- 1Departament de Ciències Ambientals, University of Girona, Girona, Spain
- 2Departament de Geografia, University of Girona, Girona, Spain
- 3CNRS, IRD, MIO (Mediterranean Institute of Oceanography), UM 110, GIS Posidonie, Aix-Marseille Univ, University of Toulon, Marseille, France
- 4Animal Demography and Ecology Unit, IMEDEA (CSIC-UIB), Esporles, Spain
- 5Departament de Biologia, Sanitat i Medi Ambient, University of Barcelona, Barcelona, Spain
- 6Chemical Oceanography Unit, FOCUS, University of Liège, Liège, Belgium
- 7Laboratory of Oceanology, FOCUS, University of Liège, Liège, Belgium
A worrying phenomenon has been affecting the common white seabream (Diplodus sargus) for near 40 years. Professional and recreational fishers from the Mediterranean coasts and the Atlantic coasts of Europe and Macaronesia have reported individuals of white seabream that became “like a tire” after cooking, and consequently inedible. The phenomenon was related neither to the freshness of the fish nor to the way it had been preserved or cooked. According to recreational fishers, this Abnormally Tough Specimen (ATS) phenomenon appeared singularly in time, in different places and to different extents. This singular, scattered appearance, with no area of origin from which to spread, de facto excluded any process of contagion. In order to compensate for the lack of knowledge and understanding related to this issue, we undertook a first study that aimed at addressing the extent of the white seabream anomaly in the western Mediterranean. To reach this objective, we carried out surveys on voluntary basis among fishers (both professional and recreational) and researchers throughout the western Mediterranean. Data from the surveys (n = 270) were then analyzed to evaluate the distribution of ATS and its possible relationship with human activities. Results showed that the anomaly affected the white seabream and very occasionally some other species, mainly of the same family Sparidae. In addition, the phenomenon did not occur simultaneously in the different areas surveyed over the last years and in some places it seems to have disappeared. We highlighted a possible link between ATS occurrence and the presence of human activities in adjacent areas. We hypothesized pollution – including by copper – could be a possible driver of ATS. Results suggested a tendency of ATS to cluster around fish farms and commercial and industrial ports, although we are aware other human factors might also influence the phenomenon. To conclude, the present study gives an overview of the importance of the white seabream anomaly in the Mediterranean and encourages further research to disentangle the exact mechanisms behind this phenomenon.
Introduction
The common white seabream Diplodus sargus (Linnaeus, 1758) (F. Sparidae) is a demersal fish, living in rocky infralittoral and circalittoral habitats. It is a very common species in the western Mediterranean and in the north-eastern coast of the Atlantic Ocean, including the Macaronesian archipelagos (Pérez et al., 2007). The individuals of this species are medium-sized with a relatively long lifespan and an omnivorous benthic diet. It is an edible species with a considerable commercial interest, representing a common catch in both professional and recreational fisheries.
In recent years, this species seems to have been affected by a strange phenomenon. Professional and recreational fishers from the Mediterranean have reported the capture of white seabream that, after cooking, became “like a tire” and inedible (hereafter called ATS: Abnormally Tough Specimens). Information collected indicates that the phenomenon is related neither to the freshness of the fish nor to the way it has been conserved or cooked. Recreational fishers advocate for a number of hypotheses, including pollution, post-mortem stress or depletion of lipid reserves during the reproductive period.
In a previous work, we studied the muscle trace-metal loads of D. sargus from the Catalan coast (North-western Mediterranean, Spain). In addition to the general high levels of mercury and other potentially harmful elements (Tramati et al., 2012; Casadevall et al., 2017; Merciai et al., 2018), copper loads were especially high in specimens landed in Roses, where a port and a fish farm are located.
Aquaculture production and consequently open cage systems have increased over the past decades (FAO, 2003). Copper, zinc, and cadmium are common ingredients in fish feeds and have been used as tracers of feed pellets (Dean et al., 2007). Moreover, with the gradual elimination of triorganotin-based formulations [e.g., tributyltin (TBT)] in anti-fouling paints, copper has become the principal biocidal component of most of these paints, usually in the form of copper oxide (Cu2O) (Yebra et al., 2004). Therefore, copper release to the environment is a characteristic that ports and farms have in common and, although the connection of the anomaly with copper is not evident, the proximity to ports and aquaculture areas could provide some clues to its origin. For this reason, the objective of this study was to investigate the spatiotemporal occurrence of the ATS phenomenon in the Western Mediterranean and the possible relationship between ATS occurrence and the presence of these facilities. To reach this goal, we first mapped the distribution of locations where ATS had been caught in the western Mediterranean, based on a voluntary survey near recreational fishers. Afterward, we analyzed the distribution of ATS observations as a function of the distance to the nearest largest (commercial) harbors and/or marine fish farms. We also considered the temporal evolution of the ATS phenomenon since its first report in the Western Mediterranean.
Materials and Methods
Fish Forums and Data Collection
A first voluntary survey of ATS occurrence at the scale of the south French littoral was performed in 2014 by Verlaque (pers. comm.). From this first regional study, 55 answers were obtained (unpubl. results). In a second step, we posted during year 2019 the same survey, with few modifications, on a platform (SurveyMonkey) to collect answers more easily from fishers’ forums and social networks. By the time we started writing up this research article, we had collected 215 positive responses that, when added to the previous 55, totalled 270 surveys, mostly answered by recreational fishers and especially by anglers. Out of these, 255 confirmed the presence of ATS. Data available from North Africa and the Atlantic were very scarce, so this work focused on data from the Mediterranean coasts of Spain, France and Italy. In addition, some locations where ATS were reported were repeated several times, and therefore considered unique. Finally, the study was centered on 96 locations for the spatial analysis of ATS occurrence. From here, therefore, ATS observations refer to these 96 locations.
The survey of recreational fishers sought information on six key topics. It included the place (or places) where ATS were caught, the year(s) and the length of the observation period, if other species with the same problem were caught and the presence of remarkable structures or elements near the fishing area (nautical port, fishing port, aquaculture facility, river, marine outfall, invasive algae, etc.). The survey (in English, French, Italian, Arabic, Spanish and Catalan) also asked about anything strange in the fish (color, behavior, parasite, etc.).
With regard to ethical issues, the work was based on observations reported from a voluntary survey. The processing of the personal data of the project informants was based on their consent, explicitly stated when sending the answers to the survey, in accordance with article 6.1 of the General Data Protection Regulation (Regulation (EU), 2016). The data from survey reports were analyzed statistically, with no links to the identifying data, and will only be processed for the time necessary to complete the ongoing project.
Our survey targeted recreational fishers because they could give us information about the capture area and the dates, and were more likely to be final consumers who would know whether or not the specimens were tough after cooking.
Regarding ports, along the Mediterranean coasts of Spain, France and Italy, the location of nautical ports is so continuous that it would have masked any trend in the survey results. Thus, given the goal of the study, the selection of ports has prioritized industrial and commercial ones because of their increased port activity with ships of deeper drafts.
The geographic information structured by layers was extracted from different sources. The industrial and commercial ports’ classification and location correspond to the World Port Index (pub. 150) of the National Geospatial-Intelligence Agency1. The database containing points representing marine finfish aquaculture facilities is updated yearly by the European Marine Observation and Data Network2. We supplemented this information by reviewing all the aerial orthophotos of the studied coastline and digitizing all the identified marine finfish aquaculture facilities. Industrial and commercial ports will be referred to as ports or commercial ports later in the paper; marine finfish aquaculture facilities will be referred to as farms or fish farms.
Distance and Temporal Analyses
Boxplots were useful to analyse ATS observations at atypical distance values to the nearest fish farm or commercial port. We then examined, using a dispersion plot, the interactions between distances to the nearest fish farm and to the nearest commercial port with two proposals. The first one was to determine if collinearity existed between the two-predictor variables. Indeed, we have to consider that ports and off-shore aquaculture facilities can be located close to each other. A Spearman’s correlation test was performed for this purpose (considering a restrictive threshold value of 0.4; Dormann et al., 2013). The second one was to analyze whether ATS observations not explained by proximity to a fish farm could be explained by proximity to a commercial port and vice versa.
Prior to the analysis of the occurrence of ATS observations as a function of distance to nearest fish farm and to nearest commercial port, data were grouped according to ‘distance classes’. The grouping distance was 2.5 km, giving a total number of 16 classes for distance to farm (maximum distance from nearest farm for ATS observation = 39.2 km) and 26 classes for distance to port (maximum distance from nearest port for ATS observation = 63.1 km). With increasing distance to the nearest farm or port, a few 2.5 km distance classes (one for distance to farm, seven for distance to port) containing no ATS observations were filled in with zero. After grouping, we analyzed the spatial distribution of the occurrence of ATS observations as a function of distance to nearest farm or port. A generalized linear model (glm) with Poisson distribution and log-link function was fit to the distance to farm data (Cameron and Trivedi, 1998; Warton et al., 2016) and a three-parameter asymptotic exponential regression model fit by non-linear least squares (nls) was fit to the distance to port data (Wooldridge, 2002; Winkelmann, 2008). The same 2.5 km distance grouping method and glm analysis (with robust parameter estimation; Cantoni and Ronchetti, 2001) were performed for a third distance factor, calculated as the average distance for ATS observations between the nearest farm and the nearest port. The first distance class 0–2.5 km – out of the 16 from the average – with little ATS observations reported was ignored in the glm analysis (i.e., this required the farm and the port to be very close, and that the ATS fish was caught close to both, which was unlikely to happen). Finally, the effect of the two distance class factors to nearest port and to nearest farm on the occurrence of ATS observations was analyzed using generalized additive model (gam) with Poisson distribution and log-link function (Zuur et al., 2009). The gam model allows for more flexible association patterns for the response variable, ATS observation, to the predictor variables, distance to nearest farm and distance to nearest port. Grouping distance was set to 5 km before gam analysis, giving 38 classes for distance to both nearest farm and nearest port (61 classes if grouping distance of 2.5 km, so close to the number of locations where ATS fish were reported; 17 classes if grouping distance of 10 km). Model assumptions (including residual distribution, variance homoscedasticity and overdispersion) and model fit were checked with diagnostic plots and tests. Akaike information criterion and Bayesian information criterion were used to select the best fitting model (Brewer et al., 2016).
Finally, the occurrence of ATS observations over time was determined by summing the number of observations reported by fishers on a yearly basis, to see if the chronological distribution contributed with some other clues.
Data analysis and statistics were performed in RStudio version 1.1.383 (R Studio Team, 2016), using R’s base functions (R Core Team, 2019) and functions of packages dplyr (Wickham et al., 2019), mgvc (Wood, 2017), nlstools (Baty et al., 2015), AER (Kleiber and Zeileis, 2008), and robustbase (Maechler et al., 2019).
Results
We collected a total of 270 survey reports, taking into account the 55 of Verlaque’s 2014 survey (unpubl. results) and the 215 from the present survey. From among all the responses, 255 (94.4%) were positive, which is to say that respondents indicated they had occasionally captured one or several species or individuals affected by the ATS anomaly. This confirmed the ATS anomaly is widespread. Only 15 respondents (5.6%) had never observed the phenomenon, but some indicated they had heard about it. Figure 1 represents all sites where the presence of ATS was reported in the Mediterranean from survey reports, together with major port infrastructures and marine farms. Only 4 rivers were mentioned in the answers: Rhône, Llobregat, Ebre, and Guadalete, which are also highlighted in Figure 1.
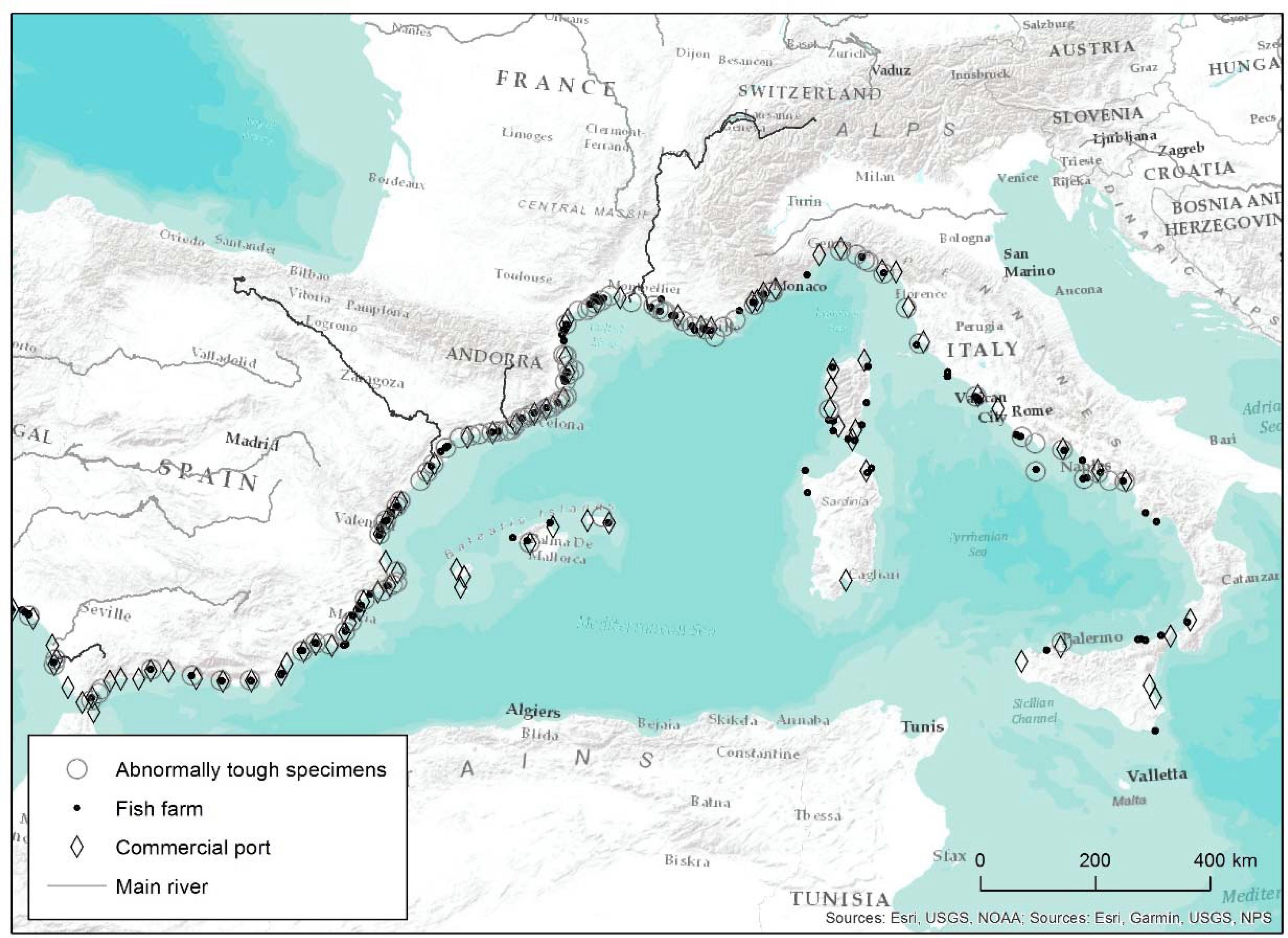
Figure 1. Mediterranean locations where abnormally tough specimens of white seabream were captured according to survey data. The map includes the major port infrastructures and marine farms. The only four rivers mentioned in survey reports are also highlighted (Rhône, Llobregat, Ebre, and Guadalete).
Answers that were inaccurate and could not be added to the survey dataset, compared to those properly filled in reports, were discarded prior to the analysis.
ATS specimens of D. sargus were reported 237 times. The anomaly was observed mainly in the Mediterranean (132 reports from Spain, 61 from France, 1 from Monaco, 22 from Italy, and 1 from Tunisia), but also in the European Atlantic (3 reports from Spain and 4 from France) and in Macaronesia (5 reports). Some respondents (8) did not indicate the locality of caught ATS. The information collected was quite variable in the different areas (Table 1).
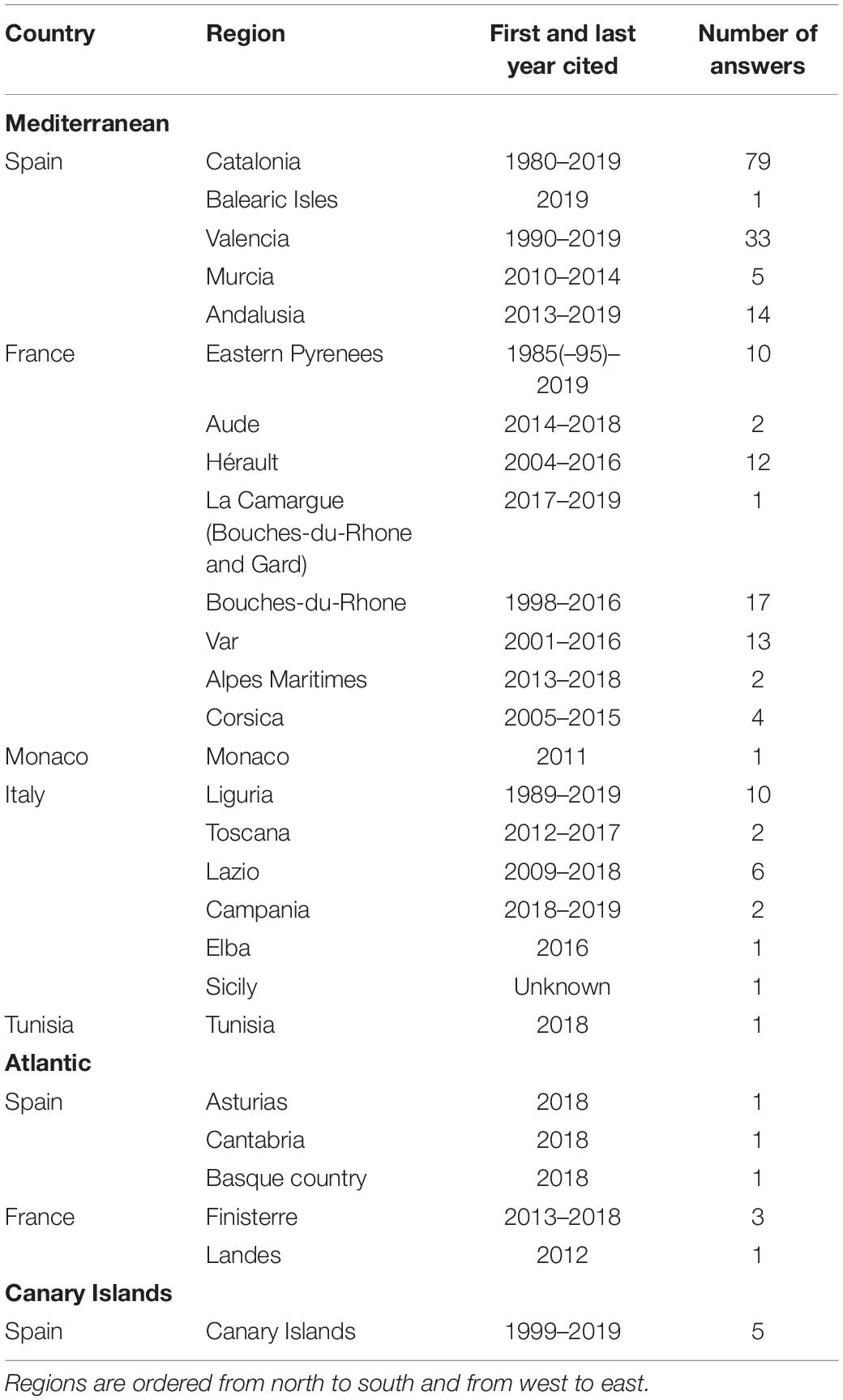
Table 1. Summary of the regions grouped by country and marine area where fishers reported they captured anomalous white seabream, and years of the oldest and most recent report.
In some localities, ATS only appeared from time to time, while in others, fishers said that most specimens showed the anomaly. In addition, the presence of ATS specimens did not appeared simultaneously in the area covered by the survey, neither correlatively. Many fishers reported the anomaly from specific periods, and some noticed that, in sites where they had always fished, the problem had appeared in recent years. Fishers mentioned that the ATS phenomenon has been known for about 40 years in Catalonia (Spain), for 25–35 years in the Gulf of Lyon (Eastern Pyrenees, France), and for 30 years in Italy.
Regarding size (except in one case), all the responses referred to individuals of D. sargus longer than 15 cm (total length), with 81.3% of the specimens longer than 20 cm.
With respect to other affected fish, most were other species of Diplodus, such as D. puntazzo (Cetti, 1777) (6), D. cervinus (Lowe, 1838) (4) and D. vulgaris (Geoffrey Saint-Hilaire, 1817) (3). However, the anomaly was also observed in Sparus aurata Linnaeus, 1758 (2), Sarpa salpa (Linnaeus, 1758) (2), Epinephelus sp. (1), Lithognatus mormyrus (Linnaeus, 1758) (1), Pomatomus saltatrix (Linnaeus, 1766) (1), Seriola dumerili (Risso, 1810) (1), Sciaena umbra Linnaeus, 1758 (1), Spondyliosoma cantharus (Linnaeus, 1758) (1), and Zeus faber Linnaeus, 1758 (1). We ignored some other responses because the common name given to caught fish by fishers could refer to two or more species.
Distance and Temporal Analyses
We finally analyzed 96 locations (i.e., 96 ATS observations; Figure 1), a number lower than the total surveys reporting ATS catches since some locations were repeated more than once. The furthest distance of ATS observation from a fish farm was 39.2 km, but half of the ATS observations were closer than 7.3 km, as shown on the boxplot (Figure 2). Two atypical values were found (38.3 and 39.2 km). However, both were quite close to a commercial port (less than 1 km to Tarragona and 5.1 km to Genova, respectively) (Table 2).
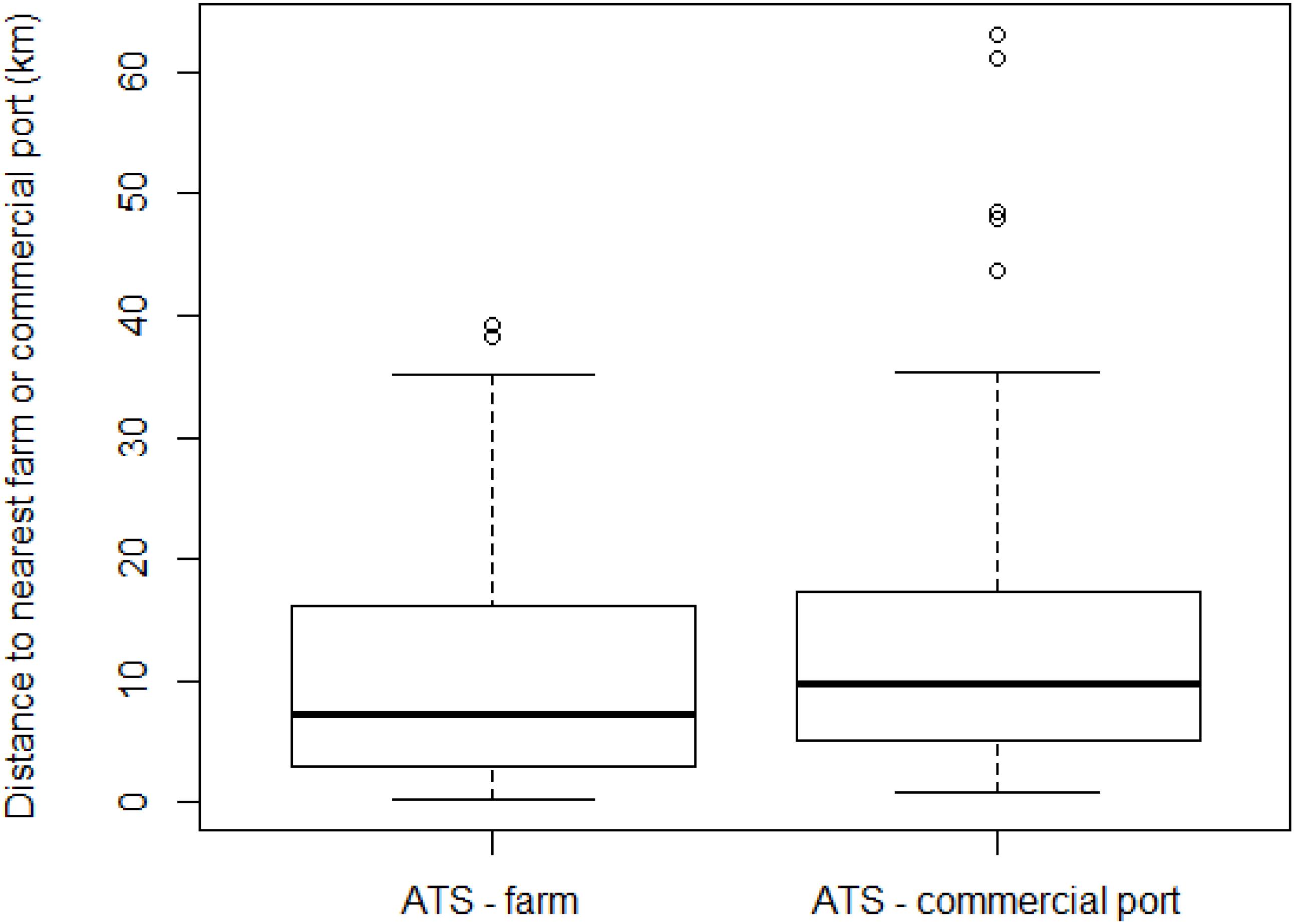
Figure 2. Boxplot of distance of Abnormally Tough Specimen (ATS) observations to the nearest farm or commercial port, with median, first and third quartiles (boxes), least values (dotted bars), and outliers (dots).
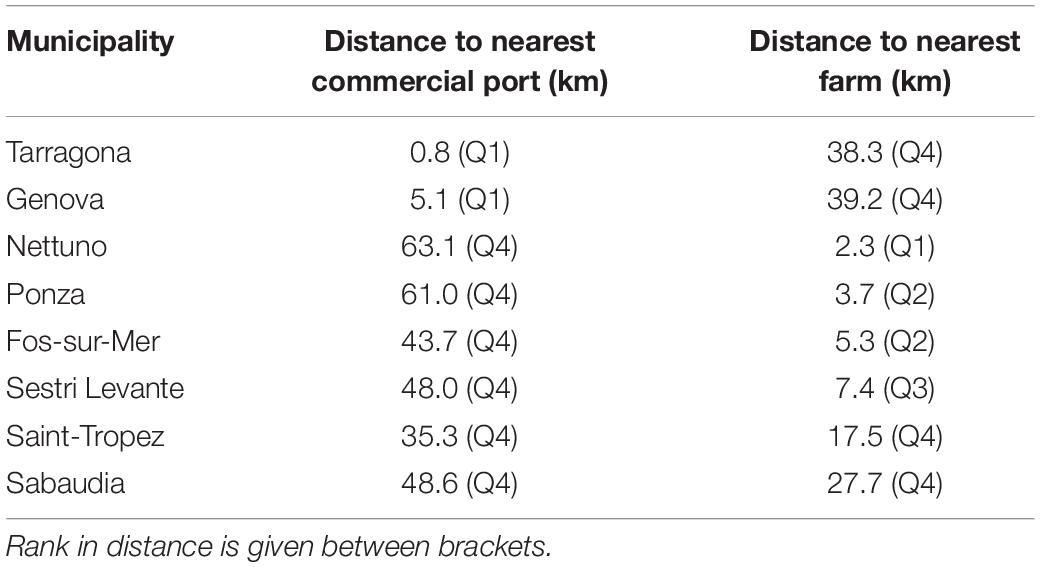
Table 2. Municipalities which present atypical values for distance to the nearest fish farm or commercial port.
The furthest ATS observation from a commercial port was 63.1 km, but the median distance was only 9.7 km. The distance to the nearest commercial port showed more atypical values than the distance to the nearest fish farm; specifically, six municipalities were classified as atypical values (Table 2).
Atypical values ranged from 35.3 to 63.1 km. Four of them were closer to a fish farm than the median distance (7.3 km). Hence, whereas atypical values of distance to the nearest fish farm were clearly explained by their distance to a commercial port, the other way round did not apply.
The interaction between both variables (distances to nearest fish farm and commercial port) was examined for ATS observations. No collinearity was observed between the two predictor variables distance to nearest farm and distance to nearest commercial port (r = 0.18; p = 0.08). The dispersion diagram (Figure 3) shows that ATS observations far from a fish farm tend to be close to a commercial port and vice versa. Only one point fell clearly outside of this tendency: Sabaudia (Lazio, Italy), which was classified as an atypical value regarding distance to the nearest commercial port and was located in the fourth quartile regarding distance to the nearest fish farm (but not as an atypical value).
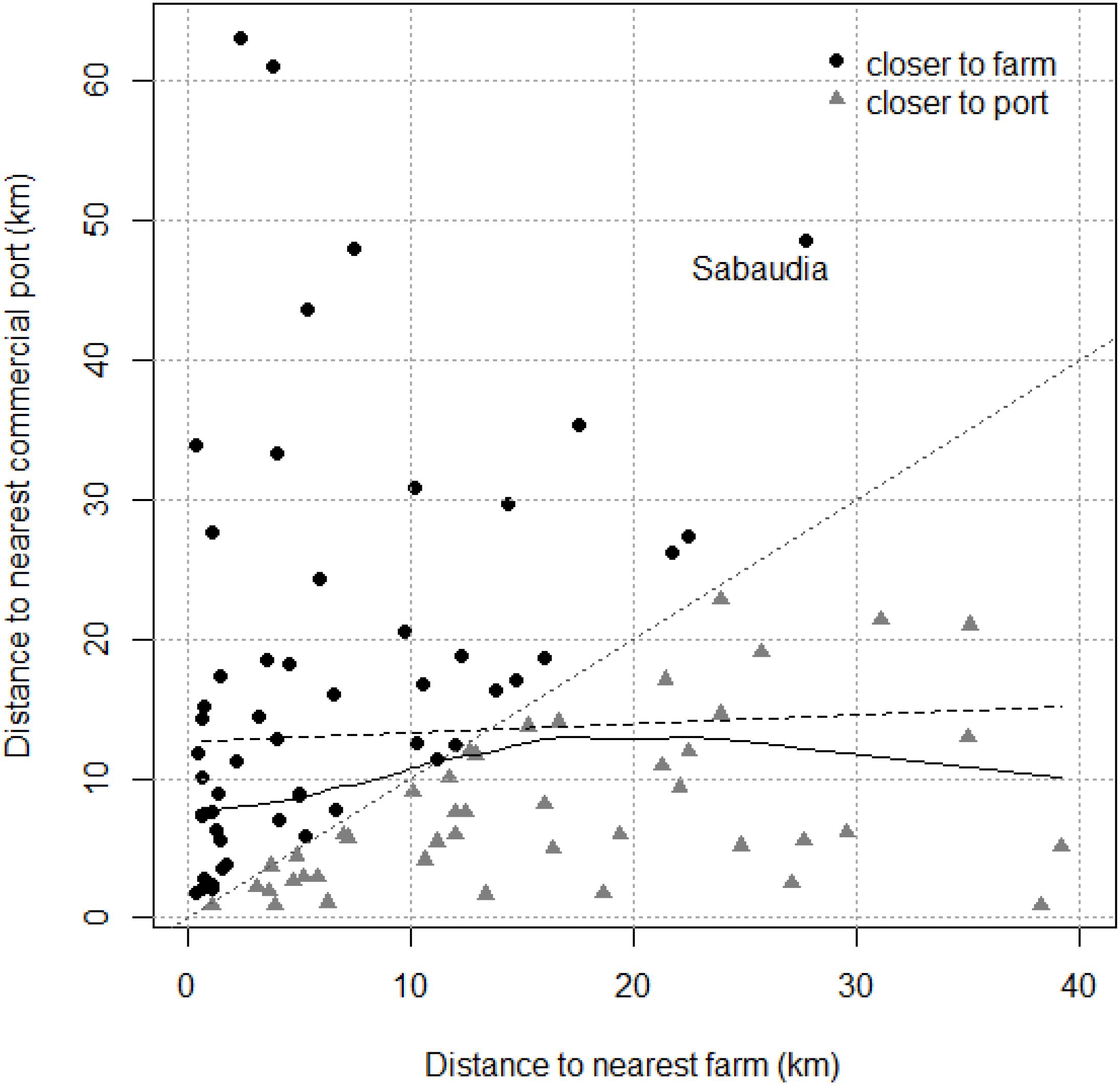
Figure 3. Dispersion diagram for Abnormally Tough Specimen (ATS) observations. The dashed and full black lines are linear and smooth relationships between distance to the nearest farm and distance to the nearest commercial port, respectively. Black dots above the dotted 0–1 gray line are ATS observations closer to farms, gray triangles under are closer to commercial ports. Sabaudia ATS observation is highlighted.
The decrease of ATS observations with increasing distance to nearest commercial port or nearest farm was obvious when plotted according to the 2.5 km distance classes (Figure 4). According to model fitting and ATS caught distribution, the decrease was rapid in the first 10 km away from farms; number of ATS observations in that distance interval remained similar for ports. Beyond this distance of 10 km, the continuous decreases in ATS observations with the increase in distance was similar for both distance factors. The occurrence of ATS observations became zero or close to zero beyond 40 km from the nearest commercial port or farm.
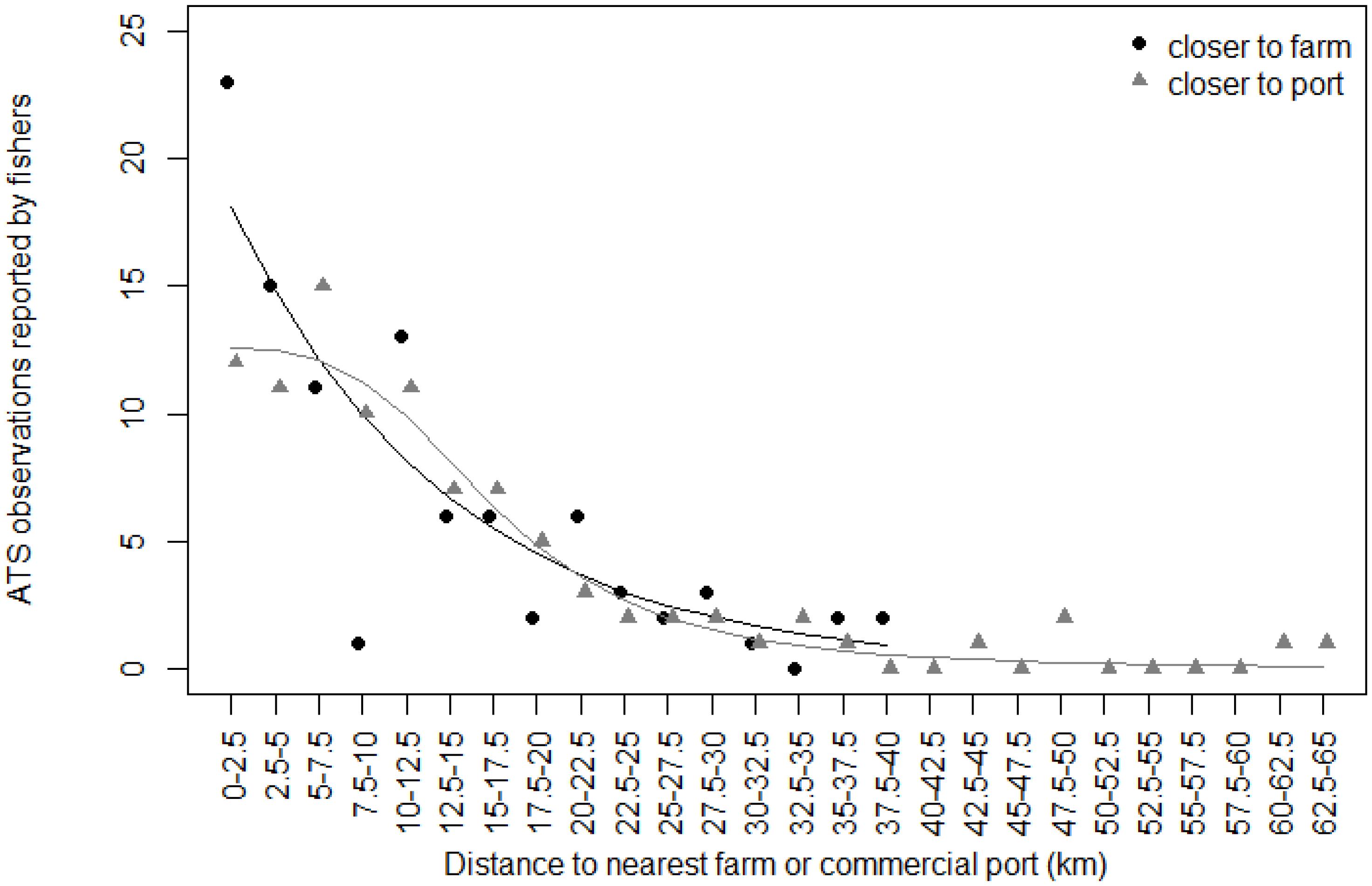
Figure 4. Relationship between Abnormally Tough Specimen (ATS) observations reported by fishers and distance to the nearest farm (black dots) or commercial port (gray triangles). ATS observations are grouped according to distance classes of 2.5 km. The black line is prediction from glm on farm data. The gray line is prediction from exponential nls on port data.
The same ascertainment was made as to the number of ATS observations as a function of the average distance to the nearest farm and to the nearest commercial port. The decrease in the number of ATS observations was obvious once fishers moved away from both farms and ports. It reached zero when the average distance between the nearest farm and the nearest port was 40 km. The few ATS observations in distance class 0–2.5 km was explained by the low probability of catching an ATS at a very short distance from both a farm and a port (Figure 5).
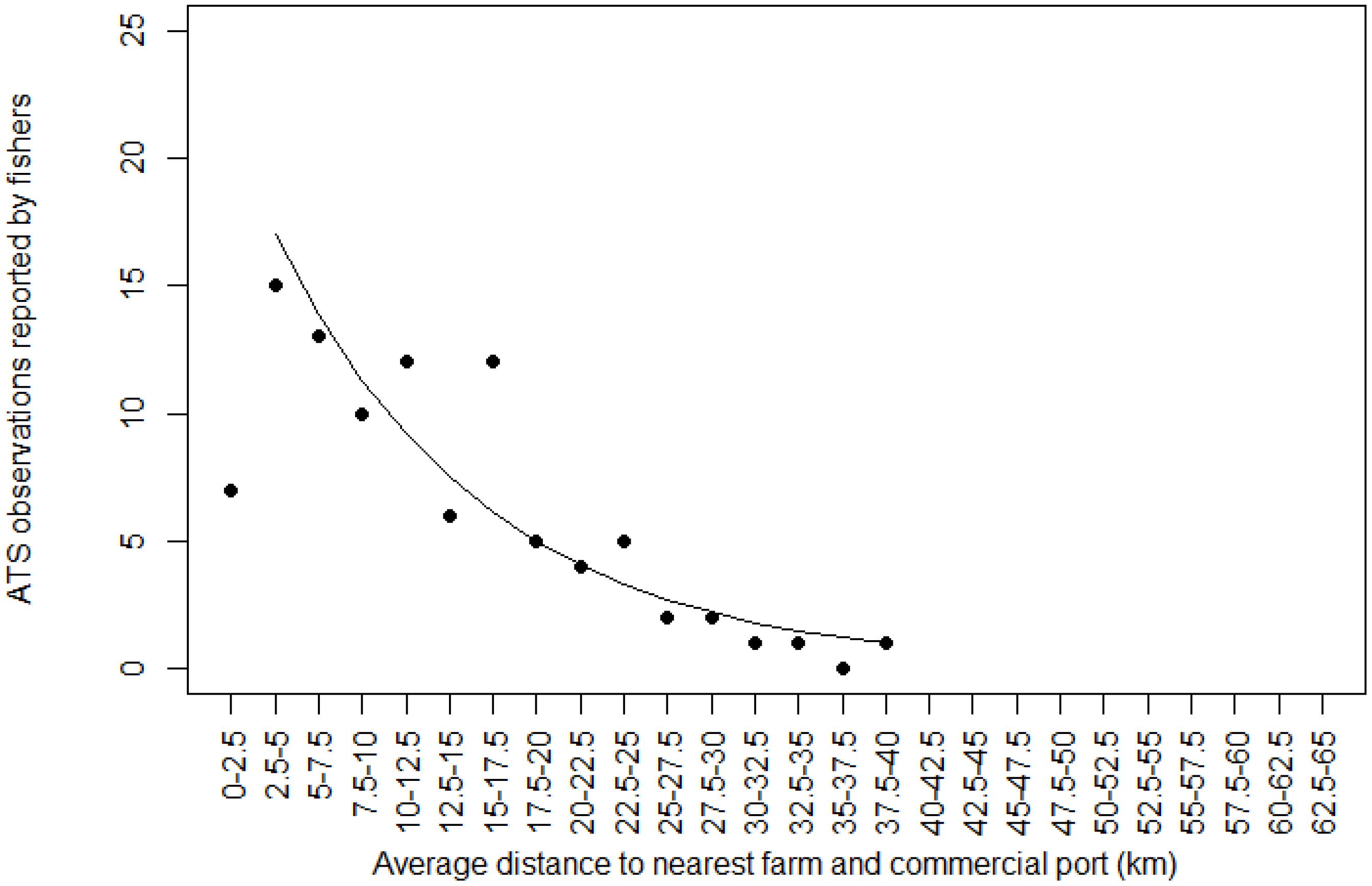
Figure 5. Relationship between Abnormally Tough Specimen (ATS) observations reported by fishers and average distance between the nearest farm and the nearest commercial port. The black line is prediction from glm (first distance class 0–2.5 km count removed).
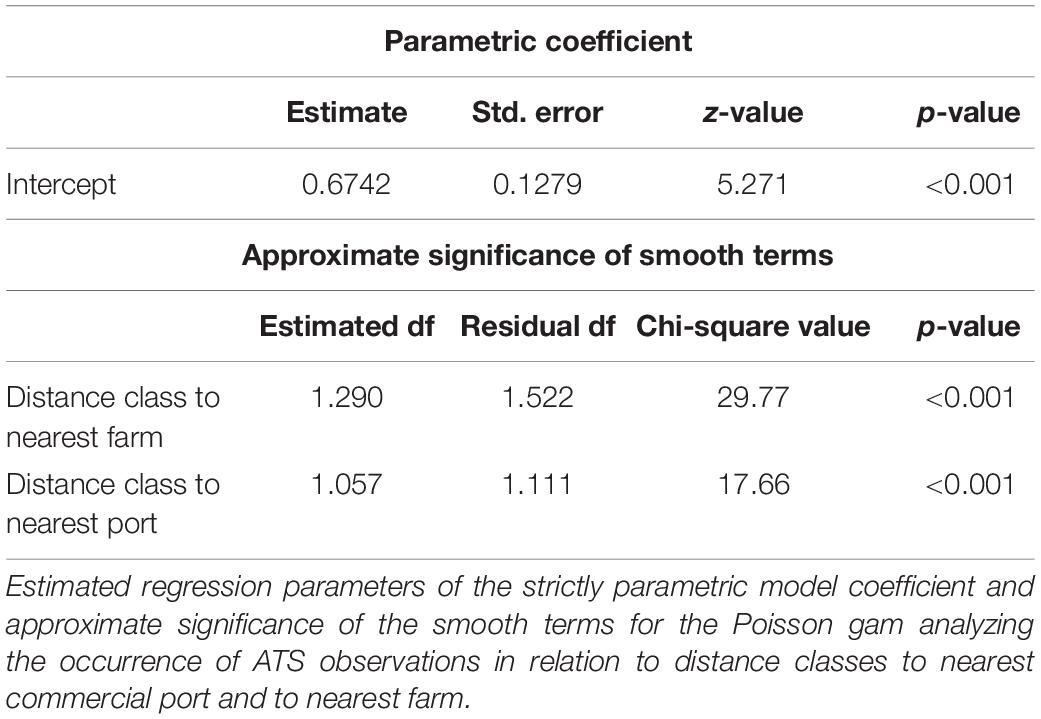
The Poisson gam analyzing the occurence of ATS observations in relation to distance classes to nearest commercial port and to nearest fish farm confirmed the significant effect of the two distance factors (Table 3). The model explained 67.1% of deviance.
Finally, Figure 6 illustrates the temporal evolution of ATS observations over time, since their first report. ATS observations reported by fishers peaked in recent years, with a net increase since the beginning of 2000s. The increase was almost continuous since then, and showed net year-to-year variability like, e.g., between years 2003–2004, 2008–2009, or 2016–2018.
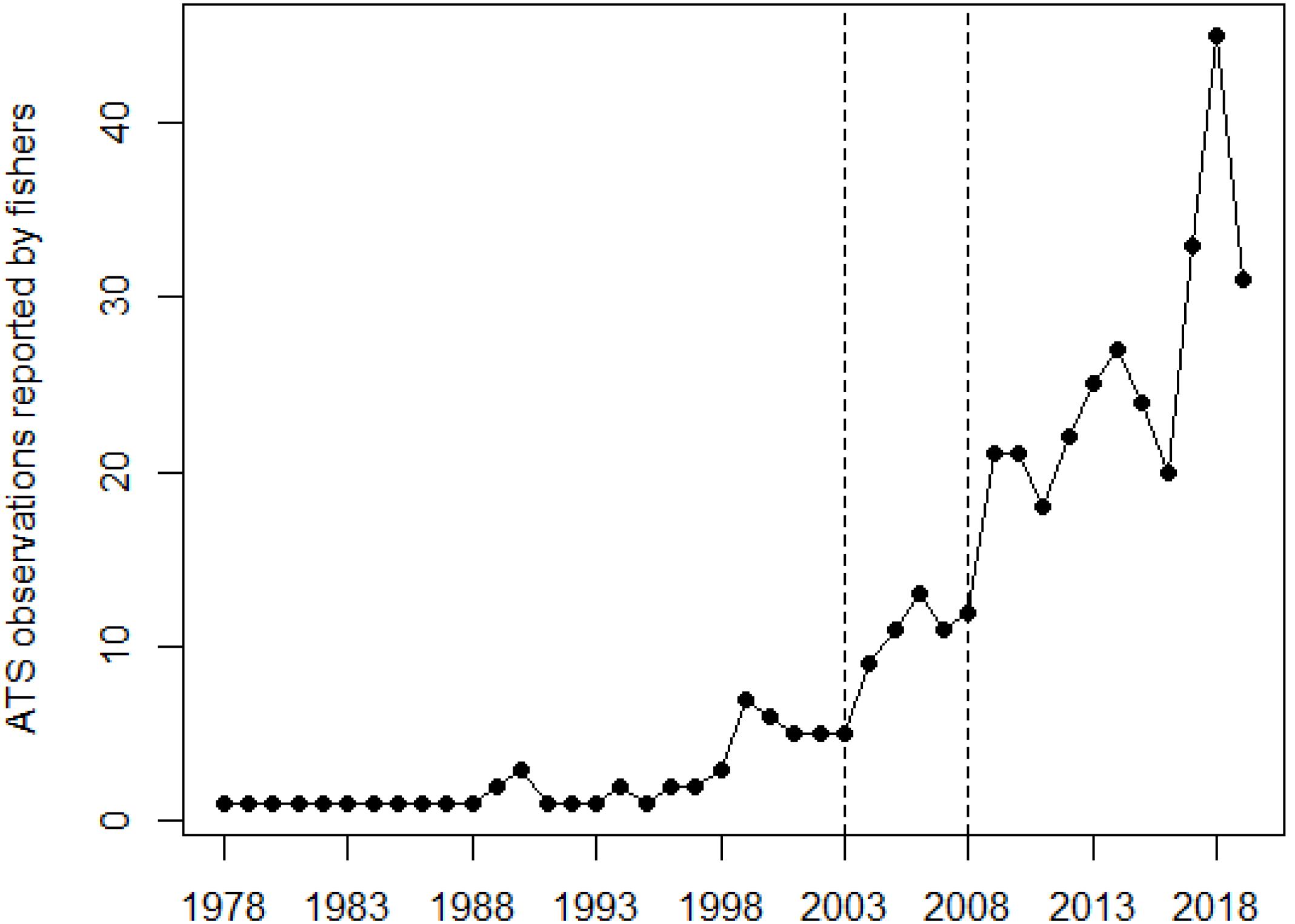
Figure 6. Occurrence of Abnormally Tough Specimen (ATS) observations over time, summing the number of ATS observations reported by fishers on a yearly basis. Vertical dashed lines mark the years when organotin compounds in anti-fouling paints were no longer allowed and copper-based started to be used on vessels and mariculture cages (2003), and when the EU banned the entry of vessels carrying paints with organotin compounds (2008).
Discussion
This work explores a phenomenon which has been underestimated until now and that, as evidenced by our results, is distributed at least throughout the western Mediterranean, the Atlantic coast of Europe and the Canary Islands. The first step of this survey work on ATS occurrence in the Mediterranean was performed in 2014 by Verlaque (unpubl. results) along the French littoral. Data collected from his preliminary work showed that the inedible white seabream phenomenon was known and common in the western Mediterranean for at least the past 35–40 years. In 2012–2013, the anomaly was also reported on the French Atlantic coast (Landes and Finistère), and in 2018 on the Atlantic coast of Spain. The first observation we have from the Canary Islands dates from 1999. The ATS anomaly affects mainly the white seabream, but its incidence in other species has also been reported in collected survey reports, mostly after 2014. The occurrence of ATS appears to be a widespread phenomenon that deserves investigation.
For the white seabream, although the phenomenon had initially a low incidence in the Mediterranean (around 1% of caught fish in Catalonia), it reached 40–50% of individuals in the eastern Pyrenees in the last 20–30 years and 20–30% of individuals in Catalonia around 2007–2010. Elsewhere, the phenomenon has also become locally important. For example, in 2015 in Naples, 70% of the white seabream presented the anomaly, leading to a drop of about 50% of its commercial value on the local fish markets (Tundo, 2015).
Results suggest a tendency of ATS to cluster around fish farms and commercial ports. Although distance data to commercial ports showed more atypical values than distance data to farms, nautical and fishing port breakwaters, and sometimes commercial ports are preferential recreational fishing sites. Considering this, apparent ATS clustering around ports is very likely to occur. We have also considered that ports and off-shore aquaculture facilities can be located close to each other. For this reason, we explored the collinearity between distances to ports and farms. In the literature, collinearity diagnostics relying on the determination of correlation coefficients rho often consider as threshold values 0.5–0.7 (Dormann et al., 2013). Some authors also used more restrictive values, e.g., 0.4. With a rho of 0.18, we can consider that collinearity has very limited impact in this work.
Despite white seabream exhibit high site fidelity (Abecasis et al., 2009; D’Anna et al., 2011; Belo et al., 2016) in both the reproductive and non-reproductive seasons, they have also shown the ability to cover large distances (e.g., 20 km) (D’Anna et al., 2004; Di Franco et al., 2012), proving a remarkable behavioral plasticity in habitat use (Di Lorenzo et al., 2016). Abecasis et al. (2015) observed daily displacement up to 4.97 km (which corresponds to percentile 39 of the distance to the nearest farm in our results), although trips of around 100 km have occasionally been recorded (Abecasis et al., 2009; Belo et al., 2016). It is known that marine fish farms have also an aggregation effect on wild fish populations (e.g., Valle et al., 2007; Sánchez-Jerez et al., 2011). The median distance for ATS capture sites to the nearest fish farm (7.30 km) is, therefore, of the same order of magnitude, although a little bigger than the possible daily displacement reported for that species (Abecasis et al., 2015). Most ATS observations reported in the present study referred to individuals longer than 20 cm following fishers’ comments. As shown by Figueiredo et al. (2005) and Merciai et al. (2018), there is a size-related diet shift from approximately 25 cm onwards, which implies a wider home range in larger specimens that feed both in the infra- and circalittoral zones. Therefore, there is some variability in the home range, related to both the topography of the area and the size of the individuals that can explain these differences.
Searching for a common denominator for ports and fish farms, and taking into account the results of our previous work on copper concentrations in D. sargus (Merciai et al., 2018), we suggest antifouling paints (as a copper source) to be a possible cause of ATS anomaly. An increase in ATS observations has apparently occurred in recent years, more obvious since around 2003. In that same year, organotin compounds were no longer allowed (Regulation (EC), 2003), and copper-based anti-fouling paints started to be used on vessels and mariculture cages. Since 2008, EU ships and other ships visiting EU ports are obliged either not to bear anti-fouling systems containing organotin compounds, or to bear a barrier coating to prevent such compounds from leaching from the underlying non-compliant antifouling system (Regulation (EC), 2008). The Regulation is further supplemented firstly by the Council Directive 76/769/EEC (European Commission, 1976) as amended that bans the marketing and use of organostannic compounds within the EU, secondly by the Comission Regulation (EC) No 536/2008 (Regulation (EC), 2008) comprising measures enabling ships flying the flag of a third State to demonstrate their compliance and procedures for control. Prior to 2003, the phenomenon of ATS was less frequent. Assuming a possible relationship between copper and ATS, the earlier cases may be related to fish farms.
Much research has been conducted on the effect of copper on aquatic organisms. Related to copper-based antifouling paints, copper oxide leaches from the boat surfaces and enters the water as free copper ion (Cu+), which is immediately oxidized to Cu2+ and forms complexes with inorganic and organic ligands (Thomas and Brooks, 2010). In aquaculture systems, copper is regularly used in the form of copper sulfate (CuSO4) to control algal blooms and aquatic macrophyte infestations (Garcia Sampaio et al., 2008). Copper is also a common component of feed pellets used in aquaculture. Beneath aquaculture installations, waste feed originating from the cages and feces enhances the attractive effect of farms (Tuya et al., 2006), but also lead to potential organic and metal pollution of the sediment, the benthic fauna and through the food chain their predators.
It is known that copper becomes toxic to cells when its concentration surpasses certain threshold levels (Theophanides and Anastassopoulou, 2002). Baldwin et al. (2003) related copper with adverse effects such as reduced olfaction (sense of smell) that leads to reduced appetite and food intake, which, in turn, contribute to reduced growth (McIntyre et al., 2008). Gioda et al. (2007) showed that copper exposure induces lipid peroxidation in the muscle of the fish Leporinus obtusidens (Valenciennes, 1836). Maharajan et al. (2016) also observed that the histopathology of muscles shows progressive damage in their structure with increasing concentrations of copper. Indeed, ferrous iron (Fe2+) and Cu (I) promote the oxidation reaction called Fenton (Kanner, 1994) in fish muscle (Decker and Hultin, 1990).
In a recent study conducted in the Bay of Toulon (France), Bouchoucha et al. (2018) analyzed metal concentrations in juveniles of D. sargus from four sites, two representative of the different types of ports present in the bay and two representative of the other types of coastal habitats available to the juveniles of local rocky fishes. The highest muscle concentrations for copper were observed in the Saint Mandrier-sur-Mer marina, where the levels of contamination by copper in water were also the highest, and clearly higher than the concentrations found in the second port, the biggest naval port of the Mediterranean, the port of Toulon. Saint Mandrier is one of the locations where we have evidence of ATS presence, next to an area of shellfish culture and fish farming, the Lazaret and Balaguier bays [with 28 shellfish farms and 18 fish farms, according to the information available at the Direction Départementale des Territoires et de la Mer du Var (2019)].
The aforementioned evidence suggests that the exposure of fish to copper can be mediated via aquaculture farms and ports, leading to its accumulation in the flesh of fish and ATS. Copper proposed as likely responsible for ATS clearly deserves further research. In addition, many chemical substances used in aquaculture can be harmful. Aquaculture plants periodically discharges wastes from farm activities. These waste products include other trace metals, detergents, effluent from net washing, antifouling chemicals, and even chemicals such as drugs (Read and Fernandes, 2003), which also deserve to be considered.
In the present study, only two locations among all where ATS were reported were not close to either of the two facilities (farms and commercial ports), namely Saint Tropez (France) and Sabaudia (Italy) (see Table 2). Nautical ports, however, are present in both locations (see Saint Madrier example), and because white seabream are able to cover long distances (D’Anna et al., 2004; Abecasis et al., 2009; Di Franco et al., 2012; Belo et al., 2016) they could also come from areas close to other facilities. Finally, there is another possible consideration for these two specific cases. These ATS records corresponded to fish caught in winter, at the beginning of the reproductive season of D. sargus (Martínez and Villegas, 1996; Gonçalves and Erzini, 2000; Morato et al., 2003; Mouine et al., 2007). Some recreational fishers reported that white seabream are particularly tough during the reproduction period, when fish invest their fat reserves in the maturation of gonads and eggs.
Recent studies (Terlizzi et al., 2011; Felline et al., 2012; Gorbi et al., 2014) have linked a depletion of energetic reserves and a decrease in the condition factor of D. sargus with the uptake of lipophilic secondary metabolites of an invasive alga, Caulerpa cylindracea Sonder, 1845 (Bryopsidales and Chlorobionta) frequently consumed by the white seabream. This alteration represents a real threat to the health of the fish since affected individuals cannot biosynthesize essential fatty acids (Felline et al., 2014). Vitale et al. (2018) candidated caulerpin as a causal factor of the metabolic disorders observed in D. sargus. Caulerpin taken with diet is directly responsible of changes observed in the metabolic profile of fish flesh, including alteration of lipid metabolism, in particular with a reduction of ω3 PUFA content (Del Coco et al., 2018).
The consumption of C. cylindracea by fish species such as Spondyliosoma cantharus, Boops boops, Sarpa salpa, and D. sargus, D. vulgaris, or Siganus luridus (Ruitton et al., 2006; Box et al., 2009; Terlizzi et al., 2011; Tomas et al., 2011; Felline et al., 2017; Vitale et al., 2018) and by sea urchins (Ruitton et al., 2006; Bulleri et al., 2011) has been extensively documented. C. cylindracea is a species of Australian origin introduced in the Mediterranean Sea and reported as a major invader since the early 1990s (Klein and Verlaque, 2008; Verlaque et al., 2015). It can modify the structure of the ecosystems it invades as well as the associated fauna and the food chain (Deudero et al., 2011; Felline et al., 2017). Another important invasive Caulerpa species in the Mediterranean Sea is C. taxifolia (M. Vahl) C. Agardh, 1817. Like its congeneric, it produces caulerpin and other similar terpenoid compounds. This species, also of Australian origin, was detected in 1984 in Monaco, from where it quickly spread in the Mediterranean (Meinesz and Hesse, 1991; Verlaque et al., 2015). A third taxon of invasive exotic Caulerpa, first detected in 2006 in Turkey, C. taxifolia var. distichophylla (Sonder) Verlaque, Huisman & Procaccini, 2013 is spreading in the central Mediterranean (Cevik et al., 2007; Verlaque et al., 2015).
The problem of ATS seems to be concentrated in very specific areas and its relationship with the introduction of invasive Caulerpa species appears unclear (Verlaque et al., 2015). For example, in Catalonia, the first known cases of ATS (1980) are prior to the (known) presence of C. cylindracea. C. taxifolia as well as its variety distichophylla have never been found in Catalonia. C. cylindracea was first observed in the south of Catalonia (Vilanova i la Geltrú) in 2009 and only recently along its northern coast (Cap de Creus and Ses Negres, unpubl. data). Along the Mediterranean coast of Spain, C. cylindracea was first sighted in the Balearic Islands in 1998 (Ballesteros et al., 1999), where there had been no cases of ATS until 2019. Besides, the algae reached the east coast of the Iberian Peninsula (Castellón) in 1999 (Aranda et al., 1999) and began to spread rapidly to the south, where it was sighted in Alicante (SE Spain) in 2000 (Aranda et al., 2003); but ATS were already reported a few years earlier. The same is true for the southwest coast of France, where the anomaly was already reported in 1985, while C. taxifolia was sighted for the first time around 1992–1994 in the port of St. Cyprien (Boudouresque et al., 1992), the only known locality colonized by that species, while C. cylindracea has not been observed in the region. In addition, no invasive Caulerpa species are present in the Atlantic coastal regions of France and continental Spain where ATS have been reported. In brief, although (a) the first reports of an invasive species can occur sometimes after the species’ settlement, and (b) a synergetic effect between caulerpin and other toxicity sources cannot be excluded, a clear relationship cannot be established between ATS and Caulerpa species invasions. This hypothetical caulerpin relationship with the ATS anomaly in D. sargus, like the exposure to copper requires more investigation, including experimental works under control conditions.
Although D. sargus seems to be the species most affected by the ATS phenomenon, other species have been reported in the present survey. They are mostly species of the same F. Sparidae that share habitat and therefore can be subjected to the same stressors and physiological anomalies. The higher propensity of D. sargus to be affected by the anomaly compared to other species sharing the same habitat may depend on its feeding habits. The diet of D. sargus is based on algae and hard-shelled benthic invertebrates widely acknowledged to accumulate trace metals (Sala and Ballesteros, 1997; Linde et al., 2004; Merciai et al., 2018), including those living close to structures treated with antifouling chemicals and affected by copper-enriched feed pellets. It is known that D. sargus can use their strong teeth to crush hard-shelled prey (Vandewalle et al., 1995). Linde et al. (2004) suggested that larger, rounded, protruding and robust incisors of D. sargus would be preferentially useful to pick tough invertebrates adhered to the substrate. By feeding on prey including directly on facility treated surfaces, it could incorporate more easily toxic components of anti-fouling paints than other species sharing the same environment, or even fish in aquaculture cages. Overfed, ‘fat’ farmed fish may be less affected by the anomaly as they never deplete their fat reserves, unlike wild fish. If environmental factors affecting fish result in a progressive loss of their lipid reserves, D. sargus with different degrees of affection should be observed within a same population, implying the existence of specimens hardly recognizable as ATS, which would not be detected through a superficial examination.
According to fishers, the ATS phenomenon appeared singularly in time in different places. This scattered, diffuse appearance, with no area of origin to spread from, de facto excluded any process of contagion. We disregarded too the hypothesis of a stress-induced ATS syndrome driven by (a) the noise from boats and nautical activities peaking in the 0–10 m depth zone, the main feeding zone of the white seabream, or (b) by the stress due to catch and landing. The reason to exclude those explanations is that fishers reported to have caught both ordinary specimens and ATS in the same outing, with the same gear. The stress is expected to be the same for all caught fish, making this hypothesis implausible. Nor would it explain why one fish species is more affected than others, be the reason an environmental issue or a fishing gear. Furthermore, in the survey, we asked about the presence of remarkable features near the fishing area (e.g., nautical port, fishing port, aquaculture facility, river, marine outfall, and invasive algae). Water courses and marine outfalls are important pollution sources to the coastal marine environment, but very few fishers reported their presence close to the locations of ATS captures. On the other hand, many nautical-recreational ports were reported, whose pollution in some periods of the year may exceed that of industrial or fishing ports. Nevertheless, all these potential impacts on ATS occurrence will have to be investigated in future studies.
The fact that 20% of the entire Mediterranean basin and 60–99% of the territorial waters of EU member states are heavily affected by intense human impacts occurring in all ecoregions and territorial waters (Micheli et al., 2013) suggests pollution as one of the global threats that could explain the scattered appearance of the phenomenon. Results show that the ATS occurrence is a general phenomenon along all the western Mediterranean coasts. The drivers of the ATS anomaly could be multiple and synergistic; therefore, potential explanations to the phenomenon need to be investigated in the next field surveys of the ATS anomaly.
Being aware of the spread of the ATS phenomenon will be necessary to perform quantitative and qualitative analyses of its environmental drivers, general physiological conditions of fish, flesh biochemistry and degree of flesh alteration in both ATS and unaffected fish, to better understand it. The dietborne uptake of copper and other pollutants may be investigated, e.g., the role of metal bioaccumulator preys like sea urchins. This work, therefore, opens up numerous research possibilities in order to understand, next, the causes of the ATS anomaly.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
MC, CR-P, and RM designed the new survey, distributed it, analyzed the results, and prepared this article. ER, JT, and JR helped in the collection of data, evaluation of results, and the revision of the manuscript. MV made the first survey and the first assessment of the case and helped in the revision of the manuscript. CM, JP, and JR performed the spatial analysis and also helped in the revision of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the fishers who provided us with the information, specially Mr. Pere Casademont who was the first to draw our attention to this phenomenon. We also thank Mr. Peter Redmond for the revision of the English text and reviewers whose comments have greatly improved the quality of the manuscript.
Footnotes
References
Abecasis, D., Bentes, L., and Erzini, K. (2009). Home range, residency and movements of Diplodus sargus and Diplodus vulgaris in a coastal lagoon: connectivity between nursery and adult habitats. Estuar. Coast. Shelf Sci. 85, 525–529.
Abecasis, D., Horta Costa, B., Afonso, P., Gonçalves, E. J., and Erzini, K. (2015). Early reserve effects linked to small home ranges of a commercial fish, Diplodus sargus, Sparidae. MEPS 518, 255–266. doi: 10.3354/meps11054
Aranda, A., Bueno, M., Solano, I., and Guillén, J. E. (2003). Red de vigilancia del litoral valenciano frente al peligro de invasión de especies ex ticas del género Caulerpa. Actas del I Congreso Nacional sobre Especies Ex ticas Invasoras (EEI 2003), 160–161.
Aranda, A., Mallol, J., and Solano, I. (1999). Presencia del alga Caulerpa racemosa Forsskål J. Agardh. (Chlorophyta, Caulerpales) en el Mediterráneo ibérico. Actas XIII Congreso Nacional Botánica Criptogámica, 53.
Baldwin, D. H., Sandahl, J. F., Labenia, J. S., and Scholz, N. L. (2003). Sublethal effects of copper on Coho salmon: impacts on non-overlapping receptor pathways in the peripheral olfactory nervous system. Environ. Toxicol. Chem. 22, 2266–2274. doi: 10.1897/02-428
Ballesteros, E., Grau, M., and Riera, F. (1999). Caulerpa racemosa (Forsskål) J. Agardh (Caulerpales, Chlorophyta) a Mallorca. Boll. Soc. Hist. Nat. Balears 42:68.
Baty, F., Ritz, C., Charles, S., Brutsche, M., Flandrois, J.-P., and Delignette-Muller, M. L. (2015). A toolbox for nonlinear regression in R: the package nlstools. J. Stat. Softw. 66, 1–21.
Belo, A. F., Pereira, T. J., Quintella, B. R., Castro, N., Costa, J. L., and de Almeida, P. R. (2016). Movements of Diplodus sargus (Sparidae) within a portuguese coastal marine protected area: are they really protected? Mar. Environ. Res. 114, 80–94. doi: 10.1016/j.marenvres.2016.01.004
Bouchoucha, M., Brach-Papa, C., Gonzalez, J. L., Lenfant, P., and Darnaude, A. M. (2018). Growth, condition and metal concentration in juveniles of two Diplodus species in ports. Mar. Pollut. Bull. 126, 31–42. doi: 10.1016/j.marpolbul.2017.10.086
Boudouresque, C. F., Meinesz, A., Verlaque, M., and Knoepffler-Peguy, M. (1992). The expansion of the tropical alga Caulerpa taxifolia (Chlorophyta) in the Mediterranean. Crypt. Algol. 13, 144–145.
Box, A., Deudero, S., Sureda, A., Blanco, A., Alòs, J., Terrados, J., et al. (2009). Diet and physiological responses of Spondyliosoma cantharus (Linnaeus, 1758) to the Caulerpa racemosa var. cylindracea invasion. J. Exp. Mar. Biol. Ecol. 380, 11–19.
Brewer, M. J., Butler, A., and Cooksley, S. L. (2016). The relative performance of AIC, AICC and BIC in the presence of unobserved heterogeneity. Methods Ecol. Evol. 7, 679–692.
Bulleri, F., Alestra, T., Ceccherelli, G., Tamburello, L., Pinna, S., Sechi, N., et al. (2011). Determinants of Caulerpa racemosa distribution in the north-western Mediterranean. Mar. Ecol. Prog. Ser. 431, 55–67.
Cameron, A. C., and Trivedi, P. K. (1998). Regression Analysis of Count Data. Cambridge, MA: Cambridge University Press.
Cantoni, E., and Ronchetti, E. (2001). Robust inference for generalized linear models. JASA 96, 1022–1030.
Casadevall, M., Rodríguez-Prieto, C., and Torres, J. (2017). The importance of the age when evaluating mercury pollution in fishes: the case of Diplodus sargus (Pisces, Sparidae) in the NW Mediterranean. AIMS Environ. Sci. 4, 17–26. doi: 10.3934/environsci.2017.1.17
Cevik, C., Yokes, M. B., Cavas, L., Erkol, L. L., Derici, O. B., and Verlaque, M. (2007). First report of Caulerpa taxifolia (Bryopsidales, Chlorophyta) on the Levantine coast (Turkey, eastern Mediterranean). Estuar. Coast. Shelf Sci. 74, 549–556.
D’Anna, G., Giacalone, V. M., Badalamenti, F., and Pipitone, C. (2004). Releasing of hatchery-reared juveniles of the white seabream Diplodus sargus (L., 1758) in the Gulf of Castellammare artificial reef area (NW Sicily). Aquaculture 233, 251–268.
D’Anna, G., Giacalone, V. M., Pipitone, C., and Badalamenti, F. (2011). Movement pattern of white seabream, Diplodus sargus (L., 1758) (Osteichthyes, Sparidae) acoustically tracked in an artificial reef area. Ital. J. Zool. 78, 255–263. doi: 10.1080/11250000903464059
Dean, R. J., Shimmield, T. M., and Black, K. D. (2007). Copper, zinc and cadmium in marine cage fish farm sediments: an extensive survey. Env. Pollut. 145, 84–95. doi: 10.1016/j.envpol.2006.03.050
Decker, E. A., and Hultin, H. O. (1990). Factors influencing catalysis of lipid oxidation by the soluble fraction of mackerel muscle. J. Food Sci. 55, 947–950. doi: 10.1016/0891-5849(92)90056-m
Del Coco, L., Felline, S., Girelli, C. R., Angilè, F., Magliozzi, L., Almada, L., et al. (2018). 1H NMR spectroscopy and MVA to evaluate the effects of caulerpin-based diet on Diplodus sargus lipid profiles. Mar. Drugs 16:390. doi: 10.3390/md16100390
Deudero, S., Box, A., Alós, J., Arroyo, N. L., and Marbà, N. (2011). Functional changes due to invasive species: food web shifts at shallow Posidonia oceanica seagrass beds colonized by the alien macroalga Caulerpa racemosa. Estuar. Coas. Shelf Sci. 93, 106–116.
Di Franco, A., Gillanders, B. M., De Benedetto, G., Pennetta, A., De Leo, G. A., and Guidetti, P. (2012). Dispersal patterns of coastal fish: implications for designing networks of marine protected areas. PLoS One 7:e31681. doi: 10.1371/journal.pone.0031681
Di Lorenzo, M., Vega Fernández, T., Badalamenti, F., Guidetti, P., Starr, R. M., Giacalone, V. M., et al. (2016). Diel activity and variability in habitat use of white sea bream in a temperate Marine Protected Area. Mar. Environ. Res. 116, 1–9. doi: 10.1016/j.marenvres.2016.02.007
Direction Départementale des Territoires et de la Mer du Var (2019). Zones-de-cultures-marines-dans-le-Var. Available online at: https://www.data.gouv.fr/fr/datasets/zones-de-cultures-marines-dans-le-var/ (accessed July 22, 2019).
Dormann, C. F., Elith, J., Bacher, S., Buchmann, C., Carl, G., Carré, G., et al. (2013). Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 027–046.
European Commission (1976). Council Directive 76/769/EEC of 27 July 1976 on The Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations. Brussels: European Commission.
FAO (2003). Review of the State of World Aquaculture, Fisheries Circular No. 886, Rev. 2. Rome: Food and Agriculture Organization of the United Nations.
Felline, S., Caricato, R., Cutignano, A., Gorbi, S., Lionetto, M. G., and Mollo, et al. (2012). Subtle effects of biological invasions: cellular and physiological responses of fish eating the exotic pest Caulerpa racemosa. PLoS One 7:e38763. doi: 10.1371/journal.pone.0038763
Felline, S., Mollo, E., Cutignano, A., Grauso, L., Andaloro, F., Castriota, L., et al. (2017). Preliminary observations of caulerpin accumulation from the invasive Caulerpa cylindracea in native Mediterranean fish species. Aquat. Biol. 26, 27–31.
Felline, S., Mollo, E., Ferramosca, A., Zara, V., Regoli, F., Gorbi, S., et al. (2014). Can a marine pest reduce the nutritional value of Mediterranean fish flesh? Mar. Biol. 161, 1275–1283.
Figueiredo, M., Morato, T., Barreiros, J. P., Afonso, P., and Santos, R. S. (2005). Feeding ecology of the white seabream, Diplodus sargus, and the ballan wrasse, Labrus bergylta, in the Azores. Fish. Res. 75, 107–119. doi: 10.1016/j.fishres.2005.04.013
Garcia Sampaio, F., de Lima Boijink, C., Tie Oba, E., Romagueira Bichara Dos Santos, L., Kalinin, A. L., and Tadeu Rantin, F. (2008). Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp. Biochem. Phys. C 147, 43–51. doi: 10.1016/j.cbpc.2007.07.009
Gioda, C. R., Lissner, L. A., Pretto, A., Da Rocha, J., Schetinger, M., Neto, J. R., et al. (2007). Exposure to sublethal concentrations of Zn (II) and Cu (II) changes biochemical parameters in Leporinus obtusidens. Chemosphere 69, 170–175. doi: 10.1016/j.chemosphere.2007.04.008
Gonçalves, J. M. S., and Erzini, K. (2000). The reproductive biology of the two-banded sea bream (Diplodus vulgaris) from the SW Coast of Portugal. J. Appl. Ichthyol. 16, 110–116.
Gorbi, S., Giuliani, M. E., Pittura, L., d’Errico, G., Terlizzi, A., Felline, S., et al. (2014). Could molecular effects of Caulerpa racemosa metabolites modulate the impact on fish populations of Diplodus sargus? Mar. Environ. Res. 96, 2–11. doi: 10.1016/j.marenvres.2014.01.010
Kanner, J. (1994). Oxidative processes in meat and meat products: quality implications. Meat Sci. 36, 169–189. doi: 10.1016/0309-1740(94)90040-X
Klein, J., and Verlaque, M. (2008). The Caulerpa racemosa invasion: a critical review. Mar. Pollut. Bull. 56, 205–225. doi: 10.1016/j.marpolbul.2007.09.043
Linde, M., Palmer, M., and Gómez-Zurita, J. (2004). Differential correlates of diet and phylogeny on the shape of the premaxilla and anterior tooth in sparid fishes (Perciformes: Sparidae). J. Evol. Biol. 17, 941–952. doi: 10.1111/j.1420-9101.2004.00763.x
Maechler, M., Rousseeuw, P., Croux, C., Todorov, V., Ruckstuhl, A., Salibian-Barrera, M., et al. (2019). Package “robustbase”: Basic Robust Statistics R package version 0.93-5.
Maharajan, A., Kitto, M. R., Paruruckumani, P. S., and Ganapiriya, V. (2016). Histopathology biomarker responses in Asian sea bass, Lates calcarifer (Bloch) exposed to copper. J. Basic Appl. Zool. 77, 21–30.
Martínez, C., and Villegas, M. L. (1996). Edad, crecimiento y reproducción de Diplodus sargus Linnaeus, 1758 (Sparidae) en aguas asturianas (norte de España). Bol. Inst. Esp. Oceanogr. 12, 65–76.
McIntyre, J. K., Baldwin, D. H., Meador, J. P., and Scholz, N. L. (2008). Chemosensory deprivation in juvenile coho salmon exposed to dissolved copper under varying water chemistry conditions. Environ. Sci. Technol. 42, 1352–1358. doi: 10.1021/es071603e
Meinesz, A., and Hesse, B. (1991). Introduction et invasion de l’algue tropicale Caulerpa taxifolia en Méditerranée nord-occidentale. Oceanol. Acta 14, 415–426.
Merciai, R., Rodríguez-Prieto, C., Torres, J., and Casadevall, M. (2018). Bioaccumulation of mercury and other trace elements in bottom-dwelling omnivorous fishes: the case of Diplodus sargus (L.) (Osteichthyes: Sparidae). Mar. Pollut. Bull. 136, 10–21. doi: 10.1016/j.marpolbul.2018.08.061
Micheli, F., Halpern, B. S., Walbridge, S., Ciriaco, S., Ferretti, F., Fraschetti, S., et al. (2013). Cumulative human impacts on mediterranean and black sea marine ecosystems: assessing current pressures and opportunities. PLoS One 8:e79889. doi: 10.1371/journal.pone.0079889
Morato, T., Afonso, P., Lourinho, P., Nash, R. D. M., and Santos, R. S. (2003). Reproductive biology and recruitment of the sea bream in the Azores. J. Fish. Biol. 63, 59–72.
Mouine, N., Francour, P., Ktari, M. H., and Chakroun-Marzouk, N. (2007). The reproductive biology of Diplodus sargus sargus in the Gulf of Tunis (central Mediterranean). Sci. Mar. 71, 461–469.
Pérez, M. J., Rodríguez, C., Cejas, J. R., Martín, M. V., Jerez, S., and Lorenzo, A. (2007). Lipid and fatty acid content in wild white seabream (Diplodus sargus) broodstock at different stages of the reproductive cycle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 146, 187–196. doi: 10.1016/j.cbpb.2006.10.097
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Read, P., and Fernandes, T. (2003). Management of environmental impacts of marine aquaculture in Europe. Aquaculture 226, 139–163.
Regulation (EC) (2003). Regulation (EC) No 782/2003 of the European Parliament and of the Council of 14 April 2003 on the Prohibition of Organotin Compounds on Ships. Available online at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:115:0001:0011:EN:PDF (accessed September, 2019).
Regulation (EC) (2008). Regulation (EC) No 536/2008 of 13 June 2008 giving effect to Article 6(3) and Article 7 of Regulation (EC) No 782/2003 of the European Parliament and of the Council on the Prohibition of Organotin Compounds on Ships and Amending that Regulation. Available online at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:156:0010:0011:EN:PDF (accessed September, 2019).
Regulation (EU) (2016). Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation). Available online at: https://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed September, 2019).
Ruitton, S., Verlaque, M., Aubin, G., and Boudouresque, C. (2006). Grazing of the introduced Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in Mediterranean Sea by herbivorous fish and sea urchins. Vie Milieu 56, 33–41.
Sala, E., and Ballesteros, E. (1997). Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar. Ecol. Prog. Ser. 152, 273–283. doi: 10.3354/meps152273
Sánchez-Jerez, P., Fernández-Jover, D., Uglem, I., Arechavala-Lopez, P., Dempster, T., Bayle-Sempere, J. T., et al. (2011). Coastal fish farms as fish aggregation devices (FADs). Artificial Reefs in Fishery Management. CRC Press, 187–208.
Terlizzi, A., Felline, S., Lionetto, M. G., Caricato, R., Perfetti, V., Cutignano, A., et al. (2011). Detrimental physiological effects of the invasive alga Caulerpa racemosa on the Mediterranean white seabream Diplodus sargus. Aquat. Biol. 12, 109–117.
Theophanides, T., and Anastassopoulou, J. (2002). Copper and carcinogenesis. Crit. Rev. Oncol. Hematol. 42, 57–64.
Thomas, K. V., and Brooks, S. (2010). The environmental fate and effects of antifouling paint biocides. Biofouling 26, 73–88. doi: 10.1080/08927010903216564
Tomas, F., Box, A., and Terrados, T. (2011). Effects of seaweeds on feeding preference and performance of keystone Mediterranean herbivore. Biol. Invasions 13, 1559–1570.
Tramati, C., Vizzini, S., Maci, S., Basset, A., and Mazzola, A. (2012). Trace metal contamination in a Mediterranean coastal pond (Acquatina, Puglia). Transit. Water Bull. 5, 124–137. doi: 10.1285/i1825229Xv5n1p124
Tundo, A. (2015). Napoli, Saraghi Immangiabili e Prezzo Crollato. “Colpa di Un’alga Che Distrugge I Grassi. Forse Utile Contro Il Colesterolo”. Available online at: https://www.ilfattoquotidiano.it/2015/08/28/napoli-saraghi-immangiabili-e-prezzo-crollato-colpa-di-unalga-che-distrugge-i-grassi-forse-utile-contro-il-colesterolo/1988381/ (accessed July, 2018).
Tuya, F., Sánchez-Jerez, P., Dempster, T., Boyra, A., and Haroun, R. (2006). Changes in demersal wild fish aggregations beneath a sea-cage fish farm after the cessation of farming. J. Fish Biol. 69, 682–697.
Valle, C., Bayle-Sempere, J., Dempster, T., Sanchez-Jerez, P., and Giménez-Casalduero, F. (2007). Temporal variability of wild fish assemblages associated with a sea-cage fish farm in the south-western Mediterranean Sea. Est. Coas. Shelf Sci. 72, 299–307.
Vandewalle, P., Saintin, P., and Chardon, M. (1995). Structures and movements of the buccal and pharyngeal jaws in relation to feeding in Diplodus sargus. J. Fish. Biol. 46, 623–656.
Verlaque, M., Ruitton, S., Mineur, F., and Boudouresque, C. F. (2015). “Macrophytes,” in CIESM Atlas of Exotic Species in the Mediterranean, ed. F. Briand, (Monaco: CIESM Publishers), 360.
Vitale, R. M., D’Aniello, E., Gorbi, S., Martella, A., Silvestri, C., Giuliani, M. E., et al. (2018). Fishing for targets of alien metabolites: a novel peroxisome proliferator-activated receptor (PPAR) agonist from a marine pest. Mar. Drugs 16:431. doi: 10.3390/md16110431
Warton, D. I., Lyons, M., Jakub, S., and Ives, A. R. (2016). Three points to consider when choosing a LM or GLMtest for count data. Methods Ecol. Evol. 7, 882–890.
Wickham, H., François, R., Henry, L., and Müller, K. (2019). dplyr: A Grammar of Data Manipulation. R package version 0.8.3.
Wood, S. N. (2017). Generalized Additive Models: An Introduction with R, 2nd Edn. London: Chapman and Hall.
Wooldridge, J. M. (2002). Econometric Analysis of Cross Section and Panel Data. Cambridge, MA: The MIT Press.
Yebra, D. M., Kiil, S., and Dam-Johansen, K. (2004). Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 50, 75–104.
Keywords: white seabream, Diplodus sargus, Mediterranean Sea, pollution, copper, anti-fouling paint
Citation: Casadevall M, Rodríguez-Prieto C, Pueyo J, Martí C, Merciai R, Verlaque M, Real E, Torres J and Richir J (2020) The Strange Case of Tough White Seabream (Diplodus sargus, Teleostei: Sparidae): A First Approach to the Extent of the Phenomenon in the Mediterranean. Front. Mar. Sci. 7:387. doi: 10.3389/fmars.2020.00387
Received: 28 October 2019; Accepted: 05 May 2020;
Published: 19 June 2020.
Edited by:
Tomaso Fortibuoni, Higher Institute for Environmental Protection and Research (ISPRA), ItalyReviewed by:
Antonio Terlizzi, University of Trieste, ItalyMichele Gristina, National Research Council (CNR), Italy
Sanja Matic-Skoko, Institute of Oceanography and Fisheries, Croatia
Copyright © 2020 Casadevall, Rodríguez-Prieto, Pueyo, Martí, Merciai, Verlaque, Real, Torres and Richir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margarida Casadevall, bWFyZ2FyaWRhLmNhc2FkZXZhbGxAdWRnLmVkdQ==
†ORCID: Margarida Casadevall, orcid.org/0000-0002-7172-3682 Conxi Rodríguez-Prieto, orcid.org/0000-0003-4935-1250 Josep Pueyo, orcid.org/0000-0002-1236-5651 Carolina Martí, orcid.org/0000-0003-4189-5878 Roberto Merciai, orcid.org/0000-0003-4051-5730 Marc Verlaque, orcid.org/0000-0001-6644-805X Enric Real, orcid.org/0000-0002-6190-6303 Jordi Torres, orcid.org/0000-0002-4999-0637 Jonathan Richir, orcid.org/0000-0001-5890-5724
 Margarida Casadevall
Margarida Casadevall Conxi Rodríguez-Prieto
Conxi Rodríguez-Prieto Josep Pueyo
Josep Pueyo Carolina Martí
Carolina Martí Roberto Merciai
Roberto Merciai Marc Verlaque
Marc Verlaque Enric Real
Enric Real Jordi Torres
Jordi Torres Jonathan Richir
Jonathan Richir