- 1Institute of Regenerative and Reconstructive Medicine, Med-X Institute, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2National Local Joint Engineering Research Center for Precision Surgery & Regenerative Medicine, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 3Shaanxi Provincial Center for Regenerative Medicine and Surgical Engineering, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 4Department of Hepatobiliary Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Irreversible electroporation (IRE), a novel non-thermal ablation technique, is utilized to ablate unresectable solid tumors and demonstrates favorable safety and efficacy in the clinic. IRE applies electric pulses to alter the cell transmembrane voltage and causes nanometer-sized membrane defects or pores in the cells, which leads to loss of cell homeostasis and ultimately results in cell death. The major drawbacks of IRE are incomplete ablation and susceptibility to recurrence, which limit its clinical application. Recent studies have shown that IRE promotes the massive release of intracellular concealed tumor antigens that become an “in-situ tumor vaccine,” inducing a potential antitumor immune response to kill residual tumor cells after ablation and inhibiting local recurrence and distant metastasis. Therefore, IRE can be regarded as a potential immunomodulatory therapy, and combined with immunotherapy, it can exhibit synergistic treatment effects on malignant tumors, which provides broad application prospects for tumor treatment. This work reviewed the current status of the clinical efficacy of IRE in tumor treatment, summarized the characteristics of local and systemic immune responses induced by IRE in tumor-bearing organisms, and analyzed the specific mechanisms of the IRE-induced immune response. Moreover, we reviewed the current research progress of IRE combined with immunotherapy in the treatment of solid tumors. Based on the findings, we present deficiencies of current preclinical studies of animal models and analyze possible reasons and solutions. We also propose possible demands for clinical research. This review aimed to provide theoretical and practical guidance for the combination of IRE with immunotherapy in the treatment of malignant tumors.
Introduction
Irreversible electroporation (IRE), a novel physical ablation technique, applies a high-voltage pulsed electric field (PEF) to alter the cell transmembrane voltage, causes nanometer-sized membrane defects or pores in the cell, and eventually leads to loss of homeostasis and cell death (1, 2). It has been used to treat arrhythmic diseases and inactivate microorganisms (3–5). In 2005, Davalos et al. first applied IRE to destroy cancer cells (6). Recently, IRE has been widely utilized to ablate unresectable solid tumors in the clinic with favorable safety and efficacy (7–9).
As opposed to thermal ablative techniques, IRE induces cell death via the delivery of high-voltage short electrical pulses (EPs) and possesses several advantages as a non-thermal ablation technique: a) less collateral thermal damage, especially for vital nerves, vessels, and cavity structures; b) no heat sink effect, avoiding incomplete ablation due to the energy reduction caused by blood flow; and c) preservation of the extracellular matrix (ECM) scaffold, promoting rapid postoperative recovery (10, 11). Currently, IRE is performed mostly for solid tumors such as liver cancer (12), pancreatic cancer (13), and prostate cancer (8) and provides an advantageous palliative treatment for advanced tumors in the vicinity of important ductal structures, such as large blood vessels, the intestines, bile ducts, or the urinary tract. However, the uneven distribution of the PEF, resulting from the heterogeneous electrical properties of tumor tissue (14, 15), leads to incomplete ablation and increases the risk of tumor recurrence, which limits the popularity of IRE in clinical practice. Notably, the membrane perforation resulting from IRE can promote the massive release of intracellular concealed tumor antigens, inducing a potential antitumor immune response to kill residual tumor cells after ablation and inhibit the local recurrence of tumors (16).
Recent studies have shown that IRE also induces an excellent effect on activating local and systematic immune responses (9, 17). Therefore, IRE can be regarded as a potential immunomodulatory therapy.
Cancer treatment has entered the era of clinical multidisciplinary comprehensive treatment, and the prognosis of patients with a variety of tumors has been significantly improved (18). Recently, the immune response induced by IRE has gained much interest among researchers (19, 20). Several clinical studies have confirmed that IRE can induce a significant immune response in cancer patients and significantly improve antitumor efficacy (21–23). Therefore, IRE combined with immunotherapy might have synergistic effects on malignant tumor treatment. This review aimed to elucidate the characteristics and mechanisms of the IRE-induced immune response and its potential in combination with immunotherapy for the treatment of tumors. This study will provide theoretical and practical guidance for the clinical application of IRE combined with immunotherapy in the treatment of solid tumors.
Effect of IRE on Tumors
The Development of IRE as a Tumor Therapy
Electroporation, an old technique, applies an external PEF to increase cell membrane permeability, inducing the development of nanoscale metastable structure defects or “pores”, which are considered to be the source of enhanced permeability (24). The application of electroporation in the biomedical field began in 1982, and Neumann et al. first applied this strategy to introduce exogenous DNA into cells (25). During electrotransfection, the pores in the cell membrane may persist for a few seconds to a few minutes. After the entry of exogenous substances, cell membrane integrity can be restored without affecting cell survival, which is known as reversible electroporation (RE) (26). Later, many studies reported the important role of RE in introducing foreign molecules into living cells, and this strategy has been used for molecular and gene transfer in vitro for many years (27, 28).
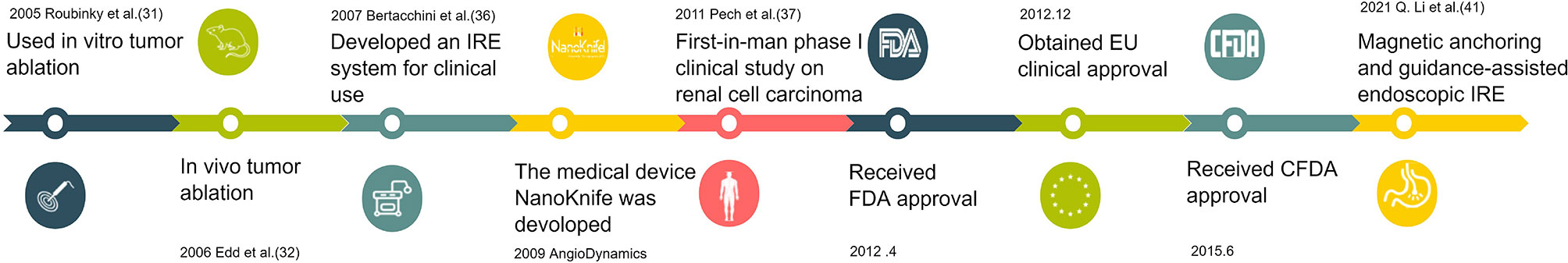
Electroporation technology for cancer therapy, known as electrochemotherapy (ECT), begins with chemotherapeutic drug delivery and promotes the absorption of drug molecules by tumor cells (29). However, when electric field strength (EFS) is higher than a certain threshold, excessive leakage of intracellular substances or slow closure of the cell membrane will cause irreversible damage to cells. Cell membrane surface perforation cannot be repaired, eventually leading to cell death (30). In 2005, Davalos et al. first announced that IRE can be used as a single therapy (without combined cytotoxic drugs or thermal effects) to destroy cancer tissue and named it “irreversible electroporation”. They found that IRE alone showed an excellent ability to destroy undesirable tissues in a similar manner to traditional focal thermal therapies (31). Edd et al. first used IRE in vivo for liver ablation in Sprague–Dawley rats in 2006 (32). Since then, a growing number of animal trials have investigated the safety and efficacy of IRE for oncology treatment (33–35). In 2007, Bertacchini et al. developed an irreversible electroporator approved for clinical use (36). This technology, marketed as NanoKnife, the first IRE tumor therapeutic apparatus, was developed by AngioDynamics in 2009. Pech et al. reported a first-in-human phase I clinical study of IRE in renal cell carcinoma in 2011 (37). Later, NanoKnife was approved by the FDA for clinical trials in April 2012. The tool was clinically licensed in the European Union in December of the same year, with CFDA approval to enter the Chinese market for clinical trials in June 2015, and is being used clinically for selected patients with locally advanced pancreatic cancer (38–40). Currently, researchers are devoted to developing therapeutic devices with customized IRE catheter-based electrodes delivered under endoscopy for surgical trauma reduction (41, 42). Figure 1 shows the timeline for the development of IRE for tumor treatment. As the precise digital control system of IRE ensures accurate output parameters for EP (43) and various electrodes have been designed based on tumors in different organs, and even based on tumor morphology (44, 45), the ablation effect of IRE on tumors is more accurate and controllable than that of other ablation therapies (46, 47).
Clinical Application of IRE on Tumors
The clinical trials of IRE ablation on tumors were often performed under computed tomography or ultrasound guidance using the commercially available NanoKnife system, which has three lengths of electrodes that are inserted into the tumor to achieve ablation (48). The number of electrodes used in the treatment procedure is determined by the size of the tumor. For lesions smaller than 2 cm, three electrodes are placed at the periphery of the lesion, and four electrodes are always placed at the periphery of the lesion for lesions between 2 and 3 cm. Four to six electrodes are used, including one to two electrodes in the center of the lesion, for lesions which are larger than 3 cm (7, 49, 50). However, a maximum of six electrodes can be used in the treatment process, as it is the maximum number of electrodes allowed by the IRE generator (7, 50). Compared with the electrodes used in most thermal ablation techniques, the electrodes used in IRE are relatively thin and allow complete destruction of the tumor and healthy liver tissue within a safe range of 1 cm around the tumor (49), and the optimal distance between the two electrodes is 0.7–2.0 cm (51). The maximum voltage and current that the generator can provide are 3,000 V and 50 A (52). Almost all clinical trials adopt an EFS of 1,500–1,800 V/cm and current of 20–50 A as the treatment parameters (52, 53). The pulse duration chosen for clinical trials is 70–100 μs, most commonly 90 or 100 μs (54). Usually, the efficacy is judged after a treatment of 90 pulses (50, 53, 55). If an insufficient extent of the ablation zone was suspected, IRE probes were repositioned, and another pulse application was performed (52). One study on pancreatic adenocarcinoma adjusted the pulse number from 90 pulses in one cycle to 30 pulses in three cycles because the patients developed interstitial edematous pancreatitis after IRE-1d (56). Technical success was defined as the ability to deliver the complete set of electrical pulses as planned (49). The above treatment parameters are not significantly different between various tumor types. Additionally, almost all clinical trials were performed percutaneously, and only some trials (54, 56, 57) used an open surgical route in a subset of cases, possibly related to the operability of the operation.
Clinical Efficacy of IRE on Tumors
Recently, many clinical trials have evaluated the therapeutic effect of IRE as tumor therapy (Supplementary Table 1). Twelve studies (7, 48–55, 58–61) involving 295 cases explored the efficacy and safety of IRE on primary or secondary liver tumors. Some of the results indicated that the effectivity rate of IRE therapy ranged from 74% to 100% (49, 54, 55, 61). The main complications were bleeding, gastric ulceration, liver abscess, and myocardial infarction, and the highest incidence of complications was 40%. The average recurrence rate in these studies was higher than 20%. Only one study (62) reported no recurrence during the follow-up period of 7 months. A pilot study (63) demonstrated that IRE is a viable treatment option for hilar cholangiocarcinoma. Regarding the effect of IRE on locally advanced pancreatic cancer (LAPC), 13 studies (38, 56, 57, 62, 64–72) involving 391 patients have shown that the effectivity rate is more than 80% and even 100% (56, 62, 64, 66, 70) in some documents. The most attainable goal of IRE in the management of LAPC is the obvious palliation of symptoms. Investigators have carefully weighed the survival benefit and treatment-related complications (50, 54, 56, 67). Complications occurred in the studies, including upper gastrointestinal bleeding (38, 65, 67, 70, 73), bleeding duodenal ulcers (68), pancreatic fistulas (70), acute pancreatitis (57, 64, 67, 69), internal fistulas in the duodenum (66), and portal vein thrombosis (70), with incidence rates ranging from 10% to 40%. However, the recurrence rate was documented to be as high as 58% with a follow-up time of 18 months (65). Additionally, IRE is also increasingly being noted by researchers as a treatment for small-cell renal cancer. Two studies involving 22 patients reported effectivity rates of 57.1% and 93.3% (74, 75). One study on prostate cancer showed comparable efficacy of IRE to standard radical prostatectomy in terms of 5-year recurrence rate, and IRE showed better preservation of urogenital function (76). Two studies found good therapeutic value of IRE in metastatic cancer, including bilateral lung metastasis of osteosarcoma (77) and retroperitoneal metastasis of ovarian, gastric, and pancreatic cancer (78). Another study confirmed that IRE possesses an acceptable safety profile and is effective in eradicating difficult-to-reach colorectal liver metastases (CRLMs) (61), while another study showed that IRE is not effective for the treatment of lung malignancies (59).
Among the clinical trials, most studies considered CT or MRI as the primary modality for assessing IRE efficacy and possible complications. The complication incidence and tumor recurrence rates were associated with the duration of follow-up and the situation of the patients, but the effectiveness and safety of IRE in the treatment of hepatocellular carcinoma (HCC) and LAPC have been verified in extensive clinical trials. However, the high recurrence rate requires caution, as recurrent tumors always exhibit more aggressive behaviors (79). Therefore, combining IRE with other therapies may be an important strategy for achieving extended survival benefits.
Characteristics of the Immune Response in Tumors Induced by IRE
IRE Induces a Much Stronger Immune Response Than Other Ablation Therapies
The biological effects of IRE are based on the microsecond PEF. The microsecond EP action is extremely short, and rare heat is generated during this process, which can be diffused and absorbed rapidly (80). Therefore, IRE, independent of thermal effects, is a non-thermal ablation technique that only acts on the lipid bilayer of the cell membrane and has little effect on other molecules, such as membrane proteins and intracellular macromolecules (2, 11). In contrast, cryoablation and thermal ablation lead to protein denaturation, resulting in changes in tumor antigenicity (81). One study collected and analyzed cell lysates of B16 melanoma cells after exposure to heat (50°C, 30 min), cold (−80°C, 30 min), and IRE (1,250 V/cm, 99 pulses, 50 ms pulses, 1 Hz interval). The researchers found that IRE released the most protein and tyrosinase-related protein-2 antigen (TRP-2). IRE dramatically outperformed both cold and heat in T-cell activation (82). Another clinical study found that the levels of macrophage migration inhibitory factor (MIF) in the serum of liver patients increased significantly after IRE treatment compared with those in patients in the radiofrequency ablation (RFA) group. The axial diameter and area of the tumor ablation zone of the IRE group were significantly smaller than those of the RFA group after 1 year. This result was attributed to the immediate increase in IRE-mediated release of MIF, which promoted early tissue repair and shrinkage of the ablation zone (83). Bulvik et al. ablated normal liver tissues via RFA or IRE and found that RFA-treated liver tissues formed a clear inflammatory margin around the ablated area, whereas in IRE-ablated tissues, inflammatory cells could infiltrate and penetrate into the entire ablated area, with immune cells distributed along the residual blood vessels. The level of secretion of IL-6 in the IRE group was 3.3 times higher than that in the RFA group. The researchers further investigated the ablation effects of IRE and RFA in a mouse model of hepatocellular carcinoma. The results showed that IRE was more effective than RFA in ablating localized liver tumor tissue, and it also inhibited subcutaneously transplanted tumors in distant sites. The density of infiltrating immune cells was positively correlated with serum IL-6 levels (84). A subcutaneous pancreatic cancer mouse model study found that the number of infiltrating CD3+ T cells was significantly higher in the ablated tumor tissue at 6, 12, and 24 h in the IRE group than in the cryoablation group, and there were many more infiltrating macrophages in the IRE-ablated tumor tissue at 12 and 24 h than in the cryoablated tumor tissue (85). These results suggested that IRE can arouse a more robust immune response than other ablative therapies, and this focal ablated therapy can be designed to prime the immune system to function in concert with immunotherapies to eventually achieve improved and durable cancer treatment in vivo.
The Local Immune Responses in Tumors Induced by IRE
Table 1 summarizes the characteristics of local and systematic immune responses induced by IRE in preclinical and clinical studies in different solid tumors. Exploration of the change in the local tumor immune microenvironment (TIME) requires in-situ tumor tissue, the obtainment of which may result in new trauma to patients, and studies analyzing the local TIME have mostly used animal models. Several studies (86–89) of liver tumor model rats and mice (86–89) and dogs (90) showed an increased density of localized CD3+ T cells (90), CD8+ T cells (86, 87, 89), dendritic cells (DCs) (86), and macrophages (88) and a decreased number of Treg cells (86, 87) and PD-1+ T cells (86) in the tumor tissue after IRE treatment. Two studies of IRE ablation of pancreatic cancer in mice (85, 91) indicated increased infiltration of CD3+ T cells (85), CD45+ T cells (91), CD8+ T cells (91), and macrophages (85) and higher levels of IFN-γ (91), CCL1 (91), and IL-2 (91) in local residual tumors of the IRE group. In contrast, the levels of cells such as myeloid-derived suppressor cells (MDSCs) (91) and cytokines such as IL-4 (91) and IL-6 (91) with tumor-promoting effects were decreased in the IRE-treated group.
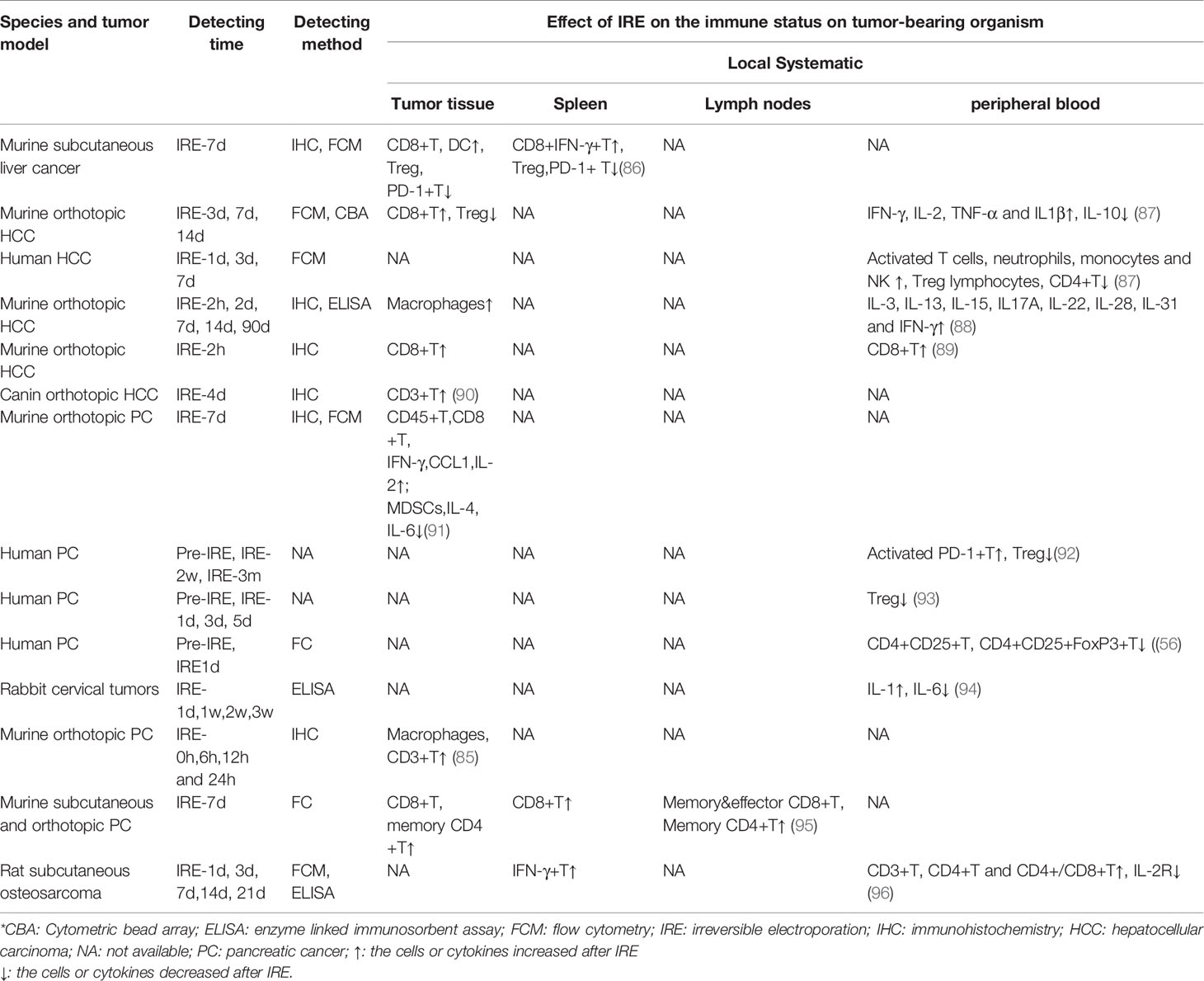
Table 1 The characteristics of local and systematic immune responses induced by IRE in preclinical and clinical study of different tumor.
Systematic Immune Responses in Tumors Induced by IRE
We summarized the changes in immune cells and cytokines in the peripheral blood, lymph nodes, and spleen of tumor-bearing organisms after IRE treatment to analyze the characteristics of the IRE-mediated systemic immune response. There have been five studies (86–90) involving mouse and rat liver cancer models (86–89) and a dog liver cancer model (90) and one clinical study on liver cancer patients (87). The results showed an increased frequency of CD8+IFN-γ+ T cells (86) and a decreased number of Treg and PD-1+ T cells (86) in spleens in the IRE group. There was a higher proportion of CD8+ T cells (89), IFN-γ+ T cells (87, 88), neutrophils, monocytes, and NK cells (87) as well as higher levels of IL-2 (87), IL-3 (88), IL-13 (88), IL-15 (88), IL-17a (88), IL-22 (88), IL-28 (88), IL-31 (88), TNF-α (87), and IL-1β (87) and lower concentrations of CD4+ T cells (87), Treg cells (87), and IL-10 (87) in the peripheral blood of the IRE-treated group than in the control group.
Three murine pancreatic cancer model studies and three clinical studies of pancreatic cancer patients found that activated PD-1+ T cells (92) were increased and Treg cells (92, 93) were decreased in the peripheral blood. Moreover, there was an increased number of memory CD4+ T and CD8+ T cells as well as effector CD8+ T cells in the lymph nodes (94) after IRE treatment. A study of 20 rabbits with cervical cancer (95) found increased levels of IL-1 and IL-6 in peripheral blood after IRE ablation of tumors. Another study of osteosarcoma in rats (96) showed increased IFN-γ+ cells in the spleen and increased CD3+ T, CD4+ T, and CD4+/CD8+ T cells as well as decreased levels of IL-2R in the peripheral blood.
These results acknowledged that IRE induces obviously cellular immune responses in the local TIME as well as in systemic immune organs in tumor-bearing organisms. Most of the results suggested that IRE enhanced the density of immune cells and cytokines with antitumor effects while reducing the level of these cytokines with tumor-promoting effects. From the view of cancer-immune phenotypes (97), IRE can induce an immune-inflamed phenotype in the local tumor microenvironment (TME), and this profile suggests a clinical response to anti-PD-L1/PD-1 therapy (98, 99).
IRE Induces an “In-Situ Tumor Vaccine” Effect
Ablative techniques induce antitumor immune responses by increasing the availability of tumor-specific antigens in an inflammatory context (100, 101). The specific antigens released from tumor cells are processed and presented by antigen-presenting cells, which enhance or induce an antitumor T-cell response (102). IRE was reported to significantly improve antitumor efficacy in immunocompetent mice but not in immunodeficient mice (91, 103). The immunocompetent tumor-bearing mice were rechallenged with the same cell line after 18 days of IRE treatment, and the growth of the second tumors was shown to be significantly reduced or entirely prevented. There was robust CD3+ cell infiltration in some treated mice, with immunocytes focused at the transition between viable and dead tumor cells. However, none of this was observed in immunodeficient mice (103). A hepatocellular carcinoma animal study showed that IRE-treated mice were tumor free after secondary tumor injection and showed increased splenic CD8+IFN-γ+ T cells. Depletion of CD8+ T cells induces local tumor regrowth and distant metastasis after IRE. In addition, inoculation of IRE-processed H22 (hepatocellular carcinoma cell line) cell lysates also prevented tumorigenesis in mice (86). Another study of orthotopic pancreatic cancer models found that an abscopal antitumor effect can be achieved after in-situ tumor ablation with IRE or exposure to the tumor culture supernatant of IRE-treated Panc02 cells (pancreatic cancer cell line) (95). The same animal model study showed that IRE can act as an “in-situ vaccine,” generating neoantigen-specific T cells that confer protection against tumor growth by adoptive cell transfer into treatment-naive immunocompromised mice (91). These results suggest that IRE can enhance tumor immunogenicity and increase the recognition of tumor antigens by the immune system to serve as a tumor vaccine.
Mechanisms Related to the IRE-Induced Immune Response In Tumors
IRE Mediates the Release of Damage-Related Molecular Patterns
Studies have proven that IRE can increase the synthesis and secretion of endogenous danger signaling molecules, namely, damage-associated molecular patterns (DAMPs), from damaged cells. DAMPs include ATP, high-mobility group B1 (HMGB1), calreticulin, and heat shock proteins (HSPs) and induce immunogenic cell death (ICD) (95, 104, 105). DCs resident in tumor tissues can take up these DAMPs and migrate to draining lymph nodes, activate tumor antigen-specific T cells, and affect the expansion of immunosuppressive T cells. Activated T cells home to remote sites to eliminate metastases and inhibit tumor progression (106–108). A study verified that the release of DAMPs increases with increasing EFS, and the DAMP secretion level is positively correlated with cell death (105). He et al. found that IRE increased the release of HMGB1, which activated the MAPK–p38 (mitogen activated protein kinases–p38) pathway by binding to the receptor for advanced glycation end products (RAGE), resulting in M1 macrophage polarization. In addition, M1 macrophage polarization was enhanced by the positive feedback-induced release or expression of HMGB1 and RAGE through the MAPK–ERK (MAPK–extracellular signal-regulated kinase) pathway in macrophages (104). The researchers also found that IRE-induced synthesis and secretion of HMGB1 promote specific T-cell infiltration and enhance immune memory. IRE not only led to complete tumor regression in situ but also induced an abscopal effect, suppressing the growth of latent lesions (95). Thymic stromal lymphopoietin (TSLP) has been demonstrated to drive immune cell polarization into the cancer-promoting Th2 immunophenotype in a variety of tumors (109, 110). Goswami et al. found that IRE inhibited the expression of TSLP in the TME of breast cancer in mice and humans and prevented immunosuppressive evolution of the TME (111). Figure 2 depicts the reported mechanisms involved in IRE-induced immune responses.
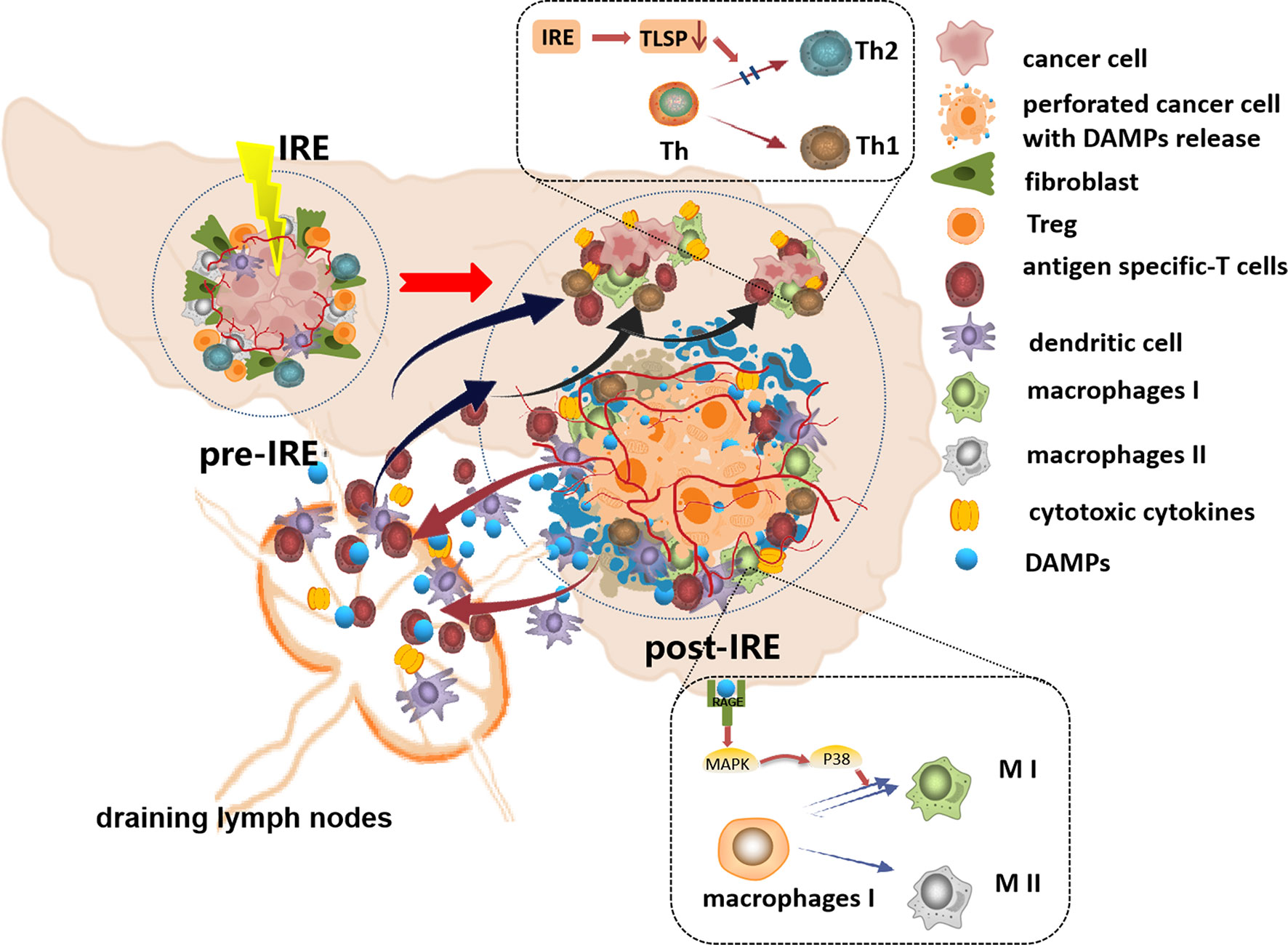
Figure 2 The schematic diagram of the reported mechanisms involved in IRE-induced immune responses. IRE increases the synthesis and secretion of damage-associated molecular patterns (DAMPs), and the DC cells take up these DAMPs, migrate to draining lymph nodes, and then activate tumor antigen-specific T cells, and the activated T cells home to tumor sites to eliminate the residual tumor cells. The DAMPs activated the MAPK–p38 pathway by binding to RAGE, resulting in M1 macrophage polarization. IRE inhibited TSLP in the TME preventing Th2 polarization. Additionally, IRE softens the ECM, increases the density and permeability of tumor vessels, and facilitates the infiltration of immune cells into residual tumor tissues.
IRE Remodels the Tumor Microenvironment
As IRE is non-thermal, it maintains important ECM structures and preserves the complete structure of blood vessels in the tumor tissue, which can prompt the infiltration of subsequently primed effector T cells to the residual ablated tumor site (30, 84). A recent study found that microvessel density and permeability increased in the viable margin of tumor tissue after IRE. The microvessel density gradually decreased after 6 days of IRE ablation and returned to the baseline level on IRE-9d. In addition, the percentages of cells expressing the hypoxia markers hypoxia-inducible factor 1-α (HIF1-α) and carbonic anhydrase-9 (CA-IX) in the residual tumor area after IRE-4d were 53% and 24%, respectively, compared with the control group, respectively. The percentages of cells expressing hyaluronate-binding protein-1 (HABP1) (a marker of stromal hyaluronic acid) and lysine oxidase (LOX) (a marker of extracellular matrix stiffness) were 70% and 41%, respectively, compared with the control group. These proteins gradually increased after IRE-6d (108), suggesting that IRE can transiently improve the TME by increasing the density and permeability of tumor vessels, softening the ECM, and relieving hypoxia, which facilitated the infiltration of immune cells into residual tumor tissues (112, 113).
One study found that IRE triggered reactive oxygen species (ROS)-dependent apoptosis in pancreatic cancer cells mediated by inhibition of the PI3K–Akt pathway. This efficacy was synergistically enhanced by IRE combined with M1 virus administration (114). Another study on liver cancer showed that nsPEF induced the translocation and release of PD-L1 from the hepatoma cell membrane and promoted CD8+ T-cell dysfunction, and blocking PD-L1 effectively inhibited tumor growth and improved the survival of tumor-bearing mice (115). IRE-induced immune responses have only recently been of interest to researchers, and the number of relevant studies is fewer than studies of the therapeutic effect of IRE. The various mechanisms should be a focus of future studies to provide a sufficient theoretical basis for clinical application.
Therapeutic Effects of IRE Combined With Immunotherapy on Solid Tumors
A clinical study (92) evaluated the immunomodulatory effect of IRE to identify an ideal time point for potential adjuvant immunotherapy. The result suggested that most IRE treatment-mediated Treg attenuation occurred between 3 and 5 days after IRE ablation, which provided a window for potentiating clinical efficacy in combination with immunotherapy. Many preclinical and clinical studies have confirmed the favorable efficacy of combination therapy. Table 2 summarizes the detailed information on the effect of IRE combined with different immunotherapies on the survival and immune status of tumor-bearing models. Murine orthotopic pancreatic cancer studies explored the efficacy of IRE combined with DC vaccination (116), a PD-1 inhibitor (91), and a PD-1 inhibitor combined with a Toll-like receptor-7 (TLR7) agonist (108). The results showed that the combination therapy significantly prolonged overall survival and improved the immune status, enabling antitumor effects in the tumor-bearing mice. Similarly, four clinical studies also verified the outcome benefits and enhanced immune status induced by IRE combined with immunotherapy agents or cells in advanced pancreatic cancer patients (23, 117–119). In addition, some animal studies have shown that IRE combined with immune checkpoint inhibitors (ICIs) and immunostimulants can augment immune status and inhibit the growth of tumors transplanted in the liver (120, 121), skin (121), and prostate (21), yielding extended survival benefits. Two clinical studies found that the IRE and allogenic natural killer cell immunotherapy combination is a promising strategy to enhance antitumor efficacy in advanced hepatocellular carcinoma patients (122, 123). These encouraging results will prompt the development of a combination therapeutic strategy for the treatment of cancer patients refractory to other therapies.
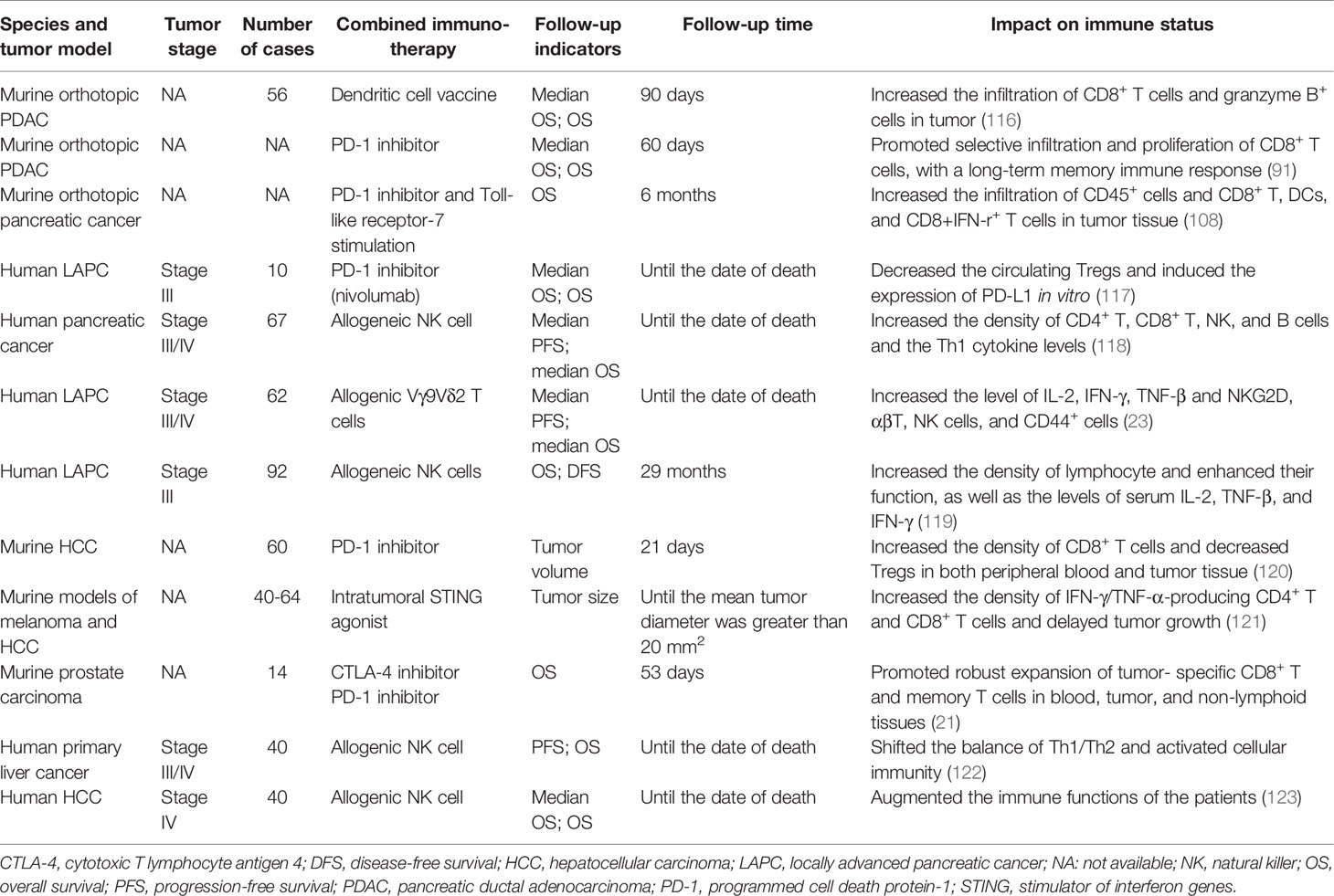
Table 2 The information of the effect of IRE combined with different immunotherapies on the survival and immune status of tumor-bearing body.
Concluding Remarks
By analyzing the characteristics and related mechanisms of the IRE-mediated immune response, we found that IRE can overcome an immunosuppressive TME, enhance tumor immunogenicity, and activate the cellular and humoral antitumor immune responses of the body, which induces an “in-situ vaccination” effect. However, there are still some issues that deserve attention in future studies.
The immune response induced by IRE is significantly different or even contradictory in many preclinical studies. The main reasons for these differences may result from the following reasons. a) The methods for establishing tumor models are different. Subcutaneous and orthotopic xenograft models are mostly used in preclinical studies, while skin and digestive tract tissues originate from the ectoderm and endoderm, respectively. Therefore, their TMEs are significantly different, which leads to different immune responses induced by IRE in the same type of tumor with the same treatment parameters (95, 124). Undoubtedly, animal orthotopic xenograft models are more accurate and suitable for exploring the immune response of tumor-bearing organisms in subsequent studies. b) Different tumor cell lines and mouse strains are used in some studies. In addition, the immune rejection response was not considered in these studies. For example, the mouse hepatoma H22 cell line originates from C3HA mice but is mostly established in C57BL/6 and BALB/c mice to generate hepatoma models. Coincidentally, IRE can always induce significant immune responses in such animal models (86, 120, 124). Therefore, mouse strains and cell-derived mouse strains for animal research must be cautiously selected. c) Several studies have explored the immune response of tumor-bearing models after IRE combined with other therapies (91, 108), while the effect of other therapies on the immune response remains to be further refined and distinguished. In addition, the tumor tissue sampling site, the detection time after IRE treatment, the detection method used, and the immune markers selected to evaluate the immune status can lead to different results. Therefore, investigators should comprehensively consider the relevant influencing factors to ensure the reliability and accuracy of the study results.
Some clinical studies have shown that IRE combined with immunotherapy is effective in prolonging OS in cancer patients (21, 23, 108, 118). However, there is still a lack of large-scale clinical data to support the clinical application of IRE. Future preclinical studies still need to deeply explore the related mechanisms of IRE to determine the optimal strategy of IRE combined with immunotherapy. In addition, for the application of IRE combined immunotherapy, clinical studies need to focus on the selection of patients and explore the best strategy (including timing and dosage) for combining the two approaches.
In summary, with the increasing number of ongoing animal and clinical studies on IRE and its combination with immunotherapy, as well as the development and optimization of IRE instruments, IRE combined with immunotherapy possesses great potential to become a promising choice for patients with unresectable tumors that can benefit more cancer patients.
Author Contributions
NZ and ZQL retrieved, sorted, and summarized all the related literature and drafted the manuscript. XH and ZZ reviewed and edited the manuscript. YZ and ZhujL helped trace the related paper and edited the manuscript. ZhijL and YL conceived the topic and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China under Grant No. 81727802.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the website of https://www.iconfont.cn/ and ScienceSlides software for providing free icons and cell morphological elements that used to complete our Figures 1 and 2.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.811726/full#supplementary-material
References
1. Geboers B, Scheffer HJ, Graybill PM, Ruarus AH, Nieuwenhuizen S, Puijk RS, et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology (2020) 295(2):254–72. doi: 10.1148/radiol.2020192190
2. Kotnik T, Rems L, Tarek M, Miklavčič D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu Rev Biophys (2019) 48:63–91. doi: 10.1146/annurev-biophys-052118-115451
3. Reddy VY, Anic A, Koruth J, Petru J, Funasako M, Minami K, et al. Pulsed Field Ablation in Patients With Persistent Atrial Fibrillation. J Am Coll Cardiol (2020) 76(9):1068–80. doi: 10.1016/j.jacc.2020.07.007
4. Di Biase L, Diaz JC, Zhang X-D, Romero J. Pulsed Field Catheter Ablation in Atrial Fibrillation. Trends Cardiovasc Med (2021) 21:S1050–738. doi: 10.1016/j.tcm.2021.07.006
5. Garner AL. Pulsed Electric Field Inactivation of Microorganisms: From Fundamental Biophysics to Synergistic Treatments. Appl Microbiol Biotechnol (2019) 103(19):7917–29. doi: 10.1007/s00253-019-10067-y
6. Miller L, Leor J, Rubinsky B. Cancer Cells Ablation With Irreversible Electroporation. Technol Cancer Res Treat (2005) 4(6):699–705. doi: 10.1177/153303460500400615
7. Sutter O, Calvo J, N’Kontchou G, Nault J-C, Ourabia R, Nahon P, et al. Safety and Efficacy of Irreversible Electroporation for the Treatment of Hepatocellular Carcinoma Not Amenable to Thermal Ablation Techniques: A Retrospective Single-Center Case Series. Radiology (2017) 284(3):877–86. doi: 10.1148/radiol.2017161413
8. Collettini F, Enders J, Stephan C, Fischer T, Baur ADJ, Penzkofer T, et al. Image-Guided Irreversible Electroporation of Localized Prostate Cancer: Functional and Oncologic Outcomes. Radiology (2019) 292(1):250–7. doi: 10.1148/radiol.2019181987
9. Ruarus AH, Vroomen LGPH, Geboers B, van Veldhuisen E, Puijk RS, Nieuwenhuizen S, et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology (2020) 294(1):212–20. doi: 10.1148/radiol.2019191109
10. DeWitt MR, Latouche EL, Kaufman JD, Fesmire CC, Swet JH, Kirks RC, et al. Simplified Non-Thermal Tissue Ablation With a Single Insertion Device Enabled by Bipolar High-Frequency Pulses. IEEE Trans BioMed Eng (2020) 67(7):2043–51. doi: 10.1109/TBME.2019.2954122
11. Lv Y, Zhang Y, Huang J, Wang Y. Rubinsky B. A Study on Nonthermal Irreversible Electroporation of the Thyroid. Technol Cancer Res Treat (2019) 18:1533033819876307. doi: 10.1177/1533033819876307
12. Liu Z-G, Chen X-H, Yu Z-J, Lv J, Ren Z-G. Recent Progress in Pulsed Electric Field Ablation for Liver Cancer. World J Gastroenterol (2020) 26(24):3421–31. doi: 10.3748/wjg.v26.i24.3421
13. Al Efishat M, Wolfgang CL, Weiss MJ. Stage III Pancreatic Cancer and the Role of Irreversible Electroporation. BMJ (2015) 350:h521. doi: 10.1136/bmj.h521
14. Lochab V, Jones TH, Alkandry E, West JD, Abdel-Rahman MH, Subramaniam VV, et al. Evaluation of Electrical Properties of Ex Vivo Human Hepatic Tissue With Metastatic Colorectal Cancer. Physiol Meas (2020) 41(8):085005. doi: 10.1088/1361-6579/abaa55
15. Wang Y, Shao Q, Van de Moortele P-F, Racila E, Liu J, Bischof J, et al. Mapping Electrical Properties Heterogeneity of Tumor Using Boundary Informed Electrical Properties Tomography (BIEPT) at 7T. Magn Reson Med (2019) 81(1):393–409. doi: 10.1002/mrm.27414
16. Geboers B, Ruarus AH, Nieuwenhuizen S, Puijk RS, Scheffer HJ, de Gruijl TD, et al. Needle-Guided Ablation of Locally Advanced Pancreatic Cancer: Cytoreduction or Immunomodulation by In Vivo Vaccination? Chin Clin Oncol (2019) 8(6):61. doi: 10.21037/cco.2019.10.05
17. Geboers B, Timmer FEF, Ruarus AH, Pouw JEE, Schouten EAC, Bakker J, et al. Irreversible Electroporation and Nivolumab Combined With Intratumoral Administration of a Toll-Like Receptor Ligand, as a Means of In Vivo Vaccination for Metastatic Pancreatic Ductal Adenocarcinoma (PANFIRE-III). A Phase-I Study Protocol. Cancers (Basel) (2021) 13(15):3902. doi: 10.3390/cancers13153902
18. Boshuizen J, Peeper DS. Rational Cancer Treatment Combinations: An Urgent Clinical Need. Mol Cell (2020) 78(6):1002–18. doi: 10.1016/j.molcel.2020.05.031
19. Brock RM, Beitel-White N, Davalos RV, Allen IC. Starting a Fire Without Flame: The Induction of Cell Death and Inflammation in Electroporation-Based Tumor Ablation Strategies. Front Oncol (2020) 10:1235. doi: 10.3389/fonc.2020.01235
20. Tian G, Guan J, Chu Y, Zhao Q, Jiang T. Immunomodulatory Effect of Irreversible Electroporation Alone and Its Cooperating With Immunotherapy in Pancreatic Cancer. Front Oncol (2021) 11:712042. doi: 10.3389/fonc.2021.712042
21. Alnaggar M, Lin M, Mesmar A, Liang S, Qaid A, Xu K, et al. Allogenic Natural Killer Cell Immunotherapy Combined With Irreversible Electroporation for Stage IV Hepatocellular Carcinoma: Survival Outcome. Cell Physiol Biochem (2018) 48(5):1882–93. doi: 10.1159/000492509
22. Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, et al. Short-Term Clinical Efficacy of Percutaneous Irreversible Electroporation Combined With Allogeneic Natural Killer Cell for Treating Metastatic Pancreatic Cancer. Immunol Lett (2017) 186:20–7. doi: 10.1016/j.imlet.2017.03.018
23. Lin M, Zhang X, Liang S, Luo H, Alnaggar M, Liu A, et al. Irreversible Electroporation Plus Allogenic Vγ9vδ2 T Cells Enhances Antitumor Effect for Locally Advanced Pancreatic Cancer Patients. Signal Transduct Target Ther (2020) 5(1):215. doi: 10.1038/s41392-020-00260-1
24. Kumar P, Nagarajan A, Uchil PD. Electroporation. Cold Spring Harb Protoc (2019) 2019(7):519–25. doi: 10.1101/pdb.top096271
25. Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene Transfer Into Mouse Lyoma Cells by Electroporation in High Electric Fields. EMBO J (1982) 1(7):841–5. doi: 10.1002/j.1460-2075.1982.tb01257.x
26. Kotnik T, Frey W, Sack M, Haberl Meglič S, Peterka M, Miklavčič D. Electroporation-Based Applications in Biotechnology. Trends Biotechnol (2015) 33(8):480–8. doi: 10.1016/j.tibtech.2015.06.002
27. Gehl J. Electroporation: Theory and Methods, Perspectives for Drug Delivery, Gene Therapy and Research. Acta Physiol Scand (2003) 177(4):437–47. doi: 10.1046/j.1365-201X.2003.01093.x
28. Mir LM. Therapeutic Perspectives of In Vivo Cell Electropermeabilization. Bioelectrochemistry (2001) 53(1):1–10. doi: 10.1016/S0302-4598(00)00112-4
29. Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu Rev BioMed Eng (2014) 16:295–320. doi: 10.1146/annurev-bioeng-071813-104622
30. Rubinsky B, Onik G, Mikus P. Irreversible Electroporation: A New Ablation Modality–Clinical Implications. Technol Cancer Res Treat (2007) 6(1):37–48. doi: 10.1177/153303460700600106
31. Davalos RV, Mir ILM, Rubinsky B. Tissue Ablation With Irreversible Electroporation. Ann BioMed Eng (2005) 33(2):223–31. doi: 10.1007/s10439-005-8981-8
32. Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In Vivo Results of a New Focal Tissue Ablation Technique: Irreversible Electroporation. IEEE Trans BioMed Eng (2006) 53(7):1409–15. doi: 10.1109/TBME.2006.873745
33. Al-Sakere B, Bernat C, Andre F, Connault E, Opolon P, Davalos RV, et al. A Study of the Immunological Response to Tumor Ablation With Irreversible Electroporation. Technol Cancer Res Treat (2007) 6(4):301–6. doi: 10.1177/153303460700600406
34. Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible Electroporation of the Pancreas in Swine: A Pilot Study. HPB (Oxford) (2010) 12(5):348–51. doi: 10.1111/j.1477-2574.2010.00174.x
35. Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, et al. Tumor Ablation With Irreversible Electroporation. PloS One (2007) 2(11):e1135. doi: 10.1371/journal.pone.0001135
36. Bertacchini C, Margotti PM, Bergamini E, Lodi A, Ronchetti M, Cadossi R. Design of an Irreversible Electroporation System for Clinical Use. Technol Cancer Res Treat (2007) 6(4):313–20. doi: 10.1177/153303460700600408
37. Pech M, Janitzky A, Wendler JJ, Strang C, Blaschke S, Dudeck O, et al. Irreversible Electroporation of Renal Cell Carcinoma: A First-in-Man Phase I Clinical Study. Cardiovasc Intervent Radiol (2011) 34(1):132–8. doi: 10.1007/s00270-010-9964-1
38. Martin RCG, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, et al. Treatment of 200 Locally Advanced (Stage III) Pancreatic Adenocarcinoma Patients With Irreversible Electroporation: Safety and Efficacy. Ann Surg (2015) 262(3):486–94. doi: 10.1097/SLA.0000000000001441
39. Kluger MD, Rashid MF, Rosario VL, Schrope BA, Steinman JA, Hecht EM, et al. Resection of Locally Advanced Pancreatic Cancer Without Regression of Arterial Encasement After Modern-Era Neoadjuvant Therapy. J Gastrointest Surg (2018) 22(2):235–41. doi: 10.1007/s11605-017-3556-1
40. Holland MM, Bhutiani N, Kruse EJ, Weiss MJ, Christein JD, White RR, et al. A Prospective, Multi-Institution Assessment of Irreversible Electroporation for Treatment of Locally Advanced Pancreatic Adenocarcinoma: Initial Outcomes From the AHPBA Pancreatic Registry. HPB (Oxford) (2019) 21(8):1024–31. doi: 10.1016/j.hpb.2018.12.004
41. Li Q, Gao X, Zhang Y, Han X, Li Z, Zhang Y, et al. Magnetic Anchoring and Guidance-Assisted Endoscopic Irreversible Electroporation for Gastric Mucosal Ablation: A Preclinical Study in Canine Model. Surg Endosc (2021) 35(10):5665–74. doi: 10.1007/s00464-020-08245-5
42. Jeon HJ, Choi HS, Keum B, Bang EJ, Lee KW, Kim SH, et al. Feasibility and Effectiveness of Endoscopic Irreversible Electroporation for the Upper Gastrointestinal Tract: An Experimental Animal Study. Sci Rep (2021) 11(1):15353. doi: 10.1038/s41598-021-94583-w
43. Maor E, Ivorra A, Rubinsky B. Non Thermal Irreversible Electroporation: Novel Technology for Vascular Smooth Muscle Cells Ablation. PloS One (2009) 4(3):e4757. doi: 10.1371/journal.pone.0004757
44. Jiang C, Davalos RV, Bischof JC. A Review of Basic to Clinical Studies of Irreversible Electroporation Therapy. IEEE Trans BioMed Eng (2015) 62(1):4–20. doi: 10.1109/TBME.2014.2367543
45. Wood LSY, Dunn JCY. Irreversible Electroporation for De-Epithelialization of Murine Small Intestine. J Surg Res (2020) 256:602–10. doi: 10.1016/j.jss.2020.07.034
46. Rossmeisl JH, Garcia PA, Pancotto TE, Robertson JL, Henao-Guerrero N, Neal RE, et al. Safety and Feasibility of the NanoKnife System for Irreversible Electroporation Ablative Treatment of Canine Spontaneous Intracranial Gliomas. J Neurosurg (2015) 123(4):1008–25. doi: 10.3171/2014.12.JNS141768
47. Chang WC, Hawkes EA, Kliot M, Sretavan DW. In Vivo Use of a Nanoknife for Axon Microsurgery. Neurosurgery (2007) 61(4):683–91. doi: 10.1227/01.NEU.0000298896.31355.80
48. Giorgio A, Amendola F, Calvanese A, Ingenito E, Santoro B, Gatti P, et al. Ultrasound-Guided Percutaneous Irreversible Electroporation of Hepatic and Abdominal Tumors Not Eligible for Surgery or Thermal Ablation: A Western Report on Safety and Efficacy. J Ultrasound (2019) 22(1):53–8. doi: 10.1007/s40477-019-00372-7
49. Kalra N, Gupta P, Gorsi U, Bhujade H, Chaluvashetty SB, Duseja A, et al. Irreversible Electroporation for Unresectable Hepatocellular Carcinoma: Initial Experience. Cardiovasc Intervent Radiol (2019) 42(4):584–90. doi: 10.1007/s00270-019-02164-2
50. Frühling P, Nilsson A, Duraj F, Haglund U, Norén A. Single-Center Nonrandomized Clinical Trial to Assess the Safety and Efficacy of Irreversible Electroporation (IRE) Ablation of Liver Tumors in Humans: Short to Mid-Term Results. Eur J Surg Oncol (2017) 43(4):751–7. doi: 10.1016/j.ejso.2016.12.004
51. Niessen C, Thumann S, Beyer L, Pregler B, Kramer J, Lang S, et al. Percutaneous Irreversible Electroporation: Long-Term Survival Analysis of 71 Patients With Inoperable Malignant Hepatic Tumors. Sci Rep (2017) 7:43687. doi: 10.1038/srep43687
52. Distelmaier M, Barabasch A, Heil P, Kraemer NA, Isfort P, Keil S, et al. Midterm Safety and Efficacy of Irreversible Electroporation of Malignant Liver Tumors Located Close to Major Portal or Hepatic Veins. Radiology (2017) 285(3):1023–31. doi: 10.1148/radiol.2017161561
53. Sugimoto K, Moriyasu F, Kobayashi Y, Saito K, Takeuchi H, Ogawa S, et al. Irreversible Electroporation for Nonthermal Tumor Ablation in Patients With Hepatocellular Carcinoma: Initial Clinical Experience in Japan. Jpn J Radiol (2015) 33(7):424–32. doi: 10.1007/s11604-015-0442-1
54. Cannon R, Ellis S, Hayes D, Narayanan G, Martin RCG. Safety and Early Efficacy of Irreversible Electroporation for Hepatic Tumors in Proximity to Vital Structures. J Surg Oncol (2013) 107(5):544–9. doi: 10.1002/jso.23280
55. Kingham TP, Karkar AM, D’Angelica MI, Allen PJ, Dematteo RP, Getrajdman GI, et al. Ablation of Perivascular Hepatic Malignant Tumors With Irreversible Electroporation. J Am Coll Surg (2012) 215(3):379–87. doi: 10.1016/j.jamcollsurg.2012.04.029
56. Sugimoto K, Moriyasu F, Tsuchiya T, Nagakawa Y, Hosokawa Y, Saito K, et al. Irreversible Electroporation for Nonthermal Tumor Ablation in Patients With Locally Advanced Pancreatic Cancer: Initial Clinical Experience in Japan. Intern Med (2018) 57(22):3225–31. doi: 10.2169/internalmedicine.0861-18
57. Lambert L, Horejs J, Krska Z, Hoskovec D, Petruzelka L, Krechler T, et al. Treatment of Locally Advanced Pancreatic Cancer by Percutaneous and Intraoperative Irreversible Electroporation: General Hospital Cancer Center Experience. Neoplasma (2016) 63(2):269–73. doi: 10.4149/213_150611N326
58. Eller A, Schmid A, Schmidt J, May M, Brand M, Saake M, et al. Local Control of Perivascular Malignant Liver Lesions Using Percutaneous Irreversible Electroporation: Initial Experiences. Cardiovasc Intervent Radiol (2015) 38(1):152–9. doi: 10.1007/s00270-014-0898-x
59. Ricke J, Jürgens JHW, Deschamps F, Tselikas L, Uhde K, Kosiek O, et al. Irreversible Electroporation (IRE) Fails to Demonstrate Efficacy in a Prospective Multicenter Phase II Trial on Lung Malignancies: The ALICE Trial. Cardiovasc Intervent Radiol (2015) 38(2):401–8. doi: 10.1007/s00270-014-1049-0
60. Melenhorst MCAM, Scheffer HJ, Vroomen LGPH, Kazemier G, van den Tol MP, Meijerink MR. Percutaneous Irreversible Electroporation of Unresectable Hilar Cholangiocarcinoma (Klatskin Tumor): A Case Report. Cardiovasc Intervent Radiol (2016) 39(1):117–21. doi: 10.1007/s00270-015-1126-z
61. Meijerink MR, Ruarus AH, Vroomen LGPH, Puijk RS, Geboers B, Nieuwenhuizen S, et al. Irreversible Electroporation to Treat Unresectable Colorectal Liver Metastases (COLDFIRE-2): A Phase II, Two-Center, Single-Arm Clinical Trial. Radiology (2021) 299(2):470–80. doi: 10.1148/radiol.2021203089
62. Wichtowski M, Nowaczyk P, Kocur J, Murawa D. Irreversible Electroporation in the Treatment of Locally Advanced Pancreas and Liver Metastases of Colorectal Carcinoma. Contemp Oncol (Pozn) (2016) 20(1):39–44. doi: 10.5114/wo.2016.57815
63. Hsiao C-Y, Yang P-C, Li X, Huang K-W. Clinical Impact of Irreversible Electroporation Ablation for Unresectable Hilar Cholangiocarcinoma. Sci Rep (2020) 10(1):10883. doi: 10.1038/s41598-020-67772-2
64. Kwon JH, Chung MJ, Park JY, Lee HS, Hwang HK, Kang CM, et al. Initial Experience of Irreversible Electroporation for Locally Advanced Pancreatic Cancer in a Korean Population. Acta Radiol (2021) 62(2):164–71. doi: 10.1177/0284185120917118
65. Kluger MD, Epelboym I, Schrope BA, Mahendraraj K, Hecht EM, Susman J, et al. Single-Institution Experience With Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann Surg Oncol (2016) 23(5):1736–43. doi: 10.1245/s10434-015-5034-x
66. Paiella S, Butturini G, Frigerio I, Salvia R, Armatura G, Bacchion M, et al. Safety and Feasibility of Irreversible Electroporation (IRE) in Patients With Locally Advanced Pancreatic Cancer: Results of a Prospective Study. Dig Surg (2015) 32(2):90–7. doi: 10.1159/000375323
67. Scheffer HJ, Vroomen LGPH, de Jong MC, Melenhorst MCAM, Zonderhuis BM, Daams F, et al. Ablation of Locally Advanced Pancreatic Cancer With Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology (2017) 282(2):585–97. doi: 10.1148/radiol.2016152835
68. Flak RV, Stender MT, Jensen TM, Andersen KL, Henriksen SD, Mortensen PB, et al. Treatment of Locally Advanced Pancreatic Cancer With Irreversible Electroporation - a Danish Single Center Study of Safety and Feasibility. Scand J Gastroenterol (2019) 54(2):252–8. doi: 10.1080/00365521.2019.1575465
69. Narayanan G, Hosein PJ, Beulaygue IC, Froud T, Scheffer HJ, Venkat SR, et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J Vasc Interv Radiol (2017) 28(3):342–8. doi: 10.1016/j.jvir.2016.10.023
70. Yan L, Chen Y-L, Su M, Liu T, Xu K, Liang F, et al. A Single-Institution Experience With Open Irreversible Electroporation for Locally Advanced Pancreatic Carcinoma. Chin Med J (Engl) (2016) 129(24):2920–5. doi: 10.4103/0366-6999.195476
71. Tarantino L, Nasto A, Busto G, Iovino V, Fristachi R, Bortone S. Irreversible Electroporation of Locally Advanced Solid Pseudopapillary Carcinoma of the Pancreas: A Case Report. Ann Med Surg (Lond) (2018) 28:11–5. doi: 10.1016/j.amsu.2018.01.009
72. Månsson C, Bergenfeldt M, Brahmstaedt R, Karlson B-M, Nygren P, Nilsson A. Safety and Preliminary Efficacy of Ultrasound-Guided Percutaneous Irreversible Electroporation for Treatment of Localized Pancreatic Cancer. Anticancer Res (2014) 34(1):289–93. doi: 10.1016/j.canlet.2020.09.015
73. Martin RCG, Philips P, Ellis S, Hayes D, Bagla S. Irreversible Electroporation of Unresectable Soft Tissue Tumors With Vascular Invasion: Effective Palliation. BMC Cancer (2014) 14:540. doi: 10.1186/1471-2407-14-540
74. Wang Z, Lu J, Huang W, Wu Z, Gong J, Wang Q, et al. A Retrospective Study of CT-Guided Percutaneous Irreversible Electroporation (IRE) Ablation: Clinical Efficacy and Safety. BMC Cancer (2021) 21(1):124. doi: 10.1186/s12885-021-07820-w
75. Wendler JJ, Pech M, Fischbach F, Jürgens J, Friebe B, Baumunk D, et al. Initial Assessment of the Efficacy of Irreversible Electroporation in the Focal Treatment of Localized Renal Cell Carcinoma With Delayed-Interval Kidney Tumor Resection (Irreversible Electroporation of Kidney Tumors Before Partial Nephrectomy [IRENE] Trial-An Ablate-And-Resect Pilot Study). Urology (2018) 114:224–32. doi: 10.1016/j.urology.2017.12.016
76. Guenther E, Klein N, Zapf S, Weil S, Schlosser C, Rubinsky B, et al. Prostate Cancer Treatment With Irreversible Electroporation (IRE): Safety, Efficacy and Clinical Experience in 471 Treatments. PloS One (2019) 14(4):e0215093. doi: 10.1371/journal.pone.0215093
77. Harris JC, Chen A, Macias V, Mahon B, Chiu B, Pillai S. Irreversible Electroporation as an Effective Technique for Ablating Human Metastatic Osteosarcoma. J Pediatr Hematol Oncol (2016) 38(3):182–6. doi: 10.1097/MPH.0000000000000516
78. Jiang TA, Zhao Q, Tian G, Chen X, Wu L. Irreversible Electroporation Ablation of End-Stage Metastatic Retroperitoneal Lesions: Report on Three Cases and Literature Review. Exp Ther Med (2019) 18(3):2243–9. doi: 10.3892/etm.2019.7780
79. D’Alterio C, Scala S, Sozzi G, Roz L, Bertolini G. Paradoxical Effects of Chemotherapy on Tumor Relapse and Metastasis Promotion. Semin Cancer Biol (2020) 60:351–61. doi: 10.1016/j.semcancer.2019.08.019
80. Ahmed M, Brace CL, Lee FT, Goldberg SN. Principles of and Advances in Percutaneous Ablation. Radiology (2011) 258(2):351–69. doi: 10.1148/radiol.10081634
81. Li T, Bu G, Xi G. Effects of Heat Treatment on the Antigenicity, Antigen Epitopes, and Structural Properties of β-Conglycinin. Food Chem (2021) 346:128962. doi: 10.1016/j.foodchem.2020.128962
82. Shao Q, O’Flanagan S, Lam T, Roy P, Pelaez F, Burbach BJ, et al. Engineering T Cell Response to Cancer Antigens by Choice of Focal Therapeutic Conditions. Int J Hyperthermia (2019) 36(1):130–8. doi: 10.1080/02656736.2018.1539253
83. Sugimoto K, Kakimi K, Takeuchi H, Fujieda N, Saito K, Sato E, et al. Irreversible Electroporation Versus Radiofrequency Ablation: Comparison of Systemic Immune Responses in Patients With Hepatocellular Carcinoma. J Vasc Interv Radiol (2019) 30(6):845–53. doi: 10.1016/j.jvir.2019.03.002
84. Bulvik BE, Rozenblum N, Gourevich S, Ahmed M, Andriyanov AV, Galun E, et al. Irreversible Electroporation Versus Radiofrequency Ablation: A Comparison of Local and Systemic Effects in a Small-Animal Model. Radiology (2016) 280(2):413–24. doi: 10.1148/radiol.2015151166
85. White SB, Zhang Z, Chen J, Gogineni VR, Larson AC. Early Immunologic Response of Irreversible Electroporation Versus Cryoablation in a Rodent Model of Pancreatic Cancer. J Vasc Interv Radiol (2018) 29(12):1764–9. doi: 10.1016/j.jvir.2018.07.009
86. Dai Z, Wang Z, Lei K, Liao J, Peng Z, Lin M, et al. Irreversible Electroporation Induces CD8 T Cell Immune Response Against Post-Ablation Hepatocellular Carcinoma Growth. Cancer Lett (2021) 503:1–10. doi: 10.1016/j.canlet.2021.01.001
87. Guo X, Du F, Liu Q, Guo Y, Wang Q, Huang W, et al. Immunological Effect of Irreversible Electroporation on Hepatocellular Carcinoma. BMC Cancer (2021) 21(1):443. doi: 10.1186/s12885-021-08176-x
88. Chen X, Ren Z, Yin S, Xu Y, Guo D, Xie H, et al. The Local Liver Ablation With Pulsed Electric Field Stimulate Systemic Immune Reaction Against Hepatocellular Carcinoma (HCC) With Time-Dependent Cytokine Profile. Cytokine (2017) 93:44–50. doi: 10.1016/j.cyto.2017.05.003
89. Nuccitelli R, Berridge JC, Mallon Z, Kreis M, Athos B, Nuccitelli P. Nanoelectroablation of Murine Tumors Triggers a CD8-Dependent Inhibition of Secondary Tumor Growth. PloS One (2015) 10(7):e0134364. doi: 10.1371/journal.pone.0134364
90. Partridge BR, O’Brien TJ, Lorenzo MF, Coutermarsh-Ott SL, Barry SL, Stadler K, et al. High-Frequency Irreversible Electroporation for Treatment of Primary Liver Cancer: A Proof-Of-Principle Study in Canine Hepatocellular Carcinoma. J Vasc Interv Radiol (2020) 31(3):482–91. doi: 10.1016/j.jvir.2019.10.015
91. Narayanan JSS, Ray P, Hayashi T, Whisenant TC, Vicente D, Carson DA, et al. Irreversible Electroporation Combined With Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol Res (2019) 7(10):1714–26. doi: 10.1158/2326-6066.CIR-19-0101
92. Scheffer HJ, Stam AGM, Geboers B, Vroomen LGPH, Ruarus A, de Bruijn B, et al. Irreversible Electroporation of Locally Advanced Pancreatic Cancer Transiently Alleviates Immune Suppression and Creates a Window for Antitumor T Cell Activation. Oncoimmunology (2019) 8(11):1652532. doi: 10.1080/2162402X.2019.1652532
93. Pandit H, Hong YK, Li Y, Rostas J, Pulliam Z, Li SP, et al. Evaluating the Regulatory Immunomodulation Effect of Irreversible Electroporation (IRE) in Pancreatic Adenocarcinoma. Ann Surg Oncol (2019) 26(3):800–6. doi: 10.1245/s10434-018-07144-3
94. He C, Huang X, Zhang Y, Lin X, Li S. T-Cell Activation and Immune Memory Enhancement Induced by Irreversible Electroporation in Pancreatic Cancer. Clin Transl Med (2020) 10(2):e39. doi: 10.1002/ctm2.39
95. Chai W, Xu Y, Zhang W, Wei Z, Li J, Shi J, et al. Irreversible Electroporation in the Eradication of Rabbit VX2 Cervical Tumors. BioMed Microdevices (2017) 19(4):90. doi: 10.1007/s10544-017-0231-y
96. Li X, Xu K, Li W, Qiu X, Ma B, Fan Q, et al. Immunologic Response to Tumor Ablation With Irreversible Electroporation. PloS One (2012) 7(11):e48749. doi: 10.1371/journal.pone.0048749
97. Chen DS, Mellman I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
98. Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive Correlates of Response to the Anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature (2014) 515(7528):563–7. doi: 10.1038/nature14011
99. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in Patients With Locally Advanced and Metastatic Urothelial Carcinoma Who Have Progressed Following Treatment With Platinum-Based Chemotherapy: A Single-Arm, Multicentre, Phase 2 Trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
100. Leuchte K, Staib E, Thelen M, Gödel P, Lechner A, Zentis P, et al. Microwave Ablation Enhances Tumor-Specific Immune Response in Patients With Hepatocellular Carcinoma. Cancer Immunol Immunother (2021) 70(4):893–907. doi: 10.1007/s00262-020-02734-1
101. Liu J, Chen X, Zheng S. Immune Response Triggered by the Ablation of Hepatocellular Carcinoma With Nanosecond Pulsed Electric Field. Front Med (2021) 15(2):170–7. doi: 10.1007/s11684-020-0747-z
102. Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, et al. Radiofrequency Ablation Induces Antigen-Presenting Cell Infiltration and Amplification of Weak Tumor-Induced Immunity. Radiology (2009) 251(1):58–66. doi: 10.1148/radiol.2511072175
103. Neal RE, Rossmeisl JH, Robertson JL, Arena CB, Davis EM, Singh RN, et al. Improved Local and Systemic Anti-Tumor Efficacy for Irreversible Electroporation in Immunocompetent Versus Immunodeficient Mice. PloS One (2013) 8(5):e64559. doi: 10.1371/journal.pone.0064559
104. He C, Sun S, Zhang Y, Xie F, Li S. The Role of Irreversible Electroporation in Promoting M1 Macrophage Polarization via Regulating the HMGB1-RAGE-MAPK Axis in Pancreatic Cancer. Oncoimmunology (2021) 10(1):1897295. doi: 10.1080/2162402X.2021.1897295
105. Polajzer T, Jarm T, Miklavcic D. Analysis of Damage-Associated Molecular Pattern Molecules Due to Electroporation of Cells In Vitro. Radiol Oncol (2020) 54(3):317–28. doi: 10.2478/raon-2020-0047
106. Santos PM, Butterfield LH. Dendritic Cell-Based Cancer Vaccines. J Immunol (2018) 200(2):443–49. doi: 10.4049/jimmunol.1701024
107. Garg AD, Romano E, Rufo N, Agostinis P. Immunogenic Versus Tolerogenic Phagocytosis During Anticancer Therapy: Mechanisms and Clinical Translation. Cell Death Differ (2016) 23(6):938–51. doi: 10.1038/cdd.2016.5
108. Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, et al. Irreversible Electroporation Reverses Resistance to Immune Checkpoint Blockade in Pancreatic Cancer. Nat Commun (2019) 10(1):899. doi: 10.1038/s41467-019-08782-1
109. De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T Helper Type 2 Cell Infiltrate Correlates With Cancer-Associated Fibroblast Thymic Stromal Lymphopoietin Production and Reduced Survival in Pancreatic Cancer. J Exp Med (2011) 208(3):469–78. doi: 10.1084/jem.20101876
110. Kitajima M, Lee H-C, Nakayama T, Ziegler SF. TSLP Enhances the Function of Helper Type 2 Cells. Eur J Immunol (2011) 41(7):1862–71. doi: 10.1002/eji.201041195
111. Goswami I, Coutermarsh-Ott S, Morrison RG, Allen IC, Davalos RV, Verbridge SS, et al. Irreversible Electroporation Inhibits Pro-Cancer Inflammatory Signaling in Triple Negative Breast Cancer Cells. Bioelectrochemistry (2017) 113:42–50. doi: 10.1016/j.bioelechem.2016.09.003
112. Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-Induced Lysyl Oxidase is a Critical Mediator of Bone Marrow Cell Recruitment to Form the Premetastatic Niche. Cancer Cell (2009) 15(1):35–44. doi: 10.1016/j.ccr.2008.11.012
113. Cox TR, Bird D, Baker A-M, Barker HE, Ho MWY, Lang G, et al. LOX-Mediated Collagen Crosslinking is Responsible for Fibrosis-Enhanced Metastasis. Cancer Res (2013) 73(6):1721–32. doi: 10.1158/0008-5472.CAN-12-2233
114. Sun S, Liu Y, He C, Hu W, Liu W, Huang X, et al. Combining NanoKnife With M1 Oncolytic Virus Enhances Anticancer Activity in Pancreatic Cancer. Cancer Lett (2021) 502:9–24. doi: 10.1016/j.canlet.2020.12.018
115. Qian J, Chen T, Wu Q, Zhou L, Zhou W, Wu L, et al. Blocking Exposed PD-L1 Elicited by Nanosecond Pulsed Electric Field Reverses Dysfunction of CD8 T Cells in Liver Cancer. Cancer Lett (2020) 495:1–11. doi: 10.1016/j.canlet.2020.09.015
116. Yang J, Eresen A, Shangguan J, Ma Q, Yaghmai V, Zhang Z. Irreversible Electroporation Ablation Overcomes Tumor-Associated Immunosuppression to Improve the Efficacy of DC Vaccination in a Mice Model of Pancreatic Cancer. Oncoimmunology (2021) 10(1):1875638. doi: 10.1080/2162402X.2021.1875638
117. O’Neill C, Hayat T, Hamm J, Healey M, Zheng Q, Li Y, et al. A Phase 1b Trial of Concurrent Immunotherapy and Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Adenocarcinoma. Surgery (2020) 168(4):610–6. doi: 10.1016/j.surg.2020.04.057
118. Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, et al. Percutaneous Irreversible Electroporation Combined With Allogeneic Natural Killer Cell Immunotherapy for Patients With Unresectable (Stage III/IV) Pancreatic Cancer: A Promising Treatment. J Cancer Res Clin Oncol (2017) 143(12):2607–18. doi: 10.1007/s00432-017-2513-4
119. Pan Q, Hu C, Fan Y, Wang Y, Li R, Hu X. Efficacy of Irreversible Electroporation Ablation Combined With Natural Killer Cells in Treating Locally Advanced Pancreatic Cancer. J BUON (2020) 25(3):1643–9.
120. Hou S, Wang W, Zhong Z, Ni J, Chen Y, Xu L. Preliminary Study on the Effect of Irreversible Electroporation Ablation Combined With Pd-1 Inhibitor in the Treatment of Mouse Liver Cancer. J Vasc Interv Radiol (2019) 028(005):454–8. doi: 10.3969/j.issn.1008-794X.2019.05.011
121. Yang Y, Qin Z, Du D, Wu Y, Qiu S, Mu F, et al. Safety and Short-Term Efficacy of Irreversible Electroporation and Allogenic Natural Killer Cell Immunotherapy Combination in the Treatment of Patients With Unresectable Primary Liver Cancer. Cardiovasc Intervent Radiol (2019) 42(1):48–59. doi: 10.1007/s00270-018-2069-y
122. Lasarte-Cia A, Lozano T, Cano D, Martín-Otal C, Navarro F, Gorraiz M, et al. Intratumoral STING Agonist Injection Combined With Irreversible Electroporation Delays Tumor Growth in a Model of Hepatocarcinoma. BioMed Res Int (2021) 2021:8852233. doi: 10.1155/2021/8852233
123. Burbach BJ, O’Flanagan SD, Shao Q, Young KM, Slaughter JR, Rollins MR, et al. Irreversible Electroporation Augments Checkpoint Immunotherapy in Prostate Cancer and Promotes Tumor Antigen-Specific Tissue-Resident Memory CD8+ T Cells. Nat Commun (2021) 12(1):3862. doi: 10.1038/s41467-021-24132-6
Keywords: irreversible electroporation, in-situ tumor vaccine, immune response, immunotherapy, tumor antigens, combination therapy
Citation: Zhang N, Li Z, Han X, Zhu Z, Li Z, Zhao Y, Liu Z and Lv Y (2022) Irreversible Electroporation: An Emerging Immunomodulatory Therapy on Solid Tumors. Front. Immunol. 12:811726. doi: 10.3389/fimmu.2021.811726
Received: 09 November 2021; Accepted: 13 December 2021;
Published: 07 January 2022.
Edited by:
Linlang Guo, Zhujiang Hospital, ChinaReviewed by:
Jia Yang, University of California, San Francisco, United StatesMichael Hader, University Hospital Erlangen, Germany
Copyright © 2022 Zhang, Li, Han, Zhu, Li, Zhao, Liu and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Lv, bHV5aTE2OUAxMjYuY29t
 Nana Zhang
Nana Zhang Zhuoqun Li
Zhuoqun Li Xuan Han
Xuan Han Ziyu Zhu
Ziyu Zhu Zhujun Li
Zhujun Li Yan Zhao
Yan Zhao Zhijun Liu
Zhijun Liu Yi Lv
Yi Lv