- 1Department of Biology and Chemistry (FB2), Universität Bremen, Bremen, Germany
- 2Leibniz Centre for Tropical Marine Research, Bremen, Germany
- 3Ministry of Marine Resources, Rarotonga, Cook Islands
- 4Pacific Island Conservation Initiative, Rarotonga, Cook Islands
- 5Reef Systems Research Group, Leibniz Centre for Tropical Marine Research (ZMT), Bremen, Germany
Feeding wild animals is a regular habit in ecotourism worldwide with poorly known consequences for ecosystem functioning. This study investigates how effective bread feeding is at attracting coral reef fish in the South Pacific, which feeding groups of fish are most attracted, and how natural foraging rates of an omnivorous and a grazing-detritivorous fish are affected. Data were collected at sites where fish are regularly fed bread by snorkellers and at comparison sites where bread was only provided for this study, within the Aitutaki lagoon (Cook Islands). The fish community was censused and foraging rates of two model species (Chaetodon auriga, Ctenochaetus striatus) were quantified one hour before, during, and an hour after feeding events. Twenty-five percent of the species present at all sites (piscivores-invertivores) were effectively attracted to bread. Overall, mean fish density was higher at tourism feeding sites than at the comparison sites. During bread feeding events, taxonomic richness decreased, compared to the hours prior and after feeding across all sites. As piscivore-invertivores were consistently attracted to bread, localized shifts in their dominance over other trophic groups may be expected if bread feeding persists, likely carrying consequences for ecosystem functioning. The effect of bread feeding events on natural foraging rates differed between the model species. C. auriga ceased foraging on natural foods to feed on bread. Although C. striatus never fed on bread, its foraging rate on epilithic algal matrices decreased during bread feeding events. This indirect non-lethal ecological consequence of bread feeding contributes a previously unanticipated example relevant to the “ecology of fear” in marine fish. Stakeholder interviews revealed that locals favor feeding to sustain tourist satisfaction, whereas tourists appreciate snorkeling regardless of feeding. This indicates an opportunity for restrictions on fish feeding with minimal drawbacks for tourism. Future research on fish metabolism and cascading effects on the reef benthos may reveal further impacts of feeding on coral reef communities.
Introduction
Wild animals have always fascinated humans. Procuring encounters with non-captive wildlife is therefore a core motivation for wildlife- and eco-tourism, two booming sectors in the tourism industry (Newsome et al., 2005; Burgin and Hardiman, 2015). Tourists’ demand for prolonged encounters with elusive animals encourages tour operators globally to attract these artificially through food (Newsome et al., 2004; Milazzo et al., 2006; Trave et al., 2017). Feeding marine fauna whilst snorkeling and diving is therefore a common, yet poorly regulated practice in marine tourism (Green and Higginbottom, 2000; Moscardo and Saltzer, 2004; Corcoran et al., 2013), with potentially grave implications for the conservation of affected marine ecosystems.
Artificial feeding of marine megafauna by tourists has reportedly led to changes in population size, migration, reproduction, and behavioral patterns, as well as being detrimental to an organism’s health (Reynolds and Braithwaite, 2001; Orams, 2002; Hammerschlag et al., 2012). Less well-understood are the consequences of feeding coral reef fish, which are most commonly encountered by tourists visiting tropical oceans (Sweatman, 1996; Bessa et al., 2017a; Mattos and Yeemin, 2018). Resource management agencies in different parts of the world enforce bans on feeding of large mammals but ignore the customary feeding of small species like birds and fish at the same locations (Orams, 2002).
Although concerns have been raised over artificial foods potentially interfering with critical ecosystem functions underpinned by coral reef fish (Cole, 1994; Milazzo et al., 2005; Medeiros et al., 2007; Bessa et al., 2017b), behavioral responses of the latter to artificial feeding events remain understudied. This is of importance, as behavior of reef fish is considered a major determinant of an organism’s functional role (Bellwood et al., 2019). Not only may artificial foods prevent fish from interacting naturally with their environment, but food provisioning by tourists may also result in behavioral habituation (Harriott, 2002; Orams, 2002; Newsome and Rodger, 2008; Semeniuk et al., 2009; Brookhouse et al., 2013; Bessa et al., 2017b). Artificial feeding events aggregate predatory fish and exacerbate predatory behaviors, thus resulting in interference competition and elevated predation risk for certain species (Newsome et al., 2004; Milazzo et al., 2006; Semeniuk and Rothley, 2008). Importantly, when large species are attracted and excited through artificial foods, human safety can be compromised (Perrine, 1989; Moribe, 2000; Brookhouse et al., 2013; Trave et al., 2017).
Spatio-temporal alterations in the relative abundance of fish species and structure of the fish community, as well as in habitat use and movement patterns (e.g., diel inversion of activity), are likely to affect the structure of entire populations and communities (Milazzo et al., 2005; Corcoran et al., 2013; Bessa et al., 2017a; Geffroy et al., 2018). Despite evidence from the Mediterranean, Atlantic, and Indian Ocean (Hémery and McClanahan, 2005; Milazzo et al., 2006; Feitosa et al., 2012) indicating that fish feeding can alter the community structure of fish assemblages, only few studies have focused on community-scale effects of artificial feeding with bread (Hémery and McClanahan, 2005; Ilarri et al., 2008; Sa-nguansil et al., 2017). It follows that experimental approaches documenting the effect on the habitual foraging rates of species are scarce (e.g., Wen et al., 2018). These knowledge gaps complicate the quantification of the impacts of artificial feeding at the ecosystem level and the sustainability of this practice within the ecotourism industry (reviewed by Newsome et al., 2012; Trave et al., 2017).
In remote island countries surrounded by extensive coral reefs, tourism is an important source of income largely supported by activities on reefs (Mellor, 2003; Spalding et al., 2017). Reduced reef health may change the abundance of reef fish, decreasing the value of the experience for tourists (Jones et al., 2004; Bruno and Selig, 2007) and potentially reducing their interest in the area. This may, in turn, prompt snorkeling and dive operators to attempt maintaining the aesthetic value of the reef environment and customer satisfaction by feeding fish artificially. Customer expectations and satisfaction are fundamental driving forces of the profitability of tourism (Semeniuk et al., 2009). Further, maintaining profitability will guide the attitudes and behaviors of local tour operators (Vaske and Manfredo, 2012). Whether the perceptions of tour operators regarding the added value of artificial fish feeding match the actual levels of satisfaction tourists obtain from such activity, however, remains uncertain (Patroni et al., 2018). As drivers and consequences of food provisioning for wildlife touch the realms of social as well as ecological sciences (Newsome, 2017), approaches that consider both ecological implications of artificial fish feeding and stakeholder perceptions are crucial to guide conservation and management actions (Ziegler et al., 2015; Patroni et al., 2018).
This study investigated for the first time both, temporary changes in fish species composition and disruptions in species foraging rates in response to artificial bread feeding while considering the stakeholder perceptions regarding this practice. Focusing on Aitutaki Lagoon (Cook Islands), where fish are fed bread daily during snorkeling tours, it was hypothesized that: (1) reefs where bread feeding by groups of tourist is a well-established practice will have higher fish density and species richness compared to sites where bread was provisioned experimentally by one researcher, (2) omnivorous fish will be most attracted to bread whereas more specialized feeding functional groups will avoid it, (3) fish species that feed on bread will cease foraging on natural substrata during bread feeding events and display lower-than-usual foraging rates afterward likely due to satiation, and (4) bread feeding is considered essential for tourists’ satisfaction during lagoon snorkeling tours by both tourists and local tour operators.
Materials and Methods
Study Area
This study was conducted from 6th December 2016 to 9th March 2017 in the near-atoll Aitutaki, located in the South Pacific Ocean, 18°51′28′′ S, 159°47′7′′ W. With an area of 18.1 km2 and ∼2000 inhabitants, Aitutaki is surrounded by a triangular carbonate forereef that encloses an approximately 50-km2 shallow, sandy lagoon, spiked with coral pinnacles (Loubersac et al., 1991; Berno, 1999; Hoffmann, 2002; Rankey and Reeder, 2009, Figure 1). An estimated 29,261 tourists visited Aitutaki in 2015, thus showing a tourists-to-residents ratio of nearly 15:1 (Cook Islands Tourism, 2019). Fish feeding has been practised regularly for more than 15 years, as a part of lagoon tours to areas where wildlife is naturally abundant and diverse. Two-hour snorkeling tours are offered daily by 10 different operators mostly within the marine protected area around One Foot Island (1–5 tours d–1) and at Maina (1–3 tours d–1).
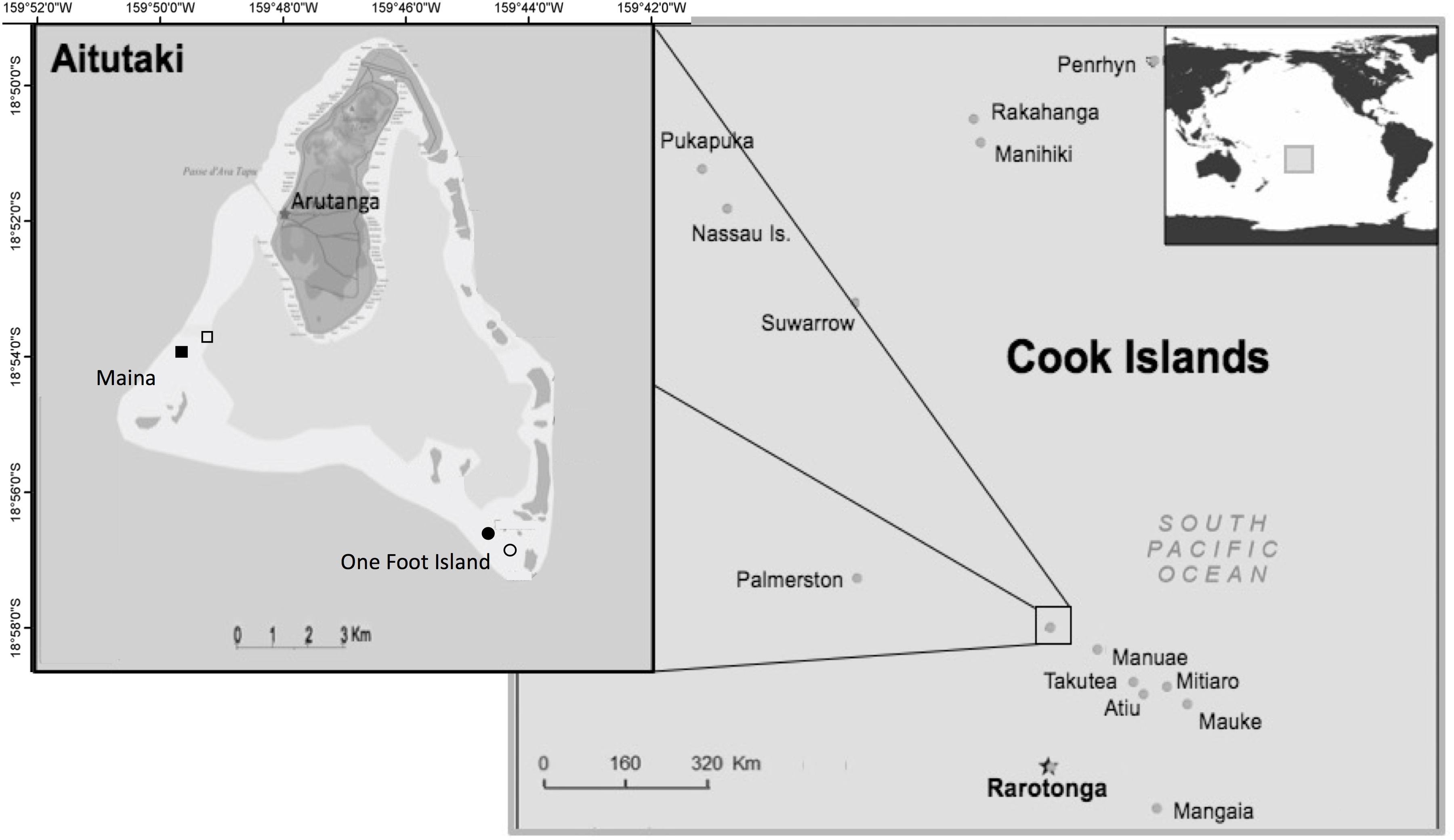
Figure 1. Location of Aitutaki in the southern group of the Cook Islands (right) in the South Pacific Ocean. Map of Aitutaki near-atoll with its shallow lagoon (left). Tourism feeding sites (filled symbols) and experimental feeding sites (open symbols) are indicated in One Foot (southeast) and Maina (southwest). Images adapted from Nevers (2008), creative commons.
This study was conducted in accordance with the research permit issued by the Foundation of National Research and Office of the Prime Minister (reference number: 24- 16), Ministry of Marine Resources and the Aitutaki Island Council, which covered all underwater surveys and stakeholder interviews. It was carried out in accordance with the regulations of Guideline 2010/63 of the European Commission, and was approved by the local authorities (Secretary, Ministry of Marine Resources, Cook Islands, December 2016).
Field Experiment
Effects of Bread Feeding Events on Fish
The field experiment spanned four sites located on lagoonal pinnacles. Two of these sites had been established as bread feeding locations by tourism operators and regularly visited by groups of snorkellers for 15 years and throughout this study. These represented the characteristics of fish communities habituated to a well-established food provisioning practice (i.e., hereafter referred to as tourism feeding sites). Two comparative adjacent sites that had never been used for food provisioning were also studied. At these sites, experimental bread feeding was conducted by one of the authors (NP) for the duration of the experiment (i.e., experimental feeding sites). Experimental feeding sites would therefore resemble the situation that would emerge immediately following the initiation of an incipient food provision practice. Experimental feeding sites were located between 600 and 850 m apart from the corresponding tourism feeding sites (Figure 1). This separation ensured no exchange of individual fish between tourism and experimental feeding sites at least for small species, including the focal species which commonly move over distances < 20 m (Krone et al., 2008; Matis, 2018). Although published home range sizes of large-bodied fish may exceed the separation between sites, in practice, these tend to be constrained by the extensive flat sandy areas separating pinnacles and reef patches in lagoonal habitats (Jordan et al., 2005). It is therefore unlikely that confounding effects would have emerged due to the exchange of fish between tourism and experimental feeding sites. The spacing between sites also avoided competitive foraging halos of snappers (Strelcheck et al., 2007). All sites were similar to each other in substratum composition, mean depth and topographic complexity (Wilson et al., 2007, Supplementary Material S1). One loaf of bread (approximately 500 g) was used per feeding treatment across all sites.
To compare the effects of bread feeding events between tourism-established and experimentally-established artificial feeding practices, the 15-min surveys were conducted in tourism and experimental feeding sites at three time points, namely 1 h before, during, and 1 h after bread feeding episodes (Feitosa et al., 2012). Surveys focused on: (a) quantifying changes in fish density, fish community composition (by feeding group), and taxonomic richness in response to bread feeding events, (b) identifying fish species with high and low affinity for bread, and (c) detecting changes in natural foraging rates of two model species attributable to bread feeding. All surveys were conducted ± 2 h from high tide between 11:00 and 15:00 to capture diurnally active fish (English et al., 1997). No tourists were present at any time when surveys were conducted.
Quantifying the fish density, fish species composition (by feeding groups), and taxonomic richness is important because of the ecological relevance of these metrics, but also because they are important contributors to the aesthetic value of snorkeling tours for tourists. Fish density and taxonomic richness were quantified using stationary underwater visual censuses (Bohnsack and Bannerot, 1986), consistently conducted by the same observer (NP) to avoid inter-observer bias (Albuquerque et al., 2015). A 3 m radius (area of 28.27 m2) was observed from a fixed position on the sea surface for 15 min while counting all active, non-cryptic fish. The maximum number of fish per species was recorded. At all sites, five censuses per site per feeding episode (before, during, after) were completed over 5 days. Species were assigned to feeding groups following Green and Bellwood (2009) and Pratchett et al. (2011) (Table 1). Mobile pelagic fish species, cryptic species and large wrasses were excluded from further analysis due to the bias that either their high variability and little attachment to single reef patches.
Species that fed on or avoided bread were identified through direct behavioral observations during the five bread feeding events initiated at both tourism and experimental feeding sites. Species were considered to display a high affinity for bread when more than 10 individuals fed on bread. Species were noted as having low affinity for bread when individuals were indifferent to or tried and rejected it.
Effects of Bread Feeding on Fish Foraging Rates
Two model species were selected for foraging rates observations given their ubiquity and important ecosystem function. Here, the species’ function is defined as the species’ role in the movement or storage of energy or material (Bellwood et al., 2019). Functionally, the omnivorous butterflyfish, Chaetodon auriga (facultative corallivore), consumes from very little to high amounts of live coral (Harmelin-Vivien, 1989; Pratchett, 2005; Cole et al., 2008), but also feeds on a variety of small invertebrates and algae (Myers, 1991). The detritivorous surgeonfish, Ctenochaetus striatus, targets the detritus entrapped within epilithic algal matrices, incidentally ingesting algal turfs and sediments (Crossman et al., 2001; Marshell and Mumby, 2012; Tebbett et al., 2017a). C. striatus plays a major role in benthic community composition structure dynamics and is one of the most important detritivorous fish species on Indo-Pacific coral reefs (Tebbett et al., 2017a). Species foraging rates (bites minute–1) were considered a valid proxy for ingestion (Choat and Clements, 1993; Bellwood, 1995; Streit et al., 2015; Tebbett et al., 2017b). Gut content analyses were avoided as the study was designed to be minimally intrusive and target species were not found in fish markets. Foraging rates were further considered representative of the animal’s ecological trophic function (Bellwood et al., 2006a; Fox and Bellwood, 2007, 2008). The extent to which fish trophic functions were affected by bread feeding was evaluated by quantifying foraging rates via 5-min focal follows of individuals of comparable size (i.e., ∼15 cm TL) conducted by a snorkeller from a conservative distance of ∼3 m (Lehner, 1996). The observers ensured the selected fish displayed no signs of disturbance, wariness, flight or hiding and continued behaving normally (Bellwood, 1995). As video recordings provide more accurate bite counts than those recorded visually by Scuba divers (Goatley and Bellwood, 2010), visual bite counts were supplemented here by recordings of the 5-min focal follow made with a GoPro video camera. Bouts of rapid consecutive bites that could not be discerned as individual bites were classed as single bites (Bellwood and Choat, 1990). Bites were not counted if dislodged material was ejected (Mantyka and Bellwood, 2007). Fifteen focal follows per species, site, and bread feeding event were completed over 5 days.
Stakeholder Perceptions
Perceptions regarding bread feeding of fish were investigated through a total of 104 questionnaires distributed among Cook Island nationals (50%, n = 52) and overseas-born stakeholders (50%, n = 52), including Aitutakian lagoon tour operators (n = 4), students (n = 28), employees of other sectors (n = 20), as well as foreign residents (n = 13) and tourists (n = 39) after obtaining prior informed consent (Questionnaire layout in Supplementary Material S2). The survey was conducted over a period of 2 weeks in March 2017 and respondents were selected by opportunity sampling. Questions assessed whether the person agreed or not with feeding bread to fish and why. Tourists were asked to respond whether their level of satisfaction would decrease or remain unchanged if bread was not provided during snorkeling tours.
Data Processing and Statistical Analysis
To investigate responses in fish density, species composition by feeding group, and taxonomic richness to bread feeding and to determine whether the magnitude of these responses differed between places where the food provisioning is well-established or experimentally initiated, linear mixed-effects models (LMEs) were fitted (Pinheiro et al., 2015). Initial models included treatment (tourism or experimental feeding site) and timing relative to the bread feeding event (before, during, after) as fixed effects, and site as a random effect to account for the repeated observations. Model residuals were plotted against the fitted values to check for homogeneity of variance, and against each explanatory variable to check for violations of independence (Zuur et al., 2007). Stepwise model selection and AIC further identified the best-fit models. Models were followed by Tukey’s post hoc tests to test for pair-wise differences whenever significant effects of multilevel factors were detected (Day and Quinn, 1989). Fish density was modeled using an LME, a fixed variance structure, and maximum likelihood estimates to account for heteroscedasticity (Zuur et al., 2009). Taxonomic richness was modeled using a generalized linear mixed-effects model (GLMM) with Poisson distribution verifying that the scale parameter φ was not significantly different from that assumed in a Poisson distribution (i.e., 1) (Crawley, 2007). To test the differences in fish species composition by feeding group, the densities were also compared between treatment (tourism or experimental feeding site) and timing relative to the bread feeding events (before, during, after) as fixed effects, and site as a random effect. To assess the degree of a species’ affinity toward, or avoidance of bread, the absolute difference between its density during and before, during and after, and before and after bread feeding events were computed in both tourism and experimental feeding sites. This analysis focused on nine species commonly observed throughout all sites, including four carnivorous fish and five herbivorous fish that are considered to fulfill important functional roles in the reef.
To determine whether bread feeding disrupts fish trophic functions, we tested whether foraging rates of the model species changed before, during, and after bread feeding events, and whether such changes occurred both at sites where bread feeding is well-established or experimentally initiated. As foraging rates did not differ between the location Maina and One Foot, these were pooled together such that 10 feeding observations were available for C. striatus and nine for C. auriga. LMEs were fitted with treatment (tourism or experimental feeding site) and timing relative to the bread feeding event (before, during, after) as fixed effects, and site as a random effect. After model residual against fitted values were tested for homogeneity of variance, stepwise model selection and AIC further identified the best-fit models (Zuur et al., 2009). To test a posteriori pairwise differences, the Tukey’s post hoc test was performed (Day and Quinn, 1989).
To obtain differences in (Yes/No) answers of stakeholders, questionnaire data were visualized in R-Studio and statistical analysis of frequency of particular responses among stakeholders was performed, using a Chi-square test (expected and observed values in Supplementary Material S4, and Supplementary Table S3). All statistical tests were performed using R-Studio (R Core Team, 2015).
Results
A total of 5128 individuals of 71 species, belonging to 14 families, were recorded throughout the study (Supplementary Material S3). Overall, carnivores and herbivores were the dominant feeding groups, whereas corallivores were least represented across sites.
Effects of Bread Feeding on Fish Species Composition
Bread feeding events had significant short-term effects on fish assemblages. Mean fish density was significantly higher at tourism feeding sites compared to experimental feeding sites (density: p = 0.046; Figure 2). At tourism feeding sites, fish density increased by 29% during bread feeding events compared to 1 h prior to feeding (p = 0.01074). One hour after feeding events, the visible fish density remained high across sites (Figure 2A). At experimental feeding sites fish density increased after feeding compared to before (p = 0.0136). Taxonomic richness decreased during feeding events by an average of ∼20% compared to 1 h before feeding (p = 0.001, Figure 2B), but recovered 1 h after (p < 0.00188). This pattern was consistent through sites and treatments.
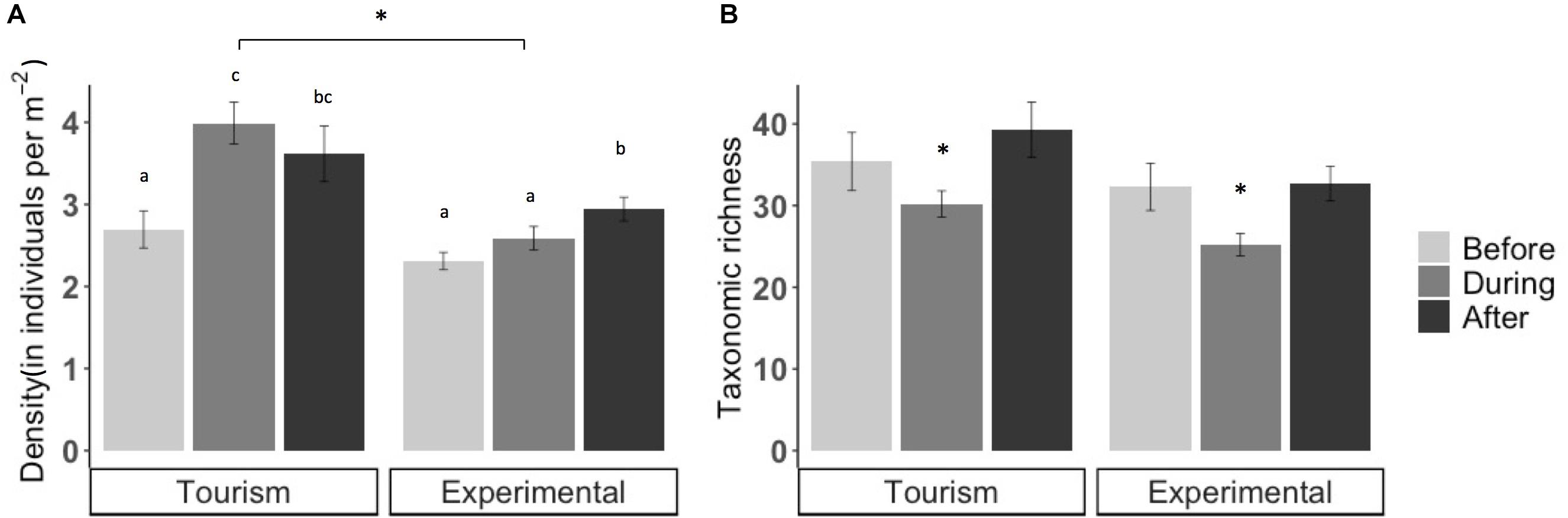
Figure 2. Mean (A) fish density (± SE) (B) and taxonomic richness (± SE) before, during, and after artificial feeding events at tourism and experimental feeding sites. Note that the scale of y axes differs and significant differences are indicated by asterisks and letters.
Piscivores-invertivores were consistently and strongly attracted to bread during feeding events at tourism feeding sites, but not at experimental feeding sites [p < 0.001, Tukey Post hoc (during Tourism – during Experimental: p = 0.00205, Table 2)]. With the exception of large groups of planktivores (Abudefduf sexfasciatus, A. vaigiensis), which were consistently attracted to bread during feeding events and remained an hour after in only one of the tourism feeding sites (Figure 3), the density of all other groups remained unchanged by bread feeding events.
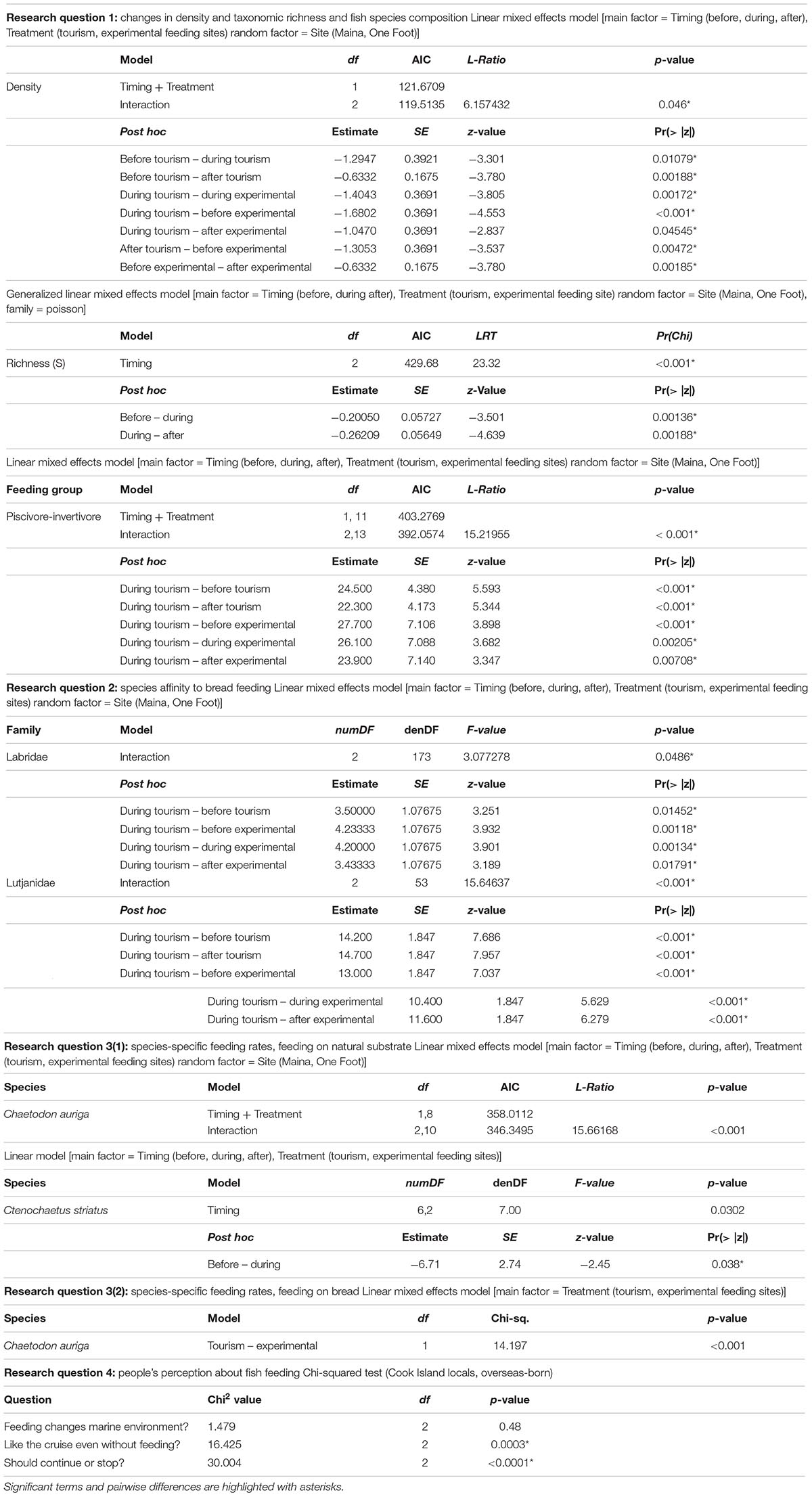
Table 2. Statistical outputs of mixed-effects models accounting for the temporal correlation among site observations and Tukey’s post hoc test results for each research question and according models.
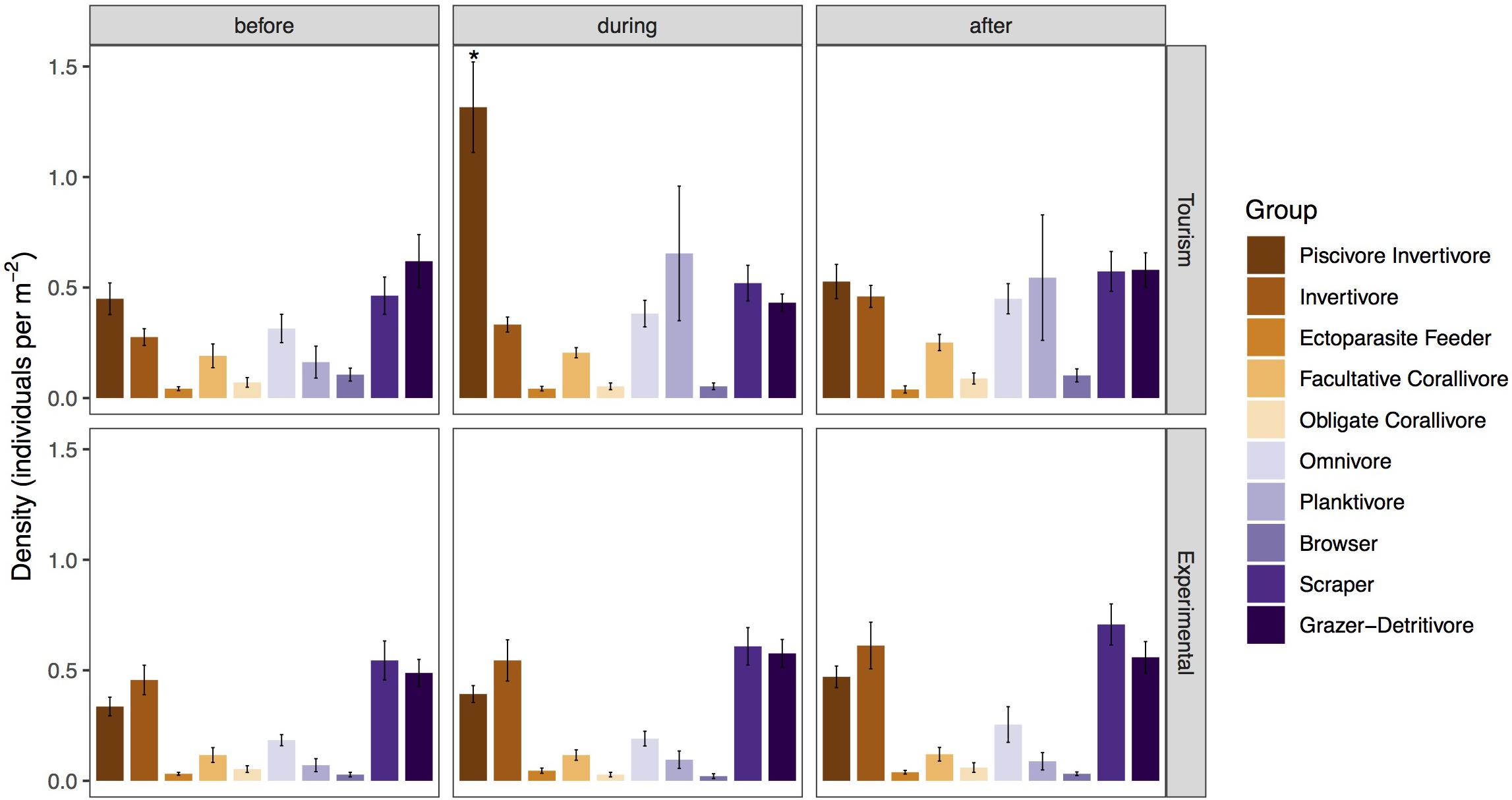
Figure 3. Mean density (± SE) of fish classified in feeding groups before, during, and after bread feeding events at tourism and experimental feeding sites. Significant differences are indicated by asterisks.
Affinity of Fish Species for Bread
During bread feeding events 25% of species fed on bread across sites, whereas 70% of species were indifferent to bread feeding episodes, and 5% tested but subsequently rejected or avoided the bread (Table 3). The majority of species that fed on bread were piscivore-invertivores (44%) and omnivores (28%). The relatively more specialized feeding groups, obligate corallivores, scrapers, macroalgal browsers, and grazer-detritivores did not feed on bread. About 40% of all species that fed on bread were non-scarine labrids (wrasses). Labrids (p = 0.0486) and lutjanids (p < 0.0001) congregated significantly when bread was supplied at tourism but not at experimental feeding sites (Figure 4 and Table 2). For both non-bread-feeding families Acanthuridae and Scaridae the magnitude of the difference in density between before and during bread feeding events was not significantly different between tourism and experimental feeding sites.
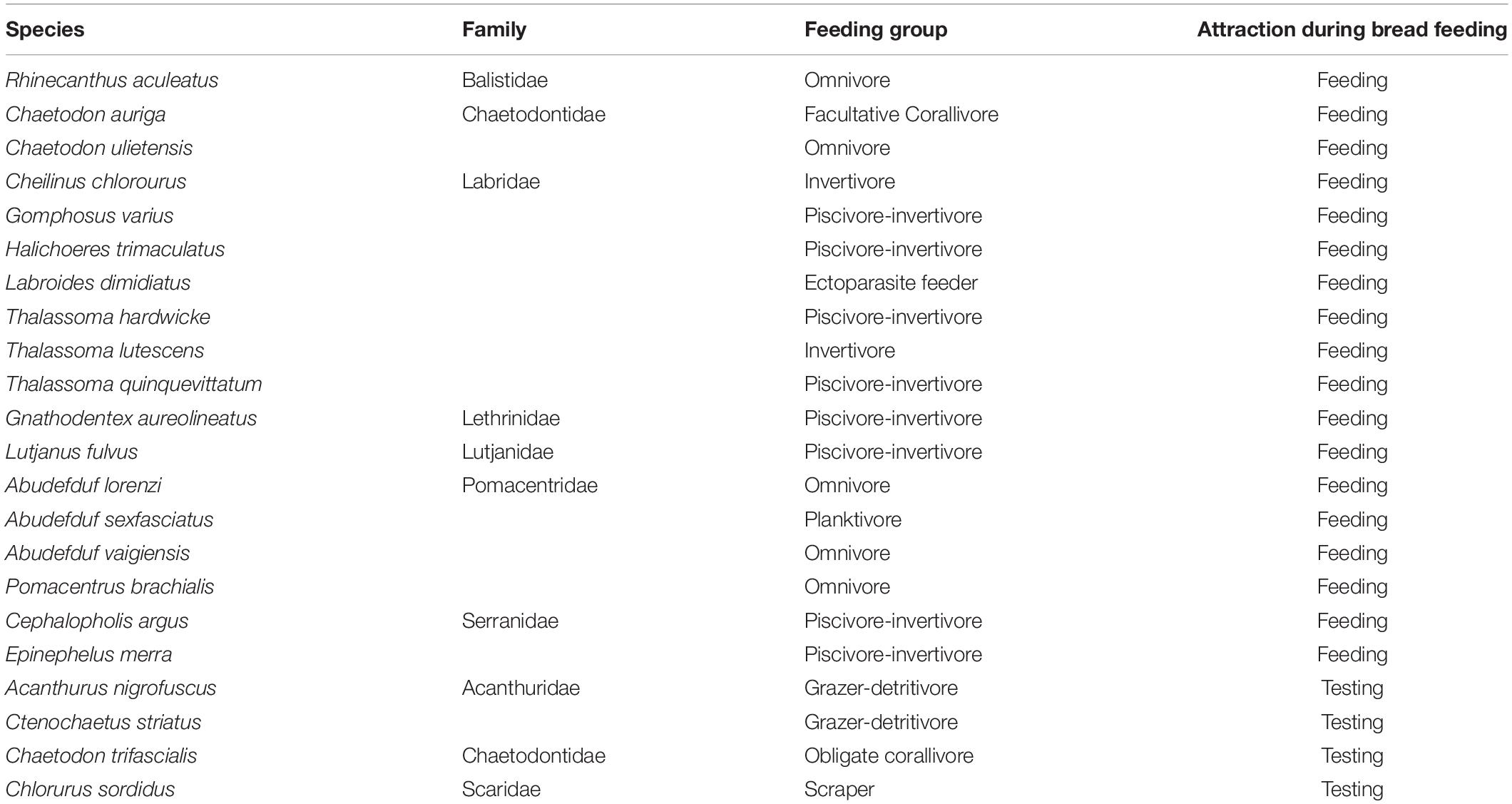
Table 3. Fish species feeding on or testing bread when it was provided during artificial feeding event.
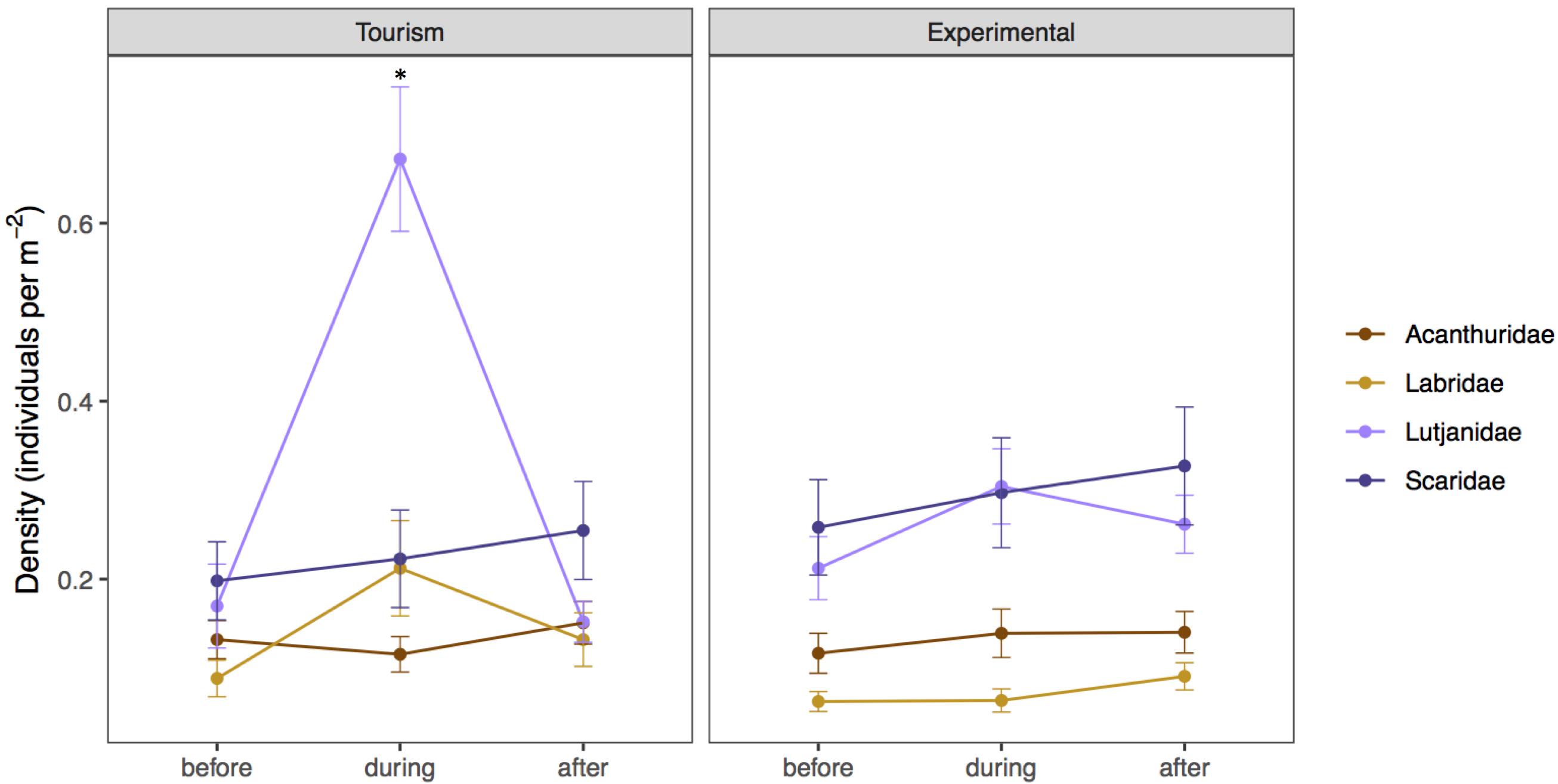
Figure 4. Mean density (± SE) of the most ubiquitous species (grouped by family for graphical succinctness) before, during, and after bread feeding events at tourism and experimental feeding sites. Acanthuridae includes Ctenochaetus striatus, Acanthurus nigrofuscus, Naso lituratus, Labridae includes Thalassoma hardwicke, Thalassoma lutescens, and Thalassoma quinquevittatum, Lutjanidae corresponds to Lutjanus fulvus, and Scaridae includes Chlorurus sordidus and Scarus frenatus. Significant differences are indicated by asterisks.
Effects of Bread Feeding on Fish Foraging Rates
Across sites, foraging rates of C. striatus on the benthos significantly decreased by 22% during bread feeding events compared to an hour before (p = 0.038, Figure 5A). One hour after bread feeding events C. striatus resumed foraging rates similar to those observed prior to bread feeding (Figure 5A). C. striatus only tested the bread with consistently low rates across sites (Figure 6).
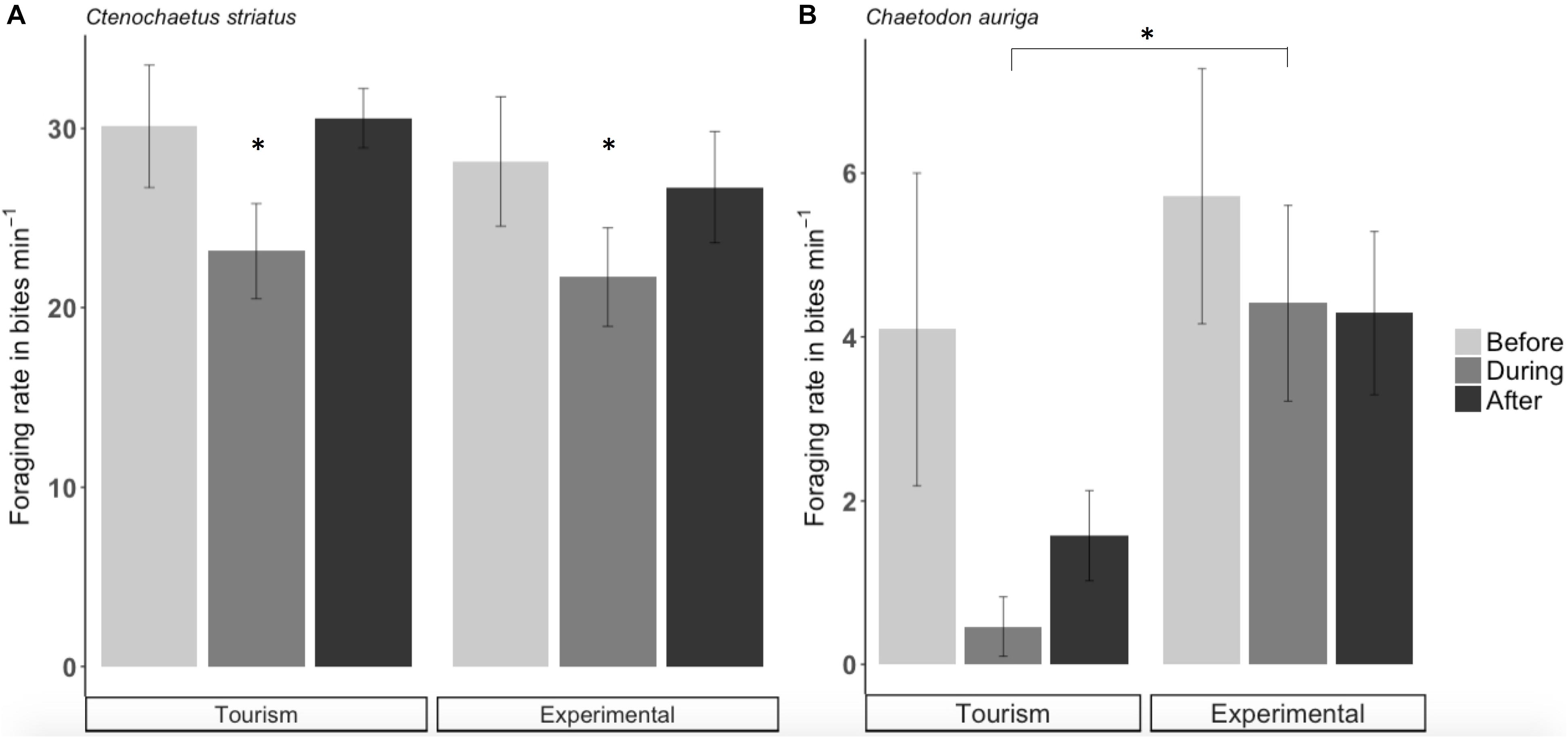
Figure 5. Foraging rate (± SE) of Ctenochaetus striatus (A) and Chaetodon auriga (B) on natural food at tourism and experimental bread feeding sites before, during and after bread feeding events. Note that the scale of y axes differs and significant differences are indicated by asterisks.
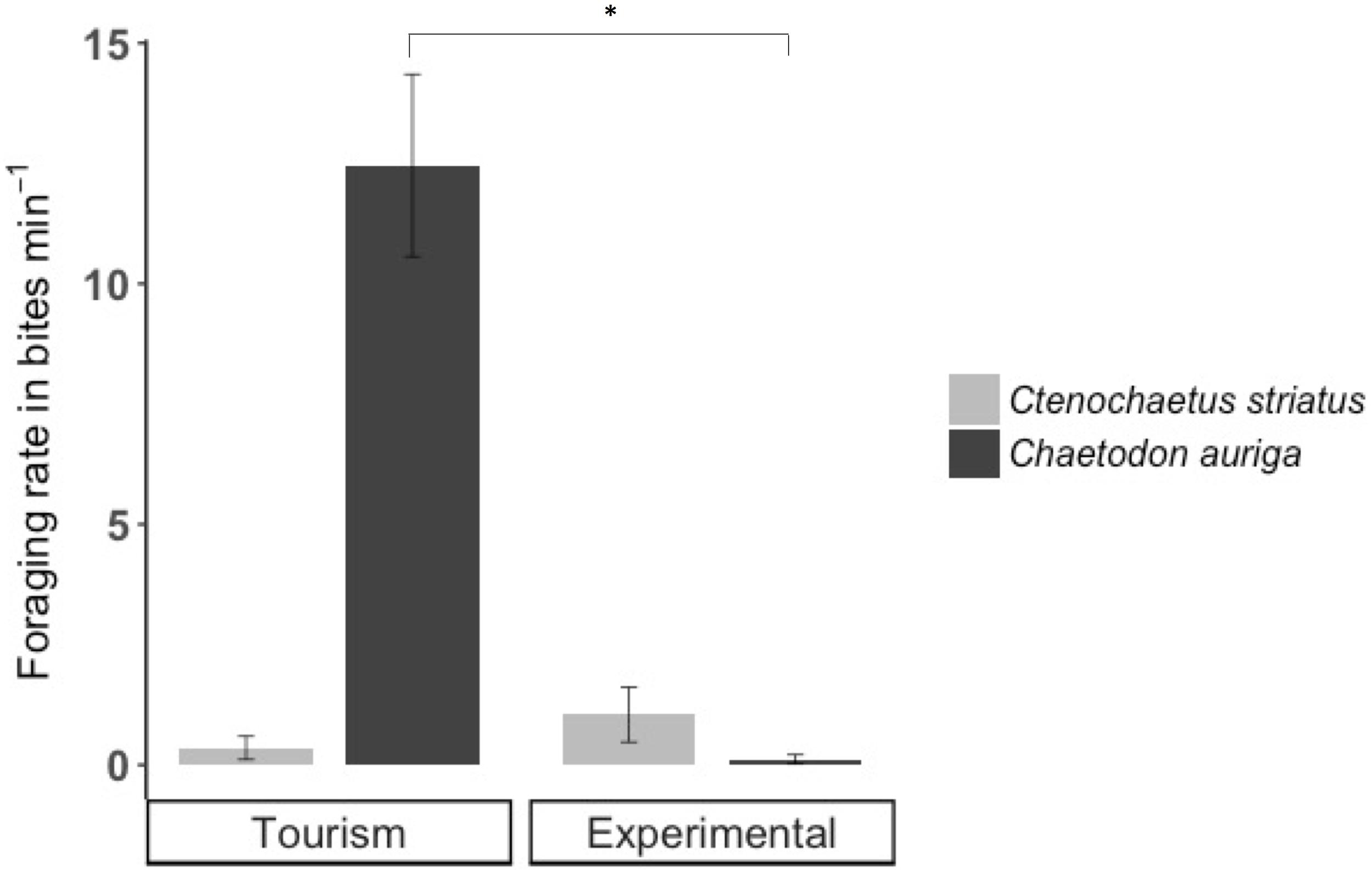
Figure 6. Foraging rates (± SE) of Ctenochaetus striatus (light gray) and Chaetodon auriga (dark gray) on bread during bread feeding events. Significant differences are indicated by asterisks.
Foraging rates of C. auriga on natural substrata were significantly different between tourism and experimental feeding sites. The magnitude of the effect of bread feeding events differed between tourism feeding sites and experimental feeding sites (p = 0.001). At tourism feeding sites, foraging rates of C. auriga were eight times lower during bread feeding events (0.46 ± 0.34 bites min–1) compared to the hour before (4.09 ± 1.8 bites min–1, Figure 5B). This effect remained 1 h after the feeding episode (1.57 ± 0.5 bites min–1). At sites where bread feeding was experimentally initiated, the foraging rates of C. auriga remained stable before (5.72 ± 1.5 bites min–1), during (4.41 ± 1.1 bites min–1), and after (4.29 ± 0.9 bites min–1) bread feeding events. C. auriga fed on bread at very high rates (12.46 ± 1.8 bites min–1) at tourism feeding sites, whereas it did not feed on bread at experimental feeding sites (p < 0.001, Figure 6).
Stakeholder Perceptions
Of all respondents (n = 104), 54% agreed that feeding may have a negative impact on the marine environment and modify natural fish feeding behaviors. All interviewed tourists that participated in a lagoon cruise declared they would appreciate these tours without bread feeding events, whereas only 54% of local stakeholders conceded that bread feeding was likely non-essential for the satisfaction of their guests (p = 0.0003, Table 2, Chi-squared expected and observed values in Supplementary Material S4, and Supplementary Table S3). Correspondingly, 75% of all local stakeholders (including tour operators) argued that bread feeding should continue, while 21% of all local stakeholders were in favor of stopping feeding fish artificially (p < 0.0001).
Discussion
This study investigated for the first time both the ecological consequences of artificial feeding for coral reef fish in a Pacific Ocean lagoon, and whether this practice is considered necessary by local and foreign stakeholders. Although feeding fish artificially during recreational activities is commonly regarded as a possible cause for changes in fish behavior this had not yet been tested. Using a controlled field experiment this study demonstrates that bread feeding of coral reef fish influences short-term density and taxonomic richness at sites where bread feeding is a well-established practice, compared to sites where feeding is experimentally initiated. Species with relatively more generalist diets were consistently attracted to bread and drove the observed changes in fish species composition. Species-specific foraging rates were significantly altered by bread feeding events, and the magnitude of these alterations differed between tourism feeding and experimental feeding sites. As a result, the functional role of key species may be incompletely fulfilled in areas where fish are artificially fed. Whether these effects are strong enough to cause long term changes in ecosystem function remains to be tested. Perceptions of local tour operators regarding bread feeding being necessary to enhance tourists’ satisfaction were not shared by tourists themselves. This indicates a potential opportunity to implement restrictions on bread feeding practices causing minimal drawbacks for local tourism.
Fish Density and Richness Are Modified at Well-Established Artificial Feeding Sites
The higher fish density observed at sites where artificial feeding practices are well-established compared to sites where the practice was experimentally initiated, confirms an influence of bread feeding on the fish community structure, in accordance with previous studies (Hémery and McClanahan, 2005; Milazzo et al., 2006; Medeiros et al., 2007). At tourism feeding sites fish density peaked during bread feeding events, whereas at experimental feeding sites density was highest on the hour after. This suggests that well-established artificial feeding practices may alter diel cycles of fish abundance that are otherwise driven by tidal changes or time of day (Corcoran et al., 2013). On Brazilian reefs where fishes are regularly fed with bread and crackers by divers, fish abundance and taxonomic richness remained higher than usual 1 h after feeding, indicating that the effect of artificial feeding can last several hours or days (Milazzo et al., 2006; Feitosa et al., 2012). Habituation of fish to artificial feeding can lead to the dominance of a few species and a long-term reduction of taxonomic richness (Medeiros et al., 2007). At several tourism feeding sites, fish also anticipate feeding events and congregate in response to the noise of boat engines (Newsome et al., 2004, reviewed by Whitfield and Becker, 2014). Although arguably possible, it is unlikely that the differences observed here between tourism and experimental feeding sites are confounded by fish moving across sites. Small-bodied reef fish tend to move over very small home ranges (i.e., <3 m for C. auriga and Abudefduf sexfasciatus, and <16.4 m C. striatus, Krone et al., 2008; Matis, 2018), and wide-ranging lethrinids and lutjanids were repeatedly observed at the same sites (NP, personal observation).
Taxonomic richness of fish assemblages decreased significantly during bread feeding events compared to an hour prior and an hour after at both tourism and experimental feeding sites, consistently with previous studies (Ilarri et al., 2008; Albuquerque et al., 2015). It is therefore argued here, that regular artificial feeding may account at least partially for an overall decrease in species richness at tourism feeding sites here and elsewhere. Although not necessarily permanent, this phenomenon is likely not in the interest of operators or tourists, as speciose fish assemblages are generally preferred over those dominated by a few species (Salim et al., 2015; Tribot et al., 2018). This decrease is also undesired from an ecosystems’ perspective, given that decreases in taxonomic richness are usually accompanied by reductions in functional richness thus posing risks to reef health and ecosystem services other than those associated to tourism (Worm et al., 2006; Burkepile and Hay, 2008).
Carnivorous and Omnivorous Fish Were Consistently Attracted to Bread
Results from this study support the notion that trophic generalists are most attracted to bread compared to relatively more specialist feeding groups (e.g., macroalgal browsers, grazer-detritivores). Bread feeding events favored generalist trophic groups such as carnivores and omnivores, as observed elsewhere (Albuquerque et al., 2015; Bessa et al., 2017b; Mattos and Yeemin, 2018). Fish species composition by trophic groups in Brazil, for instance, was also significantly altered during artificial feeding events compared to before and after (Feitosa et al., 2012). In the present study, piscivore-invertivores showed the steepest change in response to bread feeding events and fed profusely on bread. This was mainly driven by the immediate congregation of Lutjanus fulvus, Thalassoma hardwicke, and Cephalopholis argus, which dissipated when bread feeding ceased. Almost 40% of all species that fed on bread were non-scarine labrids (wrasses). These, together with Pomacentrids, were also the most representative family of bread feeding fish on reefs in Kenya and Taiwan (Hémery and McClanahan, 2005; Wen et al., 2018). Labridae are one of the most speciose, ecologically, and functionally diverse group of fish inhabiting the world’s coral reefs feeding opportunistically as generalist predators (Thresher, 1979; Wainwright, 1991; Bellwood et al., 2006b).
Short-term variability in fish community composition is natural (McClanahan et al., 2007). Yet, the prevalence of omnivores (e.g., labrids) above the reef, which peaked during bread feeding events and persisted after feeding ceased, may have long-term consequences on the reef fish community. If repeated bread feeding events lead to a persistent dominance of predatory fish on certain reefs, important changes in the trophic structure of fish assemblages may be expected (Beukers-Stewart and Jones, 2004). The consistent prevalence of high-trophic-level fish at bread-feeding sites may equate to high predation risk for small-bodied fish, but may also lead to higher fish excretion rates, which might cascade into changes in algal productivity and benthic dynamics (Allgeier et al., 2017; Wen et al., 2018).
Artificial feeding events may influence key biological interactions, such as aggressive exclusion, predation pressure, competition for local resources, and grazing (Coker et al., 2009; Brookhouse et al., 2013). Importantly, this is one of the few studies detecting non-lethal ecological consequences of artificial marine fish feeding linked to pulse increases in predator abundance (see also Milazzo et al., 2006). In this present case, snorkelers cause a sudden increase in (artificial) food that congregates predatory fish in a feeding frenzy around the bread. This likely generates a momentary “landscape of fear” where both predation risk and competition levels increase for smaller fish. Although fish may not necessarily feed on bread, their behavior may be indirectly affected by the bread feeding event (Brookhouse et al., 2013; Paula et al., 2018). Wary species that are regularly spear-fished may react solely to the presence of snorkelers. Macroalgal browsers (Naso lituratus and N. unicornis), for instance, tended to flee during bread feeding events, yet returned to their home reef an hour after. Changes observed in community composition during feeding events can therefore be attributed to both the congregation of opportunistic species habituated to feed on bread, and the departure of specialists that show alarm responses (Geffroy et al., 2017).
Bread Feeding Events Disrupted Natural Fish Foraging Rates
Foraging rates of both model species changed in response to bread feeding events across sites, yet the nature of these changes differed between species. At tourism feeding sites, the facultative corallivore C. auriga fed on bread whilst substantially decreasing foraging rates on natural prey. An hour after the tourism bread-feeding events, foraging rates of C. auriga remained lower than usual, suggesting possible satiation. During the hour prior to bread feeding events at experimental feeding sites, natural foraging rates of C. auriga on the benthos resembled those recorded in the Great Barrier Reef (Gregson et al., 2008). At experimental feeding sites, C. auriga disregarded bread and foraged on the benthos consistently throughout bread feeding events. This indicates that habituation of C. auriga to bread likely occurs beyond the time frame covered by this study (i.e., 12 weeks). This also corroborates previous findings, where none of the fish identified as bread consumers at tourism feeding sites in Thailand fed on bread when supplied by researchers at experimental feeding sites (Sa-nguansil et al., 2017). The ecological implications of the responses of C. auriga to bread feeding may not be immediately obvious, given the broad range of food items it consumes. The consumption of bread by C. auriga likely reflects its opportunistic feeding behavior and may not strongly affect predator-prey interactions (Pratchett, 2005; Cole et al., 2008). Yet, the high carbohydrate content of bread provokes unhealthy high glucose levels in the blood of carnivorous fish (Moon, 2001). Consequences of artificial feeding on growth, survival or reproductive success of animals feeding on unnatural diets such as bread are still to be evaluated (Rodgers, 2017). Further physiological effects of bread feeding on C. auriga, as well as changes in its natural diel foraging rates should be investigated. As a consequence of bread feeding, foraging ranges may be spatially restricted as fish concentrate within a few meters from the usual bread provisioning places. This could lead to artificially-enhanced levels of competition and feeding pressure on prey and other density-dependent processes, such as disease and parasite transmission (Vignon et al., 2010; Brookhouse et al., 2013). It is suggested here that these impacts may however be limited to generalist butterflyfishes, given that several obligate corallivore species observed during this study (e.g., Chaetodon bennetti, Chaetodon lunulatus, Chaetodon reticulatus) were not attracted to bread at all.
Natural foraging rates of the detritivore C. striatus were similar to rates recorded previously elsewhere (Bellwood and Choat, 1990). Likely reasons for the indifference of C. striatus toward bread may be the specialization of C. striatus to feed on benthic detritus of high nutritional value (Crossman et al., 2001). Interestingly, reduced foraging rates of C. striatus on the benthos during bread feeding compared to before and after, indicate a form of indirect disruption of its feeding activity. It is suggested here, that the feeding activity of C. striatus decreases during the bread feeding frenzy due to an increase in perceived predation risk. This alludes to the concept behind the “ecology of fear” which refers to the family of studies considering the ecosystemic consequences of prey fear responses (Brown, 2019). Previous research indicated that predation risk perceived by coral reef herbivorous fishes increased with distance from refuge habitat and affected herbivory rates (Gil et al., 2017). More generally, a number of studies on escape behavior yielded important implications for the reef fishes’ ecology of fear (e.g., the influence of fishing, marine protected areas, surveyors, prey body size, proximity of refugia, mutualism between preys, and group size) (Madin et al., 2011; Januchowski-Hartley et al., 2012; Lyons, 2013; Nunes et al., 2018). This study therefore contributes the first example linking a coral reef tourism-driven increase in perceived predation risk with potential ecosystemic consequences relevant for the reef fishes’ ecology of fear. A similar finding for freshwater fish, which reduced foraging rates in response to tourism visitation and increased them again at the end of the day, was interpreted as a rebound effect of compensatory feeding following disturbance (Wu et al., 2002; Rubio et al., 2010; Bessa et al., 2017a). The bread feeding effect observed here in C. striatus may indirectly alter the way it fulfils its ecosystem function as an important detritivore, eroder, and transporter of sediments (Schuhmacher et al., 2008). Short-term alterations of foraging intensity may not drastically influence the functionality of the reef ecosystem, yet long-term effects may include C. striatus moving their feeding grounds or even abandoning the site entirely. This effect on the presence of C. striatus and its functional role may result in a more profound alteration of reef health through locally-reduced detritivory. Further research into the response of specific feeding groups to bread is required to substantiate that assumption, as a study from Taiwan found no effect on the bite rates of herbivorous fishes (Acanthurus nigrofuscus and Scarus schlegeli) between provisioned and non-provisioned sites (Wen et al., 2018). Bread feeding of fish may be a short-term intrusion, but where repeated consistently it may have significant implications for the ecosystem function of different feeding groups (Albuquerque et al., 2015). In light of increasing tourist numbers, artificial feeding practices may pose a long-term threat to the lagoon environment through lasting physiological and behavioral changes in fish (Geffroy et al., 2018). Fish may potentially “learn” to feed on bread and this habit may cause chronic satiation, have physiological consequences, and affect their metabolic pathways. This laid beyond the scope of this study and should be further investigated. Arguably, this study would have benefited from comparisons of tourism and experimental feeding sites with sites where no bread feeding was provided. Such comparisons would have disentangled more clearly the effect of well-established artificial feeding practices from those introduced by diurnal variability in fish biodiversity and feeding behavior. However, in this case, preliminary observations (NP) and the knowledge of local tour operators indicated that diurnal variability in fish community structure was low in all sites selected for experimental bread feeding exercises. In the face of logistical limitations preventing from adding more survey sites, preference was given to the establishment of experimental bread feeding sites. This allowed for the fully factorial comparison among before, during, and after artificial feeding episodes, and revealed important information on the process of fish habituation at the onset of artificial feeding practices. Ecological effects of bread feeding practices are likely shaped by multiple mechanisms including changes in fish behavior and community structure, but may also be mediated by the distribution of food, duration of bread artificial feeding events, number of tourists, and their behavior (Feitosa et al., 2012).
Tourists Did Not Consider Bread Feeding Essential
In order to regulate the frequency and intensity of fish feeding in coral reef systems, understanding the perceptions of stakeholders is essential (Newsome, 2017; Breckwoldt et al., 2018). A stakeholder’s perception is largely dependent on their socio-demographic background, cultural context, knowledge, attitudes, norms, and personality (Beyerl et al., 2016). The respondents’ perception that “bread feeding can impact fish feeding behavior and the marine environment” shows a relatively high level of awareness of the ecological consequences of this practice. A primary human motivation in the context of ecotourism is to observe nature in as natural a state as possible (Orams, 2002). The question remains whether tourists want to see wild animals completely uninfluenced by humans or “domesticated” by continued feeding over years (Orams, 2002). Orams (1999) cautioned that “fish feeding turns the sub-aquatic world into an aquarium without walls, a zoo without bars.” Studies on the perception of whale-shark feeding tourism showed tourists supported the practice despite many being aware of the ethical complications of animal feeding for tourism purposes (Ziegler et al., 2018). In the present study, the question whether to feed or not to feed resulted in significantly different opinions between stakeholder groups. All overseas participants of snorkeling cruises declared that they would have enjoyed the activity with or without bread feeding, whereas local stakeholders highlighted the need for continued bread feeding practices in order to guarantee tourists’ satisfaction during snorkeling cruises. This suggests that implementing restrictions on bread feeding practices in Aitutaki may meet some resistance from tour operators, but will likely not harm tourist satisfaction levels. It could be argued that overseas snorkelers regarded bread feeding as superfluous only because they had already been satisfied by the proximity of abundant fish during the cruises. Whether their opinion on banning bread feeding would change if they had encountered less fish when snorkeling remains to be tested in controlled experiments. In practice the link between bread feeding activities and tourism satisfaction is driven by tourists’ behaviors and expectations, which are often to see high numbers of fish. Tourists’ satisfaction in Australia, Thailand and Malaysia was directly related to fish abundance and taxonomic richness (Moscardo et al., 2001; Topelko, 2007; Salim and Mohamed, 2014). Arguably, sustainable tourism activities provide a high-quality experience for visitors, which encourages them to be concerned about the conservation of the observed animals (Higginbottom et al., 2001). In the Cook Islands, environmental sustainability already constitutes a constraint to tourism growth, which in turn places a high priority on the protection of the environment in order to support tourism (Mellor, 2003).
Recommendations
In light of these findings, this study suggests regulation of artificial feeding practices, supporting conservation measures to protect fish communities and functions in Aitutaki lagoon or elsewhere, being subject to local management priorities. This study highlights a difference between long-term and short-term effects of bread feeding on fish assemblages. The following recommendations are given:
Initially, restrictions to bread feeding activities could be issued in the form of guidelines and rules that allow local tour operators to supply bread strictly (i) at designated feeding sites, (ii) using appropriate food types (Murray et al., 2016; Birnie-Gauvin et al., 2017), (iii) providing limited food amounts per person and boat (Great Barrier Reef Marine Park Authority, 1994; Murray et al., 2016), and (iv) at unpredictable times (Murray et al., 2016).
In lieu of a ban, it would be relevant to (1) work on transferring the perceptions of snorkellers to operators of snorkeling tours, (2) inform tour operators and dive guides on how the bread may impact fish behavior and function, and (3) involve education programs for tourists (Wiener et al., 2009; Patroni et al., 2018). Environmental education during tourism activities will result in positive attitudes of visitors toward wildlife conservation (Higginbottom et al., 2001), and may thus assist in a voluntary reduction of feeding (Bessa et al., 2017a). Lastly, pressure to feed wild animals may, in many places, come from tourists rather than from tourism operators. Avenues of non-scientific communication (i.e., tourism magazines, airline magazines, signs, flyers) to educate visitors could be helpful worldwide. On coral reefs, further research should aim to improve the understanding of long-term harmful effects of this previously overlooked activity on ecosystem health and on feeding behaviors of more fish species. In addition, potential indirect cascading effects of unconsumed bread on reef benthos, e.g., in terms of oxygen consumption and nutrient release, should also receive attention (Turner and Ruhl, 2007; Brookhouse et al., 2013).
These recommendations were delivered, and are relevant for, the currently-developed Aitutaki Management Plan. The continuous management of touristic activities in the reef requires active participation of governments, tour operators, scientists, and local communities in order to ensure its long-term ecological sustainability and incentives for conservation (Hawkins et al., 1999; Trave et al., 2017). The results of the current study suggest that no major impacts on tourism-dependent livelihoods are expected if the activity was modified by regulating the artificial feeding of fish. These findings underline the potential for sustainable management interventions in the form of reduced fish feeding activities in the Cook Islands.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
NP and SB conceptualized and designed this study with input of SF. NP procured funding and conducted the field experiment and stakeholder interviews with the support of RS and SL. This study took place within the frame of the project Resilience of South Pacific coral reef social-ecological systems in times of global change (REPICORE) led by SF. NP and SB performed the statistical analysis of the data. NP and SB led the writing of the manuscript with input of SF. All authors approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Funding for this work was provided by the German National Academic Foundation (Studienstiftung des Deutschen Volkes) and the German Academic Exchange Service (Deutscher Akademischer Auslandsdienst) through the PROMOS Scholarship and Johannes and Monika Zurnieden. The REPICORE project was funded by the (German) Federal Ministry of Education and Research (BMBF) through the ‘Nachwuchsgruppen Globaler Wandel 4 + 1’ (Grant Number 01LN1303A). The research reported in this paper contributes to the Programme on Ecosystem Change and Society (www.pecs-science.org). Further public work was kindly funded by the foundation Kellner & Stoll Stiftung.
Acknowledgments
The authors thank field volunteers Joe Kaukura, Alice Mitchell, Silke Janßen, Polly Johnson, Meegan O’Callaghan, Richard Bloomfield, and Terito Story. Thanks to the Aitutaki Island Council, Bishop Cruises, Vaka Cruises, Pacific Resort, Aitutaki Village, Tamanu Beach Resort and Aitutaki Airport Authority for their support. This study was permitted by the Office of the Prime Minister Cook Islands and Ben Ponia, Ministry of Marine Resources. Gratitude belongs to the reviewers and the editor for their valuable recommendations.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00145/full#supplementary-material
References
Albuquerque, T., Loiola, M., Nunes, J. A. C. C., Reis-Filho, J. A., Sampáio, C. L. S., and Leduc, A. O. H. C. (2015). In situ effects of human disturbances on coral reef-fish assemblage structure: temporary and persisting changes are reflected as a result of intensive tourism. Mar. Freshw. Res. 66, 23–32. doi: 10.1071/MF13185
Allgeier, J. E., Burkepile, D. E., and Layman, C. A. (2017). Animal pee in the sea: consumer-mediated nutrient dynamics in the world’s changing oceans. Glob. Chang. Biol. 23, 2166–2178. doi: 10.1111/gcb.13625
Bellwood, D. R. (1995). Carbonate transport and within-reef patterns of bioerosion and sediment release by parrotfishes (family Scaridae) on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 117, 127–136. doi: 10.3354/meps117127
Bellwood, D. R., and Choat, J. H. (1990). A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Environ. Biol. Fish 28, 189–214. doi: 10.1007/bf00751035
Bellwood, D. R., Hughes, T. P., and Hoey, A. S. (2006a). Sleeping functional group drives coral reef recovery. Curr. Biol. 16, 2434–2439. doi: 10.1016/j.cub.2006.10.030
Bellwood, D. R., Streit, R. P., Brandl, S. J., and Tebbett, S. B. (2019). The meaning of the term ‘function’ in ecology: a coral reef perspective. Funct. Ecol. 33, 948–961.
Bellwood, D. R., Wainwright, P. C., Fulton, C. J., and Hoey, A. S. (2006b). Functional versatility supports coral reef biodiversity. Proc. R. Soc. B Biol. Sci. 273, 101–107. doi: 10.1098/rspb.2005.3276
Berno, T. (1999). When a guest is a guest. Ann. Tour. Res. 26, 656–675. doi: 10.1016/S0160-7383(99)00002-X
Bessa, E., Geffroy, B., and Gonçalves-De-Freitas, E. (2017a). Tourism impact on stream fish measured with an ecological and a behavioural indicator. Aquat. Conserv. Mar. Freshw. Ecosyst. 27, 1281–1289. doi: 10.1002/aqc.2804
Bessa, E., Silva, F., and Sabino, J. (2017b). “Impacts of Fish Tourism,” in Ecotourism’s Promise and Peril, eds D. Blumstein, B. Geffroy, D. Samia, and E. Bessa, (Cham: Springer International Publishing), 59–72. doi: 10.1007/978-3-319-58331-0_5
Beukers-Stewart, B. D., and Jones, G. P. (2004). The influence of prey abundance on the feeding ecology of two piscivorous species of coral reef fish. J. Exp. Mar. Biol. Ecol. 299, 155–184. doi: 10.1016/j.jembe.2003.08.015
Beyerl, K., Putz, O., and Breckwoldt, A. (2016). The role of perceptions for community-based marine resource management. Front. Mar. Sci. 3:238. doi: 10.3389/fmars.2016.00238
Birnie-Gauvin, K., Peiman, K. S., Raubenheimer, D., and Cooke, S. J. (2017). Nutritional physiology and ecology of wildlife in a changing world. Conserv. Physiol. 5:cox030. doi: 10.1093/conphys/cox030
Bohnsack, J. A., and Bannerot, S. P. (1986). A stationary visual census technique for quantitatively assessing community structure of coral reef shes. NOAA Tech. Rep. 41, 1–15.
Breckwoldt, A., Ratter, B. M., and Wang, W. C. (2018). Fishing for human perceptions in coastal and Island marine resource use systems. Front. Mar. Sci. 5:62. doi: 10.1371/journal.pone.0143516
Brookhouse, N., Bucher, D. J., Rose, K., Kerr, I., and Gudge, S. (2013). Impacts, risks and management of fish feeding at neds beach, lord howe Island marine park, Australia: a case study of how a seemingly innocuous activity can become a serious problem. J. Ecotour. 12, 165–181. doi: 10.1080/14724049.2014.896369
Brown, J. S. (2019). “Ecology of fear,” in Encycopledia of Animal Behaviour, Vol. 1, 2nd Edn, ed. C. Chun, (London: Academic Press), 196–202. doi: 10.1016/b978-0-12-809633-8.20870-4
Bruno, J. F., and Selig, E. R. (2007). Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS One 2:e711. doi: 10.1371/journal.pone.0000711
Burgin, S., and Hardiman, N. (2015). Effects of non-consumptive wildlife-oriented tourism on marine species and prospects for their sustainable management. J. Environ. Manage. 151, 210–220. doi: 10.1016/j.jenvman.2014.12.018
Burkepile, D. E., and Hay, M. E. (2008). Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc. Natl. Acad. Sci. U.S.A. 105, 16201–16206. doi: 10.1073/pnas.0801946105
Choat, J. H., and Clements, K. D. (1993). Daily feeding rates in herbivorous labroid fishes. Mar. Biol. 117, 205–211. doi: 10.1007/BF00345664
Coker, D. J., Pratchett, M. S., and Munday, P. L. (2009). Coral bleaching and habitat degradation increase susceptibility to predation for coral-dwelling fishes. Behav. Ecol. 20, 1204–1210. doi: 10.1093/beheco/arp113
Cole, A. J., Pratchett, M. S., and Jones, G. P. (2008). Diversity and functional importance of coral-feeding shes on tropical coral reefs. Fish Fish. 9, 286–307. doi: 10.1111/j.1467-2979.2008.00290.x
Cole, R. G. (1994). Abundance, size structure and diver oriented behavior of three large benthic carnivorous fishes in a marine reserve in northeastern New Zealand. Biol. Conserv. 70, 93–99. doi: 10.1016/0006-3207(94)90276-3
Cook Islands Tourism (2019). Available online at: http://www.mfem.gov.ck/statistics/social-statistics/tourism-and-migration (accessed September 7, 2019).
Corcoran, M. J., Wetherbee, B. M., Shivji, M. S., Potenski, M. D., Chapman, D. D., and Harvey, G. M. (2013). Supplemental feeding for ecotourism reverses diel activity and alters movement patterns and spatial distribution of the southern stingray, Dasyatis americana. PLoS One 8:e59235. doi: 10.1371/journal.pone.0059235
Crossman, D. J., Choat, J. H., Clements, K. D., Hardy, T., and Mcconochie, J. (2001). Detritus as food for grazing shes on coral reefs. Limnology 46, 1596–1605. doi: 10.4319/lo.2001.46.7.1596
Day, R. W., and Quinn, G. P. (1989). Comparisons of treatments after an analysis of variance in ecology. Ecol. Monogr. 59, 433–463. doi: 10.1111/j.1523-1739.2008.01130.x
English, S. S., Wilkinson, C. C., and Baker, V. V. (1997). Survey Manual for Tropical Marine Resources. Townsville, QLD: Australian Institute of Marine Science.
Feitosa, C. V., Chaves, L. D. C. T., Ferreira, B. P., and de Araújo, M. E. (2012). Recreational fish feeding inside Brazilian MPAs: impacts on reef fish community structure. J. Mar. Biolog. Assoc. U.K. 92, 1525–1533. doi: 10.1017/S0025315412000136
Fox, R. J., and Bellwood, D. R. (2007). Quantifying herbivory across a coral reef depth gradient. Mar. Ecol. Prog. Ser. 339, 49–59. doi: 10.1098/rsbl.2012.0770
Fox, R. J., and Bellwood, D. R. (2008). Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 27, 605–615. doi: 10.1007/s00338-008-0359-6
Geffroy, B., Sadoul, B., Bouchareb, A., Prigent, S., Bourdineaud, J. P., Gonzalez-Rey, M., et al. (2018). Nature-based tourism elicits a phenotypic shift in the coping abilities of fish. Front. Physiol. 9:13. doi: 10.3389/fphys.2018.00013
Geffroy, B., Sadoul, B., and Ellenberg, U. (2017). “Physiological and behavioral consequences of human visitation,” in Ecotourism’s Promise and Peril, eds D. Blumstein, B. Geffroy, D. Samia, and E. Bessa, (Cham: Springer International Publishing), 59–72.
Gil, M. A., Zill, J., and Ponciano, J. M. (2017). Context−dependent landscape of fear: algal density elicits risky herbivory in a coral reef. Ecology 98, 534–544. doi: 10.1002/ecy.1668
Goatley, C. H., and Bellwood, D. R. (2010). Biologically mediated sediment fluxes on coral reefs: sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar. Ecol. Prog. Ser. 415, 237–245. doi: 10.3354/meps08761
Great Barrier Reef Marine Park Authority (1994). Guidelines for Fish Feeding in the Great Barrier Reef (Brochure). Townsville, QLD: Great Barrier Reef Marine Park Authority.
Green, A. L., and Bellwood, D. R. (2009). Monitoring Functional Groups of Herbivorous Reef Fishes as Indicators of Coral Reef Resilience—a Practical Guide for Coral Reef Managers in the Asia Pacific Region (No. 7). Gland: IUCN Working Group on Climate Change and Coral Reefs, 70.
Green, R. J., and Higginbottom, K. (2000). The effects of non-consumptive wildlife tourism on free- ranging wildlife: a review. Pac. Conserv. Biol. 6, 183–197.
Gregson, M. A., Pratchett, M. S., Berumen, M. L., and Goodman, B. A. (2008). Relationships between butterflyfish (Chaetodontidae) feeding rates and coral consumption on the Great Barrier Reef. Coral Reefs 27, 583–591. doi: 10.1007/s00338-008-0366-7
Hammerschlag, N., Gallagher, A. J., Wester, J., Luo, J., and Ault, J. S. (2012). Don’t bite the hand that feeds: assessing ecological impacts of provisioning ecotourism on an apex marine predator. Funct. Ecol. 26, 567–576. doi: 10.1111/j.1365-2435.2012.01973.x
Harmelin-Vivien, M. L. (1989). “Reef fish community structure: an Indo-Pacific comparison,” in Vertebrates in Complex Tropical Systems, eds M. L. Harmelin-Vivien and F. Bourlière, (New York, NY: Springer), 21–60. doi: 10.1007/978-1-4612-3510-1_2
Harriott, V. J. (2002). Marine Tourism Impacts and Their Management on the Great Barrier Reef. Technical Report 41. Townsville, QLD: CRC Reef Research Centre
Hawkins, J. P., Roberts, C. M., Van’t Hof, T., De Meyer, K., and Tratalos, J. (1999). Effects of recreational scuba diving on Caribbean coral and fish communities. Biol. Conserv. 13, 888–897. doi: 10.1046/j.1523-1739.1999.97447.x
Hémery, G., and McClanahan, T. R. (2005). Effect of recreational fish feeding on reef fish community composition and behaviour. West. Indian Ocean J. Mar. Sci. 4, 123–133.
Higginbottom, K., Rann, K., Moscardo, G., Davis, D., and Muloin, S. (2001). Status Assessment of Wildlife Tourism in Australia: An overview. Gold Coast, QLD: CRC for Sustainable Tourism.
Hoffmann, T. C. (2002). Coral reef health and effects of socio-economic factors in Fiji and Cook Islands. Mar. Pollut. Bull. 44, 1281–1293. doi: 10.1016/S0025-326X(02)00260-6
Ilarri, I., De Souza, A. T., De Medeiros, P. R., Grempel, R. G., and De Lucena Rosa, I. M. (2008). Effects of tourist visitation and supplementary feeding on fish assemblage composition on a tropical reef in the Southwestern Atlantic. Neotropical. Ichthyol. 6, 651–656. doi: 10.1590/S1679-62252008000400014
Januchowski-Hartley, F. A., Nash, K. L., and Lawton, R. J. (2012). Influence of spear guns, dive gear and observers on estimating fish flight initiation distance on coral reefs. Mar. Ecol. Prog. Ser. 469, 113–119. doi: 10.3354/meps09971
Jones, G. P., McCormick, M. I, Srinivasan, M., and Eagle, J. V. (2004). Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. U.S.A. 101, 8251–8253. doi: 10.1073/pnas.0401277101
Jordan, L. K., Gilliam, D. S., and Spieler, R. E. (2005). Reef fish assemblage structure affected by small-scale spacing and size variations of artificial patch reefs. J. Exp. Mar. Biol. Ecol. 326, 170–186. doi: 10.1016/j.jembe.2005.05.023
Krone, R., Bshary, R., Paster, M., Eisinger, M., van Treeck, P., and Schuhmacher, H. (2008). Defecation behaviour of the lined bristletooth surgeonfish Ctenochaetus striatus (Acanthuridae). Coral Reefs 27, 619–622. doi: 10.1007/s00338-008-0365-8
Loubersac, L., Burban, P. −Y., Lemaire, O., Varet, H., and Chenon, F. (1991). Integrated study of aitutaki’s lagoon (cook islands) using spot satellite data and in situ measurements: bathymetric modelling. Geocarto Int. 6, 31–37. doi: 10.1080/10106049109354304
Lyons, P. J. (2013). The benefit of obligate versus facultative strategies in a shrimp–goby mutualism. Behav. Ecol. Sociobiol. 67, 737–745. doi: 10.1007/s00265-013-1497-6
Madin, E. M. P., Madin, J. S., and Booth, D. J. (2011). Landscape of fear visible from space. Sci. Rep. 1:14. doi: 10.1038/srep00014
Mantyka, C. S., and Bellwood, D. R. (2007). Direct evaluation of macroalgal removal by herbivorous coral reef fishes. Coral Reefs 26, 435–442. doi: 10.1007/s00338-007-0214-1
Marshell, A., and Mumby, P. J. (2012). Revisiting the functional roles of the surgeonfish Acanthurus nigrofuscus and Ctenochaetus striatus. Coral Reefs 31, 1093–1101. doi: 10.1007/s00338-012-0931-y
Matis, P. A. (2018). Habitat Associations of Tropical Fishes Across Latitudes: Implications for Ocean Warming and Species Range Expansion. Doctoral dissertation, University of Technology Sydney, Ultimo, NSW.
Mattos, F. M. G., and Yeemin, T. (2018). Worldwide recreational fish feeding: a review on ecological impacts. Ramkhamhaeng Int. J. Sci. Technol. 1, 8–15.
McClanahan, T. R., Graham, N. A. J., Maina, J., Chabanet, P., Bruggemann, J. H., and Polunin, N. V. C. (2007). Influence of instantaneous variation on estimates of coral reef fish populations and communities. Mar. Ecol. Prog. Ser. 340, 221–234. doi: 10.3354/meps340221
Medeiros, P. R., Grempel, R. G., Souza, A. T., Ilarri, M. T., and Sampáio, C. L. S. (2007). Effects of recreational activities on the fish assemblage structure in a northeastern Brazilian reef. Pan Am. J. Aquat. Sci. 2, 288–300.
Mellor, C. S. (2003). Towards new tourism development strategies in Cook Islands. Pac. Econ. Bull. 18, 100–107.
Milazzo, M., Anastasi, I., and Willis, T. J. (2006). Recreational fish feeding affects coastal fish behavior and increases frequency of predation on damselfish Chromis chromis nests. Mar. Ecol. Prog. Ser. 310, 165–172. doi: 10.3354/meps310165
Milazzo, M., Badalamenti, F., Vega Fernández, T., and Chemello, R. (2005). Effects of fish feeding by snorkellers on the density and size distribution of fishes in a Mediterranean marine protected area. Mar. Biol. 146, 1213–1222. doi: 10.1007/s00227-004-1527-z
Moon, T. W. (2001). Glucose intolerance in teleost fish: fact or fiction? Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129, 243–249. doi: 10.1016/s1096-4959(01)00316-5
Moribe, J. T. (2000). Visitor attitudes toward the prohibition of fish feeding in the Hanauma Bay marine life conservation district. Master thesis, The University of Washington, Seattle.
Moscardo, G., and Saltzer, R. (2004). “Understanding wildlife tourism markets,” in Wildlife Tourism: Impacts, Management and Planning, ed. H. Karen, (Altona, VIC: Common Ground Publishing), 167–185.
Moscardo, G., Woods, B., and Greenwood, T. (2001). Understanding Visitor Perspectives on Wildlife Tourism. Gold Coast, QLD: CRC for Sustainable Tourism.
Murray, M. H., Becker, D. J., Hall, R. J., and Hernandez, S. M. (2016). Wildlife health and supplemental feeding: a review and management recommendations. Biol. Conserv. 204, 163–174. doi: 10.1098/rstb.2017.0102
Nevers (2008). Carte Topographique d’Aitutaki/Topographic Map of Aitutaki. Public Domain. Available at: https://upload.wikimedia.org/wikipedia/commons/4/42/Aitutakitopo.png (accessed April 1, 2017).
Newsome, D. (2017). “A brief consideration of the nature of wildlife tourism,” in Wilderness of Wildlife Tourism, ed. J. K. Fatima, (Palm Bay, FL: Apple Academic Press), 21–26.
Newsome, D., Dowling, R. K., and Moore, S. A. (2005). Wildlife Tourism, Vol. 24. Bristol: Channel View Publications.
Newsome, D., Lewis, A., and Moncrieff, D. (2004). Impacts and risks associated with developing, but unsupervised, stingray tourism at Hamelin Bay, Western Australia. Int. J. Tour. Res. 6, 305–323. doi: 10.1002/jtr.491
Newsome, D., Moore, S. A., and Dowling, R. K. (2012). Natural Area Tourism: Ecology, Impacts and Management, Vol. 58. Bristol: Channel view publications.
Newsome, D., and Rodger, K. (2008). To feed or not to feed: a contentious issue in wildlife tourism. Aust. Zool. 34, 255–270. doi: 10.7882/FS.2008.029
Nunes, J. A. C., Costa, Y., Blumstein, D. T., Leduc, A. O., Dorea, A. C., Benevides, L. J., et al. (2018). Global trends on reef fishes’ ecology of fear: flight initiation distance for conservation. Mar. Environ. Res. 136, 153–157. doi: 10.1016/j.marenvres.2018.02.011
Orams, M. B. (2002). Feeding wildlife as a tourism attraction: a review of issues and impacts. Tour. Manag. 23, 281–293. doi: 10.1016/S0261-5177(01)00080-2
Patroni, J., Simpson, G., and Newsome, D. (2018). Feeding wild fish for tourism—a systematic quantitative literature review of impacts and management. Int. J. Tour. Res. 20, 286–298. doi: 10.1002/jtr.2180
Paula, Y. C. D., Schiavetti, A., Sampaio, C. L., and Calderon, E. (2018). The effects of fish feeding by visitors on reef fish in a marine protected area open to tourism. Biota Neotrop. 18. doi: 10.1590/1676-0611-bn-2017-0339
Pinheiro, J., Bates, D., DebRoy, S., and Sarkar, D. (2015). R Core Team. nlme: Linear and Nonlinear Mixed Effects Models; 2015. R Package Version 3.1-120. R Core Team. R: A language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Pratchett, M. S. (2005). Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at Lizard Island, northern Great Barrier Reef. Mar. Biol. 148, 373–382. doi: 10.1007/s00227-005-0084-4
Pratchett, M. S., Hoey, A. S., Wilson, S. K., Messmer, V., and Graham, N. A. J. (2011). Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 3, 424–452. doi: 10.3390/d3030424
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rankey, E. C., and Reeder, S. L. (2009). Holocene ooids of Aitutaki Atool, Cook Islands, south pacific. Geology 37, 971–974. doi: 10.1130/G30332A.1
Reynolds, P. C., and Braithwaite, D. (2001). Towards a conceptual framework for wildlife tourism. Tour. Manag. 22, 31–42. doi: 10.1016/S0261-5177(00)00018-2
Rodgers, E. M. (2017). Foraging for fast food: the changing diets of wildlife. Conserv. Physiol. 5:cox046. doi: 10.1093/conphys/cox046
Rubio, V. C., Saìnchez, E., and Cerdaì−Reverter, J. M. (2010). Compensatory feeding in the sea bass after fasting and physical stress. Aquaculture 298, 332–337. doi: 10.1016/j.aquaculture.2009.10.031
Salim, N., and Mohamed, B. (2014). The relationship between socio-demographic characteristics and snorkeling satisfaction in pulau payar marine park, kedah. Int. J. Built Environ. Sustain. 1, 38–44. doi: 10.11113/ijbes.v1.n1.6
Salim, N., Mohamed, B., and Abdullah, A. L. (2015). An evaluation of snorkelling satisfaction at pulau payar marine park, kedah, Malaysia. Adv. Environ. Biol. 9, 35–38.
Sa-nguansil, S., Tantichodok, P., Darumas, U., Lheknim, V., and Goh, B. P. L. (2017). Coral reef fishes attracted by recreational feeding in Thailand. Phuket Mar. Biol. Cent. Res. Bull. 74, 13–22.
Schuhmacher, H., Krone, R., and van Treeck, P. (2008). “Underestimated eroder among reef fishes – experimental comparison between Ctenochaetus striatus and Acanthurus nigrofuscus (Acanthuridae),” in Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida, 331–334.
Semeniuk, C. A. D., Haider, W., Beardmore, B., and Rothley, K. D. (2009). A multi-attribute trade-off approach for advancing the management of marine wildlife tourism: a quantitative assessment of heterogeneous visitor preferences. Aquat. Conserv. Mar. Freshw. Ecosyst. 19, 194–208. doi: 10.1002/aqc
Semeniuk, C. A. D., and Rothley, K. D. (2008). Costs of group-living for a normally solitary forager: effects of provisioning tourism on southern stingrays Dasyatis americana. Mar. Ecol. Prog. Ser. 357, 271–282. doi: 10.3354/meps07299
Spalding, M., Burke, L., Wood, S. A., Ashpole, J., Hutchison, J., and zu Ermgassen, P. (2017). Mapping the global value and distribution of coral reef tourism. Mar. Policy 82, 104–113. doi: 10.1016/j.marpol.2017.05.014
Streit, R. P., Hoey, A. S., and Bellwood, D. R. (2015). Feeding characteristics reveal functional distinctions among browsing herbivorous fishes on coral reefs. Coral Reefs 34, 1037–1047. doi: 10.1007/s00338-015-1322-y
Strelcheck, A. J., Cowan, J. H., and Patterson, W. F. (2007). “Site fidelity, movement, and growth of red snapper: implications for artificial reef management,” in Proceedings of the American Fisheries Society Symposium, Vol. 60 (Bethesda, MD: American Fisheries Society), 147.
Sweatman, H. P. A. (1996). Impact of Tourist Floating Docks on Fish Assemblages on the Great Barrier Reef. Technical Report 5. Townsville, QL: CRC Reef Research Centre, 54.
Tebbett, S. B., Goatley, C. H., and Bellwood, D. R. (2017b). The effects of algal turf sediments and organic loads on feeding by coral reef surgeonfishes. PLoS One 12:e0169479. doi: 10.1371/journal.pone.0169479
Tebbett, S. B., Goatley, C. H. R., and Bellwood, D. R. (2017a). Clarifying functional roles: algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus. Coral Reefs 36, 803–813. doi: 10.1007/s00338-017-1571-z
Thresher, R. E. (1979). Social behavior and ecology of two sympatric wrasses (Labridae: Halichoeres spp.) off the coast of Florida. Mar. Biol. 53, 161–172. doi: 10.1007/bf00389187
Topelko, K. (2007). Understanding the Environmental and Social Impacts of Coral Reef Use: A Study of the Snorkeling Environment and Experience in Koh Chang Marine National Park, Thailand. Victoria, BC: University of Victoria.
Trave, C., Brunnschweiler, J. M., Sheaves, M., Diedrich, A., and Barnett, A. (2017). Are we killing them with kindness? Evaluation of sustainable marine wildlife tourism. Biol. Conserv. 209, 211–222. doi: 10.1016/j.biocon.2017.02.020
Tribot, A.-S., Deter, J., and Mouquet, N. (2018). Integrating the aesthetic value of landscapes and biological diversity. Proc. R. Soc. B Biol. Sci. 285:20180971. doi: 10.1098/rspb.2018.0971
Turner, A. M., and Ruhl, N. (2007). Phosphorus loadings associated with a park tourist attraction: limnological consequences of feeding the fish. Environ. Manag. 39, 526–533. doi: 10.1007/s00267-005-0155-9
Vaske, J. J., and Manfredo, M. J. (2012). “Social psychological considerations in wildlife management,” in Human Dimensions of Wildlife Management, eds D. J. Decker, S. Riley, and W. F. Siemer, (Baltimore, MD: The Johns Hopkins University Press), 43–57.
Vignon, M., Sasal, P., Johnson, R. L., and Galzin, R. (2010). Impact of shark-feeding tourism on surrounding fish populations off Moorea Island (French Polynesia). Mar. Freshw. Res. 61, 163–169.
Wainwright, P. C. (1991). Ecomorphology: exprimental functional anatomy for ecological problems. Am. Zool. 31, 680–693. doi: 10.1093/icb/31.4.680
Wen, C. K., Chen, K. S., Tung, W. C., Chao, A., Wang, C. W., Liu, S. L., et al. (2018). The influence of tourism-based provisioning on fish behavior and benthic composition. Ambio 48, 779–789. doi: 10.1007/s13280-018-1112-1
Whitfield, A. K., and Becker, A. (2014). Impacts of recreational motorboats on fishes: a review. Mar. Pollut. Bull. 83, 24–31. doi: 10.1016/j.marpolbul.2014.03.055
Wiener, C. S., Needham, M. D., and Wilkinson, P. F. (2009). Hawaii’s real life marine park: interpretation and impacts of commercial marine tourism in the Hawaiian Islands. Curr. Issues Tour. 12, 489–504. doi: 10.1080/13683500902736855
Wilson, S. K., Graham, N. A. J., and Polunin, N. V. C. (2007). Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 151, 1069–1076. doi: 10.1007/s00227-006-0538-3
Worm, B., Barbier, E. B., Beaumont, N., Duffy, J. E., Folke, C., Halpern, B. S., et al. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. doi: 10.1126/science.1132294
Wu, L., Xie, S., Zhu, X., Cui, Y., and Wootton, R. J. (2002). Feeding dynamics in fish experiencing cycles of feed deprivation: a comparison of four species. Aquacult. Res. 33, 481–489. doi: 10.1046/j.1365-2109.2002.00733.x
Ziegler, J. A., Dearden, P., and Rollins, R. (2015). Participant crowding and physical contact rates of whale shark tours on Isla Holbox, Mexico. J. Sustain. Tour. 24, 616–636. doi: 10.1080/09669582.2015.1071379
Ziegler, J. A., Silberg, J. N., Araujo, G., Labaja, J., Ponzo, A., Rollins, R., et al. (2018). A guilty pleasure: tourist perspectives on the ethics of feeding whale sharks in Oslob, Philippines. Tour. Manag. 68, 264–274. doi: 10.1016/j.tourman.2018.04.001
Keywords: coral reef, tourism, ecosystem function, foraging rates, provisioning, supplementary feeding, recreation, conservation evaluation
Citation: Prinz N, Story R, Lyon S, Ferse SCA and Bejarano S (2020) To Feed or Not to Feed? Coral Reef Fish Responses to Artificial Feeding and Stakeholder Perceptions in the Aitutaki Lagoon, Cook Islands. Front. Mar. Sci. 7:145. doi: 10.3389/fmars.2020.00145
Received: 22 November 2019; Accepted: 25 February 2020;
Published: 24 March 2020.
Edited by:
Maria Grazia Pennino, Instituto Español de Oceanografía (IEO), SpainReviewed by:
Robert Steneck, University of Maine, United StatesPedro Henrique Pereira, Federal Institute of Pernambuco, Brazil
Copyright © 2020 Prinz, Story, Lyon, Ferse and Bejarano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalie Prinz, bnByaW56QHVuaS1icmVtZW4uZGU=
†These authors share senior authorship
 Natalie Prinz
Natalie Prinz Richard Story3
Richard Story3 Sebastian C. A. Ferse
Sebastian C. A. Ferse Sonia Bejarano
Sonia Bejarano