
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mar. Sci. , 13 March 2020
Sec. Marine Biogeochemistry
Volume 7 - 2020 | https://doi.org/10.3389/fmars.2020.00144
Mixotrophy, understood as food ingestion and photosynthesis occurring in the same organism, is a nutrition mode relatively common in marine protists. Among these, pigmented nanoflagellates 2–20 μm in size (PNF) are now known to be responsible for a significant part of consumption of bacteria in the open ocean. However, knowledge about the importance of the mixotrophic nutrition of these organisms in coastal upwelling systems, where autotrophy prevails, is very limited. Here we compile the limited available information about mixotrophy of PNF in coastal upwelling systems, focusing on the NW Iberian upwelling, to show that this type of nutrition is relevant in these productive systems and to urge for further studies. Several indirect approaches allow inferring that mixotrophy is significant for PNF in the NW Iberian upwelling, with heterotrophy supplying approximately seventy-five percent of the total carbon requirements in this plankton group. This new insight has major implications for our view of marine food webs in coastal upwelling regions, and must be taken into account to improve biogeochemical models of the transfer of matter and energy in these marine areas.
The traditional and rather simple view of marine microbial food webs based on the autotrophic-heterotrophic dichotomy is nowadays outdated (Flynn et al., 2013). Currently, it is known that several nutrition modes coexist in the marine microbial realm (Worden et al., 2015). Mixotrophy, defined as the ability of a single cell to photosynthesize and to ingest organic particles, stands out because it confers to cells the capability of exploiting all environments in a eutrophic-oligotrophic continuum. Thus, it could be thought that mixotrophs could rely on heterotrophy only under scarcity of inorganic nutrients or light limitation (Arenovski et al., 1995; Unrein et al., 2007; Zubkov and Tarran, 2008; Stukel et al., 2011; Hartmann et al., 2012). Nevertheless, it is well known that some mixotrophs can also obtain part of their carbon requirements from food even when inorganic nutrients are abundant and photosynthesis is light saturated (Sanders et al., 1990; Skovgaard et al., 2000). Moreover, mixotrophs are not only limited to oligotrophic waters, being also found in eutrophic coastal zones (Cloern and Dufford, 2005; Burkholder et al., 2008; Farnelid et al., 2016). All this information indicates that mixotrophy is widespread and offers to marine protists an important advantage even in productive regions, allowing them to persist under the variable conditions of light, nutrients and food of these environments. However, generalizations about mixotrophy are difficult to perform due to the high functional diversity among mixotrophs. Within this group, we can find organisms originally classified as phytoplankton that eat or zooplankton that photosynthesizes harboring prey chloroplasts or endosymbionts. Mixotrophs also include organisms that have specific prey preferences and ones more generalists in their grazing (Jones, 1997; Stoecker, 1999; Mitra et al., 2016; Stoecker et al., 2017).
Quantifying mixotrophy in marine ecosystems is a challenging task. It is difficult to identify a mixotrophic species in the sea, but it is still more problematic to establish if this species is acting mixotrophically (Stoecker et al., 2017). Virtually all knowledge about the importance of mixotrophy in marine ecosystems comes from laboratory studies done with cultures and/or from incubations on board where the heterotrophy of pigmented species was determined using labeled bacteria (e.g., Havskum and Riemann, 1996; Sanders et al., 2000; Smalley et al., 2003; Park et al., 2006; Unrein et al., 2007; Kim et al., 2008; Riisgaard and Hansen, 2009; Stukel et al., 2011; Tsai et al., 2011). This means that estimates obtained for heterotrophic activity of pigmented cells are probably minimum estimates because mixotrophs in the field would consume preys additional to those supplied in the experiments. However, these studies have informed us that mixotrophy is relatively common within pigmented dinoflagellates, especially within those that form harmful blooms (Stoecker et al., 2006; Burkholder et al., 2008) and in pigmented flagellates 2–20 μm in size (pigmented nanoflagellates, PNF) (Unrein et al., 2007; Zubkov and Tarran, 2008). In this way, it has been established that mixotrophic microplankton (dinoflagellates and ciliates) and mixotrophic nanoplankton are important in the microbial community of eutrophic coastal zones, while in most oligotrophic zones mixotrophic nanoplankton, mostly haptophytes, predominates (Liu et al., 2009; Unrein et al., 2014).
Coastal upwelling systems, due to the occurrence of diatoms and also because primary production generally exceeds respiration in the water column (e.g., Daneri et al., 2000; Arbones et al., 2008), are commonly viewed as autotrophic environments (Chavez et al., 1991). Mixotrophy in these eutrophic zones is considered as subsidiary (Stoecker et al., 2017), taking place in large dinoflagellates (Ceratium spp., Dinophysis spp., etc.) and ciliates (Mesodinium rubrum) during specific periods of low levels of inorganic nutrients (e.g., Smalley et al., 2003). However, recent studies in some coastal upwelling systems have demonstrated that the group of pigmented flagellates 2–20 μm in size (PNF) is an important component of the microbial planktonic community, with its biomass exceeding that of diatoms when upwelling relaxes (e.g., Iriarte et al., 2000; Lorenzo et al., 2005; Böttjer and Morales, 2007). As mixotrophy in this plankton group has been described on only one occasion for coastal upwelling systems (Vargas et al., 2012), there is an urgent need to know the occurrence and magnitude of this type of nutrition in PNF to better understand the flows of matter and energy in these productive coastal waters.
Here we compile published results to show the importance of mixotrophy in PNF in the coastal upwelling system of the NW Iberian Peninsula. Specifically we review the spatial and temporal scales of mixotrophy in PNF based on two previous samplings (Crespo et al., 2011; Figueiras et al., 2014). One of them corresponds to a spring upwelling along the coastline of the NW Iberian Peninsula. The other covers weekly observations over an entire seasonal cycle at a continental shelf position.
The first indication of the importance of PNF in the NW Iberian upwelling was obtained during a research cruise conducted in the spring 1991 along several sections perpendicular to the coastline during a relaxation event following strong upwelling (Crespo et al., 2011). High values of autotrophic biomass were mainly observed at the most oceanic stations (Figure 1A), basically composed of PNF (Figure 1B). Diatoms were also present but confined to the coastal stations where their biomass was appreciably lower than PNF biomass (Figure 1C). According to this distribution of autotrophs, the region was divided into two domains: a coastal domain with presence of diatoms and an oceanic domain with absence of diatoms. PNF were present in both domains (Figure 1B). In fact, PNF accounted for 89 ± 6% of total autotrophic biomass integrated over the water column in the oceanic domain, and accounted for 62 ± 16% of total autotrophic biomass in the coastal domain, where diatoms only represented 27 ± 17% of total autotrophic biomass.
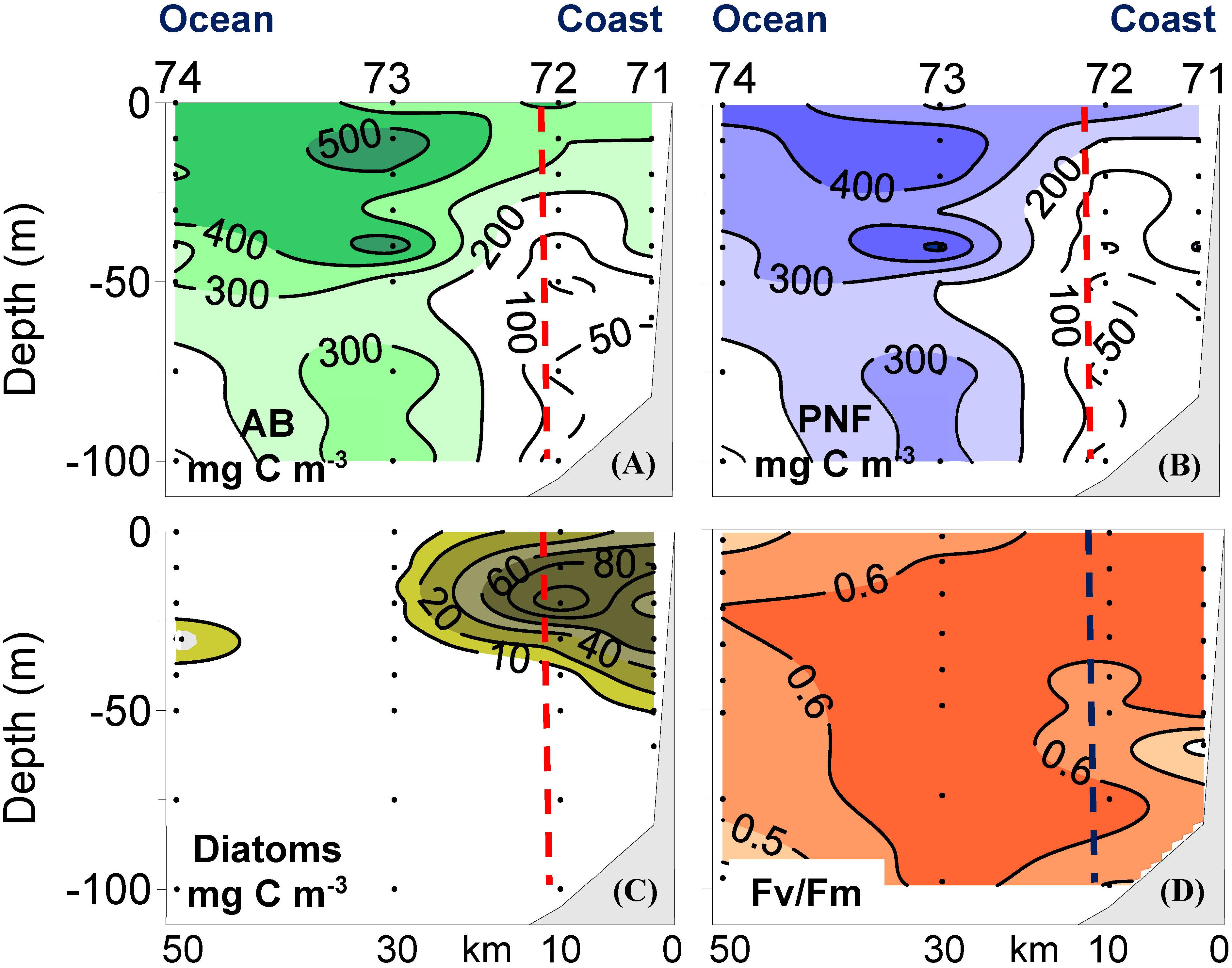
Figure 1. Vertical distributions of (A) total autotrophic biomass (AB), (B) biomass of pigmented nanoflagellates (PNF), (C) biomass of diatoms, and (D) variable fluorescence to maximum in vivo fluorescence ratio (Fv/Fm, dimensionless) at 1 selected transect sampled during the spring 1991 in the NW Iberian upwelling. The vertical dashed line separates coastal and oceanic domains. Redrawn from Crespo et al. (2011).
The phytoplankton carbon to chlorophyll a (Cph:Chl a) ratio was completely different in these two domains (Figure 2A), despite the photic layer being similar in both environments (34 ± 12 m and 36 ± 8 m in coastal and oceanic domain, respectively; P = 0.59, t-test for two samples). Therefore, the high ratio found at the oceanic domain should be due to variations in Cph since the small differences in light within similar photic layers should not affect the Chl a content. Moreover, Cph is expected to rise where nutrients are low because photosynthesis leads to production of reserve material. Consequently, the low ratio in the coastal domain, where nutrients were abundant (Crespo et al., 2011), suggests that phytoplankton were growing without limitation, while the high ratio found at the oceanic domain with low sea surface nutrient concentrations (Crespo et al., 2011) points to nutrient limitation of PNF (Buck et al., 1996; Geider et al., 1998; Marañón, 2005). The same conclusion was obtained by consideration of mean growth rates in the photic layer determined from photosynthetic carbon fixation and phytoplankton carbon biomass. The high growth rates measured in the coastal domain indicate that phytoplankton were growing at about one doubling per day without any type of limitation, whereas low growth rates at the oceanic domain suggest nutrient limitation in an environment where carbon fixation was still high (>3 g C m–2 d–1; Crespo et al., 2011).
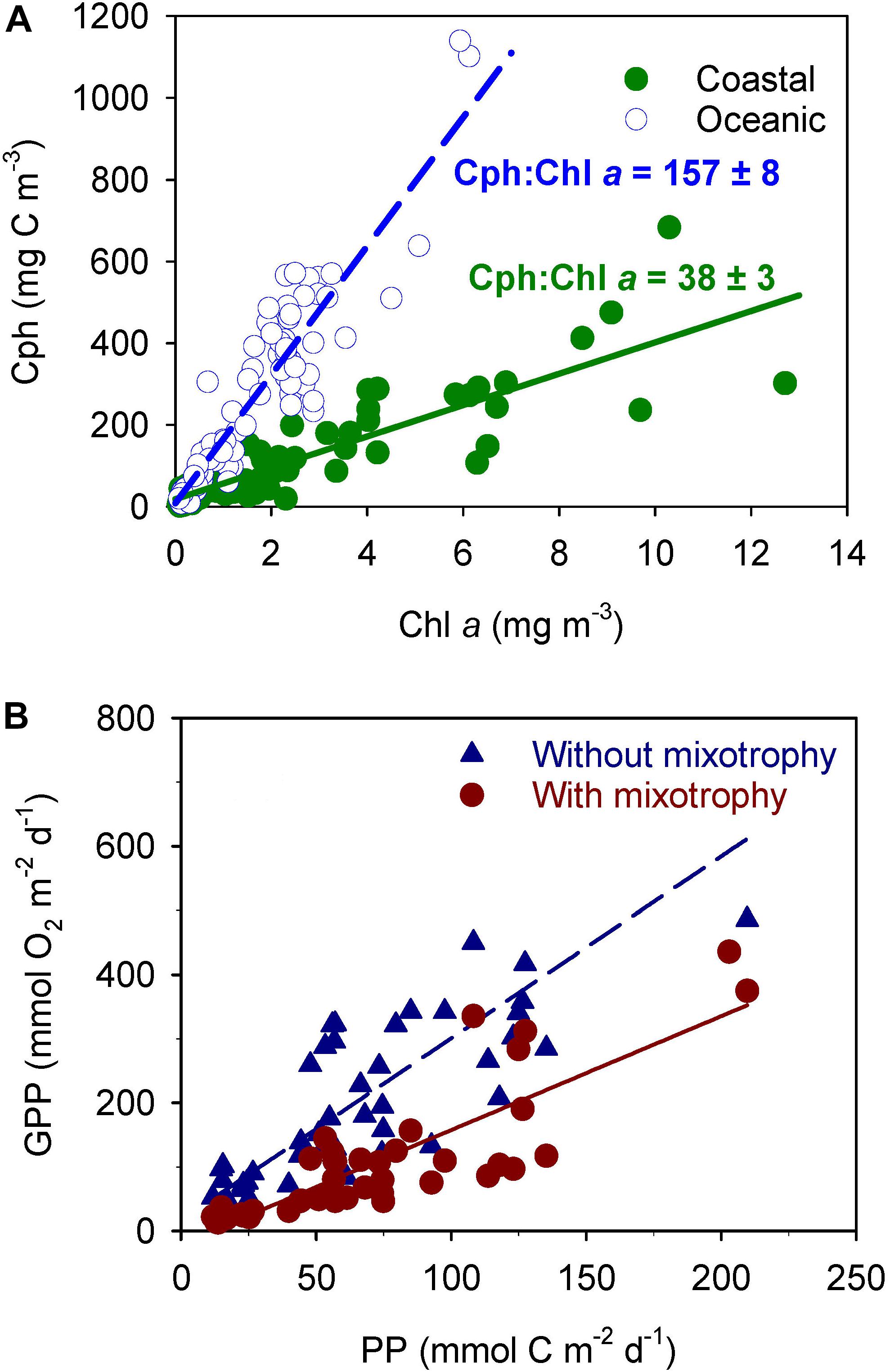
Figure 2. (A) Phytoplankton carbon to chlorophyll a relationships (Cph:Chl a) at the oceanic and costal domains identified in Figure 1. (B) Relationship between gross primary production estimated from 14C uptake (PP) and gross primary production predicted following the metabolic theory of ecology (GPP) without considering mixotrophy (blue triangles) and considering mixotrophy (brown circles) in nanophytoplankton at the station sampled on the continental shelf of NW Iberia from May 2001 to April 2002. Without correction for mixotrophy (blue dashed line) the relationship is: Y = (17.6 ± 22.6) + (2.83 ± 0.27)X; r2 = 0.71; n = 46; P < 0.001. Considering 76% of mixotrophy in nanophytoplankton (continuous brown line) the relationship is: Y = (–20.0 ± 14.1) + (1.78 ± 0.17)X; r2 = 0.71; n = 46; P < 0.001. (A) Adapted from Crespo et al. (2011) and (B) from Figueiras et al. (2014).
Nevertheless, a different conclusion was achieved examining fluorescence measurements, specifically the Fv/Fm ratio (Figure 1D). This is a ratio that provides an estimate of nutrient limitation of photosynthesis (Kolber et al., 1990; Falkowski et al., 1991; Geider et al., 1993), with values around 0.6 under sufficient nutrient conditions and values dropping to 0.3 when nutrients become limiting. According to this, the relatively high Fv/Fm values recorded during this spring cruise indicated that nutrient limitation of PNF at the oceanic domain did not occur (Figure 1D), even though sea surface nitrate levels were very low (Crespo et al., 2011).
This apparent contradiction can be resolved by postulating mixotrophic nutrition of PNF, which would allow PNF to obtain part of their carbon requirements from photosynthesis and the remaining part from heterotrophy. A rough estimation of carbon assimilated by heterotrophy was attained following Skovgaard et al. (2000), who reported Cph:Chl a ratios ∼40 for Fragilidium subglobosum cells growing exclusively with photosynthesis and Cph:Chl a ratios ∼150 for fed cells. Decreases in chlorophyll content have also been described (e.g., Wilken et al., 2013) for other mixotrophs (Ochromonas sp.) under heterotrophic nutrition.
Based on the low Cph:Chl a ratio at the coastal domain (38 ± 3; Figure 2A) it was considered that nearshore PNF nutritional mode was mainly phototrophic and also assumed that the increase in Cph:Chl a ratio at the oceanic domain was largely due to heterotrophy (Cph:Chl a = 157 ± 8; Figure 2A). From here, it was estimated that about 76 ± 11% [(157-38)/157 = 0.76] of PNF carbon requirements at the oceanic domain came through heterotrophy, and this estimate varied between 65% [(149-41)/165] and 87% [(165-35)/149] within the standard deviations of the two Cph:Chl a ratios. In spite of being a rough estimate, this provides indirect evidence for heterotrophy of PNF in a coastal upwelling system and delivers a value for carbon fraction acquired by heterotrophy similar to values reported in laboratory experiments (Sanders et al., 1990; Skovgaard et al., 2000). This estimated range of heterotrophy is also similar to levels provided by Vargas et al. (2012) for an upwelling area off central Chile.
Dominance of PNF within the phytoplankton community was also recorded during a sampling conducted on the western continental shelf in a variety of environmental conditions over an entire year (Figueiras et al., 2014).
Despite the seasonal variability observed in phytoplankton biomass, pigmented nanoplankton (essentially PNF, Espinoza-González et al., 2012), with mean biomass of 2.5 ± 1.4 g C m–2 in the photic layer, clearly dominated the phytoplankton community, accounting for 73 ± 16% of total autotrophic biomass. Microphytoplankton (mostly diatoms) with a mean biomass of 0.7 ± 1.2 g C m–2 accounted only for 14 ± 19% of total autotrophic biomass and showed high short-term variability. The contribution (12 ± 8%) and average biomass (0.4 ± 0.4 g C m–2) of picophytoplankton were similar to those of microphytoplankton but with lower variability. Both pigmented nanoplankton and diatoms shared importance in the community during summer upwelling and the spring bloom (Figueiras et al., 2014).
Size-fractionated primary production showed high short-term variability in the microphytoplankton fraction (0.35 ± 0.36 g C m–2 d–1) that, however, was not so evident in the other two fractions; 0.22 ± 0.16 g C m–2 d–1 by nanophytoplankton and 0.19 ± 0.13 g C m–2 d–1 by picophytoplankton. Nevertheless, the mean contribution of each fraction to total primary production was similar; 36 ± 28% by microphytoplankton, 35 ± 18 by nanophytoplankton and 26 ± 14 by picophytoplankton, which strongly contrasted with differences in biomass and in contributions of each fraction to total biomass. Consequently, the average turnover rates (PP/B, d–1) of microphytoplankton (1.3 ± 1.3 d–1) and picophytoplankton (0.71 ± 0.66 d–1) indicated that phytoplankton belonging to these two fractions were growing well at more than 1 doubling per day. In contrast, the low turnover rate of nanophytoplankton (0.09 ± 0.05 d–1) suggests that this phytoplankton fraction, which accounted for the highest phytoplankton biomass, obtained part of their carbon requirements from sources other than photosynthesis, mixotrophy being the best candidate (Unrein et al., 2007, 2014; Zubkov and Tarran, 2008; Hartmann et al., 2012).
The existence of mixotrophy in pigmented nanophytoplankton was indirectly proven by comparing gross primary production in oxygen units with gross primary production in carbon units. While primary production in carbon units (PP) was experimentally determined by 14C short-term incubations (Figueiras et al., 2014) corresponding to the sum of primary production of the three fractions, gross primary production in oxygen units (GPP) was estimated following the metabolic theory of ecology (López-Urrutia et al., 2006; Espinoza-González et al., 2012; Figueiras et al., 2014).
The gross primary production estimated in this way (GPP) compared fairly well with primary production (PP) determined from 14C incubations (Figure 2B) but delivered an unrealistic photosynthetic quotient (PQ = 2.83 ± 0.27 mol O2 mol C–1). However, this GPP was estimated considering that all nanophytoplankton biomass was performing photosynthesis, whereas from the spring cruise it was inferred that only 24% of PNF carbon comes from photosynthesis and the remaining carbon from heterotrophy. Therefore, GPP was re-estimated considering that 24% of the biomass of nanophytoplankton was obtained by photosynthesis and, consequently, producing oxygen. The new relationship brought a lower PQ = 1.78 ± 0.17 mol O2 mol C–1 that is not significantly different (P = 0.76, t-test for paired samples) to PQ = 1.4 mol O2 mol C–1 given by Laws (1991) as maximum value for phytoplankton growing with enough nutrients and hence predominantly synthesizing proteins. Even more, a PQ = 1.78 is nearly identical to PQ = 1.73 experimentally determined in the region using both oxygen and 14C incubations simultaneously (Arbones et al., 2008).
Mixotrophy of PNF has been well described for oligotrophic zones of the North Atlantic (Zubkov and Tarran, 2008; Hartmann et al., 2012), where bacterivory by this plankton group can represent up to 95% of all bacterivory. Likewise, it is important in oligotrophic coastal zones (Havskum and Riemann, 1996; Unrein et al., 2007; Tsai et al., 2011), accounting for between 35 and 86% of all bacterivory. In all these cases, it is assumed that heterotrophy confers to mixotrophic PNF the ability to acquire nutrients from their prey when nutrients are scarce in the environment.
However, mixotrophy of PNF has also been described in areas where nutrients are abundant. Thus, PNF can account for 50% of all bacterivory in High Nutrient-Low Chlorophyll (HNLC) areas of the equatorial Pacific (Stukel et al., 2011), where limitation by Fe has been suggested to trigger heterotrophy. Mixotrophic PNF can represent between 8 and 42% of all bacterivorous nanoflagellates in the Ross Sea, south of the Polar Front (Moorthi et al., 2009), with rates of bacteria ingestion sometimes higher than those rates shown by strict heterotrophs (McKie-Krisberg et al., 2015). In the same way, Czypionka et al. (2011) has described that mixotrophic cryptophytes in a Chilean fjord (Aysén Fjord) can get 20–60% of their carbon through heterotrophy induced by light limitation in winter.
Although mixotrophy in all these studies has been estimated from the ingestion of bacteria, there are other studies (Frias-Lopez et al., 2009) indicating that mixotrophic PNF can ingest autotrophic picoplankton (Synechococcus and Prochlorococcus), which would expand the recognized role of this plankton group in the functioning of microbial food webs and in biogeochemical cycles.
The presence and at times dominance of PNF in the phytoplankton community of the NW Iberian upwelling seems to be a characteristic feature of the system, being observed not only in the study cases detailed in this work, but also in other occasions. At the end of the upwelling season (Crespo et al., 2012), PNF accounted for 53 ± 9% of the total autotrophic biomass, when the water column was strongly stratified, nutrient levels in the surface layer were extremely low and the Cph:Chl a ratio was 170 ± 6; conditions that also point to the occurrence of mixotrophy. Under strong upwelling and high nutrient levels, PNF were also significant components of the phytoplankton community (Figueiras et al., 2002; Crespo et al., 2007; Froján et al., 2014). In addition, PNF have been also reported as important components of the phytoplankton community in other coastal upwelling systems (Iriarte and González, 2004; Böttjer and Morales, 2007; Baltar et al., 2009) which reinforces the idea that PNF are always present in the water column, from winter to summer and from downwelling to upwelling.
As PNF are habitual and important components of the phytoplankton community in coastal upwelling systems and after viewing that mixotrophy could be a common nutrition mode within this group, it is essential to consider mixotrophy when building carbon budgets or developing biogeochemical models in coastal upwelling systems. The next research step should be to quantify irrefutably this type of nutrition, not only within PNF as a group but also within the different components of PNF. It would be expected to find changes in species composition within the PNF group, similar to those observed within microplankton, in response to environmental variability. Therefore, determining this biological variability is essential to understanding PNF diversity in coastal upwelling systems. Classical and new omics approaches (Collado-Fabbri et al., 2011; Hernández-Ruiz et al., 2018) would help understand the dynamics and role of these organisms in relation to the highly variable oceanographic conditions that usually prevail in these marine ecosystems. Indirect approaches similar to the ones reported here could also be applied analyzing older databases, providing further evaluations of this type of nutrition even if resources are limited for further experimental work.
FF conceived and wrote the manuscript, following discussion and exchange of ideas with the other authors. All authors reviewed the manuscript and agreed with the final version submitted for publication.
This work was funded by the Spanish project i-SMALL (CTM2014-56119-R). IT thanks the financial support provided by a Post-doctoral grant by FCT (SFRH/BPD/108485/2015) through national and European funds (POCH programme) and that provided by FCT/MCTES to CESAM (UID/AMB/50017/2019) through national funds.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank D. Barton for the revision of the manuscript. We also like to thank the two reviewers of this article.
Arbones, B., Castro, C. G., Alonso-Pérez, F., and Figueiras, F. G. (2008). Phytoplankton size structure and water column metabolic balance in a coastal upwelling system: the Ría de Vigo, NW Iberia. Aquat. Microb. Ecol. 50, 169–179. doi: 10.3354/ame01160
Arenovski, A. L., Lim, E. L., and Caron, D. A. (1995). Mixotrophic nanoplankton in oligotrophic surface waters of the Sargasso Sea may employ phagotrophy to obtain major nutrients. J. Plankton Res. 17, 801–820. doi: 10.1093/plankt/17.4.80
Baltar, F., Arístegui, J., Montero, M. F., Espino, M., Gasol, J. M., and Herndl, G. J. (2009). Mesoscale variability modulates seasonal changes in the trophic structure of nano- and picoplankton communities across the NW Africa-Canary Islands transition zone. Prog. Oceanogr. 83, 180–188. doi: 10.1016/j.pocean.2009.07.016
Böttjer, D., and Morales, C. E. (2007). Nanoplanktonic assemblages in the upwelling area off Concepción (∼36°S), central Chile: abundance, biomass, and grazing potential during the annual cycle. Prog. Oceanogr. 75, 415–434. doi: 10.1016/j.pocean.2007.08.024
Buck, K. R., Chavez, F. P., and Campbell, L. (1996). Basin-wide distributions of living carbon components and the inverted trophic pyramid of the central gyre of the North Atlantic Ocean, summer 1993. Aquat. Microb. Ecol. 10, 283–298. doi: 10.3354/ame010283
Burkholder, J. M., Glibert, P. M., and Skelton, H. M. (2008). Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8, 77–93. doi: 10.1016/j.hal.2008.08.010
Chavez, F. P., Barber, R. T., Kosro, P. M., Huyer, A., Ramp, S. R., Stanton, T. P., et al. (1991). Horizontal transport and the distribution of nutrients in the coastal trasition zone off Northern California: effects on the primary production, phytoplankton biomass and species composition. J. Geophys. Res. 96, 14833–14848. doi: 10.1029/91JC01163
Cloern, J. E., and Dufford, R. (2005). Phytoplankton community ecology: principles applied in San Francisco Bay. Mar. Ecol. Prog. Ser. 285, 11–28. doi: 10.3354/meps285011
Collado-Fabbri, S., Vaulot, D., and Ulloa, O. (2011). Structure and seasonal dynamics of the eukaryotic picophytoplankton community in a wind-driven coastal upwelling ecosystem. Limnol. Oceanogr. 56, 2334–2346. doi: 10.4319/lo.2011.56.6.2334
Crespo, B. G., Espinoza-González, O., Teixeira, I. G., Castro, C. G., and Figueiras, F. G. (2011). Possible mixotrophy of pigmented nanoflagellates: microbial plankton biomass, primary production and phytoplankton growth in the NW Iberian upwelling in spring. Estuar. Coast. Shelf Sci. 94, 172–181. doi: 10.1016/j.ecss.2011.06.008
Crespo, B. G., Espinoza-González, O., Teixeira, I. G., Castro, C. G., and Figueiras, F. G. (2012). Structure of the microbial plankton community in the NW Iberian margin at the end of the upwelling season. J. Mar. Syst. 95, 50–60. doi: 10.1016/j.jmarsys.2012.01.013
Crespo, B. G., Figueiras, F. G., and Groom, S. (2007). Role of across-shelf currents in the dynamics of harmful dinoflagellate blooms in the northwestern Iberian upwelling. Limnol. Oceanogr. 52, 2668–2678. doi: 10.4319/lo.2007.52.6.2668
Czypionka, T., Vargas, C. A., Silva, N., Daneri, G., González, H. E., and Iriarte, J. L. (2011). Importance of mixotrophic nanoplankton in Aysén Fjord (Southern Chile) during austral winter. Cont. Shelf Res. 31, 216–224. doi: 10.1016/j.csr.2010.06.014
Daneri, G., Dellarossa, V., Quiñones, R., Jacob, B., Montero, P., and Ulloa, O. (2000). Primary production and community respiration in the humboldt current system off chile and associated oceanic areas. Mar. Ecol. Prog. Ser. 197, 41–49. doi: 10.3354/meps197041
Espinoza-González, O., Figueiras, F. G., Crespo, B. G., Teixeira, I. G., and Castro, C. G. (2012). Autotrophic and heterotrophic microbial plankton biomass in the NW Iberian upwelling: seasonal assessment of metabolic balance. Aquat. Microb. Ecol. 67, 77–89. doi: 10.3354/ame01584
Falkowski, P. G., Ziemann, D., Kolber, Z., and Bienfang, P. K. (1991). Role of eddy pumping in enhancing primary production in the ocean. Nature 352, 55–58. doi: 10.1038/352055a0
Farnelid, H. M., Turk-Kudo, K. A., and Zehr, J. P. (2016). Identification of associations between bacterioplankton and photosynthetic picoeukaryotes in coastal waters. Front. Microbiol. 7:339. doi: 10.3389/fmicb.2016.00339
Figueiras, F. G., Espinoza-González, O., Arbones, B., Garrido, J. L., Teixeira, I. G., and Castro, C. G. (2014). Estimating size-fractionated primary production in the northwestern Iberian upwelling: is mixotrophy relevant in pigmented nanoplankton? Prog. Oceanogr. 128, 88–97. doi: 10.1016/j.pocean.2014.08.011
Figueiras, F. G., Labarta, U., and Fernández-Reíriz, M. J. (2002). Coastal upwelling, primary production and mussel growth in the Rías Baixas of Galicia. Hydrobiologia 484, 121–131. doi: 10.1023/A:1021309222459
Flynn, K., Stoecker, D. K., Mitra, A., Raven, J. A., Glibert, P. M., Hansen, P. J., et al. (2013). Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. J. Plankton Res. 35, 3–11. doi: 10.1093/plankt/fbs062
Frias-Lopez, J., Thompson, A., Waldbauer, J., and Chisholm, S. W. (2009). Use of stable isotope labelled cells to identify active grazers of picocyanobacteria in ocean surface waters. Environ. Microbiol. 11, 512–525. doi: 10.1111/j.1462-2920.2008.01793.x
Froján, M., Arbones, B., Zúñiga, D., Castro, C. G., and Figueiras, F. G. (2014). Microbial plankton community in the Ría de Vigo (NW Iberian upwelling system): impact of the culture of Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 498, 43–54. doi: 10.3354/meps10612
Geider, R. J., Green, R. M., Kolber, Z., MacIntyre, H. L., and Falkowski, P. G. (1993). Fluorescence assessment of the maximum quantum efficiency of photosynthesis in the western North Atlantic. Deep Sea Res. I 40, 1205–1224. doi: 10.1016/0967-0637(93)90134-O
Geider, R. J., MacIntyre, H. L., and Kana, T. M. (1998). A dynamic regulatory model of phytoplankton acclimation to light, nutrients and temperature. Limnol. Oceanogr. 43, 679–694. doi: 10.4319/lo.1998.43.4.0679
Hartmann, M., Grob, C., Tarran, G. A., Martin, A. P., Burkill, P. H., Scanlan, D. J., et al. (2012). Mixotrophic basis of Atlantic oligotrophic ecosystems. Proc. Natl. Acad. Sci. U.S.A. 109, 5756–5760. doi: 10.1073/pnas.1118179109
Havskum, H., and Riemann, B. (1996). Ecological importance of bacteriovorous, pigmented flagellates (mixotrophs) in the Bay of Aarhus, Denmark. Mar. Ecol. Prog. Ser. 137, 251–263. doi: 10.3354/meps137251
Hernández-Ruiz, M., Prieto, A., Barber-Lluch, E., and Teira, E. (2018). Amino acid utilization by eukaryotic picophytoplankton in a coastal upwelling system. Mar. Ecol. Prog. Ser. 588, 43–57. doi: 10.3354/meps12435
Iriarte, J. L., and González, H. E. (2004). Phytoplankton size structure during and after the 1997/98 El Niño in a coastal upwelling area of the northern humboldt current system. Mar. Ecol. Prog. Ser. 269, 83–90. doi: 10.3354/meps269083
Iriarte, J. L., Pizarro, G., Troncoso, V. A., and Sobarzo, M. (2000). Primary production and biomass of size-fractionated phytoplankton off Antofagast, Chile (23-24 °S) during pre-El Niño and El Niño 1997. J. Mar. Syst. 26, 37–51. doi: 10.1016/S0924-7963(00)00037-3
Jones, H. L. J. (1997). A classification of mixotrophic protists based on their behavior. Freshw. Biol. 37, 35–43. doi: 10.1046/j.1365-2427.1997.00138.x
Kim, S., Kang, Y. G., Kim, H. S., Yih, W., and Coats, D. W. (2008). Growth and grazing responses of the mixotrophic dinoflagellate Dinophysis acuminata as a function of light intensity and prey concentration. Aquat. Microb. Ecol. 51, 301–310. doi: 10.3354/ame01203
Kolber, Z., Wyman, K., and Falkowski, P. G. (1990). Natural variability in photosynthetic energy conversion efficiency. A field study in the Gulf of maine. Limnol. Ocenaogr. 35, 72–79. doi: 10.4319/lo.1990.35.1.0072
Laws, E. A. (1991). Photosynthetic quotients, new production and net community production in the open ocean. Deep Sea Res. 38, 143–167. doi: 10.1016/0198-0149(91)90059-O
Liu, H., Probert, I., Uitz, J., Claustre, H., Aris-Brosou, S., Frada, M., et al. (2009). Extreme diversity in noncalcifying haptophytes explains a major pigment paradox in open oceans. Proc. Natl. Acad. Sci. U.S.A. 106, 12803–12808. doi: 10.1073/pnas.0905841106
López-Urrutia, A., San Martin, E., Harris, R. P., and Irigoien, X. (2006). Scaling the metabolic balance of the oceans. Proc. Natl. Acad. Sci. U.S.A. 103, 8739–8744. doi: 10.1073/pnas.0601137103
Lorenzo, L. M., Arbones, B., Tilstone, G. H., and Figueiras, F. G. (2005). Across-shelf variability of phytoplankton composition, photosynthetic parameters and primary production in the NW Iberian upwelling system. J. Mar. Syst. 54, 157–173. doi: 10.1016/j.jmarsys.2004.07.010
Marañón, E. (2005). Phytoplankton growth rates in the Atlantic subtropical gyres. Limnol. Oceanogr. 50, 299–310. doi: 10.4319/lo.2005.50.1.0299
McKie-Krisberg, Z. M., Gast, R. J., and Sanders, R. W. (2015). Physiological responses of three species of Antarctic mixotrophic phytoflagellates to changes in light and dissolved nutrients. Microb. Ecol. 70, 21–29. doi: 10.1007/s00248-014-0543-x
Mitra, A., Flynn, K. J., Tillmann, U., Raven, J. A., Caron, D., Stoecker, D. K., et al. (2016). Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: incorporation of diverse mixotrophic strategies. Protist 167, 106–120. doi: 10.1016/j.protis.2016.01.003
Moorthi, S., Caron, D. A., Gast, R. J., and Sanders, R. W. (2009). Mixotrophy; a widespread and important ecological strategy for planktonic and sea-ice nanoflagellates in the Ross Sea, Antarctica. Aquat. Microb. Ecol. 54, 269–277. doi: 10.3354/ame01276
Park, M. G., Kim, S., Kim, H. S., Myung, G., Kang, Y. G., and Yih, W. (2006). First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 45, 101–106. doi: 10.3354/ame045101
Riisgaard, K., and Hansen, P. J. (2009). Role of food uptake for photosynthesis, growth and survival of the mixotrophic dinoflagellate Dinophysis acuminata. Mar. Ecol. Prog. Ser. 381, 51–62. doi: 10.3354/meps07953
Sanders, R. W., Berninger, U. G., Lim, E. L., Kemp, P. F., and Caron, D. A. (2000). Heterotrophic and mixotrophic nanoplankton predation on picoplankton in the Sargasso Sea and on Georges Bank. Mar. Ecol. Prog. Ser. 192, 103–118. doi: 10.3354/meps192103
Sanders, R. W., Porter, K. G., and Caron, D. A. (1990). Relationship between phototrophy and phagotrophy in the mixotrophic chrysophyte Poterioochromonas malhamensis. Microb. Ecol. 19, 97–109. doi: 10.1007/BF02015056
Skovgaard, A., Hansen, P. J., and Stoecker, D. K. (2000). Physiology of the mixotrophic dinoflagellate Fragilidium subglobosum. I. Effects of phagotrophy and irradiance on photosynthesis and carbon content. Mar. Ecol. Prog. Ser. 201, 129–136. doi: 10.3354/meps201129
Smalley, G. W., Coats, D. W., and Stoecker, D. K. (2003). Feeding in the mixotrophic dinoflagellate Ceratium furca is influenced by intracellular nutrient concentrations. Mar. Ecol. Prog. Ser. 262, 137–151. doi: 10.3354/meps262137
Stoecker, D., Tillmann, U., and Granéli, E. (2006). “Phagotrophy in harmful algae,” in Ecology of Harmful Algae, eds E. Granéli, and J. T. Turner, (Berlin: Springer), 177–187. doi: 10.1007/978-3-540-32210-8_14
Stoecker, D. K. (1999). Mixotrophy among dinoflagellates. J. Eukaryot. Microbial. 46, 397–401. doi: 10.1111/j.1550-7408.1999.tb04619.x
Stoecker, D. K., Hensen, P. J., Caron, D. A., and Mitra, A. (2017). Mixotrophy in the marine plankton. Annu. Rev. Mar. Sci. 9, 311–335. doi: 10.1146/annurev-marine-010816-060617
Stukel, M. R., Landry, M. R., and Selph, K. E. (2011). Nanoplankton mixotrophy in the eastern equatorial Pacific. Deep Sea Res. II 58, 378–386. doi: 10.1016/j.dsr2.2010.08.016
Tsai, A. Y., Gong, G. C., Sanders, R. W., Chen, W. H., Chao, C. F., and Chiang, K. P. (2011). Importance of bacterivory by pigmented and heterotrophic nanoflagellates during the warm season in a subtropical western Pacific coastal ecosystem. Aquat. Microb. Ecol. 63, 9–18. doi: 10.3354/ame01470
Unrein, F., Gasol, J. M., Not, F., Forn, I., and Massana, R. (2014). Mixotrophic haptophytes are key bacterial grazers in oligotrophic coastal waters. ISME J. 8, 164–176. doi: 10.1038/ismej.2013.132
Unrein, F., Massana, R., Alonso-Sáez, L., and Gasol, J. M. (2007). Significant year-round effect of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnol. Oceanogr. 52, 456–469. doi: 10.4319/lo.2007.52.1.0456
Vargas, C. A., Contreras, P. Y., and Iriarte, J. E. (2012). Relative importance of phototrophic, heterotrophic and mixotrophic nanoflagellates in the microbial food web of a river-influenced coastal upwelling area. Aquat. Microb. Ecol. 65, 233–248. doi: 10.3354/ame01551
Wilken, S., Huisman, J., Naus-Wiezer, S., and Van Donk, E. (2013). Mixotrophic organisms become more heterotrophic with rising temperature. Ecol. Lett. 16, 225–233. doi: 10.1111/ele.12033
Worden, A. Z., Follows, M. J., Giovannoni, S. J., Wilken, S., Zimmerman, A. E., and Keeling, P. J. (2015). Rethinking the marine carbon cycle: factoring in the multifarious lifestyles of microbes. Science 347, 735–745. doi: 10.1126/science.1257594
Keywords: pigmented nanoflagellates, fluorescence, growth and turnover rates, mixotrophy, coastal upwelling systems, NW Iberia
Citation: Figueiras FG, Arbones B, Castro CG, Froján M and Teixeira IG (2020) About Pigmented Nanoflagellates and the Importance of Mixotrophy in a Coastal Upwelling System. Front. Mar. Sci. 7:144. doi: 10.3389/fmars.2020.00144
Received: 05 November 2019; Accepted: 25 February 2020;
Published: 13 March 2020.
Edited by:
Javier Arístegui, University of Las Palmas de Gran Canaria, SpainReviewed by:
Rebecca Gast, Woods Hole Oceanographic Institution, United StatesCopyright © 2020 Figueiras, Arbones, Castro, Froján and Teixeira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco G. Figueiras, cGFjb0BpaW0uY3NpYy5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.