
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 18 September 2018
Sec. Marine Megafauna
Volume 5 - 2018 | https://doi.org/10.3389/fmars.2018.00333
This article is part of the Research Topic Future Directions in Research on Marine Megafauna View all 20 articles
One of the lesser known species of baleen whales, the Bryde's whale, also known as Eden's whale (Balaenoptera edeni edeni and B. edeni brydei), although hunted as part of a North Pacific Japanese research programme1, was not heavily exploited by commercial whaling and remains a data deficient species. Their taxonomic status is not fully resolved and they are often mistaken for other species leading to uncertainty about their true distribution, behavior and conservation status. Some populations are critically endangered, whilst others are small but have high genetic diversity suggesting wider connectivity. The species' unpredictable coastal and offshore global distribution throughout warm-temperate waters has led to populations with unknown genetic variation, and facing different threats. Few areas are well-studied, but each study reveals often contrasting movement patterns, foraging strategies, and vocal repertoires; there are considerable knowledge gaps for Bryde's whales. There are few Bryde's populations with abundance estimates but they typically number in the mid- to high-hundreds of individuals, with other populations small, <100 mature individuals, and exposed to high levels of anthropogenic impacts. Future research should focus on understanding the diversity within and between populations. Here, we suggest an integrative, comparative approach toward future work on Bryde's whales, including acoustic monitoring, trophic interactions, telemetry tools, understanding their novel behaviors, and resolving their species status. This will inform conservation management of this unusual species of whale vulnerable to anthropogenic impacts.
Bryde's whales, also called Eden's whale, are currently classified as a single species, Balaenoptera edeni (Committee on Taxonomy, 2017). After much debate, two provisional subspecies were recently recognized, B.edeni edeni and B.edeni brydei, referring to the small, coastal form and larger, oceanic form respectively (Kershaw et al., 2013; Rosel and Wilcox, 2014). However, when combined with ecological and morphological data, there is strong evidence to suggest the two forms could be separated at the species level and perhaps even disconnected from their coastal and oceanic descriptors that can lead to incorrect species assignment [e.g., the New Zealand coastal population is B. e. brydei, the offshore form (Wiseman, 2008)].
Taxonomic clarity within the Bryde's whale group is hampered by the lack of a type specimen and accurate description of B. brydei, and verification of the genetic identity of the B. edeni holotype (Anderson, 1879). The realization that Olsen's description of B. brydei was incorrect resulted from the discovery of two ecotypes off South Africa (Best, 1977). Olsen's description of B. brydei included features from both the South African inshore and offshore forms (Best, 1977, 2001; Kanda et al., 2007; Yamada et al., 2008). These two forms were hunted concurrently in the early 1900's when the existence of the species was not yet known and they were reported as sei whales (Balaenoptera borealis) due to their similar morphology. Little did we know that multiple forms of this new large whale species would subsequently be discovered, resulting in the current taxonomic tangle.
Several regional studies have been conducted on Bryde's whale populations from different geographic regions resulting in a plethora of suggestions regarding the genetic identity, phylogenetic position, and proposed nomenclature of the populations in those areas (Yoshida and Kato, 1999; Wada et al., 2003; Sasaki et al., 2006; Kanda et al., 2007; Kershaw et al., 2013; Rosel and Wilcox, 2014; Luksenburg et al., 2015; Penry et al., 2018). For example, Wada et al. (2003) found that the number of nucleotide differences in the complete mitochondrial DNA control region (bp 901) between B. edeni (coastal Japan) and B. brydei (pelagic North Pacific) was greater than that between B. brydei and the sei whale. They also separated B. edeni from the sei-Bryde's group. Sasaki et al. (2006) supported this differentiation using complete mtDNA sequences and short interspersed nuclear elements insertion patterns. It is becoming increasingly apparent that Eden's whale and Bryde's whale are likely to be two separate species, but with sub-species differentiation; as seen off South Africa (Penry et al., 2016) and the Gulf of Mexico (Rosel and Wilcox, 2014). The taxonomic confusion may act against the interests of protecting vulnerable, isolated populations, perhaps most urgently in the Gulf of Mexico (Rosel and Wilcox, 2014; Soldevilla et al., 2017; Corkeron and Kraus, 2018).
The molecular markers, analytical techniques and sample sizes from the studies mentioned above vary and are not necessarily comparable, however the results present strong cases for the identification of discrete genetic units. Recommendations on the level at which these units should be recognized, their respective nomenclature, and phylogenetic position within the Balaneopteridae, conclude the majority of these studies. Regardless of the strength of these studies, the suggestions made cannot be adopted until such time as the type specimen for B. brydei has been described, and all available molecular data on Bryde's whales are included in a global analysis. To do this it is necessary to consolidate the available genetic material and supporting information on ecology for each region and population. A first step would be to establish a working group of key geneticists, taxonomists and Bryde's whale researchers to identify and consolidate all available molecular and morphometric knowledge to date, then agree upon a standardized approach to analyzing and interpreting the results. The establishment of an IUCN Specialist Group and/or an International Whaling Commission (IWC) working group to resolve the status, threats and conservation actions for these whales would be a valuable step forward. This would enable a pathway forward for Bryde's whale research and management throughout their range.
Passive acoustic monitoring (PAM) has become a powerful tool in understanding the movements and distribution of cryptic but vocal species. This is especially true for highly mobile marine animals such as cetaceans, where acoustic monitoring has provided much needed information on the migratory movements of endangered species such as blue whales (Balaenoptera musculus; Stafford et al., 2001). However, effective use of this technology requires thorough knowledge of the vocal repertoire of the species of interest and visual confirmation of the species producing the sounds.
Worldwide Bryde's whale vocalizations are very low-frequency, like other baleen whales, but are also quite short (<5 s long) making them difficult to identify in acoustic data. Bryde's whales produce a variety of sounds, including <100 Hz tonal calls, often with harmonics or overtones, frequency modulated (FM) downsweeps (<1,000 Hz), and amplitude modulated sounds (Figure 1). Calls are typically lower amplitude than other baleen whales, but when sounds are produced in long sequences they are more identifiable in acoustic data archives (SLN pers. obs.). Although there are similarities among Bryde's whale calls around the world, there appear to be regional call differences. When combined with genetic data, these regional differences may be useful in identifying stock or population boundaries (Mellinger and Barlow, 2003).
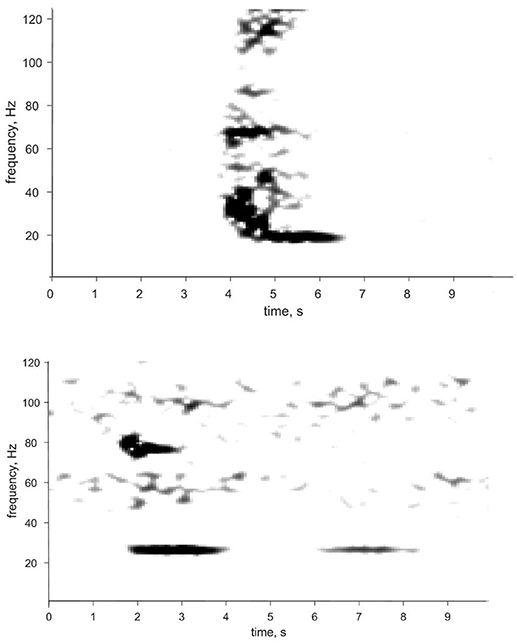
Figure 1. Spectrogram and time series of two calls attributed to Bryde's whales in the eastern tropical Pacific. The 18 Hz low-burst tonal (top) and 25 Hz high burst tonal (bottom) calls are described in Heimlich et al. (2004) and are very similar to the Be2 calls described in Oleson et al. (2003). Spectrogram parameters: 0.512-s (128-sample) frame size, 1.024-s (256 sample) FFT size, 87.5% overlap, and Hanning window, for a filter bandwidth of 7.9 Hz.
Information on the vocal repertoire of Bryde's whales has been expanding but is still limited. Studies linking vocalizations to in situ observations of Bryde's whales include Cummings et al. (1986) and Viloria-Gómora et al. (2015), Edds et al. (1993) in the Gulf of California and in the Gulf of Mexico, Oleson et al. (2003) in the northwest Pacific (off Japan), in the Eastern Tropical Pacific (ETP) and the southern Caribbean, Širović et al. (2014) in the Gulf of Mexico and Figueiredo and Simão (2014) from southeast Brazil. Others have used these confirmed Bryde's whale sounds as a powerful reference for identifying vocalizations in archival acoustic data from moored hydrophones (e.g., Heimlich et al., 2004; Rice et al., 2014; Širović et al., 2014; Putland et al., 2018), while Kerosky et al. (2012) used passive acoustic data to document a range expansion of Bryde's whales in the Southern California Bight. More recently, Bryde's whale movements were remotely tracked through a cabled observatory off Hawai'i (Helble et al., 2016).
The tools used to collect passive acoustic data are diverse, constantly improving and are chosen based on the research question. Animal-borne passive acoustic tags, including the D-tag2 and the Acousonde3 are usually attached via suction cups and provide a means of making short-term (hours—days), detailed recordings of the vocalizations of the animal wearing the tag, as well as those nearby. This provides a definitive link between a species and the recorded vocalization, information on the acoustic repertoire of the animal and the behavioral context of those vocalizations. Limpet style acoustic tags have an embedded attachment and can collect very basic acoustic data for periods of days to weeks. Sonobuoys can passively record sounds produced by whales vocalizing within range of the buoy; when buoys are directional, acoustic data can be coupled with boat-based visual observations to confirm which whale is vocalizing in situ (e.g., Širović et al., 2014). Autonomous moored recorders are routinely deployed in nearshore and remote oceans of the world, providing a continuous record of the soundscape around the deployment area for long periods spanning months to years.
To move forward in the conservation of this species, we need to define and refine the Bryde's whale acoustic repertoire around the world and collect long-term acoustic datasets to identify movements within ocean basins. This is best accomplished by collecting passive acoustic data with multiple tools and technology. Based on previous studies, acoustic data should be collected at a sample rate of at least 2,000 Hz; using a sample rate of ~16 kHz could potentially answer lingering questions regarding unidentified calls recorded in places such as the Marianas Trench (Nieukirk et al., 2016) that are potentially produced by Bryde's whales. In an area of interest, an array of 2+ moored autonomous hydrophones could be positioned on– and off–shore to collect year-long datasets to answer movement and stock questions. Simultaneously, to confirm the calls collected via moored hydrophones are indeed from Bryde's whales, in situ acoustic data could be collected, during a time of year when vocalizations are likely, via sonobuoys, underwater gliders, and animal-borne tags and could provide information on the behavior associated with vocalizations (feeding and breeding). Eventually, tags could be used to collect information on calling rates so that acoustic data can possibly be used to estimate the density of vocalizing Bryde's whales, the degree of acoustic masking from anthropogenic noise, and co-occurrence with sympatric species. With a species like the Bryde's whale that has, to date, been difficult to study, passive acoustic data are a vital component in understanding migratory movements and population boundaries of this species.
Bryde's whales do not undertake the long-range seasonal migrations typically associated with most other baleen whales, but they may travel widely throughout ocean basins as they move through tropical and warm-temperate waters (Kato and Perrin, 2018). It is widely assumed that there are inshore and offshore species of Bryde's whales but their movement patterns are almost certainly more complex both within and between populations. In some areas with low abundance estimates there is high genetic diversity suggesting wider connectivity with whales from other regions, and/or dispersal by the surveyed population (Wiseman, 2008; Tezanos-Pinto et al., 2017). Most Bryde's whale populations found away from the easily accessible near-coastal waters remain un-surveyed with little or no knowledge of their connectivity or genetic diversity.
Longer migratory movements of whales of approximately 2,000–3,500 km in distance have been reported off the west coast of South Africa (Best, 2001). Differences in residency patterns of whales suggest there are migratory movements at several sites, but the distances remain unknown (Alves et al., 2010; Penry et al., 2011) and may cover only short distances of hundreds of kilometers (Wiseman, 2008; Lodi et al., 2015). This may be in response to prey movements, as found off Brazil, Venezuela and the Gulf of California (Notarbartolo di Sciara, 1983; Tershy, 1992; Zerbini et al., 1997). To date, there are no studies using satellite telemetry data to reveal long-range movements of Bryde's whales. Tags with short prongs, such as LIMPET tags should be used as Bryde's have a thin blubber layer (see use on fin whales (Balaenoptera physalus) Panigada et al., 2017). Satellite telemetry studies would be particularly effective in oceanic regions where there are inshore and offshore whale populations [e.g., South Africa (Best, 2001)], but also where there are small, but genetically diverse populations [e.g., Hauraki Gulf, New Zealand (Tezanos-Pinto et al., 2017)] to determine connectivity.
Studies on different populations have used short-term suction cup tags (Alves et al., 2010; Soldevilla et al., 2017; Izadi et al., 2018). These have revealed vertical movements including shallow (Izadi et al., 2018), mid-water (Soldevilla et al., 2017), and deep-dive patterns (Alves et al., 2010), reflective of the environment they live in and highlighting the whales' ability to exploit a variety of surface and deeper water prey (Kato and Perrin, 2018). A range of oceanographic, physical and biological variables influence Bryde's whale movements but there is no clear pattern across populations (Corkeron et al., 2011; Weir et al., 2012; Soldevilla et al., 2017; Tardin et al., 2017). A multi-disciplinary, comparative approach to determine oceanography, prey movements and availability, triggers that drive whale movements and predictions under change scenarios would enable us to better understand whether Bryde's are the ultimate flexible baleen whale. We have improved capabilities to understand the trophic ecology of open ocean organisms through tools such as stable isotopes, fatty acids, radio isotopes, and isoscape models which can reveal spatial and foraging niche shifts over space and time (e.g., Newsome et al., 2010; Quillfeldt et al., 2010; Eisenmann et al., 2017). Determining the processes that influence whale movements, their interaction with the environment and how they are affected by change is an area of future importance.
With no large-scale connectivity studies to date, our current, limited understanding of the variability in site fidelity suggest long-range satellite tagging would be valuable. There are indications that some Bryde's populations are expanding their local range, perhaps in response to prey shifts [e.g., Gulf of California (Kerosky et al., 2012)]. Similar small-scale shifts have been observed in response to La Niña events in New Zealand (RC unpub. data), suggesting a reduction in sightings in areas where whales are typically observed. Bryde's have a preferred thermal range (Kato and Perrin, 2018) that may influence future movements and distribution patterns as ocean temperature increases; a recent phenomenon observed in other cetaceans (see review in MacLeod, 2009).
Balaenopteridae including Bryde's whales employ a foraging strategy, lunge feeding, characterized by the engulfment of a large volume of water at high speed and subsequent filtering with the mouth closed (Goldbogen et al., 2017). Bryde's whales commonly use lunge feeding behaviors throughout their range (e.g., Miyazaki and Wada, 1978; Best et al., 1984; Tershy, 1992; Anderson, 2005; Steiner et al., 2008; Alves et al., 2010; Penry et al., 2011; Lodi et al., 2015; Iwata et al., 2017). They have a broad diet of pelagic and mesopelagic fishes, squids, krill, and other zooplankton which varies by location (Olsen, 1913; Notarbartolo di Sciara, 1983; Best et al., 1984; Tershy, 1992; Best, 2001; Anderson, 2005; Murase et al., 2007; Gonçalves et al., 2015; Lodi et al., 2015; Iwata et al., 2017; Izadi, 2018; Kato and Perrin, 2018). Bryde's whales are efficient and adaptable predators, adopting behaviors in relation to prey species, feeding grounds and oceanographic environment.
Bryde's whales have diel patterns in foraging behavior. In the Gulf of California, whales fed more often at dawn and dusk when fish schools are less likely to detect predators (Tershy, 1992). In Madeira whale dive patterns of up to 250 m depth appear to mirror the diel vertical migration of zooplankton (Alves et al., 2010). In the shallow waters of the Hauraki Gulf, whales showed foraging behavior during the day and rest during the night-time (Izadi et al., 2018). In the Gulf of Thailand, they perform passive tread-water feeding (Iwata et al., 2017). This foraging strategy takes advantage of the behavior of the prey whereby the hypoxic environment in the upper Gulf of Thailand limits fish to the water surface where there is some oxygenated water. The whales tread water with their mouth open wide at the sea surface as fish spill from the surface into the whales' mouths. In New Zealand, Bryde's whales perform head-slaps to aggregate zooplankton prey, but the same individuals will switch strategies and lunge at speed from underneath fish schools (Izadi, 2018). Lunges also occur at depth (e.g., Alves et al., 2010).
Bryde's whales have a wide array of novel behaviors to catch prey, perhaps more than any other species of baleen whale. They forage at the sea-surface and at depth, during day-time and night-time, and feed on pelagic and mesopelagic prey. Behaviors are often specific to an area with many never observed in Bryde's whales in any another area, nor by any other species of baleen whale. These specializations may leave them vulnerable if a preferred prey is over-fished or affected by environmental shifts, requiring the whales to relocate to find other prey or rapidly develop a new foraging strategy. Bryde's whales have characteristics of income breeders, feeding regularly rather than relying on stored reserves, so determining their energetic requirements needs consideration if protecting important prey or feeding areas. Ascertaining how the novel foraging behaviors are developed and transmitted, especially between mothers and calves and between wider ranging populations of whales would change our traditional thoughts about baleen whales.
Bryde's whales are listed as Least Concern by the IUCN (Cooke and Brownell, 2018), but the Gulf of Mexico population is listed as Critically Endangered. Bryde's whales face similar threats to other baleen whales with entanglement, ship strike and prey depletion already reported. Entanglement in a variety of fishing gear such as rock lobster and octopus fisheries in South Africa (Penry et al., 2016) long-line interactions in the endangered Gulf of Mexico population (Soldevilla et al., 2017) and mussel aquaculture lines in New Zealand (Constantine et al., 2015) are of concern, in particular where these fisheries are expanding and/or poorly managed. These need to be managed to avoid population decline, especially as the few known abundance estimates are in the mid- to high-hundreds of individuals (Best et al., 1984; Urbán and Flores, 1996; Carretta et al., 2015), or small with <100 mature individuals (Cherdsukjai et al., 2015; Rosel et al., 2016).
As with many baleen whale populations, they are vulnerable to ship strike, especially inshore populations. Bryde's whales are less frequently represented in the ship strike statistics than other Balaenopterids (Laist et al., 2001) but in some areas, ship strike was the primary cause of whale mortality (e.g., New Zealand, Constantine et al., 2015). As with other species, the threat may be mitigated through re-routing traffic or reducing vessel speed, as implemented in the Hauraki Gulf, New Zealand. As shipping traffic increases, coastal populations of Bryde's whales are most vulnerable. Future establishment of new ports, oil and gas operations, or shipping routes need to consider whale presence to mitigate the mortality risk. Areas of high shipping traffic also mask the low frequency communications of whales through ship noise. Slowing ships can reduce their acoustic footprint, lessening another potential impact (Putland et al., 2017).
Bryde's whales were not extensively hunted during the commercial whaling era, but up to 50 whales were killed per year until 2016 as part of the Japanese whale research programme4. There may be other areas, possibly throughout the south-east Asian region where Bryde's whales are hunted opportunistically but these may also consist of Omura's whales (Balaenoptera omurai). Overall, hunting currently poses a low risk to whale populations but resumption of the Japanese programme should be monitored carefully.
With recent genetic studies revealing sub-species status for the South African and Gulf of Mexico populations, immediate action is needed to reduce and mitigate all anthropogenic mortality to ensure their future viability. In general, compared to many baleen whales, we know little about the Bryde's whales so a precautionary approach to managing threats is recommended as it is likely that there are isolated populations in other locations.
Whilst broadly distributed throughout warm-temperate waters, Bryde's whales have high variability in their distribution, foraging behavior, movement patterns and threats. With their flexible and diverse foraging strategies and broad prey preferences, Bryde's whales may be one of the more mobile baleen whales as our oceans change. As recent research has revealed the genetic isolation of some populations, we cannot assume that all Bryde's whale populations are secure. Future work should focus on defining who Bryde's and Eden's whales are, what defines populations and ascertain the real breadth of behavioral plasticity exhibited by the species. We suggest that the IUCN and IWC establish working groups to resolve the taxonomic and threat status to known populations, so we can manage Bryde's whales to avoid future conservation crises, as currently occurring in the Gulf of Mexico (Corkeron and Kraus, 2018). Given the prevalence of offshore populations, we recommend passive acoustic monitoring as a useful tool in determining stock status, possibly for abundance and distribution, and to assess potential levels of anthropogenic acoustic threats. As the environment changes, a multi-disciplinary, comparative approach will enable an ocean-wide assessment of shifts in habitat use, trophic interactions, distribution, and overall status of this lesser known baleen whale.
RC coordinated this work. All authors (RC, TI, SLN, and GSP) contributed to writing and editing the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank our many colleagues for thoughtful conversations over the years about whales and their oceanic realm. This work is PMEL contribution #4826 (SLN).
1. ^iwc.int/total-catches
2. ^http://www.whoi.edu/website/marine-mammal-behavior-lab/dtag
3. ^http://www.acousonde.com/index.html
4. ^iwc.int/total-catches
Alves, F., Dinis, A., Cascão, I., and Freitas, L. (2010). Bryde's whale (Balaenoptera brydei) stable associations and dive profiles: new insights into foraging behavior. Mar. Mamm. Sci. 26, 202–212. doi: 10.1111/j.1748-7692.2009.00333.x
Anderson, J. (1879). Anatomical and zoological Researchers: Comprising an Account of the Zoological Results of the Two Expeditions to Western Yunnan in 1868 and 1875. B. London: Quaritch. 551–564.
Anderson, R. C. (2005). Observations of cetaceans in the Maldives, 1990-2002. J. Cetacean Res. Manage 7, 119–135.
Best, B. P. (2001). Distribution and population separation of Bryde's whale Balaenoptera edeni off southern Africa. Mar. Ecol. Prog. Ser. 220, 277–289. doi: 10.3354/meps220277
Best, P. B. (1977). Two allopatric forms of Bryde's whale off South Africa. Report of the International Whaling Commission Special Issue. 1, 10–38.
Best, P. B., Butterworth, D. S., and Rickett, L. H. (1984). An assessment cruise for the South African inshore stock of Bryde's whales (Balaenoptera edeni). Report of the International Whaling Commission. 34, 403–423.
Carretta, J. V., Oleson, E. M., Weller, D. W., Lang, A. R., Forney, K. A., Baker, J., et al. (2015). U.S. Pacific Marine Mammal Stock Assessments: 2014. U.S. Department of Commerce. NOAA Technical Memorandum NMFS-SWFSC-549.
Cherdsukjai, P., Thongsukdee, S., Passada, S., and Prempree, T. (2015). “Population size of Brydes' whales (Balaenoptera edeni) in the upper Gulf of Thailand, estimated by mark and recapture method,” Proceedings of the Design Symposium on Conservation of Ecosystem. 3, 1–5. doi: 10.14989/198821
Committee on Taxonomy (2017). List of Marine Mammal Species and Subspecies. Society for Marine Mammalogy. www.marinemammalscience.org. May 2018.
Constantine, R., Johnson, M., Riekkola, L., Jervis, S., Kozmian-Ledward, L., Dennis, T., et al. (2015). Mitigation of vessel-strike mortality of endangered Bryde's whales in the Hauraki Gulf, New Zealand. Biol. Cons. 186, 149–157. doi: 10.1016/j.biocon.215.03.008
Cooke, J. G., and Brownell, R. L. Jr. (2018). Balaenoptera edeni. The IUCN red list of threatened species 2018: e.T2476A50349178. doi: 10.2305/IUCN.UK.2018-1.RLTS.T2476A50349178.en
Corkeron, P., and Kraus, S. D. (2018). Baleen whale species at risk of extinction. Nature 554:169. doi: 10.1038/d41586-018-01672-4
Corkeron, P. J., Minton, G., Collins, T., Findlay, K., Wilson, A., and Baldwin, R. (2011). Spatial models of sparse data to inform cetacean conservation planning: an example from Oman. Endanger. Species Res. 15, 39–52. doi: 10.3354/esr00367
Cummings, W. C., Thompson, P. O., and Ha, S. J. (1986). Sounds from Bryde, Balaenoptera edeni, and finback, Balaenoptera physalus, whales in the Gulf of California. Fish. Bull. 84, 359–370.
Edds, P. L., Odell, D. K., and Tershy, B. R., (1993). Vocalizations of a captive juvenile and free-ranging adult-calf pairs of Bryde's whales, Balaenoptera edeni. Mar. Mamm. Sci. 9, 269–284.
Eisenmann, P., Fry, B., Mazumder, D., Jacobsen, G., Holyoake, C. S., Coughran, D., et al. (2017). Radiocarbon as a novel tracer of extra-Antarctic feeding in Southern Hemisphere humpback whales. Sci. Rep. 7:4366. doi: 10.1038/s41598-017-04698-2
Figueiredo, L. D., and Simão, S. M. (2014). Bryde's whale (Balaenoptera edeni) vocalizations from Southeast Brazil. Aquat. Mamm. 40, 225–231. doi: 10.1578/AM.40.3.2014.225
Goldbogen, J. A., Cade, D. E., Calambokidis, J., Friedlaender, A. S., Potvin, J., Segre, P. S., et al. (2017). How baleen whales feed: the biomechanics of engulfment and filtration. Annu. Rev. Mar. Sci. 9, 367–386. doi: 10.1146/annurev-marine-122414-033905
Gonçalves, L. R., Augustowski, M., and Andriolo, A. (2015). Occurrence, distribution and behaviour of Bryde's whales (Cetacea: Mysticeti) off south-east Brazil. J. Mar. Biol. Assoc. 96, 943–954. doi: 10.1017/S0025315415001812
Heimlich, S. L., Nieukirk, S. L., Mellinger, D. K., Dziak, R., Matsumoto, H., and Fowler, M. (2004). Bryde's whale (Balaenoptera edeni) sounds collected from autonomous hydrophones in the Eastern Tropical Pacific, 1999–2001. J. Acoust. Soc. Am. 116:2614. doi: 10.1121/1.4785426
Helble, T. A., Henderson, E., Ierley, G. R., and Martin, S. W. (2016). Swim track kinematics and calling behavior attributed to Bryde's whales on the Navy's Pacific Missile Range Facility. J. Acoust. Soc. Am. 140, 4170–4177. doi: 10.1121/1.4967754
Iwata, T., Akamatsu, T., Thongsukdee, S., Cherdsukjai, P., Adulyanukosol, K., and Sato, K. (2017). Tread-water feeding of Bryde's whales. Curr. Biol. 27, R1154–1155. doi: 10.1016/j.cub.2017.09.045
Izadi, S. (2018). Flexible foraging behaviour of Bryde's whales. Ph.D. dissertation,University of Auckland, Auckland.
Izadi, S., Johnson, M., de Soto, N. A., and Constantine, R. (2018). Night-life of Bryde's whales: ecological implications of resting in a baleen whale. Behav. Ecol. Sociobiol. 72:78. doi: 10.1007/s00265-018-2492-8
Kanda, N., Goto, M., Kato, H., McPhee, M. V., and Pastene, L. A. (2007). Population genetic structure of Bryde's whales (Balaenoptera brydei) at the inter-oceanic and trans-equatorial levels. Cons. Gen. 8, 853–864. doi: 10.1007/s10592-006-9232-8
Kato, H., and Perrin, W. F. (2018). “Bryde's whales Balaenoptera edeni,” in Encyclopedia of marine mammals,” 3rd Edn, eds B. Würsig, J. G. M. Thewissen, and K. Kovacs (London: Academic Press Books Elsevier), 143–145.
Kerosky, S. M., Širović, A., Roche, L. K., Baumann-Pickering, S., Wiggins, S. M., and Hildebrand, J.A. (2012). Bryde's whale seasonal range expansion and increasing presence in the Southern California Bight from 2000 to 2010. Deep Sea Res. Part I Oceanogr Res Pap. 65, 125–132. doi: 10.1016/j.dsr.2012.03.013
Kershaw, F., Leslie, M. S., Collins, T., Mansur, R. M., Smith, B. D., Minton, G., et al. (2013). Population differentiation of 2 forms of Bryde's whales in the Indian and Pacific Oceans. J. Hered. 104, 755–764. doi: 10.1093/jhered/est057
Laist, D. W., Knowlton, A. R., Mead, J. G., Collet, A. S., and Podesta, M. (2001). Collisions between ships and whales. Mar. Mamm. Sci. 17, 35–75. doi: 10.1111/j.1748-7692.2001.tb00980.x
Lodi, L., Tardin, R. H., Hetzel, B., Maciel, I. S., Figueiredo, L. D., and Simão, S. M. (2015). Bryde's whale (Cetartiodactyla: Balaenopteridae) occurrence and movements in coastal areas of southeastern Brazil. Zoologia. 32, 171–175. doi: 10.1590/S1984-46702015000200009
Luksenburg, J. A., Heniquez, A., and Sangster, G. (2015). Molecular and morphological evidence for the subspecific identity of Bryde's whales in the southern Caribbean. Mar. Mamm. Sci. 31, 1568–1579. doi: 10.1111/mms.12236
MacLeod, C. (2009). Oceanic climate change, range changes and implications for the conservation of marine cetaceans: a review and synthesis. Endanger. Species Res. 7, 125–136. doi: 10.3354/esr00197
Mellinger, D. K., and Barlow, J. (2003). Future Directions for Acoustic Marine Mammal Surveys: Stock Assessment and habitat use. Seattle, WA: Report of a workshop held in La Jolla, CA 20–22 November 2002, NOAA OAR Special Report, NOAA/PMEL Contribution No. 2557, 37.
Miyazaki, N., and Wada, S. (1978). Observation of Cetacea during whale marking cruise in the western tropical Pacific, 1976. Sci. Rep. Whales Res. Inst. 30, 179–195.
Murase, H., Tamura, T., Kiwada, H., Fujise, Y., Watanabe, H., Ohizumi, H., et al. (2007). Prey selection of common minke (Balaenoptera acutorostrata) and Bryde's (Balaenoptera edeni) whales in the western North Pacific in 2000 and 2001. Fish. Oceanogr. 16, 186–201. doi: 10.1111/j.1365-2419.2006.00426.x
Newsome, S. D., Clementz, M. T., and Koch, P. L. (2010). Using stable isotope biogeochemistry to study marine mammal ecology. Mar. Mamm. Sci. 26, 509–572. doi: 10.1111/j.1748-7692.2009.00354.x
Nieukirk, S. L., Fregosi, S., Mellinger, D. K., and Klinck, H. (2016). A complex baleen whale call recorded in the Mariana trench marine national monument. J. Acoust. Soc. Am. 140, 274–278. doi: 10.1121/1.4962377
Notarbartolo di Sciara, G. (1983). Bryde's whales (Balaenoptera edeni Anderson, 1878) off eastern Venezuela (Cetacea, Balaenopteridae). San Diego, CA: Hubbs-SeaWorld Research Institute Technical Report. 83–153.
Oleson, E. M., Barlow, J., Gordon, J., Rankin, S., and Hildebrand, J. A. (2003). Low frequency calls of Bryde's whales. Mar. Mamm. Sci. 19, 407–419. doi: 10.1111/j.1748-7692.2003.tb01119.x
Olsen, Ø. (1913). On the external characters and biology of Bryde's whale (Balaenoptera brydei), a new rorqual from the coast of South Africa. Proc. Zool. Soc. Lond. 1913, 1073–1090.
Panigada, S., Donovan, G. P., Druon, J.-N., Lauriano, G., Pierantonio, N., Pirotta, E., et al. (2017). Satellite tagging of Mediterranean fin whales: working towards the identification of critical habitats and the focussing of mitigation measures. Sci. Reports. 7:3365. doi: 10.1038/s41598-017-03560-9
Penry, G. S., Cockcroft, V. G., and Hammond, P. S. (2011). Seasonal fluctuations in occurrence of inshore Bryde's whales in Plettenberg Bay, South Africa, with notes on feeding and multispecies associations. Afr. J. Mar. Sci. 33, 403–414. doi: 10.2989/1814232X.2011.637617
Penry, G. S., Findlay, K., and Best, P (2016). “A conservation assessment of Balaenoptera edeni,” in The Red List of Mammals of South Africa, Swaziland and Lesotho, eds M. F. Child, L. Roxburgh, E. Do Linh San, D. Raimondo, and H.T. Davies-Mostert (Gauteng: South African National Biodiversity Institute and Endangered Wildlife Trust, South Africa).
Penry, G. S., Hammond, P. S., Cockcroft, V. G., Best, P. B., Thornton, M., and Graves, J. A. G. (2018). Phylogenetic relationships in southern AfricanBryde's whales inferred frommitochondrial DNA: futher support for subspecies delineation between the two allopatric populations. Con. Gen. doi: 10.1007/s10592-018-1105-4
Putland, R. L., Merchant, N. D., Farcas, A., and Radford, C. A. (2017). Vessel noise cuts down communication space for vocalizing fish and marine mammals. Global Change Biol. 24,1708–1721. doi: 10.1111/gcb.13996
Putland, R. L., Ranjard, L., Constantine, R., and Radford, C. A. (2018). A hidden Markov model approach to indicate Bryde's whale acoustics. Ecol. Ind. 84, 479–487. doi: 10.1016/j.ecolind.2017.09.025
Quillfeldt, P., Masello, J. F., McGill, A. R., Adams, M., and Furness, R. W. (2010). Moving polewards in winter: a recent change in the migratory strategy of a pelagic seabird? Front. Zoo. 7:15. doi: 10.1186/1742-9994-7-15
Rice, A. N., Palmer, K. J., Tielens, J. T., Muirhead, C. A., and Clark, C. W. (2014). Potential Bryde's whale (Balaenoptera edeni) calls recorded in the northern Gulf of Mexico. J. Acoust. Soc. Am. 135:3066. doi: 10.1121/1.4870057
Rosel, P. E., Corkeron, P., Engleby, L., Epperson, D., Mullin, K. D., Soldevilla, M. S., et al. (2016). Status Review of Bryde's Whales (Balaenoptera edeni) in the Gulf of Mexico Under the Endangered Species Act. U.S. Louisiana: Department of Commerce. NOAA Technical Memorandum NMFS-SEFSC-692.
Rosel, P. E., and Wilcox, L. A. (2014). Genetic evidence reveals a unique lineage of Bryde's whales in the northern Gulf of Mexico. Endanger. Species Res. 25, 19–34. doi: 10.3354/esr00606
Sasaki, T., Nikaido, M., Wada, S., Yamada, T. K., Cao, Y., Hasegawa, M., et al. (2006). Balaenoptera omurai is a newly discovered baleen whale that represents an ancient evolutionary lineage. Mol. Phylogen. Evol. 41, 40–52. doi: 10.1016/j.ympev.2006.03.032
Širović, A., Bassett, H. R., Johnson, S. C., Wiggins, S. M., and Hildebrand, J. A. (2014). Bryde's whale calls recorded in the Gulf of Mexico. Mar. Mamm. Sci. 30, 399–409. doi: 10.1111/mms.12036
Soldevilla, M. S., Hildebrand, J. A., Frasier, K. E., Dias, L. A., Martinez, A., Mullin, K. D., et al. (2017). Spatial distribution and dive behaviour of Gulf of Mexico Bryde's whales: potential risk of vessel strikes and fisheries interactions. Endanger. Species Res. 32, 533–550. doi: 10.3354/esr00834
Stafford, K. M., Nieukirk, S. L., and Fox, C. G. (2001). Geographic and seasonal variation of blue whale calls in the North Pacific. J. Cetacean Res. Manage. 3, 65–76.
Steiner, L., Silva, M. A., Zereba, J., and Leal, M. J. (2008). Bryde's whales, Balaenoptera edeni, observed in the Azores: A new species record for the region. Mar. Biodivers. Rec. 1:e66. doi: 10.1017/S1755267207007282
Tardin, R. H., Chun, Y., Simão, S. M., and Alves, M. A. S. (2017). Modeling habitat use by Bryde's whale Balaenoptera edeni off southeastern Brazil. Mar. Ecol. Prog. Series 576, 89–103. doi: 10.3354/meps12228
Tershy, B. R. (1992). Body size, diet, habitat use, and social behavior of Balaenoptera whales in the Gulf of California. J. Mammal. 73, 477–486. doi: 10.2307/1382013
Tezanos-Pinto, G., Hupman, K., Wiseman, N., Dwyer, S. L., Baker, C. S., et al. (2017). Local abundance, apparent survival and site fidelity of Bryde's whales in the Hauraki Gulf (New Zealand) inferred from long-term photo-identification. Endanger. Species Res. 34, 61–73. doi: 10.3354/esr00839
Urbán, J. R., and Flores, S. R. (1996). A note on Bryde's whales (Balaenoptera edeni) in the Gulf of California, Mexico. Rep. Int. Whal. Comm. 46, 453–457.
Viloria-Gómora, L., Romero-Vivas, E., and and, Urbán, J. R. (2015). Calls of Bryde's whale (Balaenoptera edeni) recorded in the Gulf of California. J. Acoust. Soc. Am. 138, 2722–2725. doi: 10.1121/1.4932032.
Wada, S., Oishi, M., and Yamada, T. K. (2003). A newly discovered species of living baleen whale. Nature. 426, 278–281. doi: 10.1038/nature02103
Weir, C. R., MacLeod, C. D., and Pierce, G. J. (2012). Habitat preferences and evidence for niche partitioning amongst cetaceans in the waters between Gabon and Angola, eastern tropical Atlantic. J. Mar. Biol. Assoc. 92, 1735–1749. doi: 10.1017/S0025315412000148
Wiseman, N. (2008). Genetic Identity and Ecology of Bryde's Whales in the Hauraki Gulf, New Zealand. Ph.D. dissertation ,University of Auckland,Auckland.
Yamada, T. K., Kakuda, T., and Tajima, Y. (2008). Middle sized balaenopterid whale specimens in the Philippines and Indonesia. Mem. Natl. Mus. Nat. Sci. 45, 75–83.
Yoshida, H., and Kato, H. (1999). Phylogenetic relationships of Bryde's whales in the western North Pacific and adjacent waters inferred from mitochondrial DNA sequences. Mar. Mamm. Sci. 15, 1269–1286. doi: 10.1111/j.1748-7692.1999.tb00890.x
Keywords: Bryde's whale, Eden's whale, Balaenoptera edeni, taxonomy, acoustics, foraging behavior, movement ecology, conservation
Citation: Constantine R, Iwata T, Nieukirk SL and Penry GS (2018) Future Directions in Research on Bryde's Whales. Front. Mar. Sci. 5:333. doi: 10.3389/fmars.2018.00333
Received: 06 July 2018; Accepted: 28 August 2018;
Published: 18 September 2018.
Edited by:
Rob Harcourt, Macquarie University, AustraliaReviewed by:
Filipe Alves, Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação (ARDITI), PortugalCopyright © 2018 Constantine, Iwata, Nieukirk and Penry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rochelle Constantine, ci5jb25zdGFudGluZUBhdWNrbGFuZC5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.