Corrigendum: N2 Fixation in the Eastern Arabian Sea: Probable Role of Heterotrophic Diazotrophs
- 1Physical Research Laboratory, Geosciences Division, Ahmedabad, India
- 2National Institute of Ocean and Technology, Chennai, India
Biogeochemical implications of global imbalance between the rates of marine dinitrogen (N2) fixation and denitrification have spurred us to understand the former process in the Arabian Sea, which contributes considerably to the global nitrogen budget. Heterotrophic bacteria have gained recent appreciation for their major role in marine N budget by fixing a significant amount of N2. Accordingly, we hypothesize a probable role of heterotrophic diazotrophs from the 15N2 enriched isotope labeling dark incubations that witnessed rates comparable to the light incubations in the eastern Arabian Sea during spring 2010. Maximum areal rates (8 mmol N m−2 d−1) were the highest ever observed anywhere in world oceans. Our results suggest that the eastern Arabian Sea gains ~92% of its new nitrogen through N2 fixation. Our results are consistent with the observations made in the same region in preceding year, i.e., during the spring of 2009.
Introduction
Reactive nitrogen (e.g., ) is an important substrate for marine primary producers because dinitrogen (N2), though the most abundant gas in the Earth's atmosphere, is unassimilable by most photosynthetic organisms (Middelburg and Nieuwenhuize, 2000). However, marine diazotrophic cyanobacteria (e.g., Trichodesmium) have an enzymatic advantage to convert atmospheric N2 gas to a bioavailable form of nitrogen (such as ). These diazotrophs are distributed over the oligotrophic tropical and sub-tropical marine environments, e.g., in the Arabian Sea located in the northwest Indian Ocean (Capone and Carpenter, 1982; Jickells et al., 2017).
The Arabian Sea is a hotspot for studying N2 fixation. Strong summer monsoonal winds cause intense upwelling over the western Arabian Sea enhancing primary productivity over the Somali coast (Prasannakumar et al., 2001). Further, the northern and central Arabian Seas are known for high productivity during winter, caused by the cooling-driven deep convection (Madhupratap et al., 1996; Singh and Ramesh, 2015). High biological production in the surface layers and its subsequent export leads to oxygen depletion in the subsurface layers (Naqvi and Jayakumar, 2000) that further triggers denitrification process, i.e., the release of N2 and N2O back to the atmosphere from nitrate () reduction. Denitrification in the oxygen minimum zones would deplete only thereby lowering the nitrogen: phosphorus (N:P) ratio in dissolved nutrient pool (Deutsch et al., 2007). An imbalance between N2 fixation and denitrification rates based on N:P stoichiometry is an imperative problem in the marine nitrogen budget (Codispoti, 2007). N2 fixation and denitrification dominate ocean N sources and sinks processes, respectively (Codispoti et al., 2001). Global nitrogen loss and gain rates are in imbalance, indicating either an overestimation of the loss processes or an underestimation of the gain processes (Codispoti, 2007). The recent recognition of greater diversity (Zehr et al., 2001, 2003) and wider distribution (Hewson et al., 2007; Mulholland, 2007) of marine diazotrophs than had been appreciated hitherto (Mahaffey et al., 2005) suggested an underestimation of N2 fixation. Contribution of atmospheric deposition and riverine nutrients to primary production is minor in the Arabian Sea (Singh and Ramesh, 2011; Singh et al., 2012) which further supports the fact that N2 fixation is a major process in this region. The Arabian Sea witnesses diverse group of diazotrophs, which may fix N2 at varying rates (Mulholland and Capone, 2009). During spring and autumn seasons, calmer winds, warmer waters, and shallower mixed layers make the Arabian Sea oligotrophic, thus creating a niche for Trichodesmium blooms (Gandhi et al., 2011). The seasonal occurrence of N2 fixation over the Arabian Sea makes it an unique region for studying nitrogen budget (Naqvi, 1987; Capone et al., 1998).
Trichodesmium is not the only species which fixes N2, as there are some other fixers such as γ- Proteobacteria, and other small heterotrophs also contribute substantially to N2 fixation rates (Zehr et al., 1995). N2 fixation might be mediated by a variety of auto and heterotrophic bacterial community in the eastern Arabian Sea, a region of rather limited information. Previously estimated rates (Gandhi et al., 2011) were surprisingly high; so in this study, we revisited the N2 fixation and carbon uptake rates over the eastern Arabian Sea to verify the veracity of the reported higher rates, using the 15N2 gas tracer technique (Montoya et al., 1996). We report measured N2 fixation rates for dark and light conditions and discuss the possible reasons for this estimated rates.
Materials and Methods
Sampling for Incubation Experiments
Water samples were collected using Niskin bottles (bottles were closed by a messenger) from the four different depths (0, 5, 10, and 20 m) within the euphotic zone at three locations (NF-a, NF-b and NF-c that have station depths 42, 37, and 37 m, respectively) over the eastern Arabian Sea, during ORV Sagar Manjusha cruise during 10–14 May 2010 (Figure 1). Duplicate samples were taken from each depth in 1.225 L polycarbonate Nalgene bottles. All the bottles filled without headspace followed by the addition of 15N2 gas (bubble method) with the chromatographic gas tight syringe (Montoya et al., 1996). Two milliliters of 15N2 gas (99% 15N enriched gas from Cambridge Isotope Laboratories, Inc. USA) and 1 ml of 0.2 mmol ml−1 NaH13CO3 (99% 13C enriched) tracers were injected to each bottle (final enrichment of 16.6% for 15N and 8.5% for 13C) before the start of the incubations, which were performed during 10:00–14:00 h, i.e., symmetric to local noon. Tracer added bottles were covered with the calibrated neutral density filters to simulate the irradiance at the depths from which the samples were taken. After the incubations, the samples were filtered sequentially through pre-combusted (4 h at 400°C) Whatmann GF/F filters (25 mm diameter and 0.7 μm pore size), washed with filtered sea water, dried in an oven at 50°C overnight and stored for further mass spectrometric analysis. At each station, 2 L surface seawater was collected for measuring the nitrogen isotopic composition of natural particulate organic nitrogen (PON) and carbon (POC).
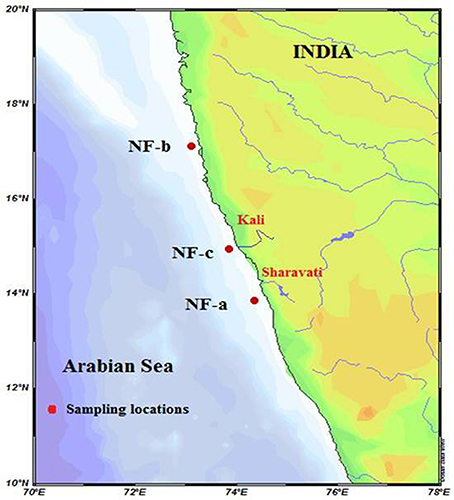
Figure 1. Sea water sampling locations (filled circles) NF-a, NF-b and NF-c, during ORV Sagar Manjusha cruise May-2010.
Mass Spectrometric Analysis and Calculation of Rates
Elemental analyzer interface with continuous flow mass spectrometer at the Physical Research Laboratory, Ahmedabad was used to measure the PON, POC, atom % 15N and atom % 13C in the samples. Volumetric rate of N2 fixation were calculated following (Montoya et al., 1996).
Where, APN0 = 15N atom% in PON at the start of experiment, APNf = 15N atom% in PON at the end of experiment, t = time of incubation (4 hrs), [PON]f = concentration of PON at the end of the experiment and ANenrich = 15N enrichment in the dissolved form after tracer addition at the start of the incubation, which is estimated as:
Surface water samples (at 0–1 m depth) were also incubated in complete dark condition and these estimates are attributed to the presence of heterotrophic diazotrophs and their contribution to N2 fixation. It has been discovered that in the “bubble method,” only 40% enrichment can be achieved in 4 h incubations, which further results in 40% underestimation in the rates (Mohr et al., 2010). Hence, we multiplied N2 fixation rates by a constant factor of 2.5 to avoid possible underestimation in the bubble gas technique. Later, it was discovered that the underestimation of rates is community dependent-there is less underestimation for Trichodesmium compared to that for other diazotrophs. Our sampling area witnesses Trichodesmium so underestimation in bubble method was less (Großkopf et al., 2012; White, 2012; Klawonn et al., 2015). We still multiplied our rates by 2.5 because we compared our results with Gandhi et al. (2011), who multiplied by the same factor. Carbon uptake rate is calculated by substituting N and 15N by C and 13C, respectively, in Equations (1) and (2) (Slawyk et al., 1977). Areal rates were calculated from the volumetric rates using the trapezoidal rule of integration, i.e., mean of volumetric rates were multiplied by the corresponding depth interval and then summed for all the depth intervals. Hundred milliliters of each sample was separately collected for nutrient measurements using a SKALAR auto analyzer at the offshore laboratory.
Spatial distribution of sea surface temperature (SST) was plotted using the Gridded High Resolution Sea Surface Temperature: Operational sea surface temperature and sea ice analysis (GHRSST: OSTIA) satellite data (Donlon et al., 2012). The mixed layer depth (MLD) was estimated based on the temperature criterion (0.2°C difference from the SST; de Boyer Montégut et al., 2004). Salinity values were obtained from the CTD data, values ranged from 35.23 to 35.56 at the sampling locations (Figure 2).
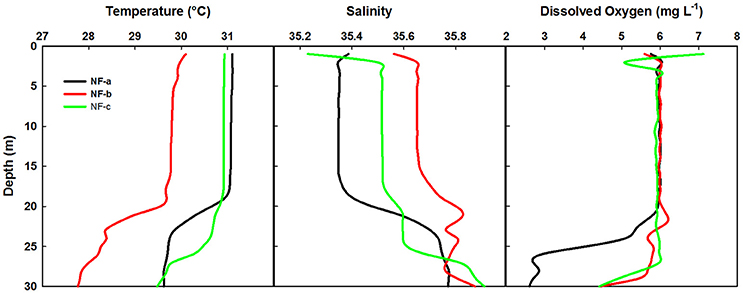
Figure 2. Temperature, salinity and dissolved O2 profiles at the three sampling locations based on the CTD data.
Results and Discussion
Hydrography
Temperature profiles from a portable CTD at sampling locations NF-a, NF-b, and NF-c showed that MLD varied between 17 and 20 m (Figure 2). Surface temperature at NF-b was ~1.4°C less than that at the other two stations. Temperature measurements obtained from CTD at surface level were reproduced by the satellite remote sensing data images (Figure 3). Salinity values obtained from CTD varied from 35.23 to 35.56 at the surface with maximum at NF-b and minimum at NF-c. NF-b is possibly influenced by Western India Coastal Current (WICC), which brings convecting mixing driven colder water from the north to the south during pre-monsoon (Schott and McCreary, 2001) as evidenced in the temperature profile (Figure 1).
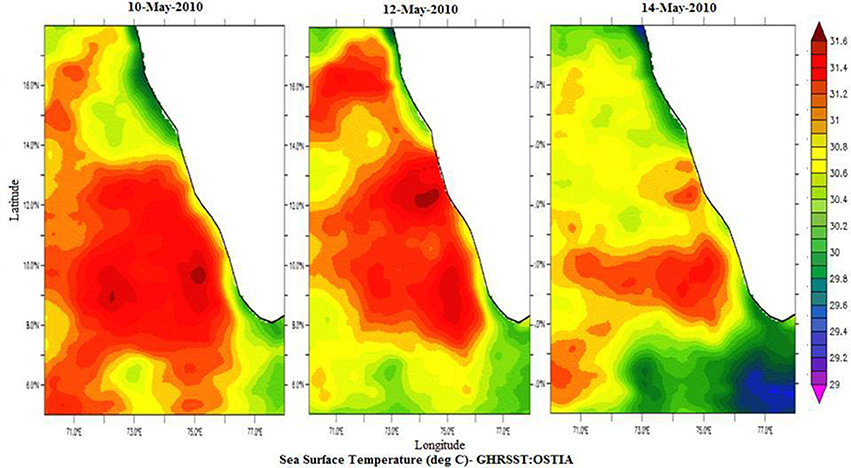
Figure 3. Spatial distribution of Sea Surface Temperature (°C) for 10th May-2010, 12th May-2010, and 14th May-2010 from GHRSST: OSTIA, http://apdrc.soest.hawaii.edu/las/v6/constrain?var=4928.
Dissolved oxygen from the same CTD casts showed a dip of 2 mg L−1 at 3 m depth at sampling location NF-c, whereas there was no vertical gradient in oxygen in the upper 20 m at locations NF-a and NF-b (Figure 2). Oxygen showed a sudden decline at all the stations below 20 m depth. Lower temperature at NF-b was associated with the detectable SiO4 values, whereas the dip in the dissolved oxygen was associated with the maximum NOx ( + ), phosphate () values and higher N:P ratios at NF- c (Table 1).
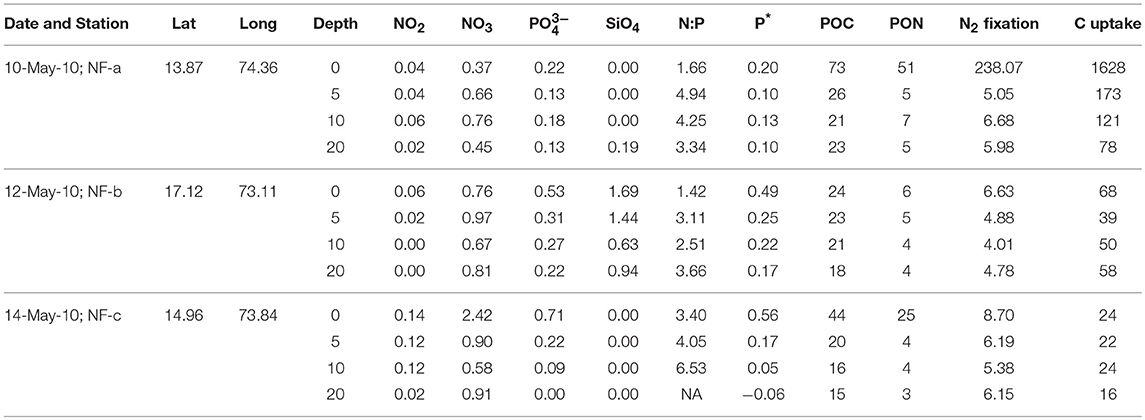
Table 1. Sampling Date, Latitude (°N), Longitude (°E), water depth (m), Nutrients concentrations (μM), N:P, P* (μM), particulate organic carbon (POC, μM) and nitrogen (PON, μM), N2 fixation (nM N h−1), and carbon (C) uptake (nM C h−1) at the three locations sampled in the eastern Arabian Sea.
Nutrients
Nutrient concentrations were higher at the surface and decreased with depth, with maximum surface value at NF-c (Figure 4). varied from 0.37 to 2.42 μM while ranged from 0 to 0.71 μM (Table 1). At sampling station NF-b, SiO4 values were readily detectable with maximum at surface (1.69 μM) and decreased with depth (Table 1). We calculated P* that indicates concentration deviations from the Redfield ratio (N:P = 16):
Positive P* indicates concentrations in excess compared to (Deutsch et al., 2007). Surface water P*-values were mostly in excess (except at 20 m at NF-c) and varied from −0.06 to 0.56 μM (Table 1). P*-values at surface showed increasing trend from NF-a to NF-c, and decreased with depth at all the three locations. and maxima at NF-c could be due to the upwelling of subsurface water. Low P* at NF-a could also be either due to the consumption of phosphate by diazotrophy or due to the upwelling, which starts during May (Gupta et al., 2016) as evidenced in the temperature profile at NF-a (Figure 2). Upwelling would create low oxygen just below MLD (Gupta et al., 2016; Sudheesh et al., 2016), as observed at NF-a (Figure 2). However, high P*-values would have been expected in low oxygen water but deeper water, as evidenced from the P* profiles (Table 1), has low P*—possibly because of the remineralization of diazotrophy dominated organic matter (high N:P ratio) in the deeper depths. was below the detection limit at 20 m depth at NF-c yet there was N2 fixation. This could again be attributed to the consumption by diazotrophs.
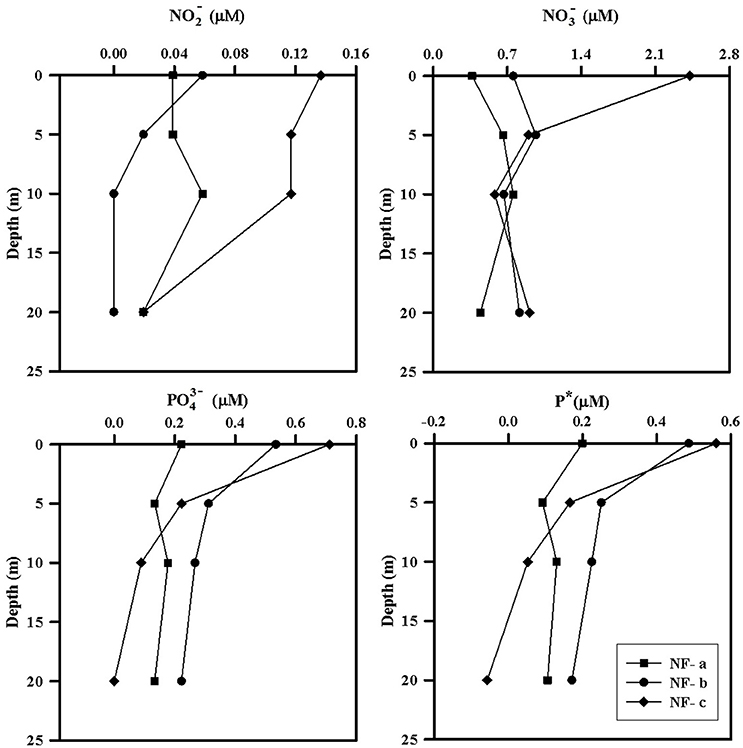
Figure 4. Depth profiles of measured nutrients (, , ) and P*, at sampling locations NF-a, NF-b, and NF-c.
N:P ratio was less than the Redfield ratio (positive P*, Table 1) at all stations with maximum at NF- c, which could have resulted in nitrogen limitation and further would have facilitated of N2 fixation. N2 fixation is controlled by iron and availability (Wu et al., 2000; Capone, 2001; Sañudo-Wilhelmy et al., 2001; Mulholland, 2007). At these sampling locations, N2 fixation may be regulated by iron input as is not the limiting nutrient in the northern Indian Ocean as suggested by P*-values (Shiozaki et al., 2014). Iron limitation is likely, as some part of the western Arabian Sea has been highlighted as a high nutrient low chlorophyll (HNLC) region (Naqvi et al., 2010; Moffett et al., 2015). Changes in the response of external forcing (e.g., seasonal monsoon and upwelling events) and inputs (e.g., aeolian and river influx) have specific control on N2 fixation over the basin (Mulholland and Capone, 2009). POC and PON varied between 15 and 73 μM and 3–51 μM, respectively (Table 1), but did not show any correlation with C and N2 fixation rates. However, the highest values of POC, PON, C, and N2 fixation rates were observed at the surface at NF-a.
N2 Fixation Rate and Carbon Uptake
N2 fixation rates varied from 4 to 238 nM N h−1, while carbon uptake rate ranged between 16 and 1628 nM C h−1 (Figure 5). Our N2 fixation rates were higher than those synthesized by Capone et al. (2008) in the world oceans (0–5.4 nM N h−1). The highest values for both carbon uptake and N2 fixation rate for light incubation fixation (Figure 5) were observed at NF-a lying south of the other two sampling locations (Figure 1). N2 fixation rates were higher in dark incubations compared to the light incubations at the surface level, except for NF-a (44.93, 63.32, and 164.7 nM N h−1 at NF-a, NF-b, and NF-c, respectively, Figure 6). Whereas carbon uptake rates in the light incubations were an order of magnitude higher than in the dark incubations (Figure 6). Higher N2 fixation rates for the dark incubations compared to light might be attributable to the presence of heterotrophic species at the surface. Heterotrophs contributed up to 52% to the total N2 fixation (estimated from light and dark incubations, assuming light incubations correspond to phototrophic and dark to heterotrophic). Heterotrophic contribution to N2 fixation could be even higher in the deeper waters due to favorable conditions for them. These higher values associated with dark incubation suggest that heterotrophic N2 fixers might play an important role in fixing N2 over the eastern Arabian Sea. In the surface waters of the Arabian Sea, the DNA and RNA recovered during the southwest monsoon periods were also categorized as those of heterotrophic bacteria (Jayakumar et al., 2012; Bird and Wyman, 2013). Therefore, our results suggest that an active N2 fixation by heterotrophic bacteria could occur in the surface water of the eastern Arabian Sea (Shiozaki et al., 2014).
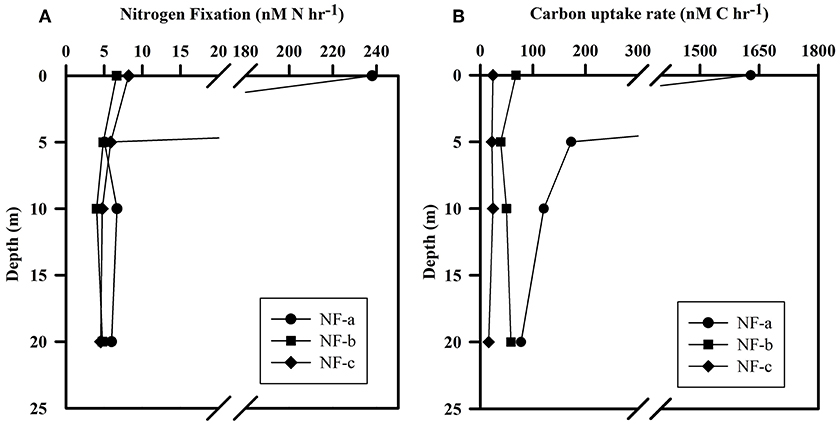
Figure 5. (A) N2 fixation rate and (B) Carbon uptake rate at the sampling location (NF-a, NF-b, and NF-c) for light (at ambient conditions) incubation for samples at different depths.
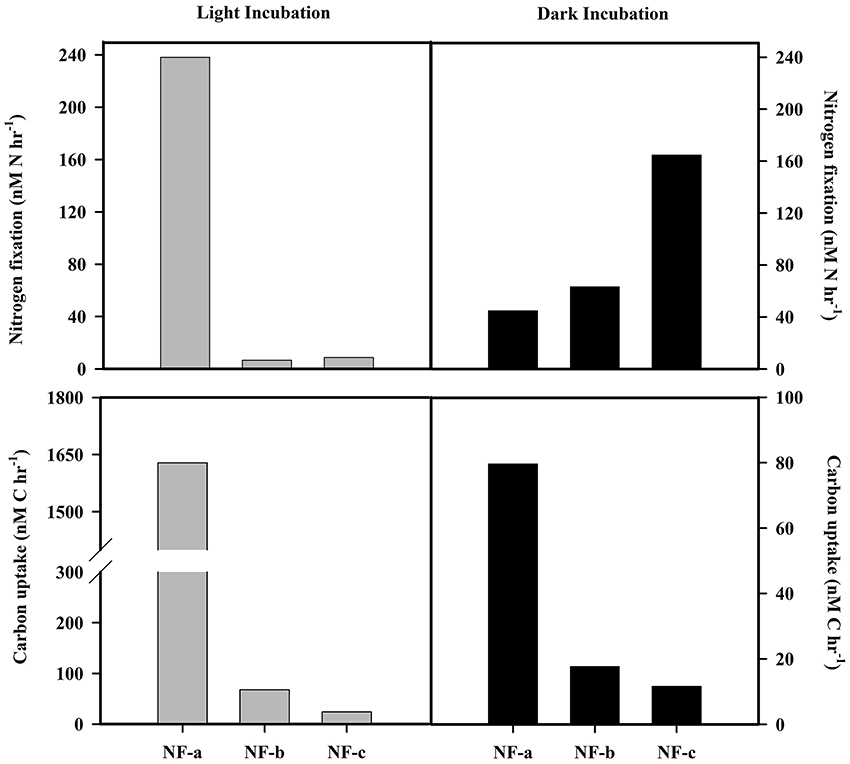
Figure 6. N2 fixation and carbon uptake rate at surface for light (gray bars) and dark (black bars) incubation.
Euphotic-depth integrated autotrophic N2 fixation and carbon uptake rates were highest at NF-a, where N2 fixation rate was 8.4 ± 2.8 mmol N m−2 d−1 (standard deviation of duplicate samples) and carbon uptake rate was 74.7 ± 17.7 mmol C m−2 d−1. Our areal rates (8 mmol N m−2 d−1) were the highest ever observed in anywhere in world oceans (Table 2) and comparable to those measured by Gandhi et al. (2011) in the similar region-suggesting that the high rates during the spring 2009 were not episodic rather it could be a regular phenomenon during the spring in the Arabian Sea. High rates in the eastern Arabian Sea are probably due to the availability of both the essential nutrients for diazotrophs, i.e., iron and . Iron is available because this region unlike the western Arabian Sea is close to the Thar desert, whereas the western Arabian Sea is recognized to be an HNLC region (Naqvi et al., 2010). is available because upwelled water brings rich (compared to ) water as is lost in denitrification. So the Arabian Sea is unique basin for sustaining diazotrophy and the higher rates of N2 fixation. On the other hand, the other major oceans, like the Atlantic does not have enough phosphate (Wu et al., 2000), while the Pacific does not have enough iron (Behrenfeld and Kolber, 1999).
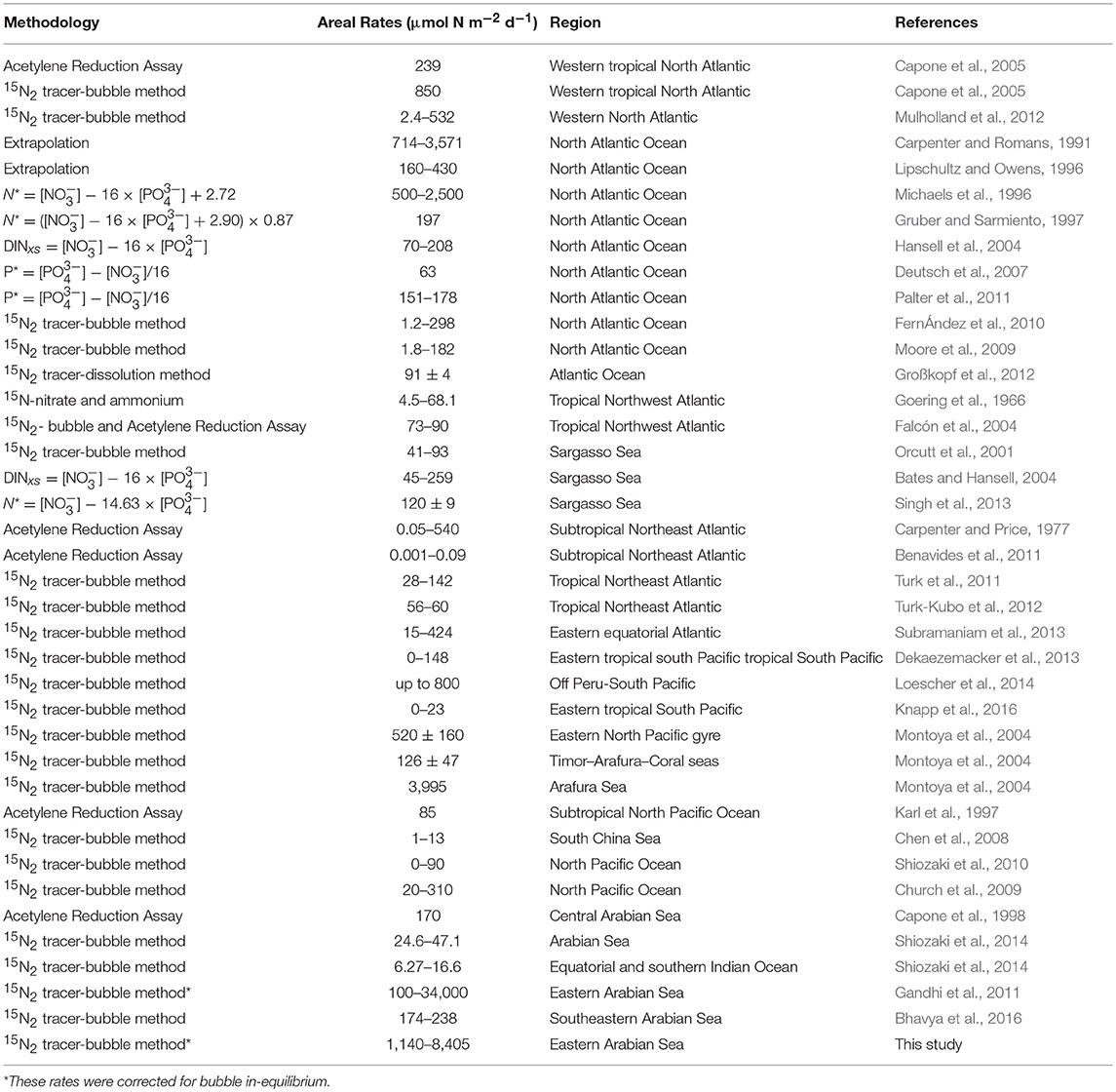
Table 2. Summary of Photic N2 fixation rates in the world oceans (Table updated after Singh et al., 2013; Benavides and Voss, 2015).
Gandhi et al. (2011) showed an overall increasing trend in the N2 fixation rate from south to north, whereas in this present study, it was in the opposite direction. N2 fixation supplies a large portion of the new N that supports marine productivity (Capone, 1997; Karl et al., 2002; LaRoche and Breitbarth, 2005; Mahaffey et al., 2005). Estimated contribution of new nitrogen to N2 fixation in the present study is about 92% of new nitrogen, which is the same as that reported by the previous study (Gandhi et al., 2011).
Most observations around the world oceans are indirectly derived from geochemical estimates that are based on sub-surface nutrient distribution (Table 2). We have directly measured N2 fixation using 15N2 tracer, which is still the best available method despite its inherent problems of incomplete dissolution of the bubble (Großkopf et al., 2012). Our findings of high N2 fixation in this region have large implications in understanding marine C, N, and cycles. If we extrapolate these high rates over the Arabian Sea, then we might find the missing nitrogen inputs (Codispoti, 2007). But we require to conduct more N2 fixation experiments using the dissolution method in this region for extrapolation. In addition to seasonal upwelling, N2 fixation might play a role in making the Arabian Sea perennially productive (Singh and Ramesh, 2015). Higher N2 fixation and its degradation in the subsurface waters may also be useful in understating the phosphorous cycle-low P* at the deeper waters and higher P* at the surface-contrary to what is expected from the denitrified waters.
Conclusion
Results from the three sampling locations in the eastern Arabian Sea suggest that the Arabian Sea witnessed the highest ever rates of the N2 fixation among the world ocean for the two consecutive springs. Out of all nitrogen gain processes, about 92% of new nitrogen is gained through N2 fixation only, with highest areal rates (8 mmol N m−2 d−1). N2 fixation rate for the dark was higher than the light incubation at the surface except for NF-a, which alluded to the presence of heterotrophic species. Based on the higher N2 fixation values at the surface for dark incubation, we hypothesize that heterotrophic fixers dominantly (about 52% of total N2 fixation is by heterotrophs) play an important role in fixing N2. There is a consistency between the higher N2 fixation rates and the carbon uptake rate.
Author Contributions
AS and RR designed research; AS, RR, and NT performed research; KK and AS analyzed data; and KK and AS wrote the manuscript with major inputs from all the co-authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Indian Space Research Organisation Geosphere- Biosphere program (ISRO-IGBP) for funding, and all the participants and the crew members of ORV Sagar Manjusha for their assistance onboard. We thank Suhas Shetye for nutrients measurements.
References
Bates, N. R., and Hansell, D. A. (2004). Temporal variability of excess nitrate in the subtropical mode water of the North Atlantic Ocean. Mar. Chem. 84, 225–241. doi: 10.1016/j.marchem.2003.08.003
Behrenfeld, M. J., and Kolber, Z. S. (1999). Widespread iron limitation of phytoplankton in the South Pacific Ocean. Science 283, 840–843. doi: 10.1126/science.283.5403.840
Benavides, M., Agawin, N. S. R., Arístegui, J., Ferriol, P., and Stal, L. J. (2011). Nitrogen fixation by Trichodesmium and small diazotrophs in the subtropical northeast Atlantic. Aquat. Microb. Ecol. 65, 45–53. doi: 10.3354/ame01534
Benavides, M., and Voss, M. (2015). Five decades of N2 fixation research in the North Atlantic Ocean. Front. Mar. Sci. 2:40. doi: 10.3389/fmars.2015.00040
Bhavya, P., Kumar, S., Gupta, G., Sudheesh, V., Sudharma, K., Varrier, D., et al. (2016). Nitrogen uptake dynamics in a tropical eutrophic estuary (Cochin, India) and adjacent coastal waters. Estuar. Coasts 39, 54–67. doi: 10.1007/s12237-015-9982-y
Bird, C., and Wyman, M. (2013). Transcriptionally active heterotrophic diazotrophs are widespread in the upper water column of the Arabian Sea. FEMS Microbiol. Ecol. 84, 189–200. doi: 10.1111/1574-6941.12049
Capone, D. G. (1997). Trichodesmium, a globally significant marine Cyanobacterium. Science 276, 1221–1229. doi: 10.1126/science.276.5316.1221
Capone, D. G. (2001). Marine nitrogen fixation: what's the fuss? Curr. Opin. Microbiol. 4, 341–348. doi: 10.1016/S1369-5274(00)00215-0
Capone, D. G., Bronk, D. A., Mulholland, M. R., and Carpenter, E. J. (2008). Nitrogen in the Marine Environment. Burlington, MA: Academic Press.
Capone, D. G., Burns, J. A., Montoya, J. P., Subramaniam, A., Mahaffey, C., Gunderson, T., et al. (2005). Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Glob. Biogeochem. Cycles 19, 1–17. doi: 10.1029/2004GB002331
Capone, D. G., and Carpenter, E. J. (1982). Nitrogen fixation in the marine environment. Science 217, 1140–1142. doi: 10.1126/science.217.4565.1140
Capone, D. G., Subramaniam, A., Montoya, J. P., Voss, M., Humborg, C., Johansen, A. M., et al. (1998). An extensive bloom of the N2-fixing cyanobacterium Trichodesmium erythraeum in the central Arabian Sea. Mar. Ecol. Prog. Ser. 172, 281–292. doi: 10.3354/meps172281
Carpenter, E. J., and Price, C. C. (1977). Nitrogen fixation, distribution, and production of Oscillatoria (Trichodesmium) spp. in the western Sargasso and Caribbean Seas. Limnol. Oceanogr. 22, 60–72. doi: 10.4319/lo.1977.22.1.0060
Carpenter, E. J., and Romans, K. (1991). Major role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254, 1356–1358. doi: 10.1126/science.254.5036.1356
Chen, Y. L., Chen, H.-Y., Tuo, S., and Ohki, K. (2008). Seasonal dynamics of new production from Trichodesmium N2 fixation and nitrate uptake in the upstream Kuroshio and South China Sea basin. Limnol. Oceanogr. 53, 1705–1721. doi: 10.4319/lo.2008.53.5.1705
Church, M. J., Mahaffey, C., Letelier, R. M., Lukas, R., Zehr, J. P., and Karl, D. M. (2009). Physical forcing of nitrogen fixation and diazotroph community structure in the North Pacific subtropical gyre. Glob. Biogeochem. Cycles 23:GB2020. doi: 10.1029/2008gb003418
Codispoti, L. A. (2007). An oceanic fixed nitrogen sink exceeding 400 TgN a-1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences 4, 233–253. doi: 10.5194/bg-4-233-2007
Codispoti, L., Brandes, J. A., Christensen, J., Devol, A., Naqvi, S., Paerl, H. W., et al. (2001). The oceanic fixed nitrogen and nitrous oxide budgets: moving targets as we enter the anthropocene? Sci. Mar. 65, 85–105. doi: 10.3989/scimar.2001.65s285
de Boyer Montégut, C., Madec, G., Fischer, A. S., Lazar, A., and Iudicone, D. (2004). Mixed layer depth over the global ocean: an examination of profile data and a profile-based climatology. J. Geophys. Res. Oceans 109:C12003. doi: 10.1029/2004jc002378
Dekaezemacker, J., Bonnet, S., Grosso, O., Moutin, T., Bressac, M., and Capone, D. (2013). Evidence of active dinitrogen fixation in surface waters of the eastern tropical South Pacific during El Ni-o and La Ni-a events and evaluation of its potential nutrient controls. Glob. Biogeochem. Cycles 27, 768–779. doi: 10.1002/gbc.20063
Deutsch, C., Sarmiento, J. L., Sigman, D. M., Gruber, N., and Dunne, J. P. (2007). Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445, 163–167. doi: 10.1038/nature05392
Donlon, C. J., Martin, M., Stark, J., Roberts-Jones, J., Fiedler, E., and Wimmer, W. (2012). The operational sea surface temperature and sea ice analysis (OSTIA) system. Remote Sens. Environ. 116, 140–158. doi: 10.1016/j.rse.2010.10.017
Falcón, L. I., Carpenter, E. J., Cipriano, F., Bergman, B., and Capone, D. G. (2004). N2 fixation by unicellular bacterioplankton from the Atlantic and Pacific Oceans: phylogeny and in situ rates. Appl. Environ. Microbiol. 70, 765–770. doi: 10.1128/AEM.70.2.765-770.2004
Fernández, A., Mouriño-Carballido, B., Bode, A., Varela, M., and Marañón, E. (2010). Latitudinal distribution of Trichodesmium spp. and N 2 fixation in the Atlantic Ocean. Biogeosciences 7, 3167–3176. doi: 10.5194/bg-7-3167-2010
Gandhi, N., Singh, A., Prakash, S., Ramesh, R., Raman, M., Sheshshayee, M., et al. (2011). First direct measurements of N2 fixation during a Trichodesmium bloom in the eastern Arabian Sea. Glob. Biogeochem. Cycles 25:GB4014. doi: 10.1029/2010GB003970
Goering, J., Dugdale, R., and Menzel, D. W. (1966). Estimates of in situ rates of nitrogen uptake by Trichodesmium sp. in the tropical Atlantic Ocean. Limnol. Oceanogr. 11, 614–620. doi: 10.4319/lo.1966.11.4.0614
Großkopf, T., Mohr, W., Baustian, T., Schunck, H., Gill, D., Kuypers, M. M., et al. (2012). Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488, 361–364. doi: 10.1038/nature11338
Gruber, N., and Sarmiento, J. L. (1997). Global patterns of marine nitrogen fixation and denitrification. Glob. Biogeochem. Cycles 11, 235–266. doi: 10.1029/97GB00077
Gupta, G., Sudheesh, V., Sudharma, K., Saravanane, N., Dhanya, V., Dhanya, K., et al. (2016). Evolution to decay of upwelling and associated biogeochemistry over the southeastern Arabian Sea shelf. J. Geophys. Res. Biogeosciences 121, 159–175. doi: 10.1002/2015JG003163
Hansell, D. A., Bates, N. R., and Olson, D. B. (2004). Excess nitrate and nitrogen fixation in the North Atlantic Ocean. Mar. Chem. 84, 243–265. doi: 10.1016/j.marchem.2003.08.004
Hewson, I., Moisander, P. H., Achilles, K. M., Carlson, C. A., Jenkins, B. D., Mondragon, E. A., et al. (2007). Characteristics of diazotrophs in surface to abyssopelagic waters of the Sargasso Sea. Aquat. Microb. Ecol. 46, 15–30. doi: 10.3354/ame046015
Jayakumar, A., Al-Rshaidat, M. M., Ward, B. B., and Mulholland, M. R. (2012). Diversity, distribution, and expression of diazotroph nifH genes in oxygen-deficient waters of the Arabian Sea. FEMS Microbiol. Ecol. 82, 597–606. doi: 10.1111/j.1574-6941.2012.01430.x
Jickells, T., Buitenhuis, E., Altieri, K., Baker, A., Capone, D., Duce, R., et al. (2017). A re-evaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean. Glob. Biogeochem. Cycles 31, 289–305. doi: 10.1002/2016GB005586
Karl, D., Letelier, R., Tupas, L., Dore, J., Christian, J., and Hebel, D. (1997). The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388, 533–538. doi: 10.1038/41474
Karl, D., Michaels, A., Bergman, B., Capone, D. G., Carpenter, E. J., Letelier, R., et al. (2002). Dinitrogen fixation in the world's oceans. Biogeochemistry 57–58, 47–98. doi: 10.1023/A:1015798105851
Klawonn, I., Lavik, G., Böning, P., Marchant, H. K., Dekaezemacker, J., Mohr, W., et al. (2015). Simple approach for the preparation of 15-15N2-enriched water for nitrogen fixation assessments: evaluation, application and recommendations. Front. Microbiol. 6:769. doi: 10.3389/fmicb.2015.00769
Knapp, A. N., Casciotti, K. L., Berelson, W. M., Prokopenko, M. G., and Capone, D. G. (2016). Low rates of nitrogen fixation in eastern tropical South Pacific surface waters. Proc. Natl. Acad. Sci. U.S.A. 113, 4398–4403. doi: 10.1073/pnas.1515641113
LaRoche, J., and Breitbarth, E. (2005). Importance of the diazotrophs as a source of new nitrogen in the ocean. J. Sea Res. 53, 67–91. doi: 10.1016/j.seares.2004.05.005
Lipschultz, F., and Owens, N. J. (1996). An assessment of nitrogen fixation as a source of nitrogen to the North Atlantic Ocean. Biogeochemistry 35, 261–274. doi: 10.1007/BF02179830
Loescher, C. R., Großkopf, T., Desai, F. D., Gill, D., Schunck, H., Croot, P. L., et al. (2014). Facets of diazotrophy in the oxygen minimum zone waters off Peru. ISME J. 8, 2180–2192. doi: 10.1038/ismej.2014.71
Madhupratap, M., Kumar, S. P., Bhattathiri, P., Kumar, M. D., Raghukumar, S., Nair, K., et al. (1996). Mechanism of the biological response to winter cooling in the northeastern Arabian Sea. Nature 384, 549–552. doi: 10.1038/384549a0
Mahaffey, C., Michaels, A. F., and Capone, D. G. (2005). The conundrum of marine N2 fixation. Am. J. Sci. 305, 546–595. doi: 10.2475/ajs.305.6-8.546
Michaels, A. F., Olson, D., Sarmiento, J., Ammerman, J. W., Fanning, K. A., Jahnke, R. A., et al. (1996). Inputs, losses and transformations of nitrogen and phosporus in the pelagic North Atlantic Ocean. Biogeochemistry 35, 181–226. doi: 10.1007/BF02179827
Middelburg, J. J., and Nieuwenhuize, J. (2000). Nitrogen uptake by heterotrophic bacteria and phytoplankton in the nitrate-rich Thames estuary. Mar. Ecol. Prog. Ser. 203, 13–21. doi: 10.3354/meps203013
Moffett, J. W., Vedamati, J., Goepfert, T. J., Pratihary, A., Gauns, M., and Naqvi, S. (2015). Biogeochemistry of iron in the Arabian Sea. Limnol. Oceanogr. 60, 1671–1688. doi: 10.1002/lno.10132
Mohr, W., Grosskopf, T., Wallace, D. W., and LaRoche, J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 5:e12583. doi: 10.1371/journal.pone.0012583
Montoya, J. P., Holl, C. M., Zehr, J. P., Hansen, A., Villareal, T. A., and Capone, D. G. (2004). High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430, 1027–1032. doi: 10.1038/nature02824
Montoya, J. P., Voss, M., Kahler, P., and Capone, D. G. (1996). A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl. Environ. Microbiol. 62, 986–993.
Moore, C. M., Mills, M. M., Achterberg, E. P., Geider, R. J., LaRoche, J., Lucas, M. I., et al. (2009). Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat. Geosci. 2, 867–871. doi: 10.1038/ngeo667
Mulholland, M., Bernhardt, P., Blanco-Garcia, J., Mannino, A., Hyde, K., Mondragon, E., et al. (2012). Rates of dinitrogen fixation and the abundance of diazotrophs in North American coastal waters between Cape Hatteras and Georges Bank. Limnol. Oceanogr. 57, 1067–1083. doi: 10.4319/lo.2012.57.4.1067
Mulholland, M. R. (2007). The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences 4, 37–51. doi: 10.5194/bg-4-37-2007
Mulholland, M. R., and Capone, D. G. (2009). Dinitrogen fixation in the Indian Ocean. Indian Ocean Biogeochem. Process. Ecol. Var. 185, 167–186. doi: 10.1029/2009GM000850
Naqvi, S. (1987). Some aspects of the oxygen-deficient conditions and denitrification in the Arabian Sea. J. Mar. Res. 45, 1049–1072. doi: 10.1357/002224087788327118
Naqvi, S. W. A., and Jayakumar, D. A. (2000). Ocean biogeochemistry and atmospheric composition: significance of the Arabian sea. Curr. Sci. 78, 289–299.
Naqvi, S., Moffett, J. W., Gauns, M., Narvekar, P., Pratihary, A., Naik, H., et al. (2010). The Arabian Sea as a high-nutrient, low-chlorophyll region during the late Southwest Monsoon. Biogeosciences 7, 2091–2100. doi: 10.5194/bg-7-2091-2010
Orcutt, K. M., Lipschultz, F., Gundersen, K., Arimoto, R., Michaels, A. F., Knap, A. H., et al. (2001). A seasonal study of the significance of N 2 fixation by Trichodesmium spp. at the Bermuda Atlantic Time-series Study (BATS) site. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 1583–1608. doi: 10.1016/S0967-0645(00)00157-0
Palter, J. B., Lozier, M. S., Sarmiento, J. L., and Williams, R. G. (2011). The supply of excess phosphate across the Gulf Stream and the maintenance of subtropical nitrogen fixation. Glob. Biogeochem. Cycles 25:GB4007. doi: 10.1029/2010gb003955
PrasannaKumar, S., Madhupratap, M., and DileepKumar, M. (2001). High biological productivity in the central Arabian Sea during the summer monsoon driven by Ekman pumping and lateral advection. Curr. Sci. 81, 1633–1638.
Sañudo-Wilhelmy, S. A., Kustka, A. B., Gobler, C. J., Hutchins, D. A., Yang, M., Lwiza, K., et al. (2001). Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature 411, 66–69. doi: 10.1038/35075041
Schott, F. A., and McCreary, J. P. (2001). The monsoon circulation of the Indian Ocean. Prog. Oceanogr. 51, 1–123. doi: 10.1016/S0079-6611(01)00083-0
Shiozaki, T., Furuya, K., Kodama, T., Kitajima, S., Takeda, S., Takemura, T., et al. (2010). New estimation of N2 fixation in the western and central Pacific Ocean and its marginal seas. Glob. Biogeochem. Cycles 24:GB1015. doi: 10.1029/2009GB003620
Shiozaki, T., Ijichi, M., Kodama, T., Takeda, S., and Furuya, K. (2014). Heterotrophic bacteria as major nitrogen fixers in the euphotic zone of the Indian Ocean. Glob. Biogeochem. Cycles 28, 1096–1110. doi: 10.1002/2014GB004886
Singh, A., Gandhi, N., and Ramesh, R. (2012). Contribution of atmospheric nitrogen deposition to new production in the nitrogen limited photic zone of the northern Indian Ocean. J. Geophys. Res. Oceans 117:C06004. doi: 10.1029/2011jc007737
Singh, A., Lomas, M., and Bates, N. (2013). Revisiting N2 fixation in the North Atlantic Ocean: significance of deviations from the Redfield Ratio, atmospheric deposition and climate variability. Deep Sea Res. Part II Top. Stud. Oceanogr. 93, 148–158. doi: 10.1016/j.dsr2.2013.04.008
Singh, A., and Ramesh, R. (2011). Contribution of riverine dissolved inorganic nitrogen flux to new production in the coastal northern Indian Ocean: an assessment. Int. J. Oceanogr. 2011:983561. doi: 10.1155/2011/983561
Singh, A., and Ramesh, R. (2015). Environmental controls on new and primary production in the northern Indian Ocean. Prog. Oceanogr. 131, 138–145. doi: 10.1016/j.pocean.2014.12.006
Slawyk, G., Collos, Y., and Auclair, J. (1977). The use of the 13C and 15N isotopes for the simultaneous measurement of carbon and nitrogen turnover rates in marine phytoplankton. Limnol. Oceanogr. 22, 925–932. doi: 10.4319/lo.1977.22.5.0925
Subramaniam, A., Mahaffey, C., Johns, W., and Mahowald, N. (2013). Equatorial upwelling enhances nitrogen fixation in the Atlantic Ocean. Geophys. Res. Lett. 40, 1766–1771. doi: 10.1002/grl.50250
Sudheesh, V., Gupta, G., Sudharma, K., Naik, H., Shenoy, D., Sudhakar, M., et al. (2016). Upwelling intensity modulates N2O concentrations over the western Indian shelf. J. Geophys. Res. Oceans 121, 8551–8565. doi: 10.1002/2016JC012166
Turk, K. A., Rees, A. P., Zehr, J. P., Pereira, N., Swift, P., Shelley, R., et al. (2011). Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic. ISME J. 5, 1201–1212. doi: 10.1038/ismej.2010.205
Turk-Kubo, K. A., Achilles, K. M., Serros, T. R., Ochiai, M., Montoya, J. P., and Zehr, J. P. (2012). Nitrogenase (nifH) gene expression in diazotrophic cyanobacteria in the Tropical North Atlantic in response to nutrient amendments. Front. Microbiol. 3:386. doi: 10.3389/fmicb.2012.00386
White, A. E. (2012). Oceanography: the trouble with the bubble. Nature 488, 290–291. doi: 10.1038/nature11481
Wu, J., Sunda, W., Boyle, E. A., and Karl, D. M. (2000). Phosphate depletion in the western North Atlantic Ocean. Science 289, 759–762. doi: 10.1126/science.289.5480.759
Zehr, J. P., Jenkins, B. D., Short, S. M., and Steward, G. F. (2003). Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ. Microbiol. 5, 539–554. doi: 10.1046/j.1462-2920.2003.00451.x
Zehr, J. P., Mellon, M., Braun, S., Litaker, W., Steppe, T., and Paerl, H. W. (1995). Diversity of heterotrophic nitrogen fixation genes in a marine cyanobacterial mat. Appl. Environ. Microbiol. 61, 2527–2532.
Keywords: dinitrogen fixation, 15N, 13C, nitrogen budget, carbon uptake rate, nutrients, biogeochemistry, Arabian Sea
Citation: Kumar PK, Singh A, Ramesh R and Nallathambi T (2017) N2 Fixation in the Eastern Arabian Sea: Probable Role of Heterotrophic Diazotrophs. Front. Mar. Sci. 4:80. doi: 10.3389/fmars.2017.00080
Received: 22 December 2016; Accepted: 08 March 2017;
Published: 23 March 2017.
Edited by:
Selvaraj Kandasamy, Xiamen University, ChinaReviewed by:
Il-Nam Kim, Incheon National University, South KoreaGupta GVM, Centre for Marine Living Resources and Ecology, Ministry of Earth Sciences, India
Copyright © 2017 Kumar, Singh, Ramesh and Nallathambi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arvind Singh, YXJ2aW5kc0BwcmwucmVzLmlu
†Present Address: R. Ramesh, School of Earth and Planetary Sciences, NISER, Jatni, India
 P. Kiran Kumar
P. Kiran Kumar Arvind Singh
Arvind Singh R. Ramesh1†
R. Ramesh1†