- 1Centre for Ecology and Conservation, University of Exeter, Penryn, United Kingdom
- 2ProDelphinus, Lima, Peru
- 3Facultad de Biologia Marina, Universidad Científica del Sur, Facultad de Biología Marina, Lima, Peru
There is widespread evidence that small-scale fisheries (SSF) bycatch threatens many populations of small cetaceans, yet conservation efforts are often limited by a lack of basic knowledge regarding their abundance, distribution, and habitat use. Here, we used passive acoustic monitoring from an SSF platform-of-opportunity to better characterize the distribution and habitat use of small cetaceans in northern Peru, focussing on the little-known Burmeister's porpoise Phocoena spinipinnis. From 2009 to 2012, acoustic click detectors (C-PODs) were attached to fishing nets for the duration of 116 fishing sets (30 fishing trips). Dolphins (unspecified delphinids) and porpoises were recorded around 71 and 22% of fishing sets, respectively. The probability of occurrence and buzzing behavior (a proxy for foraging), and time spent, were linked to both static and dynamic environmental variables to examine the drivers of habitat use. Dolphin activity was spread evenly throughout the fishing area and was not linked to any habitat variables. In contrast, porpoises were detected in neritic waters, and habitat models performed well, identifying preferences for shallow (<200 m depth) and cooler (17–18°C) waters, close (<50 km) to shore. The high bycatch rate of small cetaceans in Peruvian SSF gave us the unique opportunity to investigate the link between bycatch and cetacean activity around vessels. We found a positive relationship between the likelihood of a bycatch event and acoustic presence for both dolphins and porpoises, however as we did not know the timing of entanglement, we could not link vocalization rates to mortality events. Nonetheless, as small cetaceans (particularly dolphins) frequently encounter fishing boats, the likelihood of entanglements may be reduced through effective efforts to alert animals to the presence of the net, either acoustically (using acoustic alarms) or visually. This study demonstrates that passive acoustic monitoring from a fisheries platform can provide insights into the distribution and habitat use of small cetaceans at relatively low cost, and is likely to be suitable in regions with low monitoring effort and high fishing pressure.
Introduction
Artisanal or small-scale fisheries (hereafter SSF) play a vital role in global food production and employment, particularly in developing countries (Berkes et al., 2001; Chuenpagdee et al., 2006; Pauly, 2006). However, there is growing evidence that they have widespread impacts on non-target animals such as marine mammals, seabirds and sea turtles, which are incidentally caught as bycatch (e.g., Awkerman et al., 2006; Peckham et al., 2007; Moore et al., 2010; Alfaro-Shigueto et al., 2011). In particular, many species of small cetaceans (dolphins and porpoises) are subjected to both direct take and bycatch, as their coastal, estuarine or freshwater, and often restricted, ranges co-occur with large gillnet fleets (Dawson, 1991; Jefferson and Curry, 1994; Reeves et al., 2013). Indeed, bycatch in SSF is thought to have contributed to the extinction of the Baiji or Yangtze River dolphin Lipotes vexillifer (Turvey et al., 2007) and threatens several (sub)species of small cetaceans such as the vaquita porpoise Phocoena sinus (Jaramillo-Legorreta et al., 2017; Taylor et al., 2017) and Maui's dolphin Cephalorhynchus hectori maui (Slooten et al., 2006). Small-scale fisheries have traditionally received relatively little attention from fisheries managers, while conservation measures are often not implemented due to lack of political will or viable fishing alternatives (Read, 2008; Mangel et al., 2010; Reeves et al., 2013). Additionally, research on the abundance and distribution of captured species is often lacking, so the impacts of SSF on cetacean populations are generally poorly understood (Reeves et al., 2005; Read, 2008).
While there is a need for focussed survey effort in regions with historically high levels of bycatch (Read, 2008), monitoring cetacean abundance and distribution can be costly, logistically challenging and labor intensive (Mellinger et al., 2007; Kyhn et al., 2012). Passive acoustic techniques are a lower cost alternative to visual surveys (Mellinger et al., 2007) and are effective at sampling the behavior of dolphins and porpoises as they are highly vocal (Marques et al., 2013). Unlike visual surveys, which are usually limited by daylight and weather conditions, passive acoustic data can be collected year-round, at night, and under most sea states (Kyhn et al., 2012). Passive acoustic methods have proven particularly effective at detecting coastal species that are hard to observe, are known to avoid boats or exist at extremely low densities, providing novel insights into their habitat use and abundance (Rayment et al., 2011; Gallus et al., 2012; Jaramillo-Legorreta et al., 2017).
Generally, passive acoustic devices are towed behind a survey vessel or moored in one location (usually to buoys) for extended time periods (Mellinger et al., 2007; Sousa-Lima et al., 2013). Indeed, static acoustic arrays enable continuous measurements to be collected over long time periods, enabling the quantification of fine-scale temporal patterns in behavior (e.g., Leeney et al., 2011). However, their limitations are that moorings can be challenging to set-up and maintain (Sousa-Lima et al., 2013), and as the maximum detection ranges of devices are usually a few 100 m (e.g., Philpott et al., 2007), in the majority of cases, the spatial coverage of the sampled area is extremely limited (except see Pirotta et al., 2014; Mikkelsen et al., 2016). While this limitation may be offset to some degree by the highly mobile nature of many cetacean species, the use of mobile platforms-of-opportunity, such as passenger ferries (e.g., Kiszka et al., 2007; MacLeod et al., 2008) or fishing vessels (e.g., Lopez et al., 2004), provide a means to sample large areas. Data collected from platforms-of-opportunity are generally not spatially or temporally randomized and so can present analytical challenges (Isojunno et al., 2012); nonetheless, in regions where traditional monitoring methods are prohibitively costly or logistically challenging, platforms-of-opportunity may provide novel insights into relative spatial distributions of cetaceans (e.g., Kiszka et al., 2007; MacLeod et al., 2008). As SSF are ubiquitous in many of the world's coastal regions (Chuenpagdee et al., 2006; Pauly, 2006), fishing vessels could presumably be used as platforms for monitoring marine species, particularly in data deficient regions (Braulik et al., 2018).
Here, we take advantage of the large spatial extent of the Peruvian driftnet fleet (described in Alfaro-Shigueto et al., 2010; Mangel et al., 2010), to examine the distribution and habitat use of small cetaceans off northern Peru. We focus on the little-known Burmeister's porpoise Phocoena spinipinnis, a predominantly coastal Phocoenid endemic to South America, with a range spanning from northern Peru to southern Chile in the Pacific and from Tierra del Fuego, Argentina to southern Brazil in the Atlantic (Brownell and Praderi, 1984; Goodall et al., 1995a). While it is unclear whether it has a continuous distribution throughout its range due to a lack of survey effort (Brownell and Clapham, 1999), the Peruvian population appears to be genetically differentiated from populations in Chilean and Argentine waters (Rosa et al., 2005). Observations are challenging in anything but calm conditions due to the low, posterior located dorsal fin, and its shy and elusive swimming behavior (Brownell and Praderi, 1982, 1984), and there have been remarkably few sightings, with most of them occurring off the coast of Peru (reviewed in Van Waerebeek et al., 2002). While there are no abundance estimates (Jefferson and Curry, 1994), information about the species' biology has largely been obtained from incidentally or directly captured animals (Reyes and Van Waerebeek, 1995; García-Godos et al., 2007); these studies indicating that it is not uncommon (Brownell and Praderi, 1982; Van Waerebeek and Reyes, 1994; Van Waerebeek et al., 1997; Rosa et al., 2005).
Burmeister's porpoise are caught in gillnets throughout the species range (Jefferson and Curry, 1994). In particular, large numbers of individuals have been captured over the last four decades by SSF in Peru (Read et al., 1988; Van Waerebeek and Reyes, 1990, 1994; Van Waerebeek et al., 1997; Majluf et al., 2002; Mangel et al., 2010). Despite a national law introduced in 1996 banning the capture and trade of dolphins and porpoises, the Peruvian small-scale driftnet fishery, which predominantly targets elasmobranchs, still has one of the highest rates of small cetacean bycatch in the world, due in part to its vast capacity, with an estimated 10,000–20,000 animals killed per year (Read et al., 1988; Van Waerebeek and Reyes, 1990, 1994; Alfaro-Shigueto et al., 2010; Mangel et al., 2010). Market surveys and on-board observer schemes have indicated that porpoises are caught less frequently than they once were, possibly suggesting population declines in Peruvian waters (Rosa et al., 2005; Tzika et al., 2010). Burmeister's porpoise is listed as “Data Deficient” by the International Union for the Conservation of Nature (IUCN) (Hammond et al., 2012), while the IUCN Cetacean Specialist Group and International Whaling Commission (IWC) have listed the Peru population as a priority for bycatch reduction, and recommended increased research on its distribution and population status (Reeves et al., 2005).
In this study, acoustic click detectors or C-PODs (Chelonia Ltd., Mousehole, Cornwall, UK) were attached to fishing nets over a three-year period, providing information on the acoustic activity of dolphins and porpoises in the vicinity of SSF vessels. Over the duration of the study, the fishery sampled a large geographic area, encompassing a range of marine habitats, from coastal to oceanic waters (Mangel et al., 2010). The aim of the study was to relate acoustic detections of vocalizing small cetaceans (Burmeister's porpoise and unspecified delphinids) to habitat variables to better understand their space use. Specifically, we (1) briefly describe the acoustic characteristics of Burmeister's porpoise off the coast of Peru, and (2) identify areas with elevated dolphin and porpoise activity, and their associated habitat characteristics. As the fishery has naturally high bycatch rates, particularly of dolphins (Mangel et al., 2010, 2013), we also (3) link acoustic activity of dolphins and porpoises to bycatch events, in order to better understand how small cetaceans become entangled in fishing gear.
Materials and Methods
Data Collection
Between April 2009 and December 2012, fishing trips were monitored by observers on SSF vessels from the port of Salaverry (8°14′S, 78°59′W) in northern Peru. SSF are defined according to Peruvian fisheries regulations as boats of <15 m in length, with a maximum of 32.6 m3 of storage capacity and principally based on manual fishing techniques throughout fishing operations (Diario Oficial El Peruano, 2001). Fishing vessels set multifilament driftnets at the sea surface to capture sharks and rays, but they also incidentally capture small cetaceans including Burmeister's porpoise (Mangel et al., 2010). Vessels depart on fishing trips for approximately 1 week at a time with a set of nets laid and hauled every 24 hr. Nets are typically 1.5–2 km long and are set during the late afternoon and hauled the following morning with an average soak time of 13 h (Mangel et al., 2013).
C-PODs are autonomous loggers used to detect echolocation clicks of odontocetes at distances of up to 400 m for porpoises and 500–1,000 m for dolphins (Tougaard et al., 2006; Philpott et al., 2007; Rayment et al., 2009). A C-POD was deployed on each fishing set, in order to detect dolphin and porpoise activity in the vicinity of fishing nets. A device was attached to the lead-line of the net, at approximately 12 m depth (in order to reduce noise interference at the sea surface), and recorded acoustic activity for the duration of the fishing set. Each device was recovered at the start of the following haul. Five C-PODs were used throughout the study by different fishing vessels, but no more than one was used at the same time by the same vessel. Fisheries observers were trained how to maintain and deploy C-PODs, as well as relevant data collection methods including marine mammal identification, gear used, the timings of net setting and hauling, as well as bycatch events. Additionally, at the start of each set, observers recorded the GPS position of the vessel, the sea surface temperature (hereafter SST) using a hand-held Enviro-Safe thermometer, and sea state using the Beaufort scale.
Data Processing
Clicks produced by echolocating animals are logged by devices if they show a sufficiently high peak sound pressure level and distinct spectral peak in the frequency range (Tregenza, 2014), and the accompanying C-POD.exe software (v 2.032) applies a click train detection algorithm that detects and classifies trains into categories based on how likely they are to be of cetacean origin. The classification software can only distinguish between species groups; narrow-band high frequency (NBHF) echolocation characteristic of all porpoise and some small dolphin species, and broad-band echolocation of all other toothed whale species; boat sonars are also identified. We used the standard KERNO classifier to categorize click trains as dolphin or porpoise in origin, however initial visual screening indicated that some broadband click trains had high modal frequencies and were being wrongly identified as NBHF (Nick Tregenza, pers. comm.). To remedy this, we also used an additional classifier (GENENC) developed specifically for this project; its use is recommended both to reduce false positive NBHF clicks in areas where porpoises coexist with broadband species, and to reduce false positive broadband clicks in noisy environments (Tregenza, 2014; Robbins et al., 2016). Only acoustic detections classified in the top reliability categories (“Cet Hi” and “Cet Mod”) were used (Rayment et al., 2009; Pirotta et al., 2014). All click trains were also inspected visually on screen and validated using guidelines provided by the CPOD manufacturer (Gallus et al., 2012; Tregenza, 2014; Jaramillo-Legorreta et al., 2017).
For each fishing set, data were exported as detection positive minutes (DPM), the number of minutes in which click trains were detected, separately for dolphins and porpoises. We also exported the time series of clicks and calculated the inter-click interval (ICI) for each logged click. During approach and capture of prey items, odontocetes produce clicks with shorter and decreasing ICIs, known as feeding “buzzes” (Akamatsu et al., 2005). As such, clicks associated with low ICIs (usually <10 ms; Carlström, 2005) can be used as proxies of foraging behavior (Leeney et al., 2011; Pirotta et al., 2014). Following Pirotta et al. (2014), we used Gaussian mixture-models fitted to log-transformed ICIs to identify multimodal peaks, representative of different behaviors: (1) low ICIs associated with buzzing behavior, (2) regular clicks, and 3) intervals between click trains (see Supplementary Material for details; Pirotta et al., 2014).
Environmental Data
Previous studies have indicated that small cetaceans have preferences for habitats influenced by bathymetry and distance to the shore (Kiszka et al., 2007; Embling et al., 2010; Isojunno et al., 2012). As a result, for each fishing set location, we extracted the following variables in ArcGIS 10.1: (1) ocean floor depth (hereafter Depth) was obtained from the General Bathymetric Chart of the Oceans (GEBCO, www.gebco.net; IOC 2003) and (2) ocean floor slope (Slope) was calculated using the slope function in the Spatial Analyst toolbox. We also calculated (3) the Euclidean distance from the nearest coast (Distance). The spatial resolution of the bathymetry layer was one arc second, which corresponds to ca. 30 m at the equator.
Statistical Analysis
Analyses were conducted separately for porpoises and dolphins to determine the influence of habitat variables on variation in their presence-absence (1 = present, 0 = absent), and once present (DPM > 0), the drivers of time spent in the local area. The data were modeled in a two-step process using hurdle generalized linear mixed models (GLMMs) in the R package glmmADMB (Fournier et al., 2012): for the first (zero) part, we estimated the probability of presence using a binomial error structure and logit link function; and for the second (count) part we modeled DPM as the response variable using only positive values, and specifying a zero-truncated negative binomial error distribution and log link function (Zuur et al., 2009). Negative binomial was chosen over a Poisson distribution due to the presence of a few extremely large and many small observations. Each data point corresponded to one fishing set, which we considered a discrete monitoring unit in space and time. However, as sets within fishing trips were non-independent, trip identity was included as a random effect. As recording duration varied with each set, we initially included the log-transformed duration of time recorded as an offset in count models, and as a fixed effect in presence-absence models as it is not possible to include offsets in binomial error models. In a third set of models, we also investigated the drivers of presence-absence of buzzing behavior using GLMMs with a binomial error structure and logit link in the R package lme4 (Bates et al., 2015).
The following habitat variables were considered as covariates (summarized in Table 1): (1) SST, (2) Depth, (3) Slope, (4) Distance, and (5) year and (6) season (quarter 1 = January–March, 2 = April–June, 3 = July–September, and 4 = October–December) to account for disparities in sampling effort. We also included the following variables relating to operational characteristics: (7) bait use, as the presence of bait may influence cetacean behavior, (8) C-POD identity to control for variation in detection capabilities among devices, and (9) sea state. Finally, we also included both (10) a factor corresponding to the bycatch (entanglement) events during the set, and (11) the number of individuals captured. These variables were tested to determine a relationship between acoustic activity and bycatch, however we cannot determine causality between the two as we do not have precise information on the timing of entanglement events.
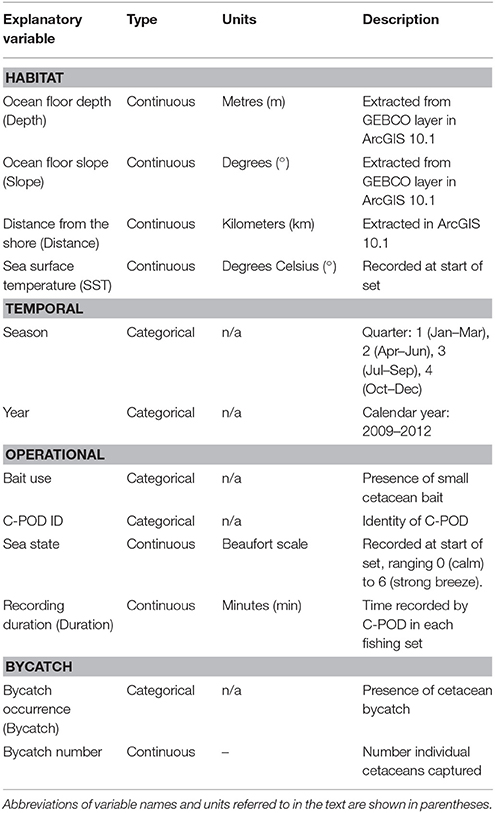
Table 1. Explanatory variables included in models of Burmeister's porpoise Phocoena spinipinnis and dolphin (unspecified delphinids) activity around fishing vessels in northern Peru.
Prior to modeling, Depth and Slope were log-transformed to improve data spread, while in the porpoise count model, Distance was square-root-transformed. We checked for collinearity between explanatory variables using variance inflation factors (VIF). Due to the small number of observations, we initially limited the number of explanatory variables included by testing the significance of all variables (and where appropriate, their quadratic terms), in standalone models, and compared them to null (intercept only) models using likelihood ratio tests (LRTs; see Table S1 in Supplementary Material). For several models, this process still yielded a large number of covariates; as such, the most parsimonious models were selected by the sequential stepwise addition of significant variables, in order of decreasing importance (ranked using X2 values; Table S1). Significance was assessed with LRTs and variable importance was determined as the proportional deviance explained by calculating the percentage reduction in residual deviance upon removal from the most parsimonious model (Zuur et al., 2009). We evaluated the performance of binomial GLMMs by computing the area under the Receiver Operator Characteristic (ROC) curve (AUC) in the R package pROC (Robin et al., 2017). Values of 0.5–0.7, 0.7–0.9, and >0.9 represent poor, reasonable and very good model performance, respectively. All analyses were conducted in the statistical program R 3.3.1 (R Core Team, 2014) and means are given ± standard deviation (SD), unless specified otherwise.
Results
Between April 2009 and December 2012, 116 sets were observed across 30 fishing trips (mean of 3.9 ± 1.6 sets per trip). Logistical constraints prevented monitoring in the first half of 2010 and for parts of 2012, and so a greater number of sets were observed in 2009 and 2011 (Figure 1). Effort was also not spread evenly across seasons with a greater number of sets observed in quarters 2 and 4 (autumn and spring, respectively). C-PODs attached to fishing nets logged 1,279 h (53.3 d) of data, with a mean of 11.0 ± 3.6 h per set. While the recording duration was occasionally cut short by device malfunction, there did not appear to be any spatial pattern in the monitoring duration (Figure S1).
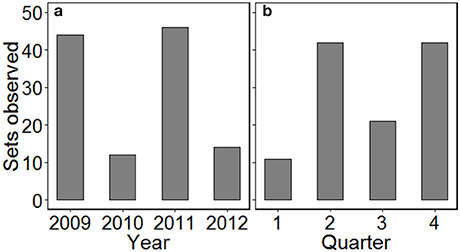
Figure 1. The distribution of gillnet fishing sets observed by (a) year and (b) quarter, from the port of Salaverry in northern Peru, over the study duration. Quarter 1 = January–March, 2 = April–June, 3 = July–September, 4 = October–December.
Acoustic Characteristics
The acoustic presence of small cetaceans (dolphins or porpoises) was recorded by C-PODs in 76% (n = 88) of fishing sets; dolphins (71%, n = 82) were recorded in a higher number of sets than porpoises (22%, n = 25). Over the study duration, 56,222 and 190,976 clicks were classified in 4,687 and 3,231 click trains, as belonging to dolphins and porpoises, respectively. Dolphins were detected in 4,101 min, a mean of 50 ± 50 (range: 0–463) per set and 4.6 ± 6.2 h−1, and porpoises detected in 1,002 min, a mean of 40 ± 50 (0–206) per set and 3.0 ± 4.1 h−1. The average modal frequency of click trains was 133.9 ± 4.4 (121–144) kHz and 117.6 ± 23.6 (30–146) kHz for porpoises and dolphins, respectively. Dolphin and porpoise ICIs ranged from ca. 1 μs to ca. 30 ms and from ca. 8 μs to ca. 15 ms, respectively. Buzz ICIs were identified by Gaussian mixture models with an average ICI of 46 ± 0.2 and 98.2 ± 0.5 μs for dolphins and porpoises, respectively, and buzzing activity was recorded in 57% (n = 66) and 8% (n = 9) of sets for dolphins and porpoises, respectively, constituting 26% (n = 49,182) and 17% (n = 9,413) of total clicks detected, respectively.
Distribution and Habitat Use
The minimum convex polygon encompassing the distribution of fishing effort covered an area of 61,557 km2, which included a range of marine habitats (coastal, continental shelf, shelf-break and oceanic waters; Figure 2) and a large range in SST values (15–25°C). Burmeister's porpoises were predominantly detected in coastal regions slightly offshore from Salaverry and a near-shore region around 50 km northwards (Figure 2a). In contrast, dolphins were detected throughout the sampled area (Figure 2b).
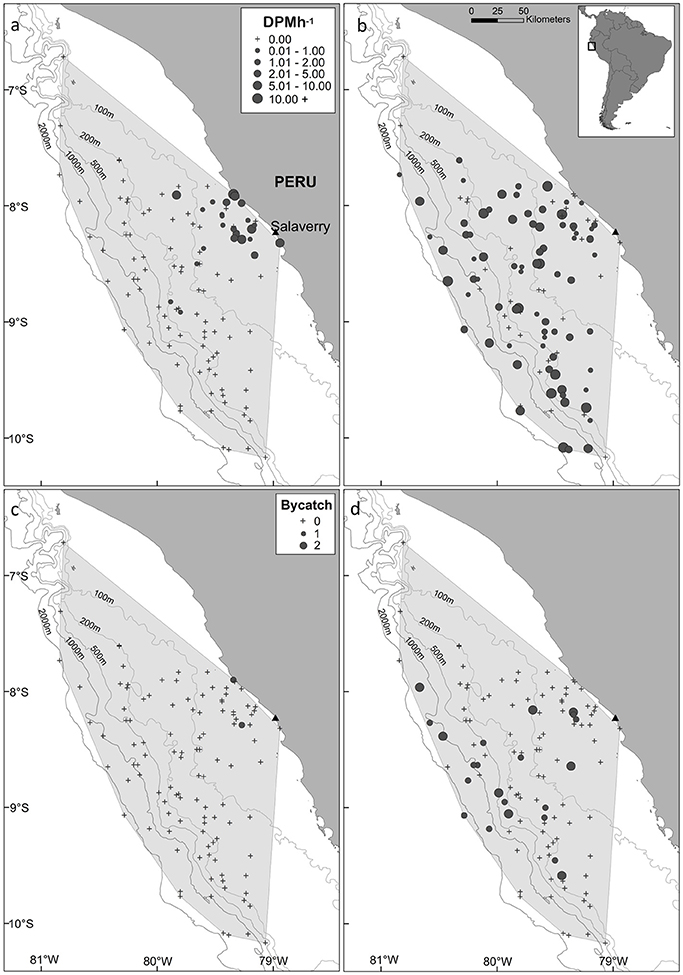
Figure 2. Map of the study area, (a,b) acoustic activity recorded by C-PODs attached to gillnets and (c,d) bycatch, of Burmeister's porpoise (left panels) and dolphins (unspecified delphinids; right panels) in fishing vessels operating from the port of Salaverry, northern Peru. For plots of acoustic activity and bycatch, the locations of sets where small cetaceans were present and absent are shown by circles and crosses, respectively, with the size of circles representing time spent [detection positive minutes (DPM) per hour] and the number of individuals captured, respectively. The shaded area represents the minimum convex polygon of fishing sets, and the position of the 100, 200, 500, 1,000, and 2,000 m isobaths are shown with gray lines.
For some sets, observers did not record certain variables, such as bait use, sea state, SST or the GPS position, and so sample sizes varied depending on which explanatory variables were included (Table S1). The variables Depth, Slope and Distance were correlated, so if more than one was deemed significant, only that which resulted in the highest deviance explained, was chosen. A similar procedure was used for the categorical variables C-POD ID and year, which were correlated in porpoise count models. Initial fitting of standalone models for each habitat variable indicated that several were important predictors of porpoise but not dolphin activity (Table S1). Final models were then assessed through forwards selection of significant variables; performance was very good for both the porpoise occurrence and buzzing occurrence models (AUCs = 0.94 and 0.98, respectively), while performance was poor and reasonable for dolphin occurrence (0.65) and buzzing occurrence (0.77) models, respectively.
The most parsimonious models included the effect of Depth on porpoise occurrence ( = 36.9, p < 0.001; Table 2), SST on time spent ( = 12.2, p < 0.001; Table 2) and Distance on the occurrence of buzzing activity ( = 16.4, p < 0.001; Table 3). In particular, Depth and Distance explained a large percentage of deviance (>20%) in their respective models (Tables 2, 3). Porpoises were detected in shallow waters up to 200 m depth, and predicted probability of presence was highest in waters of 50 m depth or shallower (Figure 3a). Porpoises spent longer periods of time in cooler waters of 17–18°C, and appeared to avoid areas with SSTs > 19°C (Figure 3b). Porpoise buzzing behavior was detected within 50 km from the shore, but occurrence was greatest within the first 20–30 km, which suggests that porpoises preferentially forage in shallow waters close to the shore (Figure 3c).
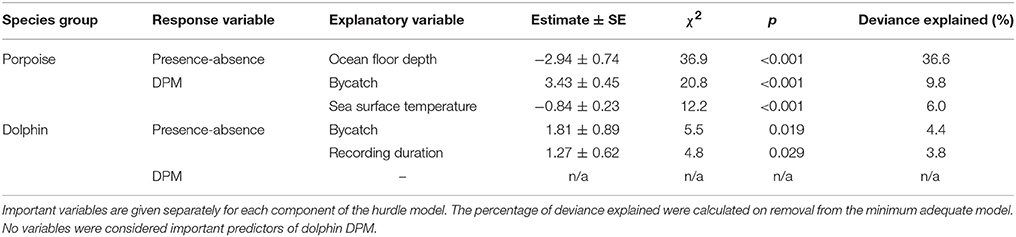
Table 2. Explanatory variables retained in the minimum adequate hurdle models of Burmeister's porpoise Phocoena spinipinnis and dolphin occurrence (presence-absence; binomial) and time spent (detection positive minutes, DPM; count).
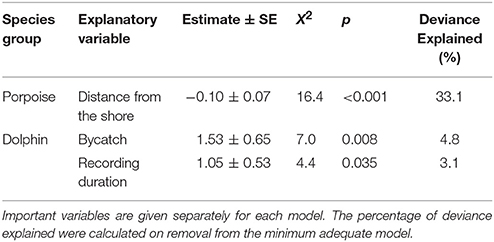
Table 3. Explanatory variables retained in the minimum adequate models of Burmeister's porpoise Phocoena spinipinnis and dolphin buzzing occurrence (presence-absence; binomial).
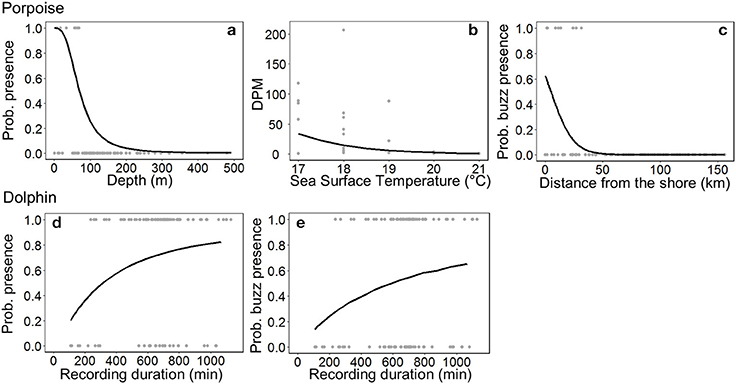
Figure 3. Responses of small cetaceans to important variables included in models: (a) ocean floor depth on Burmeister's porpoise Phocoena spinipinnis probability of presence, (b) sea surface temperature on porpoise time spent (detection positive minutes, DPM), (c) distance from the shore on the probability of porpoise buzzing activity, and the C-POD recording duration on the (d) probability of presence and (e) buzzing activity of dolphins (unspecified delphinids). For (a) the depth range is much greater (ca. 2,000 m), but only depths up to 500 m are plotted. Observed values are shown by gray dots, and the predicted relationship by a black line.
No habitat variable explained dolphin occurrence, time spent or buzzing occurrence (Tables 2, 3). The C-POD recording duration was a significant predictor of dolphin occurrence ( = 4.8, p = 0.029; Table 2) and buzzing activity ( = 4.4, p = 0.035; Table 2), such that with longer durations, there was an increased probability of presence and buzzing activity, which appeared to plateau at around 800 min (Figures 3d,e). The recording duration was not important for porpoises, while the variables bait use, C-POD ID, sea state, slope, quarter and year were not significant predictors in either dolphin or porpoise models (Tables 2, 3, Table S2).
Acoustic Activity and Bycatch
Dolphins were incidentally captured in 19% of sets (n = 22) and a total of 30 individuals were captured, consisting of 18 common dolphins Delphinus capensis, six dusky dolphins Lagenorhynchus obscurus, five bottlenose dolphins Tursiops truncates, and one Risso's dolphin Grampus griseus (Figure 2d). The dolphin bycatch per unit effort (BPUE) was 1.4 ± 0.5 animals per set for sets with bycatch and 0.3 ± 0.6 individuals per set for all sets. Burmeister's porpoises were caught in 2% of sets (n = 2) (Figures 2c, 4b), with a mean BPUE of 0.02 ± 0.1 individuals per set for all sets; both these sets registered acoustic activity of porpoises. Of those 22 sets with dolphin bycatch, 20 recorded acoustic presence. Porpoise bycatch was associated with increased acoustic time spent ( = 20.8, p < 0.001; Table 2, Figure 4a), but was not linked to the probability of presence or occurrence of buzzing (Tables 2, 3). Similarly, the bycatch of dolphins was significantly linked to higher probability of presence ( = 5.5, p = 0.019; Table 2, Figure 4c) and occurrence of buzzing activity ( = 7.0, p = 0.008; Table 3, Figure 4d), but not to time spent around fishing vessels (Table 2). Dolphins and porpoises were still detected in sets where no bycatch was recorded (53 and 17% of sets, respectively). As we do not know the timing of bycatch in relation to the acoustic detections, or the number of animals around the net, we cannot determine whether some bycatch is of silent animals. Finally, bycatch as a categorical variable explained activity metrics better than the continuous variable, suggesting no link between the number of individuals caught and acoustic activity.
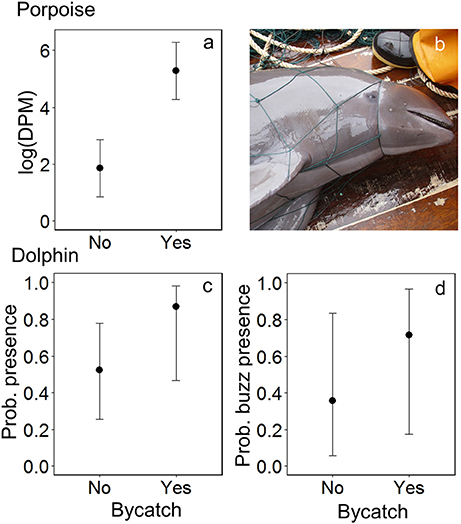
Figure 4. The modeled relationship between bycatch of Burmeister's porpoises Phocoena spinipinnis and dolphins (unspecified delphinids) and the acoustic activity recorded by C-PODs attached to fishing nets: for porpoises, (a) time spent (detection positive minutes, DPM), shown on the natural log scale, and for dolphins, (c) the probability of presence and (d) of buzzing activity. Error bars represent the 95% confidence intervals around modeled estimates. (b) A Burmeister's porpoise Phocoena spinipinnis hauled onto the deck of an SSF vessel after becoming entangled in a driftnet in northern Peru. Photo credit: ProDelphinus.
Discussion
Knowledge of the distribution and habitat use of small cetaceans is crucial for informing conservation efforts, particularly in regions where they overlap or interact with human activities such as fisheries. Our study demonstrates that passive acoustic monitoring from a SSF platform can provide novel insights into the distribution and habitat use of small cetaceans. We identified a coastal region that is likely to be important for the little-studied Burmeister's porpoise in the northern extent of its range. In contrast, as dolphins were detected in equal measure throughout the fishery area, we were unable to link occurrence or time spent to habitat variables. The latter result is likely to be confounded as passive acoustic techniques make distinguishing between different broadband echolocating species extremely challenging (see Robbins et al., 2016). Dolphin activity was likely to belong to a number of sympatric species, including those recorded as bycatch (common, bottlenose, dusky, and Risso's dolphins; Mangel et al., 2010, 2013).
It is generally recommended that acoustic recordings be supplemented with visual sightings (Leeney et al., 2011; Rayment et al., 2011), however this was not possible as nets were set predominantly during darkness. Nonetheless, observations of Burmeister's porpoise are difficult in anything but calm conditions due to its posterior located dorsal fin and neophobic behavior (Brownell and Praderi, 1982, 1984), and as a result of this, as well as scarce monitoring, there are fewer than 20 documented sightings in Peru (reviewed in Van Waerebeek et al., 2002). Porpoises are characterized by their similar vocalizations: high frequencies centerd around 125–140 kHz narrow-band (10 kHz) clicks (Akamatsu et al., 1994). A recent study has provided the first characterization of Burmeister's porpoise sounds, and confirmed they use NBHF clicks, with a peak frequency of 135 ± 2 kHz (Reyes Reyes et al., 2018), which is similar to the average modal frequency of click trains documented here (133.9 ± 4.4 kHz; range 121–144 kHz). While species verification is an important future step, we are confident that NBHF clicks belonged to Burmeister's porpoise as it is the only NBHF species in Peruvian coastal waters, unlike in other parts of the species range such as southern Chile and Argentina, where it co-occurs with Cephalorhynchus dolphins (which also use NBHF echolocation; Heinrich, 2006; Morisaka and Connor, 2007). This is supported by the fact that the two fishing sets in our study to have porpoise bycatch, also recorded NBHF activity.
Distribution and Habitat Use of Burmeister's Porpoise
Through collaboration with fishers, we were able to acoustically sample a range of habitats from near-shore and continental shelf to shelf-edge and oceanic regions, at relatively low cost. However, we acknowledge that like other opportunistic surveys, our methodology had associated limitations (Isojunno et al., 2012); for example, the activity of small cetaceans around fishing vessels may not be considered natural behavior. In other regions, dolphins are known to depredate from fishing nets (e.g., Read et al., 2003), however Peruvian dolphins and porpoises do not appear to be attracted to bait and have never been observed by fishers depredating from or feeding in nets (Pro Delphinus, pers. comm.). Indeed, dietary analyses of bycaught cetaceans indicate their main prey species are forage fish such as the Peruvian anchovy Engraulis ringens and squid (García-Godos et al., 2007); in contrast, the drift-net fishery captures large fish (predominantly sharks and rays) and the large mesh size of nets prevents capture of smaller fish (Alfaro-Shigueto et al., 2010; Mangel et al., 2010). It is more likely that cetaceans were detected while passing through the area or while feeding on nearby prey, however we acknowledge that dolphins (but probably not porpoises) may be attracted to fishing boats due to their inquisitive nature, which may bias our estimates of habitat use. As this bias is likely to be similar across fishing vessels and habitats, our main conclusion that dolphins (unspecified delphinids) were detected throughout the fishing area and porpoises were limited to a neritic and near-shore waters, remains unchanged.
Past research on the foraging ecology of Burmeister's porpoise has largely been anecdotal, with the majority of information derived from studies of bycaught individuals (Reyes and Van Waerebeek, 1995; Rosa et al., 2005; García-Godos et al., 2007; Tzika et al., 2010); nonetheless, our results corroborate the limited number of documented sightings (Van Waerebeek et al., 2002) and studies of SSF bycatch (Mangel et al., 2010, 2013) that suggest it is predominantly a neritic species. We found several habitat variables to have a significant influence on porpoise activity: porpoises were detected in regions with <200 m bottom depth and <50 km from the shore, with greater time spent in cooler waters (17–18°C). Important relationships with topographic variables such as bottom depth and distance to the shore have been observed in many other coastal cetaceans (e.g., Garaffo et al., 2007; Embling et al., 2010). While no habitat variables predicted dolphin occurrence, there appeared to be subtle partitioning by distance from the shore, whereby dolphin activity was lower in sets closest to the coast, where porpoise activity was highest. Burmeister's porpoise use similar depths in other parts of its range, however the manner in which habitats are partitioned varies between regions (Heinrich, 2006).; For example, in southern Chile spatial segregation with Chilean Cephalorhynchus eutropia and Peale's dolphins Lagenorhynchus australis has resulted in porpoises using slightly deeper waters (20–50 m compared to 0–25 m for the two dolphin species) (Heinrich, 2006), whereas in Golfo San Jose, Argentina, porpoises use the intermediate depths (5–15 m) in between inshore bottlenose dolphins (<10 m) and dusky dolphins (35 m) (Goodall et al., 1995b).
For both species groups we found evidence of buzzing activity, which is generally thought to represent foraging behavior (Akamatsu et al., 2005; Pirotta et al., 2014) In particular, the higher probability of porpoise buzzing activity in near-shore habitats likely represents less time spent traveling (or other activities) and more time spent feeding. This could reflect the distribution of their preferred prey; despite similarity between diets of Peruvian small cetaceans, Burmeister's porpoises were found to have a higher reliance on anchovy than other species (occurrence of 88% in stomachs of bycatch individuals, García-Godos et al., 2007). The Humboldt Current upwelling is one of the most productive marine ecosystems in the world, and in particular, the continental shelf off Salaverry hosts large densities of anchovy, particularly in December–April (Bertrand S. et al., 2004), and a large industrial fishery, based out of numerous ports in central and northern Peru (Fréon et al., 2008). Anchovy aggregations exhibit large spatial variability depending on the year and season (Fréon et al., 2008), yet while our sampling effort was uneven across years and seasons, we did not find any evidence of temporal variation in porpoise or dolphin activity. Visual observations and catch records indicate little seasonality in movements (Van Waerebeek and Reyes, 1990, 1994; Van Waerebeek et al., 2002), however our limited sampling for a given season prevents a robust comparison. Anchovy distribution is tightly linked to cold and productive coastal waters (Bertrand A. et al., 2004), which may explain why porpoise time spent was in the coolest waters sampled. Indeed, this preference for cooler waters in the northern part of their distribution indicates that porpoises might be at the limits of their thermal tolerance, and are likely to be particularly affected by increases in SSTs and shifts in anchovy distributions during El-Niño years (García-Godos et al., 2007), as demonstrated by high numbers of stranded individuals on Peruvian shores during these periods (Van Waerebeek et al., 1997).
Links Between Small Cetacean Acoustic Activity and Bycatch
Little is known about how small cetaceans become entangled in gillnets, due to the difficulty of underwater observations (Read et al., 2003; Martin and Crawford, 2015), and their high bycatch rate in the Peruvian driftnet fishery gave us the unique opportunity to investigate links between entanglement events and acoustic activity. Firstly, we found small cetaceans to be in the vicinity of gillnets for a large proportion (76%) of fishing sets. As bycatch is both the product of exposure (driven by distribution of animals and fishing activities and magnitude of effort) and vulnerability of a species (ecological characteristics of a species, e.g., behavior) (Lewison et al., 2014), the high encounter rate, particularly of dolphins (71% of sets had dolphin activity), goes some way to explaining why tens of thousands of individuals are likely caught by this fleet annually (Van Waerebeek and Reyes, 1990, 1994; Mangel et al., 2010). While the bycatch rate of this fishery is among the highest globally (Mangel et al., 2010), much fewer sets (<20%) recorded bycatch of small cetaceans than acoustic presence. These results indicate that small cetaceans are present in the vicinity of gillnets for long periods of time without becoming entangled, mirroring the observations of Read et al. (2003) that bottlenose dolphins frequently encounter and interact with nets without becoming entangled.
We also found a positive relationship between the likelihood of a bycatch event and acoustic activity for both dolphins and porpoises. Although we only recorded two porpoise bycatch events, DPM in these sets was significantly higher. We were not able to record the timing of entanglement events and so the temporal relationship between vocalization and entanglement lacks precision. Also, it is important to note that gillnets were generally longer than the likely detection range of small cetaceans by C-PODs (Tougaard et al., 2006; Philpott et al., 2007; Rayment et al., 2009), and so there were likely to be portions of the net that were not detected. Our findings support previous suggestions that entanglement results either from traveling animals not detecting the net as a barrier, or from entanglements while feeding in the vicinity of the net (Cox and Read, 2004). As dolphins and porpoises have a sophisticated sonar, entanglement in nets is generally thought to occur as a result of mistakes made, rather than their sonar not detecting the net (Dawson, 1991; Nielsen et al., 2012). However, a monofilament net may be hard to detect at some distance (Kastelein et al., 2000). Dolphins are not always actively engaged in echolocation, and may become entangled when not vocalizing (Dawson et al., 2013), particularly in low light levels such as at night (Akamatsu et al., 1991) or in particularly turbid or noisy environments (Martin and Crawford, 2015; Northridge et al., 2017). Indeed, there is anecdotal evidence from Peruvian fishers that dolphin bycatch is higher in windier conditions (Pro Delphinus, pers. comm.), which may be the result of increased mixing of sediments or reduced acoustic detectability of nets due to high levels of ambient noise, however this requires further investigation.
Conservation Implications
Coastal cetaceans are exposed to a range of human activities including fisheries, aquaculture, shipping, pollution and climate change (Mann, 2000), yet for many species, still little is known about their abundance and distribution (Reeves et al., 2005; Read, 2008). Using a relatively low-cost monitoring tool (Braulik et al., 2018), our study provides a quantitative assessment of the distribution and habitat use of Burmeister's porpoise, adding substantially to the knowledge base of a “Data Deficient” species (Hammond et al., 2012). One of our key findings was that porpoises use a narrow coastal region (<50 km from the shore); indeed, their distribution studied here in the northern extent of their range completely overlaps the extent of a small-scale gillnet fishery. Given historically large capture rates of small cetaceans and the large capacity of the gillnet fleet (Read et al., 1988; Van Waerebeek and Reyes, 1994; Alfaro-Shigueto et al., 2010; Mangel et al., 2010), our finding that dolphins and porpoises regularly encounter gillnet vessels, raises serious concerns about the long-term impact of this fishery on local cetacean populations (Read et al., 1988; Van Waerebeek et al., 1997; Reeves et al., 2005; Rosa et al., 2005), and highlights the chronic nature of these interactions. While no abundance estimates exist, information gathered from onboard observer schemes and in-port and market surveys suggests Burmeister's porpoises used to be more common in Peruvian waters, but their numbers relative to other small cetaceans have decreased over the last 30 years (Mangel et al., 2010; Tzika et al., 2010). The Peruvian population is not thought to undergo long-range movements (Reyes and Van Waerebeek, 1995) and is reproductively isolated from the Chilean population (Rosa et al., 2005). Furthermore, Burmeister's porpoise is believed to be particularly susceptible to changes in the abundance and distribution of anchovy, its main prey (García-Godos et al., 2007). Given the lack of information on movements and population trends, we strongly encourage formal monitoring efforts, including the establishment of passive acoustic arrays, ideally in combination with boat-based surveys or satellite tracking (e.g., Mikkelsen et al., 2016).
The results of our study suggest that the protection of key habitats for porpoises may be more straightforward than for dolphins, given their more restricted range. Successful mitigation of SSF bycatch is likely to be achieved through a combination of multiple approaches; for example, restricting fishing activities in near-shore waters, or use of acoustic alarms (pingers) on nets (e.g., van Beest et al., 2017). Indeed, a previous study has shown that pingers reduce bycatch of Peruvian small cetaceans (Mangel et al., 2013), and future work should refine the efficacy of using pingers to deter porpoises and dolphins from nets (Dawson et al., 2013). These approaches do not come without social, economic or logistical constraints, and the cost of pingers remains a major impediment to their implementation (Dawson et al., 2013; Mangel et al., 2013). As such, widespread use of pingers or other bycatch mitigation solutions will likely require concerted political will or market-based solutions that reward fishers for responsible practices (Jacquet and Pauly, 2008; José Alava et al., in press).
Author Contributions
JCM, JA-S, and BJG designed the study. JCM and JA-S performed the study. TAC processed the data and performed the statistical analysis with assistance from DJH. TAC wrote the manuscript with contributions from all authors. All authors gave final approval for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We warmly thank the fishers, field observers and ProDelphinus staff who kindly supported the data collection, in particular Natalia Ortiz, who helped with fieldwork. We would like to thank Nick Tregenza who advised on the deployment of C-PODs and assisted with data processing, Enrico Pirotta for methodological discussions, and Elizabeth Campbell, Nick Tregenza and two referees, who gave insightful comments on an earlier version of the manuscript. Funding and equipment for the project were provided by Chelonia Ltd. and the Darwin Initiative Project 18-001. DJH is supported by the Natural Environment Research Council and the European Union. BJG receives funding from the Darwin Initiative, the Natural Environment Research Council and the European Union.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00220/full#supplementary-material
References
Akamatsu, T., Hatakeyama, Y., and Ishii, K. (1991). Process of harbour Porpoise's entanglement in the gill net. Technical report of the national research institute of fisheries engineering. Fishing Gear Methods 5, 25–36.
Akamatsu, T., Hatakeyama, Y., Kojima, T., and Soeda, H. (1994). Echolocation rates of two harbor porpoises (phocoena Phocoena). Mar. Mammal Sci. 10, 401–411. doi: 10.1111/j.1748-7692.1994.tb00497.x
Akamatsu, T., Wang, D., Wang, K., and Naito, Y. (2005). Biosonar behaviour of free-ranging porpoises Proc. Biol. Sci. 272, 797–801. doi: 10.1098/rspb.2004.3024
Alfaro-Shigueto, J., Mangel, J. C., Bernedo, F., Dutton, P. H., Seminoff, J. A., and Godley, B. J. (2011). Small-scale fisheries of Peru: a major sink for marine turtles in the Pacific. J. Appl. Ecol. 48, 1432–1440. doi: 10.1111/j.1365-2664.2011.02040.x
Alfaro-Shigueto, J., Mangel, J. C., Pajuelo, M., Dutton, P. H., Seminoff, J. A., and Godley, B. J. (2010). Where small can have a large impact: structure and characterization of small-scale fisheries in Peru. Fish. Res. 106, 8–17. doi: 10.1016/j.fishres.2010.06.004
Awkerman, J. A., Huyvaert, K. P., Mangel, J., Shigueto, J. A., and Anderson, D. J. (2006). Incidental and intentional catch threatens Galápagos waved albatross. Biol. Conserv. 133, 483–489. doi: 10.1016/j.biocon.2006.07.010
Bates, D., Maechler, M., Bolker, B., Walker, S., Christensen, R. H. B., Singmann, H., et al. (2015). lme4: Linear Mixed-Effects Models Using “Eigen” and S4. Available online at: https://cran.r-project.org/web/packages/lme4/index.html (Accessed September 18, 2015).
Berkes, F., Mahon, R., McConney, P., Pollnac, R., and Pomeroy, R. (2001). Managing Small-scale Fisheries: Alternative Directions and Methods. Ottawa, ON: International Development Research Centre.
Bertrand, A., Segura, M., Gutierrez, M., and Vasquez, L. (2004). From small-scale habitat loopholes to decadal cycles: a habitat-based hypothesis explaining fluctuation in pelagic fish populations off Peru. Fish Fish. 5, 296–316. doi: 10.1111/j.1467-2679.2004.00165.x
Bertrand, S., Díaz, E., and Ñiquen, M. (2004). Interactions between fish and fisher's spatial distribution and behaviour: an empirical study of the anchovy (Engraulis ringens) fishery of Peru. ICES J. Mar. Sci. 61, 1127–1136. doi: 10.1016/j.icesjms.2004.07.016
Braulik, G. T., Kasuga, M., Wittich, A., Kiszka, J. J., MacCaulay, J., Gillespie, D., et al. (2018). Cetacean rapid assessment: An approach to fill knowledge gaps and target conservation across large data deficient areas. Aquat. Conserv. Mar. Freshw. Ecosyst. 216–230. doi: 10.1002/aqc.2833
Brownell, R. L. Jr., and Clapham, P. J. (1999). “Burmeister's porpoise - Phocoena spinipinnis Burmeister, 1865,” in Handbook of Marine Mammals, Vol. 6, The Second Book of Dolphins and Porpoises, eds S. Ridgway and R. Harrison (New York, NY: Academic Press), 393–410.
Brownell, R. L. Jr, and Praderi, R. (1982). “Status of Burmeister's porpoise Phocoena spinipinnis in southern South American waters,” in Mammals in the Seas, VoL 4: Small cetaceans and sirenians. FAO Fisheries Ser, 5, 91–96.
Carlström, J. (2005). Diel variation in echolocation behavior of wild harbor porpoises. Mar. Mammal Sci. 21, 1–12. doi: 10.1111/j.1748-7692.2005.tb01204.x
Chuenpagdee, R., Liguori, L., Palomares, M. L. D., and Pauly, D. (2006). Bottom-Up, Global Estimates of Small-Scale Marine Fisheries Catches. Vancouver, BC: Fisheries Centre Research Reports. Available online at: https://open.library.ubc.ca/cIRcle/collections/facultyresearchandpublications/52383/items/1.0074761 (Accessed September 8, 2017).
Cox, T. M., and Read, A. J. (2004). Echolocation behavior of harbor porpoises Phocoena phocoena around chemically enhanced gill nets. Mar. Ecol. Prog. Ser. 279, 275–282. doi: 10.3354/meps279275
Dawson, S. M. (1991). Modifying gillnets to reduce entanglement of cetaceans. Mar. Mammal Sci. 7, 274–282. doi: 10.1111/j.1748-7692.1991.tb00102.x
Dawson, S. M., Northridge, S., Waples, D., and Read, A. J. (2013). To ping or not to ping: the use of active acoustic devices in mitigating interactions between small cetaceans and gillnet fisheries. Endanger. Species Res. 19, 201–221. doi: 10.3354/esr00464
Embling, C. B., Gillibrand, P. A., Gordon, J., Shrimpton, J., Stevick, P. T., and Hammond, P. S. (2010). Using habitat models to identify suitable sites for marine protected areas for harbour porpoises (Phocoena phocoena). Biol. Conserv. 143, 267–279. doi: 10.1016/j.biocon.2009.09.005
Fournier, D. A., Skaug, H. J., Ancheta, J., Ianelli, J., Magnusson, A., Maunder, M. N., et al. (2012). AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 27, 233–249. doi: 10.1080/10556788.2011.597854
Fréon, P., Bouchon, M., Mullon, C., García, C., and Ñiquen, M. (2008). Interdecadal variability of anchoveta abundance and overcapacity of the fishery in Peru. Prog. Oceanogr. 79, 401–412. doi: 10.1016/j.pocean.2008.10.011
Gallus, A., Dähne, M., Verfuß, U. K., Bräger, S., Adler, S., Siebert, U., et al. (2012). Use of static passive acoustic monitoring to assess the status of the ‘Critically Endangered’ Baltic harbour porpoise in German waters. Endanger. Species Res. 18, 265–278. doi: 10.3354/esr00448
Garaffo, G. V., Dans, S. L., Pedraza, S. N., Crespo, E. A., and Degrati, M. (2007). Habitat use by dusky dolphin in patagonia: how predictable is their location? Mar. Biol. 152, 165–177. doi: 10.1007/s00227-007-0686-0
García-Godos, I., Waerebeek, K. V., Reyes, J. C., Alfaro-Shigueto, J., and Arias-Schreiber, M. (2007). Prey occurrence in the stomach contents of four small cetacean species in Peru. Lat. Am. J. Aquat. Mamm. 6, 171–183. doi: 10.5597/lajam00122
Goodall, R. N. P., Norris, K. S., Harris, G., Oporto, J. A., and Castello, H. P. (1995a). Notes on the biology of Burmeister's porpoise Phocoena spinipinnis off southern South America. Rep. Int. Whal. Comm. 16:347.
Goodall, R. N. P., Wursig, B., Wursig, M., Harris, G., and Norris, K. S. (1995b). Sightings of Burmeister's porpoise, Phooena Spinipinnis, off Southern South America. Rep. Int. Whal. Comm. 16, 297–316.
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K. A., Karkzmarski, L., Kasuya, T., Perrin, W. F., et al. (2012). Phocoena Spinipinnis. The IUCN Red List of Threatened Species 2012:e.T17029A17117957.
Heinrich, S. (2006). Ecology of Chilean Dolphins and Peale's Dolphins at Isla Chiloe, southern Chile. Ph.D., dissertation, University of St Andrews, St Andrews, UK.
Isojunno, S., Matthiopoulos, J., and Evans, P. G. H. (2012). Harbour porpoise habitat preferences: robust spatio-temporal inferences from opportunistic data. Mar. Ecol. Prog. Ser. 448, 155–170. doi: 10.3354/meps09415
Jacquet, J., and Pauly, D. (2008). Funding priorities: big barriers to small-scale fisheries. Conserv. Biol. 22, 832–835. doi: 10.1111/j.1523-1739.2008.00978.x
Jaramillo-Legorreta, A., Cardenas-Hinojosa, G., Nieto-Garcia, E., Rojas-Bracho, L., Ver Hoef, J., Moore, J., et al. (2017). Passive acoustic monitoring of the decline of Mexico's critically endangered vaquita. Conserv. Biol. 31, 183–191. doi: 10.1111/cobi.12789
Jefferson, T. A., and Curry, B. E. (1994). A global review of porpoise (Cetacea: Phocoenidae) mortality in gillnets. Biol. Conserv. 67, 167–183. doi: 10.1016/0006-3207(94)90363-8
José Alava, J., Tatar, B., José Barragán, M., Castro, C., Rosero, P., Denkinger, J., et al. (in press). Mitigating cetacean bycatch in coastal Ecuador: governance challenges for small-scale fisheries. Mar. Policy. doi: 10.1016/j.marpol.2017.05.025
Kastelein, R. A., Au, W. W., and de Haan, D. (2000). Detection distances of bottom-set gillnets by harbour porpoises (Phocoena phocoena) and bottlenose dolphins (Tursiops truncatus). Mar. Environ. Res. 49, 359–375. doi: 10.1016/S0141-1136(99)00081-1
Kiszka, J., MacLeod, K., Van Canneyt, O., Walker, D., and Ridoux, V. (2007). Distribution, encounter rates, and habitat characteristics of toothed cetaceans in the Bay of Biscay and adjacent waters from platform-of-opportunity data. ICES J. Mar. Sci. 64, 1033–1043. doi: 10.1093/icesjms/fsm067
Kyhn, L. A., Tougaard, J., Thomas, L., Duve, L. R., Stenback, J., Amundin, M., et al. (2012). From echolocation clicks to animal density–acoustic sampling of harbor porpoises with static dataloggers. J. Acoust. Soc. Am. 131, 550–560. doi: 10.1121/1.3662070
Leeney, R., Carslake, D., and Elwen, S. H. (2011). Using static acoustic monitoring to describe echolocation behaviour of heaviside's dolphins (Cephalorhynchus heavisidii) in Namibia. Aquat. Mamm. 37, 151–160. doi: 10.1578/AM.37.2.2011.151
Lewison, R. L., Crowder, L. B., Wallace, B. P., Moore, J. E., Cox, T. M., Zydelis, R., et al. (2014). Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proc. Natl. Acad. Sci. U.S.A. 111, 5271–5276. doi: 10.1073/pnas.1318960111
Lopez, A., Pierce, G. J., Valeiras, X., Santos, M. B., and Guerra, A. (2004). Distribution patterns of small cetaceans in Galician waters. J. Mar. Biol. Assoc. UK 84, 283–294. doi: 10.1017/S0025315404009166h
MacLeod, C. D., Mandleberg, L., Schweder, C., Bannon, S. M., and Pierce, G. J. (2008). A comparison of approaches for modelling the occurrence of marine animals. Hydrobiologia 612, 21–32. doi: 10.1007/s10750-008-9491-0
Majluf, P., Babcock, E. A., Riveros, J. C., Schreiber, M. A., and Alderette, W. (2002). Catch and bycatch of sea birds and marine mammals in the small-scale fishery of punta san juan, Peru. Conserv. Biol. 16, 1333–1343. doi: 10.1046/j.1523-1739.2002.00564.x
Mangel, J. C., Alfaro-Shigueto, J., Van Waerebeek, K., Cáceres, C., Bearhop, S., Witt, M. J., et al. (2010). Small cetacean captures in Peruvian artisanal fisheries: high despite protective legislation. Biol. Conserv. 143, 136–143. doi: 10.1016/j.biocon.2009.09.017
Mangel, J. C., Alfaro-Shigueto, J., Witt, M. J., Hodgson, D. J., and Godley, B. J. (2013). Using pingers to reduce bycatch of small cetaceans in Peru's small-scale driftnet fishery. Oryx 47, 595–606. doi: 10.1017/S0030605312000658
Mann, J. (2000). Cetacean Societies: Field Studies of Dolphins and Whales. Chicago, IL: University of Chicago Press.
Marques, T. A., Thomas, L., Martin, S. W., Mellinger, D. K., Ward, J. A., Moretti, D. J., et al. (2013). Estimating animal population density using passive acoustics. Biol. Rev. 88, 287–309. doi: 10.1111/brv.12001
Martin, G. R., and Crawford, R. (2015). Reducing bycatch in gillnets: a sensory ecology perspective. Glob. Ecol. Conserv. 3, 28–50. doi: 10.1016/j.gecco.2014.11.004
Mellinger, D. K., Stafford, K. M., Moore, S. E., Dziak, R. P., and Matsumoto, H. (2007). An overview of fixed passive acoustic observation methods for cetaceans. Oceanography 20, 36–45. doi: 10.5670/oceanog.2007.03
Mikkelsen, L., Rigét, F. F., Kyhn, L. A., Sveegaard, S., Dietz, R., Tougaard, J., et al. (2016). Comparing distribution of harbour porpoises (Phocoena phocoena) derived from satellite telemetry and passive acoustic monitoring. PLoS ONE 11:e0158788. doi: 10.1371/journal.pone.0158788
Moore, J. E., Cox, T. M., Lewison, R. L., Read, A. J., Bjorkland, R., McDonald, S. L., et al. (2010). An interview-based approach to assess marine mammal and sea turtle captures in artisanal fisheries. Biol. Conserv. 143, 795–805. doi: 10.1016/j.biocon.2009.12.023
Morisaka, T., and Connor, R. C. (2007). Predation by killer whales (Orcinus orca) and the evolution of whistle loss and narrow-band high frequency clicks in odontocetes. J. Evol. Biol. 20, 1439–1458. doi: 10.1111/j.1420-9101.2007.01336.x
Nielsen, T. P., Wahlberg, M., Heikkilä, S., Jensen, M., Sabinsky, P., and Dabelsteen, T. (2012). Swimming patterns of wild harbour porpoises Phocoena phocoena show detection and avoidance of gillnets at very long ranges. Mar. Ecol. Prog. Ser. 453, 241–248. doi: 10.3354/meps09630
Northridge, S., Coram, A., Kingston, A., and Crawford, R. (2017). Disentangling the causes of protected-species bycatch in gillnet fisheries. Conserv. Biol. 31, 686–695. doi: 10.1111/cobi.12741
Pauly, D. (2006). Major trends in small scale marine fisheries, with an emphasis on developing countries and some implications for the social sciences. Marit. Stud. 4, 7–22. Available online at: http://www.marecentre.nl/mast/documents/Pauly_Mast2006vol_4no_2_new.pdf
Peckham, S. H., Maldonado Diaz, D. M., Walli, A., Ruiz, G., Crowder, L. B., and Nichols, W. J. (2007). Small-scale fisheries bycatch jeopardizes endangered pacific loggerhead turtles. PLoS ONE 2:e1041. doi: 10.1371/journal.pone.0001041
Philpott, E., Englund, A., Ingram, S., and Rogan, E. (2007). Using T-PODs to investigate the echolocation of coastal bottlenose dolphins. J. Mar. Biol. Assoc. UK 87, 11–17. doi: 10.1017/S002531540705494X
Pirotta, E., Thompson, P. M., Miller, P. I., Brookes, K. L., Cheney, B., Barton, T. R., et al. (2014). Scale-dependent foraging ecology of a marine top predator modelled using passive acoustic data. Funct. Ecol. 28, 206–217. doi: 10.1111/1365-2435.12146
Rayment, W., Dawson, S., Scali, S., and Slooten, L. (2011). Listening for a needle in a haystack: passive acoustic detection of dolphins at very low densities. Endanger. Species Res. 14, 149–156. doi: 10.3354/esr00356
Rayment, W., Dawson, S., and Slooten, L. (2009). Trialling an automated passive acoustic detector (T-POD) with Hector's dolphins (Cephalorhynchus hectori). J. Mar. Biol. Assoc. UK 89, 1015–1022. doi: 10.1017/S0025315409003129
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Read, A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mammal. 89, 541–548. doi: 10.1644/07-MAMM-S-315R1.1
Read, A. J., Van Waerebeek, K., Reyes, J. C., McKinnon, J. S., and Lehman, L. C. (1988). The exploitation of small cetaceans in coastal Peru. Biol. Conserv. 46, 53–70. doi: 10.1016/0006-3207(88)90108-5
Read, A. J., Waples, D. M., Urian, K. W., and Swanner, D. (2003). Fine-scale behaviour of bottlenose dolphins around gillnets. Proc. Biol. Sci. 270(Suppl. 1), S90–S92. doi: 10.1098/rsbl.2003.0021
Reeves, R. R., Berggren, P., Crespo, E. A., Gales, N., Northridge, S. P., Notarbartolo diSciara, G., et al. (2005). Global Priorities for Reduction of Cetacean Bycatch. Report to the World Wide Fund for Nature. 29pp.
Reeves, R. R., McClellan, K., and Werner, T. B. (2013). Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endanger. Species Res. 20, 71–97. doi: 10.3354/esr00481
Reyes, J. C., and Van Waerebeek, K. (1995). Aspects of the biology of Burmeister's Porpoise from Peru. Rep. Int. Whal. Comm. 349–364.
Reyes Reyes, M. V., Marino, A., Dellabianca, N. A., Hevia, M., Torres, M., Raya Rey, A., et al. (2018). Clicks of wild Burmeister's porpoises (Phocoena spinipinnis) in Tierra del Fuego, Argentina. Mar. Mammal Sci. doi: 10.1111/mms.12489. [Epub ahead of print].
Robbins, J. R., Brandecker, A., Cronin, M., Jessopp, M., McAllen, R., and Culloch, R. (2016). Handling dolphin detections from C-PODs, with the development of acoustic parameters for verification and the exploration of species identification possibilities. Bioacoustics 25, 99–110. doi: 10.1080/09524622.2015.1125789
Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J.-C., et al. (2017). pROC: Display and Analyze ROC Curves. Available online at: https://cran.r-project.org/web/packages/pROC/index.html
Rosa, S., Milinkovitch, M. C., Waerebeek, K. V., Berck, J., Oporto, J., Alfaro-Shigueto, J., et al. (2005). Population structure of nuclear and mitochondrial DNA variation among South American Burmeister's porpoises (Phocoena spinipinnis). Conserv. Genet. 6, 431–443. doi: 10.1007/s10592-005-4988-9
Slooten, E., Dawson, S., Rayment, W., and Childerhouse, S. (2006). A new abundance estimate for Maui's dolphin: what does it mean for managing this critically endangered species? Biol. Conserv. 128, 576–581. doi: 10.1016/j.biocon.2005.10.013
Sousa-Lima, R. N., Norris, T. F., Oswald, J. N., and Fernandes, D. P. (2013). A review and inventory of fixed autonomous recorders for passive acoustic monitoring of marine mammals. Aquat. Mamm. 39, 23–53. doi: 10.1578/AM.39.1.2013.23
Taylor, B. L., Rojas-Bracho, L., Moore, J., Jaramillo-Legorreta, A., Ver Hoef, J. M., Cardenas-Hinojosa, G., et al. (2017). Extinction is imminent for Mexico's endemic porpoise unless fishery bycatch is eliminated. Conserv. Lett. 10, 588–595. doi: 10.1111/conl.12331
Tougaard, J., Rosager Poulsen, L., Amundin, M., Larsen, F., Rye, J., and Teilmann, J. (2006). “Detection function of T-PODs and estimation of porpoise densities,” in Proceedings of the Workshop; Static Acoustic Monitoring of Cetaceans (Gdynia: ECS Newsletter).
Tregenza, N. (2014). Manual for CPOD.exe. Available online at: www.chelonia.co.uk/downloads/CPOD.pdf
Turvey, S. T., Pitman, R. L., Taylor, B. L., Barlow, J., Akamatsu, T., Barrett, L. A., et al. (2007). First human-caused extinction of a cetacean species? Biol. Lett. 3, 537–540. doi: 10.1098/rsbl.2007.0292
Tzika, A. C., D'Amico, E., Alfaro-Shigueto, J., Mangel, J. C., Waerebeek, K. V., and Milinkovitch, M. C. (2010). Molecular identification of small cetacean samples from Peruvian fish markets. Conserv. Genet. 11, 2207–2218. doi: 10.1007/s10592-010-0106-8
van Beest, F. M., Kindt-Larsen, L., Bastardie, F., Bartolino, V., and Nabe-Nielsen, J. (2017). Predicting the population-level impact of mitigating harbor porpoise bycatch with pingers and time-area fishing closures. Ecosphere 8:e01785. doi: 10.1002/ecs2.1785
Van Waerebeek, K., and Reyes, J. C. (1990). Catch of small cetaceans at Pucusana Port, central Peru, during 1987. Biol. Conserv. 51, 15–22. doi: 10.1016/0006-3207(90)90028-N
Van Waerebeek, K., and Reyes, J. (1994). Post-Ban Small Cetacean Takes Off Peru: A Review. Report of the International Whaling Commission (Special issue 15), 503–519.
Van Waerebeek, K., Santillan, L., and Reyes, J. C. (2002). An unusually large aggregation of Burmeister's porpoise Phocoena spinipinnis off Peru, with a review of sightings from the Eastern South Pacific. Not. Mens. 350, 12–17. Available online at: www.vliz.be/imisdocs/publications/243227.pdf
Keywords: artisanal fisheries, bycatch, C-POD, echolocation, gillnet, passive acoustic monitoring, Peru, Phocoena spinipinnis
Citation: Clay TA, Mangel JC, Alfaro-Shigueto J, Hodgson DJ and Godley BJ (2018) Distribution and Habitat Use of a Cryptic Small Cetacean, the Burmeister's Porpoise, Monitored From a Small-Scale Fishery Platform. Front. Mar. Sci. 5:220. doi: 10.3389/fmars.2018.00220
Received: 06 April 2018; Accepted: 08 June 2018;
Published: 03 July 2018.
Edited by:
Jeremy Kiszka, Florida International University, United StatesReviewed by:
Louisa Shobhini Ponnampalam, MareCet Research Organization, MalaysiaDanielle Kreb, Conservation Foundation for Rare Aquatic Species of Indonesia, Indonesia
Copyright © 2018 Clay, Mangel, Alfaro-Shigueto, Hodgson and Godley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Alfaro-Shigueto, amFzXzI2QHlhaG9vLmNvbQ==; amFsZmFyb3NAY2llbnRpZmljYS5lZHUucGU=
†Present Address: Thomas A. Clay, School of Environmental Sciences, University of Liverpool, Liverpool, United Kingdom
 Thomas A. Clay
Thomas A. Clay Jeffrey C. Mangel1,2
Jeffrey C. Mangel1,2 David J. Hodgson
David J. Hodgson Brendan J. Godley
Brendan J. Godley