand Ministry for Primary Industries, 2017). Another example of a local-scale action mitigating the short-term effects of climate change for megafauna is building shade to cool turtle nests and help to keep maintain hatchling sex ratios (Saba et al., 2012). But there will inevitably be cases where a difficult choice must be faced when megafauna predators move into a new range and prey upon locally protected species.
Local action, however, often requires political will and commitment from the people immediately affected, especially local stakeholders (Serrao-Neumann et al., 2014). Because climate change may bring marine megafauna to places they were not previously found, cultural and political enabling conditions, normally important to generate political will, may not exist. Environmental and ocean literacy campaigns need to start working now at the grassroots level to help communities understand their new megafauna neighbors and their responsibility to help them thrive.
Finally, NGO’s, aid organizations, and development agencies need to work with local communities and economies to start planning now for the potential loss of marine megafauna that could affect human nutritional well-being, economic income, livelihood support, and even lost cultural values. This means taking action to secure alternative livelihoods, other sources of income, and possibly nutrition. Adapting to the loss of culturally important marine megafauna, however, will likely be complicated. Cultural anthropologists, sociologists, Elders, and members of the local religious communities need to be consulted now to begin to prepare for the new reality of the local extinction of culturally important marine megafauna.
Conclusion
Range shifts in marine megafauna will be an inevitable and undeniable consequence of climate change. These range shifts will occur due to climate driven factors that include a loss in quality of the places where marine megafauna now live, a loss in population resilience and growth due to impacts on reproductive success and disease, and finally a change in the areas where food can be found reliably and in sufficient quantity. These range shifts will create ecological and economic winners and losers. While we work to reduce our carbon emissions and to mitigate climate change, shifting ranges of marine megafauna have already begun.
To help better manage the impacts of climate change on marine megafauna, the following will be required:
(1) the tagging and tracking of more individuals of more species over longer terms, to better understand changes in ranges,
(2) more and better modeling to identify where to begin planning for new protected areas, management, and regulation,
(3) the creation of corridors and expanded zones of management to accommodate potential range shifts,
(4) targeted social and cultural research to understand which communities, especially those in the Arctic and tropical regions, are most likely to be affected by range shifts and the loss of megafauna resources, and
(5) education and outreach in the affected communities, to help raise their awareness of what is happening, what they can do to help prevent it, and how they can adapt to better deal with the changes.
Even the best research and ocean literacy agenda will be unable to completely mitigate the impacts of climate change on megafauna and people, but starting now to develop careful, targeted research and planning is essential to mitigate the impacts to humans and can help create ecological room for megafauna who will need to seek new places to live in a changing ocean.
Author Contributions
SG contributed to the text. LP led the development of the conceptual design and contributed text to all sections of the manuscript. AL contributed to the text, in particular providing content for species in Aotearoa-NZ. AC provided input on the content. SW provided the text for the cultural connection between Ngāti Kuri and whales. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the ISblue project, Interdisciplinary graduate school for the blue planet (ANR-17-EURE-0015) and co-funded by a grant from the French government under the program “Investissements d’Avenir.”
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Melanie Lancaster of WWF-Canada for input regarding arctic species. Figure 1 was drawn by Sharon O’Brien, and the artwork in Figure 2 provided by Gregory O’Brien. We would also like to thank our editor and reviewers for their invaluable commentary.
References
Anderson, C. R., Moore, S. K., Tomlinson, M. C., Silke, J., and Cusack, C. K. (2015). “Living with harmful algal blooms in a changing world: strategies for modeling and mitigating their effects in coastal marine ecosystems,” in Coastal and Marine Hazards, Risks, and Disasters eds J. T. Ellis and D. J. Sherman (Amsterdam: Elsevier), 495–561. doi: 10.1016/B978-0-12-396483-0.00017-0
Baker, S., Harbers, R., Harbers, B., Hickman, G., Garg, R., and Constantine, R. (2018). Surveys of Māui Dolphins in 2018: a preliminary report on sighting rates and local distribution. Appl. Environ. Microbiol. 76, 6821–6828.
Barbier, E. B. (2015). Climate change impacts on rural poverty in low-elevation coastal zones. Estuar. Coast. Shelf Sci. 165, A1–A13. doi: 10.1016/j.ecss.2015.05.035
Barbieri, M. M. (2005). Physiological and Behavioral Thermoregulation in Bottlenose Dolphins (Tursiops truncatus) in Sarasota, Florida. Ph.D. thesis, University of North Carolina Wilmington, Wilmington.
Barbosa, A., and Palacios, M. J. (2009). Health of Antarctic birds: a review of their parasites, pathogens and diseases. Polar Biol. 32, 1095–1115. doi: 10.1007/s00300-009-0640-3
Barbraud, C., and Weimerskirch, H. (2006). Antarctic birds breed later in response to climate change. Proc. Natl. Acad. Sci. U.S.A. 103, 6248–6251. doi: 10.1073/PNAS.0510397103
Bérubé, M., Aguilar, A., Dendanto, D., Larsen, F., Di Sciara, G. N., Sears, R., et al. (1998). Population genetic structure of North Atlantic. Mediterranean Sea and Sea of Cortez Fin whales, Balaenoptera physalus (Linnaeus 1758): analysis of mitochondrial and nuclear loci. Mol. Ecol. 7, 585–599. doi: 10.1046/j.1365-294x.1998.00359.x
Bindoff, N. L., Cheung, W. W. L., and Kairo, J. G. (2019). “Changing Oean, Marine Ecosystems, and Dependent Communities,” in Special Report: The Ocean and Cryosphere in a Changing Climate Summary for Policymakers, eds H.-O. Portner, D. C. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, et al. (Geneva: IPCC).
Boersma, P. D., and Rebstock, G. A. (2014). Climate change increases reproductive failure in Magellanic Penguins. PLoS One 9:e85602. doi: 10.1371/journal.pone.0085602
Bonebrake, T. C., Brown, C. J., Bell, J. D., Blanchard, J. L., Chauvenet, A., Champion, C., et al. (2018). Managing consequences of climate-driven species redistribution requires integration of ecology, conservation and social science. Biol. Rev. 93, 284–305. doi: 10.1111/brv.12344
Bornatowski, H., Navia, A. F., Rennó Braga, R., Abilhoa, V., and Maia Correa, M. F. (2014). Ecological importance of sharks and rays in a structural foodweb analysis in southern Brazil. ICES J. Mar. Sci. 71, 1586–1592. doi: 10.1093/icesjms/fsu025
Bradshaw, C. J. A., Fitzpatrick, B. M., Steinberg, C. C., Brook, B. W., and Meekan, M. G. (2008). Decline in whale shark size and abundance at Ningaloo Reef over the past decade: the world’s largest fish is getting smaller. Biol. Conserv. 141, 1894–1905. doi: 10.1016/j.biocon.2008.05.007
Breed, G. A., Matthews, C. J. D., Marcoux, M., Higdon, J. W., LeBlanc, B., Petersen, S. D., et al. (2017). Sustained disruption of narwhal habitat use and behavior in the presence of Arctic killer whales. Proc. Natl. Acad. Sci. U.S.A. 114, 2628–2633. doi: 10.1073/pnas.1611707114
Briscoe, D. K., Hobday, A. J., Carlisle, A., Scales, K., Eveson, J. P., Arrizabalaga, H., et al. (2017). Ecological bridges and barriers in pelagic ecosystems. Deep Res. Part II Top. Stud. Oceanogr. 140, 182–192. doi: 10.1016/j.dsr2.2016.11.004
Burek, K. A., Gulland, F. M. D., and O’Hara, T. M. (2008). Effects of climate change on arctic marine mammal health. Ecol. Appl. 18, S126–S134. doi: 10.1890/06-0553.1
Clark, M. R., Trnski, T., Constantine, R., Aguirre, J. D., Barker, J., Betty, E., et al. (2017). Biodiversity of the Kermadec Islands and Offshore Waters of the Kermadec Ridge: Report of a Coastal, Marine Mammal and Deep-Sea Survey (TAN1612). Wellington: Ministry for Primary Industries.
Constable, A. J., Melbourne-Thomas, J., Corney, S. P., Arrigo, K. R., Barbraud, C., Barnes, D. K. A., et al. (2014). Climate change and Southern Ocean ecosystems I: how changes in physical habitats directly affect marine biota. Glob. Chang. Biol. 20, 3004–3025. doi: 10.1111/gcb.12623
Dell’Apa, A., Carney, K., Davenport, T. M., and Carle, M. V. (2018). Potential medium-term impacts of climate change on tuna and billfish in the Gulf of Mexico: a qualitative framework for management and conservation. Mar. Environ. Res. 141, 1–11. doi: 10.1016/j.marenvres.2018.07.017
Derville, S., Torres, L. G., Albertson, R., Andrews, O., Baker, C. S., Carzon, P., et al. (2019). Whales in warming water: assessing breeding habitat diversity and adaptability in Oceania’s changing climate. Glob. Chang. Biol. 25, 1466–1481. doi: 10.1111/gcb.14563
Deutsch, S. (2017). The struggle of a marginalized community for ethnic renewal: the whale hunters of Neah Bay. Environ. Sociol. 3, 186–196. doi: 10.1080/23251042.2017.1298183
Di-Méglio, N., David, L., and Monestiez, P. (2018). Sperm whale ship strikes in the Pelagos Sanctuary and adjacent waters: assessing and mapping collision risks in summer. J. Cetacean Res. Manag. 18, 135–147.
Doney, S. C., Ruckelshaus, M., Emmett Duffy, J., Barry, J. P., Chan, F., English, C. A., et al. (2012). Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37. doi: 10.1146/annurev-marine-041911-111611
Durner, G. M., Douglas, D. C., Albeke, S. E., Whiteman, J. P., Amstrup, S. C., Richardson, E., et al. (2017). Increased Arctic sea ice drift alters adult female polar bear movements and energetics. Glob. Chang. Biol. 23, 3460–3473. doi: 10.1111/gcb.13746
Escajeda, E., Laidre, K. L., Born, E. W., Wiig, Ø., Atkinson, S., Dyck, M., et al. (2018). Identifying shifts in maternity den phenology and habitat characteristics of polar bears (Ursus maritimus) in Baffin Bay and Kane Basin. Polar Biol. 41, 87–100. doi: 10.1007/s00300-017-2172-6
Forcada, J., and Hoffman, J. I. (2014). Climate change selects for heterozygosity in a declining fur seal population. Nature 511, 462–465. doi: 10.1038/nature13542
Fox, C. J., Benjamins, S., Masden, E. A., and Miller, R. (2018). Challenges and opportunities in monitoring the impacts of tidal-stream energy devices on marine vertebrates. Renew. Sustain. Energy Rev. 81, 1926–1938. doi: 10.1016/j.rser.2017.06.004
Frantzis, A., Alexiadou, P., Paximadis, G., Politi, E., Gannier, A., and Corsini-Foka, M. (2003). Current knowledge of the Cetacean fauna of the Greek Seas. J. Cetacean Res. Manag. 5, 219–232.
Galicia, M. P., Thiemann, G. W., Dyck, M. G., Ferguson, S. H., and Higdon, J. W. (2016). Dietary habits of polar bears in Foxe Basin, Canada: possible evidence of a trophic regime shift mediated by a new top predator. Ecol. Evol. 6, 6005–6018. doi: 10.1002/ece3.2173
Gambaiani, D. D., Mayol, P., Isaac, S. J., and Simmonds, M. P. (2009). Potential impacts of climate change and greenhouse gas emissions on Mediterranean marine ecosystems and cetaceans. J. Mar. Biol. Assoc. U.K. 89, 179–201. doi: 10.1017/s0025315408002476
Gattuso, J.-P., Magnan, A. K., Bopp, L., Cheung, W. W. L., Duarte, C. M., Hinkel, J., et al. (2018). Ocean solutions to address climate change and its effects on marine ecosystems. Front. Mar. Sci. 5:337. doi: 10.3389/fmars.2018.00337
Gauffier, P., Verborgh, P., Giménez, J., Esteban, R., Sierra, J. M. S., and De Stephanis, R. (2018). Contemporary migration of fin whales through the Strait of Gibraltar. Mar. Ecol. Prog. Ser. 588, 215–228. doi: 10.3354/meps12449
Gittman, R. K., Scyphers, S. B., Smith, C. S., Neylan, I. P., and Grabowski, J. H. (2016). Ecological consequences of shoreline hardening: a meta-analysis. Bioscience 66, 763–773. doi: 10.1093/biosci/biw091
Graham, R. T. (2015). Whale sharks of the western Caribbean: an overview of current research and conservation efforts and future needs for effective management of the species. Gulf Caribb. Res. 19, 149–159. doi: 10.18785/gcr.1902.18
Guerra, Á., González, Á. F., Pascual, S., and Dawe, E. G. (2011). The giant squid Architeuthis: an emblematic invertebrate that can represent concern for the conservation of marine biodiversity. Biol. Conserv. 144, 1989–1997. doi: 10.1016/j.biocon.2011.04.021
Hamann, M., Fuentes, M. M. P. B., Ban, N. C., and Mocellin, V. J. L. (2013). “Climate change and marine turtles,” in The Biology of Sea Turtles, eds J. Wyneken, K. J. Lohmann, and J. A. Musick (Boca Raton, FL: Taylor & Francis Group), 353–378. doi: 10.1201/b13895
Hamilton, C. D., Kovacs, K. M., Ims, R. A., Aars, J., and Lydersen, C. (2017). An Arctic predator–prey system in flux: climate change impacts on coastal space use by polar bears and ringed seals. J. Anim. Ecol. 86, 1054–1064. doi: 10.1111/1365-2656.12685
Harrison, A. L., Costa, D. P., Winship, A. J., Benson, S. R., Bograd, S. J., Antolos, M., et al. (2018). The political biogeography of migratory marine predators. Nat. Ecol. Evol. 2, 1571–1578. doi: 10.1038/s41559-018-0646-8
Hawkes, L. A., Broderick, A. C., Godfrey, M. H., and Godley, B. J. (2009). Climate change and marine turtles. Endanger. Species Res. 7, 137–154.
Higdon, J. W., and Ferguson, S. H. (2009). Loss of Arctic sea ice causing punctuated change in sightings of killer whales (Orcinus orca) over the past century. Ecol. Appl. 19, 1365–1375. doi: 10.1890/07-1941.1
Hilton, G. M., Thompson, D. R., Sagar, P. M., Cuthbert, R. J., Cherel, Y., and Bury, S. J. (2006). A stable isotopic investigation into the causes of decline in a sub-Antarctic predator, the Rockhopper penguin. Glob. Chang. Biol. 12, 611–625. doi: 10.1111/j.1365-2486.2006.01130.x
Hindell, M. A., Reisinger, R. R., Ropert-Coudert, Y., Hückstädt, L. A., Trathan, P. N., Bornemann, H., et al. (2020). Tracking of marine predators to protect Southern Ocean ecosystems. Nature 580, 87–92. doi: 10.1038/s41586-020-2126-y
Hobday, A. J., Arrizabalaga, H., Evans, K., Nicol, S., Young, J. W., and Weng, K. C. (2015). Impacts of climate change on marine top predators: advances and future challenges. Deep. Res. Part II Top. Stud. Oceanogr. 113, 1–8. doi: 10.1016/j.dsr2.2015.01.013
Hoegh-Guldberg, O., Jacob, D., Taylor, M., Bindi, M., Brown, S., Camilloni, I., et al. (2018). “Impacts of 1.5oC global warming on natural and human systems,” in Global Warming of 1.5 °C An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, eds P. Z. V. Masson-Delmotte, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, et al. (Geneva: IPCC). doi: 10.1093/aje/kwp410
Hueter, R. E., Tyminski, J. P., and de la Parra, R. (2013). Horizontal Movements, Migration Patterns, and Population Structure of Whale Sharks in the Gulf of Mexico and Northwestern Caribbean Sea. PLoS One 8:e71883. doi: 10.1371/journal.pone.0071883
IPCC (2019). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, eds H.-O. Portner, D. C. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, et al. (Geneva: IPCC).
Jenouvrier, S., Caswell, H., Barbraud, C., Holland, M., Strśve, J., and Weimerskirch, H. (2009). Demographic models and IPCC climate projections predict the decline of an emperor penguin population. Proc. Natl. Acad. Sci. U.S.A. 106, 1844–1847. doi: 10.1073/pnas.0806638106
Jensen, A. S., Silber, G. K., Calambokidis, J., Campbell, C., Cordaro, J., Deiter, R., et al. (2004). Large Whale Ship Strike Database (NMFS-OPR-25). Available online at: http://www.nmfs.noaa.gov/pr/pdfs/shipstrike/lwssdata.pdf (accessed December 10, 2019).
Katselidis, K. A., Schofield, G., Stamou, G., Dimopoulos, P., and Pantis, J. D. (2014). Employing sea-level rise scenarios to strategically select sea turtle nesting habitat important for long-term management at a temperate breeding area. J. Exp. Mar. Biol. Ecol. 450, 47–54. doi: 10.1016/j.jembe.2013.10.017
Kovacs, K. M., Lemons, P., MacCracken, J. G., and Lysersen, C. (2015). “Walruses in a Time of Climate Change. Arctic Report Card. Available online at: http://www.arctic.noaa.gov/Report-Card (accessed November 21, 2019).
Last, P. R., White, W. T., Gledhill, D. C., Hobday, A. J., Brown, R., Edgar, G. J., et al. (2011). Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Glob. Ecol. Biogeogr. 20, 58–72. doi: 10.1111/j.1466-8238.2010.00575.x
Lescroel, A., Ballard, G., Grémillet, D., Authier, M., and Ainley, D. G. (2014). Antarctic climate change: extreme events disrupt plastic phenotypic response in Adélie Penguins. PLoS One 9:e85291. doi: 10.1371/journal.pone.0085291
Lone, K., Kovacs, K. M., Lydersen, C., Fedak, M., Andersen, M., Lovell, P., et al. (2018). Aquatic behaviour of polar bears (Ursus maritimus) in an increasingly ice-free Arctic. Sci. Rep. 8:9677. doi: 10.1038/s41598-018-27947-4
López, M. E. J., Palacios, D. M., Legorreta, A. J., Urbán, J. R., and Mate, B. R. (2019). Fin whale movements in the Gulf of California, Mexico, from satellite telemetry. PLoS One 14:e0209324. doi: 10.1371/journal.pone.0209324
MacLeod, C. D., Bannon, S. M., Pierce, G. J., Schweder, C., Learmonth, J. A., Herman, J. S., et al. (2005). Climate change and the cetacean community of north-west Scotland. Biol. Conserv. 124, 477–483. doi: 10.1016/j.biocon.2005.02.004
Marsh, H., Arraut, E. M., Diagne, L. K., Edwards, H., and Marmontel, M. (2017). “Impact of climate change and loss of habitat on Sirenians,” in Marine Mammal Welfare, Animal Welfare, ed. A. Butterworth (Berlin: Springer), 333–357. doi: 10.1007/978-3-319-46994-2
Martin, J., Runge, M. C., Flewelling, L. J., Deutsch, C. J., and Landsberg, J. H. (2017). An Expert Elicitation Process to Project the Frequency and Magnitude of Florida Manatee Mortality Events Caused by Red Tide (Karenia brevis). Reston, VA: United States Geological Survey. doi: 10.3133/OFR20171132
Mattern, T., Meyer, S., Ellenberg, U., Houston, D. M., Darby, J. T., Young, M., et al. (2017). Quantifying climate change impacts emphasises the importance of managing regional threats in the endangered Yellow-eyed penguin. PeerJ 5:e3272. doi: 10.7717/peerj.3272
Matthews, C. J. D., Breed, G. A., LeBlanc, B., and Ferguson, S. H. (2020). Killer whale presence drives bowhead whale selection for sea ice in Arctic seascapes of fear. Proc. Natl. Acad. Sci. U.S.A. 117, 6590–6598. doi: 10.1073/pnas.1911761117
McCoy, E., Burce, R., David, D., Aca, E. Q., Hardy, J., Labaja, J., et al. (2018). Long-term photo-identification reveals the population dynamics and strong site fidelity of adult whale sharks to the coastal waters of Donsol, Philippines. Front. Mar. Sci. 5:271. doi: 10.3389/fmars.2018.00271
McDavitt, M. T. (2005). The Cultural Significance of Sharks and Rays in Aboriginal Societies across Australia’s Top End. Available online at: http://www.mesa.edu.au/seaweek2005/pdf_senior/is08.pdf (accessed January 9, 2020).
McIntyre, T., Ansorge, I. J., Bornemann, H., Plötz, J., Tosh, C. A., and Bester, M. N. (2011). Elephant seal dive behaviour is influenced by ocean temperature: implications for climate change impacts on an ocean predator. Mar. Ecol. Prog. Ser. 441, 257–272. doi: 10.3354/meps09383
McKeon, C. S., Weber, M. X., Alter, S. E., Seavy, N. E., Crandall, E. D., Barshis, D. J., et al. (2016). Melting barriers to faunal exchange across ocean basins. Glob. Chang. Biol. 22, 465–473. doi: 10.1111/gcb.13116
Meyer-Gutbrod, E., Greene, C., and Davies, K. (2018). Marine species range shifts necessitate advanced policy planning: the case of the north Atlantic right whale. Oceanography 31, 19–23. doi: 10.5670/oceanog.2018.209
Monnahan, C. C., Branch, T. A., and Punt, A. E. (2015). Do ship strikes threaten the recovery of endangered eastern North Pacific blue whales? Mar. Mammal Sci. 31, 279–297. doi: 10.1111/mms.12157
Moore, A. M., Ambo-Rappe, R., and Ali, Y. (2017). “The Lost Princess (putri duyung)” of the Small Islands: dugongs around Sulawesi in the Anthropocene. Front. Mar. Sci. 4:284. doi: 10.3389/fmars.2017.00284
Nelson, J. D., and Eckert, S. A. (2007). Foraging ecology of whale sharks (Rhincodon typus) within Bahía de Los Angeles, Baja California Norte, México. Fish. Res. 84, 47–64. doi: 10.1016/j.fishres.2006.11.013
New Zealand Department of Conservation, and New Zealand Ministry for Primary Industries (2017). New Zealand Sea lion/rāpoka Threat Management Plan. Wellington: New Zealand Department of Conservation.
Newell, M., Wanless, S., Harris, M. P., and Daunt, F. (2015). Effects of an extreme weather event on seabird breeding success at a North Sea colony. Mar. Ecol. Prog. Ser. 257–268. doi: 10.3354/meps11329
Niella, Y., Smoothey, A. F., Peddemors, V., and Harcourt, R. (2020). Predicting changes in distribution of a large coastal shark in the face of the strengthening East Australian Current. Mar. Ecol. Prog. Ser. 642, 163–177. doi: 10.3354/meps13322
Oosthuizen, W. C., De Bruyn, P. J. N., Wege, M., and Bester, M. N. (2016). Geographic variation in subantarctic fur seal pup growth: linkages with environmental variability and population density. J. Mammal. 97, 347–360. doi: 10.1093/jmammal/gyv181
Owen, C., Rendell, L., Constantine, R., Noad, M. J., Allen, J., Andrews, O., et al. (2019). Migratory convergence facilitates cultural transmission of humpback whale song. R. Soc. Open Sci. 6. doi: 10.1098/rsos.190337
Panigada, S., Donovan, G. P., Druon, J. N., Lauriano, G., Pierantonio, N., Pirotta, E., et al. (2017). Satellite tagging of Mediterranean Fin whales: working towards the identification of critical habitats and the focussing of mitigation measures. Sci. Rep. 7:3365. doi: 10.1038/s41598-017-03560-9
Pecl, G. T., Araújo, M. B., Bell, J. D., Blanchard, J., Bonebrake, T. C., Chen, I. C., et al. (2017). Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355:eaai9214. doi: 10.1126/science.aai9214
Pirotta, V., Grech, A., Jonsen, I. D., Laurance, W. F., and Harcourt, R. G. (2019). Consequences of global shipping traffic for marine giants. Front. Ecol. Environ. 17, 39–47. doi: 10.1002/fee.1987
Pistevos, J. C. A., Nagelkerken, I., Rossi, T., Olmos, M., and Connell, S. D. (2015). Ocean acidification and global warming impair shark hunting behaviour and growth. Sci. Rep. 5:16293. doi: 10.1038/srep16293
Poloczanska, E. S., Burrows, M. T., Brown, C. J., García Molinos, J., Halpern, B. S., Hoegh-Guldberg, O., et al. (2016). Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3:62. doi: 10.3389/fmars.2016.00062
Polovina, J. J., Dunne, J. P., Woodworth, P. A., and Howell, E. A. (2011). Projected expansion of the subtropical biome and contraction of the temperate and equatorial upwelling biomes in the North Pacific under global warming. ICES J. Mar. Sci. 68, 986–995. doi: 10.1093/icesjms/fsq198
Ramp, C., Delarue, J., Palsbøll, P. J., Sears, R., and Hammond, P. S. (2015). Adapting to a warmer ocean—seasonal shift of baleen whale movements over three decades. PLoS One 10:e0121374. doi: 10.1371/journal.pone.0121374
Ripple, W. J., Wolf, C., Newsome, T. M., Betts, M. G., Ceballos, G., Courchamp, F., et al. (2019). Are we eating the world’s megafauna to extinction? Conserv. Lett. 12:e12627. doi: 10.1111/conl.12627
Roberts, J., and Doonan, I. (2016). Quantitative Risk Assessment of Threats to New Zealand Sea Lions. New Zealand Aquatic Environment and Biodiversity Report No. 166. Available online at: http://fs.fish.govt.nz (accessed November 27, 2019).
Roberts, J., O’driscoll, R. L., Hart, A., and Graham, B. (2018). Survey of New Zealand sea Lion Prey at the Auckland Islands and Stewart Snares Shelf New Zealand Aquatic Environment and Biodiversity Report No. 204. Available online at: http://www.mpi.govt.nz/news-and-resources/publications (accessed November 27, 2019).
Roberts, J. O., Webber, D. N., Roe, W. D., Edwards, C. T. T., and Doonan, I. J. (2019). Spatial Risk Assessment of Threats to Hector’s and Māui dolphins (Cephalorhynchus hectori). Available online at: http://fs.fish.govt.nz (accessed November 27, 2019).
Robillard, M., and Séret, B. (2006). Cultural importance and decline of sawfish (Pristidae) populations in West Africa. Cybium 30, 23–30.
Rohner, C. A., Armstrong, A. J., Pierce, S. J., Prebble, C. E. M., Cagua, E. F., Cochran, J. E. M., et al. (2015). Whale sharks target dense prey patches of sergestid shrimp off Tanzania. J. Plankton Res. 37, 352–362. doi: 10.1093/plankt/fbv010
Roman, J., Estes, J. A., Morissette, L., Smith, C., Costa, D., McCarthy, J., et al. (2014). Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. doi: 10.1890/130220
Ropert-Coudert, Y., Chiaradia, A., Ainley, D., Barbosa, A., Boersma, P. D., Brasso, R., et al. (2019). Happy Feet in a hostile world? The future of penguins depends on proactive management of current and expected threats. Front. Mar. Sci. 6:248. doi: 10.3389/FMARS.2019.00248
Rosa, R., Rosa, R., Rummer, J. L., Munday, P. L., and Rosa, R. (2017). Biological responses of sharks to ocean acidification. Biol. Lett. 13:20160796. doi: 10.1098/rsbl.2016.0796
Rudrud, R. W. (2010). Forbidden sea turtles: traditional laws pertaining to sea turtle consumption in Polynesia (including the Polynesian outliers). Conserv. Soc. 8, 84–97. doi: 10.4103/0972-4923.62669
Saba, V. S., Stock, C. A., Spotila, J. R., Paladino, F. V., and Tomillo, P. S. (2012). Projected response of an endangered marine turtle population to climate change. Nat. Clim. Chang. 2, 814–820. doi: 10.1038/nclimate1582
Salinger, M. J., Renwick, J., Behrens, E., Mullan, A. B., Diamond, H. J., Sirguey, P., et al. (2019). The unprecedented coupled ocean-atmosphere summer heatwave in the New Zealand region 2017/18: drivers, mechanisms and impacts. Environ. Res. Lett. 14:044023. doi: 10.1088/1748-9326/ab012a
Schumann, N., Gales, N. J., Harcourt, R. G., and Arnould, J. P. Y. (2013). Impacts of climate change on Australian marine mammals. Aust. J. Zool. 61:146. doi: 10.1071/zo12131
Scott, R., Marsh, R., and Hays, G. (2014). Ontogeny of long distance migration. Ecology 95, 2840–2850. doi: 10.1890/13-2164.1
Serrao-Neumann, S., Crick, F., Harman, B., Sano, M., Sahin, O., van Staden, R., et al. (2014). Improving cross-sectoral climate change adaptation for coastal settlements: insights from South East Queensland, Australia. Reg. Environ. Chang. 14, 489–500. doi: 10.1007/s10113-013-0442-6
Sharp, S., McLellan, W., Rotstein, D., Costidis, A., Barco, S., Durham, K., et al. (2019). Gross and histopathologic diagnoses from North Atlantic right whale Eubalaena glacialis mortalities between 2003 and 2018. Dis. Aquat. Organ. 135, 1–31. doi: 10.3354/dao03376
Sievers, M., Brown, C. J., Tulloch, V. J. D., Pearson, R. M., Haig, J. A., Turschwell, M. P., et al. (2019). The role of vegetated coastal wetlands for marine megafauna conservation. Trends Ecol. Evol. 34, 807–817. doi: 10.1016/j.tree.2019.04.004
Thaman, R. R., Puia, T., Tongabaea, W., Namona, A., and Fong, T. (2010). Marine biodiversity and ethnobiodiversity of Bellona (Mungiki) Island, Solomon Islands. Singap. J. Trop. Geogr. 31, 70–84. doi: 10.1111/j.1467-9493.2010.00391.x
Thomas, P. O., Reeves, R. R., and Brownell, R. L. (2016). Status of the world’s baleen whales. Mar. Mammal Sci. 32, 682–734. doi: 10.1111/mms.12281
Tisdell, C., and Wilson, C. (2005). Perceived impacts of ecotourism on environmental learning and conservation: turtle watching as a case study. Environ. Dev. Sustain. 7, 291–302. doi: 10.1007/s10668-004-7619-6
Traisnel, G., and Pichegru, L. (2018). Possible drivers of nest usurpation in African penguins (Spheniscus demersus). Mar. Ornithol. 46, 85–88.
Turner, J., Phillips, T., Marshall, G. J., Hosking, J. S., Pope, J. O., Bracegirdle, T. J., et al. (2017). Unprecedented springtime retreat of Antarctic sea ice in 2016. Geophys. Res. Lett. 44, 6868–6875. doi: 10.1002/2017GL073656
van den Hoff, J., McMahon, C. R., Simpkins, G. R., Hindell, M. A., Alderman, R., and Burton, H. R. (2014). Bottom-up regulation of a pole-ward migratory predator population. Proc. R. Soc. B Biol. Sci. 281:20132842. doi: 10.1098/rspb.2013.2842
VanWormer, E., Mazet, J. A. K., Hall, A., Gill, V. A., Boveng, P. L., London, J. M., et al. (2019). Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction. Sci. Rep. 9:15569. doi: 10.1038/s41598-019-51699-4
Vargas, H., Lougheed, C., and Snell, H. (2005). Population size and trends of the Galápagos Penguin Spheniscus mendiculus. Ibis 147, 367–374. doi: 10.1111/j.1474-919x.2005.00412.x
Weir, J. (2018). Review of Hector’s and Māui dolphin Diet, Nutrition and Potential Mechanisms of Nutritional Stress. Wellington: WWF-New Zealand and the Department of Conservation
Whimp, G. (2008). Cetaceans and citations: a survey of the English literature on the role of cetaceans in South Pacific island cultures. Tuhinga 19, 169–184.
Whitehead, H., and Moore, M. J. (1982). Distribution and movements of West Indian humpback whales in winter. (Megaptera novaeangliae). Can. J. Zool. 60, 2203–2211.
Keywords: climate change, marine megafauna, habitat loss, disease, range shifts, prey
Citation: Grose SO, Pendleton L, Leathers A, Cornish A and Waitai S (2020) Climate Change Will Re-draw the Map for Marine Megafauna and the People Who Depend on Them. Front. Mar. Sci. 7:547. doi: 10.3389/fmars.2020.00547
Received: 30 January 2020; Accepted: 15 June 2020;
Published: 10 July 2020.
Edited by:
Rob Harcourt, Macquarie University, AustraliaReviewed by:
Daniel Paul Costa, University of California, Santa Cruz, United StatesRebecca Ruth McIntosh, Phillip Island Nature Parks, Australia
Copyright © 2020 Grose, Pendleton, Leathers, Cornish and Waitai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susan O. Grose, c3VzYW4uaGV5ZGxlckB1bml2LWJyZXN0LmZy; cy5ncm9zZWhleWRsZXJAZ21haWwuY29t; Linwood Pendleton, bGlud29vZC5wZW5kbGV0b25Ad3dmLm9yZw==
 Susan O. Grose
Susan O. Grose Linwood Pendleton
Linwood Pendleton Amanda Leathers
Amanda Leathers Andrew Cornish
Andrew Cornish Sheridan Waitai8
Sheridan Waitai8