- 1Large Marine Vertebrates Research Institute Philippines, Bohol, Philippines
- 2WWF-Philippines, Manila, Philippines
Donsol in the Philippines is the longest running community-based whale shark (Rhincodon typus) ecotourism site in Southeast Asia, with peak visitation in 2012 of over 27,000 tourists. In order to understand this aggregation and the importance of the area to whale sharks, dedicated photographic identification (photo-ID) research began in 2007. In-water photo-ID surveys were conducted from tourism boats, weather and operator permitting, from December to June between 2007 and 2016. Visual matches of the unique spot patterns of each individual shark were validated by the pattern-recognition software Interactive Individual Identification System (I3S), and on the online database Wildbook for Whale Sharks (www.whaleshark.org). A total of 1,985 photo-ID trips over 895 survey days resulted in 6,786 encounters with R. typus. Combined with encounters from both dedicated research and citizen science dating back to 1998, 479 individual whale sharks were identified, making up 44% of the known whale shark population in the Philippines (n = 1,095). Of these, photographs of the pelvic region confirmed the sex for 158 males and 22 females. Visual size estimates ranged from 2 to 10 m (mean ± SD = 6.5 ± 1.6 m). Maturity in males (LT50) was estimated at 6.8 ± 0.2 m total length, with 53% of males considered mature. Annually, the total number of individuals sighted varied between 15 and 185 (mean ± SD = 104 ± 55.53), with a recruitment of 3–90 new individuals yearly (mean ± SD = 46.8 ± 36.29). Modeled residency using maximum likelihood methods suggested whale sharks spent 49.8 ± S.E. 14.5 [95% CI (32.3–78.6)] days in Donsol each season, with 47.1–60.8 whale sharks at any one time during the season. Twenty individuals were recorded through photo-ID at other sites across the Philippines. The extended residency of whale sharks at Donsol, paired with the presence of sexually mature animals and the economic value of the tourism industry, highlights the importance of Donsol for this endangered species.
Introduction
The whale shark, Rhincodon typus Smith 1828, a large planktivorous elasmobranch, is found in tropical and warm-temperate waters worldwide (Rowat and Brooks, 2012). The largest of the shark species, whale sharks are highly mobile (e.g., Wilson et al., 2006; Sleeman et al., 2010; Berumen et al., 2014; Robinson et al., 2017), but form predictable seasonal aggregations in hotspots around the world, predominantly associated with the presence of food (e.g., Motta et al., 2010; Robinson et al., 2013; Rohner et al., 2015a). Some whale sharks display a degree of site fidelity on an annual and inter-annual basis (Graham and Roberts, 2007; Holmberg et al., 2008; Fox et al., 2013; Araujo et al., 2017), and this predictability makes the whale shark an ideal target species for wildlife tourism (Catlin and Jones, 2010; Rowat and Brooks, 2012).
Historically, the Philippines was home to targeted whale shark fisheries with two major landing sites in the Bohol Sea, though more sites were reported extending through the Sulu Sea and southern Mindanao (Alava et al., 1997). In Donsol, a municipality in Sorsogon province, hunting did not traditionally occur. However, publicity about a large aggregation led to the fishing of at least six individuals in 1997 (Yaptinchay, 1999). In response, the municipal waters of Donsol were declared a whale shark sanctuary, and this was soon followed by a national ban on whale shark hunting, imposed in 1998 (FAO, 193, Department of Agriculture-Bureau of Fisheries and Aquatic Resources). Due to depletion of whale shark populations noted in the Indo-Pacific, the category of the species was upgraded to “Endangered” (Pierce and Norman, 2016), as well as being included in Appendix I of the Convention on Migratory Species in 2017 and in Appendix II of the Convention on International Trade of Endangered Species in 2003. China is known to still catch whale sharks through unregulated fisheries, many caught in the South China Sea (Li et al., 2012), an area that whale sharks from Taiwan and the Philippines visit (Hsu et al., 2007; WWF-Philippines, Unpub. data). Despite regulations and bans on direct hunting in several countries, the late onset of sexual maturity (Bradshaw et al., 2007) hinders population recovery and leaves whale sharks susceptible to overexploitation and anthropological impacts (Bradshaw et al., 2008).
Different techniques can be employed to assess the status of whale shark populations. Photo identification (henceforth photo-ID) is a minimally invasive tool for mark-recapture studies that relies on time-stable markings on animals so that they can be distinguished amongst other individuals in a population. This technique has been employed across different marine taxa, such as with the facial scutes of green turtles Chelonia mydas (e.g., Schofield et al., 2008), the dorsal fins of cetaceans (e.g., Hammond, 1990) or the natural spot patterns of manta rays Mobula alfredi (e.g., Marshall et al., 2011). Whale sharks have unique spot patterns on their bodies that allow for minimally invasive mark-recapture studies through photo-ID (Arzoumanian et al., 2005). It is an effective research technique for population demographics, and has been successfully employed in all sites at which they aggregate (Norman et al., 2017). Given the slow, surface-dwelling nature of the whale shark, photos of individuals captured by tourists has made citizen science contributions an active part of their research and conservation (e.g., Araujo et al., 2016; Norman et al., 2017). The use of an online-based tool, Wildbook for Whale Sharks (www.whaleshark.org), enables the comparison of identification data on a global scale.
Whale shark aggregations worldwide are mostly dominated by juvenile males (e.g., Meekan et al., 2006; Rowat et al., 2007, 2009; Riley et al., 2010; Ramírez-Macías et al., 2012a,b; Araujo et al., 2014, 2016; Himawan et al., 2015; Rohner et al., 2015b; McKinney et al., 2017). Few aggregations dominated by adults have been identified to date, namely the Galapagos Islands (Acuña-Marrero et al., 2014), St. Helena Island (Clingham et al., 2016) and the Revillagigedo Islands off Baja California (Ramírez-Macías et al., 2012b), and a large proportion of adults has been reported at an offshore aggregation in Qatar (Robinson et al., 2016) and in the mid-equatorial Atlantic off Brazil (Macena and Hazin, 2016). Some evidence exists that adults spend most of their time in the open ocean (Ramírez-Macías et al., 2017), which might explain why coastal sites tend to be juvenile dominated. Norman and Stevens (2007) reported size at maturity (TL50) of 8.1 m at Ningaloo Reef in Western Australia using a rope of known length, whereas Rohner et al. (2015b) reported 9.2 m in Mozambique using paired-laser photogrammetry. Other studies have also used photogrammetry and visual estimates to determine maturity in males (Qatar, 7.3 m, Robinson et al., 2016; Gulf of Mexico, c. 7.0 m, Ramírez-Macías et al., 2012b, respectively), yet concerns remain about the inaccuracy of such approaches (see Sequeira et al., 2016). Caution should therefore be taken when using these approaches to determine the demographics of whale shark aggregations. Maturity in females is believed to occur at around 9.0 m based on visual and photogrammetry estimates of visibly pregnant individuals (Ramírez-Macías et al., 2012b; Acuña-Marrero et al., 2014), yet it is difficult to determine maturity externally in the absence of pregnancy.
Here, we use photo-ID data collected by our team from 2007 to 2016, along with data uploaded to Wildbook for Whale Sharks from citizen science since 1998 to describe the aggregation of whale sharks at Donsol, Sorsogon, Philippines. We use maximum likelihood methods to estimate their residency patterns, mortality rate and permanent emigration, and an open population model to estimate their population size. We discuss how these results fit into our current understanding of this endangered species and how Donsol might be a unique and important site for whale sharks.
Materials and Methods
Study Site
The municipality of Donsol, province of Sorsogon, is located in the south-eastern tip of Luzon Island (Figure 1). Bordered by 11 coastal barangays, the municipal waters of Donsol span 27,780 hectares (Pine et al., 2007), situated at the mouth of the Donsol and Ogod rivers, and on the edge of the Burias Pass, a waterway that reaches >500 m leading to the Ragay Gulf with water from the Pacific Ocean entering through the San Bernardino Strait (Calumpong et al., 2013). Whale shark tourism started in 1998 with 900 visitors and rose to over 27,000 visitors in 2012 (Local Government Unit, Donsol). Tourists board outrigger boats, locally called bangkas, and go out on 3 h tours in search of whale sharks along the coastline. A maximum of six tourists are allowed per boat with a maximum of 30 boats allowed out at any one time.
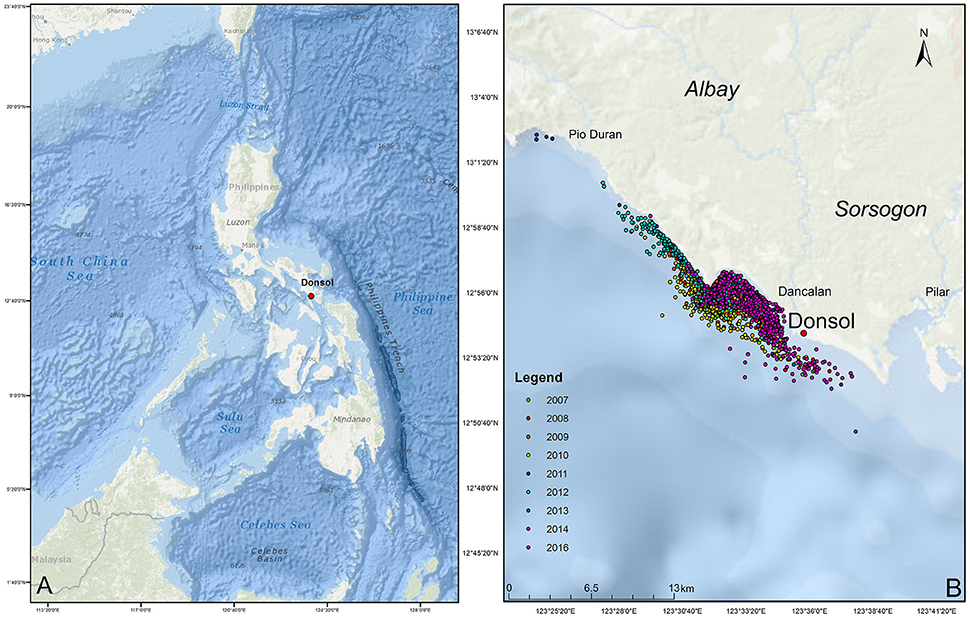
Figure 1. Map of the Philippines (A) and study site (B). Dots represent individual whale shark sightings during surveys between 2007 and 2016, whereas colors represent the different years in which they were sighted.
Photographic-Identification
Dedicated photographic-identification data collection was started by WWF-Philippines researchers and volunteers in 2007 and was complemented by LAMAVE researchers in 2015 and 2016. In-water work was conducted in collaboration with, and under permit from, the Local Government Unit of Donsol and the Department of Agriculture-Bureau of Fisheries and Aquatic Resources, under whose management the whale shark falls, following international standards for whale shark photo-ID procedures.
Researchers boarded tourist vessels daily during the season, weather and availability permitting. Effort was based on previous days' sightings in the vicinity, and researchers only went out when tourists did. Although this altered effort, generally if there were no sharks in the area, there were no trips. When two researchers overlapped with the same individual whale shark, one left the water to avoid doubling effort and unnecessary potential disturbance. Vessels haphazardly searched Donsol waters to find whale sharks at the surface between the months of November and June (Table 1). Once sighted, researchers entered the water and collected photo-ID data. Photos of the left flank of the animal were prioritized. Only identification images of the left flank were used to confirm an individual whale shark and used in the present study. Photos of the right flank of the animals were also taken where possible to further confirm the identity of an individual, where a left flank identification existed.
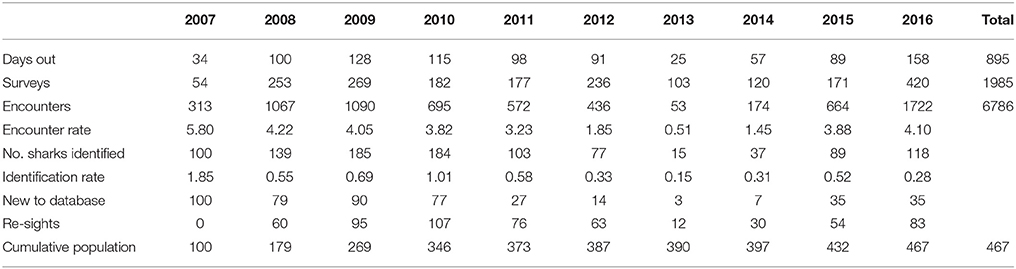
Table 1. Survey effort, encounter and identification rates of whale sharks in Donsol from 2007 to 2016.
Size Estimates
Turbidity in Donsol (normally 2–4 m visibility) hinders the use of stereo-photogrammetry or paired laser-photogrammetry (Authors, pers. obs.). We therefore recorded visual estimates in 0.5 m increments of the total length (LT) of each individual after each encounter, although this method holds a degree of error (Rohner et al., 2011; Sequeira et al., 2016). Researchers used boats and swimmers of known lengths to estimate the size of the animals. Given the longitudinal nature of the dataset and variability in size estimates from multiple researchers, we discarded intra- and inter-seasonal size estimates that differed by >2.5 m for individual whale sharks (n = 44), based on maximum growth estimates (Rowat and Brooks, 2012). To ensure this did not discard larger sharks only, we tested the relationship between visual estimate differences (2–10 m) and mean LT and found it to be not significant (r2 = 0.038, p > 0.05). We thus used the mean size over multiple encounters.
Photographs of the pelvic region were used to identify the sex based on the presence (male) or absence (female) of claspers, and sex was only assigned to an individual when a clear photo of the pelvic region was available. Males were considered mature when claspers extended beyond the pelvic fins and had a cauliflower appearance (Norman and Stevens, 2007; Rohner et al., 2015b). The total length at which 50% of males were considered mature (LT50) was calculated using a Generalized Linear Model (GLM) with a binary logit function. Scars on individuals were also photographed where possible, and their origin determined based on Speed et al. (2008) and Araujo et al. (2016) to identify potential anthropogenic pressures on the whale sharks visiting Donsol. All statistical analyses were done in program R 3.2.1 (R Core Team, 2014).
Whale sharks were visually matched against a library of images from the Donsol region and confirmed by a second researcher before being inputted into a presence spreadsheet. The software I3S (Van Tienhoven et al., 2007) was used to create a virtual fingerprint for every individual, and further confirm the identity of an individual. All whale shark encounters between 2007 and 2016, and any newly identified individuals, were uploaded onto the online open database Wildbook for Whale Sharks (www.whaleshark.org) to further confirm identity and check if that individual had previously been sighted elsewhere.
Citizen Science
Submissions from the public onto Wildbook for Whale Sharks were also run against a localized I3S database to confirm identity, date and location. Newly reported sightings were then added to the presence spreadsheet when the date and location were confirmed. These data were used to model residency and lagged identification rate (see below) as this modeling approach uses sightings data to determine effort (Whitehead, 2007). Donsol whale sharks identified elsewhere and submitted to Wildbook for Whale Sharks are also reported herein.
Residency, Lagged Identification Rate and Population Estimates
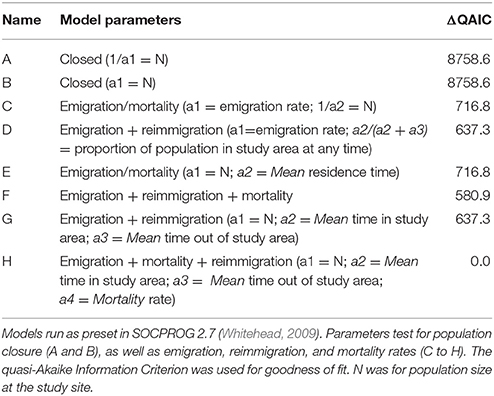
Maximum likelihood methods were used to estimate residency times of whale sharks at Donsol using program SOCPROG 2.7 (Whitehead, 2009). We calculated the lagged identification rate (LIR), defined as the probability that an individual will be resighted in Donsol after a certain time lag (Whitehead, 2001), using the “Movement” module of the software. Eight models (Table 2), using a combination of preset parameters that test for closed and open population models, including various combinations of emigration, reimmigration and mortality, were used to test the empirical dataset. The quasi-Akaike information criterion (QAIC) was used to evaluate each model's goodness of fit and account for over-dispersion of data (Whitehead, 2007). The best-fit model (Model H, Table 2) was bootstrapped for 100 repetitions to estimate standard errors and 95% confidence intervals (Buckland and Garthwaite, 1991).
We applied an open-population Jolly-Seber model (Schwarz and Arnason, 1996) using the POPAN option in program MARK (White and Burnham, 1999). For this model, only captures and recaptures from February, March, April and May were used, as effort was consistent throughout these months between years. The model uses t capture occasions (10 here) to calculate capture probability (p), t-1 to estimate apparent survival (ϕ), probability of entry into the population per occasion (β), and super-population size (N). The model was fitted with a logit link function to avoid convergence (White and Burnham, 1999). Only N was estimated, as other parameters were not central to the aim of this study.
Results
Population Structure
A total of 479 individual whale sharks were identified in Donsol, representing 44% of the currently identified whale sharks in the Philippines (n = 1,095 Wildbook for Whale Sharks, Apr 28th 2017). Sex could only be confirmed for 158 males and 22 females, highlighting a significant male bias (χ2 = 58.2, p < 0.001). Overall, whale sharks ranged in size from 2.0 to 10.0 m (n = 396, mean = 6.5 m, SD = 1.6 m; Figure 2). A total of 83 males (53% of males) were considered mature based on clasper morphology, but it was not possible to determine the females' maturity status. Mature males with available size data ranged from 6.0 to 10.0 m (n = 76, mean = 7.6 m, SD = 0.8 m). Maturity in males (LT50) was estimated to be attained at 6.8 m ± 0.2 m S.E. (Residual Deviance = 118.3; p < 0.001; AIC = 122.3). Fin truncations or amputations were observed on 79 individuals (16%), and propeller-originated scars were observed on 89 individuals (19%).
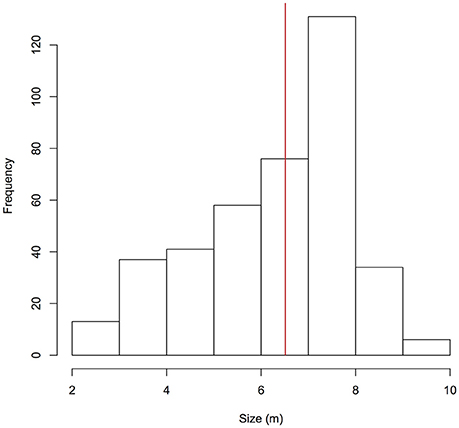
Figure 2. Size distribution of whale sharks identified in Donsol. The red line indicates the mean size of individuals (6.5 m).
Effort
A total of 1,985 surveys were conducted in 895 days over the 10 whale shark seasons (2007–2016), resulting in 6,786 encounters with R. typus. From these encounters, 467 individual R. typus were confirmed through photo-ID. Survey effort varied greatly between seasons, as these were dependent on sightings in the vicinity. There was a strong correlation between the number of surveys and the number of encounters (r2 = 0.84, p < 0.05), however, the encounter rate or identification rate did not follow a similar correlation (r2 = 0.03, p > 0.05, and r2 = 0.18, p > 0.05, respectively), reflecting the seasonal abundance of whale sharks off Donsol. Encounters occurred predominantly in the shallow coastal waters between barangay San Raphael and barangay Poblacion, although some encounters occurred as far north as Pio Duran, Albay, to as far south as barangay San Antonio, Pilar, Sorsogon (Figure 1). A further 12 individual whale sharks were added to the database through citizen science contributions from Donsol waters.
The total number of individuals identified per season (Nov–Jun) varied greatly, ranging from 15 to 185 individuals (mean = 104, SD = 55.53; Table 1), with a recruitment of 3–90 new individuals yearly (mean = 46.8, SD = 36.29). In 2013 and 2014, sightings of whale sharks were comparatively low, with 15 individuals in 2013, compared to 77 in 2012 and 89 in 2015 (Table 1). Forty-seven percent (n = 225) of whale sharks were only seen in one season in Donsol, whereas 53% were seen in at least two seasons. Some individuals (15%) returned in at least five different seasons (Nov–Jun), and two individuals were sighted in 10 different seasons (Figure 3). The longest match at Donsol was by individual P-375, which was first identified by a citizen scientist in 1998, and resighted again in 2011, 14 years later, but not since then. Similarly, individual P-135 was first identified in 2004 by a citizen scientist and was last seen in 2016, having been sighted in 2007, 2008, 2009, 2010, 2011, 2012, and 2014. A total of 20 whale sharks identified in Donsol were sighted at least once at one other location (Supplementary Table 1), and 31 whale sharks had a span of ≥ 10 years between their first and last identification at Donsol (Supplementary Table 2).
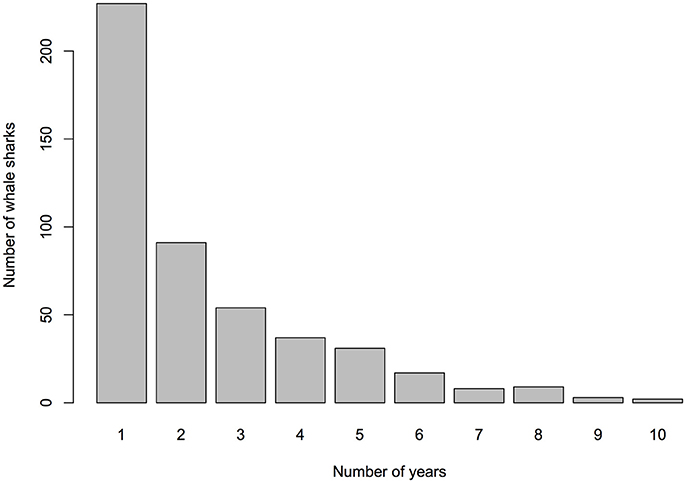
Figure 3. Number of different years individual whale sharks were sighted in Donsol through photo-ID (2007–2016).
Whale sharks were sighted as early as October and into June, with large seasonal variations. Peak season (February to May) remained consistent throughout all years of the study (Figure 4), with a maximum of 118 different individual whale sharks during April 2009 (mean = 57.1, SD = 34.5).
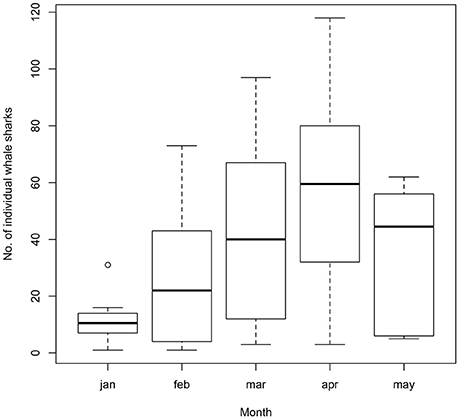
Figure 4. Boxplot depicting the number of individual whale sharks sighted monthly at Donsol from 2007 to 2016.
Residency, Lagged Identification Rate and Population Estimates
Model H (Table 2) was the best-fit model by integrating emigration, reimmigration and mortality. The modeled LIR showed that whale sharks spent a mean of 49.8 ± S.E. 14.5 [95% CI (32.3–78.6)] days in Donsol, whilst spending 56.4 ± S.E. 17.7 [95% CI (31.2–99.4)] days away. Mean mortality or permanent emigration was estimated at 0.000593 ± S.E. 0.000092 [95% CI (0.000418–0.000807)]. Estimates of aggregation size indicated a mean of 52.5 ± S.E. 4.3 [95% CI (47.1–60.8)] whale sharks present in Donsol at any one time during the season. The LIR decreased steadily from 1.0 to 205.3 days, and continued to decrease over time, but never quite reached zero (Figure 5). The LIR increased between 366.2 and 753.1 days, and then again between 1455.8 and 2623.1 days, suggesting some periodicity in the visitation by some whale sharks to Donsol over time.
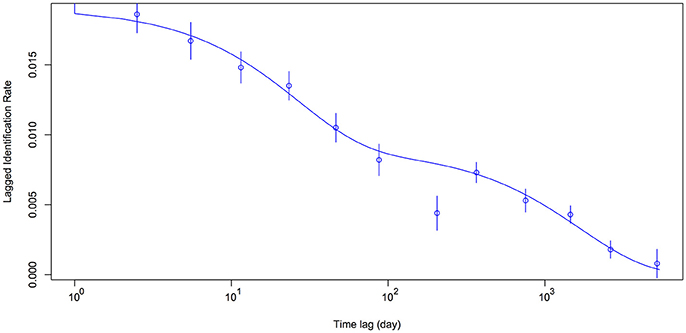
Figure 5. Lagged Identification Rate (mean ± S.E.) for whale sharks at Donsol. Model parameters included emigration, reimmigration and mortality as preset in SOCPROG 2.7 (Whitehead, 2009).
The open population model in program MARK with the POPAN extension (White and Burnham, 1999) for the photo-ID dataset (2007–2016) converged to provide a super-population estimate at Donsol of 1766.6 ± S.E. 40.7 [95% CI (1688.6–1848.1)] individuals.
Discussion
Donsol hosts a substantial aggregation of whale sharks on a seasonal basis, currently accounting for almost half of all whale sharks identified in the Philippines (Wildbook for Whale Sharks, December 2017). There was significant male bias (87%) for those individuals whose sex could be confirmed, though it could not be determined for 62.5% of individuals. Such male bias is consistent with coastal aggregations from across the globe (Meekan et al., 2006; Rowat et al., 2007, 2009; Riley et al., 2010; Ramírez-Macías et al., 2012a,b; Himawan et al., 2015; Rohner et al., 2015b) and with others from within the Philippines (Araujo et al., 2014, 2017). Adult-dominated sites remain a rarity, namely at Darwin's Arch in the Galapagos Islands (ECU), Gorda Banks in Baja California (MEX), at an offshore aggregation in Qatar (QAT), and at St Helena Island (GBR) in the South Atlantic, and at a newly identified area in the mid-equatorial Atlantic off Brazil (Ramírez-Macías et al., 2012b; Acuña-Marrero et al., 2014; Clingham et al., 2016; Macena and Hazin, 2016; Robinson et al., 2016). Whale sharks at Donsol are uncharacteristically larger than those found elsewhere in the Philippines (e.g., 5.2 m mean total length in the Bohol Sea, Authors, unpub. data.). These sites also employed visual estimates, with some laser photogrammetry validation (Araujo et al., 2014), yet there are likely large errors in estimates (Sequeira et al., 2016). However, using the same techniques, whale sharks in Donsol were larger than those in the Bohol Sea.
The average size of individuals in the Bohol Sea area was c. 5.2 m, with <20 mature males identified out of 565 individuals (Authors, unpub. data). By comparison, whale sharks at Donsol were considerably larger (c. 6.5 m) and most of the males were mature. Interestingly, only 5 out of >1,000 individuals identified at Donsol and in the Bohol Sea have been matched between these nearby locations (~400 km apart). The relatively low mixing between these regions might suggest that these groups of whale sharks utilize different areas on at least a seasonal basis. Alternatively, the lack of mixing could indicate size/age segregation within the Philippines, which would be essential information for managing critical habitats for the species (e.g., foraging, developmental, migratory corridors). A recent global photo-ID study across multiple sites revealed little movement between aggregations (Norman et al., 2017), as did a separate study looking at multiple sites in the Indian Ocean where only 1 whale shark out of 1,724 was sighted between two countries (Andrzejaczek et al., 2016). Telemetry tracking studies might help elucidate the movement patterns and connectivity of whale sharks in the Philippines. It is also important to understand if these whale sharks are moving into international waters, where hunting still occurs in relatively close proximity to Donsol (Li et al., 2012).
Although maturity could not be determined for females, over 50% of males examined were mature with large and calcified claspers. The size at which 50% of the population reached maturity was c. 6.8 m, considerably smaller than that previously reported in the western Indian Ocean using laser photogrammetry (9.2 m, Rohner et al., 2015b) and visually estimated at Ningaloo Reef, Western Australia (8.1 m, Norman and Stevens, 2007). However, it is similar to the size at maturity from the Gulf of Mexico and Qatar, where 50% maturity in males was estimated at 7.0 m and 7.3 m, respectively (Ramírez-Macías et al., 2012b; Robinson et al., 2016). In the present study we used visual estimates, which have inherent errors (Rohner et al., 2011; Sequeira et al., 2016), and thus our results are indicative more than absolute. However, differences in size-at-maturity between aggregations could indicate different in stocks or better feeding opportunities that lead to faster development or maturity. Further studies are necessary to elucidate this. Nonetheless, coupled with the fact that two of the smallest whale sharks ever recorded were found within close proximity to Donsol (Aca and Schmidt, 2011), the general occurrence of larger sharks and the large number of mature males underlines the importance of the area for this endangered species, as it might not only be a pupping and foraging ground, but also be a potential mating ground, which has thus far eluded the scientific community and the general public to date. Whale shark tourist guides operating in Donsol since 1998 have reported mating-like behavior by large individuals, but these reports have not yet been verified (Authors, pers. comm.).
Whale sharks visiting Donsol appear to spend c. 50 days in the area, as estimated using maximum likelihood methods (Whitehead, 2007), and as observed through intra-season resightings. This is considerably longer than that reported elsewhere. Using the same methodology, whale sharks at Panaon Island, Southern Leyte spent c. 27 days in the area (Araujo et al., 2016), and at an offshore aggregation in Qatar, whale sharks spent c. 29 days there (Robinson et al., 2016). In Ningaloo Reef, Western Australia, Holmberg et al. (2009) estimated whale sharks spent 33 days in the area using different methods. Contrastingly, at Utila Bay, Honduras and at an aggregation in Saudi Arabia's Red Sea, whale sharks spent c. 12 days in these areas, suggesting whale sharks are likely more transient there (Fox et al., 2013; Cochran et al., 2016). Residency patterns estimated through conventional mark-recapture techniques are a useful, non-invasive method for determining how whale sharks use certain sites. Employing acoustic telemetry, Cagua et al. (2015) showed that whale sharks were residing in the vicinity of Mafia Island, Tanzania, year-round. Some whale sharks therefore exhibit strong site fidelity and this should be considered when developing local management plans. For instance, the exclusion of fishing gear known to interact frequently with whale sharks could be temporarily restricted, or speed limits could be incorporated into local legislation to reduce the potential collisions with whale sharks, which is clearly a problem, given that 89 individuals had propeller scars on them. This number is higher in other parts of the Philippines (47 and 45% of individuals, Araujo et al., 2014, 2016 respectively), but similar to other sites, such as Isla Holbox, Mexico (Ramírez-Macías et al., 2012a).
Whale sharks displayed strong site fidelity to Donsol. Most whale sharks (53%) visited Donsol in at least two separate seasons, with some individuals returning to the site spanning over 10 years. By contrast, only 32% of individuals were resighted at Panaon Island, Southern Leyte (Araujo et al., 2016), similar to Ningaloo Reef, Western Australia, where 35% of individuals were sighted in different years (Holmberg et al., 2009). Whale sharks at Gladden Spit, Belize and in the Seychelles had a lower inter-year resight proportion of 22 and 28%, respectively (Rowat et al., 2011; Fox et al., 2013). The strong philopatry displayed by whale sharks at Donsol further highlights the importance of the site for the species.
The main driver of whale shark occurrence at Donsol appears to be prey related as sightings coincide with periods of higher productivity in the region (Gordon et al., 2011; Stewart et al., 2017). Diatom blooms appear to dominate the plankton composition in Donsol between January and August. Large surface mats of Trichodesmium were also reported, though they could be linked to an El Niño event during sampling (WWF-Philippines, unpub. data). Whale shark abundance appears to peak c. 1 month prior to these plankton communities' highest yearly densities in the area, and coincides with the dry season in the Philippines resulting in reduced water output from the rivers into Donsol waters (Lapitan-Tandang, 2010). Plankton tows were conducted in the general area year-round, but not in close proximity to feeding whale sharks as recommended by (Rohner et al., 2015a). The high density of phytoplankton is likely supporting complex zooplankton compositions, but the links remain unclear. Whale sharks were observed feeding at Donsol, as previously reported by Quiros (2007) and Yaptinchay (1999). It is thus possible that whale sharks are targeting high-density patches of zooplankton in Donsol, supported by phytoplankton species, which would explain their seasonal visits there. However, further work to understand their feeding ecology in Donsol is necessary to test this idea.
The open population model estimated that 1,767 whale sharks make up the population visiting Donsol. Although effort was inconsistent across years, encounter and individual rates were not correlated to effort. This is a considerable number of whale sharks, and makes it the largest seasonal reported aggregation in Southeast Asia. However, seasons have been variable, with 2013 and 2014 having had very few individuals. Although variability in whale shark occurrence is likely linked to prey distribution and primary productivity, it is important to monitor changes over time to detect possible threats to this already endangered species (Pierce and Norman, 2016). The population estimate at Donsol of 1,767 ± 41 is comparable to estimates from the Gulf of Mexico of 2,167 individuals (McKinney et al., 2017), although different methods were applied. It is considerably larger though than estimates from Ningaloo with 320–440 individuals (Meekan et al., 2006), the Seychelles with 348–488 individuals (Rowat et al., 2009), the Maldives with 68–81 individuals (Riley et al., 2010) or Holbox Island, Mexico, with 521–809 individuals (Ramírez-Macías et al., 2012a) using the same methods. Although mark-recapture approaches provide an estimate of population size, these numbers are more indicative than absolute, particularly in light of a lack of standardized methods for estimating whale shark abundance across sites. Genetically derived estimates across different aggregations might provide more accurate estimates for management purposes.
Citizen science contributions were valuable, with 12 newly identified individuals from the general public's photo submissions to Wildbook for Whale Sharks. Consistent with Araujo et al. (2014, 2016), whale sharks appear to be moving broadly through the Philippines. Donsol receives up to 27,000 tourists seasonally, and the potential for further harnessing citizen science for cost-effective population monitoring needs to be explored. Donsol has attracted tourists since 1998, and the tourism interaction with whale sharks has been regulated by a number of local and national legislations (Pine et al., 2007; Quiros, 2007). Some of the concerns previously highlighted by Quiros (2007) have been addressed, though others remain, such as the number of motorized vessels around a single whale shark and the proximity of swimmers to the animals (Authors, pers. obs.). Although tourism now brings over U$S 1.5 M in revenue per season to the area (Local Government Unit, Donsol), it is important to strictly regulate interactions with whale sharks at Donsol, given the importance of the area to the species and their endangered status (Pierce and Norman, 2016). Sustainable practices are recommended when engaging tourism with an endangered species, ensuring the durability of the industry for the local communities that benefit from it, and minimizing disturbance in an important habitat (Quiros, 2007; Araujo et al., 2017).
Conclusion
Donsol is a unique whale shark site, hosting the largest known whale shark aggregation in Southeast Asia. It is economically and ecologically important for both communities and whale sharks alike. Whale sharks visiting Donsol stay longer than at other aggregating sites and display yearly periodicity. There is also a considerable proportion of adult males within this aggregation, uniquely worldwide, which might provide some insight into the reproductive life history of the species. Furthermore, Donsol presents a good opportunity for implementation of citizen science approaches, given the ease of photographing surface-dwelling whale sharks, and that these data can cost-effectively help population monitoring over time.
Ethics Statement
This study was carried out in accordance with the recommendations and in collaboration with the Department of Agriculture-Bureau of Fisheries and Aquatic Resources of the Republic of the Philippines. No animal was constrained and the methods employed were non-invasive in nature.
Author Contributions
EM, RB, DD, EA, JH, JL, SS, AP, and GA designed the study, collected data, validated, and analyzed data. EM and GA prepared figures and tables. All authors wrote and reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was possible through the support of DA-BFAR, DENR and DOT Region 5, the Local Government Unit of Donsol and mayor Josephine Alcantara-Cruz. The work of LAMAVE and WWF-Philippines was supported by dedicated staff members and volunteers throughout the seasons. This research has made use of data and software tools provided by Wildbook for Whale Sharks, an online mark-recapture database operated by the non-profit scientific organization Wild Me with support from public donations and the Qatar Whale Shark Research Project. Data collection was made possible thanks to the support and collaboration of Donsol's Butanding Interaction Officers and the BBOA association. We extend our gratitude to Dr. Brad Norman and Dr. Brent Stewart for the training and support to WWF-Philippines' whale shark work, as well as to Dr. AA Yaptinchay and Carina Escudero for their essential role in the development of tourism in Donsol. Authors would like also to acknowledge Lene and Claus Topp of WWF-Denmark for their unwavering support of WWF-Philippines whale shark research, and ecotourism in the Philippines through the Protection of the bigger migrating sea-animals in the Coral Triangle project. We also thank two reviewers for their time and helping strengthen this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00271/full#supplementary-material
References
Aca, E. Q., and Schmidt, J. V. (2011). Revised size limit for viability in the wild: neonatal and young of the year whale sharks identified in the Philippines. Asia Life Sci. 20, 361–367.
Acuña-Marrero, D., Jiménez, J., Smith, F., Doherty, P. F Jr, Hearn, A, Green, J. R., et al. (2014). Whale shark (Rhincodon typus) seasonal presence, residence time and habitat use at Darwin Island, Galapagos Marine Reserve. PLoS ONE 9:e115946. doi: 10.1371/journal.pone.0115946
Alava, E. R. Z., Dolumbaló, E. R., Yaptinchay, A. A., and Trono, R. B. (1997). “Fishery and trade of whale sharks and manta rays in the Bohol Sea, Philippines,” in Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop (Sabah), 132–148.
Andrzejaczek, S., Meeuwig, J. J., Rowat, D., Pierce, S. J., Davies, T. K., Fisher, R., et al. (2016). Establishing the ecological connectivity of whale shark aggregations across the Indian Ocean–a photo-identification approach. R. Soc. Open Sci. 3:160455. doi: 10.1098/rsos.160455
Araujo, G., Lucey, A., Labaja, J., So, C. L., Snow, S., and Ponzo, A. (2014). Population structure and residency patterns of whale sharks, Rhincodon typus, at a provisioning site in Cebu, Philippines. Peer J. 2:e543. doi: 10.7717/peerj.543
Araujo, G., Snow, S., So, C. L., Labaja, J., Murray, R., Colucci, A., et al. (2016). Population structure, residency patterns and movements of whale sharks in Southern Leyte, Philippines: results from dedicated photo-ID and citizen science. Aquat. Conserv. Mar. Freshw. Ecosys. 27, 237–252. doi: 10.1002/aqc.2636
Araujo, G., Vivier, F., Labaja, J. J., Hartley, D., and Ponzo, A. (2017). Assessing the impacts of tourism on the world's largest fish Rhincodon typus at Panaon Island, Southern Leyte, Philippines. Aquat.Conserv. Mar. Freshw. Ecosys. 27, 986–994. doi: 10.1002/aqc.2762
Arzoumanian, Z., Holmberg, J., and Norman, B. (2005). An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus. J. Appl. Ecol. 42, 999–1011. doi: 10.1111/j.1365-2664.2005.01117.x
Berumen, M. L., Braun, C. D., Cochran, J. E., Skomal, G. B., and Thorrold, S. R. (2014). Movement patterns of juvenile whale sharks tagged at an aggregation site in the Red Sea. PLoS ONE 9:e103536. doi: 10.1371/journal.pone.0103536
Bradshaw, C. J., Fitzpatrick, B. M., Steinberg, C. C., Brook, B. W., and Meekan, M. G. (2008). Decline in whale shark size and abundance at Ningaloo Reef over the past decade: the world's largest fish is getting smaller. Biol. Conserv. 141, 1894–1905. doi: 10.1016/j.biocon.2008.05.007
Bradshaw, C. J., Mollet, H. F., and Meekan, M. G. (2007). Inferring population trends for the world's largest fish from mark–recapture estimates of survival. J. Anim. Ecol. 76, 480–489. doi: 10.1111/j.1365-2656.2006.01201.x
Buckland, S. T., and Garthwaite, P. H. (1991). Quantifying precision of mark-recapture estimates using the bootstrap and related methods. Biometrics 47, 255–268.
Cagua, E. F., Cochran, J. E., Rohner, C. A., Prebble, C. E., Sinclair-Taylor, T. H., Pierce, S. J., et al. (2015). Acoustic telemetry reveals cryptic residency of whale sharks. Biol. Lett. 11:20150092. doi: 10.1098/rsbl.2015.0092
Calumpong, H. P., Sienes, P. M., Santos, T. R., Padin, J. I., and Sabater, E. R. (2013). Plankton abundance in Ticao Pass, Masbate, Philippines. Ecol. Environ. Conserv. 19, 47–51.
Catlin, J., and Jones, R. (2010). Whale shark tourism at Ningaloo Marine Park: a longitudinal study of wildlife tourism. Tour. Manage. 31, 386–394. doi: 10.1016/j.tourman.2009.04.004
Clingham, E., Webb, H. D., de la Parra Venegas, R., Schreiber, C., Reid, J., Pierce, S., et al. (2016). “Further evidence of the importance of St. Helena as habitat for whale sharks,” in QScience Proceedings, The 4th International Whale Shark Conference (Doha), 11.
Cochran, J. E. M., Hardenstine, R. S., Braun, C. D., Skomal, G. B., Thorrold, S. R., Xu, K., et al. (2016). Population structure of a whale shark Rhincodon typus aggregation in the Red Sea. J. Fish Biol. 89, 1570–1582. doi: 10.1111/jfb.13054
Fox, S., Foisy, I., De La Parra Venegas, R., Galván Pastoriza, B. E., Graham, R. T., Hoffmayer, E. R., et al. (2013). Population structure and residency of whale sharks Rhincodon typus at Utila, Bay Islands, Honduras. J. Fish Biol. 83, 574–587. doi: 10.1111/jfb.12195
Gordon, A. L., Sprintall, J., and Ffield, A. (2011). Regional oceanography of the Philippine Archipelago. Oceanography 24, 14–27. doi: 10.5670/oceanog.2011.01
Graham, R. T., and Roberts, C. M. (2007). Assessing the size, growth rate and structure of a seasonal population of whale sharks (Rhincodon typus Smith 1828) using conventional tagging and photo identification. Fish. Res. 84, 71–80. doi: 10.1016/j.fishres.2006.11.026
Hammond, P. S. (1990). Capturing whales on film–estimating cetacean population parameters from individual recognition data. Mamm. Rev. 20, 17–22.
Himawan, M. R., Tania, C., Noor, B. A., Wijonarno, A., Subhan, B., and Madduppa, H. (2015). Sex and size range composition of whale shark (Rhincodon typus) and their sighting behaviour in relation with fishermen lift-net within Cenderawasih Bay National Park, Indonesia. AACL Bioflux 8, 123−133.
Holmberg, J., Norman, B., and Arzoumanian, Z. (2008). Robust, comparable population metrics through collaborative photo? Monitoring of whale sharks Rhincodon typus. Ecol. Appl. 18, 222–233. doi: 10.1890/07-0315.1
Holmberg, J., Norman, B., and Arzoumanian, Z. (2009). Estimating population size, structure, and residency time for whale sharks Rhincodon typus through collaborative photo-identification. Endanger. Species Res. 7, 39–53. doi: 10.3354/esr00186
Hsu, H. H., Joung, S. J., Liao, Y. Y., and Liu, K. M. (2007). Satellite tracking of juvenile whale sharks, Rhincodon typus, in the Northwestern Pacific. Fish. Res. 84, 25–31. doi: 10.1016/j.fishres.2006.11.030
Lapitan-Tandang, K. J. (2010). Physico-chemical Characteristics of Water Visited by Whale Sharks in Bicol Region, Philippines. Terminal Report. Partido State University.
Li, W., Wang, Y., and Norman, B. (2012). A preliminary survey of whale shark Rhincodon typus catch and trade in China: an emerging crisis. J. Fish Biol. 80, 1608–1618. doi: 10.1111/j.1095-8649.2012.03250.x
Macena, B. C., and Hazin, F. H. (2016). Whale shark (Rhincodon typus) seasonal occurrence, abundance and demographic structure in the mid-equatorial Atlantic Ocean. PLoS ONE 11:e0164440. doi: 10.1371/journal.pone.0164440
Marshall, A. D., Dudgeon, C. L., and Bennett, M. B. (2011). Size and structure of a photographically identified population of manta rays Manta alfredi in southern Mozambique. Mar. Biol. 158, 1111–1124. doi: 10.1007/s00227-011-1634-6
McKinney, J. A., Hoffmayer, E. R., Holmberg, J., Graham, R. T., Driggers, W. B. III, de la Parra-Venegas, R., et al. (2017). Long-term assessment of whale shark population demography and connectivity using photo-identification in the Western Atlantic Ocean. PLoS ONE 12:e0180495 doi: 10.1371/journal.pone.0180495
Meekan, M. G., Bradshaw, C. J., Press, M., McLean, C., Richards, A., Quasnichka, S., et al. (2006). Population size and structure of whale sharks Rhincodon typus at Ningaloo Reef, Western Australia. Mar. Ecol. Prog. Ser. 319, 275–285. doi: 10.3354/meps319275
Motta, P. J., Maslanka, M., Hueter, R. E., Davis, R. L., De la Parra, R., Mulvany, S. L., et al. (2010). Feeding anatomy, filter-feeding rate, and diet of whale sharks Rhincodon typus during surface ram filter feeding off the Yucatan Peninsula, Mexico. Zoology 113, 199–212. doi: 10.1016/j.zool.2009.12.001
Norman, B. M., Holmberg, J. A., Arzoumanian, Z., Reynolds, S. D., Wilson, R. P., Rob, D., et al. (2017). Undersea constellations: the global biology of an endangered marine megavertebrate further informed through citizen science. BioScience 67, 1029–1043. doi: 10.1093/biosci/bix127
Norman, B. M., and Stevens, J. D. (2007). Size and maturity status of the whale shark (Rhincodon typus) at Ningaloo Reef in Western Australia. Fish. Res. 84, 81–86. doi: 10.1016/j.fishres.2006.11.015
Pierce, S. J., and Norman, B. M. (2016). Rhincodon typus. The IUCN Red List of Threatened Species. 2016: e.T19488A2365291.
Pine, R., Alava, M. N. R., and Yaptinchay, A. A. (2007). “Challenges and lessons learned in setting-up a community-based whale shark eco-tourism program: the case in Donsol, Philippines,” in The First International Whale Shark Conference: Promoting International Collaboration in Whale Shark Conservation, Science and Management. Conference Overview, Abstracts and Supplementary Proceedings (CSIRO Wembley:Marine and Atmospheric Research), 36–44.
Quiros, A. L. (2007). Tourist compliance to a code of conduct and the resulting effects on whale shark (Rhincodon typus) behavior in Donsol, Philippines. Fish. Res. 84, 102–108. doi: 10.1016/j.fishres.2006.11.017
Ramírez-Macías, D., Meekan, M., De La Parra-Venegas, R., Remolina-Suárez, F., Trigo-Mendoza, M., and Vázquez-Juárez, R. (2012a). Patterns in composition, abundance and scarring of whale sharks Rhincodon typus near Holbox Island, Mexico. J. Fish Biol. 80, 1401–1416. doi: 10.1111/j.1095-8649.2012.03258.x
Ramírez-Macías, D., Queiroz, N., Pierce, S. J., Humphries, N. E., Sims, D. W., and Brunnschweiler, J. M. (2017). Oceanic adults, coastal juveniles: tracking the habitat use of whale sharks off the Pacific coast of Mexico. Peer J. 5:e3271.doi: 10.7717/peerj.3271
Ramírez-Macías, D., Vázquez-Haikin, A., and Vázquez-Juárez, R. (2012b). Whale shark Rhincodon typus populations along the west coast of the Gulf of California and implications for management. Endanger. Species Res. 18, 115–128. doi: 10.3354/esr00437
R Core Team (2014). R: A Language And Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing.
Riley, M., Hale, M., Harman, A., and Rees, R. (2010). Analysis of whale shark Rhincondon typus aggregations near South Ari Atoll, Maldives Archipelago. Aquat. Biol. 8, 145–150. doi: 10.3354/ab00215
Robinson, D. P., Jaidah, M. Y., Bach, S., Lee, K., Jabado, R. W., Rohner, C. A., et al. (2016). Population structure, abundance and movement of whale sharks in the Arabian Gulf and the Gulf of Oman. PLoS ONE 11:e0158593. doi: 10.1371/journal.pone.0158593
Robinson, D. P., Jaidah, M. Y., Bach, S. S., Rohner, C. A., Jabado, R. W., Ormond, R., et al. (2017). Some like it hot: repeat migration and residency of whale sharks within an extreme natural environment. PLoS ONE 12:e0185360. doi: 10.1371/journal.pone.0185360
Robinson, D. P., Jaidah, M. Y., Jabado, R. W., Lee-Brooks, K., El-Din, N. M., Malki, A. A., et al. (2013). Whale sharks, Rhincodon typus, aggregate around offshore platforms in Qatari waters of the Arabian Gulf to feed on fish spawn. PLoS ONE 8:e58255. doi: 10.1371/journal.pone.0058255
Rohner, C. A., Armstrong, A. J., Pierce, S. J., Prebble, C. E., Cagua, E. F., Cochran, J. E., et al. (2015a). Whale sharks target dense prey patches of sergestid shrimp off Tanzania. J. Plankton Res. 37, 352–362. doi: 10.1093/plankt/fbv010
Rohner, C. A., Richardson, A. J., Marshall, A. D., Weeks, S. J., and Pierce, S. J. (2011). How large is the world's largest fish? Measuring whale sharks Rhincodon typus with laser photogrammetry. J. Fish Biol. 78, 378–385. doi: 10.1111/j.1095-8649.2010.02861.x
Rohner, C. A., Richardson, A. J., Prebble, C. E., Marshall, A. D., Bennett, M. B., Weeks, S. J., et al. (2015b). Laser photogrammetry improves size and demographic estimates for whale sharks. Peer J. 3:e886. doi: 10.7717/peerj.886
Rowat, D., Brooks, K., March, A., McCarten, C., Jouannet, D., Riley, L., et al. (2011). Long-term membership of whale sharks (Rhincodon typus) in coastal aggregations in Seychelles and Djibouti. Mar. Freshw. Res. 62, 621–627. doi: 10.1071/MF10135
Rowat, D., and Brooks, K. S. (2012). A review of the biology, fisheries and conservation of the whale shark Rhincodon typus. J. Fish Biol. 80, 1019–1056. doi: 10.1111/j.1095-8649.2012.03252.x
Rowat, D., Meekan, M. G., Engelhardt, U., Pardigon, B., and Vely, M. (2007). Aggregations of juvenile whale sharks (Rhincodon typus) in the Gulf of Tadjoura, Djibouti. Environ. Biol. Fishes 80, 465–472. doi: 10.1007/s10641-006-9148-7
Rowat, D., Speed, C. W., Meekan, M. G., Gore, M. A., and Bradshaw, C. J. (2009). Population abundance and apparent survival of the vulnerable whale shark Rhincodon typus in the Seychelles aggregation. Oryx 43, 591–598. doi: 10.1017/S0030605309990408
Schofield, G., Katselidis, K. A., Dimopoulos, P., and Pantis, J. D. (2008). Investigating the viability of photo-identification as an objective tool to study endangered sea turtle populations. J. Exp. Mar. Biol. Ecol. 360, 103–108. doi: 10.1016/j.jembe.2008.04.005
Schwarz, C. J., and Arnason, A. N. (1996). A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 52, 860–873.
Sequeira, A. M., Thums, M., Brooks, K., and Meekan, M. G. (2016). Error and bias in size estimates of whale sharks: implications for understanding demography. R. Soc. Open Sci. 3:150668. doi: 10.1098/rsos.150668
Sleeman, J. C., Meekan, M. G., Wilson, S. G., Polovina, J. J., Stevens, J. D., Boggs, G. S., et al. (2010). To go or not to go with the flow: Environmental influences on whale shark movement patterns. J. Exp. Mar. Biol. Ecol. 390, 84–98. doi: 10.1016/j.jembe.2010.05.009
Speed, C. W., Meekan, M. G., Rowat, D., Pierce, S. J., Marshall, A. D., and Bradshaw, C. J. A. (2008). Scarring patterns and relative mortality rates of Indian Ocean whale sharks. J. Fish Biol. 72, 1488–1503. doi: 10.1111/j.1095-8649.2008.01810.x
Stewart, J. D., Rohner, C. A., Araujo, G., Avila, J., Fernando, D., Forsberg, K., et al. (2017). Trophic overlap in mobulid rays: insights from stable isotope analysis. Mar. Ecol. Prog. Ser. 580, 131–151. doi: 10.3354/meps12304
Van Tienhoven, A. M., Den Hartog, J. E., Reijns, R. A., and Peddemors, V. M. (2007). A computer-aided program for pattern-matching of natural marks on the spotted raggedtooth shark Carcharias taurus. J. Appl. Ecol. 44, 273–280. doi: 10.1111/j.1365-2664.2006.01273.x
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
Whitehead, H. (2001). Analysis of animal movement using opportunistic individual identifications: application to sperm whales. Ecology 82, 1417–1432. doi: 10.1890/0012-9658(2001)082[1417:AOAMUO]2.0.CO;2
Whitehead, H. (2007). Selection of models of lagged identification rates and lagged association rates using AIC and QAIC. Communic. Stat. Simul. Comput. 36, 1233–1246. doi: 10.1080/03610910701569531
Whitehead, H. (2009). SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. doi: 10.1007/s00265-008-0697-y
Wilson, S. G., Polovina, J. J., Stewart, B. S., and Meekan, M. G. (2006). Movements of whale sharks (Rhincodon typus) tagged at Ningaloo Reef, Western Australia. Mar. Biol. 148, 1157–1166. doi: 10.1007/s00227-005-0153-8
Keywords: Rhincodon typus, residency, LIR, maximum-likelihood models, population structure, philopatry
Citation: McCoy E, Burce R, David D, Aca EQ, Hardy J, Labaja J, Snow SJ, Ponzo A and Araujo G (2018) Long-Term Photo-Identification Reveals the Population Dynamics and Strong Site Fidelity of Adult Whale Sharks to the Coastal Waters of Donsol, Philippines. Front. Mar. Sci. 5:271. doi: 10.3389/fmars.2018.00271
Received: 27 February 2018; Accepted: 18 July 2018;
Published: 07 August 2018.
Edited by:
Mark Meekan, Australian Institute of Marine Science (AIMS), AustraliaReviewed by:
Eric Hoffmayer, Southeast Fisheries Science Center (NOAA), United StatesGail Schofield, Queen Mary University of London, United Kingdom
Copyright © 2018 McCoy, Burce, David, Aca, Hardy, Labaja, Snow, Ponzo and Araujo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gonzalo Araujo, Zy5hcmF1am9AbGFtYXZlLm9yZw==
 Emer McCoy1
Emer McCoy1 Raul Burce
Raul Burce David David
David David Jennifer Hardy
Jennifer Hardy Alessandro Ponzo
Alessandro Ponzo Gonzalo Araujo
Gonzalo Araujo