- 1ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD, Australia
- 2Biodiversity and Geosciences Program, Museum of Tropical Queensland, Queensland Museum, Townsville, QLD, Australia
Elevated carbon dioxide (CO2) levels can alter ecologically important behaviors in a range of marine invertebrate taxa; however, a clear mechanistic understanding of these behavioral changes is lacking. The majority of mechanistic research on the behavioral effects of elevated CO2 has been done in fish, focusing on disrupted functioning of the GABAA receptor (a ligand-gated ion channel, LGIC). Yet, elevated CO2 could induce behavioral alterations through a range of mechanisms that disturb different components of the neurobiological pathway that produces behavior, including disrupted sensation, altered behavioral choices and disturbed LGIC-mediated neurotransmission. Here, we review the potential mechanisms by which elevated CO2 may affect marine invertebrate behaviors. Marine invertebrate acid–base physiology and pharmacology is discussed in relation to altered GABAA receptor functioning. Alternative mechanisms for behavioral change at elevated CO2 are considered and important topics for future research have been identified. A mechanistic understanding will be important to determine why there is variability in elevated CO2-induced behavioral alterations across marine invertebrate taxa, why some, but not other, behaviors are affected within a species and to identify which marine invertebrates will be most vulnerable to rising CO2 levels.
Introduction
Human activity is resulting in unprecedented amounts of carbon dioxide (CO2) being released into the atmosphere. Since the Industrial Revolution, atmospheric CO2 levels have increased by over 45%, from approximately 280 ppm (Joos and Spahni, 2008) to over 410 ppm today (Dlugokencky and Tans, 2019), higher than any time in the past several million years (Masson-Delmotte et al., 2013). In the worst case scenario, following the business-as-usual representative concentration pathway (RCP) 8.5, atmospheric CO2 levels will increase to over 900 ppm by the end of this century. Even if substantial efforts are made to curb global CO2 emissions to keep warming below 2°C, atmospheric CO2 levels will still likely exceed 600 ppm by 2100 (Betts and McNeall, 2018). The ocean has absorbed 20–30% of anthropogenic CO2 emissions since the mid-1980s (Bindoff et al., 2019), causing a reduction in seawater pH referred to as ocean acidification. Furthermore, CO2 in the surface ocean is increasing at the same rate as in the atmosphere (Bindoff et al., 2019); therefore, marine organisms will need to cope with higher CO2 levels as well as declining seawater pH. Finally, due to a decrease in the ocean’s buffering capacity as CO2 content rises, natural CO2 fluctuations in the ocean are projected to amplify dramatically at future higher CO2 levels (Shaw et al., 2013; McNeil and Sasse, 2016). Natural diel (Hofmann et al., 2011; Santos et al., 2011; Shaw et al., 2012) and seasonal CO2 fluctuations (McNeil et al., 2007; Feely et al., 2008) will be amplified by up to three times in the future (McNeil and Sasse, 2016; Gallego et al., 2018), meaning that marine organisms will experience elevated CO2 levels for certain periods of time (daily or seasonally) much earlier than predictions based on atmospheric CO2 alone.
Elevated CO2 has been found to affect a range of processes in marine organisms, including altering calcification (Ries et al., 2009; Kroeker et al., 2013), growth and survival (Fabry et al., 2008; Kurihara et al., 2008), and behavior (Briffa et al., 2012; Clements and Hunt, 2015; Nagelkerken and Munday, 2015). Projected future CO2 levels were first found to alter animal behavior in orange clownfish Amphiprion percula larvae reared in a partial pressure of CO2 (pCO2) of ∼1,050 μatm (Munday et al., 2009). In laboratory experiments, the olfactory discriminatory abilities of 11-day-old clownfish larvae were tested in a two-channel flume. Most strikingly, larvae reared in control seawater (∼390 μatm pCO2) avoided the side of the flume with chemical cues from pungent tree leaves compared to the seawater control side. However, larvae reared in elevated CO2 spent nearly all their time in the side with these odors. Larval clownfish reared in ∼1,050 μatm CO2 were also unable to discriminate between the odor of parents and non-parents, whereas control larvae avoided the odor of their parents (Munday et al., 2009). Elevated CO2 has since been found to affect a variety of behavioral traits in a wide spectrum of fishes, including tropical and temperate reef species, eels, salmon and sharks (Munday et al., 2019). Behavioral alterations at elevated CO2 have also been demonstrated in a variety of marine invertebrates, including cnidaria, polychaetes, echinoderms, arthropods and mollusks, from a range of environments, including the intertidal zone, coastal and offshore waters and the deep-sea (Clements and Hunt, 2015; Nagelkerken and Munday, 2015; Wang and Wang, 2019). Marine invertebrates exhibit alterations in a range of behaviors at elevated CO2, including activity levels (Rosa and Seibel, 2008; Ellis et al., 2009; Spady et al., 2014), feeding rates (Saba et al., 2012; Vargas et al., 2014), settlement and metamorphosis behaviors (Albright et al., 2010; Doropoulos et al., 2012; Guo et al., 2015), burrowing behaviors (Green et al., 2013; Clements and Hunt, 2014), shelter selection (de la Haye et al., 2011), predatory behaviors (behaviors related to finding and eating prey) (Kim et al., 2015; Queirós et al., 2015; Spady et al., 2018) and predator avoidance (Bibby et al., 2007; Manríquez et al., 2013, 2014a; Spady et al., 2014; Watson et al., 2014). Behavioral categorization is often ambiguous as one behavior may actually include multiple behaviors or decision-making processes. For example, predator avoidance behaviors include multiple decisions such as mode of avoidance (including crypticity versus escape), flight-initiation distance and mode of escape (Lima and Dill, 1990). In this review, we use the behavioral category that was reported in the corresponding research paper.
Since the review by Clements and Hunt (2015) at least 61 additional papers have assessed the impact of elevated CO2 on marine invertebrate behaviors (Supplementary Table S1). Research has continued to focus on mollusks, arthropods and echinoderms, however, cnidarian settlement and metamorphosis (Foster et al., 2015; Olsen et al., 2015; Viyakarn et al., 2015; Fabricius et al., 2017; Yuan et al., 2018), the settlement behavior and swimming activity of a bryozoan (Pecquet et al., 2017), and settlement of an annelid (Nelson et al., 2020) have also been studied. In addition to continuing to assess the impact of elevated CO2 on the range of behaviors previously studied (above), a few new behaviors have also been investigated. For example, the first study assessing the effect of elevated CO2 on marine invertebrate reproductive behavior was recently published (Borges et al., 2018). Exposure of male amphipods Gammarus locusta to elevated CO2 (800 μatm pCO2) for two generations disrupted the chemosensory detection of potential mates (Borges et al., 2018). A light/dark test on swimming crabs Portunus trituberculatus exposed to control (485 μatm pCO2) or elevated (750 μatm and 1,500 μatm pCO2) CO2 was the first to assess the impact of elevated CO2 on anxiety-like behavior in a marine invertebrate. Crabs exposed to elevated CO2 levels spent significantly more time in the dark zone (Ren et al., 2018).
There appears to be large variability in behavioral responses to elevated CO2; across taxonomic groups, the same behavior can respond differently to elevated CO2, and within a species, some behaviors but not others can be affected (Nagelkerken and Munday, 2015) (Supplementary Table S1). As the phylogenetic variation among invertebrate taxa is enormous, it may account for a large component of the variability in behavioral responses across taxonomic groups; some taxa may be more tolerant to elevated CO2 than others. At the same time, various behaviors are likely associated with different processes, such as specific circuits in the nervous system. These processes may be affected differently by elevated CO2, accounting for the effects of elevated CO2 on some, but not all, behaviors within a species. It must also be noted that variability may be due to differences in experimental techniques and conditions.
Animal behavior is, to a large extent, the functional output of the nervous system (Simmons and Young, 1999); therefore, behavioral changes induced by elevated CO2 are likely caused by neurobiological mechanisms. In the nervous system, simplistically, the pathway that produces behavior involves sensory receptors that detect environmental stimuli (e.g., chemical cues, light waves) and internal stimuli (e.g., spatial orientation of the body). The received information is transduced into electrical impulses and neurotransmission relays these electrical impulses between neurons. Neurotransmission must be rapid to produce timely behavioral responses, and this is achieved via ligand-gated ion channel (LGIC) mediated neurotransmission (Dent, 2010). LGICs are transmembrane protein complexes that, upon binding of a specific neurotransmitter, allow ion flow which results in excitation or inhibition of neuronal firing depending on the ion charge and direction of flow (Tovar and Westbrook, 2012). When the sensory information arrives at higher centers of the nervous system, this information is processed and a behavioral output is produced (Blom, 1978; Kreher et al., 2008) (Figure 1).
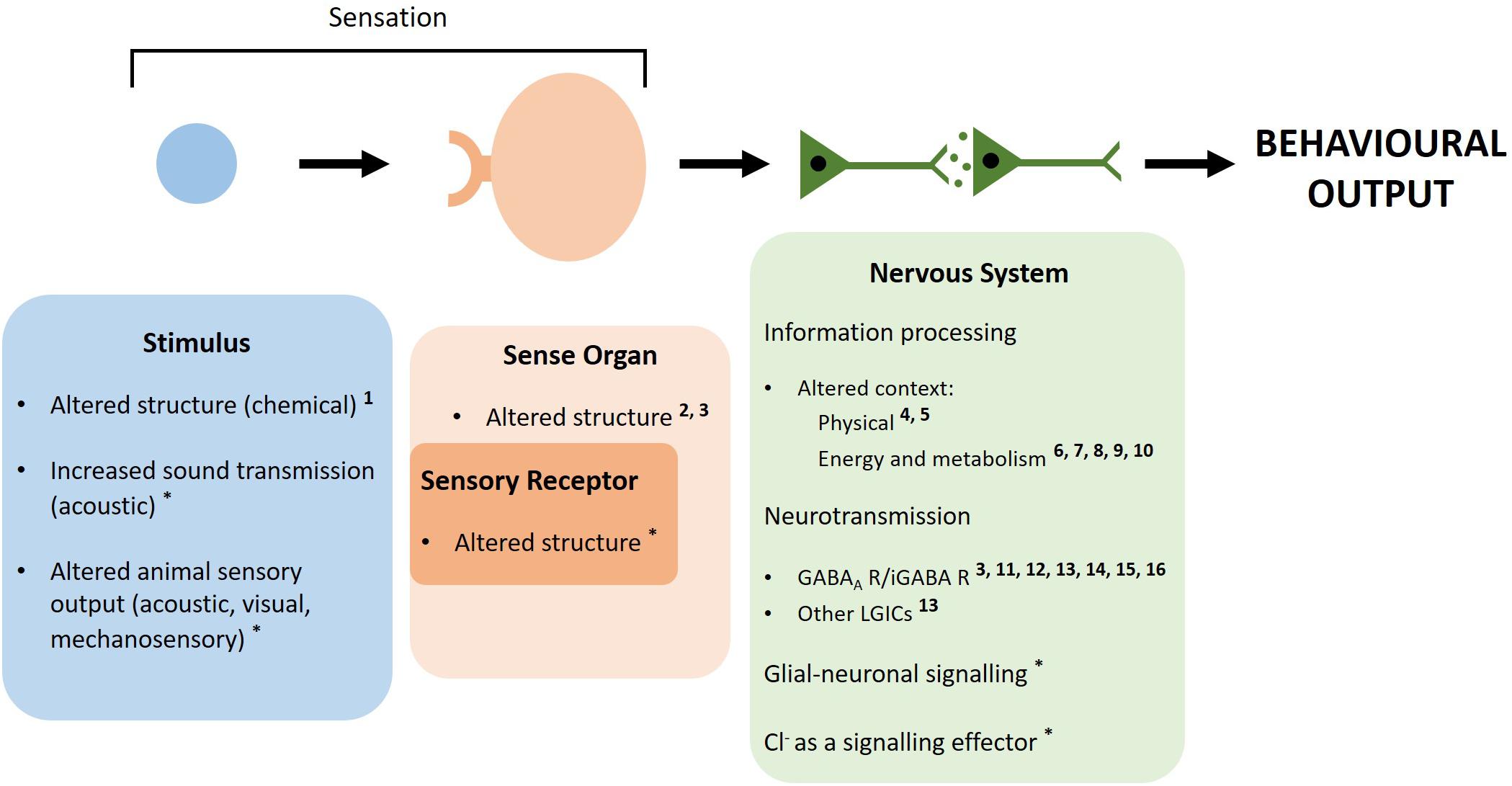
Figure 1. Simplistic pathway of how the nervous system produces behaviors and potential ways elevated CO2 could alter marine invertebrate behavior. An external or internal stimulus is detected by sensory receptors located on a sense organ. A physical stimulus binding to a receptor, e.g., a chemical cue binding to the corresponding receptor (chemoreception), is depicted. However, stimuli and receptors may range from photoreceptors detecting light energy to stretch receptors detecting body movement or hair cells detecting vibrations. The detected sensory information is then transduced into electrical impulses that are relayed between neurons to higher centers of the nervous system. Here, the information is processed which includes using external and internal contextual factors to make behavioral choices. The information is transmitted between neurons to the motor system and a behavioral response is produced. Throughout this process, neurotransmission is used to relay the electrical impulses from neuron to neuron. Elevated CO2 may alter behavior by interfering at multiple points along this pathway. Sensation may be disrupted by changes to sensory stimuli at elevated CO2 through structural change of chemical cues, increased sound transmission and altered sensory output from animals. Sensation may also be disrupted on the receiving end via altered structure of sensory receptors or physical change to sensory organs. Elevated CO2 may change the context within which decision-making is carried out, thus influencing behavioral choices. The change in ion gradients across neuronal membranes, due to acid–base regulation at elevated CO2, may disrupt LGIC-mediated neurotransmission, glial-neuronal signaling and the role of Cl– as a signaling effector. Numbers represent references providing evidence for each mechanism in marine invertebrates, while a * indicates this mechanism is based on theory with no experimental evidence in marine invertebrates. 1 Roggatz et al. (2016), 2 Maneja et al. (2011), 3 de la Haye et al. (2012), 4 Bibby et al. (2007), 5 Chan et al. (2011), 6 Dissanayake and Ishimatsu (2011), 7 Li and Gao (2012), 8 Peng et al. (2017), 9 Wang et al. (2018), 10 (Rich et al., 2018), 11 Watson et al. (2014), 12 Charpentier and Cohen (2016), 13 Moya et al. (2016), 14 Clements et al. (2017), 15 Ren et al. (2018), 16 Zlatkin and Heuer (2019).
Elevated CO2 could induce behavioral alterations through a range of mechanisms in the nervous system that disturb different components of the pathway that produces behavior [Briffa et al. (2012), as described in Figure 1]. (1) Sensation may be disrupted via changes to sensory stimuli at elevated CO2, such as structural alteration of chemical cues, disturbed transmission of acoustic cues and altered sensory output from animals experiencing behavioral changes (Roggatz et al., 2016; Nagelkerken et al., 2019). (2) Alternatively, elevated CO2 may disrupt sensation via physical change to sensory organs or structural alteration of sensory receptors (Maneja et al., 2011; Briffa et al., 2012; Bignami et al., 2013). (3) Morphological and respiratory changes at elevated CO2 may alter the context within which decision making is carried out, influencing behavioral responses (Bibby et al., 2007; Chan et al., 2011; Peng et al., 2017; Rich et al., 2018). (4) Elevated CO2 may induce change in ion gradients across neuronal membranes, due to acid–base regulation to prevent acidosis at elevated CO2, which may disrupt LGIC-mediated neurotransmission via the γ-aminobutyric acid (GABA) type A receptor (GABAA R) (Nilsson et al., 2012). These mechanisms are not necessarily mutually exclusive and may interact to alter behavior at elevated CO2.
Despite the growth in literature demonstrating elevated CO2-induced behavioral alterations in marine invertebrates, the mechanisms responsible for these behavioral alterations are still poorly understood. Due to the diversity of invertebrate nervous and neurobiological systems, it is likely that a suite of different processes underlie these behavioral changes. Here, we discuss the potential mechanisms by which elevated CO2 may alter marine invertebrate behavior; (1) disturbed sensation, (2) altered context within which behavioral choices are made, and (3) disrupted LGIC-mediated neurotransmission. Since the prominent hypothesis for altered LGIC-mediated transmission is the GABA hypothesis proposed in fish by Nilsson et al. (2012), here we discuss evidence for the GABA hypothesis in marine invertebrates and propose research to expand our understanding of the GABA hypothesis in marine invertebrates. We demonstrate that the effects of altered GABAA receptor function are likely to be widespread, including non-behavioral effects. Finally, we identify other neurobiological mechanisms that should be affected if the GABA hypothesis is correct, propose alternative neurobiological mechanisms by which behavior could be altered by elevated CO2 and suggest techniques to be utilized for future study of elevated CO2-induced behavioral alterations.
Mechanisms for Elevated Co2-Induced Behavioral Changes
Altered Sensory Stimuli at Elevated CO2
Elevated CO2 may influence behavior by altering an animal’s ability to sense the environment (Briffa et al., 2012; Draper and Weissburg, 2019). A range of sensory stimuli may be disrupted at elevated CO2 via differing mechanisms, thereby affecting associated behaviors (Figure 1). Structural alteration of chemical cues at elevated CO2 may affect chemoreception – the detection of chemical cues by binding to sensory receptors, e.g., odor molecules binding to olfactory receptors (Tierney and Atema, 1988). Impaired chemo-responsive behavior was first shown to be due to structural alteration of the chemical cue at low pH in a freshwater system (Brown et al., 2002) and the same mechanism has since been demonstrated in a marine invertebrate; the shore crab Carcinus maenas. Near-future pH levels altered the structure and charge of signaling molecules that mediate egg ventilation behavior in the shore crab C. maenas and the threshold of signaling molecule concentration required to induce egg ventilation behavior in this species increased when tested at pH 7.7 compared to pH 8.1 (Roggatz et al., 2016). Receptor alteration, such as change in ionization state, could also conceivably occur at low pH disrupting chemoreception (Tierney and Atema, 1988), and may be an additional explanation for behavioral changes observed in the shore crab (Roggatz et al., 2016). However, to date receptor structure has never been directly tested at different CO2 levels in conjunction with a behavioral assay.
Changes in ocean chemistry associated with rising CO2 levels will directly affect acoustic cues, potentially altering auditory driven behaviors. Sound absorption, in the low frequency range of ∼0.01–10 kHz, is reduced by decreasing pH due to shifts in the chemical reactions of sound absorbing compounds (e.g., magnesium sulfate, boric acid and carbonate ions) in seawater (Hester et al., 2008). Sound absorption (decibels per kilometer) is predicted to decrease by over 20% and almost 40% with a pH drop from 8.1 to 7.95 and 7.8, respectively (Hester et al., 2008). Thus, as CO2 levels rise, transmission of low-frequency sounds will increase and ecologically relevant acoustic cues, within this affected frequency range, will be transmitted further at elevated CO2 levels. For example, compared to off reef-locations, oyster reefs have higher acoustic energy levels within the frequency range of 1.5–20 kHz. This acoustic signature of reefs is used as a settlement cue by larval oysters (Lillis et al., 2013). As CO2 levels rise, oyster larvae may thus be able to detect appropriate settlement habitats from greater distances, however, it remains unknown whether the magnitude of change is sufficient to be of biological relevance. Elevated CO2 will also increase the transmission of abiotic sounds produced naturally (e.g., waves, raindrops) and by human activity (e.g., shipping, sonar and construction) (Ilyina et al., 2010). This will create a noisier environment in which it is harder for marine invertebrates to detect ecologically relevant sounds, such as those used for communication (Popper et al., 2001; Buscaino et al., 2011) as well as navigation and habitat selection for settlement (Jeffs et al., 2003; Stanley et al., 2009; Vermeij et al., 2010; Lillis et al., 2013). In a coral reef fish, predatory behavior decreased when exposed to boat noise or elevated CO2 (925 μatm pCO2), however, there was no additive effect when these stressors co-occurred (McCormick et al., 2018). Studies in marine invertebrates to determine how increased transmission of biologically relevant cues and background noise will interact as CO2 levels rise, and if this will be biologically relevant will be important.
Behavioral changes induced by elevated CO2 may alter the sensory output of an animal, affecting whether and how this animal is sensed by other animals (Draper and Weissburg, 2019). For example, increased activity of a prey animal could enhance how much or how often sound, visual and mechanosensory cues are produced, strengthening predatory sensory detection of the prey and increasing the chance of predation (Draper and Weissburg, 2019). Elevated CO2 reduced the intensity and frequency of snaps produced by snapping shrimp (Rossi et al., 2016). As these snaps are commonly present in the soundscapes used by marine invertebrate larvae as settlement cues (Stanley et al., 2009; Vermeij et al., 2010; Lillis et al., 2013), altered snapping behavior of snapping shrimp at elevated CO2 may in turn alter marine invertebrate settlement behavior.
Physical Change of Sensory Organs
Sensation may also be disrupted by physical change of sensory organs at elevated CO2. Due to lower saturation states of seawater with respect to calcium carbonate at elevated CO2, animals can have difficulty maintaining calcium carbonate structures (Orr et al., 2005) which may damage sensory organ structures (Briffa et al., 2012). Alternatively, active acid–base regulation to maintain a steady internal pH may alter the concentrations of ions that are fundamental for the formation of calcified sensory organs (Grosell, 2019). For example, many marine invertebrates use statocysts, which contain mineralized statoliths, to detect gravity to maintain orientation (Cohen, 1960; Clarke, 1978; Spangenberg, 1986), as well as vibrational stimuli for hearing in cephalopods (Mooney et al., 2010). Statocysts are also involved in motor programs that underlie hunting behavior in mollusks (Levi et al., 2004). Statolith size was reduced and morphology altered in cephalopods exposed to ∼1,300 μatm (Zakroff et al., 2019), 2,200 μatm (Kaplan et al., 2013), and 4,000 μatm (Maneja et al., 2011) pCO2. Abalone exposed to ∼700 and ∼1,000 μatm pCO2 also exhibited decreased statolith size compared to control conditions (Manríquez et al., 2014b). Conversely, statolith size was increased, and chemical composition altered, in squid exposed to 850 and 1,500 μatm pCO2 (Lacoue-Labarthe et al., 2011). Cuttlefish exposed to 4,000 μatm pCO2 exhibited reduced statolith calcification, altered statolith microstructure and decreased prey capture efficiency compared to squid in control conditions (700 μatm pCO2) (Maneja et al., 2011). Furthermore, computer modeling showed that an increased statolith mass, similar to that seen in the otoliths of fish exposed to 2,500 μatm pCO2, would alter cephalopod hearing below 10 Hz (Zhang et al., 2015). However, squid exposed to ∼1,300 μatm pCO2 had smaller statoliths with an altered morphology (Zakroff et al., 2019) but no impairment in swimming orientation (Zakroff et al., 2018). Therefore, elevated CO2-induced alteration of statoliths may disturb hearing but not gravity detection in cephalopods, impacting auditory-driven behavioral outputs but not the ability to maintain orientation. Decapod crustaceans possess calcified antennules, housing chemoreceptors, which are used for long range chemoreception. Rapid antennule flicking is used to gather chemical cue information, much in the way sniffing increases our ability to determine smells (Schmitt and Ache, 1979; Koehl, 2005). Hermit crabs with disrupted chemo-sensory responses at extremely high levels of CO2 (c. >12,000+ μatm pCO2) showed no damage to their antennules (de la Haye et al., 2012), suggesting that other mechanisms must be responsible for the observed response.
Altered Behavioral Choices
The physiological and ecological context, including external factors (e.g., presence of predators or temperature) and internal factors (e.g., hunger or reproductive state) can influence behavioral choices (Palmer and Kristan, 2011). Elevated CO2 may alter both external and internal factors, changing contextual modulation of behavioral choice and resulting in altered behavioral output. Physical changes induced by elevated CO2 may alter an animal’s behavioral choice. For example, predator-induced shell thickening observed in control periwinkles Littorina littorea did not occur in periwinkles exposed to extremely high levels of CO2 (c. >12,000+ μatm). However, predator avoidance behavior increased at elevated CO2 conditions, compared to control, which suggests behavioral compensation for the lack of morphological defense at extremely high levels of CO2 (Bibby et al., 2007). In another example, the swimming performance of larval sand dollars Dendraster excentricus was maintained at elevated CO2 (∼1,000 μatm pCO2) despite impaired arm and body morphology, likely due to a behavioral change in ciliary beat patterns (Chan et al., 2011). By contrast, both predator cue-induced byssal thread production and protective clustering behavior was decreased in mussels exposed to elevated CO2 (1,100 μatm pCO2) (Kong et al., 2019), indicating no behavioral compensation for the lack of morphological defense at elevated CO2.
The influence of elevated CO2 on respiration, energy turnover and mode of metabolism (Pörtner et al., 2004) may also alter behavioral choice. Depressed metabolism at elevated CO2 levels may reduce energy production, reducing the energy available to meet other demands and constraining performance of some behaviors. For example, metabolic scope and swimming ability were reduced in shrimp exposed to ∼1,000 μatm pCO2 (Dissanayake and Ishimatsu, 2011) and oxygen consumption rate and digging depth were decreased in razor clams exposed to 1,900 and 3,000 μatm pCO2 (Peng et al., 2017). Increased metabolism can indicate an increased energy demand at elevated CO2 and may decrease the energy available for other costly processes. For example, crabs exposed to 1,200 and 2,300 μatm pCO2 exhibited an increased metabolic rate but a decreased feeding rate (Wang et al., 2018). Alternatively, organisms may alter behaviors to meet the high energy demand. For example, respiration and feeding rate were increased in a copepod exposed to 1,000 μatm pCO2 (Li and Gao, 2012) and a sea urchin exposed to 1,300 μatm pCO2 (Rich et al., 2018). However, other studies show a change in metabolism with no associated behavioral change at 750 and 1,200 μatm pCO2 in an echinoderm (Carey et al., 2016) and at 1,500 μatm pCO2 in a mollusk (Benítez et al., 2018), or no metabolic change but altered behavior at 960 μatm pCO2 in a mollusk (Watson et al., 2014) and at 1,000, 2,000, and 3,000 μatm pCO2 in a crustacean (Menu-Courey et al., 2018). Therefore, it seems that altered metabolism in elevated CO2 may be responsible for some instances of altered behaviors, but not others.
Altered Functioning of the GABAA Receptor
Elevated CO2 has been found to alter a range of behaviors across different sensory modalities, as well as behaviors that involve higher order processing, such as decision making (de la Haye et al., 2011) and anxiety-like behaviors (Ren et al., 2018). This suggests that not only sensory detection, but also other neuronal processes are altered by elevated CO2. Neurotransmission is crucial to all components of the pathway producing behavior, from relaying electrical signals from sensory receptors to (and between) higher-order neurons for information processing, to motor neurons for the production of a behavioral response. Therefore, altered neurotransmission at elevated CO2 may underlie a variety of behavioral disturbances.
The prominent hypothesis for altered neurotransmission at elevated CO2 is the GABA hypothesis, proposed to occur in fish (Nilsson et al., 2012; Heuer and Grosell, 2014) and also suggested to apply to marine invertebrates (Watson et al., 2014). In vertebrates, GABA acts on the GABA type A receptor (GABAA R), a LGIC permeable to chloride (Cl–) and bicarbonate (HCO3–) ions, as the main inhibitory neurotransmitter in the central nervous system (DeFeudis, 1975; Bormann et al., 1987). Under normal conditions, binding of GABA opens the GABAA R channel allowing a net influx of negative charge resulting in hyperpolarization and inhibition of neuronal firing. Nilsson et al. (2012) proposed that the change in HCO3– and Cl– gradients across the neuronal membrane, due to acid–base regulation at increased CO2 levels, results in a net efflux of negative charge from the GABAA R upon GABA binding (Figure 2A). This could cause depolarization and excitation of neurons, thereby altering behavioral responses. Pharmacological studies administering GABAA R antagonists and agonists (Nilsson et al., 2012; Hamilton et al., 2013; Chivers et al., 2014; Chung et al., 2014), and measurements of brain ion gradients (Heuer et al., 2016) have supported this hypothesis in fish.
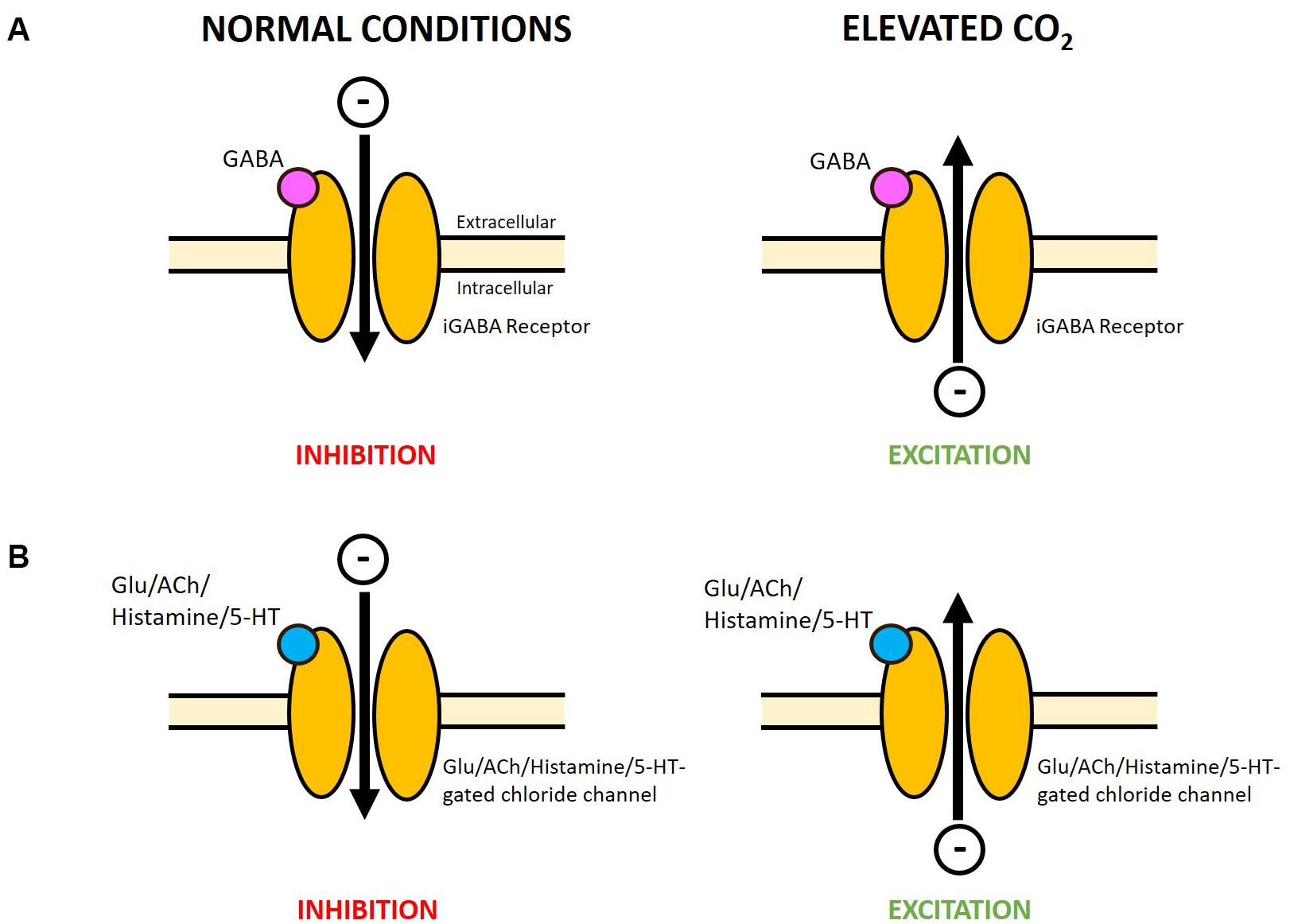
Figure 2. Potential reversal in function of varying LGICs at elevated CO2 in marine invertebrates. (A) A change in HCO3– and Cl– gradients across the neuronal membrane, due to acid–base regulation at elevated CO2, was proposed to reverse the net flow of negative charge through the GABAA R (Nilsson et al., 2012) and likely applies to the marine invertebrate iGABA R. Under normal conditions the net influx of negative charge is primarily carried by Cl–, while at elevated CO2 the net efflux of negative charge may be primarily carried by HCO3– (see Heuer et al., 2019 for a detailed explanation). (B) A range of invertebrates also possess glutamate, acetylcholine, histamine and serotonin-gated chloride channels. The flow of Cl– through these channels may be similarly altered at elevated levels of CO2. Research is needed to determine whether these receptors are also permeable to HCO3– which could also contribute to the reversal of the net movement of negative charge. A net influx of negative charge will result in hyperpolarization and inhibition of neuronal firing, while a net efflux of negative charge will cause depolarization and excitation. Glu, glutamate; ACh, acetylcholine; 5-HT, serotonin.
The Gaba Hypothesis in Marine Invertebrates
GABA is the main inhibitory neurotransmitter in the invertebrate peripheral and central nervous systems (Lummis, 1990; Lunt, 1991), acting on the ionotropic GABA receptor (iGABA R), which is also permeable to Cl– and HCO3– ions (Kaila and Voipio, 1987). Thus, the GABA hypothesis likely applies to marine invertebrates. The GABA hypothesis has been tested in marine arthropods and mollusks using pharmacological studies (Watson et al., 2014; Charpentier and Cohen, 2016; Clements et al., 2017), measurement of ion concentrations (de la Haye et al., 2012; Charpentier and Cohen, 2016) and molecular studies (Moya et al., 2016; Ren et al., 2018) (Table 1).
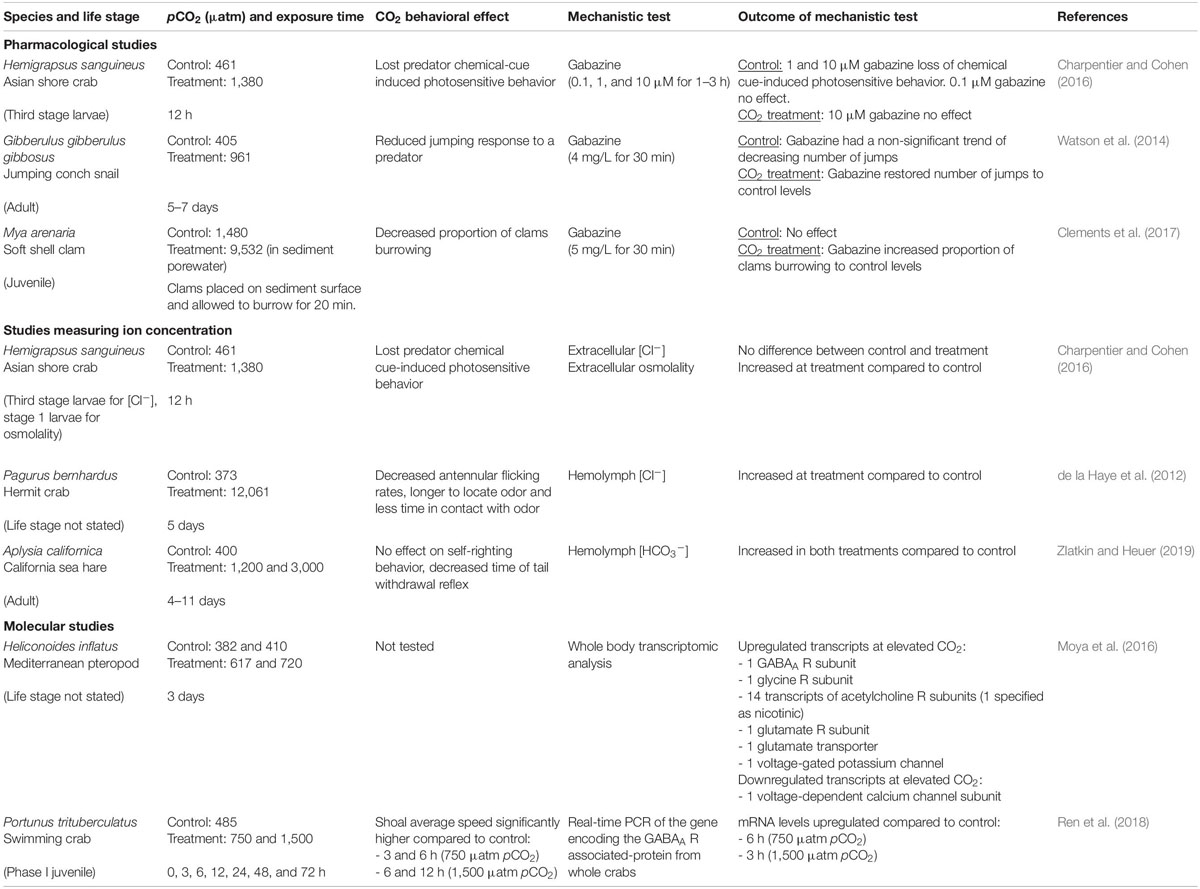
Table 1. Summary of publications that have mechanistically tested whether LGIC-mediated neurotransmission is altered in marine invertebrates at elevated CO2.
One method of assessing the GABA hypothesis involves administering the GABAA R antagonist gabazine (SR-95531) (Heaulme et al., 1986). If GABAA R functioning is altered at elevated CO2, gabazine administration should reverse elevated CO2-induced behavioral alterations by inhibiting channel opening and thus blocking the altered ion flow (Nilsson et al., 2012). Indeed, impaired escape behavior caused by exposure to 961 μatm pCO2 was restored to control levels by gabazine in the jumping conch snail Gibberulus gibbosus (Watson et al., 2014). In the soft shell clam Mya arenaria, burrowing behaviors altered by CO2 sediment levels representing present day variation were restored by gabazine (Clements et al., 2017). By contrast, in Asian shore crab larvae Hemigrapsus sanguineus, the loss of chemical cue-induced photosensitive behavior at elevated CO2 conditions (1,380 μatm pCO2) was not restored by gabazine (Charpentier and Cohen, 2016). These contrasting results initially appear to suggest that the GABA hypothesis applies to some, but not other, marine invertebrate taxa. However, crustacean iGABA Rs are commonly insensitive to gabazine (El Manira and Clarac, 1991; Jackel et al., 1994; Pearstein et al., 1996; Barry, 2002), meaning that gabazine may be inadequate for testing the GABA hypothesis in crustaceans.
It is interesting to note that the action of gabazine differed across control animals; gabazine significantly altered the behavior of control crab larvae (Charpentier and Cohen, 2016), had a non-significant trend of altering the behavior of control snails (Watson et al., 2014), and did not alter control clam burrowing behavior (Clements et al., 2017). As gabazine also blocks ion flow under normal conditions, preventing inhibition, over-excitation and behavioral alterations should occur in control animals. As gabazine does not appear to affect crustacean iGABA Rs, the behavioral change observed in crabs held at control CO2 levels may be through the action of gabazine on a different pathway, such as a different receptor type. Characterizing the pharmacology of gabazine in the studied species and using a range of GABAA R drugs will be important to confirm the GABA hypothesis is actually being tested (see the section “Pharmacological Considerations”).
Mechanistic support for the GABA hypothesis in marine invertebrates also comes from recent studies indicating changes in ion concentration and altered behavior in the same species at elevated CO2 (Table 1). Hermit crabs Pagurus bernhardus exhibited impaired chemosensory responses to a food odor and increased hemolymph Cl– concentration ([Cl–]) at extremely high (12,061 μatm pCO2) compared to control (373 μatm pCO2) conditions (de la Haye et al., 2012). Asian shore crab larvae had altered chemical cue-induced photosensitive behavior and increased extracellular osmolality, but similar extracellular [Cl–] at elevated CO2 (1,380 μatm pCO2) compared to controls (461 μatm pCO2) (Charpentier and Cohen, 2016). However, the [Cl–] measurements were very close to the limit of detection, which may be why no difference was observed. The increased extracellular osmolality was suggested to be due to an increase in HCO3– concentration ([HCO3–]), however, [HCO3–] was not directly measured. In a more recent study, sea hares Aplysia californica exposed to elevated CO2 (1,200 and 3,000 μatm pCO2) showed a reduced antipredator response and increased hemolymph [HCO3–] compared to control (400 μatm pCO2) (Zlatkin and Heuer, 2019). Together, these studies support the hypothesis of altered [HCO3–] and [Cl–] underlying altered iGABA R function and behavioral change at elevated CO2.
Molecular studies also provide support for the GABA hypothesis in marine invertebrates (Table 1). Transcriptomic analysis of the Mediterranean pteropod Heliconoides inflatus exposed to elevated CO2 (617–720 μatm pCO2) for 3 days showed upregulation of the transcript encoding a GABAA R subunit (Moya et al., 2016). However, RNA was extracted from the whole animal, potentially masking differential expression within the nervous system, and behavioral assays were not carried out. Ren et al. (2018) isolated the gene encoding the GABAA R associated-protein (GABARAP) in the crab Portunus trituberculatus. Real-time PCR found that GABARAP mRNA levels were significantly upregulated by 4.34-fold after 6 h at 750 μatm pCO2 and by 2.89-fold after 3 h at 1,500 μatm pCO2, compared to control (485 μatm pCO2). Showing a similar trend, average speed of the crab shoal’s movement was significantly higher after 3 and 6 h at 750 μatm pCO2, and six and 12 h at 1,500 μatm pCO2, compared to control. The increase in the GABARAP gene was suggested to assist more GABAA Rs to cluster on neuronal membranes, which may exaggerate the impaired function of GABAA Rs at elevated CO2 and lead to the altered behavior (Ren et al., 2018).
iGABA R Subtypes and Variability in Elevated CO2-Induced Behavioral Alterations
Inter- and intra-species variation in the behavioral effects of elevated CO2 may be due to the presence and variability of iGABA R subtypes in invertebrates. The ion permeable pore of iGABA Rs is made up of five subunits (Olsen and Sieghart, 2008). There is large variation in gene structure and the number of genes encoding iGABA R subunits between invertebrate species. For example, 5 GABA R-like genes have been found in the sea-squirt Ciona intestinalis, 12 in the fruit fly Drosophila melanogaster and 39 in the roundworm Caenorhabditis elegans (Tsang et al., 2006). Differing subunit composition forms iGABA R subtypes (Olsen and Sieghart, 2008) which vary in a range of functional properties including GABA binding affinity, and Cl– and HCO3– permeability (Lee and Maguire, 2014). Differences in iGABA R subunits may account for the variability in behavioral alterations at elevated CO2 observed between invertebrate species. Furthermore, subunit composition can vary between regions in the nervous system and cell types (Lee and Maguire, 2014). As different behaviors are driven by different nervous system regions, this may explain why some behaviors, but not others, are disrupted by elevated CO2 within a species.
Pharmacological Considerations
Studies using gabazine have provided a useful starting point to understand the mechanisms underlying behavioral alterations at elevated CO2 in marine invertebrates. However, the pharmacological profile of invertebrate iGABA Rs differs from that of vertebrate GABAA Rs (Rauh et al., 1990), and have not been characterized as extensively as in vertebrates. The majority of invertebrate research has been in non-marine invertebrates, with invertebrate iGABA R pharmacology being best studied in insects due to their potential target for insecticides (Hosie et al., 1995; Bloomquist, 2003). Gabazine inhibits a cloned planthopper iGABA R subunit expressed in a cell line (Narusuye et al., 2007) and two cloned fruit fly iGABA R subunits expressed in Xenopus laevis oocytes (Hosie and Sattelle, 1996). Gabazine also inhibits iGABA Rs in native neurons of locusts (Janssen et al., 2010) and moths (Satoh et al., 2005), but not in cockroaches (Aydar and Beadle, 1999). The few studies in other diverse invertebrates are conflicting; gabazine inhibits iGABA Rs in a freshwater hydrozoan (Concas et al., 1998), only weakly antagonizes GABA responses in a terrestrial nematode (Duittoz and Martin, 1991) and has no effect on crustaceans, including a freshwater crayfish (El Manira and Clarac, 1991; Pearstein et al., 1996) and a marine lobster (Jackel et al., 1994). These non-marine examples are more taxonomically relevant to marine invertebrates than comparisons with evolutionarily divergent marine vertebrate taxa. They indicate the wide variability in invertebrate iGABA R responses to gabazine. As iGABA R pharmacology can differ by subunit composition (Lee and Maguire, 2014; Sieghart, 2015) and there is large variation in subunit genes between invertebrates (Tsang et al., 2006) it will be useful to characterize the pharmacology of gabazine on iGABA Rs in the studied marine invertebrate species.
It is also important to note that antagonists are commonly not completely specific. For example, in insects gabazine partially and bicuculline (a GABAA R antagonist) fully inhibits locust nicotinic acetylcholine receptors (Jackson et al., 2002), and the GABAA R antagonist bicuculline inhibits and the GABAA R agonist muscimol activates a model of insect GABA-gated cation channels (Gisselmann et al., 2004). Less research has studied the off-target effects of GABA drugs in marine invertebrates, though bicuculline and picrotoxin both inhibit acetylcholine-gated chloride channels in the sea hare Aplysia californica (Yarowsky and Carpenter, 1978). To ensure the low affinity, alternative effects of drugs do not occur, careful consideration of concentration administered must be made. Using a range of GABAA R drugs with differing side effects will provide further evidence for or against the role of iGABA Rs in behavioral alterations at elevated CO2.
Marine Invertebrate Acid–Base Regulatory Mechanisms and the GABA Hypothesis
Acid–base regulatory mechanisms in marine invertebrates indicate that extra- and intra-cellular [HCO3–] and [Cl–] will alter at elevated CO2, providing theoretical support for the GABA hypothesis. Such mechanisms have best been studied in crustaceans [see reviews by Henry and Wheatly (1992) and Wheatly and Henry (1992)]. The primary mechanism to maintain extracellular pH (pHe) is via ion exchange with the external water environment (Cameron, 1985; Wheatly and Henry, 1992; Pörtner et al., 1998), including HCO3– influx in exchange for Cl– efflux (Truchot, 1983; Wheatly and Henry, 1992). Indeed, in all marine invertebrates studied so far, [HCO3–]e in the blood/hemolymph increases upon exposure to elevated seawater CO2. Exposure to pCO2 of 15 and 30 mm Hg (∼2,000 and 4,000 μatm pCO2) increased hemolymph [HCO3–] and decreased hemolymph [Cl–] compared to control conditions in the blue crab Callinectes sapidus (Cameron and Iwama, 1987). At a pCO2 of 45 mm Hg (∼6,000 μatm pCO2) in the same species, hemolymph [HCO3–] also increased, however, hemolymph [Cl–] increased (Cameron and Iwama, 1987), and 12,061 μatm pCO2 increased hemolymph [Cl–] compared to control (373 μatm pCO2) in the hermit crab Pagurus bernhardus (de la Haye et al., 2012). In palemonid shrimps exposure to 0.3 kPa CO2 (∼3,000 μatm pCO2) decreased hemolymph [Cl–] in the high shore Palaemonidae elegans, but increased hemolymph [Cl–] in the subtidal Palaemonidae serratus (Dissanayake et al., 2010). Thus, changes in both [HCO3–]e and [Cl–]e at elevated CO2 support the role of compensatory acid–base regulation as a key part of the GABA hypothesis, although studies at CO2 levels more relevant to future scenarios, such as ∼1,000 μatm CO2, would be valuable.
Intracellular pH (pHi) is also regulated via ion exchange (Walsh and Milligan, 1989), including Na+ dependent Cl–/HCO3– exchange (Roos and Boron, 1981), as seen in muscle fibers of the sipunculid worm (Pörtner et al., 2000), crayfish (Galler and Moser, 1986) and barnacle (Boron, 1977; Boron et al., 1981), as well as in crayfish neurons (Moody, 1981) and the squid giant axon (Russell and Boron, 1976; Boron and Russell, 1983). This suggests that [HCO3–]i increases and [Cl–]i decreases in order to maintain pHi. To date, neural [HCO3–]i has not been measured in a marine invertebrate exposed to elevated CO2. However, in the sipunculid worm Sipunculus nudus muscle [HCO3–]i significantly increased over 96 h in 1% CO2 (∼10,000 μatm pCO2) in air (Pörtner et al., 1998). A net efflux of Cl– is observed from the squid giant axon at a pHi of 6.5 reached by intracellular acid administration (Boron and Russell, 1983), and in crayfish isolated abdominal ganglia the resting [Cl–]i (35 mM) decreased by 3–5 mM when exposed to Ringer’s solution equilibrated with 5% CO2 (∼10,000 μatm pCO2) (Moody, 1981). Therefore, changes in [HCO3–]i and [Cl–]i occur in marine invertebrates exposed to extremely high levels of CO2. Again, studies using CO2 levels more relevant to future scenarios will be important to understand the theory underlying the GABA hypothesis.
It is unknown whether the above changes in [Cl–]i/e and [HCO3–]i/e are sufficient to alter iGABA R functioning. For elevated CO2 to disrupt GABA functioning, it is not simply altered [HCO3–] and [Cl–], but a difference in ion gradients across neuronal membranes that will alter ion flow through the iGABA R, i.e., [Cl–]i and [HCO3–]i within neurons must change by a different amount to [Cl–]e and [HCO3–]e present in the fluid bathing the neurons (Nilsson and Lefevre, 2016). A useful way to determine whether the changes in [HCO3–] and [Cl–] can alter ion flow through the iGABA R is by determining the GABA reversal potential (EGABA) and comparing it to the neuronal resting membrane potential (see Tresguerres and Hamilton (2017) and Heuer et al. (2019) for a detailed explanation). This approach has previously been employed to demonstrate that altered [Cl–] and [HCO3–] at elevated CO2 could change GABAA R function in the spiny damselfish Acanthochromis polyacanthus (Heuer et al., 2016).
Calculating EGABA requires knowledge of the HCO3–/Cl– permeability ratio of the iGABA R, and [HCO3–]i, [Cl–]i, [HCO3–]e and [Cl–]e at both control and elevated CO2 conditions. The HCO3–/Cl– permeability ratio is estimated to be between 0.2 and 0.6 in crayfish muscle fibers (Kaila and Voipio, 1987; Kaila et al., 1989; Farrant and Kaila, 2007), indicating that the iGABA R is more permeable to Cl– than it is to HCO3–. However, this permeability ratio is unknown for other invertebrates. It is important to ensure ion concentration measurements are taken in the correct fluids. In most marine invertebrates no blood brain barrier is present (Cserr and Bundgaard, 1984) and measuring extracellular ion concentration in the hemolymph may be adequate. However, structural organization of nervous tissue may provide some regulation of the neuronal microenvironment (Cserr and Bundgaard, 1984). Cephalopods have a blood brain barrier separating the blood from the brain (Cserr and Bundgaard, 1984) and extracellular measurements should be made in the brain interstitial fluid that bathes the neurons. It is also vital intracellular measurements are done on neuronal cytoplasm, as pHi regulatory mechanisms can differ between different cell types (Wheatly and Henry, 1992). Studies measuring these parameters and calculating EGABA in a marine invertebrate exposed to elevated CO2 will be useful to understand if altered [Cl–] and [HCO3–] could change iGABA R function.
The Effects of Altered iGABA Receptor Functioning
Altered iGABA R functioning is likely to disrupt behaviors due to the role of iGABA R-mediated neurotransmission in invertebrate sensation and a range of behavioral outputs. In mollusks, GABA is present in the olfactory, chemoreceptive (Nezlin and Voronezhskaya, 1997; Ito et al., 2001; Kobayashi et al., 2008), nociceptive (Kavaliers et al., 1999), visual and vestibular (Yamoah and Kuzirian, 1994) systems. iGABA R signaling mediates molluskan nociception (Kavaliers et al., 1999) and visual–vestibular interaction (Alkon et al., 1993), while mollusk photoreceptors respond to iGABA R signaling (Yamoah and Kuzirian, 1994). iGABA R-mediated signaling is important for feeding and prey-capture behaviors in mollusks (Arshavsky et al., 1993; Norekian and Satterlie, 1993; Romanova et al., 1996; Jing et al., 2003; Norekian and Malyshev, 2005) and a cnidarian (Pierobon et al., 1995, 2004; Concas et al., 1998), and GABAergic neurons are associated with effectors of feeding in a sea urchin (Bisgrove and Burke, 1987). iGABA R signaling mediates swimming of larval sea urchins (Katow et al., 2013), the righting response in a sea urchin (Shelley et al., 2019) and locomotion of a mollusk (Romanova et al., 1996). GABA mediates settling and metamorphosis, including associated behavioral changes in a range of mollusks (Morse et al., 1979; Morse et al., 1980; García-Lavandeira et al., 2005; Stewart et al., 2011; Biscocho et al., 2018), an echinoderm (Pearce and Scheibling, 1990) and a urochordate (Danqing et al., 2006). GABA is thought to mimic ligands from the environment (Morse et al., 1979) which may be detected by the iGABA R (Stewart et al., 2011) to initiate settlement and metamorphosis. Internal iGABA R mediated neurotransmission is also suggested to regulate metamorphosis (Biscocho et al., 2018). Thus altered iGABA R function will likely affect a variety of behaviors in a range of marine invertebrates.
Neural processes other than behavior may also be affected by altered iGABA R functioning. In vertebrates, GABA can act as a trophic factor (a molecule supporting cell survival) through the GABAA R, influencing cell proliferation, migration and differentiation (Owens and Kriegstein, 2002; Sernagor et al., 2010). In a fish, the three-spined stickleback Gasterosteus aculeatus, genes involved in neurogenesis and neuroplasticity were upregulated after exposure to ∼1,000 μatm pCO2 compared to control (∼330 μatm pCO2) (Lai et al., 2017). Similarly, GABA induces cellular differentiation and proliferation in abalone larvae (Morse et al., 1980). Thus, elevated CO2 may alter neurogenesis in marine invertebrates.
GABA can also have effects in non-neural tissue, playing an important role in the vertebrate immune system (Barragan et al., 2015; Wu et al., 2017). Invertebrate GABA also appears to play an immunomodulatory role. The iGABA R associated protein is implicated in the immune response of the abalone (Bai et al., 2012), GABA in the immune response of an oyster (Li et al., 2016a) and mussel (Nguyen et al., 2018), and a homolog of the glutamic acid decarboxylase (a rate limiting enzyme in GABA production) in immune regulation of an oyster (Li et al., 2016b). Thus, altered iGABA R functioning at elevated CO2 may have widespread effects.
Cross-talk between neurotransmitter receptors may also result in widespread effects of altered iGABA R functioning. Different types of neurotransmitters can be co-released from the same nerve terminal, resulting in simultaneous activation of their specific receptors, co-localized at the same post-synaptic site. This simultaneous activation can result in cross-talk between the receptors, modulating signal transmission. For example, negative cross-talk occurs when two different neurotransmitters simultaneously bind to their specific receptors, resulting in a current smaller than the sum of the currents of these two neurotransmitters acting separately (Li et al., 2003). Cross-talk involving GABAA Rs is well documented in mammals. For example, GABAA R activation suppresses the function of a dopamine receptor (de la Mora et al., 1997) and negative cross-talk occurs in both directions between GABAA Rs and glycine Rs (Trombley et al., 1999; Li et al., 2003) and GABAA Rs and the adenosine-triphosphate R P2X (Karanjia et al., 2006; Toulmé et al., 2007). Neurotransmitter cross-talk is yet to be studied in an invertebrate, but co-release of neurotransmitters occurs in the marine invertebrate nervous system, such as proctolin and GABA in crabs (Blitz et al., 1999), and dopamine and GABA in the sea hare Aplysia californica (Díaz-Ríos et al., 2002; Díaz-Ríos and Miller, 2005; Svensson et al., 2014). Furthermore, GABA has been found to post-synaptically increase dopamine currents in the sea hare Aplysia californica (Svensson et al., 2014). If cross-talk is present between invertebrate iGABA Rs and other neurotransmitter Rs, altered functioning of the iGABA R at elevated CO2 may alter cross-talk mechanisms. Thus, altered iGABA R function at elevated CO2 would not only alter the GABAergic pathway, but other pathways as well.
Alternative Mechanisms for Behavioral Change at Elevated Co2
If elevated CO2 does alter HCO3– and Cl– gradients across neuronal membranes, it is likely that functioning of LGICs other than the iGABA/GABAA R, that are also permeable to these ions, will also be disrupted (Figure 2). In vertebrates, altered glycine receptor functioning at elevated CO2 has been suggested due to its similarity to the GABAA R (Tresguerres and Hamilton, 2017). Many invertebrates lack glycine receptors (Tsang et al., 2006); however, invertebrates possess a larger variety of LGICs than vertebrates (Dent, 2010). Glutamate-gated chloride channels have been found in mollusks (Kehoe and Vulfius, 2000; Kehoe et al., 2009) and crustaceans (Marder and Paupardin-Tritsch, 1978), and are suggested to be the invertebrate equivalent of vertebrate glycine Rs (Vassilatis et al., 1997; Kehoe and Vulfius, 2000). There is also evidence for acetylcholine-gated chloride channels in mollusks (Kehoe, 1972). Moya et al. (2016) found a range of nervous system transcripts differentially expressed at elevated CO2 in the pteropod Heliconoides inflatus, including genes encoding for the LGICs (and associated proteins) of cholinergic, GABAergic, glutamatergic and glycinergic-like synapses (Moya et al., 2016) (Table 1). Thus, altered [Cl–] can conceivably disrupt functioning of a range of LGICs (Figure 2). Furthermore, taxa specific differences in the presence of specific LGICs (Dent, 2010) may explain the variability of the effects of elevated CO2 on marine invertebrate behavior.
Elevated CO2 may not only disrupt neurotransmission, but also neuronal-glial and glial-glial signaling. Glia are non-neuronal cells present in the vertebrate and invertebrate nervous system (Pentreath, 1989; Laming et al., 2000). Initially thought to be restricted to supporting neurons, the role of glia is now understood to include active participation in nervous system functioning, thus contributing to behavior (Laming et al., 2000; Jackson and Haydon, 2008). Like many other cell types, glial cells regulate pHi via ion exchange, including HCO3–/Cl– exchange (Deitmer and Rose, 1996). GABAA Rs are present in vertebrate glial cells (Butt and Jennings, 1994; Fraser et al., 1994). Less research has been carried out on invertebrate glia, with no research on the presence of iGABA Rs on marine invertebrate glial cells. However, leech glial cells reportedly respond to GABA [unpublished work reported in Deitmer and Rose (1996)]. If marine invertebrates are found to express iGABA Rs, elevated CO2 may also affect information processing through glial cells. Furthermore, fluxes in H+ ions have been found to contribute to neuron-glia signaling (Deitmer and Rose, 1996; Laming et al., 2000), which may be disrupted by exposure to elevated CO2 (and the resultant increase in H+ ions).
Changes in [Cl–] due to acid–base regulatory mechanisms at elevated CO2 may affect LGIC-mediated neurotransmission through a different mechanism, as well as having alternative effects on the nervous system. Cl– has a role as a signaling effector, with changes in [Cl–]i affecting a range of processes including gene expression, protein activity and cell proliferation (Valdivieso and Santa-Coloma, 2019). Mammalian work has supported the role of Cl– as a signaling effector in the nervous system, including [Cl–]i regulation of GABAA R expression (Succol et al., 2012) and growth of neuronal processes (Nakajima and Marunaka, 2016). The role of Cl– as a signaling anion has also been observed in bacterial cells, suggesting a conserved function (Valdivieso and Santa-Coloma, 2019). Thus, invertebrate Cl– is also likely to act as a signaling effector. If altered [Cl–]i has similar effects in marine invertebrates, elevated CO2 may impact nervous system functioning not only by altered iGABA R function but also changes in iGABA R expression, as well as altered growth of neuronal projections. Thus, altered [Cl–]i at elevated CO2 may have effects additional to altered LGIC function, having widespread consequences.
Directions for Future Research
Mechanistic studies have used targeted approaches to assess the GABA hypotheses in marine invertebrates. These approaches will also be useful to assess potential alternative mechanisms by which elevated CO2 may alter marine invertebrate behavior (Figure 3). The GABA hypothesis has been pharmacologically assessed by administering the GABAA R antagonist gabazine to marine invertebrates (Watson et al., 2014; Clements et al., 2017). Likewise, the administration of pharmacological agents targeting different LGICs will be useful to assess whether altered functioning of alternative LGICs may underlie behavioral changes at elevated CO2 conditions. Due to the diversity of invertebrates and the potential for off-target effects, it will be important to use a range of pharmacological agents, particularly those shown to work in the invertebrate taxa being studied. Furthermore, pilot studies to determine the optimal drug concentration to use will be important. For example, Charpentier and Cohen (2016) tested three gabazine concentrations. Measurement of ion concentrations at control and elevated levels of CO2 in conjunction with behavioral tests has been carried out in crabs (de la Haye et al., 2012; Charpentier and Cohen, 2016) and a mollusk (Zlatkin and Heuer, 2019). Future studies in other invertebrate taxa, with measurements made within the correct intra- and extra-cellular fluids, as well as determining the HCO3–/Cl– permeability ratio will be important to calculate EGABA at elevated CO2 to theoretically assess the GABA hypothesis. Real-time PCR, measuring the expression level of a specific gene, has been employed to assess the GABA hypothesis in a crab (Ren et al., 2018) and will also be useful to assess alternative mechanisms, e.g., measuring the expression of genes encoding for alternative LGIC subunits, and genes involved in neuronal growth and proliferation.
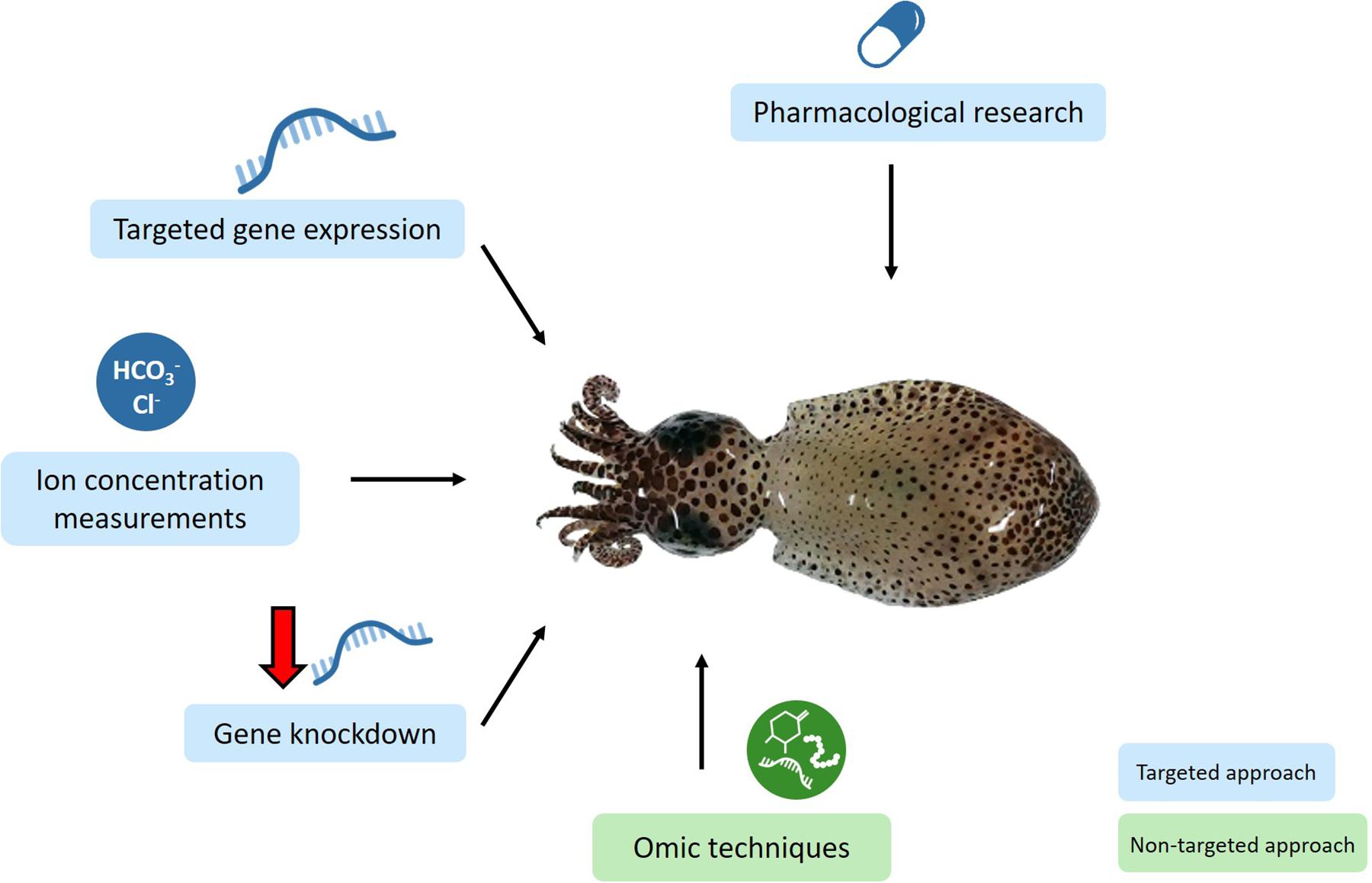
Figure 3. Conceptual diagram illustrating the techniques that will be useful for future research to assess the neurobiological mechanisms underlying behavioral change at elevated CO2 in marine invertebrates. Targeted approaches will test specific hypotheses and include pharmacological research administering drugs that target a specific receptor, measuring the expression of specific genes, measuring the concentration of HCO3– and Cl– ions in the relevant intra- and extra-cellular fluids, and knocking down the expression of specific genes. Omic techniques such as transcriptomics, proteomics and epigenomics will provide a non-targeted approach which does not require a priori hypotheses and will likely lead to the development of new hypotheses. Squid image taken by the author, JT.
These targeted approaches, however, may leave potentially relevant information unexplored. Omic technologies, such as transcriptomics and proteomics, provide a non-targeted approach in which a priori hypotheses are not required (Figure 3). Thus, data from omic approaches could unveil patterns leading to the development of novel hypotheses. For example, transcriptomics and proteomics have already been employed in fish nervous tissue, providing support for the GABA hypothesis as well as new avenues to pursue (Schunter et al., 2016, 2018; Porteus et al., 2018; Williams et al., 2019).
In marine invertebrates exposed to control and elevated CO2 levels, transcriptomic studies so far have analyzed the whole animal and have focused on pteropods (Koh et al., 2015; Maas et al., 2015; Moya et al., 2016; Thabet et al., 2017) and sea urchins (Todgham and Hofmann, 2008; Evans et al., 2013; Padilla-Gamiño et al., 2013; Clark et al., 2019). Likewise, proteomic studies have analyzed the whole body of tubeworm larvae (Mukherjee et al., 2013), oyster larvae (Dineshram et al., 2012; Dineshram et al., 2013), barnacle larvae (Wong et al., 2011), sea snail larvae (Di et al., 2019) and clam larvae (Timmins-Schiffman et al., 2019), and a metabolomics study analyzed the whole body of a crab (Trigg et al., 2019) exposed to control or elevated CO2. To understand the neurobiological mechanisms underlying behavioral changes, rather than the general molecular response to elevated CO2, it will be important to carry out omic techniques on the nervous tissue, as measurements at the whole body level may mask differential expression in the nervous system due to the heterogeneity and complexity of gene/protein expression. This is exemplified by Liu et al. (2019) who found region-specific regulation of neuropeptides in the nervous tissue of crabs Callinectes sapidus exposed to elevated CO2.
Omic technologies will provide a powerful, holistic approach to explore neurobiological mechanisms underlying behavioral change, potentially leading to the development of novel hypotheses. However, omic approaches can only determine correlational, and not causative, links between expression and behavior. Gene knockdown, in which the expression of a specific gene is reduced, will be a promising avenue for future research to determine a causative link between gene expression and behavioral change at elevated CO2. Gene knockdown is yet to be used in elevated CO2 behavioral research, however, gene knockdown of a heat shock protein assessed the stress tolerance of the white leg shrimp Litopenaeus vannamei to high CO2 (Aishi et al., 2019).
Conclusion
There is large variability in the effects of elevated CO2 on marine invertebrate behavior, which is likely due to the incredible diversity of marine invertebrates. Elevated CO2 likely alters behavior via a range of mechanisms that disrupt the nervous system pathway producing behavior; from sensory input to behavioral output. These mechanisms are not necessarily mutually exclusive, and interactions between mechanisms may account for the diversity in responses. Many of these mechanisms are based on theory and lack solid experimental evidence. Mechanistic research addressing these gaps will be important, for example linking altered sensation at elevated CO2 to behavioral change. Mechanistic fish research has focused on altered neurotransmission via disrupted GABAA R functioning at elevated CO2. The GABA hypothesis, as well as altered functioning of other LGICs, likely applies to marine invertebrates. Further research into the ionic properties of the iGABA R and other LGICs, including whether they are also permeable to HCO3–, and measuring intra- and extra-cellular ion levels in the relevant fluids at near-future CO2 levels will be beneficial for advancing our understanding of this mechanism. The diversity of LGIC subtypes between invertebrate species, and even between nervous system regions, may explain the variability in behavioral responses. Investigating the presence of LGICs on invertebrate glial cells, other modes of neuronal-glial and glial-glial transmission, and the role of Cl– as a signaling effector in invertebrates will help us understand the wider impact elevated CO2 may have on nervous system functioning.
The interconnectivity of the nervous system, such as receptor cross-talk, suggests that even disruption of one component or pathway will have widespread effects. This will make understanding the neurobiological mechanisms underlying elevated CO2-induced behavioral change extremely complex. Omics approaches will be useful in providing an untargeted, holistic approach to understand the response of the nervous system to elevated CO2, provide support or opposition for proposed mechanisms, and likely provide new avenues to explore. Exploring the mechanisms underlying behavioral change at elevated CO2 will help us to understand the variability in behavioral responses to elevated CO2 and predict which marine invertebrates are likely to be the most vulnerable to rising CO2 levels.
Author Contributions
All authors contributed to the conception and design of the review. JT wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
JT was supported by a Prestige Research Training Program Stipend from James Cook University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00345/full#supplementary-material
References
Aishi, K., Sinnasamy, S., MacRae, T. H., Muhammad, T. S. T., Lv, A., Sun, J., et al. (2019). Hsp70 knockdown reduced the tolerance of Litopenaeus vannamei post larvae to low pH and salinity. Aquaculture 512:734346. doi: 10.1016/j.aquaculture.2019.734346
Albright, R., Mason, B., Miller, M., and Langdon, C. (2010). Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl. Acad. Sci. U.S.A. 107, 20400–20404. doi: 10.1073/pnas.1007273107
Alkon, D., Anderson, M., Kuzirian, A., Rogers, D., Pass, D., Collin, C., et al. (1993). GABA-mediated synaptic interaction between the visual and vestibular pathways of Hermissenda. J. Neurochem. 61, 556–566. doi: 10.1111/j.1471-4159.1993.tb02159.x
Arshavsky, Y. I., Deliagina, T., Gamkrelidze, G., Orlovsky, G., Panchin, Y. V., Popova, L., et al. (1993). Pharmacologically induced elements of the hunting and feeding behavior in the pteropod mollusk Clione limacina, I. Effects of GABA. J. Neurophysiol. 69, 512–521. doi: 10.1152/jn.1993.69.2.512
Aydar, E., and Beadle, D. (1999). The pharmacological profile of GABA receptors on cultured insect neurones. J. Insect Physiol. 45, 213–219. doi: 10.1016/s0022-1910(98)00114-0
Bai, R., You, W., Chen, J., Huang, H., and Ke, C. (2012). Molecular cloning and expression analysis of GABAA receptor-associated protein (GABARAP) from small abalone, Haliotis diversicolor. Fish Shellfish Immunol. 33, 675–682. doi: 10.1016/j.fsi.2012.05.003
Barragan, A., Weidner, J. M., Jin, Z., Korpi, E., and Birnir, B. (2015). GABAergic signalling in the immune system. Acta Physiol. 213, 819–827. doi: 10.1111/apha.12467
Barry, M. J. (2002). Progress toward understanding the neurophysiological basis of predator-induced morphology in Daphnia pulex. Physiol. Biochem. Zool. 75, 179–186. doi: 10.1086/339389
Benítez, S., Lagos, N. A., Osores, S., Opitz, T., Duarte, C., Navarro, J. M., et al. (2018). High pCO2 levels affect metabolic rate, but not feeding behavior and fitness, of farmed giant mussel Choromytilus chorus. Aquac. Environ. Interact. 10, 267–278. doi: 10.3354/aei00271
Bibby, R., Cleall-Harding, P., Rundle, S., Widdicombe, S., and Spicer, J. (2007). Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol. Lett. 3, 699–701. doi: 10.1098/rsbl.2007.0457
Bignami, S., Enochs, I. C., Manzello, D. P., Sponaugle, S., and Cowen, R. K. (2013). Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc. Natl. Acad. Sci. U.S.A. 110, 7366–7370. doi: 10.1073/pnas.1301365110
Bindoff, N. L., Cheung, W. W., Kairo, J. G., Arístegui, J., Guinder, V. A., Hallberg, R., et al. (2019). “Chapter 5: changing ocean, marine ecosystems, and dependent communities,” in IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, eds H.-O. Pörtner, D. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, et al. (in press).
Biscocho, D., Cook, J. G., Long, J., Shah, N., and Leise, E. M. (2018). GABA is an inhibitory neurotransmitter in the neural circuit regulating metamorphosis in a marine snail. Dev. Neurobiol. 736–753. doi: 10.1002/dneu.22597
Bisgrove, B. W., and Burke, R. D. (1987). Development of the nervous system of the pluteus larva of Strongylocentrotus droebachiensis. Cell Tissue Res. 248, 335–343.
Blitz, D. M., Christie, A. E., Coleman, M. J., Norris, B. J., Marder, E., and Nusbaum, M. P. (1999). Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J. Neurosci. 19, 5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999
Blom, F. (1978). Sensory input behavioural output relationships in the feeding activity of some Lepidopterous larvae. Entomol. Exp. Appl. 24, 258–263.
Bloomquist, J. R. (2003). Chloride channels as tools for developing selective insecticides. Arch. Insect Biochem. Physiol. 54, 145–156. doi: 10.1002/arch.10112
Borges, F. O., Sampaio, E., Figueiredo, C., Rosa, R., and Grilo, T. F. (2018). Hypercapnia-induced disruption of long-distance mate-detection and reduction of energy expenditure in a coastal keystone crustacean. Physiol. Behav. 195, 69–75. doi: 10.1016/j.physbeh.2018.07.023
Bormann, J., Hamill, O. P., and Sakmann, B. (1987). Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. 385, 243–286. doi: 10.1113/jphysiol.1987.sp016493
Boron, W., and Russell, J. (1983). Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J. Gen. Physiol. 81, 373–399. doi: 10.1085/jgp.81.3.373
Boron, W. F. (1977). Intracellular pH transients in giant barnacle muscle fibers. Am. J. Physiol. Cell Physiol. 233, C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61
Boron, W. F., McCormick, W. C., and Roos, A. (1981). pH regulation in barnacle muscle fibers: dependence on extracellular sodium and bicarbonate. Am. J. Physiol. Cell Physiol. 240, C80–C89. doi: 10.1152/ajpcell.1981.240.1.C80
Briffa, M., de la Haye, K., and Munday, P. L. (2012). High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar. Pollut. Bull. 64, 1519–1528. doi: 10.1016/j.marpolbul.2012.05.032
Brown, G. E., Adrian, J., James, C., Lewis, M. G., and Tower, J. M. (2002). The effects of reduced pH on chemical alarm signalling in ostariophysan fishes. Can. J. Fish. Aquat. Sci. 59, 1331–1338.
Buscaino, G., Filiciotto, F., Gristina, M., Bellante, A., Buffa, G., Di Stefano, V., et al. (2011). Acoustic behaviour of the European spiny lobster Palinurus elephas. Mar. Ecol. Prog. Ser. 441, 177–184. doi: 10.3354/meps09404
Butt, A. M., and Jennings, J. (1994). Response of astrocytes to γ-aminobutyric acid in the neonatal rat optic nerve. Neurosci. Lett. 168, 53–56. doi: 10.1016/0304-3940(94)90414-6
Cameron, J. N. (1985). Compensation of hypercapnic acidosis in the aquatic blue crab, Callinectes sapidus: the predominance of external sea water over carapace carbonate as the proton sink. J. Exp. Biol. 114, 197–206.
Cameron, J. N., and Iwama, G. K. (1987). Compensation of progressive hypercapnia in channel catfish and blue crabs. J. Exp. Biol. 133, 183–197.
Carey, N., Harianto, J., and Byrne, M. (2016). Sea urchins in a high-CO2 world: partitioned effects of body size, ocean warming and acidification on metabolic rate. J. Exp. Biol. 219, 1178–1186. doi: 10.1242/jeb.136101
Chan, K. Y. K., Grünbaum, D., and O’Donnell, M. J. (2011). Effects of ocean-acidification-induced morphological changes on larval swimming and feeding. J. Exp. Biol. 214, 3857–3867. doi: 10.1242/jeb.054809
Charpentier, C. L., and Cohen, J. H. (2016). Acidification and γ-aminobutyric acid independently alter kairomone-induced behaviour. Open Sci. 3:160311. doi: 10.1098/rsos.160311
Chivers, D. P., McCormick, M. I., Nilsson, G. E., Munday, P. L., Watson, S. A., Meekan, M. G., et al. (2014). Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob. Change Biol. 20, 515–522. doi: 10.1111/gcb.12291
Chung, W.-S., Marshall, N. J., Watson, S.-A., Munday, P. L., and Nilsson, G. E. (2014). Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. J. Exp. Biol. 217, 323–326. doi: 10.1242/jeb.092478
Clark, M. S., Suckling, C. C., Cavallo, A., Mackenzie, C. L., Thorne, M. A., Davies, A. J., et al. (2019). Molecular mechanisms underpinning transgenerational plasticity in the green sea urchin Psammechinus miliaris. Sci. Rep. 9:952. doi: 10.1038/s41598-018-37255-6
Clarke, M. R. (1978). The cephalopod statolithan-introduction to its form. J. Mar. Biol. Assoc. U.K. 58, 701–712.
Clements, J. C., Bishop, M. M., and Hunt, H. L. (2017). Elevated temperature has adverse effects on GABA-mediated avoidance behaviour to sediment acidification in a wide-ranging marine bivalve. Mar. Biol. 164:56. doi: 10.1007/s00227-017-3085-1
Clements, J. C., and Hunt, H. L. (2014). Influence of sediment acidification and water flow on sediment acceptance and dispersal of juvenile soft-shell clams (Mya arenaria L.). J. Exp. Mar. Biol. Ecol. 453, 62–69.
Clements, J. C., and Hunt, H. L. (2015). Marine animal behaviour in a high CO2 ocean. Mar. Ecol. Prog. Ser. 536, 259–279. doi: 10.3354/meps11426
Cohen, M. (1960). The response patterns of single receptors in the crustacean statocyst. Proc. R. Soc. Lond. Ser. B Biol. Sci. 152, 30–49. doi: 10.1098/rspb.1960.0020
Concas, A., Pierobon, P., Mostallino, M., Marino, G., Minei, R., and Biggio, G. (1998). Modulation of γ-aminobutyric acid (GABA) receptors and the feeding response by neurosteroids in Hydra vulgaris. Neuroscience 85, 979–988. doi: 10.1016/s0306-4522(97)00515-0
Cserr, H. F., and Bundgaard, M. (1984). Blood-brain interfaces in vertebrates: a comparative approach. Am. J. Physiol. Regul. Integr. Comp. Physiol. 246, R277–R288. doi: 10.1152/ajpregu.1984.246.3.R277
Danqing, F., Ying, H., Caihuan, K., Shiqiang, Z., and Shaojing, L. (2006). Settlement and metamorphosis of Styela canopus Savigny larvae in response to some neurotransmitters and thyroxin. Acta Oceanol. Sin. 25, 90–97.
de la Haye, K., Spicer, J., Widdicombe, S., and Briffa, M. (2011). Reduced sea water pH disrupts resource assessment and decision making in the hermit crab Pagurus bernhardus. Anim. Behav. 82, 495–501. doi: 10.1016/j.anbehav.2011.05.030
de la Haye, K. L., Spicer, J. I., Widdicombe, S., and Briffa, M. (2012). Reduced pH sea water disrupts chemo-responsive behaviour in an intertidal crustacean. J. Exp. Mar. Biol. Ecol. 412, 134–140. doi: 10.1016/j.jembe.2011.11.013
de la Mora, M. P., Ferré, S., and Fuxe, K. (1997). GABA-dopamine receptor-receptor interactions in neostriatal membranes of the rat. Neurochem. Res. 22, 1051–1054. doi: 10.1023/a:1022439212836
Deitmer, J. W., and Rose, C. R. (1996). pH regulation and proton signalling by glial cells. Prog. Neurobiol. 48, 73–103. doi: 10.1016/0301-0082(95)00039-9
Dent, J. A. (2010). “The evolution of pentameric ligand-gated ion channels,” in Insect Nicotinic Acetylcholine Receptors, ed. S. H. Thany (New York, NY: Springer-Verlag), 11–23.
Di, G., Li, Y., Zhu, G., Guo, X., Li, H., Huang, M., et al. (2019). Effects of acidification on the proteome during early development of Babylonia areolata. FEBS Open Bio 9, 1503–1520. doi: 10.1002/2211-5463.12695
Díaz-Ríos, M., and Miller, M. W. (2005). Rapid dopaminergic signaling by interneurons that contain markers for catecholamines and GABA in the feeding circuitry of Aplysia. J. Neurophysiol. 93, 2142–2156. doi: 10.1152/jn.00003.2004
Díaz-Ríos, M., Oyola, E., and Miller, M. W. (2002). Colocalization of γ-aminobutyric acid-like immunoreactivity and catecholamines in the feeding network of Aplysia californica. J. Comp. Neurol. 445, 29–46. doi: 10.1002/cne.10152
Dineshram, R., Thiyagarajan, V., Lane, A., Ziniu, Y., Xiao, S., and Leung, P. T. (2013). Elevated CO2 alters larval proteome and its phosphorylation status in the commercial oyster, Crassostrea hongkongensis. Mar. Biol. 160, 2189–2205. doi: 10.1007/s00227-013-2176-x
Dineshram, R., Wong, K. K., Xiao, S., Yu, Z., Qian, P. Y., and Thiyagarajan, V. (2012). Analysis of Pacific oyster larval proteome and its response to high-CO2. Mar. Pollut. Bull. 64, 2160–2167. doi: 10.1016/j.marpolbul.2012.07.043
Dissanayake, A., Clough, R., Spicer, J., and Jones, M. (2010). Effects of hypercapnia on acid–base balance and osmo-/iono-regulation in prawns (Decapoda: Palaemonidae). Aquat. Biol. 11, 27–36. doi: 10.3354/ab00285
Dissanayake, A., and Ishimatsu, A. (2011). Synergistic effects of elevated CO2 and temperature on the metabolic scope and activity in a shallow-water coastal decapod (Metapenaeus joyneri; Crustacea: Penaeidae). ICES J. Mar. Sci. 68, 1147–1154. doi: 10.1093/icesjms/fsq188
Dlugokencky, E., and Tans, P. (2019). Trends in Atmospheric Carbon Dioxide. Available online at: https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed May 17, 2019).
Doropoulos, C., Ward, S., Diaz-Pulido, G., Hoegh-Guldberg, O., and Mumby, P. J. (2012). Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol. Lett. 15, 338–346. doi: 10.1111/j.1461-0248.2012.01743.x
Draper, A. M., and Weissburg, M. (2019). Impacts of global warming and elevated CO2 on sensory behavior in predator-prey interactions: a review and synthesis. Front. Ecol. Evol. 7:72. doi: 10.3389/fevo.2019.00072
Duittoz, A., and Martin, R. (1991). Effects of the arylaminopyridazine-GABA derivatives, sr95103 and SR95531 on the Ascaris muscle GABA receptor: the relative potency of the antagonists in Ascaris is different to that at vertebrate GABAA receptors. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 98, 417–422.
El Manira, A., and Clarac, F. (1991). GABA-mediated presynaptic inhibition in crayfish primary afferents by non-A, non-B GABA receptors. Eur. J. Neurosci. 3, 1208–1218. doi: 10.1111/j.1460-9568.1991.tb00055.x
Ellis, R. P., Bersey, J., Rundle, S. D., Hall-Spencer, J. M., and Spicer, J. I. (2009). Subtle but significant effects of CO2 acidified seawater on embryos of the intertidal snail, Littorina obtusata. Aquat. Biol. 5, 41–48. doi: 10.3354/ab00118
Evans, T. G., Chan, F., Menge, B. A., and Hofmann, G. E. (2013). Transcriptomic responses to ocean acidification in larval sea urchins from a naturally variable pH environment. Mol. Ecol. 22, 1609–1625. doi: 10.1111/mec.12188
Fabricius, K. E., Noonan, S. H., Abrego, D., Harrington, L., and De’ath, G. (2017). Low recruitment due to altered settlement substrata as primary constraint for coral communities under ocean acidification. Proc. R. Soc. B Biol. Sci. 284:20171536. doi: 10.1098/rspb.2017.1536
Fabry, V. J., Seibel, B. A., Feely, R. A., and Orr, J. C. (2008). Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432. doi: 10.1093/icesjms/fsn048
Farrant, M., and Kaila, K. (2007). The cellular, molecular and ionic basis of GABAA receptor signalling. Prog. Brain Res. 160, 59–87. doi: 10.1016/S0079-6123(06)60005-8
Feely, R. A., Sabine, C. L., Hernandez-Ayon, J. M., Ianson, D., and Hales, B. (2008). Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320, 1490–1492. doi: 10.1126/science.1155676
Foster, T., Gilmour, J., Chua, C., Falter, J., and McCulloch, M. (2015). Effect of ocean warming and acidification on the early life stages of subtropical Acropora spicifera. Coral Reefs 34, 1217–1226. doi: 10.1111/gcb.13848
Fraser, D. D., Mudrick-Donnon, L. A., and Macvicar, B. A. (1994). Astrocytic GABA receptors. Glia 11, 83–93.
Gallego, M., Timmermann, A., Friedrich, T., and Zeebe, R. (2018). Drivers of future seasonal cycle changes in oceanic pCO2. Biogeosciences 15, 5315–5327.
Galler, S., and Moser, H. (1986). The ionic mechanism of intracellular pH regulation in crayfish muscle fibres. J. Physiol. 374, 137–151. doi: 10.1113/jphysiol.1986.sp016071
García-Lavandeira, M., Silva, A., Abad, M., Pazos, A. J., Sánchez, J. L., and Pérez-Parallé, M. L. (2005). Effects of GABA and epinephrine on the settlement and metamorphosis of the larvae of four species of bivalve molluscs. J. Exp. Mar. Biol. Ecol. 316, 149–156. doi: 10.1016/j.jembe.2004.10.011
Gisselmann, G., Plonka, J., Pusch, H., and Hatt, H. (2004). Drosophila melanogaster GRD and LCCH3 subunits form heteromultimeric GABA-gated cation channels. Br. J. Pharmacol. 142, 409–413. doi: 10.1038/sj.bjp.0705818
Green, M. A., Waldbusser, G. G., Hubazc, L., Cathcart, E., and Hall, J. (2013). Carbonate mineral saturation state as the recruitment cue for settling bivalves in marine muds. Estuaries Coast. 36, 18–27. doi: 10.1007/s12237-012-9549-0
Grosell, M. (2019). “CO2 and calcification processes in fish,” in Carbon Dioxide, eds M. Grosell, P. L. Munday, A. P. Farrell, and C. J. Brauner (San Diego, CA: Academic Press), 133–159.
Guo, X., Huang, M., Pu, F., You, W., and Ke, C. (2015). Effects of ocean acidification caused by rising CO2 on the early development of three mollusks. Aquat. Biol. 23, 147–157. doi: 10.3354/ab00615
Hamilton, T. J., Holcombe, A., and Tresguerres, M. (2013). CO2-induced ocean acidification increases anxiety in rockfish via alteration of GABAA receptor functioning. Proc. R. Soc. B 281:20132509. doi: 10.1098/rspb.2013.2509
Heaulme, M., Chambon, J.-P., Leyris, R., Molimard, J.-C., Wermuth, C. G., and Biziere, K. (1986). Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site. Brain Res. 384, 224–231. doi: 10.1016/0006-8993(86)91158-3
Henry, R. P., and Wheatly, M. G. (1992). Interaction of respiration, ion regulation, and acid-base balance in the everyday life of aquatic crustaceans. Am. Zool. 32, 407–416.
Hester, K. C., Peltzer, E. T., Kirkwood, W. J., and Brewer, P. G. (2008). Unanticipated consequences of ocean acidification: a noisier ocean at lower pH. Geophys. Res. Lett. 35:L19601. doi: 10.1029/2008GL034913
Heuer, R., Welch, M., Rummer, J., Munday, P., and Grosell, M. (2016). Altered brain ion gradients following compensation for elevated CO2 are linked to behavioural alterations in a coral reef fish. Sci. Rep. 6:33216. doi: 10.1038/srep33216
Heuer, R. M., and Grosell, M. (2014). Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R1061–R1084. doi: 10.1152/ajpregu.00064.2014
Heuer, R. M., Hamilton, T. J., and Nilsson, G. E. (2019). “The physiology of behavioural impacts of high CO2,” in Carbon Dioxide, eds M. Grosell, P. L. Munday, A. P. Farrell, and C. J. Brauner (San Diego, CA: Academic Press), 161–194.
Hofmann, G. E., Smith, J. E., Johnson, K. S., Send, U., Levin, L. A., Micheli, F., et al. (2011). High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS One 6:e28983. doi: 10.1371/journal.pone.0028983
Hosie, A., Shirai, Y., Buckingham, S., Rauh, J., Roush, R., Baylis, H., et al. (1995). Blocking actions of BIDN, a bicyclic dinitrile convulsant compound, on wild-type and dieldrin-resistant GABA receptor homo-oligomers of Drosophila melanogaster expressed in Xenopus oocytes. Brain Res. 693, 257–260. doi: 10.1016/0006-8993(95)00605-p
Hosie, A. M., and Sattelle, D. B. (1996). Agonist pharmacology of two Drosophila GABA receptor splice variants. Br. J. Pharmacol. 119, 1577–1585. doi: 10.1111/j.1476-5381.1996.tb16075.x
Ilyina, T., Zeebe, R. E., and Brewer, P. G. (2010). Future ocean increasingly transparent to low-frequency sound owing to carbon dioxide emissions. Nat. Geosci. 3, 18–22. doi: 10.1038/NGEO719
Ito, I., Kimura, T., and Ito, E. (2001). Odor responses and spontaneous oscillatory activity in tentacular nerves of the terrestrial slug, Limax marginatus. Neurosci. Lett. 304, 145–148. doi: 10.1016/s0304-3940(01)01775-x
Jackel, C., Krenz, W., and Nagy, F. (1994). Bicuculline/baclofen-insensitive GABA response in crustacean neurones in culture. J. Exp. Biol. 191, 167–193.
Jackson, C., Bermudez, I., and Beadle, D. J. (2002). Pharmacological properties of nicotinic acetylcholine receptors in isolated Locusta migratoria neurones. Microsc. Res. Tech. 56, 249–255. doi: 10.1002/jemt.10028
Jackson, F. R., and Haydon, P. G. (2008). Glial cell regulation of neurotransmission and behavior in Drosophila. Neuron Glia Biol. 4, 11–17. doi: 10.1017/S1740925X09000027
Janssen, D., Derst, C., Rigo, J.-M., and Van Kerkhove, E. (2010). Cys-loop ligand-gated chloride channels in dorsal unpaired median neurons of Locusta migratoria. J. Neurophysiol. 103, 2587–2598. doi: 10.1152/jn.00466.2009
Jeffs, A., Tolimieri, N., and Montgomery, J. C. (2003). Crabs on cue for the coast: the use of underwater sound for orientation by pelagic crab stages. Mar. Freshw. Res. 54, 841–845. doi: 10.1071/MF03007
Jing, J., Vilim, F. S., Wu, J.-S., Park, J.-H., and Weiss, K. R. (2003). Concerted GABAergic actions of Aplysia feeding interneurons in motor program specification. J. Neurosci. 23, 5283–5294. doi: 10.1523/JNEUROSCI.23-12-05283.2003
Joos, F., and Spahni, R. (2008). Rates of change in natural and anthropogenic radiative forcing over the past 20,000 years. Proc. Natl. Acad. Sci. U.S.A. 105, 1425–1430. doi: 10.1073/pnas.0707386105
Kaila, K., Pasternack, M., Saarikoski, J., and Voipio, J. (1989). Influence of GABA-gated bicarbonate conductance on potential, current and intracellular chloride in crayfish muscle fibres. J. Physiol. 416, 161–181. doi: 10.1113/jphysiol.1989.sp017755
Kaila, K., and Voipio, J. (1987). Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature 330, 163–165. doi: 10.1038/330163a0
Kaplan, M. B., Mooney, T. A., McCorkle, D. C., and Cohen, A. L. (2013). Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PLoS One 8:e63714. doi: 10.1371/journal.pone.0063714
Karanjia, R., García-Hernández, L. M., Miranda-Morales, M., Somani, N., Espinosa-Luna, R., Montano, L. M., et al. (2006). Cross-inhibitory interactions between GABAA and P2X channels in myenteric neurones. Eur. J. Neurosci. 23, 3259–3268. doi: 10.1111/j.1460-9568.2006.04861.x
Katow, H., Abe, K., Katow, T., Zamani, A., and Abe, H. (2013). Development of the γ-amino butyric acid (GABA)-ergic signaling system and its role in larval swimming in sea urchin. J. Exp. Biol. 216, 1704–1716. doi: 10.1242/jeb.074856
Kavaliers, M., Perrot-Sinal, T. S., Desjardins, D. C., Cross-Mellor, S. K., and Wiebe, J. P. (1999). Antinociceptive effects of the neuroactive steroid, 3α-hydroxy-5α-pregnan-20-one and progesterone in the land snail, Cepaea nemoralis. Neuroscience 95, 807–812. doi: 10.1016/S0306-4522(99)00499-6
Kehoe, J. (1972). Ionic mechanism of a two-component cholinergic inhibition in Aplysia neurones. J. Physiol. 225, 85–114. doi: 10.1113/jphysiol.1972.sp009930
Kehoe, J., Buldakova, S., Acher, F., Dent, J., Bregestovski, P., and Bradley, J. (2009). Aplysia cys-loop glutamate-gated chloride channels reveal convergent evolution of ligand specificity. J. Mol. Evol. 69, 125–141. doi: 10.1007/s00239-009-9256-z
Kehoe, J., and Vulfius, C. (2000). Independence of and interactions between GABA-, glutamate-, and acetylcholine-activated Cl conductances in Aplysia neurons. J. Neurosci. 20, 8585–8596. doi: 10.1523/JNEUROSCI.20-23-08585.2000
Kim, T. W., Taylor, J., Lovera, C., and Barry, J. P. (2015). CO2-driven decrease in pH disrupts olfactory behaviour and increases individual variation in deep-sea hermit crabs. ICES J. Mar. Sci. 73, 613–619. doi: 10.1093/icesjms/fsv019
Kobayashi, S., Hattori, M., and Ito, E. (2008). The effects of GABA on the network oscillations of the procerebrum in Limax valentianus. Acta Biol. Hung. 59(Suppl. 2), 77–79. doi: 10.1556/ABiol.59.2008.Suppl.12
Koehl, M. (2005). The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem. Sen. 31, 93–105. doi: 10.1093/chemse/bjj009
Koh, H. Y., Lee, J. H., Han, S. J., Park, H., Shin, S. C., and Lee, S. G. (2015). A transcriptomic analysis of the response of the arctic pteropod Limacina helicina to carbon dioxide-driven seawater acidification. Polar Biol. 38, 1727–1740.
Kong, H., Clements, J. C., Dupont, S., Wang, T., Huang, X., Shang, Y., et al. (2019). Seawater acidification and temperature modulate anti-predator defenses in two co-existing Mytilus species. Mar. Pollut. Bull. 145, 118–125. doi: 10.1016/j.marpolbul.2019.05.040
Kreher, S. A., Mathew, D., Kim, J., and Carlson, J. R. (2008). Translation of sensory input into behavioral output via an olfactory system. Neuron 59, 110–124. doi: 10.1016/j.neuron.2008.06.010
Kroeker, K. J., Kordas, R. L., Crim, R., Hendriks, I. E., Ramajo, L., Singh, G. S., et al. (2013). Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896. doi: 10.1111/gcb.12179
Kurihara, H., Matsui, M., Furukawa, H., Hayashi, M., and Ishimatsu, A. (2008). Long-term effects of predicted future seawater CO2 conditions on the survival and growth of the marine shrimp Palaemon pacificus. J. Exp. Mar. Biol. Ecol. 367, 41–46. doi: 10.1016/j.jembe.2008.08.016
Lacoue-Labarthe, T., Reveillac, E., Oberhänsli, F., Teyssié, J.-L., Jeffree, R., and Gattuso, J. (2011). Effects of ocean acidification on trace element accumulation in the early-life stages of squid Loligo vulgaris. Aquat. Toxicol. 105, 166–176. doi: 10.1016/j.aquatox.2011.05.021
Lai, F., Fagernes, C. E., Bernier, N. J., Miller, G. M., Munday, P. L., Jutfelt, F., et al. (2017). Responses of neurogenesis and neuroplasticity related genes to elevated CO2 levels in the brain of three teleost species. Biol. Lett. 13:20170240. doi: 10.1098/rsbl.2017.0240
Laming, P., Kimelberg, H., Robinson, S., Salm, A., Hawrylak, N., Müller, C., et al. (2000). Neuronal–glial interactions and behaviour. Neurosci. Biobehav. Rev. 24, 295–340.
Lee, V., and Maguire, J. (2014). The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circuits 8:3. doi: 10.3389/fncir.2014.00003
Levi, R., Varona, P., Arshavsky, Y. I., Rabinovich, M. I., and Selverston, A. I. (2004). Dual sensory-motor function for a molluskan statocyst network. J. Neurophysiol. 91, 336–345. doi: 10.1152/jn.00753.2003
Li, M., Qiu, L., Wang, L., Wang, W., Xin, L., Li, Y., et al. (2016a). The inhibitory role of γ-aminobutyric acid (GABA) on immunomodulation of Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 52, 16–22. doi: 10.1016/j.fsi.2016.03.015
Li, M., Wang, L., Qiu, L., Wang, W., Xin, L., Xu, J., et al. (2016b). A glutamic acid decarboxylase (CgGAD) highly expressed in hemocytes of Pacific oyster Crassostrea gigas. Dev. Comp. Immunol. 63, 56–65. doi: 10.1016/j.dci.2016.05.010
Li, W., and Gao, K. (2012). A marine secondary producer respires and feeds more in a high CO2 ocean. Mar. Pollut. Bull. 64, 699–703. doi: 10.1016/j.marpolbul.2012.01.033
Li, Y., Wu, L.-J., Legendre, P., and Xu, T.-L. (2003). Asymmetric cross-inhibition between GABAA and glycine receptors in rat spinal dorsal horn neurons. J. Biol. Chem. 278, 38637–38645. doi: 10.1074/jbc.M303735200
Lillis, A., Eggleston, D. B., and Bohnenstiehl, D. R. (2013). Oyster larvae settle in response to habitat-associated underwater sounds. PLoS One 8:e79337. doi: 10.1371/journal.pone.0079337
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640.
Liu, Y., Buchberger, A. R., DeLaney, K., Li, Z., and Li, L. (2019). Multifaceted mass spectrometric investigation of neuropeptide changes in Atlantic blue crab, Callinectes sapidus, in response to low pH stress. J. Proteome Res. 18, 2759–2770. doi: 10.1021/acs.jproteome.9b00026
Lummis, S. C. (1990). GABA receptors in insects. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 95, 1–8.
Maas, A. E., Lawson, G. L., and Tarrant, A. M. (2015). Transcriptome-wide analysis of the response of the thecosome pteropod Clio pyramidata to short-term CO2 exposure. Comp. Biochem. Physiol. Part D Genomics Proteomics 16, 1–9. doi: 10.1016/j.cbd.2015.06.002
Maneja, R., Piatkowski, U., and Melzner, F. (2011). Effects of ocean acidification on statolith calcification and prey capture in early life cuttlefish, Sepia officinalis. J. Shellfish Res. 30, 989–1023. doi: 10.2983/035.030.0342
Manríquez, P. H., Jara, M. E., Mardones, M. L., Navarro, J. M., Torres, R., Lardies, M. A., et al. (2013). Ocean acidification disrupts prey responses to predator cues but not net prey shell growth in Concholepas concholepas (loco). PLoS One 8:e68643. doi: 10.1371/journal.pone.0068643
Manríquez, P. H., Jara, M. E., Mardones, M. L., Torres, R., Navarro, J. M., Lardies, M. A., et al. (2014a). Ocean acidification affects predator avoidance behaviour but not prey detection in the early ontogeny of a keystone species. Mar. Ecol. Prog. Ser. 502, 157–167. doi: 10.3354/meps10703
Manríquez, P. H., Jara, M. E., Torres, R., Mardones, M. L., Lagos, N. A., Lardies, M. A., et al. (2014b). Effects of ocean acidification on larval development and early post-hatching traits in Concholepas concholepas (loco). Mar. Ecol. Prog. Ser. 514, 87–103. doi: 10.3354/meps10951
Marder, E., and Paupardin-Tritsch, D. (1978). The pharmacological properties of some crustacean neuronal acetylcholine, gamma-aminobutyric acid, and L-glutamate responses. J. Physiol. 280, 213–236. doi: 10.1113/jphysiol.1978.sp012381
Masson-Delmotte, V., Sculz, M., Abe-Ouchi, A., Beer, J., Ganopolski, A., González Rouco, J., et al. (2013). “Information from paleoclimate archives,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. Allen, J. Boschung, et al. (Cambridge: Cambridge University Press).
McCormick, M. I., Watson, S.-A., Simpson, S. D., and Allan, B. J. (2018). Effect of elevated CO2 and small boat noise on the kinematics of predator–prey interactions. Proc. R. Soc. B Biol. Sci. 285:20172650. doi: 10.1098/rspb.2017.2650
McNeil, B. I., Metzl, N., Key, R. M., Matear, R. J., and Corbiere, A. (2007). An empirical estimate of the Southern Ocean air-sea CO2 flux. Glob. Biogeochem. Cycles 21:GB3011. doi: 10.1029/2007GB002991
McNeil, B. I., and Sasse, T. P. (2016). Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle. Nature 529, 383–386. doi: 10.1038/nature16156
Menu-Courey, K., Noisette, F., Piedalue, S., Daoud, D., Blair, T., Blier, P. U., et al. (2018). Energy metabolism and survival of the juvenile recruits of the American lobster (Homarus americanus) exposed to a gradient of elevated seawater pCO2. Mar. Environ. Res. 143, 111–123. doi: 10.1016/j.marenvres.2018.10.002
Moody, W. Jr. (1981). The ionic mechanism of intracellular pH regulation in crayfish neurones. J. Physiol. 316, 293–308. doi: 10.1113/jphysiol.1981.sp013788
Mooney, T. A., Hanlon, R. T., Christensen-Dalsgaard, J., Madsen, P. T., Ketten, D. R., and Nachtigall, P. E. (2010). Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to low-frequency particle motion and not pressure. J. Exp. Biol. 213, 3748–3759. doi: 10.1242/jeb.048348
Morse, D. E., Duncan, H., Hooker, N., Baloun, A., and Young, G. (1980). GABA induces behavioral and developmental metamorphosis in planktonic molluscan larvae. Fed. Proc. 39, 3237–3241.
Morse, D. E., Hooker, N., Duncan, H., and Jensen, L. (1979). γ-Aminobutyric acid, a neurotransmitter, induces planktonic abalone larvae to settle and begin metamorphosis. Science 204, 407–410. doi: 10.1126/science.204.4391.407
Moya, A., Howes, E. L., Lacoue-Labarthe, T., Forêt, S., Hanna, B., Medina, M., et al. (2016). Near-future pH conditions severely impact calcification, metabolism and the nervous system in the pteropod Heliconoides inflatus. Glob. Change Biol. 22, 3888–3900. doi: 10.1111/gcb.13350
Mukherjee, J., Wong, K. K., Chandramouli, K. H., Qian, P.-Y., Leung, P. T., Wu, R. S., et al. (2013). Proteomic response of marine invertebrate larvae to ocean acidification and hypoxia during metamorphosis and calcification. The J. Exp. Biol. 216, 4580–4589. doi: 10.1242/jeb.094516
Munday, P. L., Dixson, D. L., Donelson, J. M., Jones, G. P., Pratchett, M. S., Devitsina, G. V., et al. (2009). Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. U.S.A. 106, 1848–1852. doi: 10.1073/pnas.0809996106
Munday, P. L., Jarrold, M. D., and Nagelkerken, I. (2019). “Ecological effects of elevated CO2 on marine and freshwater fishes: from individual to community effects,” in Carbon Dioxide, eds M. Grosell, P. L. Munday, A. P. Farrell, and C. J. Brauner (San Diego, CA: Academic Press), 323–368.
Nagelkerken, I., Doney, S. C., and Munday, P. (2019). “Consequences of anthropogenic changes in the sensory landscape of marine animals,” in Oceanography and Marine Biology: An Annual Review, eds S. J. Hawkins, A. L. Allcock, A. E. Bates, and L. B. Firth I P. Smith, S. E. Swearer, et al. (London: CRC Press).
Nagelkerken, I., and Munday, P. L. (2015). Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob. Change Biol. 22, 974–989. doi: 10.1111/gcb.13167
Nakajima, K.-I., and Marunaka, Y. (2016). Intracellular chloride ion concentration in differentiating neuronal cell and its role in growing neurite. Biochem. Biophys. Res. Commun. 479, 338–342. doi: 10.1016/j.bbrc.2016.09.075
Narusuye, K., Nakao, T., Abe, R., Nagatomi, Y., Hirase, K., and Ozoe, Y. (2007). Molecular cloning of a GABA receptor subunit from Laodelphax striatella (Fallén) and patch clamp analysis of the homo-oligomeric receptors expressed in a Drosophila cell line. Insect Mol. Biol. 16, 723–733. doi: 10.1111/j.1365-2583.2007.00766.x
Nelson, K. S., Baltar, F., Lamare, M. D., and Morales, S. E. (2020). Ocean acidification affects microbial community and invertebrate settlement on biofilms. Sci. Rep. 10:3274. doi: 10.1038/s41598-020-60023-4
Nezlin, L., and Voronezhskaya, E. (1997). GABA-immunoreactive neurones and interactions of GABA with serotonin and FMRFamide in a peripheral sensory ganglion of the pond snail Lymnaea stagnalis. Brain Res. 772, 217–225. doi: 10.1016/s0006-8993(97)00835-4
Nguyen, T. V., Alfaro, A. C., Young, T., Ravi, S., and Merien, F. (2018). Metabolomics study of immune responses of New Zealand greenshellTM mussels (Perna canaliculus) infected with pathogenic Vibrio sp. Mar. Biotechnol. 20, 396–409. doi: 10.1007/s10126-018-9804-x
Nilsson, G. E., Dixson, D. L., Domenici, P., McCormick, M. I., Sørensen, C., Watson, S.-A., et al. (2012). Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Change 2, 201–204. doi: 10.1038/NCLIMATE1352
Nilsson, G. E., and Lefevre, S. (2016). Physiological challenges to fishes in a warmer and acidified future. Physiology 31, 409–417. doi: 10.1152/physiol.00055.2015
Norekian, T. P., and Malyshev, A. Y. (2005). Coordinated excitatory effect of GABAergic interneurons on three feeding motor programs in the mollusk Clione limacina. J. Neurophysiol. 93, 305–315. doi: 10.1152/jn.00722.2004
Norekian, T. P., and Satterlie, R. A. (1993). FMRFamide and GABA produce functionally opposite effects on prey-capture reactions in the pteropod mollusk Clione limacina. Biol. Bull. 185, 248–262. doi: 10.2307/1542005
Olsen, K., Paul, V. J., and Ross, C. (2015). Direct effects of elevated temperature, reduced pH, and the presence of macroalgae (Dictyota spp.) on larvae of the Caribbean coral Porites astreoides. Bull. Mar. Sci. 91, 255–270. doi: 10.5343/bms.2014.1050
Olsen, R. W., and Sieghart, W. (2008). International union of pharmacology. LXX. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 60, 243–260. doi: 10.1124/pr.108.00505
Orr, J. C., Fabry, V. J., Aumont, O., Bopp, L., Doney, S. C., Feely, R. A., et al. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686. doi: 10.1038/nature04095
Owens, D. F., and Kriegstein, A. R. (2002). Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 3, 715–727. doi: 10.1038/nrn919
Padilla-Gamiño, J. L., Kelly, M. W., Evans, T. G., and Hofmann, G. E. (2013). Temperature and CO2 additively regulate physiology, morphology and genomic responses of larval sea urchins, Strongylocentrotus purpuratus. Proc. R. Soc. Lond. B Biol. Sci. 280:20130155. doi: 10.1098/rspb.2013.0155
Palmer, C. R., and Kristan, W. B. Jr. (2011). Contextual modulation of behavioral choice. Curr. Opin. Neurobiol. 21, 520–526. doi: 10.1016/j.conb.2011.05.003
Pearce, C. M., and Scheibling, R. E. (1990). Induction of metamorphosis of larvae of the green sea urchin, Strongylocentrotus droebachiensis, by coralline red algae. Biol. Bull. 179, 304–311. doi: 10.2307/1542322
Pearstein, E., Cattaert, D., and Clarac, F. (1996). Crayfish sensory terminals and motor neurones exhibit two distinct types of GABA receptors. J. Comp. Physiol. A 180, 71–79.
Pecquet, A., Dorey, N., and Chan, K. Y. K. (2017). Ocean acidification increases larval swimming speed and has limited effects on spawning and settlement of a robust fouling bryozoan, Bugula neritina. Mar. Pollut. Bull. 124, 903–910. doi: 10.1016/j.marpolbul.2017.02.057
Peng, C., Zhao, X., Liu, S., Shi, W., Han, Y., Guo, C., et al. (2017). Ocean acidification alters the burrowing behaviour, Ca2+/Mg2+-ATPase activity, metabolism, and gene expression of a bivalve species, Sinonovacula constricta. Mar. Ecol. Prog. Ser. 575, 107–117. doi: 10.3354/meps12224
Pierobon, P., Concas, A., Santoro, G., Marino, G., Minei, R., Pannaccione, A., et al. (1995). Biochemical and functional identification of GABA receptors in Hydra vulgaris. Life Sci. 56, 1485–1497. doi: 10.1016/0024-3205(95)00111-i
Pierobon, P., Tino, A., Minei, R., and Marino, G. (2004). Different roles of GABA and glycine in the modulation of chemosensory responses in Hydra vulgaris (Cnidaria, Hydrozoa). Hydrobiologia 530, 59–66.
Popper, A. N., Salmon, M., and Horch, K. W. (2001). Acoustic detection and communication by decapod crustaceans. J. Comp. Physiol. A 187, 83–89. doi: 10.1007/s003590100184
Porteus, C. S., Hubbard, P. C., Webster, T. M. U., van Aerle, R., Canário, A. V., Santos, E. M., et al. (2018). Near-future CO2 levels impair the olfactory system of a marine fish. Nat. Clim. Change 8, 737–743. doi: 10.1038/s41558-018-0224-8
Pörtner, H.-O., Bock, C., and Reipschlager, A. (2000). Modulation of the cost of pHi regulation during metabolic depression: a 31P-NMR study in invertebrate (Sipunculus nudus) isolated muscle. J. Exp. Biol. 203, 2417–2428.
Pörtner, H.-O., Langenbuch, M., and Reipschläger, A. (2004). Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718. doi: 10.1007/s10872-004-5763-0
Pörtner, H.-O., Reipschläger, A., and Heisler, N. (1998). Acid-base regulation, metabolism and energetics in Sipunculus nudus as a function of ambient carbon dioxide level. J. Exp. Biol. 201, 43–55.
Queirós, A. M., Fernandes, J. A., Faulwetter, S., Nunes, J., Rastrick, S. P., Mieszkowska, N., et al. (2015). Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Glob. Change Biol. 21, 130–143. doi: 10.1111/gcb.12675
Rauh, J. J., Lummis, S. C., and Sattelle, D. B. (1990). Pharmacological and biochemical properties of insect GABA receptors. Trends Pharmacol. Sci. 11, 325–329. doi: 10.1016/0165-6147(90)90236-2
Ren, Z., Mu, C., Li, R., Song, W., and Wang, C. (2018). Characterization of a γ-aminobutyrate type A receptor-associated protein gene, which is involved in the response of Portunus trituberculatus to CO2-induced ocean acidification. Aquac. Res. 49, 2393–2403. doi: 10.1111/are.13699
Rich, W. A., Schubert, N., Schläpfer, N., Carvalho, V. F., Horta, A. C., and Horta, P. A. (2018). Physiological and biochemical responses of a coralline alga and a sea urchin to climate change: implications for herbivory. Mar. Environ. Res. 142, 100–107. doi: 10.1016/j.marenvres.2018.09.026
Ries, J. B., Cohen, A. L., and McCorkle, D. C. (2009). Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134. doi: 10.1130/G30210A.1
Roggatz, C. C., Lorch, M., Hardege, J. D., and Benoit, D. M. (2016). Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules. Glob. Change Biol. 22, 3914–3926. doi: 10.1111/gcb.13354
Romanova, E. V., Rubakhin, S., and S-rózsa, K. (1996). Behavioral changes induced by GABA-receptor agonists in Lymnaea stagnalis L. Gen. Pharmacol. 27, 1067–1071. doi: 10.1016/0306-3623(95)00122-0
Rosa, R., and Seibel, B. A. (2008). Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc. Natl. Acad. Sci. U.S.A. 105, 20776–20780. doi: 10.1073/pnas.0806886105
Rossi, T., Connell, S. D., and Nagelkerken, I. (2016). Silent oceans: ocean acidification impoverishes natural soundscapes by altering sound production of the world’s noisiest marine invertebrate. Proc. R. Soc. B Biol. Sci. 283:20153046. doi: 10.1098/rspb.2015.3046
Russell, J. M., and Boron, W. F. (1976). Role of chloride transport in regulation of intracellular pH. Nature 264, 73–74. doi: 10.1038/264073a0
Saba, G. K., Schofield, O., Torres, J. J., Ombres, E. H., and Steinberg, D. K. (2012). Increased feeding and nutrient excretion of adult Antarctic krill, Euphausia superba, exposed to enhanced carbon dioxide (CO2). PLoS One 7:e52224. doi: 10.1371/journal.pone.0052224
Santos, I. R., Glud, R. N., Maher, D., Erler, D., and Eyre, B. D. (2011). Diel coral reef acidification driven by porewater advection in permeable carbonate sands, Heron Island, Great Barrier Reef. Geophys. Res. Lett. 38:L03604. doi: 10.1029/2010GL046053
Satoh, H., Daido, H., and Nakamura, T. (2005). Preliminary analysis of the GABA-induced current in cultured CNS neurons of the cutworm moth, Spodoptera litura. Neurosci. Lett. 381, 125–130. doi: 10.1016/j.neulet.2005.02.007
Schmitt, B. C., and Ache, B. W. (1979). Olfaction: responses of a decapod crustacean are enhanced by flicking. Science 205, 204–206. doi: 10.1126/science.205.4402.204
Schunter, C., Welch, M. J., Nilsson, G. E., Rummer, J. L., Munday, P. L., and Ravasi, T. (2018). An interplay between plasticity and parental phenotype determines impacts of ocean acidification on a reef fish. Nat. Ecol. Evol. 2, 334–342. doi: 10.1038/s41559-017-0428-8
Schunter, C., Welch, M. J., Ryu, T., Zhang, H., Berumen, M. L., Nilsson, G. E., et al. (2016). Molecular signatures of transgenerational response to ocean acidification in a species of reef fish. Nat. Clim. Change 6, 1014–1018. doi: 10.1038/NCLIMATE3087
Sernagor, E., Chabrol, F., Bony, G., and Cancedda, L. (2010). GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Front. Cell. Neurosci. 4:11. doi: 10.3389/fncel.2010.00011
Shaw, E. C., McNeil, B. I., and Tilbrook, B. (2012). Impacts of ocean acidification in naturally variable coral reef flat ecosystems. J. Geophys. Res. Oceans 117:C03038. doi: 10.1029/2011JC007655
Shaw, E. C., Mcneil, B. I., Tilbrook, B., Matear, R., and Bates, M. L. (2013). Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob. Change Biol. 19, 1632–1641. doi: 10.1111/gcb.12154
Shelley, C., Dyar, A., and Schieber, M. (2019). Neurotransmitter receptor antagonism inhibits the sea urchin righting response. FASEB J. 33:849.3.
Sieghart, W. (2015). “Allosteric modulation of GABAA receptors via multiple drug-binding sites,” in Advances in Pharmacology, ed. U. Rudolph (San Diego, CA: Academic Press), 53–96. doi: 10.1016/bs.apha.2014.10.002
Simmons, P., and Young, D. (1999). Nerve Cells and Animal Behaviour. Cambridge: Cambridge University Press.
Spady, B. L., Munday, P. L., and Watson, S. A. (2018). Predatory strategies and behaviours in cephalopods are altered by elevated CO2. Glob. Change Biol. 24, 2585–2596. doi: 10.1111/gcb.14098
Spady, B. L., Watson, S.-A., Chase, T. J., and Munday, P. L. (2014). Projected near-future CO2 levels increase activity and alter defensive behaviours in the tropical squid Idiosepius pygmaeus. Biol. Open 3, 1063–1070. doi: 10.1242/bio.20149894
Spangenberg, D. (1986). Statolith formation in Cnidaria: effects of cadmium on Aurelia statoliths. Scan. Electron Microsc. 1986, 1609–1616.
Stanley, J. A., Radford, C. A., and Jeffs, A. G. (2009). Induction of settlement in crab megalopae by ambient underwater reef sound. Behav. Ecol. 21, 113–120. doi: 10.1093/beheco/arp159
Stewart, P., Williams, E. A., Stewart, M. J., Soonklang, N., Degnan, S. M., Cummins, S. F., et al. (2011). Characterization of a GABAA receptor β subunit in the abalone Haliotis asinina that is upregulated during larval development. J. Exp. Mar. Biol. Ecol. 410, 53–60. doi: 10.1016/j.jembe.2011.10.005
Succol, F., Fiumelli, H., Benfenati, F., Cancedda, L., and Barberis, A. (2012). Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat. Commun. 3:738. doi: 10.1038/ncomms1744
Svensson, E., Proekt, A., Jing, J., and Weiss, K. R. (2014). PKC-mediated GABAergic enhancement of dopaminergic responses: implication for short-term potentiation at a dual-transmitter synapse. J. Neurophysiol. 112, 22–29. doi: 10.1152/jn.00794.2013
Thabet, A. A., Maas, A. E., Saber, S. A., and Tarrant, A. M. (2017). Assembly of a reference transcriptome for the gymnosome pteropod Clione limacina and profiling responses to short-term CO2 exposure. Mar. Genomics 34, 39–45. doi: 10.1016/j.margen.2017.03.003
Tierney, A. J., and Atema, T. (1988). Amino acid chemoreception: effects of pH on receptors and stimuli. J. Chem. Ecol. 14, 135–141. doi: 10.1007/BF01022537
Timmins-Schiffman, E., Guzmán, J. M., Thompson, R. E., Vadopalas, B., Eudeline, B., and Roberts, S. B. (2019). Dynamic response in the larval geoduck (Panopea generosa) proteome to elevated pCO2. Ecol. Evol. 10, 185–197. doi: 10.1002/ece3.5885
Todgham, A. E., and Hofmann, G. E. (2008). Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J. Exp. Biol. 212, 2579–2594. doi: 10.1242/jeb.032540
Toulmé, E., Blais, D., Léger, C., Landry, M., Garret, M., Séguéla, P., et al. (2007). An intracellular motif of P2X3 receptors is required for functional cross-talk with GABAA receptors in nociceptive DRG neurons. J. Neurochem. 102, 1357–1368. doi: 10.1111/j.1471-4159.2007.04640.x
Tovar, K. R., and Westbrook, G. L. (2012). “Ligand-gated ion channels,” in Cell Physiology Source Book, ed. N. Sperelakis (San Diego, CA: Academic Press), 549–562.
Tresguerres, M., and Hamilton, T. J. (2017). Acid–base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 220, 2136–2148. doi: 10.1242/jeb.144113
Trigg, S. A., McElhany, P., Maher, M., Perez, D., Busch, D. S., and Nichols, K. M. (2019). Uncovering mechanisms of global ocean change effects on the Dungeness crab (Cancer magister) through metabolomics analysis. Sci. Rep. 9:10717. doi: 10.1038/s41598-019-46947-6
Trombley, P. Q., Hill, B. J., and Horning, M. S. (1999). Interactions between GABA and glycine at inhibitory amino acid receptors on rat olfactory bulb neurons. J. Neurophysiol. 82, 3417–3422. doi: 10.1152/jn.1999.82.6.3417
Truchot, J. (1983). “Regulation of acid–base balance,” in The Biology of Crustacea. Internal Anatomy and Physiological Regulation, ed. L. Mantel (New York, NY: Academic Press), 431–457.
Tsang, S.-Y., Ng, S.-K., Xu, Z., and Xue, H. (2006). The evolution of GABAA receptor–like genes. Mol. Biol. Evol. 24, 599–610. doi: 10.1093/molbev/msl188
Valdivieso, Á. G., and Santa-Coloma, T. A. (2019). The chloride anion as a signalling effector. Biol. Rev. 94, 1839–1856. doi: 10.1111/brv.12536
Vargas, C. A., Aguilera, V. M., San Martín, V., Manríquez, P. H., Navarro, J. M., Duarte, C., et al. (2014). CO2-driven ocean acidification disrupts the filter feeding behavior in Chilean gastropod and bivalve species from different geographic localities. Estuaries Coast. 38, 1163–1177. doi: 10.1007/s12237-014-9873-7
Vassilatis, D. K., Elliston, K. O., Paress, P. S., Hamelin, M., Arena, J. P., Schaeffer, J. M., et al. (1997). Evolutionary relationship of the ligand-gated ion channels and the avermectin-sensitive, glutamate-gated chloride channels. J. Mol. Evol. 44, 501–508. doi: 10.1007/PL00006174
Vermeij, M. J., Marhaver, K. L., Huijbers, C. M., Nagelkerken, I., and Simpson, S. D. (2010). Coral larvae move toward reef sounds. PLoS One 5:e10660. doi: 10.1371/journal.pone.0010660
Viyakarn, V., Lalitpattarakit, W., Chinfak, N., Jandang, S., Kuanui, P., Khokiattiwong, S., et al. (2015). Effect of lower pH on settlement and development of coral, Pocillopora damicornis (Linnaeus, 1758). Ocean Sci. J. 50, 475–480. doi: 10.1007/s12601-015-0043-z
Walsh, P. J., and Milligan, C. L. (1989). Coordination of metabolism and intracellular acid–base status: ionic regulation and metabolic consequences. Can. J. Zool. 67, 2994–3004. doi: 10.1139/z89-422
Wang, T., and Wang, Y. (2019). Behavioral responses to ocean acidification in marine invertebrates: new insights and future directions. J. Oceanol. Limnol. (in press). doi: 10.1007/s00343-019-9118-5
Wang, Y., Hu, M., Wu, F., Storch, D., and Pörtner, H.-O. (2018). Elevated pCO2 affects feeding behavior and acute physiological response of the brown crab Cancer pagurus. Front. Physiol. 9:1164. doi: 10.3389/fphys.2018.01164
Watson, S.-A., Lefevre, S., McCormick, M. I., Domenici, P., Nilsson, G. E., and Munday, P. L. (2014). Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proc. R. Soc. Lond. B Biol. Sci. 281:20132377. doi: 10.1098/rspb.2013.2377
Wheatly, M. G., and Henry, R. P. (1992). Extracellular and intracellular acid-base regulation in crustaceans. J. Exp. Zool. 263, 127–142. doi: 10.1002/jez.1402630204
Williams, C. R., Dittman, A. H., McElhany, P., Busch, D. S., Maher, M. T., Bammler, T. K., et al. (2019). Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob. Change Biol. 25, 963–977. doi: 10.1111/gcb.14532
Wong, K. K., Lane, A. C., Leung, P. T., and Thiyagarajan, V. (2011). Response of larval barnacle proteome to CO2-driven seawater acidification. Comp. Biochem. Physiol. Part D Genomics Proteomics 6, 310–321. doi: 10.1016/j.cbd.2011.07.001
Wu, C., Qin, X., Du, H., Li, N., Ren, W., and Peng, Y. (2017). The immunological function of GABAergic system. Front. Biosci. 22, 1162–1172.
Yamoah, E., and Kuzirian, A. M. (1994). Effects of GABA on outward currents in Hermissenda photoreceptors. Biol. Bull. 187, 265–266. doi: 10.1086/BBLv187n2p265
Yarowsky, J., and Carpenter, D. (1978). A comparison of similar ionic responses to gamma-aminobutyric acid and acetylcholine. J. Neurophysiol. 41, 531–541. doi: 10.1152/jn.1978.41.3.531
Yuan, X., Yuan, T., Huang, H., Jiang, L., Zhou, W., and Liu, S. (2018). Elevated CO2 delays the early development of scleractinian coral Acropora gemmifera. Sci. Rep. 8:2787. doi: 10.1038/s41598-018-21267-3
Zakroff, C., Mooney, T. A., and Berumen, M. L. (2019). Dose-dependence and small-scale variability in responses to ocean acidification during squid, Doryteuthis pealeii, development. Mar. Biol. 166:62. doi: 10.1007/s00227-019-3510-8
Zakroff, C., Mooney, T. A., and Wirth, C. (2018). Ocean acidification responses in paralarval squid swimming behavior using a novel 3D tracking system. Hydrobiologia 808, 83–106. doi: 10.1007/s10750-017-3342-9
Zhang, Y., Shi, F., Song, J., Zhang, X., and Yu, S. (2015). Hearing characteristics of cephalopods: modeling and environmental impact study. Integr. Zool. 10, 141–151. doi: 10.1111/1749-4877.12104
Keywords: marine invertebrate, behavior, carbon dioxide, ocean acidification, GABA, ligand-gated ion channel, mechanisms, GABAA receptor
Citation: Thomas JT, Munday PL and Watson S-A (2020) Toward a Mechanistic Understanding of Marine Invertebrate Behavior at Elevated CO2. Front. Mar. Sci. 7:345. doi: 10.3389/fmars.2020.00345
Received: 18 December 2019; Accepted: 23 April 2020;
Published: 29 May 2020.
Edited by:
Maria Lourdes D. Palomares, The University of British Columbia, CanadaReviewed by:
Anwesha Ghosh, Indian Institute of Science Education and Research Kolkata, IndiaThiago Mendes, Federal University of São Paulo, Brazil
Copyright © 2020 Thomas, Munday and Watson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jodi T. Thomas, am9kaS50aG9tYXNAbXkuamN1LmVkdS5hdQ==
†ORCID: Jodi T. Thomas, orcid.org/0000-0002-7622-4479; Philip L. Munday, orcid.org/0000-0001-9725-2498; Sue-Ann Watson, orcid.org/0000-0002-9818-7429
 Jodi T. Thomas
Jodi T. Thomas Philip L. Munday
Philip L. Munday Sue-Ann Watson
Sue-Ann Watson