
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 06 March 2019
Sec. Marine Ecosystem Ecology
Volume 6 - 2019 | https://doi.org/10.3389/fmars.2019.00084
This article is part of the Research TopicAdvances in Understanding Marine Heatwaves and Their ImpactsView all 23 articles
 Mads S. Thomsen1,2*
Mads S. Thomsen1,2* Luca Mondardini1
Luca Mondardini1 Tommaso Alestra1
Tommaso Alestra1 Shawn Gerrity1
Shawn Gerrity1 Leigh Tait3
Leigh Tait3 Paul M. South4
Paul M. South4 Stacie A. Lilley1
Stacie A. Lilley1 David R. Schiel1
David R. Schiel1Detailed research has documented gradual changes to biological communities attributed to increases in global average temperatures. However, localized and abrupt temperature anomalies associated with heatwaves may cause more rapid biological changes. We analyzed temperature data from the South Island of New Zealand and investigated whether the hot summer of 2017/18 affected species of bull kelp, Durvillaea antarctica, D. poha, and D. willana. Durvillaea spp. are large iconic seaweeds that inhabit the low intertidal zone of exposed coastlines, where they underpin biodiversity and ecosystem functioning. Sea surface temperatures (SST) during the summer of 2017/18 included the strongest marine heatwaves recorded in 38 years of existing oceanic satellite data for this region. Air temperatures were also high, and, coupled with small wave heights, resulted in strong desiccation stress during daytime low tides. Before-After analysis of drone images of four reef platforms (42, 42, 44, and 45°S) was used to evaluate changes to bull kelp over the hot summer. Bull kelp loss varied among species and reefs, with the greatest (100%) loss of D. poha at Pile Bay in Lyttelton Harbor (44°S). In Pile Bay, SST exceeded 23°C and air temperatures exceeded 30°C, while Durvillaea was exposed for up to 3 h per day during low tide. Follow-up surveys showed that all bull kelps were eliminated from Pile Bay, and from all reefs within and immediately outside of Lyttelton Harbor. Following the localized extinction of bull kelp in Pile Bay, the invasive kelp Undaria pinnatifida recruited in high densities (average of 120 m-2). We conclude that bull kelps are likely to experience additional mortalities in the future because heatwaves are predicted to increase in magnitude and durations. Losses of the endemic D. poha are particularly concerning due to its narrow distributional range.
Between 1950 and 2009, mean sea surface temperature (SST) of the Atlantic, Indian, and Pacific Oceans increased by 0.41, 0.65, and 0.31°C, respectively (Hoegh-Guldberg et al., 2014). This increase has brought creeping changes to species distributions around the world (Hoegh-Guldberg and Bruno, 2005; Wernberg et al., 2011; Poloczanska et al., 2013). Superimposed on slow and gradual temperature increases are discrete heating events covering smaller regions (1–1000 km) and occurring over shorter durations (days–months). These events are referred to as “heatwaves” (Perkins and Alexander, 2013; Hobday et al., 2016; Oliver et al., 2018) and can have more instantaneous and conspicuous effects (Wernberg et al., 2016; Le Nohaïc et al., 2017; Hobday et al., 2018; Smale et al., 2019). For example, in 2011 an extensive heat wave in Western Australia caused large scale southward range contraction of the canopy-forming fucoid Scytothalia dorycarpa (Smale and Wernberg, 2013) and kelp Ecklonia radiata (Wernberg et al., 2016). During the heatwave, temperatures exceeded the physiological tolerance of these species (Wernberg et al., 2010; Smale and Wernberg, 2013). Similarly, in northern Spain, range contractions have been reported for several canopy-forming seaweeds, including Fucus serratus and Himanthalia elongata (Duarte et al., 2013).
New Zealand recently experienced an exceptionally hot summer with unusually high sea water temperatures in coastal regions of the South Island (Megan, 2018). However, nearshore rocky intertidal marine species on the South Island of New Zealand are embedded within a wide temperate biogeographical region, are not near a convergence with warm subtropical waters, and are therefore expected to be relatively robust to short-term temperature anomalies (Schiel, 2004, 2011; Ayers and Waters, 2005; Schiel et al., 2016).
In southern New Zealand waters, the largest seaweeds over most of the coastline are southern bull kelps (Durvillaea spp.), the world’s largest fucoid algae. Because of their occurrence at the intertidal-subtidal margin they can experience both elevated air and sea temperatures during periods of emersion and immersion. On the South Island there are three co-occurring bull kelp species with either narrow endemic (Durvillaea willana, Durvillaea poha) or wide (Durvillaea antarctica) latitudinal ranges (Fraser et al., 2009, 2012). Individuals of these species can reach up to 10 m long, weigh up to 70 kg, and live for up to 10 years (Hurd, 2003). D. poha and D. antarctica are exposed during low-water on spring tides and D. willana is mostly subtidal with only some individuals being exposed on the biggest spring tides. Together, they form dense stands with extensive canopy cover. These bull kelps are important foundation species which control local community structure, affect biodiversity and provide food and habitat to culturally and economically important species, including lobsters and abalone (Smith and Simpson, 1995; Taylor and Schiel, 2005; Schiel et al., 2018b).
The objective of this study was to quantify changes in the abundance of Durvillaea spp. and associated short-term ecological changes following the hot summer of 2017/18 in New Zealand. To address the objective, we first quantified temperature changes in New Zealand, focusing on 42.5–45.4°S on the east coast of the South Island. Second, we quantified changes in abundances of bull kelp on reefs from the same regions before and after the 2017/18 summer. We hypothesized that bull kelp generally would decrease in abundance and that D. poha, with the most southern northern range limit, would be most severely affected (Smale and Wernberg, 2013; Bennett et al., 2015; Thomson et al., 2015; Wernberg et al., 2016; Arias-Ortiz et al., 2018). Finally, we quantified effects of bull kelp loss, by comparing changes in abundances of key organisms before and after the hot summer in Pile Bay, to changes that followed from a manipulative bull kelp removal experiment. We hypothesized that bull kelp loss would be followed by rapid colonization of weedy macroalgae, irrespective of whether the loss of bull kelp occurred through high temperatures or experimental removals, and that abundances of weedy macroalgae are correlated negatively with abundances of juvenile bull kelp (Wernberg and Connell, 2008; Flukes et al., 2014; Benes and Carpenter, 2015; Wernberg et al., 2016).
This study was done in the rocky intertidal zone on the east coast of the South Island of New Zealand. Surveys were conducted before and after the heatwave on reefs at Oaro (-42.516396, 173.510030), Pile Bay in the semi-protected Lyttelton Harbor (-43.615418, 172.765899) and two reefs in Moeraki (Kaik, -45.356039, 170.861386 and Point, -45.363833, 170.863349). These reefs extend 40–150 m from the upper intertidal to the subtidal zones, are generally protected from severe wave action by offshore reefs, and have a coastal topography that deflects swells. Following this survey (which showed bull kelp elimination from Pile Bay; see Results), other nearby reefs within and outside Lyttelton Harbor were surveyed to identify the wider area of bull kelp loss. Finally, we also used data from a canopy-loss experiment at Oaro and the two Moeraki reefs to estimate wider ecological impacts from the heatwave.
First we used offshore sea-surface temperature (SST) to characterize heatwaves at the three main study sites using the R marine heatwave package (Smit et al., 2018). A heatwave was defined as a period of 5 or more consecutive days where the SST was greater than the 90th percentile calculated from > 30 years of data (Hobday et al., 2016). This analysis was based on NOAA high resolution blended analysis of daily SST data in 1/4 degree grids derived from satellites and in situ data1 (Reynolds et al., 2007). Data were used from 30 August 1980 to 20 April 2018 from Oaro (grid centered on 42.625°S, 173.625°E), Pile Bay (43.375°S, 173.125°E) and Moeraki (45.375°S, 171.125°E). The distance from the reef sites to the grid center was < 25 km. To compare the summer of 2017/18 with past temperature anomalies we calculated (1) duration (in days), (2) maximum intensity (temperatures above the 90% threshold) and (3) cumulative intensity (duration × intensity) of all heatwaves, as defined by Smit et al. (2018) during the 38 years of available satellite SST. This analysis was based on daily mean temperatures from offshore waters and was carried out for each of the three sites separately. However, Pile Bay is located within the outer reaches of a harbor so this location may experience higher temperature fluctuations. We therefore also analyzed hourly SST from 4 nearby inshore buoys within the harbor from August 2016 to April 2018 (all sites were less than 4 km from Pile Bay2). The offshore and Lyttelton Harbor temperature data were analyzed graphically. Finally, we provide additional analyses and data in online Supplementary Material to better understand the environmental context and impacts of the hot summer. We analyzed the large-scale extent of the temperature anomaly Supplementary Figure S1, visualized marine heatwaves at the three main sites with different graphs (Supplementary Figures S2, S3) plotted 38 years of maximum air temperature for the same regions (recorded at the nearest airport, Supplementary Figure S4) and corresponding atmospheric heatwaves (Supplementary Figure S5), measured rocky shore intertidal high-resolution temperature from Hobo-pendant loggers at Oaro over the summer of 17/18 (Supplementary Figure S6), and plotted high resolution wave data from January 2014 to July 2018 from an offshore buoy near Lyttelton harbor (Supplementary Figure S7).
Four reef platforms from Oaro to Moeraki were sampled before the summer of 2017/18 with an Advanced Phantom 3 drone equipped with a 12 MP HD camera. Geo-tagged images were taken at low tide 10 m above the reef, with each image covering c. 95 m2 that was estimated from survey tapes and 1 m2 fixed quadrats positioned on each reef (Murfitt et al., 2017). Drone images were collected in 2017 before the hot summer, with 20 images collected from Oaro (5 April), 20 from Pile Bay (24 August), 20 from Kaik reef (29 April), and 31 from Point reef (29 May). These reefs were re-visited in April 2018, where each entire reef was photographed (“after” data). Note that these surveys of coastal platforms may have underestimated the abundance of D. willana, because it occurs mostly in the subtidal zone. In the laboratory, Orthorectified and georeferenced maps of the reefs were created and the exact locations of the 2017 images were identified using a combination of the geotagged coordinates and visual land markers (boulders, channels, etc.). Percent cover of the three bull kelp species was estimated visually for each image (with a super-imposed grid of 100 cells), differentiating among the three species based on color and width of the blades and stipe characteristics (Adams, 1994; Fraser et al., 2009, 2012; Nelson, 2013). Data contained many zeroes and could not be transformed to variance homogeneity. We therefore tested our hypotheses that bull kelp would decrease in abundance and that D. poha would be most severely affected by the hot summer, using Wilcoxon Signed Paired Rank Tests for each of the three species (pooling data across reefs). For this analysis we only included paired before-after images where bull kelps were observed in at least one of the two sampling events.
The regional drone survey showed that Durvillaea spp. had become locally extinct at Pile Bay. We therefore visited 19 reefs in March–May 2018 around Pile Bay, where we had information about the existence of healthy bull kelp in the years prior to the hot summer, based on a combination of geotagged photos and personal observations (by co-authors and locals). At each reef we recorded whether bull kelp blades were present (i.e., Durvillaea were alive), only holdfasts remained (“ghost holdfasts,” i.e., Durvillaea were dying, see Figure 1), or if we could see fresh “rock scars” that are typically visible following the recent detachment of Durvillaea holdfasts (i.e., Durvillaea have recently died) (Schiel et al., 2018a). We classified each reef as having experienced 100 (not a single blade left), >90 (a few blades were left), 90–30 (patches of blades were left) or <30% (many blades remained, only a few ghost holdfasts were found) loss of bull kelp. In addition to visiting these 19 reefs, we also surveyed the entire Lyttelton Harbor for any traces of surviving bull kelp blades using a boat that was slowly driven along the coastline. These surveys were all done during low tides when emergent bull kelp blades and ghost holdfasts are easy to see. The results from these semi-quantitative surveys were analyzed by visually inspecting maps that showed spatial locations of reefs with surviving bull kelp vs. localized extinctions.
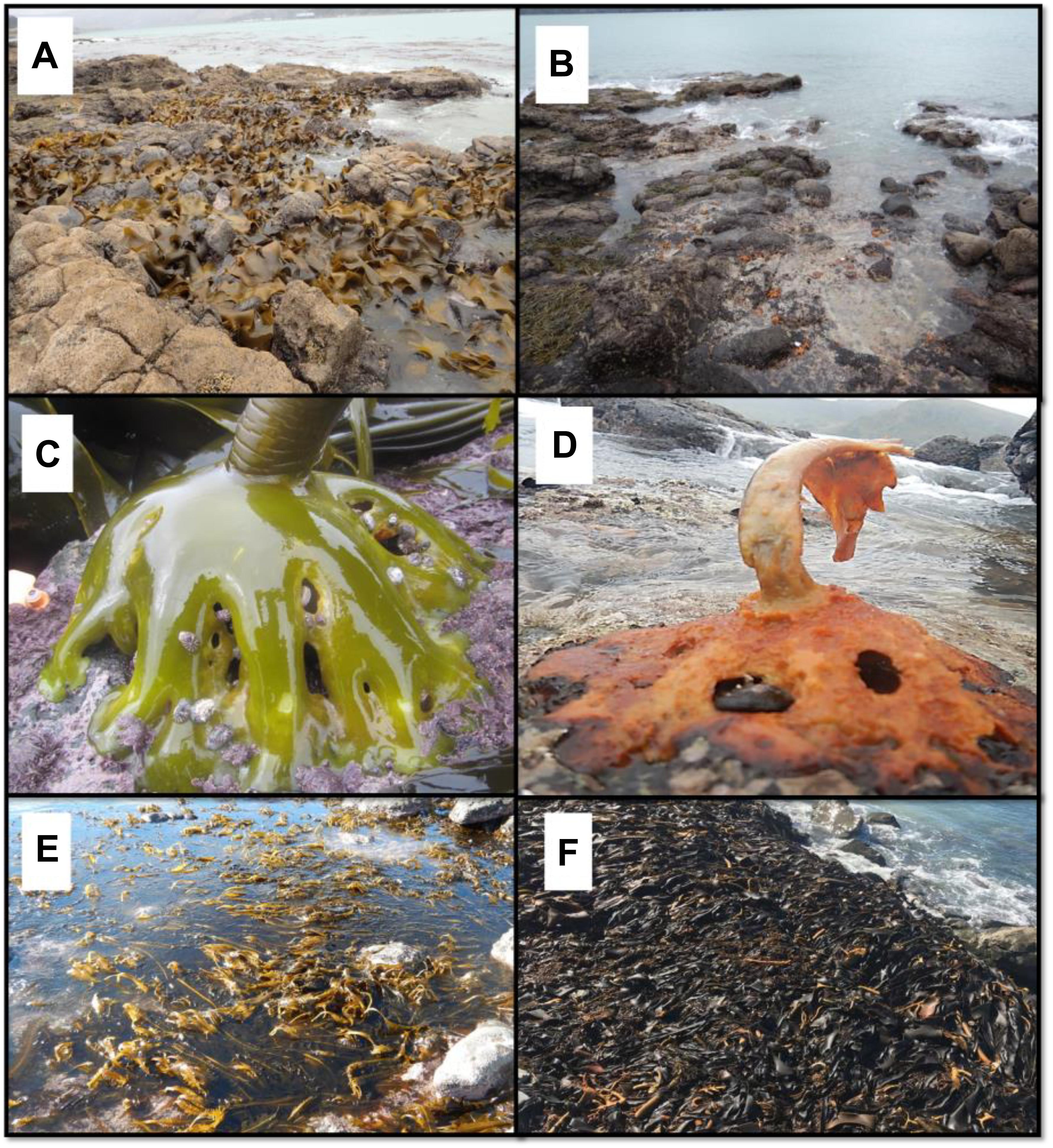
Figure 1. (A) Durvillaea poha bed in Pile Bay, November 2017. (B) The same area in March 2018 where only remnants of holdfasts are visible. (C) Healthy bull kelp holdfasts inhabited by Siphonaria limpets, November 2017. (D) Decaying bull kelp in March 2018 (“ghost holdfast”). (E) Undaria pinnatifida in high densities invaded the lower parts of the reef shown in (A,B) in May 2018. (F) Large wrack accumulation of D. willana observed on many reefs after the marine heatwave (photo near Oaro, southern New Zealand).
We photographed 10 0.25 m2 plots with >90% bull kelp cover in Pile Bay (where the canopy was moved to make understory species visible) in August 2017 (“before” data). We then also photographed 13 0.25 m2 plots at the same sites in March 2018 after bull kelp had been eliminated from the reef (“after” data). From these photos we estimated cover of conspicuous sessile organisms that were easy to identify. Data included many zeros and could not be transformed to variance homogeneity. We therefore used Mann–Whitney ranked U-tests on before-after data to test the hypothesis that weedy macroalgae would be more abundant after bull kelp were lost from the reef. We also tested whether mussels changed abundances because mussels were the most common and conspicuous understory species in these plots prior to the hot summer.
Prior to the summer 2017/18 we had set up a bull kelp removal experiment at Oaro, Kaik and Point reefs, to simulate different intensities of bull kelp loss: no removals (control plots with 100% canopy-cover), removals of blades (cutting blades off 10 cm above the end of the stipe), removals of stipes (cutting the middle of the stipe), and removals of holdfasts (i.e., removing the entire plant, very small holdfasts with diameters less than 2 cm were left undisturbed in all plots). Each plot was 1 m2 and each treatment was replicated four times. Kaik and Point reef plots were established in May 2017 and Oaro plots in June 2017, just prior to the reproductive period of bull kelp (Taylor and Schiel, 2003, 2005). After 4 months we counted the number of juvenile bull kelps and estimated the percent cover of weedy macroalgae (the green sheet-forming Ulva spp. and U. pinnatifida), two taxa that were absent in the plots prior to removals. Variances were homogenous after arcsin-square root transformation (Cochran’s C for the removal treatment and Reef factor; p = 0.78 and 0.51, respectively). Nested Anova (3 reefs nested within 4 removal levels) was therefore used to test the hypothesis that the loss of fronds, stipes or holdfast would result in colonization of weedy macroalgae. A significant effect of removals was followed by SNK post hoc tests to identify differences among treatments. Note that the experimental and the before-after impact-analyses supplemented each other; colonization by macroalgae in Pile Bay following the hot summer could be caused by either loss of bull kelp and/or because of high summer temperature, whereas colonization following experimental removals could only be caused by loss of bull kelp. Finally, we were also interested in examining whether weedy macroalgae inhibited bull kelp recovery and vice versa, that is, if bull kelp recruits inhibit weedy macroalgae. These variables were not manipulated in the experiment so that causation cannot be inferred. We therefore tested the hypothesis that the cover of weedy macroalgae correlated negatively with density of juvenile bull kelp across the 48 plots using Spearman’s rank correlation coefficient.
Oaro, Lyttelton, and Moeraki have experienced many heatwaves since 1980, but with increasing durations and intensities since 2014 (Figure 2). The heatwaves over the 2017/18 summer were particular strong with maximum and cumulative intensities being approximately two times greater than any recorded event since 1980 (Figures 2B,C). Satellite-derived oceanic SSTs were generally lowest at the most southern site (Moeraki) and highest at the mid-latitude region (Lyttelton) (Supplementary Material), but because data were derived from offshore satellite images they may not have captured temperature extremes at inshore protected bays and inlets or intertidal rocky shores (see discussion in Smale and Wernberg, 2009). Analysis of SST data near Pile Bay within Lyttelton Harbor showed many days with water temperatures exceeding 23°C during the summer of 2017/18 (Figure 3) and intertidal in situ loggers at Oaro showed several days where atmospheric temperatures on the reef platforms exceeded >45°C (when cloudless days co-occurred with big, mid-day low tides, see Supplementary Figure S6).
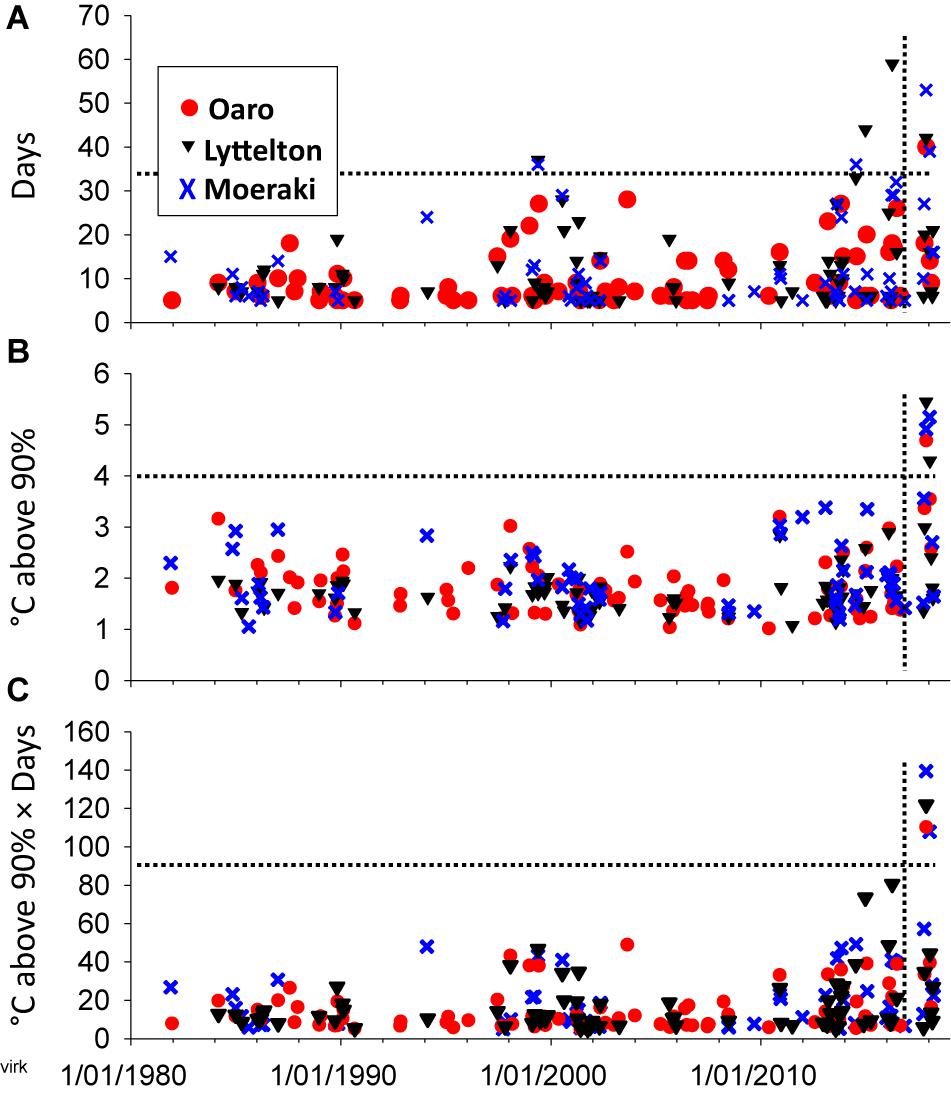
Figure 2. Duration (A), maximum intensity (B), and cumulative intensities (C) of marine heatwaves recorded from Oaro, Lyttelton and Moeraki from 1/1-1980 to 20/4-2018 Heatwaves were calculated from satellite derived daily sea surface temperatures in the r package Marine Heatwaves; 90% = the 90% climatic quantile calculated from 38 years of data. Dotted lines were inserted to highlight the long durations and extreme maximum and cumulative intensities of the 2017/18 summer heatwaves.
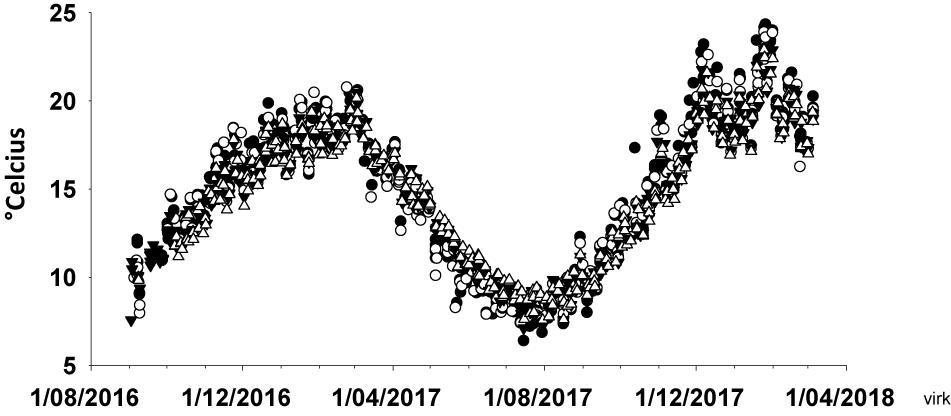
Figure 3. Sea surface temperature in Lyttelton Harbor from August 2016 to April 2018 at four stations (shown with different symbols) within five km from Pile Bay.
Prior to the summer of 2017/18, the four intertidal platforms were dominated by D. poha, with smaller patches of D. willana and D. antarctica at the Point and Kaik reefs (Figure 4). We found a strong reduction in abundances of D. poha (Z-Statistic = 7.154, p < 0.001, n = 87), smaller reductions in D. willana (Z-Statistic = 2.032, p = 0.042, n = 11), and no change in abundance of D. antarctica (Z-Statistic = 1.342, p = 0.180, n = 5). Importantly, D. poha from Pile Bay decreased from 12 to 0 percent cover (Figure 4), suggesting dramatic population-wide effects for this species on this particular reef.
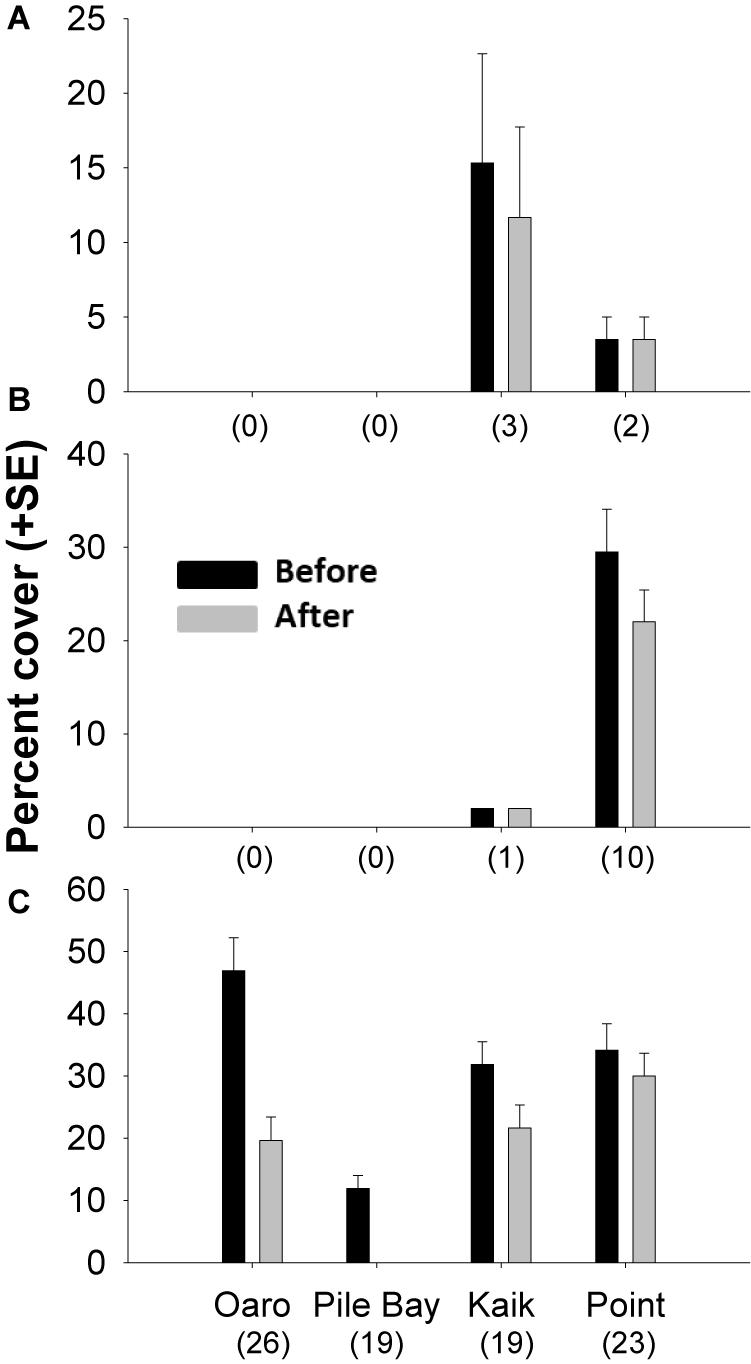
Figure 4. Percent cover (+ SE) of Durvillaea antarctica (A), D. willana (B), and D. poha (C) before (black) and after (gray) the summer of 2017/18 on four intertidal reef platforms. Cover was estimated from paired 95 m2 drone images that showed presence of bull kelp either before and/or after the hot summer of 2017/18. Replication levels are shown in brackets.
Detailed and targeted follow-up reef-surveys revealed total elimination of bull kelp on 12 out of 19 reefs, including the entire Pile Bay reef (see above, Figure 5). All 12 reefs that experienced total loss of bull kelp were within, or immediately north and south of, Lyttelton Harbor. We were assured that each of these reefs were inhabited by bull kelp before the hot summer of 2017/18 because abundant remnant and decaying ghost holdfasts (Figure 1) attested to very recent living bull kelp populations. It was not possible to distinguish D. antarctica and D. poha using ghost holdfasts, but our prior qualitative observations and collections from many of these reefs suggested they were mainly D. poha. A few surviving blades of D. poha were found at one site close to Lyttelton Harbor, and relatively abundant populations were found at reefs > 10 km north and south of the Harbor (Figure 5).
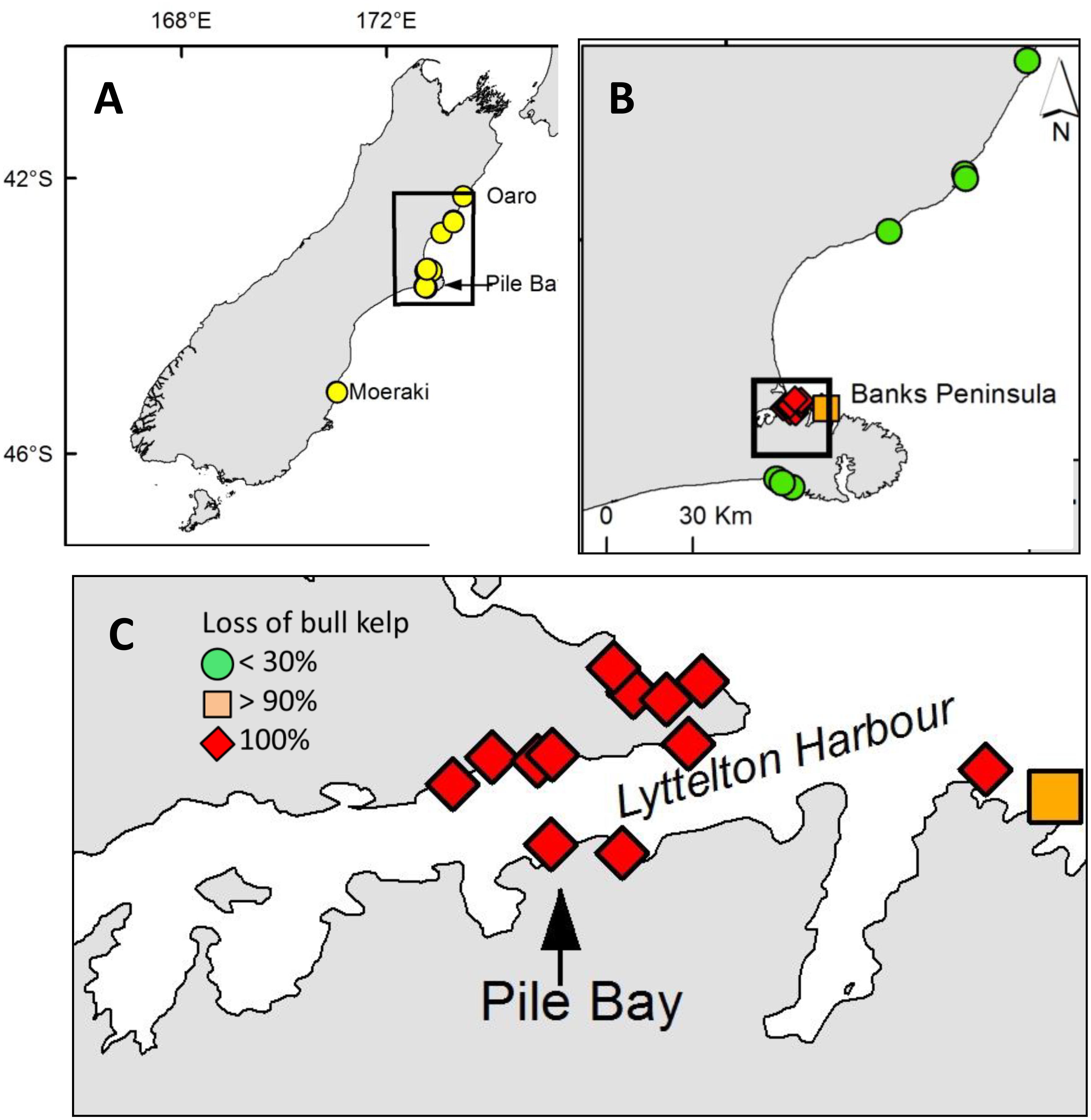
Figure 5. (A) Surveyed bull kelp sites on the South Island of New Zealand; drone surveys were done at Oaro, Pile Bay and Point and Kaik reefs in Moeraki. (B) Close-up of reefs around Banks Peninsula where detailed searches for live bull kelp blades and dead “ghost holdfasts” were done. (C) Close-up of sites with reef-wide extinctions of bull kelp around Pile Bay in Lyttelton Harbor.
There were no Undaria under bull kelp canopies in Pile Bay in August 2017. However, in March 2018, after the elimination of bull kelp, densities increased from 0 ( ± 0) to 121 m-2 ( ± 20 SE) juvenile Undaria in the area previously occupied by Durvillaea, whereas cover increased from 0 ( ± 0) to 20.9% ( ± 5.6 SE). Cover of other weedy macroalgae also increased, including Colpomenia sinuosa that increased from 0 ( ± 0) to 1.6% ( ± 0.5) and Dictyota sp. that increased from 0.02% ( ± 0.01) to 1.6% ( ± 0.6). Finally we also found that mussels (mainly Perna canaliculus) declined from 7.3% ( ± 2.0) to 0.7 ( ± 0.1)%. These changes in both cover and densities were all significant (all Z-scores > 1.98, all p < 0.05).
There were no weedy macroalgae (Undaria or Ulva) under healthy bull kelp canopies in any of the 48 plots prior to removal treatments (data not shown). However, 4 months after the removals we found significant differences in cover of weedy macroalgae (Fremoval = 8.257, p < 0.001) across the three reefs [Freef(removal) = 0.760, p = 0.639]. Post hoc SNK tests showed significantly more weedy macroalgae in the three removal treatments (which had similar cover) compared to the control plots (Figure 6). Ulva generally colonized removal plots at Oaro whereas Undaria colonized removal plots at Kaik and Point reefs in Moeraki (Figure 6). We also found a significant negative relationship between cover of weedy macroalgae and density of bull kelp recruits (r = -0.414, p = 0.003, Figure 7).
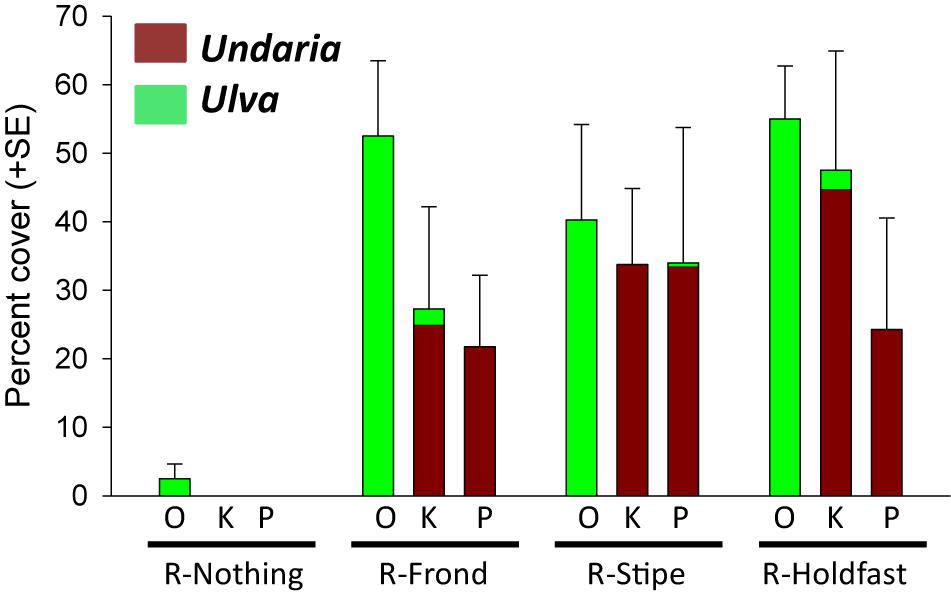
Figure 6. Percent cover (+ SE) of weedy macroalgae (the non-native kelp Undaria pinnatifida and green sheet-forming Ulva spp.) 4 months after bull kelp fronds, stipes or holdfasts were removed (R) from plots in Oaro (O), Kaik (K), and Point (P) reefs (n = 4). R-Nothing = intact control plots.
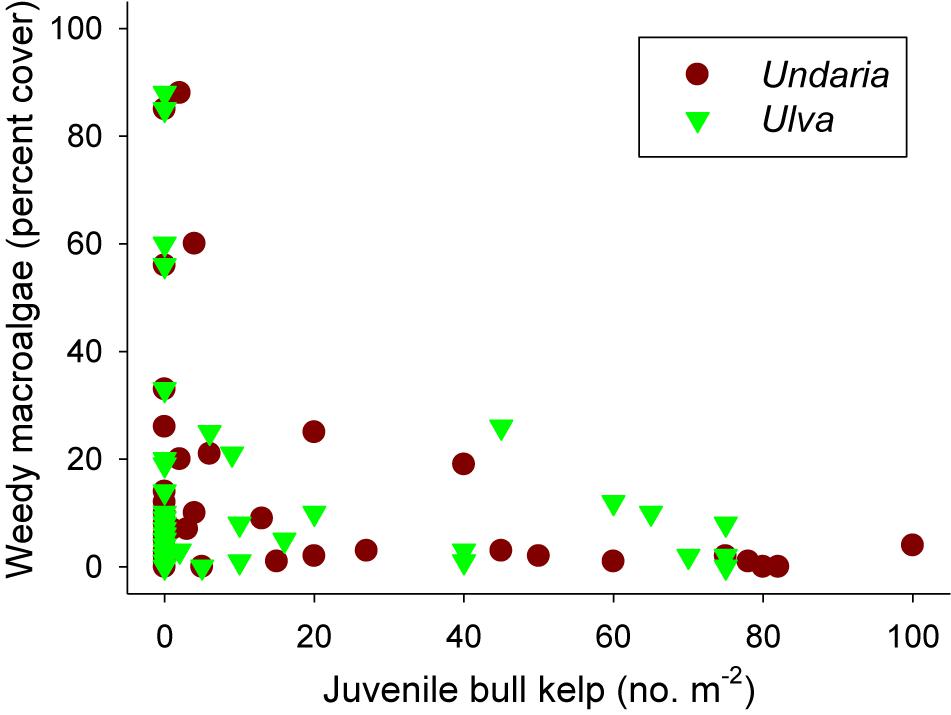
Figure 7. Relationship between cover of weedy macroalgae (the non-native kelp Undaria pinnatifida and green sheet-forming Ulva spp.) and recruit densities of Durvillaea in 48 plots 4 months after a removal experiment was initiated (with 12 undisturbed control plots and 36 plots with different intensities of bull kelp removals, see Figure 6 for details).
We documented the loss of bull kelp following the unusually hot summer of 2017/18, characterized by high sea and air temperatures, low daytime tides, and reduced wave action (Figures 2,3 and Supplementary Figures S1–S6), so that Durvillaea could have been stressed at both high and low tide. Elevated SST alone can cause similar losses, as has been shown for subtidal kelp and seagrasses around their northern range limits in Western Australia following the Ningaloo 2010/11 marine heatwave (Smale and Wernberg, 2013; Thomson et al., 2015; Wernberg et al., 2016; Arias-Ortiz et al., 2018). However, kelp and seagrasses are typically considered to be more robust to sea temperature anomalies within their range limits (Diaz-Almela et al., 2007; Reed et al., 2016; Wernberg et al., 2016). Our results differ because D. poha were eliminated from reefs more than 200 km south of its northern range limit (Fraser et al., 2012). This mid-range local extinction event (Bennett et al., 2015) occurred in inshore bay-waters, which were several degrees warmer than the coastal waters closer to the species northern range limit (Adams, 1994; Fraser et al., 2012). Durvillaea species are not tolerant to long periods of desiccation (Hay, 1979; Taylor and Schiel, 2005) and inhabit exposed coasts where wave splash keeps them moist. Typically, individuals have a bleached appearance as they deteriorate, which was observed in earthquake uplifted and exposed Durvillaea populations north of Oaro (Schiel et al., 2018a) and also in Lyttelton Harbor (this study). It seems likely that at least D. poha was near its ecophysiological limit. Indeed, there appeared to be a relationship between maximum observed temperature and bull kelp loss (Pile Bay > Oaro > Moeraki), as seen for Ecklonia radiata in Western Australia following the Ningaloo 2010/11 heatwave (Wernberg et al., 2016).
The three Durvillaea species typically occupy the intertidal-subtidal fringe with their down-shore distributions shifting from D. poha that is exposed on all spring tides, to D. antarctica and then D. willana which is normally subtidal and exposed only on the largest spring tides (Adams, 1994; Fraser et al., 2012). The ocean temperatures analyzed in this study do not account for air-temperature stress occurring during emersion that can correlate with fucoid canopy-cover (Schiel et al., 2016). Coastal air and water temperature typically co-vary over larger and longer time-scales (Rayner et al., 2003; Schiel et al., 2016) and air temperature was also high during the 2017/18 summer (Supplementary Figures S4–S6). This almost certainly contributed to the die-off of D. poha, which is typically exposed to the air for longer than other species of Durvillaea (Fraser et al., 2012). It is likely that the die-off of bull kelp therefore reflects the net effects of duration and intensity of extreme water and air temperatures, their timing relative to diurnal and lunar cycles, but also the configuration of the landscape (e.g., slope, aspect). Finally, after being exposed to desiccation and high air temperatures at low tides (exceeding 45°C, see Supplementary Figure S6), the elevated seawater temperature increased stress and offered little opportunity for recovery.
Our results suggest that D. poha was more affected than D. willana that again was more affected than D. antarctica, a pattern that co-varies with their northern range limits. D. poha inhabits only the South Island of New Zealand, D. willana is found on the South Island and in the southern North Island, whereas D. antarctica is common around the coast of New Zealand as well as in temperate Australia and Chile (Adams, 1994; Fraser et al., 2009, 2012). Similar relationships between poleward range limits and resistance to temperature stress have been shown for both terrestrial and aquatic organisms (Hickling et al., 2006; Wernberg et al., 2011; Poloczanska et al., 2013; Smale et al., 2019). As previously noted, our drone survey prior to the heatwave was not designed to sample the more wave-exposed D. antarctica and more subtidal D. willana so their losses were calculated from fewer drone images and require further studies. However, qualitative observations support our quantitative analyses showing some loss of both species at Moeraki, and we observed unusually large wrack accumulations, particularly of D. willana, at many sites from Oaro to Moeraki (cf. Figure 1).
Our study was, in essence, a natural experiment (Hargrove and Pickering, 1992) like other marine heatwave impact studies (Garrabou et al., 2009; Short et al., 2015; Wernberg et al., 2016; Smale et al., 2019). Other factors could have contributed to the loss of bull kelp, but we argue that temperature effects were the most significant region-wide stressor. First, bull kelp are long-lived plants with relatively low seasonal variation in the cover of adult canopies that maintain consistent beds for long time periods (Hay, 1979; Santelices et al., 1980; Westermeier et al., 1994; Hurd, 2003; Taylor and Schiel, 2005). This implies that large scale die-offs are unusual events that previously only have been reported following dramatic seismic uplifts (Castilla, 1988; Schiel et al., 2018a). Second, there were no unusually large storms during the 2017/18 summer (Supplementary Figure S7). Given that storms rarely cover 600 km coastline and that bull kelp are adapted to high wave action and can survive massive storms, it seems unlikely to have caused the extensive large-scale mortalities recorded in this study (Taylor and Schiel, 2003, 2005; Thomsen and Wernberg, 2005; Taylor et al., 2010). Third, we did not observe any unusual turbidity or sedimentation events at any of our regular sampling sites during the summer of 2017/18. Fourth, bull kelps are typically not limited by grazers along the east coast of New Zealand, even though the odacid fish Odax pullus can affect juvenile recruitment at some sites and have characteristic bite marks (Taylor et al., 2010). Finally, we are not aware of any bull kelp pathogens, except for gall-forming parasites, which are relatively harmless and clearly visible and were not observed to occur (Goecke et al., 2012; Murúa et al., 2017). Nevertheless, it is plausible that temperature stressed bull kelp became more susceptible to infectious diseases and thereby accelerated the decay (Campbell et al., 2011, 2014; Case et al., 2011; Marzinelli et al., 2015). Similarly, temperature stressed and bacteria-coated bull kelp may also have experienced increased grazing from limpet, snails and herbivorous fish (Kristensen et al., 1992; Taylor et al., 2010; Campbell et al., 2014). However, these indirect effects are proximate stressors that would only effectuate after bull kelp were stressed by high temperatures.
The loss of an algal canopy can have positive and negative effects on sub-canopy species (Lilley and Schiel, 2006; Schiel, 2006; Wernberg and Connell, 2008; Wernberg et al., 2013; Flukes et al., 2014). At Pile Bay the dominant understory taxa, encrusting coralline algae, did not appear to be immediately affected but weedy macroalgae, including ephemeral (Ulva, Colpomenia), opportunistic (Undaria) or low-lying (Dictyota) species increased, probably due to competitive release from Durvillaea (Taylor and Schiel, 2005; Arkema et al., 2009; Benes and Carpenter, 2015; Schiel et al., 2018b). Colonization by Undaria pinnatifida was also seen in the removal plots in Moeraki, supporting past studies done within stands of Durvillaea spp. and other canopy-forming algae in New Zealand (Thompson and Schiel, 2012; South et al., 2016; South and Thomsen, 2016; Schiel et al., 2018b), Australia (Valentine and Johnson, 2003, 2004), and the United Kingdom (De Leij et al., 2017). In places where Undaria was not present, other opportunistic fast-growing seaweeds, especially Ulva, colonized disturbed plots. This was also seen after the extensive canopy loss of Durvillaea and other fucoid algae after seismic uplift along 100 km of coastline (Schiel et al., 2018b). We also found that mussels decreased dramatically following bull kelp loss, possibly due to increased predation from shore birds, fish and crabs (Rilov and Schiel, 2006), or increased exposure to light and decreased humidity – stress-factors exaggerated by the high summer temperatures. It is likely that more complex effects will follow over time. For example, calcareous encrusting algae may be outcompeted by turf-forming taxa (Wernberg et al., 2013; Connell et al., 2014; Filbee-Dexter and Wernberg, 2018) with cascading negative effects on species that depend on these algae, such as abalone (Haliotis spp.) that cue in on this substrate for larval settlement (Morse and Morse, 1984; Daume et al., 1999; Roberts et al., 2004). It is also possible that expansion of weedy macroalgae creates an unfavorable habitat for Durvillaea to recolonize, should it be dispersed into the harbor by drifting individuals (Taylor and Schiel, 2003; Taylor et al., 2010; Fraser et al., 2011). Indeed, past studies, and our new correlation analysis, suggests that weedy macroalgae can inhibit recruitment of large and long-lived macroalgae, that instead require rocky substratum to attach firmly to – and thereby withstand years of strong hydrodynamic wave-forces (Taylor and Schiel, 2003; Taylor et al., 2010; Wernberg et al., 2013; Connell et al., 2014; Filbee-Dexter and Wernberg, 2018).
Extensive losses of bull kelp were recorded at many reefs along three degrees of latitude following an unusually warm summer with Durvillaea being eliminated from the warmest areas. Following the die-off, weedy macroalgae, especially the non-native kelp Undaria pinnatifida and sheet-forming Ulva spp. rapidly colonized empty spaces. The long-term implications are not yet clear, but if the trend of warming seas and increased intensities of marine heatwaves continues (Oliver et al., 2018), especially when coincident with hot air temperatures, there may be “press” effects that habitat-forming species such as Durvillaea cannot overcome (Martínez et al., 2018). Where appropriate micro-habitats still exist, there may be a need to enhance connectivity between remaining populations by the use of novel techniques, such as transplantations, restoration, and strain selection, to ensure the long-term survival of these iconic foundation species in affected areas.
The datasets generated for this study are available on request to the corresponding author.
Drone surveys were done with high sensitivity to wildlife. In particular, sites and timing of surveys were chosen to avoid potential effects on seals and seabirds. If these species were present, flights were postponed to another day.
MT conceived the idea for the manuscript, collected some of the data, analyzed most of the data, and wrote the manuscript. LM, TA, SG, LT, and PS collected some of the data and commented on the manuscript. SL analyzed some data and commented on the manuscript. DS commented on the manuscript.
This research was supported by a grant from Brian Mason (Impacts of an unprecedented marine heatwave on bull kelp forests and implications for conservation and restoration) and with support from the Ministry of Primary Industries and the Ministry of Business, Innovation and Employment (earthquake impacts and recovery).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Eric C. J. Oliver for producing marine heatwave maps (seen in Supplementary Figure S1).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00084/full#supplementary-material
Arias-Ortiz, A., Serrano, O., Masqué, P., Lavery, P. S., Mueller, U., Kendrick, G. A., et al. (2018). A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat. Clim. Chang. 8:338. doi: 10.1038/s41558-018-0096-y
Arkema, K. K., Reed, D. C., and Schroeter, S. C. (2009). Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology 90, 3126–3137. doi: 10.1890/08-1213.1
Ayers, K., and Waters, J. (2005). Marine biogeographic disjunction in central New Zealand. Mar. Biol. 147, 1045–1052. doi: 10.1007/s00227-005-1632-7
Benes, K. M., and Carpenter, R. C. (2015). Kelp canopy facilitates understory algal assemblage via competitive release during early stages of secondary succession. Ecology 96, 241–251. doi: 10.1890/14-0076.1
Bennett, S., Wernberg, T., Joy, B. A., De Bettignies, T., and Campbell, A. H. (2015). Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6:10280. doi: 10.1038/ncomms10280
Campbell, A. H., Harder, T., Nielsen, S., Kjelleberg, S., and Steinberg, P. D. (2011). Climate change and disease: bleaching of a chemically defended seaweed. Glob. Change Biol. 17, 2958–2970. doi: 10.1111/j.1365-2486.2011.02456.x
Campbell, A. H., Vergés, A., and Steinberg, P. D. (2014). Demographic consequences of disease in a habitat-forming seaweed and impacts on interactions between natural enemies. Ecology 95, 142–152. doi: 10.1890/13-0213.1
Case, R. J., Longford, S. R., Campbell, A. H., Low, A., Tujula, N., Steinberg, P. D., et al. (2011). Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga. Environ. Microbiol. 13, 529–537. doi: 10.1111/j.1462-2920.2010.02356.x
Castilla, J. C. (1988). Earthquake-caused coastal uplift and its effects on rocky intertidal kelp communities. Science 242, 440–443. doi: 10.1126/science.242.4877.440
Connell, S., Foster, M., and Airoldi, L. (2014). What are algal turfs? Towards a better description of turfs. Mar. Ecol. Prog. Ser. 495, 299–307. doi: 10.3354/meps10513
Daume, S., Brand-Gardner, S., and Woelkerling, W. J. (1999). Settlement of abalone larvae (Haliotis laevigata Donovan) in response to non-geniculate coralline red algae (Corallinales, Rhodophyta). J. Exp. Mar. Biol. Ecol. 234, 125–143. doi: 10.1016/S0022-0981(98)00143-9
De Leij, R., Epstein, G., Brown, M. P., and Smale, D. A. (2017). The influence of native macroalgal canopies on the distribution and abundance of the non-native kelp Undaria pinnatifida in natural reef habitats. Mar. Biol. 164:156. doi: 10.1007/s00227-017-3183-0
Diaz-Almela, E., Marba, N., and Duarte, C. M. (2007). Concequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob. Chang. Biol. 13, 224–235. doi: 10.1111/j.1365-2486.2006.01260.x
Duarte, L., Viejo, R. M., Martínez, B., deCastro, M., Gomez-Gesteira, M., and Gallardo, T. (2013). Recent and historical range shifts of two canopy-forming seaweeds in North Spain and the link with trends in sea surface temperature. Acta. Oecol. 51, 1–10. doi: 10.1016/j.actao.2013.05.002
Filbee-Dexter, K., and Wernberg, T. (2018). Rise of turfs: a new battlefront for globally declining kelp forests. Bioscience 68, 64–76. doi: 10.1093/biosci/bix147
Flukes, E., Johnson, C., and Wright, J. (2014). Thinning of kelp canopy modifies understory assemblages: the importance of canopy density. Mar. Ecol. Prog. Ser. 514, 57–70. doi: 10.3354/meps10964
Fraser, C. I., Hay, C. H., Spencer, H. G., and Waters, J. M. (2009). Genetic and morphological analyses of the southern bull kelp Durvillaea antartica (Phaeophycea: Durvillaeales) in New Zealand reveal cryptic species. J. Phycol. 45, 436–443. doi: 10.1111/j.1529-8817.2009.00658.x
Fraser, C. I., Nikula, R., and Waters, J. M. (2011). Oceanic rafting by a coastal community. Proc. R. Soc. Lond. B Biol. Sci. 278, 649–655. doi: 10.1098/rspb.2010.1117
Fraser, C. I., Spencer, H. G., and Waters, J. M. (2012). Durvillaea poha sp. nov.(Fucales, Phaeophyceae): a buoyant southern bull-kelp species endemic to New Zealand. Phycologia 51, 151–156. doi: 10.2216/11-47.1
Garrabou, J., Coma, R., Bensoussan, N., Bally, M., Chevaldonné, P., Cigliano, M., et al. (2009). Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob. Chang. Biol. 15, 1090–1103. doi: 10.1111/j.1365-2486.2008.01823.x
Goecke, F., Wiese, J., Núñez, A., Labes, A., Imhoff, J. F., and Neuhauser, S. (2012). A novel phytomyxean parasite associated with galls on the bull-kelp Durvillaea antarctica (Chamisso) Hariot. PLoS One 7:e45358. doi: 10.1371/journal.pone.0045358
Hargrove, W. W., and Pickering, J. (1992). Pseudoreplication: a sine qua non for regional ecology. Landsc. Ecol. 6, 251–258. doi: 10.1007/BF00129703
Hay, C. H. (1979). Some factors affecting the upper limit of the southern bull kelp Durvillaea antarctica (Chamisso) Hariot on two New Zealand shores. J. R. Soc. N. Z. 9, 279–287. doi: 10.1080/03036758.1979.10419408
Hickling, R., Roy, D. B., Hill, J. K., Fox, R., and Thomas, C. D. (2006). The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 12, 450–455. doi: 10.1111/j.1365-2486.2006.01116.x
Hobday, A., Oliver, E. C. J., Gupta, A. S., Benthuysen, J. A., Burrows, M. T., Donat, M., et al. (2018). Categorizing and naming marine heatwaves. Oceanography 31, 63–73. doi: 10.5670/oceanog.2018.205
Hobday, A. J., Alexander, L. V., Perkins, S. E., Smale, D. A., Straub, S. C., Oliver, E. C., et al. (2016). A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 141, 227–238. doi: 10.1016/j.pocean.2015.12.014
Hoegh-Guldberg, O., and Bruno, J. F. (2005). The impact of climate change on the worlds marine ecosystems. Science 308:541.
Hoegh-Guldberg, O., Cai, R., Poloczanska, E. S., Brewer, P. G., Sundby, S., Hilmi, K., et al. (2014). “The Ocean,” in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds V. R. Barros, C. B. Field, D. J. Dokken, M. D. Mastrandrea, K. J. Mach, T. E. Bilir, et al. (Cambridge: Cambridge University Press), 1655–1731.
Hurd, C. (2003). “Bull kelp,” in The Living Reef - The Ecology of New Zealand’s Rocky Reefs, eds N. Andrew and M. Francis (Nelson: Craig Potton Publishing), 56–63.
Kristensen, E., Andersen, F. Ø, and Blackburn, T. H. (1992). Effects of benthic macrofauna and temperature on degradation of macroalgal detritus: the fate of organic carbon. Limnol. Oceanogr. 37, 1404–1419. doi: 10.4319/lo.1992.37.7.1404
Le Nohaïc, M., Ross, C. L., Cornwall, C. E., Comeau, S., Lowe, R., McCulloch, M. T., et al. (2017). Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci. Rep. 7:14999. doi: 10.1038/s41598-017-14794-y
Lilley, S. A., and Schiel, D. R. (2006). Community effects following the deletion of a habitat-forming alga from rocky marine shores. Oecologia 148, 672–681. doi: 10.1007/s00442-006-0411-6
Martínez, B., Radford, B., Thomsen, M. S., Connell, S. D., Carreño, F., Bradshaw, C. J., et al. (2018). Distribution models predict large contractions of habitat-forming seaweeds in response to ocean warming. Div. Distribut. 24, 1350–1366. doi: 10.1111/ddi.12767
Marzinelli, E. M., Campbell, A. H., Zozaya Valdes, E., Vergés, A., Nielsen, S., Wernberg, T., et al. (2015). Continental-scale variation in seaweed host-associated bacterial communities is a function of host condition, not geography. Environ. Microbiol. 17, 4078–4088. doi: 10.1111/1462-2920.12972
Megan, G. (2018). It Was Officially New Zealand’s Hottest Summer on Record. Available at: https://www.stuff.co.nz/environment/101996439/it-was-officially-new-zealands-hottest-summer-on-record
Morse, A. N., and Morse, D. E. (1984). Recruitment and metamorphosis of Haliotis larvae induced by molecules uniquely available at the surfaces of crustose red algae. J. Exp. Mar. Biol. Ecol. 75, 191–215. doi: 10.1016/0022-0981(84)90166-7
Murfitt, S. L., Allan, B. M., Bellgrove, A., Rattray, A., Young, M. A., and Ierodiaconou, D. (2017). Applications of unmanned aerial vehicles in intertidal reef monitoring. Sci. Rep. 7:10259. doi: 10.1038/s41598-017-10818-9
Murúa, P., Goecke, F., Westermeier, R., van West, P., Küpper, F. C., and Neuhauser, S. (2017). Maullinia braseltonii sp. nov. (Rhizaria, Phytomyxea, Phagomyxida): a cyst-forming parasite of the bull kelp Durvillaea spp. (Stramenopila, Phaeophyceae, Fucales). Protist 168, 468–480. doi: 10.1016/j.protis.2017.07.001
Oliver, E. C., Donat, M. G., Burrows, M. T., Moore, P. J., Smale, D. A., Alexander, L. V., et al. (2018). Longer and more frequent marine heatwaves over the past century. Nat. Commun. 9:1324. doi: 10.1038/s41467-018-03732-9
Perkins, S., and Alexander, L. (2013). On the measurement of heat waves. J. Clim. 26, 4500–4517. doi: 10.1175/JCLI-D-12-00383.1
Poloczanska, E. S., Brown, C. J., Sydeman, W. J., Kiessling, W., Schoeman, D. S., Moore, P. J., et al. (2013). Global imprint of climate change on marine life. Nat. Clim. Chang. 3:919. doi: 10.1038/nclimate1958
Rayner, N., Parker, D. E., Horton, E., Folland, C., Alexander, L., Rowell, D., et al. (2003). Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. J. Geophys. Res. 108:4401. doi: 10.1029/2002JD002670
Reed, D., Washburn, L., Rassweiler, A., Miller, R., Bell, T., and Harrer, S. (2016). Extreme warming challenges sentinel status of kelp forests as indicators of climate change. Nat. Commun. 7:13757. doi: 10.1038/ncomms13757
Reynolds, R. W., Smith, T. M., Liu, C., Chelton, D. B., Casey, K. S., and Schlax, M. G. (2007). Daily high-resolution-blended analyses for sea surface temperature. J. Clim. 20, 5473–5496. doi: 10.1175/2007JCLI1824.1
Rilov, G., and Schiel, D. R. (2006). Trophic linkages across seascapes: subtidal predators limit effective mussel recruitment in rocky intertidal communities. Mar. Ecol. Prog. Ser. 327, 83–93. doi: 10.3354/meps327083
Roberts, R. D., Kaspar, H. F., and Barker, R. J. (2004). Settlement of abalone (Haliotis iris) larvae in response to five species of coralline algae. J. Shellfish Res. 23, 975–988.
Santelices, B., Castilla, J., Cancino, J., and Schmiede, P. (1980). Comparative ecology of Lessonia nigrescens and Durvillaea antarctica (Phaeophyta) in central Chile. Mar. Biol. 59, 119–132. doi: 10.1007/BF00405461
Schiel, D. R. (2004). The structure and replenishment of rocky shore intertidal communities and biogeographic comparisons. J. Exp. Mar. Biol. Ecol. 300, 309–342. doi: 10.1016/j.jembe.2004.01.001
Schiel, D. R. (2006). Rivets or bolts? When single species count in the function of temperate rocky reef communities. J. Exp. Mar. Biol. Ecol. 338, 233–252. doi: 10.1016/j.jembe.2006.06.023
Schiel, D. R. (2011). Biogeographic patterns and long-term changes on New Zealand coastal reefs: non-trophic cascades from diffuse and local impacts. J. Exp. Mar. Biol. Ecol. 400, 33–51. doi: 10.1016/j.jembe.2011.02.026
Schiel, D. R., Gerrity, S., Alestra, T., Pirker, J., Marsden, I., Dunmore, R. A., et al. (2018a). “Kaikoura earthquake: summary of impacts and changes in nearshore marine communities,” in Shaky Shores – Coastal Impacts & Responses to the 2016 Kaikôura Earthquakes, eds C. Hendtlass, J. Borrero, and T. Shand (Thorndon: NZCS), 25–28.
Schiel, D. R., Lilley, S. A., and South, P. M. (2018b). Ecological tipping points for an invasive kelp in rocky reef algal communities. Mar. Ecol. Prog. Ser. 587, 93–104. doi: 10.3354/meps12429
Schiel, D. R., Lilley, S. A., South, P. M., and Coggins, J. H. (2016). Decadal changes in sea surface temperature, wave forces and intertidal structure in New Zealand. Mar. Ecol. Prog. Ser. 548, 77–95. doi: 10.3354/meps11671
Short, J., Foster, T., Falter, J., Kendrick, G. A., and McCulloch, M. T. (2015). Crustose coralline algal growth, calcification and mortality following a marine heatwave in Western Australia. Cont. Shelf Res. 106, 38–44. doi: 10.1016/j.csr.2015.07.003
Smale, D., Wernberg, T., Oliver, E., Thomsen, M. S., Harvey, B., Straub, S., et al. (2019). Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. doi: 10.1038/s41558-019-0412-1
Smale, D. A., and Wernberg, T. (2009). Satellite-derived SST data as a proxy for water temperature in nearshore benthic ecology. Mar. Ecol. Prog. Ser. 387, 27–37. doi: 10.3354/meps08132
Smale, D. A., and Wernberg, T. (2013). Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B Biol. Sci. 280, 1–9. doi: 10.1098/rspb.2012.2829
Smit, A. J., Oliver, E. C. J., and Schlegal, R. W. (2018). Detect Marine Heat Waves and Marine Cold Spells – Package ‘RMarineHeatWaves’. Available at: https://cran.r-project.org/web/packages/RmarineHeatWaves/RmarineHeatWaves.pdf.
Smith, S., and Simpson, R. (1995). Effects of the ’Nella Dan’oil spill on the fauna of Durvillaea antarctica holdfasts. Mar. Ecol. Prog. Ser. 121, 73–89. doi: 10.3354/meps121073
South, P. M., Lilley, S., Tait, L. W., Alestra, T., Hickford, M. J. H., Thomsen, M. S., et al. (2016). Transient effects of an invasive kelp on the community structure and primary productivity of an intertidal assemblage. Mar. Freshw. Res. 67, 103–112. doi: 10.1071/MF14211
South, P. M., and Thomsen, M. S. (2016). The ecological role of invading Undaria pinnatifida: an experimental test of the driver-passenger models. Mar. Biol. 163, 1–15. doi: 10.1007/s00227-016-2948-1
Taylor, D., Delaux, S., Stevens, C., Nokes, R., and Schiel, D. (2010). Settlement rates of macroalgal propagules: cross-species comparisons in a turbulent environment. Limnol. Oceanogr. 55:66. doi: 10.4319/lo.2010.55.1.0066
Taylor, D. I., and Schiel, D. R. (2003). Wave-related mortality in zygotes of habitat-forming algae from different exposures in southern New Zealand: the importance of ’stickability’. J. Exp. Mar. Biol. Ecol. 290, 229–245. doi: 10.1016/S0022-0981(03)00094-7
Taylor, D. I., and Schiel, D. R. (2005). Self-replacement and community modification by the southern bull kelp Durvillaea antarctica. Mar. Ecol. Prog. Ser. 288, 87–102. doi: 10.3354/meps288087
Thompson, G. A., and Schiel, D. R. (2012). Resistance and facilitation by native algal communities in the invasion success of Undaria pinnatifida. Mar. Ecol. Prog. Ser. 468:95. doi: 10.3354/meps09995
Thomsen, M. S., and Wernberg, T. (2005). What affects the forces required to break or dislodge macroalgae? A minireview. Eur. J. Phycol. 40, 1–10.
Thomson, J. A., Burkholder, D. A., Heithaus, M. R., Fourqurean, J. W., Fraser, M. W., Statton, J., et al. (2015). Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Glob. Chang. Biol. 21, 1463–1474. doi: 10.1111/gcb.12694
Valentine, J. P., and Johnson, C. R. (2003). Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J. Exp. Mar. Biol. Ecol. 295, 63–90. doi: 10.1016/S0022-0981(03)00272-7
Valentine, J. P., and Johnson, C. R. (2004). Establishment of the introduced kelp Undaria pinnatifida following dieback of the native macroalga Phyllospora comosa in Tasmania, Australia. Mar. Freshw. Res. 55, 223–230. doi: 10.1071/MF03048
Wernberg, T., Bennett, S., Babcock, R. C., Bettignies, T. D., Cure, K., Depczynski, M., et al. (2016). Climate driven regime shift of a temperate marine ecosystem. Science 353, 169–172. doi: 10.1126/science.aad8745
Wernberg, T., and Connell, S. D. (2008). Physical disturbance and subtidal habitat structure on open rocky coasts: effects of wave exposure, extent and intensity. J. Sea Res. 59, 237–248. doi: 10.1016/j.seares.2008.02.005
Wernberg, T., Russell, B., Thomsen, M. S., Gurgel, F. G., Bradshaw, C. J. A., Poloczanska, E. S., et al. (2011). Seaweeds in retreat from ocean warming. Curr. Biol. 21, 1–5. doi: 10.1016/j.cub.2011.09.028
Wernberg, T., Smale, D. A., Tuya, F., Thomsen, M. S., Langlois, T. J., de Bettignies, T., et al. (2013). An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 3, 78–82. doi: 10.1038/nclimate1627
Wernberg, T., Thomsen, M. S., Tuya, F., Kendrick, G. A., Staehr, P. A., and Toohey, B. D. (2010). Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecol. Lett. 13, 685–694. doi: 10.1111/j.1461-0248.2010.01466.x
Keywords: canopy forming seaweed, temperature anomaly, marine heatwave, extinction, endemic species, foundation species
Citation: Thomsen MS, Mondardini L, Alestra T, Gerrity S, Tait L, South PM, Lilley SA and Schiel DR (2019) Local Extinction of Bull Kelp (Durvillaea spp.) Due to a Marine Heatwave. Front. Mar. Sci. 6:84. doi: 10.3389/fmars.2019.00084
Received: 05 November 2018; Accepted: 13 February 2019;
Published: 06 March 2019.
Edited by:
Ke Chen, Woods Hole Oceanographic Institution, United StatesReviewed by:
Ezequiel Miguel Marzinelli, University of Sydney, AustraliaCopyright © 2019 Thomsen, Mondardini, Alestra, Gerrity, Tait, South, Lilley and Schiel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mads S. Thomsen, bWFkcy50aG9tc2VuQGNhbnRlcmJ1cnkuYWMubno=; bWFkcy5zb2xnYWFyZC50aG9tc2VuQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.