
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 02 October 2019
Sec. Aquatic Microbiology
Volume 6 - 2019 | https://doi.org/10.3389/fmars.2019.00581
The Baltic Sea is prone to oxygen deficiency due to the restricted water exchange with the North Sea in coincidence with a high biological oxygen demand. The partitioning of organic carbon between respiration, accumulation and export is co-determined by phytoplankton primary production and its subsequent bacterial remineralization. Here, we investigated net phytoplankton primary production, heterotrophic bacterial biomass production and dark CO2 fixation by on-board incubations with radiolabeled tracers in the Baltic Proper and in the Gulf of Riga after the main spring bloom. Results show that low phytoplankton standing stocks of ≤1.6 μg chlorophyll a L–1 sustained net primary production of 161–724 mg C m–2 d–1 under nitrogen limitation. Estimates of bacterial carbon remineralization suggest that freshly produced organic carbon was supplied to the aphotic zone at all stations. In the southern Baltic Proper, net primary production exceeded the bacterial carbon demand in the surface mixed layer, suggesting that organic matter derived from nutrient-limited primary production was available for export to bacterial communities below the oxycline. On average, 46% of heterotrophic bacterial production was mediated in oxygen minimum zones, revealing the high importance of organic matter recycling under hypoxic and anoxic conditions for the carbon budget. Dark CO2 fixation of up to 4.33 μg C L–1 d–1 in sulfide-free waters equaled 9–54% of the co-inciding heterotrophic bacterial carbon demand and may have provided another organic carbon source for heterotrophic activity. Substantially higher dark CO2 fixation up to 25.46 μg C L–1 d–1 was determined in sulfidic waters. Since our study was conducted 5 months after the major Baltic inflow event in winter 2014/2015, potential effects of deep water ventilation could be investigated. In the Gotland Basin, heterotrophic bacterial production in renewed oxygen-rich bottom water was similar to that in the uplifted oxygen-deficient former bottom water, while it was significantly reduced in sulfidic waters. Hence, our results suggest that the removal of hydrogen sulfide by inflow events has a high potential to increase bacterial carbon remineralization.
The Baltic Sea is a brackish ecosystem enclosed by Scandinavia, Finland, the Baltic countries, and the North European Plain. The ecosystem is put under anthropogenic pressure by agricultural and industrial development, increasing ship traffic, and its highly populated drainage basin (Elmgren, 2001; Reusch et al., 2018). Two shallow narrow straits connect the Baltic Sea with the North Sea and provide sporadic inflow of saline oxygenated seawater. The lateral advection of North Sea water results in a vertical salinity gradient and a permanent pycnocline in 60–90 m depth. As a consequence of stagnation periods between deep-water renewals, which occurred approximately once per decade in the recent past, hypoxia spreads in the water column and in sediments (Stigebrandt and Wulff, 1987; Gräwe et al., 2015). After more than 10 years without major inflow events, the third strongest event since 1880 was observed in late December 2014, about 5 months before this study took place (Mohrholz et al., 2015). The inflow of 198 km3 North Sea water, containing 2.04 × 106 t of oxygen, caused massive changes in deep water oxygen concentrations of the southern Baltic Proper and the eastern Gotland Basin (Gräwe et al., 2015; Mohrholz et al., 2015).
Biological consumption is an important oxygen sink and co-determines the expansion of anoxic zones and the duration of seasonal oxygen depletion at coastal sites (Diaz and Rosenberg, 2008). It is largely determined by the activity of heterotrophic bacterioplankton that degrade and metabolize organic matter originating from phytoplankton primary production (Robinson, 2019). In the Baltic Sea during early spring, replete nutrients in combination with increasing light availability induce the development of intense phytoplankton blooms at low seawater temperature that are often dominated by diatoms (Wasmund et al., 2008, 2013). The termination of these early spring blooms is characterized by high sedimentation of phytoplankton biomass (Heiskanen and Leppänen, 1995). In contrast to spring, the summer communities are strongly limited by inorganic nitrogen. As a consequence, small-sized phytoplankton species <10 μm dominate primary production based on regenerated nutrients, before blooms of large nitrogen-fixing cyanobacteria with a patchy distribution develop in late summer (Kahru et al., 1991). During the transition phase between spring and summer blooms, the phytoplankton community sustains only low chlorophyll a concentrations of ≤2 μg L–1 (Kahru et al., 1991). The coupling of phytoplankton primary production and heterotrophic bacterial activity during non-bloom phases is only poorly investigated. Consequently, little is known about the relevance of nutrient-limited organic matter production and degradation for carbon cycling and biological oxygen consumption in the Baltic Sea.
Recent studies have shown that the quality of organic matter strongly affects the turnover of organic matter in hypoxic and anoxic waters, while oxygen depletion in oceanic oxygen minimum zones and coastal bottom waters do not hamper heterotrophic bacterial activity (Pantoja et al., 2009; Liu et al., 2013). Experiments and field work conducted in the oxygen minimum zone of the Pacific coastal upwelling regimes imply that the lability of organic carbon is potentially an important factor co-determining oxygen consumption in the oxygen-deficient parts of the ocean (Pantoja et al., 2009; Maßmig et al., 2019). The role of organic matter supply for the anaerobic metabolism of bacterioplankton in bottom waters of coastal sites and in the deep basins of the Baltic Sea is largely unexplored. While several studies provide evidence for chemolithotrophic denitrification by hydrogen sulfide oxidation (Brettar and Rheinheimer, 1991; Hietanen et al., 2012; Dalsgaard et al., 2013), recent experimental results have shown for the first time heterotrophic denitrification in anoxic sulfide-free waters of the Baltic Sea (Bonaglia et al., 2016). The addition of labile organic carbon stimulated denitrification and dissimilatory nitrate reduction to ammonium in a natural oxycline community of the Western Gotland Basin (Bonaglia et al., 2016). Therefore, the supply of fresh and easily degradable organic matter to the deeper and oxygen-deficient water column might be of high relevance for oxygen consumption and nitrogen remineralization.
We analyzed phytoplankton primary production, heterotrophic bacterial production and chemolithoautotrophic dark CO2 fixation along with concentrations of oxygen and inorganic nutrients in the Baltic Proper and in the Gulf of Riga during June 2015. At the time of our study, North Sea water derived from a major Baltic inflow during the previous winter season had propagated to the Gotland Basin. The former bottom water in the Eastern Gotland Basin was uplifted and renewed by saline oxygen rich North Sea water. In this context, we investigated
(1) nutrient-limited phytoplankton production
(2) bacterial carbon remineralization in the surface mixed layer
(3) heterotrophic bacterial activity in renewed, uplifted and sulfidic bottom water, and
(4) dark CO2 fixation as a potential source of reactive organic carbon in oxygen minimum zones.
Twenty stations in the Baltic Proper and the Gulf of Riga were sampled during a cruise with RV Alkor from June, 3rd to June, 19th, 2015 (Figure 1). All stations, except stations T1 and GOR, are HELCOM monitoring stations1. The cruise track included several coastal stations up to 90 m depth as well as stations in the Bornholm Basin and the Gotland Basin with up to 430 m depth. At each station, samples for chemical analysis were collected in 4–12 depths by a rosette sampler equipped with Niskin bottles. Samples for primary production and heterotrophic bacterial production were taken at 11 and 19 stations, respectively. A CTD system was used to determine depth profiles of temperature and salinity. Surface water temperature ranged from 10.2 to 14.0°C and the cruise track spanned a surface salinity gradient from 5.3 at station 142 to 8.3 at station OMTF0102. The CTD system was further equipped with sensors for chlorophyll a fluorescence and oxygen (OxyGuard). Time-series data of the Helsinki commission (HELCOM) reveal that sampling took place at low chlorophyll concentrations after the decline of the spring bloom and before the onset of summer blooms (Figure 2A).
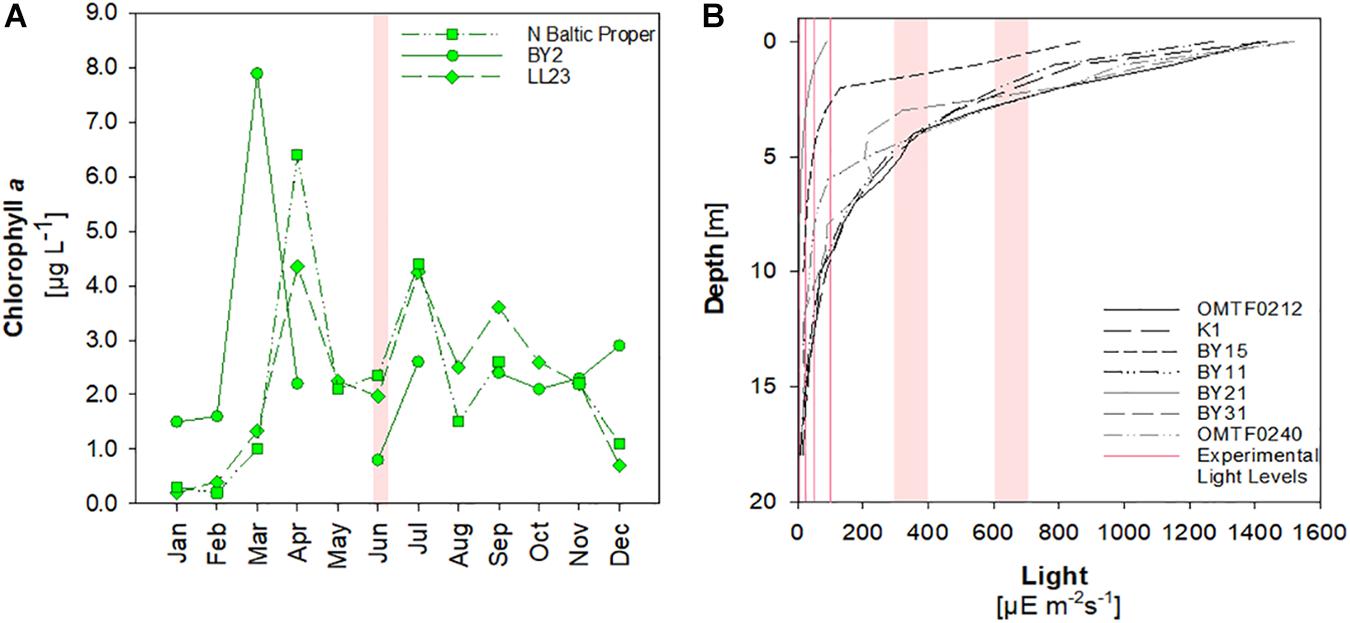
Figure 2. (A) Seasonal development of chlorophyll a concentrations at selected HELCOM stations. The red shaded area indicates the time of sampling during June, 2015 (BY2: 55°N,14.1°E; LL23: 58.6°N, 18.2°E; N Baltic Proper: 58.8°N,20.5°E) (Data are retrieved from ICES Dataset on Ocean Hydrography. The International Council for the Exploration of the Sea, Copenhagen.). (B) Light profiles recorded during this study in June 2015 and experimental light levels.
Dissolved oxygen was measured in discrete samples with optodes (Fibox, PreSens, Germany), validated with standard Winkler titrations. For the optode measurements, a sensor spot was glued to the inner wall of a Winkler bottle. An optical fiber was aligned with the sensor spot from the outside of the bottle and oxygen concentrations could be determined by luminescence emitted by the sensor spot. Measurements were corrected for salinity and temperature. Optode measurements were used to calibrate the OxyGuard sensor connected to the CTD-rosette.
Nitrate, nitrite and ammonium were analyzed according to Hansen and Koroleff (1999). Samples for nitrate and nitrite were filtered with 0.2 μm syringe filters and stored frozen at −20°C until analysis by an autoanalyzer (QuAAtro; Seal Analytical) in the lab. Ammonium was photometrically analyzed as the indophenol blue complex on board immediately after sampling.
Hydrogen sulfide was analyzed by the methylene blue method according to Fonselius et al. (1999). A zinc acetate solution was added to the samples immediately after sampling to form a zinc sulfide precipitate and to preserve the in situ concentration until analysis.
Samples for chlorophyll a analysis were collected by filtering 0.5–2.0 L of seawater through Whatman GF/F filters that were stored at −20°C. The filters were extracted in 90% acetone and homogenized with glass beads in a cell mill. After centrifugation, samples were analyzed with a fluorometer (Turner, 10-AU) according to Edler (1979) and Evans et al. (1987). Calibration of the fluorometer was carried out with standard solutions of chlorophyll a (Sigma).
Samples for dissolved inorganic carbon (DIC) were taken according to Dickson et al. (2007) and analyzed with a Single Operator Multi-parameter Metabolic Analyzer (SOMMA) (Johnson et al., 1993).
At 11 out of the 20 stations, 14 cell culture bottles were filled with 260 mL of surface sample after pre-screening through a 200 μm mesh to exclude large grazers. Each bottle was spiked with 50 μL of a 8 μCi Na14CO3 solution and mixed carefully but thoroughly. Immediately after mixing, 200 μL of spiked samples were transferred to 200 μL 2 N NaOH and 4 mL of scintillation cocktail (Ultima Gold AB) were added to determine the initial Na14CO3 concentration in each incubation bottle. After that, duplicate bottles were placed in a temperature-controlled incubator adjusted to surface temperature and light intensities of 5–7 μE m–2 s–1, 20–30 μE m–2 s–1, 50 μE m–2 s–1, 100 μE m–2 s–1, 300–400 μE m–2 s–1, and 600–700 μE m–2 s–1, respectively. Two bottles were incubated in the dark. The temperature decrease from surface to the base of the euphotic zone was <1.5°C except for stations BY21 and BY39, where higher deviations of 2.3 and 4.8°C occurred. The experimental light levels represented the range of irradiance in the euphotic zone (Figure 2B). During days with high solar radiation, in situ irradiance recorded in the upper 4 m of the water column during early afternoon exceeded the maximum experimental irradiance of 600–700 μE m–2 s–1. Apart from station BY21, the maximum values at solar noon (highest position of the sun) was above 700 μE m–2 s–1 at BY15 and greater than 1200 μE m–2 s–1 at all other stations with available light measurements, according to our calculations of the diurnal variations of solar irradiance. Incubations were run for 16 h under light followed by 8 h in the dark adjusted to the in situ day-night cycle. An incubation time of 24 h was chosen to detect the release of freshly assimilated carbon into the pool of dissolved organic matter (PP-DOC). At the same time, long incubations allow bacterial carbon remineralization and, therefore, may underestimate primary production. After 24 h, incubations were stopped by filtering the sample through 0.4 μm-polycarbonate filters. Thereby, 14C incorporated into phytoplankton cells, referred to as net particulate primary production (PP-POC), was retained on the filters and 14C in organic compounds derived from dissolved primary production (PP-DOC) was collected in the filtrate. To remove leftover concentrations of Na14CO3 both filters and filtrate were acidified with 1 N HCl. Dried filters were placed in 200 μL of 2 N NaOH and 5 mL of scintillation cocktail. Furthermore, 4 mL of filtrate were added to 800 μl 2 N NaOH and 15 mL scintillation cocktail. Samples were shaken and radioassayed after 24 h to ensure homogenization.
Primary production of particulate and dissolved organic carbon (PP-POC, PP-DOC; μmol C L–1 d–1) was calculated from scintillation results according to Gargas (1975):
where a is the number of desintegrations per minute (DPM) measured after incubation and corrected for the dark incubations, DIC is the concentration of DIC in the sample, v is the incubation volume (L), A is the initial number of DPM of added Na14CO3 and t is the incubation time (d). The factor 1.05 is a correction factor for the discrimination between 12C and 14C.
Based on the measurements of particulate and dissolved primary production the percentage of extracellular release (PER) could be calculated by
Total net primary production (PP-TOC) was calculated by the summation of PP-POC and PP-DOC. Depth-integrated primary production rates of PP-TOC and PP-DOC were derived in consideration of the measured irradiance profiles, integrated over the euphotic zone (Baltic Proper: 12.6–19.0 m, Gulf of Riga: 7.7–9.2 m), see section “Calculations” as well as Supplementary Material.
At 19 stations, bacterial production was estimated from the incorporation of 3H-leucine (specific activity 100 Ci mmol–1) at a saturating final concentration of 20 nmol L–1. Rates were determined in 4–12 depths per station, including the surface (≤5 m), the depth of maximum chlorophyll a concentration, the oxycline, suboxic/anoxic waters below the oxycline and the bottom depth. Sampling depths were adjusted at individual stations after inspecting the vertical profiles of chlorophyll a fluorescence and oxygen. Samples were transferred from Niskin bottles of the rosette sampler into 5 mL-glass vials. Glass vials were filled to overflowing with gas-tight Teflon tubing and closed with a gas-tight septum to incubate samples close to ambient oxygen concentrations. Samples were spiked with a N2-aerated 3H-leucine solution through the septum with a Hamilton syringe and thoroughly shaken. After that, samples were kept close to in situ temperature in a cooling incubator for 1–3 h. Incubation was stopped by the addition of trichloroacetic acid at a final concentration of 5% and subsamples of 2 mL were processed by the centrifugation method according to Smith and Azam (1992). Samples were analyzed by liquid scintillation counting after the addition of scintillation cocktail (Ultima Gold AB, Perkin Elmer). A conversion factor of 1.5 kg C mol leucine–1 was used to convert leucine uptake into bacterial biomass production in carbon units (μg C L–1 d–1) (Simon and Azam, 1989). Cell-specific rates were calculated by dividing the volumetric biomass production by the abundance of bacteria.
Rates of chemolithoautotrophic CO2 fixation were estimated by the uptake of Na14CO3 in the dark in selected samples collected at the oxycline and in oxygen-deficient waters. For each sample, duplicate 50 mL-glass bottles with a gas-tight stopper were filled after an overflow of at least two times the bottle volume to avoid air bubbles. Bottles were spiked with 25 μL of a 6.25 μCi Na14CO3 solution. Immediately after mixing, 200 μL of spiked samples were transferred to 200 μL 2 N NaOH and 4 mL scintillation cocktail were added to determine the initial Na14CO3 concentration in each incubation bottle. Two additional replicates were immediately poisoned with 3% formalin and served as control. Samples and controls were incubated for 24 h close to in situ temperature in the dark. Incubation was stopped by filtration through 0.2 μm-polycarbonate filters, thereby collecting 14C incorporated into cells. Filters were acidified and dried before they were added to 200 μl 2 N NaOH and 5 mL scintillation cocktail for liquid scintillation counting. Dark CO2 fixation (μmol C L–1 d–1) was calculated according to
where a is the number of DPM measured in the sample after incubation and corrected for counts in the poisoned control, A is the initial number of DPM of added Na14CO3, DIC is the concentration of DIC in the sample and t is the incubation time (d).
Samples were fixed on board with glutardialdehyde at 2% final concentration and stored at −20°C until analysis by flow cytometry (FACSCalibur, Becton Dickinson) within 3 months after the cruise. Bacterial cells were counted after staining with the DNA-binding dye SybrGreen I (Invitrogen). Cell numbers were estimated after visual inspection and manual gating of the populations in the cytogram using the software CellQuest Pro (Becton Dickinson). Fluorescent latex beads (Polyscience, Becton Dickinson) were used to normalize the counted events to volume (Gasol and Del Giorgio, 2000).
In order to estimate the fraction of net primary production that was remineralized by heterotrophic bacterial activity in the euphotic zone (PPremin–eu) and in the surface mixed layer (PPremin–ML), net primary production (PPint) and the bacterial carbon demand (BCDint) were integrated over depth of the euphotic zone and the surface mixed layer, respectively. Density profiles reveal that the euphotic zone was completely mixed at all stations except BY2. At station BY2, the euphotic zone was about 2 m deeper than the surface mixed layer. For the estimation of local PPint we combine the results of the incubation experiments with the available light measurements at respective sites. Details of the calculations are described in the Supplementary Material. Briefly, in our approach we assign the total light levels (irradiance integrated over 24 h period) of the incubation experiments to those observed at depth. Light measurements with depth, their dates, times, and spatial coordinates are considered for the derivation of the diurnal light cycles (Supplementary Figure S1). Once the measured PP-TOC and PP-DOC rates are assigned to depths of identical levels of (daily) total irradiance (Supplementary Figure S2) we apply an exponential function and fit it to four subsets of these data, minimizing least-squares (Supplementary Figure S3). For every single fit we compute the vertical integral over the euphotic zone. The mean of these integrals provides a robust estimate of PPint for those stations where data from the incubation experiments are available.
BCDint was estimated from bacterial production assuming a bacterial growth efficiency of 20%. Like for other marine systems, the limited number of published values for bacterial growth efficiency suggest high variation in the Baltic Sea (Zweifel et al., 1993; Donali et al., 1999; Herlemann et al., 2014). Our assumption of 20% corresponds to and oceanic mean value that has been determined in a cross-system comparison of natural aquatic habitats (del Giorgio and Cole, 1998). The value also resembles the mean bacterial growth efficiency of 27% that was determined in untreated seawater cultures of the Baltic Sea (Zweifel et al., 1993). Furthermore, it corresponds to bacterial growth efficiencies at low chlorophyll a concentrations in a temperate Atlantic shelf sea (García-Martín et al., 2017).
PPremin [%] could then be estimated by
Stations in the southern Baltic Proper and in the eastern Gotland Basin were strongly affected by intense inflow of highly saline, oxygen-rich North Sea water during winter 2014/15, about 5 months before our cruise took place.
Oxygen profiles in the southern Baltic Sea (stations OMTF0212, BMPK2, PL-P3, and K1) showed oxygen concentrations of 58.5–72.8 μmol O2 L–1 at bottom depth, revealing recent ventilation by the major inflow event. Also two stations in the Bornholm Basin (stations OMTF0212, BMPK2) showed increasing oxygen concentrations below the pycnocline, originating from the lateral supply of North Sea water (Figure 3). Nitrate was available below the oxycline at stations in the southern Baltic Sea with concentrations up to 13.1 μmol L–1. At the same time, lowest ammonium concentrations of 0.39 μmol L–1 were measured (Supplementary Table S3).
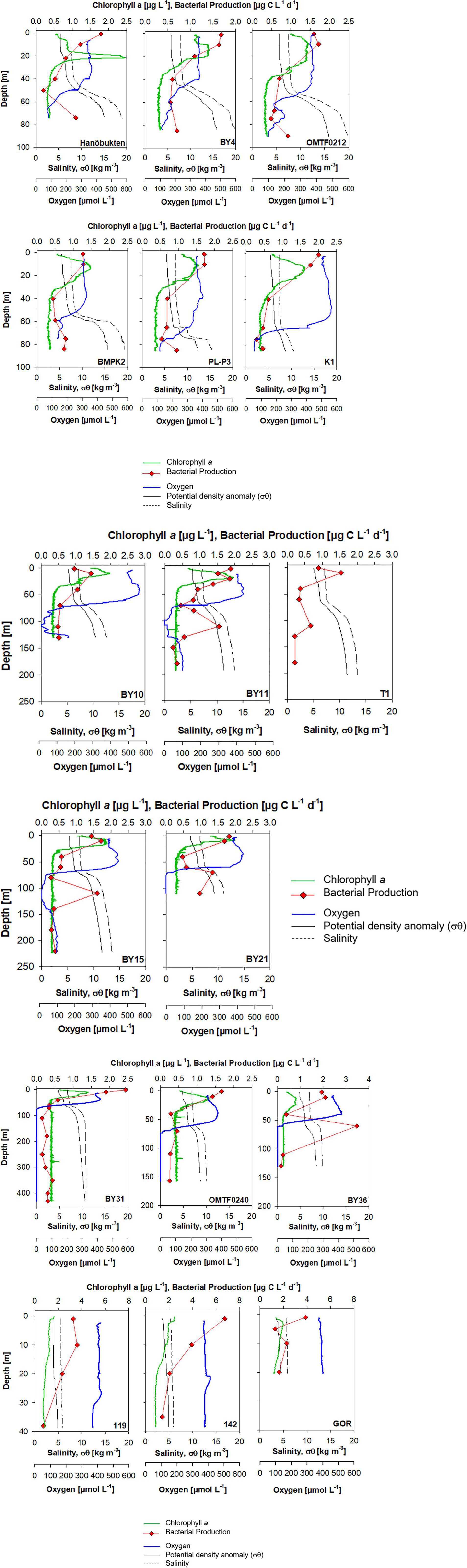
Figure 3. Individual depth profiles of chlorophyll a, heterotrophic bacterial production, oxygen, salinity, and density in the Baltic Sea during June 2015.
The deep stations in the Eastern Gotland Basin (stations BY10, T1, BY11, BY15) revealed an oxygen minimum zone below the pycnocline with oxygen-depleted cores between ca. 90 and 125 m (Figure 3). No hydrogen sulfide was detected in anoxic waters of stations BY11 and BY15, while the anoxic core of station BY10 contained 2.3 μmol H2S at 110 m depth. Below 130 m depth, stations showed an increase in oxygen up to ca. 76 μmol O2 L–1, supplied by North Sea water. The water column at the shallower station BY21, located at the northern edge of the eastern Gotland Basin, was completely anoxic below 70 m during our cruise and a hydrogen sulfide concentration of 6.45 μmol L–1 was analyzed in a discrete sample of 110 m depth (Figure 3 and Supplementary Figure S4). Oxygen-depleted cores of stations BY11 and BY15 in the eastern Gotland Basin showed nitrate concentrations of 0.12 to 0.26 μmol L–1 and nitrite accumulation of 0.09 to 0.11 μmol L–1. In contrast, nitrate was not detectable and the nitrite concentration of 0.03 μmol L–1 was substantially lower at station BY21, where anoxia expanded to bottom depth (Supplementary Table S1 and Supplementary Figure S4).
The depth profiles in the western Gotland Basin (stations BY31, OMTF0240, and BY36) showed an anoxic water column expanding from the oxycline to bottom depth. The water column at the Landsort Deep (BY31) was anoxic from 75 m to the bottom depth of 430 m (Figure 3). Hydrogen sulfide was detected at all stations in the western Gotland Basin with a maximum of 17.74 μmol L–1 at 110 m depth at station BY36. It is noteworthy, that the water column at the Landsort Deep (BY31) showed a sulfide-free zone with nitrate concentrations of 1.1–3.3 μmol L–1 at 300–350 m depth (Supplementary Table S1 and Supplementary Figure S4).
Shallow stations in the Gulf of Riga (119, 142, GOR) had a completely mixed water column and homogenous oxygen concentrations from surface to bottom (Figure 3).
At all stations in the Baltic Proper and in the Gulf of Riga, nitrate concentrations at the surface and at 10 m depth were ≤0.11 μmol L–1, revealing strong nitrogen-limitation of phytoplankton. Surface phosphate concentrations ranged from 0.20 to 2.39 μmol L–1 in the Baltic Proper, while lower concentrations of 0.01–0.17 μmol L–1 suggest phosphate deficiency in the Gulf of Riga. Surface concentrations of 5.53–36.16 μmol L–1 revealed silicate repletion in the Baltic Proper, while stations in the Gulf of Riga showed lower silicate concentrations of 0.69–1.68 μmol L–1 (Supplementary Table S1).
Surface chlorophyll a concentrations in the Baltic Proper were low during our cruise, ranging from 0.8 to 1.6 μg chlorophyll a L–1. Higher concentrations of 2.4–2.6 μg chlorophyll a L–1 were detected in the Gulf of Riga (stations 119, 142, GOR) (Table 1). Stations located in the Gotland Basin showed a high proportion of cyanobacteria, while large phytoplankton in the Gulf of Riga was dominated by cryptophytes and dinoflagellates (Supplementary Table S2). Small-sized phytoplankton communities in the Gulf of Riga included higher shares of phycoerythrin-containing cells, suggesting elevated abundances of Synechococcus sp., than stations in the Baltic Proper (Supplementary Table S3).
PP-TOC in the Baltic Proper and in the Gulf of Riga averaged 28.0 μg C L–1 d–1 and 86.6 μg C L–1 d–1, respectively (Table 1). Photosynthetically fixed carbon is partly not incorporated into phytoplankton biomass but released by the cells as dissolved organic matter. In our study, the PER could be derived from the proportion of PP-DOC (Eq. 2). PER ranged from 6.6% at station K1 to 20.3% at station BY11, corresponding to rates of PP-DOC of 2.55 to 6.11 μg C L–1 d–1 (Table 1). While PP-POC was directly related to chlorophyll a concentration (r2 = 0.76, p = 0.001, n = 11), no significant correlation between PP-DOC and chlorophyll a could be determined.
PP-POC was significantly lower in incubations at 5–7 μE m–2 s–1 compared to rates determined at 20–30 μE m–2 s–1, 50 μE m–2 s–1, 100 μE m–2 s–1, and 300–400 μE m–2 s–1, suggesting light limitation of primary production in the lowermost 0.5–7.5 m of the euphotic zone (Figure 4A). Likewise, PP-DOC was significantly reduced at the minimum experimental irradiance of 5–7 m–2 s–1 (ANOVA on ranks, Dunn’s post hoc test, p < 0.05) (Figure 4B).
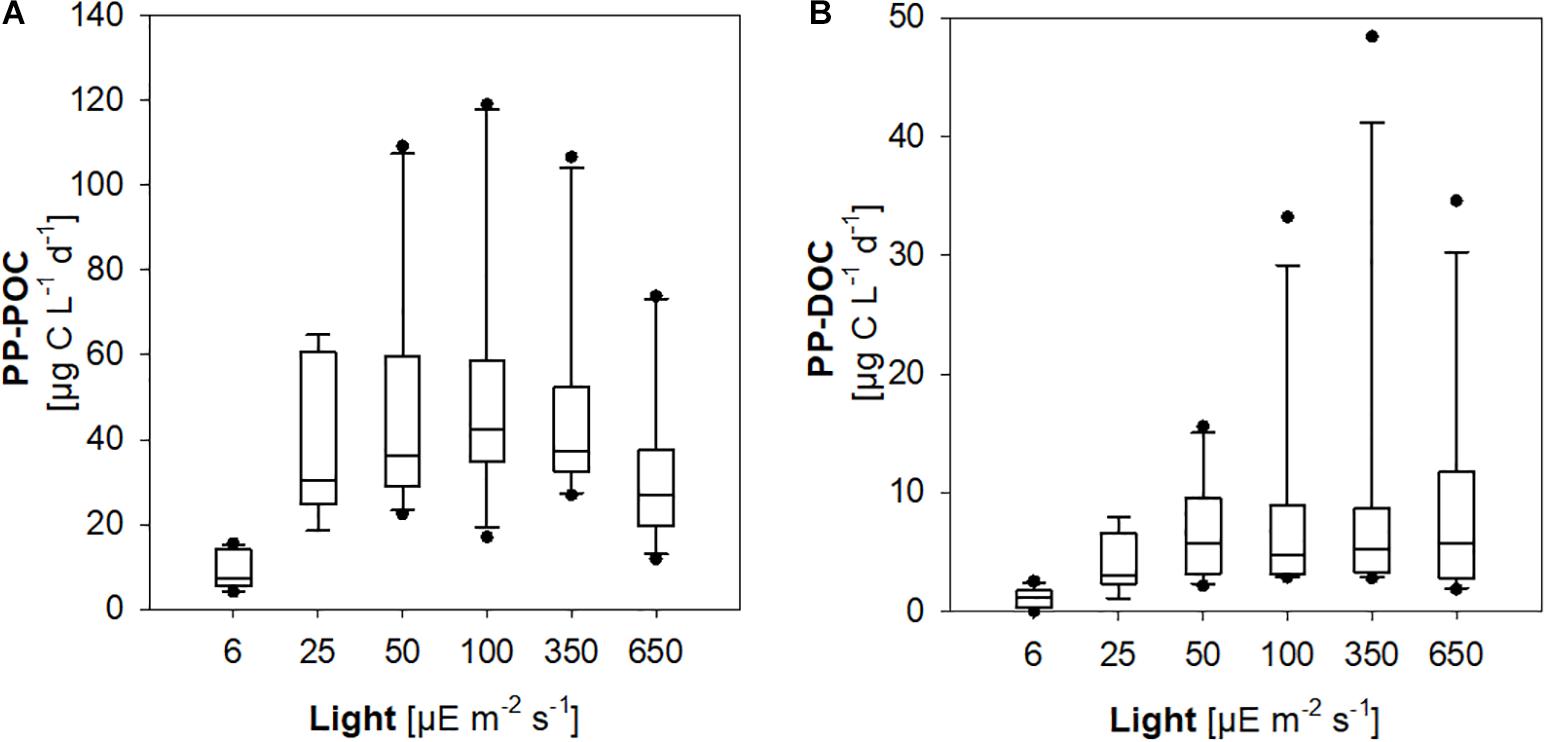
Figure 4. Primary production of phytoplankton communities at different experimental light levels. (A) Particulate primary production (PP-POC). (B) Dissolved primary production (PP-DOC).
Depth profiles of heterotrophic bacterial biomass production revealed high rates in the euphotic zone and a strong decrease with depth that was interrupted by pronounced secondary maxima at the oxic-anoxic interface and in anoxic waters at several stations (Figure 3).
In the Baltic Proper, a maximum bacterial production of 2.43 μg C L–1 d–1 was detected at the surface of station BY31, while stations BY10 and T1 showed minima of 0.90–0.96 μg C L–1 d–1 in surface waters (Figure 3). Higher surface bacterial production ranging from 3.26 to 6.74 μg C L–1 d–1 was determined in the Gulf of Riga (Figure 3). High bacterial production was determined in oxygen-depleted waters at and below the oxycline at stations BY11, T1, BY15, and BY21 in the eastern Gotland Basin and at station BY36 in the western Gotland Basin (Figure 3). At station BY36, bacterial production at the oxycline at 60 m depth reached 3.47 μg C L–1 d–1 and even exceeded rates in surface waters (Figure 3). Depth-integrated rates revealed that 41–68% of bacterial production in the Gotland Basin occurred in oxygen minimum zones (Table 2).
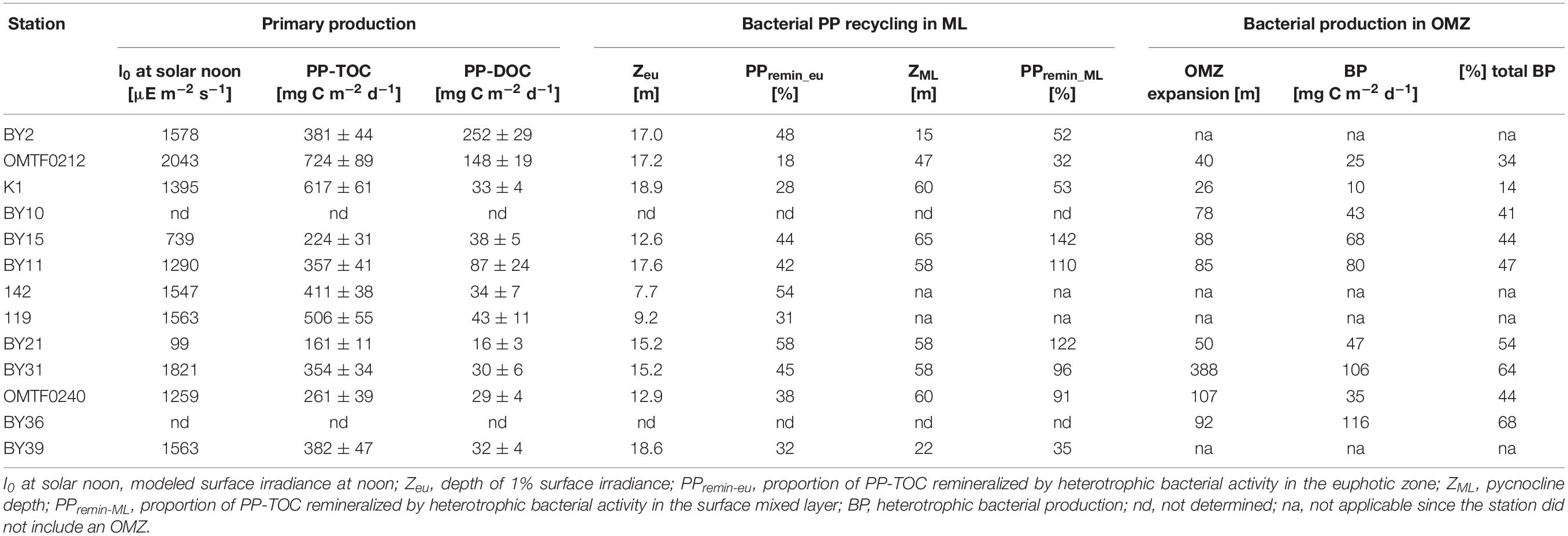
Table 2. Depth-integrated primary production (PP), bacterial carbon remineralization in the surface mixed layer (ML) and heterotrophic bacterial production in oxygen minimum zones (OMZ).
In order to compare heterotrophic bacterial biomass production in different water masses below the oxycline, four groups of samples can be distinguished (Figures 3, 5): Hanöbukten, BY4, OMTF0212, BMPK2, PL-P3, and K1 are shallow stations located in the southern Baltic Proper (shallow bottom water). At stations BY10, BY11 and BY15, located in the Eastern Gotland Basin, the bottom water was renewed by oxygen-rich North Sea (renewed bottom water) and the spreading North Sea water inflow lifted the oxygen-deficient former bottom water to 80–140 m depth (uplifted bottom water). Station BY21, the northernmost station in the Eastern Gotland Basin, and all stations in the Western Gotland Basin showed sulfidic zones (sulfidic waters). Cell-specific bacterial production was significantly lower in sulfidic waters than in uplifted and shallow bottom water (p < 0.05), while rates in renewed bottom water were not significantly different from the other sample groups (ANOVA on ranks, Dunn’s post hoc test, p < 0.05) (Figure 5). Likewise, renewed bottom water was not significantly different from the other sample groups and sulfidic waters showed significantly lower bacterial biomass production when volumetric rates were compared (ANOVA on ranks, Dunn’s post hoc test, p < 0.05).
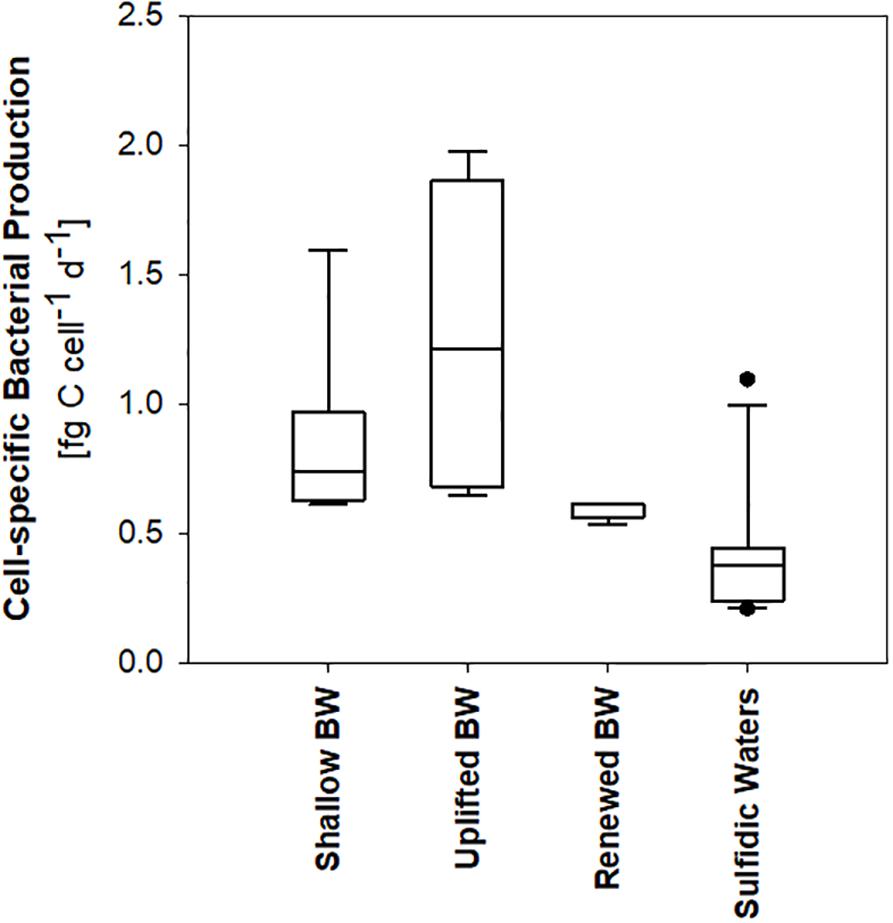
Figure 5. Heterotrophic bacterial production in different types of bottom water (BW) and in sulfidic waters.
Depth-integrated PP-TOC ranged from 161–724 mg C m–2 d–1 in the Baltic Proper. PP-TOC in the southern Baltic Sea was significantly higher than in the Gotland Basin (t-test, p = 0.02) (Table 2). PP-TOC in the Gulf of Riga, where 710 and 685 mg C m–2 d–1 were estimated at stations 119 and 142, respectively, was about 13% lower than in the southern Baltic Proper and about 70% higher than in the Gotland Basin. Depth-integrated PP-DOC was on average 74 mg C m–2 d–1, with exceptionally high values of 252 mg C m–2 d–1 and 148 mg C m–2 d–1 at stations BY2 and OMTF0212, respectively. In contrast to PP-TOC, PP-DOC in the southern Baltic Sea and in the Gotland Basin were not significantly different (t-test, p > 0.05) (Table 2).
To estimate the share of current primary production that was remineralized by heterotrophic bacterial activity in the euphotic zone (PPremin–eu) and in the surface mixed layer (PPremin–ML), respectively, the depth-integrated bacterial carbon demand was estimated and subtracted from depth-integrated PP-TOC (Eq. 4) (Table 2). PPremin–eu revealed that 18–58% of net primary production was remineralized by heterotrophic bacteria in the euphotic zone, suggesting that fresh primary production was supplied to the aphotic part of the water column. PPremin–ML of 91 and 96% showed that primary production and the bacterial carbon demand were roughly balanced in the surface mixed layer of the Western Gotland Basin (BY31, OMTF0240). In the Eastern Gotland Basin (BY15, BY11, BY21), the bacterial carbon demand in the surface mixed layer even exceeded ongoing primary production as revealed by PPremin–ML >100%. In contrast, PPremin–ML ranged from 35 to 53% in the southern Baltic Sea, indicating that reactive organic matter derived from ongoing primary production was supplied to heterotrophic communities below the pycnocline. In case of stations OMTF0212 and K1 in the southern Baltic Sea, the low bacterial remineralization of primary production in the surface mixed layer further suggests that labile organic matter was available for bacterial utilization in oxygen-deficient waters (Table 2 and Figure 3). Our estimates, however, do not consider biological processes mediated by non-bacterial heterotrophic plankton and physical processes that may alter the flux of organic matter to deeper waters. These unknown gains and losses may include lateral advection and zooplankton grazing and respiration. On the other hand, there is no direct approach to quantify downward fluxes of fresh organic matter that originates from current primary production.
Dark fixation of CO2 was determined in selected samples collected at an below the oxycline to assess the potential for the chemolithotrophic fixation of inorganic carbon. Low dark CO2 fixation of ≤2.04 μg C L–1 d–1 was determined in hypoxic samples with oxygen concentrations of 2.55–127 μmol L–1 (Figure 6A). Assuming 20% as heterotrophic bacterial growth efficiency, dark CO2 fixation in hypoxic samples equaled 9–54% of the estimated heterotrophic bacterial carbon demand in the same sample (Figure 6B). Substantially higher dark CO2 fixation of 3.89–25.46 μg C L–1 d–1 was determinded at oxygen concentrations ≤2.26 μmol L–1, with maxima determined in anoxic samples containing sulfide concentrations of 1.41–3.70 μmol L–1 (Figures 6A,B). Overall, dark CO2 fixation was positively correlated to ammonium concentrations (Figure 6C).
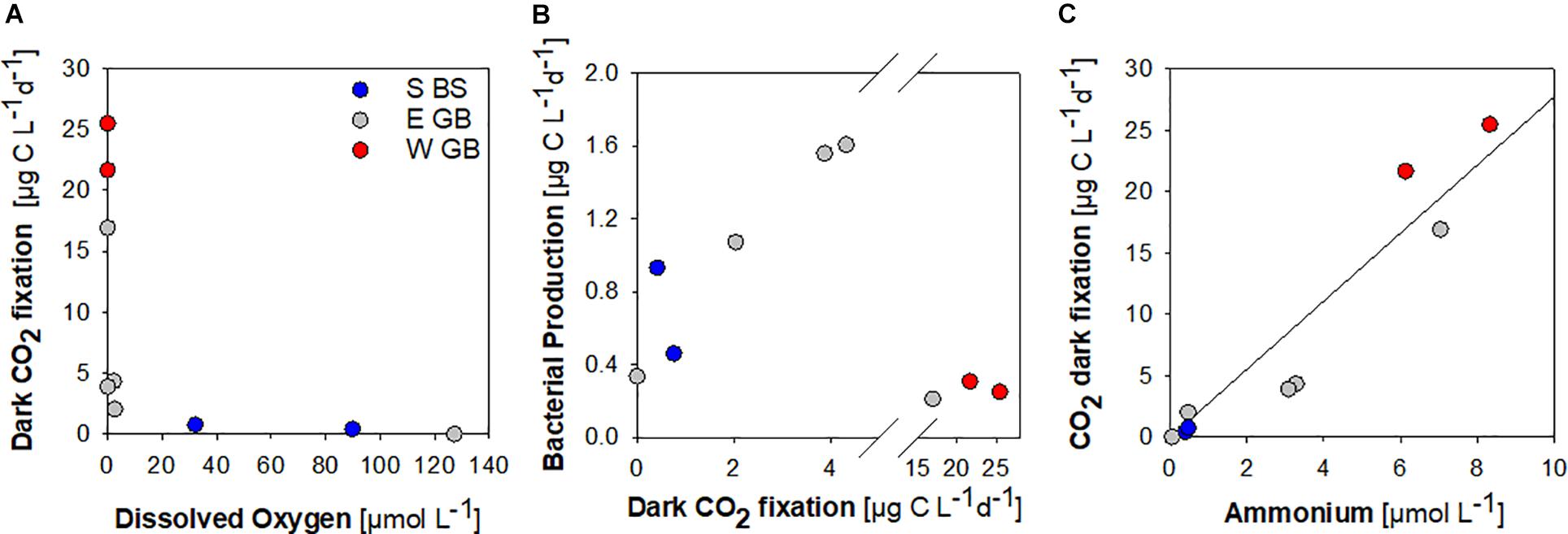
Figure 6. Dark CO2 fixation in the southern Baltic Sea (S BS), in the Eastern (E GB) and Western (W GB) Gotland Basin shown in relation to dissolved oxygen (A), bacterial production (B) and ammonium (r2 = 0.91, p < 0.0001) (C).
Oxygen deficiency in subsurface waters arises when the respiratory oxygen demand of organic matter remineralization exceeds the oxygen re-supply by air-sea exchange, physical ventilation and oxygenic photosynthesis (Rabalais et al., 2009). The Baltic Sea is naturally prone to hypoxia due to a restricted water exchange with the North Sea that leads to a long residence time above 30 years (Stigebrandt and Gustafsson, 2003; Döös et al., 2004). At the same time, the anthropogenic nutrient input increases primary production and export of organic matter to the poorly ventilated deep waters, thereby disrupting the balance between oxygen supply from physical processes and oxygen demand from heterotrophic organic matter remineralization (Carstensen et al., 2014). Little is known about the role of organic matter quality for the bacterial remineralization and the related utilization of oxygen and alternative terminal electron acceptors (Robinson, 2019). The major annual phytoplankton blooms in the Baltic Sea, occurring in spring and late summer, provide massive but transient input of fresh and reactive organic matter. Highest export fluxes have been determined during spring blooms, while sedimentation was shown to strongly decrease during early summer (Höglander et al., 2004; Gustafsson et al., 2013). This study examines the coupling between primary production and bacterial organic matter recycling under various oxygen regimes during early summer. Our results show that
(1) oxyclines in the southern Baltic Sea may receive supply of fresh organic matter during nutrient-limited phytoplankton production.
(2) heterotrophic bacterial activity is tolerant to oxygen-deficiency, and
(3) the removal of hydrogen sulfide by inflow events has a high potential to increase bacterial carbon remineralization.
The following discussion addresses these findings in more detail and evaluates their relevance for biogeochemical cycles in hypoxic and anoxic waters of the Baltic Sea.
The early summer season in the Baltic Proper is characterized by low concentrations of inorganic nutrients and chlorophyll a. While chlorophyll a concentrations up to 7 μg L–1 and 12 μg L–1 are reached during spring in the northern and southern Baltic Proper (Kahru et al., 1991; Wasmund et al., 1998), respectively, concentrations below 2 μg L–1 as determined in our study are representative for early summer (Kahru et al., 1991). Although similar to winter chlorophyll concentrations, low concentrations in early summer coincide with substantially higher levels of primary production that result in net production after nitrate exhaustion (Kaczmarek et al., 1997; Schneider et al., 2009). In our study, total primary production integrated over the euphotic zone showed considerable spatial variability. The range of primary production is in good agreement with previous studies conducted in the Baltic Sea during the same season (Kaczmarek et al., 1997; Renk and Ochocki, 1998). Similarly to previous studies, primary production in the Gulf of Riga was higher than in the Baltic Proper. Within the Baltic Proper, stations OMTF0212 and K1 in the Bornholm Basin and east of the basin, respectively, showed elevated rates. It has been suggested that upwelling of nutrients at the edges of the basins may locally sustain high primary production in the Baltic Sea (Kaczmarek et al., 1997).
Net primary production (PP-TOC) includes formation of biomass as well as dissolved photosynthesis products that are released by the phytoplankton cells. This dissolved primary production (PP-DOC) is a quantitatively important and easily accessible source of labile and semilabile organic matter for heterotrophic marine bacterioplankton. The PER depends on intrinsic capabilities of phytoplankton species (López-Sandoval et al., 2013) as well as on environmental factors like nutrient concentrations (Myklestad, 1977; López-Sandoval et al., 2011), temperature (Claquin et al., 2008) and light availability (Zlotnik and Dubinsky, 1989; Verity et al., 2000). Stations OMTF0212, BY11, and BY15 located in the Bornholm Basin and in the Eastern Gotland Basin, respectively, showed elevated PER of 17.0–20.3%. The reason, however, remains unclear. The phytoplankton composition at these stations was similar to stations with low PER. Since inorganic nitrogen exhaustion prevailed at all stations in the Baltic Proper, it seems unlikely that nutrient limitation was the primary factor controlling phytoplankton exudation. Our results further suggest that phytoplankton exudation during our study was largely unaffected by light.
Rates of heterotrophic bacterial biomass production determined during our field survey are in the range of previously published summer data for the northern and southern Baltic Proper (Tuomi, 1997; Feuerpfeil et al., 2004; Dahlgren et al., 2010).
Overall, our data show that heterotrophic bacterioplankton maintained high levels of activity in anoxic waters as long as nitrate was present and hydrogen sulfide did not accumulate. Peaks in bacterial production were determined in the sulfide-free anoxic zone in the Eastern Gotland Basin, while no elevated bacterial production under anoxia was observed in the sulfidic Western Gotland Basin. Moreover, the comparision of different water masses below the oxycline revealed that oxygenated North Sea water entrained into the Eastern Gotland Basin did not enhance heterotrophic bacterial activity and, consequently, bacterial organic matter remineralization at the time of our study. Our results suggest that bacterial communities may have adapted their carbon metabolism and growth to spatially and seasonally occurring oxygen fluctuations. Efficient respiration at nanomolar oxygen concentrations and the use of nitrate and/or other alternative electron acceptors could explain the high tolerance of the communities to different oxygen regimes. Previously published experimental results that do not show any inhibition of glucose and leucine uptake by natural bacterioplankton communities of the Baltic Sea under non-sulfidic anoxic conditions underpin the independence of metabolic activity from oxygen (Hoppe et al., 1990).
Similar bacterial activity in renewed and uplifted bottom water was probably also the result of diminishing differences in community composition. Four months before our study took place, the composition of bacterial communities in uplifted and renewed bottom water was analyzed. The successive dilution of inflowing North Sea water with Baltic Sea water during its passage to the Eastern Gotland Basin resulted in increasingly similar communities (Bergen et al., 2018). With regard to heterotrophic bacterial activity, our results suggest that these newly combined communities perform equally well under oxic, hypoxic and sulfide-free anoxic conditions.
Oxygen-depleted waters in the Baltic Sea often harbor bacterioplankton specialized for chemolithoautrophic metabolism (Grote et al., 2012). Chemolithoautotrophy in Baltic Sea communities can be supported by several electron donors and acceptors (Jost et al., 2010). Rates of CO2 dark fixation determined in our study are in the range of previous studies conducted in the Baltic Proper and other oxygen-deficient marine systems (Gocke, 1989; Detmer et al., 1993; Jost et al., 2010; Taylor et al., 2011). High dark CO2 fixation of 16.90–25.46 μg C L–1 d–1 was detected in samples containing hydrogen sulfide concentrations of 1.77–3.70 μmol L–1. It can be suggested that sulfide oxidation largely supported chemolithoautotrophic growth in these samples as concentrations were substantially lower than 20 μmol H2S L–1 that has been determined as threshold for toxic effects (Jost et al., 2010). In two out of three sulfidic samples (stations BY10 and BY31), nitrate was present and likely served as electron acceptor. At station OMTF0240, high dark CO2 fixation was determined in a sulfidic sample with nitrate and nitrite close to the detection limit. It has been suggested that also particulate manganese, which was detected up to 220 m depth in the Eastern Gotland Basin, may serve as electron acceptor in sulfidic waters of the Baltic Sea (Jost et al., 2010). Lower dark CO2 fixation up to 4.33 μg C L–1 d–1 was determined in sulfide-free samples collected at the oxycline in the southern Baltic Sea and in the uplifted bottom water in the Eastern Gotland Basin. Rates in hypoxic samples of the southern Baltic Sea were likely driven by nitrification. Several studies proved active nitrification at the oxic–anoxic interface in the water column of the Baltic Sea after mixing of ammonium-rich deep water with oxic water but also under oxygen concentrations below the detection limit (Enoksson, 1986; Hietanen et al., 2012). Whether anammox contributed to dark CO2 fixation in the uplifted bottom water in our study cannot be resolved with the available data. However, anammox has been detected after inflow events at re-establishing redoxclines (Hannig et al., 2007).
The coupling between nitrification and chemolithoautotrophic denitrification via the exchange of nitrate and ammonium at oxic-anoxic interfaces has been previously discussed as an important feedback loop in the Baltic Sea (Brettar and Rheinheimer, 1991, 1992). Furthermore, it has been shown that nitrifiers benefit from the heterotrophic bacterial remineralization of organic matter. Archaeal nitrifiers are largely dependent on the ammonia supplied by heterotrophic processes (Francis et al., 2005; Wuchter et al., 2006). Also anammox in the Gotland Basin is potentially sustained by ammonium derived from organic nitrogen remineralization (Le Moigne et al., 2017). Little is known, however, about the relevance of organic carbon produced by chemolithoautotrophic bacteria as substrate for heterotrophic microbial communities. Our results are in line with previous studies showing that hydrogen sulfide hampers growth of heterotrophic bacteria. Hence, it can be suggested that heterotrophic bacteria cannot benefit from organic matter derived from chemolithoautotrophic carbon fixation driven by sulfide oxidation. CO2 dark fixation of 2.04–4.33 μg C L–1 d–1 were determined in sulfide-free samples and likely driven by ammonium oxidation. In these samples, organic carbon produced by chemolithoautotrophic production equalled 9–54% of the heterotrophic bacterial carbon demand. Further studies on the chemical composition and the utilization of organic matter derived from chemolithoautotrophic production in food webs are required. If a significant share of chemolithoautotrophically produced organic carbon is transferred to heterotrophic bacterioplankton, the exchange of ammonium from protein degradation and organic carbon produced by nitrification may sustain another relevant feedback loop in oxygen-deficient waters of the Baltic Sea.
Time-series data and field surveys often reveal the spatial and temporal coupling between phytoplankton primary production and heterotrophic bacterial production in marine systems (Hoppe et al., 2002; Morán et al., 2002; von Scheibner et al., 2018). In the Baltic Sea, the succession of early phytoplankton blooms during February and March followed by distinct peaks in bacterial abundance in spring (March–April) shows a strong coupling of phytoplankton and heterotrophic bacterioplankton during productive periods (Samuelsson et al., 2006; Hoppe et al., 2008; von Scheibner et al., 2014). In contrast, our data on primary production and bacterial production reveal only a weak coupling in the surface mixed layer during the early summer season. While total primary production showed a north-south gradient in the Baltic Proper, heterotrophic bacterial production was quite uniform. Hence, heterotrophic bacterial production in the surface mixed layer could not scale up with high primary production in the southern Baltic Proper. A potential reason is that spatial differences in primary production were largely driven by particulate production, while stations in the southern Baltic Proper and in the Gotland Basin showed similar dissolved primary production. Like previously shown for other marine systems (Morán et al., 2002), dissolved organic matter released by phytoplankton exudation was potentially a regulating factor of heterotrophic bacterial production in our study. A weak coupling of primary production and heterotrophic bacterial production was further supported by the fact that on average 40% of total primary production escaped rapid bacterial remineralization in the euphotic zone. The seasonal succession of plankton in the Baltic Proper shows that abundances of bacterivorous nanoflagellates peak during summer (May–July), suggesting strong grazing pressure and an efficient top-down control of bacteria at the time of our study (Samuelsson et al., 2006; Zöllner et al., 2009).
Organic carbon derived from recent primary production is largely composed of labile compounds and, therefore, strongly affects growth rates of heterotrophic bacterial communities (Larsson and Hagström, 1979; Morán et al., 2002). Our estimates of PPremin–ML suggest that organic carbon derived from nutrient-limited primary production was supplied to heterotrophic communities in hypoxic waters of the southern Baltic Sea. Considering only the bacterial carbon demand, however, simplifies complex pelagic food web processes and likely leads to an overestimation of organic carbon available for export out of the surface mixed layer. In particular zooplankton consume organic particles, thereby reducing particle fluxes in the water column. Hence, our calculation provides a potential upper estimate of organic matter supply to bacterioplankton at and below the pycnocline. Studies that simultaneously investigated carbon remineralization by zooplankton and heterotrophic bacterial activity are scarce. In the North Atlantic Ocean, heterotrophic bacterial activity clearly dominated carbon remineralization in the presence of large, fast-sinking particles. A share of 70 to 92% of remineralization between 50 and 1000 m depth could be assigned to heterotrophic prokaryotes when compared with zooplankton respiration (Giering et al., 2014). Even if a strong impact of zooplankton grazing and a further reduction of primary production by 30% is assumed, our results suggest that fresh organic matter was exported to below the pycnocline in the southern Baltic Sea during summer.
In contrast to the southern Baltic Sea, PPremin–ML suggests that fresh organic matter was completely remineralized by heterotrophic bacteria in the surface mixed layer at stations in the Gotland Basin. In line with our results, particles collected by a sediment trap at station BY15 during our cruise included a high contribution of diatom-derived detritus that probably originated from the spring bloom, since diatoms showed low abundances during our field survey (Cisternas-Novoa et al., 2019). Hence, it seems likely that heterotrophic bacterial activity below the oxycline in the Gotland Basin was mainly sustained by semilabile organic matter derived from particle solubilization.
Declining dissolved oxygen levels combined with expanding sulfidic zones was noted as early as in the 1930s (Fonselius and Valderrama, 2003), before these zones began to spread in the 1950s (Karlson et al., 2002). Human-induced riverine nutrient input is the most important regional factor that increases primary production and, subsequently, leads to severe oxygen limitation. Societal effects in consequence of political changes led to a reduction of human nutrient inputs after 1989. However, the trend of declining oxygen concentrations continued despite decreasing nutrient and chlorophyll a concentrations (Lennartz et al., 2014). Our results show that even under nutrient-limited conditions freshly produced organic matter may be supplied to below the pycnocline in the southern Baltic Sea, where it will fuel bacterial oxygen consumption. Hence, also the response of heterotrophic bacterial activity to environmental changes needs to be considered in projections of the future Baltic Sea. It has been suggested that temperature-enhanced bacterial oxygen consumption combined with a prolonged stratification period will counteract the decline in eutrophication (Lennartz et al., 2014).
Our field survey in early summer 2015 took place after a major inflow of oxygen-rich North Sea water during the previous winter season that ventilated the deep basins of the Baltic Proper after more than 10 years of stagnation (Mohrholz et al., 2015). For larger complex animals the availability of oxygen is an essential prerequisite for survival. Hence, anoxia and strong oxygen deficiency eliminate higher trophic levels in food webs, thereby impacting the turnover of organic carbon. Our study suggests that oxygen availability is of minor importance for the bacterial turnover of organic carbon. Bonaglia et al. (2016) have shown that heterotrophic bacterial communities in the Baltic Proper have the metabolic potential to remineralize organic carbon in anoxic zones of the Baltic Sea by the use of nitrate. In line with this study, our results show that bacterial organic matter remineralization proceeds at high rate as long as nitrate is available and hydrogen sulfide did not accumulate. Hence, it is likely that the removal of hydrogen sulfide by inflow events has a stronger impact on bacterial carbon remineralization than the oxygenation of bottom waters.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
JP conducted most of the rate measurements, analyzed the data, and wrote the manuscript. SE and FL collected the samples and conducted the measurements of oxygen, ammonium, and hydrogen sulfide. MS modeled the depth-integrated net primary production. AE designed the cruise program and collected the samples. All authors provided input for the data analysis and contributed to the writing of the manuscript.
This work was supported by the institutional funds of the GEOMAR Helmholtz Centre for Ocean Research Kiel and by the Wirtschaftsförderung und Technologietransfer Schleswig-Holstein GmbH (Project “CoastSens”).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Tania Klüver, Carolina Cisternas Novoa, Marie Maßmig, Jon Roa, and Manuel Olivares Requena for chemical and biological analyses on board and in the laboratory. Tobias Steinhoff is gratefully acknowledged for the analysis of dissolved inorganic carbon concentrations. We also thank the captain and crew of RV Alkor for their support during our cruise. Comments of the two reviewers improved the manuscript substantially.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00581/full#supplementary-material
Bergen, B., Naumann, M., Herlemann, D. P. R., Gräwe, U., Labrenz, M., and Jürgens, K. (2018). Impact of a major inflow event on the composition and distribution of bacterioplankton communities in the baltic sea. Front. Mar. Sci. 5:383. doi: 10.3389/fmars.2018.00383
Bonaglia, S., Klawonn, I., De Brabandere, L., Deutsch, B., Thamdrup, B., and Brüchert, V. (2016). Denitrification and DNRA at the Baltic sea oxic–anoxic interface: substrate spectrum and kinetics. Limnol. Oceanogr. 61, 1900–1915. doi: 10.1002/lno.10343
Brettar, I., and Rheinheimer, G. (1991). Denitrification in the central baltic: evidence for H2S-oxidation as motor of denitrification at the oxic-anoxic interface. Mar. Ecol. Prog. Ser. 77, 157–169. doi: 10.3354/meps077157
Brettar, I., and Rheinheimer, G. (1992). Influence of carbon availability on denitrification in the central Baltic Sea. Limnol. Oceanogr. 37, 1146–1163. doi: 10.4319/lo.1992.37.6.1146
Carstensen, J., Conley, D. J., Bonsdorff, E., Gustafsson, B. G., Hietanen, S., Janas, U., et al. (2014). Hypoxia in the baltic sea: biogeochemical cycles, benthic fauna, and management. Ambio 43, 26–36. doi: 10.1007/s13280-013-0474-7
Cisternas-Novoa, C., Le Moigne, F. A. C., and Engel, A. (2019). Composition and vertical flux of particulate organic matter to the oxygen minimum zone of the central Baltic Sea: impact of a sporadic North Sea inflow. Biogeosciences 16, 927–947. doi: 10.5194/bg-16-927-2019
Claquin, P., Probert, I., Lefebvre, S., and Veron, B. (2008). Effects of temperature on photosynthetic parameters and TEP production in eight species of marine microalgae. Aquat. Microb. Ecol. 51, 1–11. doi: 10.3354/ame01187
Dahlgren, K., Andersson, A., Larsson, U., Hajdu, S., and Båmstedt, U. (2010). Planktonic production and carbon transfer efficiency along a north-south gradient in the Baltic Sea. Mar. Ecol. Prog. Ser. 409, 77–94. doi: 10.3354/meps08615
Dalsgaard, T., De Brabandere, L., and Hall, P. O. J. (2013). Denitrification in the water column of the central Baltic Sea. Geochim. Cosmochim. Acta 106, 247–260. doi: 10.1016/j.gca.2012.12.038
del Giorgio, P. A., and Cole, J. J. (1998). Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29, 503–541. doi: 10.1146/annurev.ecolsys.29.1.503
Detmer, A. E., Giesenhagen, H. C., Trenkel, V. M., Auf Dem Venne, H., and Jochem, F. J. (1993). Phototrophic and heterotrophic pico- and nanoplankton in anoxic depths of the central Baltic Sea. Mar. Ecol. Prog. Ser. 99, 197–203. doi: 10.3354/meps099197
Diaz, R. J., and Rosenberg, R. (2008). Spreading dead zones and consequences for marine ecosystems. Science 321, 926–930. doi: 10.1126/science.1156401
Dickson, A., Sabine, C., and Christian, J. (eds) (2007). Guide to Best Practices for Ocean CO2 Measurements. Sidney: North Pacific Marine Science Organization.
Donali, E., Olli, K., Heiskanen, A., and Andersen, T. (1999). Carbon flow patterns in the planktonic food web of the Gulf of Riga, the Baltic Sea: a reconstruction by the inverse method. J. Mar. Syst. 23, 251–268. doi: 10.1016/s0924-7963(99)00061-5
Döös, K., Meier, H. E. M., and Döscher, R. (2004). The baltic haline conveyor belt or the overturning circulation and mixing in the Baltic. AMBIO Am. J. Hum. Environ. 33, 261–266. doi: 10.1579/0044-7447-33.4.261
Edler, L. (1979). Recommendations on methods for marine biological studies in the Baltic Sea. phytoplankton and chlorophyll. Publ. Balt. Mar. Biol. BMB 5, 1–38.
Elmgren, R. (2001). Understanding human impact on the Baltic ecosystem: changing views in recent decades. Ambio 30, 222–231. doi: 10.1579/0044-7447-30.4.222
Enoksson, V. (1986). Nitrification rates in the Baltic Sea: comparison of three isotope techniques. Appl. Environ. Microbiol. 51, 244–250.
Evans, C. A., O’Reilly, J. E., and Thomas, J. P. (1987). A Handbook for the Measurement of Chlorophyll-a and Primary Productivity. College Station, TX: Texas A&M University.
Feuerpfeil, P., Rieling, T., Estrum-Youseff, S. R., Dehmlow, J., Papenfuß, T., Schoor, A., et al. (2004). Carbon budget and pelagic community compositions at two coastal areas that differ in their degree of eutrophication, in the Southern Baltic Sea. Estuar. Coast. Shelf Sci. 61, 89–100. doi: 10.1016/j.ecss.2004.04.006
Fonselius, S., Dyrssen, D., and Yhlen, B. (1999). “Determination of hyrogen sulfide,” in Methods of Seawater Analysis, eds K. Grasshoff, K. Kremling, and M. Ehrhardt, (Weinheim: Wiley-VCH).
Fonselius, S., and Valderrama, J. (2003). One hundred years of hydrographic measurements in the Baltic Sea. J. Sea Res. 49, 229–241. doi: 10.1016/S1385-1101(03)00035-2
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E., and Oakley, B. B. (2005). Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. U.S.A. 102, 14683–14688. doi: 10.1073/pnas.0506625102
García-Martín, E. E., Daniels, C. J., Davidson, K., Lozano, J., Mayers, K. M. J., McNeill, S., et al. (2017). Plankton community respiration and bacterial metabolism in a North Atlantic shelf Sea during spring bloom development. Prog. Oceanogr. 2017:101873. doi: 10.1016/J.POCEAN.2017.11.002
Gargas, E. (1975). A manual for phytoplankton primary production studies in the baltic. Baltic Mar. Biol. 2:88.
Gasol, J. M., and Del Giorgio, P. A. (2000). Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci. Mar. 64, 197–224. doi: 10.3989/scimar.2000.64n2197
Giering, S. L. C., Sanders, R., Lampitt, R. S., Anderson, T. R., Tamburini, C., Boutrif, M., et al. (2014). Reconciliation of the carbon budget in the ocean’s twilight zone. Nature 507, 480–483. doi: 10.1038/nature13123
Gocke, K. (1989). Forschungen der abteilung marine mikrobiologie des instituts für meereskunde an der universität kiel. Berichte Aus Dem Inst. Für Meereskd. An Der Cau Kiel 188, 40–47.
Gräwe, U., Naumann, M., Mohrholz, V., and Burchard, H. (2015). Anatomizing one of the largest saltwater inflows into the Baltic Sea in december 2014. J. Geophys. Res. Ocean Res. 120, 7676–7697. doi: 10.1002/2015JC011269
Grote, J., Schott, T., Bruckner, C. G., Glockner, F. O., Jost, G., Teeling, H., et al. (2012). Genome and physiology of a model epsilonproteobacterium responsible for sulfide detoxification in marine oxygen depletion zones. Proc. Natl. Acad. Sci. U.S.A. 109, 506–510. doi: 10.1073/pnas.1111262109
Gustafsson, O., Gelting, J., Andersson, P., Larsson, U., and Roos, P. (2013). An assessment of upper ocean carbon and nitrogen export fluxes on the boreal continental shelf: a 3-year study in the open Baltic Sea comparing sediment traps, 234Th proxy, nutrient, and oxygen budgets. Limnol. Oceanogr. Methods 11, 495–510. doi: 10.4319/lom.2013.11.495
Hannig, M., Lavik, G., Kuypers, M. M. M., Martens-Habbena, W., and Jürgens, K. (2007). Shift from denitrification to anammox after inflow events in the central Baltic Sea. Limnol. Oceanogr. 52, 1336–1345. doi: 10.4319/lo.2007.52.4.1336
Hansen, H. P., and Koroleff, F. (1999). “Determination of nutrients,” in Methods of Seawater Analysis, eds K. Grasshoff, K. Kremling, and M. Ehrhardt, (Weinheim: Wiley-VCH), 159–228. doi: 10.1002/9783527613984.ch10
Heiskanen, A. S., and Leppänen, J. M. (1995). Estimation of export production in the coastal Baltic Sea: effect of resuspension and microbial decomposition on sedimentation measurements. Hydrobiologia 316, 211–224. doi: 10.1007/BF00017438
Herlemann, D. P. R., Manecki, M., Meeske, C., Pollehne, F., and Labrenz, M. (2014). Uncoupling of bacterial and terrigenous dissolved organic matter dynamics in decomposition experiments. PLoS One 9:e93945. doi: 10.1371/journal.pone.0093945
Hietanen, S., Jäntti, H., Buizert, C., Jürgens, K., Labrenz, M., Voss, M., et al. (2012). Hypoxia and nitrogen processing in the Baltic Sea water column. Limnol. Oceanogr. 57, 325–337. doi: 10.4319/lo.2012.57.1.0325
Höglander, H., Larsson, U., and Hajdu, S. (2004). Vertical distribution and settling of spring phytoplankton in the offshore NW baltic sea proper. Mar. Ecol. Prog. Ser. 283, 15–27. doi: 10.3354/meps283015
Hoppe, H. G., Breithaupt, P., Walther, K., Koppe, R., Bleck, S., Sommer, U., et al. (2008). Climate warming in winter affects the coupling between phytoplankton and bacteria during the spring bloom: a mesocosm study. Aquat. Microb. Ecol. 51, 105–115. doi: 10.3354/ame01198
Hoppe, H. G., Gocke, K., Koppe, R., and Begler, C. (2002). Bacterial growth and primary production along a north-south transect of the Atlantic Ocean. Nature 416, 168–171. doi: 10.1038/416168a
Hoppe, H. G., Gocke, K., and Kuparinen, J. (1990). Effect of H2S on heterotrophic substrate uptake, extracellular enzyme activity and growth of brackish water bacteria. Mar. Ecol. Prog. Ser. 64, 157–167. doi: 10.3354/meps064157
Johnson, K. M., Wills, K. D., Butler, D. B., Johnson, W. K., and Wong, C. S. (1993). Coulometric total carbon dioxide analysis for marine studies: maximizing the performance of an automated gas extraction system and coulometric detector. Mar. Chem. 44, 167–187. doi: 10.1016/0304-4203(93)90201-X
Jost, G., Martens-Habbena, W., Pollehne, F., Schnetger, B., and Labrenz, M. (2010). Anaerobic sulfur oxidation in the absence of nitrate dominates microbial chemoautotrophy beneath the pelagic chemocline of the eastern gotland basin. Baltic Sea. FEMS Microbiol. Ecol. 71, 226–236. doi: 10.1111/j.1574-6941.2009.00798.x
Kaczmarek, S., Koblentz-Mishke, O. J., Ochocki, S., Nakonieczny, J., and Renk, H. (1997). Primary production in the eastern and southern Baltic Sea. Oceanologia 39, 117–135.
Kahru, M., Kaasik, E., and Leeben, A. (1991). annual cycle of particle-size fractions and phytoplankton biomass in the Northern Baltic proper. Mar. Ecol. Ser. 69, 117–124. doi: 10.3354/meps069117
Karlson, K., Rosenberg, R., and Bonsdorff, E. (2002). “Temporal and spatial large-scale effects of eutrophication and oxygen deficiency on benthic fauna in scandinavian and Baltic waters - a review,” in Oceanography and Marine Biology, eds R. J. Gibson, R. N. Barnes, and M. Atkinson, (Sweden: University of Gothenburg), 427–489. doi: 10.1201/9780203180594.ch8
Larsson, U., and Hagström, A. (1979). Phytoplankton exudate release as an energy source for the growth of pelagic bacteria. Mar. Biol. 52, 199–206. doi: 10.1007/bf00398133
Le Moigne, F. A. C., Cisternas-Novoa, C., Piontek, J., Maßmig, M., and Engel, A. (2017). On the effect of low oxygen concentrations on bacterial degradation of sinking particles. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-16903-3
Lennartz, S. T., Lehmann, A., Herrford, J., Malien, F., Hansen, H. P., Biester, H., et al. (2014). Long-term trends at the Boknis Eck time series station (Baltic Sea), 1957-2013: does climate change counteract the decline in eutrophication? Biogeosciences 11, 6323–6339. doi: 10.5194/bg-11-6323-2014
Liu, Z., Liu, S., Liu, J., and Gardner, W. S. (2013). Differences in peptide decomposition rates and pathways between hypoxic and oxic coastal environments. Mar. Chem. 157, 67–77. doi: 10.1016/j.marchem.2013.08.003
López-Sandoval, D. C., Fernández, A., and Marañón, E. (2011). Dissolved and particulate primary production along a longitudinal gradient in the Mediterranean Sea. Biogeosciences 8, 815–825. doi: 10.5194/bg-8-815-2011
López-Sandoval, D. C., Rodríguez-Ramos, T., Cermeño, P., and Marañón, E. (2013). Exudation of organic carbon by marine phytoplankton: dependence on taxon and cell size. Mar. Ecol. Prog. Ser. 477, 53–60. doi: 10.3354/meps10174
Maßmig, M., Piontek, J., Le Moigne, F. A. C., Cisternas-Novoa, C., and Engel, A. (2019). Potential role of oxygen and inorganic nutrients on microbial carbon turnover in the Baltic Sea. Aquat. Microb. Ecol. 83, 95–108. doi: 10.3354/ame01902
Mohrholz, V., Naumann, M., Nausch, G., Krüger, S., and Gräwe, U. (2015). Fresh oxygen for the Baltic Sea - an exceptional saline inflow after a decade of stagnation. J. Mar. Syst. 148, 152–166. doi: 10.1016/j.jmarsys.2015.03.005
Morán, X. A. G., Estrada, M., Gasol, J. M., and Pedrós-Alió, C. (2002). Dissolved primary production and the strength of phytoplankton-bacterioplankton coupling in contrasting marine regions. Microb. Ecol. 44, 217–223. doi: 10.1007/s00248-002-1026-z
Myklestad, S. (1977). Production of carbohydrates by marine planktonic diatoms II. Influence of the N/P ration in the growth medium on the assimilation ratio, growth rate, and production of cellular and extracellular carbohydrates by chaetoceros affinis var. Willei Hust. 29, 161–179. doi: 10.1016/0022-0981(77)90046-6
Pantoja, S., Rossel, P., Castro, R., Cuevas, L. A., Daneri, G., and Córdova, C. (2009). Microbial degradation rates of small peptides and amino acids in the oxygen minimum zone of chilean coastal waters. Deep Res. Part II Top. Stud. Oceanogr. 56, 1019–1026. doi: 10.1016/j.dsr2.2008.09.007
Rabalais, N. N., Díaz, R. J., Levin, L. A., Turner, R. E., Gilbert, D., and Zhang, J. (2009). Dynamics and distribution of natural and human-caused coastal hypoxia. Biogeosci. Discuss. 6, 9359–9453. doi: 10.5194/bgd-6-9359-2009
Renk, H., and Ochocki, S. (1998). Photosynthetic rate and light curves of phytoplankton in the southern Baltic. Oceanologia 40, 331–344.
Reusch, T. B. H., Dierking, J., Andersson, H. C., Bonsdorff, E., Carstensen, J., Casini, M., et al. (2018). The Baltic Sea as a time machine for the future coastal ocean. Sci. Adv. 4:eaar8195. doi: 10.1126/sciadv.aar8195
Robinson, C. (2019). Microbial respiration, the engine of ocean deoxygenation. Front. Mar. Sci. 5:533. doi: 10.3389/fmars.2018.00533
Samuelsson, K., Berglund, J., and Andersson, A. (2006). Factors structuring the heterotrophic flagellate and ciliate community along a brackish water primary production gradient. J. Plankton Res. 28, 345–349. doi: 10.1093/plankt/fbi118
Schneider, B., Kaitala, S., Raateoja, M., and Sadkowiak, B. (2009). A nitrogen fixation estimate for the Baltic Sea based on continuous pCO2 measurements on a cargo ship and total nitrogen data. Cont. Shelf Res. 29, 1535–1540. doi: 10.1016/j.csr.2009.04.001
Simon, M., and Azam, F. (1989). Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 51, 201–213. doi: 10.3354/meps051201
Smith, D. C., and Azam, F. (1992). A simple, economical method for measuring bacterial protein synthesis rates in seawater using tritiated-leucine. Mar. Microb. Food Webs 6, 107–114.
Stigebrandt, A., and Gustafsson, B. G. (2003). Response of the Baltic Sea to climate change - Theory and observations. J. Sea Res. 49, 243–256. doi: 10.1016/S1385-1101(03)00021-2
Stigebrandt, A., and Wulff, F. (1987). A model for the dynamics of nutrients and oxygen in the Baltic proper. J. Mar. Res. 45, 729–759. doi: 10.1357/002224087788326812
Taylor, G. T., Iabichella, M., Ho, T., Scranton, M. I., Robert, C., Muller-karger, F., et al. (2011). Chemoautotrophy in the redox transition zone of the cariaco basin: asignificant midwater source of organic carbon production. Limnol. Oceanogr. 46, 148–163. doi: 10.4319/lo.2001.46.1.0148
Tuomi, P. (1997). Bacterial carbon production in the northern Baltic: a comparison of thymidine incorporation and FDC based methods. Mar. Ecol. Prog. Ser. 153, 59–66. doi: 10.3354/meps153059
Verity, P. G., Williams, S. C., and Hong, Y. (2000). Formation, degradation, and mass:volume ratios of detritus derived from decaying phytoplankton. Mar. Ecol. Prog. Ser. 207, 53–68. doi: 10.3354/meps207053
von Scheibner, M., Dörge, P., Biermann, A., Hoppe, H. G., Sommer, U., and Jürgens, K. (2014). Impact of warming on phyto-bacterioplankton coupling and bacterial community composition in experimental mesocosms. Environ. Microbiol. 16, 718–733. doi: 10.1111/1462-2920.12195
von Scheibner, M., Herlemann, D. P. R., Lewandowska, A. M., and Jürgens, K. (2018). Phyto- and bacterioplankton during early spring conditions in the baltic sea and response to short-term experimental warming. Front. Mar. Sci. 5:231. doi: 10.3389/fmars.2018.00231
Wasmund, N., Göbel, J., and Bodungen, B. V. (2008). 100-years-changes in the phytoplankton community of Kiel Bight (Baltic Sea). J. Mar. Syst. 73, 300–322. doi: 10.1016/j.jmarsys.2006.09.009
Wasmund, N., Nausch, G., and Feistel, R. (2013). Silicate consumption: an indicator for long-term trends in spring diatom development in the Baltic Sea. J. Plankton Res. 35, 393–406. doi: 10.1093/plankt/fbs101
Wasmund, N., Nausch, G., and Matthaus, W. (1998). Phytoplankton spring blooms in the southern Baltic Sea-spatio-temporal development and long-term trends. J. Plankton Res. 20, 1099–1117. doi: 10.1093/plankt/20.6.1099
Wuchter, C., Abbas, B., Coolen, M. J. L., Herfort, L., van Bleijswijk, J., Timmers, P., et al. (2006). Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. U.S.A. 103, 12317–12322. doi: 10.1073/pnas.0600756103
Zlotnik, I., and Dubinsky, Z. (1989). The effect of light and temperature on dissolved organic carbon excretion by phytoplankton. Limnol. Ocean 34, 831–839. doi: 10.4319/lo.1989.34.5.0831
Zöllner, E., Hoppe, H.-G., Sommer, U., and Jürgens, K. (2009). Effect of zooplankton-mediated trophic cascades on marine microbial food web components (bacteria, nanoflagellates, ciliates). Limnol. Oceanogr. 54, 262–275. doi: 10.4319/lo.2009.54.1.0262
Zweifel, U. L., Norrman, B., and Hagstrom, A. (1993). Consumption of dissolved organic matter by marine bacteria and demand for inorganic nutrients. 1: 23-32 consumption of dissolved organic carbon by marine bacteria and demand for inorganic nutrients. Mar. Ecol. Prog. Ser. 101, 23–32. doi: 10.3354/meps101023
Keywords: primary production, bacterial biomass production, dark CO2 fixation, organic matter, oxygen deficiency
Citation: Piontek J, Endres S, Le Moigne FAC, Schartau M and Engel A (2019) Relevance of Nutrient-Limited Phytoplankton Production and Its Bacterial Remineralization for Carbon and Oxygen Fluxes in the Baltic Sea. Front. Mar. Sci. 6:581. doi: 10.3389/fmars.2019.00581
Received: 08 May 2019; Accepted: 30 August 2019;
Published: 02 October 2019.
Edited by:
Tony Gutierrez, Heriot-Watt University, United KingdomReviewed by:
Alex J. Poulton, The Lyell Centre, United KingdomCopyright © 2019 Piontek, Endres, Le Moigne, Schartau and Engel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anja Engel, YWVuZ2VsQGdlb21hci5kZQ==
†Present address: Judith Piontek, Leibniz Institute for Baltic Sea Research Warnemünde, Rostock, Germany; Frédéric A. C. Le Moigne, Mediterranean Institute of Oceanography, UM110, Aix Marseille Univ., CNRS, IRD, Marseille, France
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.