- 1Blue Economy, The Scottish Association for Marine Science, Oban, United Kingdom
- 2Department of Animal and Aquacultural Sciences, Faculty of Biosciences, Norwegian University of Life Sciences, Ås, Norway
- 3Seaweed Energy Solutions AS, Trondheim, Norway
Cultivation of kelp has been well established throughout Asia, and there is now growing interest in the cultivation of macroalgae in Europe to meet future resource needs. If this industry is to become established throughout Europe, then balancing the associated environmental risks with potential benefits will be necessary to ensure the carrying capacity of the receiving environments are not exceeded and conservation objects are not undermined. This is a systematic review of the ecosystem changes likely to be associated with a developing seaweed aquaculture industry. Monitoring recommendations are made by risk ranking environmental changes, highlighting the current knowledge gaps and providing research priorities to address them. Environmental changes of greatest concern were identified to include: facilitation of disease, alteration of population genetics and wider alterations to the local physiochemical environment. Current high levels of uncertainty surrounding the true extent of some environmental changes mean conservative risk rankings are given. Recommended monitoring options are discussed that aim to address uncertainty and facilitate informed decision-making. Whilst current small-scale cultivation projects are considered ‘low risk,’ an expansion of the industry that includes ‘large-scale’ cultivation will necessitate a more complete understanding of the scale dependent changes in order to balance environmental risks with the benefits that seaweed cultivation projects can offer.
Seaweed Production Across Europe
Throughout the world, high demands on many natural resources necessitates the development of alternate resources to produce important commodities such as food, feed, fuel, cosmetics, and pharmaceuticals. The development of large-scale seaweed aquaculture in Europe has the potential to play an important role in meeting future resource needs, but must do so in a manner that does not undermine the use and value of existing marine resources.
Large-scale cultivation of seaweeds has been practiced in Asia for decades (Cheng, 1969), but has only recently been a commercial activity in Europe (FAO, 2014; Bostock et al., 2016). High demand has driven a rapid expansion in Asia in this form of aquaculture. Global production has increased at a rate of 7.6% year-1 between 2004 and 2015 when an estimated 28.1 million tons were produced (FAO, 2015). China is the biggest producer of brown algae, mainly cultivated kelp species. Although a large proportion of the Chinese crop is sold dried for the food market (McHugh, 2003), extracts derived from cultivated macroalgal species are now in a growing number of global consumer products (Smit, 2004; Bixler and Porse, 2011) such as; cosmetics, pharmaceuticals and foods. The top species produced are the brown algae Saccharina japonica (Japanese Kelp) and the red algae Eucheuma sp. Together these species account for 66% of the global production (FAO, 2015).
Asian cultivation of brown seaweeds are characterized by a range of long-line techniques with vertical droppers; similar growing techniques have been trialed successfully in Europe (Peteiro and Freire, 2009, 2011, 2012, 2013b; Sanderson et al., 2012; Handa et al., 2013; Marinho et al., 2015; Peteiro et al., 2016). Growing systems for kelp species in China and the rest of Asia are very effective. However, due to the labor intensive nature of these systems and the low costs of such a large work force in China, technological modifications to reduce labor costs associated with cultivation will need to be developed in emerging seaweed producing countries in Europe (Edwards and Watson, 2011). Required technological modifications include mechanization of seeding and harvesting, year-round production based on a number of co-cultured species and scales that create running costs economies. With the global drive to find sustainable sources of food, feeds, fuels and other products, attention has turned to developing and adapting large-scale suspended cultivation methods used in Asia for European waters (Bruton et al., 2009; Kraan, 2010; Borines et al., 2011; Hughes et al., 2012b).
The environmental conditions required for growing different species of seaweed are variable (Kerrison et al., 2015). In general, seaweed production requires areas with sufficient nutrients and light for growth and salinity and temperatures that are not limiting to the species being cultivated. The mesotrophic boreal temperate coastal ocean is ideal for growing many species, of which large brown kelp species are most commonly grown.
The principle cultivated species in Europe are large brown kelp species (e.g., Saccharina latissima and Undaria pinnatifida), and the production cycle currently employed generally follows that of Chinese methods (Figure 1). Kelp production begins in the autumn, when plant reproductive material is induced to release spores by temperature and/or osmotic shock. Gametophytes and/or sporophytes are then cultured on small twine in a nursery before being transferred to sea (Rolin et al., 2017). Alternately sporophytes may be directly seeded on materials aided by a binder before being deployed the same day. The latter approach is in development at many cultivation sites throughout Europe to address the need for automation and cost-reduction to achieve greater financial viability. The seeded materials (rope, nets and other hard wearing rough surfaces) are then suspended from a mooring structure (e.g., grid or long line) at a depth where light is optimal so that growth can take place. This depth will depend on local water transparency and insolation, but is likely to be in the range of 1–5 m water depth (Kerrison et al., 2015). In late spring to early summer, the mature plants are harvested and brought to shore where they are processed for a range of markets including, human and animal feeds, soil conditioners, nutraceuticals, cosmetic ingredients or pharmaceuticals (McHugh, 2003). Seaweed may also be grown for energy where the carbohydrate-rich biomass is fermented to produce alcohol (Wargacki et al., 2012), or subjected to methanogenic anaerobic digestion (Fasahati et al., 2017). Where biomass is produced for low cost commodities such as fuel, a bio-refinery process will likely be used to maximize returns by extracting more valuable compounds first before the remaining biomass is converted to energy.
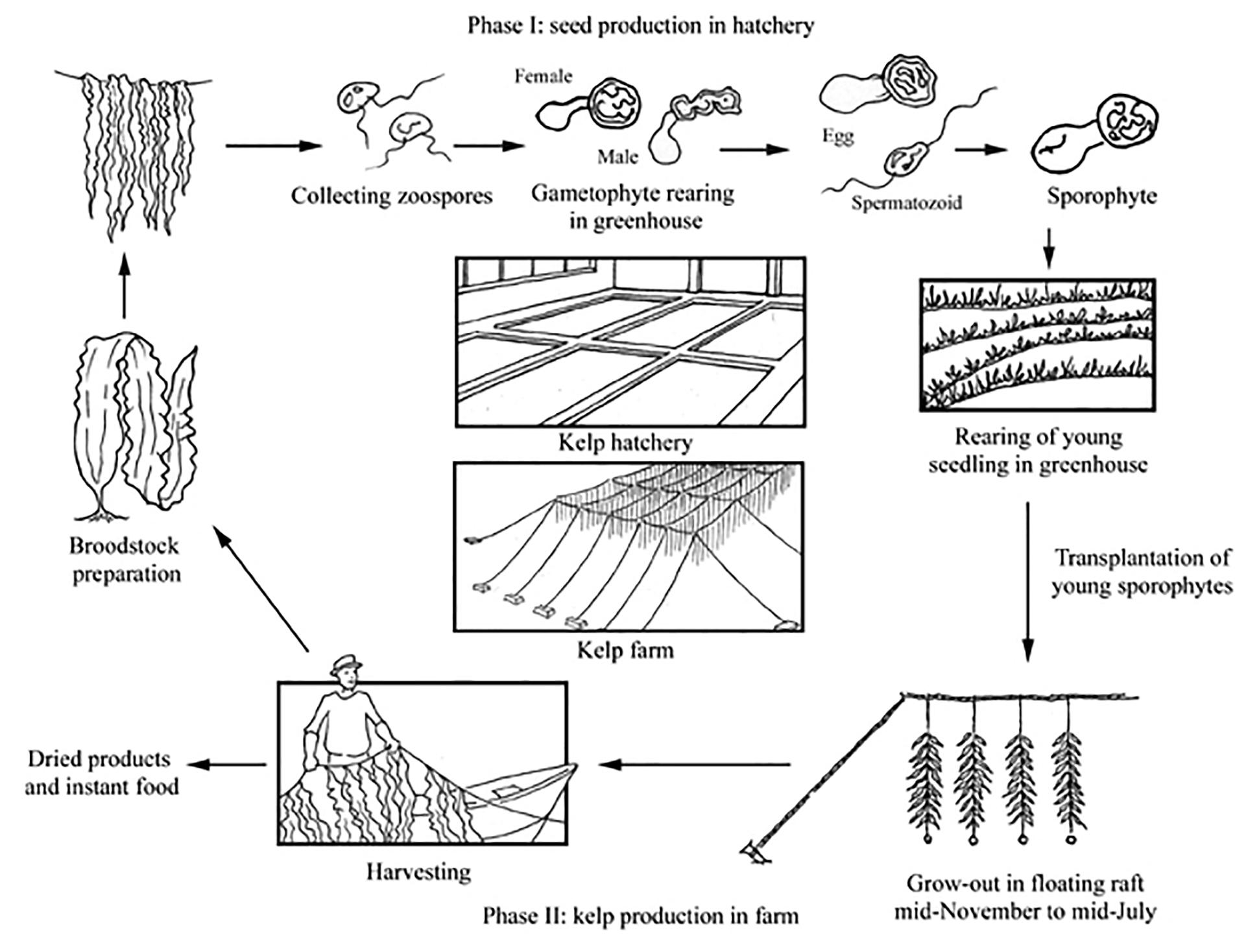
Figure 1. Production cycle of Japanese Kelp (Saccharina japonica) in China, from FAO (2004).
Cultivation systems (surface structures and moorings) currently being trialed throughout Europe are varied. They represent divergent approaches to similar problems of seeding and maintain growing structures in a way that will maximize returns (e.g., growth) whilst minimizing costs (e.g., handling and infrastructure costs). For the purpose of this review, we make the assumption that future cultivation systems will be modular and contain surface structures designed to facilitate automated seeding and harvesting whilst cultivating seaweed at an optimized stocking density (see section “Defining Scale”) using mooring components designed to reduce costs where appropriate.
Current production is largely restricted to periods where light and nutrients are sufficient to allow growth (principally autumn and spring). High levels of fouling during summer months can cause problems with crop quality (Andersen et al., 2011). The use of techniques such as coppicing and growing different algae groups such as green and red seaweeds may allow for a more continues supply whilst increasing overall production. Methods for seeding both green and red species are still in the early stages of development and therefore this review will focus in part on the cultivation of kelps.
Cultivation models used to grow seaweed depend on a large number of considerations (e.g., scale and siting) (Kraan, 2017; Marine Scotland, 2017), and an assessment of the environmental risks will change depending on these factors. However, similar environmental legislation and policies throughout Europe dictate a set of common farm management principles. These include: siting that minimizes damage to sensitive environments; seed sources that maintain the genetic diversity of wild stocks; no cultivation of non-native species; biosecurity measures to control the spread of diseases, parasites and non-natives; no fertilization; and infrastructure which is well maintained (e.g., The Water Framework Directive- Council Directive 2000/60/EC, and Council Regulation EC No. 708/2007 of 11 June 2007 concerning the use of alien and local absent species in aquaculture). However, it should be noted that variation from these assumptions is possible within other European countries currently developing policy governing seaweed cultivation practices. For example, the cultivation of non-native species (e.g., U. pinnatifida) at locations where this species has already become established. When discussing the likely consequence of environmental changes associated with seaweed cultivation, we assume that these common principles have been followed when undertaking cultivation projects.
The scale of the cultivation activities within a particular area has important implications for the magnitude of environmental changes and potentially the consequences of such changes on the receiving environment. We apply terminology from the Scottish government’s seaweed cultivation policy document that identifies two scales of commercial seaweed cultivation (Marine Scotland, 2017). Where ‘Small-medium’ refers to seaweed farms of a similar size to a typical mussel farm (0–50 × 200 m lines), and ‘large-scale’ refers to sites that require different equipment to a mussel long line system (>50 × 200 m lines).
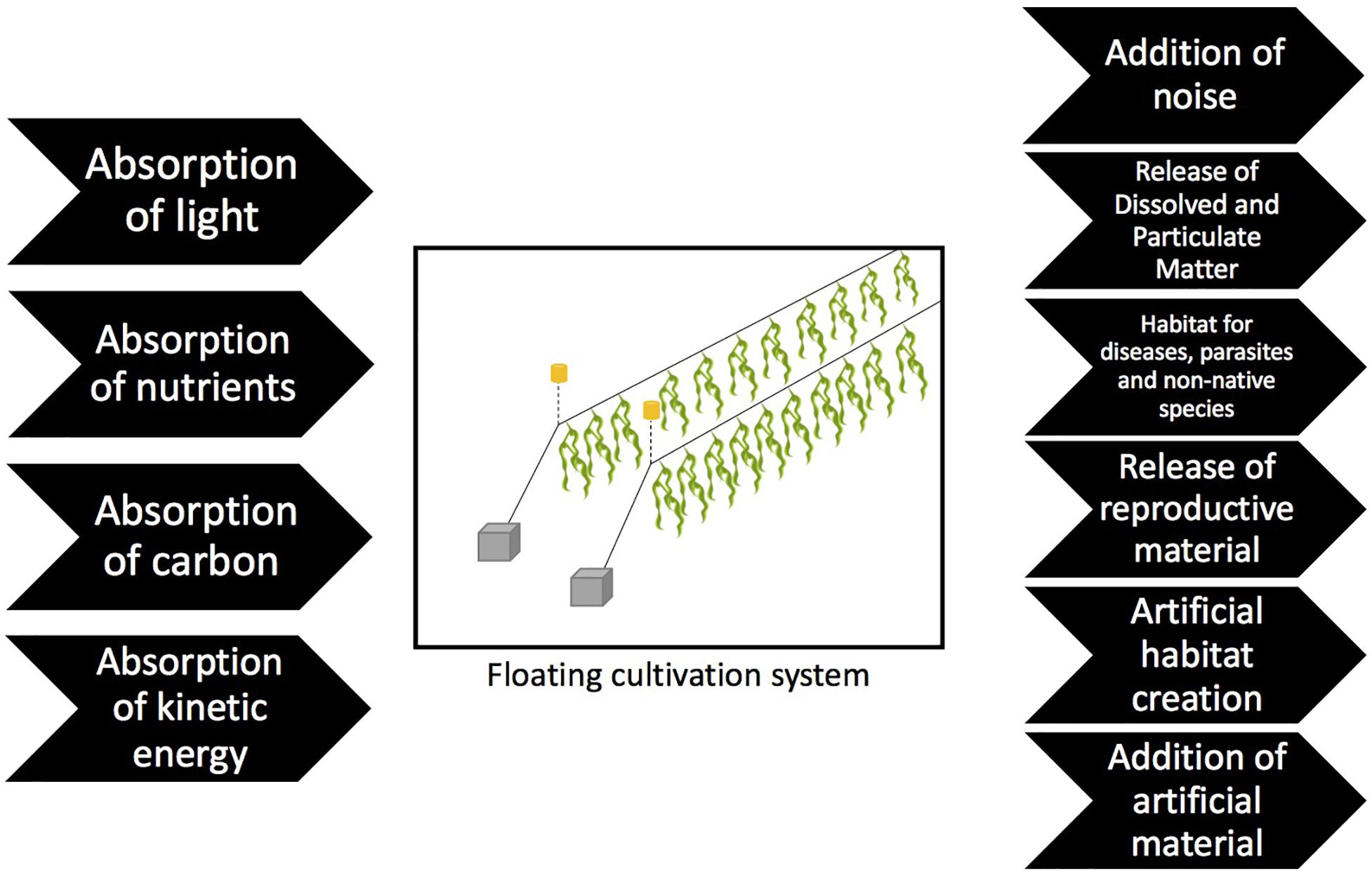
This review aims to explore potential site specific considerations that could be made to minimize negative environmental interactions when selecting appropriate sites and methods to cultivate seaweed. Assuming standard practice and siting, the key drivers of environmental change have been identified (Figure 2), and the likely consequence of each driver is summarized, although it is recognized that in practice each driver is unlikely to be operating independently. Based on this, a risk assessment is presented, indicating the prioritization of future monitoring and research objectives to address knowledge gaps that contribute to uncertainty in the consenting process.
Drivers of Environmental Change
Absorption of Light
Competition for light is important in structuring aquatic algal communities, and this has been demonstrated in the understory algal communities shaded by kelps (Reed and Foster, 1984; Clark et al., 2004; Flukes et al., 2014; Benes and Carpenter, 2015). Light intensity and its quality is directly altered by the water column itself (Morel, 1978; Platt et al., 1988), as well as indirectly by vegetation (Reed and Foster, 1984; Clark et al., 2004).
The vertical bottom up structure of giant kelp habitats has been compared to that of terrestrial forests (Dayton and Tegner, 1984). Benthic shading by kelp can affect understory algae, as kelp canopies are capable of reducing light that reaches the benthos by <3% of surface influx (Reed and Foster, 1984). Natural macroalgae communities are limited by available habitat where light conditions are suitable for growth (Burrows, 2012). Cultivated seaweed habitats differ from natural macroalgal habitats as the crops must be cultivated in surface waters at depths that optimizes levels of Photosynthetically Active Radiation (PAR). Excessive light can cause photo-oxidative stress, resulting in reduced photosynthetic efficiency (Heinrich et al., 2012). Whereas levels of PAR which are suboptimal for the species being cultivated result in low levels of photosynthesis and growth. Cultivation of seaweeds on surface waters may therefore shade underlying habitats containing autotrophic organisms (e.g., pelagic phytoplankton and benthic macroalgae). Therefore, it is important to understand whether cultivation systems overlap with habitats containing autotrophic species.
The up scaling of cultivation practices in Europe, may require a modular approach similar to other aquaculture activities. Rocky, shallow (e.g., less than 10 m) water environments are technically challenging places to deploy cultivation systems as there is more potential for breaking waves causing excessive wear and infrastructure failures. Deeper (e.g., more than 60 m) water cultivation systems have other technical challenges including longer mooring systems possibly subjected to greater drag forces. Given these considerations it is unlikely that cultivation systems currently being developed in Europe will overlap with shallow habitats supporting productive benthic macroalgae communities. Maerl beds and seagrass communities should be avoided when considering possible sites as such species are afforded a high level of protection in Europe and may be sensitive to shading effects and/or disturbance (Wilson et al., 2004). However, these communities are typically adapted to low irradiances and are generally tolerant to periods of low light irradiances. That said, the possibility of negative benthic shading effects should be considered when siting projects. Assuming that cultivation projects will have limited overlap with sensitive benthic environments and avoid habitats that are afforded high levels of protection, cultivation projects are unlikely to cause significant detrimental effects by benthic shading at small-medium and large scales.
Shading has implications for the pelagic environment as cultivation systems will be designed to efficiently absorb irradiance at the water’s surface. Similar shading can be observed in giant kelp communities where floating kelp fronds reduce irradiance in the upper meter of the canopy. Light penetration is exponentially related to canopy density, but can be higher than expected due to transmission through heterogeneous kelp blades (Gerard, 1984). In well vegetated areas, average irradiances at 1 m depth is low enough to limit macroalgal photosynthesis even under sunny conditions (Gerard, 1984). Despite the possibility of shading effects on sessile organisms located under cultivation projects, water movement required for efficient nutrient and gas exchange among cultivated species mean that phytoplankton communities will only experience shading for the length of time it takes to travel through the site. Therefore, significant shading effects on pelagic communities are highly unlikely at small-medium scales for individual cultivation sites but may act cumulatively within a site with multiple cultivation systems.
At large-scales, changes to planktonic communities are possible as phytoplankton will experience increased competition for light from cultivated species. A large-scale kelp cultivation site in Sanggou Bay (Yellow Sea, China) has been shown to suppress the abundance of phytoplankton during the growing season (Shi et al., 2011). Changes in primary productivity can affect trophic flow through affected marine food webs. Ecosystem structure was studied in an area of intense kelp cultivation by using Ecopath to model trophic structure (Wu et al., 2016). Kelp cultivation in these areas was shown to have restricted trophic flow into the water column primary production and strengthened the benthic food webs by provision of habitat and food resources directly and indirectly through enhancing detrital biomass (Wu et al., 2016).
Determining the causes and consequences of changes in the phytoplankton community is complex and must consider a number of factors including, competition for nutrients, increased grazing pressure from epibenthic species and altered hydrodynamics in addition to elevated competition for light. Phytoplankton communities have limited protection in Europe under the Marine Strategy Framework Directive, which requires maintenance of the marine environment to “Good Ecological Status” on a reginal scale. It is unlikely that shading will be sufficient enough to cause significant negative environmental effects at small-medium scales. However, a focused monitoring program assessing phytoplankton changes and resulting effects would be required to determine the potential negative interactions at larger scales.
Absorption of Nutrients
Depending on their connections with the ocean, coastal seas receive nutrients from a range of natural marine and atmospheric sources (Paerl, 1995; Prospero et al., 1996; Jickells, 1998; Baker, 2003). In addition, nutrients are added to the marine environment from anthropogenic sources (e.g., finfish aquaculture, agriculture, and urban wastewater) (Smith, 2003). These sources of nutrient fluxes are often related to increasing occurrences of harmful algal blooms (Anderson et al., 2008; Heisler et al., 2008). Human induced changes to sources and sinks of nutrients can have negative impacts on coastal ecosystems altering local ecology and ecosystem services (Shumway, 1990; Anderson et al., 2002; Heisler et al., 2008).
Seaweeds in suspended cultivation remove inorganic nutrients from the marine environment during growth (Kerrison et al., 2015; Marinho et al., 2015). Positive remedial effects will occur when the quantity and proportion of nutrients removed are equal to those added by anthropogenic activities (Seghetta et al., 2016b). However, undesirable effects could occur if nutrient removal by cultivation results in concentrations which fall below that required for natural primary productivity, and very large-scale culture of macroalgae will extract proportionate amounts of nutrients from the surrounding water body (Lüning and Pang, 2003).
Suspended aquaculture systems used to cultivate seaweed affect local hydrodynamic movements by increasing surface drag. Alterations to water flow can affect the carrying capacity of a water body through reducing water exchange necessary for maintaining levels of nutrients required for growth (primarily Dissolved Inorganic Nitrogen) (Shi et al., 2011). This effect is intensified in the interior of large-scale cultivation sites where flow is decreased by the drag effects and growth can become nutrient limited as a result (Shi et al., 2011). Models of nutrient regimes in simulated large-scale macroalgae farms (20 km2, 20 t/ha dry weight production) indicate there will be a reduction in phytoplankton biomass within the cultivation area, and >10% reductions in chlorophyll concentration over 7.5 km away from farms (Aldridge et al., 2012). As well as nutrient models, hydrodynamically driven models simulating nutrient uptake by a large-scale farm (112 km2) in the North Sea at various stocking densities, only observed significant changes in nutrient availability at the highest stocking densities, and that realistic stocking densities were classified as marginally significant (Aldridge et al., 2012).
Current realistic stocking densities in Europe are generally lower than that reported in current models (Aldridge et al., 2012) and those observed in China where nutrient depletion has been observed. China produces up to 18 tons of dry kelp per hectare in the most productive areas (Aldridge et al., 2012). Assuming similar water content between kelp species this equates to approximately 151 wet tons per hectare (10,000 m2) (Saccharina latissima dry/fresh = 0.12). Although it should be noted that dry vs. fresh weight ratios vary with species and throughout the growing season (Broch and Slagstad, 2011; Peteiro and Freire, 2013a). The grid systems employed in China use either vertical or horizontal (preferred) rope raft culture methods that are densely packed (stocking density estimated at approximately 0.66 linear meters of growing line per meter squared of cultivation area) (Shi et al., 2011). Reported growing systems in Europe are generally less space efficient (Peteiro and Freire, 2013a). Using the above figure, China is therefore able to produce 22.9 kg per line meter of growing line to achieve a biomass of 151 tons per hectare. To achieve similar yields within European sites, growing systems must first increase the density of seeded materials whilst increasing yields per liner meter from current levels [average 9.1 kg wet weight per linear meter (Seghetta et al., 2016a)]. For example, observed biomass of S. latissima cultivated at a site in Spain produced approximately 16 kg m-1 on growing lines in one season (Peteiro and Freire, 2013a). Production at this site was 4.7 t/ha dry weight (4 m spacing between lines). To achieve greater stocking densities more effective cultivation infrastructure will need to be developed whilst mitigating competition for nutrients and other resources such as light.
A model of the nitrogen requirements for a hypothetical large scale farm (20 km2) in the Clyde estuary in Scotland estimated extraction of nitrogen at 480 tons per year for a site producing 20 t/ha dry weight (Aldridge et al., 2012), suggesting there is potential for a significant reduction in local nitrogen resources on this scale. At the time of a typical harvest the nitrogen content of dry material is approximately 1.2% (Broch and Slagstad, 2011; Schiener et al., 2015). Even where we assume future productivity per meter of seeded materials would be equivalent to those observed in China (22.9 kg m-1 per line). Small-medium scale operations (<50 200 m lines) would produce up to 229 tons of biomass (27 tons dry weigh) and extract 0.33 tons of nitrogen. At these scales negative environmental effects from diminished nitrogen resources are highly unlikely assuming cultivation practices are located in areas with artificially elevated nitrogen resources during times when crop growth rate is high.
Anthropogenic sources of nitrogen in the marine environment can be significant. For example, 7,500 tons of nitrogen were estimated to be released by the Scottish salmon farming industry in 2010 (Aldridge et al., 2012). If careful consideration is given to the siting of seaweed farms, ensuring that carrying capacity of the environments are not exceeded, negative environmental effects of localized nitrogen depletion may be avoided.
At larger regional scales, cultivation projects may contribute substantially to remediation of excess nitrogen if co-located in suitable areas of high anthropogenic nitrogen input. A Life Cycle Impact Assessment (LCIA) of seaweed cultivation and nutrient extraction in Europe, indicates that at large scales (208 km2), seaweed cultivation can have a positive effect through bioextraction of N and P from anthropogenic activities in the marine environment and aid management strategies at the water body level (Seghetta et al., 2016b).
The timing of effluent release and uptake, dispersal characteristics of the site along with and knowledge of nitrogen cycling in the environment, are required to develop nitrogen mass balances for each cultivation site. Such information would support a more holistic approach to managing nutrient levels and allow for the scaling of cultivation projects to the characteristics of the specific water body. Present seaweed biomass required to remove the nitrogen effluent from a typical salmon farm is much greater than small-medium seaweed farm operations can produce [approximately 1000 wet tons (Broch and Slagstad, 2011)], and negative interactions associated with cultivation infrastructure (e.g., reduced flow) may diminish the overall benefits of such an approach if poorly sited. Therefore, the development of coupled hydrodynamical-biological models at industry realistic stocking densities will support future developments by providing more clarity to estimated sources and sinks of nitrogen.
Competition between cultivated algae and phytoplankton can be expected at time intervals in the production cycle where algae growth is rapid and natural renewal of nitrogen resources is affected by altered water exchange. Where projects are large-scale and have high stocking densities, depletion of phytoplankton communities could have negative implications for some species in affected areas. The feasibility of large-scale cultivation projects will require a degree of site specific modeling and monitoring work to ensure a strong evidence-base to determine the trade-offs and interactions associated with large-scale macroalgae production versus protecting, conserving and enhancing biodiversity.
Absorption of Carbon
Aquaculture of fed species such as finfish contribute carbon dioxide to the global carbon cycle primarily through reliance on capture fisheries and terrestrial agricultural production (Pelletier et al., 2009). In contrast, large-scale aquaculture of un-fed invertebrates and macroalgae can remove large amounts of carbon from the coastal environment (Tang et al., 2011; Hughes et al., 2012a) and offer alternative low carbon food and energy resource if managed efficiently.
The removal of carbon dioxide by cultivated algae is unlikely to lead to any detrimental effects within cultivation sites and surrounding areas. When CO2 reacts with water it forms a balance of ionic and non-ionic chemical species including free carbon dioxide, carbonic acid, bicarbonate and carbonate, the ratio of which depend on many factors such as temperature and pH. In an open freely moving water body the negative effects of carbon removal from large scale cultivation is likely to be negligible due to marine waters chemistry and inherent buffering capacity. Conversely, large bodies of photosynthetic material may absorb enough carbon to increase the pH locally and mitigate impacts caused as a result of ocean acidification, similar to shellfish calcification downstream of highly vegetated areas (Krause-Jensen and Duarte, 2016). Although kelp habitats remain an important source of organic carbon for marine food webs (Burrows et al., 2017), the implications of cultivation on carbon cycling are poorly understood and will require further research.
The contribution to carbon sequestration (blue carbon) that cultivation losses could make when buried in sediments or exported into the deep sea needs to be assessed in a cultivation context (Krause-Jensen and Duarte, 2016; Duarte et al., 2017). However, continuing trends toward increased seaweed aquaculture could still provide a significant contribution to climate change mitigation and adaptation though providing additional benefits through carbon capture (estimate 1,500 tons CO2 km-2 year-1), animal feed supplements that reduce levels of methane production, substituting synthetic fertilizers and mitigating coastal erosion through absorbing wave energy (Duarte et al., 2017).
Absorption of Kinetic Energy
Seaweed farms require water flow to encourage growth, and will absorb and deflect tidal and wave energy altering flow conditions in connected habitats (including local geomorphology at large scales). How cultivation structures alter coastal hydrology will be an important factor in determining the ecological implications at different scales. Relevant observational studies on wild kelp beds have confirmed that standing crops of wild kelp dampen natural currents and cause microclimates within the canopy (Jackson and Winant, 1983), reducing average current speed to a third of the surrounding area. In some cases this microclimate can occur vertically beyond the extend of kelp fronds (Andersen et al., 1996). Natural kelp beds are anchored in the seabed and therefore have a bottom up effect on currents rather than the predominantly surface impacting structure of suspended kelp culture.
As part of a suspended structure, cultivated kelps can experience increased water motion, which increases the rate of nutrient uptake (Neushul et al., 1992). Flow rates along the open channels and within Sanggou Bay- a large-scale Chinese kelp cultivation site- have been simulated using a two-dimensional vertically averaged model (Grant and Bacher, 2001). In this model, increased seabed friction simulated the presence of aquaculture structures. By increasing the drag coefficient of the seabed to simulate the frictional effects of suspended aquaculture structures, flow along the open channels within the farm was reduced by 20%, and within cultivation areas was reduced by 54%. In addition to reduced current speeds, the vertical structure of tidal currents in Sanggou Bay is predicted to be affected by the strengthening of a surface boundary layer created by suspended cultivation systems (Fan et al., 2009). This is supported by field measurements of tidal currents taken in Sanggou Bay, which demonstrate clear vertical structure of the observed tidal currents (Zeng et al., 2015). Observations show that although total tidal exchange volume remains unchanged, there is a reduction in tidal flow at the surface where kelp is suspended, which causes the maximum flow point to occur below the suspended kelp fronds. The depth between the lower limits of suspended kelp and the seabed will determine where the maximum velocity point will occur as a result of the increased drag by kelp at the surface. This could have implications for the benthic and pelagic habitats below, which would experience altered flow dynamics resulting from changes to surface boundary conditions.
Alterations to water flow can affect the cultivation carrying capacity of a water body through potential reduction in water exchange necessary for maintaining levels of nutrients required for growth (Shi et al., 2011). Careful consideration must be given to the siting of cultivation projects in areas and at times where alterations of natural hydrodynamics could result in significant changes to marine chemistry (e.g., peak biomass would cause greatest friction coefficients), sediment transport and associated biological communities. Risk will most likely increase with larger scale projects and siting in areas important for water exchange, such as the entrance to enclosed water bodies. Assuming sites are well located, negative environmental effects are unlikely at small to medium scales, and it is unlikely that farms of this scale will have the resources to carry out detailed hydrodynamic impact assessments. However, the assessment of potential negative environmental effects must be made on a case-by-case basis and incorporate cumulative effects of other marine projects. Therefore, it is not possible to make general predictions regarding the extent and consequences of altered local and reginal hydrodynamics. A strategic siting and modeling approach may be required to ensure licensing authorities are able to make informed decisions about the consequences of large-scale projects as well as cumulative level assessment of smaller co-located projects.
Addition of Artificial Material
Large-scale cultivation of seaweed requires the addition of artificial materials to provide a secure substrate for growing seaweed. A range of systems and configurations are currently being tested within Europe, and will require further development to improve the overall efficiency of the growing phase. All systems are comprised of a mixture of moorings, lines and floats with varying degrees of complexity.
The largest proportion of material added to the marine environment will likely be comprised of a mixture of synthetic polymer rope (e.g., polypropylene). These materials are typically designed to be highly resistant to degradation in the marine environment. Pollution caused by discarded or lost components may contribute to marine pollution if seaweed farms are improperly managed. Once lost from the farm, debris may contribute to existing environmental pollution issues such as increasing levels of plastics in marine food webs (Derraik, 2002; Andrady, 2011) or social concerns such as the reduction in coastal amenities due to drifting debris (Sheavly and Register, 2007). Assuming cultivation activities are managed responsibly, are well maintained and fit for purpose, accidental loss of infrastructure at sea should be minimal.
Loss of infrastructure to the marine environment can result in the mortality of marine megafauna (e.g., marine mammals, marine turtles, sharks, rays, and large bony fish) caused by entanglement in subsurface mooring lines and fishing gears, and is already a significant conservation problem throughout the world (Benjamins et al., 2014). There are a number of risk factors which are associated with a greater likelihood of entanglement. These include: moorings and lines that have low tension, poor visibility leading to reduced avoidance and moorings and components that are unable to resist the forces of an encounter (e.g., gray seal [≈0.1 KN] or Minke whale [≈16 KN]) (Benjamins et al., 2014). The use of nets to cultivate algae may pose a significant threat of entanglement to both small and large megafauna species. The diving behavior of marine mammals puts them at risk of interaction with infrastructure, as it may not be possible to avoid infrastructure when resurfacing for air.
The true extent of entanglement risk from well-established marine activities is poorly understood. A study into the cause of death of 422 cetacean carcasses across England and Wales found that entanglement of megafauna in fishing gear (by-catch) was the principle cause of death in most cases (Kirkwood et al., 1997). The global estimate of marine mammal by-catch is approximately 600,000 animals and entanglement with stationary gear is more likely where nets and pot-type gear are used (Read et al., 2006). The contribution that an emerging cultivation industry might have to mortality within megafauna populations is currently unknown.
Entanglement of animals cannot be ruled out, even when assuming cultivation practices will be managed to reduce the likelihood of entanglement. Small-medium scale cultivation projects pose a similar threat of entanglement to many existing aquaculture activities as mooring and cultivation equipment will utilize similar technologies, and as large-scale cultivation projects will inherently require a greater infrastructure the risk will be increased.
Many marine megafauna species are slow-growing, have low reproductive rates and are commonly afforded a high level of protection within many European countries. Entanglement-related injuries and mortalities are a critical conservation problem. Siting of cultivation activities is a crucial consideration to avoid negative environmental interactions. There is limited evidence to suggest whether marine mammals and other megafauna will avoid or be attracted to cultivation activities and any responses are likely to be location- and species-specific. Cultivation activities may enhance foraging opportunities for some species, and although this would be a positive interaction it could lead to a greater risk of entanglement if poorly managed. Larger species of marine mammals are more often observed in deeper offshore areas (Ried et al., 2003). Therefore, cultivation activities that are sited in deeper offshore areas may have to take extra precautions to avoid entanglement.
Licensing authorities should ensure cultivation activities and infrastructure are well designed to avoid entanglement and sited to avoid important areas for foraging, reproduction and migration. If these considerations are made significant effects to megafauna populations is highly unlikely at small-medium cultivation scales, and could reduce entanglement risk at larger scales. The assessment of risk associated with projects is complex and uncertainty is increased by a current lack of information regarding the likelihood of entanglement of different megafauna species with seaweed cultivation systems. As the overall consequence of large-scale cultivation is currently unknown cultivation activities must be managed responsibly to ensure that infrastructure deployed is well maintained and fit for purpose to avoid accidental loss of infrastructure at sea, as is required of other aquaculture activities across Europe through multiple regulations (EC, 2016).
Addition of Noise
Cultivation sites will result in a localized increase in vessel traffic and machinery required for site activities including installation, maintenance, seeding and harvesting. The extent that cultivation activities will elevate local noise above background is currently unknown but it can be considered proportionate to the scale of operations. Vessel engines are a source of anthropogenic noise and negative environmental effects (e.g., habitat displacement and barrier affects) could be observed where noise produced causes behavioral responses that contribute to local or regional population decline. However, the sensitivity of marine species to small vessel noise is likely to be low assuming the location of the farm has been considered with respect to sensitive features (e.g., avoiding protected seal hall-out areas) (Southall et al., 2008; De Robertis and Handegard, 2013).
At small-medium scales, the increase in magnitude of vessel traffic associated with project is likely to be small and therefore unlikely to cause significant ecological changes assuming cultivation project are sited away from sensitive features. Elevated risk associated with larger cultivation projects will require additional consideration during the consenting process.
Release of Dissolved and Particulate Matter
Particulate Organic Matter (POM)
Organic matter (OM) can be released by macroalgae as either Particulate or Dissolved Organic Matter (POM and DOM, respectively). In kelp cultivation sites, POM tends to result from wave action and decomposition of plant tissue matter, and is often suspended in the water column before settlement on the benthos (Ren et al., 2014).
Natural kelp beds already play an important role in providing organic matter to the coastal ecosystem (Duggins et al., 1990; Steneck et al., 2002; Leclerc et al., 2013), and can export organic matter beyond the immediate kelp habitat (Harrold et al., 1998; Wada and Hama, 2013). Similarly, POM is exported from seaweed cultivation sites. At an existing large-scale site (several km2) in Sanggou Bay in China, three modes of kelp tissue loss occur: fall-off from kelps, where the holdfast becomes detached or there is a break in the stipe; break-off, where there is a clear break leaving part of the blade and distal erosion which occurs at the edges and tip of the kelp blade where there is continual decay (Zhang et al., 2011). The proportions of each mode of loss at this site is dependent on seasonality and stage of growth. Fall-off occurs early in the grow-out season (January–February), and can result in an estimated 4.2% of overall loss of kelp from sampled long lines. Break-off peaks later in the grow-out season (June–July) resulting in approximately 4.5% of overall loss. Distal erosion increases through earlier growth months (January–April), and remains high in the months after and equated to 91.5% of loss at the sampled cultivation site. This suggests the release of POM on a large-scale cultivation site will be strongly seasonal, increase with increasing biomass and will consist primarily of smaller tissue fragments. Losses during harvesting operations may also contribute to the release of POM. Maximizing crop biomass is in the interest of the cultivator; however, the extent of losses from mechanical harvesting is currently unknown.
Depending on its buoyancy/settling velocity, lost plant tissue may deposit on the seabed and stimulate benthic microbial metabolism and affect macrobenthic community structure. The scale of impact will be related to the distance that solid material lost from the farm is advected (directly and after any resuspension) before its remineralization is complete, with low settling-velocity fragments traveling long distances. For example, organic enrichment of a submarine canyon (153–454 m) observed by a Remotely Operated Vehicle (ROV), estimated that 20% of drift parcels were comprised of kelp tissue derived from an adjacent Macrocystis pyrifera bed (Harrold et al., 1998). At a distance of 9 km away from standing crops, in a continental shelf habitat (87–357 m) very few drift parcels were observed, however, of those observed 50% were composed of kelp particles. This emphasizes the importance of understanding the true extent of OM drift from large-scale kelp culture and its interaction with benthic environments. The biogeochemical consequences of large amounts of material decomposing in depositional areas might include sedimentary anoxia and hypoxia in bottom waters, together with enhanced sediment nutrient fluxes, particularly in areas with long water residence times (see section “Interactions With Benthic Species”). Further scientific investigations to assess the true extent and fate of POM release from seaweed aquaculture are required to provide perspective on what scales and environmental conditions released POM could have negative consequences.
Dissolved Organic Matter
A large proportion of Dissolved Organic Matter (DOM) is observed as photosynthates in the seaweed tissue, and these photosynthates are released by kelps as DOM into the water column (Khailov and Burlakova, 1969; Sieburth, 1969; Fankboner and de Burgh, 1977; Abdullah and Fredriksen, 2004; Wada et al., 2007; Hulatt et al., 2009). This released DOM is thought to be a complex mixture of mainly carbohydrates which can enter the oceanic Dissolved Organic Carbon (DOC) pool (Wada et al., 2007). It is unknown whether this release occurs as a passive or active function in the tissue. However, it has been suggested that exudation is increased during times of greater growth when excess photosynthates are assimilated (Abdullah and Fredriksen, 2004).
Seaweed exudate studies have mainly identified and monitored the carbon content of exudates as DOC. A proportion of this released DOC is thought to be refractory DOC (rDOC), due to the long turnover rates in coastal seawater (Wada et al., 2008). This suggests that a proportion of kelp exudates may be resistant to biological breakdown, and rDOC will join the oceanic carbon pool which is estimated to be 4,000–6,000 years old (Bauer and Druffel, 1998). As carbon entering this pool from seaweed cultivation will effectively be sequestered (Hughes et al., 2012a), the potential long-term environmental consequences of the refractory portion of seaweed exudates may be less direct, but in the short-term exudates may alter light attenuation due to their colorimetric nature (Hulatt et al., 2009).
The alternate fraction of these carbohydrate rich exudates will be bioavailable to microplankton such as bacterioplankton and phytoplankton, and could be rapidly utilized (Azam et al., 1983). Dissolved substrates are an important intermediates in the rapid cycling of bioactive compounds by bacterioplankton in the “microbial loop” (Azam et al., 1983). At high concentrations, these bioavailable exudates have the potential to alter the balance and composition of the local microbial assemblages. However, the extent and significance of this change would likely be negligible for small-medium cultivation projects when compared against naturally occurring levels of bioactive compounds from other sources. The scale and wider ecological implications of large-scale projects are currently unknown, and will be dependent on the hydrodynamics and seasonality of the site.
Habitat for Diseases, Parasites and Non-native Species
Diseases and Pests
The prevalence of diseases and pests affecting aquaculture production worldwide is a major global concern (Kim et al., 2014; Stentiford et al., 2017). This issue is intensified by a reduction in genetic diversity associated with the domestication of wild seaweed species making crops more susceptible to abiotic stressors, disease and parasites (Valero et al., 2017). Unlike terrestrial agriculture, a reduction of genetic diversity of open sea cultivated marine species in favor of a few selected traits cannot be supported by the use of pesticides and fertilizers to support growth. Cultivated stands will likely experience a large reduction in yield where diseases and pests are prevalent, and may also act as a reservoir for diseases which could impact natural populations (Loureiro et al., 2015; Valero et al., 2017). For example, carrageenophyte (Kappaphycus) producing countries have seen a dramatic decline in production following rising sea water temperatures which cause bleaching of the thallus making cultivated individuals more susceptible to infection from viruses and bacteria (Vairappan et al., 2008). Protocols that mitigate crop losses are often rudimentary (centered on removal of affected crops) and chemical treatments are known to reduce crop quality (Loureiro et al., 2015). Knowledge regarding the epidemiology of seaweed pathogens in European species is very poor and in many cases pathogens responsible for diseases are difficult to identify and study using current microbial methods (Gachon et al., 2010). Further investigation is required to inform appropriate mitigation measures and prevent significant ecological impacts. Mandatory biosecurity planning will ensure actions are taken that mitigate risk where practical and will benefit all stakeholders.
Mitigation measures hinge on developing and enhancing biosecurity programs through capacity building (Cottier-Cook et al., 2016). This must include training in quarantine procedures and farm management practices to enhance biosecurity measures, as well as the development of diagnostic techniques to rapidly detect disease to inform management practices. Finally, breeding programs for farmed species should be developed to ensure sufficient genetic diversity and disease resistance, for both current and future production.
Non-native Species
Non-native species (NNS) are classified as organisms that have been intentionally or unintentionally introduced outside their native range as a consequence of human activity. NNS may cause ecological damage to the receiving environments and may also be associated with economic losses within affected marine industries, including aquaculture (Pimentel et al., 2001). Once established, species that threaten biodiversity and/or cause economic damage are referred to as ‘Invasive’ (INNS). It is widely accepted that once a NNS has been introduced to a new environment, is it very challenging, and in the majority of cases practically unfeasible, to eradicate. As a result, preventing the introduction of new NNS and restricting the likelihood of secondary introductions is typical of current marine management policies in Europe [e.g., Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species].
The relatively recent boom in aquaculture has often contributed to the global spread of non-native marine organisms (Naylor et al., 2001). Despite the largely sedentary life history of macroalgae, they have often been the subject of invasive spread through aquaculture (Fletcher and Farrell, 1999). The deliberate introduction of reproductively active species in addition to the creation of possible introduction pathways greatly increases the chance for spread and establishment of NNS (Schaffelke et al., 2006). In a global assessment of invasive macroalgae introductions, 121 of 223 introductions were derived from aquaculture either through macroalgae cultivation or indirectly through shellfish farming (Williams and Smith, 2007). In some areas, the introduction of non-native species through abandoned cultivation efforts has had a serious effect on local ecosystems and the economy. In Hawaii, a number of invasive species have been recorded within reef areas and have caused phase-shifts from coral to algae (Smith et al., 2002). In particular, the previously cultivated red alga Gracilaria salicornia subsequently colonized the prized reefs of Waikiki (Smith et al., 2004).
In 1983 the brown kelp U. pinnatifida native to Asia was introduced to the French Atlantic coast for commercial cultivation (Kraan, 2017). Although it was believed that it could not reproduce, it soon became established in the local environment and since then has spread widely, typically becoming the dominant biofouling species on artificial substrate (Fletcher and Farrell, 1999). To date this species has been farmed along the Brittany coast where it has been established for the last 33 years (Kraan, 2017). Allowing farming of this species in the North Atlantic undermines efforts to control the spread of this species within other parts of Europe adopting a more precautionary approach to controlling the spread on NNS in general.
Despite a history of species introductions associated with the global seaweed production practices, the introduction of species outside their native range are unlikely to be permitted within a European context. However, it is important that there is more clarity regarding which target cultivation species are permitted throughout Europe to ensure comparable approaches across neighboring countries. Furthermore, if growing cultivars present in the “local” environment is considered best practice in the future, consideration of what “local” means in different countries for different species is necessary as the degree of genetic variation varies greatly between countries. For example, some seaweed species are characterized by low intra-specific genetic diversity in the northern extent of their range due to founder effects of range expansion caused by global climate change (Assis et al., 2016).
Artificial structures used to cultivate seaweed may provide a novel habitat that will favor the establishment of NNS (Glasby et al., 2007; Mineur et al., 2012). In addition the presence of vectors (e.g., movement of biofouling associated with vessels and other structures) must be managed in such a way as to reduce the potential risk that cultivation activities will result in the spread on NNS. Although the risk of unintentional introductions can never be managed fully, cultivation practices are unlikely to cause significant environmental effects assuming native species are cultivated and operations are managed to reduce the potential risks of introducing NNS.
European countries typically restrict the introduction of NNS to avoid documented negative environmental consequences [e.g., Regulation no. 708/2007; no. 535/2008 and Regulation (EC) no. 506/2008 amending Annex IV to Council Regulation (EC) no. 708/2007]. Current advice in the United Kingdom now promotes the use of Biosecurity Planning as a way to assess and manage any potential risks created by marine activities that may lead to the accidental introduction and/or spread of NNS (Cook et al., 2014). The principle component of any Biosecurity Plan is a record of the actions that will be taken in order to minimize the spread of NNS, and can be combined with disease management plans to increase efficiency. A Biosecurity Plan may consist of four principle stages (1) a description of the activity (2) a risk assessment (3) resulting actions after risk assessment and (4) a contingency plan.
Release of Reproductive Material
The increasing requirement for marine based commodities, along with the difficulty in sustainably exploiting natural populations is driving a shift from humans as hunters of the marine environment to cultivators (Valero et al., 2017). The domestication of wild seaweed cultivars will be an unavoidable consequence of large-scale seaweed cultivation practices (Valero et al., 2017). Cultivated seaweeds will most likely be characterized by a human imposed shift in their reproductive strategy (e.g., from outcrossing to self-fertilizing and from sexual reproduction to vegetative reproduction) introducing genetic bottlenecks that may narrow the genetic diversity of cultivated stands potentially making them more susceptible to environmental changes and disease as observed in vegetative propagation of domesticated Gracilaria (Leonardi et al., 2006; Valero et al., 2017). Studies have resulted in the production of improved varieties of kelps with respect to commercially valuable traits (e.g., stipe length, frond length, width and thickness, and iodine content) (Liu et al., 2014; Li et al., 2016) and these have been widely applied in cultivation activities (Li et al., 2007, 2008, 2016). The consequences of producing cultivars that are genetically and phenotypically distinct from natural populations is unknown but there is the potential for significant environmental effects through both direct competition with wild populations and hybridization with natural stands (Halling et al., 2013; Loureiro et al., 2015; Valero et al., 2017).
Cultivation practices supported by the supply of locally sourced cultivars still have the potential to genetically depress natural populations through so called “crop-to-wild” gene flow (Loureiro et al., 2015; Valero et al., 2017). Breeders must focus on strategies that optimize the selection of desirable traits whilst maintaining the domesticates evolutionally potential to ensure good yield in variable environmental conditions whilst reducing impacts on natural populations (Valero et al., 2017). Such a task will require a paradigm shift in breading strategies that will demand the maintenance of a large number of locally sourced cultivars phenotypically optimized to ensure suitable genetic variance.
The effect of gene flow from cultivated seaweed species are as yet unknown. Focused monitoring and research activities will be required to understand both variability in natural populations and the effect of cultivated domesticates on surrounding population fitness and associated ecosystems (Loureiro et al., 2015; Valero et al., 2017). The widespread production of sterile cultivars may be technically feasible and should be considered as an important step to mitigating the effects of gene depression and introducing locally absent cultivars and species (Loureiro et al., 2015). Furthermore the establishment of national seed banks which are responsible for maintaining a high health status of seed stock has been recommended to ensure that breading strategies are appropriate to reduce negative environmental effects (Cottier-Cook et al., 2016).
Artificial Habitat Creation
Cultivation sites will replace existing habitats with novel man-made habitats by virtue of physical and biological changes associated with suspended cultivation infrastructure. Habitats created may be characterized by; increased complexity including the physical presence of the structure itself, the addition of hard artificial substrate, pulses of seaweed growth consistent with growing cycles, as well as altered physical and chemical properties of the surrounding water. To summarize some of the potential changes associated with this type of habitat creation this review focuses on three major species groups (plankton, benthic species and epifauna and megafauna species).
Interactions With Plankton
There have been several studies on the interactions between macroalgae and microalgae (see section “Absorption of Nutrients”). While a range of species specific effects have been observed, some major interactions have been identified. Generally, in both low and high-nutrient situations macroalgae can affect the composition of the phytoplankton assemblages through competition for nutrient resources (Fong et al., 1993). In addition, macroalgae can inhibit microalgal growth both through allelopathy (Jeong et al., 2000; Nan et al., 2004, 2008), and through shading of the water column by dense macroalgal canopies (Borchers and Field, 1981). More complex interactions are also observed to occur as a result of nutrient competition, and resource availability. For example when nutrients are low, macroalgae may outcompete microalgae by utilizing previously stored nutrients in tissues (Solidoro et al., 1995; Lüning and Pang, 2003). Under high nutrient concentrations microalgae may benefit from having a larger surface to volume ratio than microalgae (Fong et al., 1993). A recent study on picoplankton abundance in an Integrated Multi-trophic Aquaculture (IMTA) site in Sanggou Bay, China, observed abundance to be lower within the kelp cultivation area than the shellfish area (Zhao et al., 2016), and attributed changes in abundance and distribution largely to grazing by protists, as opposed to higher nutrients in shellfish growing areas. This indicates that more complex interactions are occurring in large-scale kelp cultivation sites, and is reflected in emerging work from wild kelp forests. The microbial community structure and function within a kelp forest on Vancouver Island, Canada (Clasen and Shurin, 2015), was altered within the kelp forest studied. Bacteria were subject to increased viral-mediated mortality, and effects correlated with kelp forest size. Kelp cultivation sites may have similar effects on microplankton assemblage and function, and will need to be determined not only in relation to the size of the cultivation site, but also through a cascade of indirect effects, which will require further investigation.
Interactions With Benthic Species
As with finfish (Cromey et al., 2012) and shellfish (Chamberlain, 2001; Weise et al., 2009) farming, large areas of suspended kelp may alter sedimentation patterns (see section “Absorption of Kinetic Energy”) as well as the delivery of POM to the seabed [see section “Particulate Organic Matter (POM)”]. These drivers may change benthic community structure in affected areas.
In an area of extensive macroalgal cultivation in China (Sangou Bay), benthic species diversity was generally low, but can be greater in summer-autumn than in winter–spring (Zhang et al., 2009). The Norwegian Modelling - Ongrowing fish farms- Monitoring (MOM) system (Hansen et al., 2001) was used to assess benthic impacts of long-term large-scale aquaculture of shellfish and seaweed on the benthic environment and were considered to be low impact (Zhang et al., 2009). In a fish/kelp polyculture system in Sandu Bay, East China Sea sedimentary acid volatile sulfide content under kelp culture were observed to be greater (1.22 mg/g) than a control station (0.14 mg/g dw), but slightly lower than that recorded at a fish farm station (1.4 mg/g) (Zhou, 2012). Both the fish farm and kelp farm (separated by ca. 10 km) showed reduced benthic biodiversity compared to a control station (Zhou, 2012).
The impacts of benthic organic enrichment have been studied extensively for fish and shellfish sites, from the immediate changes in biogeochemical processes (Chamberlain, 2001; Holmer et al., 2005), to the subsequent changes in fauna where species abundance and richness can be reduced with proximity to finfish cage sites (Hamoutene et al., 2015). The dilution and loss of POM is currently minimized and monitored using hydrodynamic models in other aquaculture sectors [e.g., DEPOMOD (Cromey et al., 2002)]. Where releases of POM are considered sufficient to cause environmental harm, the magnitude and severity of environmental changes associated with seaweed cultivation may be modeled using similar tools to aid management decisions.
Interactions With Epifauna and Megafauna Species
Sublittoral kelps are recognized as important habitats for a range of invertebrate macrofauna (Christie et al., 2009; Norderhaug and Christie, 2011), which in turn supports a diverse ichthyofaunal assemblage (Norderhaug et al., 2005). One argument for cultivation rather than wild harvest relates to the ecological importance of kelp forests. Large-scale intensive cultivation of kelps is likely to provide additional habitat for a range of invertebrate and fish species, and kelp farms will naturally act as fish aggregating devices, as do shellfish (Davenport et al., 2003) and finfish farms (Dempster et al., 2009, 2011).
Extensive information exists on the macroinvertebrates which live in close association with wild kelps (Ojeda and Santelices, 1984; Dayton, 1985; Duggins et al., 1990; Anderson et al., 1997; Hepburn and Hurd, 2005; Christie et al., 2009; Zahn et al., 2016). This relationship is thought to be a result of increased habitat size and complexity in addition to increased grazing and filter feeding opportunities (Christie et al., 2009).
The creation of novel kelp habitats through cultivation could support positive changes to local ecosystems through the provision of habitat as well as increased food resources, traits that are also associated with wild kelp beds. However, as cultivated kelp is held in suspension and harvested frequently, it is likely to facilitate a different benthic assemblage than that associated with natural kelp beds. For example, holdfast communities of Laminaria digitata cultivated in Ireland provided habitat for a different and more diverse macroinvertebrate assemblage compared to wild kelp beds although both had similar volumes of epifauna (Walls et al., 2016). Furthermore, kelp cultivation which occurs in energetic coastal marine environments may result in physical abrasion and removal of some macroinvertebrates, offsetting the positive effects that kelps can have on macroinvertebrate recruitment (Connell, 2003).
There is limited evidence to suggest whether marine mammals and other megafauna will avoid or be attracted to cultivation activities and any responses are likely to be location- and species-specific. The consequence of displacement effects from cultivated area will depend on the relative importance of that habitat for foraging and migration and breeding (Markowitz et al., 2004). Avoidance of poorly sited operations may interfere with and restrict normal migration routes leading to ‘barrier effects.’ Conversely, cultivation activities may enhance foraging opportunities for some species. Larger transient megafauna including adult female bottlenose dolphins (Tursiops sp.) in Shark Bay Western Australia, avoid shellfish culture longlines (Watsoncapps and Mann, 2005), and Dusky dolphins (Lagenorhynchus obscurus) in New Zealand generally avoid areas occupied by longline structures (Markowitz et al., 2004). This is most probably due to the lines and buoys restricting the normal movement of schooling fish and making it difficult for the dolphins to carry out fish aggregation maneuvers (Wiirsig and Gailey, 2002). Thus it is possible that there will be some exclusion of cetaceans from large-scale macroalgal farms. In contrast, common seals are observed around mussel long lines (Roycroft et al., 2004) and the diet of young common seals can include crustacea and fish (Anderson, 1990), which are known to occupy macroalgal habitats. In most jurisdictions, marine mammals are protected and there is a statutory responsibility to consider interactions when planning marine developments. Little is known about the interactions of marine mammals with large-scale macroalgal farms but, given their potential to attract fish, these may present marine mammals with foraging opportunities.
It is likely that many bird species would benefit from increased foraging opportunities around kelp farms and research will be required to understand this interaction and to optimize management practices with respect to birds. However, in contrast to both finfish farms (Nemtzov and Olsvig-Whittaker, 2003) and shellfish farms (Zydelis et al., 2008) where birds may be a nuisance, kelp farms are unlikely to be negatively impacted by birds and it is probable that they would become useful habitat for several species by providing foraging opportunities.
In a scenario where seaweed cultivation becomes widespread, it will be necessary to characterize the artificial habitats created in order to assess the extent of positive ecosystem services whilst identifying negative interactions with associated species to determine suitable mitigation measures.
Key Gaps and Recommendations
Defining Scale
The sustainable development of seaweed farming in Europe will depend on the magnitude of potential environmental impacts, the scale of farm operations, and the sensitivity of the receiving environment (receptors) which will require site specific consideration (e.g., prevailing environmental conditions, habitats and other features of the surrounding environment).
The scale of the cultivation activities within a particular area has important implications for the magnitude of environmental changes and potentially the consequences of such changes on the receiving environment. This review had adopted terminology from the Scottish government’s seaweed cultivation policy document which identifies two scales of commercial seaweed cultivation (Marine Scotland, 2017): small-medium (0–50 × 200 m lines) and large (>50 × 200 m lines). At present, these scales are rather arbitrary with significant variation between cultivation site size and infrastructure. For example, when giving cultivation licenses the Norwegian government have recently practiced a limit of 10 hectares, above which additional requirements for environmental monitoring are required. The 10 ha limit is similar to the maximum extent of small-medium scale assuming 10 m of separation between growing lines. It is likely that as more information regarding the scale dependent environmental changes become available a clearer definition of policies linked to scale will be possible.
The variation in cultivation practices currently employed to grow seaweed in Europe make direct comparisons between sites difficult. However, the relative simplicity of the cultivation process means that attempts to correlate scale dependent environmental changes could be made against a few key features of a cultivation project. Equation 1 gives a simplified explanation for the relationship between the overall seaweed harvest removed from a site (site production), key features of the site and the growing system used. Thus allowing comparison between sites utilizing different cultivation practices.
Equation 1. Yield = the average weight of seaweed produced per linear meter of growing line, and lines are defined as the structures attached to the mooring system which may be used directly to grow seaweed or support other structures (e.g., nets) to grow seaweed. Stocking density = the amount of growing line per m2 of cultivation area. Area = the total cultivation area used in a growing season and should include the full extent of surface and/or sub-surface structures.
It is likely that negative and/or positive environmental changes may be correlated with one or more of the variables expressed in equation 1. Future studies must work to reduce any negative environmental changes whilst maximizing economies from cultivation sites. Standardizing principle features of cultivation practices in this way may be beneficial, allowing for comparisons to be made between studies at different sites. Whilst a better understanding of the relationship between these variables can be used to determine the most economic and sustainable growing systems (e.g., determining the optimum stocking density for growth of a target species with well-defined environmental conditions).
Prioritizing Key Knowledge Gaps
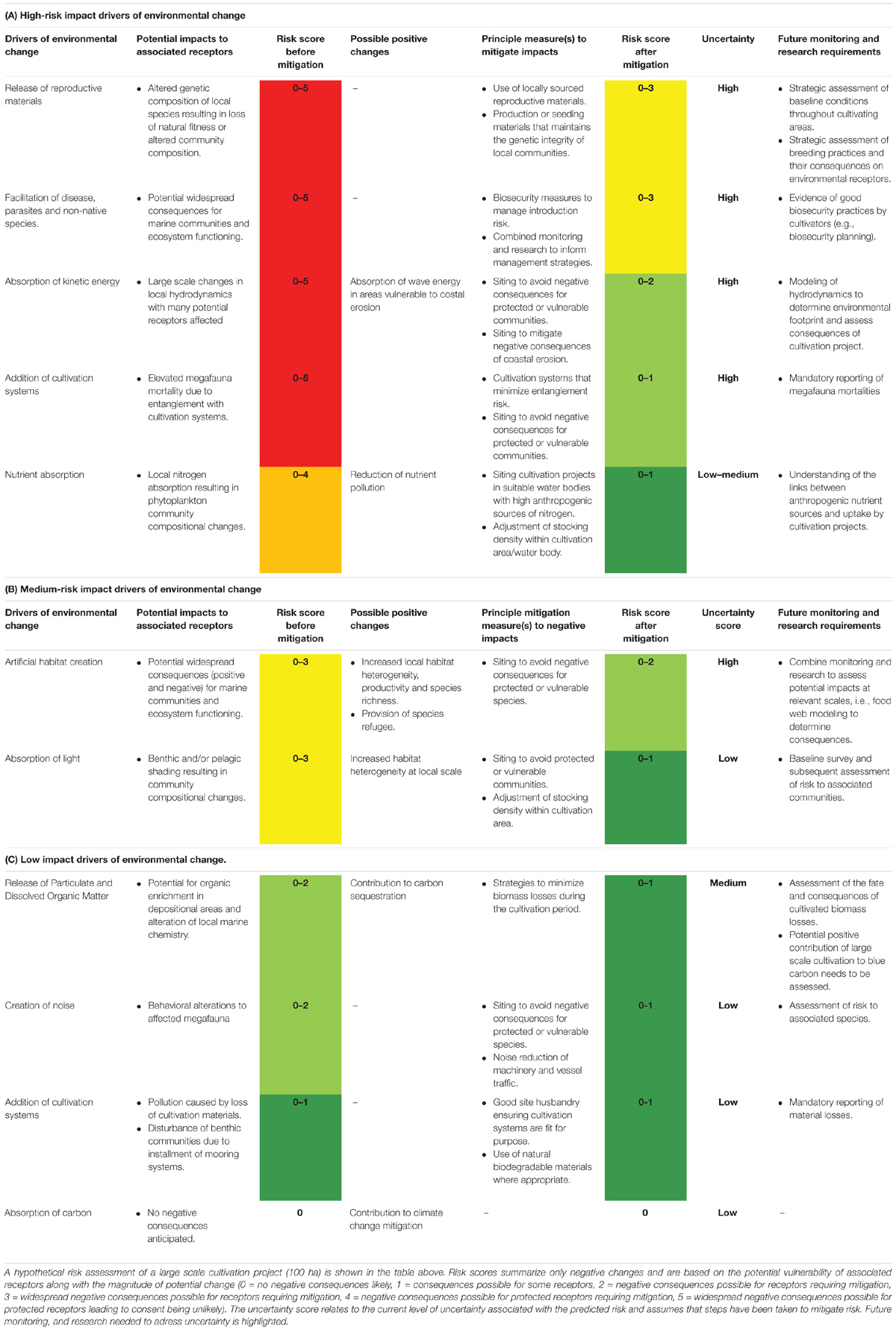
Prioritizing research and monitoring objectives will facilitate the effective allocation of resources and with planning, support future managers and decision makers. Undertaking a qualitative assessment of anticipated environmental changes with respect to scale, legislative obligations, and standard environmental practices is challenging due to incomplete knowledge of the wider ecological systems that future seaweed cultivation projects are deployed. This has led to a high level of uncertainty for many impact pathways producing conservative upper limits for anticipated risk. It has, however, been possible to identify several important environmental risks based on this systematic review. These risks can be considered as either high, medium or low before mitigation options are in place. Those that have been identified as high risk include: genetic depression of natural algal populations, facilitation of algal diseases, changes to the physical environment through alteration of hydrodynamic regimes, entanglement of mega-fauna, and depletion of natural nitrogen pools in enclosed water bodies. Impact pathways which are identified as medium risk include: artificial habitat creation and absorption of light. Finally, those impact pathways that are considered here to be low risk include: release of particulate and dissolved organic matter, creation of noise, and pollution and disturbance caused by the addition of cultivation structures. A hypothetical risk assessment for each of the identified impact pathways in this review is presented in Table 1. This serves as a qualitative assessment to identify principle concerns and should be updated as new data becomes available. Risk assesment relating to specific cultivation activities must be assessed on a case-by-case basis. As the presence of receptors is not known in this hypothetical example it is assumed that both sensitive and non-sensitive receptors are present giving a range of results. Furthermore, assumed magnitude of change is based on the possible geographical and temporal extent of changes.
After mitigation, only genetic depression of natural algal populations and facilitation of algal diseases are considered to have the potential for widespread negative consequences for receptors requiring further mitigation. If compared to large-scale seaweed cultivation in countries such as; China, Indonesia, Philippines, and South Korea, the risks identified here are in line with the challenges limiting growth of the sector in the rest of the global industry (Cottier-Cook et al., 2016). Despite these challenges the environmental consequences of large-scale seaweed cultivation in these regions has remained relatively benign when compared to other form of aquaculture and can even offset risks by providing positive ecosystem services (Kim et al., 2017). Life Cycle Analysis of the environmental demands of global aquaculture systems revealed seaweed and mussel cultivation result in fewer demands on the environment per unit production than other form of aquaculture (Hall et al., 2011). Suspended shellfish cultivation is well-established and is managed across Europe with few environmental risks. If a European industry takes into account of the challenges currently being faced elsewhere in the global seaweed industry, and establishes monitoring and management systems to prevent the same problems from occurring, then seaweed cultivation could offer a low risk addition to the growth of the blue economy.
For most impact pathways good site selection to avoid sensitive areas, farm design and farm management are important considerations in the mitigation of risk. Furthermore, monitoring by growers may be undertaken for some impact pathways through the mandatory reporting of issues encountered within cultivation sites (e.g., entanglement events or infrastructure loss) to establish whether there is indeed a cause for concern. Furthermore, biosecurity planning for controlling the prevalence of disease and non-native species could also be considered standard mitigation practice.
Predicting scale-dependent environmental changes to habitats both within the farm and surrounding areas should be given careful consideration. Discerning which environmental changes are effects (encompassing both positive and negative) and which should be considered as significant impacts will require more investigation ensuring that complex interactions are resolved through focused research efforts spanning a range of geographical locations. Separate to this, many of the monitoring options available to growers and environmental managers center around ecosystem monitoring and it is important to consider what components should be monitored and why. For example, if maintaining the composition and abundance of existing benthic communities is considered important, what metrics should we then use to describe change, what scale is important when considering change, and what is an acceptable level of change all need to be clearly defined.
For many impact pathways the siting of cultivation sites in areas which minimize risk to sensitive marine features will be a critical step in minimizing the overall environmental cost, if any, of the proposed growing site (Table 1C). Many impact pathways created by the absorption of nutrients (namely nitrogen) or through alteration of the hydrodynamic conditions can be modeled to select areas that promote the absorption of anthropogenic sources of nitrogen whilst selecting productive sites for cultivation (Table 1A). The development of models used to determine the ‘carrying capacity’ of coastal areas will allow for the minimization of negative environmental interactions whilst supporting the industry to develop successful cultivation projects (Seghetta et al., 2016b).
Recommendations for Future Monitoring
Each new cultivation site will have a responsibility to demonstrate that existing conservation objectives will not be undermined and any potential environmental risks identified at the consenting stage are kept within acceptable limits throughout the lifetime of the project. Seaweed cultivation offers the potential for positive ecosystem services if managed correctly (e.g., bioextraction of nutrients at the water body level), but the net effect on the surrounding ecosystem could be negative where risks that have been identified in this review are not monitored appropriately. It will be necessary for governing bodies to agree on levels of environmental change that should trigger different management options (i.e., mitigation). Targeted monitoring programs can then be designed to understand the likelihood that thresholds have been exceeded with a known degree of scientific certainty. This avoids collecting monitoring data to assess the significance of changes against a null hypothesis of no-change. This later approach is highly limited as even a well-designed monitoring survey which details a statistically significant change tells us very little about whether to interoperate the result as a ‘significant impact’ requiring management. Justified thresholds are necessary to facilitate effective decision-making when managing important marine resources (Wilding et al., 2017). Conversely, setting monitoring obligations that are poorly defined and without first agreeing, where possible, levels of acceptable environmental charge will facilitate the production of ‘data-rich, information-poor’ (DRIP) data (Wilding et al., 2017). This situation has been observed in other marine industries where current monitoring programs are extensive and costly yet many provide little useful data in relation to ecosystem-scale changes necessary for the assessment of ‘impact’ for new projects (Wilding et al., 2017). It is recommended that when considering the appropriateness of a monitoring program to achieve a certain goal that a decision making process similar to Figure 3 be used. If the effort/cost of a monitoring program required to assess a change is not feasible then it is suggested that the monitoring program will result in inconclusive data as a result of limited resources. In the context of seaweed cultivation, the consideration of thresholds is extremely important given the complexity of a number of environmental changes many of which could be considered positive.

Figure 3. A recommended example of the decision making process to a rationalized monitoring program with identifiable thresholds with agreed spatial and temporal domains [reprinted and adapted from Wilding et al. (2017) with permission from Elsevier].
Collaborative research with developers with emphasis on initial data collection, focusing, among other things, on high priority impact pathways is recommended. Much of this research may be regarded as monitoring (e.g., describing the genetic characteristics of natural populations as well as the prevalence of disease). However, if undertaken as a strategic collaboration between stakeholders, similar to research, it may benefit the industry as a whole and should focus initially at providing a robust description of baseline conditions in key growing areas to support future monitoring and decision making.
Following this review, current small-medium scale monitoring activities undertaken by developers as part of their consent agreement are only necessary where specific issues have been identified such as site specific features which may be sensitive to the activity. Large-scale projects will likely require additional monitoring to be undertaken and these must be informed by improved definition of the magnitude of environmental changes along with the severity of that change. Agreeing acceptable limits of change where possible will be necessary to design robust monitoring procedures, especially given a number of site specific ‘positive’ and ‘negative’ changes are likely to occur simultaneously in cultivation areas.
Conclusion
Seaweed cultivation if properly managed can provide ecosystem services whilst developing marine resources currently underexploited throughout Europe. Current cultivation sites are typically small-medium scale and if located and managed with consideration to environmental receptors pose a low risk to the receiving environment and associated features. However, an expansion of the industry necessitates a more complete understanding of the scale dependent changes in order to fully assess and manage risk. Targeted research and monitoring is therefore required to address knowledge gaps and facilitate informed decision making during consenting of larger projects. The production of biologically coupled hydrodynamic models to support the assessment of risk, understand carrying capacity of water bodies and select suitable sites which minimize negative environmental changes should be considered a top priority in cultivating countries.
General recommendations for standard monitoring include a focus on ensuring farm management is fit for purpose and an understanding of the baseline conditions, most notably natural population genetic diversity and algae disease prevalence is in place. Mandatory monitoring imposed at cultivation sites should be justifiable and based on an assessment of risk specific to the site characteristics and the operation. In time, a complete set of regulations for seaweed farming will likely be developed and implemented. This may include several different types of regulation managing features such as the genetic characteristics of cultivated crops, farm design, size, stocking density and materials used.
Author Contributions
IC and AM contributed equally to the conception and design of the review, and co-wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
The authors would like to acknowledge the Macrofuels project. This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 654010. BIOFEED Novel salmon feed by integrated bioprocessing of non-food biomass (the Research Council of Norway; Grant No. 239003/O30) and Foods of Norway, a Centre for Research-based Innovation (the Research Council of Norway; Grant No. 237841/030). Global Challenge Research Fund (Biotechnology and Biological Sciences Research Council; Grant No. BB/P027806/1).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdullah, M. I., and Fredriksen, S. (2004). Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway. J. Mar. Biol. Assoc. UK 84, 887–894. doi: 10.1017/S002531540401015Xh
Aldridge, J., van de Molen, J., and Forster, R. (2012). Wider Ecological Implications of Macroalgae Cultivation. London: The Crown Estate, 95.
Andersen, G. S., Steen, H., Christie, H., Fredriksen, S., and Moy, F. E. (2011). Seasonal patterns of sporophyte growth, fertility, fouling, and mortality of Saccharina latissima in skagerrak, norway: implications for forest recovery. J. Mar. Biol. 2011:690375. doi: 10.1155/2011/690375
Andersen, K. H., Mork, M., and Nilsen, J. E. O. (1996). Measurement of the velocity-profile in and above a forest of Laminaria hyperborea. Sarsia 81, 193–196. doi: 10.1080/00364827.1996.10413621
Anderson, D. M., Burkholder, J. M., Cochlan, W. P., Glibert, P. M., Gobler, C. J., Heil, C. A., et al. (2008). Harmful algal blooms and eutrophication: examining linkages from selected coastal regions of the United States. Harmful Algae 8, 39–53. doi: 10.1016/j.hal.2008.08.017
Anderson, D. M., Glibert, P. M., and Burkholder, J. M. (2002). Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25, 704–726. doi: 10.1007/BF02804901
Anderson, R. J., Carrick, P., Levitt, G. J., and Share, A. (1997). Holdfasts of adult kelp Ecklonia maxima provide refuges from grazing for recruitment of juvenile kelps. Mar. Ecol. Prog. Ser. 159, 265–273. doi: 10.3354/meps159265
Andrady, A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605. doi: 10.1016/j.marpolbul.2011.05.030
Assis, J., Lucas, A. V., Bárbara, I., and Serrão, E. Á. (2016). Future climate change is predicted to shift long-term persistence zones in the cold-temperate kelp Laminaria hyperborea. Mar. Environ. Res. 113, 174–182. doi: 10.1016/j.marenvres.2015.11.005
Azam, F., Fenchel, T., Field, J. G., Gray, J. S., Meyer-Reil, L. A., and Thingstad, F. (1983). The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263. doi: 10.3354/meps010257
Baker, A. R. (2003). Atmospheric deposition of nutrients to the Atlantic Ocean. Geophys. Res. Lett. 30:2296. doi: 10.1029/2003GL018518
Bauer, J. E., and Druffel, E. R. M. (1998). Ocean margins as a significant source of organic matter to the deep open ocean. Nature 392, 20–23. doi: 10.1038/33122
Benes, K. M., and Carpenter, R. C. (2015). Kelp canopy facilitates understory algal assemblage via competitive release during early stages of secondary succession. Ecology 96, 241–251. doi: 10.1890/14-0076.1
Benjamins, S., Harnois, V., Smith, H. C. M., Johanning, L., Greenhill, L., Carter, C., et al. (2014). Understanding the Potential for Marine Megafauna Entanglement Risk from Marine Renewable Energy Developments. Inverness: Scottish Natural Heritage.
Bixler, H., and Porse, H. (2011). A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 23, 321–335. doi: 10.1007/s10811-010-9529-3
Borchers, P., and Field, J. G. (1981). The effect of kelp shading on phytoplankton production. Bot. Mar. 24, 89–92. doi: 10.1515/botm.1981.24.2.89
Borines, M. G., McHenry, M. P., and de Leon, R. L. (2011). Integrated macroalgae production for sustainable bioethanol, aquaculture and agriculture in Pacific island nations. Biofuel. Bioprod. Biorefin. 5, 599–608. doi: 10.1002/bbb.310
Bostock, J., Lane, A., Hough, C., and Yamamoto, K. (2016). An assessment of the economic contribution of EU aquaculture production and the influence of policies for its sustainable development. Aquac. Int. 24, 699–733. doi: 10.1007/s10499-016-9992-1
Broch, O. J., and Slagstad, D. (2011). Modelling seasonal growth and composition of the kelp Saccharina latissima. J. Appl. Phycol. 24, 759–776. doi: 10.1007/s10811-011-9695-y
Bruton, T., Lyons, H., Lerat, Y., Stanley, M. S., and Rasmussen, M. B. (2009). A Review of the potential of marine algae as a source of biofuel in Ireland. Dublin: Sustainable Energy Ireland.
Burrows, M. T. (2012). Influences of wave fetch, tidal flow and ocean colour on subtidal rocky communities. Mar. Ecol. Prog. Ser. 445, 193–207. doi: 10.3354/meps09422
Burrows, M. T., Hughes, A. D., Austin, W. E. N., Smeaton, C., Hicks, N., Howe, J. A., et al. (2017). Assessment of Blue Carbon Resources in Scotland’s Inshore Marine Protected Area Network. Scottish Natural Heritage Commissioned Report No. 957. Inverness.
Chamberlain, J. (2001). Impacts of biodeposits from suspended mussel (Mytilus edulis L.) culture on the surrounding surficial sediments. ICES J. Mar. Sci. 58, 411–416. doi: 10.1006/jmsc.2000.1037
Cheng, T. H. (1969). Production of kelp-a major aspect of China’s exploitation of the sea. Econ. Bot. 23, 215–236. doi: 10.1007/BF02860454
Christie, H., Norderhaug, K. M., and Fredriksen, S. (2009). Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 396, 221–233. doi: 10.3354/meps08351
Clark, R. P., Edwards, M. S., and Foster, M. S. (2004). Effects of shade from multiple kelp canopies on an understory algal assemblage. Mar. Ecol. Prog. Ser. 267, 107–119. doi: 10.3354/meps267107
Clasen, J. L., and Shurin, J. B. (2015). Kelp forest size alters microbial community structure and function on Vancouver Island, Canada. Ecology 96, 862–872. doi: 10.1890/13-2147.1
Connell, S. D. (2003). Negative effects overpower the positive of kelp to exclude invertebrates from the understorey community. Oecologia 137, 97–103. doi: 10.1007/s00442-003-1312-6
Cook, E. J., Payne, R., and Macleod, A. (2014). Marine Biosecurity Planning-Identification of Best Practice: A Review. Scotland: Scottish Natural Heritage.
Cottier-Cook, E. J., Nagabhatla, N., Badis, Y., Campbell, M. L., Chopin, T., Dai, W., et al. (2016). Safeguarding the Future of the Global Seaweed Aquaculture Industry. United Nations University (INWEH) and Scottish Association for Marine Science Policy Brief. Available at: http://inweh.unu.edu/safeguarding-the-future-of-the-global-seaweed-aquaculture-industry-policy-brief/
Cromey, C. J., Nickell, T. D., and Black, K. D. (2002). DEPOMOD- modelling the deposition and biological effects of waste solids from marine cage farms. Aquaculture 214, 211–239. doi: 10.1016/S0044-8486(02)00368-X
Cromey, C. J., Thetmeyer, H., Lampadariou, N., Black, K. D., Kögeler, J., and Karakassis, I. (2012). MERAMOD: predicting the deposition and benthic impact of aquaculture in the eastern Mediterranean Sea. Aquac. Environ. Interact. 2, 157–176. doi: 10.3354/aei00034
Davenport, J. C., Black, K., Burnell, G., Cross, T., Culloty, S., Ekaratne, S., et al. (2003). Aquaculture: the Ecological Issues. Hoboken, NJ: John Wiley & Sons.
Dayton, P. K. (1985). Ecology of kelp communities. Annu. Rev. Ecol. Syst. 16, 215–245. doi: 10.1146/annurev.es.16.110185.001243
Dayton, P. K., and Tegner, M. J. (1984). “The importance of scale in community ecology: a kelp forest example with terrestrial analogs,” in A New Ecology: Novel Approaches to Interactive Systems, eds P. W. Price, C. N. Slobodchikoff, and W. S. Gaud (New York, NY: Wiley), 457–481.
De Robertis, A., and Handegard, N. O. (2013). Fish avoidance of research vessels and the efficacy of noise-reduced vessels: a review. ICES J. Mar. Sci. 70, 34–45. doi: 10.1093/icesjms/fss155
Dempster, T., Sanchez-Jerez, P., Fernandez-Jover, D., Bayle-Sempere, J., Nilsen, R., Bjørn, P. A., et al. (2011). Proxy measures of fitness suggest coastal fish farms can act as population sources and not ecological traps for wild gadoid fish. PLoS One 6:e15646. doi: 10.1371/journal.pone.0015646
Dempster, T., Uglem, I., Sanchez-Jerez, P., Fernandez-Jover, D., Bayle-Sempere, J., Nilsen, R., et al. (2009). Coastal salmon farms attract large and persistent aggregations of wild fish: an ecosystem effect. Mar. Ecol. Prog. Ser. 385, 1–14. doi: 10.3354/meps08050
Derraik, J. G. (2002). The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 44, 842–852. doi: 10.1016/S0025-326X(02)00220-5
Duarte, C. M., Wu, J., Xiao, X., Bruhn, A., and Krause-Jensen, D. (2017). Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 4:100. doi: 10.3389/fmars.2017.00100
Duggins, D. O., Eckman, J. E., and Sewell, A. T. (1990). Ecology of understory kelp environments. II. Effects of kelps on recruitment of benthic invertebrates. J. Exp. Mar. Biol. Ecol. 143, 27–45. doi: 10.1016/0022-0981(90)90109-P
Edwards, M., and Watson, L. (2011). Aquaculture Explained. No. 26 Cultivating Laminaria Digitata. Dublin: Irish Sea Fisheries Board, 26.
Fan, X., Wei, H., Yuan, Y., and Zhao, L. (2009). Vertical structure of tidal current in a typically coastal raft-culture area. Cont. Shelf Res. 29, 2345–2357. doi: 10.1016/j.csr.2009.10.007
Fankboner, P. V., and de Burgh, M. E. (1977). Diurnal exudation of C-14 labelled compounds by the large kelp Macrocystis integrifolia Bory. J. Exp. Mar. Biol. Ecol. 28, 151–162. doi: 10.1016/0022-0981(77)90114-9
FAO (2004). Cultured Aquatic Species Information Programme. Laminaria japonica. Rome: Food and Agriculture Organization of the United Nations.
Fasahati, P., Saffron, C. M., Woo, H. C., and Liu, J. J. (2017). Potential of brown algae for sustainable electricity production through anaerobic digestion. Energy Convers. Manag. 135, 297–307. doi: 10.1016/j.enconman.2016.12.084
Fletcher, R. L., and Farrell, P. (1999). Introduced brown algae in the North East Atlantic, with particular respect to Undaria pinnatifida (Harvey) Suringar. Helgolander Meeresuntersuchungen 52, 259–275. doi: 10.1007/BF02908901
Flukes, E. B., Johnson, C. R., and Wright, J. T. (2014). Thinning of kelp canopy modifies understory assemblages: the importance of canopy density. Mar. Ecol. Prog. Ser. 514, 57–70. doi: 10.3354/meps10964
Fong, P., Donohoe, R. M., and Zedler, J. B. (1993). Competition with macroalgae and benthic cyanobacterial mats limits phytoplankton abundance in experimental microcosms. Mar. Ecol. Prog. Ser. 100, 97–102. doi: 10.3354/meps100097
Gachon, C. M., Sime-Ngando, T., Strittmatter, M., Chambouvet, A., and Kim, G. H. (2010). Algal diseases: spotlight on a black box. Trends Plant Sci. 15, 633–640. doi: 10.1016/j.tplants.2010.08.005
Gerard, V. A. (1984). The light environment in a giant kelp forest: influence of Macrocystis pyrifera on spatial and temporal variability. Mar. Biol. 84, 189–195. doi: 10.1007/BF00393004
Glasby, T. M., Connell, S. D., Holloway, M. G., and Hewitt, C. L. (2007). Nonindigenous biota on artificial structures: could habitat creation facilitate biological invasions? Mar. Biol. 151, 887–895. doi: 10.1007/s00227-006-0552-5
Grant, J., and Bacher, C. (2001). A numerical model of flow modification induced by suspended aquaculture in a Chinese bay. Can. J. Fish. Aquat. Sci. 58, 1003–1011. doi: 10.1139/f01-027
Hall, S. J., Delaporte, A., Phillips, M. J., Beveridge, M., and O’Keefe, M. (2011). Blue Frontiers: Managing the Environmental Costs of Aquaculture. Penang: The WorldFish Center.
Halling, C., Wikström, S. A., Lilliesköld-Sjöö, G., Mörk, E., Lundsør, E., and Zuccarello, G. C. (2013). Introduction of Asian strains and low genetic variation in farmed seaweeds: indications for new management practices. J. Appl. Phycol. 25, 89–95. doi: 10.1007/s10811-012-9842-0
Hamoutene, D., Salvo, F., Bungay, T., Mabrouk, G., Couturier, C., Ratsimandresy, A., et al. (2015). Assessment of finfish aquaculture effect on newfoundland epibenthic communities through video monitoring. N. Am. J. Aquac. 77, 117–127. doi: 10.1080/15222055.2014.976681
Handa, A., Forbord, S., Wang, X., Jacob, O., Wiborg, S., Røvik, T., et al. (2013). Seasonal- and depth-dependent growth of cultivated kelp (Saccharina latissima) in close proximity to salmon (Salmo salar) aquaculture in Norway. Aquaculture 414–415, 191–201. doi: 10.1016/j.aquaculture.2013.08.006
Hansen, P. K., Ervik, A., Schaanning, M., Johannessen, P., Aure, J., Jahnsen, T., et al. (2001). Regulating the local environmental impact of intensive marine fish farming II. The monitoring programme of the MOM system (Modelling-Ongrowing fish farms-Monitoring). Aquaculture 194, 75–92. doi: 10.1016/S0044-8486(97)00186-5
Harrold, C., Light, K., and Lisin, S. (1998). Organic enrichment of submarine-canyon and continental-shelf benthic communities by macroalgal drift imported from nearshore kelp forests. Limnol. Oceanogr. 43, 669–678. doi: 10.4319/lo.1998.43.4.0669
Heinrich, S., Valentin, K., Frickenhaus, S., John, U., and Wiencke, C. (2012). Transcriptomic analysis of acclimation to temperature and light stress in Saccharina latissima (Phaeophyceae). PLoS One 7:e44342. doi: 10.1371/journal.pone.0044342
Heisler, J., Glibert, P. M., Burkholder, J. M., Anderson, D. M., Cochlan, W., Dennison, W. C., et al. (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8, 3–13. doi: 10.1016/j.hal.2008.08.006
Hepburn, C. D., and Hurd, C. L. (2005). Conditional mutualism between the giant kelp Macrocystis pyrifera and colonial epifauna. Mar. Ecol. Prog. Ser. 302, 37–48. doi: 10.3354/meps302037
Holmer, M., Wildish, D., and Hargrave, B. (2005). Organic enrichment from marine finfish aquaculture and effects on sediment biogeochemical processes. Environ. Effect. Mar. Finfish Aquac. 5, 181–206. doi: 10.1007/b136010
Hughes, A. D., Black, K. D., Campbell, I., Davidson, K., Kelly, M. S., and Stanley, M. S. (2012a). Does seaweed offer a solution for bioenergy with biological carbon capture and storage? Greenhouse Gases Sci. Technol. 2, 402–407. doi: 10.1002/ghg
Hughes, A. D., Kelly, M. S., Black, K. D., and Stanley, M. S. (2012b). Biogas from Macroalgae: is it time to revisit the idea? Biotechnol. Biofuels 5:86. doi: 10.1186/1754-6834-5-86
Hulatt, C. J., Thomas, D. N., Bowers, D. G., Norman, L., and Zhang, C. (2009). Exudation and decomposition of Chromophoric Dissolved Organic Matter (CDOM) from some temperate macroalgae. Estuar. Coast. Shelf Sci. 84, 147–153. doi: 10.1016/j.ecss.2009.06.014
Jackson, G. A., and Winant, C. D. (1983). Effect of a kelp forest on coastal currents. Cont. Shelf Res. 2, 75–80. doi: 10.1016/0278-4343(83)90023-7
Jeong, J. H., Jin, H. J., Sohn, C. H., Suh, K. H., and Hong, Y. K. (2000). Algicidal activity of the seaweed Corallina pilulifera against red tide microalgae. J. Appl. Phycol. 12, 37–43. doi: 10.1023/A:1008139129057
Jickells, T. D. (1998). Nutrient biogeochemistry of the coastal zone. Science 281, 217–223. doi: 10.1126/science.281.5374.217
Kerrison, P. D., Stanley, M. S., Edwards, M. D., Black, K. D., and Hughes, A. D. (2015). The cultivation of European kelp for bioenergy: site and species selection. Biomass Bioenergy 80, 229–242. doi: 10.1016/j.biombioe.2015.04.035
Khailov, K. M., and Burlakova, Z. P. (1969). Release of dissolved organic matter by marine seaweeds and distribution of their total organic production to inshore communities. Limnol. Oceanogr. 14, 521–527. doi: 10.4319/lo.1969.14.4.0521
Kim, G. H., Moon, K.-H., Kim, J.-Y., Shim, J., and Klochkova, T. A. (2014). A revaluation of algal diseases in Korean Pyropia (Porphyra) sea farms and their economic impact. Algae 29, 249–265. doi: 10.4490/algae.2014.29.4.249
Kim, J. K., Yarish, C., Hwang, E. K., Park, M., and Kim, Y. (2017). Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. Algae 32, 1–13. doi: 10.4490/algae.2017.32.3.3
Kirkwood, J. K., Bennett, P. M., Jepson, P. D., Kuiken, T., Simpson, V. R., and Baker, J. R. (1997). Entanglement in fishing gear and other causes of death in cetaceans stranded on the coasts of England and Wales. Vet. Rec. 141, 94–98. doi: 10.1136/vr.141.4.94
Kraan, S. (2010). Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 18, 27–46. doi: 10.1007/s11027-010-9275-5
Kraan, S. (2017). Undaria marching on; late arrival in the Republic of Ireland. J. Appl. Phycol. 29, 1107–1114. doi: 10.1007/s10811-016-0985-2
Krause-Jensen, D., and Duarte, C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 9, 737–742. doi: 10.1038/ngeo2790
Leclerc, J. C., Riera, P., Leroux, C., Lévêque, L., and Davoult, D. (2013). Temporal variation in organic matter supply in kelp forests: linking structure to trophic functioning. Mar. Ecol. Prog. Ser. 494, 87–105. doi: 10.3354/meps10564
Leonardi, P. I., Miravalles, A. B., Faugeron, S., Flores, V., Beltrán, J., and Correa, J. A. (2006). Diversity, phenomenology and epidemiology of epiphytism in farmed Gracilaria chilensis (Rhodophyta) in northern Chile. Eur. J. Phycol. 41, 247–257. doi: 10.1080/09670260600645659
Li, X., Cong, Y., Yang, G., Shi, Y., Qu, S., Li, Z., et al. (2007). Trait evaluation and trial cultivation of Dongfang No. 2, the hybrid of a male gametophyte clone of Laminaria longissima (Laminariales, Phaeophyta) and a female one of L. japonica. J. Appl. Phycol. 19, 139–151. doi: 10.1007/s10811-006-9120-0
Li, X., Liu, J., Cong, Y., Qu, S., Zhang, Z., Dai, H., et al. (2008). Breeding and trial cultivation of Dongfang No. 3, a hybrid of Laminaria gametophyte clones with a more than intraspecific but less than interspecific relationship. Aquaculture 280, 76–80. doi: 10.1016/j.aquaculture.2008.05.005
Li, X., Zhang, Z., Qu, S., Liang, G., Sun, J., Zhao, N., et al. (2016). Improving seedless kelp (Saccharina japonica) during its domestication by hybridizing gametophytes and seedling-raising from sporophytes. Sci. Rep. 6:21255. doi: 10.1038/srep21255
Liu, F., Sun, X., Wang, F., Wang, W., Liang, Z., Lin, Z., et al. (2014). Breeding, economic traits evaluation, and commercial cultivation of a new Saccharina variety “Huangguan No. 1.”. Aquac. Int. 22, 1665–1675. doi: 10.1007/s10499-014-9772-8
Loureiro, R., Gachon, C. M. M., and Rebours, C. (2015). Seaweed cultivation: potential and challenges of crop domestication at an unprecedented pace. New Phytol. 206, 489–492. doi: 10.1111/nph.13278
Lüning, K., and Pang, S. (2003). Mass cultivation of seaweeds: current aspects and approaches. J. Appl. Phycol. 15, 115–119. doi: 10.1023/A:1023807503255
Marine Scotland (2017). Seaweed Cultivation Policy Statement. Available at: https://www.gov.scot/publications/seaweed-cultivation-policy-statement-2017/
Marinho, G. S., Holdt, S. L., Birkeland, M. J., and Angelidaki, I. (2015). Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. J. Appl. Phycol. 27, 1963–1973. doi: 10.1007/s10811-014-0519-8
Markowitz, T. M., Harlin, A. D., Würsig, B., and McFadden, C. J. (2004). Dusky dolphin foraging habitat: overlap with aquaculture in New Zealand. Aquat. Conserv. Mar. Freshw. Ecosyst. 14, 133–149. doi: 10.1002/aqc.602
Mayor, D. J., Zuur, A. F., Solan, M., Paton, G. I., and Killham, K. E. N. (2010). Factors affecting benthic impacts at scottish fish farms. Environ. Sci. Technol. 44, 2079–2084. doi: 10.1021/es903073h
Mineur, F., Cook, E. J., Minchin, D., Bohn, K., MacLeod, A., and Maggs, C. A. (2012). Changing coasts: marine aliens and artificial structures. Oceanogr. Mar. Biol. 50, 189–234. doi: 10.1201/b12157-5
Morel, A. (1978). Available, usable, and stored radiant energy in relation to marine photosynthesis. Deep Sea Res. 25, 673–688. doi: 10.1016/0146-6291(78)90623-9
Nan, C., Zhang, H., Lin, S., Zhao, G., and Liu, X. (2008). Allelopathic effects of Ulva lactuca on selected species of harmful bloom-forming microalgae in laboratory cultures. Aquat. Bot. 89, 9–15. doi: 10.1016/j.aquabot.2008.01.005
Nan, C., Zhang, H., and Zhao, G. (2004). Allelopathic interactions between the macroalga Ulva pertusa and eight microalgal species. J. Sea Res. 52, 259–268. doi: 10.1016/j.seares.2004.04.001
Naylor, R. L., Williams, S. L., and Strong, D. R. (2001). Ecology. Aquaculture–a gateway for exotic species. Science 294, 1655–1656. doi: 10.1126/science.1064875
Nemtzov, S. C., and Olsvig-Whittaker, L. (2003). The use of netting over fishponds as a hazard to waterbirds. Waterbirds 26, 416–423. doi: 10.1675/1524-4695(2003)026[0416:TUONOF]2.0.CO;2
Neushul, M., Benson, J., Harger, B. W. W., and Charters, A. C. (1992). Macroalgal farming in the sea: water motion and nitrate uptake. J. Appl. Phycol. 4, 255–265. doi: 10.1007/BF02161211
Norderhaug, K. M., and Christie, H. (2011). Secondary production in a Laminaria hyperborea kelp forest and variation according to wave exposure. Estuar. Coast. Shelf Sci. 95, 135–144. doi: 10.1016/j.ecss.2011.08.028
Norderhaug, K. M., Christie, H., Fossô, J. H. O., and Fredriksen, S. P. (2005). Fish - macrofauna interactions in a kelp (Laminaria hyperborea) forest. J. Mar. Biol. Assoc. UK 85, 1279–1286. doi: 10.1017/S0025315405012439
Ojeda, F. P., and Santelices, B. (1984). Invertebrate communities in holdfasts of the kelp Macrocystis pyrifera from southern Chile. Mar. Ecol. Prog. Ser. Oldend 16, 65–73. doi: 10.3354/meps016065
Paerl, H. W. (1995). Coastal eutrophication in relation to atmospheric nitrogen deposition: current perspectives. Ophelia 41, 237–259. doi: 10.1080/00785236.1995.10422046
Pelletier, N., Tyedmers, P., Sonesson, U., Scholz, A., Ziegler, F., Flysjo, A., et al. (2009). Not all salmon are created equal: life cycle assessment (LCA) of global salmon farming systems. Environ. Sci. Technol. 43, 8730–8736. doi: 10.1021/es9010114
Peteiro, C., and Freire, Ó. (2009). Effect of outplanting time on commercial cultivation of kelp Laminaria saccharina at the southern limit in the Atlantic coast, N.W. Spain. Chin. J. Oceanol. Limnol. 27, 54–60. doi: 10.1007/s00343-009-0054-7
Peteiro, C., and Freire, Ó. (2011). Offshore cultivation methods affects blade features of the edible seaweed Saccharina latissima in a Bay of Galicia, northwest Spain. Russ. J. Mar. Biol. 37, 319–323. doi: 10.1134/S1063074011040110
Peteiro, C., and Freire, Ó. (2012). Observations on fish grazing of the cultured kelps Undaria pinnatifida and Saccharina latissima (Phaeophyceae, Laminariales) in Spanish Atlantic waters. AACL Bioflux 5, 189–196.
Peteiro, C., and Freire, Ó. (2013a). Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. J. Appl. Phycol. 25, 205–213. doi: 10.1007/s10811-012-9854-9
Peteiro, C., and Freire, Ó. (2013b). Epiphytism on blades of the edible kelps Undaria pinnatifida and Saccharina latissima farmed under different abiotic conditions. J. World Aquac. Soc. 44, 706–715. doi: 10.1111/jwas.12065
Peteiro, C., Sánchez, N., and Martínez, B. (2016). Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe?: an overview. Algal Res. 15, 9–23. doi: 10.1016/j.algal.2016.01.012
Pimentel, D., McNair, S., Janecka, J., Wightman, J., Simmonds, C., O’connell, C., et al. (2001). Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 84, 1–20. doi: 10.1016/S0167-8809(00)00178-X
Platt, T., Sathyendranath, S., Caverhill, C., and Lewis, M. R. (1988). Ocean primary production and available light: further algorithms for remote sensing. Deep Sea Res. 35, 855–879. doi: 10.1016/0198-0149(88)90064-7
Prospero, J. M., Barrett, K., Church, T., Dentener, F., Duce, R. A., Galloway, J. N., et al. (1996). Atmospheric deposition of nutrients to the North Atlantic Basin. Biogeochemistry 35, 27–73. doi: 10.1007/BF02179824
Read, A. J., Drinker, P., and Northridge, S. (2006). Bycatch of marine mammals in U.S. and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Reed, D. C., and Foster, M. S. (1984). The effects of canopy shadings on algal recruitment and growth in a giant kelp forest. Ecology 65, 937–948. doi: 10.2307/1938066
Ren, L., Zhang, J., Fang, J., Tang, Q., Zhang, M., and Du, M. (2014). Impact of shellfish biodeposits and rotten seaweed on the sediments of Ailian Bay, China. Aquac. Int. 22, 811–819. doi: 10.1007/s10499-013-9709-7
Ried, J., Evans, P., and Northridge, S. (2003). Atlas of Cetacean Distribution in North-West European Waters. Peterborough: Joint Nature Conservation Committee.
Rolin, C., Inkster, R., Laing, J., and McEvoy, L. (2017). Regrowth and biofouling in two species of cultivated kelp in the Shetland Islands, UK. J. Appl. Phycol. 29, 2351–2361. doi: 10.1007/s10811-017-1092-8
Roycroft, D., Kelly, T. C., and Lewis, L. J. (2004). Birds, seals and the suspension culture of mussels in Bantry Bay, a non-seaduck area in Southwest Ireland. Estuar. Coast. Shelf Sci. 61, 703–712. doi: 10.1016/j.ecss.2004.07.012
Sanderson, J. C., Dring, M. J., Davidson, K., and Kelly, M. S. (2012). Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) C. E. Lane, C. Mayes, Druehl & G. W. Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 354–355, 128–135. doi: 10.1016/j.aquaculture.2012.03.019
Schaffelke, B., Smith, J. E., and Hewitt, C. L. (2006). Introduced Macroalgae- a growing concern. J. Appl. Phycol. 18, 529–541. doi: 10.1007/s10811-006-9074-2
Schiener, P., Black, K. D., Stanley, M. S., and Green, D. H. (2015). The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 27, 363–373. doi: 10.1007/s10811-014-0327-1
Seghetta, M., Hou, X., Bastianoni, S., Bjerre, A.-B., and Thomsen, M. (2016a). Life cycle assessment of macroalgal biorefinery for the production of ethanol, proteins and fertilizers – A step towards a regenerative bioeconomy. J. Clean. Prod. 137, 1158–1169. doi: 10.1016/j.jclepro.2016.07.195
Seghetta, M., Tørring, D., Bruhn, A., and Thomsen, M. (2016b). Bioextraction potential of seaweed in Denmark - An instrument for circular nutrient management. Sci. Total Environ. 563–564, 513–529. doi: 10.1016/j.scitotenv.2016.04.010
Sheavly, S. B., and Register, K. M. (2007). Marine debris & plastics: environmental concerns, sources, impacts and solutions. J. Polym. Environ. 15, 301–305. doi: 10.1007/s10924-007-0074-3
Shi, J., Wei, H., Zhao, L., Yuan, Y., Fang, J., and Zhang, J. (2011). A physical–biological coupled aquaculture model for a suspended aquaculture area of China. Aquaculture 318, 412–424. doi: 10.1016/j.aquaculture.2011.05.048
Shumway, S. E. (1990). A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquac. Soc. 21, 65–104. doi: 10.1111/j.1749-7345.1990.tb00529.x
Sieburth, J. M. (1969). Studies on algal substances in the sea. III. The production of extracellular organic matter by littoral marine algae. J. Exp. Mar. Biol. Ecol. 3, 290–309. doi: 10.1016/0022-0981(69)90052-5
Smit, A. J. (2004). Medicinal and pharmaceutical uses of seaweed natural products: a review. J. Appl. Phycol. 16, 245–262. doi: 10.1023/B:JAPH.0000047783.36600.ef
Smith, J. E., Hunter, C. L., Conklin, E. J., Most, R., Sauvage, T., Squair, C., et al. (2004). Ecology of the invasive red alga Gracilaria salicornia (Rhodophyta) on O’ahu, Hawai’i. Pac. Sci. 58, 325–343. doi: 10.1353/psc.2004.0023
Smith, J. E., Hunter, C. L., and Smith, C. M. (2002). Distribution and reproductive characteristics of nonindigenous and invasive marine algae in the Hawaiian Islands. Pac. Sci. 56, 229–315. doi: 10.1353/psc.2002.0030
Smith, V. H. (2003). Eutrophication of freshwater and coastal marine ecosystems- A global problem. Environ. Sci. Pollut. Res. 10, 126–139. doi: 10.1065/espr2002.12.142
Solidoro, C., Dejak, C., France, D., Pastres, R., and Pecenik, G. (1995). A model for macroalgae and phytoplankton growth in the Venice Lagoon. Environ. Int. 21, 619–626. doi: 10.1016/0160-4120(95)00080-5
Southall, B. L., Bowles, A. E., Ellison, W. T., Finneran, J. J., Gentry, R. L., Greene, C. R. Jr., et al. (2008). Marine mammal noise-exposure criteria: initial scientific recommendations. Bioacoustics 17, 273–275. doi: 10.1080/09524622.2008.9753846
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459. doi: 10.1017/S0376892902000322
Stentiford, G. D., Sritunyalucksana, K., Flegel, T. W., Williams, B. A., Withyachumnarnkul, B., Itsathitphaisarn, O., et al. (2017). New paradigms to help solve the global aquaculture disease crisis. PLoS Pathog. 13:e1006160. doi: 10.1371/journal.ppat.1006160
Tang, Q., Zhang, J., and Fang, J. (2011). Shellfish and seaweed mariculture increase atmospheric CO2 absorption by coastal ecosystems. Mar. Ecol. Prog. Ser. 424, 97–104. doi: 10.3354/meps08979
Vairappan, C. S., Chung, C. S., Hurtado, A. Q., Soya, F. E., Lhonneur, G. B., and Critchley, A. (2008). Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J. Appl. Phycol. 20, 477–483. doi: 10.1007/s10811-007-9299-8
Valero, M., Guillemin, M.-L., Destombe, C., Jacquemin, B., Gachon, C. M. M., Badis, Y., et al. (2017). Perspectives on domestication research for sustainable seaweed aquaculture. Perspect. Phycol. 4, 33–46. doi: 10.1127/pip/2017/0066
Wada, S., Aoki, M. N., Mikami, A., Komatsu, T., Tsuchiya, Y., Sato, T., et al. (2008). Bioavailability of macroalgal dissolved organic matter in seawater. Mar. Ecol. Prog. Ser. 370, 33–44. doi: 10.3354/meps07645
Wada, S., Aoki, M. N., Tsuchiya, Y., Sato, T., Shinagawa, H., and Hama, T. (2007). Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan. J. Exp. Mar. Biol. Ecol. 349, 344–358. doi: 10.1016/j.jembe.2007.05.024
Wada, S., and Hama, T. (2013). The contribution of macroalgae to the coastal dissolved organic matter pool. Estuar. Coast. Shelf Sci. 129, 77–85. doi: 10.1016/j.ecss.2013.06.007
Walls, A. M., Kennedy, R., Fitzgerald, R. D., Blight, A. J., Johnson, M. P., and Edwards, M. D. (2016). Potential novel habitat created by holdfasts from cultivated Laminaria digitata: assessing the macroinvertebrate assemblages. Aquac. Environ. Interact. 8, 157–169. doi: 10.3354/aei00170
Wargacki, A. J., Leonard, E., Win, M. N., Regitsky, D. D., Santos, C. N. S., Kim, P. B., et al. (2012). An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335, 308–313. doi: 10.1126/science.1214547
Watsoncapps, J., and Mann, J. (2005). The effects of aquaculture on bottlenose dolphin (Tursiops sp.) ranging in Shark Bay, Western Australia. Biol. Conserv. 124, 519–526. doi: 10.1016/j.biocon.2005.03.001
Weise, A. M., Cromey, C. J., Callier, M. D., Archambault, P., Chamberlain, J., and McKindsey, C. W. (2009). Shellfish-DEPOMOD: modelling the biodeposition from suspended shellfish aquaculture and assessing benthic effects. Aquaculture 288, 239–253. doi: 10.1016/j.aquaculture.2008.12.001
Wiirsig, B., and Gailey, G. A. (2002). “Marine mammals and aquaculture: conflicts and potential resolutions,” in Responsible Marine Aquaculture, eds R. R. Stickney and J. P. McVay (New York, NY: CAP International Press), 45–59.
Wilding, T. A., Gill, A. B., Boon, A., Sheehan, E., Dauvin, J.-C., Pezy, J.-P., et al. (2017). Turning off the DRIP (“Data-rich, information-poor”)–rationalising monitoring with a focus on marine renewable energy developments and the benthos. Renew. Sustain. Energy Rev. 74, 848–859. doi: 10.1016/j.rser.2017.03.013
Williams, S. L., and Smith, J. E. (2007). A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu. Rev. Ecol. Evol. Syst. 38, 327–359. doi: 10.1146/annurev.ecolsys.38.091206.095543
Wu, Z., Zhang, X., Lozano-Montes, H. M., and Loneragan, N. R. (2016). Trophic flows, kelp culture and fisheries in the marine ecosystem of an artificial reef zone in the Yellow Sea. Estuar. Coast. Shelf Sci. 182, 86–97. doi: 10.1016/j.ecss.2016.08.021
Wilson, S., Blake, C., Berges, J. A., and Maggs, C. A. (2004). Environmental tolerances of free-living coralline algae (maerl): implications for European marine conservation. Biol. Conserv. 120, 279–289. doi: 10.1016/j.biocon.2004.03.001
Zahn, L. A., Claisse, J. T., Williams, J. P., Williams, C. M., and Pondella, D. J. (2016). The biogeography and community structure of kelp forest macroinvertebrates. Mar. Ecol. 37, 770–785. doi: 10.1111/maec.12346
Zeng, D., Huang, D., Qiao, X., He, Y., and Zhang, T. (2015). Effect of suspended kelp culture on water exchange as estimated by in situ current measurement in Sanggou Bay, China. J. Mar. Syst. 149, 14–24. doi: 10.1016/j.jmarsys.2015.04.002
Zhang, J., Fang, J., Wang, W., Du, M., Gao, Y., and Zhang, M. (2011). Growth and loss of mariculture kelp Saccharina japonica in Sungo Bay, China. J. Appl. Phycol. 24, 1209–1216. doi: 10.1007/s10811-011-9762-4
Zhang, J., Hansen, P. K., Fang, J., Wang, W., and Jiang, Z. (2009). Assessment of the local environmental impact of intensive marine shellfish and seaweed farming—application of the MOM system in the Sungo Bay, China. Aquaculture 287, 304–310. doi: 10.1016/j.aquaculture.2008.10.008
Zhao, L., Zhao, Y., Xu, J., Zhang, W., Huang, L., Jiang, Z., et al. (2016). Distribution and seasonal variation of picoplankton in Sanggou Bay, China. Aquac. Environ. Interact. 8, 261–271. doi: 10.3354/aei00168
Zhou, J. (2012). Impacts of mariculture practices on the temporal distribution of macrobenthos in Sandu Bay, South China. Chin. J. Oceanol. Limnol. 30, 388–396. doi: 10.1007/s00343-012-1150-7
Keywords: seaweed, aquaculture, environment, ecosystem, risks
Citation: Campbell I, Macleod A, Sahlmann C, Neves L, Funderud J, Øverland M, Hughes AD and Stanley M (2019) The Environmental Risks Associated With the Development of Seaweed Farming in Europe - Prioritizing Key Knowledge Gaps. Front. Mar. Sci. 6:107. doi: 10.3389/fmars.2019.00107
Received: 12 April 2018; Accepted: 21 February 2019;
Published: 22 March 2019.
Edited by:
Simone Libralato, Istituto Nazionale di Oceanografia e di Geofisica Sperimentale (OGS), ItalyReviewed by:
Annalisa Falace, University of Trieste, ItalyMarianne Thomsen, Aarhus University, Denmark
Copyright © 2019 Campbell, Macleod, Sahlmann, Neves, Funderud, Øverland, Hughes and Stanley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iona Campbell, aW9uYS5jYW1wYmVsbEBzYW1zLmFjLnVr Adrian Macleod, YWRyaWFuLm1hY2xlb2RAc2Ftcy5hYy51aw==
 Iona Campbell
Iona Campbell Adrian Macleod
Adrian Macleod Christian Sahlmann
Christian Sahlmann Luiza Neves3
Luiza Neves3 Margareth Øverland
Margareth Øverland Michele Stanley
Michele Stanley