- 1Stranding Response Program, Research and Conservation Section, Virginia Aquarium & Marine Science Center, Virginia Beach, VA, United States
- 2Marine Mammal Pathology Services, Olney, MD, United States
Peracute underwater entrapment (PUE) is a recognized cause of death associated with anthropogenic trauma in marine mammals. We describe internal lesions likely resulting from extreme agonal exertion in bottlenose dolphins due to entanglement and forced submergence in fishing gear during PUE. We reviewed necropsy findings from bottlenose dolphins with known PUE statuses in Virginia, United States from 2016–2019 (n = 31) for the presence of five lesions: pulmonary petechiae, pulmonary perivascular edema, hemorrhagic pulmonary lymph, separation of the rectus abdominis muscles, and acute abdominal hernias. Of the 31 cases, 23 were considered PUE cases due to the presence of external ligature marks consistent with entanglement in fishing gear. Of the animals examined, pulmonary perivascular edema, pulmonary petechiae, and hemorrhagic pulmonary lymph were found in both PUE and non-PUE cases. Though found in one non-PUE case, pulmonary perivascular edema was significantly related to PUE. There was no significant relationship between PUE and pulmonary petechiae or hemorrhagic pulmonary lymph. Rectus abdominis muscle separations and acute abdominal hernias were only found in PUE cases and nine animals exhibited either one (n = 7) or both (n = 2) of these traumatic lesions. Although these two lesions were relatively rare, there was a statistically significant relationship between the presence of one or both of the lesions and positive PUE status. This study suggests that pulmonary perivascular edema, acute hernias and separations of the rectus abdominis muscles may be useful for diagnosing PUE in the absence of external fishery interaction lesions, and highlights the severity of agonal fisheries interactions.
Introduction
Wild cetaceans and other marine mammals can experience anthropogenic trauma via vessel, gunshot, or entanglement in debris or fishing gear, among other scenarios (Moore et al., 2013). One type of anthropogenic trauma is peracute (brief and intense) underwater entrapment (PUE), which describes an agonal entanglement/entrapment event that involves extreme physical struggle and asphyxiation (Moore et al., 2013). PUE can occur via entanglement in active fishing gear, marine debris, or any material that can lead to restraint and forced submersion. In our study, fishing gear was used as a representative for this mechanism because: (1) it can be more easily confirmed via external gross examination than other types of PUE, and (2) since fishing practices can be directly managed by various authorities (Read et al., 2006; Geijer and Read, 2013; Hayes et al., 2018), increased understanding and diagnosis of PUE cases could have management implications.
Though it can be difficult to determine true numbers, previous studies have indicated that anthropogenic trauma, and specifically bycatch, can be a significant source of mortality in small cetacean populations (Read et al., 2006; Brownell et al., 2019). Despite its contribution to mortality, the number of animals that are observed and reported as fatally taken in fishery interactions is generally under representative of the true total number. Few animals are reported as bycaught, partially due to low observer coverage (Hayes et al., 2018) and a disincentive for fishers to report bycatch. Furthermore, many never wash ashore to be recovered (Peltier et al., 2012). Of the stranded carcasses recovered, the only currently accepted lesions diagnostic of fishery interaction are external gear lesions (Kuiken, 1996; Read and Murray, 2000; Moore and Barco, 2013; Moore et al., 2013). However, decompositional sloughing of the epidermis, desiccation, scavenging, and impermanence of ligature lesions often results in undiagnosed PUE cases.
One of the primary goals of a necropsy on stranded cetaceans is to determine a cause of death/stranding, sometimes in conjunction with histopathological diagnosis. Although external examination may not always lead to a conclusive diagnosis or diagnoses, there are several previously published internal lesions that have been shown to be consistent with PUE. These include pulmonary edema, vascular congestion, undigested fish in the forestomach, froth in airways, edema/hemorrhage, and unhealed fractures (Kuiken, 1996; Jepson et al., 2000; Duignan et al., 2003; Moore et al., 2013; Bernaldo de Quirós et al., 2018). Some of these can be directly attributed to the fishing gear itself, such as skin lesions, fractures, and subcutaneous edema and/or hemorrhage (Kuiken, 1996; Duignan et al., 2003). Other lesions, such as pulmonary edema, pulmonary petechiae, and froth in the airways, can be caused by internal forces or imbalances (Lunde and Waaler, 1969; Farber et al., 1983; Kuiken, 1996; Goodman, 2001). However, none of these internal lesions on their own are diagnostic of PUE. Most of these lesions can be attributed to trauma and other pathologies, or can be confused with post-mortem changes such as lividity. For example, traumatic findings such as contusions, hemorrhagic edema, and fractures can be seen in cetaceans with a cause of death/stranding of blunt vessel strike, chronic entanglement, or infanticide (Moore et al., 2013; Estrade and Dulau, 2017). Therefore, diagnosis of PUE often depends on the weight of internal and external evidence.
In this study, we investigated the prevalence of five internal findings that we hypothesized were present in PUE cases: pulmonary petechiae, pulmonary perivascular edema, hemorrhagic pulmonary lymph, separation of the rectus abdominis muscles, and acute abdominal hernias. None of these lesions, with the exception of pulmonary petechiae, have been previously described in relation to PUE in cetaceans. Both pulmonary petechiae and pulmonary perivascular edema involve bloody fluid (presumptive hemorrhage) within the lung parenchyma, so we suspected that the pulmonary lymph channels would reflect this as hemorrhagic pulmonary lymph. To investigate this possible relationship we chose to include pulmonary petechiae in our review. We examined each lesion’s potential to be diagnostic of peracute underwater entrapment in the absence of external fishery lesions.
Materials and Methods
Cases
All necropsies were permitted by the federal government and were performed by staff of the Virginia Aquarium & Marine Science Center. Included cases were limited to bottlenose dolphins that stranded in Virginia from January 2016 through September 2019, and were fresh or in moderate decomposition at the time of necropsy (Geraci and Lounsbury, 2005). They also must have received a full external and internal examination, anthropogenic trauma evaluation (Moore and Barco, 2013) and a gross necropsy report. Necropsies were performed using standard technique (Geraci and Lounsbury, 2005) except for the use of parasagittal ventral incisions as opposed to the routine ventral midline incision. This method facilitated better examination of the linea alba and rectus abdominis muscles. In order to eliminate confounding variables associated with ontogenetic differences in physiology and behavior, neonates were excluded (Mead and Potter, 1990; Noren et al., 2006). Additionally, only cases that were either positive or negative for signs of PUE (no cases for which PUE could not be determined) were included. Positive signs of PUE were defined as external lesions consistent with interaction with fishing gear and a cause of death associated with that interaction. Negative signs of PUE were defined as no external lesions consistent with interaction with fishing gear, and a cause of death not associated with fishery interaction.
Lesion Presence
Two reviewers retrospectively examined internal photographs and necropsy reports for the presence of the five internal lesions: pulmonary petechiae, pulmonary perivascular edema, hemorrhagic pulmonary lymph, separation of the rectus abdominis muscles, and acute abdominal hernias. Lesions were scored as “present” if a photograph of the lesion was present or if the lesion was mentioned in the necropsy report. Lesions were scored as “not present” if photographic and/or written documentation was available for the full anatomical area where the lesion is found, but the lesion was not present in the photographs, or absence of the lesion was specifically mentioned in the necropsy report. Lesion presence was scored as CBD if the anatomical area where the lesion is found was poorly documented and neither presence nor absence were mentioned in the necropsy report. The anatomical area where the lesion is found was defined as the lungs for pulmonary petechiae, pulmonary perivascular edema, and hemorrhagic pulmonary lymph and as the ventral abdominal wall for hernias and separation of the rectus abdominis muscles. If both reviewers agreed on the presence/absence of the lesion, no further action was necessary. If the reviewers did not agree on a lesion, a third reviewer was consulted and the majority opinion was selected. All lesions were evaluated grossly. When available, hernias and separation of the rectus abdominis muscles were evaluated histopathologically by a veterinary pathologist to validate gross diagnoses of chronicity (e.g., acute vs. chronic, ante- vs. post-mortem).
Lesion Definitions
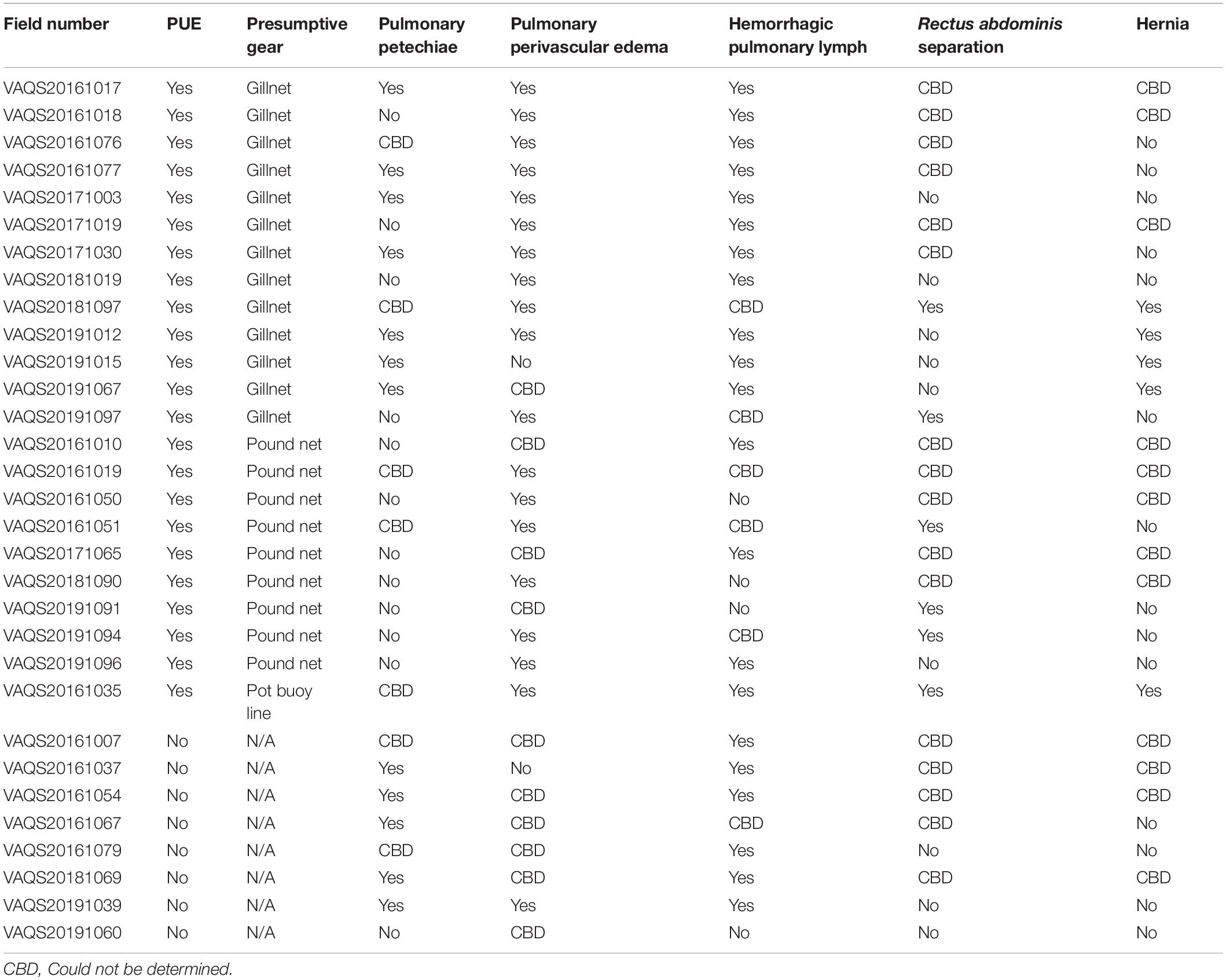
The definitions used in this paper were based primarily on gross observations, but were validated using histopathological observations when available (Costache et al., 2014). Pulmonary petechiae were defined as small, subserosal, multifocal, diffuse to coalescing hemorrhages of various sizes visible on the serosal surfaces of the lungs (Figures 1A,B). Pulmonary perivascular edema was defined as presence of edema and/or diffuse hemorrhage in the adventitia and/or parenchyma surrounding pulmonary vessels (and occasionally airways) (Figures 1C,D). Hemorrhagic pulmonary lymph was defined as blood-tinged fluid present within the superficial pulmonary lymph channels visible on the serosal surfaces of the lungs (Figures 1E,F). Separation of the rectus abdominis muscles was defined as rupture of the linea alba with concomitant separation of the left and right muscles from each other (Figures 2A,B). Abdominal hernia was defined as entrapment of the peritoneum, often in addition to mesentery (including the omentum, medial umbilical ligaments, median umbilical ligament, and/or falciform ligament) through the internal lamina of the rectus sheath or linea alba that showed evidence of an acute response (Figures 2C,D).
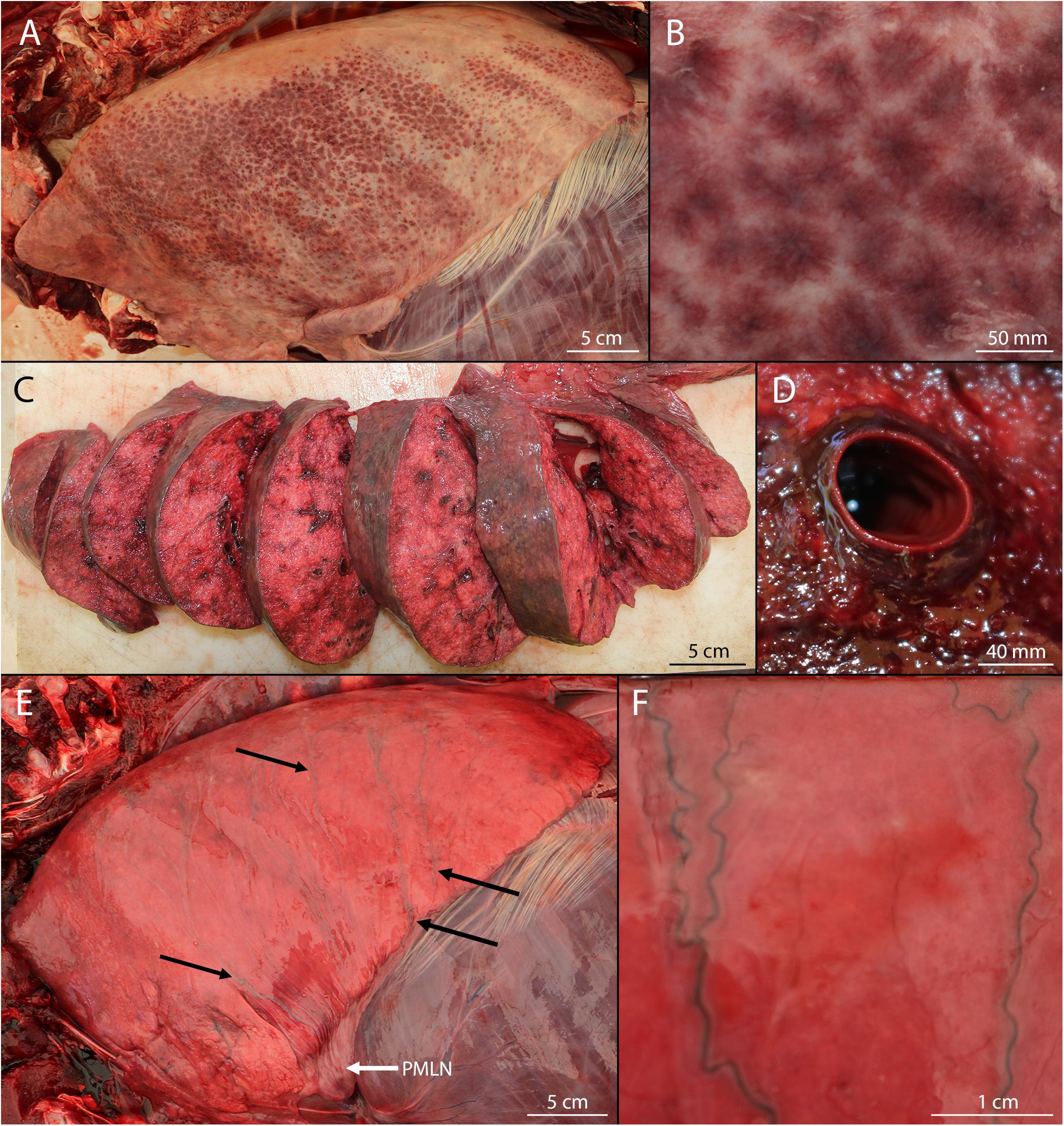
Figure 1. Pulmonary lesions in bycaught bottlenose dolphins. (A) Pulmonary petechiae. (B) Detail of pulmonary petechiae. (C) Pulmonary perivascular edema and/or hemorrhage. (D) Detail of pulmonary perivascular edema and/or hemorrhage. (E) Hemorrhagic lymph draining (black arrows) to the pulmonary marginal lymph node (PMLN; white arrow). (F) Detail of hemorrhagic pulmonary lymph.
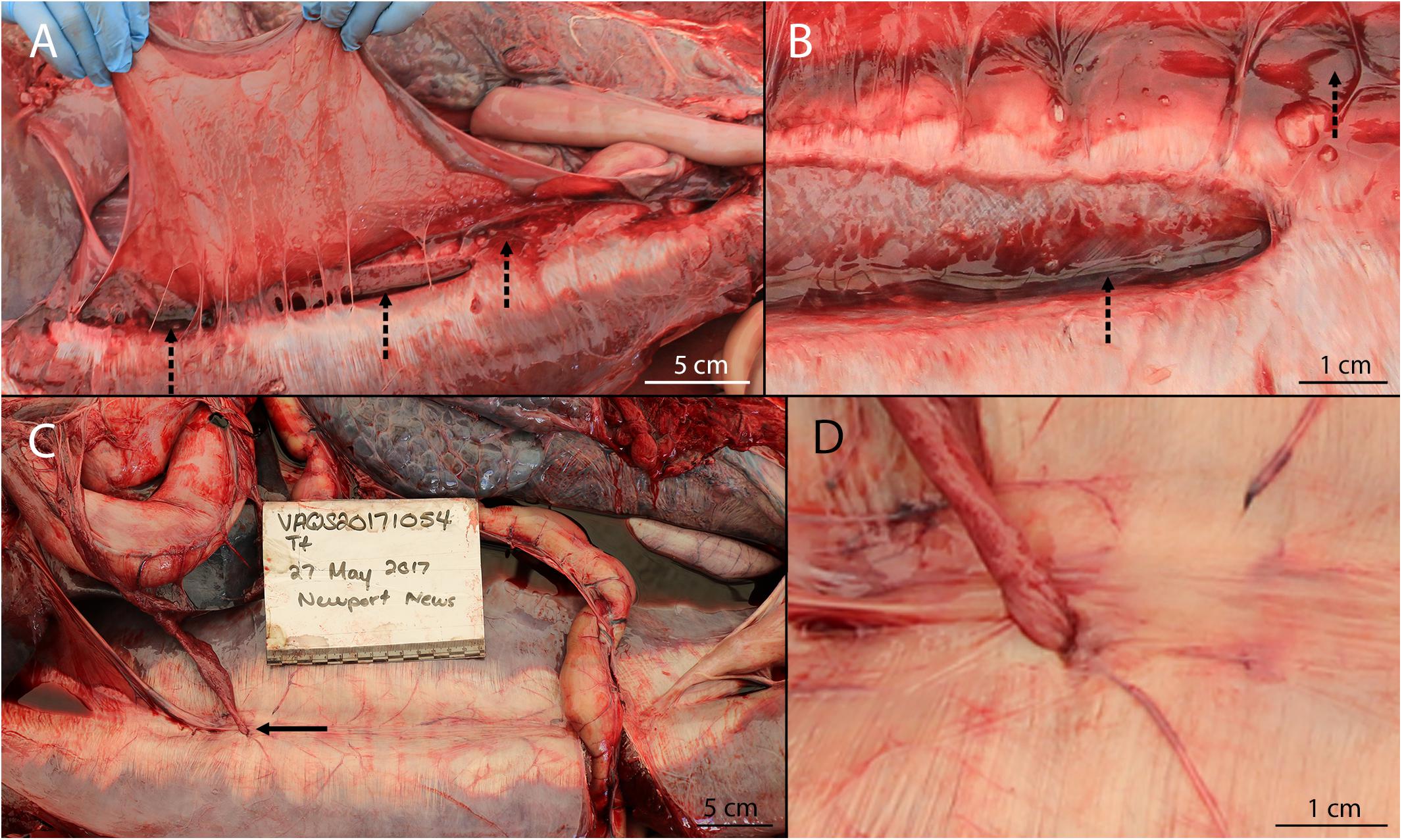
Figure 2. Abdominal lesions in bycaught bottlenose dolphins. (A) Separation of the rectus abdominis muscles with intact peritoneum reflected back. Emissary blood vessels from cranial epigastric artery and veins visible as long strands. Note the hemorrhagic edema present along the length of the separation (dotted arrows). (B) Detail of caudal margin of rectus abdominis separation showing hemorrhagic edema (dotted arrows) and irregular margins consistent with tearing. (C) Omental hernia through rectus sheath (solid arrow). This hernia is chronic, however, gross presentation of acute hernias did not differ. Note that the caudal incision (to the right of the image) with the intestinal loop is an artifact of dissection. (D) Detail of omental hernia. Note the emissary vessel emanating from the hernia location and an additional emissary vessel to the right of the hernia.
Gross and Histopathologic Criteria for Evidence of Chronicity
Morphological diagnoses of chronicity were based predominantly on gross diagnosis but often bolstered with histopathological diagnosis. Since edema and/or hemorrhage are considered acute ante-mortem processes, all pulmonary lesions (pulmonary petechiae, pulmonary perivascular edema, hemorrhagic pulmonary lymph), which by definition included edema and/or hemorrhage, were considered acute based on gross diagnosis. Gross evidence for acute separation of the rectus abdominis muscles included retroperitoneal and intralesional edema and/or hemorrhage and evidence of tendinous rupture without overt tissue response (ex: adhesions, remodeling of irregular rupture margins such as fiber fringing, lack of wound bridging, etc.). No chronic separations of the rectus abdominis muscles were present in our study, so this was not defined. Gross evidence for acute hernias included congestion (vascular engorgement or bloody discoloration), and lack of firm adhesion. Histopathological evidence for acute processes included the presence of edema, hemorrhage, fibrin, and/or neutrophils. Gross evidence for chronic hernias included green-gray to black discoloration (presumptive necrosis) of tissue at the site of herniation and adhesion of the entrapped tissue to the surrounding tissue. Histopathological evidence for chronic processes included lymphoplasmacytic infiltrates, fibroblasts, and/or mature collagen.
Gear Type and Anatomical Entanglement Location
Photographs, necropsy reports, and anthropogenic trauma evaluations were reviewed to determine anatomical entanglement locations and gear type based on external lesions. When possible, presumptive gear type was determined based on seasonal fisheries prosecuted in Virginia waters (Hayes et al., 2018). When animals exhibited evidence of monofilament net or both monofilament and larger diameter multifilament line, they were presumed to have interacted with a gillnet. When animals exhibited evidence of fine twisted twine impressions, they were presumed to have interacted with a pound net. When animals exhibited only thick braided multifilament line, they were presumed to have interacted with pot buoy line (consistent with crab or whelk pots). Anatomical entanglement locations were separated into the following categories: rostrum/mandible, head, pectoral flippers, dorsal fin, body, peduncle, and flukes.
Analyses
Stranding demographics and internal findings were compiled in Microsoft Excel (RRID:SCR_016137). The anatomical entanglement location was examined in relation to gear type and internal lesion type. To evaluate size-based relationships, animals were placed into 25 cm total length bins, starting at 150 cm and ending at 250+ cm. R Project for Statistical Computing (RRID:SCR_001905) was used to perform 2 × 2 Pearson’s Chi-squared contingency tests (Pearson, 1900; Fisher, 1922) to determine if there was a significant relationship between the presence of lesion(s) and positive PUE status, with p ≤ 0.05 considered significant.
Results
Selected cases (n = 31) ranged in total length from 151 to 302 cm and consisted of an almost equal distribution of sexes (see Supplementary Material). All carcasses ranged from fresh to moderate decomposition. Eight cases were scored as not having any external signs of PUE, and 23 cases were scored as having external signs of PUE (Table 1). Lesion presence and presumptive gear type were determined for all cases (Table 1).
Pulmonary Petechiae
Pulmonary petechiae were present in both PUE (n = 7, 30%) and non-PUE cases (n = 5, 63%) but were observed in a higher proportion of non-PUE cases. There was not a significant relationship between PUE and pulmonary petechiae (χ2 = 2.572; p = 0.108).
Pulmonary Perivascular Edema
Pulmonary perivascular edema was more prevalent in PUE cases (78%, n = 18) than non-PUE cases (13%, n = 1) and this relationship was significant (χ2 = 10.819; p = 0.001). However, 75% of non-PUE cases scored as CBD for the presence of this lesion.
Hemorrhagic Pulmonary Lymph
Hemorrhagic pulmonary lymph was a common finding in this study and was present in 15 (65%) PUE and 6 (75%) non-PUE cases. There was not a significant relationship between PUE and hemorrhagic pulmonary lymph (χ2 = 0.260; p = 0.610). Of those 21 cases, 17 exhibited one or more of the other pulmonary lesions (six exhibited pulmonary perivascular edema, five exhibited pulmonary petechiae, and an additional six exhibited both pulmonary perivascular edema and pulmonary petechiae). Although four hemorrhagic pulmonary lymph cases exhibited neither pulmonary perivascular edema nor pulmonary petechiae, each case had at least one CBD for pulmonary perivascular edema or pulmonary petechiae.
Separation of the Rectus abdominis Muscles
Separation of the rectus abdominis muscles occurred in six PUE animals (26%) ranging in length from 151 to 200 cm. Although no non-PUE cases in our study exhibited separation of the rectus abdominis muscles, there was not a significant relationship between PUE and separation of the rectus abdominis muscles (χ2 = 2.588; p = 0.108). For all cases, the peritoneum overlying the rupture was intact (Figure 2A). Separations ranged from partial to full thickness (down to the ventral subdermal connective sheath) and ranged in length from a few centimeters to the majority of the rectus abdominis muscles. All of the separations were at least partially centrally located (peri-umbilical) (i.e., none of the separations were only caudal sections or cranial sections). For all cases of separation of the rectus abdominis muscles, ante-mortem tissue reaction was observed grossly, including irregular tearing margins, sometimes with intermittent remnant tendinous fibers bridging the separation (Figure 3). Additionally, all separations of the rectus abdominis muscles exhibited intralesional retroperitoneal hemorrhage and edema, most pronounced at the apices of the separation. Histopathological diagnoses were obtained for two cases, which indicated disruption of myofiber bundles, edema, hemorrhage, and neutrophilic infiltrates which aligned with the ante-mortem gross diagnoses.
Figure 3. Rectus abdominis separation in a bycaught bottlenose dolphin. Intact superficial midline and irregular margins are consistent with tearing due to tension, rather than incision. Note the hemorrhagic edema present on the left of the image (cranial aspect), the remnant tendinous fibers bridging the wound, the lack of healing wound bridging fibers, and the presence of emissary vessels.
Acute Hernias
Acute abdominal hernias occurred in five PUE cases (22%) and animals exhibiting this lesion ranged in length from 194 to 282.2 cm. Although no non-PUE cases exhibited acute hernias, there was not a significant relationship between PUE and hernias (χ2 = 2.588; p = 0.108). All cases of acute hernias were observed in animals with a cause of death/stranding of PUE. Histology results were obtained for three out of five hernias and confirmed that the hernias exhibited acute changes (Figure 4A and Table 2). In addition to the acute changes, two cases revealed additional underlying chronic changes associated with the sampled tissue (Figure 4B).
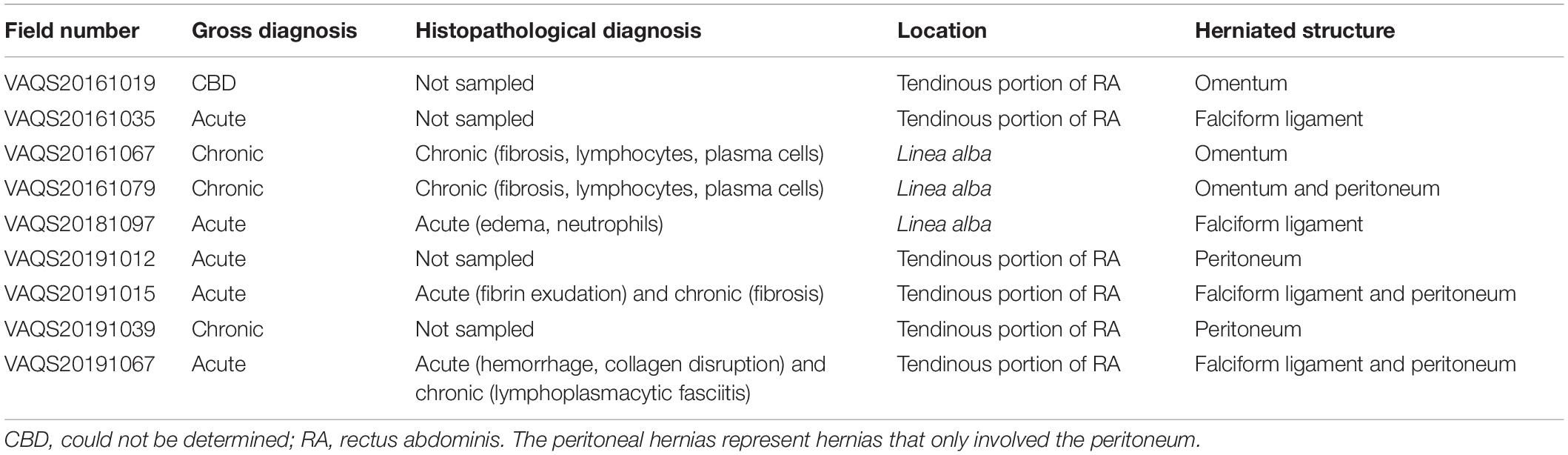
Table 2. Hernia chronicity determined by gross diagnosis and histopathological diagnosis, location of hernia, and herniated structure(s).
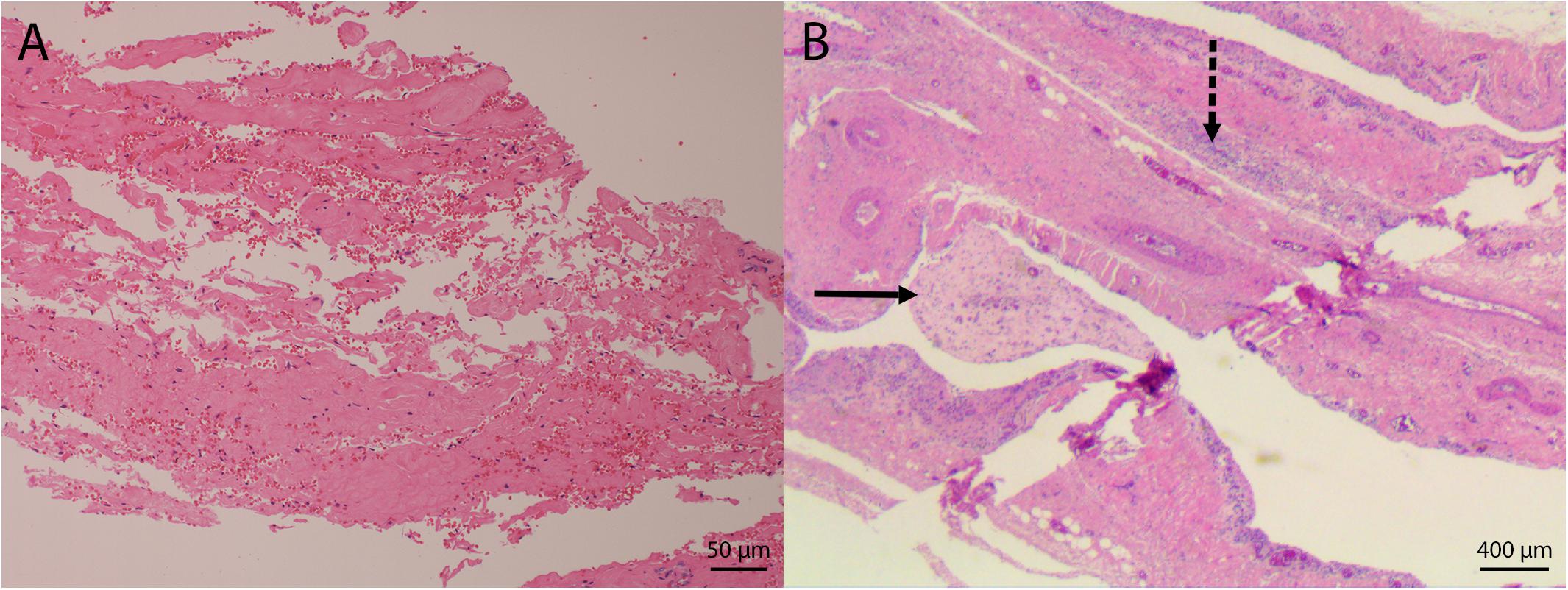
Figure 4. Microphotographs of hernias in bottlenose dolphins. (A) Disruption of collagen bundles with associated hemorrhage in an acute hernia. (B) Fibrovascular fronds extend from the site of the chronic hernia (solid arrow). Inflammatory cells infiltrate the wall (dashed arrow).
Though not considered acute hernias, one additional hernia was observed in a PUE positive animal which had undetermined chronicity and three chronic hernias were found in non-PUE animals (Table 2). These chronic hernia cases were assigned a natural cause of death/stranding unrelated to PUE. Histopathologic evaluation was obtained on two out of three of the chronic hernias, which confirmed solely chronic processes in both cases. The total length of these animals ranged from 193 to 214 cm.
All hernias were either within the linea alba (n = 3) or within the tendinous portion of the rectus abdominis sheath a few centimeters lateral to the linea alba (n = 6; Table 2). All hernias by necessity included the peritoneum, however, entrapped tissue sometimes also included mesenteric tissue such as the omentum or falciform ligament.
Separation of the Rectus abdominis Muscles and Acute Hernias Combined
Separation of the rectus abdominis muscles and hernias were relatively rare lesions compared to others discussed above, but they were the only lesions that occurred exclusively in PUE cases. Thus, we tested the relationship between PUE and the presence of either of the two lesions. When combined, there was a significant relationship between the presence of at least one of these muscular lesions and PUE status (χ2 = 4.411; p = 0.036).
External Gear Evidence
It was possible to infer gear type in 22 out of 23 PUE cases (Table 1), using previously established guidelines (Moore and Barco, 2013). Most animals exhibited evidence of interaction with a gillnet (n = 13) or a pound net (n = 9). One animal exhibited only signs of multifilament line entanglement, which was suspected pot buoy line. Preliminary analysis of entanglement location indicated that there were no obvious relationships among entanglement location, presence of each lesion, and gear type. However, due to low sample size, a potential association cannot be eliminated (see Supplementary Material for further information on entanglement locations).
Summary
Out of 23 PUE cases, pulmonary perivascular edema was the most common lesion and was present in 78% of cases (n = 18), followed by hemorrhagic pulmonary lymph, which was present in 65% (n = 15; Figure 5). Out of eight non-PUE cases, hemorrhagic pulmonary lymph was the most common lesion and present in 75% of cases (n = 6), followed by pulmonary petechiae (63%; n = 5). Separations of the rectus abdominis muscles and hernias were not present in any non-PUE cases. Separations of the rectus abdominis muscles were present in 26% of the PUE cases (n = 6) and hernias were present in 22% of the PUE cases (n = 5). Overall, the total number of lesions in non-PUE cases was lower (range: 0–2) than the total number of lesions in PUE cases (range: 1–4, Figure 6) and only PUE cases exhibited three or more lesions.
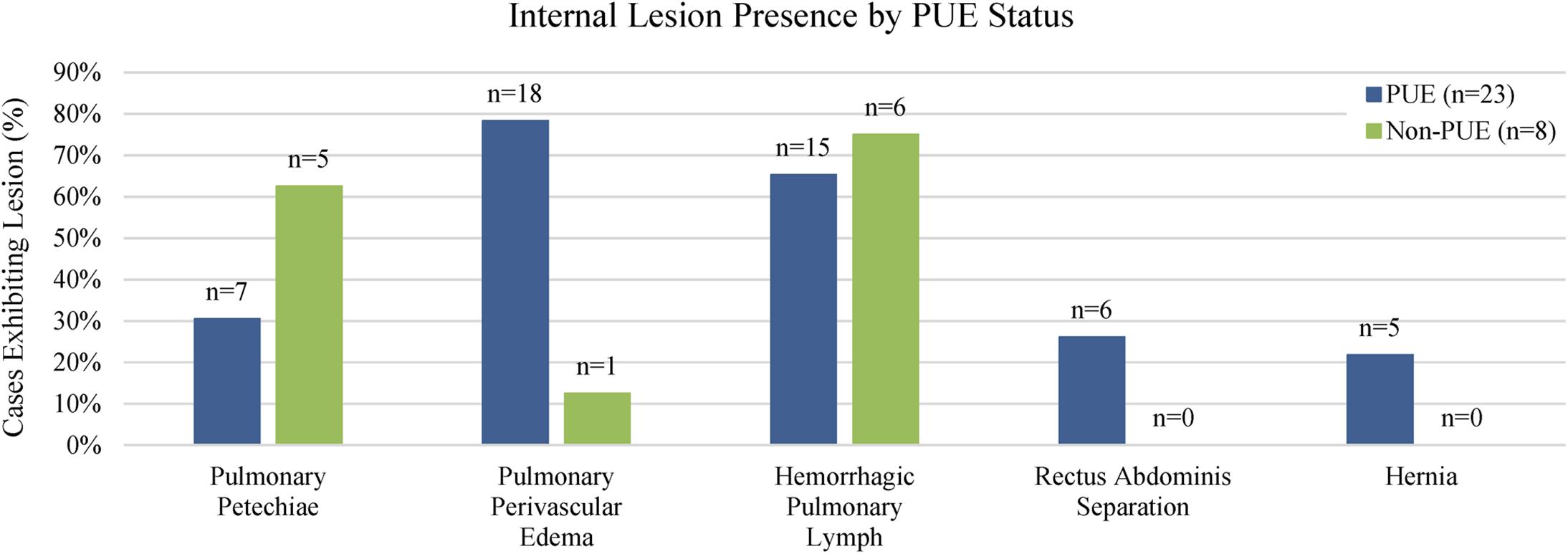
Figure 5. Lesions present in bottlenose dolphins. Pulmonary petechiae and hemorrhagic pulmonary lymph were more common in non-PUE cases, while rectus abdominis separations and hernias were only found in PUE cases. Pulmonary perivascular edema was the most common lesion observed in PUE cases.
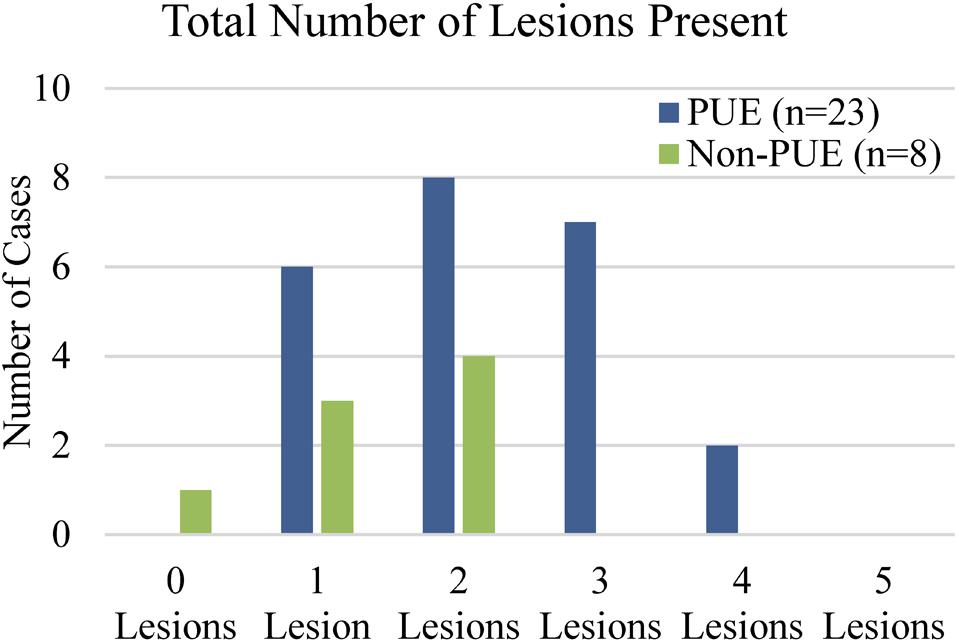
Figure 6. Lesions present in bottlenose dolphins. PUE cases exhibited a higher total number of abdominal and/or pulmonary lesions (range: 1–4) than non-PUE cases (range: 0–2). Only PUE cases exhibited three or more lesions.
In addition to the lesions of interest, many PUE and non-PUE cases exhibited other significant findings, including infectious disease, trauma, and lesions of unknown etiology.
A preliminary analysis of length indicated no clear relationships between each lesion and length, but pulmonary petechiae appeared slightly more common in larger animals and separation of the rectus abdominis muscles appeared slightly more common in smaller animals (see Supplementary Material for additional information). However, low sample size did not allow for confident analysis of significant relationships between length and lesion presence.
Discussion
Pulmonary Petechiae
Pulmonary petechiae have been used previously as a bycatch evidence criterion in cetaceans (Kuiken, 1996; Moore et al., 2013), however, the mechanism is poorly understood. In humans, petechiae are caused by hemorrhages from capillaries or small blood vessels in the lungs which can rupture due to traumatic asphyxia or hypoxia (Guntheroth et al., 1973; Farber et al., 1983; Ambade et al., 2009). Previous studies in rats indicated that pulmonary petechiae were uncommon with respiratory paralysis and that vigorous respiratory effort was necessary for their production (Guntheroth et al., 1973). A forensic study of strangulation indicated that petechiae on conjunctivae, mucosal surfaces, and facial skin were an indication of the severity of the strangulation and the result of vessel occlusion and rupture of capillaries (Plattner et al., 2005).
In rabbits, there is a positive relationship between the progression of left heart failure and presence of pulmonary petechiae (Farber et al., 1983). Airway occlusion and hypoxia can result in increased pulmonary circulatory pressures, which cause small pulmonary hemorrhages in human infants (Farber et al., 1983). We suggest that an increase in pulmonary intravascular pressure during agonal struggle and hypoxia lead to petechial hemorrhages in dolphins during PUE, but that other mechanisms can also result in pulmonary petechiae formation.
This is the only lesion included in this study that had been previously suggested as supportive of PUE as the cause of death/stranding when present in association with other PUE supportive lesions (Kuiken, 1996; Moore et al., 2013). However, in our study pulmonary petechiae were present in a higher percentage of non-PUE cases than PUE cases, indicating that this lesion may be associated with many other pathologies and should be interpreted with caution.
Pulmonary Perivascular Edema
There is limited mention of this lesion in existing literature in relation to other species, and its prevalence in marine mammals is even more poorly documented. One study in humpback whales and another in bycaught dolphins and porpoises mention pulmonary perivascular edema as a histological finding (Knieriem and Hartmann, 2001; Groch et al., 2018) but its etiology and mechanism were not determined. Pressure differences in the perivascular interstitial space and hypoxic physical exertion have been mentioned as possible contributors of this lesion in other species (Whayne and Severinghaus, 1968; Timby et al., 1990). Edema surrounding the vasculature of the lungs was present in rats both swimming and relaxing in a hypoxic environment (Whayne and Severinghaus, 1968). This finding was also associated with acute altitude change and vigorous exercise in humans (Whayne and Severinghaus, 1968). The aforementioned study also suggested that pulmonary perivascular edema was due to a “focal rupture or separation of elements of the arterial wall that permits the passage of blood, plasma, or both into extravascular spaces.”
The widely accepted Starling hypothesis states that the transmural movement of fluid is dictated by the hydrostatic pressure gradient, which normally favors fluid leaving a vessel, and the osmotic pressure gradient, which normally favors fluid retention in the vessel (Lunde and Waaler, 1969; Goodman, 2001). Therefore, an increase in transmural hydrostatic pressure has been shown to cause the net outward movement of fluid from the pulmonary vessels into the surrounding lung parenchyma (Lunde and Waaler, 1969; Goodman, 2001). We suggest that in the case of PUE, this increased hydrostatic transmural pressure is caused by increased systemic blood pressure resulting from amplified abdominal muscle contractions. Changes in thoracic and intra-abdominal pressure caused by muscle contractions have been previously documented in humans (Kawabata et al., 2010) and cetaceans (Slijper, 1962; Lillie et al., 2017). Additionally, effects of the mammalian dive response (Scholander, 1940; Davis and Williams, 2012) and stress related peripheral vasoconstriction and tachycardia (Bonanno, 2011) may contribute to detrimental systemic blood pressure, but the effects are not well-understood and there may be compensatory mechanisms.
In the cases examined during this study, pulmonary perivascular edema was significantly associated with PUE cases. Most often, this lesion was observed around veins, and less often around airways and arteries. This supports the hypothesis that transmural pressure plays a role in the pathogenesis of this lesion as veins are thin walled and more vulnerable to elevated pressures. The one non-PUE case with pulmonary perivascular edema exhibited numerous gross pulmonary findings (including severe pneumonia and diffuse interstitial pulmonary edema) as well as signs of a systemic infectious process. It is possible these conditions may have contributed to the pulmonary perivascular edema.
Due to the significant association with PUE, it is our belief that pulmonary perivascular edema is generated under conditions of extreme agonal exertion typically encountered in PUE, and is a useful finding for supporting PUE as the cause of death/stranding.
Hemorrhagic Pulmonary Lymph
Pulmonary edema increases lymphatic drainage (Ware and Matthay, 2005). As the lymph channels drain fluid from the lung interstitium, hemorrhage in the lungs will be reflected as dark red pulmonary lymph channels visible on serosal surfaces. We were unable to find a published example of this lesion in cetaceans.
Hemorrhagic pulmonary lymph was a common finding in this study and was present in 21 cases, including both PUE (n = 15) and non-PUE cases (n = 6). Though we hypothesized that hemorrhagic pulmonary lymph would co-occur with pulmonary petechiae or pulmonary perivascular edema since both lesions result in hemorrhage and interstitial edema, it is unclear if they were related in this study group. Though 17 of the 21 cases had either pulmonary perivascular edema, pulmonary petechiae, or both, four cases had neither. However, all four of these cases had at least one CBD for pulmonary perivascular edema or pulmonary petechiae, so their association cannot be ruled out. Additionally, many other pulmonary findings can contribute to the presence of hemorrhagic pulmonary lymph, including interstitial hemorrhage, severe infection, or blunt trauma. Due to the commonality of pulmonary disease in cetaceans (Ridgway, 1972) and the lack of significant association with PUE, we suggest that this lesion should be noted, but interpreted with caution.
Separation of the Rectus abdominis Muscles
Each rectus abdominis muscle is encased in tendinous aponeurotic fibers which intertwine with the contralateral fibers along the ventral midline forming the linea alba (Askar, 1977; Cotten et al., 2008; Figure 7). In delphinids, these muscles play a major role in the downward stroke of the tail during locomotion (Cotten et al., 2008). When the muscles contract, the abdominal or thoracic cavity of the dolphin may be compressed which assists with expiration. We suggest that if an animal is entangled and anchored or restrained, locomotory effort becomes amplified, but results in isometric-like contractions. The agonal struggle associated with PUE likely results in muscle hypercontraction with elevated forces and pronounced muscle swelling or bulging (Baskin and Paolini, 1967; Dick and Wakeling, 2018; Eng et al., 2018). This bulging increases the cross sectional surface area of the muscles, which tears the aponeuroses along the linea alba and separates the two muscles.
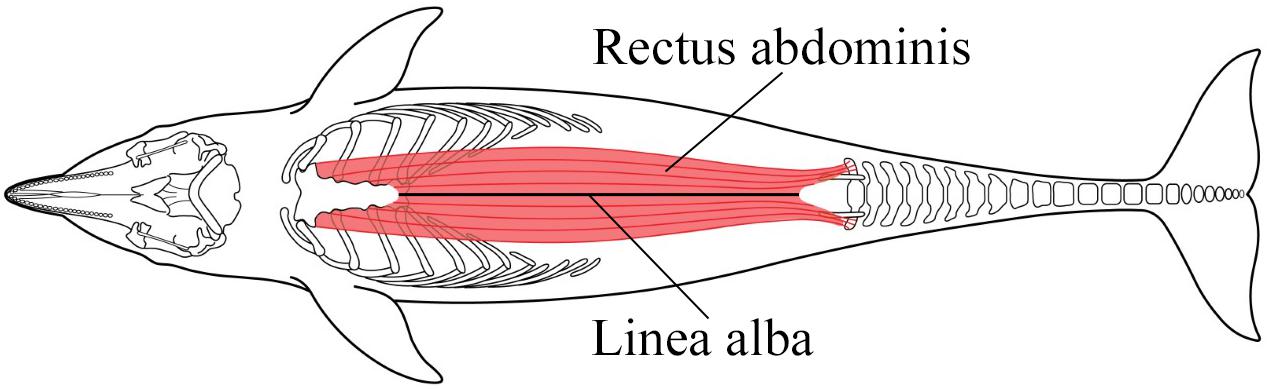
Figure 7. In bottlenose dolphins each rectus abdominis muscle stretches from the sternal ribs and sternum to the pelvic bones and transverse processes of caudal vertebrae (Cotten et al., 2008). Each muscle is enveloped by the rectus sheath, formed by aponeuroses of the external and internal abdominal oblique and transverse abdominal muscles (Schaller et al., 2007). The fibers of the internal and external laminae wrap around the rectus muscles and intertwine with the contralateral fibers along the ventral midline, forming the linea alba. Figure adapted from Cotten et al. (2008) and Cozzi et al. (2017).
We were unable to find examples in existing literature of this lesion. However, observation of this lesion requires a non-standard parasagittal incision along the ventrum, in place of the common midline incision (Geraci and Lounsbury, 2005). We recommend careful examination of the midline prior to examination or excision of abdominal organs, followed by reflection of the peritoneum to reveal potential separation of the rectus abdominis muscles. Frequently hemorrhagic edema can be observed retroperitoneally along the midline, prior to reflection of the peritoneum.
There are several published examples of extremely forceful muscle contractions during events such as seizures, electrocution, and weight lifting leading to fractures and muscle tears in humans (Finelli and Cardi, 1989; Naffaa et al., 2014; Lana et al., 2017; Rushworth et al., 2018). This literature suggests that internal forces alone generated by extreme muscle contractions can lead to catastrophic musculoskeletal injuries. We believe that separation of the rectus abdominis muscles in dolphins may, similarly, represent a catastrophic self-inflicted injury resulting from extreme agonal exertion.
We have not seen severe bloating, advanced decomposition, or any external bloating-related defects (e.g., urogenital and lingual prolapse) in the study cases with separation of the rectus abdominis muscles. All cases that exhibited a rectus abdominis separation also had a completely intact peritoneum overlaying the separation. We also have observed cases with separation of the rectus abdominis muscles that exhibited some intact rectus sheath fibers dispersed intermittently along the length of the separation, likely precluding decompositional-induced forces (e.g., bloating) as the mechanism of generation. These areas of remnant fibers have included the umbilicus, which is a weak point in the abdominal wall and often the first area to experience decompositional related defects. These, combined with histologic results, indicate that this lesion is not related to decomposition and is an ante-mortem lesion, likely related to the traumatic struggle.
The location and morphology of many of the rectus abdominis muscle separations suggest that peri-umbilical weaknesses play a role in the pathogenesis of this lesion. Separations ranged from minimal separation to full thickness separation. It is possible that these differences were related to the intensity/duration of the struggle or the extent of the restraint, and that partial and/or short separations represent a lesser intensity or duration of struggle.
Though we are not aware of literature on the microstructure of the linea alba of the dolphin, there is ample evidence in other species of differential morphology and orientation of the linea alba fibers cranial and caudal to the umbilicus (Askar, 1977). In humans, pregnancy can increase the risk and incidence of defects within the linea alba, including rectus abdominis diastasis (Reinpold et al., 2019). Though pregnancy did not play a role in the formation of lesions in these particular cases (all were sexually immature and two were males), the effect of mechanical stress on the linea alba should always be considered as a differential contributing factor.
As all animals that exhibited separation of the rectus abdominis muscles were ≤200 cm, it is possible that size or ontogenetic differences (e.g., linea alba development) may play a role in the formation of this lesion. However, the sample size was too small to establish any clear relationship between separation of the rectus abdominis muscles and total length. Two neonate cases that were not PUE positive and did not qualify for inclusion in this study, however, exhibited separation of the rectus abdominis muscles which we believe was associated with conspecific or interspecific aggression resulting in blunt trauma. It is not known if the separation of the rectus abdominis muscles in either case was the direct result of blunt trauma or the self-inflicted result of agonal exertion due to the traumatic event.
Although the low numbers in our preliminary data did not show a significant relationship between PUE and separation of the rectus abdominis muscles, the presence of this lesion only in PUE cases within this study and the relative rarity of the lesion (6 of 23 PUE cases) suggest that, though not common, it may be a good indicator of PUE. Additionally, when separation of the rectus abdominis muscles was combined with acute abdominal hernias, there was a significant relationship between presence of either of these lesions and PUE.
Acute Hernias
Hernias occur when an organ or tissue is displaced through muscle or connective tissue into another space in the body (Jubb and Kennedy, 1970). This may occur when there is an increase in intra-abdominal pressures (Light and Routledge, 1965; Smith et al., 1999; Jensen et al., 2015) and/or weakening in the surrounding tissue (Zelel et al., 1992; Smith et al., 1999; Moles et al., 2005). In cetaceans, there are two published examples of diaphragmatic hernias: a chronic hernia with unknown etiology and a congenital hernia (Stephen, 1993; Kastelein et al., 2009). Diaphragmatic, umbilical, and inguinal hernias have also been documented in pinnipeds (Beekman, 2010; Colegrove et al., 2010; Greene et al., 2015), manatees (Gerlach et al., 2012), and terrestrial mammals (Hayes, 1974) and all were associated with congenital or unknown causes. We were unable to find any published examples of acute traumatic hernias in pinnipeds or cetaceans or any hernias associated with a fishery interaction or entanglement. However, manipulation of the peritoneum, mesentery, or other abdominal organs and tissues prior to examination of the midline may displace acute abdominal hernias. Additionally, the ventral abdominal wall is an often overlooked structure that is casually removed.
We have observed peritoneum, omentum, and falciform ligament herniated in bottlenose dolphins of various PUE determinations. These tissues have protruded through the linea alba into the midline cavity, or between fibers of the internal lamina of the rectus sheath into the rectus abdominis muscle. Chronic hernias exhibited gross and histological findings such as firm adhesions, fibrosis, inflammation, and neovascularization. This could support a past fisheries interaction, congenital defect, or other trauma, but was not considered directly related to PUE. Since acute abdominal hernias do not exhibit these chronic findings, we suggest they occur as a result of large muscular excursions, increased force of muscle contraction, and elevated intra-abdominal pressures forcing peritoneum and mesentery between the rectus fibers. As the rectus abdominis muscles contract and bulge with increased force, space is created between the fibers for tissue to become entrapped. Although the low numbers in our preliminary data did not show a significant relationship between PUE and acute abdominal hernias, the presence of this lesion only in PUE cases and the relative rarity of the lesion (5 of 23 PUE cases) suggest that, though not common, it may be a good indicator of PUE. Additionally, when acute abdominal hernias were combined with separation of the rectus abdominis muscles, presence of either lesion was significantly related to PUE.
Previously described hernias are often associated with weak points in tissues, like the linea semilunaris or esophageal hiatus (Zelel et al., 1992; Smith et al., 1999; Moles et al., 2005). Under normal circumstances, small emissary vessels from the cranial and caudal epigastric arteries and veins travel between the fibers of the internal lamina of the rectus sheath to the ventral mesentery (Schummer et al., 1981; Schaller et al., 2007). We have observed sequential hernias in the same individual containing these emissary vessels and surrounding tissue (Figure 8). At least one of these hernias also exhibited gross acute evidence of congestion/hemorrhage of the entrapped peritoneum. We believe that emissary vessels may create weak points within the tendinous sheath, making these spaces more susceptible to herniation. Many hypotheses exist for the etiology of non-traumatic abdominal wall hernias in humans (Moles et al., 2005). One of the earlier hypotheses for the pathogenesis of spigelian hernias suggested that hernias were occurring at openings created by perforating vessels and nerves (Cooper, 1804). However, a more recent hypothesis suggested that defects at the arcuate line lead to the weaknesses in the muscle layers (Zimmerman et al., 1944). We suggest an additional hypothesis regarding the relationship between sites of herniation and locations of vessels. As the rectus abdominis muscles bulge during contraction, the epigastric vessels are stretched and may create tension on the peritoneum, pulling it toward and through the fibers of the rectus sheath to create a hernia.
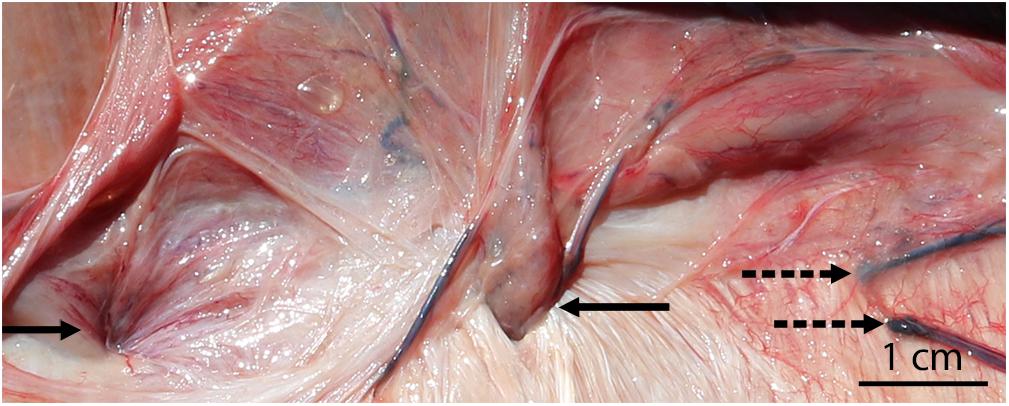
Figure 8. Acute abdominal hernias in a bycaught bottlenose dolphin (with the peritoneum reflected back). Blood vessels are visible at the site of both hernias (solid arrows), and congestion/hemorrhage is present surrounding the hernia on the left. Additional emissary veins (not associated with hernias) are also present (dashed arrows) and may predispose the region to herniation.
In addition to inherent defects or weaknesses, trauma has been shown to induce hernias, including those caused by both internally generated forces (Smith et al., 1999) and external forces (Dreyfuss et al., 1986). The bulging of the rectus abdominis muscle during agonal PUE may further accentuate weak points within the rectus sheath, such as the site of emissary vessels. The increased abdominal pressure caused by the increased intensity/effort during isometric contractions (Harman et al., 1988; Hagins et al., 2004; Kawabata et al., 2010) provides additional opportunity for herniation. In humans, the inhalation form of breath control has been shown to produce greater intra-abdominal pressure than other forms of breath hold (Hagins et al., 2004). Cetaceans normally breath hold on inhalation (Kooyman and Cornell, 1981) so the contribution of this to intra-abdominal pressure in cetaceans and its potential contribution to hernia development is unknown. Though a single defect is sometimes at work, hernias are often caused by a combination of factors (Light and Routledge, 1965). We propose that the combined effects of the forceful contractions (leading to muscle bulging, fiber stretching, and increased intra-abdominal pressure) and the vascular weak points contribute to the formation of hernias in PUE cases. In this study, all hernias were either within the linea alba or within the medial half of the tendinous portion of the rectus abdominis sheath. This finding is consistent with the previously mentioned emissary vessel hypothesis since those vessels are located medially.
All three cases with chronic hernias had a cause of death/stranding unrelated to PUE. These chronic hernias were presumptively related to other causes (ex: previous non-fatal entanglement, previous trauma, congenital factors, infectious processes, etc.). The chronic components of acute hernias are of unknown origin. However, they may be associated with chronic mesenteric changes unrelated to the acute hernia. For example, fibrosis of the falciform ligament could be a normal sequel of aging and hepatic tension in an acrobatic species, or may be associated with a traumatic birth or a previous hernia.
Though not included in our study, two bottlenose dolphins in North Carolina that were observed beachcast from gillnets exhibited several peritoneal hernias. These cases provide examples of confirmed bycaught dolphins exhibiting abdominal hernias with no gross evidence of chronicity, providing support for this being a PUE-related traumatic lesion. Both animals were recently deceased, ∼210 cm in length and were in robust body condition, suggesting lack of long term debilitation or decompositional artifact. Interestingly, both animals also exhibited moderate to severe lungworm infections. It is unknown to what degree pulmonary compromise could affect the degree of agonal exertion and therefore the presence of the lesions in this study. All other gross pathology exhibited by these two animals was consistent with previously published criteria for PUE diagnosis (Moore et al., 2013).
Separation of the Rectus abdominis Muscles and Acute Hernias Combined
One potential mechanistic hypothesis for separation of the rectus abdominis muscles and acute abdominal hernias is that entanglement at both the cranial and caudal ends (resulting in the animal being “hogtied”), may immobilize the animal in extension or flexion. This immobilization would result in forceful isometric muscle contractions, which in turn contribute to the formation of the traumatic musculoskeletal lesions. A length-dependent relationship exists between muscle damage and isometric contractions at maximal effort, with maximal damage occurring during the plateau phase of contraction, rather than the rising or relaxation phases (Allen et al., 2017). Perhaps most importantly, this same study found that the extent of muscle damage was more closely related to length of the muscle than to force generated, whereby extended muscle states resulted in more damage than shortened, more powerful muscle states (Allen et al., 2017). An additional study showed significant increases in cross-sectional surface area of muscles with increased isometric contraction effort, with peak increases around the middle of the muscle belly (Raiteri et al., 2016). In the six animals with separation of the rectus abdominis muscles, evidence for being hogtied was present in three, could not be determined in two, and was not present in one. In the five animals with acute abdominal hernias, evidence of being hogtied was present in four, and could not be determined in one. However, if presumed that all animals with missing flukes/peduncle due to human mutilation experienced entanglement at the flukes, one CBD for each lesion above would be counted as a yes. Therefore, all hernia cases and four out of six separation of the rectus abdominis muscles cases would have exhibited evidence of being hogtied. Though our data do not suggest hogtied animals exclusively develop these traumatic musculoskeletal lesions, we consider it a likely mechanistic contributor.
External Gear Evidence
The most frequent types of gear involved in the cases examined for this study were gillnets (n = 13), closely followed by pound nets (n = 9). In the geographic region where the strandings occurred, gillnets are anchored, making both pound nets and gillnets fixed gear. Fixed gear configurations may facilitate separation of the rectus abdominis muscles and hernias due to the physics of the interaction (e.g., fixed gear forces opposing locomotory effort during agonal struggle). The relatively high prevalence of separation of the rectus abdominis muscles & hernias in Virginia PUE cases may, therefore, be due to prevalence of bottlenose dolphin interactions with fixed gear in Virginia rather than lack of detection in other regions. Targeted examinations of the abdomen in other geographic regions may shed light on the prevalence of those lesions outside of Virginia.
Only cases for which PUE status could be determined were included in the study. However, this represents a very small portion of the total strandings in Virginia. During the study period, findings of anthropogenic trauma and thus PUE could not be determined for more than 200 stranded bottlenose dolphins. Since PUE detection rates likely underrepresent actual number of interactions, it is likely that numerous cases go undetected each year. If a constellation of internal lesions could be a diagnostic indicator of PUE in the absence of external lesions (such as in decomposed cases), stranding network members may observe more PUE positive cases.
Summary
Of the examined lesions, pulmonary perivascular edema, separation of the rectus abdominis muscles and acute abdominal hernias had the strongest association with PUE cases. Neither separation of the rectus abdominis muscles nor hernias were frequent findings, and individually did not have a significant relationship with PUE. However, when presence of either or both lesions was combined, they were significantly related to PUE status. Additionally, separation of the rectus abdominis muscles and acute abdominal hernias were both solely present in PUE study cases, and we believe them to be the result of extreme agonal struggle that does not commonly occur. Thus, though not the most common lesion present in PUE cases, separation of the rectus abdominis muscles and acute abdominal hernias are more likely to be indicative of PUE than other lesions discussed here. Despite a small sample size, two animals exhibited both acute abdominal hernias and separation of the rectus abdominis muscles, suggesting that similar physiologic processes may result in their formation. Though present in both PUE and non-PUE cases, pulmonary perivascular edema was significantly associated with PUE cases, and we recommend it for inclusion in future PUE investigations. There was no significant association between PUE and pulmonary petechiae or hemorrhagic pulmonary lymph and both lesions were present at higher rates in non-PUE animals compared with PUE animals. Therefore, we suggest that these lesions should be interpreted with caution and may not provide robust support for PUE diagnosis.
Multiple lesions were present in varying degrees of severity and in different combinations. It is possible that these represent different levels of intensity and/or duration of struggle. Physical forces at work during an entanglement (ex: degree of extension and flexion, intensity of contracture, and duration of struggle) all may contribute to different manifestations of these lesions. Additionally, unrelated pathologies may have compounding or confounding effects. For example, an animal with a severe lungworm infection may expire more rapidly or be weakened and unable to generate maximal force, thereby avoiding the more catastrophic injuries. Furthermore, the impact of ontogenetic differences and variations in size and/or strength is unknown, but may also contribute to the formation and/or presentation of the lesions. Gear type and its ability to anchor or immobilize an animal may also influence the pathogenesis. Finally, behavioral and physiologic differences between species are likely to play a role in how animals manifest agonal struggle and to what degree these lesions occur.
All dolphins included in this study stranded after the 2013 bottlenose dolphin unusual mortality event, attributed to dolphin morbillivirus (Morris et al., 2015) had ended. Though some symptoms of active morbillivirus have been documented (Di Guardo et al., 2005), the effects of post-morbillivirus exposure pathology are not well-understood. Both PUE and non-PUE cases exhibited evidence of underlying disease and its contribution to the presence of the pulmonary lesions in this study is unknown.
For prosectors interested in examining for the presence of separation of the rectus abdominis muscles and hernias, we suggest the following approach, in order: (1) parasagittal ventral incision, (2) examination of the ventral peritoneum to look for hernias and median retroperitoneal hemorrhage and edema prior to substantial abdominal viscera examination, (3) examination of abdominal organs, and (4) blunt dissection and reflection of the peritoneum to look for separation of the rectus abdominis muscles (see example in Figure 2A). As the pulmonary lesions (pulmonary petechiae, pulmonary perivascular edema, hemorrhagic pulmonary lymph) are not highly affected by manipulation and dissection artifact, they can be examined in situ or ex situ.
Future Work
Though all necropsies included in the study were considered full internal examinations, 52 lesions of interest were scored as CBD in this retrospective review due to insufficient documentation. In order to combat that in the future, our program has already implemented a standardized PUE checklist to enhance documentation and increase consistent evaluation of the lesions discussed above, which has resulted in fewer CBD determinations during 2019 than previous years. This checklist includes the novel lesions introduced in this study, additional lesions that the authors have observed that may be relevant to PUE, and previously published lesions.
Though these additional lesions are of interest, our research into them was still in its early stages at the time of publication. However, they are factors to consider when conducting necropsies. One such lesion is self-inflicted fractures and/or luxations. Intense physical events like electrocution and seizures have been shown to create self-inflicted fractures in humans (Finelli and Cardi, 1989; Kotak et al., 2000; Nekkanti et al., 2016). One of the two cases that exhibited both an acute abdominal hernia and separation of the rectus abdominis muscles during the study period also presented with numerous skeletal fractures, hemorrhage, and multi-organ fractures. Morphologic and histopathological reviews agreed that these findings were acute and consistent with extreme internal forces/agonal muscle contractions resulting from PUE. Additionally, one unpublished lesion with possible association to PUE is cutaneous ventral bruising/erythema, likely associated with the violent muscle excursions during agonal struggle. Based on preliminary observations, this lesion appears primarily focused in the gular and sternal regions, but can also present more subtly along the abdomen, and becomes more evident with time (see Supplementary Material for images). We are continuing to examine the patterns associated with this bruising/erythema and its relationship to entanglement locations and musculoskeletal attachments.
Within our study animals we observed several previously published PUE supportive lesions, such as intravascular gas bubbles, recently ingested GI contents, intramuscular bruising/hemorrhage, and vascular congestion, which are all included in our PUE checklist. After employing our full checklist with additional cases, we hope to combine both the novel and previously published lesions for a comprehensive analysis to present as many facets of PUE diagnosis as possible. Approximately 75% of all bottlenose dolphins that stranded in VA over the past 10 years were undiagnosed as to whether anthropogenic trauma was present (unpublished data). It is likely that some of those were PUE positive, but could not be diagnosed due to lack of external evidence (frequently due to decomposition). Though not all cases outside of our study group were reviewed, we know of at least six cases that had a hernia or separation of the rectus abdominis muscles during our 2016–2019 study period, but were undiagnosed as to whether anthropogenic trauma was present (and therefore did not qualify for inclusion in the current study). With additional future development, if internal lesions in the absence of external lesions could be used to diagnosis PUE, it may be possible to identify additional animals whose mortality could be directly attributed to fisheries. This data could then be included in stock assessments and fishery management plans. Though there is still no single internal lesion that is pathognomonic of PUE, our hope is that the PUE checklist and larger sample size will help us determine if the lesions in this study can be used, alone or in combination with other lesions, to diagnose PUE.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by Virginia Aquarium & Marine Science Center’s Animal Care Committee.
Author Contributions
AC conceived the study. AC, AE, JD, and SB designed the study. AE and JD organized and analyzed the data and wrote the manuscript. DR provided histopathological analysis and interpretation. All authors contributed to the article and approved the submitted version.
Funding
This work was partially funded by the John H. Prescott Marine Mammal Rescue Assistance Grant Program (Grant Numbers NA15NMF4390025 and NA18NMF4390025) and the Virginia Aquarium Batten Fund for Professional Development.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MM declared a past co-authorship with several of the authors SB, DR, and AC to the handling Editor.
Acknowledgments
We thank all of the past and present staff, volunteers, and interns at the Virginia Aquarium Stranding Response Program. We also thank the University of North Carolina Wilmington, North Carolina Stranding Network, NOAA Marine Mammal Health and Stranding Response Program, the editor SR, and Antonio Fernandez.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00503/full#supplementary-material
References
Allen, T. J., Jones, A., David, L., Morgan, R., and Proske, U. (2017). Muscle damage produced by isometric contractions in human elbow flexors. J. Appl. Physiol. 124, 388–399. doi: 10.1152/japplphysiol.00535.2017
Ambade, V., Namdeorao, L., and Wankhede, A. (2009). Blunt trauma and petechiae. J. Indian Acad. Forensic Med. 31, 156–159.
Askar, O. M. (1977). Surgical anatomy of the aponeurotic expansions of the anterior abdominal wall. Ann. R. Coll. Surg. Engl. 59, 313–321.
Baskin, R. J., and Paolini, P. J. (1967). Volume change and pressure development in muscle during contraction. Am. J. Physiol. 213, 1025–1030. doi: 10.1152/ajplegacy.1967.213.4.1025
Beekman, G. K. (2010). Type III hiatal hernia in a harbor seal (Phoca vitulina concolor). Aquat. Mamm. 34, 178–181. doi: 10.1578/AM.34.2.2008.178
Bernaldo de Quirós, Y., Hartwick, D., Rotstein, M., Garner, A., Bogomolni, W., Greer, M. E., et al. (2018). Discrimination between bycatch and other causes of cetacean and pinniped stranding. Dis. Aquat. Organ 127, 83–95. doi: 10.3354/dao03189
Bonanno, F. G. (2011). Physiopathology of shock. J. Emerg. Trauma Shock 4, 222–232. doi: 10.4103/0974-2700.82210
Brownell, R. L., Reeves, A. J., Read, B. D., Smith, P. O., Thomas, K., Ralls, M., et al. (2019). Bycatch in gillnet fisheries threatens Critically Endangered small cetaceans and other aquatic megafauna. Endang. Spec. Res. 40, 285–296. doi: 10.3354/esr00994
Colegrove, K. M., Denise, J., and Gulland, F. (2010). Cause of live strandings in Northern elephant seals (Mirounga angustirostris) and Pacific harbor seals (Phoca vitulina) along the central California Coast, 1992-2001. Aquat. Mamm. 1, 1–10. doi: 10.1578/AM.31.1.2005.1
Cooper, A. (1804). The Anatomy and Surgical Treatment of Inguinal and Congenital Hernia. London: Johnson, Longman and Rees.
Costache, M., Lazaroiu, A., Contolenco, D., Costache, S., George, M., Sajin, R., et al. (2014). Clinical or postmortem? The importance of the autopsy; a retrospective study. Maedica 9, 261–265.
Cotten, P. B., Piscitelli, M., McLellan, W., Rommel, S., Dearolf, J., and Pabst, D. (2008). The gross morphology and histochemistry of respiratory muscles in bottlenose dolphins, Tursiops truncatus. J. Morphol. 269, 1520–1538. doi: 10.1002/jmor.10668
Cozzi, B., Huggenberger, S., and Oelschläger, H. (2017). Anatomy of Dolphins: Insights into Body Structure and Function. Cambridge, MA: Elsevier Inc.
Davis, R. W., and Williams, T. M. (2012). The marine mammal dive response is exercise modulated to maximize aerobic dive duration. J. Comp. Physiol. A 198, 583–591. doi: 10.1007/s00359-012-0731-4
Di Guardo, G., Marruchella, U., Agrimi, S., and Kennedy, S. (2005). Morbillivirus infections in aquatic mammals: a brief overview. J. Vet. Med. Ser. A 52, 88–93. doi: 10.1111/j.1439-0442.2005.00693.x
Dick, T. J. M., and Wakeling, J. (2018). Geometric models to explore mechanisms of dynamic shape change in skeletal muscle. R. Soc. Open Sci. 5:172371. doi: 10.1098/rsos.172371
Dreyfuss, D. C., Flancbaum, I., Krasna, B., Tell, R., and Trooskin, S. (1986). Acute trans-rectus traumatic hernia. J. Trauma 26, 1134–1136. doi: 10.1097/00005373-198612000-00016
Duignan, P. J., Gibbs, N., and Jones, G. (2003). Autopsy of Cetaceans Incidentally Caught in Fishing Operations, 1997/98, 1999/2000, and 2000/01. Wellington: Department of Conservation.
Eng, C. M., Azizi, E., and Roberts, T. J. (2018). Structural determinants of muscle gearing during dynamic contractions. Integr. Comp. Biol. 58:12.
Estrade, V., and Dulau, V. (2017). First case of spinner dolphin (Stenella longirostris) infanticide off reunion island: necropsy’s findings and post mortem inter-species interactions. Oceanogr. Fish. Open Access J. 3:555615. doi: 10.19080/OFOAJ.2017.03.555615
Farber, J. P., Catron, A. C., and Krous, H. F. (1983). Pulmonary petechiae: ventilatory-circulatory interactions. Pediatr. Res. 17, 230–233. doi: 10.1203/00006450-198303000-00013
Finelli, P. F., and Cardi, J. K. (1989). Seizure as a cause of fracture. Neurology 39, 858–860. doi: 10.1212/WNL.39.6.858
Fisher, R. A. (1922). On the interpretation of χ 2 from contingency tables, and the calculation of P. J. R. Stat. Soc. 85, 87–94. doi: 10.2307/2340521
Geijer, C. K. A., and Read, A. (2013). Mitigation of marine mammal bycatch in U.S. fisheries since 1994. Biol. Conserv. 159, 54–60. doi: 10.1016/j.biocon.2012.11.009
Geraci, J., and Lounsbury, V. (2005). Marine Mammals Ashore: A Field Guide for Strandings, 2 Edn. Maryland: E. John Schmitz & Sons Inc.
Gerlach, T. J., de Wit, M., and Landolfi, J. (2012). Diaphragmatic hernia and right-sided heart enlargement in a florida manatee (Trichechus manatus latirostris). J. Wildl. Dis. 48, 1102–1104. doi: 10.7589/2011-12-344
Goodman, B. E. (2001). Pulmonary and renal pressure-flow relationships: what should be taught? Adv. Physiol. Educ. 25, 15–28. doi: 10.1152/advances.2001.25.2.15
Greene, R., Van Bonn, W., Dennison, S., Greig, D., and Gulland, F. (2015). Laparoscopic gastropexy for correction of a hiatal hernia in a northern elephant seal (Mirounga angustirostris). J. Zoo Wildl. Med. 46, 414–416. doi: 10.1638/2014-0226r.1
Groch, K. R., Díaz-Delgado, M., Marcondes, A., Colosio, E., Santos-Neto, V., Carvalho, G., et al. (2018). Pathology and causes of death in stranded humpback whales (Megaptera novaeangliae) from Brazil. PLoS One 13:e0194872. doi: 10.1371/journal.pone.0194872
Guntheroth, W. G., Breazeale, D., and McGough, G. (1973). The significance of pulmonary petechiae in crib death. Pediatrics 52, 601–603.
Hagins, M., Pietrek, M., Sheikhzadeh, A., Nordin, M., and Axen, K. (2004). The effects of breath control on intra-abdominal pressure during lifting tasks. Spine 29, 464–469. doi: 10.1097/01.brs.0000092368.90019.d8
Harman, E. A., Frykman, P., Clagett, E., and Kraemer, W. (1988). Intra-abdominal and intra-thoracic pressures during lifting and jumping. Med. Sci. Sports Exerc. 20, 195–201. doi: 10.1249/00005768-198820020-00015
Hayes, H. M. (1974). Congenital umbilical and inguinal hernias in cattle, horses, swine, dogs, and cats: risk by breed and sex among hospital patients. Am. J. Vet. Res. 35, 839–842.
Hayes, S. A., Josephson, K., Maze-Foley, P., and Rosel, P. (2018). US Atlantic and Gulf of Mexico Marine Mammal Stock Assessments - 2017 (second edition). Woods Hole, MA: Department of Commerce and Northeast Fisheries Science Center.
Jensen, K. K., Henriksen, N., and Jorgensen, L. (2015). Abdominal wall hernia and pregnancy: a systematic review. Hernia 19, 689–696. doi: 10.1007/s10029-015-1373-6
Jepson, P. D., Kuiken, P. M., Bennett, J. R., Baker, V. R., Simpson, R., and Kennedy, S. (2000). Pulmonary pathology of harbour porpoises (Phocoena phocoena) stranded in England and Wales between 1990 and 1996. Vet. Rec. 146, 721–728. doi: 10.1136/vr.146.25.721
Jubb, K. V. F., and Kennedy, P. C. (1970). Pathology of Domestic Animals, 2nd Edn, Vol. 2. New York: Academic Press, Inc.
Kastelein, R. A., van Dooren, M., and Tibboel, D. (2009). A case study of congenital diaphragmatic hernia in a juvenile striped dolphin (Stenella coeruleoalba). Aquat. Mamm. 35, 32–35. doi: 10.1578/am.35.1.2009.32
Kawabata, M., Shima, H., Hamada, I., Natamura, P., and Nishizono, H. (2010). Changes in intra-abdominal pressure and spontaneous breath volume by magnitude of lifting effort: highly trained athletes versus healthy men. Eur. J. Appl. Physiol. 109, 279–286. doi: 10.1007/s00421-009-1344-7
Knieriem, A., and Hartmann, M. (2001). Comparative histopathology of lungs from by-caught Atlantic white-sided dolphins (Leucopleurus acutus). Aquat. Mamm. 27, 73–81.
Kooyman, G. L., and Cornell, L. H. (1981). Flow properties of expiration and inspiration in a trained bottle-nosed porpoise. Physiol. Zool. 54, 55–61. doi: 10.1086/physzool.54.1.30155804
Kotak, B. P., Haddo, O., Iqbal, M., and Chissell, H. (2000). Bilateral scapular fractures after electrocution. J. R. Soc. Med. 93, 143–144. doi: 10.1177/014107680009300310
Lana, D., Tarallo, L., and Catani, F. (2017). A rare case of triceps brachii injury after electrocution. J. Hand Microsurg. 10, 46–48.
Light, H. G., and Routledge, J. (1965). Intra-abdominal pressure, factor in hernia disease. Arch. Surg. 90, 115–117.
Lillie, M. A., Vogl, S., Raverty, M., Haulena, W., McLellan, G., and Shadwick, R. (2017). Controlling thoracic pressures in cetaceans during a breath-hold dive: importance of the diaphragm. J. Exp. Biol. 220, 3464–3477. doi: 10.1242/jeb.162289
Lunde, P. K. M., and Waaler, B. A. (1969). Transvascular fluid balance in the lung. J. Physiol. 205, 1–18. doi: 10.1113/jphysiol.1969.sp008947
Mead, J. G., and Potter, C. W. (1990). “Natural history of bottlenose dolphins along the Central Atlantic Coast of the United States,” in The Bottlenose Dolphin, eds S. Leatherwood and R. Reeves (San Diego, CA: Academic Press, Inc), 165–195.
Moles, L., Durántez, J., Robles, M., and de Quinta, F. (2005). Spigelian hernia in Spain. An analysis of 162 cases. Rev. Espanola Enfermedades Digestivas 97, 338–347. doi: 10.4321/s1130-01082005000500006
Moore, K. T., and Barco, S. (2013). Handbook for Recognizing, Evaluating, and Documenting Human Interaction in Cetaceans and Pinnipeds. NOAA-TM-NMFS-SWFSC-510:102. Silver Spring, MA: NOAA.
Moore, M. J., van der Hoop, J., Barco, S., Costidis, A., Gulland, F., Jepson, P., et al. (2013). Criteria and case definitions for serious injury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aquat. Organ. 103, 229–264. doi: 10.3354/dao02566
Morris, S. E., Zelner, J., Fauquier, D., Rowles, T., Rosel, P., Gulland, F., et al. (2015). Partially observed epidemics in wildlife hosts: modelling an outbreak of dolphin morbillivirus in the northwestern Atlantic, June 2013–2014. J. R. Soc. Interface 12:20150676. doi: 10.1098/rsif.2015.0676
Naffaa, L. N., Tandon, Y., and Rubin, M. (2014). Myotendinous rupture of temporalis muscle: a rare injury following seizure. World J. Radiol. 6, 388–391. doi: 10.4329/wjr.v6.i6.388
Nekkanti, S., Vijay, C., Theja, J., RaviShankar, R., and Raj, S. (2016). An unusual case of simultaneous bilateral neck of femur fracture following electrocution injury-A case report and review of literature. J. Orthopaed. Case Rep. 6, 70–72. doi: 10.13107/jocr.2250-0685.514
Noren, S. R., Biedenbach, G., and Edwards, E. F. (2006). Ontogeny of swim performance and mechanics in bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 209, 4724–4731. doi: 10.1242/jeb.02566
Pearson, K. (1900). X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Lond. Edinburgh Dublin Philos. Magaz. J. Sci. 50, 157–175. doi: 10.1080/14786440009463897
Peltier, H., Dabin, P., Daniel, O., Van Canneyt, G., Dorémus, M., Huon, M., et al. (2012). The significance of stranding data as indicators of cetacean populations at sea: modelling the drift of cetacean carcasses. Ecol. Indic. 18, 278–290. doi: 10.1016/j.ecolind.2011.11.014
Plattner, T., Bolliger, S., and Zollinger, U. (2005). Forensic assessment of survived strangulation. Forensic Sci. Int. 153, 202–207. doi: 10.1016/j.forsciint.2004.09.106
Raiteri, B. J., Cresswell, A., and Lichtwark, G. (2016). Three-dimensional geometrical changes of the human tibialis anterior muscle and its central aponeurosis measured with three-dimensional ultrasound during isometric contractions. PeerJ 4:e2260. doi: 10.7717/peerj.2260
Read, A. J., Drinker, P., and Northridge, S. (2006). Bycatch of marine mammals in U.S. and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Read, A. J., and Murray, K. T. (2000). Gross Evidence of Human-Induced Mortality in Small Cetaceans. Silver Spring, MA: NOAA.
Reinpold, W., Köckerling, R., Bittner, J., Conze, R., Fortelny, A., Koch, J., et al. (2019). Classification of rectus diastasis—a proposal by the german hernia society (DHG) and the international endohernia society (IEHS). Front. Surg. 6:1. doi: 10.3389/fsurg.2019.00001
Ridgway, S. H. (1972). “Homeostasis in the aquatic environment.,” in Mammals of the Sea: Biology and Medicine, ed. S. H. Ridgway (Springfield, IL: Charles C. Thomas), 590–747.
Rushworth, B., Doumas, S., and Kanatas, A. (2018). Tear of the sternocleidomastoid muscle: a rare complication of lifting weights that can be managed conservatively. Br. J. Oral Maxillof. Surg. 56, 645–646. doi: 10.1016/j.bjoms.2018.03.024
Schaller, O., Gheorghe, M., Habel, W., Sack, P., Simoens, P., and de Vos, N. (2007). Illustrated Veterinary Anatomical Nomenclature, 2nd Edn. Stuttgart: Ferdinand Enke.
Scholander, P. F. (1940). Experimental Investigations on the Respiratory Function in Diving Mammals and Birds, Vol. 22. Oslo: Scientific Results of Marine Biological Research.
Schummer, A., Wilkens, B., Vollmerhaus, R., and Habermehl, K.-H. (1981). “The anatomy of the domestic animals,” in The Circulatory System, the Skin, and the Cutaneous Organs of the Domestic Mammals, eds W. G. Siller and P. A. L. Wight (Berlin: Springer).
Smith, A. B., Dickerman, C. S., McGuire, J. W., East, W. J., McConathy, R., and Pearson, H. F. (1999). Pressure-overload-induced sliding hiatal hernia in power athletes. J. Clin. Gastroenterol. 28, 352–354. doi: 10.1097/00004836-199906000-00014
Stephen, C. (1993). Hiatal hernia in a harbor porpoise (Phocoena phocoena). J. Wildl. Dis. 29, 364–366. doi: 10.7589/0090-3558-29.2.364
Timby, J., Reed, C., Zeilender, S., and Glauser, F. (1990). Mechanical causes of pulmonary edema. Chest 98, 973–979. doi: 10.1378/chest.98.4.973
Whayne, T. F., and Severinghaus, J. (1968). Experimental hypoxic pulmonary edema in the rat. J. Appl. Physiol. 25, 729–732. doi: 10.1152/jappl.1968.25.6.729
Zelel, Y., Shalev, S., Romano, M., Ben-Ami, U., Dan, P., and Weiner, E. (1992). Incarcerated spigelian hernia in pregnancy: an ultrasonic diagnosis. J. Clin. Ultrasound 20, 146–148. doi: 10.1002/jcu.1870200213
Keywords: anthropogenic trauma, fishery interaction, bycatch, stranding, cetacean, entanglement
Citation: Epple AL, Daniel JT, Barco SG, Rotstein DS and Costidis AM (2020) Novel Necropsy Findings Linked to Peracute Underwater Entrapment in Bottlenose Dolphins (Tursiops truncatus). Front. Mar. Sci. 7:503. doi: 10.3389/fmars.2020.00503
Received: 14 February 2020; Accepted: 03 June 2020;
Published: 02 July 2020.
Edited by:
Stephen Raverty, Animal Health Center, CanadaReviewed by:
Michael John Moore, Woods Hole Oceanographic Institution, United StatesTodd Schmitt, SeaWorld Entertainment, United States
Sam Ridgway, National Marine Mammal Foundation, United States
Copyright © 2020 Epple, Daniel, Barco, Rotstein and Costidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra L. Epple, QWxleGFuZHJhRXBwbGVAZ21haWwuY29t
 Alexandra L. Epple
Alexandra L. Epple Joanna T. Daniel
Joanna T. Daniel Susan G. Barco
Susan G. Barco David S. Rotstein
David S. Rotstein Alexander M. Costidis
Alexander M. Costidis