- 1Griffith School of Environment and Science, Griffith University, Southport, QLD, Australia
- 2Australian Rivers Institute, Griffith University, Southport, QLD, Australia
- 3National Oceanography Centre Southampton, University of Southampton Waterfront Campus, Southampton, United Kingdom
- 4Young Scientist Academy, Wilmington, NC, United States
- 5Red Sea Research Center, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
The perception that anthropogenic stressors cause jellyfish blooms is widespread within the scientific literature and media but robust evidence in support of these claims appears scarce. We used a citation analysis of papers published on “jellyfish blooms” to assess the extent to which such claims are made and the robustness of the evidence cited to support claims. Our search of the Web of Science returned 365 papers on “jellyfish blooms.” Each paper was searched for statements linking jellyfish blooms to specific anthropogenic stressors. For each statement we recorded the affirmation afforded to the claim, identified the stressors purported to cause blooms, the sources cited to support the statement, the type of study cited and the species studied in the cited source. Almost half the papers contained statements claiming that blooms were facilitated by anthropogenic stressors but most (70%) afforded a low degree of affirmation to the claim. We identified three major limitations in the evidence cited to support claims: (1) it was dominated by studies of two wide-spread and highly invasive taxa (Aurelia aurita and Mnemiopsis leidyi) that may not represent the responses of jellyfishes more generally; (2) the empirical evidence cited was dominated by correlative studies which, whilst useful for generating hypotheses, cannot attribute causation; and (3) the reviews most commonly-cited as evidence mostly cited circumstantial evidence and other reviews and provided conceptual models of how stressors could influence blooms, rather than robust evidence. We conclude that, although anthropogenic stressors could enhance jellyfish blooms, robust evidence is limited. Claims that strongly affirm anthropogenic stressors as causes of jellyfish blooms appear to be amplifying the evidence beyond that available. As a community we need to qualify the statements we make about jellyfish to strike a better balance between perpetuating perception and accurately portraying the state of knowledge.
Introduction
Ideas that gain widespread acceptance within a scientific discipline need to be supported by a substantial body of robust primary data. The formation and propagation of these ideas throughout the literature should be easily traced back to the primary data through the network of citations. Often the link to the primary data will be direct (i.e., when the primary data are directly cited) although it may be indirect, such as when reviews or meta-analyses that cite the primary data are cited instead of the primary data itself.
Scientific ideas, however, can become distorted as they are propagated throughout the literature. Distortion can take different forms, including bias (i.e., selective citation of evidence that supports a claim and failure to cite evidence contrary to the claim) and amplification [where the idea gains widespread acceptance despite supporting evidence being limited Greenberg, 2009]. Sometimes a small number of papers that are heavily cited have a disproportionate influence on the propagation of ideas. These papers have the potential to distort ideas if they exhibit bias or make statements that are not fully supported by robust primary data (Greenberg, 2009). Finally, hypotheses may become accepted as “fact” through sheer repetition (Greenberg, 2009).
Despite some contrary evidence (Condon et al., 2013), an idea that is widespread in the marine ecology literature is that jellyfish blooms are increasing. Indeed, >85% of papers that issued statements about jellyfish population trends claimed (with greater or lesser affirmation) that jellyfish blooms were increasing and none claimed blooms were decreasing (Sanz-Martin et al., 2016). Problematic jellyfish blooms certainly occur in some coastal regions of the world and often generate substantial scientific and media attention due to their negative interactions with tourism, power generation and fishing industries (Graham et al., 2014). These blooms are often portrayed as symptoms of degraded ocean environments (e.g., Jackson et al., 2001) and claims that they are caused by anthropogenic stressors such as climate change, eutrophication and overfishing pervade the scientific (e.g., Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012) and mainstream popular literature (e.g., Gershwin, 2013) and media (Condon et al., 2012).
More often than not, authors make generic statements about the responses of “jellyfish” (defined here as cnidarian medusae and ctenophores) to anthropogenic stressors rather than statements about specific species or taxa (e.g., Richardson et al., 2009). Jellyfish, however, are an ecologically diverse group of animals that occupy different geographic regions and habitats (Lucas et al., 2014), exhibit substantial trophic diversity and have diverse, and usually complex, life histories. The broad ecological and physiological diversity of these taxa suggests that not all species will respond similarly to environmental perturbations. The portrayal, therefore, of jellyfish as a group that will uniformly benefit by exposure to anthropogenic stressors is at odds with their diversity and distribution in the global ocean.
Sanz-Martin et al. (2016) recently identified that bias, through the selective citation of studies reporting increasing jellyfish populations contributed to the (as yet) unsubstantiated claim that jellyfish blooms are increasing globally (Condon et al., 2013). However, in addition to common assertions that jellyfish blooms are increasing globally, the published literature also frequently contains statements that anthropogenic stressors cause bloom events (e.g., Richardson et al., 2009; Purcell, 2012). We asked, therefore, whether claims that anthropogenic stressors cause jellyfish blooms are supported by robust primary data or whether such claims have also become distorted as they have propagated throughout the literature. We were particularly interested in assessing the types of evidence people cited to support their claims and whether the claims were supported by studies of just a few, or a broad suite of taxa. We, therefore, undertook a citation analysis of the literature to test the robustness of the evidence that underpins the perception that jellyfish blooms are facilitated by anthropogenic stressors. First, we determined the extent to which claims that anthropogenic stressors cause blooms are made within the scientific literature, we identified the stressors most commonly cited as causing blooms, assessed the types of evidence used to support each claim and determined the species that were studied in the cited sources. Finally, we critically analyzed the robustness of the evidence presented in the three most influential (i.e., highly cited) reviews on the topic.
Methods
Literature Search
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was used as a guide for undertaking this systematic review (Moher et al., 2015). We searched the Web of Science for papers published until Dec 31st, 2015 using the search term “jellyfish blooms.” Our aim was to return a representative sub-set of relevant literature to search rather than a comprehensive analysis of all literature on jellyfish blooms. We chose “jellyfish blooms” as the search term because this phrase is used very widely in the literature to describe bloom events and we wanted to focus on bloom-forming species. Moreover, since a recent citation analysis found no published papers stating that jellyfish blooms are decreasing (Sanz-Martin et al., 2016), we were not concerned about the potential for our search term to bias results toward studies of increasing rather than decreasing blooms. Papers that were not published in English or that could not be accessed after extensive searching were excluded. Each paper was searched for statements claiming that anthropogenic stressors cause jellyfish blooms or increases in jellyfish populations (Figure 1). It is important to note that we did not report on the findings of studies, rather only on statements issued regarding the drivers of blooms. Most statements occurred in the introduction and/or discussion and were supported by references to other studies. For inclusion in the citation network, statements needed to refer to specific types of stressors (e.g., eutrophication, overfishing) and the drivers needed to be linked to increasing (rather than just varying) jellyfish populations. Hence statements such as “It has been suggested that, in different places, jellyfish abundance may have increased in response to eutrophication, overfishing, and/or climate change” (Bastian et al., 2011) were included, but non-specific ones such as “….blooms may be increasing globally in response to changing ocean conditions” (Lalande and Fortier, 2011) were not, because such statements did not specifically state that the changing conditions were anthropogenic. A list of papers used in the network is provided in Tables S1, S2.
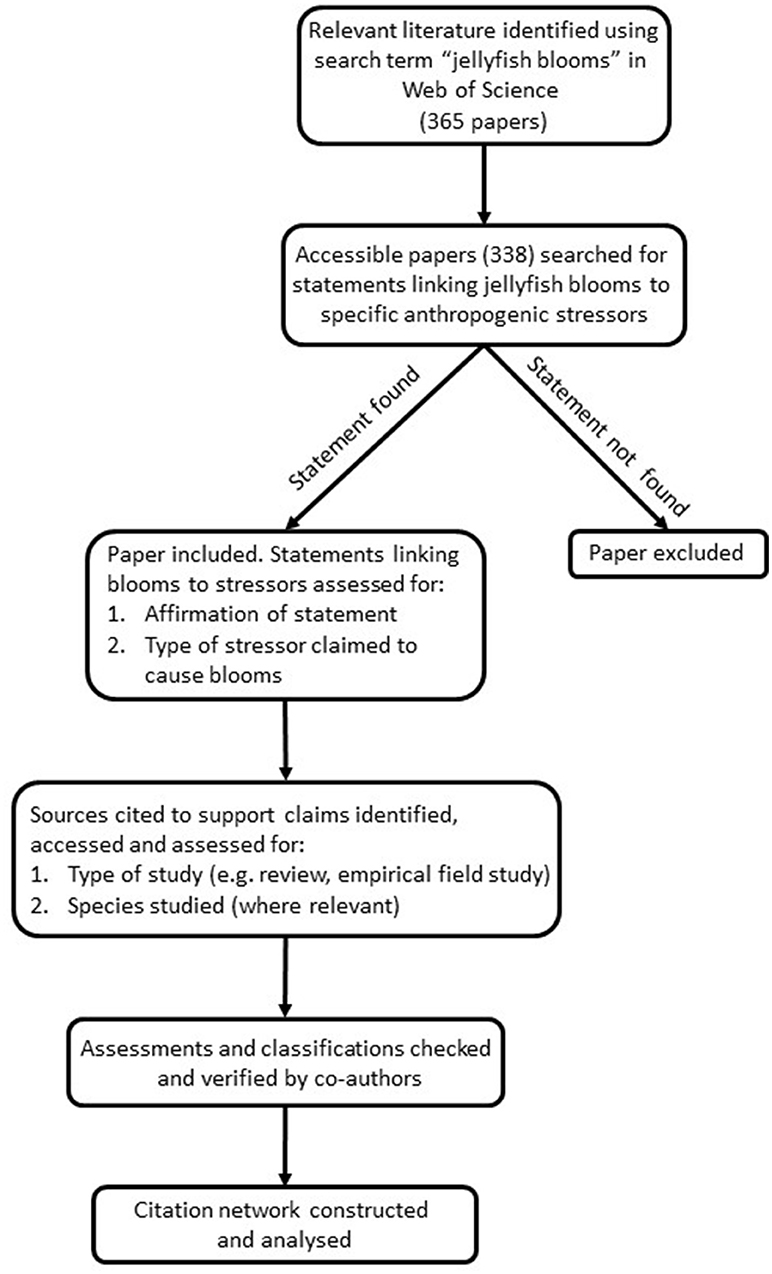
Figure 1. Flow diagram of the methodology used to select and assess papers in this systematic review.
For each statement we recorded the affirmation afforded to the claim (i.e., whether the author asserted that it was possible, probable or definite that anthropogenic stressors caused blooms; Table S1), identified the stressors purported to cause blooms, the sources cited to support the claim, the type of study cited (i.e., whether it was a review, a correlative study, a mensurative study, an experimental laboratory or field study, a model, a phylogenetic study or a social study), and, if applicable, the species studied by the cited source (Table S2). Often the source cited to support the attribution of blooms to a particular stressor was readily identified but sometimes (18.6% of sources) it was not possible to assign the cited source to a specific stressor. This situation usually occurred when multiple stressors were listed in the statement but all sources were cited at the end of the sentence, rather than after each stressor the source was used to support.
The initial selection and classification of statements was undertaken by one author and their decisions were verified by a second author. Whenever these two authors differed in opinion a third author was consulted and the statement was discussed until a consensus was reached. Approximately 6% of the original classifications were queried.
Construction of Citation Network
Using the citation threads, we constructed a citation network that identified the types of evidence being used to support claims and the papers that were the most influential (defined as having the highest citation rates) in supporting the idea that anthropogenic stressors are increasing jellyfish populations. Each node in the network represented a paper that either contained statements linking jellyfish blooms to anthropogenic stressors (“citing papers”) or that was cited as evidence that anthropogenic stressors are increasing jellyfish blooms (“cited papers”). Some papers were classified as both “citing and cited” because those papers cited other papers to support statements they made about links between jellyfish populations and anthropogenic stressors, but were also cited by others as evidence that anthropogenic stressors are increasing jellyfish populations. Links from node i to node j indicated that paper i was cited by paper j. The colors of the nodes of the cited papers depicted the type of study (e.g., a review or a correlational study) and the transition of the colors of the links from black to green indicated the direction of citing paper to cited paper. The network was created using the igraph package (Csardi and Nepusz, 2006) in the R statistical environment (R Core Team, 2016).
Analyses of the Three Most Influential Reviews
Four papers were identified as having had the greatest influence (defined as highest annual citation rate) in forming the perception that anthropogenic stressors cause jellyfish blooms, including three reviews (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012) and one study that combined empirical experiments in the field and laboratory with field-based observations (Duarte et al., 2013). The three reviews were further analyzed for the evidence they provided in support of specific stressors causing blooms.
For each of the three reviews, the sources cited in support of each the following categories and sub-categories of stressors were assessed: overfishing (predation on jellyfish, overfishing of competitors of jellyfish, and fishing down food webs), eutrophication (changes to food web-structure, hypoxia and turbidity), anthropogenic climate change (warming and acidification) and artificial structures (Table S3). Only sources that referred directly to jellyfish were included. For example, sources cited as evidence that eutrophication increases production of microplankton were not included but sources cited as evidence that jellyfish can prey on microplankton were included. The sources cited were classified as being circumstantial or direct. Circumstantial evidence relies on inference to connect it to a conclusion of fact whereas direct evidence supports the assertion directly. Examples of circumstantial evidence included observations that jellyfish could feed on microplankton (e.g., Colin et al., 2005; the inference being that eutrophication increases relative abundances of microplankton, therefore, eutrophication can stimulate jellyfish production); evidence that jellyfish are preyed upon by fish (e.g., Mianzan et al., 1996) or that their diets overlap with those of zooplanktivorous fish (e.g., Purcell and Sturdevant, 2001; the inference being that overfishing of jellyfish predators or competitors will enhance jellyfish blooms) and observations that jellyfish were more abundant during warming periods of climate cycles (e.g., Molinero et al., 2005; the inference being that human-induced ocean warming will enhance jellyfish numbers).
Results
Synthesized Findings
Our literature search returned 365 unique papers on the topic of “jellyfish blooms,” of which 338 were accessible and searched for statements that linked increasing jellyfish populations to anthropogenic stressors. The earliest papers returned by our search were published in 1995 (Olesen, 1995; Riisgard et al., 1995) but only two (Mills, 2001; Lynam et al., 2004) of the 15 papers published from 1995 to 2004 contained statements about anthropogenic drivers of blooms (Figure 2). The year 2005 heralded the start of an almost exponential increase in the number of papers published on the topic of jellyfish blooms and almost half (45 ± 6% SE) the papers published each year since then have included statements that associate increases in jellyfish populations with specific anthropogenic stressors (Figure 2).
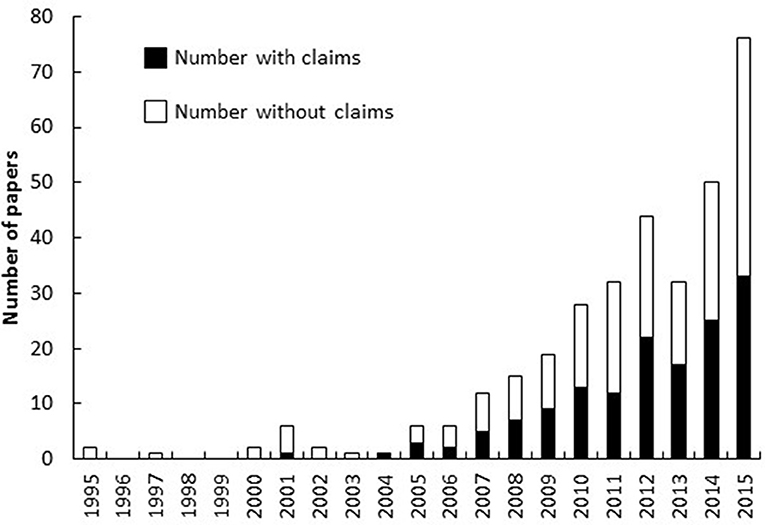
Figure 2. Number of papers published on the topic of “jellyfish blooms” each year that do and do not make claims that anthropogenic stressors cause jellyfish blooms.
Analysis of Citation Network
The citation network contained 283 papers: 150 papers made statements linking jellyfish increases to anthropogenic stressors (“citing papers”) and they cited 170 different papers to substantiate their claims (“cited papers”; Figure 3). Thirty-seven papers were “citing and cited.” Reviews were the most commonly cited (60% of all sources cited) type of paper for evidence of anthropogenic drivers of jellyfish blooms (Figure 3). Studies that correlated blooms with anthropogenic variables comprised 20% of all citations and experimental studies in the laboratory and field were cited relatively rarely (each comprised 5% of all citations).
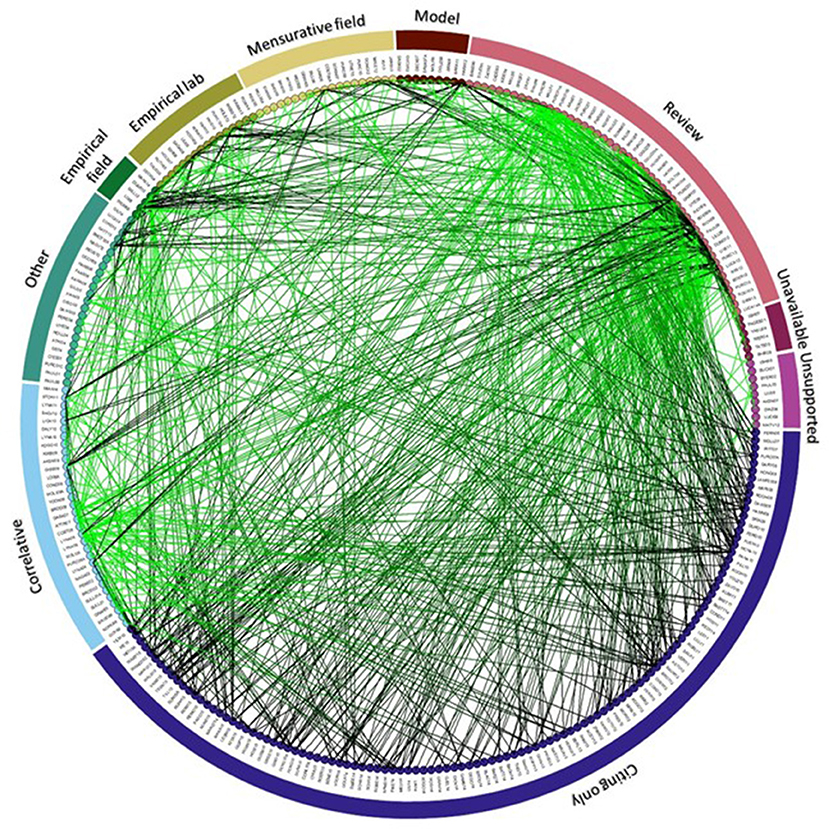
Figure 3. Network of citations. Nodes represent papers and lines connect citing and cited papers; the color of the line transitions from black (citing paper) to green (cited paper). Cited papers are arranged according to type of study and then chronologically (arranged clockwise). Individual papers are identified using a code available in Table S1.
More than half (60%) of the 170 papers that were cited in support of claims that blooms are caused by anthropogenic stressors were cited only once in the network and only thirteen papers were cited >10 times, indicating that a handful of papers have had a disproportionately large influence on conforming the perception that anthropogenic stressors cause jellyfish blooms (Figures 3, 4). The nine most frequently cited papers in support of such claims were: Purcell et al. (2007; 55 citations), Mills (2001; 44 citations), Purcell (2005; 40 citations), Richardson et al. (2009; 37 citations), Arai (2001; 31 citations), Purcell (2012; 29 citations), Lynam et al. (2006; 22 citations), Duarte et al. (2013; 16 citations), and Attrill et al. (2007) and Parsons and Lalli (2002); (15 citations each) but when the number of citations were standardized for the number of years since publication, three review papers (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012) and one empirical / observational study (Duarte et al., 2013) were identified as being most influential (Figure 5).
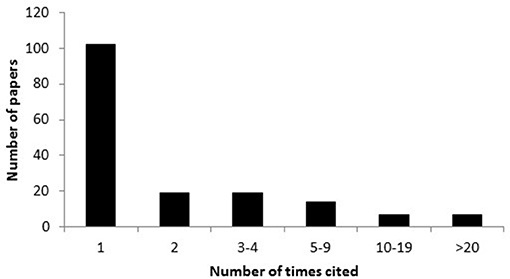
Figure 4. Frequency with which papers cited in support of anthropogenic stressors causing jellyfish blooms have been cited.
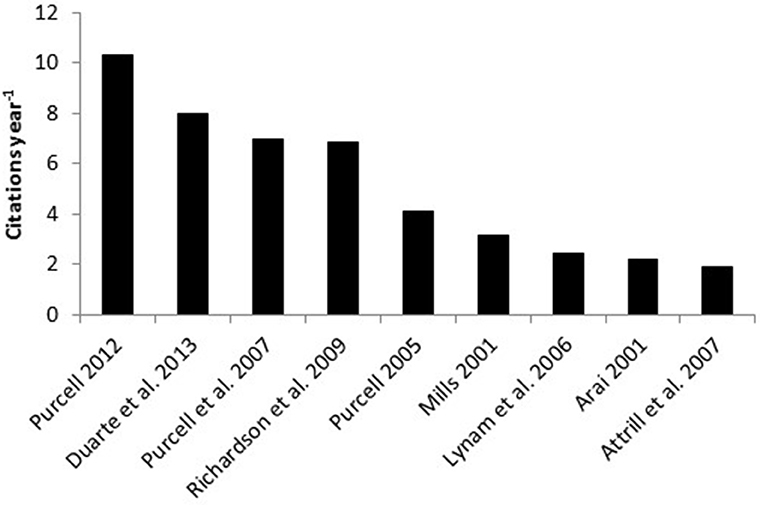
Figure 5. Average citation rate (number per year) of the papers most commonly cited in support of claims that anthropogenic stressors cause jellyfish blooms.
Most authors (68%) were cautious about attributing jellyfish blooms to anthropogenic stressors but 15% of authors considered it probable and 17% asserted that jellyfish blooms had anthropogenic causes. Eutrophication (including the associated hypoxia and ocean darkening), anthropogenic climate change (including ocean warming and acidification), and overfishing were the most commonly cited causes of blooms, comprising 25, 25, and 23% of citations, respectively. Ocean sprawl and invasive species accounted for 13 and 10% of claims, respectively (Figure 6).
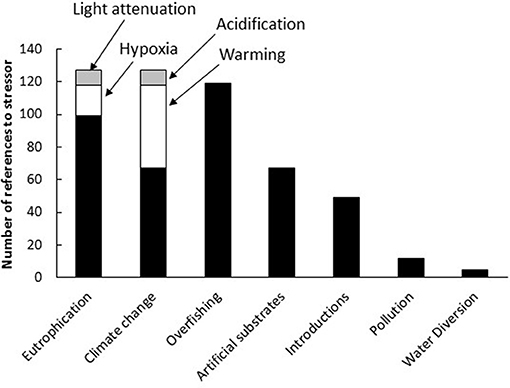
Figure 6. Frequency with which various anthropogenic stressors have been attributed to causing jellyfish blooms.
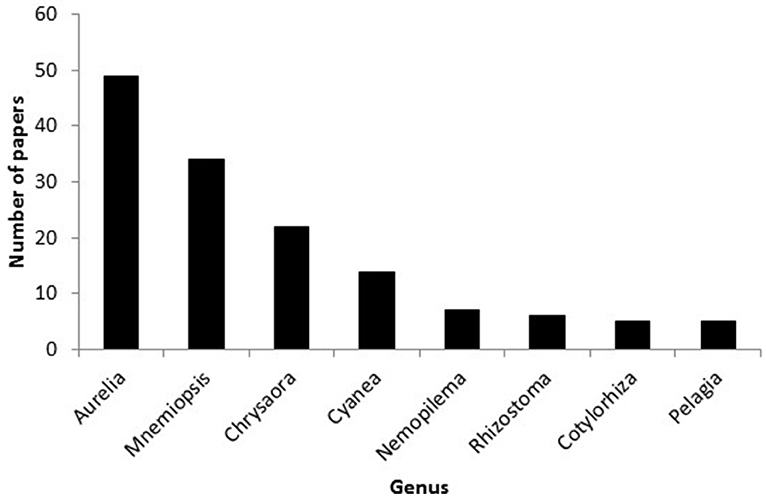
Studies of 46 different taxa derived from 17 genera of Hydrozoa; 13 genera of Scyphozoa, and three genera of Ctenophora were used to support claims relating anthropogenic stressors to jellyfish blooms. Scyphozoa were studied in 116 of the 170 different papers cited as evidence that anthropogenic stressors cause blooms, with Ctenophora and Hydrozoa featuring in the remaining 40 and 24, respectively. Studies of the scyphozoan genus Aurelia and the ctenophore species Mnemiopsis leidyi, however, dominated the literature, and were represented in almost half (47.6%) of all studies cited in support of claims. Species in the genera Chrysaora and Cyanea featured a moderate amount (21 and 13 studies respective) but the remaining taxa featured in a maximum of seven papers (Figure 7). Studies of just a few taxa, therefore, have had a disproportionate influence on forming perceptions about the response of jellyfish to anthropogenic stressors.
Analysis of the Three Most Influential Reviews
Two-thirds (67%) of the evidence cited in support of anthropogenic stressors causing blooms in the influential reviews of Purcell et al. (2007), Richardson et al. (2009) and Purcell (2012) was circumstantial (Figure 8). This was particularly apparent for claims relating to overfishing of jellyfish predators (9 of 11 papers cited were deemed to be circumstantial). These generally cited a combination of reviews (e.g., Arai, 2005) and mensurative studies (e.g., Link and Ford, 2006) to demonstrate that jellyfish are preyed on by vertebrates but did not demonstrate any direct link between overfishing of predators and increases in jellyfish numbers. Similarly, the circumstantial evidence cited for eutrophication-induced changes in food webs causing blooms mostly comprised laboratory-based feeding experiments that showed that some species of jellyfish ingest microplankton (e.g., Colin et al., 2005) and that jellyfish reproduce faster when more prey is available (e.g., Purcell et al., 1999) but did not demonstrate that jellyfish could grow and reproduce when consuming microplankton characteristic of eutrophic food webs. Notably, these three reviews also relied heavily on former reviews as evidence for claims that anthropogenic stressors cause blooms (25% of sources cited were other reviews).
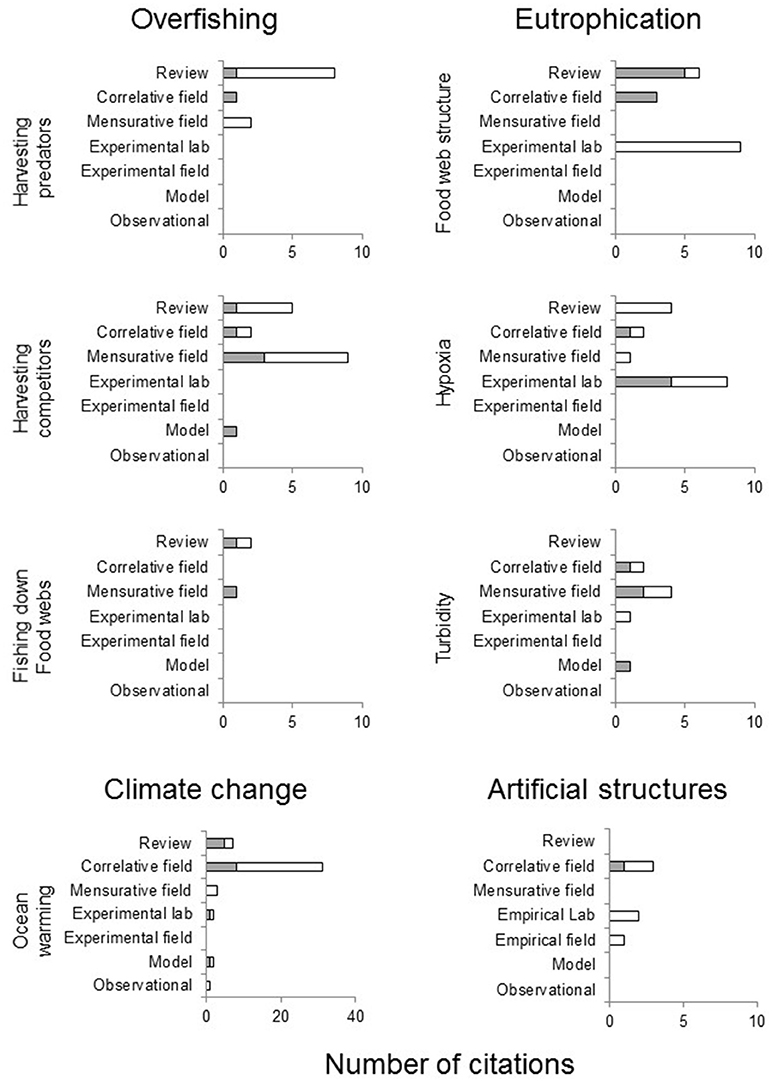
Figure 8. Types of studies cited as evidence by the three most influential reviews (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012) for the anthropogenic stressors most commonly cited to cause jellyfish blooms. White, circumstantial evidence; gray, non-circumstantial evidence.
Discussion
Summary of Main Findings
The perception that jellyfish blooms are caused by anthropogenic stressors appears to have been amplified in the literature beyond what can be reliably inferred from the primary data. Our conclusion is based primarily on three major limitations we identified in the evidence being cited in support of anthropogenic stressors causing blooms. These are: (1) many of the large-scale phenomena linked to jellyfish blooms (such as overfishing or climate change) are evidenced by correlative studies that cannot attribute causation; (2) most evidence is dominated by studies of just two taxa: Aurelia spp. and Mnemiopsis leidyi; (3) the three reviews (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012) and one empirical study (Duarte et al., 2013) that have had the greatest influence in forming the perception have relied heavily on circumstantial evidence. Consequently, whilst the hypothesis that anthropogenic stressors cause blooms is certainly plausible (and may yet be substantiated), currently it is not well-supported by robust evidence.
The primary data cited in support of claims that stressors cause blooms was dominated by correlative studies. This reflects the substantial challenges associated with studying pelagic ecosystems and taxa that are difficult to house in the laboratory or manipulate in field-based mescosms. Well-conducted correlative studies are certainly valuable for generating hypotheses and testing some hypotheses. Nevertheless, they cannot attribute causation and so must be interpreted cautiously. Indeed, the long-term (decadal) cycling that occurs in many jellyfish populations (Condon et al., 2013) poses a particular challenge for interpreting correlative studies because potentially spurious relationships may be identified if environmental variables correlate with the rising or falling phases of the cycle. For example, the inverse correlation between the biomass of jellyfish and forage fish in the Bering Sea during the 1990s (Brodeur et al., 2002), which has been cited frequently as evidence that overfishing may cause jellyfish blooms, coincided with the rising phase of a much longer-term jellyfish cycle (Brodeur et al., 2008). The relationship between jellyfish and forage fish subsequently broke down when the jellyfish population entered a declining phase in the early 2000s (Brodeur et al., 2008). Indeed correlations between environmental variables and population dynamics frequently break down when retested with new data (Myers, 1998) and inferring mechanistic understanding from correlational data can be problematic.
Studies of the scyphozoan medusa Aurelia spp. and the ctenophore Mnemiopsis leidyi, accounted for almost half of all taxa studied in papers cited to support anthropogenic stressors causing blooms. Aurelia spp. and Mnemiopsis leidyi are bloom-forming taxa (e.g., Sullivan et al., 2001; Hamner and Dawson, 2009), are widely distributed (Dawson, 2004) and highly invasive (Bayha and Graham, 2014). Invasive species generally exhibit greater phenotypic plasticity than non-invasive species and this trait can allow them to persist in unfavorable environmental conditions (e.g., Richards et al., 2006). Moreover, invasive populations of Mnemiopsis leidyi mature earlier and at much smaller body sizes than native populations of M. leidyi (Jaspers et al., 2018) and Aurelia sp 1 polyps appear to compete more successfully for space within benthic communities than other scyphozoans (Feng et al., 2017). Aurelia spp. and Mnemiopsis leidyi, therefore, may poorly represent the responses to anthropogenic stressors of other scyphozoans and ctenophores. Over-representation of these two species in the literature cited to support claims that jellyfish are robust to stressors may have fueled the perception that jellyfish, in general, are robust to anthropogenic stressors. Indeed, the response of individual species to anthropogenic stressors is probably determined by a complex interaction of their physiologies, life histories, and ecological relationships and jellyfish exhibit diversity across all these traits (e.g., Arai, 1997; Rutherford and Thuesen, 2005; Flemming et al., 2015). The World Register of Marine Species (http://www.marinespecies.org) recognizes (at 26/1/2018) 191 species and 69 genera of Scyphozoa, 47 species of Cubozoa, 3,684 species of hydromedusae and 200 species of Ctenophora. We have studied the responses to anthropogenic stressors of just a small proportion of the total biodiversity of jellyfish and many more taxa need to be studied before concluding that jellyfish, in general, are robust to anthropogenic stressors.
Reviews were most commonly cited in support of statements linking blooms to stressors. Reviews are often appropriate sources to cite because they summarize the status of the knowledge at the time of publication. Most of the evidence provided to support claims in the three most commonly cited reviews (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2012), however, was circumstantial and these three reviews often cited other reviews to support their statements. Moreover, the highly cited empirical and observational study by Duarte et al. (2013) also relied on circumstantial evidence to support conclusions. For example, they inferred that artificial substrates could promote jellyfish blooms because polyps were observed to settle prolifically (and sometimes preferentially) on artificial surfaces. They did not demonstrate, however, that prolific settlement of polyps on artificial surfaces would result in more medusae. The observation that the four most influential papers in the network relied primarily on circumstantial evidence, highlights the paucity of robust data that can unambiguously link proliferations of jellyfish to anthropogenic stressors.
The limitations of the evidence provided in the three most-cited reviews were clearly acknowledged by their authors but papers citing these studies often overlooked the caveats issued. Purcell et al. (2007) emphasized that only a few scyphozoan species are responsible for problematic blooms and provided examples of populations that may be negatively affected by anthropogenic stressors. Purcell et al. (2007) and Purcell (2012) clearly stated that anthropogenic causes of jellyfish blooms had not been demonstrated and that inferences were often drawn from correlative studies. Richardson et al. (2009) acknowledged that some of the evidence cited was circumstantial. Twenty-six percent of authors that cited these reviews, however, proffered strong assertions (i.e., affirmations were “probable” or “definite”) that anthropogenic stressors caused blooms. These three reviews, therefore, may have unintentionally amplified the perception that anthropogenic stressors cause blooms.
Robust citation practices and thorough evaluation of evidence are fundamental for ensuring accurate portrayal of the state of knowledge of a discipline. Our citation analysis of the literature used to support claims of a global rise in jellyfish (Sanz-Martin et al., 2016) identified selective citation of studies favoring increasing jellyfish populations facilitated a perception that was not substantiated when subsequently tested with quantitative data (Condon et al., 2013). Our current study has identified a different problem with citation practices; specifically that claims that anthropogenic stressors cause blooms have been amplified beyond what should be reasonably inferred given the types of studies and evidence available. Hence two major perceptions associated with jellyfish ecology appear to have been distorted, at least partially, by poor citation practices.
Problems of Perpetuating Perception
Only a relatively small number of jellyfish species form blooms. Scyphozoan jellyfish appear to account for most of the species that interfere with human enterprise but less than half the scyphozoan genera have been reported to aggregate, bloom or swarm (Hamner and Dawson, 2009). That only a subset of jellyfish species even form blooms (and that only some of these are problematic) is rarely acknowledged, however, in the statements published in the scientific literature (but see Purcell et al., 2007) and often entirely ignored when these results are communicated to the public and policy makers. Jellyfish ecologists should be vigilant in specifying that only a subset of species form problematic blooms to avoid perpetuating the perception that jellyfish, in general, are problematic. Qualifying the statements we issue about jellyfish, for example, by referring to “invasive jellyfish” or “problematic jellyfish” instead of “jellyfish” in general may help to more accurately convey the behavior of jellyfish populations to our peers and the public.
Conclusion
The idea that anthropogenic stressors cause jellyfish blooms appears to have been amplified beyond the evidence provided by the primary data. As a research community we should qualify the statements we issue about jellyfish, more critically evaluate the evidence we cite and strike a better balance between perpetuating perception and accurately portraying the state of knowledge, both in our scientific papers and in the way we convey our results to the public and policy makers.
Author Contributions
KP, CL, RC, and CD conceived the ideas and designed the methodology. KP, CL, and RC collated the data, BS-K undertook the network analysis, KP interpreted the data, KP led the writing of the manuscript and all authors contributed critically to drafts and gave final approval for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00451/full#supplementary-material
References
Arai, M. N. (2001). Pelagic coelenterates and eutrophication: a review. Hydrobiologia 451, 69–87. doi: 10.1023/A:1011840123140
Arai, M. N. (2005). Predation on pelagic coelenterates: a review. J. Mar. Biol. Assoc. UK 85, 523–536. doi: 10.1017/S0025315405011458
Attrill, M. J., Wright, J., and Edwards, M. (2007). Climate-related increases in jellyfish frequency suggest a more gelatinous future for the North Sea. Limnol. Oceanogr. 52, 480–485. doi: 10.4319/lo.2007.52.1.0480
Bastian, T., Stokes, D., Kelleher, J. E., Hays, G. C., Davenport, J., and Doyle, T. K. (2011). Fisheries bycatch data provide insights into the distribution of the mauve stinger (Pelagia noctiluca) around Ireland. Ices J Mar Sci. 68, 436–443. doi: 10.1093/icesjms/fsq178
Bayha, K. M., and Graham, W. M. (2014). “Nonindigenous marine jellyfish: invasiveness, invasibility, and impacts,” in Jellyfish Blooms, eds K.A. Pitt & C.H. Lucas. (Dordrecht; Heidelberg; New York, NY; London: Springer), 45–77.
Brodeur, R., Sugisaki, H., and Hunt, G. J. (2002). Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Mar. Ecol. Prog. Ser. 233, 89–103. doi: 10.3354/meps233089
Brodeur, R. D., Decker, M. B., Ciannelli, L., Purcell, J. E., Bond, N. A., Stabeno, P. J., et al. (2008). Rise and fall of jellyfish in the eastern Bering Sea in relation to climate regime shifts. Prog. Oceanogr. 77, 103–111. doi: 10.1016/j.pocean.2008.03.017
Colin, S. P., Costello, J. H., Graham, W. M., and Higgins, J. (2005). Omnivory by the small cosmopolitan hydromedusa Aglaura hemistoma. Limnol. Oceanogr. 50, 1264–1268. doi: 10.4319/lo.2005.50.4.1264
Condon, R. H., Duarte, C. M., Pitt, K. A., Robinson, K. L., Lucas, C. H., Sutherland, K. R., et al. (2013). Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. U.S.A. 110, 1000–1005. doi: 10.1073/pnas.1210920110
Condon, R. H., Graham, W. M., Duarte, C. M., Pitt, K. A., Lucas, C. H., Haddock, S. H. D., et al. (2012). Questioning the rise of gelatinous zooplankton in the world's oceans. Bioscience 62, 160–169. doi: 10.1525/bio.2012.62.2.9
Csardi, G., and Nepusz, T. (2006). The igraph software package for complex network research. InterJ. Complex Syst. 1695, 1–9.
Dawson, M. N. (2004). Macro-morphological variation among cryptic species of the moon jellyfish, Aurelia (Cnidaria: Scyphozoa) (vol 143, pg 369, 2003). Mar. Biol. 144, 203–203. doi: 10.1007/s00227-003-1230-5
Duarte, C. M., Pitt, K. A., Lucas, C. H., Purcell, J. E., Uye, S-i., Robinson, K., et al. (2013). Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 11:246. doi: 10.1890/110246
Feng, S., Wang, S. W., Zhang, G. T., Sun, S., and Zhang, F. (2017). Selective suppression of in situ proliferation of scyphozoan polyps by biofouling. Mar. Pollut. Bull. 114, 1046–1056. doi: 10.1016/j.marpolbul.2016.10.062
Flemming, N. E., Harrod, C., Newton, J., and Houghton, J. D. (2015). Not all jellyfish are equal: isotopic evidence for inter- and intraspecific variation in jellyfish trophic ecology. PeerJ. 3:e1110. doi: 10.7717/peerj.1110
Gershwin, L-A. (2013). Stung!: on Jellyfish Blooms and the Future of the Ocean. Chicago: The University of Chicago Press.
Graham, W. M., Gelcich, S., Robinson, K. L., Duarte, C. M., Brotz, L., Purcell, J. E., et al. (2014). Linking human well-being and jellyfish: ecosystem services, impacts, and societal responses. Front. Ecol. Environ. 12, 515–523. doi: 10.1890/130298
Greenberg, S. A. (2009). How citation distortions create unfounded authority: analysis of a citation network. Br. Med. J. 339:b2680. doi: 10.1136/bmj.b2680
Hamner, W. M., and Dawson, M. N. (2009). A review and synthesis on the systematics and evolution of jellyfish blooms: advantageous aggregations and adaptive assemblages. Hydrobiologia 616, 161–191. doi: 10.1007/s10750-008-9620-9
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–638. doi: 10.1126/science.1059199
Jaspers, C., Marty, L., and Kiørboe, T. (2018). Selection for life-history traits to maximize population growth in an invasive marine species. Glob. Chang. Biol. 24, 1164–1174. doi: 10.1111/gcb.13955
Lalande, C., and Fortier, L. (2011). Downward particulate organic carbon export and jellyfish blooms in southeastern Hudson Bay. J. Mar. Syst. 88, 446–450. doi: 10.1016/j.jmarsys.2010.12.005
Link, J. S., and Ford, M. D. (2006). Widespread and persistent increase of Ctenophora in the continental shelf ecosystem off NE USA. Mar. Ecol. Prog. Ser. 320, 153–159. doi: 10.3354/meps320153
Lucas, C. H., Jones, D. O. B., Hollyhead, C. J., Condon, R. H., Duarte, C. M., Graham, W. M., et al. (2014). Gelatinous zooplankton biomass in the global oceans: geographic variation and environmental drivers. Glob. Ecol. Biogeogr. 23, 701–714. doi: 10.1111/geb.12169
Lynam, C. P., Gibbons, M. J., Axelsen, B. E., Sparks, C. A., Coetzee, J., and Heywood, B. G. (2006). Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 16, R492–R493. doi: 10.1016/j.cub.2006.09.012
Lynam, C. P., Hay, S. J., and Brierley, A. S. (2004). Interannual variability in abundance of North Sea jellyfish and links to the North Atlantic Oscillation. Limnol. Oceanogr. 49, 637–643. doi: 10.4319/lo.2004.49.3.0637
Mianzan, H., Mari, N., Prenski, B., and Sanchez, F. (1996). Fish predation on neritic ctenophores from the Argentine continental shelf: a neglected food resource? Fish. Res. 27, 69–79. doi: 10.1016/0165-7836(95)00459-9
Mills, C. E. (2001). Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451, 55–68. doi: 10.1023/A:1011888006302
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1–9. doi: 10.1186/2046-4053-4-1
Molinero, J. C., Ibanez, F., Nival, P., Buecher, E., and Souissi, S. (2005). North Atlantic climate and northwestern Mediterranean plankton variability. Limnol. Oceanogr. 50, 1213–1220. doi: 10.4319/lo.2005.50.4.1213
Myers, R. A. (1998). When do environment-recruitment correlations work? Rev. Fish Biol. Fish 8, 285–305. doi: 10.1023/A:1008828730759
Olesen, N. J. (1995). Clearance potential of jellyfish “Aurelia aurita”, and predation impact on zooplankton in a shallow cove. Mar. Ecol. Prog. Ser. 124, 63–72. doi: 10.3354/meps124063
Parsons, T. R., and Lalli, C. M. (2002). Jellyfish population explosions: revisiting a hypothesis of possible causes. La mer 40, 111–121.
Purcell, J. E. (2005). Climate effects on formation of jellyfish and ctenophore blooms: a review. J. Mar. Biol. Assoc. UK 85, 461–476. doi: 10.1017/S0025315405011409
Purcell, J. E. (2012). Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Ann. Rev. Mar. Sci. 4, 209–235. doi: 10.1146/annurev-marine-120709-142751
Purcell, J. E., and Sturdevant, M. V. (2001). Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 210, 67–83. doi: 10.3354/meps210067
Purcell, J. E., Uye, S., and Lo, W-T. (2007). Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Mar. Ecol. Prog. Ser. 350, 153–174. doi: 10.3354/meps07093
Purcell, J. E., White, J. R., Nemazie, D. A., and Wright, D. A. (1999). Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Mar. Ecol. Prog. Ser. 180, 187–196. doi: 10.3354/meps180187
R Core Team (2016). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Staistical Computing. Available online at: https://www.R-project.org/
Richards, C. L., Bossdorf, O., Muth, N. Z., Gurevitch, J., and Pigliucci, M. (2006). Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 9, 981–993. doi: 10.1111/j.1461-0248.2006.00950.x
Richardson, A. J., Bakun, A., Hays, G. C., and Gibbons, M. J. (2009). The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol. 24, 312–322. doi: 10.1016/j.tree.2009.01.010
Riisgard, H. U., Bondo Christensen, P., Olsen, N. J., Petersen, J. K., Moller, M. M., and Andersen, P. (1995). Biological structure in a shallow cove (Kertinge Nor, Denmark) - control by benthic nutrient fluxes and suspension-feeding ascidians and jellyfish. Ophelia 41, 329–344. doi: 10.1080/00785236.1995.10422051
Rutherford, L. D., and Thuesen, E. V. (2005). Metabolic performance and survival of medusae in estuarine hypoxia. Mar. Ecol. Prog. Ser. 294, 189–200. doi: 10.3354/meps294189
Sanz-Martin, M., Pitt, K. A., Condon, R. H., Lucas, C. H., de Santana, C. N., and Duarte, C. M. (2016). Flawed citation practices facilitate the unsubstantiated perception of a global trend toward increased jellyfish blooms. Glob. Ecol. Biogeogr. 25, 1039–1049. doi: 10.1111/geb.12474
Keywords: citation analysis, network analysis, gelatinous zooplankton, medusae, ctenophore
Citation: Pitt KA, Lucas CH, Condon RH, Duarte CM and Stewart-Koster B (2018) Claims That Anthropogenic Stressors Facilitate Jellyfish Blooms Have Been Amplified Beyond the Available Evidence: A Systematic Review. Front. Mar. Sci. 5:451. doi: 10.3389/fmars.2018.00451
Received: 07 July 2018; Accepted: 12 November 2018;
Published: 29 November 2018.
Edited by:
Paul E. Renaud, Akvaplan-niva, NorwayReviewed by:
Erik Caroselli, Università degli Studi di Bologna, ItalyGuillem Chust, Centro Tecnológico Experto en Innovación Marina y Alimentaria (AZTI), Spain
Copyright © 2018 Pitt, Lucas, Condon, Duarte and Stewart-Koster. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kylie A. Pitt, ay5waXR0QGdyaWZmaXRoLmVkdS5hdQ==
 Kylie A. Pitt
Kylie A. Pitt Cathy H. Lucas
Cathy H. Lucas Robert H. Condon
Robert H. Condon Carlos M. Duarte
Carlos M. Duarte Ben Stewart-Koster
Ben Stewart-Koster