
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci. , 07 May 2020
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00251
Number of attempts have been made to cryopreserve fish and shellfish gametes. Success has been achieved to establish only sperm banks in case of some commercially important fish. In shellfish, also particularly in shrimps, though the sperm cryopreservation was successful, no attempts were made to develop sperm banks. As far as cryopreservation of egg and embryos of fish and shellfish is concerned, less research efforts were made with limited success. Number of reasons have been given for the failure of egg/embryo cryopreservation and the main barriers speculated are low membrane permeability in the eggs, the large yolk mass of the oocyte, and the presence of compartments in early developing embryos. These factors result in ice crystal formation during the freezing process. In addition, the oocytes and embryos are prone to chilling injuries unrelated to ice crystal damage. There are number of other problems reported by several researchers in the egg/embryo cryobanking protocols which are elaborately discussed in the present paper. There is an urgent need to develop a viable cryobanking technology for fish egg/embryos to enhance fish production in captive condition. Attempts to cryopreserve larvae of aquatic animals is another challenge occurring in the recent past. The aim of the present review is to collect comprehensive information on the efforts so far made on fish and shellfish egg and embryo cryobanking; and to assess the challenges in the development of viable technology and plan for future research for making this technology viable and cost effective.
In order to enhance fish stocking through aquaculture technologies and reduce the dependency on wild stock for fish production, cryobanking of egg and embryos becomes a very important. Successful cryobanking of egg and embryos will support not only the increasing the fish production, but also help the restoration of important genetic resources and create an opportunity to ensure species survival and possible restocking ability. Whilst the cryobanking of fish sperm has been relatively successful, the cryobanking of egg and embryos has not achieved much success. Two main reasons in cryobanking technology have been speculated i.e., low membrane permeability of cryoprotective agents in the egg and embryos and the presence of a large yolk mass which results in an ice crystal formation during the freezing process. In addition, the oocytes and embryos are prone to chilling injuries under extreme low temperatures. At present, research on fish egg/embryo cryobanking is currently focused on the three new important aspects, i.e., permeability of the cryoprotectants in the egg/embryo membranes through media modification and ultra-sound treatment, direct modification of the yolk mass by micro-manipulation and the use of impedance spectroscopy for rapid assessment of embryo membrane permeability (Rawson and Zhang, 2005).
Studies on cryopreservation of fish egg/embryos have been carried out by number of researchers but due to multi-compartmental arrangements in the structure of the egg and the embryos, the cryopreservation was not successful (Kimmel and Law, 1985; Zhang and Rawson, 1993, 1995a,b, 1996a,b; Hagedorn et al., 1996, 1997, 1998; Harvey, 1983; Harvey et al., 1983; Suzuki et al., 1995; Zhang et al., 1998; Chao and Liao, 2001; Zhang et al., 2005a, b, 2007; Zhang Y.Z. et al., 2005; Diwan et al., 2010). Among several cryoprotectants used, it has been reported that methanol was the most effective than either DMSO or ethanediol for zebra fish embryos (Zhang et al., 1993). It has been observed that methanol penetrated the entire embryo within 15 min and other cryoprotectants exhibited little or no penetration into yolk even after 2.5 h (Hagedorn et al., 1997). In zebra fish embryo, the permeability of the methanol appeared to decrease during embryo development at 22°C (Zhang and Rawson, 1998). The use of ultrasound technique has been reported to enhance the embryo permeability in zebra fish (Bart, 2000). However, methanol was demonstrated to show a limited degree of penetration into prim-6 stage of zebra fish embryos, but it did not penetrate in the subsequent stages (Liu et al., 2000). Aril et al. (1987) however, observed that DMSO is a good cryoprotectant for medaka (Oryzias latipes) fish embryos. In common carp, the morulae stage embryos are found to be partially protected against chilling in DMSO and sucrose mixture, half-epiboly in DMSO, sucrose and methanol solution and heart beat stage in methanol and glycerol solution (Dinnyes et al., 1998). Using isotope labeled DMSO and glycerol, it has been found that these solutes penetrated into both dichorionated and intact zebra fish embryos after a duration of 5 h Harvey et al. (1983). Ahammad et al. (2003b) while working on the hatching of common carp (Cyprinus carpio) embryos stored at 4 and –2°C in different concentrations of methanol and sucrose, found that the hatching performance was at its maximum (41%) in sucrose at 4°C. No survival was observed at –2°C in any concentration of sucrose solutions. Further, it was reported that the combination of methanol and sucrose produced best results among all concentrations tested at both temperatures. Same researchers (Ahammad et al., 2002, 2003a,b, 2004) while studying the hatching responses of rohu embryos at different concentrations of cryoprotectants and temperatures, reported that the hatching performance of embryos stored at –4°C temperature in combination of methanol and trehalose showed the highest percentage of hatch out (72%). Furthermore, it was mentioned that cryopreservation of pluripotent blastomere and the grafting of thawed cells into recipient embryos has opened an alternate pathway for the production of improved strains of fish and shellfish (Tsai and Lin, 2012). Tsai and Lin (2012) while discussing advantages and applications of cryopreservation in fish and shellfish reported that successful attempts have been made on cryopreservation of fish sperm for more than 200 fish species for the purpose of developing sperm cryobanks. However, it is further mentioned that cryopreservation of fish egg/embryos could not be made successful because of high chilling sensitivity and low membrane permeability. They further suggested that the cryopreservation of an isolated embryonic cell is one of the options to preserve both maternal and paternal genome. Relatively, attempts to cryopreserve invertebrates, especially crustacean egg, embryos and larvae, have been more successful than the finfish (Diwan et al., 2010). For example in penaeid shrimp, the successful survival of thawed egg and larvae have been reported up to freezing temperature of –10°C (Diwan and Kandasami, 1997; Diwan, 2000).
Although sperm cryopreservation has been carried out successfully in a number of commercial important aquatic species, particularly in some teleost fish (Muir and Roberts, 1993) and also shellfish (Subramoniam and Newton, 1993; Diwan and Joseph, 1998), the technology is still not at the stage of advanced commercial application that is seen in domestic mammals. Asahina and Takahashi (1978) made the first successful attempt on the cryopreservation of sea urchin egg and embryos. Later Zell (1978) and Erdahl and Graham (1980) carried out the work on cryopreservation of the eggs of rainbow trout. In subsequent years, efforts have been made to cryopreserve the embryos of Japanese medaka fish, Oryzias letipes (Aril et al., 1987), rainbow trout, Oncorhynchus mykiss (Nilsson and Cloud, 1993), and zebra fish Brachydanio rerio (Zhang et al., 1993). During the same period, some attempts have also been made to cryopreserve the embryos and nauplii of shrimp, Penaeus indicus (Subramoniam and Newton, 1993; Simon et al., 1994; Subramoniam, 1994). Subramoniam and Newton (1993) were the first to successfully preserve nauplii of P. indicus. They held them at −30°C using liquid nitrogen and reported survival of 82% for nauplii frozen to −30°C and 63% at −40°C. Diwan and Kandasami (1997) also reported success of freezing viable embryos and larvae of shrimp Penaeus semisulcatus.
Number of researchers have pointed out the difficulties that they have encountered while doing cryrobanking studies of fish and shellfish egg/embryos and also the oocytes at their different developmental stages. In spite of many efforts, cryobanking of fish egg/embryos constantly failed because of the presence of large amounts of yolk. Rall (1993) while discussing the cryopreservation of salmonids fishes mentioned that there are five major hindrances in the cryopreservation of egg and embryos of teleost fishes.
1. The size of fish egg and embryos is very large and divided by multiple compartments. This makes the embryos difficult to cool and warm uniformly without damage and ice formation.
2. Large size of the egg and embryos results in low surface/volume ratio and lower membrane permeability to water and cryoprotectant solutions.
3. Because of the presence of multi-layered membrane structure, it hinders the osmotic properties for each compartment of the egg/embryos which finally affects the transport of the cryoprotectant solutions.
4. Due to the presence of chorionic layer, there is a low permeability of the membrane.
5. The fish egg and embryos are very sensitive to low temperatures, particularly at sub-zero degrees.
In order to address these issues, a number of studies have been carried out by different researchers. Among several efforts, one of the attempts was on the microinjection of cryoprotectants directly into the cytoplasm (Leung and Jamieson, 1991). Another attempt was the use of negative pressure on the egg/embryos to increase permeability of the cryoprotectants (Routray et al., 2002). Trials were also made on the microinjection of anti-freeze protein (Robles et al., 2006) and some of the researchers even tried the application of hydrostatic pressure on the egg/embryos (Zhang and Rawson, 1995; Hagedorn et al., 1997; Dinnyes et al., 1998; Zhang et al., 2003a, b; Valdez et al., 2005). It has been reported that in spite of all these efforts, success could not be achieved in developing viable technology for the cryobanking of egg/embryos. It is emphasized that for successful cryopreservation of egg/embryos, precise knowledge of embryo permeability would be one of the major factors which is most essential (Danilo et al., 2014). There are also reports that the microinjection of cryoprotectants and laser irradiation for rewarming has shown promise toward achieving this long sought goal. It is also reported that some researchers have tried by injecting cryoprotectants along with plasmonic gold nanoparticles into egg/embryos of the zebra fish. Such embryos with plasmonic gold nanoparticles were later plunged into liquid nitrogen for few seconds, then removed, and immediately exposed to the laser irradiation to heat up the nanoparticles which were uniformly distributed inside the embryos at an ultra-fast rate. Many of the cryopreserved embryos with plasmonic gold nanoparticles were revived and the development process continued until they started to wiggle (American Chemical Society (ACS) News, 2017; Figure 1).
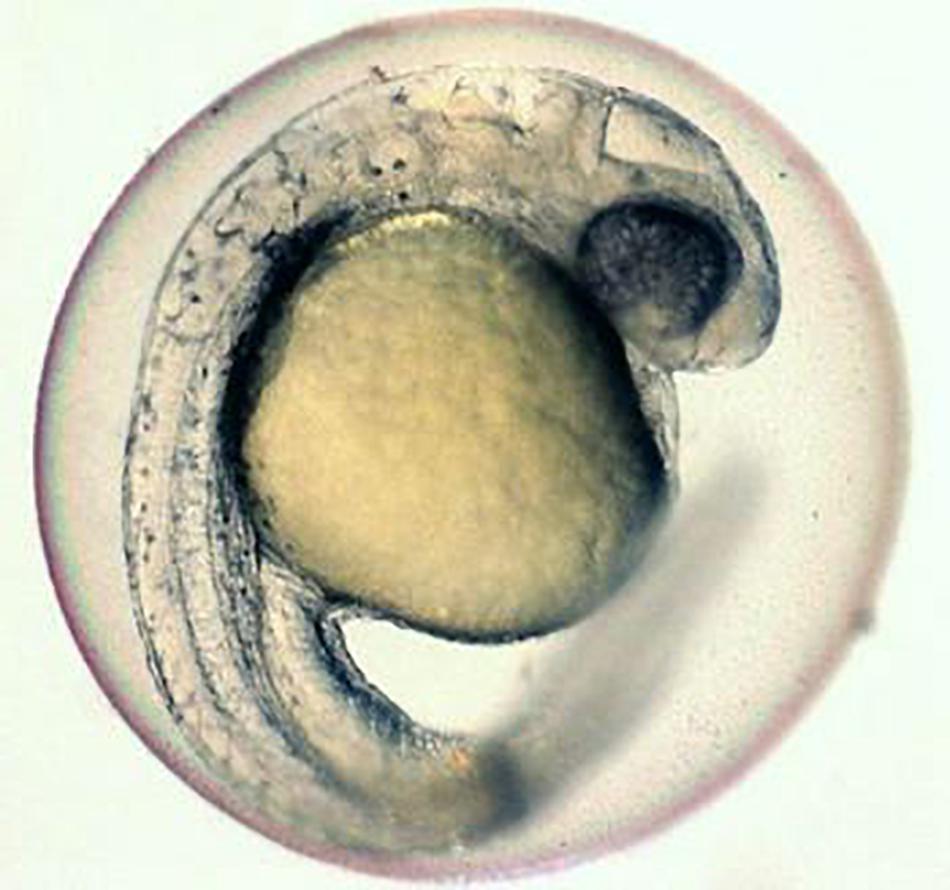
Figure 1. Zebra fish embryo cryopreservation by using gold nano-particles and laser. Source: (University of Guelph published in ACS Nano).
In spite of number efforts made so far, cryobanking of eggs and embryos of aquatic animals has really not received an appreciable amount of attention. Attempts to cryopreserve shrimp egg and embryos have not been successful, although standard protocols for several mammalian embryos are available. In shrimp, though the size of their eggs and embryos is small, the eggs have a tendency to absorb water soon after their release, they swell and become activated for fertilization. After fertilization, a strong hatching envelope forms around the egg (Seymour, 1994). Therefore, the presence of water and the thick protective envelope surrounding the eggs are some of the disadvantages for successful freezing of viable eggs and embryos (Clark and Griffin, 1993). It has been also reported that the large volume of absorbed water present in the eggs and embryos tends to develop crystals while freezing and damages the internal parts (Simon et al., 1994). According to Martinez et al. (2016) in the cryobanking studies of shellfish egg and embryos, there are three very important aspects. First, the assessment of embryonic and larval survival, second, the survival of the embryo after the thawing process, and third, the long term rearing performance of the thawed larvae.
In order to address the issue of permeability of cryoprotectants into the egg while freezing at low temperatures, it is essential to know the composition of the layers surrounding the egg. As far as the structure of the fish egg is concerned, considerable literature is available attempting both histological, as well as electron microscopical studies (Guraya, 1986). Cotelli et al. (1988) worked on the structure and composition of the chorionic layer of fish (Carassius auratus) egg and reported the presence of three layers of chorion surrounding the matured egg starting from the outer side to the inner side close to the egg plasma membrane. Further, they studied the biochemical composition of these chorionic layers using gel electrophoresis technique. It is mentioned that all these three coats of chorionic layers have different functional properties and these properties have been studied in details by different researchers (Bleil and Wassarman, 1980; Rosati et al., 1982). Rawson et al. (1997, 2000) and Kalicharan et al. (1997) studied the chorion, plasma membrane and syncytial layers of the gastrula stage embryos of the zebra fish, B. rerio to find out the functional and structural relationship, with respect to cryoprotectant penetration. These studies were carried out by using field emission scanning electron microscopy. The study revealed that at the gastrula stage developing embryo has outer chorionic layer which is thick and consists of three sub-layers, i.e., electron dense outer layer, the inner most layer, and in-between these two layers, there is another layer which is electron lucent mid layer. They observed that the middle and inner layer are pierced by the pore canal. Ninhaus et al. (2008) while studying the effect of freezing temperatures on the structural composition of the egg/embryos of the fish, Prochilodus lineatus, observed that the nuclei in the eggs have a distorted chromatin with the cracks in the plasma membrane and microvilli causing the death of the developing embryos. Similarly Neves et al. (2012), while investing the effect of injuries on the embryos of fish, Piaractus mesopotamicus, after freezing and thawing, found that the chorionic layer was broken and blastoderm stage in the developing embryo was missing. While studying the vitrification impact on the embryos of the fish Colossoma macropomum, Fornari et al. (2012) reported that by using a mixture of methanol, DMSO, ethylene glycol and glycerol at concentration of 10, 20, and 30% there was no damage to the chorionic layers and some cellular structures were found to be intact and well preserved. Digmayer (2013) studied the structure of the oocytes of fish C. macropomum after exposing to the cryo-solutions containing 1.6 M methanol and two concentrations of non-permeating cryoprotectants (glucose, sucrose, trehalose and fructose) using slow cooling protocols following storage in liquid nitrogen. It is reported that after the thawing process, the oocytes preserved in methanol showed that the layer of zona radiate was intact but there was a displacement of this structure from the oocyte membrane. These studies indicate that by modifying the cryosolutions and following proper cooling rate protocol, it is possible to preserve the composition of the egg structure.
In case of shell fish particularly in crustaceans, the fertilized egg is surrounded by two coats, outer and inner, and the inner coat originates from the ovary. This kind of structure has been reported in H. americanus and H. vulgaris (Yonge, 1937) and Crangon vulgaris (Yonge, 1955). Cheung (1966) has described the outermost coat of the fertilized oocyte of the crab, Carcinus maenas, and noted that the ovary gives rise to the new additional coats which surround the oocytes. Adding to the above information, Talbot and Goudeau (1988) have also reported that the lobster (H. americanus) egg coat undergoes a swelling process during spawning and the formation of the inner coat takes place through a complex cortical reaction. In penaeid shrimps, oocytes show extracellular crypts with feathery cortical rods in them (Clark et al., 1974, 1980; Drouslet et al., 1975). These cortical rods are said to be of oocytes origin, as reported by Lynn and Clark (1975) and Clark and Lynn (1977). The extracellular crypts with cortical rods are delimited from external environment by forming a thin vitelline membrane (Clark et al., 1980; Pillai and Clark, 1987). In contrast, oocytes of the caridean shrimp, M. rosenbergii, does not show any extracellular crypts. These oocytes surrounded by a thick investment coat and even the germinal vesicle have been reported to be absent in the oocytes (Lynn and Clark, 1983).
Currently our knowledge involving the molecular events and other key factors accompanying the egg activation process in fish is very limited and restricted to a few species only. However, egg activation is a very important physiological process because the fertilization of an egg depends on activation event. If the egg activation process is blocked, there are reports that it affects the process of fertilization (Coward et al., 2002; Coward and Parrington, 2003). Coward et al. (2002) while working on gamete physiology of teleost fish, mentioned the mechanism of egg activation and fertilization. At present, studies on egg activation in fish are confined to medaka fish, O. latipes and zebra fish, B. rerio. In both species, it is reported that an increase in the intracellular Ca+ appears to trigger the process of egg activation but the mechanism of Ca2+ release may be quite different. In medaka fish, egg activation occurs when egg comes in contact with the sperm suggesting the involvement of a sperm is a specific factor for the release of intracellular Ca2+. In zebra fish, eggs appear to require only contact with external spawning medium for the activation process. Similar observations have been reported by Kinsey et al. (2007) regarding the mechanism of fertilization and egg activation in different fishes. Coward and Parrington (2003) also reported the molecular mechanism of egg activation in several fishes. However, while discussing the importance of intracellular Ca2+ and its role in activation of eggs, they further mentioned that there is another compound, i.e., inositol 1, 4, 5 triphosphate (IP3) which also triggers the intracellular release of Ca2+. There are several studies supporting the evidence of the involvement of IP3 in the egg activation process. It is also reported that if the binding of IP3 to the IP3-receptors is prevented by injecting the IP3 antagonist heparin or IP3 receptor antibody, which is a functionally inhibitor agent, then the release of Ca2+ is prevented at the time of fertilization, which in turn also blocks the egg activation process (Miyazaki et al., 1993).
Supporting literature pertaining to egg activation among crustaceans is very limited. Clark and his team (Clark et al., 1974; Lynn and Clark, 1975; Clark and Lynn, 1977; Lynn and Clark, 1987) have done detailed studies on egg activation in penaeid shrimps, viz., Penaeus japonicus, Penaeus aztecus, and Sicyonia ingentis. In fact, egg activation means such events like the release of eggs from meiotic arrest, immediately after the ovulation process, the formation of the hatching envelope around a newly created zygote and switching on the bio-synthetic machinery are necessary for embryonic development. Fertilization is not a requisite to bring out the activation changes in eggs. The eggs can even be activated upon contact with seawater at the time of spawning. Therefore, an egg viability assay, similar to that of sperm viability, becomes an important tool in cryopreservation technology (Kalicharan et al., 1997; Keivanloo and Sudagar, 2013).
Although the development of the sperm banks has been done successfully in a number of commercially important aquatic species, particularly teleost fishes, the technology has not yet reached an advanced level suitable for commercial applications in shellfish. Cryopreservation of gametes is one of the important ex situ methods for not only for the conservation of germplasm, but also for improving the quality of gene resources and enhancing fish production in captive condition (Martinez et al., 2013, 2016; Maria et al., 2015). The main purpose of cryobanking of gametes is to increase the longevity of gametes without any drastic changes in the fertilizing capacity. Even though the need for cryopreservation of fish eggs and embryos assumes a lot of significance in the light of the role played by the mitochondrial DNA, however, such attempts have been met with no or limited success (Ahammad et al., 2003a, b, 2004). There are a few successful case studies regarding cryobanking of invertebrate eggs, embryos and larvae particularly in penaeid shrimps (Diwan and Kandasami, 1997; Diwan, 2000). Sperm cryobanking protocols are available now for over 200 species of finfish and shellfish as reported earlier also (Horton and Ott, 1976; Legendre and Billard, 1980; Kerby, 1983; Leung and Jamieson, 1991; Holtz, 1993; Lakra, 1993; McAndrew et al., 1993; Billard et al., 1995; Diwan and Nandakumar, 1998; Lerveroni and Maisee, 1998, 1999; Horvath and Urbanyi, 2000; Horvath et al., 2003; Chao and Liao, 2001; Huang and Tiersch, 2004; Liu et al., 2006; Horvath et al., 2009; Ding et al., 2011, 2012; Mims et al., 2011; Fauvel et al., 2012; Li et al., 2013; Aramli et al., 2015; Liu Q. et al., 2015; Liu Q.H. et al., 2015; Yamaner et al., 2015; Yildiz et al., 2015). A few sperm banks for finfish have been created, notably for groupers, salmonids and some commercial and endangered fish species (Dziewulska et al., 2011; Sanches et al., 2013; Judycka et al., 2015). Though cryopreservation of fish sperm has been considerably studied using a number of teleost as models (Linhart et al., 2000), successful cryopreservation of fish eggs and embryos still remains elusive. Several attempts to cryopreserve unfertilized eggs of fish were not successful (Horton and Ott, 1976) due to dehydration problems, because of relatively large size of eggs and different water permeability of membranes (Loeffler and Lovtrup, 2000). While carrying out studies on the cryopreservation of rainbow trout (O. mykiss) semen by Tekin et al. (2003), it is reported that the sperm of rainbow trout can be successfully cryopreserved in a glucose based extender containing 10% DMSO with afertilization rate similar to that of fresh spermatozoa. Nahiduzzaman et al. (2012) carried out studies on sperm cryopreservation of the Indian major carp, Labeo calbasu. Here the attempts were made to study the effect of cryoprotectants, cooling and thawing rates of cryopreserved sperm on egg fertilization. It is reported that the sperm of L. calbasu can be cryopreserved in 10% DMSO or 10% methanol with cooling rate of 10°C/min from 5 to −80°C and stored in liquid nitrogen. The preserved sperm can be retrieved and thawed at 40°C for 15 s to fertilize egg after gamete activation. It is further mentioned that this protocol can be used for commercial hatchery operations by using cryopreserved fish sperm. Successful cryopreservation of the sperm of Japanese eel fish Anguilla japonica and European eel fish Anguilla anguilla has been carried out recently by Jusdado et al. (2019) by using methanol as cryoprotectant. It is reported that the success rate of fertilization with methanol cryopreserved sperm was considerably high and therefore even for larger scale fertilization the protocol developed by them is quite useful. By using DMSO as cryoprotectant, it is found that the fertilization rate in both species of eel fish was low and this was mainly due to damaging effects of DMSO on epigenetic changes in the eel fish sperm. However, it is also reported that by using methanol, besides improving the fertilization rate of the sperm, there was an improvement in the motility values also.
Xin et al. (2019) while working on cryoinjuries of the fish spermatozoa reported that the use of antioxidant enzymes, in appropriate proportion, when added to the cryoprotectants can improve or prevent damage to the spermatozoa caused by freezing temperatures. In addition to the antioxidants, it is also suggested that the usage of anti-freeze proteins and anti-freeze glycoproteins can also help stabilizing the cell membrane integrity and inhibit ice crystal growth. This, in turn, reduces damage related to osmotic stress and ice solidification. Figueroa et al. (2013, 2014, 2015a, 2015b) and Zilli and Vilella (2012) while studying the effects of cryopreservation on mitochondria of fish sperm reported that freezing temperatures have the ability to damage the mitochondrial structure. These observations have been drawn by studying several teleost finfishes earlier. The category of the damages to the sperm mitochondria recorded were damage to the membrane integrity, loss of membrane, damage to the mid piece of the spermatozoa, alterations in cellular respiration and bioenergy and disturbance in the mitochondrial DNA integrity and nuclear genes. Further, it is mentioned that the sperm motility and the rate of fertilization depends up on the extent of damage to the mitochondria, which in turn alters the biochemical process, reducing the energy production and affecting the motility of the sperm. Several researchers have confirmed and reported similar observations while working on fish sperm cryopreservation studies (Boryshpolets et al., 2009; Carton et al., 2012, 2013). Asturiano et al. (2017) in their review paper on fish gamete cryopreservation discussed the progress made so far regarding sperm, oocytes and embryo cryopreservation of different fish species. In the discussion, they emphasized the need of developing a cryobanking of genome and genetic resources for endangered fish species.
Liu et al. (2019) while discussing the development of germplasm repositories of the aquatic species of fish and shellfish, gave a detailed account on the practical methods and strategies to be adopted for making the sperm repositories. This paper particularly deals with the development of germplasm repositories of viviparous fishes as a model and further mentions that these repositories contain other basic components like a facility for cryopreservation of sperm and also a provision for genetic assessment and information systems. In recent years, several researchers have developed repositories of cryopreserved sperm of different species of fishes like blue catfish, Ictalurus furcatus (Hu et al., 2011), Atlantic salmon, Salmo salar (Yang et al., 2017), zebra fish, Danio rerio (Yang et al., 2007a, b), medaka fish, O. latipes (Yang and Tiersch, 2009) and Xiphophorus species (Yang et al., 2012).
While working on cryobanking of fish sperm Che-Zulkifli et al. (2019a, b) developed an innovative method of cryobanking of sperm wherein they used dry ice for shipping and packaging of cryopreserved sperm of giant grouper fish Epinephelus lanceolatus. Their investigations initially were mainly to determine the temperature changes during transferring the cryopreserved sperm from liquid nitrogen to dry ice and to study the effect on the viability and motility rate. Here, the sperms were transferred from liquid nitrogen to a closed Styrofoam box containing dry ice for shipping. It is observed that while transferring the sperm from liquid nitrogen to dry ice, there was a drop in the temperature to almost −30°C and the sperm showed a high percentage of viability and motility rate in the dry ice as a cooling medium for 48 h. Further, these researchers have also tested the performance of dry iced cryopreserved sperm of fish, E. lanceolatus for artificial insemination studies with the female grouper E. fuscoguttatus and it was observed that the dry iced cryopreserved sperm could successfully fertilize the egg and produced normal hybrid larvae (Che-Zulkifli et al., 2019a, b).
Though for cryopreservation of sperm of different species of fish several protocols have been developed by different researchers, as far as the commercial application of these protocols is concerned, there are a number of difficulties in making the technology viable and successful. In addressing the issues for developing cryobanking of fish sperm, further research efforts are needed on the effects of cryo-freezing temperatures on mitochondrial DNA, enzymatic or metabolic modifications of the citric acid cycle, and the oxidative phosphorylation process in the inner membrane, as well as studies on the ultrastructure of the mitochondrion of the fish spermatozoa.
For the first time, Chow (1982) reported the successful preservation of spermatophores of the fresh water prawn Macrobrachium rosenbergii. Ishida et al. (1986) later developed a technique for long term storage of lobster (Homarus) spermatophores. Spermatozoa of the penaeid shrimp S. ingentis have been successfully preserved for a period of 2 months in liquid nitrogen by Anchordoguy et al. (1988). Jeyalectumie and Subramoniam (1989) developed a method to cryopreserve viable spermatophores of the mud crab Scylla serrata. Subramoniam and Newton (1993); Subramoniam (1994) carried out studies on cryopreservation of gametes and embryos of a few cultivable crustaceans, particularly on penaeid shrimps. Diwan and Joseph (1998) did elaborate studies on cryopreservation of spermatozoa of the penaeid shrimp P. indicus and P. monodon and they found that among the various cryoprotectants tested, DMSO (dimethyl sulfoxide) and glycerol have been reported to be the best protective media at ultra-low temperatures. Successful preservation of spermatophores of shrimp in glycerol at −196°C has also been reported by Chow et al. (1985). Similar observations have been made by Behlmer and Brown (1984) for Limulus sp. spermatozoa. When DMSO was used for spermatophores preservation in M. Rosenbergii the post thawing survival was reported to be nil (Chow et al., 1985). While on contrary, Anchordoguy et al. (1988) reported that sperm survival was higher in S. ingentis when glycerol was used as a cryoprotectant for the preservation of sperm even though DMSO gave the highest percentage of survival. In S. serrata glycerol and DMSO+, trehalose gave the highest sperm survival (Jeyalectumie and Subramoniam, 1989). To improve the sperm longevity and quality during long term cryopreservation, number of efforts have been made recently in the modification for the protocol of cryopreservation technology. Cell membrane studies including the mitochondrial status and antioxidant status are generally considered as main factors for evaluation using different cryo methodologies, and chromatin integrity is also considered as an important check point (Cabrita et al., 2014). The proteomic studies of the sperm have also been given as a priority to understand the physiology of protein changes during cryopreservation (Li et al., 2012, 2013; Zilli et al., 2014; Nynca et al., 2015). Several improvements have taken place in modifying freezing media by addition of different compounds in order to increase cryo-resistance in the sperm (Yildiz et al., 2015). Vuthiphandchai et al. (2007) while working on the development of a cryopreservation protocol for long term storage of the spermatophores of the shrimp P. monodon reported that they could store the spermatophores in liquid nitrogen successfully for 210 days with more than 90% viability. Further, it is mentioned that thawed spermatophores held in liquid nitrogen for less than 62 days, fertilized the eggs and hatching process also proceeded. The rate of fertilization and hatching of the cryopreserved spermatophores has been reported almost similar to the freshly collected spermatophores. In recent years, sperm cryopreservation of the penaeid shrimps has been successfully carried out by few researchers (Nimrat et al., 2005, 2006; Castelo-Branco et al., 2014, 2015, 2018). The real issue in cryopreservation technology in the shrimps is long term cryopreservation of the sperm with greater viability to fertilize the egg either in vitro or in vivo operations (Alfaro et al., 2007; Braga et al., 2014). In recent times, Claudet et al. (2016) worked on the effects of cryoprotectants and cooling rates on the fertility of the sperm of giant freshwater prawn, M. rosenbergii. In this particular study sperm were cryopreserved using different cryoprotectants like DMSO, propylene glycol, methanol, glycerol and ethylene glycol at different concentrations. The freezing rates used were −1.5°C, −3°C, and −5°C/m and finally the sperm were plunged into liquid nitrogen for a period of 90 days duration. The results indicated the sperm fertility was more than 50% when compared to the fertility registered with normal freshly collected sperm (90%). The cryoprotectants like DMSO and ethylene glycol were found to be more amenable agents for cryopreservation studies. At present, there is not much knowledge on the sperm action when they are deposited into the thelycum of the female shrimps. Braga et al. (2013) reported that once the sperm are deposited into the thelycum there can be an exchange of ions between spermatozoa and thelycum, leading to biochemical changes which results in morphological rearrangements. Sperm cryobanking protocol in case of mud spiny lobster, Panulirus polyphagus has been developed with a cooling period of 15 min at temperatures (25, 20, 16, 4, 2, −4, −20, −80, and −150°C) followed by plunging the same into the liquid nitrogen (−196°C). It is observed that the success rate of the revival of the sperm was more than 90% for those sperm preserved at cooling temperatures of −20 and −80°C and cryopreserved in liquid nitrogen for a period of 6 h. The cryoprotectant used here was 10% glycine with saline as an extender (Fatihah et al., 2016).
Though cryopreservation of fish sperm has been studied considerably using a number of teleost fishes (Linhart et al., 2000), successful cryopreservation of fish eggs and embryos still remains elusive. Several attempts to cryopreserve unfertilized eggs of fish were not successful (Horton and Ott, 1976) due to dehydration problems, the large size of eggs and different water permeability of the membranes (Loeffler and Lovtrup, 2000). It is stated that the storage of zebra fish embryos using propylene glycol in the liquid nitrogen even for 1 min resulted in damage of mitochondria, disorganization of ribosomes and plasma membrane of the yolk syncytial layers (Anchordoguy et al., 1987). There was 100% mortality of some teleost (C. carpio, Labeo rohia, and B. rerio) embryos when stored in liquid nitrogen temperature even for short duration of up to 3 h (Harvey et al., 1983). On the other hand, Zhang et al. (1989) reported successful cryopreservation of common carp embryos at freezing temperatures; however, these results have not been duplicated. Whittingham and Rosenthal (1978) showed that herring embryos did not survive after cooling below –10°C when protected with dimethyl sulfoxide (DMSO; Me2SO). Studies on cryopreservation of fish egg/embryos have been carried out by a number of researchers but due to the presence of multilayers in their structure and the technology of cryopreserving, viable egg/embryos were not successful (Hagedorn et al., 1998; Zhang and Rawson, 1996a, b; Suzuki et al., 1995; Zhang et al., 1998, Zhang et al., 2005a; Danilo et al., 2014; Keivanloo and Sudagar, 2013, 2016).
Zhang et al. (1993) carried out cryopreservation studies of pre-hatch embryos of zebra fish B. rerio in details and reported that the pre-hatch embryos when exposed to a range of cryoprotectants (methanol, DMSO, glycerol, ethanediol and sucrose) at different concentrations for 30 min at room temperature, heart beat stage embryos survived in methanol up to 5 h. Ethanediol was found to be toxic after 3 and 1 h exposure. Further they observed that the effect of cooling up to −30°C temperature on poor survival rate of pre-hatch embryos. Here the concentrations of cryoprotectants, equilibrium time and cooling rate have been reported to be the main factors while doing the cryopreservation studies. The best embryo survival rate reported was at −10°C (94%), at −15°C survival rate was 72% and at −20°C it was 43% and at −25°C it was only 8%. The embryos did not hatch out at −30°C due to ice formation within the eggs affecting the survival of the embryos. Lui et al. (1992) attempted to cryopreserve goldfish embryos by passing fertilized eggs through a series of DMSO dilutions, viz. 5, 8, 10, and 12%, for 5 min in each dilution. The embryos were frozen at −15 and −25°C for 60 min. Survival rates observed were 20% at −15°C and 5% at −25°C, respectively and the hatching rate reported was 15 and 3% at −15 and −25°C, respectively.
Zhang and Rawson (1995a, b) carried out studies on chilling sensitivity of the embryos of the zebra fish during early developmental stages and it has been reported that 50% of the embryos died at heart beat stage at 0°C during different periods of cooling, i.e., 3, 7, 15, 20 and 27 h. Heart beat stage embryos tolerated a temperature of 0°C up to 10 h without any adverse effect. However, survival was significantly decreased at sub-zero temperature. Advanced stages of embryos showed more sensitivity to cooling temperatures. Among different cryoprotectants tested, methanol proved to be optimal cryoprotectants for non-freezing storage of embryos at 0°C and sub-zero temperature. No embryos survived after exposure for 72 h at 0°C and 1 h at −20°C. It is further observed that loss of embryo viability may be related to freezing injury, cryoprotectants toxicity, osmotic stress and other factors. Liu et al. (1999a, b) studied the effect of partial removal of yolk on chilling sensitivity of zebra fish embryos. Here, they removed partial yolk from dichorionated embryos by multiple punctures with a sharp micro needle. With this technique, approximately 50–75% of the yolk was removed. The survival of the yolk reduced embryos was found to be dependent on the development stage of the embryos. It is observed that the survival rate of 26-somite stage embryos was only 7.9%, whereas prim-6 stage was 56.7%, prim-15 stage was 62.4 and 81.3% survival was for high-pec embryo stage. As far as chilling sensitivity, there was no sign of any injury to the high-pec stage of embryos at 0°C up to 6 h. When the period was extended up to 24 h, the recovery of high-pec stage embryos of reduced yolk was significantly high compared to the chilled control embryos. These results showed that partial removal of yolk in zebra fish embryos at different stages of development will facilitate not only survival, but also their sensitivity to chilling can be reduced. Zhang et al. (2003a, b) studied the effect of methanol and developmental arrest on chilling injury on zebra fish embryos during different phases of development (64 cell stage, 50% epiboly, 6-somite, prim-6 and long budded stages). In their experiment embryos were exposed to methanol before cooling at −50°C with slow cooling rates and at different time periods. It has been reported that the addition of methanol to embryo medium has increased embryo survival significantly at all developmental stages under all cooling rates. As the concentration of methanol increased, it has improved the survival rate of embryos. As far as chilling sensitivity is concerned 50% epiboly and prim-6 stages embryos underwent developmental arrest after 15 min due to oxygen deprivation.
Liu et al. (1998a, b) studied the impact of vitrification on the survival of zebra fish (D. rerio) embryos by using methanol as cryoprotectants. It has been reported that at 10M concentration, methanol showed toxic effect with early stage embryos compared to an advanced stages. Early stage embryos survived after exposure for only 3.0 min to the 10M concentration of methanol at 0°C. However, advanced stages of embryos like epiboly, 6-somite and prim-6 stages when exposed for 5–10 min to methanol did not show any detrimental effects and no embryos survived after 40 min exposure. Chen and Tian (2005) while working on cryopreservation of flounder fish embryos by vitrification method, reported that propylene glycol and methanol are less toxic to the embryos than dimehtylformamide (DMF) and DMSO; whereas the use of ethylene glycol and glycerol were found to be more toxic. The embryos between 4-somite and tail bud stages have been reported to be more tolerant to vitrifying solutions than the embryos in other developmental stages. Further, it is mentioned that investigation was continued by using different vitrifying solutions in combination with propylene glycol and DMSO on the flounder fish embryos. It has been mentioned that out several experiments conducted on vitrification of flounder fish embryos, fourteen larvae with normal morphology hatched successfully out of 292 cryopreserved embryos and there was a recovery of 20 viable embryos. Further, it is also reported that the tail bud stage embryos exhibited greater tolerance to vitrification than the embryos at other stages. These results indicated that cryopreservation of founder fish embryos by vitrification is possible. Sharifuddin and Azizah (2014) carried out studies on cryopreservation of snakehead fish (Channa striata) embryos. Their objective was to study the sensitivity of embryos hatching rate to low temperature in relation to four different cryoprotectants. They selected morula stage and heartbeat stage embryos for the study using DMSO, ethylene glycol, methanol and sucrose solutions. Embryos were kept at 15, 4, and −2°C for 5 min, 1 and 3 h, respectively. It has been reported that there has been significant decrease in hatching rate in both the developmental stages of the embryos after exposure to 4 and −2°C at 1 and 3 h. Further, it was observed that heartbeat stage embryos were more tolerant against chilling at −2°C for 3 h exposure in DMSO followed by methanol, sucrose and ethylene glycol.
Isayeva et al. (2004) while studying chilling sensitivity of zebra fish oocytes reported that the oocytes are very sensitive to cooling temperatures and their survival decreased with decreasing temperature and increasing exposure time periods. Further, it is mentioned that stage III oocytes are found to be more susceptible to chilling than stage IV oocytes and that individual females had a significant influence on oocytes chilling sensitivity. This freeze cooling sensitivity factor may also be one of the hurdles for the development of protocol of cryopreservation technology. Tsai et al. (2009) carried out studies on chilling sensitivity on early stage ovarian follicles of zebra fish. For this purpose, they selected different stages of developing ovarian tissues particularly stage I (primary growth), stage II (Cortical alveolus) and stage III (Vitellogenic). The ovarian tissues of these stages were first chilled in KCL buffer and L-18 medium for up to 144 h at −1°C in low temperature bath. Later these tissues were exposed to 2M methanol or 2M DMSO and chilled at −1°C and −5°C temperature for a period of 168 h. Control follicles were also maintained at 28°C. It is reported that the ovarian tissues of stage I and II are found to be less sensitive to chilling than stage III ovarian follicles. Later, they confirmed these results following in vitro maturation of the chilled ovarian follicles. Guan et al. (2010) while doing cryopreservation studies on zebra fish oocytes viability, investigated the effect of different cryopreservation media, cryoprotectants and freezing temperature before plunging the ovarian tissue in to liquid nitrogen. In their observation, it is found that the viability of oocytes frozen in KCL buffer was significantly higher than the oocytes frozen in L-medium. They also reported that fast thawing and stepwise removal of cryoprotectants improved oocyte survival significantly with highest viability of 88.0%. However, after 2 h incubation at −22°C the viability of freeze-thawed oocytes decreased to 29%. They emphasized that new oocyte viability assessment methods are urgently needed.
Fish embryo cryopreservation, no doubt, is very difficult due to number of hurdles during cryopreservation process. Therefore, recently, more focus has been laid on fish oocyte cryopreservation. The advantage in the oocyte cryopreservation is because of their small size, high membrane permeability and less chilling sensitivity features (Isayeva et al., 2004; Zhang Y.Z. et al., 2005). Several researchers have carried out the studies on cryopreservation of fish oocytes particularly on zebra fish (Guan et al., 2010; Anil et al., 2011) and also on other marine and freshwater fish species (Streit et al., 2014). Leandro et al. (2013) while studying cryopreservation of stage IV oocytes of zebra fish, reported that vitrification ability of different cryosolutions when used with oocytes showed different visible changes. For vitrification purpose they used different devices like plastic strawsvitrification block, andfibre-plug, while cryosolutions used were propylene glycol, methanol and DMSO. The changes recorded in the oocytes after vitrification were, in the fiber-plug material, the membrane integrity was intact in the oocytes which were preserved in a mixture of methanol and propylene glycol. However, when vitrified in cryosolutions like methanol and DMSO, the membrane integrity of the oocytes was found to be decreased. It was observed that the follicles located in the middle part of the ovarian tissue were more protected from the cryo-injuries. In granular cells, mitochondrial integrity was found to be damaged by the vitrification protocol. Further, it is mentioned that this kind of information will help in the development of optimal protocol for cryopreservation of fish oocytes. Marques et al. (2015) also studied the viability of ovarian follicles in zebra fish after vitrification in a metal container. They used whole ovarian tissue as well as fragmented parts of the tissue for this purpose. In one of the experiments they tested the follicular viability of five developmental stages following vitrification using different cryosolutions. It is reported that highest viability rates were obtained with immature follicles with methanol and propylene glycol mixture. In another experiment, fragmented parts of the ovarian tissue were kept in two different carriers like plastic cryo-tube or a metal container. Here, it was noticed that after 24 h, cryopreservation morphological features of the follicles under vitrified condition were found to be well preserved. Follicular survival rate was also higher in vitrified fragments of the ovarian tissue when compared to the whole intact ovary. There are studies which deal with successful technological protocols for in vitro maturation of oocytes after cryopreservation. Seki et al. (2008, 2011) reported that they were able to do successfully in vitro maturation of late stage III zebra fish oocytes until they were fertilized and developed up to the hatching process. These studies clearly indicate that better results can be obtained by cryopreserving early stage oocytes.
Toxicity studies of cryoprotectants to zebra fish oocytes were investigated by Plachinta et al. (2004). In this study they used DMSO, methanol, ethylene glycol (EG), propylene glycol (PG), sucrose and glucose as cryoprotectants. These cryoprotectants were tested on different phases of ovarian development like vitellogenic ovary, maturation ovary and matured egg stage oocytes which were incubated first in Hank’s medium containing different concentrations of cryoprotectants for 30 min at room temperature. Toxicity test was measured using different staining methods and also on the observation of germinal vesicle breakdown. It has been reported that toxicity effect of cryoprotectants increased through the concentration and the effect could be seen more on the advanced stages of maturation for the ovary. The sensitivity of oocytes to cryoprotectants appeared to increase with development stages with stage IV oocytes being the most sensitive. In another study attempts to cryopreserve eggs and embryos of the carp L. rohita (Ponniah et al., 1999) has been reported as unsuccessful and in this particular investigation toxicity tests were carried out with DMSO and methanol. Methanol has been mentioned to be the least toxic with a hatching rate of 18% but post-thaw survival has been reported to be nil. In an attempt to cryopreserve the embryos of common carp and rohu, John et al. (1993) reported that the tail bud stage embryo of the common carp showed more tolerance to cryoprotectants than the morulae stage. Nilsson and Cloud (1992, 1993) made efforts to cryopreserve isolated blastomere from rainbow trout blastulae. In this investigation, individual blastomere was frozen in a medium containing 12% DMSO. Three rates of cooling (3, 1 and 0.3°C/min) through the critical period were compared using a Cryomedical controlled Rate Freezer. All cells were thawed at the same rate in a 4°C water bath for 3 min. Blastomere frozen at the highest cooling rate (3°C/min) had the lowest percentage (6.6%) of viable cells upon thawing. In contrast, the blastomere frozen at other two cooling rates had been observed to have 31 and 51% viability, respectively. Studies on cryoprotectant toxicity to early stage ovarian follicles of zebra fish, B. rerio have been carried out by Tsai et al. (2008). It is mentioned that cryoprotectant toxicity test when conducted with ovarian follicles stage I and II, the test indicated that the impact of toxicity increased in the order of methanol, DMSO, propylene glycol and ethylene glycol whereas stage III ovarian follicles appeared to be more sensitive. For assessment of toxicity test, two staining methods were used viz., trypan blue and fluorescein diacetate along with propidium iodide. Here the ovarian follicles of stage I and II were incubated in 50% L-15 medium containing different concentrations of cryoprotectants (0.25–5M) for 30 min at room temperature. While evaluating the suitability of cryoprotectants for olive barb fish, Puntius sarana embryos, Rahman et al. (2015) mentioned that hatching rates of gastrula, somite and tail stage embryos decreased with increasing cryoprotectant concentration and toxicity to the embryos varied in the order of propylene glycol, ethylene glycol, DMSO and dimehtylformamide. It is further reported that they used thirteen cryoprotectant mixture solutions comprising five cryoprotectants and tested the toxicity with the embryos at different concentrations and found that tail stage embryos were more tolerant to all these mixtures than the earlier stages.
Kopeika et al. (2005) while carrying out studies on cryopreservation of genome of fish reproductive cells reported that the survival of fertilized eggs of Loach incubated in caffeine solution was better when compared to the same incubated in 3-aminobenzamide solution. Further, it is mentioned that freezing of zebra fish embryos at blastomere stage has damaged the mtDNA and there was also an increase in the mutation frequency. From these findings it was concluded that the cryopreservation might potentially increase the instability of mtDNA genome and subsequent function of the cells. Kopeika et al. (2005) reported that when DMSO is used as cryoprotectants to preserve zebra fish egg/embryos, mtDNA did not show many changes and the mutation process was under control. It is also stated that while designing the protocol of cryopreservation of egg and embryos these factors have to be taken into consideration as precautions. In recent years, Martinez et al. (2016) in their review paper on cryobanking of aquatic species, discussed in detail about the application of cryobanking of genome of several aquatic species including freshwater and marine fish as well as invertebrates. In this review, it is reported about the State of the Art of cryopreservation of different cellular types (sperm, oocytes, embryos, somatic cells, and primordial germ cells of early spermatogonia) and also about the advantages and disadvantages of each protocol. Several cryobanks which have been established in Europe, United States, Brazil, Australia and New Zealand were also discussed in detail in this review.
A number of researchers have carried out studies on the permeability of cryoprotectants during different developmental stages of oocytes and developed eggs (Zhang et al., 1998; Liu et al., 2000, 2001a,b). While discussing the characterization of major permeability barriers in the zebra fish embryo, Hagedorn et al. (1996, 1997, 1998) carried out in-depth studies on the characterization permeability barriers of various cryoprotectants in the embryos of zebra fish as a model. They considered permeability of water and cryoprotectants as the major components in this multilayered embryological system. It is reported that they first measured shrink/swell dynamics using optical volumetric method and second, they measured dimethyl sulfoxide (DMSO) and propylene glycol uptake with diffusion weighted nuclear magnetic resonance spectroscopy (NMR). It is observed that for the first few minutes DMSO uptake in the embryo was rapid, but then this uptake gradually reduced thereafter. It is further mentioned that the yolk and blastoderm had very similar water permeability that can be seen in the presence of DMSO, the DMSO permeability is different as it is separated by three orders of magnitude. The low solute permeability of yolk indicated that yolk and yolk layers surrounding the compartment are more susceptible to cryodamage.
In order to overcome permeability barrier of the cryoprotectants, Janik et al. (2000) developed a technique of microinjection of cryoprotectants in to zebra fish embryos. The aim of microinjection of cryoprotectants was to discover not only the survival of the embryos, but also to assess toxicity and cryo-damage to the yolk layers after vitrification trials. It is reported that embryos after microinjection of propylene glycol showed better survival rate (70%) than the DMSO (60%) after 5 days. Further, they mentioned that due to dehydration of the yolk while vitrification has resulted in low survival rate of the embryos; other factors needed to be examined for successful vitrification. Zhang et al. (2005a, b) studied the membrane permeability of zebra fish oocytes in the presence of different cryoprotectants. The study was conducted on stage IV and stage V developing oocytes. The cryoprotectants used were DMSO, propylene glycol and methanol. Oocytes were exposed to these cryoprotectants for 40 min at −22°C. It is reported that membrane permeability of the stage IV oocytes decreased significantly. No significant changes in the cell volume during methanol treatment have been recorded. Further, they mentioned that it was not possible to estimate membrane permeability parameters for stage V oocytes because these oocytes experienced the separation of outer oolemma membrane from inner vitelline during exposure to cryopreservation. In the recent past, Sawitri and Amrit (2010) carried out studies on permeability of methanol into zebra fish embryos by using ultrasound technique. For this purpose, they selected three of embryos (dichorionated, with soft chorion and intact embryos). It is mentioned that with an ultrasound treatment there was 100% recovery of DMSO in embryo samples, whereas in those embryos without an ultrasound the recovery of DMSO was found to be 3.1%. In soft chorion embryos, penetration was more and in dichorionated embryos it was very less. From the studies it was concluded that novel and non-invasive methods are needed to achieve greater methanol penetration for successful vitrification of the embryos.
After analyzing the available literature, it is observed that the studies on fish embryo cryopreservation have been carried out under different categories. There are studies involving cryopreservation protocols in relation to toxicity of different cryoprotectants with different freezing times and thawing rates. Some studies have focused their research aiming only to find out membrane permeability of different cryoprotectants. There are still other studies which are related to the development of new methods for evaluating fish embryo survival and developmental process. Some other works are again related to evolve new technologies for the improvement of the fish embryo’s ability for survival to extreme cold and chilling conditions. In recent years, de Carvalho et al. (2014) in their review paper on the efficacy of fish embryo vitrification protocols in terms of embryo morphology, discussed in details about new vitrification methods in relation to toxicity of different cryoprotectants and the developmental stages embryos and conditions under which embryos are exposed for vitrification process. In an effort to develop cryobanking of the fish genome, number of researchers used different genomic materials like spermatozoa, primordial germ cells, oocytes and embryos in the different stages of development (Labbé et al., 2013). As mentioned earlier, the cryopreservation of egg and embryos and larvae has some limitations due to so many factors affecting the technology development. Several researchers have suggested the development of cryo methods for in vitro maturation of ovarian follicles (Seki et al., 2008, 2011; Tsai et al., 2010) but however, development of such protocols require lot of experimental studies. It is mentioned that while developing the biotechnological techniques for preserving the fish genome material, care has to be taken to devise the methods to address the problems like cellular DNA integrity, cell membrane protection, types of cryodamage on the chromatin and cellular structures and their epigenetics consequences, regarding the production and effects of reactive oxygen species, to guarantee the embryo survival etc. (Chenais et al., 2014).
Tiersch and Green (2011) mentioned that cryopreservation technology is quite useful and potential tool for preservation of genetic material. Gwo (2000) also described earlier that cryobanking of milt of several aquatic species has helped in carrying out hybridization process, Gynogenesis and also domestication and conservation of important traits in the context of commercial aquaculture. In recent past, a number of researchers have carried out artificial insemination studies in various shrimp species and reported different success rates in the production of nauplii (Nimrat et al., 2005; Bart et al., 2006; Vuthiphandchai et al., 2007). However, there are many shortfalls in this particular technology which requires attention and need further research. In order to avoid dependency on artificial insemination for the production of nauplii, cryopreservation of embryos, nauplii and larvae has been considered as alternate tool. Kuleshova et al. (2001) while working on cryopreservation of embryos in shrimp reported that dehydration of the embryos during cryopreservation is very important factor in the failure of the technology than the toxicity of the cryoprotectants.
Two methods have been suggested to produce osmotic dehydration during cryopreservation, i.e., one is vitrification process in which the cells are immersed directly into liquid nitrogen, changing rapidly their state from liquid to glassy and other one is controlled and slow freezing and thawing. Both methods require the removal of water from the cells, the introduction of cryoprotectants and the subsequent removal of these compounds from the embryos (Saragusty and Arav, 2011). Dong et al. (2004) while working on embryo cryopreservation of several shrimp species reported that advanced stages of the embryos and larvae shows higher resistance to the cryoprotectant toxicity than the embryos of early developmental stages.
Cryopreservation of shellfish embryos compared to fish embryos is easy because of their smaller size which helps in effective permeability of water and cryoprotectants, low yolk content and holoblastic cleavage of the developing embryos (Robles et al., 2008). Among shellfishes cryopreservation studies on embryos and larvae have been carried out mainly on Pacific oyster because of its high commercial value (Renard, 1991; Gwo, 1995; Usuki et al., 2002; Suquet et al., 2014). Asahina and Takahashi (1978) reported that late developing embryos of sea urchin could survive at least for a short period of time at −196°C in the presence of cryoprotectant like DMSO and ethylene glycol. Among shellfishes clam and oyster eggs were cryopreserved (Utting et al., 1990) at −196°C and thawed after 30–60 min and their viability and growth was compared with non-frozen control larvae from the same parents. Growth of Manila clam larvae was monitored for 35 days and there was no significant difference between the shell length of the frozen and control larvae. Survival of frozen larvae has been reported to be high at 84% compared to that of the control. In an early trial, Pacific oyster larvae, which had been frozen, were reared successfully to settlement, but survival was reported to be poor. However, after modification of the freezing process, survival of larvae was much higher (Rana et al., 1992). Rana et al. (1992) reported that cryopreservation of egg and embryos of the oyster, Crassostrea gigas, were not very much successful. However, varying degrees of success have been reported using early trocophore (7 h) larvae. In all the trials they found that DMSO at concentrations of 1.0–1.5M gave consistently better thaw viability of larvae than glycerol. At least, 40–50% of the larvae were recovered after thawing. The post-thaw viability was found to increase by 15% using larger embryos. Taylor (1990) reported that frozen nauplii of Penaeus vannamei at −30°C, when thawed recovered and metamorphosed successfully to protozoeal stage larvae. Cryopreservation of cirripede nauplii was attempted by Kurokura et al. (1992) who examined the prospects of this technique for other crustaceans like prawns and crabs. It is reported that when the larvae were transferred to seawater containing 2M DMSO and 0.1M saccharose as cryoprotectants and frozen to −196°C the nauplii failed to survive.
Preston and Coman (1998) studied the effects of cryoprotectants, chilling and freezing on the embryos and nauplii of shrimp, P. esculentus. From their findings, it was concluded that there are several barriers in the success of cryopreservation of penaeid shrimp embryos. The embryos are found to be very sensitive to chilling and osmotic change and its tolerance to −1°C was seldom more than 20 min. Rapid exposure to hyperosmotic or hypo-osmotic conditions has been reported to be lethal to the developing embryos. Further, they mentioned that embryo tolerance to cryoprotectants varied according to the molecular weight and concentration of the cryoprotectants (Preston and Coman, 1998). For the first time, the nauplii of the shrimp P. indicus were frozen to −30 and −40°C using liquid nitrogen vapor. The high percentage survival of the nauplii frozen to −30°C (82%) and −40°C (63%) was recorded using 15% v/v ethylene glycol which suggested that rapid thawing and slow dilution is necessary in the protocol for cryopreservation of penaeid shrimp nauplii (Subramoniam and Newton, 1993; Subramoniam, 1994). Simon et al. (1994) attempted to determine a suitable freezing medium for cryopreservation of P. indicus embryos. Among the five developmental stages studied, gastrulae and 5-h embryos were found to be resistant to a prolonged contact with cryoprotectants and it is observed that of the 11 cryoprotectants used, the combination of methanol with ethylene glycol or DMSO gave hatching rates equal to those of the control. Diwan and Kandasami (1997) and Diwan (2000) while working on cryopreservation of embryos and nauplii of the shrimp P. semisulcatus reported that when DMSO and glycerol were used independently as cryoprotectants, the viability of freeze-thaw embryos was nil. But 40–50% of the embryos hatched out successfully to nauplii when cryopreserved in mixtures of DMSO and glycerol prepared in grades of 5 to 20%. Similar success has been reported by the same authors with regard to cryopreservation of nauplii also. Screening of stages of embryonic development for resistance to chilling temperatures (+7 and 0°C) by McLellan et al. (1992) indicated that the fifth nauplius (N5) and first zoea stage of P. vannamei were able to withstand chilling stress. Alfaro et al. (2001) worked on the sensitivity studies of the developing embryos and larvae of the shrimps, Trachypenaeus byrdi and Penaeus stylirostris to cooling, cryoprotectant exposure and hyper saline treatment in order to gain basic knowledge of cryopreservation procedures. The cryoprotectants used were DMSO, sucrose, methanol and glycerol. It has been reported that morulae and advanced stage embryos showed more tolerance to DMSO to cooling at 10°C but they were more sensitive to 0°C exposures. Methanol exposure at 12°C up to 2M concentration was found to be non-toxic for the advanced embryos. Morulae stage embryos have been reported to be more resistant to hyper saline treatment at 55 ppt than advanced stage embryos. It is further mentioned that nauplii stage larvae showed better tolerance to cooling in DMSO exposure than the embryos of any stage. Effects of cryoprotectant toxicity on the embryos and larvae of the white shrimp, L. vannamei have been studied by Dong et al. (2004). The cryoprotectants used were methanol, ethylene glycol, propylene glycol and DMSO. The developmental stages of embryos and larvae selected were pre-nauplius embryos, nauplius (N I), nauplius (N II), nauplius (N V), and protozoea I and protozoea III. Three different concentrations of cryoprotectants were used and animals were exposed to each cryoprotectant for 5–60 min. It is reported that for protozoea larvae (Z 1) ethylene glycol was least toxic as survival rate of the larvae was more than 83%. Advanced stage larvae (Z 3) were found to be more tolerant to higher concentrations of cryoprotectants to long exposure time. Pre-nauplius embryos are found to be most sensitive stage to the cryoprotectant. These observations become the base line data for attempts to cryopreserve embryos and larvae of the penaeid shrimps. In recent past Tsai and Lin (2009) investigated the toxicity of cryoprotectants to the embryos of banded coral shrimp, Stenopus hispidus. For the purpose of this study, they selected three stages of embryonic development, i.e., eye formation stage, heart-beat stage and pre-hatch stage and exposed to different concentrations of cryoprotectants. It has been mentioned that the intensity of toxicity of cryoprotectants increased in the order of methanol, ethylene glycol, DMSO, glycerol and dimethylacetamide. Pre-hatch stage embryos appeared to be more tolerant to cryoprotectants toxicity than eye formation and heart beat stage embryos.
Developments of gamete banks for aquatic animals would open new perspectives in any culture operations and this would also serve as an important insurance for research and breeding programs. In aquaculture industry, the identification of parental strains and progeny and management of better quality of wild stocks for farming are main issues which are difficult to overcome. The ability to freeze the gametes of aquatic organisms and to store them for longer periods without deterioration would be of considerable value for the genetic improvement of aquaculture. In recent years, due to realization of the importance of development of cryobanks of fertile gametes to augment animal production, elaborate studies are being undertaken to determine the sperm-egg characteristics of several economically important aquatic species. In most cases, female and male release their gametes into aquatic environment and egg–sperm interaction is expedited by a closely timed release of gametes. Once contact between an egg and sperm is established, sperm of most species are induced to undergo an activation reaction as a prerequisite to fertilization.
Penetration of the eggs by fertilizing sperm stimulates the egg to develop (Clark et al., 1973). The gametes of aquatic animals are quite unique in many ways and show remarkable differences from terrestrial animals. Research pertinent to gamete structure and induction of gamete activity in such animals, especially of invertebrates, is limited and not much information is available. The summary of the cryobanking status of fish and shellfish egg, embryos and larvae is given in the Table 1.
Cryopreservation of gametes of aquatic animals in contrast to the situation in terrestrial vertebrates, particularly mammals, has been met with very limited success. Sperm cryopreservation has been successful with a number of commercially important aquatic species, particularly some teleost fishes. However, the reproductive success with cryopreserved sperm in general is still poor and the technology involved requires further refinement. The development of the sperm cryobanking in a number of other aquatic animals is not at the stage of advanced commercial application as seen with domestic mammals. This may partly due to the problems related to the need for a relatively large volume of sperm to fertilize the larger number of eggs produced by aquatic animals. There is also a need to undertake innovative research to address some of the priority areas like possible effects of cryopreservation on mitochondrial DNA, enzymatic or metabolic modifications of the citric acid cycle, and the oxidative phosphorylation process in the inner membrane of the sperm, as well as studies of the ultrastructure of the mitochondrion.
In gamete preservation, eggs are fundamentally more difficult to freeze successfully than sperm. The main reason is that due to the large size of eggs, there will be some interference with penetration of cryoprotectants and uniform cooling during the cryopreservation process. Sometimes eggs with a large yolk sac tend to develop crystals which damage the egg as it freezes. It has been also stated that the chromosomes in the egg are more vulnerable to damage those in sperm. Also the loss of membrane integrity both in sperm and eggs is a critical damaging factor incurred during the freeze/thaw process. Recent evidence has shown that certain key enzymes in the cells get altered/broken down upon freezing. More innovative research is needed particularly in the areas of membrane permeability of the egg/embryos for different cryosolutions, freezing impact on the retention of structural composition, microinjections of various cryoprotectants and antifreeze protein, and also the application of osmotic and hydrostatic pressures to enhance permeability of cryosolutions. Very few attempts have been made on cryopreservation of sperm in decapods crustaceans in general and marine crustaceans in particular. Therefore, further efforts are very much needed particularly for improving this technology not only for cryopreservation of spermatozoa, but also for eggs, embryos and larvae of economically important fish and shellfish species. For developing an aquaculture industry, one of the major constraints is non-availability of sufficient seed and spawners to produce seed at the desired time. Even in the event of the availability of spawners, their maintenance and management become difficult and expensive. Therefore, to ease this problem there is an urgent need to devise a suitable technology of cryopreservation and cryobanking of viable gametes so that production of aquatic animals in captive condition can be made as per the need.
AD has articulated the idea of writing this article and prepared the draft of the manuscript. SH was responsible for the collection of relevant literature and helping in articulation of the manuscript. Gopalkrishna has critically gone through the draft and offered suggestion for improvement. AP has helped in collection of the data and also in the analysis of the interpretation in the discussion part of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We wish to express our sincere thanks to Shri Ankushrao Kadam, Secretary, Mahatma Gandhi Mission (MGM) University, Aurangabad, Maharashtra, India for giving constant encouragement and support for writing the present review.
Ahammad, M. M., Bhattacharyya, D., and Jana, B. (2002). The hatching of common carp (Cyprinus carpio L.) embryos in response to exposure to different concentrations of cryoprotectant at low temperatures. Cryobiology 44, 114–121. doi: 10.1016/s0011-2240(02)00012-3
Ahammad, M. M., Bhattacharyya, D., and Jana, B. B. (2003a). Hatching of common carp (Cyprinus carpio L.) embryos stored at 4 and–2°C in different concentrations of methanol and sucrose. Theriogenology 60, 1409–1422. doi: 10.1016/s0093-691x(03)00118-3
Ahammad, M. M., Bhattacharyya, D., and Jana, B. B. (2003b). Stage dependent hatching responses of rohu (Labeo rohita) embryos to different concentration of cryoprotectants and temperatures. Cryobiology 46, 2–16. doi: 10.1016/s0011-2240(02)00138-4
Ahammad, M. M., Bhattacharyya, M., Banerjee, D., and Bandyopadhyay, P. K. (2004). Toxicity of Protectants to embryos of silver carp (Hypophthalmichthys molitrix). After Cold Storage at different storage time periods. Cell Preserv. Technol. 2, 227–233.
Alfaro, J., Komen, J., and Huisman, E. M. A. (2001). Cooling, cryoprotectant and hyper saline sensitivity of penaeid shrimp embryos and nauplius larvae. Aquaculture 16, 353–366.
Alfaro, J., Ulate, K., and Vargas, M. (2007). Sperm maturation and capacitation in the open thelycum shrimp Litopenaeus (Crustacea: Decapoda: Penaeoidea). Aquaculture 270:436442.
American Chemical Society (ACS) News (2017). Zebra Fish Embryo Cryopreservation Achieved by Using Gold Nanotechnology and Laser. Tokyo: Fish Information Services (FIS).
Anchordoguy, T. J., Crowe, J. H., Griffin, F. J., and Clark, W. H. Jr. (1988). Cryopreservation of sperm from the marine shrimp Sicyonia ingentis. Cryobiology 25, 238–243. doi: 10.1016/0011-2240(88)90031-4
Anchordoguy, T. J., Rudolph, A. S., Carpenter, J. F., and Crowe, J. H. (1987). Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 24, 324–331. doi: 10.1016/0011-2240(87)90036-8
Anil, S., Zampolla, T., and Zhang, T. (2011). Development of in vitro culture method for zebra fish ovarian tissue fragment. Cryobiology 63, 311–312.
Aramli, M. S., Golshahi, K., Nazari, R. M., and Aramli, S. (2015). Effect of freezing rate on motility, adenosine triphosphate content and fertilizability in beluga sturgeon (Huso huso) spermatozoa. Cryobiology 70, 170–174. doi: 10.1016/j.cryobiol.2015.02.001
Aril, N., Namai, K., Gomi, F., and Nakazawa, T. (1987). Cryopreservation of medaka embryos during development. Zool. Sci. 4, 813–818.
Asahina, E., and Takahashi, T. (1978). Freezing tolerance in embryos and spermatozoa if the sea urchin. Cryobiology 15, 122–127. doi: 10.1016/0011-2240(78)90016-0
Asturiano, J. F., Cabrita, E., and Ákos, H. (2017). Progress, challenges and perspectives on fish gamete cryopreservation: a mini review general and comparative. Endocrinology 245, 69–76. doi: 10.1016/j.ygcen.2016.06.019
Bart, A. (2000). “New approaches in cryopreservation of fish embryos,” in Cryopreservation in Aquatic Species, 8, eds T. R. Terrence and P. M. Mazik (Baton Rouge: World Aquaculture Society), 179–187. doi: 10.1006/cryo.1997.2014
Bart, A. N., Choosuk, S., and Thakur, D. P. (2006). Spermatophore cryopreservation and artificial insemination of black tiger shrimp, Penaeus monodon (Fabricius). Aquac. Res. 37:523528.
Behlmer, S. D., and Brown, G. (1984). Viability of cryopreserved spermatozoa of the crab Limulus polyphemus. Int. J. Invert. Rep. Dev. 7, 193–199.
Billard, R., Cosson, J., Crim, L. W., and Suquet, M. (1995). “Sperm physiology and quality,” in Brood Stock Management and Egg and Larval Quality, eds N. R. Bromage and R. J. Roberts (Cambridge, MA: Cambridge University Press), 53–76.
Bleil, J. D., and Wassarman, P. M. (1980). Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc. Natl. Acad. Sci. 77, 1029–1033.
Boryshpolets, S., Dzyuba, B., Stejskal, V., and Linhart, O. (2009). Dynamics of ATP and movement in Eurasian perch (Perca fluviatilis L.) sperm in conditions of decreasing osmolality. Theriogenology 72, 851–859. doi: 10.1016/j.theriogenology.2009.06.005
Braga, A., Nakayama, C. L., Poerch, L., and Wasielesky, W. (2013). Unistellate spermatozoa of decapods: comparative evaluation and evolution of the morphology. Zoomorphology 132, 261–284.
Braga, A., Suita, de Castro, L. A., Poersch, L. H., and Wasielesky, W. (2014). Spermatozoal capacitation of pink shrimp Farfantepenaeus paulensis. Aquaculture 430, 207–210.
Cabrita, E., Martinez, P. S., Gavaia, P. J., Riesco, M. F., Valcarce, D. G., Sarasquete, C., et al. (2014). Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 432, 389–401.
Carton, G. F., Riesco, M., Cabrita, E., Herraez, M. P., and Robles, V. (2013). Quantification of lesions in nuclear and mitochondrial genes of Sparus aurata cryopreserved sperm. Aquaculture 402–403, 106–112.
Carton, G. F., Riesco, M. F., Cabrita, E., Martınez, P. F., Herraez, M. P., and Robles, V. (2012). Quantification of DNA damage on specific sequences after gilthead sea bream sperm cryopreservation. Cryobiology 65:363.
Castelo-Branco, T., Bambozzi, A., Mello, M. R. B., and Oshiro, L. M. Y. (2014). Cryopreservation and sperm storage of the white shrimp. Boletim Instit. Pesca 40, 17–22. doi: 10.1016/j.anireprosci.2018.03.008
Castelo-Branco, T., Batista, A. M., Guerra, M. M. P., Soares, R., and Peixoto, S. (2015). Sperm vitrification in the white shrimp Litopenaeus vannamei. Aquaculture 436, 110–113.
Castelo-Branco, T., Guerra, M. M. P., Soares, R., and Peixoto, S. (2018). Sperm vitrification of Litopenaeus vannamei: effect of cryoprotectant solutions on sperm viability and spawning quality after artificial insemination. Aquac. Int. 26, 913–920.
Chao, N. H., and Liao, I. C. (2001). Cryopreservation of finfish and shellfish gametes and embryos. Aquaculture 197, 161–189.
Chen, S. L., and Tian, Y. S. (2005). Cryopreservation of flounder (Paralichthys olivaceus) embryos by vitrification. Theriogenology 63, 1207–1219. doi: 10.1016/j.theriogenology.2004.06.007
Chenais, N., Depince, A., Le Bail, P. Y., and Labbe, C. (2014). Fish cell cryopreservation and fish reconstruction by nuclear transfer stand as promising technologies for preservation of finfish genetic resources. Aquac. Int. 22, 63–76.
Cheung, T. S. (1966). The development of the egg membrane and egg attachment in the shore crab Carcinus maenas, and some related decapods. J. Mar. Biol. Assoc. U.K. 46, 373–400.
Che-Zulkifli, C. I., Chu, K. C., Mustafa, S., Md Sheriff, S., and Ikhwanuddin, M. H. D. (2019a). Use of dry ice for the shipping and packaging of cryopreserved giant grouper Epinephelus lanceolatus spermatozoa. Egyp. J. Aquat. Res. 46, 85–90.
Che-Zulkifli, C. I., Koh, C. C., Sufian, M., Muhammad, T., Muhammad, N. S., Adnan, A. S., et al. (2019b). The performance of dry ice-transported cryopreserved spermatozoa in the artificial insemination of the hybrid grouper (Epinephelus fuscoguttatus ♀× Epinephelus lanceolatus ♂). J. Appl. Aquac. 41:33. doi: 10.1080/10454438.2019.1689217
Chow, S. (1982). Artificial insemination using preserved spermatophores in the palaemonid shrimp, Macrobrachium rosenbergii. Bull. Jpn Soc. Sci. Fish. 48, 177–183.
Chow, S., Taki, T., and Ogassawara, T. (1985). Cryopreservation of spermatophores of the freshwater shrimp Macrobrachium rosenbergii. Biol. Bull. 168, 471–475.
Clark, W. H. Jr., and Griffin, F. J. (1993). “Acquisition and manipulation of penaeoidean gametes,” in Handbook of Techniques for Crustacean Aquaculture, 2nd Edn, Vol. 1, ed. J. McVew (Boca Raton: CRC Press), 133–152.
Clark, W. H. Jr., Ho, P., and Yudin, A. I. (1974). A microscopic analysis of the cortical specialization and their activation in the egg of the prawn, Penaeus sp. Am. Zool. 14:45.
Clark, W. H. Jr., and Lynn, J. W. (1977). A Mg++dependent cortical reaction in the eggs of penaeid shrimp. J. Exp. Zool. 200, 177–183.
Clark, W. H. Jr., Lynn, J. W., Yudin, A. I., and Ho, P. (1980). Morphology of the cortical reaction in the eggs of penaeid shrimp. Biol. Bull. 158, 175–186.
Clark, W. H. Jr., Talbot, P., Neal, R. A., Mock, C. R., and Salser, B. R. (1973). In vitro fertilization with on-motile spermatozoa of the brown shrimp Penaeus aztecus. Mar. Biol. 22, 353–354.
Claudet, P. V., Selvakumar, N., and Natesan, M. (2016). Effect of cryoprotectants and cooling rates on fertility potential of sperm in the giant freshwater prawn, Macrobrachium rosenbergii (De Man). Anim. Reprod. Sci. 171, 40–57. doi: 10.1016/j.anireprosci.2016.05.013
Cotelli, F., Andronico, F., Brivio, M. L., and Lamia, C. (1988). Structure and composition of the fish egg chorion (Carassius auratus). J. Ultrastruct. Mol. Struct. Res. 99, 70–80.
Coward, K., Bromage, N. R., Hibbitt, O., and Parrington, J. (2002). Gametogenesis, fertilization and egg activation in teleost fish. Rev. Fish Biol. Fish. 12, 33–58.
Coward, K., and Parrington, J. (2003). New insights into the mechanism of egg activation in fish. Aquat. Living Resour. 16, 395–398.
Danilo, P. S. Jr., Leandro, C. G., Ricardo, P. R., Darci, C. F., Melanie, D., and Zhang, T. (2014). “Cryopreservation of embryos and oocytes of South American fish species,” in Recent Advances in Cryopreservation, ed. H. Yamashiro (Rijeka: InTech).
de Carvalho, A. F. S., Ramos, S. E., de Carvalho, T. S. G., de Souza, Y. C. P., Zangeronimo, M. G., Pereira, L. J., et al. (2014). Efficacy of fish embryo vitrification protocols in terms of embryo morphology-a systematic review. Cryoletters 35, 361–370.
Digmayer, M. (2013). Influência Da Baixa Temperatura e Diferentes Crioprotetores em Oócitos e Embriões de Colossoma Macropomum e Piaractus Brachypomus. Ph.D. Thesis, Universidade Estadual de Maringá, Maringá.
Ding, F., Lall, S. P., Li, J., Lei, J., Rommens, M., and Milley, J. E. (2011). Cryopreservation of sperm from Atlantic halibut (Hippoglossus hippoglossus L.) for commercial application. Cryobiology 63, 56–60. doi: 10.1016/j.cryobiol.2011.04.009
Ding, F., Milley, J. E., Rommens, M., Li, J., Lei, J., and Lall, S. P. (2012). Effect of hormone implantation on cryopreservation of Atlantic halibut (Hippoglossus hippoglossus L.) sperm. Cryobiology 65, 51–55. doi: 10.1016/j.cryobiol.2012.04.001
Dinnyes, A., Urbanyi, B., Baranyai, B., and Magyary, I. (1998). Chilling sensitivity of carp (Cyprinus carpio) embryos at different developmental stages in the presence or absence of cryoprotectants: work in progress. Theriogenology 50, 1–13. doi: 10.1016/s0093-691x(98)00108-3
Diwan, A. D., Ayyappan, S., Lal, K. K., and Lakra, W. S. (2010). Cryopreservation of fish gametes and embryos. Indian J. Anim. Sci. 80, 109–124.
Diwan, A. D., and Joseph, S. (1998). Cryopreservation of spermatophores of the marine shrimp Penaeus indicus H. Milne edwards. Fish Gen. Biodiversity Conserv. 5, 243–252.
Diwan, A. D., and Kandasami, K. (1997). Freezing of viable embryos and larvae of marine shrimp, Penaeus semisulcatus de Haan. Aquac. Res. 28, 101–104.
Diwan, A. D., and Nandakumar, A. (1998). Studies on cryogenic preservation of sperms of certain cultivable marine fishes. Indian J. Fish 45, 387–397.
Dong, Q., Lin, J., and Huang, C. (2004). Effects of cryoprotectant toxicity on the embryos and rvae of pacific white shrimp Litopenaeus vannamei. Aquaculture 242, 655–670.
Drouslet, J. J., Yudin, A. I., Wheeler, R. S., and Clark, W. H. Jr. (1975). “Light and fine structural studies of natural and artificially induced egg growth of penaeid shrimp,” in Proceedings of the 6th Ann. Meet World Mariculture Society, Seattle.
Dziewulska, K., Rzemieniecki, A., Czerniawski, R., and Domagała, J. (2011). Post-thawed motility and fertility from Atlantic salmon (Salmo salar L.) sperm frozen with four cryodiluents in straws or pellets. Theriogenology 76, 300–311. doi: 10.1016/j.theriogenology.2011.02.007
Erdahl, D. A., and Graham, E. F. (1980). “Preservation of gametes of freshwater fish,” in Proceedings of International Conference of Animal Reproduction and Artificial Insemination, Madrid, 317–326.
Fatihah, S. N., Abol-Munafi, A. B., Noorbaiduri, H., Muhd-Farouk, I., and Khwanuddin, M. (2016). Development of a sperm cryopreservation protocol for the mud S spiny lobster, Panulirus polyphagus. Aquaculture 462, 56–63.
Fauvel, C., Boryshpolets, S., Cosson, J., Wilson Leedy, J. G., Labbe, C., Haffray, P., et al. (2012). Improvement of chilled sea bass sperm conservation using a cell culture medium. J. Appl. Ichthyol. 28, 961–966.
Figueroa, E., Ivan, V., Andrea, B. Z., Carolina, A. F., Kelly, D., Rodrigo, L. C., et al. (2015a). Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 9, 76–87.
Figueroa, E., Merino, O., Risopatron, J., Isachenko, V., Sanchez, R., Effer, B., et al. (2015b). Effect of seminal plasma on Atlantic salmon (Salmo salar) sperm vitrification. Theriogenology 83, 238–245. doi: 10.1016/j.theriogenology.2014.09.015
Figueroa, E., Risopatron, J., Sanchez, R., Isachenko, E., Merino, O., Valdebenito, I., et al. (2013). Sperm vitrification of sex-reversed rainbow trout (Oncorhynchus mykiss): effect of seminal plasma on physiological parameters. Aquaculture 37, 119–126.
Figueroa, E., Valdebenito, I., and Farias, J. G. (2014). Technologies used in the study of sperm function in cryopreserved fish spermatozoa. Aquac. Res. 47, 1691–1705. doi: 10.1111/are.12630
Fornari, D. C., Ribeiro, R. P., Streit, D. P. Jr., Vargas, L., Godoy, L. C., Oliveira, C. A. L., et al. (2012). Increasing storage capability of pacu (Piaractus mesopotamicus) embryos by chilling: development of a useful methodology for hatcheries management. CryoLetters 33, 125–133.
Guan, M., Rawson, D. M., and Zhang, T. (2010). Cryopreservation of zebra fish (Danio rerio) oocytes by vitrification. Cryoletters 31, 230–238.
Gwo, J. C. (1995). Cryopreservation of oyster (Crassostrea gigas) embryos. Theriogenology 43, 1163–1174.
Gwo, J. C. (2000). Cryopreservation of aquatic invertebrate semen: a review. Aquac. Res. 31, 259–271.
Hagedorn, M., Hsu, E., Kleinhans, F. W., and Wildt, D. E. (1997). New approaches for studying the permeability of fish embryos: toward successful cryopreservation. Cryobiology 34, 335–347. doi: 10.1006/cryo.1997.2014
Hagedorn, M., Kleinhans, F. W., Artemov, D., and Pilatus, U. (1998). Characterization of a major permeability barrier in the zebra fish embryo. Biol. Reprod. 59, 1240–1250.
Hagedorn, M., Kleinhans, F. W., and Wildt, D. E. (1996). Water permeability studies in dichorionated zebra fish embryos. Cryobiology 33:646.
Harvey, B. (1983). Cryopreservation of Sarotherodon mossambicus spermatozoa. Aquaculture 32, 313–320.
Harvey, B., Kelly, R. N., and Ashwood-Smith, M. J. (1983). Cryobiology Permeability of intact and dichorionated zebra fish embryos to glycerol and dimethyl sulfoxide. Aquaculture 20, 432–439. doi: 10.1016/0011-2240(83)90033-0
Holtz, W. (1993). Cryopreservation of rainbow trout (Oncorhynchus mykiss) sperm: practical recommendations. Aquaculture 110, 97–100.
Horton, H. F., and Ott, A. G. (1976). Cryopreservation of fish spermatozoa and ova. J. Fish Res. Board Can. 33, 995–1000.
Horvath, A., Miskolczi, E., and Urbanyi, B. (2003). Cryopreservation common carp sperm. Aquat. Living Resour. 16, 457–460.
Horvath, A., and Urbanyi, B. (2000). The effect of cryoprotectants on the motility and fertilizing capacity of cryopreserved of African catfish sperm, Clarius gariepinus (Burchell 1822). Aquac. Res. 31, 317–324.
Horvath, A., Urbanyi, B., and Mims, S. D. (2009). “Cryopreservation of sperm from species of the order acipenseriformes,” in Methods in Reproductive Aquaculture: Marine and freshwater species, eds E. Cabrita, V. Robles, and P. Herraez (Florida: CRC Press), 409–414.
Hu, E., Yang, H., and Tiersch, T. R. (2011). High-throughput cryopreservation of spermatozoa of blue catfish (Ictalurus furcatus): establishment of an approach for commercial-scale processing. Cryobiology 62, 74–82. doi: 10.1016/j.cryobiol.2010.12.006
Huang, Q. D., and Tiersch, T. R. (2004). Sperm cryopreservation of a live-bearing fish, the platy fish Xiphophorus couchianus. Theriogenology 62, 971–989. doi: 10.1016/j.theriogenology.2003.12.022
Isayeva, A., Zhang, T., and Rawson, D. M. (2004). Studies on chilling sensitivity of zebra fish (Danio rerio) oocytes. Cryobiology 49, 114–122. doi: 10.1016/j.cryobiol.2004.05.005
Ishida, T., Talbot, P., and Kooda-Cisco, M. (1986). Technique for long term storage of Lobster (Homarus) spermatophores. Gametes Res. 14, 183–195. doi: 10.1002/jmor.1051720206
Janik, M., Kleinhans, F. W., and Hagedorn, M. (2000). Overcoming permeability barriers by microinjecting cryoprotectants into zebra fish embryos (Brachydanio rerio). Cryobiology 41, 25–35. doi: 10.1006/cryo.2000.2261
Jeyalectumie, C., and Subramoniam, T. (1989). Cryopreservation of spermatophores of the edible crab, Scylla serrata. Biol. Bull. 177, 247–253.
John, G., Ponniah, A. G., Lakra, W. S., Gopalkrishnan, A., and Barat, A. (1993). Preliminary attempts to cryopreserve embryos of Cyprinus carpio (L) and Labeo rohita (Ham). J. Inland Fish. Soc. India 25, 55–57.
Judycka, S., Szczepkowski, M., Ciereszko, A., and Dietrich, G. J. (2015). New extender for cryopreservation of Siberian sturgeon (Acipenser baerii) semen. Cryobiology 70, 184–189. doi: 10.1016/j.cryobiol.2015.02.005
Jusdado, J. G., Victor, G., Marina, M., Christoffer, R., Luz, P., Tamás, M., et al. (2019). Eel sperm cryopreservation: an overview. Theriogenology 133, 210–215. doi: 10.1016/j.theriogenology.2019.03.033
Kalicharan, D., Jongebloed, W. L., Zhang, T., and Rawson, D. M. (1997). “The zebra fish (Brachydanio rerio) egg/embryo inner and outer membrane: a FE-SEM and TEM investigation. Part 1: chorion,” in Jaarboek NVvM’97, ed. W. Belendsen (Leiden: Karstens Drukker), 96–98.
Keivanloo, S., and Sudagar, M. (2013). Preliminary studies on the cryopreservation of Persian Sturgeon (Acipenser persicus) embryos. Open Access Sci. Rep. 2:674. doi: 10.4172/scientific reports: 674
Keivanloo, S., and Sudagar, M. (2016). Cryopreservation of Persian sturgeon (Acipenser persicus) embryos by DMSO-based vitrificant solutions. Theriogenology 85, 1013–1018. doi: 10.1016/j.theriogenology.2015.11.012
Kerby, J. H. (1983). Cryogenic preservation of sperm from striped bass. Trans. Am. Fish. Soc. 112, 86–94.
Kimmel, C. B., and Law, R. D. (1985). Cell lineage of zebra fish blastomeres. II. Formation of the yolk syncytial layer. Dev. Biol 108, 86–93. doi: 10.1016/0012-1606(85)90011-9
Kinsey, W. H., Sharma, D., and Kinsey, S. C. (2007). “Fertilization and egg activation in fishes,” in The Fish Oocyte: From Basic Studies to Biotechnological Applications, eds P. J. Babin, J. Cerdà, and E. Lubzens (Berlin: Springer), 397–409.
Kopeika, J., Zhang, T., Rawson, D. M., and Elgar, G. (2005). Effect of cryopreservation on mitochondrial DNA of zebra fish (Danio rerio) blastomere cells. Mutat. Res. Fundament. Mol. Mech. Mutagen. 570, 49–61. doi: 10.1016/j.mrfmmm.2004.09.007
Kuleshova, L. L., Shaw, J. M., and Trounson, A. O. (2001). Studies on replacing most of the penetrating cryoprotectant by polymers for embryo cryopreservation. Cryobiology 43:2131. doi: 10.1006/cryo.2001.2335
Kurokura, H., Wu, J. G., Anil, A. C., and Kado, R. (1992). “Preliminary studies on the cryopreservation of the cirripede nauplius,” in Proceedings of the Workshop on gametes and Embryos Storage and Preservation of Aquatic organism, Mary Le Roi.
Labbé, C., Robles, V., and Herráez, M. P. (2013). “Cryopreservation of gametes for aquaculture and alternative cell sources for genome preservation,” in Advances in Aquaculture Hatchery Technology, eds G. Allan and G. Burnell (Sawston: Wood Head Publishing), 76–116.
Lakra, W. S. (1993). Cryogenic preservation of fish spermatozoa and its application to aquaculture. Indian J. Cryogenics 18, 171–176.
Leandro, C. G., Danilo, P. S., Zampolla, T., Mikich, A. B., and Zhang, T. (2013). A study on the vitrification of stage III zebra fish (Danio rerio) ovarian follicles. Cryobiology 67, 347–354. doi: 10.1016/j.cryobiol.2013.10.002
Legendre, M., and Billard, R. (1980). Cryopreservation of rainbow trout sperm by deep freezing. Reprod. Nutr. Dev. 20, 1859–1868. doi: 10.1051/rnd:19801012
Lerveroni, C. S., and Maisee, G. (1998). Cryopreservation of carp (Cyprinus carpio) blastomere: influence of Embryo stage on post thaw survival rate. Cryobiology 36, 255–262.
Lerveroni, C. S., and Maisee, G. (1999). Cryopreservation of Rainbow Trout (Oncorhynchus mykiss)) blastomere. Aquat. Living Resour. 12, 71–74. doi: 10.1006/cryo.1998.2084
Leung, L. K. B., and Jamieson, B. G. M. (1991). “Live preservation of fish gametes,” in Fish Evolution and Systematic: Evidence from Spermatozoa, ed. B. G. M. Jamieson (Cambridge, MA: Cambridge University Press), 245–269.
Li, L., Zhang, X., Zhao, L., Xia, X., and Wang, W. (2012). Comparison of DNA apoptosis in mouse and human blastocysts after vitrification and slow freezing. Mol. Reprod. Dev. 79, 229–236. doi: 10.1002/mrd.22018
Li, P., Hulak, M., Li, Z. H., Sulc, M., Psenicka, M., Rodina, M., et al. (2013). Cryopreservation of common carp (Cyprinus carpio L.) sperm induces protein phosphorylation in tyrosine and threonine residues. Theriogenology 80, 84–89. doi: 10.1016/j.theriogenology.2013.03.021
Linhart, O., Rodina, M., and Cosson, J. (2000). Cryopreservation of sperm in common carp Cyprinus carpio: sperm motility and hatching success of embryos. Cryobiology 41, 241–250. doi: 10.1006/cryo.2000.2284
Liu, L., Wei, Q., Guo, F., Zhang, J., and Zhang, T. (2006). Cryopreservation of Chinese sturgeon (Acipenser sinensis) sperm. J. Appl. Ichthyol. 22, 384–388.
Liu, Q., Wang, X., Wang, W., Zhang, X., Xu, S., Ma, D., et al. (2015). Effect of the addition of six antioxidants on sperm motility, membrane integrity and mitochondrial function in red sea bream (Pagrus major) sperm cryopreservation. Fish Physiol. Biochem. 41, 413–422. doi: 10.1007/s10695-014-9993-9
Liu, Q. H., Ma, D. Y., Xu, S. H., Xiao, Z. Z., Xiao, Y. S., Song, Z. C., et al. (2015). Summer flounder (Paralichthys dentatus) sperm cryopreservation and application in interspecific hybridization with olive flounder (P. olivaceus). Theriogenology 83, 703–710. doi: 10.1016/j.theriogenology.2014.11.004
Liu, X. H., Zhang, T., and Rawson, D. M. (1998a). Effects of cooling rates and anoxia on the chilling sensitivity of zebra fish (Danio rerio) embryos. CryoLetters 19, 309–318. doi: 10.1016/s0093-691x(02)01199-8
Liu, X. H., Zhang, T., and Rawson, D. M. (1998b). Feasibility of vitrification of zebra fish (Danio rerio) embryos using methanol. Cryo Lett. 19, 309–318.
Liu, X. H., Zhang, T., and Rawson, D. M. (1999a). Effects of cooling rates and anoxia on the chilling sensitivity of zebra fish (Danio rerio) embryos. Cryobiology 39, 318–319.
Liu, X. H., Zhang, T., and Rawson, D. M. (1999b). The effect of partial removal of yolk on the chilling sensitivity of zebra fish (Danio rerio) embryos. Cryobiology 39, 236–242. doi: 10.1006/cryo.1999.2206
Liu, X. H., Zhang, T., and Rawson, D. M. (2000). Differential scanning calorimetry study on the characterization of intra-embryonic freezing and cryoprotectant penetration in zebra fish (Danio rerio) embryos. Cryobiology 41, 355–356. doi: 10.1002/jez.1060
Liu, X. H., Zhang, T., and Rawson, D. M. (2000). D.Sc. studies on characterization of Intra embryonic freezing and cryoprotectant penetration in zebra fish embryos. Cryobiology 41, 355–356.
Liu, X. H., Zhang, T., and Rawson, D. M. (2001a). Differential scanning calorimetry studies of intra-embryonic freezing and cryoprotectant penetration in zebra fish (Danio rerio) embryos. J. Exp. Zool. 290, 299–310.
Liu, X. H., Zhang, T., and Rawson, D. M. (2001b). Effect of cooling rate and partial removal of yolk on the chilling injury in zebra fish (Danio rerio) embryos. Theriogenology 55, 1719–1731. doi: 10.1016/s0093-691x(01)00515-5
Liu, Y., Harvey, B., and Terrence, R. T. (2019). Development of germplasm repositories to assist conservation of endangered fishes: examples from small-bodied live bearing fishes. Theriogenology 135, 138–151. doi: 10.1016/j.theriogenology.2019.05.020
Lui, K. C., Chu, T. C., and Lin, H. D. (1992). “Cryo-survival of goldfish embryos between the stages optical vesicle and heart formation under subzero freezing,” in Proceedings of the Workshop on Gamete and Embryo Storage and cryopreservation of Aquatic Organisms, Marly Le Roi.
Lynn, J. W., and Clark, W. H. Jr. (1975). A Mg++ dependent cortical reaction in the egg of penaeid shrimp. J. Cell. Biol. 251, 177–183.
Lynn, J. W., and Clark, W. H. Jr. (1983). A morphological examination of sperm egg interaction in the fresh water prawn Macrobrachium rosenbergii. Biol. Bull. 164, 446–458.
Lynn, J. W., and Clark, W. H. Jr. (1987). Physiological and biochemical investigations of the egg jelly release in Penaeus aztecus. Biol. Bull. 173, 451–460. doi: 10.2307/1541692
Maria, A. N., Carvalho, A. C. M., Araujo, R. V., Santos, J. P., Carneiro, P. C. F., and Azevedo, H. C. (2015). Use of cryo-tubes for the cryopreservation of Tambaqui fish semen (Colossoma macropomum). Cryobiology 70, 109–114. doi: 10.1016/j.cryobiol.2015.02.004
Marques, L. S., Mikich, A. B., Leandro, G., Laura, A. S., Daniel, M., Zhang, T., et al. (2015). Viability of zebra fish (Danio rerio) ovarian follicles after vitrification in a metal container. Cryobiology 71, 1–7. doi: 10.1016/j.cryobiol.2015.09.004
Martinez, P. S., Diogo, P., Dinis, M. T., Soares, F., Sarasquete, C., and Cabrita, E. (2013). Effect of two sulfur-containing amino acids, and hypotaurine in European sea bass (Dicentrarchus labrax) sperm cryopreservation. Cryobiology 66, 333–338. doi: 10.1016/j.cryobiol.2013.04.001
Martinez, P. S., Horváth, Á, Labbé, C., Zhang, T., Robles, V., Herráez, P., et al. (2016). Cryobanking of aquatic species. Aquaculture 472, 156–177.
McAndrew, B. J., Rana, K. J., and Penman, D. J. (1993). “Conservation and genetic variation in aquatic organisms,” in Recent Advances in Aquaculture, Vol. IV, eds J. F. Muir and R. J. Roberts (Oxford: Blackwell Science), 295–236.
McLellan, M. R., Mac Fadzen, I. R. B., Morris, G. J., Hartinez, G., and Wyban, J. A. (1992). “Recovery of shrimp embryos (Penaeus vannamei and Penaeus monodon) from -30°C,” in Proceedings of the Workshop on Gamete and Embryo Storage and cryopreservation of Aquatic Organisms, Marly Le Roi.
Mims, S. D., Tsvetkova, L. I., Brown, G. G., and Gomelsky, B. J. (2011). “Cryopreservation of sperm of sturgeon and paddlefish,” in Cryopreservation in Aquatic Species, eds T. R. Tiersch and P. M. Mazik (Baton Rouge: World Aquaculture Society), 123–129.
Miyazaki, S., Shirakawa, H., Nakad, K., and Honda, Y. (1993). Essential role of the inositol 1,4,5–triphosphate/Ca2+ channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev. Biol. 158, 62–78. doi: 10.1016/j.ydbio.2007.12.012
Muir, J. E., and Roberts, R. J. (1993). “Conservation and preservation of genetic variation,” in Recent Advances in Aquaculture, IV, eds F. James and M. R. J. Roberts (London: Blackwell Scientific Publication), 305–307.
Nahiduzzaman, M., Mahbubul, H. M., Roy, P. K., Hossain, A. M., Hossain, M. A. R., and Tiersch, T. R. (2012). Sperm cryopreservation of the Indian major carp, Labeo calbasu: effects of cryoprotectants, cooling rates and thawing rates on egg fertilization. Anim. Reprod. Sci. 136, 133–138. doi: 10.1016/j.anireprosci.2012.10.023
Neves, P. R., Ribeiro, R. P., Streit, D. P. Jr., Natali, M. R. M., Fornari, D. C., Santos, A. I., et al. (2012). Injuries in pacu embryos (Piaractus mesopotamicus) after freezing and thawing. Zygote 20, 1–7. doi: 10.1017/S096719941200024X
Nilsson, E., and Cloud, J. G. (1992). “Cryopreservation of isolated blastomeres from rainbow trout blastulae,” in Proceedings of the Workshop on Gamete and Embryo Storage and cryopreservation of Aquatic Organisms, Marly Le Roi.
Nilsson, E., and Cloud, J. G. (1993). Cryopreservation of rainbow trout (Oncorhynchus mykiss) blastomeres. Aquat. Living Resour. 6, 77–80. doi: 10.1006/cryo.1998.2084
Nimrat, S., Sangnawakij, T., and Vuthiphandchai, V. (2005). Preservation of black tiger shrimp Penaeus monodon spermatophores by chilled storage. J. World Aquacult. Soc. 36, 76–86.
Nimrat, S., Siriboonlamon, S., Zhang, S., Xu, Y., and Vuthiphandchai, V. (2006). Chilled storage of white shrimp (Litopenaeus vannamei) spermatophores. Aquaculture 261:951.
Ninhaus, S. A., Foresti, F., Azevedo, A., Agostinho, C. A., and Silveira, R. V. (2008). Cryogenic preservation of embryos of Prochilodus lineatus (Valenciennes, 1836) (Characiforme; Prochilodontidae). Zygote 17:45. doi: 10.1017/S0967199408004991
Nynca, J., Dietrich, G. J., Dobosz, S., Zalewski, T., and Ciereszko, A. (2015). Effect of Post-thaw storage time and sperm-to-egg ratio on fertility of cryopreserved brook trout sperm. Theriogenology 83, 253–256. doi: 10.1016/j.theriogenology.2014.09.009
Pillai, M. C., and Clark, W. H. Jr. (1987). Oocytes activation in the marine shrimp, Sicyonia ingentis. J. Exp. Zool. 244, 325–329.
Plachinta, M., Zhang, T., and Rawson, D. M. (2004). Studies on cryoprotectant toxicity to zebra fish (Danio rerio) oocytes. Cryoletters 25, 415–424.
Ponniah, A. G., Sahoo, P. K., Dayal, R., and Barat, A. (1999). Cryopreservation of Tor putitora spermatozoa. Effect of extender composition, activation solution, cryoprotectant and equilibration time. Proc. Natl. Acad. Sci. U.S.A. 1, 53–59.
Preston, N. P., and Coman, F. E. (1998). “The effects of cryoprotectants, chilling and freezing on Penaeus esculentus embryos and nauplii,” in Advances in Shrimp Biotechnology, ed. T. W. Flegel (Khlong Nueng: National Center for Genetic Engineering and Biotechnology).
Rahman, S. M., Ahsan, M. N., Abdullah, A. M., Hossain, Q. Z., Rahman, M. M., Hasan, M. M., et al. (2015). Evaluating the suitability of cryoprotectants and cryopreservation solutions for olive barb, Puntius sarana embryos. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 85, 175–180.
Rall, W. F. (1993). “Recent advances in the cryopreservation of salmonids fishes,” in Genetic conservation of Salmonids fishes, eds J. G. Cloud and G. H. Thorgard (New York: Plenum Press), 138–158.
Rana, K. J., McAndrew, B. J., and Musa, A. (1992). “Cryopreservation of oyster, Crassostrea gigas eggs and embryos,” in Proceedings of the Workshop on Gamete and Embryo Storage and cryopreservation of Aquatic Organisms, Marly Le Roi. doi: 10.1016/0093-691x(95)00088-p
Rawson, D. M., and Zhang, T. (2005). New Approaches to the Cryopreservation of Fish Oocytes and Embryos. Turin: Villa Gualino, 209–210.
Rawson, D. M., Zhang, T., Kalicharan, D., and Jongebloed, W. L. (2000). FE-SEM and TEM studies of the chorion, plasma membrane and syncytial layers of the gastrula stage embryo of the zebra fish (Brachydanio rerio): a consideration of the structural and functional relationship with respect to cryoprotectant penetration. Aquac. Res. 31, 325–336.
Rawson, D. M., Zhang, T. T., and Jongebloed, W. (1997). Structure-permeability relationships of the chorion and vitelline membranes of zebra fish (Brachydanio rerio) embryos. Cryo Lett. 18:67.
Renard, P. (1991). Cooling and freezing tolerances in embryos of the Pacific oyster, Crassostrea gigas: methanol and sucrose effects. Aquaculture 92, 43–57.
Robles, V., Cabrita, E., Acker, J. P., Herraez, M. P. (2008). “Embryo cryopreservation: what we know until now?,” in Methods in Reproductive Aquaculture, eds E., Cabrita, V., Robles, and M. P., Herraez (Boca Raton: CRC Press), 261–294.
Robles, V., Cabrita, E., Anel, L., and Herráez, M. P. (2006). Micro injection of the antifreeze protein type III (AFPIII) in turbot (Scophthalmus maximus) embryos: toxicity and protein distribution. Aquaculture 261, 1299–1306.
Rosati, F., Cotelli, F., De Santis, R., Monroy, C., and Pinto, M. R. (1982). Synthesis of fucosyl containing glycoproteins of the vitelline coat in the oocytes of Ciona intestinalis (Ascidia). Proc. Natl. Acad. Sci. U.S.A. 79, 1908–1911. doi: 10.1073/pnas.79.6.1908
Routray, P., Suzuki, T., and Strüssmann, C. A. (2002). Factors affecting the uptake of DMSO by the eggs and embryos of medaka, Oryzias latipes. Theriogenology 58, 1483–1496. doi: 10.1016/s0093-691x(02)01076-2
Sanches, E. G., Oliveira, I. R., Serralheiro, P. C. D. S., and Cerqueira, V. R. (2013). Cryopreservation of mutton snapper (Lutjanus analis) sperm. An. Acad. Bras. Cienc. 85, 1083–1092. doi: 10.1590/S0001-37652013005000047
Saragusty, J., and Arav, A. (2011). Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 141, 1–19. doi: 10.1530/REP-10-0236
Sawitri, S., and Amrit, B. (2010). Ultrasound enhanced permeation of methanol in to zebra fish, Danio rerio, embryos. Aquaculture 303, 71–76.
Seki, S., Kouya, T., Tsuchiya, R., Valdez, D. M., Jin, B., Hara, T., et al. (2008). Development of a reliable in-vitro maturation system for zebra fish oocytes. Reproduction 135, 285–292. doi: 10.1530/REP-07-0416
Seki, S., Kouya, T., Tsuchiya, R., Valdez, D. M., Jin, B., Koshimoto, C., et al. (2011). Cryobiological properties of immature zebra fish oocytes assessed by their ability to be fertilized and develop into hatching embryos. Cryobiology 62, 8–14. doi: 10.1016/j.cryobiol.2010.11.003
Sharifuddin, M. M., and Azizah, M. N. S. (2014). Preliminary studies on cryopreservation of snakehead (Channa striata) embryos. Cryobiology 69, 1–9. doi: 10.1016/j.cryobiol.2014.04.001
Simon, C., Dumont, P., Cuende, F., and Dilter, A. (1994). Determination of suitable freezing media for cryopreservation of Penaeus indicus embryos. Cryobiology 31, 245–253.
Streit, D. P., Godoy, L. C., Ribeiro, R. P., Fornari, D. C., Digmayer, M., and Zhang, T. (2014). “Cryopreservation of embryos and oocytes of South American fish species,” in Recent Advances in Cryopreservation, ed. H. Yamashiro (London: IntechOpen).
Subramoniam, T. (1994). Cryopreservation of crustacean gametes and embryos. Proc. Indian Natl. Sci. Acad. B 60, 229–236.
Subramoniam, T., and Newton, S. S. (1993). Cryopreservation of embryos of the penaeid prawn Penaeus indicus. Curr. Sci. 65, 176–177.
Suquet, M., Labbe, C., Puyo, S., Mingant, C., Quittet, B., Boulais, M., et al. (2014). Survival, growth and reproduction of cryopreserved larvae from a marine Invertebrate, the Pacific oyster (Crassostrea gigas). PLoS One 9:e93486. doi: 10.1371/journal.pone.0093486
Suzuki, T., Komada, H., Takai, R., Arii, K., and Kozima, T. T. (1995). Relation between toxicity of cryoprotectant DMSO and its concentration in several fish embryos. Fish. Sci. 61, 193–197.
Talbot, P., and Goudeau, M. (1988). A complex cortical reaction leads to formation of the fertilization envelope in the lobster Homarus. Gamete Res. 19, 1–18. doi: 10.1002/mrd.1120190102
Tekin, N., Selçu, K., Sakcay, E., and Bozkurt, Y. (2003). Cryopreservation of rainbow trout (Oncorhynchus mykiss) semen. Israeli J. Aquac. Bamidgeh 55, 208–212. doi: 10.1016/j.cryobiol.2014.10.005
Tiersch, T. R., and Green, C. C. (2011). Cryopreservation in Aquatic Species, 2nd Edition. Baton Rouge: World Aquaculture Society.
Tsai, S., and Lin, C. (2009). Effects of cryoprotectant on the embryos of banded coral shrimp (Stenopus hispidus), preliminary studies to establish freezing protocols. CryoLetters 30, 373–381.
Tsai, S., and Lin, C. (2012). Advantage and applications of cryopreservation in fisheries science. Braz. Arch. Biol. Technol. 55, 1–15.
Tsai, S., Rawson, D. M., and Zhang, T. (2008). Studies on cryoprotectant toxicity to early stage zebra fish (Danio rerio) ovarian follicle. CryoLetters 29, 477–483.
Tsai, S., Rawson, D. M., and Zhang, T. (2009). Development of cryopreservation protocols for early stage zebra fish (Danio rerio) ovarian follicles using controlled slow cooling. Theriogenology 71, 1226–1233. doi: 10.1016/j.theriogenology.2009.01.014
Tsai, S., Rawson, D. M., and Zhang, T. (2010). Development of in vitro culture method for early stage zebra fish (Danio rerio) ovarian follicles for use in cryopreservation studies. Theriogenology 74, 290–303. doi: 10.1016/j.theriogenology.2010.02.013
Usuki, H., Hamaguchi, M., and Ishioka, H. (2002). Effects of developmental stage, seawater concentration and rearing temperature on cryopreservation of Pacific oyster Crassostrea gigas larvae. Fish. Sci. 68, 757–762.
Utting, S., Como, Y., and Mc Fadzen, I. (1990). Frozen larvae, bivalve-hatcheries could soon reap benefits. Fish Farmer 13, 45–47.
Valdez, M. D., Miyamoto, A., Hara, T., Edashige, K., and Kasai, M. (2005). Sensitivity to chilling of medaka (Orysias latipes) embryos at various developmental stages. Theriogenology 64, 112–122. doi: 10.1016/j.theriogenology.2004.11.006
Vuthiphandchai, V., Nimrat, S., Kotcharat, S., and Bart, A. N. (2007). Development of a cryopreservation protocol for long-term storage of black tiger shrimp (Penaeus monodon) spermatophores. Theriogenology 68, 1192–1199. doi: 10.1016/j.theriogenology.2007.08.024
Whittingham, D. G., and Rosenthal, H. (1978). Attempts to preserve herring embryos at subzero temperatures. Arch. Fish. Wiss. 29, 75–79.
Xin, M., Hamid, N., Anna, S. K., Mohammad, A. M. S., Jan, S., Sergii, B., et al. (2019). Molecular and subcellular cryoinjury of fish spermatozoa and approaches to improve cryopreservation. Rev. Aquac. 6, 1–16. doi: 10.1111/raq.12355
Yamaner, G., Memiş, D., and Baran, A. (2015). Sperm quality and effects of different cryomedia on spermatozoa motility in first-time spawning of cultured Russian sturgeon (Acipenser gueldenstaedtii Brandt & Ratzeburg, 1833). J. Appl. Ichthyol. 31, 71–74.
Yang, H., Carmichael, C., Varga, Z. M., and Tiersch, T. R. (2007a). Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebra fish, Danio rerio. Theriogenology 68, 128–136. doi: 10.1016/j.theriogenology.2007.02.015
Yang, H., Cuevas-Uribe, R., Savage, M. G., Walter, R. B., and Tiersch, T. R. (2012). Sperm cryopreservation in live-bearing Xiphophorus fishes: offspring production from Xiphophorus variatus and strategies for establishment of sperm repositories. Zebra Fish. 9, 126–134. doi: 10.1089/zeb.2012.0737
Yang, H., Hazelwood, L., Heater, S. J., Guerrero, P. A., Walter, R. B., and Tiersch, T. R. (2007b). Production of F1 interspecies hybrid offspring with cryopreserved sperm from a live-bearing fish, the swordtail Xiphophorus helleri. Biol. Reprod. 76, 401–406. doi: 10.1095/biolreprod.106.056549
Yang, H., Hu, E., Buchanan, J. T., and Tiersch, T. R. (2017). A strategy for sperm cryopreservation of Atlantic Salmon, Salmo salar, for remote commercial-scale high-throughput processing. J. World Aquac. Soc. 49, 694–696. doi: 10.1111/jwas.12431
Yang, H., and Tiersch, T. (2009). Sperm motility initiation and duration in a euryhaline fish, medaka (Oryzias latipes). Theriogenology 72, 386–392. doi: 10.1016/j.theriogenology.2009.03.007
Yildiz, C., Yavas, I., Bozkurt, Y., and Aksoy, M. (2015). Effect of cholesterol-loaded cyclodextrin on Cryosurvival and fertility of cryopreserved carp (Cyprinus carpio) sperm. Cryobiology 70, 190–194. doi: 10.1016/j.cryobiol.2015.01.009
Yonge, C. M. (1937). The nature and significance of the membrane surrounding the developing eggs of Homarus vulgaris and other decapods. Proc. Zool. Soc. Lond. Ser. A 107, 499–517.
Yonge, C. M. (1955). Egg attachment in Crangon vulgaris and other caridean. Proc. R. Soc. Edinburgh 65, 369–400.
Zell, S. R. (1978). Cryopreservation of gametes and embryos of salmonids fishes. Ann. Biol. Anim. Biochem. Biophys. 18, 1089–1099.
Zhang, T., Isayeva, A., Adams, S. L., and Rawson, D. M. (2005a). Studies on membrane permeability of zebra fish (Danio rerio) oocytes in the presence of different cryoprotectants. Cryobiology 50, 285–293. doi: 10.1016/j.cryobiol.2005.02.007
Zhang, T., Plachinta, M., Kopeika, J., Rawson, D. M., Cionna, C., Tosti, L., et al. (2005b). Membrane integrity and cathepsin activities of zebra fish (Danio rerio) oocytes after freezing to -196°C using controlled slow cooling. Cryobiology 51, 385–386. doi: 10.1016/j.cryobiol.2008.01.002
Zhang, Y. Z., Zhang, S. C., Liu, X. Y., Xu, Y. Z., Hu, J. H., Xu, Y. Y., et al. (2005). Toxicity and protective efficiency of cryoprotectant to flounder (Paralichthys olivaceus) embryos. Theriogenology 63, 765–773. doi: 10.1016/j.theriogenology.2004.04.011
Zhang, T., Liu, X.-H., and Rawson, D. M. (2003a). Effects of methanol and developmental arrest on chilling injury in zebra fish (Danio rerio) embryos. Theriogenology 59, 1545–1556.
Zhang, T., Plachinta, M., Isayeva, A., and Rawson, D. M. (2003b). Studies on zebra fish (Danio rerio) oocytes sensitivity to chilling and cryoprotectant toxicity. Cryobiology 47, 259.
Zhang, T., Rawson, D., Pekarsky, I., Blais, I., and Lubzens, E. (2007). “Low-temperature preservation of fish gonad cells and oocytes,” in The Fish Oocyte, eds P. Babin, J. Cerda, and E. Lubzens (Netherlands: Springer), 411–436. doi: 10.1007/978-1-4939-3795-0_12
Zhang, T., and Rawson, D. M. (1993). Cryoprotectant toxicity and permeability studies on zebra fish (Brachydanio rerio) embryos. Cryobiology 6:640. doi: 10.1006/cryo.1997.2002
Zhang, T., and Rawson, D. M. (1995). Studies on chilling sensitivity of zebra fish (Brachydanio rerio) embryos. Cryobiology 32, 239–246.
Zhang, T., and Rawson, D. M. (1995a). Preliminary studies on vitelline membrane permeability of zebra fish (Brachydanio rerio) embryos. CryoLetters 16:319.
Zhang, T., and Rawson, D. M. (1995b). Studies on chilling sensitivity of zebra fish (Brachydanio rerio) embryos. Cryobiology 32, 239–246.
Zhang, T., and Rawson, D. M. (1996a). Feasibility studies on vitrification of zebra fish (Brachydanio rerio) embryos. Cryobiology 33, 1–13. doi: 10.1262/jrd.09-136e
Zhang, T., and Rawson, D. M. (1996b). Permeability of the vitelline membrane of zebra fish (Brachydanio rerio) embryos to methanol and propane-1, 2-diol. CryoLetters 17, 273–280.
Zhang, T., and Rawson, D. M. (1998). Permeability of dichorionated 1-cell and 6-somite stage zebra fish (Brachydanio rerio) embryos to water and methanol. Cryobiology 32, 239–246. doi: 10.1006/cryo.1998.2093
Zhang, T., Rawson, D. M., and Jongebloed, W. L. (1998). Further studies on zebra fish (Brachydanio rerio) embryo membranes: permeability and ultra-structure. CryoLetters 3:65.
Zhang, T., Rawson, D. M., and Morris, G. J. (1993). Cryopreservation of pre-hatch embryos of zebra fish (Brachydanio rerio). Aquat. Living Resour. 6, 145–153.
Zhang, X. S., Zhao, L., Hua, T. C., and Zhu, H. Y. (1989). A study on the cryopreservation of common carp (Cyprinus carpio) embryos. CryoLetters 10, 271–278. doi: 10.1016/j.cryobiol.2007.02.003
Zilli, L., Beirao, J., Schiavone, R., Herraez, M. P., Gnoni, A., and Vilella, S. (2014). Comparative proteome analysis of cryopreserved fiagella and head plasma membrane proteins from sea bream spermatozoa: effect of antifreeze proteins. PLoS One 9:e99992. doi: 10.1371/journal.pone.0099992
Keywords: cryobanking, fish, shellfish, egg, embryos, larvae
Citation: Diwan AD, Harke SN, Gopalkrishna and Panche AN (2020) Cryobanking of Fish and Shellfish Egg, Embryos and Larvae: An Overview. Front. Mar. Sci. 7:251. doi: 10.3389/fmars.2020.00251
Received: 14 September 2019; Accepted: 30 March 2020;
Published: 07 May 2020.
Edited by:
Seyed Hossein Hoseinifar, Gorgan University of Agricultural Sciences and Natural Resources, IranReviewed by:
E Hu, Center for Aquaculture Technologies, United StatesCopyright © 2020 Diwan, Harke, Gopalkrishna and Panche. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arvind D. Diwan, YXJ2aW5kZGl3YW5AeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.