
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mar. Sci. , 11 October 2018
Sec. Marine Biogeochemistry
Volume 5 - 2018 | https://doi.org/10.3389/fmars.2018.00341
This article is part of the Research Topic Facing Marine Deoxygenation View all 15 articles
The shelf sediments off Namibia are some of the most unusual and extreme marine habitats because of their extremely high hydrogen sulphide concentrations. High surface productivity of the northern Benguela upwelling system provides benthic life with so much carbon that biotic processes must rely on innovative mechanisms to cope with perennial anoxia and toxic hydrogen sulphide. Bottom dwelling communities are forced to adapt lifestyles to deal physiologically and behaviourally with these stressful conditions. The upside of hydrogen sulphide is that it fuels extensive mats of large sulphide-oxidizing bacteria on the seabed, which create detoxified habitat niches and food for the animals living there. The threat of hypoxic stress exacerbated by hydrogen sulphide is largely overcome in the water column by microbes that detoxify sulphide, allowing animals in the upper water layers to thrive in this productive upwelling area. The bearded goby Sufflogobius bibarbatus is a cornerstone species that successfully couples the inhospitable benthic environment with the pelagic. Benthic studies have as yet not characterized the sulphidic shelf communities, which have the potential to uncover biotic adaptations to toxic sulphide. This ancient shelf upwelling system has long operated under hypoxic pressure, balancing always the abundance of particulate food against oxygen limitation and hydrogen sulphide toxicity. Challenges faced by this unique system could include environmental changes related to climate change, or man-made physical disturbances of the anoxic, sulphide-rich seabed sediments.
The highly productive Benguela Upwelling Ecosystem plays a major role in the circulation and fisheries production of the South Atlantic Ocean (Currie, 1953; Shannon, 1985; Shannon and Nelson, 1996). The upwelling regime has persisted for millenia (Diester-Haass et al., 2002). Despite the Namibian shelf being considered one of the most inhospitable, oxygen depleted, and sulphidic open shelf environments on earth (Baturin, 2002), it has sustained one of the world's most spectacular concentrations of marine life (Howarth et al., 2014). Palaeo-construction from inner shelf sediment cores reveals abundant fish populations over the last 3,200 years (Struck et al., 2002).
Much has been learned of how this ancient system successfully couples biological abundance with severe oxygen limitation, exacerbated by hydrogen sulphide. In this synthesis we review relevant knowledge regarding the biological integration of naturally occurring hydrogen sulphide into the system.
The broad continental shelf off central Namibia slopes gently to a shelf break at 300–350 m (Shannon, 1985). Intense euphotic productivity has long been observed (summarised in (Shannon and Pillar, 1986)), with primary production for the Benguela estimated at 0.37 Gt C yr−1 (Carr, 2002). A near-constant supply of organic material sinks towards the ocean floor adding to a diatomaceous, sulphidic mud belt that spans the inner Namibian shelf for >700 km in waters < 200 m water depth (Bremner, 1983; Emeis et al., 2004). Extraordinarily high organic carbon accumulation [up to 23% dry weight (Bremner, 1978; Inthorn et al., 2006; Mollenhauer et al., 2007 and references therein] promotes bacterial production of hydrogen sulphide (H2S). Compared to other Eastern Boundary Upwelling Systems, these features combined with a lack of reactive iron in the sediments to precipitate sulphide, concurrent build-up of methane, and low-oxygen upwelling source water, promote regular and frequent occurrences of H2S in the water column so characteristic of Namibian waters. Extensive mats of Large Sulphide-oxidizing Bacteria (LSB) cover the mud, fueled by a continual, plentiful supply of H2S from the sediment. Although not initially identified for their key role in the ecosystem when recorded as “slimy grass” from historical grab samples (von Bonde, 1928), these were almost certainly the Sulphide-oxidizing Bacteria described over 70 years later (Schulz et al., 1999). Historical records designated these areas to an “azoic zone” and also describe “sulphur eruptions” along the central coast (Gilchrist, 1914; Marchand, 1928; von Bonde, 1928; Copenhagen, 1934, 1953; Hart and Currie, 1960).
Most of Namibia's coast experiences perennial coastal upwelling (Shannon and Nelson, 1996). Oxygen-poor water from the Angola gyre (Hart and Currie, 1960; Stander, 1964; Bubnov, 1972) is entrained into South Atlantic Central Water, which dominates the mix of upwelling source water onto the shelf, particularly during late summer to autumn (Chapman and Shannon, 1987; Mohrholz et al., 2008). Although low in oxygen, this remotely-formed source water is neither sulphidic nor anoxic, varying intra- and inter-annually in oxygen content (Mohrholz et al., 2008). It intercepts the upper slope and shelf of the central coast to contribute to a permanent Oxygen Minimum Zone (OMZ: < 0.5 ml O2 L−1; Helly and Levin, 2004). Microbial break-down from high organic loading over the shelf increases oxygen demand (Hart and Currie, 1960; Calvert and Price, 1971; Chapman and Shannon, 1987; Bailey, 1991). Direct contact of bottom water with the seabed influences dissolved components of the OMZ further (van der Plas et al., 2007). When H2S diffuses into bottom water it becomes totally anoxic (Brüchert et al., 2003).
High concentrations of H2S characterize the inner shelf surface sediments between 19°S and 27°S (Brüchert et al., 2006). Here bacterial sulphate reduction rates in water depths 28–200 m vary between 3.1 and 62.7 mmol m−2 day−1. Dissolved H2S in porewaters can reach 22 mM just 10 cm below the sediment surface, consistently exceeding 2 mM at 6 cm sediment-depth. Limited oxidative precipitation of H2S occurs, mainly due to low reactive iron in the diatomaceous mud (Brüchert et al., 2003, 2006; Borchers et al., 2005). The sulphide-rich muds favour trace metal enrichment, with some precipitation of the metals by H2S (Borchers et al., 2005).
Intense microbial decay succession in the sediments leads to biogenic production of free methane gas within 100 cm of the sediment surface. These gas accumulations are patchy within the sediment, but significantly cover at least 1,350 km2 of the mud belt (Emeis et al., 2004).
Occasional occurrences of H2S in bottom water are accompanied by extreme oxygen depletion. H2S concentrations can reach >100 μM total H2S (e.g., Copenhagen, 1934; Brüchert et al., 2003, 2006, 2009; Emeis et al., 2004; Lavik et al., 2009). Compared to sedimentary input, in situ generation of H2S in the water column likely contributes minimally to these events, as measurements of sulphate reduction rates in bottom water showed no correlation to observed amounts of H2S (Brüchert et al., 2006).
Various mechanisms responsible for transport of H2S from the sediment into the overlying water have been suggested, mainly by diffusion and ebullition (Emeis et al., 2004; Weeks et al., 2004; Brüchert et al., 2006, 2009; van der Plas et al., 2007). The temporal and spatial variability of water column H2S suggests that multiple mechanisms are active on the shelf, with gas ebullition closer inshore and diffusive supply in deeper waters (Brüchert et al., 2006, 2009). Whatever transport mechanism(s) are involved (discussed in Weeks et al., 2002, 2004; Emeis et al., 2004; Brüchert et al., 2006, 2009; Altenbach and Struck, 2006; van der Plas et al., 2007; Ohde and Dadou, 2018), the reality is that relatively high concentrations of H2S do regularly occur in the water column, which pelagic organisms have to contend with.
During episodic, ephemeral “sulphur eruptions” described since the late nineteenth century (summarised in Hart and Currie, 1960) large amounts of H2S rapidly pervade the whole water column. The H2S oxidizes to colloidal sulphur that is clearly visible as milky turquoise surface water, which can be photographed and identified from space (Weeks et al., 2002, 2004; Ohde et al., 2007; Ohde and Dadou, 2018). Severe episodes have co-occurred with mass mortalities of marine life (Gilchrist, 1914; Copenhagen, 1953; Currie, 1953). Their true impact to the ecosystem (apart from obvious onshore wash-ups of dead littoral animals and fish) has not been quantified.
Methane in the water column is common (Scranton and Farrington, 1977; Monteiro et al., 2006). Ebullition of a mixture of H2S and methane can explain sudden high concentrations of sulphide in the water (Emeis et al., 2004; Brüchert et al., 2006, 2009). Occasionally sedimentary methane dislodges whole chunks of mud, as evidenced by floating islands (Waldron, 1900; summary in Rogers and Bremner, 1991), and craters on the seabed (Brüchert et al., 2006).
Large Sulphide-oxidizing Bacteria (LSB) belonging to the Beggiatoceae fuel their metabolism with H2S (Schulz and Jorgensen, 2001), converting H2S into non-toxic sulphur that accumulates as distinctive shiny white micro-granules in their cytoplasm. During anoxic conditions, nitrate stored in large vacuoles is used as the electron acceptor for anaerobic oxidation of sulphide.
Discovery of extensive mats of active LSB covering the Namibian inner shelf were reported in 1999 (Schulz et al., 1999). Dominated by Thiomargarita namibiensis, nicknamed the “sulphur pearl of Namibia,” these enormous, spherical bacteria reach a biomass of 47 g m−2 wet weight. As described in Salman et al. (2011, 2013) several members of the Beggiatoacea are abundant on the Namibian shelf. They flexibly use oxygen or nitrate as electron acceptors (Schulz et al., 1999; Schulz and De Beer, 2002; Brock and Schulz-Vogt, 2011; Salman et al., 2013), so are ideally suited to thrive on the plentiful sedimentary H2S supply coupled with fluctuating bottom-water oxygen conditions. The bacterial mats are considered effective to stop most of the upward-diffusing hydrogen sulphide from entering the water column (Brüchert et al., 2006), and provide a detoxified microhabitat for eukaryotic benthic communities (Levin, 2003; Levin et al., 2009; Figure 1). These bacteria produce polyphosphates, resulting in high concentrations of inorganic phosphate in sediment pore waters during anoxic periods (Schulz and Schulz, 2005; van der Plas et al., 2007; Goldhammer et al., 2010; Brock and Schulz-Vogt, 2011). This sedimentary phosphate has the potential to enrich the upwelling water that passes over the shelf (Currie, 1953; van der Plas et al., 2007).
Despite the bacterial barrier, H2S can comprise up to 25% of the total oxygen consumption in water on the shelf (Brüchert et al., 2006). When H2S does diffuse into the water column, a consortium of anaerobic chemolithotrophic bacteria take on the detoxifying role. They metabolize H2S using nitrate in the anaerobic waters to catalyze the conversion of H2S to harmless sulphur (Lavik et al., 2009; Figure 1). Such events of H2S in the lower water column may go unnoticed in surface water, because bacteria consume sulphide before it reaches the air–sea interface (Vaquer-Sunyer and Duarte, 2010).
Contrary to the historical misnomer of “azoic,” the diatomaceous mud belt is not barren of metazoan life (Edelman-Furstenberg and Kidwell, 2015). H2S imposes severe respiratory stress on benthic animals that differ in tolerance at both species and population levels (Jahn and Theede, 1997; Vaquer-Sunyer and Duarte, 2010). The benthic invertebrate fauna of the Namibian sulphidic muds have not yet been characterized; critically this should include the small-sized (< 300 μm) component. A pioneer study of macrofaunal diversity on a transect through the sulphidic mud from Walvis Bay (23°S) showed a 10-fold increase in diversity from 100 to 200–300 m water depths, as oxygen shifted from just under 2% saturation to 11–15% saturation (Sanders, 1969). Leiter and Altenbach (2010) found the heterotrophic foraminiferan Virgulinella fragilis largely restricted to the sulphidic shelf environment, co-occurring with less numerous Nonionella stella and Discammina compressa. Suggested possible survival strategies for V. fragilis included symbiotic sulphide-oxidizing bacteria, functional kleptoplasts, and peroxisome proliferation.
Macrofaunal components include annelids, molluscs and crustaceans (Copenhagen, 1953; Levin, 2003; Zettler et al., 2009, 2013; Eisenbarth and Zettler, 2016). LSB mats offer a potential abundant food supply for species that can tolerate the sulphur (Levin, 2003, 2005), but diets have not yet been examined. Polychaetes associated with the Namibian microbial mats have elaborate appendages to maximize oxygen uptake: Diopatra sp. has long spiral branchiae, nereids have posterior branchial proliferations and a tube-dwelling pectinariid has anterior gill filaments (Levin et al., 2009). Mollusc deposits at 133 m water depth on the shelf at 20°S reveal chemoautotrophic bivalves Lucinoma capensis, found also in recent samples (Edelman-Furstenberg, 2014). Lucinids are characteristic of upwelling systems with high, steady organic supply to H2S-rich sediments, as discussed in Edelman-Furstenberg and Kidwell (2015). Other large-sized (>1 mm) taxa recorded from sulphidic muds are widely distributed over the Namibian shelf e.g., the gastropod Nassarius vinctus that extends its siphon to oxic waters (Figure 1); the polychaete Paraprionospia pinnata and the cumacean Iphinoe africana (Zettler et al., 2009, 2013; Edelman-Furstenberg, 2014; Steffani et al., 2015; Eisenbarth and Zettler, 2016). With the broad distribution of these species, their presence in sulphidic areas is considered not directly related to H2S, but possibly due to a detoxified habitat niche provided by the bacteria.
Even in low concentrations, H2S is usually toxic to vertebrates by inhibiting cytochrome c oxidase in the mitochondria (Bagarinao, 1992; Jahn and Theede, 1997). To survive fluctuating sulphidic environments, vertebrates require behavioural and physiological flexibility (Childress, 1995; Hagerman, 1998; Vaquer-Sunyer and Duarte, 2010). Namibian shelf fish distributions have not been attributed directly to H2S, but given that dissolved H2S reduces oxygen, tolerances of fish to gradients of oxygen-depletion are important (Gallo and Levin, 2016). The low diversity of the central shelf demersal fish assemblage between 19 and 27°S is ascribed to very low oxygen conditions (Hamukuaya et al., 1998). It is dominated by a few species, namely Cape hake (Merluccius capensis), horse mackerel (Trachurus capensis), and bearded goby (Sufflogobius bibarbatus). For adult and juvenile horse mackerel, critical oxygen levels are reported as 10% and 11.2–13.2% air saturation, respectively (Ekau et al., 2010; Geist et al., 2013). Hake tolerate oxygen concentrations as low as 10.9 μmol kg−1 (0.2 ml L−1; Woodhead et al., 1998). The bearded goby has a critical oxygen level of 5.3% air saturation (Utne-Palm et al., 2010) and tolerates oxygen levels as low as 0.3 μmol kg −1 or < 0.12% air saturation (Salvanes et al., 2011). The goby's oxygen consumption is unaffected by 100–200 μM total sulphide (corresponding to 6–12 μM H2S), dropping to a few percent at 11 to 14 μM H2S, but shuts off at 500 μM total sulphide (30 μM H2S; Utne-Palm et al., 2010). Gobies surviving high H2S levels appear to rely on deep metabolic depression with extreme anoxia tolerance facilitated by anaerobic respiration, to cope with sulphide induced respiratory stress, rather than H2S tolerant cytochrome c or other respiratory strategies such as fermentation or specialized blood pigment (but see discussion in Utne-Palm et al., 2010).
High-resolution acoustic surveys of animal activity over the shelf OMZ visualize an empty area (no back scattering) directly over the sulphidic mud where severely hypoxic conditions prevail (0–0.2 ml L1, 8.6 μmol kg −1, 3.4% of surface air saturation; (Utne-Palm et al., 2010); Figure 2). During daytime large densities of gobies only, were present on the seabed, whilst predator species hake and horse mackerel were trawled deeper on the outer shelf where bottom water oxygen levels increased to >0.3 ml L−1, 13.02 μmol kg −1, >5% air saturation (Utne-Palm et al., 2010; Figure 2A). During hours of anaerobic exposure, gobies generate a lactate buildup or “oxygen debt” (Utne-Palm et al., 2010), likely explaining their migration into oxygen-sufficient (>20% air saturation) pelagic environments at night to replenish oxygen and feed in dense pelagic plankton layers from 60 m and higher above the sediment (Figure 2C). They return to the seabed at dawn to avoid visual predation by burying in the sulphidic mud (Salvanes et al., 2011). Gobies feed opportunistically on both pelagic and benthic organisms (Cedras et al., 2011; Hundt et al., 2011). Fatty acid and stable isotope signatures indicate that the diatom- and bacteria-rich sulphidic sediments contribute to approximately 15% of the gobies' diet (Van der Bank et al., 2011).
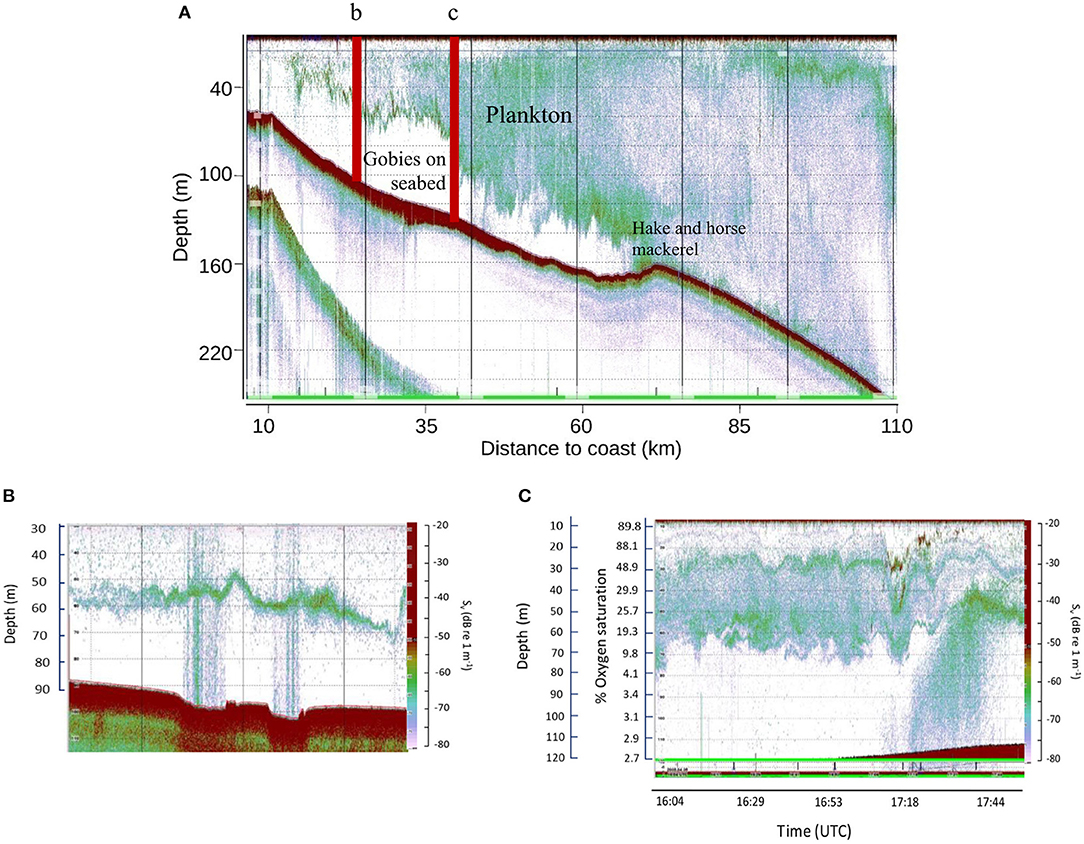
Figure 2. Linking of respiratory niches on the Namibian shelf. (A) Composite 10-day high resolution acoustic image across the Namibian shelf, 23°20′S to 23°40′S showing lack of pelagic animals in the severely oxygen depleted bottom waters over the sulphidic mud (dissolved oxygen 0.1–0.2 ml L−1, or 4.3–8.6 μmol kg −1; 1.7–3.4% air saturation). As bottom water oxygen increases to >0.3 ml L−1 (13 μmol kg −1; >5% air saturation) on the outer shelf in depths >160 m, plankton and fish (hake and horse mackerel) appear in bottom waters. The position of images (b) and (c) are indicated. (B) Methane bubbling from sediment. (C) Goby migration at sunset from sulphidic mud to oxygenated layers to replenish oxygen and enter the food web. Image credits: Images from (Utne-Palm, 2008), and Utne-Palm et al. (2010) Supplementary Information with permission.
Whilst in the water column, gobies themselves are eaten, comprising >50% prey of commercially important hake and horse mackerel, and top predators (Crawford et al., 1985; David, 1987; Salvanes and Gibbons, 2018). This is especially relevant following the regime shift associated with the collapse of the sardine population (Cury and Shannon, 2004; van der Lingen et al., 2006).
Benthic-pelagic coupling includes inorganic and biological pathways, though the biological links are more difficult to quantify (Marcus and Boero, 1998; Griffiths et al., 2017). In boundary upwelling OMZs, where steep vertical oxygen gradients segregate species (Levin, 2003), biological coupling is essential to overcome and fully exploit the different respiratory niches. The Namibian shelf offers an example of a mature ecosystem that exploits H2S and integrates it into biological food webs (Figures 1, 2).
• Methane coupled to microbial H2S production in the sediments plays a key role in release of H2S into the water column.
• Bacterial decay accumulates H2S in the pore water. Some H2S promotes metal burial within the sediment, beneficially keeping high metal concentrations unavailable for bio-uptake by animals in oxic waters.
• LSB on surface sediments (i) consume H2S, lessening its toxic and deoxygenating effects on pelagic organisms (ii) establish a detoxified niche/habitat for benthos (iii) enrich overlying upwelling water with phosphate.
• Nutrient-rich upwelled water supports abundant phytoplankton diatom-dominated blooms. Sinking diatoms form biogenic mud where bacterial degradation accumulates H2S in pore water. Escape of H2S into the water column is detoxified by chemolithotrophic bacteria that mitigate sulphidic damage to pelagic life.
• Metazoan specialists equipped to cope with severely hypoxic to anoxic and sulphidic conditions integrate carbon from the sulphidic environment into the shelf food web. Key players are bacteria, benthic invertebrates and the remarkable bearded goby, which as both predator and prey, constitutes a cornerstone species in this ecosystem.
Reproductive stages of metazoan organisms are usually more vulnerable than adults to oxygen stress (Levin et al., 2009). Nursery grounds of commercially important pelagic and demersal fish species coincide spatially with the highest occurrences of sulphide eruptions on the Namibian shelf (Emeis et al., 2004), making young stages particularly vulnerable to sulphide outbreaks. An example is the catastrophic loss of juvenile hake in 1994, when they fled anoxic conditions on the shelf and were heavily cannibalized by adult hake in deeper waters (Hamukuaya et al., 1998). Extended batch spawning, high tolerances of key species to low oxygen, and horizontal transport of larvae and young across the shelf from better oxygenated areas, may be key to surviving sporadic sulphide events (Sundby et al., 2001; Hutchings et al., 2002; Utne-Palm et al., 2010; Geist et al., 2013). Gobies attach their eggs demersally (Skrypzeck et al., 2014) but the tolerance of eggs and larvae to H2S remains unstudied.
Much remains to be understood of the responses of Namibian shelf biota to hydrogen sulphide. The timing, triggering, intensity and recovery of severe H2S events and episodic “eruptions” remain elusive and inconclusive, despite various theories (Weeks et al., 2002, 2004; Emeis et al., 2004; Altenbach and Struck, 2006; Brüchert et al., 2006, 2009; van der Plas et al., 2007; Ohde and Dadou, 2018). To be genuinely useful to an ecosystem approach by ocean managers, the triggering of severe sulphidic episodes needs to be known. Whilst the oceanographical studies contribute to understanding, atmospheric pressure studies possibly affecting methane ebullition, merit further investigation.
The benthic invertebrate communities of the sulphidic inner shelf are not characterized. Scanty and inadequate sampling of the sulphidic mud fringes only, is documented. Dedicated qualitative, quantitative and experimental benthic studies will be required to understand how the animals living in this risky environment survive and contribute to the ecological functioning of the Namibian shelf system. Key knowledge gaps are how resilient the species are to H2S exposure and sulphide-exacerbated hypoxic stress. What are critical levels, and sub-lethal effects from these stressors? Do the mats of LSB indeed protect benthic fauna from severe sulphide exposure in a narrow niche on the sediment? The nutritional role of LSB in the diet of the benthic animals is unknown, but are likely food (e.g., Levin, 2005) given the extensive bacterial coverage and its biomass estimates (e.g., Schulz et al., 1999). In the interest of both biogeochemistry and biology, it would be interesting to estimate how much carbon is fixed by the LSB mats. Also of interest will be investigations to examine whether the small benthic invertebrates living in the sulphidic environment are equipped with symbioses or metabolic mechanisms that allow them to survive intermittent, and sometimes high concentrations of H2S. Metabolic responses to sulphide and anoxic stress (e.g., Larade and Storey, 2002; Menon et al., 2005) are largely unexplored.
At community level, it is not known whether extinction-recolonisation by the shelf fauna occurs after intense sulphidic “eruption” events. Life history strategies must have developed to deal with this risky environment: are life histories boom-and-bust, with short, highly fecund life cycles, serial spawning and efficient larval dispersal to promote species survival through extreme events, and/or are vulnerable early life stages replenished from better oxygenated areas on the shelf, following extinctions from episodes of sulphide? Reproductive strategies of benthic and pelagic species are key to understanding how this shallow system carries on despite the stress from H2S.
As summarized by Tobler et al. (2016), organisms living in H2S-rich habitats provide unique examples that answer fundamental biological questions, such as how some organisms cope with environmental stressors considered lethal for most others; how biological processes—from cellular to ecosystem level—respond to H2S; and how H2S can shape the evolution of ecosystems. Ecological opportunities offered by sulphidic environments include resource availability, reduced competition, and reduced exposure to natural predators. Future studies could contribute to understanding human-induced environmental change and develop potential biomedical applications (Tobler et al., 2016; Breiland et al., 2018). With increased deoxygenation predicted on a global scale (Deutsch et al., 2011) and emerging as a major threat to coastal ecosystems globally (Vaquer-Sunyer and Duarte, 2010) it is relevant to examine systems that have long adjusted to extreme oxygen stress and H2S.
Eastern boundary upwelling areas, such as the northern Benguela off Namibia, serve as primary centres for fishery production. Taking modern cumulative pressures on the ocean into account as fisheries management moves towards an ecosystem-based approach in Namibia, and internationally, a better understanding of trophic interactions that couple anoxic benthic environments with productive fishing zones is important.
BC proposed the synthesis and all authors contributed to writing the manuscript. BC led manuscript production with contributions and comments from all authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge support from their parent organizations, though no funding was specifically allocated to this project. AGVS was funded by the University of Bergen and the Fullbright Foundation. Jock Currie is thanked for technical and editorial comments to the final manuscript. The authors acknowledge the multiple research cruises in Namibian waters, tainted with a strong odour of hydrogen sulphide that was well worth the exciting research findings!
Altenbach, A., and Struck, U. (2006). “Some remarks on Namibia's shelf environments, and a possible teleconnection to the hinterland,” in The Changing Culture and Nature of Namibia: Case Studies, ed H. Leser (Basel: The Sixth Namibia Workshop; Basler Afrika Bibliographien), 109–124.
Bagarinao, T. (1992). Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat. Toxicol. 24, 21–62. doi: 10.1016/0166-445X(92)90015-F
Bailey, G. W. (1991). “Organic carbon flux and development of oxygen deficiency on the modern Benguela continental shelf south of 22°S: spatial and temporal variability,” in Modern and Ancient Continental Shelf Anoxia, eds R. D. Tyson and T. H. Pearson (London: Geological Society, Special Publications), 171–183.
Baturin, G. N. (2002). Nodular fraction of phosphatic sand from the Namibia Shelf. Lithol. Mineral Resour. 37, 1–17. doi: 10.1023/A:1013628020381
Borchers, S. L., Schnetger, B., Böning, P., and Brumsack, H. J. (2005). Geochemical signatures of the Namibian diatom belt: perennial upwelling and intermittent anoxia. Geochem. Geophys. Geosyst. 6:20. doi: 10.1029/2004GC000886
Breiland, A. A., Flood, B. E., Nikrad, J., Bakarich, J., Husman, M., Rhee, T., et al. (2018). Polyphosphate-accumulating bacteria: potential contributors to mineral dissolution in the oral cavity. Appl. Environ. Microbiol. 84, e02440–e02417. doi: 10.1128/AEM.02440-17
Bremner, J. M. (1978). Sediments on the Continental Margin off South West Africa Between Latitudes 170 and 250 S, PhD thesis, University of Cape Town, 300.
Bremner, J. M. (1983). “Biogenic sediments on the SW African (Namibian) continental margin,” in Coastal Upwelling: its Sediment Record. Part B: Sedimentary Records of Ancient Coastal Upwelling, eds E. Suess and J. Thiede (New York, NY: Plenum Press), 610.
Brock, J., and Schulz-Vogt, H. N. (2011). Sulfide induces phosphate release from polyphosphate in cultures of a marine Beggiatoa strain. ISME J. 5, 497–506. doi: 10.1038/ismej.2010.135
Brüchert, V., Currie, B., and Peard, K. R. (2009). Hydrogen sulphide and methane emissions on the central Namibian shelf. Prog. Oceanogr. 83, 169–179. doi: 10.1016/j.pocean.2009.07.017
Brüchert, V., Currie, B., Peard, K. R., Lass, U., Endler, R., Dübecke, A., et al. (2006). “Biogeochemical and physical control on shelf anoxia and water column hydrogen sulphide in the Benguel a coastal upwelling system off Namibia,” in Past and Present Water Column Anoxia (Dordrecht: Springer), 161–193.
Brüchert, V., Jorgensen, B. B., Neumann, K., Riechmann, D., Schlösser, M., and Schulz, H. (2003). Regulation of bacterial sulfate reduction and hydrogen sulfide fluxes in the central Namibian coastal upwelling zone. Geochim. Cosmochim. Acta 67, 4505–4518. doi: 10.1016/S0016-7037(03)00275-8
Bubnov, V. A. (1972). Structure and characteristics of the oxygen minimum layer in the southeastern Atlantic. Oceanology 12, 193–201.
Calvert, S. E., and Price, N. B. (1971). Upwelling and nutrient regeneration in the Benguela Current, October, 1968. Deep-Sea Res. Oceanogr. Abstr. 18, 505–523. doi: 10.1016/0011-7471(71)90074-X
Carr, M.-E. (2002). Estimation of potential productivity in Eastern Boundary Currents using remote sensing. Deep Sea Res Part II Topical Studies Oceanogr. 49, 59–80. doi: 10.1016/S0967-0645(01)00094-7
Cedras, R. B., Salvanes, A.-G., and Gibbons, M. J. (2011). Investigations into the diet and feeding ecology of the bearded goby Sufflogobius bibarbatus off Namibia. Afr. J. Mar. Sci. 33, 313–320. doi: 10.2989/1814232X.2011.600431
Chapman, P., and Shannon, L. V. (1987). Seasonality in the oxygen minimum layers at the extremities of the Benguela system. South Afr. J. Mar. Sci. 5, 85–94. doi: 10.2989/025776187784522162
Childress, J. J. (1995). Life in sulfidic environments: historical perspective and current research trends. Integr. Comp. Biol. 35, 83–90. doi: 10.1093/icb/35.2.83
Copenhagen, W. J. (1934). Occurrence of Sulphides in Certain Areas of the Sea Bottom on the South African Coast. Investigational Report No 3, Fisheries and Marine Biological Survey Division, Department of Commerce and Industries, Union of South Africa.
Copenhagen, W. J. (1953). The periodic mortality of fish in the Walvis region. S. Afr. J. Sci. 49:330.
Crawford, R. J. M., Cruickshank, R. A., Shelton, P. A., and Kruger, I. (1985). Partitioning of a goby resource amongst four avian predators and evidence for altered trophic flow in the pelagic community of an intense, perennial upwelling system. South Afr. J. Mar. Sci. 3, 215–228. doi: 10.2989/025776185784461252
Cury, P., and Shannon, L. (2004). Regime shifts in upwelling ecosystems: observed changes and possible mechanisms in the northern and southern Benguela. Prog. Oceanogr. 60, 223–243. doi: 10.1016/j.pocean.2004.02.007
David, J. H. M. (1987). Diet of the South African fur seal (1974–1985) and an assessment of competition with fisheries in southern Africa. South Afr. J. Mar. Sci. 5, 693–713. doi: 10.2989/025776187784522568
Deutsch, C., Brix, H., Ito, T., Frenzel, H., and Thompson, L. (2011). Climate-forced variability of ocean hypoxia. Science 333, 336–339. doi: 10.1126/science.1202422
Diester-Haass, L., Meyers, P. A., and Vidal, L. (2002). The late Miocene onset of high productivity in the Benguela Current upwelling system as part of a global pattern. Mar. Geol. 180, 87–103. doi: 10.1016/S0025-3227(01)00207-9
Edelman-Furstenberg, Y. (2014). Distribution and paleoecology of molluscan skeletal remains along an upwelling tract: Benguela system, Namibian shelf. Mar. Geol. 353, 153–162. doi: 10.1016/j.margeo.2014.04.011
Edelman-Furstenberg, Y., and Kidwell, S. M. (2015). Chemosymbiont-dominated seafloor communities in modern and Cretaceous upwelling systems support a new, high-productivity variant of standard low-oxygen models. Geology 43, 975–978. doi: 10.1130/G37017.1
Eisenbarth, S., and Zettler, M. L. (2016). Diversity of the benthic macrofauna off northern Namibia from the shelf to the deep sea. J. Mar. Syst. 155, 1–10. doi: 10.1016/j.jmarsys.2015.10.017
Ekau, W., Auel, H., Pörtner, H.-O., and Gilbert, D. (2010). Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences 7, 1669–1699. doi: 10.5194/bg-7-1669-2010
Emeis, K. C., Brüchert, V., Currie, B., Endler, R., Ferdelman, T., Kiessling, A., et al. (2004). Shallow gas in shelf sediments of the Namibian coastal upwelling ecosystem. Cont. Shelf Res. 24, 627–642. doi: 10.1016/j.csr.2004.01.007
Gallo, N. D., and Levin, L. A. (2016). “Fish ecology and evolution in the world's oxygen minimum zones and implications of ocean deoxygenation,” in Advances in Marine Biology (London: Elsevier), 117–198.
Geist, S. J., Ekau, W., and Kunzmann, A. (2013). Energy demand of larval and juvenile Cape horse mackerels, Trachurus capensis, and indications of hypoxia tolerance as benefit in a changing environment. Mar. Biol. 160, 3221–3232. doi: 10.1007/s00227-013-2309-2
Gilchrist, J. D. F. (1914). Marine Biological Report for the Year ending 30th June, 1914. Cape Town: Union of South Africa. Available online at: http://archive.org/details/marinebiological21914cape (Accessed March 31, 2017).
Goldhammer, T., Brüchert, V., Ferdelman, T. G., and Zabel, M. (2010). Microbial sequestration of phosphorus in anoxic upwelling sediments. Nat. Geosci. 3:557. doi: 10.1038/ngeo913
Griffiths, J. R., Kadin, M., Nascimento, F. J. A., Tamelander, T., Törnroos, A., Bonaglia, S., et al. (2017). The importance of benthic–pelagic coupling for marine ecosystem functioning in a changing world. Glob. Chang. Biol. 23, 2179–2196. doi: 10.1111/gcb.13642
Hagerman, L. (1998). Physiological flexibility; a necessity for life in anoxic and sulphidic habitats. Hydrobiologia 375–376, 241–254. doi: 10.1023/A:1017033711985
Hamukuaya, H., O'Toole, M. J., and Woodhead, P. M. J. (1998). Observations of severe hypoxia and offshore displacement of Cape hake over the Namibian shelf in 1994. South Afr. J. Mar. Sci. 19, 57–59.
Helly, J. J., and Levin, L. A. (2004). Global distribution of naturally occurring marine hypoxia on continental margins. Deep Sea Res. Part I Oceanogr. Res. Papers 51, 1159–1168. doi: 10.1016/j.dsr.2004.03.009
Howarth, L. M., Roberts, C. M., Thurstan, R. H., and Stewart, B. D. (2014). The unintended consequences of simplifying the sea: making the case for complexity. Fish Fish. 15, 690–711. doi: 10.1111/faf.12041
Hundt, M., Utne-Palm, A. C., and Gibbons, M. J. (2011). Cross-shelf observations of diet and diel feeding behaviour of the bearded goby Sufflogobius bibarbatus off Namibia. Afr. J. Mar. Sci. 33, 119–126. doi: 10.2989/1814232X.2011.572365
Hutchings, L., Beckley, L. E., Griffiths, M. H., Roberts, M. J., Sundby, S., Lingen, C., et al. (2002). Spawning on the edge: spawning grounds and nursery areas around the southern African coastline. Mar. Freshwater Res. 53, 307–318. doi: 10.1071/MF01147
Inthorn, M., Wagner, T., Scheeder, G., and Zabel, M. (2006). Lateral transport controls distribution, quality, and burial of organic matter along continental slopes in high-productivity areas. Geology 34, 205–208. doi: 10.1130/G22153.1
Jahn, A., and Theede, H. (1997). Different degrees of tolerance to hydrogen sulphide in populations of Macoma balthica (Bivalvia, Tellinidae). Mar. Ecol. Prog. Ser. 154, 185–196. doi: 10.3354/meps154185
Larade, K., and Storey, K. B. (2002). A profile of the metabolic responses to anoxia in marine. Sens. Signal. Cell Adaptation 3, 27–46. doi: 10.1016/S1568-1254(02)80005-5
Lavik, G., Stührmann, T., Brüchert, V., Van der Plas, A., Mohrholz, V., Lam, P., et al. (2009). Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature 457, 581–584. doi: 10.1038/nature07588
Leipe, T. (2004). Cruise report: R/V A.v.Humboldt, cruise No. 44/04/03, leg 3. Warnemuende: Baltic Sea Research Institute.
Leiter, C., and Altenbach, A. V. (2010). Benthic foraminifera from the diatomaceous mud belt off Namibia: characteristic species for severe anoxia. Palaeontologia Electronica 13:19.
Levin, L. A. (2003). Oxygen minimum zone benthos: adaptation and community response to hypoxia. Oceanogr. Mar. Biol. 41, 1–45.
Levin, L. A. (2005). Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. Oceanogr. Mar. Biol. 43, 1–46. doi: 10.1201/9781420037449.ch1
Levin, L. A., Ekau, W., Gooday, A. J., Jorissen, F., Middelburg, J. J., Naqvi, S. W. A., et al. (2009). Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences 6, 2063–2098, doi: 10.5194/bg-6-2063-2009
Marchand, J. M. (1928). “Special reports: the nature of the sea-floor deposits in certain regions on the west coast,” in Fisheries and Marine Biological Survey. Report No. 6 for the Year Ending June, 1928, ed C. von Bonde (Pretoria: Union of South Africa), 11.
Marcus, N. H., and Boero, F. (1998). Minireview: the importance of benthic-pelagic coupling and the forgotten role of life cycles in coastal aquatic systems. Limnol. Oceanogr. 43, 763–768. doi: 10.4319/lo.1998.43.5.0763
Menon, J., Willsie, J. K., Tauscher, A., and Arp, A. J. (2005). Epidermal ultrastructure and implications for sulfide tolerance in six species of deep-sea polychaetes. Invertebrate Biol. 122, 334–346. doi: 10.1111/j.1744-7410.2003.tb00098.x
Mohrholz, V., Bartholomae, C. H., Van der Plas, A. K., and Lass, H. U. (2008). The seasonal variability of the northern Benguela undercurrent and its relation to the oxygen budget on the shelf. Continental Shelf Res. 28, 424–441. doi: 10.1016/j.csr.2007.10.001
Mollenhauer, G., Inthorn, M., Vogt, T., Zabel, M., Sinninghe Damsté, J. S., and Eglinton, T. I. (2007). Aging of marine organic matter during cross-shelf lateral transport in the Benguela upwelling system revealed by compound-specific radiocarbon dating. Geochem. Geophys. Geosyst. 8:Q09004. doi: 10.1029/2007GC001603
Monteiro, P. M., van der Plas, A. K. A., Mohrholz, V., Mabille, E., Pascal, A., and Joubert, W. (2006). Variability of natural hypoxia and methane in a coastal upwelling system: ocean physics or shelf biology? Geophys. Res. Lett. 33:L16614. doi: 10.1029/2006GL026234
Ohde, T., and Dadou, I. (2018). Seasonal and annual variability of coastal sulphur plumes in the northern Benguela upwelling system. PLoS ONE 13:e0192140. doi: 10.1371/journal.pone.0192140
Ohde, T., Siegel, H., Rei\s smann, J., and Gerth, M. (2007). Identification and investigation of sulphur plumes along the Namibian coast using the MERIS sensor. Continental Shelf Res. 27, 744–756. doi: 10.1016/j.csr.2006.11.016
Rogers, J., and Bremner, J. M. (1991). The Benguela ecosystem. Part VII. Marine-geological aspects. Oceanogr. Mar. Biol. 29, 1–85.
Salman, V., Amann, R., Girnth, A. C., Polerecky, L., Bailey, J. V., Høgsland, S., et al. (2011). A single-cell sequencing approach to the classification of large, vacuolated sulfur bacteria. Syst. Appl. Microbiol. 34, 243–259. doi: 10.1016/j.syapm.2011.02.001
Salman, V., Bailey, J. V., and Teske, A. (2013). Phylogenetic and morphologic complexity of giant Sulphur bacteria. Antonie van Leeuwenhoek, 104, 169–186. doi: 10.1007/s10482-013-9952-y
Salvanes, A. G., Utne-Palm, A. C., Currie, B., and Braithwaite, V. A. (2011). Behavioural and physiological adaptations of the bearded goby, a key fish species of the extreme environment of the northern Benguela upwelling. Mar. Ecol. Prog. Ser. 425, 193–202. doi: 10.3354/meps08998
Salvanes, A. G. V., and Gibbons, M. J. (2018). Adaptation to hypoxic environments; bearded gobies Sufflogobius bibarbatus in the Benguela upwelling ecosystem. J. Fish Biol. 92, 752–772. doi: 10.1111/jfb.13547
Sanders, H. L. (1969). “Benthic marine diversity and the stability-time hypothesis,” in Brookhaven Symposia in Biology (Upton, NY: Brookhaven National Laboratory), 71–81.
Schulz, H. N., Brinkhoff, T., Ferdelman, T. G., Mariné, M. H., Teske, A., and Jørgensen, B. B. (1999). Dense populations of a giant sulfur bacterium in Namibian shelf sediments. Science 284, 493–495. doi: 10.1126/science.284.5413.493
Schulz, H. N., and De Beer, D. (2002). Uptake rates of oxygen and sulfide measured with individual Thiomargarita namibiensis cells by using microelectrodes. Appl. Environ. Microbiol. 68, 5746–5749. doi: 10.1128/AEM.68.11.5746-5749.2002
Schulz, H. N., and Jorgensen, B. B. (2001). Big bacteria. Annu. Rev. Microbiol. 55, 105–137. doi: 10.1146/annurev.micro.55.1.105
Schulz, H. N., and Schulz, H. D. (2005). Large sulfur bacteria and the formation of phosphorite. Science 307, 416–418. doi: 10.1126/science.1103096
Scranton, M. I., and Farrington, J. W. (1977). Methane production in the waters off Walvis Bay. J. Geophys. Res. 82, 4947–4953. doi: 10.1029/JC082i031p04947
Shannon, L. V. (1985). The Benguela ecosystem. Part I. Evolution of the Benguela, physical features and processes. Oceanogr. Mar. Biol. 23, 105–182.
Shannon, L. V., and Nelson, G. (1996). “The Benguela: large scale features and processes and system variability,” in The South Atlantic: Present and Past Circulation, eds G. Wefer, W. H. Berger, G. Siedler, and D. J. Webb (Berlin: Springer-Verlag), 163–210.
Shannon, L. V., and Pillar, S. C. (1986). The Benguela ecosystem. Part III. Plankton. Oceanogr. Mar. Biol. 24, 65–170.
Skrypzeck, H., Salvanes, A. G., Currie, B., and Kotze, A. (2014). First records of reproductive behaviour and early development of the bearded goby Sufflogobius bibarbatus. J. Fish Biol. 84, 1256–1261. doi: 10.1111/jfb.12347
Stander, G. H. (1964). The Benguela Current off South West Africa. Investigational Report 2. Marine Research Laboratory, South West Africa.
Steffani, N., Sedick, S., Rogers, J., and Gibbons, M. J. (2015). Infaunal benthic communities from the inner shelf off Southwestern Africa are characterised by generalist species. PLoS ONE 10:e0143637. doi: 10.1371/journal.pone.0143637
Struck, U., Altenbach, A. V., Emeis, K.-C., Alheit, J., Eichner, C., and Schneider, R. (2002). Changes of the upwelling rates of nitrate preserved in the δ15N-signature of sediments and fish scales from the diatomaceous mud belt of Namibia. Geobios 35, 3–11. doi: 10.1016/S0016-6995(02)00004-9
Sundby, S., Boyd, A. J., Hutchings, L., O'Toole, M. J., Thorisson, K., and Thorsen, A. (2001). Interaction between Cape hake spawning and the circulation in the Northern Benguela upwelling ecosystem. South Afr. J. Mar. Sci. 23, 317–336. doi: 10.2989/025776101784528971
Tobler, M., Passow, C. N., Greenway, R., Kelley, J. L., and Shaw, J. H. (2016). The evolutionary ecology of animals inhabiting hydrogen sulfide–rich environments. Ann. Rev. Ecol. Evol. System. 47, 239–262. doi: 10.1146/annurev-ecolsys-121415-032418
Utne-Palm, A. C. (2008). Cruise Report: Gobies and Hake in the Hypoxic Waters of the Benguela Upwelling Current. Cruise reports, R/V. G.O. Sars.
Utne-Palm, A. C., Salvanes, A. G., Currie, B., Kaartvedt, S., Nilsson, G. E., Braithwaite, V. A., et al. (2010). Trophic structure and community stability in an overfished ecosystem. Science 329, 333–336. doi: 10.1126/science.1190708
Van der Bank, M. G., Utne-Palm, A. C., Pittman, K., Sweetman, A. K., Richoux, N. B., Brüchert, V., et al. (2011). Dietary success of a ‘new'key fish in an overfished ecosystem: evidence from fatty acid and stable isotope signatures. Mar. Ecol. Prog. Ser. 428, 219–233. doi: 10.3354/meps09078
van der Lingen, C. D., Shannon, L. J., Cury, P., Kreiner, A., Moloney, C. L., Roux, J.-P., et al. (2006). “Resource and ecosystem variability, including regime shifts, in the Benguela Current System,” in Large Marine Ecosystems Benguela - Predicting a Large Marine Ecosystem, eds V. Shannon, G. Hempel, P. Malanotte-Rizzoli, C. Moloney, and J. Woods (Elsevier), 147–184.
van der Plas, A. K., Monteiro, P. M. S., and Pascall, A. (2007). Cross-shelf biogeochemical characteristics of sediments in the central Benguela and their relationship to overlying water column hypoxia. Afr. J. Mar. Sci. 29, 37–47. doi: 10.2989/AJMS.2007.29.1.3.68
Vaquer-Sunyer, R., and Duarte, C. M. (2010). Sulfide exposure accelerates hypoxia-driven mortalit. Limnol. Oceanogr. 55, 1075–1082. doi: 10.4319/lo.2010.55.3.1075
von Bonde, C. (1928). Fisheries and Marine Biological Survey. Report No. 5 for the Years 1925-1927. Pretoria: Union of South Africa.
Waldron, F. W. (1900). On the appearance and disappearance of a mud island at Walfish Bay. Trans. South Afr. Philos. Soc. 11, 185–188. doi: 10.1080/21560382.1900.9525965
Weeks, S. J., Currie, B., and Bakun, A. (2002). Satellite imaging: massive emissions of toxic gas in the Atlantic. Nature 415:493. doi: 10.1038/415493b
Weeks, S. J., Currie, B., Bakun, A., and Peard, K. R. (2004). Hydrogen sulphide eruptions in the Atlantic Ocean off southern Africa: implications of a new view based on SeaWiFS satellite imagery. Deep Sea Res. Part I Oceanogr. Res. Papers 51, 153–172. doi: 10.1016/j.dsr.2003.10.004
Woodhead, P. M., Hamukuaya, H., O'Toole, M. J., and McEnroe, M. (1998). “Effects of oxygen depletion in shelf waters on hake populations off central and northern Namibia,” in International Symposium, Environmental Variability in the South East Atlantic, eds L. V. Shannon and M. J. O'Toole (Swakopmund: NATMIRC), 10.
Zettler, M. L., Bochert, R., and Pollehne, F. (2009). Macrozoobenthos diversity in an oxygen minimum zone off northern Namibia. Mar. Biol. 156, 1949–1961. doi: 10.1007/s00227-009-1227-9
Keywords: hydrogen sulphide, Namibia, benthic-pelagic coupling, Northern Benguela, shelf ecosystem
Citation: Currie B, Utne-Palm AC and Salvanes AGV (2018) Winning Ways With Hydrogen Sulphide on the Namibian Shelf. Front. Mar. Sci. 5:341. doi: 10.3389/fmars.2018.00341
Received: 01 June 2018; Accepted: 05 September 2018;
Published: 11 October 2018.
Edited by:
Arthur Capet, MAST-University of Liège, BelgiumReviewed by:
Dirk De Beer, Max-Planck-Gesellschaft (MPG), GermanyCopyright © 2018 Currie, Utne-Palm and Salvanes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bronwen Currie, Y3VycmllMzJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.